Summary
Cardiac angiography and coronary/vascular interventions utilising intravascular contrast agents (CA) are being widely performed in a growing number of elderly patients with multiple comorbidities. In spite of improvements in their chemical structure, CA still possess kidney toxicity and represent one of the main causes of contrast-induced nephropathy (CIN) and hospital-acquired renal failure. These iatrogenic clinical complications are associated with increased in-hospital and long-term morbidity and mortality. Development of CIN prevention strategies is ongoing, but efforts have been hampered by an incomplete understanding of CIN pathophysiology. The most popular theories include a combination of decreased renal medullary blood flow, resulting in medullary ischaemia, free radical formation, and a direct toxic effect on tubular cells. The definition of CIN includes absolute (>0.5mg/dL/ >44micromol/l) or relative (>25%) increase in serum creatinine at 48-72 hours after exposure to a CA compared to baseline serum creatinine values, when alternative explanations for renal impairment have been excluded. Although the risk of renal function impairment associated with radiological procedures is low (0.6-2.3%) in the general population, it may be very high (up to 50%) in some subsets, especially in patients with major risk factors such as advanced chronic kidney disease (CKD) and diabetes mellitus. Because no effective treatment exists for CIN, prevention remains the key strategy. The use of the smallest possible dose of low-osmolar or iso-osmolar CA, volume expansion, stopping nephrotoxic drugs and avoiding repeat contrast injections within 72 hours remain the simplest and most effective approaches to reduce the risk of CIN. N-acetylcysteine, particularly when associated with adequate hydration, may be a useful drug for CIN prevention in patients with renal impairment. A possible dose-dependent protective effect has been suggested by more recent studies which included patients undergoing coronary interventional procedures requiring large contrast volume. The purpose of this chapter is to review the contemporary literature regarding CIN and to provide a comprehensive and evidence-based analysis of trials on prevention strategies.
Introduction
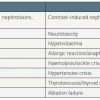
In recent years, the use of contrast agents (CA) has increased steadily in routine medical practice. In the last two decades, the number of computed tomography studies has increased by 800%, while an increase of 390% in cardiac catheterisation has been reported [11. Katzberg RW, Haller C. Contrast-induced nephrotoxicity: clinical landscape. Kidney Int Suppl. 2006;100:S3–S7. ]. A number of CA are currently available and these differ in chemical structure as well as in key properties, such as available iodine concentrations, osmolality and viscosity ( Table 1 ). Despite their indisputable benefits in terms of diagnosis and therapy of cardiovascular diseases, CA can cause adverse events that range in severity from mild to severe and even life-threatening ( Table 2 ). One of the most important and well-known complications of CA administration is contrast-induced nephropathy (CIN). With the growing complexity of diagnostic and interventional procedures, which require large doses of CA and are performed in an ever-expanding number of elderly patients with a high prevalence of chronic kidney disease (CKD) and other comorbidities, CIN is becoming an increasingly important issue in cardiovascular medicine from an epidemiological, clinical and prognostic perspective. Indeed, CIN is the third most common cause of hospital-acquired renal failure [22. Gupta R, Binbaum Y, Uretsky BF. The renal patient with coronary artery disease. Current concepts and dilemmas. J Am Coll Cardiol. 2004;44:1343-135. , 33. Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930-936. ], increasing both short-term [44. Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality: a cohort analysis. JAMA. 1996;275:1489-94.
This is a large cohort analytic study in patients undergoing radio-contrast procedures showing that subjects who develop contrast-induced renal failure have a significantly higher risk of mortality and severe non-renal complications as compared to a matched group without renal failure., 55. Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR Jr. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259-64.
A retrospective analysis of the Mayo Clinic PCI registry including 7,586 patients showing that patients with baseline elevation of serum creatinine are at high risk of acute renal failure after PCI and that acute renal failure is highly correlated with in-hospital and long-term mortality., 66. Gruberg L, Mintz GS, Mehran R, Dangas G, Lansky AJ, Kent KM, Pichard AD, Satler LF, and Leon MB. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542-1548. ] and long-term [55. Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR Jr. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259-64.
A retrospective analysis of the Mayo Clinic PCI registry including 7,586 patients showing that patients with baseline elevation of serum creatinine are at high risk of acute renal failure after PCI and that acute renal failure is highly correlated with in-hospital and long-term mortality., 66. Gruberg L, Mintz GS, Mehran R, Dangas G, Lansky AJ, Kent KM, Pichard AD, Satler LF, and Leon MB. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542-1548. , 77. McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368-375. ] risk for adverse events, including the need for renal replacement therapy, myocardial infarction, congestive heart failure, stroke and death.
Types of contrast agents approved for intravascular use
The initial generation of iodinated CA was used primarily for gastrointestinal and genitourinary studies. Early iodinated CA were poorly soluble in water and not well tolerated. Beginning in the 1950s, ionic CA based on a tri-iodinated benzene ring structure with substituted side chains at positions 3 and 5, iodine atoms at positions 2, 4 and 6, and a cation at position 1 were introduced. Thus, for every 3 iodine atoms, 2 particles were present in solution (i.e., a ratio of 3:2). In these so-called high-osmolar CA (HOCA), the negatively charged iodine moiety was balanced in solution with positively charged anions, leading to solutions that were high in osmolality and viscosity and dissociated in solution. At normally used concentrations, the osmolality of HOCA is 5-6 times higher than that of human plasma. While HOCA were better tolerated than earlier CA for urographic examinations, when applied in patients undergoing angiography they produced unpleasant heat sensations and undesirable effects on cardiac electrophysiology. A new generation of non-ionic CA was introduced in the 1980s and 1990s, the so-called low-osmolar CA (LOCA). These agents, substantially better tolerated than HOCA, are the clinical standard for use today and are of two types ( Table 1 ): non-ionic monomers and ionic dimers. In non-ionic monomers, the tri-iodinated benzene ring is made water-soluble by the addition of hydrophilic hydroxyl groups to organic side chains placed at the 1, 3, and 5 positions. Lacking a carboxyl group, non-ionic monomers do not ionise in solution. Thus, for every 3 iodine atoms, only 1 particle is present in solution (i.e., a ratio of 3:1). At a given iodine concentration, non-ionic monomers have approximately one half the osmolality of ionic monomers in solution. At normally used concentrations, their osmolality is 290-860 mOsm/kg, i.e., 1-3 times that of human plasma. Ionic dimers are formed by joining 2 ionic monomers and eliminating 1 carboxyl group. These agents contain 6 iodine atoms for every 2 particles in solution (i.e., a ratio of 6:2). The only commercially available ionic dimer is ioxaglate (Hexabrix®, Mallinckrodt, Inc., Saint Louis, MO, USA). At an iodine concentration of 320 mgI/mL, Hexabrix® has an osmolality which is two times that of human plasma. Because of its high viscosity, Hexabrix® is not manufactured at higher concentrations. Iso-osmolar (or “isotonic”) CA (IOCM) consist of 2 joined non-ionic monomers (“non-ionic dimers”). These substances contain 6 iodine atoms for every 1 particle in solution (i.e., ratio of 6:1). For a given iodine concentration, they have the lowest osmolality of all the contrast agents. At approximately 60% concentration by weight, they are iso-osmolar with plasma. Their viscosity is also very high, so that they are not manufactured at concentrations higher than 320 mgI/mL. Two IOCM are available on the European marketplace, iotrolan (Isovist®, Bayer Schering Pharma AG, Berlin, Germany) and iodixanol (Visipaque®, GE Healthcare Ltd, Little Chalfont, Buckinghamshire, UK), though the only IOCM approved for intravascular use is iodixanol.
Contrast-induced nephropathy
DEFINITION
Although there is no universally agreed upon definition, CIN is usually defined as an otherwise unexplained acute decline in renal function, characterised by an absolute rise of at least 0.5 mg/dL (44 μmol/L) in serum creatinine (SCr) or by a relative increase of at least 25% over the baseline value [88. Barrett BJ, Parfrey PS. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379-386. ]. In the majority of patients, this rise occurs within the first 24 hours, peaking 3-4 days after CA administration, and is associated with a reduction in creatinine clearance (CrCl) [77. McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368-375. ]. Based on this definition, the overall incidence of CIN in the general population is estimated to be lower than 3%, while it can rise up to 50% or more in patients with multiple risk factors. However, the reported frequencies probably underestimate the magnitude of the problem, because SCr is not measured routinely following CA exposure. Moreover, SCr is an imperfect marker of renal function and SCr measurements are an insensitive method to monitor it as a more than 50% reduction in glomerular filtration rate (GFR) may occur before any SCr increase is observed. More recently, a lower threshold of absolute SCr increase (≥0.3 mg/dl) has been proposed to define acute kidney injury (AKI), both in patients with cardiovascular and non-cardiovascular diseases [165165. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012; 2:1-138. ].
PATHOPHYSIOLOGY
CIN is a well-known cause of acute renal failure; however, despite more than 30 years of research, underlying mechanisms are incompletely understood. There is increasing evidence that a combination of direct toxic effects on tubular epithelial cells and renal ischaemia play a pathogenetic role [99. Persson PB, Tepel M. Contrast medium-induced nephropathy: the pathophysiology. Kidney Int. 2006;69 (Suppl 100):S8-S10. , 1010. . Persson PB. Pathophysiology of contrast-agent-induced nephropathy.
In “Contrast-induced nephropathy”] ( Figure 1 ). Direct cytotoxic effects of CA on the proximal convoluted tubular cells and the inner cortex of the kidney have been demonstrated. This injury can be reproduced in vitro by incubating cells of the proximal tubule with CA [1111. Romano G, Briguori C, Quintavalle C, Zanca C, Rivera NV, Colombo A, Condorelli G. Contrast agents and renal cell apoptosis. Eur Heart J. 2008; 29:2569–2576. ]. Interestingly, both LOCA and IOCA have been shown to produce dose-dependent and time-dependent renal cell apoptosis [1111. Romano G, Briguori C, Quintavalle C, Zanca C, Rivera NV, Colombo A, Condorelli G. Contrast agents and renal cell apoptosis. Eur Heart J. 2008; 29:2569–2576. ]. Epithelial cell vacuolisation, interstitial inflammation and cellular necrosis have been observed following CA exposure. Studies in animals suggest that oxidant-mediated injury, due to enhanced production of oxygen-free radicals and lipid peroxidation of biological membranes may also be implicated. Concerning ischaemic injury, studies have shown that immediate vasoconstriction and decreased renal blood flow after CA administration are not uniform. Indeed, CA appear to exert regional effects within the kidney, with an increase in blood flow to the renal cortex and simultaneous flow reduction to the renal medulla. The deeper portion of the kidney outer medulla is particularly vulnerable to ischaemic injury. This area is maintained at low oxygen tension, with pO2 levels often as low as 20 mmHg, whereas its active sodium transport is associated with high metabolic activity and oxygen requirements. Two possible mechanisms by which medullary hypoxia and ischaemia may occur in response to CA exposure have been proposed. First, CA may cause renal vasoconstriction and both increased activity of several intrarenal mediators (adenosine, vasopressin, angiotensin II, dopamine and endothelin), and decreased activity of renal vasodilators (nitric oxide and prostaglandins). Secondly, they may decrease renal blood flow indirectly by causing erythrocyte aggregation.
Probably as a consequence of their high viscosity, dimeric IOCA have been reported to cause more erythrocyte aggregation, cessation of flow in the renal microcirculation, and greater reduction of renal blood flow than monomeric LOCA [1212. Schrader R. Contrast material-induced renal failure: an overview. J Interven Cardiol. 2005;18:417-423. ]. Other experimental studies have suggested that dimeric IOCA may worsen medullary hypoxaemia more than LOCA [1313. Liss P, Nygren A, Erikson U, Ulfendahl HR. Injection of low and iso-osmolar contrast medium decreases oxygen tension in the renal medulla. Kidney Int. 1998;53:698-702. ]. A diminished transit time of the higher-viscosity dimeric IOCA in the tubule might lead to decreased GFR and renal blood flow by compression of peritubular vessels. Moreover, the reduced tubular transit time of the non-ionic dimers may result in a longer time for solute transport and increased oxygen utilisation. Although clinical trials indicate a lower incidence of CIN with LOCA, experimental studies on the role of osmolality per se in the pathogenesis of CIN provide conflicting data. Physiochemical properties of CA other than osmolality (i.e., viscosity, hydrophilicity) may contribute substantially to their toxic effect.
In summary, several mechanisms can contribute to kidney function impairment after CA administration. The direct toxic effect of CA on renal cells is exacerbated by the reduction in blood flow to the medullary portions of the kidney.
RISK FACTORS AND PATIENTS AT RISK
A large body of data indicates that the risk of CIN is related to patient characteristics, clinical setting, and other modifiable factors ( Table 3 ).
Thus, identification of patients at increased risk is of major importance. The highest risk for CIN is seen in those with pre-existing renal impairment [77. McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368-375. , 1414. Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R and Berg KJ Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003; 348: 491-499. , 1515. Thomsen HS, Morcos SK. Contrast media and the kidney: European Society of Urogenital Radiology (ESUR) guidelines. Br J Radiol. 2003; 76:513-518. , 1616. Morcos SK, Thomsen HS, Webb JAW and members of contrast media safety committee of the European Society of Urogenital Radiology (ESUR). Contrast Media Induced Nephrotoxicity: A consensus report. Eur Radiol. 1999; 9: 1602-1613. , 1717. Rudnick MR, Goldfarb S, Wexler L, Ludbrook PA, Murphy MJ, Halpern ET, Hill JA, Winniford M, Cohen MB, Vanfossen DB. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. The Iohexol Cooperative Study. Kidney Int. 1995; 47: 254-261. ]. The higher the baseline SCr value, the greater is the risk [1818. McCullough PA, Dandberg KR. Epidemiology of contrast-induced nephropathy. Rev Cardiovasc Med. 2003;4 (suppl. 5):1172-1181. ]. Indeed, a decreased GFR results in a greater load of contrast to be excreted by each nephron. Diabetes mellitus per se is not a strong risk factor [1515. Thomsen HS, Morcos SK. Contrast media and the kidney: European Society of Urogenital Radiology (ESUR) guidelines. Br J Radiol. 2003; 76:513-518. , 1717. Rudnick MR, Goldfarb S, Wexler L, Ludbrook PA, Murphy MJ, Halpern ET, Hill JA, Winniford M, Cohen MB, Vanfossen DB. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. The Iohexol Cooperative Study. Kidney Int. 1995; 47: 254-261. ]. However, its presence may significantly increase the risk in patients with pre-existing renal dysfunction. Diabetes is associated with impaired endothelial function with loss of ability to generate nitric oxide. This compromises the ability of the kidney vasculature to maintain blood flow in the presence of medullary vasoconstriction induced by CA [1919. Persson BP, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005;68:14–22. ]. Studies have shown that patients with diabetes and renal impairment have a 4-fold higher rate of CIN as compared to those without these conditions [77. McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368-375. ]. Additional risk factors include advanced age (likely related to the normal decline in renal function with ageing), congestive heart failure, reduced effective arterial volume (as in the case of dehydration or procedure-related blood loss), nephrosis, cirrhosis, anaemia, and concurrent use of potentially nephrotoxic drugs, such as diuretics and aminoglycosides, as well as drugs impairing the renovascular autoregulation, such as non-steroidal anti-inflammatory drugs [77. McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368-375. , 2020. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas GA. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393-9.
This single-centre study demonstrated that individual patient risk for contrast-induced nephropathy after PCI can be globally assessed with the calculation of a simple risk score based on readily available information.]. Finally, large doses of CA and multiple injections within 72 hours are associated with increased risk. The route of administration is also important and CA seem less nephrotoxic when injected intravenously than when given intra-arterially, particularly when administered into the renal arteries or into the aorta proximal to the origin of these vessels [2121. Katzberg R, Barrett B. Risk of contrast-induced nephropathy with the intravenous administration of iodinated contrast media. Radiology. 2007; 243:622-628. ]. There are several possible explanations for the higher incidence of CIN with intra-arterial CA administration:
- acute kidney concentration of CA is much higher after intra-arterial than after intravenous administration;
- intra-arterial injections tend to be larger and repeated during percutaneous coronary intervention (PCI) and other percutaneous procedures;
- patients receiving intra-arterial CA often have a greater burden of underlying cardiovascular disease;
- there is a greater likelihood of haemodynamic compromise at the time of intra-arterial injection, particularly in patients undergoing urgent procedures;
- the occurrence of atherothrombotic embolisation to the kidney vessels due to the manipulation of catheters in the aorta.
Apart from the known unfavourable association of diabetes and renal insufficiency, the presence of two or more risk factors is additive, possibly by a variety of interacting mechanisms, and the likelihood of CIN rises sharply as the number of risk factors increases. Information derived from multiple large-scale studies has led to the development of multivariate prediction scoring schemes for patients undergoing PCI [77. McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368-375. , 2020. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas GA. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393-9.
This single-centre study demonstrated that individual patient risk for contrast-induced nephropathy after PCI can be globally assessed with the calculation of a simple risk score based on readily available information., 2222. Cochran ST, Wong WS, Roe DJ. Predicting angiography-induced acute renal function impairment: clinical risk model. Am J Roentgenol. 1983;141:1027-1033. , 2323. Rich MW, Crecelius CA. Incidence, risk factors and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. A prospective study. Arch Intern Med. 1990;150:1237–1242. , 2424. Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93:1515-1519. ] ( Figure 2 ). Application of these risk scores showed that patients with multiple risk factors have a very high, if not certain, likelihood of developing CIN after CA exposure. It should be noted, however, that these risk scores have been evaluated retrospectively, and none of them has been prospectively validated in different populations.
CLINICAL RELEVANCE OF CIN IN PRIMARY PCI
Patients undergoing primary PCI constitute a particularly high-risk group. Sadeghi et al were the first to report the clinical and prognostic relevance of CIN in ST-elevation myocardial infarction (STEMI) patients undergoing primary PCI [2525. Sadeghi HM, Stone GW, Grines CL, Mehran R, Dixon SR, Lansky AJ, Fahy M, Cox DA, Garcia E, Tcheng JE, Griffin JJ, Stuckey TD, Turco M, and Carroll JD. Impact of renal insufficiency in patients undergoing primary angioplasty for acute myocardial infarction. Circulation. 2003;108:2769-2775. ]. They evaluated CIN incidence, defined as an absolute SCr increase of >0.5 mg/dL (>44 micromol/l), in 1,884 patients enrolled in the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. The renal complication occurred in 4.6% of patients (being 3 times more prevalent in those with CKD), and was associated with a strikingly worse prognosis. In patients with CIN, 30-day mortality was 16.2% and 1-year mortality was 23.3% as compared to 1.2% and 3.2%, respectively, in patients without CIN. However, the incidence of CIN was probably underestimated. Indeed, patients with cardiogenic shock and renal insufficiency (SCr >2 mg/dL/>166 micromol/l)), two important predictors of AKI in patients with STEMI, were excluded from this trial. Moreover, daily SCr measurement was not routinely performed. As SCr levels were assessed at admission, 24 hours after PCI, and at hospital discharge, transient SCr increase, typically occurring 48 to 72 hours after contrast exposure, may have been missed in many patients.
The impact of CIN after primary PCI has been investigated in more depth in one study carried out in our institute [2626. Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, Grazi M, Veglia F, Bartorelli AL. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004; 44:1780-5.
This prospective study enrolled an unselected population of consecutive patients undergoing primary PCI for acute myocardial infarction and found that contrast-induced nephropathy is a frequent complication, even in patients with normal renal function, and is associated with a more complicated in-hospital course and very high mortality rate.]. In 208 STEMI patients undergoing primary PCI, the incidence, clinical predictors, and clinical consequences of CIN, defined as an absolute SCr increase >0.5 mg/dL (>44 micromol/l), were evaluated. Forty patients (19%) developed CIN. This complication occurred in 40% of patients with CKD and in 13% of those with normal or mildly impaired renal function. Patients with CIN experienced a more complicated in-hospital clinical course and had an average length of hospital stay approximately 1.5 times longer than that of patients without this complication. The overall in-hospital mortality of the entire study population was 6.2%. However, mortality rate was significantly higher in patients developing CIN than in those without it (31% vs. 0.6%; p<0.001). In multivariate analysis, the following variables were significant independent correlates of CIN: age >75 years (OR 5.28), anterior STEMI (OR 2.17), time-to-reperfusion >6 hours (OR 2.51), CA volume >300 mL (OR 2.80) and use of an intra-aortic balloon pump (IABP) (OR 15.51). When these variables were included as risk indicators in a scoring system, the incidence of CIN, as well as in-hospital mortality rate, revealed a significant gradation as the risk score increased in the study population ( Figure 3 ). This score has been validated in the large Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial [166166. Narula A, Mehran R, Weisz G, Dangas GD, Yu J, Généreux P, Nikolsky E, Brener SJ, Witzenbichler B, Guagliumi G, Clark AE, Fahy M, Xu K, Brodie BR, Stone GW. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. Eur Heart J. 2014;35:1533-1540. ].
Although all STEMI patients treated with primary PCI are effectively exposed to CA toxicity, several other factors may contribute to an increased risk of AKI in this setting. Among them, hypotension, or even shock, and the lack of time to start a renal prophylactic therapy are most likely involved. Indeed, patients with STEMI who are not treated with primary PCI may also develop AKI, with the same prognostic implications as for CIN. Goldberg et al reported AKI, defined as an increase of >0.5 mg/dL (>44micromol/l) in SCr level, in about 10% of an unselected sample of more than 1,000 patients with STEMI (27). As less than one quarter (22%) of them had primary PCI, haemodynamic alterations or other extra-renal factors, such as volume overload, medications, and bleeding, were probably responsible for AKI. These authors found AKI to be a strong independent predictor of in-hospital (adjusted OR 11.4) and 1-year (adjusted hazard ratio 7.2) mortality.
The key role of haemodynamic compromise in AKI occurrence is clearly suggested by studies evaluating patients with STEMI complicated by cardiogenic shock. Indeed, cardiogenic shock, as well as its aggressive treatment, is associated with an increased risk of AKI. This is probably the result of renal hypoperfusion due to prolonged systemic hypotension, CA exposure during primary PCI, medications, bleeding, and possible atheroembolic events during catheterisation and IABP support. In the Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial, 13% of patients treated with early revascularisation and 24% of those treated with initial medical therapy developed AKI, defined as an increase in SCr exceeding 3 mg/dL (264 micromol/l) [2828. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, Picard MH, Menegus MA, Boland J, Dzavik V, Thompson CR, Wong SC, Steingart R, Forman R, Aylward PE, Godfrey E, Desvigne-Nickens P and LeJemtel TH for the SHOCK investigators. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341:625-634. ]. Moreover, in a retrospective analysis of 118 patients with cardiogenic shock, 86% of whom had STEMI, AKI developed within 24 hours of shock onset in 33% of them and was associated with a significantly higher in-hospital mortality rate when compared to patients without AKI (87% vs. 53%; OR 6.0; p<0.001) [2929. Koreny M, Delle Karth G, Geppert A, Neunteufl T. Prognosis of patients who develop acute renal failure during the first 24 hours of cardiogenic shock after myocardial infarction. Am J Med. 2002;112:115-119. ]. However, the true incidence of AKI was probably underestimated also in this study because its development was considered in the first 24 hours and after cardiogenic shock onset only. The incidence and the clinical consequences of AKI, defined as an increase in SCr >25% from baseline during the first 72 hours, have been investigated in a population of 97 consecutive patients with STEMI complicated by cardiogenic shock at hospital admission and treated in all cases with IABP and primary PCI [3030. Marenzi G, Assanelli E, Campodonico J, De Metrio M, Lauri G, Marana I, Moltrasio M, Rubino M, Veglia F, Montorsi P, Bartorelli AL. Acute kidney injury in ST-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit Care Med. 2010;38:438-444. ]. Acute kidney injury occurred in 55% of patients and required renal replacement therapy in 25% of cases. Development of AKI was associated with a longer hospital stay and a marked increase of mortality rate when compared to those without AKI (50% vs. 2.2%; p<0.001).
Acute hyperglycaemia was also found to be associated with a two-fold higher incidence of CIN and with an enhanced morbidity and mortality risk in STEMI patients undergoing primary PCI [3131. Marenzi G, De Metrio M, Rubino M, Lauri G, Cavallero A, Assanelli E, Grazi M, Moltrasio M, Marana I, Campodonico J, Discacciati A, Veglia F, Bartorelli AL. Acute hyperglycemia and contrast-induced nephropathy in primary percutaneous coronary intervention. Am Heart J. 2010;160:1170-1177. ]. The mechanisms underlying the association between acute hyperglycaemia and an increased risk for CIN remain unclear but several effects of acute hyperglycaemia may negatively impact on kidney function and increase renal toxicity of CA. Acute hyperglycaemia suppresses flow-mediated vasodilatation, likely through increased production of oxygen-derived free radicals, and increases oxidative stress. Both oxidative stress-mediated injury and renal medullary hypoxia and ischaemia have been implicated as causative factors for CIN. As a result, acute hyperglycaemia may exacerbate the deleterious effects of CA on the kidney.
In conclusion, regardless of the exact underlying mechanism (CA toxicity, acute ischaemic injury, acute hyperglycaemia-induced oxidative stress), an increase in SCr concentration during the acute phase of STEMI represents a strong independent predictor of in-hospital morbidity and mortality and a potential target for prophylactic strategies.
CLINICAL RELEVANCE OF CIN IN NON-CORONARY INTERVENTIONAL PROCEDURES
Peripheral vascular interventions
Peripheral vascular interventions represent an overlooked cause of CIN. Indeed, technical and procedural advances make this type of treatment available to higher risk patients, whose peripheral vascular disease is an indication of more severe and diffuse atherosclerosis. Moreover, it is not unusual that multiple lesions, located in different peripheral vessels, are treated simultaneously in the same patient, sometimes in combination with PCI. Thus, high CA volume may be required. Finally, because most of these patients suffer from generalised atherosclerotic vascular disease, they are also at increased risk of developing AKI secondary to atheroembolic disease [3232. Rudnick MR, Berns JS, Cohen RM, Goldfarb S. Nephrotoxic risks of renal angiography: contrast-media associated nephrotoxicity and atheroembolism – a critical review. Am J Kidney Dis. 1994;24:713-727. ].
Recently, the impact of endovascular aortic aneurysm repair (EVAR) on postoperative renal function has come under close scrutiny. Of particular concern is the demonstration by several investigators of progressive renal dysfunction over time after EVAR [3333. Alsac JM, Zarins CK, Heikkinen MA, Karwowski J, Arko FR, Desgranges P, Roudot Thoraval F, Becquemin JP. The impact of aortic endografts on renal function. J Vasc Surg. 2005;41:926-930. , 3434. Parmer SS, Carpenter JP. Endovascular aneurysm repair with suprarenal vs. infrarenal fixation: a study of renal effects. J Vasc Surg. 2006;43:19-25. , 3535. Greenberg RK, Chuter TA, Lawrence-Brown M, Haulon S, Nolte L. Analysis of renal function after aneurysm repair with a device using suprarenal fixation (Zenith AAA Endovascular Graft) in contrast to open surgical repair. J Vasc Surg. 2004;39:1219-1228. , 3636. Surowiec SM, Davies MG, Fegley AJ, Tanski WS, Pamoukian VN, Sternbach Y, Waldman DL, Green RM. Relationship of proximal fixation to postoperative renal dysfunction in patients with normal serum creatinine concentration. J Vasc Surg. 2004;39:804-810. ]. Although patients receiving EVAR are spared the ischaemic insult of aortic cross-clamping and have less perioperative haemorrhage [3737. Adriansen ME, Bosch JL, Halpern EF, Hunink MGM, Gazelle GS Elective endovascular versus open surgical repair of abdominal aortic aneurysms: systemic review of short-term results. Radiology. 2002;224:739-747. , 3838. Zeebregts CJ, Geelkerken RH, van der Palen J, Huisman AB, De Smit P, Van Det RJ. Outcome of abdominal aneurysm repair in the era of endovascular treatment. Br J Surg. 2004;91:563-568. ], the potential nephrotoxicity of large CA volume must be considered. Patients undergoing EVAR receive a higher CA dose than that used in other peripheral vascular interventions ( Table 4 ) [3939. Marenzi G, Bartorelli AL. Contrast-induced nephro-pathy. In “Peripheral Vascular Intervention”, Second Edition. R. Heuser & M. Henry editors. Informa Healthcare. 2009, Chapter 93: 799-807. ].
These data are in agreement with those of a previous study that enrolled 97 patients undergoing EVAR for thoracic aneurysms and received an average CA volume of 307±188 mL [4040. Eggebrecht H, Breuckmann F, Martini S, Baumgart D, Herold U, Kienbaum P, Peters J, Jakob H, Erbel R, Mehta RH. Frequency and outcomes of acute renal failure following thoracic aortic stent-graft placement. Am J Cardiol. 2006;98:458-463. ]. Of note, post-procedural CIN occurred in 34% of them and was associated with a significantly lower survival at 1 year as compared to the patients without this complication (65% vs. 90%). High CA volume in EVAR is the result of the multiple angiographies that are often needed for correct endograft positioning and for assessing, particularly in complex anatomy, the results of type I endoleak treatment at the proximal or distal fixation sites after endograft implantation. In addition to CA volume, other factors may be responsible for AKI after this type of endovascular intervention. Firstly, the only independent risk factor that has been linked with the development of postoperative AKI after infrarenal EVAR is preoperative CKD [4141. Parmer SS, Fairman RM, Karmacharya J, Carpenter JP, Velazquez OC, Woo EY. A comparison of renal function between open and endovascular aneurysm repair in patients with baseline chronic renal insufficiency. J Vasc Surg. 2006;44:706-711. ]. In addition, manipulation and positioning of the endograft within the aneurysm together with balloon inflation at the proximal fixation site may result in thromboembolism of the renal artery and renal infarction. Finally, repeated administration of CA during periprocedural evaluation and follow-up surveillance with multidetector computed tomography represents an additional risk for progressive renal dysfunction [3636. Surowiec SM, Davies MG, Fegley AJ, Tanski WS, Pamoukian VN, Sternbach Y, Waldman DL, Green RM. Relationship of proximal fixation to postoperative renal dysfunction in patients with normal serum creatinine concentration. J Vasc Surg. 2004;39:804-810. , 4141. Parmer SS, Fairman RM, Karmacharya J, Carpenter JP, Velazquez OC, Woo EY. A comparison of renal function between open and endovascular aneurysm repair in patients with baseline chronic renal insufficiency. J Vasc Surg. 2006;44:706-711. ]. The type of endograft used for proximal fixation in abdominal aorta aneurysms must be added to the list of factors that may have an influence on renal function. Indeed, there are still concerns regarding suprarenal fixation of the endograft and long-term adverse effects. Interference with renal artery flow, stenosis of the renal ostium, renal infarction and a biological response of the aorta may be the result of continued injury from the suprarenal fixation stent, and may play a role in renal function deterioration over time [4242. Blocker D, Krauss M, Mansmann U, Halawa M, Lange R, Probst T, Raither D. Incidence of renal infarction after endovascular AAA repair: relationship to infrarenal versus suprarenal fixation. J Endovasc Ther. 2003;10:1054-1060. , 4343. Sun Z, Stevenson G. Transrenal fixation of aortic stent-grafts: short- to midterm effects on renal function. A systematic Review. Radiology. 2006;240:65-72. ].
Transcatheter aortic valve implantation
Another emerging procedure associated with an increased risk of AKI is percutaneous transcatheter aortic valve implantation (TAVI). This is a new and less-invasive treatment option for severe aortic valve stenosis in patients at high surgical risk. Indeed, patients undergoing TAVI are older and have multiple and complex comorbidities, including renal and cardiac dysfunction. Moreover, before TAVI they undergo CA administration both for coronary anatomy assessment by coronary angiography and for evaluation of the aorto-iliofemoral vessels by angiography or multidetector computed tomography. A substantial amount of CA may also be needed during the procedure. Recently published studies report an incidence of AKI after TAVI ranging from 12% to 28% [4444. Aregger F, Wenaweser P, Hellige GJ, Kdner A, Carrel T, Windecker S, Frey FJ. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol Dial Transplant. 2009;24:2175-2179. , 4545. Strauch JT, Scherner MP, Haldenwang PL, Pfister R, Kuhn EW, Madershahian N, Rahmanian P, Wippermann J, Wahlers T. Minimally invasive transapical aortic valve implantation and the risk of acute kidney injury. Ann Thorac Surg. 2010;89:465-470. , 4646. Bagur R, Webb JC, Nietlispach F, Dumont E, De Larochelliere R, Doyle D, Masson JB, Guiterrez MJ, Clavel MA, Bertrand F, Pibarot P, Cabau JR. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010;31:865-874. ]. More importantly, AKI in these patients is a strong predictor of mortality. Bagur et al found that patients who developed AKI after TAVI had a more than 4-fold increased risk of death (46). In another study, the occurrence of AKI after TAVI was associated with a 5-fold increased risk for 30-day mortality and 6-fold increased risk for 1-year mortality [4747. Sinning JM, Ghanem A, Steinhauser H, Adenauer V, Hammerstingl C, Nickenig G, Werner N. Renal function as a predictor of mortality in patients after thanscatheter aortic valve implantation. J Am Coll Cardiol Cardiovasc Interv. 2010;3:1141-1149. ]. Beside the nephrotoxicity of CA used before and during the procedure, the mechanism of AKI is likely multifactorial. Hypotension due to rapid pacing, balloon valvuloplasty, valve deployment as well as moderate-to-severe periprosthetic regurgitation and atherothrombotic emboli caused by valvuloplasty, catheter manipulation in the aorta and valve deployment may be responsible for acute deterioration of renal function [4747. Sinning JM, Ghanem A, Steinhauser H, Adenauer V, Hammerstingl C, Nickenig G, Werner N. Renal function as a predictor of mortality in patients after thanscatheter aortic valve implantation. J Am Coll Cardiol Cardiovasc Interv. 2010;3:1141-1149. ].
CLINICAL PRESENTATION AND PROGNOSTIC IMPLICATIONS
The clinical course of CIN is usually characterised by spontaneous recovery of SCr. Although the clinical relevance of CIN may not be immediately evident, given the subclinical course and the high frequency of recovery of SCr, some degree of residual renal impairment has been reported in as many as 30% of those affected and up to 7% of patients may require temporary dialysis or progress to end-stage renal failure [77. McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368-375. ]. Serious clinical consequences, including death, may occur in patients developing CIN. Patients with CIN have been observed to be prone to several non-cardiac in-hospital complications, including haematoma formation, pseudoaneurysms, stroke, coma, adult respiratory distress syndrome, pulmonary embolism and gastrointestinal haemorrhage [55. Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR Jr. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259-64.
A retrospective analysis of the Mayo Clinic PCI registry including 7,586 patients showing that patients with baseline elevation of serum creatinine are at high risk of acute renal failure after PCI and that acute renal failure is highly correlated with in-hospital and long-term mortality.]. Patients who develop CIN after PCI have a 15-fold higher rate of major adverse cardiac events during hospitalisation than patients without CIN [55. Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR Jr. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259-64.
A retrospective analysis of the Mayo Clinic PCI registry including 7,586 patients showing that patients with baseline elevation of serum creatinine are at high risk of acute renal failure after PCI and that acute renal failure is highly correlated with in-hospital and long-term mortality.]. They also have a 6-fold increase in myocardial infarction and an 11-fold increase in coronary vessel re-occlusion [2424. Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93:1515-1519. ]. Although few (<1%) patients with CIN require dialysis, those that do have a more complicated clinical outcome than those who do not require renal replacement therapy, including a significantly higher rate of non-Q-wave myocardial infarction (46% vs. 15%), pulmonary oedema (65% vs. 3%), and gastrointestinal bleeding (16% vs. 1%). Moreover, they have a 15-fold longer stay in the intensive care unit and a 5-fold longer in-hospital stay [33. Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930-936. , 4848. Gruber L, Mehran R, Dangas G, Mintz GS, Waksman R, Kent KM, Pichard AD, Satler LF, Wu H and Leon MB. Acute renal failure requiring dialysis after percutaneous coronary interventions. Catheter Cardiovasc Interv. 2001;52:409-416. ].
It has been recognised that the development of CIN is linked to an increased risk of in-hospital and long-term mortality. In a large retrospective study including more than 16,000 patients undergoing procedures requiring CA, the risk of death during hospitalisation was 34% in patients who developed CIN, compared with 7% in matched controls who received CA but did not developed CIN [4949. Levy, EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality: a cohort analysis. JAMA. 1996:275;1489-1494. ]. The high risk of in-hospital death associated with CIN has also been noted in a retrospective analysis of 7,586 patients [55. Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR Jr. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259-64.
A retrospective analysis of the Mayo Clinic PCI registry including 7,586 patients showing that patients with baseline elevation of serum creatinine are at high risk of acute renal failure after PCI and that acute renal failure is highly correlated with in-hospital and long-term mortality.]. The hospital mortality rate was 22% in the patients developing CIN, compared with only 1.4% in patients who did not have this complication. In this study, the increased risk of death persisted over time, with significantly higher mortality rates at 1 year (12.1%) and at 5 years (44.6%), compared with rates of 3.7% and 14.5%, respectively, in patients who did not develop CIN. Other studies confirmed this observation, with in-hospital mortality rates ranging from 7.1% to 14.9%, and with 1-year mortality rates from 30% to 37%, depending on the patient’s baseline risk profile [66. Gruberg L, Mintz GS, Mehran R, Dangas G, Lansky AJ, Kent KM, Pichard AD, Satler LF, and Leon MB. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542-1548. , 77. McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368-375. ]. A significantly higher in-hospital and 1-year mortality has been reported in patients who develop CIN and require renal replacement therapy [77. McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368-375. , 4848. Gruber L, Mehran R, Dangas G, Mintz GS, Waksman R, Kent KM, Pichard AD, Satler LF, Wu H and Leon MB. Acute renal failure requiring dialysis after percutaneous coronary interventions. Catheter Cardiovasc Interv. 2001;52:409-416. , 4949. Levy, EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality: a cohort analysis. JAMA. 1996:275;1489-1494. , 5050. Marenzi G, Bartorelli AL, Lauri G, Assanelli E, Grazi M, Campodonico J, Marana I. Continuous veno-venous hemofiltration for the treatment of contrast-induced acute renal failure after percutaneous coronary interventions. Cathet Cardiovasc Intervent. 2003;58:59-64. ].
Although incomplete recovery from severe AKI is a well-recognized pathway to persistent and progressive CKD, recent data indicate that AKI, also when characterized by a transient and mild kidney dysfunction, more than likely results in persistent loss of kidney function, faster subsequent rate of decline in kidney function, and future risk of progression to end-stage renal disease (ESRD) [167167. Marenzi G, Cosentino N. Bartorelli A. Acute kidney injury in patients with acute coronary syndromes. Heart. 2015;101:1778-85. ]. Of note, the greater the severity of AKI, the higher the risk of CKD and ESRD progression. Thus, the common opinion on the reversible nature of renal damage in AKI seems unlikely and probably concerns SCr concentration only.
These speculations are supported by both clinical and experimental observations. Indeed, a recent study demonstrated that a persistent deterioration of kidney function, defined as a >25% or >0.5 mg/dL increase in SCr above baseline, was still detectable between 6 and 8 months after PCI in about 40% of patients developing AKI during acute coronary syndromes (ACS), and that it was a strong independent predictor of 5-year mortality [168168. Nemoto N, Iwasaki M, Nakanishi M, et al. Impact of continuous deterioration of kidney function 6 to 8 months after percutaneous coronary intervention for acute coronary syndrome. Am J Cardiol. 2014;113:1647-51. ]. The Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) showed that, among 14,782 patients (10,671 with ACS) undergoing coronary angiography, AKI was associated with an increased risk of death, progression to ESRD, and subsequent hospitalization for AKI, but not of hospitalization for myocardial infarction and cerebrovascular events at long-term (median 19.7 months) follow-up. [169169. James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, Pannu N, Manns BJ, Klarenbach SW, Hemmelgarn BR; Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) Investigators. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123:409-16. ]
After an acute renal insult, kidney may undergo progressive structural damage, which may then predispose to a more rapid GFR decrease. Initially, these changes can be functionally compensated for by adaptations in renal hemodynamics to maintain sufficient GFR, resulting in glomerular over-filtration in the residual nephrons and release of neuro-hormones that affect renal blood flow. However, in the setting of impaired renal function (pre-existing CKD), the kidney may lack sufficient functional reserve and is more likely to develop irreversible damage. If we transfer these data to the clinical arena, we can explain why patients with concomitant AKI and CKD are far more likely to develop ESRD. [170170. Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223-8. ] On the other hand, in patients with normal function, multiple episodes of AKI may dissipate renal reserve, promoting the development of CKD, as each additional AKI episode has been associated with an independent, cumulative increase in the risk of advanced CKD [171171. Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6:2567-72. ]. Thus, AKI may identify subjects who fail their renal “stress test” due to a limited renal reserve and who are more likely to have progressive kidney disease.
Renal protection measures
Because the occurrence of CIN can be predicted in most cases, preventive strategies represent the only effective therapeutic approach. Theoretically, CIN prophylaxis should be targeted at reducing kidney injury and the adverse outcomes associated with it. Unfortunately, no protective measure showed any evidence of reduced injury and relatively few studies addressing long-term adverse outcomes are available. Moreover, evidence regarding CIN prevention is derived from studies looking at an imperfect marker of kidney function, i.e., SCr. Preventive studies can be divided into trials which assessed the effect on CIN incidence of fluid administration, pharmacological strategies, renal replacement therapies and use of different CA. Most of the prospective randomised trials have been conducted in patients undergoing cardiac angiography, while relatively few studies have enrolled patients receiving intravenous CA. Of note, follow-up data on adverse events have been reported infrequently.
GENERAL MEASURES
All patients receiving CA should be evaluated for their risk of CIN. The patient’s history, as well as knowledge of renal function, can be used to identify “high-risk” individuals. SCr is now generally recognised as an insensitive indicator of abnormal renal function as moderate impairment can occur within the normal reference range. Direct measurement of glomerular filtration rate is the gold standard but is impractical in daily practice. Thus, formulae have been devised to help estimate abnormal renal function ( Table 5 ) [5151. National Kidney Foundation (NKF) Kidney Disease Outcome Quality Initiative (K/DOQI) Advisory Board. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Suppl.
2):S17-S31]. However, these formulae were not designed for use in cardiological practice. An estimated GFR (eGFR) below 60 mL/min should be considered as a “high-risk” condition for CIN [5252. Solomon R, Deray G, on behalf of The Consensus Panel for CIN. How to prevent contrast-induced nephropathy and manage risk patients: practical recommendations. Kidney Int. 2006;69 (Suppl 100):S51-S53. ].
Drugs that adversely affect renal function should be withheld prior to and immediately following CA exposure. In general, drugs that produce volume depletion or renal vasoconstriction should be reviewed for their risks and benefits prior to CA administration. When withdrawal of these drugs is not associated with increasing risk to the patients, it should be undertaken for at least 48 hours following CA exposure in “high-risk” patients, or until renal function is restored to baseline levels. When possible, repeated CA exposure should be delayed for 72 hours, and the smallest possible amount of CA should be used in patients with risk factors [5252. Solomon R, Deray G, on behalf of The Consensus Panel for CIN. How to prevent contrast-induced nephropathy and manage risk patients: practical recommendations. Kidney Int. 2006;69 (Suppl 100):S51-S53. ].
FLUID ADMINISTRATION
Hydration remains the cornerstone of CIN prevention despite the lack of randomised controlled trials comparing a strategy of volume expansion with no volume expansion. Hydration results in plasma volume expansion with concomitant suppression of the renin-angiotensin-aldosterone system, downregulation of the tubuloglomerular feedback, dilution of the CA, and thus attenuation of renal cortical vasoconstriction and tubular obstruction prevention [5353. Erley CM. Does hydration prevent radiocontrast-induced acute renal failure?. Nephrol Dial Transplant. 1999;14:1064-1066. ]. Multiple trials have addressed type, amount, duration, and route of volume expansion to prevent CIN [5454. Solomon R, Werner C, Mann D, D’Elia J, Silva P. Effects of saline, mannitol, and furosemide on acute decreases in renal function induced by radiocontrast agents.
This prospective randomised study compared saline hydration alone to saline hydration plus either mannitol or furosemide in patients with chronic kidney disease undergoing cardiac angiography and demonstrated that saline hydration alone provides better protection against contrast-induced nephropathy. , 5555. Taylor AJ, Hotchkis D, Morse RW, McCabe J. PREPARED: preparation for angiography in renal dysfunction. A randomized trial of inpatient vs outpatient hydration protocols for cardiac catheterization in mild to moderate renal dysfunction. Chest. 1998;114:1570-1574. , 5656. Trivedi HS, Moore H, Nasr S, Aggarwal K, Agrawal A, Goel P, Hewett J. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephropathy. Nephron Clin Pract. 2003;93:C29-C34. , 5757. Stevens MA, McCullough PA, Tobin KJ, Speck JP, Westveer DC, Guido-Allen DA, Timmis GC, and O’Neill WW. A prospective randomized trial of prevention measures in patients at high risk for contrast nephropathy: results of the PRINCE study. J Am Coll Cardiol. 1999;33:403-411. ] ( Figure 4 ).
However, many of these aspects remain undefined. Trivedi et al found that oral hydration alone appeared to be inferior to intravenous hydration with respect to the development of CIN [5656. Trivedi HS, Moore H, Nasr S, Aggarwal K, Agrawal A, Goel P, Hewett J. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephropathy. Nephron Clin Pract. 2003;93:C29-C34. ]. Conversely, a recent meta-analysis of 6 randomised studies including 513 patients suggested that the oral route of volume expansion may be as effective as the intravenous route. Given the potential advantages of oral (e.g. reduced patient burden and cost) over intravenous volume expansion, adequately powered trials comparing these strategies with clinically relevant end points are warranted [5858. Hiremath S, Akbari A, Shabana W, Fergusson DA, Knoll GA. Prevention of contrast-induced acute kidney injury: is simple oral hydration similar to intravenous?. A systematic review of the evidence. PLoS One. 2013;8:1. ].
By comparing patients treated with hydration and mannitol and hydration and furosemide, Solomon et al demonstrated that intravenous infusion of 0.45% saline (1 mL/kg/hour), starting 4-6 hours before CA administration, and continued for 24 hours afterwards, reduced the risk of CIN in patients with mild renal insufficiency undergoing cardiac angiography [5454. Solomon R, Werner C, Mann D, D’Elia J, Silva P. Effects of saline, mannitol, and furosemide on acute decreases in renal function induced by radiocontrast agents.
This prospective randomised study compared saline hydration alone to saline hydration plus either mannitol or furosemide in patients with chronic kidney disease undergoing cardiac angiography and demonstrated that saline hydration alone provides better protection against contrast-induced nephropathy. ]. Other authors suggest that hydration with isotonic saline is superior to half-isotonic saline, probably because of the enhanced ability of isotonic fluids to expand intravascular volume [5959. Mueller C, Buerkle G, Buettner HJ, Petersen J, Perruchoud AP, Eriksson U, Marsch S, Roskamm H. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329-336. ]. The advantage of isotonic hydration is certainly demonstrated in patients with normal renal function and with a low risk of CIN, but these results cannot be transferred conclusively to patients with moderate and severe CKD. Merten et al demonstrated that hydration with sodium bicarbonate (154 mEq/L of sodium bicarbonate in dextrose and water at a rate of 3 mL/kg/h per 1 hour before CA exposure, followed by 1 mL/kg/h during, and for 6 hours after the procedure) is more effective than hydration with sodium chloride and may provide additional kidney protection by alkalinising renal tubular fluid and thereby minimising tubular damage [6060. Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, Bersin RM, Van Moore A, Simonton CA 3rd, Rittase RA, Norton HJ, Kennedy TP. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328-34.
A prospective, single-centre, randomised trial conducted in patients undergoing diagnostic or interventional procedures requiring contrast media demonstrating that hydration with sodium bicarbonate before contrast exposure is more effective than hydration with sodium chloride for prophylaxis of contrast-induced renal failure.]. The generation of reactive oxygen species is facilitated in an acidic environment as might occur in the distal nephron. Administration of sodium bicarbonate will alkalinise the urine and will presumably slow down the generation of these reactive oxygen species. Multiple single-centre studies evaluating sodium bicarbonate as a prophylactic measure have produced conflicting results. However, recent meta-analyses suggest a benefit, particularly in patients undergoing emergency procedures [6161. Joannidis M, Schmid M, Wiedermann CJ. Prevention of contrast media-induced nephropathy by isotonic sodium bicarbonate: a meta-analysis. Wien Klin Wochenschr. 2008;120:742–748. , 6262. Meier P, Ko DT, Tamura A, Umesh Tamhane U, Hitinder S Gurm HS. Sodium bicarbonate-based hydration prevents contrast-induced nephropathy: a meta-analysis. BMC Med. 2009;7:23–34. ]. Nevertheless, despite a reduction in CIN rate, there was no effect on major adverse events, such as need for renal replacement therapy or mortality [6262. Meier P, Ko DT, Tamura A, Umesh Tamhane U, Hitinder S Gurm HS. Sodium bicarbonate-based hydration prevents contrast-induced nephropathy: a meta-analysis. BMC Med. 2009;7:23–34. ]. Finally, Clavijo et al reported a retrospective analysis showing that a rapid intra-arterial infusion of dextrose 5% (1 litre administered though the femoral artery sheath as a bolus in ~5 minutes immediately before angiography), was well tolerated and effective against CIN in patients with a creatinine clearance <60 mL/min [6363. Clavijo LC, Pinto TL, Kuchulakanti PK, Torguson R. Effect of a rapid intra-arterial infusion of dextrose 5% prior to coronary angiography on frequency of contrast-induced nephropathy in high-risk patients. Am J Cardiol. 2006;97:981-983. ].
Although a clearly emerging concept is that volume expansion is critical in the prevention of CIN, the prognostic impact of hydration is still controversial, and there are no definitive data on the possible advantage of this strategy on CIN-associated cardiovascular complications and mortality rate in high-risk patients. Also lacking are data from controlled clinical trials that define the most effective hydration period, infusion rate, or hydration volume. Additional studies are also required to investigate the role of hydration in patients with congestive heart failure and renal insufficiency, a population that has always been poorly represented in previous studies, and in which vigorous hydration is logistically difficult and poorly tolerated.
The AMACING (A MAstricht Contrast-Induced Nephropathy Guideline) prospective, open-label trial randomized patients at high risk of CIN (eGFR of 30–59 mL/min/1·73 m²) undergoing an elective procedure to receive intravenous 0.9% saline solution (n=332) or no prophylaxis (n=328). The primary outcome was incidence of CIN (defined as an increase in SCr from baseline >25% within 2–6 days of contrast exposure). Contrast-induced nephropathy occurred in 2.6% of non-hydrated patients and in 2.7% of hydrated patients (P=0.47) [172172. Nijssen EC, Rennenberg RJ, Nelemans PJ, Essers BA, Janssen MM, Vermeeren MA, van Ommen V, Wildberger JE. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high-risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non inferiority trial. Lancet. 2017;389:1312-22. ]. This trial and other indirect evidences suggest that the hydration volume should be commensurate to the risk of the patient, and a high-volume of controlled hydration is probably required in high-risk patients. This goal can be achieved either by exactly matching fluid removal to high-volume intravenous hydration to prevent fluid overload, as it can be done with haemofiltration (HF) [6464. Marenzi G, Marana I, Lauri G, Assanelli E, Grazi M, Campodonico J, Trabattoni D, Fabbiocchi F, Montorsi P, Bartorelli AL. The prevention of radiocontrast-agent-induced nephropathy by hemofiltration. N Engl J Med. 2003; 349:1333-40.
This is the first prospective, randomised study demonstrating the effectiveness of hemofiltration, as compared with saline hydration, for the prevention of contrast-induced nephropathy in patients with chronic renal insufficiency undergoing elective PCI. ], or by exactly matching intravenous hydration to forced urine output to avoid hypovolaemia [6565. Marenzi G, Ferrari C, Marana I, Assanelli E, De Metrio M, Teruzzi G, Veglia F, Fabbiocchi F, Montorsi P, Bartorelli AL. Prevention of contrast nephropathy with furosemide-induced diuresis and matched hydration - The MYTHOS trial. J Am Coll Cardiol Intv. 2012;5:90-7. ].
HIGH URINE OUTPUT WITH MATCHED HYDRATION
In patients with CKD, hydration is usually performed at a rate significantly lower than that shown to provide protection because of the fear of overhydration and pulmonary edema, particularly in patients with impaired left ventricular function [173173. Liu Y, Li H, Chen S, Chen J, Tan N, Zhou Y, Liu Y, Ye P, Ran P, Duan C, Chen P. Excessively high hydration volume may not be associated with decreased risk of contrast-induced acute kidney injury after percutaneous coronary intervention in patients with renal insufficiency. J Am Heart Assoc. 2016; 5. pii: e003171. doi: 10.1161/JAHA. 115. 003171. ]. In previous studies, diuretics have been combined with hydration therapy to increase urine output and prevent overhydration. In addition to increasing urine flow, resulting in greater contrast dilution within the renal tubules and reduced direct kidney toxicity, loop diuretics may protect against medullary ischemia, a potential mechanism of CIN. In these studies, however, furosemide was associated with deleterious effects that were likely the result of vasoconstriction induced by intravascular volume depletion, further exacerbating that produced by contrast itself [174174. Solomon R, Werner C, Mann D, D’Elia J, Silva P. Effects of saline, mannitol, and furosemide on acute changes in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:403-11. ]. Interestingly, the PRINCE (Prevention of Radiocontrast Induced Nephropathy Clinical Evaluation) study showed that forced diuresis, achieved with a single dose of diuretic in combination with intravenous fluid replacement matched to urine output, prevented dehydration and provided a modest protective effect against CIN [175175. Stevens M, McCullough P, Tobin KJ, Speck JP, Westveer DC, Guido-Allen DA, Timmis GC, O’Neill WW. A prospective randomized trial of prevention measures in patients at high risk for contrast nephropathy: results of the P.R.I.N.C.E. Study. Prevention of Radiocontrast Induced Nephropathy Clinical Evaluation. J Am Coll Cardiol. 1999;33:403-11. ]. More importantly, this study showed that CIN requiring dialysis did not develop in any patient with a mean urine flow rate above 150 ml/h. Thus, furosemide-induced high-volume diuresis with concurrent maintenance of intravascular volume through matched hydration may be an alternative strategy for CIN prevention in high-risk patients. Based on these data, a new prophylactic strategy has been developed in order to achieve real-time, automated fluid matching by continuously measuring urine output – accelerated by a bolus of intravenous furosemide - and replacing it with an exactly matched amount of infused fluid volume. The RenalGuard System™ (RenalGuard Solutions, Inc., Milford, MA, USA) comprises a urinary collection bag connected to a Foley catheter and hung on a digital scale that drives a high-volume fluid pump ( Figure 6). Any amount of urine entering the collection bag results in an equal volume of saline infused intravenously back to the patient. The infusion rate is adjusted milliliter for milliliter and second by second in response to changes in urine output, thus preventing net fluid loss (hypovolemia) and fluid overload (pulmonary edema). By administering a small bolus (250 ml) of fluid initially and stimulating diuresis with furosemide (0.25 to 0.5 mg/kg), urine output increases to 500-600 ml/min in about 60 minutes, and this rate is maintained during PCI and for the following 4 hours. The safety and efficacy of the RenalGuard system has been evaluated in some randomized trilas [6565. Marenzi G, Ferrari C, Marana I, Assanelli E, De Metrio M, Teruzzi G, Veglia F, Fabbiocchi F, Montorsi P, Bartorelli AL. Prevention of contrast nephropathy with furosemide-induced diuresis and matched hydration - The MYTHOS trial. J Am Coll Cardiol Intv. 2012;5:90-7. , 176176. Briguori C, Visconti G, Focaccio A, Airoldi F, Valgimigli M, Sangiorgi GM, Golia B, Ricciardelli B, Condorelli G; REMEDIAL II Investigators. Renal insufficiency after contrast media administration trial II (REMEDIAL II): RenalGuard System in high-risk patients for contrast-induced acute kidney injury. Circulation. 2011;124:1260-9. , 177177. Barbanti M, Gulino S, Capranzano P, Immè S, Sgroi C, Tamburino C, Ohno Y, Attizzani GF, Patanè M, Sicuso R, Pilato G, Di Landro A, Todaro D, Di Simone E, Picci A, Giannetto G, Costa G, Deste W, Giannazzo D, Grasso C, Capodanno D, Tamburino C. Acute kidney injury with the
RenalGuard system in patients undergoing transcatheter aortic valve replacement. The PROTECT-TAVI Trial (PROphylactic effecT of furosEmide-induCed diuresis with matched isotonic intravenous hydraTion in Transcatheter Aortic Valve Implantation). J Am Coll Cardiol. 2015;8:1595-604. ]. Moreover, a recent meta-analysis of 4 randomized trials assessed its effect in 698 patients undergoing PCI and TAVI. . This strategy was associated with a significant reduction of CIN (8% vs. 21%), a lower need for renal replacement therapy (0.6% vs. 3.4%), and a nonsignificant lower rate of mortality, post-procedural ACS, stroke, and acute pulmonary edema [178178. Putzu A, Boscolo Berto M, Belletti A, Pasotti E, Cassina T, Moccetti T, Pedrazzini G. Prevention of contrast-induced acute kidney injury by furosemide with matched hydration in patients undergoing interventional procedures: a systematic review and meta-analysis of randomized trials. JACC Cardiovasc Interv. 2017;10:355-363. ]. Some insights can be inferred from the data of the MYTHOS (Induced Diuresis With Matched Hydration Compared to Standard Hydration for Contrast Induced Nephropathy Prevention) trial [6565. Marenzi G, Ferrari C, Marana I, Assanelli E, De Metrio M, Teruzzi G, Veglia F, Fabbiocchi F, Montorsi P, Bartorelli AL. Prevention of contrast nephropathy with furosemide-induced diuresis and matched hydration - The MYTHOS trial. J Am Coll Cardiol Intv. 2012;5:90-7. ]. This trial indicates that RenalGuard-treated patients achieve a significantly higher volume of renal-targeted hydration (4,000 ml vs. 1,750 ml) in a much shorter time (6 h vs. 24 h) than those treated with standard systemic hydration,markedly amplifying the kidney protective effects of hydration.
PHARMACOLOGICAL PREVENTION STRATEGIES
Several pharmacological approaches have been devised to mitigate the risk of CIN in patients with CKD [6767. Briguori C, Marenzi G. Pharmacologic prophylaxis. Kidney Intern. 2006;69:S30-S38. ] ( Table 6 ). A number of studies have targeted renal vasoconstriction and hypoxia-induced oxidative stress that are among the mechanisms through which CA are believed to cause nephrotoxicity, and have evaluated the role of several pharmacological therapies designed to counteract them. However, with the exception of volume expansion and antioxidant agents, few of these therapies have shown any clear and consistent benefit.
Drugs - vasodilators
Endothelin receptor antagonist
Due to the potential role of the haemodynamic effects induced by CA, several vasodilator drugs have been tested for preventing acute reduction of renal function. The possible role of endothelin-induced renal vasoconstriction has led to the evaluation of a non-selective endothelin receptor antagonist in a multicentre, double-blind, randomised trial of high-risk patients undergoing coronary angiography [6868. Wang, A, Holcslaw, T, Bashore, TM, Freed MI, Miller D, Rudnick MR, Szerlip H, Thames MD, Davidson CJ, Shusterman N, Schwab SJ. Exacerbation of radiocontrast nephrotoxicity by endothelin receptor antagonism. Kidney Int. 2000;57:1675-1680. ]. Disappointingly, a significantly higher percentage of patients who received active therapy developed CIN as compared with those randomised to placebo (56% vs. 29%; p=0.002). However, this study evaluated the effects of a mixed endothelin A and B receptor antagonist, and the negative results may tentatively be explained by endothelin B receptor inhibition which favours vasoconstriction. To date, it is not known whether selective endothelin-A blockade may be beneficial in preventing CIN.
Atrial natriuretic peptide
No benefit was observed with the intravenous administration of this agent in a large multicentre, prospective, double-blind, placebo-controlled, randomised trial [6969. Kurnik, BR, Allgren, RL, Genter, FC, Solomon RJ. Prospective study of atrial natriuretic peptide for the prevention of radiocontrast-induced nephropathy. Am J Kidney Dis. 1998;31:674-680. ]. Recently, however, Morikawa et al using a more intensive treatment 254 protocol reported that ANP administration, on top of hydration, is effective in the prevention of CIN in patients with CKD. They randomised 254 patients to pre and post hydration with Ringers solution alone or pre and post hydration with Ringers solution with additional ANP (0.042 μg/kg/min 4 to 6 hours before procedure continued for 48 hours after procedure) and found a significant difference in the incidence of CIN (3% vs. 12%; p=0.015). Despite the important limitation deriving from infusion protocol duration of 48 hours, this therapy looks promising [7070. Morikawa S, Sone T, Tsuboi H, Mukawa H, Morishima I, Uesugi M, Morita Y, Numaguchi Y, Okumura K, Murohara T. Renal protective effects and the prevention of contrast-induced nephropathy by atrial natriuretic peptide. J Am Coll Cardiol. 2009;53:1040-1046. ].
Calcium-channel blockers
Verapamil, diltiazem, and amlodipine have been found to attenuate the renal vasoconstrictive response to CA and to inhibit CIN in rats. A randomised placebo-controlled study of 35 patients with renal insufficiency has shown that oral nitrendipine (20 mg/day for 3 days) is effective for preventing the decrease in GFR [7171. Neumayer HH, Junge W, Kufner A, Wenning A. Prevention of radiocontrast-media-induced nephrotoxicity by the blocker nitrendipine: a prospective randomized clinical trial. Nephrol Dial Transplant. 1989;4:1030-1036. ]. In contrast, other studies with nitrendipine, felodipine, and amlodipine did not confirm a beneficial effect of calcium antagonists. However, it must be emphasised that only dihydropyridine calcium-channel blockers have been clinically tested so far. These agents have a more potent peripheral vasodilating effect than verapamil or diltiazem and therefore the hypotensive effect caused by these drugs, which may lower renal perfusion pressure, may offset a potential protective effect of calcium-channel inhibition. Current recommendations do not include calcium-channel blockers for the prevention of CIN.
Prostaglandins
Prostaglandin E1 has been considered a promising agent for CIN prevention due to its vasodilatory effects. A randomised placebo-controlled pilot study showed that prophylactic administration of iloprost, a prostacyclin analogue, at a dose of 1 ng/kg/min, in patients with CKD undergoing coronary procedures is safe and may effectively prevent CIN [7272. Spargias K, Adreanides E, Giamouzis G, Karagiannis S, Gouziouta A. Iloprost for prevention of contrast-mediated nephropathy in high-risk patients undergoing a coronary procedure. Results of a nonrandomized pilot study. Eur J Clin Pharmacol. 2006;62:589-595. ]. However, further studies are needed to confirm the effectiveness of this agent.
Adenosine antagonists
Administration of CA stimulates intrarenal secretion of adenosine that binds to the renal adenosine receptor and acts as a potent vasoconstrictor, primarily in the efferent arterioles, reducing renal blood flow. As animal studies showed that the vasoconstrictive response can be blunted with theophylline, several investigators have evaluated aminophylline and theophylline as potential drugs for reducing CIN risk in patients. However, these studies have been limited by small sample size, variation in timing of drug administration and drug dose, and different CIN definition. Despite a meta-analysis of 7 trials including a total of 480 patients suggested a beneficial effect of theophylline [7373. Ix JH, McCulloch CE, Chertow GM. Theophylline for the prevention of radiocontrast nephropathy: a meta-analysis. Nephrol Dial Transplant. 2004;19:2747-2753. ], further studies are needed to determine the efficacy and safety of this drug definitively. In particular, the potential clinical benefit of theophylline must be weighed against possible side effects [7474. Cooling DS. Theophylline toxicity. J Emerg Med. 1993;11:415-425. ].
Dopamine
Although dopamine use is theoretically justified, studies testing the effectiveness of low (<2 μg/kg/min) doses of this drug showed negative or neutral results (75,76). This may be due to hypovolaemia and tachyarrhythmia induced by the diuretic and pro-arrhythmogenic effects of dopamine, both leading to reduced cardiac output and effective circulating arterial volume.
Fenoldopam
In contrast to dopamine, fenoldopam is a selective dopamine-1 receptor agonist with systemic and renal arteriolar vasodilatory properties that does not stimulate dopamine-2 or adrenergic receptors, even when administered at high doses. Thus, fenoldopam significantly increases renal blood flow and decreases renal vascular resistance, without altering GFR [7777. Mathur V, Swan S, Lambrecht L, Anjum S, Fellmann J, McGuire D, Epstein M, Luther R. The effects of fenoldopam, a selective dopamine receptor agonist, on systemic and renal hemodynamic in normotensive subjects. Crit Care Med. 1999;27:1832-1837. ]. Following preliminary studies showing a benefit in reducing CIN, a prospective randomised trial (CONTRAST) evaluated fenoldopam in 315 patients undergoing diagnostic and/or interventional procedures who were at risk for developing CIN. The trial showed negative results [7878. Stone GW, McCullough PA, Tumlin JA, Lepor NE, Madyoon H, Murray P, Wang A, Chu AA, Schaer GL, Stevens M, Wilensky RL, O’Neill WW, for the CONTRAST Investigators. Fenoldopam mesylate for the prevention of contrast-induced nephrotoxicity. JAMA. 2003;290:2284-2291. ].
L-Arginine
Theoretically, L-arginine might be renoprotective because it is a substrate for nitric oxide synthesis. However, a single infusion of L-arginine (300 mg/kg) immediately before coronary angiography did not prevent CIN in patients with mild-to-moderate renal failure included in a randomised, placebo controlled trial [7979. Miller HI, Dascalu A, Rassin TA, Wollman Y, Chernichowsky T, Laina A Effects of an acute dose of L-arginine during coronary angiography in patients with chronic renal failure: a randomized, parallel, double-blind clinical trial. Am J Nephrol. 2003;23:91-95. ].
Angiotensin-converting enzyme inhibitors
The role of angiotensin-converting enzyme inhibition for the prevention of CIN is still controversial. Preliminary studies suggest that abnormalities of renal perfusion, possibly mediated by the renin angiotensin system, are responsible for the development of CIN, and administration of captopril may offer protection against its development, particularly in diabetic patients [8080. Gupta RK, Kapoor A, Tewari S, Sinha N, Sharma RK. Captopril for prevention of contrast-induced nephropathy in diabetic patients: a randomized study. Indian Heart J. 1999;51:521-526. ].
Drugs-antioxidants
In recent years, several clinical studies have been performed using antioxidant compounds in an attempt to prevent CIN. The rationale for the use of these agents is based on animal experiments suggesting a pathogenetic role of reactive oxygen species in the occurrence of CIN [8181. Yoshimasa Y, Fogo A, Beckman JD. Reduced activity of antioxidant enzymes underlies contrast media-induced renal injury in volume depletion. Kidney Int. 1992;41:1008-1015. , 8282. Bakris GL, Lass N, Gaber AO, Jones JD, Burnett JC. Radiocontrast medium-induced declines in renal function: a role for oxygen free radicals. Am J Physiol Renal Physiol. 1990;258:F115-120. , 8383. Parvez Z, Rahman MA, Moncada R. Contrast media-induced lipid peroxidation in the rat kidney. Invest Radiol. 1989;24:697-702. , 8484. Hanss BG, Valencia SH, Shah SV, Vari SC. The iron chelator desferoxamine prevents contrast media induced acute renal failure in the rabbit. J Am Soc Nephrol. 1990;1:612. ].
N-acetylcysteine
In 2004, Drager et al demonstrated that after CA exposure, urinary levels of 15-isoprostane F2, a specific marker of oxidative stress, increased significantly over baseline values in patients receiving hydration only [8585. Drager LF, Andrade L, Barros de Toledo JF, Laurindo FR, Machado César LA, Seguro AC. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant. 2004;19:1803-1807. ]. Conversely, they remained essentially unchanged in patients treated with hydration and the N-acetyl derivative of cysteine, known as N-Acetylcysteine (NAC). Furthermore, NAC treatment led to lower levels of alpha-glutathione S-transferase, a specific proximal tubular injury marker, as compared to hydration only.
This drug is the most widely studied agent of all prophylaxis strategies. It has direct vasodilating effects on kidney vessels, contributing to improved renal haemodynamics, and may also attenuate endothelial dysfunction. More notably, it is also able to scavenge oxygen-free radicals, thus preventing the direct oxidative tissue damage occurring after CA administration [8585. Drager LF, Andrade L, Barros de Toledo JF, Laurindo FR, Machado César LA, Seguro AC. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant. 2004;19:1803-1807. , 8686. DiMari J, Megyesi J, Udvarbelyi N, Price P, Davis R, Safirstein R. N-acetylcysteine ameliorates ischemic renal failure. Am J Physiol. 1997;272:F292-F298. , 8787. Conesa EL, Valero F, Nadal JC, Fenoy FJ, López B, Arregui B, Salom MN. N-acetyl-L-cysteine improves renal medullary hypoperfusion in acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R730-R737. , 8888. Forman MB, Puett DW, Cates CU, McCroskey DE, Beckman JK, Greene HL, and Virmani R. Glutathione redox pathway and reperfusion injury. Effect of N-acetylcysteine on infarct size and ventricular function. Circulation. 1988;78:202-213. , 8989. Lopez BL, Snyder JW, Birenbaum DS, Ma XI. N-acetylcysteine enhances endothelium-dependent vasorelaxation in the isolated rat mesenteric artery. Ann Emerg Med. 1998;32:405-410. , 9090. Heyman SN, Goldfarb M, Shina A, Karmeli F, Rosen S. N-acetylcysteine ameliorates renal microcirculation: studies in rats. Kidney Int. 2003;63:634-641. ].
After the publication of the seminal study by Tepel et al [9191. Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180-4.
First clinical demonstration that prophylactic oral administration of N-acetylcysteine, along with hydration, prevents the reduction in renal function induced by the intravenous administration of iopromide, a non-ionic, low-osmolality contrast agent, in patients with chronic renal insufficiency.] showing that NAC offers some protection, a large number of studies, in most cases with relatively small sample sizes, were published. Indeed, Tepel et al demonstrated that NAC (600 mg orally twice daily) plus hydration before and after CA administration has a CIN preventive effect in patients with renal insufficiency undergoing computed tomography with a fixed dose (75 mL) of CA. The results were impressive: the rate of CIN was 21% in the placebo group and only 2% in the NAC group (p=0.01). This finding, however, was supported by some, but not all subsequent clinical trials investigating the efficacy of NAC as a CIN preventive agent, both in patients with pre-existing CKD and in those with normal renal function [9292. Shyu KG, Cheng JJ, Kuan P. Acetylcysteine protects against acute renal damage in patients with abnormal renal function undergoing a coronary procedure. J Am Coll Cardiol. 2002;40:1383-1388. , 9393. Kay J, Chow WH, Chan TM, Kai Lo S, Kwok OH, Yip A, Fan K, Lee CH, Lam WF. Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention: a randomized controlled trial. JAMA. 2003;289:553-558. , 9494. Briguori C, Manganelli F, Scarpato P, Elia PP, Golia B, Riviezzo G, Lepore S, Librera M, Villari B, Colombo A, Ricciardelli B. Acetylcysteine and contrast-agent associated nephrotoxicity. J Am Coll Cardiol. 2002;40:298-303. ].
Several meta-analyses have been published on this topic [9595. Birck R, Krzossok S, Markowetz F, Schnülle P, Van der Woude FJ, Braun C. Acetylcysteine for prevention of contrast nephropathy. Lancet. 2003;362:598-603. , 9696. Isenbarger D, Kent S, O’Malley P. Meta-analysis of randomized clinical trials on the usefulness of acetylcysteine for prevention of contrast nephropathy. Am J Cardiol. 2003;92:1454-1458. , 9797. Alonso A, Lau J, Jaber BL, Weintraub A. Prevention of radiocontrast nephropathy with N-acetylcysteine in patients with chronic kidney disease: a meta-analysis of randomized, controlled trials. Am J Kidney Dis. 2004;43:1-9. , 9898. Kshirsagar A, Poole C, Mottl A, Shoham D, Franceschini N, Tudor G, Agrawal M, Denu-Ciocca C, Ohman EM, and Finn WF. N-acetylcysteine for the prevention of radiocontrast induced nephropathy: a meta-analysis of prospective controlled trials. J Am Soc Nephrol. 2004;15:761-769. , 9999. Pannu N, Manns B, Lee H, Tonelli M. Systematic review of the impact of N-acetylcysteine on contrast nephropathy. Kidney Int. 2004;65:1366-1374. , 100100. Guru V, Fremes S. The role of N-acetylcysteine in preventing radiographic contrast-induced nephropathy. Clin Nephrol. 2004;62:77-83. , 101101. Bagshaw S, Ghali WA. Acetylcysteine for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. BMC Med. 2004;2:38. , 102102. Misra D, Leibowitz K, Gowda R, Shapiro M, Khan I. Role of N-acetylcysteine in prevention of contrast-induced nephropathy after cardiovascular procedures: a meta-analysis. Clin Cardiol. 2004;27:607-610. , 103103. Nallamothu BK, Shojania KG, Saint S, Hofer TP. Is acetylcysteine effective in preventing contrast-related nephropathy?. a meta-analysis. Am J Med. 2004;117:938-947. , 104104. Liu R, Nair D, Ix J, Moore D, Bent S. N-acetylcysteine for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. J Gen Intern Med. 2005;20:193-200. , 105105. Duong M, MacKenzie T, Malenka D. N-Acetylcysteine prophylaxis significantly reduces the risk of radio-contrast-induced nephropathy. Catheter Cardio-vasc Interv. 2005;64:471-479. ] ( Table 7 ). By combining the data from available prospective controlled clinical trials that used NAC, Tepel et al [106106. Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation. 2006;113:1799-1806. ] reported an overall significant relative risk reduction in patients with CKD receiving NAC. Nine meta-analyses presented pooled risk estimates suggesting benefit. However, as the available literature is greatly heterogeneous, the benefit of oral NAC among all individuals with renal insufficiency cannot be confirmed definitively [9898. Kshirsagar A, Poole C, Mottl A, Shoham D, Franceschini N, Tudor G, Agrawal M, Denu-Ciocca C, Ohman EM, and Finn WF. N-acetylcysteine for the prevention of radiocontrast induced nephropathy: a meta-analysis of prospective controlled trials. J Am Soc Nephrol. 2004;15:761-769. ]. Differences in CA type and volume, definition of CIN, patient selection, type of intervention, applied hydration regimen, NAC dose (cumulative dosage varied between 1,500 mg and >10,000 mg in the different studies) and route of administration (intravenous vs. oral), as well as the timing of the cardiovascular procedure (urgent vs. elective) may have contributed to the heterogeneity (i.e., variation of effect across studies greater than can be expected by chance) observed in the pooled analysis [107107. Kelly AM, Dwamena B, Cronin P, Bernstein SJ, Carlos RC. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008;148:284-294. ].
An interesting point was raised by Briguori et al who observed that NAC-associated renal protection was restricted to patients receiving small (<140 mL) volumes of contrast [9494. Briguori C, Manganelli F, Scarpato P, Elia PP, Golia B, Riviezzo G, Lepore S, Librera M, Villari B, Colombo A, Ricciardelli B. Acetylcysteine and contrast-agent associated nephrotoxicity. J Am Coll Cardiol. 2002;40:298-303. ]. They postulated that the discordance in results among different studies might be due to the amount of CA administered. Indeed, patients undergoing PCI, EVAR and other peripheral vascular interventions often require larger CA doses as compared to that (75 mL) used by Tepel et al [9191. Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180-4.
First clinical demonstration that prophylactic oral administration of N-acetylcysteine, along with hydration, prevents the reduction in renal function induced by the intravenous administration of iopromide, a non-ionic, low-osmolality contrast agent, in patients with chronic renal insufficiency.].
Additional studies seem to support this hypothesis. In the RAPPID study, patients with mild-to-moderate CKD undergoing PCI were randomised to receive NAC and intravenous hydration or intravenous hydration alone. NAC was given intravenously at a dose of 150 mg/kg before CA exposure, followed by 50 mg/kg over the subsequent 4 hours [108108. Baker CSR, Wragg A, Kumar S, De Palma R, Baker LRI, and Knight CJ. A rapid protocol for the prevention of contrast-induced renal dysfunction: the RAPPID study. J Am Coll Cardiol. 2003;41:2114-2118. ]. Therefore, for a 70 kg patient, the cumulative NAC dose was 14,000 mg, a value much greater than that used by most authors (2,400 mg). In the two groups, the contrast volumes were 238 mL and 222 mL and CIN occurred in 5% and 21% of cases, respectively (p=0.045). In another study by Briguori et al, two different NAC dosages were compared (600 mg vs. 1,200 mg orally twice daily) before and after CA administration in patients with mild CKD undergoing coronary and/or peripheral procedures [108108. Baker CSR, Wragg A, Kumar S, De Palma R, Baker LRI, and Knight CJ. A rapid protocol for the prevention of contrast-induced renal dysfunction: the RAPPID study. J Am Coll Cardiol. 2003;41:2114-2118. ]. The incidence of CIN was lower in patients receiving a double dose of NAC (3.5% vs. 11%; p=0.038). The benefit of double-dose NAC was greater for patients receiving a CA dose >140 mL (5.4% vs. 18.9%; p=0.039) than for those who received a CA dose <140 mL (1.7% vs. 3.6%; p=0.61). Thus, the emerging concept from these studies is that a greater dose of NAC is probably needed in CKD patients undergoing PCI, suggesting a dose-dependent protective effect of this drug. Further evidence of a possible dose-dependent effect of NAC derives from a study evaluating its use for the prevention of CIN in patients with STEMI undergoing primary PCI [110110. Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, De Metrio M, Galli S, Fabbiocchi F, Montorsi P, Veglia F and Bartorelli AL. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773-2782. ]. In this study, a total of 352 STEMI patients were randomly assigned to receive placebo (control group, n=119), an intravenous bolus of 600 mg of NAC before PCI, followed by an oral administration (600 mg twice daily) for the following 48 hours (NAC total dose = 3,000 mg) (NAC group, n=116), or an intravenous bolus of 1,200 mg of NAC before intervention, followed by an oral administration (1,200 twice daily) for the following 48 hours (NAC total dose = 6,000 mg) (high-dose NAC group, n=118). The observed rate of CIN (increase in SCr >25%) was 37% in the control group, 15% in the NAC group, and 8% in the high-dose NAC group (P<0.001). When an absolute rise in SCr (>0.5 mg/dL/>44micromol/l) was considered, the frequency of CIN was 18%, 6%, and 3%, respectively (p<0.001). A significant trend toward a reduction of in-hospital death and other clinical complications in patients receiving NAC was also observed. However, the mechanisms through which NAC may reduce CIN and improve clinical outcomes in this clinical setting remain unclear and additional studies should investigate whether the extra-renal effects of NAC play some beneficial role. Indeed, in both clinical and experimental acute myocardial infarction studies, intravenous infusion of NAC was associated with decreased infarct size and left ventricular function improvement, possibly due to the antioxidant and free radical scavenger properties of this drug [111111. Arstall MA, Yang J, Stafford I, Betts WH, Horowitz JD. N-acetylcysteine in combination with nitroglycerin and streptokinase for the treatment of evolving acute myocardial infarction: safety and biochemical effects. Circulation. 1995, 92:2855-2862. , 112112. Sochman J, Kole J, Vrana M, Fabian J. Cardioprotective effects of N-acetylcysteine: the reduction in the extent of infarction and occurrence of reperfusion arrhythmias in the dog. Int J Cardiol. 1990;28:191-196. ]. These cardiac effects may be enhanced in patients treated with primary PCI. This is a clinical setting in which oxidative stress and reperfusion injury were demonstrated to occur, and in which these deleterious phenomena are particularly pronounced due to the high coronary patency rates, with rapid and complete flow restoration. Moreover, it has been demonstrated that NAC inhibits platelet aggregation, and this effect too could be relevant during acute coronary thrombosis and mechanical thrombus fragmentation [113113. Anfossi G, Russo I, Massucco P, Mattiello L, Cavalot F, Trovati M. N-acetyl-L-cysteine exerts direct anti-aggregating effect on human platelets. Eur J Clin Invest. 2001;31:452-461. ].
Positive results were also observed in the RENO study in which hydration with sodium bicarbonate plus NAC, started just before CA injection and continued for the following 12 hours in patients undergoing emergency PCI (primary PCI in 43% of cases), reduced the incidence of CIN (1.8% vs. 21.8%; p<0.001) and anuric AKI (1.8% vs. 12.7%; p=0.032) in comparison to the standard hydration protocol consisting of intravenous isotonic saline for 12 hours after PCI [114114. Recio-Mayoral A, Chaparro M, Prado B, Cózar R, Méndez I, Banerjee D, Kaski JC, Cubero J, and Cruz JM. The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the RENO study. J Am Coll Cardiol. 2007;49:1283-8.
A prospective, controlled, randomised, single-centre trial in consecutive patients with acute coronary syndrome undergoing emergency PCI showing that rapid intravenous hydration with sodium bicarbonate plus high dose of N-acetylcysteine before contrast injection is effective and safe in the prevention of contrast-induced nephropathy.]. In both groups, two doses of oral NAC were administered the next day.
A recent study, the Prevention of Serious Adverse Events Following Angiography (PRESERVE) trial, randomized 5177 patients at high risk for renal complications undergoing mainly coronary angiography to receive i.v sodium bicarbonate or i.v. sodium chloride and 5 days of oral NAC or oral placebo. No significant between-group differences in the rates of CIN was observed. However, it is noteworthy that, despite the fact that the enrolled patients were defined as being at high risk, less than 30% of them underwent PCI and a low CA amount (median 85 ml) was administered [179179. Weisbord SD, Gallagher M, Jneid H, Garcia S, Cass A, Thwin SS, Conner TA, Chertow GM, Bhatt DL, Shunk K, Parikh CR, McFalls EO, Brophy M, Ferguson R, Wu H, Androsenko M, Myles J, Kaufman J, Palevsky PM, for the PRESERVE Trial Group. Outcomes after angiography with sodium bicarbonate and acetylcysteine. New Engl J Med. 2017;378:603-614. ].
A meta-analysis evaluating the efficacy of high-dose NAC for the prevention of CIN suggests that higher NAC dose may be beneficial in true high-risk patients [115115. Trivedi H, Daram S, Szabo A, Bartorelli AL, Marenzi G. High-dose N-acetylcysteine for the prevention of contrast-induced nephropathy. Am J Med. 2009; 122:874.
e9-e15]. High-dose NAC was a priori defined as a daily dose greater than 1,200 mg or a single periprocedural dose greater than 600 mg, periprocedural being defined as immediately or within 4 hours of planned CA exposure. Sixteen prospective studies of patients (total sample size of 1,677 subjects) randomised to NAC administered either orally or intravenously versus a control group (842 assigned to high-dose NAC, 835 to the control arm) were included in this meta-analysis. The overall effect size revealed an odds ratio of 0.46 (95% CI: 0.33–0.63; p<0.0001) for the occurrence of CIN with the use of high-dose NAC, suggesting a significant protective effect. Notably, in this meta-analysis, the definition of high-dose NAC was arbitrary. It must be noted, however, that no dose finding studies with NAC used as a CIN preventive agent were performed. Indeed, the so-called standard dosing is purely based on the original Tepel et al study that employed a 600 mg dose two times a day. Therefore, the appropriate dose remains uncertain except that a dose greater than that used by Tepel et al decreases the risk of CIN. A similar lack of clear guidance limits the choice of the optimal route of administration (oral vs. intravenous), since studies have employed varying routes. Further, and more importantly, the existing data do not address the issue of whether high-dose NAC has any impact on clinical outcome other than that on the incidence of CIN. Data from ACT (Acetylcysteine for the prevention of Contrast-induced nephropaThy), a large randomised, controlled trial evaluating 2,308 at-risk patients undergoing elective intravascular angiography at 46 centres in Brazil, showed no difference, in terms of CIN rate, need for dialysis and cardiovascular mortality between NAC and placebo [116116. ACT Ivestigators. Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized Acetylcysteine for Contrast-induced nephropathy Trial (ACT). Circulation. 2011;124:1250-9. ].
There have been some concerns that NAC may affect SCr, the surrogate marker of GFR that is routinely employed in clinical practice, without affecting GFR. In an uncontrolled study of healthy volunteers, one group of investigators found that NAC reduced SCr but not cystatin C, and speculated that NAC may have an effect on SCr independent of GFR [117117. Hoffmann U, Fischereder M, Kruger B, Drobnik W, Kramer BK. The value of N-acetyl-cysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15:407-410. ]. However, this NAC effect was never confirmed in the setting of AKI. Experimental administration of high doses of NAC (200 and 500 mg/kg) to protect against AKI showed a similar pattern of change in SCr and cystatin C, indicating no influence of NAC on SCr levels [118118. Zhu J, Yin R, Shao H, Dong G, Luo L, Jing H. N-acetylcysteine to ameliorate acute renal injury in a rat cardiopulmonary bypass model. J Thorac Cardiovasc Surg. 2007;133:696-703. ]. In another study in which SCr and cystatin C were both assayed, the NAC arm showed a significant correlation between SCr and cystatin C at baseline and a better correlation 48 hours after CA exposure [119119. Poletti PA, Saudan P, Platon A, et al. I.V. N-acetylcysteine and emergency CT: use of serum creatinine and cystatin C as markers of radiocontrast nephrotoxicity. Am J Roentgenol. 2007;189:687-692. ]. In agreement with these results, a randomised trial designed to prevent AKI in CKD patients and employing high-dose NAC (300 mg/kg IV) showed no difference in the direction of SCr and cystatin C response and ruled out a creatinine lowering effect or a difference in urinary creatinine excretion related to NAC [120120. Haase M, Haase-Fielitz A, Ratnaike S, Reade MC, Bagshaw SM, Morgera S, Dragun D, Bellomo R. N-Acetylcysteine does not artifactually lower plasma creatinine concentration. Nephrol Dial Transplant. 2008;23:1581-1587. ]. Finally, a more recent study in which a “double dose” of NAC was administered in the absence of CA to patients with stable CKD showed no effect of NAC on either SCr or cystatin C levels [121121. Rehman T, Fought J, Solomon R. N-acetylcysteine effect on serum creatinine and cystatin C levels in CKD patients. J Am Soc Nephrol. 2008;3:1610-1614. ].
Based on the evidence of the most recent literature, and given its potential benefit, low-cost and excellent safety profile, the use of high-dose of NAC should be recommended in all high-risk patients for the prevention of CIN, particularly when its grim prognosis is considered.
Ascorbic acid
Additional evidence of the effectiveness of an antioxidant strategy comes from the study by Spargias et al, who investigated the impact of ascorbic acid in a randomised, double-blind, placebo-controlled trial including 231 patients with a SCr concentration ≥1.2 mg/dL (>53 micromol/l) undergoing coronary angiography and/or intervention [122122. Spargias K, Alexopoulos E, Kyrzopoulos S, Iacovis P, Greenwood DC, Manginas A, Voudris V, Pavlides G, Buller CE, Kremastinos D, and Cokkinos DV. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation. 2004;110:2837-2842. ]. Ascorbic acid (3 g at least 2 hours before the procedure and 2 g in the night and the morning after the procedure) or placebo was administered orally. CIN occurred in 9% of the ascorbic acid group and in 20% of the placebo group (p=0.02). The antioxidant ascorbic acid has been shown to attenuate renal damage caused by a variety of insults, such as post-ischaemic stress, cisplatin, aminoglycosides, and potassium bromate in animals. When added to NAC, however, ascorbic acid did not show any improvement as compared to NAC alone [123123. Briguori C, Airoldi F, D’Andrea D, Bonizzoni E, Morici N, Focaccio A, Michev I, Montorfano M, Carlino M, Cosgrave J, Ricciardelli B, and Colombo A. Renal insufficiency following contrast adminstration trial (REMEDIAL). A randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211-1217. ]. Thus, the possible benefits of ascorbic acid deserve further investigation.
Trimezatidine
This drug has initially been described as a cellular anti-ischaemic agent. Further studies, however, demonstrated that trimezatidine exerts potent antioxidant activity in myocardial, renal, and hepatic ischaemia-reperfusion injury. In a randomised, controlled trial, trimezatidine (20 mg t.i.d. orally for 72 hours starting 48 hours before the procedure) in addition to standard intravenous saline hydration was compared with hydration alone in 82 patients with mild CKD undergoing elective coronary procedures [124124. Onbasili AO, Yeniceriglu Y, Agaoglu P, Karul A, Tekten T, Akar H, Discigil G. Trimetazidine in the prevention of contrast-induced nephropathy after coronary procedures. Heart. 2007;93:698-702. ]. The incidence of CIN was significantly lower in patients receiving trimezatidine (2.5% vs. 16.6%; p<0.05). The potential usefulness of this drug in the prevention of CIN, particularly in higher risk patients, should be investigated in larger prospective clinical studies.
Other drugs
Statins
It has been suggested that statins may reduce CIN by means of their beneficial effects on endothelial function and oxidative stress. A retrospective review of 1,002 patients with CKD undergoing coronary angiography suggested that the risk of CIN was lower in patients in whom a statin was initiated just before the procedure [125125. Attalah N, Yassine L, Musial J, Yee J, Fisher K. The potential role of statins in contrast nephropathy. Clin Nephrol. 2004;62:273-278. ]. The results of a large PCI registry study including 29,409 patients also confirmed this conclusion [126126. Khanal S, Attallah N, Smith DE, Kline-Rogers E, Share D, O’Donnell MJ, Moscucci M. Statin therapy reduces contrast-induced nephropathy: an analysis of contemporary percutaneous interventions. Am J Med. 2005;118:843-849. ]. The effect of statin therapy on CIN prevention was recently evaluated by two randomised trials. Toso et al conducted a placebo-controlled trial of high-dose atorvastatin (80 mg/day), before and after CA exposure compared with placebo added to a protocol of intravenous normal saline, oral high-dose NAC (1,200 mg), and IOCA [127127. Toso A, Maioli M, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, Manzone C, Amato M, Bellandi F. Usefulness of atorvastatin (80 mg) in prevention of contrast-induced nephropathy in patients with chronic renal disease. Am J Cardiol. 2010;105:288-292. ]. They demonstrated that high-dose atorvastatin had no effect on CIN rate. Xinwei et al compared two dosages of simvastatin (80 mg vs. 20 mg) in patients undergoing elective PCI [128128. Xinwei J, Xianghua F, Jing Z, Xinshun G. Comparison of usefulness of simvastatin 20 mg versus 80 mg in preventing contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol. 2009, 104:519–524. ]. At 48 hours, patients receiving 80 mg of simvastatin had significantly fewer CIN events than patients receiving the lower dose of this drug. Short-term pre-treatment with high-dose simvastatin did not prevent CIN after administration of CA in CKD patients undergoing coronary angiography in the PROMISS trial [129129. Jo SH, Koo BK, Park JS, Kang HJ, Cho YS, Kim YJ, Youn TJ, Chung WY, Chae IH, Choi DJ, Sohn DW, Oh BH, Park YB, Choi YS, Kim HS. Prevention of radiocontrast medium-induced nephropathy using short-term high-dose simvastatin in patients with renal insufficiency undergoing coronary angiography (PROMISS) trial--a randomized controlled study. Am Heart J. 2008;155:499.
e1-8]. Conversely, recent studies reported a potential benefit of atorvastatin and pravastatin [130130. Patti G, Nusca A, Chello M, Pasceri V, D’Ambrosio A, Vetrovec GW, Di Sciascio G. Usefulness of statin pretreatment to prevent contrast-induced nephropathy and to improve long-term outcome in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2008;101:279-285. , 131131. Ozhan H, Erden I, Ordu S, Aydin M, Caglar O, Basar C, Yalcin S, Alemdar R. Efficacy of short-term high-dose atorvastatin for prevention of contrast-induced nephropathy in patients undergoing coronary angiography. Angiology. 2010;61:711-714. , 132132. Yoshida S, Kamihata H, Nakamura S, Senoo T, Manabe K, Motohiro M, Sugiura T, Iwasaka T. Prevention of contrast-induced nephropathy by chronic pravastatin treatment in patients with cardiovascular disease and renal insufficiency. J Cardiol. 2009;54:192-198. ], and rosuvastatin [133133. Leoncini M, Toso A, Maioli M, Tropeano F, Villani S, Bellandi F. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome. Results from protective effect of rosuvastatin and antiplatelet therapy on contrast-induced acute kidney injury and myocardial damage in patients with acute coronary syndrome (PRATO-ACS Study). J Am Coll Cardiol. 2014;63:71-9. , 134134. Han Y, Zhu G, Han L, Hou F, Huang W, Liu H, Gan J, Jiang T, Li X, Wang W, Ding S, Jia S, Shen W, Wang D, Sun L, Qiu J, Wang X, Li Y, Deng J, Li J, Xu B, Mehran R, Huo Y. Short-term rosuvastatin therapy for prevention of contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease. J Am Coll Cardiol. 2014;63:62-70. ]. Three meta-analyses recently published on this topic provided conflicting results. Zhang et al. [135135. Zhang T, Shen LH, Hu LH, He B. Statins for the prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Am J Nephrol. 2011;33:344-51. ] reported a benefit of short-term, high-dose statin treatment in patients undergoing procedures requiring contrast exposure. A second meta-analysis reported a non-significant protective trend with statin pre-treatment [136136. Zhang BC, Li WM, Xu YW. High-dose statin pretreatment for the prevention of contrast-induced nephropathy: a meta-analysis. Can J Cardiol. 2011;27:851-8. ]. Finally, Zhou et al. [137137. Zhou Y, Yuan WJ, Zhu N, Wang L. Short-term, high-dose statins in the prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Clin Nephrol. 2011;76:475-83. ] found a beneficial effect in the subgroup of patients with stage >3 CKD only. Several potential confounders, such as type and dose of statin, length of pre-treatment, patients’ baseline characteristics and clinical setting may explain the heterogeneous results of randomised trials and meta-analyses performed so far. In particular, a recent meta-analysis support the use of high-dose, pre-procedural statin therapy to reduce the risk of AKI in ACS patients, while its role in non-ACS patients remain uncertain [180180. Marenzi G, Cosentino N, Werba JP, Tedesco CC, Veglia F, Bartorelli AL. A meta-analysis of randomized controlled trials on statins for the prevention of contrast-induced acute kidney injury in patients with and without acute coronary syndromes. Int J Cardiol. 2015;183:47-53. ].
Combination therapy
A recent meta-analysis reported the results of randomised controlled trials investigating the combined prophylaxis with sodium bicarbonate and NAC for the prevention of CIN [138138. Brown JR, Block CA, Malenka DJ, O’Connor GT, Schoolwerth AC, and Thompson CA. Sodium bicarbonate plus N-acetylcysteine prophylaxis: a meta-analysis. J Am Coll Cardiol Cardiovasc Interv. 2009, 2:1116–1124. ]. Among ten randomised trials analysed there was a non-significant trend toward a 35% reduction in CIN rate, with a pooled RR of 0.65 (95% CI, 0.40-1.05). A similar overall trend was found regarding dialysis (RR, 0.47; 95%CI, 0.16-1.41). Another combination therapy that has been tested is NAC plus theophylline. The combination of oral NAC and theophylline in conjunction with normal saline hydration has been shown to reduce CIN as compared to normal saline or normal saline plus NAC, with a RR of 0.48 (95% CI, 0.41-0.57) and RR of 0.49 (95% CI,0.40-0.57), respectively [139139. Baskurt M, Okcun B, Abaci O, Dogan GM, Kilickesmez K, Ozkan AA, Ersanli M and Gurmen T. N-acetylcysteine versus Nacetylcysteine + theophylline for the prevention of contrast nephropathy. Eur J Clin Invest. 2009, 39:793–799. ]. Similarly, another randomised controlled trial has shown the efficacy of theophylline added to sodium bicarbonate to significantly reduce CIN, with a RR of 0.61 (95% CI, 0.48–0.78) [140140. Malhis M, Al-Bitar S, Al-Deen Zaiat K. The role of theophylline in prevention of radiocontrast media-induced nephropathy. Saudi J Kidney Dis Transpl. 2010, 21:276–283. ].
Renal replacement therapies
Haemodialysis
On the basis of studies demonstrating its effectiveness in CA removal, haemodialysis (HD) has been proposed as a CIN preventive strategy after radiographic procedures. However, several studies have shown that the strategy of performing HD immediately after administration of CA in patients with reduced renal function does not prevent CIN [141141. Moon SS, Back SE, Kurkus J, Nilsson Ehle P. Hemodialysis for elimination of the nonionic contrast medium iohexol after angiography in patients with impaired renal function. Nephron. 1995;70:430-437. , 142142. Lehnert T, Keller E, Gondolf K, Schäffner T, Pavenstädt H, and Schollmeyer P. Effect of hemodialysis after contrast medium administration in patients with renal insufficiency. Nephrol Dial Transplant. 1998;13:358-362. , 143143. Sterner G, Frennby B, Kurkus J, Nyman U. Does post-angiographic hemodialysis reduce the risk of contrast medium nephropathy?. Scand J Urol Nephrol. 2000;34:323-326. , 144144. Vogt B, Ferrari P, Schonholzer C, Marti HP. Prophylactic hemodialysis after radiocontrast media in patients with renal insufficiency is potentially harmful. Am J Med. 2001;111:692-698. ]. The discrepancy between the effective removal of CA obtained with HD and the lack of a preventive effect against CIN may be the result of HD-related nephrotoxicity, caused by activation of inflammatory reactions, coagulation processes, and release of vasoactive substances that may induce acute hypotension [145145. Marenzi G, Bartorelli A. Recent advances in the prevention of radiocontrast-induced nephropathy. Curr Opinion Crit Care. 2004;10:205-209. ]. Furthermore, haemodynamic instability due to the osmotic shift of fluid from the intravascular to the interstitial and intracellular compartments, and to the dialysis-associated ultrafiltration, is frequently observed during HD. Hypovolaemia can induce renal hypoperfusion, vasoconstriction, and ischaemic injury. A third possible reason may be that renal injury may occur rapidly after administration of CA before HD is started. Indeed, in most of the studies, CA removal by HD was started after a relatively long time, even hours, after the initial administration of CA, whereas renal hypoperfusion has been demonstrated within 20 minutes after the injection of CA, suggesting that the renal insult may occur at their first renal haemodynamic passage. Thus, the lack of clinical benefit could be due to an overlong delay between exposure to and elimination of CA. However, this study also failed to support the hypothesis that a simultaneous HD therapy may protect the patient from CIN, presumably because plasma peak concentration of CA was not affected by this type of therapy. Indeed, the peak value of CA concentration, more than the time to which kidneys are exposed to them, is thought to be the major factor responsible for CIN.
Haemofiltration
This is a simple renal replacement therapy that can be easily performed by personnel without specific nephrological expertise. With HF, effective fluid and solute removal can be achieved with fluid volume control and haemodynamic stability greater than HD. The improved haemodynamic stability represents a clear advantage of HF over HD, especially in the treatment of patients with associated acute renal and cardiac insufficiency. A randomised study has provided evidence that HF offers protection against CIN in high-risk patients [6464. Marenzi G, Marana I, Lauri G, Assanelli E, Grazi M, Campodonico J, Trabattoni D, Fabbiocchi F, Montorsi P, Bartorelli AL. The prevention of radiocontrast-agent-induced nephropathy by hemofiltration. N Engl J Med. 2003; 349:1333-40.
This is the first prospective, randomised study demonstrating the effectiveness of hemofiltration, as compared with saline hydration, for the prevention of contrast-induced nephropathy in patients with chronic renal insufficiency undergoing elective PCI. ]. CIN occurred in only 5% of patients in the HF group, and in 50% of patients in the control group (p<0.001). Moreover, in-hospital and 1-year mortality rates were significantly reduced in patients treated with HF when compared to the control group (2% vs. 18% and 10% vs. 30%, respectively). The mechanisms involved in the prophylactic effect of HF remain unclear. The positive results may derive from its ability to remove CA from the circulation, thereby reducing kidney exposure to their nephrotoxic effects. However, this hypothesis is questioned by the results of another randomised clinical study from Italy, in which two different HF protocols for the prevention of CIN in patients with severe CKD (creatinine clearance <30 mL/min) and scheduled for elective cardiovascular procedures were compared [146146. Marenzi G, Lauri G, Campodonico J, Marana I. Comparison of two hemofiltration protocols for prevention of contrast-induced nephropathy in high-risk patients. Am J Med. 2006;119:155-162. ]. One group was treated with HF for 18 to 24 hours after the procedure (post-HF group), while another group underwent HF for 6 hours before, and for 18 to 24 hours after CA administration (pre/post-HF group). Twenty-six per cent of patients in the post-HF group experienced CIN, as compared with only 3% of the pre/post-HF group (p=0.0013). This study confirmed that HF is particularly effective in preventing CIN and the associated poor outcome in high-risk patients. It also demonstrated that a pre-procedural session is necessarily required in order to obtain the full clinical benefit of this treatment. Of note, the most recent guidelines on myocardial revascularisation of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) have recommended (Class IIa recommendation, level of evidence B) the use of prophylactic HF for the prevention of CIN in patients with severe CKD undergoing complex PCI [147147. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501-2555. ].
Other non-pharmacological strategies for CIN prevention
Electronic warning systems. Cho et al. [148148. Minsinger KD, Kassis HM, Block CA, Sidhu M, Brown JR. Meta-analysis of the effect of automated contrast injection devices versus manual injection and contrast volume on risk of contrast-induced nephropathy. Am J Cardiol. 2014;113:49-53. ] demonstrated that using a computerised alertness program able to make the physician aware by a warning message that CIN prophylactic measures are indicated may decrease the risk of CIN (3% vs. 10%; P=0.02) in hospitalised CKD patients undergoing contrast-enhanced computed tomography.
Automated contrast injector systems (ACIS). The volume of CA is a major modifiable risk factor for CIN. ACIS have been shown to be associated with a reduction of CA total volume. Indeed, in a recent meta-analysis including 79,694 patients from 10 studies, ACIS reduced CA volume delivery by 45 ml/case. In addition, CIN incidence was significantly reduced by 15% when ACIS was compared to manual injection [149149. Sekiguchi H, Ajiro Y, Uchida Y, Ishida I, Otsuki H, Hattori H, Arashi H, Kobayashi Y, Jujo K, Yamaquchi J, Li M, Iwade K, Tanaka N, Shiimamoto K, Tsurumi Y, Kawana M, Hagiwara N. Oxygen pre-conditioning prevents contrast-induced nephropathy (Option CIN Study). J Am Coll Cardiol. 2013;62:162-163. ].
Oxygenation support. Sekiguchi et al. [150150. Er F, Nia AM, Dopp H, Hellmich M, Dahlem KM, Caglayan E, Kubacki T, Benzing T, Erdmann E, Burst V, Gassanov N. Randomized pilot RenPro trial (Renal Protection Trial). Ischemic preconditioning for prevention of contrast medium-induced nephropathy. Circulation. 2012;126:296-303. ] randomly assigned 349 patients undergoing elective coronary angiography/ PCI to either an oxygenation group (oxygen administration via nasal cannula; 2 L/min of oxygen from 10 minutes before the procedure to the end of the procedure [n=174]) or to a control group (room air; n=175). Isotonic (0.9%) saline solution was administered 12 hours before and 12 hours after the procedure in both groups. There were no significant differences in patient characteristics between groups, including CA volume, sCr and GFR. The baseline PaO2 was significantly higher in the oxygen preconditioning group than in the control group (134±28 mmHg vs. 90±12 mmHg; P<0.001). The incidence of CIN was significantly lower in the oxygen preconditioning group than in the control group (0.6% vs. 5.1%; P=0.01), indicating that oxygen preconditioning in conjunction with standard hydration may reduce CIN risk. Notably, the protective effect was more pronounced in patients with CKD.
Remote ischaemic preconditioning. Er et al. [151151. Duffy SJ, Ruygrok P, Juergens CP, Sievert H, Richards M, Blake J, Whitbourn R, Farouque HO, Pertile T, Kaye DM. Removal of contrast media from the coronary sinus attenuates renal injury after coronary angiography and intervention. J Am Coll Cardiol. 2010; 56:525-526. ] demonstrated that remote ischaemic preconditioning, induced by intermittent upper-arm ischaemia prior to elective coronary angiography, markedly reduced the incidence of CIN in patients with CKD and in those at high risk of CIN (OR 0.21, 95% CI 0.07-0.57; P=0.002). Ischaemic preconditioning was performed with a standard upper-arm blood pressure cuff alternating 4 cycles of 5 minutes of inflation (cuff inflated to the individual’s systolic blood pressure plus 50 mmHg) and 5 minutes of deflation to induce transient and repetitive arm ischaemia and reperfusion. Although the protective mechanism of remote ischemic preconditioning is still unknown, it has been postulated that release into the systemic circulation of humoral factors, such as adenosine and bradykinin from the target area may protect remote organs as the kidney.
Removal of contrast media from the coronary sinus. The coronary sinus is the main drainage of myocardial venous blood and of CA after coronary angiography. Recently, a new system (CINCOR Contrast Removal System, Osprey Medical, St. Paul, Minnesota), consisting of a dedicated coronary sinus CA extraction catheter that may be inserted via the internal jugular or the femoral veins, has been proposed for CIN prevention. In brief, the catheter extracts small volumes of CA-laden blood from the coronary sinus shortly after CA injection. Early data suggest that the withdrawal of CA from the coronary sinus may reduce systemic exposure and attenuate the expected nephrotoxicity [152152. Barrett BJ, Carlisle EJ. Meta-analysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology. 1993;188:171-178. ]. These findings, however, warrant further investigation in a large-scale randomised trial to evaluate the effectiveness of coronary sinus contrast capture to reduce the risk of CIN and its complications.
Contrast media reduction device, the DyeVert™ system.
The most modifiable approach to reduce the risk of CIN for a treating physician is to minimize CA administration. The DyeVert™ system (Ospery Medical, Inc.,Minnetonka, MN, USA) is a single-use sterile device aimed at contrast reduction which is connected between a manual injection syringe and a manifold of choice, allowing modulation of CA volume during injection. During an injection, the DyeVert system diverts a portion of the injected CA through a secondary fluid pathway controlled by a pressure-compensating diversion valve. This allows a decrease in over injection of CA and less aortic reflux. The diversion valve provides variable resistance in the secondary fluid path to ensure a flow rate to the coronary artery, which results in adequate image quality. The valve is constructed in a way that the diversion pathway resistance automatically increases with higher injection pressure and decreases with lower injection pressure proportionally decreasing or increasing CA delivered to the coronary artery, respectively. The diverted CA is temporarily stored in the reservoir and is returned to the injection syringe when the physician aspirates CA for the next injection. A recent randomised controlled trial enrolled 96 patients to xamine whether the DyeVert™ system leads to a reduction in CA volume in patients undergoing diagnostic coronary angiography [181181. Corcione N, Biondi-Zoccai G, Ferraro P, Messina S, Maresca G, Avellino R, Napolitano G, Cavarretta E, Giordano A. Contrast Minimization With the New-Generation DyeVert Plus System for Contrast Reduction and Real-Time Monitoring During Coronary and Peripheral Procedures: First Experience. J Invasive Cardiol. 2017 29:259-262. ]. Use of the DyeVert™system resulted in a significant (41%) reduction in CA volume (36.9±10.9 mL vs. 62.5± 12.7 mL, p<0.001). Of note, image quality was non inferior compared to control. Similarly, the randomized AVERT Clinical Trial, presented at the Society for Cardiovascular Angiography and Interventions (SCAI) annual meeting in Orlando, Florida on May 4, 2016, enrolled 578 patients and showed significant reduction of CA delivered to patients undergoing diagnostic and interventional coronary procedures with the DyeVert™system, with adequate image quality in >99.3% of cases. Interestingly, while the overall study did not show a reduction in AKI, post-trial sub-group analysis showed a 49% reduction of AKI in patients with stage 3 CKD. A new-generation system (DyeVert™ Plus) ( Figure 7) continues to exploit the contrast-reducing feature of the first generation DyeVert™ system, while adding the ability to set predetermined thresholds and real-time monitoring. The role of the DyeVert™ Plus system was recently assessed in a small feasibility study that enrolled a consecutive series of 10 patients undergoing coronary or peripheral diagnostic and interventional procedures [182182. Desch S, Fuernau G, Pöss J, Meyer-Saraei R, Saad M, Eitel I, Thiele H, de Waha S. Impact of a novel contrast reduction system on contrast savings in coronary angiography – The DyeVert randomised controlled trial. Int J Cardiol. 2018:257:50-53. ]. Average CA volume was 79.9 ± 48.8 mL (95% CI, 53.2 to 109.4), with an absolute saving of 55.8 ± 31.9 mL (95% CI, 39.1 to 76.7; P<0.05) and a relative saving of 41.8 ± 7.3% (95% CI, 37.5 to 46.4; P<0.05). All procedures were successfully completed with high-quality angiographic images.
Contrast agents
CHOICE OF CONTRAST AGENTS
The choice of CA may influence the risk of CIN. The use of LOCA (600 to 850 mOsmkg) have been associated with fewer renal adverse effects than high-osmolar contrast agents (HOCA; 1500 to 2150 mOsm/kg). A large meta-analysis performed by Barrett and Carlyle pooled data from 31 trials and showed a significant reduction of CIN with LOCA in patients with pre-existing CKD who received intra-arterial CA [153153. Rudnick MR, Goldfarb S, Wexler L, Ludbrook PA, Murphy MJ, Halpern EF, Hill JA, Winniford M, Cohen MB, VanFossen DB. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. Kidney Int. 1995;47:254-261. ]. However, no benefit was found among patients with normal renal function, with or without diabetes, or in those receiving CA intravenously. These results were confirmed by Rudnick et al in a large prospective study of 1,196 patients [154154. Boccalandro F, Amhad M, Smalling RW, Sdringola S. Oral acetylcysteine does not protect renal function from moderate to high doses of intravenous radiographic contrast. Cathet Cardiovasc Interv. 2003;58:336-341. ]. They found a significant benefit of LOCA (iohexol) over HOCA (diatrizoate) only in patients with pre-existing CKD. Based on these data, HOCA should be avoided, particularly in patients at increased risk of CIN.
The issue is still whether the other available CA, either LOCA or IOCA, differ in terms of nephrotoxicity. Several studies have compared LOCA with IOCA. A reduced nephrotoxic effect was demonstrated with a non-ionic IOCA (iodixanol) by the NEPHRIC trial [1414. Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R and Berg KJ Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003; 348: 491-499. ]. This was a randomised, double blind, prospective, multicentre study comparing the non-ionic iodixanol (290 mOsm/kg) with the non-ionic LOCA iohexol. The study included 129 patients with diabetes and SCr >1.5 mg/dL undergoing coronary or peripheral angiography. The two groups differed significantly with regard to the interventional procedures and duration of diabetes, but were otherwise comparable in terms of baseline SCr (1.49 mg/dL[131micromol/l] vs. 1.6 mg dL[141micromol/l]) and CA volume (163 mL vs. 162 mL). The incidence of CIN was 3% in the iodixanol group and 26% in the iohexol group (p=0.002). These results were attributed to the greater osmotic diuresis induced by LOCA, which may increase the work of the medullary tubules, induce ischaemia within the renal medulla and volume depletion with activation of vasoregulatory hormones. However, the purported advantage of IOCM has not been supported by more recent and larger trials. First, other studies with iodixanol in CKD patients have shown higher rates of CIN than that observed in the NEPHRIC study (21% in the RAPPID trial, 12% in the study of Boccalandro et al, 33% and 25% with iodixanol and other CA, respectively, in the CONTRAST trial) [7878. Stone GW, McCullough PA, Tumlin JA, Lepor NE, Madyoon H, Murray P, Wang A, Chu AA, Schaer GL, Stevens M, Wilensky RL, O’Neill WW, for the CONTRAST Investigators. Fenoldopam mesylate for the prevention of contrast-induced nephrotoxicity. JAMA. 2003;290:2284-2291. , 108108. Baker CSR, Wragg A, Kumar S, De Palma R, Baker LRI, and Knight CJ. A rapid protocol for the prevention of contrast-induced renal dysfunction: the RAPPID study. J Am Coll Cardiol. 2003;41:2114-2118. , 155155. Kuhn MJ, Chen N, Sahani DV, Reimer D, Van Beek EJR, Heiken JP, So GJ. The PREDICT study: a randomized double-blind comparison of contrast-induced nephropathy after low- or iso– osmolar contrast agent exposure. AJR Am J Roentgenol. 2008;191:151–157. ]. Second, several large and randomised controlled trials have shown no difference in CIN rate when iodixanol was compared with different types of LOCA [156156. Thomsen HS, Morcos SK, Erley CM, Grazioli L, Bonomo L, Ni Z, Romano L, on behalf of Investigators in the Abdominal Computed Tomography: IOMERON 400 Versus VISIPAQUE 320 Enhancement (ACTIVE) Study. The ACTIVE Trial: comparison of the effects on renal function of iomeprol-400 and iodixanol-320 in patients with chronic kidney disease undergoing abdominal computed tomography. Invest Radiol. 2008;43:170–178. , 157157. Solomon RJ, Natarajan MK, Doucet S, Sharma SK, Staniloae CS, Katholi RE, Gelormini JL, Labinaz M, Moreyra AE; Investigators of the CARE Study. Cardiac Angiography in Renally Impaired Patients (CARE) study: a randomized double blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation. 2007;115:3189-96.
A multicentre, randomised, double-blind comparison of iopamidol and iodixanol in patients with chronic kidney disease undergoing cardiac angiography or percutaneous coronary intervention that demonstrated no significant difference in the rate of contrast-induced nephropathy after the intra-arterial administration of the two contrast media to these high-risk patients, with or without diabetes mellitus. ]. The Cardiac Angiography in Renally Impaired Patients (CARE) study was a multicentre, double-blind, randomised study designed to prospectively compare the incidence of CIN after intra-arterial administration of a LOCA (iopamidol) or a IOCA (iodixanol) in 414 patients with moderate to severe CKD (eGFR 20 to 59 mL/min per 1.73 m2) undergoing cardiac angiography or PCI [158158. Heinrich MC, Haberle L, Muller V, Bautz W, Uder M. Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast media: meta-analysis of randomized controlled trials. Radiology. 2009;250:68–86. ]. All patients received intravenous bicarbonate prophylaxis, and some patients also received NAC. The rate of CIN, defined by multiple endpoints (primary endpoint: post-dose SCr increase >0.5 mg/dL(>44micromol/l) over baseline; secondary endpoints: post-dose SCr increase >25%, post-dose eGFR decrease of >25%, and mean peak change in SCr), was not statistically different after the intra-arterial administration of iopamidol or iodixanol to high-risk patients, with or without diabetes mellitus. In addition, a recent meta-analysis comparing iodixanol to a pool of non-ionic LOCA showed no significant reduction in the rates of CIN [159159. Reed M, Meier P, Tamhane UU, Welch KB, Moscucci M, Gurm H. The relative renal safety of iodixanol compared with low-osmolar contrast media. J Am Coll Cardiol Intv. 2009;2:645-654. ]. In 2009, Reed et al published another meta-analysis in which a total of 16 trials including 2,763 patients were pooled [160160. Wessely R, Koppara T, Bradaric C, Vorpahl M, Braun S, Schulz S, Mehilli J, Schömig A, Kastrati A. Contrast Media and Nephrotoxicity Following Coronary Revascularization by Angioplasty Trial Investigators. Choice of contrast medium in patients with impaired renal function undergoing percutaneous coronary intervention. Circ Cardiovasc Intervent. 2009;2:430-437. ]. The vast majority (12 out of 16) of these trials included patients with elevated SCr values. Moreover, only trials designed to assess CIN as an endpoint, thus ensuring adequate control of confounding variables and minimising ascertainment bias, were included. The authors did not find any significant difference in CIN incidence between iodixanol and a pool of ionic and non-ionic LOCA. Interestingly, using a stratified analysis that indirectly compared different types of LOCA, it was found that some LOCA (ioxaglate, iohexol) are relatively more likely to cause CIN compared to iodixanol. Finally, the Contrast Media and Nephrotoxicity Following Coronary Revascularization by Angioplasty (CONTRAST) trial compared an IOCA (iodixanol) and a LOCA (iomeprol) in patients with CKD undergoing PCI [161161. Cigarroa RG, Lange RA, Williams RH, Hillis LD. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am J Med. 1989;86:649-652. ]. No significant differences were observed between the two groups, when CIN rate, need for dialysis, duration of hospitalisation and major adverse cardiac events at 6-month follow-up were considered. These data further support the concept that a wide spectrum of nephrotoxicity may exist among different CA, regardless of their osmolarity.
In conclusion, the available and more recent data suggest that IOCA, when compared to LOCA as a class, is not associated with reduced rate of CIN. However, the relative safety of LOCA compared to that of iodixanol may vary depending on the specific type of LOCA.
CONTRAST AGENT VOLUME
In 1989, Cigarroa et al were the first to propose a formula (5 mL CA volume x body weight [kg]/baseline SCr [mg/dL]) to calculate a safe weight and creatinine-adjusted maximal CA dose (MCAD), up to a maximum of 300 mL [162162. Freeman RV, O’Donnell M, Share D, Meengs WL, Kline-Rogers E, Clark VL, DeFranco AC, Eagle KA, McGinnity JG, Patel K, Maxwell-Eward A, Bondie D, Moscucci M; Blue Cross-Blue Shield of Michigan Cardiovascular Consortium (BMC2). Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. Am J Cardiol. 2002;90:1068-1073. ]. Exceeding the MCAD threshold was associated with an increased risk of CIN in patients with CKD (SCr >1.8 mg/dL/>158 micromol/l). In another registry of >16,000 PCI, exceeding a calculated MCAD was found to be the strongest independent predictor of nephropathy requiring dialysis in patients undergoing elective coronary interventions [163163. Marenzi G, Assanelli E, Campodonico J, Lauri G, Marana I, De Metrio M, Moltrasio M, Grazi M, Rubino M, Veglia F, Fabbiocchi F, Bartorelli AL. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann Intern Med. 2009;150:170-177. ]. Recently, the impact of CA volume, both absolute and weight- and creatinine-adjusted, on CIN incidence and clinical outcome in 561 consecutive STEMI patients undergoing primary PCI was investigated [164164. Brown JR, Robb JF, Block CA, Schoolwerth AC, Kaplan AV, O’Connor GT, Solomon RJ, Malenka DJ. Does “safe” dosing of iodinated contrast prevent contrast-induced acute kidney injury?. Circ Cardiovasc Interv. 2010;3:1–5. ]. For each patient, the MCAD was calculated, according to the Cigarroa formula and the contrast ratio (CR), defined as the ratio between the CA volume effectively administered and that calculated, was assessed. One-hundred-fifteen (20.5%) patients developed CIN after primary PCI. In-hospital mortality was higher in patients with CIN than in those without CIN (21.4% vs. 0.9%; p<0.001) ( Figure 5 ). In 130 (23%) patients, the MCAD was exceeded. Patients exceeding the MCAD (CR>1) had more complicated in-hospital clinical courses and higher mortality rates (13% vs. 2.8%; p<0.001) than patients with a CR<1. A graded association between CIN development and both CA volume and CR was found. Moreover, CA volume, particularly CR, were independent predictors of in-hospital mortality (OR 4.4, 95% CI 1.9 to 10.3; p=0.0005, for a CR>1). Brown et al confirmed these results and demonstrated a dose-dependent relationship between exceeding the MCAD threshold and risk of CIN, whereby each 50% excess use of CA volume over the threshold was associated with an additional 7% increased likelihood of CIN [164164. Brown JR, Robb JF, Block CA, Schoolwerth AC, Kaplan AV, O’Connor GT, Solomon RJ, Malenka DJ. Does “safe” dosing of iodinated contrast prevent contrast-induced acute kidney injury?. Circ Cardiovasc Interv. 2010;3:1–5. ].
These data suggest that any cardiac catheterisation laboratory should have the MCAD integrated into their computer systems to automatically calculate the safe CA volume that is appropriately tailored for each patient based on the pre-procedure SCr value or eGFR and body weight in kilograms.
Finally, the type of interventional strategy chosen for the treatment of multi-vessel coronary artery disease (ad hoc vs. staged PCI) can impact the CA volume. In an analysis of 315,241 PCI in Medicare patients, it was estimated that approximately 3% were staged PCI [183183. Curtis JP, Schreiner G, Wang Y, Chen J, Spertus JA, Rumsfeld JS, et al. All cause re-admission and repeat revascularization after percutaneous coronary interventions in a cohort of Medicare patients. J Am Coll Cardiol. 2009;54:903-7. ]. The most common reasons for staging PCI for the treatment of multi-vessel disease were poor renal function, CA dose, lesion complexity and the presence of ACS. However, the supposed nephro-protective effects of staged PCI compared to ad hoc PCI have not been rigorously studied. A recent retrospective analysis examined renal outcomes of patients undergoing staged (defined as more than one PCI within the span of 30 days on elective basis) and ad hoc PCI [184184. Shah M, Gajanana D, Wheeler DS, Punjabi C, Maludum O, Mezue K, Lerma EV, Ardati A, Romero-Corral A, Witzke C, Rangswami J. Effects of staged versus ad hoc percutaneous coronary interventions on renal function. Is there a benefit to staging?. Cardiovascular Revascularization Medicine. 2017;18:344-348. ]. While staged PCI did use less CA per procedure, the cumulative CA load was much greater than with ad hoc PCI. Moreover, patients with baseline CKD undergoing staged PCI had significantly worse renal outcomes at 4–12 weeks, which was confirmed on propensity match analysis. Thus, staged PCI seems to expose CKD patients to greater cumulative CA loads and a higher decline in renal function.
Conclusions
The incidence of CIN is growing, largely due to the increasing number of cardiac catheterisations, PCI, and procedures using CA in patients who are older and have associated comorbidities such as CKD, diabetes and cardiac failure. This serious complication is associated with prolongation of hospital stay, increase in cost of care and long-term adverse events. Because CIN is potentially preventable, risk prediction and prophylactic measures are mandatory. Despite a large number of studies, most of the prophylactic pharmacological agents that have been evaluated have not proven to be effective. Promising approaches include minimising the volume of contrast, prophylactic use of isotonic saline, sodium bicarbonate and high-dose (1,200 mg) NAC, and periprocedural HF in patients with severe CKD. There does not appear to be a difference in nephrotoxic potential between the non-ionic IOCA and LOCA as a class. Future studies are warranted to define better the specific role of each of these approaches, with particular emphasis on hard clinical endpoints, optimally customised prophylactic protocols, and their most cost-effective and potentially combined application. Finally, there is a pressing need for additional long-term follow-up data on patients developing CIN following CA administration.
Personal perspective – Antonio Bartorelli
As our population ages and the number of patients with some degree of renal impairment increases, as the prevalence of diabetes reaches epidemic proportions, and as we embark on newly introduced cardiovascular diagnostic techniques and catheter-based interventional therapies, kidney toxicity of contrast agents (CA) and the syndrome of contrast-induced nephropathy (CIN) have become recognised as major clinical problems which must be targeted with understanding and appreciation of pathophysiology, prognostic implications, and management concepts. Although several mechanisms can contribute to kidney function impairment after CA administration, there is increasing proof that direct toxic effect on renal cells and reduction in blood flow to the medullary portions of the kidney play a pathogenetic role. The clinical course of CIN is usually characterised by spontaneous recovery of renal function. However, some degree of residual impairment of kidney function has been reported in as many as 30% of those affected, and up to 7% of patients may require temporary dialysis or progress to end-stage renal failure. More importantly, it has been recognised that CIN development is linked to an increased risk of in-hospital and long-term adverse events and mortality. As a large body of data indicates that the risk of CIN is related to patient characteristics, clinical setting and other modifiable factors, its occurrence can be predicted in most cases. Thus, preventive strategies represent the only effective therapeutic approach. Unfortunately, despite a large number of trials, most of the prophylactic pharmacologic agents that were evaluated have not proven to be effective. N-acetylcysteine (NAC), the most widely studied drug of all prophylactic agents, may constitute an exception. Indeed, several studies and meta-analyses indicate a kidney protective effect particularly when a greater dose of NAC is used in higher-risk patients. Given its potential benefit, low cost and excellent safety profile, the use of high-dose NAC should be recommended in all high-risk patients for the prevention of CIN. Another emerging concept is that volume expansion by hydration is critical for prophylaxis. Several studies suggest that it should be commensurate to the patient risk, and a high volume of controlled hydration is likely to be required in patients who have the highest risk. This can be achieved with haemofiltration that exactly matches fluid removal to high-volume intravenous hydration, thus preventing fluid overload. This prophylactic strategy, which has demonstrated a significant reduction of CIN incidence and a remarkable improvement of in-hospital and long-term outcome in high-risk patients undergoing percutaneous coronary intervention, received a Class IIa recommendation (level of evidence B) in the 2014 European guidelines on myocardial revascularization [185185. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Uva MS, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial Revascularization The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-619. ]. Similarly, furosemide-induced high-volume diuresis and maintenance of euvolemia through matched fluid replacement (RenalGuard™ therapy) might become a standard of care strategy for the prevention of CI-AKI in high-risk patients or in cases where prophylactic hydration before the procedure cannot be accomplished. Indeed, also this approach received a Class IIb recommendation (level of evidence A) in the 2014 European guidelines on myocardial revascularization [185185. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Uva MS, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial Revascularization The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-619. ]. Finally, most data regarding CA nephrotoxicity suggest that use of iso-osmolar CA, when compared to low-osmolar CA as a class, is not associated with a reduced rate of CIN. What an interventionalist should strive for when treating patients at risk is a CA volume as low as possible and appropriately tailored for each patient based on the preprocedural renal function and body weight.