Definition and classification
ASSESSMENT AND TREATMENT OF CAD IN A STABLE STATE
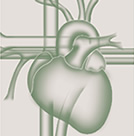
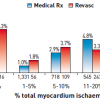
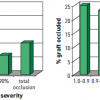
Stable ischaemic heart disease is typically characterised clinically in terms of severity of angina symptoms, physiologically with measurement of ischaemia burden, and anatomically in terms of the number of significant stenoses and coronary arteries involved. Assessment of the severity of stable CAD may be done in several ways, all of which provide complementary information with regard to prognosis and help guide evidence based therapy. Assessment of anatomical disease is most commonly performed utilising calcium scoring, computed tomographic angiography (CTA), and invasive coronary angiography. Each of these methods can provide information regarding the extent of disease. Furthermore, with each of these modalities, the burden of disease correlates with the risk of death and ischaemic events. As will be discussed later in this chapter, the assessment of the burden of disease is helpful in determining the role and type of revascularisation strategy for patients with stable CAD. Calcium scoring is a useful tool in predicting overall risk of ischaemic events and is additive to information derived from traditional risk scoring systems such as the Framingham Risk Score ( Figure 1 and Figure 2 ), ( Table 1 ). However, by itself the calcium score is not helpful in determining whether revascularisation would benefit a given patient. Coronary CTA shows anatomical details as well as the burden of CAD and has reasonable sensitivity and specificity in detecting severe CAD as well as an excellent negative predictive value in excluding significant CAD. However, it has a lower positive predictive value and does not provide any additional information related to the functional significance of coronary atherosclerotic lesions. More recently coronary CTAs have been used as imaging platforms where the functional assessment of a given coronary lesion can be precisely estimated by applying computational fluid dynamic principles. [11. Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol. 2013;61:2233-41. ] The diagnostic performance of this novel methodology which assesses non-invasively the functional significance of coronary lesions expressed as CT derived fractional flow reserve (CT-FFR) was investigated in 103 patients which underwent CTA, invasive coronary angiography and conventional FFR. [22. Koo BK, Erglis A, Doh JH et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms - Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58:1989-97. ] The study proved the feasibility of assessing non-invasively the functional significance of coronary lesions, meanwhile 1) more data are needed in this direction to prove this method as accurate alternative to invasive FFR and 2) additional FFR algorithms instead of computational fluid dynamics such as machine learning are needed to expedite the FFR calculations.
Selective invasive coronary angiography remains the gold standard in assessing the severity of CAD, with excellent reliability in estimating severe and mild disease. Catheterisation can also be used to determine the functional significance of any given coronary stenosis with measurement of FFR. Intravascular ultrasound (IVUS) can also be used at the time of coronary angiography to assess plaque morphology and vessel geometry to further guide operator’s decision regarding the approach to PCI or whether a patient may be better served with medical therapy or CABG. A recent meta-analysis involving 19,619 patients from one randomised trial and ten observational studies suggested that IVUS-guided coronary drug-eluting stent (DES) implantation was associated with a significant reduction in death, major adverse cardiovascular events (MACE) and stent thrombosis compared to angiographic guidance. Appropriately powered randomised trials are necessary to confirm the findings from this meta-analysis. [33. Zhang Y, Farooq V, Garcia-Garcia HM et al. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. 2012;8:855-65. , 44. Zhang J, Gao X, Kan J et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J Am Coll Cardiol. 2018;72:3126-3137. ]
The severity of CAD, as determined by coronary angiography, correlates well with the incidence of adverse coronary events. In the CASS study (Coronary Artery Surgery Study), patients were classified according to the number of diseased vessels with ≥70% stenosis with the 3 major epicardial coronary arteries defined as the left anterior descending (LAD), left circumflex (LCx) and right coronary artery (RCA) [55. Coronary artery surgery study (CASS): a randomized trial of coronary artery bypass surgery - Survival data. Circulation. 1983;68:939-50. ]. Further classification included left main coronary artery (LM) involvement, with ≥50% stenosis considered significant. In the CASS randomised study, those who had significant LM or 3 vessel CAD involving the LAD had significantly worse survival when treated with medical therapy compared to bypass surgery. In the CASS Registry, which included patients that were not randomised due to patient characteristics or physician or patient preference, the 12-year survival of those treated with medical therapy alone was 91% for those with mild or no angiographic disease, 74% for those with 1 vessel disease, 59% for those with 2 vessel disease, and 40% for those with 3 vessel disease. In addition, patients with >95% stenosis of the proximal LAD with 3 vessel CAD had a 59% five-year survival compared to 79% survival in those with 3 vessel disease without proximal LAD severe disease.
Clinical evaluation of CAD is based on a patient’s functional status, exercise capacity, and the severity of angina. Although there is no clear correlation between the severity of angina symptoms and the anatomical extent of CAD, anginal symptoms and functional capacity have been shown to be important predictors of mortality and the development of acute coronary syndromes. In a study with 8,908 outpatients, anginal symptoms were assessed with the Seattle Angina Questionnaire, a useful tool that involves self-reporting of symptoms by patients. At 2 years mean follow-up, patients reporting greater physical limitation due to angina had higher mortality rates compared to those who had little or no limitation: 27% higher with mild limitation (hazard ratio [HR] 1.27, 95% CI 0.98 to 1.64), 61% higher with moderate limitation (HR 1.61, 95% CI 1.27 to 2.05), and 2.5-fold higher with severe limitation (HR 2.55, 95% CI 1.97 to 3.30) (P for trend <.001) ( Figure 3 ). The initial experience with percutaneous transluminal coronary angioplasty (PTCA), done without stent placement, was largely with single-vessel CAD in patients with stable angina. In this selected group of patients, it has been clearly demonstrated that PCI can effectively relieve angina. A meta-analysis by Wijeysundera et al included 14 trials comparing medical therapy to PCI (this meta-analysis comprised 3 trials with PTCA only and the rest with stenting using bare metal stents) in 7,818 patients. [66. Wijeysundera HC, Nallamothu BK, Krumholz HM, Tu JV, Ko DT. Meta-analysis: effects of percutaneous coronary intervention versus medical therapy on angina relief. Ann Intern Med. 2010;152:370-9. ] The authors found that 73% of patients were free from angina in the PCI group and 63.9% were free from angina in the medically treated group. Although there was an overall benefit on angina relief (odds ratio, 1.69 [95% CI, 1.24 to 2.30]), the clear benefit of PCI observed in older trials (odds ratio, 3.38 [CI, 1.89 to 6.04]) was less robust in recent trials (odds ratio, 1.13 [CI, 0.76 to 1.68])
Typical assessment of severity of stable CAD
- Anginal Symptoms
- Ischaemia on stress testing and FFR measurement
- Anatomical disease by coronary angiography and IVUS
Increased adverse clinical events, including MI and death, are associated with patients presenting with severely limiting angina, significant ischaemia on stress testing or FFR measurement, and multivessel CAD
The COURAGE trial suggests that current optimal medical therapy for patients with stable CAD results in similar rates of survival and myocardial infarction compared to PCI. The trial enrolled 2,287 patients who had stable CAD by coronary angiography and objective evidence of myocardial ischaemia on stress testing or at least an 80% stenotic lesion on coronary angiography with classic angina symptoms. [77. Boden WE, O’Rourke RA, Teo KK et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503-16. ] In this trial, the PCI group consisted of 1,149 patients assigned to undergo PCI with optimal medical therapy (OMT); the OMT group included 1,138 patients who received optimal medical therapy alone (aspirin or clopidogrel, beta blocker, nitrates, amlodipine, lisinopril or losartan, and aggressive lipid control). At 4.6-year follow up, there was no significant difference in primary event rates (19.0% in the PCI group and 18.5% in the OMT group (p = 0.62). Primary events were defined as death and non-fatal myocardial infarction. As expected, those assigned to the PCI group suffered less angina in the first year and required fewer medications. However, difference in angina relief was no longer significant at 3 years. It is important to note that patients enrolled in COURAGE represent a selected subset of stable CAD patients and that approximately a third of the OMT group required revascularisation during follow-up. Since no differences in mortality were seen between the two groups, an initial strategy to optimise medical therapy for such patients with stable CAD is reasonable.
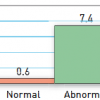
Assessment of the stable CAD patient should include an evaluation of ischaemic burden. This is often done with exercise or pharmacological stress testing with or without adjunctive imaging studies, but other modalities such as ambulatory ECG monitoring have also been tested with favourable predictive values. With all of these types of testing, the magnitude of myocardial ischaemia strongly correlates to major adverse cardiac events (MACE) including death, MI, hospitalisations for ischaemic events, and revascularisation. In a review of 14 studies involving >12,000 patients with stable symptoms of CAD who underwent single photon emission computed tomographic (SPECT) technetium-99m sestamibi imaging, Iskander and Iskandrian noted a low annual event rate (death or MI) of 0.6% in those with normal scans and a 12 fold higher rate of 7.4% in those with abnormal scans ( Figure 4 ). A greater severity of abnormality in the scans was associated with worse outcomes.
In the ACIP (Asymptomatic Cardiac Ischemia Pilot) trial, patients who were asymptomatic (67%) or mildly symptomatic (33%), but had ischaemia on ambulatory monitoring and stress testing were randomised to a revascularisation arm, an angina guided medical therapy (n=183) arm, or to a medical therapy arm with the goal of relieving ambulatory ischaemia (n=183). [88. Davies RF, Goldberg AD, Forman S et al. Asymptomatic Cardiac Ischemia Pilot (ACIP) study two-year follow-up: outcomes of patients randomized to initial strategies of medical therapy versus revascularization. Circulation. 1997;95:2037-43. ] At 2-year follow-up, those who were treated with medical therapy had a 5.5% mortality (6.6% and 4.4% for angina guided and ischaemia guided therapy respectively) compared to a 1.1% mortality for those treated with revascularisation (p<0.02).
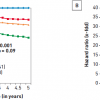
In modern day PCI with stenting, the benefits of percutaneous revascularisation have been demonstrated in patients with a moderate to large amount of ischaemia noted on stress myocardial perfusion SPECT (single photon emission computed tomography) imaging in the COURAGE trial [99. Shaw LJ, Berman DS, Maron DJ et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283-91. ]. This substudy of COURAGE assessed patients’ ischaemia with serial stress myocardial perfusion scans and showed a greater reduction of ischaemic burden in the PCI group than in the OMT group (33% versus 19% relative improvements respectively). The difference was especially marked (78% versus 52%) in patients with moderate to severe ischaemia, involving ≥10% of myocardium. Reductions in ischaemic burden correlated with significant reductions in death and myocardial infarction, especially in patients who received at least a 5% absolute reduction in ischaemic burden. The event-free survival at 5-year follow-up was directly related to the amount of residual ischaemia of the left ventricle: 100% if 0% residual ischaemia, 84.4% for 1 to 4.9%, 77.7% for 5 to 9.9%, and 60.7% for ≥10% ischaemic myocardium (p=0.001) ( Figure 5 ).
A more recent observation from the COURAGE trial suggested that the degree of ischaemia did not predict events instead the burden of anatomical disease did. A total of 621 patients enrolled in the COURAGE trial with baseline quantitative nuclear single-photon emission computed tomography (SPECT) and quantitative coronary angiography were studied. Several multiple regression models were constructed to determine independent predictors of the endpoint of death, myocardial infarction (MI) (excluding periprocedural MI) and non-ST-segment elevation acute coronary syndromes (NSTE-ACS). Ischaemic burden during stress SPECT, anatomical burden derived from angiography, left ventricular ejection fraction, and assignment to either optimal medical therapy (OMT) + percutaneous coronary intervention (PCI) or OMT alone were analysed. In nonadjusted and adjusted regression models, anatomical burden and left ventricular ejection fraction were consistent predictors of death, MI, and NSTE-ACS, whereas ischaemic burden and treatment assignment were not. [1010. Mancini GBJ, Hartigan PM, Shaw LJ et al. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv. 2014;7:195-201. ]
Following these important observations two crucial questions arise: 1. Should angiograms be restricted to patients with higher risk scores from non-invasive evaluations of ischemia, or should be considered by angiography? 2. What will be the future role of CCTA in these patients? Although this sub-study of the COURAGE trial does not answer all of these questions it certainly raises the possibility that form (anatomy) may sometimes trump function (physiology) in predicting cardiovascular events; meanwhile both form and function are important components.
Revascularisation also reduced ischaemia to a much greater extent than intensive medical therapy in a substudy of the BARI 2-D trial which assessed ischaemia via stress myocardial SPECT imaging. The BARI 2-D study randomised 2,368 patients with stable CAD and diabetes to a strategy of early revascularisation with optimal medical therapy (OMT) versus delayed or no revascularisation with OMT . [1111. Group BDS, Frye RL, August P et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503-15. ] In the substudy involving 1,505 patients, at one-year following enrolment, 59% of patients who received revascularisation had no ischaemia on stress myocardial SPECT imaging compared to 49% of patients receiving OMT alone. Those who received revascularisation had less ischaemia (3% vs. 9% of left ventricular myocardium). Patients who had an abnormality of 10% or greater on SPECT imaging had an 80% increased relative risk of cardiac death or MI.
In a small (n=407), non-randomised prospective study by Legalery et al, wherein ischaemia was assessed by fractional flow reserve (FFR), patients whose FFR was <0.80 who were treated with medical therapy had greater one-year adverse event rates (death, acute coronary syndrome, and revascularisation procedures) of 21% (7 of 34) compared to those who received PCI (6%, 6 of 99) [1212. Legalery P, Schiele F, Seronde MF et al. One-year outcome of patients submitted to routine fractional flow reserve assessment to determine the need for angioplasty. Eur Heart J. 2005;26:2623-9. ].
Noteworthy also is the observation that revascularisation appears to provide no benefit in reducing the likelyhood of death or MI when CAD is not associated with ischaemia. In the study by Legalery, those patients with FFR ≥ 0.80 who received PCI had an 11% (4 of 37) adverse event rate at 1 year compared to 7% (14 of 237) in those patients with FFR ≥ 0.80 who received medical therapy.
Another important substudy of the BARI 2D trial investigated retrospectively the angiographic burden of disease and how this affected the 5-year clinical outcomes of the patients randomized to either CABG or PCI stratum. More specific an angiographic risk score was developed from variables assessed at randomisation; independent prognostic factors were myocardial jeopardy index, total number of coronary lesions, prior coronary revascularization, and left ventricular ejection fraction. The 5-year risk of death/myocardial infarction/stroke was 36.8% for intensive medical therapy compared with 24.8% for prompt coronary revascularization among the 381 coronary artery bypass graft surgery-selected patients in the highest angiographic risk tertile (P=0.005); this treatment effect was amplified in patients with both high angiographic and high Framingham risk (47.3% intensive medical therapy versus 27.1% prompt coronary revascularisation; P=0.010; hazard ratio=2.10; P=0.009). In brief this sub study showed that among patients with diabetes mellitus and stable ischemic heart disease a strategy of prompt CABG surgery significantly reduces the rate of death/myocardial infarction/stroke in those with extensive anatomical disease burden or impaired left ventricular function. [1313. Brooks MM, Chaitman BR, Nesto RW et al. Clinical and angiographic risk stratification and differential impact on treatment outcomes in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation. 2012;126:2115-24. ]
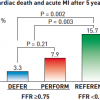
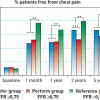
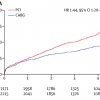
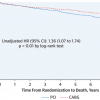
The DEFER trial studied 325 patients with intermediate stenoses in patients with single vessel CAD who were referred for PCI ( Figure 6 and Figure 7 ). Of these patients, 181 had FFR ≥ 0.75, and were subsequently randomised to PCI versus medical therapy. The remaining 144 had FFR < 0.75 and were treated with PCI and followed as the reference group. At 5-year follow-up, those patients with FFR ≥ 0.75 who received PCI had a greater combined event rate (cardiac death and acute MI) compared to those who received medical therapy (7.9% vs. 3.3%, p=0.21), although the difference was statistically not significant. Of note, the reference group who had FFR < 0.75 and subsequently underwent PCI had the greatest combined event rate of 15.7%, which reflects the repeatedly observed higher risk in patients with greater ischaemic burden. The patients in the reference group did have greater freedom from angina at all time points up to 5 years compared to the two groups with FFR ≥ 0.75.
In the FAME study, 1,005 patients with multivessel CAD were randomly assigned to one of two treatment groups. [1414. De Bruyne B, Pijls NH, Kalesan B et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991-1001. , 1515. Pijls NH, Fearon WF, Tonino PA et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177-84. ] One group received PCI based on anatomical severity alone whereas in the other group lesions were treated with PCI only if FFR was less than 0.80. At one-year follow-up, patients assigned to the FFR based therapy received fewer stents (1.9% vs. 2.7%, p<0.001) and experienced less MACE (death, nonfatal MI, and repeat revascularisation) (13.2% vs. 18.3%, p=0.02) and length of hospitalisation compared to those assigned to routine PCI. Combined death and MI rates were lower in the FFR-guided PCI group as well (7.3% vs. 11.1%, p=0.04). The follow-up period has been up to 3 years at present and longer follow-up will be required in determining whether FFR based revascularisation in multivessel CAD will continue to be effective in reducing ischaemic events due to progression of disease in non-revascularised areas.
As noted in the BARI (Bypass Angioplasty Revascularisation Investigation) trial, at five-year follow-up, 42% of patients treated with PCI had progression of disease, with over two thirds of new lesions occurring at sites not initially treated with PTCA. [1616. Alderman EL, Kip KE, Whitlow PL et al. Native coronary disease progression exceeds failed revascularization as cause of angina after five years in the Bypass Angioplasty Revascularization Investigation (BARI). J Am Coll Cardiol. 2004;44:766-74. ] Interestingly, at 2-year follow-up of the FAME study, only 10 of 513 functionally non-significant lesions (1.9%) that were not treated by PCI resulted in a repeat revascularisation and only 1 of 513 (0.2%) resulted in late myocardial infarction. The lower event rates may reflect the improvements in concurrent medical therapy: as an example, less than 15% of patients in the BARI trial were treated with lipid lowering medications. At the very least, the data from the DEFER and FAME trials strongly suggest that there is no clear clinical benefit to revascularisation in the absence of ischaemia, and that revascularisation solely based on anatomy leads to greater cost and inconvenience to the patient, if not worse clinical outcomes.
In addition to anatomy, clinical symptoms, and ischaemic burden, several other factors are associated with prognosis in patients with stable CAD. Left ventricular function was a marker of survival in CASS and also identified the patients who benefited most from CABG. Those with 3 vessel CAD and LV dysfunction had a survival benefit with CABG compared to medical therapy (the difference being almost statistically significant), but those with 3 vessel CAD and preserved LV function had similar survival rates whether they underwent CABG or were treated with medical therapy. Other tests that correlate with outcome include biomarkers such as high sensitivity C-reactive protein (HS-CRP), a marker of vascular inflammation even in a clinically stable state, and brain natriuretic peptide (BNP), which is a marker of ventricular and atrial strain even without overt heart failure. Assessments of systemic atherosclerotic disease such as ankle brachial index measurement and the burdens of peripheral vascular and cerebrovascular disease are also helpful in predicting survival and ischaemic events.
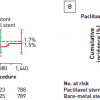
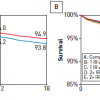
In the FAME 2 trial (Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease) patients with functionally significant stenoses, as determined by measurement of FFR, PCI plus the best available medical therapy was hypothesised to be superior to the best available medical therapy alone. Patients in whom at least one stenosis was functionally significant (FFR, ≤0.80) were randomly assigned to FFR-guided PCI plus the best available medical therapy (PCI group) or the best available medical therapy alone (medical-therapy group). Patients in whom all stenoses had an FFR of more than 0.80 were entered into a registry and received the best available medical therapy. The primary end point was a composite of death, myocardial infarction, or urgent revascularisation. The trial’s recruitment was halted prematurely after enrollment of 1220 patients (888 who underwent randomisation and 332 enrolled in the registry) because of a significant between-group difference in the percentage of patients who had a primary end-point event: 4.3% in the PCI group and 12.7% in the medical-therapy group (hazard ratio with PCI, 0.32; 95% confidence interval [CI], 0.19 to 0.53; P<0.001). The difference was driven by a lower rate of urgent revascularisation in the PCI group than in the medical-therapy group (1.6% vs. 11.1%; hazard ratio, 0.13; 95% CI, 0.06 to 0.30; P<0.001); in particular, in the PCI group, fewer urgent revascularisations were triggered by a myocardial infarction or evidence of ischemia on electrocardiography (hazard ratio, 0.13; 95% CI, 0.04 to 0.43; P<0.001). [1414. De Bruyne B, Pijls NH, Kalesan B et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991-1001. ] The 5-year clinical follow-up of patients enrolled in FAME-2 was recently conducted showing that the primary endpoint, a composite of death, myocardial infarction, or urgent revascularization was lower in the PCI group than in the medical therapy group (13,9% vs. 27,0%; hazard ratio, 0,46; 95% confidence interval [CI], 0,34 to 0,63; p<0,001). [1717. Xaplanteris P, Fournier S, Pijls NHJ et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N Engl J Med. 2018;379:250-259. ] ( Figure 8 )
Similar to the 1-year prior observations, the difference was primarily driven by urgent revascularizations, which occurred in 6,3% of the patients in the PCI group as compared with 21,1% of those in the medical therapy group (hazard ratio, 0,27; 95% CI, 0,18 to 0,41).
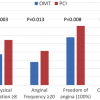
Chronic total occlusions (CTOs) remain a topic of active debate in interventional cardiology as to whether revascularization provides incremental benefit compared to optimal medical therapy (OMT). Although the 3-year follow-up of DECISION CTO trial showed no difference in the combined primary endpoint of all-cause death, myocardial infarction, stroke, and repeat revascularization in the intention-to-treat population for patients assigned to OMT alone compared to the interventional group (19,6% vs. 20,6%; p = 0,008 for noninferiority) results from the Euro-CTO trial were more encouraging. In this trial three hundred and ninety-six patients were enrolled in a 2:1 randomized fashion to compare the treatment by PCI with OMT. The primary endpoint was the change in health status assessed by the seattle angina questionnaire between baseline and 12 months follow-up. Indeed, complete freedom from angina was more frequent with PCI in 71.6% than OMT in 57.8% (p = 0.008). ( Figure 9 ) [1818. Werner GS, Martin-Yuste V, Hildick-Smith D et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39:2484-2493. ] Although Euro-CTO did not include hard clinical endpoints compared to the DECISION CTO, this trial satisfied the ultimate patient’s endpoint which is the angina relief.
Effectiveness of revascularisation therapy in stable CAD
Elective intervention in stable CAD has proven benefits.
In symptomatic patients with obstructive CAD, both PCI and CABG have been shown to relieve angina significantly, and improve exercise capacity and quality of life. Revascularisation also decreases the magnitude of ischaemia. Studies that support the benefit of ischaemia-guided interventional therapy include SWISSI, ACIP, the COURAGE nuclear substudy, and the BARI 2-D CABG arm. Likewise, data from the DEFER and FAME studies suggest that revascularisation without objective ischaemia does not improve outcomes such as anginal relief or recurrent ischaemic events.
Proven Benefits of PCI in patients with stable CAD
- Relieve anginal symptoms
- Reduce ischaemic burden
- PCI has not been shown to reduce MI or death in patients with stable CAD. Optimal medical therapy (OMT) has consistently been shown to reduce death, MI, and stroke rates. However, significant reduction in ischaemic burden have been associated with reduced myocardial infarctions and improved survival, and PCI in addition to OMT achieves a greater reduction in ischaemic burden compared to OMT alone. PCI has also been associated with improved regional left ventricular function in patients with hibernating myocardium
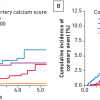
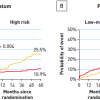
Revascularisation can also improve outcome when CAD is more advanced. In the BARI 2-D CABG stratum, wherein pateints had extensive coronary disease, coronary bypass surgery in addition to medical therapy was superior to medical therapy alone in reducing death, MI, and stroke. In the group of patients randomised to CABG versus OMT, at 5 years, patients in the OMT arm had a 30.5% rate of death, MI, and stroke compared to 22.4% of those who underwent prompt CABG (p<0.01). The BARI 2-D investigators developed an “angiographic risk score” based on several independent predictors of MACE: myocardial jeopardy index (MJI, the percentage of left ventricular myocardium jeopardized by ≥50% stenoses), total number of coronary lesions, reduced left ventricular ejection fraction, and prior revascularisation. As shown ( Figure 10 ), those patients with a high angiographic risk score received significant benefit if revascularised early with CABG with reduced MACE rates, but those with low to intermediate risk scores did not receive a significant benefit with CABG up to 5 years, although the curves appear to be slightly diverging.
EFFECTIVENESS OF PCI COMPARED TO MEDICAL THERAPY
In the 1980s, PTCA was compared with medical therapy and uniformly showed improvement in anginal symptoms and exercise time but failed to show any improvement of mortality in patients with stable CAD [1919. Henderson RA, Pocock SJ, Clayton TC et al. Seven-year outcome in the RITA-2 trial: coronary angioplasty versus medical therapy. J Am Coll Cardiol. 2003;42:1161-70. , 2020. LaRosa JC, Grundy SM, Waters DD et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425-35. ]. In comparison, coronary artery bypass graft surgery also showed improvements in anginal control and exercise time, and in the CASS study demonstrated an improvement in mortality compared to medical therapy in the subset of patients with left main or multivessel coronary disease and left ventricular systolic dysfunction. Additionally, in Yusuf’s analysis of 7 trials comparing CABG to medical therapy in the 1970s and 1980s, patients with proximal LAD disease, severe angina, and abnormal exercise tests also appeared to gain significant survival advantage with CABG. [2121. Yusuf S, Zucker D, Peduzzi P et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563-70. ] As described earlier, the COURAGE trial demonstrated no benefit of routine PCI in addition to OMT over OMT alone in reducing the incidences of death and MI during 5 years of follow-up. One of the limitations and criticisms of the COURAGE trial, however, was that patients with more advanced CAD were not uniformly enrolled as only about one-third had proximal LAD disease.
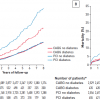
Certain observational studies have implied that revascularisation can improve outcomes in stable CAD patients. At Cedars-Sinai medical centre, 10,627 consecutive patients with no history of MI or revascularisation and who had undergone myocardial perfusion imaging (MPI) were followed for 2 years. In those with ischaemia involving more than 10% of the left ventricle, revascularisation was associated with improved survival compared to medical therapy alone ( Figure 11 ). Conversely, in those patients with minimal to moderate ischaemia, there was no benefit from revascularisation. The type of medical therapy given to the patients in this study, however, was not documented, and there are limitations with regard to the selection biases with any observational study.
Nevertheless, these studies (along with others) do generate the hypothesis that treatment of ischaemia with revascularisation coupled with OMT would improve survival and reduce ischaemic events compared to OMT alone. Based on this hypothesis, the ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial has been designed as an international randomised controlled trial which plans to enroll patients with stable CAD and moderate to severe ischaemia on stress testing. It will assess the effectiveness of two initial management strategies: cardiac catheterisation with revascularisation if feasible (with either PCI or CABG) plus OMT versus no catheterisation and OMT alone. The results from this study should improve our understanding of the role of revascularisation in those patients with stable CAD and documented ischaemia.
One of the shortcomings of comparing OMT to percutaneous or surgical revascularisation is the difference in mechanisms of action of each therapy. Whereas PCI treats the most severe lesions that are haemodynamically significant and improves ischaemic burden immediately through improved myocardial oxygen supply, the mechanism of OMT is a slower one that involves plaque stabilisation, prevention of ulceration and rupture, and sometimes regression, along with a reduction of myocardial oxygen demand and ischaemic preconditioning. The distinct mechanisms of OMT and PCI are additive and might actually be synergistic in improving ischaemic burden and reducing ischaemic events. An example of these mechanisms in play is nicely demonstrated in the AVERT (Atorvastatin versus Revascularisation Treatment) study. Study patients (n=341) had stable CAD with at least 50% stenosis and a serum low-density lipoprotein (LDL) cholesterol level of at least 115 mg per decilitre (3.0 mmol per litre). Most patients were asymptomatic or had Canadian Cardiovascular Society (CCS) class I or II angina and were able to complete at least four minutes of a treadmill test using the Bruce protocol or a bicycle exercise test at 20 W per minute without significant ischaemic ECG changes. Patients with left main CAD, triple-vessel disease, unstable angina or MI within the previous two weeks, and a left ventricular ejection fraction of less than 40% were excluded. These patients were randomised to treatment with percutaneous revascularisation (PTCA in 70% and stenting in 30%) with standard medical therapy or to standard medical therapy plus high dose atorvastatin 80 mg daily as the lipid lowering agent of choice. Patients receiving PCI had better angina control (54% had improvement compared to 41%, p=0.009) compared to the atorvastatin group, consistent with the mechanism of immediate improvement in myocardial oxygen supply achieved with PCI. However, patients assigned to the atorvastatin group had significantly less ischaemic events at 18 months (13% vs. 21%, p=0.048) compared to the PCI group. Ischaemic events were defined as death from cardiac causes, resuscitation after cardiac arrest, non-fatal MI, cerebrovascular accident, CABG, PCI, and worsening angina with objective evidence resulting in hospitalisation. LDL levels were 119 vs. 77 mg/decilitre in patients treated with PCI vs. high dose atorvastatin. The reduction in ischaemic events with high dose atorvastatin therapy is consistent with its mechanism of plaque stabilisation that is achieved with LDL reduction.
In the TNT (Treating to New Targets) trial, 10,001 patients with stable CAD were randomly assigned to treatment with 10 mg or 80 mg of atorvastatin. The primary endpoint was the occurrence of a first major cardiovascular event, which included death from CHD, non-fatal non-procedure-related MI and resuscitation after cardiac arrest or fatal or non-fatal stroke. The mean LDL cholesterol levels were 77 mg per decilitre (2.0 mmol per litre) during treatment with 80 mg of atorvastatin and 101 mg per decilitre (2.6 mmol per litre) during treatment with 10 mg of atorvastatin. A primary event occurred in 8.7% of patients receiving 80 mg atorvastatin, and 10.9% of those receiving 10 mg atorvastatin, representing a 22% relative risk reduction (P<0.001). Thus, AVERT and TNT demonstrated the importance of lipid lowering and optimal medical therapy in the treatment of patients with stable CAD. Therefore, the only reasonable comparisons in the AVERT trial are those involving the difference in ischaemic endpoints in the patients who received aggressive versus conservative lipid lowering. In this particular trial, one cannot even compare angina status fairly, since those patients not assigned to PCI did not necessarily get OMT for angina relief. In summary, it is important to understand the strengths and limitations of our interventional and medical therapies and make comparisons of efficacy based on specific mechanisms of action rather than simply grouping treatments into interventional and non-invasive arms. Individual patients would benefit most when all pathophysiological mechanisms of CAD, including plaque stabilisation, treatment of ischaemia, and treatment of symptoms, are adequately addressed and treated.
A recent randomized sham controlled trial, enrolled 230 patients with ischemic symptoms under OMT to either PCI (n=105) or placebo (n=95). The primary endpoint of ORBITA was the difference in exercise time increment between the groups. Interestingly, PCI did not increase exercise tolerance compared to the placebo group. Following these observations, debates arose questioning the feasibility of PCI in patients with stable ischemic heart disease in the era of advanced pharmacotherapy. Indeed, the ORBITA results were striking; meanwhile, during the follow-up of those patients in the placebo group there was a significant cross over in the PCI group. Small sample randomized trials such as the ORBITA trial provide limited evidence to draw any definite conclusions and we should be aware also of the many limitations of this trial such as 1) the control group does not accurately mimic practice as completely revascularized single vessel patients are not usually treated w/ CCBs and β-blockers and 2) equivalent relief of angina does not mean that selection of lifetime aggressive anti-ischemic therapy is superior to relief of coronary obstruction. [2222. Al-Lamee R, Thompson D, Dehbi HM et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. 2018;391:31-40. ]
COMPARISONS OF REVASCULARISATION STRATEGIES: PCI VS. CABG
Comparisons of PCI versus CABG in patients with stable multivessel CAD
- Outcomes with PCI are comparable to CABG for low complexity CAD
- Outcomes with CABG are superior to PCI for intermediate to high complexity CAD
- Patients treated with PCI with Taxus drug eluting stents in the SYNTAX trial had similar 3 year clinical outcomes as those who underwent CABG if the anatomical complexity of disease was low (by SYNTAX score). However, those with intermediate to high SYNTAX scores had higher 3 year MACCE rates with PCI compared to CABG
Historical comparisons: PTCA vs. CABG
Several randomised controlled trials in the 1980s have compared PTCA with CABG as revascularisation techniques for patients with multivessel CAD.
The Emory Angioplasty vs. Surgery Trial (EAST) found no difference in the composite endpoint of death, Q-wave MI, and detection of a large ischaemic defect on thallium scans at three years in-patients treated with CABG compared with PTCA. However, the incidence of repeat bypass surgery (1%) or angioplasty (13%) was significantly lower in patients undergoing CABG than those in the PTCA group (22% and 41%, respectively). Angina was more frequent in the PTCA group (20% vs. 12% for CABG), suggesting that more complete revascularisation with CABG reduces ischaemia burden more effectively. Similar trends were found in other trials, including the CABRI and GABI
The BARI (Bypass Angioplasty Revascularisation Investigation) investigators randomised patients with multivessel CAD to CABG (n=914) or PTCA (n=915), and at five years found no significant difference in survival free of Q-wave MI (80.4% versus 78.7%), but those who underwent CABG had only an 8% repeat-revascularisation rate compared to 54% of those who underwent PTCA. Importantly, diabetics who were treated with insulin or oral hypoglycaemic medications had a significantly better five-year survival with CABG than with PTCA (80.6% versus 65.5%). For all patients, ten-year survival was 71.0% for PTCA and 73.5% for CABG (p = 0.18), but the PTCA group had higher revascularisation rates than the CABG group (76.8% versus 20.3%, p <0.001). Angina control was similar in the two groups. In non-diabetic patients, survival rates were nearly identical (PTCA 77.0% versus CABG 77.3%, p = 0.59), but in diabetics, the CABG group had greater survival (PTCA 45.5% versus CABG 57.8%, p = 0.025). In the Emory Database of 2,639 diabetic patients treated with CABG or PTCA, insulin-requiring diabetics had a significantly improved five-year and ten-year survival with CABG compared to PTCA (75%, 47% versus 68%, 36%, corrected for baseline differences).
These early studies demonstrated the superiority of PTCA over CABG with regard to early survival with shorter hospitalisation duration and less frequency of in-hospital death and Q-wave myocardial infarction. However, the long-term limitations of angioplasty, namely restenosis requiring repeat revascularisation, were exposed. In addition to these shortcomings, it was clear that in selected patients with diabetes, CABG offers a survival benefit. It should also be noted that these early studies enrolled patients with less complex coronary anatomy than in later trials such as the SYNTAX trial. When patients with more complex anatomy and higher burden of disease have been compared in the past decade, there is no early survival advantage or reduction of myocardial infarctions with PCI compared to CABG, but there is a lower risk of stroke in patients treated with PCI. These data will be discussed in more detail later in this chapter.
Effectiveness of coronary stenting compared to CABG
The use of intracoronary stents improved the durability and safety of PTCA. Coronary stenting achieves a larger initial gain in lumen after balloon angioplasty, and although associated with greater neointima formation and late loss, decreases overall angiographic and clinical restenosis rates via a net gain of lumen. In the 1990s at least 4 trials compared bypass surgery to PCI with bare-metal stents (BMS).
The Arterial Revascularisation Therapies Study (ARTS), ERACI II, and Stents or Surgery (SoS) trials examined the hypothesis that a reduction in restenosis would improve long-term outcomes with PCI and narrow the gap between PCI and CABG. The MASS II (the Medicine, Angioplasty, or Surgery Study) randomised ~600 patients with multivessel CAD and preserved LV function to optimal medical therapy, PCI, or CABG.
ARTS randomised 1,205 patients to CABG (n = 605) or PCI with stenting (n = 600). There was no difference in five-year mortality between the stent and CABG groups (8.0% versus 7.6%, respectively; p = 0.83). Among 208 diabetic patients, mortality was 13.4% in the stent group and 8.3% in the CABG group (p = 0.27). Overall freedom from death, stroke, or myocardial infarction was not significantly different between groups (18.2% in the stent group versus 14.9% in the surgical group; p = 0.14). However, the repeat revascularisation rate was significantly higher in the stent group (30.3%) than in the CABG group (8.8%; p <0.001). The composite event-free survival rate was 58.3% in the stent group and 78.2% in the CABG group (p <0.0001). The rate of major adverse cardiovascular and cerebral events (MACCE) was higher in the stent group, driven primarily by the increased need for repeat revascularisation.
The ERACI II trial randomised 450 patients (225 in each group) to receive CABG or PCI with stents. Five-year survival and freedom from non-fatal MI were similar between the CABG and stent groups (92.8% versus 88.4% and 97.3% versus 94%, respectively; p = 0.16). Freedom from repeat revascularisation was significantly lower with PCI than with CABG (71.5% versus 92.4%; p = 0.0002). Freedom from MACCE was also significantly lower with PCI than with CABG (65.3% versus 76.4%; p = 0.013). At five years, similar numbers of patients in the CABG and PCI arms were asymptomatic or had class I angina.
The SoS trial randomised 988 (n = 488 PCI, n = 500 CABG) patients at 53 centres between1996 and 1999. There was no difference in mortality between the PCI group and the CABG group according to baseline angina grade, the severity of coronary disease, or diabetic status. However, mortality at a median follow-up of six years was 10.9% in the PCI group compared with 6.8% in the CABG group (hazard ratio 1.66, 95% confidence interval 1.08 to 2.55, p = 0.022). This difference in survival compared to the other stents versus CABG trials may be explained by several factors. First, the 5-year mortality rate for CABG in the SoS trial was 4.3%, which was the lowest of these 4 trials: 5-year mortality rates were 7.6%, 11.5%, and 7.9% in the ARTS, ERACI II, and MASS II trials respectively. Also, in the SoS trial, there was less complete revascularisation in the PCI arm compared to the ARTS trial (54% vs. 72% respectively) due to different criteria for enrollment. Whereas the ARTS trial selected patients who could achieve “the same extent of revascularisation” with PCI or CABG, those in the SoS trial were selected if they had multivessel CAD in whom revascularisation was considered “clinically indicated and appropriate by either strategy”. These factors may explain some of the differences in survival outcomes in the SoS trial compared to the other trials.
The MASS II study randomised 611 patients with stable angina, multivessel CAD, and preserved LV function to medical therapy, PCI, or CABG. Approximately 60% of patients in each arm had 3 vessel CAD (the remaining had 2 vessel CAD), and 90% had severe disease in the proximal LAD. Complete revascularisation (either bypassing all intended vessels or performing PCI in all major epicardial vessels with ≥70% stenosis) was achieved in 74% of patients who underwent CABG and 41% of patients undergoing PCI. At five-year follow-up, the composite primary endpoint (total mortality, Q-wave myocardial infarction, or refractory angina requiring revascularisation) occurred in 21.2% of patients who underwent CABG compared with 32.7% treated with PCI and 36% receiving medical therapy alone (p = 0.0026). There were no significant differences in overall mortality among the three groups. However, 9.4% of patients in the medical therapy group and 11.2% of PCI patients underwent repeat revascularisation compared with 3.9% of CABG patients (p = 0.021). Non-fatal myocardial infarction occurred in 15.3%, 11.2%, and 8.3% of patients in the medical therapy, PCI, and CABG groups, respectively (p <0.001).
Daemen et al performed a pooled analysis of the above four trials. [2323. Daemen J, Boersma E, Flather M et al. Long-term safety and efficacy of percutaneous coronary intervention with stenting and coronary artery bypass surgery for multivessel coronary artery disease: a meta-analysis with 5-year patient-level data from the ARTS, ERACI-II, MASS-II, and SoS trials. Circulation. 2008;118:1146-54. ] At five years, the cumulative incidence of death, MI, and stroke was similar in patients randomised to PCI with stenting versus CABG (16.7% versus 16.9%, respectively). Repeat revascularisation was more frequent after PCI (29.0% versus 7.9% after CABG). MACCE rates were significantly higher in the PCI group than in the CABG group (39.2% versus 23.0%, respectively), driven by greater repeat-revascularisation rates in the patients treated with PCI ( Figure 12 ).
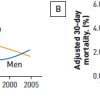
Hlatky et al performed an analysis of the individual patient data from the above four stent vs. CABG trials and six trials comparing PTCA with CABG. [2424. Hlatky MA, Boothroyd DB, Bravata DM et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009;373:1190-7. ] Their analysis found that although overall mortality is similar for PCI and CABG, diabetic patients and patients older than 65 years demonstrated a significant mortality benefit with CABG ( Figure 13 ).
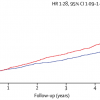
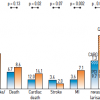
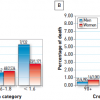
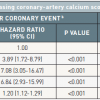
The most recent evidence in revascularization selection was recently reported by Head et al. who analyzed 11 randomized trials with over 11,000 patients assigned by a heart-team to either PCI (n=5753) or CABG (n=5765). 5 year all-cause mortality was significantly different between the interventions in patients with multivessel disease ( Figure 14 ) (11,5% after PCI vs 8,9% after CABG; HR 1,28, 95% CI 1,09–1,49; p=0.0019), including those with diabetes (15,5% vs 10,0%; 1·48, 1,19–1,84; p=0.0004). ( Figure 15 )
Interestingly, patients with left main disease and those without diabetes had comparable 5-year clinical outcomes after PCI or CABG. Indeed, PCI technology has improved significantly over the years enabling operators to treat even complex lesions that lead to excellent long-term clinical outcomes. [2525. Head SJ, Milojevic M, Daemen J et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet. 2018;391:939-948. ] Despite this substantial improvement in techniques and devices, PCI remains problematic in the clinical subsets of multivessel disease and diabetes leading to inferior long-term clinical outcomes when PCI is the selected treatment strategy. This was recently demonstrated in the BEST trial (Trial of Everolimus-Eluting Stents or Bypass Surgery for Coronary Disease) where even advanced technologic iterations of contemporary DES such as current generation everolimus-eluting stents did not show any incremental benefit in revascularization with PCI in patients with multivessel disease compared to the cohort treated with CABG. [2626. Park SJ, Ahn JM, Kim YH et al. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med. 2015;372:1204-12. ] Furthermore, in the same clinical subset the 8-year follow-up of FREEDOM trial (Strategies for Multivessel Revascularization in Patients with Diabetes) elaborated on the all-cause mortality of patients with multivessel disease and diabetes treated with either PCI or CABG. FREEDOM, initially enrolled 1,900 patients and the primary outcome occurred more frequently in the PCI group (p=0.005), with 5-year rates of 26.6% in the PCI group and 18.7% in the CABG group. The benefit of CABG was driven by differences in the rates of both myocardial infarction (p<0.001) and death from any cause (p=0.049). [2727. Farkouh ME, Domanski M, Sleeper LA et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375-84. ] The 8-year follow-up showed that CABG remained superior to PCI with a drug-eluting stent, with those undergoing PCI at a 36% increased risk of all-cause mortality when compared with surgical patients. [2828. Farkouh ME, Domanski M, Dangas GD et al. Long-term Survival following Multivessel Revascularization in Patients with Diabetes (FREEDOM Follow-On Study). J Am Coll Cardiol. 2018. ]
Drug-eluting stents vs. CABG
The limitations of restenosis caused by neointimal hyperplasia in bare metal stents have been significantly reduced by the development of drug-eluting stents (DES). The first-generation DES, including the Cypher® (sirolimus-eluting stent, Cordis, Johnson & Johnson, Warren, NJ, USA) and Taxus® (paclitaxel-eluting stent, Boston Scientific, Natick, MA, USA), have been well studied in randomised trials. Although there are some concerns regarding delayed endothelialisation of DES compared to bare metal stents and the potential for increased risk of late stent thrombosis, several meta-analyses of randomised controlled trials as well as large registries comparing these two DES have demonstrated reassuring results with regard to their safety and efficacy, particularly with dual antiplatelet therapy with aspirin and clopidogrel for at least a year [2929. Stone GW, Moses JW, Ellis SG et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998-1008. , 3030. Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020-9. ] ( Figure 16 and Figure 17 and Figure 18 ).
The ARTS-II registry enrolled patients with similar characteristics as those in the ARTS trial, and these patients underwent multivessel PCI using Cypher® sirolimus-eluting stents. At three years, among non-diabetic patients, the incidence of MACCE (the primary composite of death, cerebrovascular accident (CVA), MI, and repeat revascularisation) was significantly lower in ARTS-II than in the ARTS PCI arm (16%.3 versus 30.9%) and similar to the ARTS CABG arm (15.8%). The ARTS-II patients had significantly lower risk for death, CVA, and MI than either the ARTS PCI (7.8% versus 11.5%) or ARTS CABG patients (7.8% versus 10.3%). Among patients with diabetes, the incidence of MACCE in ARTS-II was 27.7% at 3 years, which is better than that of PCI (47.3%), but worse than CABG (17.7%) in ARTS. Conversely, the incidence of death, CVA, and myocardial infarction was significantly lower in ARTS-II than in ARTS PCI (9.4% versus 18.8%) and was similar to that of ARTS CABG (13.5%). The differences in MACCE were again driven by revascularisation rates, which were 21.4% versus 7.3% (PCI in ARTS-II versus CABG in ARTS). Improved revascularisation rates were noted in the ARTS-II PCI registry compared to the ARTS PCI arm, which had a revascularisation rate of 38.4% in patients with diabetes.
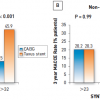
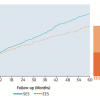
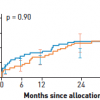
The SYNTAX trial randomised 1,800 patients with three-vessel or left main coronary artery disease (LM CAD) to undergo CABG or PCI using Taxus Express® paclitaxel-eluting stents (Boston Scientific, Natick, MA, USA). The primary endpoint was a MACCE (i.e., death from any cause, stroke, myocardial infarction, or repeat revascularisation) during the 12-month period after randomization. MACCE occurred more frequently in the PCI group (17.8% for PCI versus 12.4% for CABG; p = 0.002), in large part because of an increased rate of repeat revascularisation (13.5% versus 5.9%; p <0.001). At 12 months, the rates of death and MI were similar between the two groups; stroke was significantly more likely to occur with CABG (2.2%, vs. 0.6% with PCI; P = 0.003). Three-year follow-up data showed MACCE rates of 28% vs. 20.2% for the PCI group vs. the CABG group respectively, the difference predominantly driven by repeat revascularisation (19.7% vs. 10.7%, p<0.001) [3131. Kappetein AP, Feldman TE, Mack MJ et al. Comparison of coronary bypass surgery with drug-eluting stenting for the treatment of left main and/or three-vessel disease: 3-year follow-up of the SYNTAX trial. Eur Heart J. 2011;32:2125-34. ] ( Figure 19 ). Of note, patients who were treated with PCI had a higher MI rate (7.1% vs. 3.6%, p=0.002), but no significant differences in composite safety outcome (death, MI, and stroke rate of 14% vs. 12%, p=0.21, PCI vs CABG). Stroke rate, although significantly lower in the PCI arm at 1 year, was no longer statistically significant by 3 years (2.0% vs. 3.4%, p=0.07, PCI vs. CABG). This study also risk stratified patients based on the anatomic complexity of their coronary artery disease with a “SYNTAX score” given to each patient, with higher scores reflecting more severe and complex disease burden. [3232. Sianos G, Morel MA, Kappetein AP et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219-27. ] Components of the SYNTAX score (www.syntaxscore.com) include dominance (right vs. left coronary artery), number of lesions, location of lesions with proximal vessel location carrying greater weight, left main involvement, involvement of 3 vessel disease, presence of total occlusion and its angiographic characteristics, vessel tortuosity, involvement of bifurcation, and presence of calcification and thrombus. Figure 20 A and Figure 20 B illustrate examples of 2 cases and their calculated SYNTAX scores. One-year event rates were similar between the two treatment groups for patients with low SYNTAX scores (0 to 22) or intermediate SYNTAX scores (23 to 32). Among patients with high SYNTAX scores (≥33, indicating the most complex disease), those in the PCI group had significantly higher event rates at 12 months than those in the CABG group. Similarly at 3 years, the groups in the lowest tertile for SYNTAX score had nearly identical MACCE rates (22.7% for PCI, 22.5% for CABG, p=0.98)), whereas CABG had more favourable outcomes in patients with higher SYNTAX scores: patients with intermediate SYNTAX scores had 27.4 vs. 18.9% MACCE rates (p=0.02), and patients with high SYNTAX scores had 34.1 vs. 19.5% (p<0.001) MACCE rates for PCI and CABG respectively at 3 years. Of note, the 3 year data now shows significantly higher MACCE rates for patients with intermediate SYNTAX scores, whereas at 1 year, these differences were not statistically significant ( Figure 21 ).
The SYNTAX study included 452 patients with diabetes, including 182 who required insulin. In patients without diabetes, there was a significant survival advantage for CABG if the SYNTAX score was ≥ 33 (2.2% vs. 6.1% mortality, CABG vs. PCI respectively, p=0.04) at one-year follow-up. This difference favouring CABG was more pronounced in patients with diabetes (4.1% vs. 13.5%). Those with low and intermediate SYNTAX scores had similar survival rates with CABG or PCI regardless of diabetic status at one year. At 3-year follow-up, MACCE rates in patients with diabetes were 37% in the PCI group and 22.9% in the CABG group (p=.002), the difference being mostly driven by repeat revascularisation rates (28% for PCI and 12.9% for CABG, p=0.001). The combined 3 year-death, MI, and stroke rate was not significantly different in the overall study in the diabetic subgroup (16.3% vs. 14%, PCI vs. CABG, p=0.53). At 3 years, patients with diabetes had significantly worse MACCE rates with PCI if they had intermediate or high SYNTAX scores. MACCE rates in diabetic patients for PCI vs. CABG were: 29.8 vs. 30.5% (p=0.94), 36.2 vs. 21% (p=0.04), and 45.9 vs. 18.5% (p<0.001) respectively for the low, intermediate, and high SYNTAX score categories ( Figure 22 ).
COMPARATIVE OUTCOMES AMONG DRUG-ELUTING STENTS
Several “second generation” drug-eluting stents (DES) have been brought to the European market since introduction of the first generation Cypher® and Taxus® stents. Among these are the Xience® V (Abbott Vascular, Redwood City, CA, USA) or Promus™ (Boston Scientific, Natick, MA,USA) everolimus eluting stent, the Endeavor® zotarolimus-eluting stent, the Endeavor Resolute® zotarolimus-eluting stent (both Medtronic, Minneapolis, MN, USA), and the Biolimus™ stent (Biosensors International, Singapore). Details of the various DES, together with the results of major clinical studies, are discussed in Chapter 3.3. In general, head to head comparisons of Cypher® and Taxus® “first generation DES” to “second generation” stents have shown similar outcomes, although in the SPIRIT IV trial, patients treated with Xience® V DES had reduced primary endpoint of target lesion failure (4.2% vs. 6.8%, p=0.001) at 1 year compared to those treated with Taxus® DES. There was also a reduced incidence of stent thrombosis (0.17% vs. 0.85%, p=0.004) and MI (1.9% vs. 3.1%, p=0.02) in the Xience® V group. Target lesion failure was improved in patients treated with Xience® V DES, except in patients with diabetes, where the one-year outcome was the same (6.4% vs. 6.9% for Xience® V vs. Taxus® respectively). Similarly, in the COMPARE trial (1,800 patients, single centre) patients who received Xience® V stents had a lower incidence of target lesion failure at 1 year compared to those who received Taxus-Liberte® DES (6% vs. 9%). Other comparative trials involving DES show equivalence between the Cypher® and Xience® V (SORT OUT 4), Xience® V and Resolute® (Resolute All Comers), Cypher® and Endeavor® (SORT OUT 3, ENDEAVOR 3, ZEST), with equivalent MACE rates, although there are some trends favouring these stents compared to Taxus® stents. The SORT OUT IV trial at 5 years indicated that the overall rate of composite MACE, was lower at 5 years in the EES group than in the SES group. The difference was not apparent in the first year, but emerged thereafter. Definite stent thrombosis occurred less frequently in the EES group than the SES group (0.4% vs 2.0%; P = .0004). This endpoint also did not differ in the first year, but beyond year 1, EES was associated with lower rates of very late definite stent thrombosis than SES (0.2% vs 1.4%; P = .003). [3333. Jensen LO, Thayssen P, Christiansen EH et al. Safety and Efficacy of Everolimus- Versus Sirolimus-Eluting Stents: 5-Year Results From SORT OUT IV. J Am Coll Cardiol. 2016;67:751-62. ] ( Figure 23 )
In general, no major differences exist in outcomes among these newer generation DES that would suggest meaningful differences in outcomes between the PCI and CABG arms in modern trials such as SYNTAX. [3434. Task Force on Myocardial Revascularization of the European Society of C, the European Association for Cardio-Thoracic S, European Association for Percutaneous Cardiovascular I et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501-55. ]
Patient and disease subsets
Diabetes mellitus
Diabetes mellitus is a challenging problem for patients with CAD and is discussed in detail in Chapter 3.19. This section will provide an overview of relevant data with regard to treatment of patients with diabetes and stable CAD. Diabetes is a condition that accelerates atherosclerosis and atherothrombosis, and treatments to control the disease and associated risk factors, although successful, do not adequately reduce the increased risk of death or adverse events. The rate of death among patients with diabetes without overt CAD is as unfavourable as patients without diabetes and a history of MI. For patients with diabetes who have had an MI, their odds of having recurrent events and death is over twice that of non-diabetics. Diabetics also have higher restenosis rates after PCI, whether performed with PTCA, bare metal stents, or drug-eluting stents, and have a higher graft occlusion rate after CABG. Yet, because of the higher burden of disease in diabetics, revascularisation plays an important role in improving their survival and quality of life.
In the setting of stable ischaemic heart disease/stable CAD, as discussed earlier, the BARI 2-D trial demonstrated significant reduction of MACE, and particularly myocardial infarctions, in patients treated with CABG and intensive medical therapy compared to intensive medical therapy alone. The difference in outcomes was seen in patients with more advanced disease as defined by the “angiographic score”. Diabetic patients with a low or intermediate angiographic score did not benefit from early CABG.
There was no benefit to prompt revascularisation compared to intensive medical therapy in the PCI arm of the study. However, this trial did not compare effectiveness of CABG versus PCI. Rather, it compared the effectiveness of prompt revascularisation in diabetic patients to intensive medical therapy with provisional revascularisation. In the CABG stratum, patients had greater burden of disease and were expected to have higher event rates compared to the PCI stratum. Patients in the medical therapy arm for CABG had a five year-mortality of 16.4%, whereas those in the medical therapy arm for PCI had a five-year mortality of 10.2%. Nevertheless, those patients who had high angiographic scores and underwent PCI did not experience a reduction of MACE at 5 years compared to patients with high angiographic scores treated with medical therapy.
From these data, it is reasonable to conclude that in diabetic patients with advanced coronary disease, early revascularisation with CABG is beneficial, whereas in those with more moderate disease, medical therapy is as effective as revascularisation, although it is still inadequate in reducing adverse events in this challenging disease. CABG seems effective in reducing ischaemic events as patients who receive it have some protection against progression of proximal native disease. This is particularly the case when a patient receives a LIMA graft to the LAD, as proximal plaque rupture and occlusion of the LAD leads many fatal myocardial infarctions. On the other hand, PCI does not appear to confer adequate protection against ischaemic events in the diabetic population with stable CAD.
The case for choosing CABG in patients with diabetes who have severe disease is further corroborated by the SYNTAX trial data, where MACCE rates with CABG were no worse (and arguably better) in patients with higher SYNTAX scores compared to those with lower SYNTAX scores, and the net benefit compared to PCI increased dramatically with increasing SYNTAX scores ( Figure 22 ).
The repeat revascularisation advantages of CABG compared to PCI in patients with diabetes are evident in all of the randomised controlled trials comparing PCI to CABG. Hlatky et al have shown in a meta-analysis of 10 trials that 5-year mortality is significantly worse in diabetics with multivessel CAD treated with PCI compared to CABG (20% vs. 12.3%) ( Figure 13 ). In the SYNTAX trial patients with diabetes with high SYNTAX scores had significantly higher mortality at 1 year if they received PCI with Taxus® DES compared to CABG (13.5% vs. 4.1%, p=0.04). In comparison, patients with high SYNTAX scores without diabetes had half the mortality at 1 year whether they underwent PCI (6.1%) or CABG (2.2%). Patients with diabetes with a low SYNTAX score fared equally well whether they underwent CABG or PCI. However, diabetics comprised relatively small proportions of these trials.
The CARDia trial randomised 510 patients with diabetes with multivessel or complex single-vessel CAD to PCI or CABG. The trial planned on enrolling 600 patients, but was prematurely stopped and was underpowered for the primary composite outcome. There was no difference at one year in the primary endpoint (composite of death, myocardial infarction, or stroke), which was met in 10.5% for CABG and 13.0% for PCI (p = 0.39). [3535. Kapur A, Hall RJ, Malik IS et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients: 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol. 2010;55:432-40. ] Although mortality was the same at 3.2% for each arm, those who underwent PCI had more repeat revascularisations with a combined primary outcome plus repeat revascularisation rate of 19.3% compared to 11.3% for those who received CABG (p=0.02). 69% of patients who were treated with PCI received drug- eluting stents, with no difference in the primary endpoint when this subgroup was compared to those who underwent CABG. The FREEDOM trial randomised 1,900 patients with diabetes and multivessel CAD to PCI with DES or CABG. The patients were followed for a minimum of 2 years (median among survivors, 3.8 years). All patients were prescribed currently recommended medical therapies for the control of low-density lipoprotein cholesterol, systolic blood pressure, and glycated haemoglobin. The primary outcome measure was a composite of death from any cause, non-fatal myocardial infarction, or nonfatal stroke. The primary outcome occurred more frequently in the PCI group (P=0.005), with 5-year rates of 26.6% in the PCI group and 18.7% in the CABG group. The benefit of CABG was driven by differences in rates of both myocardial infarction (P<0.001) and death from any cause (P=0.049). Stroke was more frequent in the CABG group, with 5-year rates of 2.4% in the PCI group and 5.2% in the CABG group (P=0.03). Although the authors concluded that patients with diabetes and advanced coronary artery disease, benefited from CABG compared to PCI in that it significantly reduced rates of death and myocardial infarction, they were criticised by the SYNTAX group investigators who previously showed that in patients with a low SYNTAX score (i.e. <22) there was a minimal difference in long-term clinical outcomes between cardiac surgery and PCI. [2727. Farkouh ME, Domanski M, Sleeper LA et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375-84. ] The 8-year follow-up of FREEDOM consistently demonstrated that patients with multivessel disease and diabetes have superior clinical outcomes with significantly lower all-cause mortality when the initial strategy is CABG rather than PCI. [2828. Farkouh ME, Domanski M, Dangas GD et al. Long-term Survival following Multivessel Revascularization in Patients with Diabetes (FREEDOM Follow-On Study). J Am Coll Cardiol. 2018. , 3636. Farkouh ME, Domanski M, Dangas GD et al. Long-Term Survival Following Multivessel Revascularization in Patients With Diabetes: The FREEDOM Follow-On Study. J Am Coll Cardiol. 2019;73:629-638. ]
Similarly, the 5-year follow-up of SYNTAX showed that in the clinical subset of diabetes MACCE rates were significantly higher in those in the PCI group than in those in the CABG group (29,0% in the CABG group vs 46,5% in the PCI group; p=0,0002), with numbers increased in both groups compared with the overall population. Furthermore, patients with multivessel disease showed 50% higher MACCE rates in the PCI group compared to the CABG group and patients classified according to SYNTAX complexity demonstrated comparable clinical outcomes with both revascularization strategies when SYNTAX scores were below 32. [3737. Mohr FW, Morice MC, Kappetein AP et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629-38. ]
Left main CAD
Patients with LM CAD have significantly worse survival than those with less severe CAD, including three-vessel CAD. Patients with LM CAD who undergo CABG have improved survival compared to those undergoing medical therapy. However, these same patients have higher mortality than those who undergo CABG for two-vessel or three-vessel CAD. The debate regarding the best revascularisation treatment for LM CAD has evolved: PCI, once regarded as clearly inferior to CABG in this setting, may now be considered on a par with CABG in some clinical and anatomic situations. Park et al published the largest single-centre experience of unprotected LM CAD stenting: 1,102 patients with unprotected LM CAD treated with PCI/stenting and 1,138 patients who underwent CABG in South Korea between January 2000 and June 2006. [3838. Seung KB, Park DW, Kim YH et al. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781-92. ] Three years after revascularisation, in the overall matched cohort, there was no significant difference between the stenting and CABG groups in the risk of death (~8% for both) or MACE (death, Q-wave myocardial infarction, or stroke) (~9%). The rates of target-vessel revascularisation were significantly higher in the PCI group than in the group that underwent CABG (12.6 % PCI versus 3.6% CABG; p <0.001).
The French left main Taxus registry is a prospective multicentre real-world registry of LM PCI utilising Taxus® DES with a protocol-defined technique for LM stenting: 92% of distal LM PCI was performed using a provisional T stent technique with kissing balloon PTCA performed in 97% of these patients. [3939. Vaquerizo B, Lefevre T, Darremont O et al. Unprotected left main stenting in the real world: two-year outcomes of the French left main taxus registry. Circulation. 2009;119:2349-56. ] 291 patients with three-vessel CAD and LM CAD were treated with Taxus® stents and followed for a mean of two years. The incidence of revascularisation was only 8.7% and the incidence of LM CAD stent thrombosis was 0.7% with 99% follow-up and no routine repeat angiography. These data suggest that with appropriate PCI techniques, LM CAD stenting can be performed safely with low rates of stent thrombosis.
The SYNTAX randomised trial included 705 patients with LM CAD, who comprised nearly 40% of each group. 3-year all-cause mortality was 7.3% vs. 8.4% for PCI vs. CABG (p=0.64). MACCE rates were similar between the PCI and CABG groups in this subset of patients (13.7% and 15.8%, respectively; p = 0.44) at 1 year; by 3 years, MACCE rates were 26.8% and 22.3% respectively (p=0.2). The rate of repeat revascularisation was significantly higher in the PCI group (11.8%, versus 6.5% in the CABG group at 1 year; p = 0.02; 20% vs. 11.7% at 3 years, p=0.004), but there was a significantly higher rate of stroke in the CABG subgroup (2.7%, versus 0.3% in the corresponding PCI subgroup at 1 year; p = 0.01; 4.0% vs. 1.2% respectively at 3 years; p=0.02). Rates of MI were identical at 1 year (4.2%), but by 3 years there was a suggestion of more MI’s in the PCI group, although not statistically significant (6.9% vs. 4.1%, p=0.14). Again, the burden of disease predicts which revascularisation strategy is optimal, with patients with low or intermediate SYNTAX scores doing at least as well with PCI as CABG, but those with high SYNTAX scores doing better with CABG ( Figure 25 ). The 2010 ESC/EACTS guidelines reflect these data, with LM PCI receiving either a IIa or IIb indication unless the SYNTAX score is ≥33, in which case it receives a class III indication in patients who are eligible for CABG ( Table 8 ). It should be noted that there were a total of 1,200 patients with LM disease in the combined SYNTAX randomised trial and registry. The randomised trial enrolled nearly 60% of these patients as they were found suitable for revascularisation with either PCI or CABG. The remaining 40% of patients were candidates for only CABG as part of the registry. Taken these exclusions into account, and considering the patients who have diabetes and high SYNTAX scores, approximately 2/3 of the patients with LM disease enrolled in SYNTAX are most appropriately treated with CABG, and 1/3 of them may be appropriately treated with PCI.
The 5 year results of the SYNTAX trial were recently reported which were in line with the previous observations of 3-years. CABG should remain the standard of care for patients with complex lesions (high or intermediate SYNTAX scores). For patients with less complex disease (low SYNTAX scores) or left main coronary disease (low or intermediate SYNTAX scores), PCI is an acceptable alternative. All patients with complex multivessel coronary artery disease should be reviewed and discussed by both a cardiac surgeon and interventional cardiologist to reach consensus on optimum treatment. [3737. Mohr FW, Morice MC, Kappetein AP et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629-38. ]
The novel results of EXCEL (Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease) trial provided robust evidence in favor of PCI as treatment strategy for left main with moderate associated anatomic complexity. [4040. Stone GW, Sabik JF, Serruys PW et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N Engl J Med. 2016;375:2223-2235. ] EXCEL, randomized 1,905 eligible patients with left main disease of low or intermediate anatomical complexity to either PCI with contemporary cobalt–chromium everolimus-eluting stents (PCI group, 948 patients) or CABG (CABG group, 957 patients). Anatomic complexity was defined based on the SYNTAX score of 32 or lower. The primary end point was the rate of a composite of death from any cause, stroke, or MI at 3 years, reaching 15.4% in the PCI group and for 14.7%, in the CABG group. (p=0.02) demonstrating non-inferiority. For the secondary composite endpoint of death, stroke, or MI at 30 days, PCI was superior to CABG: 4.9 % vs. 7.9%, (p=0,008). NOBLE (Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis: a prospective, randomised, open-label, non-inferiority trial) was another trial that randomized 1201 patients with LM disease to PCI (n=598) or CABG (n=603) and demonstrated discordant results compared to EXCEL. [4141. Makikallio T, Holm NR, Lindsay M et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016;388:2743-2752. ] The 5 year estimates of MACCE were 28% for PCI and 18% for CABG, HR 1,51 (95% CI 1,13–2,00), exceeding the limit for non-inferiority, and CABG was significantly better than PCI (p=0,0044). Comparing PCI with CABG, 5 year estimates were 11% versus 9% (1,08, 0,67–1,74, p=0,84) for all-cause mortality, 6% versus 2% (2,87, 1,40–5,89, p=0,0040) for non-procedural myocardial infarction, 15% versus 10% (1,50, 1,04–2,17, p=0,0304) for any revascularisation, and 5% versus 2% (2,20, 0,91–5,36, p=0,08) for stroke. Taking the results of both trials one can see that PCI favors early clinical outcomes (from EXCEL) while longer term, CABG has incremental benefit as revascularization strategy. (In the EXCEL trial, by the time you get out over 3 years, death is beginning to split in favor of surgery). Despite these contradicting results, it has become clear that PCI in LM disease is not anymore a forbidden territory but over time with advanced technologies, optimized techniques and operators’ competency the procedure becomes available in patients with mild and moderate associated anatomic complexity. Current ESC guidelines recommend PCI as primary treatment strategy in patients with LM disease and low SYNTAX score (<22) with Class I B indication while ACC/AHA guidelines recommend IIaB indication. In moderate SYNTAX Scores of >23 and <33 both ESC and ACC/AHA recommendations give a II class indication while in high SYNTAX scores of >33 CABG is the most appropriate treatment strategy. [4242. Neumann FJ, Sousa-Uva M, Ahlsson A et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165. ]
PCI of LM lesions is discussed in greater detail in Chapter on 'Left main coronary artery disease'.
Chronic total coronary occlusions (CTO’s) and saphenous vein graft (SVG) disease
CTO’s and SVG disease pose challenging problems for patients, and these two anatomical subsets will be discussed in detail in Chapter 3.13 and Chapter 3.14.
Women
Outcomes in women have traditionally been reported as significantly worse with PCI compared to their male counterparts. Numerous registry data have consistently shown that women present at an older age and with more comorbidities including diabetes mellitus and renal insufficiency. In the 1980s and early 1990s, complications including death and bleeding have been consistently reported as higher in women than in men. However, over the past decade, outcomes have become comparable when coexisting factors such as the presence of diabetes, age, body surface area (BSA), and renal function are taken into account ( Figure 26 et Figure 27 ) [4343. Singh M, Rihal CS, Gersh BJ et al. Mortality differences between men and women after percutaneous coronary interventions: A 25-year, single-center experience. J Am Coll Cardiol. 2008;51:2313-20. , 4444. Duvernoy CS, Smith DE, Manohar P et al. Gender differences in adverse outcomes after contemporary percutaneous coronary intervention: an analysis from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) percutaneous coronary intervention registry. Am Heart J. 2010;159:677-683 e1. ] Women who underwent PCI or CABG also had similar one-year outcomes as men in the SYNTAX trial in spite of being older and with greater frequency of diabetes and other comorbidities. Changes in PCI technique and adjunctive pharmacology have made significant contributions in decreasing complications in women. The use of smaller size catheters (5 or 6 Fr rather than 8 Fr), weight based anticoagulation therapy compared to empiric uniform dosing, improvement in deliverability of balloons and stents, and experience of operators have all contributed in decreasing complication rates in women as well as men. Whereas it is encouraging that PCI has become safer for women and that the gender gap for death and other complications has closed, specific precautions in performing PCI in women involve consideration of greater comorbidities. Women (and men) with greater bleeding risk may be better suited for a radial approach, using smaller catheters, or using bivalirudin for anticoagulation. Those with renal insufficiency would benefit from contrast sparing techniques including staged interventions, use of IVUS with less angiography, use of biplane cine-angiography, and adequate hydration. With optimal planning for these patients with greater comorbidities, there is no reason to withhold PCI in women based on gender alone.
The elderly
Elderly patients present an ongoing and increasing challenge to health care systems around the world. They typically have greater comorbid conditions such as renal insufficiency and heart failure, decreased cardiovascular reserve, a greater activation of a prothrombotic state with a concomitant increase in bleeding tendency. Their coronary anatomy is typically more challenging for performing PCI due to greater calcification of the coronary arteries as well as atherosclerosis of the aorta and peripheral vasculature. They also have a greater burden of disease compared to younger patients, which may be one of the reasons why a greater benefit for CABG compared to PCI was seen in elderly patients with multivessel CAD in the meta-analysis by Hlatky et al. In general, elderly patients have significantly higher death rate and MACE rates compared to younger patients after PCI ( Figure 28 ). Mortality is particularly high in the presence of shock, acute MI, renal failure, and LV dysfunction. Although clinical trials specifically assessing the elderly with stable CAD have been limited, studies such as TACTICS-TIMI 18 have demonstrated that the elderly derive a greater benefit from PCI in acute coronary syndromes compared to younger population, albeit with greater complication rates with PCI. [4545. Bach RG, Cannon CP, Weintraub WS et al. The effect of routine, early invasive management on outcome for elderly patients with non-ST-segment elevation acute coronary syndromes. Ann Intern Med. 2004;141:186-95. ] As with women, improvements in technical factors and adjunct pharmacology have led to significant improvements in PCI outcomes in the elderly. In the National Cardiovascular Network Registry involving over 7,000 octogenarians, there was a significant decrease of death/MI/stroke between 1994 and 1997 (OR of 0.61 for death/myocardial infarction/stroke in 1997 vs. 1994; 95%CI 0.45 to 0.85). As data from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the American College of Cardiology/American Heart Association Guidelines) registry illustrate, even in the past decade, opportunities for improvement in outcomes are being identified. ( Figure 29 ) shows how anticoagulant therapy is often overdosed particularly in the elderly (as it is in women, those with chronic kidney disease, and heart failure), leading to greater bleeding complications, mortality, and length of stay. As elderly patients comprise a greater proportion of patients with CAD, PCI will continue to be a good treatment option in this population, albeit with the increased risks that need to be optimally addressed.
Heart failure
CAD is the most common aetiology for the development of heart failure. In patients with CAD and impaired left ventricular function (LV ejection fraction <40%), a meta-analysis by Baker et al showed a 30% to 50% improvement in 3-year survival with CABG compared to medical therapy, with a 5% to 30% operative mortality rate. [4646. Baker DW, Jones R, Hodges J, Massie BM, Konstam MA, Rose EA. Management of heart failure; III- The role of revascularization in the treatment of patients with moderate or severe left ventricular systolic dysfunction. JAMA. 1994;272:1528-34. ] This data is from trials from 1966 to 1983, when modern medical therapy including ACE inhibitors and specific beta-blockers were not standard methods of care. Another meta-analysis from trials in the 1990s demonstrated an 80% reduction in annual mortality in patients with CAD, left ventricular dysfunction (mean LVEF 32%), and viable myocardium who underwent revascularisation compared to medical therapy (3.2% vs. 16% annual mortality, p<0.0001) . In the Canadian APPROACH registry, 2,538 patients with heart failure and CAD were stratified on whether they received revascularisation. ( Figure 30 ) shows a significant survival benefit with revascularisation with either CABG or PCI compared to medical therapy without revascularisation. With regard to revascularisation strategy for patients with heart failure, there is more data regarding the benefit of CABG, but no direct comparisons of the two revascularisation strategies have been made in randomised controlled trials. Accordingly, the ESC/EACTS guidelines give CABG a class I indication for patients with angina and chronic heart failure and LVEF <35%, and PCI receives a IIb recommendation if coronary anatomy is suitable in the presence of viable myocardium ( Table 2 and Table 3 ). As with any other set of patients, overall burden of disease, comorbid conditions, and procedure/operative mortality all need to be considered in making the decision for patients with heart failure, particularly because the risk of complications is higher in these patients with both revascularisation strategies.
Chronic kidney disease
Patients with chronic kidney disease (CKD) have decreased survival and increased cardiovascular morbidity than those with normal renal function. They have more coexisting conditions including hypertension, diabetes, heart failure, and advanced age, leading to greater complication rates with revascularisation or medical therapies. Treatment of CAD in patients with CKD is discussed in detail in Chapter 3.20.