Summary
Percutaneous septal ablation has emerged as an interventional treatment of symptomatic patients with hypertrophic obstructive cardiomyopathy (HOCM). In the past decades, the availability of this sophisticated technique has revived the interest of cardiologists in left ventricular outflow tract obstruction, which has led to the recognition that about 70% of the patients with hypertrophic cardiomyopathy (HCM) have dynamic obstruction. Follow-up studies have already shown the safety and efficacy of the procedure, which offers symptomatic relief in most patients. Long-term survival is comparable to historical reports after surgical myectomy. Complications are rare and can be further reduced by an increase in the experience of the operators, while the theoretical concern for possible ventricular arrhythmogenicity caused by the myocardial scar has not been documented by the existing data. In experienced centers percutaneous septal ablation is a viable and safe treatment option for patients with HOCM.
Introduction
Hypertrophic cardiomyopathy (HCM) is a primary myocardial disorder which is clinically defined by the presence of left ventricular hypertrophy unexplained by abnormal loading conditions [11. Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014; 35: 2733-79.
Actual guidelines on hypertrophic cardiomyopathy of the ESC., 22. Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004; 363: 1881-91. ]. The differential diagnosis requires consideration of some congenital and acquired diseases. ( Table 1 ) HCM is inherited as an autosomal dominant trait with variable penetrance, most commonly involving sarcomeric protein mutations. HCM is considered the most common genetic cardiac disease, affecting 1 in 500 individuals [33. Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults Echocardiographic analysis of 4111 subjects in the CARDIA Study Coronary Artery Risk Development in (Young) Adults. Circulation. 1995; 92: 785-9. ]. Including new imaging techniques and genotype positve probants the prevalence may be even higher up to 1 in 200 individuals [44. Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015; 65: 1249-54. ]. The disease can be diagnosed in patients of all ages and presents a variety of clinical manifestations. Most patients remain asymptomatic, while others may have severe symptoms of exertional dyspnoea or angina and reduced exercise capacity [22. Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004; 363: 1881-91. ]. Exercise-induced syncopes are mainly caused by dynamic outflow obstruction [55. Seggewiss H, Koljaja-Batzner A, Seggewiss K, Meesmann M. Syncope in hypertrophic (obstructive) cardiomyopathy. Herzschrittmacherther Elektrophysiol. 2018; 29: 141-3. ]. Natural history of the disease may be highly heterogeneous with life expectancy ranging from normal longevity to sudden arrhythmic death, often presenting at a young age, or evolution to congestive heart failure or stroke [66. Maron BJ, Olivotto I, Spirito P, et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation. 2000; 102: 858-64. ]. Most patients present a characteristic left ventricular morphology with hypertrophy of the basal interventricular septum that is coupled with systolic anterior motion (SAM) of the anterior mitral valve leaflet and leads to dynamic left ventricular outflow tract (LVOT) obstruction and mitral regurgitation due to malcoaptation of the mitral leaflets [77. Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic Obstructive Cardiomyopathy: Surgical Myectomy and Septal Ablation. Circ Res. 2017; 121: 771-83.
The paper describes the options of gradient reduction therapies in HOCM.]. Symptomatic status depends on left ventricular obstruction, diastolic dysfunction and myocardial ischaemia [22. Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004; 363: 1881-91. ]. The existence of significant obstruction at rest or after provocation is associated with symptomatic status and has significant prognostic implications [88. Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003; 348: 295-303. ].
In general, treatment of patients with hypertrophic cardiomyopathy aims at relieving symptoms, reducing the risk of sudden death and offering genetic counselling [11. Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014; 35: 2733-79.
Actual guidelines on hypertrophic cardiomyopathy of the ESC.]. Consequently, treatment of symptomatic patients with obstructive HCM (HOCM) aims at the reduction of the pressure gradient [77. Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic Obstructive Cardiomyopathy: Surgical Myectomy and Septal Ablation. Circ Res. 2017; 121: 771-83.
The paper describes the options of gradient reduction therapies in HOCM., 99. Fifer MA, Vlahakes GJ. Management of symptoms in hypertrophic cardiomyopathy. Circulation. 2008; 117: 429-39. ]. Medical treatment with ß-blockers, disopyramide or verapamil, however, fails to relieve symptoms in a substantial subset of patients [11. Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014; 35: 2733-79.
Actual guidelines on hypertrophic cardiomyopathy of the ESC., 77. Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic Obstructive Cardiomyopathy: Surgical Myectomy and Septal Ablation. Circ Res. 2017; 121: 771-83.
The paper describes the options of gradient reduction therapies in HOCM.]. In such drug-refractory patients, alcohol septal ablation has was introduced as a less invasive treatment than surgery to reduce LVOT obstruction by creating an infarction limited to the part of the septum, either basal or mid-cavitary, involved in the development of LV obstruction [1010. Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet. 1995; 346: 211-4.
Sigwart describes the technique and results of the first patients treated with alcohol septal ablation., 1414. Seggewiss H, Gleichmann U, Faber L, Fassbender D, Schmidt HK, Strick S. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: acute results and 3-month follow-up in 25 patients. J Am Coll Cardiol. 1998; 31: 252-8. ].
Indications for septal reduction treatment
Patient selection for septal reduction therapy in HOCM is of the utmost importance in order to maximise the benefit from gradient reduction [77. Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic Obstructive Cardiomyopathy: Surgical Myectomy and Septal Ablation. Circ Res. 2017; 121: 771-83.
The paper describes the options of gradient reduction therapies in HOCM., 99. Fifer MA, Vlahakes GJ. Management of symptoms in hypertrophic cardiomyopathy. Circulation. 2008; 117: 429-39. ]. The indication for interventional treatment is present in patients with significant clinical symptoms, appropriate cardiac morphology and haemodynamically significant obstruction [1515. Seggewiss H. Current status of alcohol septal ablation for patients with hypertrophic cardiomyopathy. Curr Cardiol Rep. 2001; 3: 160-6. ]. Preprocedural evaluation of possible candidates for septal reduction therapy is based on history, cardiac imaging data (echocardiography, MRI), cardiac catheterisation and coronary arteriography. Functional capacity in patients with ambivalent symptoms or limited activities in everyday life, in whom symptoms are difficult to evaluate, may be assessed by cardiopulmonary exercise test. The presence and degree of obstruction and symptoms is highly dependent on loading conditions of the left ventricle and may vary greatly with changes of preload, afterload or contractility produced by physiological or pharmacological methods of provocation ( Table 2 ). Imaging studies can establish the diagnosis and provide clues on cardiac morphology, while echocardiography immediately after exercise may reveal latent obstruction in patients who have no significant gradient at rest [1616. Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006; 114: 2232-9.
This paper describes the influence of resting outflow obstruction on prognosis of patients with HCM.]. In symptomatic patients, LV gradient ≥30 mmHg at rest and ≥50 mmHg at provocation are accepted indications for gradient reduction procedures [11. Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014; 35: 2733-79.
Actual guidelines on hypertrophic cardiomyopathy of the ESC., 77. Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic Obstructive Cardiomyopathy: Surgical Myectomy and Septal Ablation. Circ Res. 2017; 121: 771-83.
The paper describes the options of gradient reduction therapies in HOCM.].
According to the ACC/ESC clinical expert consensus document on HCM [1717. Maron BJ, McKenna WJ, Danielson GK, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy - A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003; 42: 1687-713. ], the 2011 ACCF/AHA Guidelines for the diagnosis and treatment of HCM [1818. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy : executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011; 58: 2703-38. ] and the 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy [11. Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014; 35: 2733-79.
Actual guidelines on hypertrophic cardiomyopathy of the ESC.] patients who qualify for septal reduction therapy (surgical or interventional) are those with drug-refractory symptoms of dyspnoea or angina that limit their functional capacity during daily activities (NYHA/CCS class III or IV).
Additionally, patients with recurrent exercise-induced syncope may benefit from gradient reduction [55. Seggewiss H, Koljaja-Batzner A, Seggewiss K, Meesmann M. Syncope in hypertrophic (obstructive) cardiomyopathy. Herzschrittmacherther Elektrophysiol. 2018; 29: 141-3. , 77. Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic Obstructive Cardiomyopathy: Surgical Myectomy and Septal Ablation. Circ Res. 2017; 121: 771-83.
The paper describes the options of gradient reduction therapies in HOCM.]. Morphology of the left ventricle must be consistent with the expectation that septal reduction will reduce LVOT gradient. This typically involves asymmetric as well as symmetric septal hypertrophy with SAM of the anterior mitral valve leaflet and associated posteriorly directed mitral regurgitation. Furthermore, patients with mid-ventricular obstruction are candidates for echocardiography-guided septal ablation if they fulfil the symptomatic and haemodynamic criteria for gradient reduction [1111. Seggewiss H, Faber L. Percutaneous septal ablation for hypertrophic cardiomyopathy and mid-ventricular obstruction. Eur J Echocardiogr. 2000; 1: 277-80. ]. The question whether patients with septal thickness <17 mm can be safely treated is still under investigation.
A number of morphological characteristics will shift the preference in favour of surgical treatment and against alcohol septal ablation ( Table 3 ). Patients with thin septum and intrinsic mitral valve abnormalities (extremely long or flail leaflets) or anomalous papillary muscle morphology (anomalous papillary muscle insertion) should be treated surgically [1919. Lever HM. Selection of hypertrophic cardiomyopathy patients for myectomy or alcohol septal ablation. Anadolu Kardiyol Derg. 2006; 6 Suppl 2: 27-30.
Lever describes the influence of mitral valve morphology on decision making in HOCM.]. On the contrary, mitral valve regurgitation associated with systolic anterior motion of the mitral valve is expected to diminish after successful treatment [2020. Seggewiss H, Faber L, Ziemssen P, Gleichmann U. One-year follow-up after echocardiographically-guided percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Dtsch Med Wochenschr. 2001; 126: 424-30. ]. Existence of a sometimes inconspicuous abnormal subvalvular membrane in the outflow tract is also an indication for surgery and should be effectively identified (i.e. with transoesophageal echocardiography) before decision for invasive treatment. In the cathlab it can be suspected by the configuration of an aortic pressure curve not typical for HOCM. Coronary artery disease requiring surgical revascularisation would turn the indication towards surgery. However, if coronary artery disease is amenable to angioplasty, then alcohol ablation can be performed after persistence of symptoms has been established at follow-up. The only reason for a combined percutaneous treatment with angioplasty and alcohol ablation at the same time is when the target septal branch originates at the site of a coronary lesion in the LAD [2121. Seggewiss H, Faber L, Meyners W, Bogunovic N, Odenthal HJ, Gleichmann U. Simultaneous percutaneous treatment in hypertrophic obstructive cardiomyopathy and coronary artery disease: a case report. Cathet Cardiovasc Diagn. 1998; 44: 65-9. ]. In that case, two angioplasty guidewires are used and a stent is placed in the LAD lesion after alcohol septal ablation has been performed.
Patients with acceptable exercise capacity and trivial or no symptoms are not candidates for septal reduction therapy [11. Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014; 35: 2733-79.
Actual guidelines on hypertrophic cardiomyopathy of the ESC., 1818. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy : executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011; 58: 2703-38. ]. Nevertheless, they should be followed regularly with Holter, echocardiography and cardiopulmonary exercise testing for any sign of decreasing exercise capacity, cardiac decompensation, or episodes of atrial fibrillation. Furthermore, risk stratification for sudden death should be performed regulary [11. Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014; 35: 2733-79.
Actual guidelines on hypertrophic cardiomyopathy of the ESC., 2222. O’Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J. 2014; 35: 2010-20.
Description of a new risk stratification model in HCM patients.].
Finally, hypertrophy not involving the septum (apical hypertrophy, hypertrophy limited to the free LV wall) or absence of intraventricular obstruction are absolute contraindications for interventional treatment.
Historical perspective of alcohol septal ablation
The idea of a non-surgical interventional treatment for HOCM has grown together with the advances in interventional cardiology in the 1980s. Early in that decade, it was observed that temporary balloon occlusion of the left anterior descending artery caused left ventricular wall motion abnormalities [2323. Sigwart U, Grbic M, Essinger A, Rivier J. L’effect aigu d’une occlusion coronarienne par ballonet de la dilatation transluminale. Schweiz Med Wochenschr. 1982; 45: 1631. ]. In particular, patients with HCM and obstruction developed non-obstructive physiology after septal myocardial infarction [2424. Come PC, Riley MF. Hypertrophic cardiomyopathy - Disappearance of auscultatory, carotid pulse, and echocardiographic manifestations of obstruction following myocardial infarction. Chest. 1982; 82: 451-4. ]. On the other hand, electrophysiologists have tried a chemical septal branch ablation procedure for the treatment of ventricular arrhythmias in patients with coronary artery disease [2525. Brugada P, de Swart H, Smeets JL, Wellens HJ. Transcoronary chemical ablation of ventricular tachycardia. Circulation. 1989; 79: 475-82. ]. These observations inspired the original description of the technique of alcohol septal ablation in 1989 (G. Berghoefer, personal communication). In the early 1990s, it was reported that temporary balloon occlusion of the first septal branch led to gradient reduction in some patients [2626. Kuhn H, Gietzen F, Leuner C, Gerenkamp T. Induction of subaortic septal ischaemia to reduce obstruction in hypertrophic obstructive cardiomyopathy - Studies to develop a new catheter-based concept of treatment. Eur Heart J. 1997; 18: 846-51. ] (M. Takayama, personal communication). The first procedure of alcohol-induced septal ablation in man was performed by Sigwart in 1994 [1010. Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet. 1995; 346: 211-4.
Sigwart describes the technique and results of the first patients treated with alcohol septal ablation.]. Two years later, echocardiographic guidance with echo-contrast-mediated identification of the target septal branch was introduced [1313. Faber L, Seggewiss H, Gleichmann U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: results with respect to intraprocedural myocardial contrast echocardiography. Circulation. 1998; 98: 2415-21.
First decription of echo-guided septal ablation in HOCM.]. This was clearly the most significant improvement of the original technique.
Alcohol septal ablation has gained remarkable popularity in recent decades and it is estimated that far more patients have already been treated using the interventional technique in the first decade after its introduction than with surgical myectomy in the first 45 years [2727. Maron BJ. Controversies in cardiovascular medicine - Surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation. 2007; 116: 196-206. ]. As a benefit of the growing volume of available data, medium-term results were published in the early 2000s and long-term results have already appeared in the literature [2828. Seggewiss H, Rigopoulos A, Welge D, Ziemssen P, Faber L. Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Clin Res Cardiol. 2007; 96: 856-63.
Up to 8 years follow-up of the first 100 consecutive patients with echo-guided septal ablation., 2929. Sorajja P, Valeti U, Nishimura RA, et al. Outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2008; 118: 131-9. , 3030. Welge D, Seggewiss H, Fassbender D, Schmidt HK, Horstkotte D, Faber L. Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Dtsch Med Wochenschr. 2008; 133: 1949-54. , 3131. Fernandes VL, Nielsen C, Nagueh SF, et al. Follow-up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy the Baylor and Medical University of South Carolina experience 1996 to 2007. JACC Cardiovasc Interv. 2008; 1: 561-70. , 3232. Kuhn H, Lawrenz T, Lieder F, et al. Survival after transcoronary ablation of septal hypertrophy in hypertrophic obstructive cardiomyopathy (TASH): a 10 year experience. Clin Res Cardiol. 2008; 97: 234-43. , 3333. Kwon DH, Kapadia SR, Tuzcu EM, et al. Long-term outcomes in high-risk symptomatic patients with hypertrophic cardiomyopathy undergoing alcohol septal ablation. JACC Cardiovasc Interv. 2008; 1: 432-8. , 3434. Lyne JC, Kilpatrick T, Duncan A, Knight CJ, Sigwart U, Fox KM. Long-term follow-up of the first patients to undergo transcatheter alcohol septal ablation. Cardiology. 2010; 116: 168-73. , 3535. Jensen MK, Prinz C, Horstkotte D, et al. Alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy: low incidence of sudden cardiac death and reduced risk profile. Heart. 2013; 99: 1012-7. , 3636. Batzner A, Pfeiffer B, Neugebauer A, Aicha D, Blank C, Seggewiss H. Survival After Alcohol Septal Ablation in Patients With Hypertrophic Obstructive Cardiomyopathy. J Am Coll Cardiol. 2018; 72: 3087-94.
Up to 18 years follow-up results in a large single center cohort after alcohol septal ablation., 3737. Veselka J, Jensen MK, Liebregts M, et al. Long-term clinical outcome after alcohol septal ablation for obstructive hypertrophic cardiomyopathy: results from the Euro-ASA registry. Eur Heart J. 2016; 37: 1517-23.
Follow-up results of a multicenter European septal ablation registry., 3838. Veselka J, Krejci J, Tomasov P, Zemanek D. Long-term survival after alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a comparison with general population. Eur Heart J. 2014; 35: 2040-5. ].
Technique of alcohol septal ablation
It is better for patients to be admitted to the hospital one day before the intervention, so that medications with anti-arrhythmic potential (ß-blockers, verapamil and disopyramide) can be safely withdrawn. Moreover, earlier admittance permits an echocardiographic assessment prior to the intervention.
Indication for alcohol septal ablationCLINICAL INDICATION
- Symptomatic patients
- Drug-refractory or severe side effects of drugs
- Functional class III and IV or functional class II with objective exercise limitations
- Recurrent exercise-induced syncopes
- Failure of prior myectomy or pacemaker
- Comorbidity-related increased surgical risk
HAEMODYNAMIC INDICATION
- Intracavitary gradient >30 mmHg at rest and/or
- Provocable gradient >50 mmHg
- Valsalva manoeuvre
- Physiologic stress test (Echo)
- Post extrasystole
- No dobutamine-induced gradients
MORPHOLOGIC INDICATION
- Echocardiography
- Subaortic, SAM – associated gradient
- Mid-cavitary gradient
- Exclusion of intrinsic mitral valve apparatus disorders
- Coronary angiography
- Suitable septal branch
The femoral approach is mostly preferred, as it offers all-in-one access for two arterial and one venous sheath. A temporary pacemaker lead is mandatory and is placed through a femoral venous sheath in the right ventricular apex. In patients with a permanent pacemaker or ICD an increased pacing output is advisable, although a temporary pacemaker may also be placed in order to avoid loss of capture, especially if a high risk of significant conduction abnormalities is suspected (previous myectomy, baseline LBBB, septal pacing). The temporary pacemaker lead is usually kept on site for 24(-48) hours; however, the jugular or subclavian venous access route may be preferable if temporary pacing is required for a longer time or ambulatory monitoring is not available post procedure. Some operators have started using the subclavian access with an externalised permanent pacemaker and electrodes with active fixation for as long as this is necessary, in order to avoid complications from the femoral puncture site (in this case with radial arterial access) or to minimise the possibility of pericardial tamponade.
The use of two arterial catheters enables continuous recording of the LVOT gradient by simultaneous measurement of the left ventricular and aortic pressures. A coronary angioplasty guide catheter is positioned at the ostium of the left coronary artery, through a right femoral artery sheath and a special pigtail catheter with holes only in its distal part and not on the shaft (Cordis) is inserted through a left femoral artery sheath and remains positioned in the apex of the left ventricle. Special guide catheters offering superior backup can be used, although Judkins left catheters are the first choice in most cases, provided there is no anatomic complexity of the left coronary artery origin. Without doubt, a 6 Fr guide catheter is sufficient for the procedure, but a 7 Fr catheter allows accurate pressure measurement and optimal support. It should be emphasised that the LVOT gradient can be recorded both at rest and during provocative manoeuvres, such as Valsalva and after an extrasystolic beat, thus obviating the need for any echocardiographic Doppler measurements during the procedure [77. Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic Obstructive Cardiomyopathy: Surgical Myectomy and Septal Ablation. Circ Res. 2017; 121: 771-83.
The paper describes the options of gradient reduction therapies in HOCM.].
In order to avoid thrombo-embolic complications, weight-adjusted heparin should be administered intravenously. Besides, early analgesic medication (preferably with opiates) is essential to suppress pain during the alcohol injection.
In general, the first large septal branch is angiographically identified as the target branch and an angioplasty guidewire is advanced into this branch. In most of the patients RAO cranial views identify the target septal branch. In some patients LAO cranial or, especially in septal arteries atypically originating from diagonal or intermediate branches, LAO caudal views are necessary for identifying the target vessels. A supportive wire with good steering, with its floppy tip manually shaped to a long steep curve, is more likely to enter difficult septal branches originating at >90° angle. Softer wires should be used in order to reduce the risk of coronary artery damage, even though changing to a stiffer wire that offers increased support may facilitate the introduction of the balloon catheter into the septal branch. A short (6 - 10 mm-long) compliant, over-the-wire balloon, compatible with the injection of absolute alcohol, is advanced into the septal branch and inflated at the nominal pressure. The choice of a balloon with a slightly larger diameter than the septal branch precludes overexpansion above the nominal pressure which could lead to rupture. The balloon should be outside the parent vessel (e.g., the left anterior descending artery) but not too deep in the septal branch. At this point transthoracic baseline echos are performed with non-inflated balloon catheter. ( Figure 2A, Figure 2B, Figure 2C ) Thereafter the OTW-balloon is inflated and the angioplasty guidewire is withdrawn. Angiography of the left coronary artery can verify that the septal branch is blocked. Myocardial contrast echocardiography (MCE, see below) is performed before selective injection of angiographic contrast through the central lumen of the balloon catheter provides an ideal septal branch angiography, while it can rule out the possibility of inadequate sealing of the septal branch and identify the possible existence of collateral vessels [3939. Koljaja-Batzner A, Pfeiffer B, Seggewiss H. Septal Collateralization to Right Coronary Artery in Alcohol Septal Ablation: Solution to a Dangerous Pitfall. JACC Cardiovasc Interv. 2018; 11: 2009-11. ].
Angiography alone is not sufficient for the correct identification of the target septal branch; and MCE should always be used to demarcate the myocardium perfused by the target septal artery [1313. Faber L, Seggewiss H, Gleichmann U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: results with respect to intraprocedural myocardial contrast echocardiography. Circulation. 1998; 98: 2415-21.
First decription of echo-guided septal ablation in HOCM.]. Before angiographic contrast is given a small quantity (1-2 ml) of echocardiographic contrast agent is administered through the central lumen of the balloon catheter under real-time transthoracic two-dimensional echocardiographic and colour Doppler monitoring. Agitated gelatine polysuccinate (Gelafundin 4%®) has turned out to be the optimal contrast agent [4040. Pfeiffer B, Rigopoulos A, Seggewiss H. Myocardial contrast echocardiography guided alcohol septal ablation in hypertrophic obstructive cardiomyopathy with a new echocardiographic contrast agent. Dtsch Med Wochenschr. 2012; 137: 2093-6. ]. The area perfused by the optimal septal branch is adjacent to the colour-Doppler-estimated area of maximal flow acceleration and to the point of contact between the mitral valve and the septum during systole. Opacification of any other cardiac structure calls for selection of another septal branch. At this point, if MCE fails to identify an appropriate septal branch, the procedure has to be abandoned. Even in experienced hands, in about 5% of the patients, a target branch cannot be identified, with consequent abandonment of the procedure and referral for elective surgical myectomy [77. Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic Obstructive Cardiomyopathy: Surgical Myectomy and Septal Ablation. Circ Res. 2017; 121: 771-83.
The paper describes the options of gradient reduction therapies in HOCM., 3636. Batzner A, Pfeiffer B, Neugebauer A, Aicha D, Blank C, Seggewiss H. Survival After Alcohol Septal Ablation in Patients With Hypertrophic Obstructive Cardiomyopathy. J Am Coll Cardiol. 2018; 72: 3087-94.
Up to 18 years follow-up results in a large single center cohort after alcohol septal ablation.].
Echocardiographic assessment involves all classic views (apical 2- and 4-chamber, parasternal short- and long-axis, subcostal) which are compared with baseline echocardiograms. Only when contrast echocardiography has convincingly shown that the chosen septal branch fulfils the above criteria for optimal location (perfusion area at the area of maximal flow acceleration, in subaortic obstruction mitral-septal contact point, and no opacification of any other cardiac structure), 1-3 ml of absolute alcohol in 1 ml portions are slowly (1 ml / 1 minute) injected through the central lumen of the over-the-wire balloon. Injection of alcohol should be performed under continuous fluoroscopic surveillance while the indeflator pressure should be visible throughout the procedure in order to prevent unintended pressure loss. The total amount of alcohol injected depends mainly on the echocardiographically estimated size of the contrasted septal area. As a rule of thumb, 1 ml of alcohol should be enough for every 10 mm of echocardiographically measured septal thickness [4141. Faber L, Seggewiss H, Welge D, et al. Echo-guided percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: 7 years of experience. Eur J Echocardiogr. 2004; 5: 347-55. ]. In recent years, it has been shown that the use of less alcohol is associated with fewer complications, while small or ultra-low doses of alcohol have attained equally effective haemodynamic results [4242. Veselka J, Duchonova R, Palenickova J, et al. Impact of ethanol dosing on the long-term outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy: a single-center prospective, and randomized study. Circ J. 2006; 70: 1550-2. ]. In case of balloon catheter dislodgement, kinking or resistance in alcohol injection, the procedure should be terminated. Otherwise, the balloon can be deflated and removed 10 minutes after the last alcohol injection as shorter inflation duration are associated with higher risk of alcohol reflux into the LAD [1515. Seggewiss H. Current status of alcohol septal ablation for patients with hypertrophic cardiomyopathy. Curr Cardiol Rep. 2001; 3: 160-6. , 4343. Ruzyllo W, Chojnowska L, Demkow M, et al. Left ventricular outflow tract gradient decrease with non-surgical myocardial reduction improves exercise capacity in patients with hypertrophic obstructive cardiomyopathy. Eur Heart J. 2000; 21: 770-7. ]. A final coronary angiogram excludes left coronary artery damage and verifies septal branch occlusion and final haemodynamic measurements confirm the acute result of septal ablation. The patient will stay in the coronary care unit under haemodynamic and rhythm monitoring for at least 48 hours. It should be taken into account that delayed occurence of complete heart block may occur 4-9 days after ablation even without warning or preceding evidence of heart block during the procedure or the early post-interventional period ([4444. Kern MJ, Holmes DG, Simpson C, Bitar SR, Rajjoub H: Delayed occurrence of complete heart block without warning after alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Catheter Cardiovasc Interv. 2002; 56: 503-7. , 4545. Faber L, Seggewiss H, Welge D, et al. Predicting the risk of atrioventricular conduction lesions after percutaneous septal ablation for obstructive hypertrophic cardiomyopathy. Z Kardiol. 2003; 92: 39-47. ] own observation).
Apart from the technique described above, other variants have been referred to under different acronyms that incorporate significant technical differences [1313. Faber L, Seggewiss H, Gleichmann U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: results with respect to intraprocedural myocardial contrast echocardiography. Circulation. 1998; 98: 2415-21.
First decription of echo-guided septal ablation in HOCM., 3535. Jensen MK, Prinz C, Horstkotte D, et al. Alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy: low incidence of sudden cardiac death and reduced risk profile. Heart. 2013; 99: 1012-7. , 3636. Batzner A, Pfeiffer B, Neugebauer A, Aicha D, Blank C, Seggewiss H. Survival After Alcohol Septal Ablation in Patients With Hypertrophic Obstructive Cardiomyopathy. J Am Coll Cardiol. 2018; 72: 3087-94.
Up to 18 years follow-up results in a large single center cohort after alcohol septal ablation., 3737. Veselka J, Jensen MK, Liebregts M, et al. Long-term clinical outcome after alcohol septal ablation for obstructive hypertrophic cardiomyopathy: results from the Euro-ASA registry. Eur Heart J. 2016; 37: 1517-23.
Follow-up results of a multicenter European septal ablation registry., 3838. Veselka J, Krejci J, Tomasov P, Zemanek D. Long-term survival after alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a comparison with general population. Eur Heart J. 2014; 35: 2040-5. , 4646. Kuhn H, Gietzen FH, Leuner C, et al. Transcoronary ablation of septal hypertrophy (TASH): a new treatment option for hypertrophic obstructive cardiomyopathy. Z Kardiol. 2000; 89 Suppl 4: IV41-54. , 4747. Lakkis NM, Nagueh SF, Dunn JK, Killip D, Spencer WH. Nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy: one-year follow-up. J Am Coll Cardiol. 2000; 36: 852-5. , 4848. Boekstegers P, Steinbigler P, Molnar A, et al. Pressure-guided nonsurgical myocardial reduction induced by small septal infarctions in hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2001; 38: 846-53. , 4949. de la Torre Hernandez JM, Masotti Centol M, Lerena Saenz P, et al. Effectiveness and safety beyond 10 years of percutaneous transluminal septal ablation in hypertrophic obstructive cardiomyopathy. Rev Esp Cardiol. 2014; 67: 353-8. , 5050. Khouzam RN, Naidu SS. Current status and future perspectives on alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Curr Cardiol Rep. 2014; 16: 478. ] ( Table 4 ). Furthermore, various modalities have been used instead of alcohol, such as coils, polyvinyl alcohol particles, used angioplasty wires, cyanoacrylate glue, gelatine particles, radio-frequency ablation and cryoablation. However, all the above methods have not gained further approval, probably because alcohol-induced necrosis is the key feature in the pathophysiology of interventional septal reduction therapy. This theory is supported by the fact that mechanical occlusion with a covered stent leads to late re-occurence of the gradient due to development of septal collaterals [5151. Fifer MA, Yoerger DM, Picard MH, Vlahakes GJ, Palacios IF. Images in cardiovascular medicine - Covered stent septal ablation for hypertrophic obstructive cardiomyopathy: initial success but ultimate failure resulting from collateral formation. Circulation. 2003; 107: 3248-9. , 5252. Gaspar J, Martinez-Rios MA, Vonderwalde C, et al. Pericardium-covered stent for septal myocardial ablation in hypertrophic obstructive cardiomyopathy. Catheter Cardiovasc Interv. 1999; 47: 73-9. , 5353. Seggewiss H. Percutaneous alcohol ablation in HOCM. Catheter Cardiovasc Interv. 1999; 48: 241-2. ].
Close monitoring of patients after the intervention is probably the most important measure in order to avoid complications during the hospital stay. CCU monitoring should last at least 48 hours as in patients with acute myocardial infarction. Any serious arrhythmic event (complete heart block, ventricular tachyarrhythmia, atrial fibrillation) can be recognised by continuous monitoring of the electrocardiogram (in the CCU or in the wards with telemetry). Besides this, monitoring of the arterial blood pressure in the CCU can aid the timely recognition of a drop in blood pressure caused by pericardial tamponade or acute increase of LVOT gradient due to post-interventional oedema.
Technique of alcohol septal ablation
- 2 arterial catheters for simultaneous pressure recording
- Temporary pacemaker
- Coronary angiography with identification of target vessel
- RAO and LAO cranial
- Wiring of estimated septal branch
- Primarily the use of soft tip wires
- Use of short over-the-wire balloon
- Avoid LAD ballooning
- Exclusion of leakage
- Myocardial contrast echo
- Stepwise slow (1 ml/minute) alcohol injection
- 1 ml alcohol/1 cm septal thickness
- Balloon deflation and removal
- 10 minutes after final alcohol injection
- Monitoring at the CCU for 48 hours
Results in alcohol septal ablation
- Haemodynamic results
- >50% gradient reduction in >90% of patients
- Ongoing gradient reduction during follow-up
- Reduction of pulmonary artery pressure
- Echocardiographic results
- Septal thinning
- Posterior wall thickness reduction
- Mitral regurgitation reduction
- Left atrial size reduction
- Exercise test
- Increase of exercise capacity
- Increase of peak oxygen consumption
- Clinical results
- Improvement of functional class
- Symptomatic relief
- Reduction of syncopal episodes
Pathophysiological effects of septal ablation
Injection of alcohol during alcohol ablation causes coagulative necrosis of the myocardium and the septal arteries. Tissue oedema appears early in this process, while muscle replacement by scar formation develops only after several days [5454. Baggish AL, Smith RN, Palacios I, et al. Pathological effects of alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Heart. 2006; 92: 1773-8. ].
The haemodynamic response after alcohol septal ablation follows three separate phases that run parallel to the histological changes [5555. Keren A, Poteckin M, Mazouz B, et al. Late in-hospital pressure gradient measurements improve prediction of long-term outcome of alcohol septal ablation in hypertrophic cardiomyopathy. Isr Med Assoc. J 2007; 9: 239-42. ]. The first (perioperative) phase is characterised by significant LVOT gradient reduction due to stunning and akinesia of the ablated septum. During the second (early postoperative) phase, the gradient is increased even to preoperative levels in about half of the patients for at least a week [5656. Yoerger DM, Picard MH, Palacios IF, Vlahakes GJ, Lowry PA, Fifer MA. Time course of pressure gradient response after first alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2006; 97: 1511-4. ]. Thinning of the ablated area and scar formation lead to a permanent and significant reduction of the obstruction and the associated mitral regurgitation within the next 3-12 months [1414. Seggewiss H, Gleichmann U, Faber L, Fassbender D, Schmidt HK, Strick S. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: acute results and 3-month follow-up in 25 patients. J Am Coll Cardiol. 1998; 31: 252-8. , 2828. Seggewiss H, Rigopoulos A, Welge D, Ziemssen P, Faber L. Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Clin Res Cardiol. 2007; 96: 856-63.
Up to 8 years follow-up of the first 100 consecutive patients with echo-guided septal ablation., 4747. Lakkis NM, Nagueh SF, Dunn JK, Killip D, Spencer WH. Nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy: one-year follow-up. J Am Coll Cardiol. 2000; 36: 852-5. , 5757. Seggewiss H, Faber L, Gleichmann U. Percutaneous transluminal septal ablation in hypertrophic obstructive cardiomyopathy. Thorac Cardiovasc Surg. 1999; 47: 94-100. , 5858. Mazur W, Nagueh SF, Lakkis NM, et al. Regression of left ventricular hypertrophy after nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy. Circulation. 2001; 103: 1492-6. ]. The scar occupies <10% of the left ventricular myocardium and gradually shrinks with time, thus allowing the LVOT cross-sectional area to expand [5959. Aqel RA, Hage FG, Zohgbi GJ, et al. Serial evaluations of myocardial infarct size after alcohol septal ablation in hypertrophic cardiomyopathy and effects of the changes on clinical status and left ventricular outflow pressure gradients. Am J Cardiol. 2008; 101: 1328-33. , 6060. van Dockum WG, ten Cate FJ, ten Berg JM, et al. Myocardial infarction after percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: evaluation by contrast-enhanced magnetic resonance imaging. J Am Coll Cardiol. 2004; 43: 27-34. , 6161. Angelini P. The «1st septal unit» in hypertrophic obstructive cardiomyopathy: a newly recognized anatomo-functional entity, identified during recent alcohol septal ablation experience. Tex Heart Inst J. 2007; 34: 336-46. , 6262. Schulz-Menger J, Strohm O, Waigand J, Uhlich F, Dietz R, Friedrich MG. The value of magnetic resonance imaging of the left ventricular outflow tract in patients with hypertrophic obstructive cardiomyopathy after septal artery embolization. Circulation. 2000; 101: 1764-6. , 6363. Sitges M, Qin JX, Lever HM, et al. Evaluation of left ventricular outflow tract area after septal reduction in obstructive hypertrophic cardiomyopathy: a real-time 3-dimensional echocardiographic study. Am Heart J. 2005; 150: 852-8. ]. It has been observed that a generalised remodelling of the left ventricular wall results in additional reduction of myocardial mass far from the ablated septum [2020. Seggewiss H, Faber L, Ziemssen P, Gleichmann U. One-year follow-up after echocardiographically-guided percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Dtsch Med Wochenschr. 2001; 126: 424-30. , 6464. van Dockum WG, Beek AM, ten Cate FJ, et al. Early onset and progression of left ventricular remodeling after alcohol septal ablation in hypertrophic obstructive cardiomyopathy. Circulation. 2005; 111: 2503-8. ]. This favours the hypothesis that left ventricular hypertrophy in HOCM is in part due to the increased afterload and can therefore be reversed with effective reduction of the LV gradient.
Coronary flow reserve and myocardial flow reserve improve after septal ablation [6565. Jaber WA, Yang EH, Nishimura RA, et al. Immediate improvement in coronary flow reserve after alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. Heart. 2009; 95: 564-9. , 6666. van Dockum WG, Knaapen P, Hofman MB, et al. Impact of alcohol septal ablation on left anterior descending coronary artery blood flow in hypertrophic obstructive cardiomyopathy. Int J Cardiovasc Imaging. 2009; 25: 511-8. , 6767. Pedone C, Biagini E, Galema TW, Vletter WB, ten Cate FJ. Myocardial perfusion after percutaneous transluminal septal myocardial ablation as assessed by myocardial contrast echocardiography in patients with hypertrophic obstructive cardiomyopathy. J Am Soc Echocardiogr. 2006; 19: 982-6. , 6868. Soliman OI, Geleijnse ML, Michels M, et al. Effect of successful alcohol septal ablation on microvascular function in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2008; 101: 1321-7. ]. The left ventricular ejection fraction decreases but myocardial contractility is preserved, as is suggested by the increase in global systolic indices (shortening index, Tei index) and the changes in pressure-volume loops at six months [6969. Steendijk P, Meliga E, Valgimigli M, Ten Cate FJ, Serruys PW. Acute effects of alcohol septal ablation on systolic and diastolic left ventricular function in patients with hypertrophic obstructive cardiomyopathy. Heart. 2008; 94: 1318-22. , 7070. Meliga E, Steendijk P, Valgimigli M, Ten Cate FJ, Serruys PW. Effects of percutaneous transluminal septal myocardial ablation for obstructive hypertrophic cardiomyopathy on systolic and diastolic left ventricular function assessed by pressure-volume loops. Am J Cardiol. 2008; 101: 1179-84. ]. More importantly, the diastolic function improves significantly with reduction of end-diastolic pressure, diastolic stiffness and tau; echocardiographic indices of diastolic function remain improved at one and two-year follow-up in patients with successful alcohol ablation [7171. Jassal DS, Neilan TG, Fifer MA, et al. Sustained improvement in left ventricular diastolic function after alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Eur Heart J. 2006; 27: 1805-10. ]. As a consequence, there is an improvement in left atrial haemodynamics with decrease in pressure and volume, and increase in ejection fraction associated with gradient reduction [2020. Seggewiss H, Faber L, Ziemssen P, Gleichmann U. One-year follow-up after echocardiographically-guided percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Dtsch Med Wochenschr. 2001; 126: 424-30. , 7272. Hage FG, Karakus G, Luke WD, Jr., et al. Effect of alcohol-induced septal ablation on left atrial volume and ejection fraction assessed by real time three-dimensional transthoracic echocardiography in patients with hypertrophic cardiomyopathy. Echocardiography. 2008; 25: 784-9. ].
Apart from ECG changes indicative of myocardial infarction (new ST elevation, new Q-waves) or ischaemia (increased QRS duration), conduction abnormalities are common after alcohol septal ablation [7373. Kazmierczak J, Kornacewicz-Jach Z, Kisly M, Gil R, Wojtarowicz A. Electrocardiographic changes after alcohol septal ablation in hypertrophic obstructive cardiomyopathy. Heart. 1998; 80: 257-62. ]. The ablated area commonly includes the right bundle branch, with a new RBBB appearing in up to 50% of the patients [3636. Batzner A, Pfeiffer B, Neugebauer A, Aicha D, Blank C, Seggewiss H. Survival After Alcohol Septal Ablation in Patients With Hypertrophic Obstructive Cardiomyopathy. J Am Coll Cardiol. 2018; 72: 3087-94.
Up to 18 years follow-up results in a large single center cohort after alcohol septal ablation., 7474. Talreja DR, Nishimura RA, Edwards WD, et al. Alcohol septal ablation versus surgical septal myectomy: comparison of effects on atrioventricular conduction tissue. J Am Coll Cardiol. 2004; 44: 2329-32. ]. Furthermore, transient complete heart block will be observed during alcohol injection in almost half of the patients, whereas persistent complete heart block requiring a permanent pacemaker is nowadays reported in up to 10% [3636. Batzner A, Pfeiffer B, Neugebauer A, Aicha D, Blank C, Seggewiss H. Survival After Alcohol Septal Ablation in Patients With Hypertrophic Obstructive Cardiomyopathy. J Am Coll Cardiol. 2018; 72: 3087-94.
Up to 18 years follow-up results in a large single center cohort after alcohol septal ablation., 7575. Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol. 2006; 19: 319-27. ].
Potential complications
Procedure-related mortality has been reported to be 1.5% (0% to 5%) and is attributable to haemodynamic collapse from LV or RV ventricular failure, cardiac tamponade, LAD dissection, ventricular fibrillation or pulmonary embolism [7575. Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol. 2006; 19: 319-27. ]. Mortality rates are associated with volume and expertise of the hospitals [77. Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic Obstructive Cardiomyopathy: Surgical Myectomy and Septal Ablation. Circ Res. 2017; 121: 771-83.
The paper describes the options of gradient reduction therapies in HOCM., 7676. Kim LK, Swaminathan RV, Looser P, et al. Hospital Volume Outcomes After Septal Myectomy and Alcohol Septal Ablation for Treatment of Obstructive Hypertrophic Cardiomyopathy: US Nationwide Inpatient Database, 2003-2011. JAMA Cardiol. 2016; 1: 324-32. ]. In our large single center cohort in-hospital mortality was only 0.2% and occured only in the post-interventinal phase [3636. Batzner A, Pfeiffer B, Neugebauer A, Aicha D, Blank C, Seggewiss H. Survival After Alcohol Septal Ablation in Patients With Hypertrophic Obstructive Cardiomyopathy. J Am Coll Cardiol. 2018; 72: 3087-94.
Up to 18 years follow-up results in a large single center cohort after alcohol septal ablation.].
Non-fatal complications of alcohol septal ablation are rare in high-volume centres and mostly occur in the cathlab or during the early post-interventional period. They include coronary dissection, coronary artery spasm, cardiac tamponade, pulmonary embolism, cardiogenic shock, puncture site complications, and stroke [7575. Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol. 2006; 19: 319-27. ].
Damage to the coronary arteries is a very rare complication, mostly occurring during the learning curve, and can be avoided by proper selection and prudent use of guidewires and balloons [1515. Seggewiss H. Current status of alcohol septal ablation for patients with hypertrophic cardiomyopathy. Curr Cardiol Rep. 2001; 3: 160-6. , 3131. Fernandes VL, Nielsen C, Nagueh SF, et al. Follow-up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy the Baylor and Medical University of South Carolina experience 1996 to 2007. JACC Cardiovasc Interv. 2008; 1: 561-70. ].
Alcohol leakage to the left anterior descending artery is also an uncommon, albeit catastrophic, complication leading to a remote myocardial infarction [4343. Ruzyllo W, Chojnowska L, Demkow M, et al. Left ventricular outflow tract gradient decrease with non-surgical myocardial reduction improves exercise capacity in patients with hypertrophic obstructive cardiomyopathy. Eur Heart J. 2000; 21: 770-7. , 7777. Antolinos Pérez MJ dlMVG, Gimeno Blanes JR, Cerdán Sánchez Mdel C, Hurtado Martínez JA, Valdés Chavarri M. Balloon rupture and alcohol leakage into the left anterior descending coronary artery during percutaneous septal ablation for hypertrophic obstructive cardiomyopathy. Rev Esp Cardiol. 2005; 58(7):872-4. ]. Potential causes are retrograde flow to the LAD due to incomplete sealing of the septal branch by the inflated balloon, slippage of the balloon (melon seed effect), too early deflation and retraction of the balloon or antegrade flow due to collateralisation between septal branches that has been overlooked. Strict adhesion to a detailed interventional protocol that involves the use of a slightly oversized balloon, special attention during slow alcohol injection and timely deflation of the balloon at least 10 minutes after the last alcohol injection will eliminate the chance of any retrograde alcohol spilling [7878. Seggewiss H. Percutaneous transluminal septal myocardial ablation: a new treatment for hypertrophic obstructive cardiomyopathy. Eur Heart J. 2000; 21: 704-7. ]. Besides, collateralisation between septal branches can be revealed or ruled out by careful angiography of the target septal branch [3939. Koljaja-Batzner A, Pfeiffer B, Seggewiss H. Septal Collateralization to Right Coronary Artery in Alcohol Septal Ablation: Solution to a Dangerous Pitfall. JACC Cardiovasc Interv. 2018; 11: 2009-11. ].
The most common complications concern disruption of the conduction system. First degree AV block has been reported in 53% (0-100%) of patients while complete heart block is reported to occur temporarily during alcohol injection to last for the first hours or days after the intervention and with subsequent automatic recovery [1313. Faber L, Seggewiss H, Gleichmann U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: results with respect to intraprocedural myocardial contrast echocardiography. Circulation. 1998; 98: 2415-21.
First decription of echo-guided septal ablation in HOCM., 7575. Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol. 2006; 19: 319-27. , 7979. Reinhard W, Ten Cate FJ, Scholten M, De Laat LE, Vos J. Permanent pacing for complete atrioventricular block after nonsurgical (alcohol) septal reduction in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2004; 93: 1064-6. ]. This substantiates the need for both temporary pacing during the procedure and watchful rhythm monitoring throughout the hospital stay. Faber and colleagues have developed a scoring system for the prediction of permanent pacemaker dependency after the intervention [4545. Faber L, Seggewiss H, Welge D, et al. Predicting the risk of atrioventricular conduction lesions after percutaneous septal ablation for obstructive hypertrophic cardiomyopathy. Z Kardiol. 2003; 92: 39-47. ]. It involves assessment of electrocardiographic (QRS duration, PQ duration, atrioventricular block occurrence and persistence or recovery, heart rate) as well as haemodynamic variables (baseline gradient) and myocardial enzyme kinetics (peak SGOT time-point) and categorises patients into 3 groups: low risk of pacemaker dependency, need for prolonged monitoring, and propensity to early pacemaker implantation ( Table 5 ). It should be mentioned, however, that delayed occurrence of complete heart block up to nine days after the intervention has also been reported ([4444. Kern MJ, Holmes DG, Simpson C, Bitar SR, Rajjoub H: Delayed occurrence of complete heart block without warning after alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Catheter Cardiovasc Interv. 2002; 56: 503-7. , 4545. Faber L, Seggewiss H, Welge D, et al. Predicting the risk of atrioventricular conduction lesions after percutaneous septal ablation for obstructive hypertrophic cardiomyopathy. Z Kardiol. 2003; 92: 39-47. ] personal observation). Overall, less than 10% of patients in modern series will eventually need a permanent pacemaker [77. Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic Obstructive Cardiomyopathy: Surgical Myectomy and Septal Ablation. Circ Res. 2017; 121: 771-83.
The paper describes the options of gradient reduction therapies in HOCM., 3636. Batzner A, Pfeiffer B, Neugebauer A, Aicha D, Blank C, Seggewiss H. Survival After Alcohol Septal Ablation in Patients With Hypertrophic Obstructive Cardiomyopathy. J Am Coll Cardiol. 2018; 72: 3087-94.
Up to 18 years follow-up results in a large single center cohort after alcohol septal ablation., 8080. Fifer MA. Controversies in cardiovascular medicine. Most fully informed patients choose septal ablation over septal myectomy. Circulation. 2007; 116: 207-16; discussion 16. ].
( Figure 2 ) illustrates the anatomic relationsship of the coronary septal perforators with myocardial conduction tissue.
Ventricular arrhythmias, including ventricular fibrillation and sustained ventricular tachycardia, have been reported early after the intervention, although there have been reports of ventricular tachycardia several weeks after the procedure [7575. Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol. 2006; 19: 319-27. , 8181. Seggewiss H, Faber L, Ziemssen P. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Cardiol Rev. 1999; 7: 316-23. , 8282. Boltwood CM, Jr, Chien W, Ports T. Ventricular tachycardia complicating alcohol septal ablation. N Engl J Med. 2004; 351: 1914-5. , 8383. Antoun P, El Masry H, Breall JA. Sudden cardiac death complicating alcohol septal ablation: a case report and review of literature. Catheter Cardiovasc Interv. 2009; 73: 956-9. ]. Ventricular arrhythmias that appear in the early post-procedural period may possibly be due to ischaemia. Nonetheless, concern exists that re-entry around the myocardial scar could lead to exacerbation of ventricular arrhythmias after septal ablation [8484. Kimmelstiel CD, Maron BJ. Role of percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Circulation. 2004; 109: 452-6. ]. Up to now, this theory has not been supported by the existing data. Long follow-up studies have not shown any increased manifestation of ventricular arrhythmias [3030. Welge D, Seggewiss H, Fassbender D, Schmidt HK, Horstkotte D, Faber L. Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Dtsch Med Wochenschr. 2008; 133: 1949-54. , 3131. Fernandes VL, Nielsen C, Nagueh SF, et al. Follow-up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy the Baylor and Medical University of South Carolina experience 1996 to 2007. JACC Cardiovasc Interv. 2008; 1: 561-70. , 3636. Batzner A, Pfeiffer B, Neugebauer A, Aicha D, Blank C, Seggewiss H. Survival After Alcohol Septal Ablation in Patients With Hypertrophic Obstructive Cardiomyopathy. J Am Coll Cardiol. 2018; 72: 3087-94.
Up to 18 years follow-up results in a large single center cohort after alcohol septal ablation., 3737. Veselka J, Jensen MK, Liebregts M, et al. Long-term clinical outcome after alcohol septal ablation for obstructive hypertrophic cardiomyopathy: results from the Euro-ASA registry. Eur Heart J. 2016; 37: 1517-23.
Follow-up results of a multicenter European septal ablation registry., 8585. Faber L, Welge D, Fassbender D, Schmidt HK, Horstkotte D, Seggewiss H. Percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: managing the risk of procedure-related AV conduction disturbances. Int J Cardiol. 2007; 119: 163-7. ]. These findings are supported by pathological studies showing that septal ablation produces a sequestered and stabilised type of scar, histologically very different from that found after ischaemia-induced necrosis [8686. Raute-Kreinsen U. Morphology of necrosis and repair after transcoronary ethanol ablation of septal hypertrophy. Pathol Res Pract. 2003; 199: 121-7. ]. Besides, patients who already had an ICD before the procedure, as they were deemed high risk on clinical grounds, have not shown any increased incidence of malignant ventricular arrhythmias after alcohol septal ablation [8787. Lawrenz T, Obergassel L, Lieder F, et al. Transcoronary ablation of septal hypertrophy does not alter ICD intervention rates in high risk patients with hypertrophic obstructive cardiomyopathy. Pacing Clin Electrophysiol. 2005; 28: 295-300. , 8888. Cuoco FA, Spencer WH, 3rd, Fernandes VL, et al. Implantable cardioverter-defibrillator therapy for primary prevention of sudden death after alcohol septal ablation of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008; 52: 1718-23. , 8989. Rigopoulos AG, Daci S, Pfeiffer B, Papadopoulou K, Neugebauer A, Seggewiss H. Low occurrence of ventricular arrhythmias after alcohol septal ablation in high-risk patients with hypertrophic obstructive cardiomyopathy. Clin Res Cardiol. 2016; 105: 953-61. ]. On the contrary, in one study the risk of ventricular arrhythmias was shown to increase in relation to the post-interventional gradient, implying that arrhythmic risk may be related to a less agreeable haemodynamic outcome [9090. Noseworthy PA, Rosenberg MA, Fifer MA, et al. Ventricular arrhythmia following alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2009; 104: 128-32. ]. Alcohol septal ablation is a rather complex technique which is based on the practice and skills of percutaneous coronary angioplasty, but is further refined by myocardial contrast echocardiography. MCE guiding has improved haemodynamic results and reduced complication risk [1313. Faber L, Seggewiss H, Gleichmann U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: results with respect to intraprocedural myocardial contrast echocardiography. Circulation. 1998; 98: 2415-21.
First decription of echo-guided septal ablation in HOCM., 8585. Faber L, Welge D, Fassbender D, Schmidt HK, Horstkotte D, Seggewiss H. Percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: managing the risk of procedure-related AV conduction disturbances. Int J Cardiol. 2007; 119: 163-7. ]. However, it is evident that all steps in the procedure have to be successful in order to ensure good results and avoid complications.
Complications of alcohol septal ablation
- Hospital mortality
- Complete heart block
- Remote acute infarction
- Alcohol leakage retrogradely to LAD
- Alcohol misplacement
- Inadvertent echo-contrast misplacement
- Inadvertent intraseptal collateralization
- LMS or LAD dissection
- Emergency surgery
- Coronary damage
- Acute mitral regurgitation
- Pericardial tamponade
- Puncture site complications
- Haematoma
- Pseudoaneurysm
- AV-fistula
Clinical results
Most patients tolerate alcohol septal ablation well. Chest discomfort during the alcohol injection which may wane during 24 hours in some of them. Follow-up studies have shown a favourable result of the intervention both in the short term and longer-term. Haemodynamic success with reduction in both resting and provocable gradients is accomplished in ≥90% of patients and is associated with significant improvement in symptoms [1010. Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet. 1995; 346: 211-4.
Sigwart describes the technique and results of the first patients treated with alcohol septal ablation., 1212. Gietzen FH, Leuner CJ, Raute-Kreinsen U, et al. Acute and long-term results after transcoronary ablation of septal hypertrophy (TASH). Catheter interventional treatment for hypertrophic obstructive cardiomyopathy. Eur Heart J. 1999; 20: 1342-54. , 1313. Faber L, Seggewiss H, Gleichmann U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: results with respect to intraprocedural myocardial contrast echocardiography. Circulation. 1998; 98: 2415-21.
First decription of echo-guided septal ablation in HOCM., 1414. Seggewiss H, Gleichmann U, Faber L, Fassbender D, Schmidt HK, Strick S. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: acute results and 3-month follow-up in 25 patients. J Am Coll Cardiol. 1998; 31: 252-8. , 2020. Seggewiss H, Faber L, Ziemssen P, Gleichmann U. One-year follow-up after echocardiographically-guided percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Dtsch Med Wochenschr. 2001; 126: 424-30. , 2828. Seggewiss H, Rigopoulos A, Welge D, Ziemssen P, Faber L. Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Clin Res Cardiol. 2007; 96: 856-63.
Up to 8 years follow-up of the first 100 consecutive patients with echo-guided septal ablation., 2929. Sorajja P, Valeti U, Nishimura RA, et al. Outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2008; 118: 131-9. , 3535. Jensen MK, Prinz C, Horstkotte D, et al. Alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy: low incidence of sudden cardiac death and reduced risk profile. Heart. 2013; 99: 1012-7. , 3737. Veselka J, Jensen MK, Liebregts M, et al. Long-term clinical outcome after alcohol septal ablation for obstructive hypertrophic cardiomyopathy: results from the Euro-ASA registry. Eur Heart J. 2016; 37: 1517-23.
Follow-up results of a multicenter European septal ablation registry., 7474. Talreja DR, Nishimura RA, Edwards WD, et al. Alcohol septal ablation versus surgical septal myectomy: comparison of effects on atrioventricular conduction tissue. J Am Coll Cardiol. 2004; 44: 2329-32. , 7575. Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol. 2006; 19: 319-27. , 8585. Faber L, Welge D, Fassbender D, Schmidt HK, Horstkotte D, Seggewiss H. Percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: managing the risk of procedure-related AV conduction disturbances. Int J Cardiol. 2007; 119: 163-7. , 9191. Knight C, Sigwart U. Non-surgical ablation of the ventricular septum for the treatment of hypertrophic cardiomyopathy. Heart. 1996; 76: 92. , 9292. Lakkis NM, Nagueh SF, Kleiman NS, et al. Echocardiography-guided ethanol septal reduction for hypertrophic obstructive cardiomyopathy. Circulation. 1998; 98: 1750-5. , 9393. Qin JX, Shiota T, Lever HM, et al. Outcome of patients with hypertrophic obstructive cardiomyopathy after percutaneous transluminal septal myocardial ablation and septal myectomy surgery. J Am Coll Cardiol. 2001; 38: 1994-2000. , 9494. Nagueh SF, Ommen SR, Lakkis NM, et al. Comparison of ethanol septal reduction therapy with surgical myectomy for the treatment of hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2001; 38: 1701-6. , 9595. Gietzen FH, Leuner CJ, Obergassel L, Strunk-Mueller C, Kuhn H. Role of transcoronary ablation of septal hypertrophy in patients with hypertrophic cardiomyopathy, New York Heart Association functional class III or IV, and outflow obstruction only under provocable conditions. Circulation. 2002; 106: 454-9. , 9696. Liebregts M, Faber L, Jensen MK, et al. Outcomes of Alcohol Septal Ablation in Younger Patients With Obstructive Hypertrophic Cardiomyopathy. JACC Cardiovasc Interv. 2017; 10: 1134-43. ]. In a systematic review of 42 published studies between 1996 and 2005 involving 2,959 patients, mean NYHA class decreased from 2.9 to 1.2 and mean CCS class decreased from 1.9 to 0.4 at 1-year follow-up [7575. Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol. 2006; 19: 319-27. ]. Exercise capacity also improved on a treadmill from 5.4 to 7.3 minutes and mean peak oxygen consumption increased from 17.8 to 23.6 ml/Kg/min. In a cohort of the first 100 consecutive patients treated the overall survival was 96% at 8 years, while 74% of patients remained free of severe symptoms, atrial fibrillation, stroke or ICD implantation [2828. Seggewiss H, Rigopoulos A, Welge D, Ziemssen P, Faber L. Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Clin Res Cardiol. 2007; 96: 856-63.
Up to 8 years follow-up of the first 100 consecutive patients with echo-guided septal ablation.].
Long-term follow-up studies confirm that the favourable haemodynamic and clinical effect of alcohol septal ablation is sustained ( Table 6 ) [2828. Seggewiss H, Rigopoulos A, Welge D, Ziemssen P, Faber L. Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Clin Res Cardiol. 2007; 96: 856-63.
Up to 8 years follow-up of the first 100 consecutive patients with echo-guided septal ablation., 2929. Sorajja P, Valeti U, Nishimura RA, et al. Outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2008; 118: 131-9. , 3030. Welge D, Seggewiss H, Fassbender D, Schmidt HK, Horstkotte D, Faber L. Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Dtsch Med Wochenschr. 2008; 133: 1949-54. , 3131. Fernandes VL, Nielsen C, Nagueh SF, et al. Follow-up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy the Baylor and Medical University of South Carolina experience 1996 to 2007. JACC Cardiovasc Interv. 2008; 1: 561-70. , 3232. Kuhn H, Lawrenz T, Lieder F, et al. Survival after transcoronary ablation of septal hypertrophy in hypertrophic obstructive cardiomyopathy (TASH): a 10 year experience. Clin Res Cardiol. 2008; 97: 234-43. , 3333. Kwon DH, Kapadia SR, Tuzcu EM, et al. Long-term outcomes in high-risk symptomatic patients with hypertrophic cardiomyopathy undergoing alcohol septal ablation. JACC Cardiovasc Interv. 2008; 1: 432-8. , 33. Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults Echocardiographic analysis of 4111 subjects in the CARDIA Study Coronary Artery Risk Development in (Young) Adults. Circulation. 1995; 92: 785-9. , 3535. Jensen MK, Prinz C, Horstkotte D, et al. Alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy: low incidence of sudden cardiac death and reduced risk profile. Heart. 2013; 99: 1012-7. , 9696. Liebregts M, Faber L, Jensen MK, et al. Outcomes of Alcohol Septal Ablation in Younger Patients With Obstructive Hypertrophic Cardiomyopathy. JACC Cardiovasc Interv. 2017; 10: 1134-43. , 9797. Sorajja P, Ommen SR, Holmes DR, Jr, et al. Survival after alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2012; 126: 2374-80. ]. The benefit obtained in gradient reduction during the first year ( Figure 3 ) may even improve with further elimination of obstruction in most patients [2828. Seggewiss H, Rigopoulos A, Welge D, Ziemssen P, Faber L. Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Clin Res Cardiol. 2007; 96: 856-63.
Up to 8 years follow-up of the first 100 consecutive patients with echo-guided septal ablation.]. Once obstruction is diminished after successful treatment, it does not recur during follow-up. Depending on the primary treatment strategy redo procedures are needed in some patients, mainly due to the fact that the basal part of the septum is supplied by more than one septal branch [9898. Singh M, Edwards WD, Holmes DR, Jr, Tajil AJ, Nishimura RA. Anatomy of the first septal perforating artery: a study with implications for ablation therapy for hypertrophic cardiomyopathy. Mayo Clin Proc. 2001; 76: 799-802. ], while a minority is ultimately referred to surgery for gradient abolition. In a large serie of an experienced center long-term overall 15 years survival and survival free of cardiac events are 79.7%, respectively 96.5% ( Figure 4) [3636. Batzner A, Pfeiffer B, Neugebauer A, Aicha D, Blank C, Seggewiss H. Survival After Alcohol Septal Ablation in Patients With Hypertrophic Obstructive Cardiomyopathy. J Am Coll Cardiol. 2018; 72: 3087-94.
Up to 18 years follow-up results in a large single center cohort after alcohol septal ablation.]. Most importantly, long-term survival after alcohol septal ablation has been shown to be comparable to that in an age- and sex-matched general population [3838. Veselka J, Krejci J, Tomasov P, Zemanek D. Long-term survival after alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a comparison with general population. Eur Heart J. 2014; 35: 2040-5. , 9797. Sorajja P, Ommen SR, Holmes DR, Jr, et al. Survival after alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2012; 126: 2374-80. ].
Echocardiographic follow-up has shown reduction in septal and posterior wall thickness, SAM and mitral regurgitation, as well as pulmonary artery pressure [1212. Gietzen FH, Leuner CJ, Raute-Kreinsen U, et al. Acute and long-term results after transcoronary ablation of septal hypertrophy (TASH). Catheter interventional treatment for hypertrophic obstructive cardiomyopathy. Eur Heart J. 1999; 20: 1342-54. , 1414. Seggewiss H, Gleichmann U, Faber L, Fassbender D, Schmidt HK, Strick S. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: acute results and 3-month follow-up in 25 patients. J Am Coll Cardiol. 1998; 31: 252-8. , 2828. Seggewiss H, Rigopoulos A, Welge D, Ziemssen P, Faber L. Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Clin Res Cardiol. 2007; 96: 856-63.
Up to 8 years follow-up of the first 100 consecutive patients with echo-guided septal ablation.]. Left ventricular dimensions increase slightly with preserved systolic function. MRI studies have confirmed this remodelling effect and clearly depict the gradual increase of the LVOT cross-sectional area as the scar shrinks in the months following septal ablation [6262. Schulz-Menger J, Strohm O, Waigand J, Uhlich F, Dietz R, Friedrich MG. The value of magnetic resonance imaging of the left ventricular outflow tract in patients with hypertrophic obstructive cardiomyopathy after septal artery embolization. Circulation. 2000; 101: 1764-6. ].
Comparison with surgery
Septal myectomy has been considered the gold standard treatment of HOCM for decades. It is given a class II a recommendation in the latest American guidelines whereas interventional therapy is given a class II b recommendation unless co-morbidities preclude surgery. However, with alcohol ablation gaining ground as an attractive, less invasive alternative to surgery, the problem of choosing the best modality for the individual patient has emerged ( Table 6 ). A true comparison of septal ablation with surgery would require a randomised controlled trial, an endeavour that has thus far been difficult to organise [9999. Olivotto I, Ommen SR, Maron MS, Cecchi F, Maron BJ. Surgical myectomy versus alcohol septal ablation for obstructive hypertrophic cardiomyopathy - Will there ever be a randomized trial?. J Am Coll Cardiol. 2007; 50: 831-4. ]. Several non-randomised observational and retrospective studies have addressed this issue with most of them comparing a patient cohort of generally older patients who have undergone alcohol ablation with historical matched controls from myectomy series [9393. Qin JX, Shiota T, Lever HM, et al. Outcome of patients with hypertrophic obstructive cardiomyopathy after percutaneous transluminal septal myocardial ablation and septal myectomy surgery. J Am Coll Cardiol. 2001; 38: 1994-2000. , 9494. Nagueh SF, Ommen SR, Lakkis NM, et al. Comparison of ethanol septal reduction therapy with surgical myectomy for the treatment of hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2001; 38: 1701-6. ]. Furthermore, some non-randomised observational data of septal ablation, using higher doses of alcohol than contemporary practice, have suggested improved outcomes and fewer complications with septal myectomy and mitral leaflet extension [100100. van der Lee C, ten Cate FJ, Geleijnse ML, et al. Percutaneous versus surgical treatment for patients with hypertrophic obstructive cardiomyopathy and enlarged anterior mitral valve leaflets. Circulation. 2005; 112: 482-8. ]. Meta-analyses, however, involving 12 respectively 10 studies has shown similar short-term and long-term mortality rates and functional status outcomes for both treatment options [101101. Agarwal S, Tuzcu EM, Desai MY, et al. Updated meta-analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010; 55: 823-34. , 102102. Singh K, Qutub M, Carson K, Hibbert B, Glover C. A meta analysis of current status of alcohol septal ablation and surgical myectomy for obstructive hypertrophic cardiomyopathy. Catheter Cardiovasc Interv. 2016; 88: 107-15. ]. NYHA class is higher after alcohol ablation, but the degree of improvement of NYHA class is comparable with either treatment. Likewise, occurrence of ventricular arrhythmia, reintervention rate and mitral regurgitation after treatment in most series does not differ between alcohol ablation and myectomy. Nonetheless, alcohol septal ablation is associated with a higher need for permanent pacemaker implantation (pooled OR: 2.6, 95% CI: 1.7-3.9) and results in higher residual LVOT gradient than myectomy, although the clinical efficacy of both treatment modalities in LVOT gradient reduction is comparable. The most recent study comparing alcohol septal ablation to an age- and sex-matched cohort of patients who underwent isolated myectomy has shown similar long-term survival after both options which was comparable to that of the general population [9797. Sorajja P, Ommen SR, Holmes DR, Jr, et al. Survival after alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2012; 126: 2374-80. ]. Based on available data, the choice between alcohol ablation and myectomy in symptomatic patients with HOCM should be made after thorough evaluation of all relevant anatomical and physiological characteristics in the individual patient and taking account of the level of expertise of the individual centre [77. Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic Obstructive Cardiomyopathy: Surgical Myectomy and Septal Ablation. Circ Res. 2017; 121: 771-83.
The paper describes the options of gradient reduction therapies in HOCM., 8080. Fifer MA. Controversies in cardiovascular medicine. Most fully informed patients choose septal ablation over septal myectomy. Circulation. 2007; 116: 207-16; discussion 16. , 103103. Fifer MA. Septal Reduction Therapy for Hypertrophic Obstructive Cardiomyopathy. J Am Coll Cardiol. 2018; 72: 3095-7. ]. As many patients may prefer alcohol ablation to surgery, a detailed discussion with the patient about both therapies is warranted in order to arrive at an informed decision.
Conclusions
Alcohol septal ablation has emerged in the last two decades as a less invasive alternative to the standard surgical treatment of symptomatic patients with HOCM. The accumulated long-term results have shown an ongoing relief of symptoms in the majority of patients. Hospital mortality can be practically eliminated in experienced centres, while the need for permanent pacing has also been reduced with increased experience.
Personal perspective - Hubert Seggewiss & Angelika Batzner
From the principal standpoint a randomized trial comparing alcohol septal ablation and myectomy in symptomatic HOCM patients should be performed. However, due to the low prevalence of HOCM and low rate of hard clinbical endpoints during follow-up, especially cardiac mortality, it seems unlikely that such a study will ever be performed. Therefore, a team of experts in both treatments (an 'HCM' team) should clarify the best individual optimal therapy in an individual HOCM patient with respect to anatomical findings, concomitant cardiac and non-cardiac morbidities as well as patient choice. It should be underlined that operators' expertise and availability are the most important criteria to achieve good results in alcohol septal ablation as well as surgical myectomy.




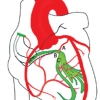







great Chapter!