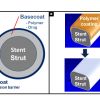
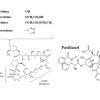
Drug-eluting stent platforms
EARLY-(FIRST) GENERATION DRUG-ELUTING STENTS
Sirolimus-eluting stents
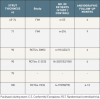
The first SES was the Cypher stent, developed by Cordis Corporation, Warren, NJ. It consisted of sirolimus in a concentration of 140 µg/cm2, incorporated in an amalgam of two biostable polymers, with the polymer/drug matrix then applied onto the tubular 316L stainless steel BX Velocity stent ( Table 3).[66. Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346(23):1773-1780. ], [5151. Morice MC, Serruys PW, Barragan P, et al. Long-term clinical outcomes with sirolimus-eluting coronary stents: five-year results of the RAVEL trial. J Am Coll Cardiol. 2007;50(14):1299-1304. , 5252. Schampaert E, Cohen EA, Schluter M, et al. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS). J Am Coll Cardiol. 2004;43(6):1110-1115. , 5353. Schofer J, Schluter M, Gershlick AH, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS). Lancet. 2003;362(9390):1093-1099. , 5454. Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315-1323. , 5555. Weisz G, Leon MB, Holmes DR, Jr., et al. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) Trial. J Am Coll Cardiol. 2009;53(17):1488-1497. , 5656. Sabate M, Jimenez-Quevedo P, Angiolillo DJ, et al. Randomized comparison of sirolimus-eluting stent versus standard stent for percutaneous coronary revascularization in diabetic patients: the diabetes and sirolimus-eluting stent (DIABETES) trial. Circulation. 2005;112(14):2175-2183. , 5757. Jimenez-Quevedo P, Sabate M, Angiolillo DJ, et al. Long-term clinical benefit of sirolimus-eluting stent implantation in diabetic patients with de novo coronary stenoses: long-term results of the DIABETES trial. Eur Heart J. 2007;28(16):1946-1952. , 5858. Jimenez-Quevedo P, Hernando L, Gomez-Hospital JA, et al. Sirolimus-eluting stent versus bare metal stent in diabetic patients: the final five-year follow-up of the DIABETES trial. EuroIntervention. 2013;9(3):328-335. , 5959. Maresta A, Varani E, Balducelli M, et al. Comparison of effectiveness and safety of sirolimus-eluting stents versus bare-metal stents in patients with diabetes mellitus (from the Italian Multicenter Randomized DESSERT Study). Am J Cardiol. 2008;101(11):1560-1566. , 6060. Baumgart D, Klauss V, Baer F, et al. One-year results of the SCORPIUS study: a German multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patients. J Am Coll Cardiol. 2007;50(17):1627-1634. , 6161. Diaz de la Llera LS, Ballesteros S, Nevado J, et al. Sirolimus-eluting stents compared with standard stents in the treatment of patients with primary angioplasty. Am Heart J. 2007;154(1):164 e161-166. , 6262. van der Hoeven BL, Liem SS, Jukema JW, et al. Sirolimus-eluting stents versus bare-metal stents in patients with ST-segment elevation myocardial infarction: 9-month angiographic and intravascular ultrasound results and 12-month clinical outcome results from the MISSION! Intervention Study. J Am Coll Cardiol. 2008;51(6):618-626. , 6363. Boden H, van der Hoeven BL, Liem SS, et al. Five-year clinical follow-up from the MISSION! Intervention Study: sirolimus-eluting stent versus bare metal stent implantation in patients with ST-segment elevation myocardial infarction, a randomised controlled trial. EuroIntervention. 2012;7(9):1021-1029. , 6464. Di Lorenzo E, De Luca G, Sauro R, et al. The PASEO (PaclitAxel or Sirolimus-Eluting Stent Versus Bare Metal Stent in Primary Angioplasty) Randomized Trial. JACC Cardiovasc Interv. 2009;2(6):515-523. , 6565. Di Lorenzo E, Sauro R, Varricchio A, et al. Benefits of drug-eluting stents as compared to bare metal stent in ST-segment elevation myocardial infarction: four year results of the PaclitAxel or Sirolimus-Eluting stent vs bare metal stent in primary angiOplasty (PASEO) randomized trial. Am Heart J. 2009;158(4):e43-50. , 6666. Menichelli M, Parma A, Pucci E, et al. Randomized trial of Sirolimus-Eluting Stent Versus Bare-Metal Stent in Acute Myocardial Infarction (SESAMI). J Am Coll Cardiol. 2007;49(19):1924-1930. , 6767. Musto C, Fiorilli R, De Felice F, et al. Long-term outcome of sirolimus-eluting vs bare-metal stent in the setting of acute myocardial infarction: 5-year results of the SESAMI trial. Int J Cardiol. 2013;166(2):399-403. , 6868. Valgimigli M, Percoco G, Malagutti P, et al. Tirofiban and sirolimus-eluting stent vs abciximab and bare-metal stent for acute myocardial infarction: a randomized trial. JAMA. 2005;293(17):2109-2117. , 6969. Tebaldi M, Arcozzi C, Campo G, et al. The 5-year clinical outcomes after a randomized comparison of sirolimus-eluting versus bare-metal stent implantation in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2009;54(20):1900-1901. , 7070. Spaulding C, Henry P, Teiger E, et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006;355(11):1093-1104. , 7171. Spaulding C, Teiger E, Commeau P, et al. Four-year follow-up of TYPHOON (trial to assess the use of the CYPHer sirolimus-eluting coronary stent in acute myocardial infarction treated with BallOON angioplasty). JACC Cardiovasc Interv. 2011;4(1):14-23. , 7272. Pache J, Dibra A, Mehilli J, et al. Drug-eluting stents compared with thin-strut bare stents for the reduction of restenosis: a prospective, randomized trial. Eur Heart J. 2005;26(13):1262-1268. , 7373. Suttorp MJ, Laarman GJ, Rahel BM, et al. Primary Stenting of Totally Occluded Native Coronary Arteries II (PRISON II): a randomized comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions. Circulation. 2006;114(9):921-928. , 7474. Van den Branden BJ, Rahel BM, Laarman GJ, et al. Five-year clinical outcome after primary stenting of totally occluded native coronary arteries: a randomised comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions (PRISON II study). EuroIntervention. 2012;7(10):1189-1196. , 7575. Teeuwen K, Van den Branden BJ, Rahel BM, et al. Late catch-up in lumen diameter at five-year angiography in MACE-free patients treated with sirolimus-eluting stents in the Primary Stenting of Totally Occluded Native Coronary Arteries: a randomised comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions (PRISON II). EuroIntervention. 2013;9(2):212-219. , 7676. Rubartelli P, Petronio AS, Guiducci V, et al. Comparison of sirolimus-eluting and bare metal stent for treatment of patients with total coronary occlusions: results of the GISSOC II-GISE multicentre randomized trial. Eur Heart J. 2010;31(16):2014-2020. , 7777. Ardissino D, Cavallini C, Bramucci E, et al. Sirolimus-eluting vs uncoated stents for prevention of restenosis in small coronary arteries: a randomized trial. JAMA. 2004;292(22):2727-2734. , 7878. Menozzi A, Solinas E, Ortolani P, et al. Twenty-four months clinical outcomes of sirolimus-eluting stents for the treatment of small coronary arteries: the long-term SES-SMART clinical study. Eur Heart J. 2009;30(17):2095-2101. , 7979. Kaiser C, Galatius S, Erne P, et al. Drug-eluting versus bare-metal stents in large coronary arteries. N Engl J Med. 2010;363(24):2310-2319. , 8080. Vermeersch P, Agostoni P, Verheye S, et al. Randomized double-blind comparison of sirolimus-eluting stent versus bare-metal stent implantation in diseased saphenous vein grafts: six-month angiographic, intravascular ultrasound, and clinical follow-up of the RRISC Trial. J Am Coll Cardiol. 2006;48(12):2423-2431. , 8181. Vermeersch P, Agostoni P, Verheye S, et al. Increased late mortality after sirolimus-eluting stents versus bare-metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC Trial. J Am Coll Cardiol. 2007;50(3):261-267. , 8282. Kelbaek H, Thuesen L, Helqvist S, et al. The Stenting Coronary Arteries in Non-stress/benestent Disease (SCANDSTENT) trial. J Am Coll Cardiol. 2006;47(2):449-455. , 8383. Kelbaek H, Klovgaard L, Helqvist S, et al. Long-term outcome in patients treated with sirolimus-eluting stents in complex coronary artery lesions: 3-year results of the SCANDSTENT (Stenting Coronary Arteries in Non-Stress/Benestent Disease) trial. J Am Coll Cardiol. 2008;51(21):2011-2016. ]
Both fast release stents with drug release in < 15 days and slow release stents with ≥ 28 day drug release were developed and tested in the FIM study in 1999 in Sao Paulo, Brazil and Rotterdam, the Netherlands. Angiographic and IVUS results from the 45 patients who were studied showed remarkable suppression of in-stent neointimal hyperplasia, which continued out to 4 years of follow-up.[8484. Sousa JE, Costa MA, Abizaid A, et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001;103(2):192-195. , 8585. Rensing BJ, Vos J, Smits PC, et al. Coronary restenosis elimination with a sirolimus eluting stent: first European human experience with 6-month angiographic and intravascular ultrasonic follow-up. Eur Heart J. 2001;22(22):2125-2130. , 8686. Sousa JE, Costa MA, Abizaid AC, et al. Sustained suppression of neointimal proliferation by sirolimus-eluting stents: one-year angiographic and intravascular ultrasound follow-up. Circulation. 2001;104(17):2007-2011. ]
The pivotal RAVEL study (RAndomised study with the sirolimus-eluting VElocity balloon-expandable stent in the treatment of patients with de novo native coronary artery Lesions) evaluated the Cypher SES by randomizing 238 patients with relatively low risk lesions to treatment with SES or BMS. At 1-year follow-up the rate of binary stenosis was 0.0% and 26.6% for patients treated with Cypher SES and BMS, respectively.[66. Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346(23):1773-1780. ] These results were subsequently confirmed in the larger SIRIUS trial (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) that enrolled 1058 patients with more complex lesions than were seen in RAVEL. Significantly lower rates of target lesion revascularization (TLR) and MACE following treatment with the Cypher SES were demonstrated when compared to BMS controls at 9-months, 2-years and 5-year follow-up.[5454. Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315-1323. , 5555. Weisz G, Leon MB, Holmes DR, Jr., et al. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) Trial. J Am Coll Cardiol. 2009;53(17):1488-1497. , 8787. Weisz G, Leon MB, Holmes DR, Jr., et al. Two-year outcomes after sirolimus-eluting stent implantation: results from the Sirolimus-Eluting Stent in de Novo Native Coronary Lesions (SIRIUS) trial. J Am Coll Cardiol. 2006;47(7):1350-1355. ] The Cypher stent was thus the first DES to receive CE-mark in April 2002 and was subsequently approved by the FDA in 2003. A meta-analysis of data from four double-blind studies with 1,784 patients found that TLR was reduced from 23.6% with BMS to 7.8% with SES (hazard ratio [HR] 0.29, 95% CI 0.22-0.39, p < 0.001) at four years ( Table 4).[8888. Stone GW, Moses JW, Ellis SG, et al. Safety and Efficacy of Sirolimus- and Paclitaxel-Eluting Coronary Stents. N Engl J Med. 2007;356(10):998-1008. , 8989. Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356(10):1030-1039. , 9090. Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937-948. , 9191. Kirtane AJ, Gupta A, Iyengar S, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119(25):3198-3206. , 9292. Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. The New England journal of medicine. 2007;356(10):998-1008. ] Although rates of death or MI were similar for both BMS and SES, the latter showed a somewhat higher propensity for late ST (5 vs. 0 events) between 1 and 4 years; efficacy remained superior with SES out to 5 years (TLR: SES 15% vs 30.1%; p < 0.0001).[9393. Caixeta A, Leon MB, Lansky AJ, et al. 5-year clinical outcomes after sirolimus-eluting stent implantation insights from a patient-level pooled analysis of 4 randomized trials comparing sirolimus-eluting stents with bare-metal stents. J Am Coll Cardiol. 2009;54(10):894-902. ]
Performance of the Cypher SES has been assessed in ‘off label’ settings and specific subgroups of patients such as diabetics,[5656. Sabate M, Jimenez-Quevedo P, Angiolillo DJ, et al. Randomized comparison of sirolimus-eluting stent versus standard stent for percutaneous coronary revascularization in diabetic patients: the diabetes and sirolimus-eluting stent (DIABETES) trial. Circulation. 2005;112(14):2175-2183. , 6060. Baumgart D, Klauss V, Baer F, et al. One-year results of the SCORPIUS study: a German multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patients. J Am Coll Cardiol. 2007;50(17):1627-1634. ] and those presenting with AMI,[6565. Di Lorenzo E, Sauro R, Varricchio A, et al. Benefits of drug-eluting stents as compared to bare metal stent in ST-segment elevation myocardial infarction: four year results of the PaclitAxel or Sirolimus-Eluting stent vs bare metal stent in primary angiOplasty (PASEO) randomized trial. Am Heart J. 2009;158(4):e43-50. , 6666. Menichelli M, Parma A, Pucci E, et al. Randomized trial of Sirolimus-Eluting Stent Versus Bare-Metal Stent in Acute Myocardial Infarction (SESAMI). J Am Coll Cardiol. 2007;49(19):1924-1930. , 7070. Spaulding C, Henry P, Teiger E, et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006;355(11):1093-1104. , 9494. Valgimigli M, Campo G, Percoco G, et al. Comparison of Angioplasty With Infusion of Tirofiban or Abciximab and With Implantation of Sirolimus-Eluting or Uncoated Stents for Acute Myocardial Infarction: The MULTISTRATEGY Randomized Trial. JAMA. 2008;299(15):1788-1799. ] In addition it has been assessed in patients with different lesion types including chronic total occlusions,[7373. Suttorp MJ, Laarman GJ, Rahel BM, et al. Primary Stenting of Totally Occluded Native Coronary Arteries II (PRISON II): a randomized comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions. Circulation. 2006;114(9):921-928. , 7474. Van den Branden BJ, Rahel BM, Laarman GJ, et al. Five-year clinical outcome after primary stenting of totally occluded native coronary arteries: a randomised comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions (PRISON II study). EuroIntervention. 2012;7(10):1189-1196. ] SVGs,[8080. Vermeersch P, Agostoni P, Verheye S, et al. Randomized double-blind comparison of sirolimus-eluting stent versus bare-metal stent implantation in diseased saphenous vein grafts: six-month angiographic, intravascular ultrasound, and clinical follow-up of the RRISC Trial. J Am Coll Cardiol. 2006;48(12):2423-2431. , 8181. Vermeersch P, Agostoni P, Verheye S, et al. Increased late mortality after sirolimus-eluting stents versus bare-metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC Trial. J Am Coll Cardiol. 2007;50(3):261-267. ] lesions in small coronary vessels,[7777. Ardissino D, Cavallini C, Bramucci E, et al. Sirolimus-eluting vs uncoated stents for prevention of restenosis in small coronary arteries: a randomized trial. JAMA. 2004;292(22):2727-2734. , 7878. Menozzi A, Solinas E, Ortolani P, et al. Twenty-four months clinical outcomes of sirolimus-eluting stents for the treatment of small coronary arteries: the long-term SES-SMART clinical study. Eur Heart J. 2009;30(17):2095-2101. ] and complex lesions.[8282. Kelbaek H, Thuesen L, Helqvist S, et al. The Stenting Coronary Arteries in Non-stress/benestent Disease (SCANDSTENT) trial. J Am Coll Cardiol. 2006;47(2):449-455. , 8383. Kelbaek H, Klovgaard L, Helqvist S, et al. Long-term outcome in patients treated with sirolimus-eluting stents in complex coronary artery lesions: 3-year results of the SCANDSTENT (Stenting Coronary Arteries in Non-Stress/Benestent Disease) trial. J Am Coll Cardiol. 2008;51(21):2011-2016. ] Irrespective of clinical situation, when compared with BMS, the use of SES results in significant reductions in angiographic in-stent late loss, in-stent angiographic stenosis, and repeat revascularization at both short and long-term 5-year follow-up, with results consistent across numerous different patient and lesion types ( Table 3 and Figure 11).
Despite the wealth of data confirming the efficacy of the Cypher stent, the manufacturer ceased production at the end of 2011.
Paclitaxel-eluting stents
The first TAXUS PES (Boston Scientific, Natick, MA) consisted of paclitaxel contained within a polyolefin derivative biostable polymer coated on the stainless steel NIR platform. A slow release (SR) formulation with an 18 µm thick coat, a moderate release (MR) with a 7 µm coat and a fast release with 4 µm coat shed 8%, 22% and 50% of the paclitaxel within 30 days respectively ( Table 2). The difference in release was achieved by changing the polymer to drug ratio while maintaining the same paclitaxel concentration (1µg/mm2).[9595. Baim DS, (Ed). Grossman’s Cardiac Catheterisation, angiography, and Intervention. Philadelphia: Lippincott Williams & Wilkins 2006. ] The TAXUS PES has been evaluated in the TAXUS series of trials which have enrolled different patient and lesion types ( Table 5): [77. Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350(3):221-231. ], [6464. Di Lorenzo E, De Luca G, Sauro R, et al. The PASEO (PaclitAxel or Sirolimus-Eluting Stent Versus Bare Metal Stent in Primary Angioplasty) Randomized Trial. JACC Cardiovasc Interv. 2009;2(6):515-523. ], [9696. Grube E, Silber S, Hauptmann KE, et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation. 2003;107(1):38-42. , 9797. Colombo A, Drzewiecki J, Banning A, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108(7):788-794. , 9898. Silber S, Colombo A, Banning AP, et al. Final 5-year results of the TAXUS II trial: a randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for de novo coronary artery lesions. Circulation. 2009;120(15):1498-1504. , 9999. Ellis SG, Stone GW, Cox DA, et al. Long-term safety and efficacy with paclitaxel-eluting stents: 5-year final results of the TAXUS IV clinical Trial (TAXUS IV-SR: Treatment of de novo coronary disease using a single paclitaxel-eluting stent). J Am Coll Cardiol Intv. 2009;2(12):1248-1259. , 100100. Stone GW, Ellis SG, Cannon L, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. Jama. 2005;294(10):1215-1223. , 101101. Ellis SG, Cannon L, Mann T, et al. Final 5-year outcomes from the TAXUS V de novo trial: Long term safety and effectiveness of the Paclitaxel-eluting TAXUS stent in complex lesions. Abstract presentation at Transcatheter Cardiovascular Therapeutics, San Francisco, 22nd September 2009. Am J Cardiol. 2009;Vol.
104(6, Supplement):135D, 102102. Dawkins KD, Grube E, Guagliumi G, et al. Clinical efficacy of polymer-based paclitaxel-eluting stents in the treatment of complex, long coronary artery lesions from a multicenter, randomized trial: support for the use of drug-eluting stents in contemporary clinical practice. Circulation. 2005;112(21):3306-3313. , 103103. Grube E, Dawkins K, Guagliumi G, et al. TAXUS VI final 5-year results: a multicentre, randomised trial comparing polymer-based moderate-release paclitaxel-eluting stent with a bare metal stent for treatment of long, complex coronary artery lesions. EuroIntervention. 2009;4(5):572-577. , 104104. Stone GW, Lansky AJ, Pocock SJ, et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009;360(19):1946-1959. , 105105. Stone GW, Witzenbichler B, Guagliumi G, et al. Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): final 3-year results from a multicentre, randomised controlled trial. Lancet. 2011;377(9784):2193-2204. , 106106. Erglis A, Narbute I, Kumsars I, et al. A randomized comparison of paclitaxel-eluting stents versus bare-metal stents for treatment of unprotected left main coronary artery stenosis. J Am Coll Cardiol. 2007;50(6):491-497. , 107107. Laarman GJ, Suttorp MJ, Dirksen MT, et al. Paclitaxel-eluting versus uncoated stents in primary percutaneous coronary intervention. N Engl J Med. 2006;355(11):1105-1113. , 108108. Vink MA, Dirksen MT, Suttorp MJ, et al. 5-year follow-up after primary percutaneous coronary intervention with a paclitaxel-eluting stent versus a bare-metal stent in acute ST-segment elevation myocardial infarction: a follow-up study of the PASSION (Paclitaxel-Eluting Versus Conventional Stent in Myocardial Infarction with ST-Segment Elevation) trial. JACC Cardiovasc Interv. 2011;4(1):24-29. ]
- The TAXUS I trial, a FIM phase I feasibility study with 61 randomised patients, reported a 3% MACE rate versus 10% in BMS at one year. Patients in the PES group had no TLR or binary stenosis, proving that paclitaxel effectively inhibited neo-intimal proliferation.[9696. Grube E, Silber S, Hauptmann KE, et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation. 2003;107(1):38-42. ]
- The TAXUS II study randomized 536 patients to treatment with BMS or SR PES, and BMS or MR PES. The reduction in percentage neointimal hyperplasia as measured by IVUS at 6 months was, 7.8% for SR and 7.8% for MR versus 23.2% and 20.5% for control BMS.[9797. Colombo A, Drzewiecki J, Banning A, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108(7):788-794. ] These results provided the foundation for the sustained reduction in TLR of 4.5% and 10.3% for the MR PES and SR PES respectively, (BMS 18.4%, BMS vs. PES p < 0.001) out to 5 years.[9898. Silber S, Colombo A, Banning AP, et al. Final 5-year results of the TAXUS II trial: a randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for de novo coronary artery lesions. Circulation. 2009;120(15):1498-1504. ] Of note, the MR formulation which was not subsequently used for commercialisation showed a better anti-restenotic effect than the SR formulation at 5 years.
- TAXUS III tested the fast release PES in 28 patients with in-stent restenosis. At 6-months the in-stent late loss was 0.54 mm with a neointimal hyperplasia volume of 20.3 mm3, and a subsequent MACE rate was 29%. Overall results suggested that PES was a potentially efficacious treatment in those with in-stent restenosis.[109109. Tanabe K, Serruys PW, Grube E, et al. TAXUS III Trial: in-stent restenosis treated with stent-based delivery of paclitaxel incorporated in a slow-release polymer formulation. Circulation. 2003;107(4):559-564. ]
- TAXUS IV. The PES platform was changed from the NIR platform to the less rigid Express platform ( Table 2) and this combination was studied in the TAXUS IV study, which randomised 1326 patients with non-complex coronary artery disease (CAD) to treatment with the TAXUS Express stent or Express BMS. Target vessel revascularization (TVR) at 9 months was significantly lower in the PES group (12.1% vs. 4.7%; p < 0.0001) and this advantage was maintained through 5 years (27.4% vs. 16.9%; p < 0.0001), despite comparable annual TVR rates for BMS and PES between years 1 and 5 (4.1%/year vs. 3.3%/year; respectively, p = 0.16).[77. Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350(3):221-231. , 9999. Ellis SG, Stone GW, Cox DA, et al. Long-term safety and efficacy with paclitaxel-eluting stents: 5-year final results of the TAXUS IV clinical Trial (TAXUS IV-SR: Treatment of de novo coronary disease using a single paclitaxel-eluting stent). J Am Coll Cardiol Intv. 2009;2(12):1248-1259. ]
- TAXUS V randomized 1156 patients, over half of whom had complex coronary lesions not studied in earlier PES trials, to treatment with PES (n = 557) and BMS (n = 579). Consistent with earlier studies, use of PES lead to significantly lower rates of angiographic stenosis, TLR, and TVR at 9-months, with comparable rates of death, MI and ST. The benefit in favour of PES was maintained out to 5-year follow-up, however PES was also associated with higher rates of MI (9.3% vs. 5.6%, p < 0.05) and definite/probable ST (2.4% vs. 1.5%, p < 0.05).[100100. Stone GW, Ellis SG, Cannon L, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. Jama. 2005;294(10):1215-1223. , 101101. Ellis SG, Cannon L, Mann T, et al. Final 5-year outcomes from the TAXUS V de novo trial: Long term safety and effectiveness of the Paclitaxel-eluting TAXUS stent in complex lesions. Abstract presentation at Transcatheter Cardiovascular Therapeutics, San Francisco, 22nd September 2009. Am J Cardiol. 2009;Vol.
104(6, Supplement):135D]
- TAXUS VI also randomized 446 patients with long complex lesions to treatment with either PES or the Express BMS. At 9-months follow-up use of PES led to significantly lower rates of binary stenosis, TLR and TVR, whilst the overall MACE rate was similar. Subsequent 5-year follow-up demonstrated the sustained anti-restenotic effect of PES on TLR (14.6% vs. 21.4%, p = 0.03), however a significantly higher rate of non-TLR was also seen in the PES group (10.9% vs. 5.1%, p = 0.03). Rates of ST and MACE were similar. [102102. Dawkins KD, Grube E, Guagliumi G, et al. Clinical efficacy of polymer-based paclitaxel-eluting stents in the treatment of complex, long coronary artery lesions from a multicenter, randomized trial: support for the use of drug-eluting stents in contemporary clinical practice. Circulation. 2005;112(21):3306-3313. , 103103. Grube E, Dawkins K, Guagliumi G, et al. TAXUS VI final 5-year results: a multicentre, randomised trial comparing polymer-based moderate-release paclitaxel-eluting stent with a bare metal stent for treatment of long, complex coronary artery lesions. EuroIntervention. 2009;4(5):572-577. ]
Patient level meta-analysis of the initial PES approval trials have confirmed the comparable safety and superior efficacy of PES, compared to BMS out to 4-year follow-up ( Table 4).[8888. Stone GW, Moses JW, Ellis SG, et al. Safety and Efficacy of Sirolimus- and Paclitaxel-Eluting Coronary Stents. N Engl J Med. 2007;356(10):998-1008. , 9090. Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937-948. ] A meta-analysis of five double-blind trials in 3,513 patients also revealed that TLR decreased from 20.0% with BMS to 10.1% with PES at 4 years (HR 0.46, 95% CI 0.38-0.55, p < 0.001).[9292. Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. The New England journal of medicine. 2007;356(10):998-1008. ] Rates of death and MI were balanced among patients treated with PES and BMS at 4 years of follow up. The incidence of ST was low owing to the non-complex underlying disease and not different between PES and BMS at one year. Between 1 and 4 years, however, there was an increase in those treated with PES (0.7% vs. 0.2%, 95% CI 0.98-21.03). TAXUS II is the first trial reporting 5-year outcome data comparing PES with BMS in patients with non-complex coronary artery disease. In this analysis, both slow- and moderate-release polymer based PES were more effective than BMS to reduce TLR (PES-MR: 4.5%; PES-SR: 10.3%, BMS: 18.4%, p < 0.001).[110110. Silber S, Colombo A, Banning AP, et al. Final 5-Year Results of the TAXUS II Trial. A Randomized Study to Assess the Effectiveness of Slow- and Moderate-Release Polymer-Based Paclitaxel-Eluting Stents for De Novo Coronary Artery Lesions. Circulation. 2009;13:1498-1504. ]
TAXUS™ Element™
A third iteration of the TAXUS stent is the TAXUS Element stent (Ion, Boston Scientific, Natick, MA) which has a PtCr platform coated with a poly(styrene-b-isobutylene-b-styrene) polymer, which facilitates controlled elution of paclitaxel (concentration 1µg/mm2) in an identical pattern to that seen on the stainless steel TAXUS Liberté and Express stent ( Table 2). The device was evaluated in the PERSEUS (A Prospective Evaluation in a Randomised Trial of the Safety and Efficacy of the use of the TAXUS Element Paclitaxel Eluting Coronary Stent System for the Treatment of De Novo Coronary Artery Lesions) clinical trial program, which includes: [111111. Kereiakes DJ, Cannon LA, Feldman RL, et al. Clinical and angiographic outcomes after treatment of de novo coronary stenoses with a novel platinum chromium thin-strut stent: primary results of the PERSEUS (Prospective Evaluation in a Randomized Trial of the Safety and Efficacy of the Use of the TAXUS Element Paclitaxel-Eluting Coronary Stent System) trial. J Am Coll Cardiol. 2010;56(4):264-271. , 112112. Allocco DJ, Cannon LA, Britt A, et al. A prospective evaluation of the safety and efficacy of the TAXUS Element paclitaxel-eluting coronary stent system for the treatment of de novo coronary artery lesions: design and statistical methods of the PERSEUS clinical program. Trials. 2010;11:1. , 113113. Cannon LA, Kereiakes DJ, Mann T, et al. A prospective evaluation of the safety and efficacy of TAXUS Element paclitaxel-eluting coronary stent implantation for the treatment of de novo coronary artery lesions in small vessels: the PERSEUS Small Vessel trial. Eurointervention. 2011;6(8):920-927, 921-922. ]
- The PERSEUS Workhorse trial which randomized 1262 patients, with lesions <28mm long, in vessels between 2.75-4.00 mm in diameter, to treatment with the TAXUS Element (n = 942) or the TAXUS Express PES (n = 320).[111111. Kereiakes DJ, Cannon LA, Feldman RL, et al. Clinical and angiographic outcomes after treatment of de novo coronary stenoses with a novel platinum chromium thin-strut stent: primary results of the PERSEUS (Prospective Evaluation in a Randomized Trial of the Safety and Efficacy of the Use of the TAXUS Element Paclitaxel-Eluting Coronary Stent System) trial. J Am Coll Cardiol. 2010;56(4):264-271. ] The study met its pre-specified criteria for non-inferiority for the primary endpoint of TVF at 12-months clinical follow-up and its secondary endpoint, per cent diameter stenosis, at 9-months angiographic follow-up. No significant differences were seen between stents with respect to late loss (Element 0.34 ± 0.55 mm vs. Express 0.26 ± 0.52 mm, p = 0.33), or other the clinical points such as MACE, mortality, MI and ST. Clinical outcomes remained similar between treatment groups through to 5-years.[114114. Kereiakes DJ, Cannon LA, Dauber I, et al. Long-term follow-up of the platinum chromium TAXUS element (ION) stent: The PERSEUS Workhorse and Small Vessel Trial Five-Year Results. Catheter Cardiovasc Interv. 2015;86(6):994-1001. ]
- The PERSEUS small vessel trial, which compared the TAXUS Element stent to historical BMS controls in patients with lesions <20 mm long, in vessels between 2.25-2.75 mm in diameter.[113113. Cannon LA, Kereiakes DJ, Mann T, et al. A prospective evaluation of the safety and efficacy of TAXUS Element paclitaxel-eluting coronary stent implantation for the treatment of de novo coronary artery lesions in small vessels: the PERSEUS Small Vessel trial. Eurointervention. 2011;6(8):920-927, 921-922. ] Overall the study enrolled 224 patients treated with the Element stent, who were compared to 125 lesion-matched historical controls treated with a BMS from the TAXUS IV study. Results at 9-months follow-up demonstrated a significantly lower in-stent late loss (the primary endpoint) with the Element stent compared to the BMS stent (0.38 ± 0.51 mm vs. 0.80 ± 0.53 mm, p < 0.001). At 12-months follow-up the rates of target lesion failure (TLF) and MACE were both significantly lower with the Element stent, whilst safety endpoints and ST were comparable between both stents. At 5-year rates of MACE, and TLF were significantly lower for the Element stent following adjustment for baseline characteristics and were primarily due to lower TLR rates (Element 14.9% vs. 27.2% BMS, p=0.049).[114114. Kereiakes DJ, Cannon LA, Dauber I, et al. Long-term follow-up of the platinum chromium TAXUS element (ION) stent: The PERSEUS Workhorse and Small Vessel Trial Five-Year Results. Catheter Cardiovasc Interv. 2015;86(6):994-1001. ]
Comparative studies of sirolimus-eluting and paclitaxel-eluting stents
Several randomized studies, which are summarized in Table 6 [115115. Lee SW, Park SW, Kim YH, et al. A randomized comparison of sirolimus- versus Paclitaxel-eluting stent implantation in patients with diabetes mellitus. J Am Coll Cardiol. 2008;52(9):727-733. , 116116. Lee SW, Park SW, Kim YH, et al. A randomized comparison of sirolimus- versus paclitaxel-eluting stent implantation in patients with diabetes mellitus 2-year clinical outcomes of the DES-DIABETES trial. J Am Coll Cardiol. 2009;53(9):812-813. , 117117. Dibra A, Kastrati A, Mehilli J, et al. Paclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patients. N Engl J Med. 2005;353(7):663-670. , 118118. Morice MC, Colombo A, Meier B, et al. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions: the REALITY trial: a randomized controlled trial. JAMA. 2006;295(8):895-904. , 119119. Windecker S, Remondino A, Eberli FR, et al. Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N Engl J Med. 2005;353(7):653-662. , 120120. Raber L, Wohlwend L, Wigger M, et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization LATE trial. Circulation. 2011;123(24):2819-2828, 2816 p following 2828. , 121121. Galloe AM, Thuesen L, Kelbaek H, et al. Comparison of paclitaxel- and sirolimus-eluting stents in everyday clinical practice: the SORT OUT II randomized trial. JAMA. 2008;299(4):409-416. , 122122. Goy JJ, Stauffer JC, Siegenthaler M, et al. A prospective randomized comparison between paclitaxel and sirolimus stents in the real world of interventional cardiology: the TAXi trial. J Am Coll Cardiol. 2005;45(2):308-311. , 123123. Berger A, Stauffer JC, Seydoux C, et al. Three-year follow-up of the first prospective randomized comparison between paclitaxel and sirolimus stents: the TAXi-LATE trial. Catheter Cardiovasc Interv. 2007;70(2):163-166. , 124124. Lee JH, Kim HS, Lee SW, et al. Prospective randomized comparison of sirolimus- versus paclitaxel-eluting stents for the treatment of acute ST-elevation myocardial infarction: pROSIT trial. Catheter Cardiovasc Interv. 2008;72(1):25-32. , 125125. Kim HS, Lee JH, Lee SW, et al. Long-term safety and efficacy of sirolimus- vs. paclitaxel-eluting stent implantation for acute ST-elevation myocardial infarction: 3-year follow-up of the PROSIT trial. Int J Cardiol. 2011;147(2):253-257. , 126126. Mehilli J, Kastrati A, Byrne RA, et al. Paclitaxel- versus sirolimus-eluting stents for unprotected left main coronary artery disease. J Am Coll Cardiol. 2009;53(19):1760-1768. , 127127. Kim YH, Park SW, Lee SW, et al. Sirolimus-eluting stent versus paclitaxel-eluting stent for patients with long coronary artery disease. Circulation. 2006;114(20):2148-2153. , 128128. Mehilli J, Dibra A, Kastrati A, et al. Randomized trial of paclitaxel- and sirolimus-eluting stents in small coronary vessels. Eur Heart J. 2006;27(3):260-266. , 129129. Kastrati A, Mehilli J, von Beckerath N, et al. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA. 2005;293(2):165-171. , 130130. Mehilli J, Byrne RA, Tiroch K, et al. Randomized Trial of Paclitaxel- Versus Sirolimus-Eluting Stents for Treatment of Coronary Restenosis in Sirolimus-Eluting Stents: The ISAR-DESIRE 2 (Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis 2) Study. J Am Coll Cardiol. 2010:j.jacc.2010. 2002. 2009. ] have directly compared outcomes between patients treated with SES or PES in: (I) unselected patients populations; (II) specific patient groups such as diabetics or those with STEMI; and (III) specific lesion types such as unprotected left main stem lesions, long lesions or lesions in small vessels.[115115. Lee SW, Park SW, Kim YH, et al. A randomized comparison of sirolimus- versus Paclitaxel-eluting stent implantation in patients with diabetes mellitus. J Am Coll Cardiol. 2008;52(9):727-733. , 116116. Lee SW, Park SW, Kim YH, et al. A randomized comparison of sirolimus- versus paclitaxel-eluting stent implantation in patients with diabetes mellitus 2-year clinical outcomes of the DES-DIABETES trial. J Am Coll Cardiol. 2009;53(9):812-813. , 117117. Dibra A, Kastrati A, Mehilli J, et al. Paclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patients. N Engl J Med. 2005;353(7):663-670. , 118118. Morice MC, Colombo A, Meier B, et al. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions: the REALITY trial: a randomized controlled trial. JAMA. 2006;295(8):895-904. , 119119. Windecker S, Remondino A, Eberli FR, et al. Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N Engl J Med. 2005;353(7):653-662. , 120120. Raber L, Wohlwend L, Wigger M, et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization LATE trial. Circulation. 2011;123(24):2819-2828, 2816 p following 2828. , 121121. Galloe AM, Thuesen L, Kelbaek H, et al. Comparison of paclitaxel- and sirolimus-eluting stents in everyday clinical practice: the SORT OUT II randomized trial. JAMA. 2008;299(4):409-416. , 122122. Goy JJ, Stauffer JC, Siegenthaler M, et al. A prospective randomized comparison between paclitaxel and sirolimus stents in the real world of interventional cardiology: the TAXi trial. J Am Coll Cardiol. 2005;45(2):308-311. , 123123. Berger A, Stauffer JC, Seydoux C, et al. Three-year follow-up of the first prospective randomized comparison between paclitaxel and sirolimus stents: the TAXi-LATE trial. Catheter Cardiovasc Interv. 2007;70(2):163-166. , 124124. Lee JH, Kim HS, Lee SW, et al. Prospective randomized comparison of sirolimus- versus paclitaxel-eluting stents for the treatment of acute ST-elevation myocardial infarction: pROSIT trial. Catheter Cardiovasc Interv. 2008;72(1):25-32. , 125125. Kim HS, Lee JH, Lee SW, et al. Long-term safety and efficacy of sirolimus- vs. paclitaxel-eluting stent implantation for acute ST-elevation myocardial infarction: 3-year follow-up of the PROSIT trial. Int J Cardiol. 2011;147(2):253-257. , 126126. Mehilli J, Kastrati A, Byrne RA, et al. Paclitaxel- versus sirolimus-eluting stents for unprotected left main coronary artery disease. J Am Coll Cardiol. 2009;53(19):1760-1768. , 127127. Kim YH, Park SW, Lee SW, et al. Sirolimus-eluting stent versus paclitaxel-eluting stent for patients with long coronary artery disease. Circulation. 2006;114(20):2148-2153. , 128128. Mehilli J, Dibra A, Kastrati A, et al. Randomized trial of paclitaxel- and sirolimus-eluting stents in small coronary vessels. Eur Heart J. 2006;27(3):260-266. , 129129. Kastrati A, Mehilli J, von Beckerath N, et al. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA. 2005;293(2):165-171. , 130130. Mehilli J, Byrne RA, Tiroch K, et al. Randomized Trial of Paclitaxel- Versus Sirolimus-Eluting Stents for Treatment of Coronary Restenosis in Sirolimus-Eluting Stents: The ISAR-DESIRE 2 (Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis 2) Study. J Am Coll Cardiol. 2010:j.jacc.2010. 2002. 2009. ] Results at short-term angiographic follow-up consistently demonstrate superior reductions in late loss with the use of SES, however long-term angiographic follow-up, indicates a greater delayed late loss with SES, such that at 5-years there was no longer a significant difference in late loss between SES and PES.[120120. Raber L, Wohlwend L, Wigger M, et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization LATE trial. Circulation. 2011;123(24):2819-2828, 2816 p following 2828. ] In terms of clinical outcomes, a meta-analysis of 16 randomized trials of SES versus PES, which included 8,695 patients and where possible patient level data, reported significant reductions in TLR (HR:0.74, 95% CI:0.63-0.87, p < 0.001) and ST (HR 0.66, 95% CI:0.46-0.94, p = 0.02) with SES, whilst no significant differences in death (HR 0.92, 95%: CI:0.74-1.13, p = 0.43), or MI (HR 0.84, 95% CI:0.69-1.03, p = 0.10) were noted at a median of 2-year follow-up.[131131. Schomig A, Dibra A, Windecker S, et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J Am Coll Cardiol. 2007;50(14):1373-1380. ] The SORT-OUT II and SIRTAX studies have both reported long-term outcomes and failed to show any between-stent differences in MACE, cardiac death, MI, clinically-indicated TLR and ST at 10-year follow-up, with attenuation of the differences in MACE noted beyond 1-year.[132132. Galloe AM, Kelbaek H, Thuesen L, et al. 10-Year Clinical Outcome After Randomization to Treatment by Sirolimus- or Paclitaxel-Eluting Coronary Stents. J Am Coll Cardiol. 2017;69(6):616-624. , 133133. Yamaji K, Raber L, Zanchin T, et al. Ten-year clinical outcomes of first-generation drug-eluting stents: the Sirolimus-Eluting vs. Paclitaxel-Eluting Stents for Coronary Revascularization (SIRTAX) VERY LATE trial. Eur Heart J. 2016;37(45):3386-3395. ]
Early-generation DES
- The stainless steel SES was the first DES to receive CE and FDA approval, shortly followed by the PES.
- Studies have confirmed consistently superior angiographic outcomes, and significantly lower rates of repeat revascularization with the use of SES or PES compared with BMS in patients with simple or complex lesions at short- and long-term follow-up.
- SES have been shown to have superior angiographic outcomes and lower rates of repeat revascularization when compared with PES.
NEW-GENERATION DRUG-ELUTING STENTS WITH DURABLE POLYMER COATING
Everolimus-eluting stents
XIENCE V® (Abbott Vascular, Santa Clara, CA, USA), PROMUS™ (Boston Scientific, Natick, MA, USA)
The cobalt chromium EES stent has a strut thickness of 81µm, and is coated with a 7.6 µm thick, non-erodable, co-polymer of poly vinylidene fluoride co-hexafluoropropylene (PVDF-HFP), and poly n-butyl methacrylate (PBMA), which facilitates elution of everolimus over 120-days ( Table 2). The feasibility of using everolimus on a DES was first assessed in the FUTURE I [134134. Costa RA, Lansky AJ, Mintz GS, et al. Angiographic results of the first human experience with everolimus-eluting stents for the treatment of coronary lesions (the FUTURE I trial). Am J Cardiol. 2005;95(1):113-116. , 135135. Grube E, Sonoda S, Ikeno F, et al. Six- and twelve-month results from first human experience using everolimus-eluting stents with bioabsorbable polymer. Circulation. 2004;109(18):2168-2171. ] and FUTURE II [136136. Tsuchiya Y, Lansky AJ, Costa RA, et al. Effect of everolimus-eluting stents in different vessel sizes (from the pooled FUTURE I and II trials). Am J Cardiol. 2006;98(4):464-469. ] studies. Numerous randomised studies have compared the performance of EES to BMS, PES, SES, R-ZES and most recently stents with biodegradable polymers. This stent was also commercially available until 2012 as the Promus™ (Boston Scientific, Natick, MA) stent.
XIENCE PRIME™ (Abbott Vascular, Santa Clara, CA, USA)
The Xience PRIME EES, represents a newer iteration of the Xience® V stent. This modified EES has a CoCr platform; however, this is mounted on a new enhanced stent delivery system that enables the stent to be more flexible and deliverable and offers longer stent lengths. Furthermore, the stent balloon has higher rate burst pressures (18 atm vs. 16 atm), and shorter balloon tapers (1-2 mm vs. 3-5 mm) to minimize the risk of edge dissections. The stent’s efficacy and safety has been demonstrated in the 510 patient SPIRIT PRIME clinical trial which met its primary endpoint of TLF at 1-year with statistical significance when compared to pre-specified performance goals derived from previous EES trials.[137137. Costa M. One-year outcomes after implantation of XIENCE PRIME and XIENCE PRIME LL stents in patients with coronary artery disease: Primary endpoint results of the SPIRIT PRIME Multi-center clinical trial. Presentation at Transcatheter Cardiovascular Therapeutics, San Francisco, November 8th 2011. ] Furthermore, the safety and effectiveness profile of the Xience PRIME EES was also similar and consistent to that of the Xience EES. On the basis of the SPIRIT PRIME clinical data the FDA approved the stent for use in the US in late 2011.
EES vs. BMS
The SPIRIT FIRST study enrolled 56 patients (EES = 27, BMS = 29) and demonstrated superior performance of EES with respect to 6-month in-stent late lumen loss (0.10 mm vs. 0.87 mm, p < 0.001), and angiographic binary restenosis (0.0 vs. 25.9, p < 0.05) ( Table 7). Similarly, clinical follow-up through to 5-years demonstrated significantly lower rates of TLR with the use of EES, with comparable rates of mortality, MI and overall MACE.[138138. Serruys PW, Ong AT, Piek JJ, et al. A randomized comparison of a durable polymer Everolimus-eluting stent with a bare metal coronary stent: The SPIRIT first trial. EuroIntervention. 2005;1(1):58-65. , 139139. Wiemer M, Serruys PW, Miquel-Hebert K, et al. Five-year long-term clinical follow-up of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo coronary artery lesions: The SPIRIT FIRST trial. Catheter Cardiovasc Interv. 2010;75(7):997-1003. ]
Contemporary studies of EES versus BMS have been conducted in specific patient groups including patients with stable angina over 80 years of age (XIMA), patients undergoing primary PCI for AMI (EXAMINATION), patients requiring stents greater than 3mm in diameter (BASKET PROVE) and patients with chronic kidney disease (RENAL-DES). Results ( Table 7) show superior efficacy with EES compared with BMS, and comparable safety. [139139. Wiemer M, Serruys PW, Miquel-Hebert K, et al. Five-year long-term clinical follow-up of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo coronary artery lesions: The SPIRIT FIRST trial. Catheter Cardiovasc Interv. 2010;75(7):997-1003. , 139139. Wiemer M, Serruys PW, Miquel-Hebert K, et al. Five-year long-term clinical follow-up of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo coronary artery lesions: The SPIRIT FIRST trial. Catheter Cardiovasc Interv. 2010;75(7):997-1003. , 139139. Wiemer M, Serruys PW, Miquel-Hebert K, et al. Five-year long-term clinical follow-up of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo coronary artery lesions: The SPIRIT FIRST trial. Catheter Cardiovasc Interv. 2010;75(7):997-1003. , 139139. Wiemer M, Serruys PW, Miquel-Hebert K, et al. Five-year long-term clinical follow-up of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo coronary artery lesions: The SPIRIT FIRST trial. Catheter Cardiovasc Interv. 2010;75(7):997-1003. , 139139. Wiemer M, Serruys PW, Miquel-Hebert K, et al. Five-year long-term clinical follow-up of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo coronary artery lesions: The SPIRIT FIRST trial. Catheter Cardiovasc Interv. 2010;75(7):997-1003. ] A meta-analysis of these studies (excluding RENAL-DES) by Valgimigli et al, which included 4896 patients followed-up for a median of 720 days, reaffirmed that compared to BMS, EES lowered MI and ST as well as cardiovascular mortality.[144144. Valgimigli M, Sabate M, Kaiser C, et al. Effects of cobalt-chromium everolimus eluting stents or bare metal stent on fatal and non-fatal cardiovascular events: patient level meta-analysis. Bmj. 2014;349:g6427. ]
EES vs. PES
Six randomised trials have compared EES to PES in 8,819 patients with increasingly complex lesions ranging from those with up to two relatively simple de novo lesions in the SPIRIT II study, to the unrestricted all-comers population in the COMPARE study ( Table 7).[145145. Serruys PW, Ruygrok P, Neuzner J, et al. A randomised comparison of an everolimus-eluting coronary stent with a paclitaxel-eluting coronary stent:the SPIRIT II trial. EuroIntervention. 2006;2(3):286-294. , 146146. Ruygrok P, Desaga M, van den Branden F, et al. One year clinical follow-up of the XIENCE V Everolimus-eluting stent system in the treatment of patients with de novo native coronary artery lesions: the SPIRIT II study. Eurointervention. 2007(3):315-320. , 147147. Claessen BE, Beijk MA, Legrand V, et al. Two-Year Clinical, Angiographic, and Intravascular Ultrasound Follow-Up of the XIENCE V Everolimus-Eluting Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions: The SPIRIT II Trial. Circ Cardiovasc Intervent. 2009;2(4):339-347. , 148148. Garg S, Serruys PW, Onuma Y, et al. Three year clinical follow up of the XIENCE V Everolimus eluting coronary stent system in the treatment of patients with de novo coronary artery lesions. The SPIRIT II Trial. J Am Coll Cardiol Intv. 2009;2(12):1190-1198. , 149149. Garg S, Serruys PW, Miquel-Hebert K. Four-year clinical follow-up of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with de novo coronary artery lesions: the SPIRIT II trial. Catheter Cardiovasc Interv. 2011;77(7):1012-1017. , 150150. Onuma Y, Miquel-Hebert K, Serruys PW. Five-year long-term clinical follow-up of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with de novo coronary artery disease: the SPIRIT II trial. EuroIntervention. 2013;8(9):1047-1051. , 151151. Stone GW, Midei M, Newman W, et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299(16):1903-1913. , 152152. Gada H, Kirtane AJ, Newman W, et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6(12):1263-1266. , 153153. Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010;362(18):1663-1674. , 154154. Stone GW, Rizvi A, Sudhir K, et al. Randomized comparison of everolimus- and paclitaxel-eluting stents. 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV trial. J Am Coll Cardiol. 2011;58(1):19-25. , 155155. Brener SJ, Kereiakes DJ, Simonton CA, et al. Everolimus-eluting stents in patients undergoing percutaneous coronary intervention: final 3-year results of the Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System in the Treatment of Subjects With de Novo Native Coronary Artery Lesions trial. Am Heart J. 2013;166(6):1035-1042. , 156156. Chevalier B. SPIRIT V Single Arm Study - 2 year follow-up. Presentation at EuroPCR, 25th-28th May 2010, Paris, France. , 157157. Kedhi E, Joesoef KS, McFadden E, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375(9710):201-209. , 158158. Smits PC, Kedhi E, Royaards KJ, et al. 2-year follow-up of a randomized controlled trial of everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice. COMPARE (Comparison of the everolimus eluting XIENCE-V stent with the paclitaxel eluting TAXUS LIBERTE stent in all-comers: a randomized open label trial). J Am Coll Cardiol. 2011;58(1):11-18. , 159159. Smits PC, Vlachojannis GJ, McFadden EP, et al. Final 5-Year Follow-Up of a Randomized Controlled Trial of Everolimus- and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice: The COMPARE Trial (A Trial of Everolimus-Eluting Stents and Paclitaxel Stents for Coronary Revascularization in Daily Practice). JACC Cardiovasc Interv. 2015;8(9):1157-1165. , 160160. Ribichini F, Romano M, Rosiello R, et al. A clinical and angiographic study of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with multivessel coronary artery disease: the EXECUTIVE trial (EXecutive RCT: evaluating XIENCE V in a multi vessel disease). JACC Cardiovasc Interv. 2013;6(10):1012-1022. , 161161. Kaul U, Bhagwat A, Pinto B, et al. Paclitaxel-Eluting Stents versus Everolimus-Eluting Coronary Stents in a Diabetic Population: 2 Years Follow-up of TUXEDO-India Trial. EuroIntervention. 2017. ] Irrespective of patient complexity or follow-up period, angiographic and clinical outcomes have consistently demonstrated superior outcomes in those treated with EES. Specifically in the SPIRIT II (0.11 mm vs. 0.36 mm) and SPIRIT III (0.16 mm vs. 0.30 mm) study in-stent late loss at 6- and 8-months, respectively were significantly lower with EES ( Figure 12).[145145. Serruys PW, Ruygrok P, Neuzner J, et al. A randomised comparison of an everolimus-eluting coronary stent with a paclitaxel-eluting coronary stent:the SPIRIT II trial. EuroIntervention. 2006;2(3):286-294. , 151151. Stone GW, Midei M, Newman W, et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299(16):1903-1913. ] Consistent with these results are findings of the EXECUTIVE study, which enrolled patients with multivessel disease, and reported in-stent late lumen losses at 9-months follow-up of 0.08 mm (95% CI: -0.01, 0.16) and 0.22 mm (95% CI: -0.13, 0.31) (p = 0.018) amongst patients randomised to EES and PES, respectively.[160160. Ribichini F, Romano M, Rosiello R, et al. A clinical and angiographic study of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with multivessel coronary artery disease: the EXECUTIVE trial (EXecutive RCT: evaluating XIENCE V in a multi vessel disease). JACC Cardiovasc Interv. 2013;6(10):1012-1022. ] Longer angiographic follow-up is only available from the SPIRIT II study, and this demonstrated evidence of catch up in late loss with EES, such that the significant difference in in-stent late loss between EES and PES which was observed at 6-months was no longer present at 2-years.[147147. Claessen BE, Beijk MA, Legrand V, et al. Two-Year Clinical, Angiographic, and Intravascular Ultrasound Follow-Up of the XIENCE V Everolimus-Eluting Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions: The SPIRIT II Trial. Circ Cardiovasc Intervent. 2009;2(4):339-347. ] Nevertheless, clinical outcomes at 3-, 4- and 5-year follow-up in the SPIRIT II study remain consistent with those seen at 6-months and 1-year ( Figure 12). Similarly, at 5-year follow-up in the SPIRIT III study, treatment with EES led to significantly lower rates of MACE.[152152. Gada H, Kirtane AJ, Newman W, et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6(12):1263-1266. ] More extensive assessment of EES took place in the SPIRIT IV trial, which randomized 3,690 patients (EES = 2,458, PES = 1,229), and the all-comers COMPARE study, which recruited 1,800 patients (EES = 897, PES = 903).[152152. Gada H, Kirtane AJ, Newman W, et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6(12):1263-1266. , 152152. Gada H, Kirtane AJ, Newman W, et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6(12):1263-1266. , 152152. Gada H, Kirtane AJ, Newman W, et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6(12):1263-1266. , 152152. Gada H, Kirtane AJ, Newman W, et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6(12):1263-1266. , 152152. Gada H, Kirtane AJ, Newman W, et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6(12):1263-1266. , 152152. Gada H, Kirtane AJ, Newman W, et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6(12):1263-1266. , 152152. Gada H, Kirtane AJ, Newman W, et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6(12):1263-1266. ] At 3- (SPIRT IV) and 5-year (COMPARE) follow-up both studies reported superior efficacy and safety with EES compared to PES. Notably rates of definite/probable ST were significantly lower with EES in both at final follow-up (SPIRIT IV 0.6% vs. 1.6%, p=0.003 and COMPARE 3.1% vs. 5.9%, p=0.005).[155155. Brener SJ, Kereiakes DJ, Simonton CA, et al. Everolimus-eluting stents in patients undergoing percutaneous coronary intervention: final 3-year results of the Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System in the Treatment of Subjects With de Novo Native Coronary Artery Lesions trial. Am Heart J. 2013;166(6):1035-1042. , 159159. Smits PC, Vlachojannis GJ, McFadden EP, et al. Final 5-Year Follow-Up of a Randomized Controlled Trial of Everolimus- and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice: The COMPARE Trial (A Trial of Everolimus-Eluting Stents and Paclitaxel Stents for Coronary Revascularization in Daily Practice). JACC Cardiovasc Interv. 2015;8(9):1157-1165. ] The TUXEDO trial compared EES vs. PES among 1830 diabetic patients. At 2-year follow-up, EES was associated with a significant reduction in the risk of TVF (4.3% vs. 6.6%, p=0.03), mainly driven by a reduction in the risk of MI, TLR, and ST.[161161. Kaul U, Bhagwat A, Pinto B, et al. Paclitaxel-Eluting Stents versus Everolimus-Eluting Coronary Stents in a Diabetic Population: 2 Years Follow-up of TUXEDO-India Trial. EuroIntervention. 2017. ]
A patient-level pooled analysis of the 6,789 patients enrolled in the SPIRIT-II, -III, -IV and COMPARE studies has confirmed the superior performance of EES compared with PES. At 12-months follow-up whilst there were no between-stent differences in mortality or cardiac death, there were significantly lower rates of MI (2.1% vs. 4.0%, p < 0.001), ischaemic TLR (2.3% vs. 4.7%, p < 0.001), MACE (4.4% vs. 7.6%), definite ST (0.4% vs. 1.2%, p < 0.001) and definite/probable ST (0.5% vs. 1.5%, p < 0.001) with EES.[163163. Stone GW, Kedhi E, Kereiakes DJ, et al. Differential clinical responses to everolimus-eluting and Paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124(8):893-900. ] Results were maintained even after adjustment of confounding factors. Following on from this, meta-analysis of the SPIRIT studies at 3-year follow-up have shown the emergence of a clear safety advantage with the use of EES compared to PES. Amongst 4,989 patients, who were prospectively randomised to EES (n=3350) or PES (n=1639), significantly lower rates of all endpoints including all-cause mortality (HR 0.65, p=0.003), MI (HR 0.64, p=0.02), TLR (HR 0.72, p=0.004), MACE (HR 0.71, p=0.0002) and definite/probable ST (HR 0.45, p=0.003) were seen with EES.[164164. Dangas GD, Serruys PW, Kereiakes DJ, et al. Meta-analysis of everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease: final 3-year results of the SPIRIT clinical trials program (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2013;6(9):914-922. ]
EES vs. SES
Several studies have reported the results from the comparison of EES with SES, which has been regarded as the most efficacious first generation DES ( Table 8).[7979. Kaiser C, Galatius S, Erne P, et al. Drug-eluting versus bare-metal stents in large coronary arteries. N Engl J Med. 2010;363(24):2310-2319. ], [165165. Park KW, Chae IH, Lim DS, et al. Everolimus-eluting versus sirolimus-eluting stents in patients undergoing percutaneous coronary intervention: the EXCELLENT (Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting) randomized trial. J Am Coll Cardiol. 2011;58(18):1844-1854. , 166166. Byrne RA, Kastrati A, Massberg S, et al. Biodegradable polymer versus permanent polymer drug-eluting stents and everolimus- versus sirolimus-eluting stents in patients with coronary artery disease: 3-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2011;58(13):1325-1331. , 167167. Kufner S, Byrne RA, Valeskini M, et al. Five-year outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease: final results from the ISAR-TEST 4 randomised trial. EuroIntervention. 2016;11(12):1372-1379. , 168168. Jensen LO, Thayssen P, Hansen HS, et al. Randomized comparison of everolimus-eluting and sirolimus-eluting stents in patients treated with percutaneous coronary intervention: the Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV). Circulation. 2012;125(10):1246-1255. , 169169. Jensen LO, Thayssen P, Christiansen EH, et al. Safety and Efficacy of Everolimus- Versus Sirolimus-Eluting Stents: 5-Year Results From SORT OUT IV. J Am Coll Cardiol. 2016;67(7):751-762. , 170170. Kimura T, Morimoto T, Natsuaki M, et al. Comparison of everolimus-eluting and sirolimus-eluting coronary stents: 1-year outcomes from the Randomized Evaluation of Sirolimus-eluting Versus Everolimus-eluting stent Trial (RESET). Circulation. 2012;126(10):1225-1236. , 171171. Shiomi H, Kozuma K, Morimoto T, et al. Long-term clinical outcomes after everolimus- and sirolimus-eluting coronary stent implantation: final 3-year follow-up of the randomized evaluation of sirolimus-eluting versus everolimus-eluting stent trial. Circ Cardiovasc Interv. 2014;7(3):343-354. , 172172. Park DW, Kim YH, Song HG, et al. Comparison of everolimus- and sirolimus-eluting stents in patients with long coronary artery lesions: a randomized LONG-DES-III (Percutaneous Treatment of LONG Native Coronary Lesions With Drug-Eluting Stent-III) Trial. JACC Cardiovasc Interv. 2011;4(10):1096-1103. , 173173. Kim WJ, Lee SW, Park SW, et al. Randomized Comparison of Everolimus-Eluting Stent Versus Sirolimus-Eluting Stent Implantation for De Novo Coronary Artery Disease in Patients With Diabetes Mellitus (ESSENCE-DIABETES): Results From the ESSENCE-DIABETES Trial. Circulation. 2011;124(8):886-892. , 174174. Hofma SH, Brouwer J, Velders MA, et al. Second-Generation Everolimus-Eluting Stents Versus First-Generation Sirolimus-Eluting Stents in Acute Myocardial Infarction: 1-Year Results of the Randomized XAMI (XienceV Stent vs. Cypher Stent in Primary PCI for Acute Myocardial Infarction) Trial. J Am Coll Cardiol. 2012;60(5):381-387. , 175175. Hofma SH, Smits PC, Brouwer J, et al. Long-term follow-up of second-generation everolimus-eluting stents versus first-generation sirolimus-eluting stents in acute myocardial infarction: three-year results of the XAMI trial. EuroIntervention. 2015. , 176176. Di Lorenzo E, Sauro R, Varricchio A, et al. Randomized comparison of everolimus-eluting stents and sirolimus-eluting stents in patients with ST elevation myocardial infarction: RACES-MI trial. JACC Cardiovasc Interv. 2014;7(8):849-856. , 177177. Raber L, Juni P, Nuesch E, et al. Long-term comparison of everolimus-eluting and sirolimus-eluting stents for coronary revascularization. J Am Coll Cardiol. 2011;57(21):2143-2151. ]
The EXCELLENT study enrolled 1,372 patients randomised 3:1 to EES (n = 1029) and SES (n = 343). The study achieved its pre-specified non-inferiority primary endpoint of in-segment late lumen loss at 9-months (EES 0.10 mm vs. SES 0.05 mm, Pnon-inferoirty = 0.023). At 12-months clinical follow-up there were no significant differences in rates of MI, TLR, and the composites of mortality/MI and MACE. Rates of ST were lower with EES (0.4% vs. 0.8%, p = 0.028).[165165. Park KW, Chae IH, Lim DS, et al. Everolimus-eluting versus sirolimus-eluting stents in patients undergoing percutaneous coronary intervention: the EXCELLENT (Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting) randomized trial. J Am Coll Cardiol. 2011;58(18):1844-1854. ]
In a sub-study of the ISAR-TEST 4 trial, late loss at 6-8 months amongst the 1,304 patients randomised to treatment with EES and SES was 0.14 mm versus 0.17 mm respectively (p = NS).[166166. Byrne RA, Kastrati A, Massberg S, et al. Biodegradable polymer versus permanent polymer drug-eluting stents and everolimus- versus sirolimus-eluting stents in patients with coronary artery disease: 3-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2011;58(13):1325-1331. ] At 2 years of follow-up with repeat angiography performed, the investigators observed a trend towards lower TLR (9.9% vs. 13.5%, HR=0.73, 0.52-1.01, p = 0.06) and a significant reduction of binary restenosis (12.7% vs. 16.9%, p = 0.03) in favour of EES in the absence of differences for safety endpoints. At 5-year clinical follow-up both efficacy and safety remained numerically lower with EES (p > 0.05 for all).[167167. Kufner S, Byrne RA, Valeskini M, et al. Five-year outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease: final results from the ISAR-TEST 4 randomised trial. EuroIntervention. 2016;11(12):1372-1379. ]
SORT OUT IV reported non-inferior outcomes with EES compared with SES in terms of MACE (4.9% vs. 5.2%, HR 0.94, 0.67-1.31) and TLR (1.4% vs. 1.7%, HR 0.87, 0.48-1.58) at 9 months among 2,774 patients randomly assigned treatment with EES or SES.[168168. Jensen LO, Thayssen P, Hansen HS, et al. Randomized comparison of everolimus-eluting and sirolimus-eluting stents in patients treated with percutaneous coronary intervention: the Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV). Circulation. 2012;125(10):1246-1255. ] Differential outcomes occurred after the first year, and at 5-years significantly lower rates of MACE were seen in those treated with EES (14.0% vs. 17.4%, HR 0.80, p=0.02), which was larger the result of significantly lower rates of definite ST with EES (0.4% vs. 2.0%, HR 0.18).[169169. Jensen LO, Thayssen P, Christiansen EH, et al. Safety and Efficacy of Everolimus- Versus Sirolimus-Eluting Stents: 5-Year Results From SORT OUT IV. J Am Coll Cardiol. 2016;67(7):751-762. ]
The largest randomised study of EES and SES is the RESET study which randomised 3197 all-comers patients and achieved its pre-specified non-inferiority primary clinical endpoint with rates of TLR at 12-months of 4.3% and 5.0% with EES and SES, respectively (Pnon-inferority<0.001). Other safety and efficacy endpoints were comparable between the stents with similar results observed at 3-years. Of note, significant between-stent differences in favour of EES were seen in the secondary composite endpoints of TLF, TVF, MACE, and the device-orientated endpoint.
BASKET PROVE randomly assigned 2,314 patients undergoing stent implantation of large vessels (stent diameter > 3.0 mm) to receive SES, EES or BMS. At 2 years of follow-up, TVR was lower with both EES (3.7%) and SES (4.3%) as compared with BMS (10.3%, p = 0.005 vs SES, p = 0.002 vs EES), however, the event rates were similar for EES and SES (3.7% vs 4.3%, p = 0.85).[7979. Kaiser C, Galatius S, Erne P, et al. Drug-eluting versus bare-metal stents in large coronary arteries. N Engl J Med. 2010;363(24):2310-2319. ]
LONG-DES III assessed outcomes in 500 patients randomised to EES and SES who had a coronary lesion which required at least 28 mm of stent.[172172. Park DW, Kim YH, Song HG, et al. Comparison of everolimus- and sirolimus-eluting stents in patients with long coronary artery lesions: a randomized LONG-DES-III (Percutaneous Treatment of LONG Native Coronary Lesions With Drug-Eluting Stent-III) Trial. JACC Cardiovasc Interv. 2011;4(10):1096-1103. ] The study failed to meet its non-inferiority primary endpoint of in-segment late loss at 9-months follow-up (EES 0.17 mm vs. SES 0.09 mm, Pnon-inferiority = 0.96, Psuperiority = 0.04). Furthermore, in-segment binary angiographic restenosis was also significantly lower with SES (EES 7.3% vs. SES 2.7%, p = 0.046). Despite these angiographic outcomes, there were no significant between-stent differences in clinical outcomes.
ESSENCE-DIABETES study showed a similar trend in the assessment of EES versus SES in patients with diabetes.[173173. Kim WJ, Lee SW, Park SW, et al. Randomized Comparison of Everolimus-Eluting Stent Versus Sirolimus-Eluting Stent Implantation for De Novo Coronary Artery Disease in Patients With Diabetes Mellitus (ESSENCE-DIABETES): Results From the ESSENCE-DIABETES Trial. Circulation. 2011;124(8):886-892. ] Specifically the study demonstrated that EES was non-inferior to SES in terms of in-segment late loss and angiographic restenosis at 8-months, with similar clinical outcomes being seen at 12-months follow-up.
The X-AMI study[174174. Hofma SH, Brouwer J, Velders MA, et al. Second-Generation Everolimus-Eluting Stents Versus First-Generation Sirolimus-Eluting Stents in Acute Myocardial Infarction: 1-Year Results of the Randomized XAMI (XienceV Stent vs. Cypher Stent in Primary PCI for Acute Myocardial Infarction) Trial. J Am Coll Cardiol. 2012;60(5):381-387. , 175175. Hofma SH, Smits PC, Brouwer J, et al. Long-term follow-up of second-generation everolimus-eluting stents versus first-generation sirolimus-eluting stents in acute myocardial infarction: three-year results of the XAMI trial. EuroIntervention. 2015. ] randomised 625 patients undergoing primary PCI for acute MI in a 2:1 ratio (EES n=404, SES n=221). The study met its non-inferiority primary endpoint of MACE, a composite of cardiac death, non-fatal MI and TVR at 1-year (EES 4.0% vs. SES 7.7%, Pnon-inferority=0.048); no individual endpoints were significantly different. Rates of ST were low considering the population, and no between-stent differences were observed. A further analysis at 3-years reported low overall events rates without identifying any significant differences between patients treated with EES or SES.
The RACES-MI study[176176. Di Lorenzo E, Sauro R, Varricchio A, et al. Randomized comparison of everolimus-eluting stents and sirolimus-eluting stents in patients with ST elevation myocardial infarction: RACES-MI trial. JACC Cardiovasc Interv. 2014;7(8):849-856. ] also compared the performance of EES and SES in the setting of primary PCI for AMI randomising 500 patients in a 1:1 fashion (EES n=250, SES n=250). The study was powered for a primary endpoint of MACE, a composite of cardiac death, reinfarction, definite or probable ST and TVR at 3-year follow-up. Results showed comparable outcomes for MACE and its components apart from ST, which was significantly lower in patients receiving EES (EES 1.6% vs. SES 5.2%, p = 0.035).
A meta-analysis of the 7,370 patients (EES = 4044, SES = 3326) enrolled in BASKET–PROVE, ESSENCE-DIABETES, EXCELLENT, SORT OUT IV and ISAR-TEST 4 has confirmed the comparable performance of EES compared with SES in terms of efficacy and safety.[178178. de Waha A, Dibra A, Byrne RA, et al. Everolimus-eluting versus sirolimus-eluting stents: a meta-analysis of randomized trials. Circ Cardiovasc Interv. 2011;4(4):371-377. ] At a median of 13.3 months follow-up rates of MACE (7.2% vs. 8.8%, p = 0.28), cardiac death (2.2% vs. 2.6%, p = 0.92), MI (1.7% vs. 1.9%, p = 0.76), repeat revascularization (3.8% vs. 4.8%, p = 0.16), and the composite of definite and probable ST (0.8% vs. 1.0%, p = 0.33) were not significantly different between EES and SES. However, this analysis did not include the most recent trial reports and therefore requires an update to include longer-term follow-up data.
A larger meta-analysis by Park et al which included 11 randomised trials and just under 13,000 patients followed-up for a median of 23.8 months, also confirmed comparable safety outcomes between EES and SES.[179179. Park KW, Kang SH, Velders MA, et al. Safety and efficacy of everolimus- versus sirolimus-eluting stents: a systematic review and meta-analysis of 11 randomized trials. Am Heart J. 2013;165(2):241-250 e244. ] In contrast to the previous meta-analysis this study was able to demonstrate significantly lower rates of repeat revascularization (OR 0.85, p=0.047) and definite ST (OR 0.44, p=0.007) with EES.
EES vs. Non-EES DES Durable Polymer DES
A meta-analysis of 13 randomised trials enrolling a total of 17101 patients treated with either EES (n = 9764) or non-EES DES (n = 7337) has confirmed a consistent benefit with the use EES out to a mean follow-up of 21.7 months.[180180. Baber U, Mehran R, Sharma SK, et al. Impact of the everolimus-eluting stent on stent thrombosis: a meta-analysis of 13 randomized trials. J Am Coll Cardiol. 2011;58(15):1569-1577. ] Specifically use of EES was associated with similar cardiac mortality (1.6% vs. 1.9%, p = 0.38) and significant reductions in rates of MI (2.9% vs. 3.9%, p = 0.02), TVR (5.7% vs. 7.7%, p = 0.004) and definite/probable ST (0.7% vs. 1.5%, p = 0.001), when compared to patients receiving non-EES DES.
Consistent with this are the results of a larger comprehensive network meta-analysis of 51 randomised studies by Palmerini et al which included just over 51,000 patients,[181181. Palmerini T, Benedetto U, Biondi-Zoccai G, et al. Long-Term Safety of Drug-Eluting and Bare-Metal Stents: Evidence From a Comprehensive Network Meta-Analysis. J Am Coll Cardiol. 2015;65(23):2496-2507. ] and demonstrated that:
- EES is the only DES to show a significant reduction in all-cause mortality compared to BMS (HR 0.81, 95% CI 0.64-1.00, p<0.05), SES (HR 0.86, 95% CI 0.70-1.00, p<0.05) and PES (HR 0.81, 95% CI 0.68-1.00, p<0.05), together with offering the greatest reduction in cardiac death versus BMS (HR 0.71, 95% CI 0.54-0.91, p<0.05). No mortality reductions have been seen in the individual comparison of other DES with BMS or between DES.
- EES significantly reduces the risk of MI compared with BMS (HR 0.66, 95% CI 0.52-0.85, p<0.05), SES (HR 0.78, 95% CI 0.64-0.95, p<0.05) and PES (HR 0.64, 95% CI 0.52-0.78, p<0.05).
- EES is the only DES to significantly reduce the rate of definite ST compared to BMS (HR 0.48, 95% CI 0.29-0.82, p<0.05). Significant reductions in ST have also been seen with EES versus PES (HR 0.42, 95% CI 0.27-0.64, p<0.05); versus SES (HR 0.41, 95% CI 0.26-0.64 p<0.05) and biolimus-eluting stents (BES, HR 0.58, 95% CI 0.31-1.00, p<0.05).
Other analyses include a mixed-treatment comparison analysis of DES (SES, PES, EES, E-ZES and R-ZES) versus BMS with 117,762 patient-years of follow-up, which reported similar findings, and concluded that EES was overall the stent with the most advantageous safety profile.[182182. Bangalore S, Kumar S, Fusaro M, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125(23):2873-2891. ]
A similar analysis in patients with diabetes treated with either SES, PES, EES, E-ZES, R-ZES or BMS by Bangalore et al also concluded that EES was the safest and most efficacious stent through 22,844 patient years of follow-up.[183183. Bangalore S, Kumar S, Fusaro M, et al. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: mixed treatment comparison analysis of 22 844 patient years of follow-up from randomised trials. BMJ. 2012;345:e5170. ] A smaller meta-analysis which only included studies using EES also reported significant reductions in ST with EES out to 2-years follow-up.[184184. Palmerini T, Kirtane AJ, Serruys PW, et al. Stent thrombosis with everolimus-eluting stents: meta-analysis of comparative randomized controlled trials. Circ Cardiovasc Interv. 2012;5(3):357-364. ]
Several factors that have been suggested to be behind the consistent superior performance of EES including the fact that everolimus is slightly more lipophilic than sirolimus, and therefore more rapidly absorbed into the arterial wall. In addition, pre-clinical data have suggested that the combination non-erodible, co-polymer of PVDF-HFP and PBMA, is potentially associated with less inflammation than seen with the polymers on SES and PES.[4646. Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52(5):333-342. ] Finally, the fluoro-polymer has been shown to have thrombo-resistant properties,[4040. Kolandaivelu K, Swaminathan R, Gibson WJ, et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123(13):1400-1409. ] which when combined with thin-struts, and the reduced polymer and drug load may contribute to the low rates of ST with EES.
Zotarolimus-Eluting ENDEAVOR Stents
The first clinical assessment of E-ZES took place in the 100 patient single arm ENDEAVOR I study, which reported a 12-months in-stent late loss and binary restenosis rate of 0.61 ± 0.44 mm and 5.4%, respectively. Clinic event rates were low, with 2 TLRs, 1 MI and 1 definite/probable ST at 1-year, and only one further TLR and no addition MIs or ST events reported out to 5-year follow-up.[185185. Meredith IT, Ormiston J, Whitbourn R, et al. First-in-human study of the Endeavor ABT-578-eluting phosphorylcholine-encapsulated stent system in de novo native coronary artery lesions: Endeavor I Trial. EuroIntervention. 2005;1(2):157-164. , 186186. Meredith IT, Ormiston J, Whitbourn R, et al. Five-year clinical follow-up after implantation of the endeavor zotarolimus-eluting stent: ENDEAVOR I, first-in-human study. Catheter Cardiovasc Interv. 2009;74(7):989-995. ]
E-ZES vs. BMS
The ENDEAVOR II trial enrolled 1197 patients (ZES = 598, BMS = 599) and demonstrated significantly lower rates of in-stent late loss (0.61 ± 0.46 mm vs. 1.03 ± 0.58 mm, p < 0.001), binary in-stent restenosis (9.4% vs. 33.5%, p < 0.001), TLR (4.6% vs. 11.8%, p < 0.001) and TVF, a composite of cardiac death, MI attributable to the target vessel, and clinically-driven TLR, (7.9% vs. 15.1%, p < 0.001) at 9-months follow-up, with additional clinical follow-up at 5-years indicating a sustained benefit in favour of E-ZES with respect to TLR and TVF. [187187. Fajadet J, Wijns W, Laarman GJ, et al. Randomized, double-blind, multicenter study of the Endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVOR II trial. Circulation. 2006;114(8):798-806. , 188188. Fajadet J, Wijns W, Laarman GJ, et al. Long-term follow-up of the randomised controlled trial to evaluate the safety and efficacy of the zotarolimus-eluting driver coronary stent in de novo native coronary artery lesions: five year outcomes in the ENDEAVOR II study. Eurointervention.
6(5):562-567] Mortality and rates of MI and ST were comparable at all time points ( Table 9).
E-ZES vs. SES
The comparison of E-ZES and SES has taken place in three randomised studies - ENDEAVOR III, SORT-OUT III and PROTECT.[189189. Kandzari DE, Leon MB, Popma JJ, et al. Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J Am Coll Cardiol. 2006;48(12):2440-2447. , 190190. Kandzari DE, Mauri L, Popma JJ, et al. Late-term clinical outcomes with zotarolimus- and sirolimus-eluting stents. 5-year follow-up of the ENDEAVOR III (A Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2011;4(5):543-550. , 191191. Rasmussen K, Maeng M, Kaltoft A, et al. Efficacy and safety of zotarolimus-eluting and sirolimus-eluting coronary stents in routine clinical care (SORT OUT III): a randomised controlled superiority trial. Lancet. 2010;375(9720):1090-1099. , 192192. Maeng M, Tilsted HH, Jensen LO, et al. 3-Year clinical outcomes in the randomized SORT OUT III superiority trial comparing zotarolimus- and sirolimus-eluting coronary stents. JACC Cardiovasc Interv. 2012;5(8):812-818. , 193193. Maeng M, Tilsted HH, Jensen LO, et al. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-eluting and sirolimus-eluting coronary stents (the SORT OUT III study): a multicentre, open-label, randomised superiority trial. Lancet. 2014;383(9934):2047-2056. , 194194. Camenzind E, Wijns W, Mauri L, et al. Stent thrombosis and major clinical events at 3 years after zotarolimus-eluting or sirolimus-eluting coronary stent implantation: a randomised, multicentre, open-label, controlled trial. Lancet. 2012;380(9851):1396-1405. , 195195. Wijns W, Steg PG, Mauri L, et al. Endeavour zotarolimus-eluting stent reduces stent thrombosis and improves clinical outcomes compared with cypher sirolimus-eluting stent: 4-year results of the PROTECT randomized trial. Eur Heart J. 2014;35(40):2812-2820. ] ENDEAVOR III compared E-ZES with SES in a non-inferiority trial with a primary angiographic endpoint (N = 436 patients).[189189. Kandzari DE, Leon MB, Popma JJ, et al. Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J Am Coll Cardiol. 2006;48(12):2440-2447. ] E-ZES was found inferior to SES regarding late loss (in-stent: 0.60 ± 0.48 mm vs. 0.15 ± 0.34 mm, p < 0.001) ( Figure 13) and binary restenosis (in-segment: 11.7% vs. 4.3%, p = 0.04). Conversely, the incidence of late acquired stent malapposition as assessed by IVUS was lower with E-ZES than SES (0.5% vs. 5.9%, p = 0.02). E-ZES had a lower rate of MI than SES (SES: 3.5% vs. E-ZES: 0.6%, RR = 0.18, 95% CI 0.03-0.96, p = 0.04) at 9 months, which was mainly due to a lower incidence of peri-procedural myonecrosis.[189189. Kandzari DE, Leon MB, Popma JJ, et al. Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J Am Coll Cardiol. 2006;48(12):2440-2447. ] There were no significant differences in rates of death, cardiac death, ST, repeat revascularization, MACE, and TVF. At 5-years[190190. Kandzari DE, Mauri L, Popma JJ, et al. Late-term clinical outcomes with zotarolimus- and sirolimus-eluting stents. 5-year follow-up of the ENDEAVOR III (A Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2011;4(5):543-550. ] the absolute difference in TLR between E-ZES and SES was small 1.6% at 5-years (E-ZES 8.1% vs. SES 6.5%). Rates of ST remained similar between both groups throughout follow-up, although the study was not powered for this endpoint.
In contrast, SORT-OUT III enrolled 2332 patients (E-ZES = 1162, SES = 1170) and reported significant differences in favour of SES with respect to MI, TLR and ST at both 9- and 18-months follow-up. At 3-years, rates of MI (E-ZES 3.8% vs. SES 3.3%, p = 0.44) and ST (1.1% vs. 1.4%, p = 0.61) were comparable between E-ZES and SES, whilst TLR remained significantly lower with SES (6.8% vs. 3.9%, p = 0.002).[192192. Maeng M, Tilsted HH, Jensen LO, et al. 3-Year clinical outcomes in the randomized SORT OUT III superiority trial comparing zotarolimus- and sirolimus-eluting coronary stents. JACC Cardiovasc Interv. 2012;5(8):812-818. ] This significant difference in TLR was no longer present at final 5-year follow-up (7.6% vs. 6.0%, p=0.15);[193193. Maeng M, Tilsted HH, Jensen LO, et al. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-eluting and sirolimus-eluting coronary stents (the SORT OUT III study): a multicentre, open-label, randomised superiority trial. Lancet. 2014;383(9934):2047-2056. ] similarly no between-stent differences in death, MI or ST was seen. Landmark analyses showed significantly lower rates of definite ST (0.1% vs. 1.8%, p=0.003), TLR (2.4% vs. 4.8%, p=0.003) and TVR (4.1% vs. 7.0%, p=0.003) with E-ZES compared with SES between 1- and 5-years, thereby reversing the significantly higher rates of these respective endpoints with E-ZES at 1-year follow-up (definite ST 1.1% vs. 0.3%, p=0.04; TLR 5.3% vs. 1.4%, p<0.001; TVR 6.7% vs. 2.8%, p<0.001).
The much larger PROTECT study recruited 8709 all-comers patients who were randomised to treatment with SES and E-ZES.30 Uniquely the study was powered to detect a 1% difference in definite/probable ST at 3-years follow-up, however consequent to event rates in the SES arm (1.8%) being lower than anticipated in the power calculation (2.5%), even this large study was somewhat underpowered. The study failed to identify any significant differences between E-ZES and SES with regards the primary endpoint of definite/probable ST (E-ZES 1.4% vs. SES 1.8%, HR:0.81) and secondary clinical safety endpoints such as death and MI at 3 years. However, in the pre-specified 4-year of follow-up there was an increase in the absolute between-stent difference in definite/probable ST from 0.4% at 3-years to 1.0%, such that rates were significantly lower with E-ZES at 4-years (1.6% vs. 2.6%, p=0.003).[195195. Wijns W, Steg PG, Mauri L, et al. Endeavour zotarolimus-eluting stent reduces stent thrombosis and improves clinical outcomes compared with cypher sirolimus-eluting stent: 4-year results of the PROTECT randomized trial. Eur Heart J. 2014;35(40):2812-2820. ] with resultant lower rates of MI as per the extended historical definition (E-ZES 4.6% vs. SES 5.8%, p=0.02). Whilst TVR was comparable at 4-years follow-up (9.0% vs. 8.6%), TLR remained significantly higher with E-ZES (5.9% vs. 4.5%, p=0.002), however there was a fall in the absolute between-stent difference (2.1% at 3-years vs. 1.4% at 4-years).
Overall these three studies confirm differential clinical outcomes over time amongst these two DES with differing abilities to suppress neointimal hyperplasia. During early follow-up E-ZES is associated with inferior outcomes compared to SES, however these differences appear to disappear or even reverse with long-term follow-up. Importantly, these contrasting short- and long-term results have implications for clinical trial design reiterating the need for long-term follow-up to fully evaluate the efficacy and safety of DES.
E-ZES vs. PES
ENDEAVOR IV compared E-ZES with PES in a non-inferiority, randomized trial enrolling 1,548 patients with a primary clinical endpoint of TVF ( Table 9).[196196. Leon MB, Mauri L, Popma JJ, et al. A randomised comparison of the Endeavor Zotarolimus-eluting stent versus the TAXUS Paclitaxel-eluting stent in de novo native coronary lesions: 12-Month outcomes from the ENDEAVOR IV Trial. J Am Coll Cardiol. 2010;55(6):543-554. , 197197. Kirtane AJ, Leon MB, Ball MW, et al. The "final" 5-year follow-up from the ENDEAVOR IV trial comparing a zotarolimus-eluting stent with a paclitaxel-eluting stent. JACC Cardiovasc Interv. 2013;6(4):325-333. ] In the angiographic arm of the trial, E-ZES did not achieve the pre-specified secondary endpoint of in-segment late loss (0.36 ± 47 mm vs. 0.23 ± 0.45 mm, p = 0.023). However, E-ZES met its primary clinical endpoint of non-inferiority on TVF at 9 months (E-ZES: 6.6% vs. PES: 7.2%, p = 0.685). While the rate of MI was lower at 30 days (0.8% vs. 2.3%, p = 0.02) largely related to fewer side-branch occlusions, there were no significant differences in rates of death, cardiac death, or MI at 9 and 12 months.[196196. Leon MB, Mauri L, Popma JJ, et al. A randomised comparison of the Endeavor Zotarolimus-eluting stent versus the TAXUS Paclitaxel-eluting stent in de novo native coronary lesions: 12-Month outcomes from the ENDEAVOR IV Trial. J Am Coll Cardiol. 2010;55(6):543-554. ] The 5 year clinical follow-up results of ENDEAVOR IV revealed an increasing safety benefit of E-ZES over PES with a lower rate of the composite of cardiac death and MI (E-ZES=6.4%, vs. PES=9.1%, p = 0.048).[197197. Kirtane AJ, Leon MB, Ball MW, et al. The "final" 5-year follow-up from the ENDEAVOR IV trial comparing a zotarolimus-eluting stent with a paclitaxel-eluting stent. JACC Cardiovasc Interv. 2013;6(4):325-333. ] Rates of definite and probable ST were no different at 9 months (E-ZES=0.8% vs. PES=0.1%, P=0.12) or 5 years (E-ZES=1.4% vs. PES=1.9%, p=0.42). Of note, the incidence of very late ARC definite and probable ST between one and five years was significantly reduced in favor of patients treated with E-ZES (E-ZES=0.4% vs. PES=1.8%, p = 0.012). In terms of efficacy, differences in rates of TLR remained unchanged among E-ZES (7.7%) and PES (8.6%, p = 0.70) treated patients.
E-ZES vs. SES vs. PES
The ZEST trial compared outcomes amongst 2640 patients randomised to E-ZES (n = 880), PES (n = 880) and SES (n = 880).[198198. Park DW, Kim YH, Yun SC, et al. Comparison of zotarolimus-eluting stents with sirolimus- and paclitaxel-eluting stents for coronary revascularization: the ZEST (comparison of the efficacy and safety of zotarolimus-eluting stent with sirolimus-eluting and paclitaxel-eluting stent for coronary lesions) randomized trial. J Am Coll Cardiol. 2010;56(15):1187-1195. ] The primary endpoint was MACE at 12-months, with the comparison of E-ZES with SES analysed as a non-inferiority analysis, whilst the comparison between E-ZES and PES was a superiority analysis ( Table 9). At 12 months, MACE rates were non-inferior between E-ZES and SES (10.2% vs. 8.3%, Pnon-inferiority = 0.01, Psuperiority = 0.17) and significantly lower with E-ZES compared with PES (10.2% vs. 14.1%, p=0.01). The incidence of death or MI was similar (E-ZES 5.8% vs. SES 6.9% vs. PES 7.6%, p = 0.31), whilst the incidence of ST was significantly lower in the SES group (E-ZES 0.7% vs. SES 0.0% vs. PES 0.8%, respectively, p = 0.02). Overall at 12-months follow-up the use of E-ZES resulted in similar rates of MACE compared with SES and fewer MACE events compared with PES.
Zotarolimus-Eluting ENDEAVOR RESOLUTE Stents
The R-ZES is the second iteration of the E-ZES ( Table 2). The first R-ZES consisted of the Driver CoCr stent platform, and a Biolinx polymer - a blend of 3 different polymers: the hydrophobic C10 polymer to control drug release; the biocompatible and hydrophilic C19 polymer; and polyvinyl pyrrolidone to allow an early burst of drug release.[199199. Meredith IT, Worthley S, Whitbourn R, et al. The next-generation Endeavor Resolute stent: 4-month clinical and angiographic results from the Endeavor Resolute first-in-man trial. EuroIntervention. 2007;3(1):50-53. ] The polymer allows delayed drug release, such that at least 85% of the zotarolimus is released within 60 days, with the remainder being released within 180 days ( Figure 14).
The second version of R-ZES was called the Resolute Integrity ZES, which only differed from its predecessor by being manufactured using continuous sinusoid technology. This method of stent manufacturing molds one single strand of wire into a sinusoidal wave which is then wrapped into a helical pattern and laser-fused at certain points, making the stent comparable to a flexible spring enhancing deliverability and conformability to the vessel wall. The latest iteration of the R-ZES stent is called the Resolute Onyx ZES, which this differs from the Resolute Integrity by its stent platform being made from core wire technology. Consequently the Onyx R-ZES has a denser core metal wrapped in a cobalt alloy outer layer, which enables thinner and stronger stent struts that enhance deliverability, improve conformability and increase radiopacity with no compromise to radial and longitudinal strength.
The initial evaluation of R-ZES took place in the 139 patient multi-centre, non-randomized, FIM RESOLUTE study which demonstrated an angiographic in-stent late loss of 0.22 mm at 9-months follow-up ( Figure 13) and respective rates of MACE, TLR and any definite/probable ST of 16.5%, 3.1% and 0.0% at 12-months follow-up, and 14.0%, 2.3% and 0.0% at 5-year follow-up.[200200. Meredith IT, Worthley S, Whitbourn R, et al. Clinical and Angiographic Results With the Next-Generation Resolute Stent System A Prospective, Multicenter, First-in-Human Trial. JACC Cardiovasc Interv. 2009;2(10):977-985. , 201201. Meredith I, Worthley S, Whitbourn R, et al. Long-term clinical outcomes with the next generation Resolute Stent System: a report of the two-year follow-up from RESOLUTE clinical trial. EuroIntervention. 2010;5:692-697. , 202202. TCT-414: Three-Year Follow-up of a New Zotarolimus-Eluting Stent: Results of the RESOLUTE First-In-Man Trial. The American Journal of Cardiology. 2009;104(6):153D-154D. , 203203. Meredith I. Four-Year Clinical Outcomes from the RESOLUTE First-In-Man Trial. Presentation at Transcatheter Cardiovascular Therapeutics, Washington, USA, September 22nd 2010. , 204204. Leon MB. Zotarolimus-eluting (RESOLUTE) stents 2011/2012: Emerging data from randomised trials and registries. Presentation at Transcatheter Cardiovascular Therapeutics, 7th November 2011. Available online at http://www.tctmd.com/txshow.aspx?. tid=1087388&id=109713&trid=1086298. [Accessed 20th November 2011]. ]
Several studies have evaluated the performance of R-ZES as summarized in Table 10:[205205. Serruys PW, Silber S, Garg S, et al. Comparison of Zotarolimus-Eluting and Everolimus-Eluting Coronary Stents. N Engl J Med. 2010;363:123-135. , 206206. Serruys P, Silber S, Windecker S. Final 5-year outcomes from the randomised comparison of Zotarolimus-eluting stents with Everolimus-eluting stents in the RESOLUTE All Comers Trial. Presentation at EuroPCR 2014, Paris, France. 2014. , 207207. Yeung AC, Leon MB, Jain A, et al. Clinical evaluation of the Resolute zotarolimus-eluting coronary stent system in the treatment of de novo lesions in native coronary arteries: the RESOLUTE US clinical trial. J Am Coll Cardiol. 2011;57(17):1778-1783. , 208208. Massberg S, Byrne RA, Kastrati A, et al. Polymer-Free Sirolimus- and Probucol-Eluting Versus New Generation Zotarolimus-Eluting Stents in Coronary Artery Disease: The Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus- and Probucol-Eluting Versus Zotarolimus-Eluting Stents (ISAR-TEST 5) Trial. Circulation. 2011;124(5):624-632. , 209209. Kufner S, Sorges J, Mehilli J, et al. Randomized Trial of Polymer-Free Sirolimus- and Probucol-Eluting Stents Versus Durable Polymer Zotarolimus-Eluting Stents: 5-Year Results of the ISAR-TEST-5 Trial. JACC Cardiovasc Interv. 2016;9(8):784-792. , 210210. Colleran R, Kufner S, Harada Y, et al. Five-year follow-up of polymer-free sirolimus- and probucol-eluting stents versus new generation zotarolimus-eluting stents in patients presenting with st-elevation myocardial infarction. Catheter Cardiovasc Interv. 2017;89(3):367-374. , 211211. von Birgelen C, Basalus MW, Tandjung K, et al. A randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients: the TWENTE trial. J Am Coll Cardiol. 2012;59(15):1350-1361. , 212212. von Birgelen C, van der Heijden LC, Basalus MW, et al. Five-Year Outcome After Implantation of Zotarolimus- and Everolimus-Eluting Stents in Randomized Trial Participants and Nonenrolled Eligible Patients: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2017;2(3):268-276. , 213213. Ahn JM, Park DW, Kim YH, et al. Comparison of resolute zotarolimus-eluting stents and sirolimus-eluting stents in patients with de novo long coronary artery lesions: a randomized LONG-DES IV trial. Circ Cardiovasc Interv. 2012;5(5):633-640. ]
The RESOLUTE All-Comers trial,[205205. Serruys PW, Silber S, Garg S, et al. Comparison of Zotarolimus-Eluting and Everolimus-Eluting Coronary Stents. N Engl J Med. 2010;363:123-135. ] which was the first randomised assessment of two new generation DES, enrolled 2,300 patients, who were randomized in a 1:1 ratio to treatment with either the R-ZES or the Xience™ V EES. At 12-months clinical follow-up in a predominantly off-label population, the R-ZES was found to be non-inferior to EES with respect to the primary clinical endpoint of TLF, a composite of cardiac death, target vessel MI and clinically indicated TLR (R-ZES 8.2% vs. EES 8.3%, Pnon-inferiority < 0.001). In addition, in a sub-group of patients who were randomised to 13-month angiographic follow-up, R-ZES was found to be non-inferior to EES with respect to the powered angiographic secondary endpoint of in-stent diameter stenosis (R-ZES 21.65 ± 14.42% versus EES 19.76 ± 14.64%, Pnon-inferiority=0.04). Considering the complex patient population, the overall rate of definite or probable ST was low at 2.3% and 1.5% for R-ZES and EES respectively (P= 0.17).[205205. Serruys PW, Silber S, Garg S, et al. Comparison of Zotarolimus-Eluting and Everolimus-Eluting Coronary Stents. N Engl J Med. 2010;363:123-135. ] Five-year results mirrored the trends seen at 1-year with no significant between-stent differences in TLR (R-ZES 17.1% vs. EES 16.3%, P=0.65) or its individual safety and efficacy components.[206206. Serruys P, Silber S, Windecker S. Final 5-year outcomes from the randomised comparison of Zotarolimus-eluting stents with Everolimus-eluting stents in the RESOLUTE All Comers Trial. Presentation at EuroPCR 2014, Paris, France. 2014. ] Only 10% of patients remained on DAPT at 5-years, however despite this rates of ST were low, with very late definite/probable ST rates of only 0.84% and 1.03% (p=0.66) for R-ZES and EES, respectively.
The ISAR-TEST 5 study enrolled 3002 patients who were randomized to treatment with the R-ZES (n = 1000) and a polymer-free rapamycin/probucol dual DES (n = 2002).[208208. Massberg S, Byrne RA, Kastrati A, et al. Polymer-Free Sirolimus- and Probucol-Eluting Versus New Generation Zotarolimus-Eluting Stents in Coronary Artery Disease: The Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus- and Probucol-Eluting Versus Zotarolimus-Eluting Stents (ISAR-TEST 5) Trial. Circulation. 2011;124(5):624-632. ] At 12-months follow-up the study achieved its non-inferiority primary endpoint following MACE rates of 13.1% for both stents (P=0.83, Pnon-inferiority = 0.012). The two stents were also comparable with respect to other clinical endpoints such as mortality, TLR and ST, and the angiographic endpoints in-stent late loss and binary angiographic restenosis. Rates of MACE (23.8% vs. 24.2%, p=0.80), its individual components and definite/probable ST (1.3% vs. 1.6%, p=0.64) remained similar out to 5-years follow-up.[209209. Kufner S, Sorges J, Mehilli J, et al. Randomized Trial of Polymer-Free Sirolimus- and Probucol-Eluting Stents Versus Durable Polymer Zotarolimus-Eluting Stents: 5-Year Results of the ISAR-TEST-5 Trial. JACC Cardiovasc Interv. 2016;9(8):784-792. ] Parallel results were seen in the cohort of AMI patients.[210210. Colleran R, Kufner S, Harada Y, et al. Five-year follow-up of polymer-free sirolimus- and probucol-eluting stents versus new generation zotarolimus-eluting stents in patients presenting with st-elevation myocardial infarction. Catheter Cardiovasc Interv. 2017;89(3):367-374. ]
The single centre TWENTE study randomly allocated in a 1:1 fashion 1,380 patients presenting with stable angina or non-ST elevation MI to treatment with EES or R-ZES.[211211. von Birgelen C, Basalus MW, Tandjung K, et al. A randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients: the TWENTE trial. J Am Coll Cardiol. 2012;59(15):1350-1361. ] The primary endpoint of this non-inferiority trial included cardiac death, target vessel related-MI and clinically-driven TVR and occurred with a similar frequency for both devices (EES 8.1% vs. ZES 8.2 %, Pnon-inferiority = 0.001). Similar to RESOLUTE All-comers, rates of definite or probable ST were low and of similar magnitude between groups (R-ZES 0.86% vs. EES 1.16%, Pnon-inferiority = 0.12). The safety and efficacy of both stents remained comparable at 5-year follow-up.[212212. von Birgelen C, van der Heijden LC, Basalus MW, et al. Five-Year Outcome After Implantation of Zotarolimus- and Everolimus-Eluting Stents in Randomized Trial Participants and Nonenrolled Eligible Patients: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2017;2(3):268-276. ]
The LONG DES IV study randomised 500 patients with coronary lesions ≥ 25mm in length to treatment with either R-ZES (n = 250) or SES (n = 250).[213213. Ahn JM, Park DW, Kim YH, et al. Comparison of resolute zotarolimus-eluting stents and sirolimus-eluting stents in patients with de novo long coronary artery lesions: a randomized LONG-DES IV trial. Circ Cardiovasc Interv. 2012;5(5):633-640. ] The study achieved its primary endpoint by demonstrating the non-inferior performance of R-ZES compared to SES for angiographic in-segment late lumen loss at 9-month follow-up (R-ZES 0.14 ± 0.38 vs. SES 0.12 ± 0.43, Pnon-inferiority = 0.03, Psuperiority = 0.6). Both stent platforms were associated with comparably low clinical events rates.
PROMUS Element
The PROMUS Element stent has a PtCr platform, a PBMA primer coating, a PVDF-HFP polymer and is loaded with 1 µg/mm2 of everolimus, 80% of which is eluted within 90-days of stent implantation ( Table 2). Initial assessment of the stent took place in the PLATINUM clinical trial programme comprising of:[214214. Meredith IT, Whitbourn R, Scott D, et al. PLATINUM QCA: a prospective, multicentre study assessing clinical, angiographic, and intravascular ultrasound outcomes with the novel platinum chromium thin-strut PROMUS Element everolimus-eluting stent in de novo coronary stenoses. EuroIntervention. 2011;7(1):84-90. , 215215. Stone GW, Teirstein PS, Meredith IT, et al. A prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a Prospective, Randomized, Multicenter Trial to Assess an Everolimus-Eluting Coronary Stent System [PROMUS Element] for the Treatment of Up to Two de Novo Coronary Artery Lesions) trial. J Am Coll Cardiol. 2011;57(16):1700-1708. , 216216. Meredith IT, Teirstein PS, Bouchard A, et al. Three-year results comparing platinum-chromium PROMUS element and cobalt-chromium XIENCE V everolimus-eluting stents in de novo coronary artery narrowing (from the PLATINUM Trial). Am J Cardiol. 2014;113(7):1117-1123. , 217217. Teirstein PS, Meredith IT, Feldman RL, et al. Two-year safety and effectiveness of the platinum chromium everolimus-eluting stent for the treatment of small vessels and longer lesions. Catheter Cardiovasc Interv. 2015;85(2):207-215. ]
- The single-arm PLATINUM QCA study which enrolled 100 patients and has reported clinical and angiographic data through to 9-months follow-up.[214214. Meredith IT, Whitbourn R, Scott D, et al. PLATINUM QCA: a prospective, multicentre study assessing clinical, angiographic, and intravascular ultrasound outcomes with the novel platinum chromium thin-strut PROMUS Element everolimus-eluting stent in de novo coronary stenoses. EuroIntervention. 2011;7(1):84-90. ] The primary endpoint of the study, which was the 30-day composite rate of cardiac death, MI, TLR and ST occurred in 1 patient, with no additional clinical events observed between 1- and 9-months. The study also achieved its primary efficacy endpoint, which was in-stent late lumen loss for workhorse lesions when compared with historical data from the TAXUS Express stent (0.17 ± 0.25 mm vs. 0.44 mm, p < 0.001). Incomplete stent apposition was also significantly lower than historical controls from SPIRIT III.
- The multicenter PLATINUM Workhorse non-inferiority study which randomised 1532 patients to treatment with the PROMUS Element or PROMUS Xience V EES stent (Boston Scientific, Natick, MA).[215215. Stone GW, Teirstein PS, Meredith IT, et al. A prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a Prospective, Randomized, Multicenter Trial to Assess an Everolimus-Eluting Coronary Stent System [PROMUS Element] for the Treatment of Up to Two de Novo Coronary Artery Lesions) trial. J Am Coll Cardiol. 2011;57(16):1700-1708. ] At 12-months follow-up the PROMUS Element stent met the primary non-inferiority endpoint of TLF compared to the PROMUS Stent (3.4% vs. 2.9%, Pnon-inferiority = 0.001). In addition rates of cardiac death (0.8% vs. 0.4%, p = 0.51), target-vessel MI (0.8% vs. 1.6%, p = 0.14), ischaemia-driven TLR (1.9% vs. 1.9%, p = 0.96), TVR (2.7% vs. 2.9%, p = 0.83) and ST (0.4% vs. 0.4%, p = 0.99) were comparable between both stents. Comparable outcomes were maintained out to 3-year follow-up.[216216. Meredith IT, Teirstein PS, Bouchard A, et al. Three-year results comparing platinum-chromium PROMUS element and cobalt-chromium XIENCE V everolimus-eluting stents in de novo coronary artery narrowing (from the PLATINUM Trial). Am J Cardiol. 2014;113(7):1117-1123. ]
- The single-arm PLATINUM Small vessel study which enrolled 94 patients with lesions in vessels between 2.25 and 2.5 mm. The study met its primary endpoint of TLF at 12-months with a rate of 2.4% for the 2.25 mm stent, which was significantly lower than the pre-specified performance goal of 21.1% derived from historical outcomes of the 2.25 mm TAXUS Express PES. Clinical events out to 2-years were low.[217217. Teirstein PS, Meredith IT, Feldman RL, et al. Two-year safety and effectiveness of the platinum chromium everolimus-eluting stent for the treatment of small vessels and longer lesions. Catheter Cardiovasc Interv. 2015;85(2):207-215. ]
- The single-arm PLATINUM Long lesion study which enrolled 102 patients with lesions between 24 and 34 mm in length. The study met its pre-specified primary endpoint at 12-months with a rate of TLF of 3.2% for the 32 and 38 mm stents compared to the performance goal of 19.4% derived from historical outcomes of the 32 mm TAXUS Express PES. Clinical outcomes at 2-years were low.[217217. Teirstein PS, Meredith IT, Feldman RL, et al. Two-year safety and effectiveness of the platinum chromium everolimus-eluting stent for the treatment of small vessels and longer lesions. Catheter Cardiovasc Interv. 2015;85(2):207-215. ]
- The multi-centre PLATINUM PLUS trial randomised 2,980 all-comers patients in a 2:1 ratio to PtCr EES (n=1,952) or the CoCr EES.[218218. Fajadet J, Neumann FJ, Hildick-Smith D, et al. Twelve-month results of a prospective, multicentre trial to assess the everolimus-eluting coronary stent system (PROMUS Element): the PLATINUM PLUS all-comers randomised trial. EuroIntervention. 2017;12(13):1595-1604. ] The study met its primary endpoint at 12-months in the intention-to-treat analysis with TVF rates of 4.6% with PtCr EES and 3.2% with CoCr EES (Pnon-inferiority p=0.012; Psuperiority, p=0.08). In the per-protocol analysis the primary endpoint was significantly more common with the PtCr EES (HR 1.64, 95% CI: 1.05-2.55, p=0.03). The individual components of TVF and rates of definite/probable ST were similar between the stents.
The PROMUS element stent platform design has undergone a small important modification; this second iteration, called the PROMUS Premier stent, has two additional proximal connectors designed to improve longitudinal strength in the area where distortion is most common.
- [Heading 4] Promus EES vs. R-ZES
Two all-comers randomised non-inferiority studies have reported outcomes comparing treatment with the Promus EES and R-ZES.[219219. von Birgelen C, Sen H, Lam MK, et al. Third-generation zotarolimus-eluting and everolimus-eluting stents in all-comer patients requiring a percutaneous coronary intervention (DUTCH PEERS): a randomised, single-blind, multicentre, non-inferiority trial. Lancet. 2014;383(9915):413-423. , 220220. van der Heijden LC, Kok MM, Lowik MM, et al. Three-year safety and efficacy of treating all-comers with newer-generation Resolute Integrity or PROMUS Element stents in the randomised DUTCH PEERS (TWENTE II) trial. EuroIntervention. 2017;12(17):2128-2131. , 221221. Park KW, Kang SH, Kang HJ, et al. A randomized comparison of platinum chromium-based everolimus-eluting stents versus cobalt chromium-based Zotarolimus-Eluting stents in all-comers receiving percutaneous coronary intervention: HOST-ASSURE (harmonizing optimal strategy for treatment of coronary artery stenosis-safety & effectiveness of drug-eluting stents & anti-platelet regimen), a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2014;63(25 Pt A):2805-2816. ]
The DUTCH PEERS[219219. von Birgelen C, Sen H, Lam MK, et al. Third-generation zotarolimus-eluting and everolimus-eluting stents in all-comer patients requiring a percutaneous coronary intervention (DUTCH PEERS): a randomised, single-blind, multicentre, non-inferiority trial. Lancet. 2014;383(9915):413-423. ] study enrolled 1811 patients in a 1:1 ratio (EES 905 vs. R-ZES 906) and met its non-inferiority endpoint of TVF, a composite of cardiac death, target-vessel MI, and TVR (EES 5% vs. R-ZES 6%, Pnon-inferiority=0.006). All components of the primary endpoint were also comparable. Definite ST rates were low (EES 0.7% vs. R-ZES 0.3%, p=0.34). Longitudinal stent deformation was identified in 9 out of 1591 implanted EES stents, with no deformed R-ZES stents; reassuringly these deformed stents were not associated with any adverse clinical outcomes. No significant between-stent differences emerged out to 3-years of clinical follow-up.[220220. van der Heijden LC, Kok MM, Lowik MM, et al. Three-year safety and efficacy of treating all-comers with newer-generation Resolute Integrity or PROMUS Element stents in the randomised DUTCH PEERS (TWENTE II) trial. EuroIntervention. 2017;12(17):2128-2131. ]
The larger HOST-ASSURE[221221. Park KW, Kang SH, Kang HJ, et al. A randomized comparison of platinum chromium-based everolimus-eluting stents versus cobalt chromium-based Zotarolimus-Eluting stents in all-comers receiving percutaneous coronary intervention: HOST-ASSURE (harmonizing optimal strategy for treatment of coronary artery stenosis-safety & effectiveness of drug-eluting stents & anti-platelet regimen), a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2014;63(25 Pt A):2805-2816. ] study randomised 3755 patients in a 2:1 ratio to treatment with Promus EES (n=2503) or R-ZES (n=1252). The primary endpoint, which was TLF, a composite of cardiac death, target vessel MI and TLR occurred in 2.9% of patients treated with EES and R-ZES, achieving the pre-specific margin of non-inferiority (Psuperiority=0.006, Pnon-inferiority=0.0025). There were no differences in the components of the primary endpoint, the patient-orientated composite endpoint or definite/probable ST. As in the DUTCH PEERS study there were no stent deformations in the R-ZES arm, however 7 out of the 3500 Promus EES stents deployed were deformed with no resultant clinical sequela.
Promus EES vs. R-ZES
Two all-comers randomised non-inferiority studies have reported outcomes comparing treatment with the Promus EES and R-ZES.[221221. Park KW, Kang SH, Kang HJ, et al. A randomized comparison of platinum chromium-based everolimus-eluting stents versus cobalt chromium-based Zotarolimus-Eluting stents in all-comers receiving percutaneous coronary intervention: HOST-ASSURE (harmonizing optimal strategy for treatment of coronary artery stenosis-safety & effectiveness of drug-eluting stents & anti-platelet regimen), a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2014;63(25 Pt A):2805-2816. , 221221. Park KW, Kang SH, Kang HJ, et al. A randomized comparison of platinum chromium-based everolimus-eluting stents versus cobalt chromium-based Zotarolimus-Eluting stents in all-comers receiving percutaneous coronary intervention: HOST-ASSURE (harmonizing optimal strategy for treatment of coronary artery stenosis-safety & effectiveness of drug-eluting stents & anti-platelet regimen), a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2014;63(25 Pt A):2805-2816. ]
The DUTCH PEERS[205205. Serruys PW, Silber S, Garg S, et al. Comparison of Zotarolimus-Eluting and Everolimus-Eluting Coronary Stents. N Engl J Med. 2010;363:123-135. ]205 study enrolled 1811 patients in a 1:1 ratio (EES 905 vs. R-ZES 906) and met its non-inferiority endpoint of target vessel failure, a composite of cardiac death, target-vessel MI, and TVR (EES 5% vs. R-ZES 6%, Pnon-inferiority=0.006). All components of the primary endpoint were also comparable. Definite ST rates were low (EES 0.7% vs. R-ZES 0.3%, p=0.34). Longitudinal stent deformation was identified in 9 out of 1591 implanted EES stents, with no deformed R-ZES stents; reassuringly these deformed stents were not associated with any adverse clinical outcomes.
The larger HOST-ASSURE[205205. Serruys PW, Silber S, Garg S, et al. Comparison of Zotarolimus-Eluting and Everolimus-Eluting Coronary Stents. N Engl J Med. 2010;363:123-135. ] study randomised 3755 patients in a 2:1 ratio to treatment with Promus EES (n=2503) or R-ZES (n=1252). The primary endpoint, which was TLF, a composite of cardiac death, target vessel MI and TLR occurred in 2.9% of patients treated with EES and R-ZES, achieving the pre-specific margin of non-inferiority (Psuperiority=0.006, Pnon-inferiority=0.0025). There were no differences in the components of the primary endpoint, the patient-orientated composite endpoint or definite/probable ST. As in the DUTCH PEERS study there were no stent deformations in the R-ZES arm, however 7 out of the 3500 Promus EES stents deployed were deformed with no resultant clinical sequelae.
Novolimus-eluting stents with durable polymer
The Elixir DESyne permanent polymer novolimus eluting stent (NES) was first assessed in the 15-patient FIM EXCELLA study, which reported an angiographic in-stent late loss of 0.31 ± 0.25 mm, and a percent volume obstruction on IVUS of 6.0 ± 4.4% at 8-months follow-up, together with no MACE through 12 months,[222222. Costa JR, Jr., Abizaid A, Feres F, et al. EXCELLA First-in-Man (FIM) study: safety and efficacy of novolimus-eluting stent in de novo coronary lesions. EuroIntervention. 2008;4(1):53-58. ] and one MACE event at 24 months ( Table 11).[223223. Abizaid A, Costa JR Jr., Feres F, Costa R, Seixas A, Maia F, Staico R, Siqueira D, Ormiston J, Sousa AGMR, Fitzgerald P, Honda Y, Otake H, Sousa JE. TCT-429: Single Center, First-In-Man Study of the Elixir Novolimus Eluting Coronary Stent System with Durable Polymer 24- Month Clinical Safety and Efficacy Results. Am J Cardiol. 2009;104(6, Supplement 1):158D-158D. ]
Further assessment of the NES has been performed in the single-blind, prospective EXCELLA-II study, which randomised 210 patients to treatment with either NES (n = 139) or E-ZES (n = 71).[224224. Serruys PW, Garg S, Abizaid A, et al. A randomised comparison of novolimus-eluting and zotarolimus-eluting coronary stents: 9-month follow-up results of the EXCELLA II study. EuroIntervention. 2010;6(2):195-205. ] At 9-month follow-up, the primary endpoint of angiographic in-stent late loss was measured at 0.11 ± 0.32 mm, and to 0.63 ± 0.42 mm in patients treated with NES and ZES, respectively (Pnon-inferiority < 0.0001, Psuperiority < 0.0001). At 12-months clinical follow-up there were no significant differences between stent groups in the device orientated composite endpoint (NES 2.9% vs. E-ZES 5.6%, p = 0.45) or its individual components. The rate of ST was comparable between both groups.[224224. Serruys PW, Garg S, Abizaid A, et al. A randomised comparison of novolimus-eluting and zotarolimus-eluting coronary stents: 9-month follow-up results of the EXCELLA II study. EuroIntervention. 2010;6(2):195-205. ] At 5-year follow-up, patients in the NES group had significantly lower rates of the patient-oriented (HR 0.53, 95% CI: 0.32-0.87, p=0.013) and device-oriented (HR 0.38, 95% CI: 0.17-0.83, p=0.011) composite endpoints. Rates of cardiac death and definite/probable stent thrombosis were similar between the two groups; however, there was a trend towards reduction in MI and repeat revascularisation in the NES group.[225225. Iqbal J, Verheye S, Abizaid A, et al. DESyne novolimus-eluting coronary stent is superior to Endeavor zotarolimus-eluting coronary stent at five-year follow-up: final results of the multicentre EXCELLA II randomised controlled trial. EuroIntervention. 2016;12(11):e1336-e1342. ]
Myolimus-eluting stents with durable polymer
The FIM study of the myolimus-eluting stent enrolled 15-patients, and at 6-months angiographic follow-up in-stent late lumen loss, binary restenosis and percent neointimal volume obstruction were 0.15 ± 0.11mm, 0.0% and 1.4%, respectively ( Table 11). Clinical events out to 9-months comprised of one MI; there was no death, TLR or ST.[226226. Rutsch W. Multi-center First-In-Man Study With the Lowest Known Limus Dose on the Elixir Medical Myolimus Eluting Coronary Stent System With a Durable Polymer: Nine Month Clinical and Six Month Angiographic and IVUS Follow-up. Presentation at EuroPCR 19th-22nd May 2009, Barcelona, Spain. ]
Ridafolimus-Eluting Stents With Durable Polymer
The BioNIR stent (Medinol, Israel) elutes ridafolimus using a bio-permanent elastomeric durable polymer, which is resistant to bending, bonding, cracking, peeling and distortions unlike other contemporary DES. The stent platform is made from CoCr and is characterised by a variable strut size and width, which ensures that drug distribution is in a more even and gradual pattern compared to other contemporary DES. The stent’s manufacturing process is also distinctive as the stents are made from a thin sheet of cobalt alloy that is laser cut, spray and coated with drug and rolled into a cylinder and laser welded. This process allows for greater efficiencies, which may lead to lower production costs compared to other DES.
The stent was first evaluated in the NIREUS FIM non-inferiority study, which randomised 302 patients in a 2:1 ratio to BioNIR (n=201) and R-ZES (n=101).[227227. Smits P. Primary endpoint results from the NIREUS trial. A prospective randomised trial comparing the novel ridaforolimus-eluting BioNIR stent to the zotarolimus-eluting Resolute stent. Presentation at TCT 2016. 2016. ] The study met its primary endpoint of in-stent late lumen loss at 6-months (BioNIR 0.04mm vs. R-ZES 0.03, Pnon-inferiority<0.001). Clinical event rates were low and compared between the stents.
Further assessment has taken place in the BIONICS multi-centre randomized trial BIONICS study which randomised 1,919 patients to BioNIR (n=958) or R-ZES (n=961). The primary endpoint was TLF at 12-months follow-up and rates were identical for both devices (5.3%), achieving the pre-specified criterion for non-inferiority (Pnon-inferiority=0.0012). No significant differences in the individual components of TLF and rates of ST were seen.[228228. David K. BIONICS: Ridaforolimus-Eluting Stent Noninferior to Zotarolimus Stent at 1 Year. 2017. ]
Newer-generation DES with durable polymer coatings
- The cobalt chromium EES is superior to BMS in terms of angiographic outcomes and clinical safety and efficacy.
- The use of EES has been shown to result in superior angiographic and clinical outcomes. EES is associated with lower rates of mortality, ST, MI and repeat revascularization compared to PES.
- In comparison with SES, EES has been shown to be non-inferior with respect to angiographic outcomes. EES has been shown to be somewhat more effective and associated with lower rates of ST, particularly very late ST.
- E-ZES has been shown to be inferior in terms of angiographic performance when compared to SES and PES, whereas R-ZES has a similar angiographic and clinical outcomes compared to EES.
- E-ZES has shown a time-dependent difference compared with SES with lower efficacy during the first year which was offset by lower rates of ST during long-term follow-up resulting in similar overall rates of ST and repeat revascularization.
- R-ZES has been shown to be non-inferior in terms of clinical safety and efficacy when compared with EES in randomized all-comers trials.
NEWER-GENERATION DRUG-ELUTING STENTS WITH BIODEGRADABLE POLYMER COATINGS
Durable polymer coatings have proven to be a successful method for drug loading and release, the key determinants of DES efficacy in clinical practice. However, an important limitation of durable coatings is their undetermined effect on arterial healing. Several animal and human studies have identified durable polymer coatings of early generation DES as a possible stimulus for hypersensitivity reactions and nidus for chronic inflammation. These patho-mechanisms may play an important role in the predisposition for very late ST and delayed restenosis.[229229. Byrne RA, Iijima R, Mehilli J, et al. Durability of antirestenotic efficacy in drug-eluting stents with and without permanent polymer. JACC Cardiovasc Interv. 2009;2(4):291-299. , 230230. Cook S, Ladich E, Nakazawa G, et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120(5):391-399. , 231231. Cook S, Wenaweser P, Togni M, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation. 2007;115(18):2426-2434. ] Several newer generation DES platforms utilize biodegradable as opposed to durable polymers and are reviewed here. In theory immediately after implantation these devices function similar to conventional DES, however after polymer breakdown, they speculatively may offer the safety benefits of a BMS. Short-term results from these stents have been encouraging, whilst recent long-term data have provided some evidence that these stents may lead to the perceived improvements in clinical safety.
There are many challenges remaining for this polymer technology, which include amongst others, establishing the optimal biocompatibility, composition, formulation, and degradation time of the polymer. In addition attention must be paid to the pharmacokinetics of the anti-proliferative agent released by the degradation of the polymer, and the variation in polymer degradation time which can be affected by production factors such as the use of long polymer chains, decreased polymer hydrophobicity and greater polymer crystallinity; together with physical and biological environmental factors.[232232. Waksman R, Pakala R. Coating bioabsorption and chronic bare metal scaffolding versus fully bioabsorbable stent. Eurointervention. 2009;5 (Supplement F):F36-F42. ] Evidence indicates that polymer breakdown can be associated with a significant inflammatory reaction which at times can create an acidic environment; moreover, complications may also occur as a result of a persistent immune response to monomer breakdown products.[233233. De Jong WH, Eelco Bergsma J, Robinson JE, et al. Tissue response to partially in vitro predegraded poly-L-lactide implants. Biomaterials. 2005;26(14):1781-1791. ] These uncertainties reiterate the need for continued research, with clinical outcomes assessed at long-term follow-up.
The currently available biodegradable polymer stents, together with those under investigation are summarized in Table 12.[234234. Dani S, Kukreja N, Parikh P, et al. Biodegradable-polymer-based, sirolimus-eluting Supralimus stent: 6-month angiographic and 30-month clinical follow-up results from the series I prospective study. EuroIntervention. 2008;4(1):59-63. , 235235. Han Y, Jing Q, Xu B, et al. Safety and Efficacy of Biodegradable Polymer-Coated Sirolimus-Eluting Stents in "Real-World" Practice: 18-Month Clinical and 9-Month Angiographic Outcomes. J Am Coll Cardiol Intv. 2009;2(4):303-309. , 236236. Ormiston JA, Abizaid A, Spertus J, et al. Six-month results of the NEVO Res-Elution I (NEVO RES-I) trial: a randomized, multicenter comparison of the NEVO sirolimus-eluting coronary stent with the TAXUS Liberte paclitaxel-eluting stent in de novo native coronary artery lesions. Circ Cardiovasc Interv. 2010;3(6):556-564. , 237237. Windecker S, Serruys PW, Wandel S, et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008;372(9644):1163-1173. , 238238. Garg S, Sarno G, Serruys PW, et al. The twelve-month outcomes of a biolimus eluting stent with a biodegradable polymer compared with a sirolimus eluting stent with a durable polymer. EuroIntervention. 2010;6(2):233-239. , 239239. Meredith IT, Verheye S, Dubois CL, et al. Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J Am Coll Cardiol. 2012;59(15):1362-1370. , 240240. Chevalier B, Silber S, Park S-J, et al. Randomized Comparison of the Nobori Biolimus A9-Eluting Coronary Stent With the Taxus Liberte Paclitaxel-Eluting Coronary Stent in Patients With Stenosis in Native Coronary Arteries: The NOBORI 1 Trial--Phase 2. Circ Cardiovasc Intervent. 2009;2(3):188-195. , 241241. Verheye S, Agostoni P, Dubois CL, et al. 9-month clinical, angiographic, and intravascular ultrasound results of a prospective evaluation of the Axxess self-expanding biolimus A9-eluting stent in coronary bifurcation lesions: the DIVERGE (Drug-Eluting Stent Intervention for Treating Side Branches Effectively) study. J Am Coll Cardiol. 2009;53(12):1031-1039. , 242242. Verheye S. Overview of novolimus eluting and myolimus elution from durable and bioabsorbable polymers. Presentation at Transcatheter Cardiovascular Interventions, Washington, USA, 22nd September 2010. , 243243. Schofer J. Multicentre, first-in-man study on the Elixir Myolimus-eluting coronary stent system with bioabsorbable polymer: 12-month clinical and angiographic/IVUS results. Presentation EuroPCR, 25th-28th May 2010, Paris, France. Available [online] http://www.pcronline. com/Lectures/2010/Multicentre-first-in-man-study-on-the-Elixir-Myolimus-eluting-coronary-stent-system-with-bioabsorbable-polymer-12-month-clinical-and-angiographic-IVUS-results. [Accessed 29th May 2010]. , 244244. Haude M, Lee SW, Worthley SG, et al. The REMEDEE trial: a randomized comparison of a combination sirolimus-eluting endothelial progenitor cell capture stent with a paclitaxel-eluting stent. JACC Cardiovasc Interv. 2013;6(4):334-343. , 245245. Lemos PA, Moulin B, Perin MA, et al. Randomized evaluation of two drug-eluting stents with identical metallic platform and biodegradable polymer but different agents (paclitaxel or sirolimus) compared against bare stents: 1-Year results of the PAINT trial. Catheter Cardiovasc Interv. 2009. , 246246. Vranckx P, Serruys PW, Gambhir S, et al. Biodegradable-polymer-based, paclitaxel-eluting Infinnium stent: 9-Month clinical and angiographic follow-up results from the SIMPLE II prospective multi-centre registry study. EuroIntervention. 2006;2(3):310-317. , 247247. Gao R. FIREHAWK Abluminal groove fillewd bioabsorbable polymer sirolimus eluting stent: Update on the first in man TARGET I and TARGET II studies. Presentation at Transcatheter Cardiovascular Therapeutics, San Francisco, 10th November 2011. , 248248. Ormiston J, Webster M, Stewart J, et al. First-in-human evaluation of a bioabsorbable polymer-coated sirolimus-eluting stent: imaging and clinical results of the DESSOLVE I Trial (DES with sirolimus and a bioabsorbable polymer for the treatment of patients with de novo lesion in the native coronary arteries). JACC Cardiovasc Interv. 2013;6(10):1026-1034. , 249249. Webster M, Harding S, McClean D, et al. First-in-human evaluation of a sirolimus-eluting coronary stent on an integrated delivery system: the DIRECT study. EuroIntervention. 2013;9(1):46-53. , 250250. Hamon M, Niculescu R, Deleanu D, et al. Clinical and angiographic experience with a third-generation drug-eluting Orsiro stent in the treatment of single de novo coronary artery lesions (BIOFLOW-I): a prospective, first-in-man study. EuroIntervention. 2013;8(9):1006-1011. , 251251. Dani S, Costa RA, Joshi H, et al. First-in-human evaluation of the novel BioMime sirolimus-eluting coronary stent with bioabsorbable polymer for the treatment of single de novo lesions located in native coronary vessels - results from the meriT-1 trial. EuroIntervention. 2013;9(4):493-500. , 252252. Lemos PA. Inspiron Sirolimus Eluting Stent. Clinical Research Program Update. Presentation at Transcatheter Therapeutics, Miami, Fl. Oct 2012. Available http://www. tctmd.
com/] An overview of most commonly used biodegradable polymer DES is provided in Figure 15. The first generations of biodegradable polymer devices (BioMatrix BES, Biosensors, Morges, Switzerland and Nobori BES, Terumo, Japan) have been in use for a decade, and have now been joined by a heterogeneous group of second-generation biodegradable polymer devices which use a platform of CoCr or PtCr instead of stainless steel; elute other macrocyclic lactone inhibitors such as sirolimus, everolimus, novolimus, and myolimus instead of biolimus; have struts which are between 61-80mm thick compared to 120-125mm and have polymers with a thickness of 2-15mm instead of 10-20mm ( Table 12).
Biolimus A9-eluting stents
Two stent platforms utilize the combination of a biodegradable PLA polymer and the elution of biolimus A9:
Biomatrix™ stent (Biosensors International PTE LTD, Singapore)
The BioMatrix stent platform has undergone several modifications since it was first developed in 2008. The original device was made of stainless steel with 125mm struts, and these have now been replaced with 84-88mm struts made of CoCr on the latest iteration, the BioMatrix Alpha stent.
The angiographic efficacy of BES was first demonstrated in the randomized STEALTH-I study which reported significantly lower in-stent late lumen loss (0.26mm vs. 0.74mm, p<0.001) and percent neointimal volume (3.2% vs. 32%, p<0.001) with BES compared to BMS at 6-months follow-up.[253253. Grube E, Hauptmann KE, Buellesfeld L, et al. Six-month results of a randomized study to evaluate safety and efficacy of a Biolimus A9 eluting stent with a biodegradable polymer coating. EuroIntervention. 2005;1(1):53-57. ] In the angiographic follow-up group of the LEADERS study (described below), BES was non-inferior to SES for the principal angiographic endpoint of in-stent percent diameter stenosis (BES: 20.9% vs. SES: 23.3%, difference -2.2%, 95%-CI -6.0 to 1.6, Pnon-inferiority = 0.001, Psuperiority = 0.26) with no significant difference in any other angiographic endpoint. Comparisons with more contemporary devices took place in the EVERBIO II study,[254254. Puricel S, Arroyo D, Corpataux N, et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J Am Coll Cardiol. 2015;65(8):791-801. ] which randomised 238 patients 1:1:1 to BES, EES or Absorb BVS, and showed comparable rates of in-stent, and in-segment late lumen loss at 9-months angiographic follow-up for all three devices.
In the LEADERS study, the BioMatrix Flex BES ( Figure 16) was compared against SES in 1,707 all comer patients undergoing PCI. BES was found non-inferior to SES for the primary clinical endpoint, a composite of cardiac death, MI, and TVR (BES: 9.2% vs. SES: 10.5%, rate ratio 0.88, 95%-CI 0.64 to 1.19, Pnon-inferiority = 0.003, Psuperiority = 0.39) ( Table 12).[237237. Windecker S, Serruys PW, Wandel S, et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008;372(9644):1163-1173. , 238238. Garg S, Sarno G, Serruys PW, et al. The twelve-month outcomes of a biolimus eluting stent with a biodegradable polymer compared with a sirolimus eluting stent with a durable polymer. EuroIntervention. 2010;6(2):233-239. ] There were no significant differences for any individual safety or efficacy endpoints between the two stents. As above, BES was also non-inferior to SES for the principal angiographic endpoint of in-stent percent diameter stenosis.[237237. Windecker S, Serruys PW, Wandel S, et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008;372(9644):1163-1173. ] Five-year follow-up data showed similar rates of MACE (BES: 22.3% vs. SES: 26.1%, HR=0.83, 95% CI 0.69-1.02, Pnon-inferiority < 0.001, Psuperiority = 0.07).[255255. Serruys PW, Farooq V, Kalesan B, et al. Improved Safety and Reduction in Stent Thrombosis Associated With Biodegradable Polymer-Based Biolimus-Eluting Stents Versus Durable Polymer-Based Sirolimus-Eluting Stents in Patients With Coronary Artery Disease: Final 5-Year Report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) Randomized, Noninferiority Trial. JACC Cardiovasc Interv. 2013;6(8):777-789. ] Beyond one year, an increasing difference in very late definite ST emerged with an annual incidence of definite ST amounting to 0.17% in BES and 0.63% in SES treated patients. This resulted in a significant relative risk reduction of 74% (HR 0.26, CI 0.10 – 0.68, p = 0.003) in favour of BES between year one and five. The reduction in very late ST translated into a lower incidence of clinical events associated with definite ST, whereas there was no reduction in clinical events not associated with ST. The long-term findings of improved clinical outcomes as well as the interaction of treatment effect with time, namely the absence of treatment effect within the first year and an emerging differential beyond one year, provide a proof of concept with respect to the long-term advantage of a stent using the biodegradable polymer technology.
In the COMFORTABLE study, 1161 patients undergoing primary PCI for a AMI were randomised to receive either BES (n=575) or BMS (n=582).[256256. Raber L, Kelbaek H, Ostojic M, et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308(8):777-787. ] Data out to 1-year demonstrated comparable rates of cardiac death and ST, whilst use of BES lead to significantly lower rates of MACE (4.3% vs. 8.7%, p=0.004). The benefit of BES over BMS in terms of MACE continued to accrue at out to 5 years (8.6 % vs. 14.9%, p=0.001) with clinical differences mainly driven by a significantly reduced risk for target vessel re-infarction (BES 2.2% vs. BMS 5.0%, p=0.02) and ischaemia-driven TLR (4.4% vs. 10.4%, p<0.001). Although approximately 10% of patients discontinued DAPT after 1 year and >80% at 2-years, comparable rates of very late ST were reported for BES and BMS.[257257. Raber L. Long-term clincial outcomes of Biolimus-eluting stents with biodegradable polymer versus bare-metal stents in patients with acute STEMI: 5 year results of the randomised COMFORTABLE AMI trial. Presentation at ESC Congress, Rome Aug 2016. 2016. ]
The all-comers SORT-OUT VI study randomised 2,999 patients to BES (n=1497) or R-ZES (n=1502).[258258. Raungaard B, Jensen LO, Tilsted HH, et al. Zotarolimus-eluting durable-polymer-coated stent versus a biolimus-eluting biodegradable-polymer-coated stent in unselected patients undergoing percutaneous coronary intervention (SORT OUT VI): a randomised non-inferiority trial. Lancet. 2015. ] The study met its non-inferiority primary endpoint of MACE, a composite of cardiac death, target vessel MI, and ischaemia-driven TLR with rates of 5.3% and 5.0% for R-ZES and BES, respectively (Pnon-inferiority =0.004). There were no significant differences in rates of efficacy or safety, including ST. No between-stent differences emerged out to 3-year follow-up.[259259. Raungaard B, Christiansen EH, Botker HE, et al. Comparison of Durable-Polymer Zotarolimus-Eluting and Biodegradable-Polymer Biolimus-Eluting Coronary Stents in Patients With Coronary Artery Disease: 3-Year Clinical Outcomes in the Randomized SORT OUT VI Trial. JACC Cardiovasc Interv. 2017;10(3):255-264. ]
Nobori™ stent (Terumo, Japan)
The Nobori™ stent uses the same PLA polymer and anti-proliferative agent as the aforementioned BioMatrix stent, however, the Nobori stent uses the S-Stent platform, which was only used on the first iteration of the BioMatrix stent. Other differences relate to the delivery system, delivery balloon, and the stent coating process. The BioMatrix stent is coated by an automated autopipette proprietary technology, whilst the Nobori stent is not. The Nobori stent has been compared with the Cypher SES, TAXUS PES and EES.
- The Nobori I study randomized 243 patients to treatment with either the Nobori™ stent (n = 153) or the TAXUS PES stent (n = 90).[240240. Chevalier B, Silber S, Park S-J, et al. Randomized Comparison of the Nobori Biolimus A9-Eluting Coronary Stent With the Taxus Liberte Paclitaxel-Eluting Coronary Stent in Patients With Stenosis in Native Coronary Arteries: The NOBORI 1 Trial--Phase 2. Circ Cardiovasc Intervent. 2009;2(3):188-195. ] Results at 9-months amongst the 86% of patients returning for follow-up demonstrated non-inferiority, and subsequent superiority, of the Nobori™ stent with respect to late loss when compared to the TAXUS PES stent (0.11 ± 0.30 mm vs. 0.32 ± 0.50 mm, Pnon-inferiority < 0.001, Psuperiority = 0.001) ( Figure 17). Although the study was not powered for clinical outcomes, no differences in the composite of death and MI and TLF were reported up to 5 years. Rates of ischemia- and non-ischemia-driven TLR were higher in the TAXUS arm whereas ARC defined ST were lower in the Nobori arm (0.0% vs. 3.2%, p=0.014).[260260. Chevalier B, Wijns W, Silber S, et al. Five-year clinical outcome of the Nobori drug-eluting coronary stent system in the treatment of patients with coronary artery disease: final results of the NOBORI 1 trial. EuroIntervention. 2014. ]
- In the all-comers non-inferiority COMPARE 2 study 2707 patients were randomised 2:1 to receive either the Nobori BES (n = 1795) or EES (n = 912).[261261. Smits PC, Hofma S, Togni M, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet. 2013;381(9867):651-660. ] The study achieved its primary endpoint of MACE (a composite of cardiac death, non-fatal MI and ischaemia driven TVR at 12-months) with rates of 5.2% and 4.8% with the Nobori BES and EES, respectively (Pnon-inferiority < 0.0001). All individual components of MACE were comparable as were rates of ST. At 5-years the study failed to demonstrate any reduction in very late adverse events with a biodegradable, as opposed to a durable polymer DES, following the absence of any significant between-stent differences for MACE, efficacy, safety or ST. [262262. Vlachojannis GJ, Smits PC, Hofma SH, et al. Biodegradable Polymer Biolimus-Eluting Stents Versus Durable Polymer Everolimus-Eluting Stents in Patients With Coronary Artery Disease: Final 5-Year Report From the COMPARE II Trial (Abluminal Biodegradable Polymer Biolimus-Eluting Stent Versus Durable Polymer Everolimus-Eluting Stent). JACC Cardiovasc Interv. 2017;10(12):1215-1221. ]
- Similar to COMPARE 2 was the all-comers NEXT study, which randomised 3235 patients to receive either Nobori BES (n = 1617) or EES (n = 1618).[263263. Natsuaki M, Kozuma K, Morimoto T, et al. Biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent: a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2013;62(3):181-190. ] The study met its pre-specified non-inferiority primary endpoint of TLR at 12-months with rates of 4.2% in each group (Pnon-inferiority < 0.0001). Clinical event rates for other outcome measures were low and comparable between devices. A sub-group of 528 patients underwent angiographic follow-up at 266 ± 43 days and met the primary endpoint of non-inferiority for in-segment late loss (0.03 mm vs. 0.06 mm, Pnon-inferiority < 0.0001). At final three year follow-up, the study also met its non-inferior primary safety endpoint of death or MI with rates of 9.9% and 10.3% for Nobori BES and EES, respectively (Pnon-inferiority < 0.0001, Psuperiority =0.7).[264264. Natsuaki M, Kozuma K, Morimoto T, et al. Final 3-Year Outcome of a Randomized Trial Comparing Second-Generation Drug-Eluting Stents Using Either Biodegradable Polymer or Durable Polymer: NOBORI Biolimus-Eluting Versus XIENCE/PROMUS Everolimus-Eluting Stent Trial. Circulation: Cardiovascular Interventions. 2015;8(10). ]
- In the all-comers SORT OUT V study, 2468 patients were randomised to the Nobori BES (n = 1229) or the Cypher SES (n = 1239).[265265. Christiansen EH, Jensen LO, Thayssen P, et al. Biolimus-eluting biodegradable polymer-coated stent versus durable polymer-coated sirolimus-eluting stent in unselected patients receiving percutaneous coronary intervention (SORT OUT V): a randomised non-inferiority trial. Lancet. 2013;381(9867):661-669. ] The study was powered for non-inferiority of MACE a composite of cardiac death, MI, definite ST and TVR at 9-months. Unlike previous studies of the Nobori BES, the study narrowly failed to meet this primary endpoint with rates of MACE of 4.1% with the Nobori BES and 3.1% with Cypher SES (Pnon-inferiority = 0.06). This difference in MACE was driven by significantly higher rates of early definite ST (0.7% vs. 0.2%, p = 0.03), MI (1.3% vs. 0.6%, p = 0.10), and TVR (3.3% vs. 2.1%, p = 0.08) with Nobori BES. Similar to COMPARE 2, this study failed to identify any significant late clinical benefit through to 5-years from using a biodegradable polymer stent with comparable event rates between both devices (MACE, OR=0.93, p=0.53; cardiac death, OR=0.81, p=0.30; MI, OR=1.05, p=0.76; TVR, OR=0.92 p= 0.54).[266266. Christiansen E. 5-year outcomes after implantation of biodegradable polymer-coated biolimus-eluting stents versus durable polymer-coated sirolimus-eluting stents in unselected patients. Presentation at ESC 2016, Rome. 2016. ] Definite ST was also comparable at 5-years, and unlike the LEADERS trial, there was no significant difference in favour of the biodegradable polymer DES for very late ST (ST>1 year, OR=0.89, p=0.77).
- The BASKET-PROVE II trial[267267. Kaiser C, Galatius S, Jeger R, et al. Long-Term Efficacy and Safety of Biodegradable-Polymer Biolimus-Eluting Stents: Main Results of the Basel Stent Kosten-Effektivitats Trial-PROspective Validation Examination II (BASKET-PROVE II), A Randomized, Controlled Noninferiority 2-Year Outcome Trial. Circulation. 2015;131(1):74-81. ] compared the performance of Nobori BES with EES and a new generation, thin strut BMS with a biocompatible coating (ProKinetik, Biotronik) in 2291 patients with stable CAD or ACS needing stenting in large vessels (≥0 mm in diameter) and treated with aspirin and prasugrel. The primary endpoint was a composite of cardiac death, MI and clinically indicated TVR within 2 years. In the intention-to-treat analysis, the Nobori BES proved non-inferior compared with the EES and more effective than thin strut BMS. However, no differences in the occurrence of the secondary safety endpoint (a composite of very late ST, MI and cardiac death) were reported among the different arms challenging the concept that durable polymers are the main drivers of ST.
- The LONG-DES V study randomised 500 patients with coronary lesions >24mm to receive either Nobori BES or Promus EES.[268268. Lee JY, Park DW, Kim YH, et al. Comparison of biolimus A9-eluting (Nobori) and everolimus-eluting (Promus Element) stents in patients with de novo native long coronary artery lesions: a randomized Long Drug-Eluting Stent V trial. Circ Cardiovasc Interv. 2014;7(3):322-329. ] The study met its non-inferiority primary endpoint of in-segment late luminal loss at 9-months angiographic follow-up (BES 0.14±0.38 vs. EES, 0.11±0.37 mm; Pnoninferiority =0.03, P superiority =0.45). There were no significant between-stent differences in binary restenosis and in-stent late lumen loss, together with clinical outcomes at 12-months.
Novolimus-eluting stents with biodegradable polymers
The FIM study of the Elixir DESyne BD biodegradable polymer NES enrolled 9-patients, and reported an in-stent late lumen loss of 0.16 ± 0.23 mm together with no binary restenosis at 6-months, and no MACE events through to 9-months ( Figure 17).[242242. Verheye S. Overview of novolimus eluting and myolimus elution from durable and bioabsorbable polymers. Presentation at Transcatheter Cardiovascular Interventions, Washington, USA, 22nd September 2010. ] In the follow-on randomized EXCELLA II BD trial 146 patients were randomised 3:1 to the Elixir DESyne BD (n=115) or the E-ZES (n=31). The study achieved its primary endpoint by demonstrating non-inferiority of the Elixir DESyn BD compared to the control stent with respect to in-stent late loss (0.12 ± 0.15 mm vs. 0.67 ± 0.47 mm, Pnon-inferiority < 0.001, Psuperiority<0.001). In addition significantly lower rates of binary angiographic stenosis (0.0% vs. 7.9%, p = 0.003) were seen in the Elixir DESyn group. Clinical events remained low through to 5 years and clinically indicated TLR was lower in the DEsyne BD group compared with the E- ZES (4.5% vs. 9.7%, p=0.11). No ST was reported at 60 months.[269269. Costa R. Device specifications and clinical program update - Desyne BD. Presentation at TCT 2016. 2016. ]
Myolimus-eluting stents with biodegradable polymers
The FIM study of the myolimus-eluting stent recruited 30 patients half of whom had angiographic follow-up at 6-months, whilst the remaining returned at 12-months. Late lumen loss and percent neointimal volume obstruction were 0.08±0.16mm and 3.2%, and 0.13 ± 0.27 mm and 5.4% at 6- and 12-months, respectively; there was no binary restenosis. Clinical events, assessed at 12-months, demonstrated no mortality or ST; there were however two MIs and two TLRs.[243243. Schofer J. Multicentre, first-in-man study on the Elixir Myolimus-eluting coronary stent system with bioabsorbable polymer: 12-month clinical and angiographic/IVUS results. Presentation EuroPCR, 25th-28th May 2010, Paris, France. Available [online] http://www.pcronline. com/Lectures/2010/Multicentre-first-in-man-study-on-the-Elixir-Myolimus-eluting-coronary-stent-system-with-bioabsorbable-polymer-12-month-clinical-and-angiographic-IVUS-results. [Accessed 29th May 2010]. ]
Sirolimus-eluting DES with biodegradable polymers
NEVO™ Stent (Cordis, Warren, NJ, USA)
The NEVO™ stent was an open-cell, cobalt chromium stent, with a PLGA biodegradable polymer which facilitated elution of sirolimus. The stent was unique in its design as the polymer and sirolimus were contained within reservoirs, which eliminate the need for a surface polymer coating, and subsequently reduce tissue-polymer contact by over 75%. This stent design was previously used on the durable polymer, paclitaxel-eluting CoStar stent (Conor MedSystems, Palo Alto, CA). Unfortunately despite promising initial results,[270270. Serruys PW, Sianos G, Abizaid A, et al. The effect of variable dose and release kinetics on neointimal hyperplasia using a novel paclitaxel-eluting stent platform: the Paclitaxel In-Stent Controlled Elution Study (PISCES). J Am Coll Cardiol. 2005;46(2):253-260. , 271271. Kaul U, Gupta RK, Mathur A, et al. Cobalt chromium stent with antiproliferative for restenosis trial in India (COSTAR I). Indian Heart J. 2007;59(2):165-172. , 272272. Dawkins KD, Verheye S, Schuhlen H, et al. The European cobalt STent with Antiproliferative for Restenosis trial (EuroSTAR): 12 month results. EuroIntervention. 2007;3(1):82-88. ] the CoStar stent failed to develop following disappointing results from the CoStar II study,[273273. Krucoff MW, Kereiakes DJ, Petersen JL, et al. A novel bioresorbable polymer paclitaxel-eluting stent for the treatment of single and multivessel coronary disease: primary results of the COSTAR (Cobalt Chromium Stent With Antiproliferative for Restenosis) II study. J Am Coll Cardiol. 2008;51(16):1543-1552. ] where it was shown not to be non-inferior to the TAXUS PES with respect to MACE, (CoStar 11.0% vs. PES 6.9%, p=0.005) and angiographic outcomes such as in-stent late loss (CoStar 0.49 mm vs. PES 0.18 mm, p < 0.0001).
The stent was evaluated in the NEVO-RES I study, which was a randomized, multi-center, non-inferiority study comparing the NEVO™ stent to the TAXUS™ Liberté PES stent in 394 patients with single de novo coronary artery lesions. The sirolimus-eluting NEVO stent was found not only to be non-inferior but also superior to PES for the endpoint in-stent late loss (0.13 ± 0.31 vs 0.36 ± 0.46, Pnon-inferiority < 0.001 and Psuperiority < 0.001) and a trend towards lower in-segment binary restenosis (3.9% vs 8.6%, p = 0.08) at 6 month of follow-up ( Figure 17, Table 12).[236236. Ormiston JA, Abizaid A, Spertus J, et al. Six-month results of the NEVO Res-Elution I (NEVO RES-I) trial: a randomized, multicenter comparison of the NEVO sirolimus-eluting coronary stent with the TAXUS Liberte paclitaxel-eluting stent in de novo native coronary artery lesions. Circ Cardiovasc Interv. 2010;3(6):556-564. ]
Despite these promising initial results, two factors have led to the withdrawal of the NEVO stent. Firstly stent dislodgements were observed in three patients (all in the NEVO group) during the early phase of ‘all-comers’ NEVO-II study, resulting in the study being stopped. Secondly, and perhaps more importantly, the stent manufacturer, Cordis (Warren, NJ), decided to withdraw from the coronary stent industry at the end of 2011. It seems unlikely that this stent technology will develop further.
The Combo Stent Platform (OrbusNeich, Fort Lauderdale, FL, USA)
The Combo stent is a 100µm thick stainless steel stent covered abluminally with a biodegradable polymer matrix allowing a controlled release of sirolimus. An additional circumferential layer of anti-CD34 antibodies is applied on the stent struts on top of the polymer aiming to accelerate endothelial coverage. Data from histology and OCT at 28 days follow-up in the porcine model indicates that this combination promotes endothelialization, while also reducing neointimal formation and inflammation when compared to the standard SES and Genous EPC stent.[4444. Granada JF, Inami S, Aboodi MS, et al. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ Cardiovasc Interv. 2010;3(3):257-266. ] Overall, the Combo Stent offers the potential to improve vascular healing whilst still maintaining effective control over neointimal proliferation. The polymer is predictably degraded within 90 days and sirolimus is applied at a dosage of 5µg/mm stent length, which corresponds to approximately half the dose of the Cypher stent platform. The resolution profile however is very similar to the one of the Cypher stent platform. The Combo stent was tested in the REMEDEE trial, an angiographic non-inferiority trial, comparing the in-stent late loss at 6 month between the Combo stent and the Taxus Liberté PES in a total of 183 patients (2:1 randomization).[244244. Haude M, Lee SW, Worthley SG, et al. The REMEDEE trial: a randomized comparison of a combination sirolimus-eluting endothelial progenitor cell capture stent with a paclitaxel-eluting stent. JACC Cardiovasc Interv. 2013;6(4):334-343. ] The primary endpoint was met with an in-stent late loss amounting to 0.39 ± 0.45 mm in the Combo as compared to 0.44 ± 0.56 mm in the Taxus Liberté stent group (p non-inferiority= 0.0012). These findings were corroborated in an IVUS sub study. Clinical event rates were low, and no ST was seen out to 5-years. Evaluation of the device in an all-comers population took place in the 1000 patient REMEDEE registry, which report low event rates out to 1-year follow-up.[274274. Woudstra P, Kalkman DN, den Heijer P, et al. 1-Year Results of the REMEDEE Registry: Clinical Outcomes After Deployment of the Abluminal Sirolimus-Coated Bioengineered (Combo) Stent in a Multicenter, Prospective All-Comers Registry. JACC Cardiovasc Interv. 2016;9(11):1127-1134. ] A recent propensity matched analysis between outcomes in patients receiving the Combo stent in the REMEDEE registry, and Promus EES or R-ZES from the Dutch Peers study, showed comparable clinical outcomes out to 2-years.[275275. Winter Rd. Clinical outcomes after PCI with the Combo stent versus Resolute Integrity and Promus Stent: A Propensity Matched Analysis. Presentation at EuroPCR 2017. 2017. ]
The EGO-COMBO reported a 9-month late lumen loss of 0.23 ± 0.36 in 61 patients and neointimal regression at OCT follow-up from 9 to 24 months. The rate of MACE beyond 36 months was low (3.28%) and no definite ST occurred.[276276. Lee S. Two-Year Sequential OCT Follow-up Findings & Beyond 3-Year Clinical Outcomes of the New Dual Therapy Endothelial Progenitor Cell Capturing Sirolimus-Eluting COMBO Stent: The EGO-COMBO Study. Presented at TCT 2014. 15th September 2014. ]
Results from the randomised HARMONEE study, of the Combo stent versus EES and the REDUCE trial, of 3 versus 12 months of DAPT post Combo stent insertion in ACS patients, are awaited.
The ISAR TEST 4 stent platform
A custom-made stent platform consisting of a mixture of a microporous stainless steel stent coated with sirolimus, a biodegradable polymer and a biocompatible resin widely used in the coating of medical tablets (n=1299) has been compared against two durable polymer DES platforms, the Cypher SES and the Xience V EES (n = 1304) in the ISAR-TEST-4 non-inferiority trial. At 1-year the biodegradable polymer SES was found to be non-inferior for the primary endpoint, a composite of cardiac death, MI, and TLR (13.8% vs. 14.4%, RR 0.96, 95% CI 0.78-1.17, Pnon-inferiority = 0.005, Psuperiority = 0.29). Likewise, there were no differences with respect to cardiac death or MI (6.3% vs 6.2%, p = 0.94), TLR (8.8% vs 9.4%, p = 0.58), and ARC definite or probable ST (1.0% vs 1.5%, p = 0.58).[277277. Byrne RA, Kastrati A, Kufner S, et al. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) Trial. Eur Heart J. 2009;30(20):2441-2449. ] Similar trends were observed at 5-year follow-up with the primary endpoint occurring in 20.5% and 19.5% of patients treated with biodegradable polymer and permanent polymer DES, respectively (p=0.71). Rates of ST were similar with the BP SES and EES but numerically higher with Cypher-SES compared with EES (2.4% vs. 1.4%, p=0.22).[278278. Kufner S, Byrne RA, Valeskini M, et al. Five-year outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease: final results from the ISAR-TEST 4 randomised trial. EuroIntervention. 2014. ]
FIREHAWK™ Stent (MicroPort, Shanghai, China)
The FIREHAWK stent is a CoCr stent with abluminal grooves containing a PLA bioabsorbable polymer which releases sirolimus.[247247. Gao R. FIREHAWK Abluminal groove fillewd bioabsorbable polymer sirolimus eluting stent: Update on the first in man TARGET I and TARGET II studies. Presentation at Transcatheter Cardiovascular Therapeutics, San Francisco, 10th November 2011. ] The safety and feasibility of the stent was confirmed in the TARGET FIM which enrolled 21 patients and showed in-stent late lumen losses of 0.13 ± 0.18 mm and 0.16 ± 0.07 mm at 4- and 13-month respectively. Furthermore, a primary OCT endpoint showed that 96.2% of struts were fully covered at 4-months, whilst low clinical events rates were seen out to 12-months. Following this in the TARGET I study 458 patients were randomised to the FIREHAWK SES (n = 227) or EES (n = 231).[279279. Gao RL, Xu B, Lansky AJ, et al. A randomised comparison of a novel abluminal groove-filled biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: clinical and angiographic follow-up of the TARGET I trial. EuroIntervention. 2013;9(1):75-83. ] The study achieved its primary endpoint of non-inferiority between the FIREHAWK SES and EES with respect to in-stent late loss at 9-month follow-up (0.13 vs. 0.13, Pnon-inferiority < 0.001). Clinical event rates remained low and comparable between both groups with no definite/probable ST events with the FIREHAWK out to 60-months.[280280. Xu B. Updates on the five-year TARGET I and TARGET II randomised clinical trials. Presentation at EuroPCR, May 2017. 2017. ] The 730 patient TARGET II registry also reported low event rates out to 60-months follow-up.[280280. Xu B. Updates on the five-year TARGET I and TARGET II randomised clinical trials. Presentation at EuroPCR, May 2017. 2017. ] The ongoing TARGET All Comers trial (NCT01196819) will test non-inferiority and subsequently superiority of the FIREHAWK SES compared with EES stents in 1656 all-comer patients for the primary endpoint of TLF at 12 months.
MiStent (Micell Technologies, Durham, NC, USA)
The MiStent is a cobalt chromium stent, coated in a PLGA bioabsorbable polymer which degrades over 45-60 days, and elutes sirolimus which is present in a crystalline formulation to facilitate modified release such that the stent’s anti-restenotic drug last three times longer than its polymer.[248248. Ormiston J, Webster M, Stewart J, et al. First-in-human evaluation of a bioabsorbable polymer-coated sirolimus-eluting stent: imaging and clinical results of the DESSOLVE I Trial (DES with sirolimus and a bioabsorbable polymer for the treatment of patients with de novo lesion in the native coronary arteries). JACC Cardiovasc Interv. 2013;6(10):1026-1034. ] The stent’s FIM study is the DESSOLVE I study which enrolled 30 patients and had a primary endpoint of in-stent late lumen loss. Angiographic and IVUS follow-up demonstrated the stent’s efficacy with an in-stent late lumen loss of 0.08 mm, and a neointimal percentage obstruction of 11.2% at 18-months.[248248. Ormiston J, Webster M, Stewart J, et al. First-in-human evaluation of a bioabsorbable polymer-coated sirolimus-eluting stent: imaging and clinical results of the DESSOLVE I Trial (DES with sirolimus and a bioabsorbable polymer for the treatment of patients with de novo lesion in the native coronary arteries). JACC Cardiovasc Interv. 2013;6(10):1026-1034. ] No binary restenosis or TLR was seen out to 18-months. Safety has been demonstrated on OCT with a mean strut coverage of >85%, and clinically at 5-years, with low MACE rates and no reported TLF or ST. Further evaluation took place in the DESSOLVE II study, which enrolled 184 patients who were randomised 2:1 between the MiStent (n = 121) and the E- ZES (n = 60).[281281. Kandzari D. DESSOLVE MiStent Clinical Trial Program. Presentation at Transcatheter Therapeutics, Miami, Fl. October 2012. Available http://www. tctmd.
com/] The primary endpoint of the study was met with a significantly lower in-stent late loss at 9-months with the MiStent compared with E-ZES (0.27 mm vs. 0.58 mm, p < 0.001). OCT performed in a selected group of patients showed a very low proportion of uncovered struts in both arms and mean neointimal thickness significantly lower in patients treated with the MiStent. Clinical event rates and ST were low and similar up to 5-year follow-up.[282282. Wijns W, Vrolix M, Verheye S, et al. Randomised study of a bioabsorbable polymer-coated sirolimus-eluting stent: results of the DESSOLVE II trial. EuroIntervention. 2014. , 283283. Kandzari D. Micell Clinical Trials Program - MiStent Sirolimus Eluting Stent. Presentation at TCT 2016. 2016. ]
The most robust evaluation of the device took place in multi-centre all-comers DESSOLVE III study which enrolled 1398 patients who were randomised 1:1 to the MiStent (n=703) or Xience EES (n=695).[284284. Winter Rd. DESSOLVE III - A randomised comparison of Xience vs MiStent, a novel DES that embeds sirolimus microcrystals in the vessel wall. Presentation at EuroPCR 2017, Paris. 2017. ] The study met its non-inferiority primary endpoint, a composite of cardiac death, target vessel MI and clinical indicated TLR at 12-months follow-up, with rates of 5.8% for MiStent and 6.5% EES (Pnon-inferiority<0.001). No significant differences were observed in rates of the individual clinical endpoints, including ST.
Svelte (Svelte Medical Systems, New Providence, NJ, USA)
The Svelte is a sirolimus-eluting cobalt chromium stent,[249249. Webster M, Harding S, McClean D, et al. First-in-human evaluation of a sirolimus-eluting coronary stent on an integrated delivery system: the DIRECT study. EuroIntervention. 2013;9(1):46-53. ] which has a combined PLGA and amino acid based coating which is highly biocompatible, non-thrombogenic, non-inflammatory and fully bioresorbable over 12-months. Data confirm that tissue concentrations are similar to the Cypher SES, with the drug elution pattern comparable to both Cypher SES and Xience EES. The stent has been designed to reduce procedural time and overall costs. This is achieved by the stent being designed for direct stenting owing to it being pre-mounted on a 0.012-inch flexible coil guidewire; hence no additional guidewire is required. The stent balloon also has distinct features including two balloon control bands located on the proximal and distal balloon shoulders which are designed to facilitate smooth stent delivery; focus pressure under the stent for controlled stent deployment; and minimise longitudinal balloon growth and contact with the vessel wall. The balloons also have a lower compliance enabling higher-pressure inflations, which is important given the inability to pre-dilate the lesion.
The first clinical assessment of the stent took place in the DIRECT FIM study, which enrolled 30 patients and had a primary safety endpoint of angiographic TVF, and a primary efficacy endpoint of in-stent late loss. There was one device failure. Angiographic, IVUS and OCT follow-up reported an in-stent late lumen loss of 0.22 mm; a neointimal percentage obstruction of 2.7%; and 97.9% strut coverage at 6-months, respectively. There was one binary restenosis, one angiographically driven TLR and no reported cardiac death, MI or ST at 60-months.
The DIRECT II trial randomised 159 patients 2:1 to Svelte or R-ZES the 6-month lumen loss showed non-inferiority of the Svelte stent to the R-ZES stent (0.09mm vs. 0.13mm, Pnon-inferiority<0.0001). Similar clinical outcomes were reported for both groups and no ST occurred.[285285. Verheye S. 6-Month Outcomes Using the Svelte Coronary Stent Integrated Delivery System (IDS) with Enzymatic BioresorbableSirolimusCoating:The DIRECT II Study. Presented at TCT 2014. 15th September 2014. ]
Orsiro stent (Biotronik, Germany)
The Orsiro SES is a cobalt chromium stent with ultrathin struts (60 or 80 µm) whose surface is fully coated with a layer of an amorphous hydrogen-rich silicon carbide, which acts as a diffusion barrier sealing the underlying bare metal surface and reducing ion release by up to 96%. The components of this silicon carbide include: silicon carbide, a ceramic material, carbon and a chemical compound of silicon. Overlying this is a PLLA polymer that elutes sirolimus over a period of approximately 100 days. The polymer matrix has an asymmetric design that allows for the release of a greater drug dose on the abluminal than luminal side. The clinical trial program has been robust comparing this second-generation biodegradable polymer DES with second-generation durable polymer DESs,[286286. Windecker S, Haude M, Neumann FJ, et al. Comparison of a Novel Biodegradable Polymer Sirolimus-Eluting Stent With a Durable Polymer Everolimus-Eluting Stent: Results of the Randomized BIOFLOW-II Trial. Circulation Cardiovascular interventions. 2015;8(2):e001441. , 287287. Ruiz-Salmeron R. BIOFLOW II, A randomized controlled trial Orsiro SES vs. Xience PRIME EES- 24 Months Clinical results. TCT 2014 September 16, 2014. 2014. , 288288. Kang SH, Chung WY, Lee JM, et al. Angiographic outcomes of Orsiro biodegradable polymer sirolimus-eluting stents and Resolute Integrity durable polymer zotarolimus-eluting stents: results of the ORIENT trial. EuroIntervention. 2017;12(13):1623-1631. , 289289. Pilgrim T, Heg D, Roffi M, et al. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. Lancet. 2014;384(9960):2111-2122. , 290290. Zbinden R, Piccolo R, Heg D, et al. Ultrathin Strut Biodegradable Polymer Sirolimus-Eluting Stent Versus Durable-Polymer Everolimus-Eluting Stent for Percutaneous Coronary Revascularization: 2-Year Results of the BIOSCIENCE Trial. Journal of the American Heart Association. 2016;5(3):e003255. ] and more recently, in the first of its kind, with other biodegradable polymer DESs.[291291. Jensen LO, Thayssen P, Maeng M, et al. Randomized Comparison of a Biodegradable Polymer Ultrathin Strut Sirolimus-Eluting Stent With a Biodegradable Polymer Biolimus-Eluting Stent in Patients Treated With Percutaneous Coronary Intervention: The SORT OUT VII Trial. Circ Cardiovasc Interv. 2016;9(7). , 292292. von Birgelen C, Kok MM, van der Heijden LC, et al. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet. 2016;388(10060):2607-2617. ]
The FIM study was the BIOFLOW-I which enrolled 30 patients, and reported a primary endpoint of in-stent late loss of 0.05 mm at 9-months.[250250. Hamon M, Niculescu R, Deleanu D, et al. Clinical and angiographic experience with a third-generation drug-eluting Orsiro stent in the treatment of single de novo coronary artery lesions (BIOFLOW-I): a prospective, first-in-man study. EuroIntervention. 2013;8(9):1006-1011. ] The 12-month MACE rate was 10% owing to one cardiac death, and two ischaemia driven TLRs. Further assessment took place in the multi-center BIOFLOW-II study, which randomised 452 stable patients to treatment with the Orsiro stent (n = 298) and Xience EES (n = 154). The study achieved its non-inferiority primary endpoint of in-stent late loss at 9-months (0.10±0.32mm vs. 0.11±0.29mm, Pnon-inferiority < 0.0001). Clinical event rates were low and comparable between both devices at one year: rates of the device-oriented endpoint, TLF, were 6.5% in the Orsiro arm vs. 8.0% in patients treated with XIENCE (p=0.58).[286286. Windecker S, Haude M, Neumann FJ, et al. Comparison of a Novel Biodegradable Polymer Sirolimus-Eluting Stent With a Durable Polymer Everolimus-Eluting Stent: Results of the Randomized BIOFLOW-II Trial. Circulation Cardiovascular interventions. 2015;8(2):e001441. ] These findings were confirmed at 24 months in the overall population and also in diabetic and small vessel cohorts.[287287. Ruiz-Salmeron R. BIOFLOW II, A randomized controlled trial Orsiro SES vs. Xience PRIME EES- 24 Months Clinical results. TCT 2014 September 16, 2014. 2014. ] Neointimal thickness assessed by OCT in a subgroup of patients was similar without differences in terms of uncovered or malapposed struts were recorded. The potent inhibitory effect on neointimal hyperplasia did not appear to affect arterial healing as no case of ST occurred in either group at one year whilst only one patient in the XIENCE arm experienced a possible very late ST.
In the randomized ORIENT study the Orsiro SES was shown to be non-inferior in terms of in-stent late lumen loss at 9-months compared to R-ZES (median 0.06 mm vs. 0.12 mm; Pnon-inferiority<0.001; Psuperiority=0.21).[288288. Kang SH, Chung WY, Lee JM, et al. Angiographic outcomes of Orsiro biodegradable polymer sirolimus-eluting stents and Resolute Integrity durable polymer zotarolimus-eluting stents: results of the ORIENT trial. EuroIntervention. 2017;12(13):1623-1631. ] Angiographic restenosis was significantly lower with Orsiro (15% vs. 20%, p=0.002), whilst adverse clinical outcomes were low in both groups.
In the BIOSCIENCE trial 2119 patients with minimal exclusion criteria, were randomly assigned to receive the Orsiro stent (n=1063) or the XIENCE EES (n=1056).[289289. Pilgrim T, Heg D, Roffi M, et al. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. Lancet. 2014;384(9960):2111-2122. ] At 12 months, the comparable rate of the combined safety and efficacy primary endpoint proved the non-inferiority of the Orsiro stent compared with XIENCE. No significant differences were reported in rates of ST. Clinical outcomes continued to be similar between groups at 2-years follow-up in the full cohort[290290. Zbinden R, Piccolo R, Heg D, et al. Ultrathin Strut Biodegradable Polymer Sirolimus-Eluting Stent Versus Durable-Polymer Everolimus-Eluting Stent for Percutaneous Coronary Revascularization: 2-Year Results of the BIOSCIENCE Trial. Journal of the American Heart Association. 2016;5(3):e003255. ] and the pre-specified diabetic sub-group.[293293. Franzone A, Pilgrim T, Heg D, et al. Clinical outcomes according to diabetic status in patients treated with biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents: prespecified subgroup analysis of the BIOSCIENCE trial. Circ Cardiovasc Interv. 2015;8(6). ] Randomisation was stratified according to patients presenting with or without an ST-elevation MI, and results from this sub-group identified a significant interaction between the device and the treatment effect.[294294. Piccolo R, Heg D, Franzone A, et al. Biodegradable-Polymer Sirolimus-Eluting Stents Versus Durable-Polymer Everolimus-Eluting Stents in Patients With Acute ST-Segment Elevation Myocardial Infarction: Insights From the 2-Year Follow-Up of the BIOSCIENCE Trial. JACC Cardiovasc Interv. 2016;9(9):981-983. ]
The all-comers SORT OUT VII study was the first randomised study to compare the performance of two biodegradable polymer DES–Orsiro SES (n=1261) and Nobori-BES (n=1264).[291291. Jensen LO, Thayssen P, Maeng M, et al. Randomized Comparison of a Biodegradable Polymer Ultrathin Strut Sirolimus-Eluting Stent With a Biodegradable Polymer Biolimus-Eluting Stent in Patients Treated With Percutaneous Coronary Intervention: The SORT OUT VII Trial. Circ Cardiovasc Interv. 2016;9(7). ] At 1-year the primary endpoint of TLF occurred in 3.8% and 4.6% of patients treated with Orsiro and Nobori, respectively, achieving the pre-specific criterion for non-inferiority (Pnon-inferiority<0.001). Overall clinical event rates were low with no between-stent differences in any clinical endpoints other than definite ST, which was significantly lower with Orsiro (0.4% vs. 1.2%, p=0.034) and driven by lower rates of sub-acute definite ST (0.1% vs. 0.6%, p=0.05). The study was not powered for ST and therefore the variances in ST should only be regarding as hypothesis generating. Notwithstanding this, these differences may be result of the different stent designs between these two generations of biodegradable polymer DES which includes, amongst others, their differing strut thickness and polymer degradation time.
The all-comers BIO-RESORT (TWENTE III) trial was a multicentre double-blinded trial, which randomised 3,514 patients to Orsiro (n=1,169), Synergy EES (Boston Scientific, Natick, MA, n=1,172) and R-ZES (n=1,173).[292292. von Birgelen C, Kok MM, van der Heijden LC, et al. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet. 2016;388(10060):2607-2617. ] The study’s two independent hypotheses were that Orsiro and Synergy would have non-inferior rates of TVF, a composite of cardiac death, target vessel MI and clinically indicated TVR at 12-months, when compared separately with R-ZES. Despite >70% of study population having acute coronary syndromes the event rate was lower than expected. At 12-months the primary endpoint of TVF occurred in 4.7% and 5.4% of patients receiving Orsiro and R-ZES, respectively (HR 0.87, Pnon-inferiority<0.001). There were no significant differences in the components of TVF or ST.
The BIOFLOW V randomized in a 2:1 ratio a total of 1334 patients to undergo PCI with the Orsiro SES or the Xience EES. The primary endpoint of TLF was significantly reduced among patients allocated to the Orsiro SES (6% vs. 10%, p=0.0399) due mainly to a lower occurrence of target-vessel MI in the experimental arm (5% vs. 8%, p=0.0155).[295295. Kandzari DE, Mauri L, Koolen JJ, et al. Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. 2017. ]
Ultimaster stent (Terumo Corporation, Tokyo, Japan)
The Ultimaster stent is made of a cobalt-chromium bare metal platform with thin struts (80 µm) and open cell design and a biodegradable polymer (poly-DL-lactic acid –PDLLA- and polycaprolactone co-polymer) applied to the abluminal side only. The polymer elutes sirolimus drug (3.9 µg/mm stent length) and degrades during a period of 3-4 months. A peculiar aspect of this device is a gradient coating consisting of a lack of drug polymer on the stent areas experiencing the highest physical stress with the aim to reduce the risk of polymer cracking and delamination. In the randomized CENTURY II trial,[296296. Saito S, Valdes-Chavarri M, Richardt G, et al. A randomized, prospective, intercontinental evaluation of a bioresorbable polymer sirolimus-eluting coronary stent system: the CENTURY II (Clinical Evaluation of New Terumo Drug-Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease) trial. Eur Heart J. 2014;35(30):2021-2031. ] it showed a safety and efficacy profile similar to a DP EES (Xience). In a population of 1123 patients with broad inclusion criteria, freedom from TLF (a composite of cardiac death, target vessel MI and TLR) was 95.6% in the BP-SES group and 95.1% in the DP-EES group (Pnon-inferiority<0.0001) at 9-month follow-up. A comparable short-term safety profile was also noted with non-significant differences in rates of cardiac death, MI and ST. At 3 years follow up from CENTURY II, there was no difference between the Ultimaster and Xience in clinical outcomes and ST.
Tivoli stent (EssenTech, Beijing, China)
The Tivoli stent is an open cell, balloon expandable, cobalt-chromium stent (80 µm) coated with a biodegradable polymer (PLGA) containing sirolimus at a dose of 8 µg per mm of stent length. Most of the drug (75%) is released in 28 days. It proved to be non-inferior for the occurrence of efficacy primary endpoint at 12 months compared with a DP sirolimus-eluting stent sharing the same cobalt-chromium platform, the FIREBIRD 2 stent (MicroPort, Shanghai, China) in the randomized I-LOVE-IT-2 trial. [297297. Han Y, Xu B, Jing Q, et al. A randomized comparison of novel biodegradable polymer- and durable polymer-coated cobalt-chromium sirolimus-eluting stents. JACC Cardiovasc Interv. 2014;7(12):1352-1360. ]
BioMime stent (Meril Life Sciences, Gujarat India)
The BioMime SES stent has a cobalt chromium ultra-thin (65 µ) platform and a hybrid cell design that is coated in a combined PLGA and PLLA biodegradable polymer. The initial clinical assessment took place in the Merit-I study, which enrolled 28 patients, and reported an in-stent late loss of 0.15mm at 8-months angiographic follow-up.[251251. Dani S, Costa RA, Joshi H, et al. First-in-human evaluation of the novel BioMime sirolimus-eluting coronary stent with bioabsorbable polymer for the treatment of single de novo lesions located in native coronary vessels - results from the meriT-1 trial. EuroIntervention. 2013;9(4):493-500. ] There were no clinical events out to 2-years.[298298. Seth A. Biomimicry in DES - BioMime SES and meriT clinical update. Presentation at Transcatheter Therapeutics, Miami, Fl. October 2012. Available http://www. tctmd.
com/] Following this was the Merit-2 study, which recruited 242 more complex patients, who had an in-stent late loss of 0.13 mm at 8-months. The rate of MACE was 6.2% at 1-year as a result of 2 cardiac deaths, 3 non-fatal MIs and 10 clinically-driven TLRs. Furthermore 3 STs were seen..[298298. Seth A. Biomimicry in DES - BioMime SES and meriT clinical update. Presentation at Transcatheter Therapeutics, Miami, Fl. October 2012. Available http://www. tctmd.
com/] Randomized comparisons with EES are ongoing (meriT4, meriT5, merit China).
Inspiron stent (Scitech, Sao Paulo, Brazil)
The Inspiron has a cobalt chromium platform coated in an abluminal PLA and PLGA polymer and elutes a low dose of sirolimus (56µg). Initial clinical evaluation took place in the INSPIRON I trial, which randomised 58 patients 2:1 to treatment with the Inspiron stent (n = 39) or a BMS control (n = 19)[299299. Ribeiro EE, Campos CM, Ribeiro HB, et al. First-in-man randomised comparison of a novel sirolimus-eluting stent with abluminal biodegradable polymer and thin-strut cobalt-chromium alloy: INSPIRON-I trial. EuroIntervention. 2014;9(12):1380-1384. ] The study achieved its primary endpoint with a significantly lower in-segment late loss with the Inspiron stent compared to the controls (0.19 vs. 0.58, p <0.001). In-stent late loss and percent neointimal obstruction were also both significantly lower with the Inspiron SES. MACE rates at 12-motnhs were comparable, whilst no TLR was seen with the SES out to a median of 878 days. In the multi-center DESTINY trial including 170 patients, the Inspiron stent was non-inferior to the Biomatrix Flex stent for the 9-months in stent lumen loss (0.20 vs. 0.15, Pnon-inferiority <0.001). IVUS and OCT findings showed a slightly higher neointimal hyperplasia and strut coverage compared with the Biomatrix stent, respectively.inspiro[300300. Lemos PA. Inspiron Stent, Clinical research program TCT 2014 September 15, 2014. 2014. ]
Everolimus-eluting DES with biodegradable polymers
SYNERGY stent (Boston Scientific, Natick, MA, USA)
The SYNERGY stent utilises the same PtCr alloy and stent design as the Element stent platform, however the stent is coated in an abluminal ultrathin rollcoat bioerodable polylactic-co-glycolic acid polymer that elutes everolimus. Drug release profiles and tissue concentrations are similar to the PROMUS Element stent. The safety and performance of the stent was assessed in the single-blind randomised, non-inferiority EVOLVE trial, which enrolled 291 patients in 29 sites in Europe, Australia, and New Zealand. The trial compared two doses of everolimus (PROMUS-like, 113 µg/20 mm stent; and half-PROMUS, 56 µg/20 mm stent) on the SYNERGY stent to the Promus EES stent in patients with a single de novo native coronary artery lesion.[239239. Meredith IT, Verheye S, Dubois CL, et al. Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J Am Coll Cardiol. 2012;59(15):1362-1370. ] At 6-months both SYNERGY stents were shown to be non-inferior to the Promus EES with respect to the primary angiographic endpoint of late loss (Standard dose 0.10 mm vs. Low dose 0.13 mm vs. EES 0.15, Pnon-inferiority < 0.001).[301301. Meredith IT, Verheye S, Weissman NJ, et al. Six-month IVUS and two-year clinical outcomes in the EVOLVE FHU trial: a randomised evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting stent. EuroIntervention. 2013;9(3):308-315. ] In addition, rates of the primary clinical endpoint of TLF, a composite of cardiac death, MI, and TLR at 30 days were comparable between stents (1.1% vs. 3.1% vs. 0.0%, p = NS), with results maintained out to 60-months.
In the EVOLVE II trial, 1684 patients scheduled for PCI due to stable CAD or Non-ST segment elevation MI were randomly assigned to receive either the SYNERGY stent or the PROMUS EES. Comparable rates of the primary composite endpoint, TLF at 12-month (6.7% vs. 6.5%, p=0.83 for difference) demonstrated the non-inferiority of the SYNERGY stent (Pnon-inferiority =0.0005). Rates of the individual components of TLF were similar for both study arms: cardiac death (0.5% vs. 0.9%, p= 0.34), MI (5.4% vs. 5.0%, p= 0.68), clinically indicated TLR (2.6% vs. 1.7%, p= 0.21). Comparable clinical safety was established in view of a similar rate of ST (0.4% vs. 0.6%, p= 0.50).[302302. Kerieakes D. Efficacy and Safety of a Novel Bioabsorbable Polymer-Coated, Everolimus-Eluting Coronary Stent: The EVOLVE II Randomized Trial.In press ] At 2 years, rates of TLF were 9.4% in the SYNERGY groups vs 8.5% in the PROMUS EES (p=0.57). Three-year follow up data support long term safety and efficacy of the SYNERGY stent with a 0.2% probable ST rate in the SYNERGY group versus 0.7% in the PROMUS EES (p=0.15). On the basis of the results from this study, the SYNERGY stent received FDA approval, and became the first biodegradable polymer DES available in the US.
As mentioned previously two arms of the BIO-RESORT (TWENTE III) trial included patients randomised to Synergy EES (n=1,172) or R-ZES (n=1,173),[292292. von Birgelen C, Kok MM, van der Heijden LC, et al. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet. 2016;388(10060):2607-2617. ] and at 12-months, as with Orsiro, the Synergy EES was shown to be non-inferior to R-ZES for TVF (Synergy 4.7% vs. R-ZES 5.4%, HR 0.87, Pnon-inferiority<0.001). As in the other arm of the study there were no significant differences in the components of TVF or ST.
Biodegradable polymer DES vs. durable polymer DES
Stefanini et al reported a patient level meta-analysis of three randomised studies (LEADERS, ISAR TEST 3, ISAR TEST 4) of biodegradable polymer stents (n = 2358) versus early-generation - SES (n=1704).[303303. Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33(10):1214-1222. ] At 4-years rates of TLR (HR 0.82, p = 0.03), and definite ST (HR 0.56, p = 0.02) were significantly lower in the biodegradable polymer group compared with the durable polymer group. Therefore, biodegradable polymer DES provide better efficacy and safety outcomes when compared with early-generation DES. Collectively, data from randomised trials suggest similar efficacy and safety outcomes between biodegradable and durable polymer new-generation DES. However, a direct comparison in the SORT OUT VII indicated that biodegradable polymer DES with thinner struts may be associated with a lower risk of ST compared with relatively thicker biodegradable polymer DES during the early period after PCI.[291291. Jensen LO, Thayssen P, Maeng M, et al. Randomized Comparison of a Biodegradable Polymer Ultrathin Strut Sirolimus-Eluting Stent With a Biodegradable Polymer Biolimus-Eluting Stent in Patients Treated With Percutaneous Coronary Intervention: The SORT OUT VII Trial. Circ Cardiovasc Interv. 2016;9(7). ] This finding has been also confirmed by a large network meta-analysis accounting also for indirect evidence.[304304. Kang SH, Chae IH, Park JJ, et al. Stent Thrombosis With Drug-Eluting Stents and Bioresorbable Scaffolds: Evidence From a Network Meta-Analysis of 147 Trials. JACC Cardiovasc Interv. 2016;9(12):1203-1212. ]
Biodegradable polymer-based stents
- Biodegradable polymer stents have been developed in response to the safety concerns associated with early generation DES rendering the stent surface more closely to a BMS following completion of biodegradation.
- Biolimus A9 eluting biodegradable polymer stents have been shown to be non-inferior in terms of clinical and angiographic outcomes when compared to durable polymer SES stents. Clinical long-term follow-up data up to five years suggests that by reducing the risk of cardiac events associated with very late ST biolimus A9 eluting biodegradable polymer stents may result in improved long-term outcomes as compared to durable polymer SES stents. More recent data suggest similar clinical outcomes of biolimus A9 eluting biodegradable polymer stents compared with durable polymer everolimus-eluting stents throughout 2 years of follow-up.
- Novolimus, myolimus, sirolimus and everolimus-eluting biodegradable polymer based stents with thin-strut cobalt-chromium platforms are replacing the early generation thick strut platforms.
POLYMER-FREE DRUG-ELUTING STENTS
Non-polymeric DES offer the potential advantages of avoiding the long-term adverse effects of a polymer, improved healing, and an improvement to the integrity of the stent’s surface. The physical properties of these polymer-free stents and angiographic follow-up results from FIM studies or randomized trials are summarized in Table 13, [279279. Gao RL, Xu B, Lansky AJ, et al. A randomised comparison of a novel abluminal groove-filled biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: clinical and angiographic follow-up of the TARGET I trial. EuroIntervention. 2013;9(1):75-83. ], [305305. Costa R. Amazonia PAX (Minvasys) Programme Update. Presentation at Transcatheter Therapeutics, Oct 23 2012, Miami, USA. Available at tctmd.
com, 306306. Grube E. BioFreedom First In Man Report. Presentation at Transcatheter Cardiovascular Therapeutics, Washington, USA, 25th September 2010. , 307307. Costa JR, Jr., Abizaid A, Costa R, et al. 1-Year Results of the Hydroxyapatite Polymer-Free Sirolimus-Eluting Stent for the Treatment of Single De Novo Coronary Lesions: The VESTASYNC I Trial. J Am Coll Cardiol Intv. 2009;2(5):422-427. , 308308. Mehilli J, Kastrati A, Wessely R, et al. Randomized trial of a nonpolymer-based rapamycin-eluting stent versus a polymer-based paclitaxel-eluting stent for the reduction of late lumen loss. Circulation. 2006;113(2):273-279. , 309309. Costa RA, Abizaid A, Mehran R, et al. Polymer-Free Biolimus A9-Coated Stents in the Treatment of De Novo Coronary Lesions: 4- and 12-Month Angiographic Follow-Up and Final 5-Year Clinical Outcomes of the Prospective, Multicenter BioFreedom FIM Clinical Trial. JACC Cardiovasc Interv. 2016;9(1):51-64. , 310310. Carrie D, Berland J, Verheye S, et al. A multicenter randomized trial comparing amphilimus- with paclitaxel-eluting stents in de novo native coronary artery lesions. J Am Coll Cardiol. 2012;59(15):1371-1376. , 311311. Zhang L, Yuan J, Liu G, et al. One-year clinical outcome of a randomized trial of polymer-free paclitaxel-eluting stents versus biodegradable polymer-based rapamycin-eluting stents in patients with coronary heart disease. J Interv Cardiol. 2012;25(6):604-610. , 312312. Seth A. Nanoparticle based stents - FOCUS np (Envision Scientific) Program Update. Presentation at Transcather Cardiovascular Interventions, Miami FL, October 23rd 2012. , 313313. Worthley SG, Abizaid A, Kirtane AJ, et al. First-in-Human Evaluation of a Novel Polymer-Free Drug-Filled Stent: Angiographic, IVUS, OCT, and Clinical Outcomes From the RevElution Study. JACC Cardiovasc Interv. 2017;10(2):147-156. ] and late loss figures are provided in Figure 18. Overall robust clinical studies that confirm the improvements in clinical safety hypothesised by their design are currently lacking.
YUKON – sirolimus eluting stent
The stainless steel YUKON SES (Translumina, Germany) was the first and the most extensively studied polymer-free DES. The stent has a micro-porous surface, with pores that are 2 µm deep and effectively function as a drug reservoir removing the need for a polymer.[314314. Wessely R, Hausleiter J, Michaelis C, et al. Inhibition of neointima formation by a novel drug-eluting stent system that allows for dose-adjustable, multiple, and on-site stent coating. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(4):748-753. ] Uniquely the dose of sirolimus is customized in the cath lab, just prior to stent implantation, in a coating process taking approximately eight minutes ( Figure 19). Initial studies have established that the optimal concentration of rapamycin to prevent restenosis is 2%.[315315. Hausleiter J, Kastrati A, Wessely R, et al. Prevention of restenosis by a novel drug-eluting stent system with a dose-adjustable, polymer-free, on-site stent coating. Eur Heart J. 2005;26(15):1475-1481. ]
After complete drug release the remaining micro-porous surface appears to favour the adhesion of endothelial cells, a hypothesis initially suggested by angiographic follow-up data,[316316. Dibra A, Kastrati A, Mehilli J, et al. Influence of stent surface topography on the outcomes of patients undergoing coronary stenting: a randomized double-blind controlled trial. Catheter Cardiovasc Interv. 2005;65(3):374-380. ] and subsequently confirmed by OCT, which has demonstrated significantly greater neointimal thickening, and stent strut coverage with the YUKON stent compared to SES at 3-months follow-up.[317317. Moore P, Barlis P, Spiro J, et al. A randomized optical coherence tomography study of coronary stent strut coverage and luminal protrusion with rapamycin-eluting stents. JACC Cardiovasc Interv. 2009;2(5):437-444. ] Clinical studies including both registry and randomized data demonstrate non-inferiority of a YUKON stent eluting 2% rapamycin when compared with PES out to 1-year follow-up ( Figure 18, Table 13).[308308. Mehilli J, Kastrati A, Wessely R, et al. Randomized trial of a nonpolymer-based rapamycin-eluting stent versus a polymer-based paclitaxel-eluting stent for the reduction of late lumen loss. Circulation. 2006;113(2):273-279. , 318318. Ruef J, Storger H, Schwarz F, et al. Comparison of a polymer-free rapamycin-eluting stent (YUKON) with a polymer-based paclitaxel-eluting stent (TAXUS) in real-world coronary artery lesions. Catheter Cardiovasc Interv. 2008;71(3):333-339. ] In the randomised ISAR-TEST study comparable rates of TLR (YUKON 16.5% vs. PES 16.4%, p = 0.89), death/MI (16.6% vs. 20.0%, p = 0.52), MACE (27.3% vs. 31.7%, p = 0.40) and ST have been seen (0.5% vs. 1.6%, p = 0.32) out to 5-year follow-up.[319319. King L, Byrne RA, Mehilli J, et al. Five-year clinical outcomes of a polymer-free sirolimus-eluting stent versus a permanent polymer paclitaxel-eluting stent: final results of the intracoronary stenting and angiographic restenosis - test equivalence between two drug-eluting stents (ISAR-TEST) trial. Catheter Cardiovasc Interv. 2013;81(1):E23-28. ]
Observational data extending out to 2-year follow-up have indicated that the YUKON stent may be less susceptible to delayed restenosis when compared to conventional DES. Byrne et al reported a significantly lower change in late loss between 6-8 months and 2 years for the YUKON stent, when compared with durable polymer based SES and PES (YUKON 0.01±0.42 mm, SES 0.17 ± 0.50 mm, and PES 0.13 ± 0.50mm, p < 0.001).[320320. Goldstein JA, Demetriou D, Grines CL, et al. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343(13):915-922. ] This finding has also been observed during similar 2-year follow-up of the ISAR-TEST 2 and ISAR-TEST 3 studies, both of which randomized patients to treatment with polymer free SESs, or either conventional polymer or biodegradable polymer DES.[321321. Byrne RA, Mehilli J, Iijima R, et al. A polymer-free dual drug-eluting stent in patients with coronary artery disease: a randomized trial vs. polymer-based drug-eluting stents. Eur Heart J. 2009;30(8):923-931. , 322322. Byrne RA, Kastrati A, Tiroch K, et al. 2-year clinical and angiographic outcomes from a randomized trial of polymer-free dual drug-eluting stents versus polymer-based Cypher and Endeavor [corrected] drug-eluting stents. J Am Coll Cardiol. 2010;55(23):2536-2543. , 323323. Mehilli J, Byrne RA, Wieczorek A, et al. Randomized trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis. Eur Heart J. 2008;29(16):1975-1982. , 324324. Byrne RA, Kufner S, Tiroch K, et al. Randomised trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis: 2-year follow-up results. Heart. 2009;95(18):1489-1494. ] Collectively these results suggest that polymer-free DES may not be subject to the “late-catch” phenomena which have been reported with durable polymer DES, and appears to be worse in those stents eluting macrocylic lactone derived anti-proliferative coatings.[120120. Raber L, Wohlwend L, Wigger M, et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization LATE trial. Circulation. 2011;123(24):2819-2828, 2816 p following 2828. , 147147. Claessen BE, Beijk MA, Legrand V, et al. Two-Year Clinical, Angiographic, and Intravascular Ultrasound Follow-Up of the XIENCE V Everolimus-Eluting Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions: The SPIRIT II Trial. Circ Cardiovasc Intervent. 2009;2(4):339-347. , 325325. Aoki J, Abizaid AC, Ong AT, et al. Serial assessment of tissue growth inside and outside the stent after implantation of drug-eluting stent in clinical trials. - Does delayed neointimal growth exist?. EuroIntervention. 2005;1(3):235-255. ]
BioFreedom™ biolimus A9-eluting stent
The polymer-free biolimus-eluting BioFreedom stent (BES-PF, Biosensors International PTE LTD, Singapore) is made of stainless steel with a strut thickness of 112 µm and a micro-structured, polymer-free abluminal surface ( Figure 6). The investigation of drug release kinetics revealed that >90% of drug is released within 50 hours with biolimus detectable in neointima and myocardium surrounding stent struts at 28 days. Pre-clinical studies of BES-PF provide support for the concept of polymer-free DES. In a porcine model Tada et al demonstrated comparable early, and more durable long-term, efficacy between BES-PF and a durable polymer SES. Furthermore, at 180-days compared with SES, BES-PF use was associated with decreased fibirn, and less inflammation suggesting superior arterial healing.[326326. Tada N, Virmani R, Grant G, et al. Polymer-free biolimus a9-coated stent demonstrates more sustained intimal inhibition, improved healing, and reduced inflammation compared with a polymer-coated sirolimus-eluting cypher stent in a porcine model. Circ Cardiovasc Interv. 2010;3(2):174-183. ]
Clinical assessment of this BES-PF has taken place in the BioFreedom FIM study which randomised patients to treatment with either BES-PF with standard dose biolimus (15.6 µg/mm stent length), BES-PF with low dose biolimus (7.8 µg/mm stent length) or Taxus Liberté PES.[309309. Costa RA, Abizaid A, Mehran R, et al. Polymer-Free Biolimus A9-Coated Stents in the Treatment of De Novo Coronary Lesions: 4- and 12-Month Angiographic Follow-Up and Final 5-Year Clinical Outcomes of the Prospective, Multicenter BioFreedom FIM Clinical Trial. JACC Cardiovasc Interv. 2016;9(1):51-64. ] In total 182 patients were enrolled, 75 into the first cohort which had a primary endpoint of in-stent late loss at 4-months; with the remaining 107 patients entering into the second cohort which had a primary endpoint of in-stent late loss at 12-months. The first cohort achieved its primary endpoint with late loss significantly reduced in both BES-PF with standard dose (late loss 0.08 mm) and BES-PF with low dose (late loss 0.12 mm) at 4 months compared with Taxus Liberté (0.37 mm, both p < 0.001) ( Figure 20, Table 13). The IVUS results confirmed the angiographic findings with a lower neointimal volume obstruction in the BES-PF standard dose (1.3%) compared to both BES-PF low dose (5.5%, p = 0.003) and PES (6.6%, p = 0.0003).
Similarly, the second cohort also achieved its primary endpoint of non-inferiority for in-stent late loss with respective median late loss values for the standard dose BES-PF, low dose BES-PF, and TAXUS PES of 0.17mm, 0.22mm, and 0.35mm (standard dose vs. TAXUS Pnon-inferiority < 0.001 and Psuperoirty = 0.11, low dose vs. TAXUS Pnon-inferiority < 0.21). At 5 years, clinical event rates were similar, with no ST observed in any group.[309309. Costa RA, Abizaid A, Mehran R, et al. Polymer-Free Biolimus A9-Coated Stents in the Treatment of De Novo Coronary Lesions: 4- and 12-Month Angiographic Follow-Up and Final 5-Year Clinical Outcomes of the Prospective, Multicenter BioFreedom FIM Clinical Trial. JACC Cardiovasc Interv. 2016;9(1):51-64. ]
Further evaluation of the BES-PF took place in the LEADERS FREE study, a randomised double blind study, which aimed to assess whether the rapid elution of biolimus in the absence of a polymer would offer a safety advantage over BMS in those patients requiring the efficacy of a DES, but who were unable to take prolonged DAPT.[327327. Urban P, Abizaid A, Chevalier B, et al. Rationale and design of the LEADERS FREE trial: A randomized double-blind comparison of the BioFreedom drug-coated stent vs the Gazelle bare metal stent in patients at high bleeding risk using a short (1 month) course of dual antiplatelet therapy. Am Heart J. 2013;165(5):704-709. ] The LEADERS FREE study enrolled 2466 patients, who due to co-morbidity, or a high-risk of bleeding (including age >75 in 64% patients), were unable to take DAPT beyond one-month, and randomised them to receive either the BES-BF or a BMS. The study had two 1-year primary endpoints: a non-inferior safety composite of cardiac death, MI and ST; and a superiority efficacy endpoint of clinically driven TLR. The primary safety endpoint occurred in 112 patients (9.4%) in the BES-PF group and in 154 patients (12.9%) in the BMS group (Pnon-inferiority < 0.001 and Psuperiority=0.005) at 390 days. Clinically driven TVR occurred in 59 patients (5.1%) in the drug coated stent group and in 113 patients (9.8%) in the BES-PF and BMS groups, respectively (p< 0.001). Results at 2-years showed that BES-PF continued to remain both significantly safer and more effective than BMS in high bleeding risk patients treated with a one month only DAPT course.[328328. Garot P, Morice MC, Tresukosol D, et al. 2-Year Outcomes of High Bleeding Risk Patients After Polymer-Free Drug-Coated Stents. J Am Coll Cardiol. 2017;69(2):162-171. ]
Observation data assessing the efficacy and safety of the stent in an all-comer’s population are available from the 1103 patients enrolled in the RUDI-FREE registry.[329329. Sardella G. RUDI-Free Study. Presentation at EuroPCR 2017, Paris. 2017. ] Clinical outcomes at 12 months confirmed device safety and efficacy, with as expected, significantly higher event rates in patients with high as opposed to non-high bleeding risk. Notably, the device’s performance in high-bleeding risk patients was comparable to those reported in the LEADERS-FREE study, whilst its performance in non-high bleeding risk patients was comparable to contemporary DES.
VESTAsync™ - sirolimus-eluting stent
The VESTAsyn SES (MIV Therapeutics, Atlanta, GA) is a polymer free stainless stent which has a nano-thin, micro-porous, hydroxyapatite surface coating impregnated with 55µg dose of sirolimus. Sirolimus is eluted over 90-days, whilst the hydroxyapatite remains stable over the first 4-months, before completely dissolving around 9-12 months after stent implantation. Pre-clinical studies indicate that the low dose of sirolimus, which is made possible by the hydroxyapatite platform, results in reduced signs of delayed vascular healing, suggesting less local toxicity, and a faster healing response.[330330. Tector AJ, Schmahl TM, Janson B, et al. The internal mammary artery graft. Its longevity after coronary bypass. JAMA. 1981;246(19):2181-2183. ]
Primary evaluation of the stent took place in the 15 patients VESTAsync I FIM study ( Figure 18, Table 13). [331331. Dimas AP, Arora RR, Whitlow PL, et al. Percutaneous transluminal angioplasty involving internal mammary artery grafts. Am Heart J. 1991;122(2):423-429. ] At 4- and 9-months angiographic follow-up effective reductions in late loss and intimal hyperplasia were observed, with no evidence of ‘late-catch’ seen on quantitative coronary angiography (QCA) or IVUS. Out to three year follow-up the only clinic event was a single TLR.[332332. Abizaid A. The MIV VESTASYNC Polymer-Free Sirolimus-Eluting Stent Program (Micorporous Hydroxyapatite Surface). Presentation at Transcatheter Cardiovascular Therapeutics, San Francisco, September 21st 2009. Available online: http://www.tctmd.com/txshow.aspx?.tid=939088&id=84036&trid=938634. [Accessed October 10th 2009]. 2009. ]
Further assessment is on-going in the VESTAsyncII study which has randomised 75 patients in a 2:1 ration to either the VESTAsyn SES (n = 50) or the Gen X stent, a control BMS with a microporous hydroxyapatite surface coating. The in-stent late loss at 8-months follow-up was 0.39±0.20mm and 0.74±0.52mm in VESTAsyn SES and BMS respectively (p=0.03), such the study achieved its primary endpoint. In addition, IVUS demonstrated significantly less neointimal hyperplasia with the VESTAsynSES stent (15.4mm3 vs. 29.4mm3, p=0.01).[333333. Costa JR, Jr., Oliveira BA, Abizaid A, et al. Clinical, angiographic, and intravascular ultrasound results of the VestSaync II trial. Catheter Cardiovasc Interv. 2014;84(7):1073-1079. ] Clinical event rates out to 2-years were low, with 3 and 5 MACE events in the VESTAsyn SES group (1 death, 1 MI, 1 TLR) and BMS (2 deaths, 1 MI and 2 TLRs) respectively; there were no ST events.[334334. Abizaid A. Two-year results form the VESTASync II randomized trial with a hydroxyapatite polymer-free sirollimus eluting stent. Presentation at Transcatheter Cardiovascular Therapeutics, San Francisco, November 8th 2011. ]
PAX – paclitaxel-eluting stent
The Amazonia Pax (Minvasys, Genevilliers, France) stent is the only polymer free stent that is made of cobalt chromium, and elutes paclitaxel. The stent has an open cell design, with 73µm thick struts, which are coated with a 5µm thick abluminal coating of polymer free paclitaxel at a dose of 2.5 µg/mm2. The pure paclitaxel is applied using a micro-drop spray crystallization process. This consistent coating ensures that 98% of the drug is eluted within 30 days, and ensures that by 45 days all that remains is a bare metal cobalt chromium stent.
The multicenter Pax A study randomized 30 patients to treatment with either the Amazonia stent or the TAXUS PES ( Figure 18, Table 13).[305305. Costa R. Amazonia PAX (Minvasys) Programme Update. Presentation at Transcatheter Therapeutics, Oct 23 2012, Miami, USA. Available at tctmd.
com] At 4-months the respective in-stent late lumen loss and %neointimal volume obstruction for the Amazonia and PES were 0.77mm versus 0.42mm (p = 0.20), and 19% versus 6% (p = 0.08). OCT analysis demonstrated significantly more stent strut coverage with the Amazonia stent compared with PES. Clinically at 2-years there were no deaths or ST events; however four patients treated with PES had a TLR, whilst two patient in the Amazonia arm had an MI, and two had a TLR.[305305. Costa R. Amazonia PAX (Minvasys) Programme Update. Presentation at Transcatheter Therapeutics, Oct 23 2012, Miami, USA. Available at tctmd.
com]
Cre8 Sirolimus eluting stent
The Cre8 stent is a polymer free stent which releases sirolimus through the use of abluminal reservoir technology such that drug elution is completed in 90 days. The stent uses an Amphilimus formulation –sirolimus formulated with a mixture of long chain fatty acids which enhances drug stability, facilitates sustained drug elution timing and modulates drug bioavailability. Uniquely the bare metal stent struts are coated in a second generation pure carbon coating which has a crystalline structure close to diamonds and provides excellent bio- and haemo-compatibiltity. Pre-clinical data indicate the absence of a chronic inflammatory response with the Cre8 stent as evidenced by reduced inflammatory scores and neointimal thickness in porcine models when compared to controls comprising of the same stent platform without the carbon coating and the Cypher SES.[335335. Moretti C, Lolli V, Perona G, et al. Cre8 coronary stent: preclinical in vivo assessment of a new generation polymer-free DES with Amphilimus formulation. EuroIntervention. 2012;7(9):1087-1094. ] Consistent with this are the results from the Demonstr8 OCT study which reported that strut coverage was non-inferior between the Cre8 stent at 3-months and a BMS at 1-month.[336336. Stella P. Demonstr8 trial results. Presentation at EuroPCR 2013, Paris, 23 May 2013. Available at http://www. pcronline.
com/]
The first clinical study was the multicentre prospective NEXT trial which randomised 323 patients to Cre8 stent (n = 162) and the TAXUS PES (n = 161).[310310. Carrie D, Berland J, Verheye S, et al. A multicenter randomized trial comparing amphilimus- with paclitaxel-eluting stents in de novo native coronary artery lesions. J Am Coll Cardiol. 2012;59(15):1371-1376. ] The study achieved its primary endpoint by demonstrating non-inferiority of the Cre8 stent with regards to in-stent late loss at 6-months (Cre8 0.14±0.36mm vs. PES 0.34±0.40mm, Pnon-inferiority < 0.0001, Psuperiority < 0.0001). The occurrence of MACE (cardiac death, all MI, all TLR) was similar in both groups (8.3% vs. 10.1%, p=0.59)
The multi-centre, all-comer’s pARTicip8 registry enrolled 1186 patients treated with Cre8 stent and reported low rates of ST, device-oriented MACE and TLR at 1-year follow-up in the entire population, and a pre-specified diabetic sub-group. The latter group also underwent 6-month angiographic follow-up, with a reported late lumen loss of 0.16mm. Specific assessment of the Cre8 stent’s performance in diabetic patient took place in the RESERVOIR study, which randomised 112 patients to the Cre8 stent (n=56) or EES (n=56). The study met its primary endpoint of %neointimal volume obstruction as assessed by OCT at 9-month follow up (Cre8 11.97+/- 5.94% vs. EES 16.11 +/- 18.18%, Pnon-inferiority=0.0003, Psuperority=0.22).[337337. Romaguera R, Gomez-Hospital JA, Gomez-Lara J, et al. A Randomized Comparison of Reservoir-Based Polymer-Free Amphilimus-Eluting Stents Versus Everolimus-Eluting Stents With Durable Polymer in Patients With Diabetes Mellitus: The RESERVOIR Clinical Trial. JACC Cardiovasc Interv. 2016;9(1):42-50. ]
The ASTUTE registry has suggested that the Cre8 stent can be used safely in patients needing a short duration of DAPT (<3 months).[338338. Godino C, Chiarito M, Donahue M, et al. Midterm and one-year outcome of amphilimus polymer free drug eluting stent in patients needing short dual antiplatelet therapy. Insight from the ASTUTE registry (AmphilimuS iTalian mUlticenTer rEgistry). Int J Cardiol. 2017;231:54-60. ] The study reported comparable rates of 1-year TVF between the 106 patients receiving <3 months DAPT (5.7%) and the 1102 patients receiving >6 months (5.1%). BARC defined major bleeding was significantly higher in the short DAPT group (3.4% vs. 0.2%, p=0.007), however this difference was not observed following a landmark analysis at 90-days (0% vs. 0.3%).
Further trials of the Cre8 stent are on-going and include the EFFICACY study in diabetic patients, and the ReCre8 study[339339. Rozemeijer R, Stein M, Frambach P, et al. Rationale and design of amphilimus sirolimus-eluting stents versus zotarolimus-eluting stents in all-comers requiring percutaneous coronary intervention (ReCre8): A multicenter randomized clinical trial. Catheter Cardiovasc Interv. 2017. ] which will randomise 1530 all-comers patients to Cre8 stent or R-ZES with DAPT given for 1-month (stable angina) or 12-months (ACS).
FOCUS np DES system
The FOCUS np DES system (Envision Scientific, Surat, India) is a DES using nanotechnology.[312312. Seth A. Nanoparticle based stents - FOCUS np (Envision Scientific) Program Update. Presentation at Transcather Cardiovascular Interventions, Miami FL, October 23rd 2012. ] The potential benefits of using this concept on a DES is to enhance long-term safety, improve stent efficacy, and reduce the long-term requirement for DAPT.[312312. Seth A. Nanoparticle based stents - FOCUS np (Envision Scientific) Program Update. Presentation at Transcather Cardiovascular Interventions, Miami FL, October 23rd 2012. ] These stents have a standard stent platform, with a nanomatrix coating, made up of nano-particles of an anti-proliferative drug combined with one or more stabilising excipients; this matrix functions as a substitute for the polymer. There are many advantages of using nanoparticles for drug-delivery: (i) they enable controlled and reproducible drug release kinetics; (ii) they increase drug stability in vivo because of the encapsulation process; (iii) they are able to penetrate deeper into the vessel wall thereby improving efficacy; (iv) they facilitate rapid release of drug into the tissue, with a drug depot or reservoir effect, which enables the stent to become drug-free in a short period of time, leading to more rapid healing; (v) they allow increased intra-cellular uptake of drug, with a prolonged residence time at site; (vi) they permit a lower overall drug dosage; and (viii) they increase bio-availability at the target site.
The FOCUS np polymer-free stent has a cobalt chromium stent platform, and is coated with nano-particles containing sirolimus within two excipients, which were selected to create a two phase programmed drug release. The first phase is a burst release from the top layer of nano-particles occurring within 60 seconds of stent deployment; whilst the second phase is a programmed release from the bottom layer. Drug delivery is completed in 28-days. Uniquely the stent balloon shoulders are also coated in the nanomatrix to allow delivery of drug at edges of the stent to avoid edge restenosis. Following successful pre-clinical studies,[340340. Takimura CK, Galon MZ, Gutierrez PS, et al. A new polymer-free drug-eluting stent with nanocarriers eluting sirolimus from stent-plus-balloon compared with bare-metal stent and with biolimus A9 eluting stent in porcine coronary arteries. Cardiovascular diagnosis and therapy. 2015;5(2):113-121. ] the Nano ActiveFIM- IN study has completed enrolment of 83 patients divided in cohort A (n= 55, simple lesions) and B (n=28, real world scenario). Data from 6-month and 1-year follow-up are expected.
Drug-filled stent
These novel polymer-free DES have 81mm struts made from a tri-layer wire:
- The outer layer is made of a cobalt alloy for radial strength
- The middle layer is made of tantalum for radio-opacity.
- The core material of the inner layer of the wire is removed to produce hollow struts which function as the reservoir for sirolimus that is present at a dose of 1.1 ug/mm2.
Sirolimus is released through an average of six laser-drilled holes on the abluminal side of each stent, each with a minimal bore diameter of 20μm (∼1,800 holes for an 18 mm stent) Drug elution commences on stent deployment and is controlled and sustained through natural diffusions via direct interaction with the vessel wall. Pre-clinical data show 68% and 93% of the sirolimus is released by 28- and 90-days respectively, with histology confirming that this drug release is effective at suppressing neointimal hyperplasia compared to BMS controls (P<0.001), with minimal inflammation. The FIM RevElution clinical trial is currently being conducted, and 9-month results from the first cohort of 50 patients are encouraging with a late loss of 0.26mm which is non-inferior to R-ZES historical controls (Pnon-inferior<0.001), a 0% binary restenosis rate, and signs of rapid early healing with >98% stent strut coverage and 0% late incomplete malapposition.[313313. Worthley SG, Abizaid A, Kirtane AJ, et al. First-in-Human Evaluation of a Novel Polymer-Free Drug-Filled Stent: Angiographic, IVUS, OCT, and Clinical Outcomes From the RevElution Study. JACC Cardiovasc Interv. 2017;10(2):147-156. ] At 12-months clinical follow-up, two patients had had experienced a cardiovascular event leading to a target vessel non-Q-wave MI, and a TLR; no ST was seen.[341341. Worthley S. RevElution: 12-months clinical outcomes with a novel polymer-free drug-filled stent. Presentation at EuroPCR 2017, Paris. 2017. ]
DES with durable polymers vs. biodegradable polymer vs. polymer free
The ISAR-TEST 3 trial is currently the only comparison of three stents with different types of polymer and the same anti-proliferative drug - sirolimus.[323323. Mehilli J, Byrne RA, Wieczorek A, et al. Randomized trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis. Eur Heart J. 2008;29(16):1975-1982. , 324324. Byrne RA, Kufner S, Tiroch K, et al. Randomised trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis: 2-year follow-up results. Heart. 2009;95(18):1489-1494. ] This non-inferiority study randomized 605 patients to SES with either a durable polymer (202 patients), a biodegradable polymer (202 patients), or a stent that was polymer free (201 patients). At 6-8 months angiographic follow-up the biodegradable polymer stent met its pre-specified criterion for non-inferiority in terms of in-stent late lumen loss (0.23 mm vs. durable polymer 0.17 mm, Pnon-inferiority < 0.001); whilst the polymer free stent failed to achieve non-inferiority (0.47 mm vs. 0.17 mm, Pnon-inferiority = 0.94) ( Figure 20). Despite these results, clinical outcomes at 1-year demonstrated a similar safety profile for the three stents; however, efficacy appeared numerically inferior with the polymer free stent, and comparable between the biodegradable and durable polymer stents. At 2-years follow-up, clinical outcomes remained comparable in terms of rates of mortality, MI, and ST. The rate of TLR was also comparable between all three stents, however the absolute increase in TLR between 1- and 2-year follow-up was notably higher with the biodegradable polymer and durable polymer stents, when compared to the polymer free stent (∆2.5% vs. ∆2.5% vs. 0.5%). Paired angiographic follow-up was available in 69% of patients, and demonstrated a delayed in-stent late lumen loss of 0.17 mm, 0.16 mm and -0.01 mm for biodegradable polymer, durable polymer and polymer-free stents, respectively (p < 0.001). Importantly, these results indicate that not only are biodegradable polymer stents still susceptible to the delayed restenosis observed previously with durable polymer stents,[120120. Raber L, Wohlwend L, Wigger M, et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization LATE trial. Circulation. 2011;123(24):2819-2828, 2816 p following 2828. , 147147. Claessen BE, Beijk MA, Legrand V, et al. Two-Year Clinical, Angiographic, and Intravascular Ultrasound Follow-Up of the XIENCE V Everolimus-Eluting Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions: The SPIRIT II Trial. Circ Cardiovasc Intervent. 2009;2(4):339-347. , 325325. Aoki J, Abizaid AC, Ong AT, et al. Serial assessment of tissue growth inside and outside the stent after implantation of drug-eluting stent in clinical trials. - Does delayed neointimal growth exist?. EuroIntervention. 2005;1(3):235-255. ] but they also indicate that polymer free stents are potentially less prone to this unwanted long-term phenomenon. This observation is consistent with that previously reported by Byrne et al, and warrants additional investigation.[320320. Goldstein JA, Demetriou D, Grines CL, et al. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343(13):915-922. ]
Dual drug-eluting polymer-free stent vs. durable polymer SES vs. durable polymer ZES
The failure of polymer-free stents to demonstrate non-inferiority compared to durable polymer stents in the ISAR-TEST 3 study prompted interest in dual drug-eluting polymer-free (Dual-PF) stents. This approach, which aims at improving the anti-restenotic performance of polymer-free stents through the use of a second anti-proliferative agent which targets a different part of the cell-cycle, was evaluated in the ISAR-TEST 2 and ISAR-TEST 5 study.[208208. Massberg S, Byrne RA, Kastrati A, et al. Polymer-Free Sirolimus- and Probucol-Eluting Versus New Generation Zotarolimus-Eluting Stents in Coronary Artery Disease: The Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus- and Probucol-Eluting Versus Zotarolimus-Eluting Stents (ISAR-TEST 5) Trial. Circulation. 2011;124(5):624-632. , 321321. Byrne RA, Mehilli J, Iijima R, et al. A polymer-free dual drug-eluting stent in patients with coronary artery disease: a randomized trial vs. polymer-based drug-eluting stents. Eur Heart J. 2009;30(8):923-931. , 322322. Byrne RA, Kastrati A, Tiroch K, et al. 2-year clinical and angiographic outcomes from a randomized trial of polymer-free dual drug-eluting stents versus polymer-based Cypher and Endeavor [corrected] drug-eluting stents. J Am Coll Cardiol. 2010;55(23):2536-2543. ]
The ISAR-TEST 2 study randomized 1007 patients to treatment with SES (n = 335), ZES (n = 339) or a Dual-PF stent (n = 333), that eluted sirolimus and the anti-oxidant probucol which has previously been shown to reduce neointimal hyperplasia.[342342. Tardif JC, Cote G, Lesperance J, et al. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. N Engl J Med. 1997;337(6):365-372. ] The rate of the primary endpoint of binary restenosis at 6-8months follow-up was Dual-PF 11.0%, ZES 19.3% (p < 0.001 vs. Dual-PF), and SES 12.0% (p = 0.68 vs. Dual PF). Clinical outcomes at 1-year follow-up demonstrated comparable safety in terms of mortality, MI and ST between the three stents, however rates of TLR were significantly lower with the Dual-PF compared to ZES (Dual-PF 6.8% vs. ZES 13.6%, p = 0.001), and comparable with SES (Dual-PF 6.8% vs. SES 7.2%, p = 0.83).
At 2-years follow-up, safety clinical outcomes remained comparable amongst the three groups.[322322. Byrne RA, Kastrati A, Tiroch K, et al. 2-year clinical and angiographic outcomes from a randomized trial of polymer-free dual drug-eluting stents versus polymer-based Cypher and Endeavor [corrected] drug-eluting stents. J Am Coll Cardiol. 2010;55(23):2536-2543. ] Similar to the 1-year results, rates of TLR were significantly lower with Dual-PF compared to ZES (p = 0.006), and comparable between Dual-PF and SES. Moreover, as seen in the ISAR-TEST 3, the absolute increase in TLR between 1- and 2-year follow-up was notably higher with the durable polymer SES compared to the Dual-PF SES (∆3.5% vs. ∆0.9%, p = 0.009). Likewise paired angiographic follow-up demonstrated a significantly greater increase in in-stent binary restenosis with the durable SES compared to the Dual-PF SES (∆6.6% vs. ∆2.9%, p = 0.002). Overall this study demonstrated that Dual-PF DES offer a reduction in delayed restenosis compared to 1st generation DES, whilst maintaining a comparable safety profile. Importantly this reduction in delayed restenosis with the polymer free stent, is consistent with other studies such as ISAR-TEST, and ISAR-TEST 3, [320320. Goldstein JA, Demetriou D, Grines CL, et al. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343(13):915-922. , 323323. Mehilli J, Byrne RA, Wieczorek A, et al. Randomized trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis. Eur Heart J. 2008;29(16):1975-1982. , 324324. Byrne RA, Kufner S, Tiroch K, et al. Randomised trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis: 2-year follow-up results. Heart. 2009;95(18):1489-1494. ] suggesting these stents may hold promise for the future.
Similarly, and as described earlier, the ISAR-TEST 5 study also demonstrated that the polymer-free sirolimus and probucol-eluting stent was non-inferior to R-ZES out to 12-months.[208208. Massberg S, Byrne RA, Kastrati A, et al. Polymer-Free Sirolimus- and Probucol-Eluting Versus New Generation Zotarolimus-Eluting Stents in Coronary Artery Disease: The Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus- and Probucol-Eluting Versus Zotarolimus-Eluting Stents (ISAR-TEST 5) Trial. Circulation. 2011;124(5):624-632. ]
Polymer-free stents
- Polymer-free stents have been developed in attempt to further improve the safety of DES by eliminating the polymer for drug release.
- At short-term follow-up late-loss values for polymer-free stents appear to be inferior to those observed for similar stents with durable or biodegradable polymers.
- Delayed late loss however appears to be somewhat less with polymer-free stents particularly when combined with dual drug release (sirolimus and probucol).
- Observational data indicate favourable safety and efficacy at 1-year follow-up of the BES-PF in all-comers patients.
- Dual-drug eluting-stents appear to provide non-inferior outcomes to permanent polymer stents, however clinical evaluation continues.
NOVEL STENT COATINGS
The physical properties of these stents with novel coatings, together with angiographic follow-up results from FIM studies or randomized trials are summarized in Table 14.[343343. Tamburino C, La Manna A, Di Salvo ME, et al. First-in-man 1-year clinical outcomes of the Catania Coronary Stent System with Nanothin Polyzene-F in de novo native coronary artery lesions: the ATLANTA (Assessment of The LAtest Non-Thrombogenic Angioplasty stent) trial. JACC Cardiovasc Interv. 2009;2(3):197-204. , 344344. Cutlip DE, Garratt KN, Novack V, et al. 9-Month Clinical and Angiographic Outcomes of the COBRA Polyzene-F NanoCoated Coronary Stent System. JACC Cardiovasc Interv. 2017;10(2):160-167. , 345345. Windecker S, Simon R, Lins M, et al. Randomized Comparison of a Titanium-Nitride-Oxide-Coated Stent With a Stainless Steel Stent for Coronary Revascularization: The TiNOX Trial. Circulation. 2005;111(20):2617-2622. , 346346. Pilgrim T, Raber L, Limacher A, et al. Comparison of titanium-nitride-oxide-coated stents with zotarolimus-eluting stents for coronary revascularization a randomized controlled trial. JACC Cardiovasc Interv. 2011;4(6):672-682. , 347347. Beijk MA, Klomp M, Verouden N, et al. Genous Endothelial progenitor cell capturing stent versus the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomised, single-centre, pilot study. Eur Heart J. 2009;31(9):1055-1064. ]
Polyzene F Coated stents (CeloNova BioSciences, Tx, USA)
Polyzene F is a biocompatible, biostatic, proprietary formulation of poly[bis(trifluoro-ethoxy)phosphazene], which has anti-inflammatory, bacteria-resistant and pro-healing qualities. Its application onto a stent was therefore proposed as a method of creating very low surface thrombogenecity, thereby potentially reducing the risk of ST. Pre-clinical studies have demonstrated reduced inflammation, neointimal hyperplasia, and thrombogenicity with stents coated with Polyzene F compared to uncoated stents.[348348. Koppara T, Sakakura K, Pacheco E, et al. Preclinical evaluation of a novel polyphosphazene surface modified stent. Int J Cardiol. 2016;222:217-225. ] Subsequently two commercial stents have been developed by applying a 40nm thick layer of Polyzene F onto the surface of cobalt chromium struts creating the Catania stent (CeloNova BioSciences, Tx, US), and its newer iteration the Cobra stent (CeloNova BioSciences, Tx, US).
The FIM ATLANTA study reported a 6-month late lumen loss of 0.60±0.48mm, whilst at 12-months follow-up there were no reported deaths or MI, and a clinically driven TLR rate of 3.6% in the 55 patients treated with the Catania stent.[343343. Tamburino C, La Manna A, Di Salvo ME, et al. First-in-man 1-year clinical outcomes of the Catania Coronary Stent System with Nanothin Polyzene-F in de novo native coronary artery lesions: the ATLANTA (Assessment of The LAtest Non-Thrombogenic Angioplasty stent) trial. JACC Cardiovasc Interv. 2009;2(3):197-204. ] No ST was observed, despite DAPT being given for only 30-days. In addition OCT, which was performed in 15 patients, showed that 99.5% of struts were fully covered at 6-months.[349349. La Manna A, Capodanno D, Cera M, et al. Optical coherence tomographic results at six-month follow-up evaluation of the CATANIA coronary stent system with nanothin Polyzene-F surface modification (from the Assessment of The LAtest Non-Thrombogenic Angioplasty Stent [ATLANTA] trial). Am J Cardiol. 2009;103(11):1551-1555. ] Registry data have also demonstrated the absence of ST events at 6-months follow-up amongst 94 patients with ACS who were treated with the Catania stent, and received only 30-days of DAPT.[350350. La Manna A, Sanfilippo A, Di Salvo ME, et al. Short and Mid-Term Benefits of CATANIA Stent in Acute Coronary Syndromes [abstract]. Am J Cardiol. 2009;104 (6):111D. ] A broader, more real world cohort were enrolled in the follow-up ATLANTA-II registry, which enrolled 300 patients, 14% of whom presented with ST-elevation MI. At 1-year follow-up, the cumulative rate of MACE was 8.8%, with individual rates of cardiac death, MI and TLR of 2.5%, 0.7% and 6.5%, respectively. DAPT was again given for only 30-days, and the rate of ST was 0.7% due to two cases of sub-acute ST.[351351. Tamburino C, Capodanno D, Di Salvo ME, et al. Safety and effectiveness of the Catania Polyzene-F coated stent in real world clinical practice: 12-month results from the ATLANTA 2 registry. EuroIntervention. 2012;7(9):1062-1068. ]
The Cobra stent has been evaluated in the non-randomised 296 patient PzF SHIELD study,[344344. Cutlip DE, Garratt KN, Novack V, et al. 9-Month Clinical and Angiographic Outcomes of the COBRA Polyzene-F NanoCoated Coronary Stent System. JACC Cardiovasc Interv. 2017;10(2):160-167. ] whose favourable results enabled the device to receive FDA approval in March 2017. The study achieved its primary endpoint with a rate of TVF, a composite of cardiac death, MI and clinically driven TLR at 9-months follow-up, of 11.5% which met the pre-specified performance goal of 19.62%, that had been derived from historical meta-analyses of BMS. The study also met its powered secondary endpoint with a late lumen loss of 0.84mm, compared with the performance goal of 1.1mm). There were no ST events. Further studies are on-going including the all-comers e-COBRA registry and the COBRA Reduce trial, which will assess the stent’s performance in patients, treated with oral anti-coagulation, who receive only 14-days of DAPT post PCI.
Titanium-nitride oxide coated stents (Hexacath, Rueil-Malmaison, France)
Titanium-nitride oxide ( Figure 21), has been shown to inhibit platelet aggregation, minimize fibrin deposition, reduce inflammation, and promote healing, and consequently has been utilised as a coating on the three iterations of the TiTAN coronary stent (Hexacath, France). The first two versions (TiTAN and TiTAN-2) had a platform of stainless steel, whilst the newest iteration, the TiTAN Optimax, uses cobalt chromium, which offers greater radio-opacity and enables the struts to be 20% thinner. The TiNOX study randomized 92 patients to treatment with either a BMS, or a BMS coated with titanium-nitride oxide and reported a significant reduction in late loss (0.55 ± 0.63 mm vs. 0.90 ± 0.76 mm, p = 0.03) at 6-months follow-up. Clinical evaluation demonstrated significantly reduced MACE, which was driven primarily by a reduction in TLR, with the titanium-coated stent at 6-months follow-up,[345345. Windecker S, Simon R, Lins M, et al. Randomized Comparison of a Titanium-Nitride-Oxide-Coated Stent With a Stainless Steel Stent for Coronary Revascularization: The TiNOX Trial. Circulation. 2005;111(20):2617-2622. ] with results indicating preservation of this out to 5-year follow-up.[352352. Moschovitis A, Simon R, Seidenstucker A, et al. Randomised comparison of titanium-nitride-oxide coated stents with bare metal stents: five year follow-up of the TiNOX trial. EuroIntervention. 2010;6:63-68. ]
Additional studies include the TiTAX-AMI trial, which randomized 425 patients with ST-elevation MI to treatment with either the TiTAN stent or the TAXUS PES. At 12-months there were no significant differences in the primary endpoint (p=0.5), however the TiTAN stent had significantly lower rates of ST (0.9% vs. 4.3%, p=0.03).[353353. Karjalainen PP, Ylitalo A, Niemela M, et al. Titanium-nitride-oxide coated stents versus paclitaxel-eluting stents in acute myocardial infarction: a 12-months follow-up report from the TITAX AMI trial. EuroIntervention. 2008;4(2):234-241. ] At 5-years, use of the TiTAN stent lead to a significantly lower incidence of MACE, cardiac death, re-infarction, and ST. Furthermore, despite the absence of an anti-proliferative drug, the rate of TLR was comparable.[354354. Tuomainen PO, Ylitalo A, Niemela M, et al. Five-year clinical outcome of titanium-nitride-oxide-coated bioactive stents versus paclitaxel-eluting stents in patients with acute myocardial infarction: long-term follow-up from the TITAX AMI trial. Int J Cardiol. 2013;168(2):1214-1219. ]
In contrast, the TiTAN-2 stent failed to demonstrate non-inferiority when compared to the E-ZES in the randomized 300 patient TIDE study.[346346. Pilgrim T, Raber L, Limacher A, et al. Comparison of titanium-nitride-oxide-coated stents with zotarolimus-eluting stents for coronary revascularization a randomized controlled trial. JACC Cardiovasc Interv. 2011;4(6):672-682. ] At 6-months angiographic follow-up, in-stent late lumen loss was 0.64 ± 0.61 mm and 0.47 ± 0.48 mm for the TiTAN-2 stent and E-ZES, respectively (Pnon-inferiority = 0.54). Of note, differences in late lumen loss were more pronounced in patients with diabetes, small vessel disease and patients over 65. Clinical outcomes assessed up to five years of follow-up were comparable. The majority of events occurred within the first year after PCI and were mostly related to clinically-indicated TVR.[355355. Pilgrim T, Raber L, Limacher A, et al. Five-year results of a randomised comparison of titanium-nitride-oxide-coated stents with zotarolimus-eluting stents for coronary revascularisation. EuroIntervention. 2015. ]
Further assessment took place in the BASE-ACS trial, which enrolled 827 patients with ACS who were randomised to either the TiTAN-2 stent (n=417) or EES (n=410).[356356. Karjalainen PP, Niemela M, Airaksinen JK, et al. A prospective randomised comparison of titanium-nitride-oxide-coated bioactive stents with everolimus-eluting stents in acute coronary syndrome: the BASE-ACS trial. EuroIntervention. 2012;8(3):306-315. ] At 1-year the study met its primary non-inferior endpoint of MACE, a composite of cardiac death, non-fatal MI or ischaemia-driven TLR (9.6% vs. EES 9.0%, Pnon-inferiority <0.001). A 5-years follow-up the TiTAN-2 stent remained non-inferior in terms of MACE; whilst its use was associated with significantly lower rates of non-fatal MI (5.9% vs. 9.7%, p=0.03), and comparable rates of cardiac death and TLR.[357357. Karjalainen PP, Nammas W, Ylitalo A, et al. Long-term clinical outcome of titanium-nitride-oxide-coated stents versus everolimus-eluting stents in acute coronary syndrome: Final report of the BASE ACS trial. Int J Cardiol. 2016;222:275-280. ]
A more contemporary study in ACS patients is the TIDES ACS trial, which randomised 1500 ACS patients in a 2:1 ratio to the TiTAN Optimax (n=1000) or Synergy EES (n=500).[358358. Kervinen K. Comparison of the Titanium-Nitride-Oxide coated Bio Active-Stent (Optimax) to the Synergy DES in ACS (TIDES-ACS Trial). Presentation at EuroPCR 2017, Paris. 2017. ] The study’s main hypotheses are that the Optimax will be non-inferior to Synergy for MACE at 12-months follow-up and superior for hard endpoints (cardiac death, MI and major bleeding) at 18-months follow up. Preliminary results from the first 1451 patients who have reached 6-months follow-up show comparable rates of MACE (4.6% vs. 4.5%, p=0.94). Full results are expect in 2018.
Genous™ Bio-engineered R-stent™ (OrbusNeich, Fort Lauderdale, FL, USA)
This bare metal stainless steel stent is unique by containing on its luminal surface immobile CD34 antibodies ( Figure 22). In pre-clinical and clinical studies these antibodies are able to bind to endothelial progenitor cells (EPC), resulting in a rapidly formed, functional endothelial covering of the stent’s struts,[359359. Larsen K, Cheng C, Tempel D, et al. Capture of circulatory endothelial progenitor cells and accelerated re-endothelialization of a bio-engineered stent in human ex vivo shunt and rabbit denudation model. Eur Heart J. 2012;33(1):120-128. ] which ultimately has the potential to reduce ST and restenosis. Unfortunately, the CD34+ markers that are used to phenotype EPCs are non-specific, and are shared by other hematopoietic stem cells. Therefore, it is possible for the EPC capture stent to sequester other bone marrow cell lines such as smooth muscle progenitor cells, which in turn can lead to neointimal proliferation.[360360. Inoue T, Sata M, Hikichi Y, et al. Mobilization of CD34-positive bone marrow-derived cells after coronary stent implantation: impact on restenosis. Circulation. 2007;115(5):553-561. , 361361. Garg S, Duckers HJ, Serruys PW. Endothelial progenitor cell capture stents: will this technology find its niche in contemporary practice?. Eur Heart J. 2010;31(9):1032-1035. ] This is reflected in published clinical studies that have shown low rates of ST despite only one month of DAPT, however late-loss at 6-month follow-up has repeated been above 0.6mm.[362362. Aoki J, Serruys PW, van Beusekom H, et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) Registry. J Am Coll Cardiol. 2005;45(10):1574-1579. , 363363. Duckers HJ, Silber S, de Winter R, et al. Circulating endothelial progenitor cells predict angiographic and intravascular ultrasound outcome following percutaneous coronary interventions in the HEALING-II trial: evaluation of an endothelial progenitor cell capturing stent. EuroIntervention. 2007;3(1):67-75. , 364364. den Dekker WK, Houtgraaf JH, Onuma Y, et al. Final results of the HEALING IIB trial to evaluate a bio-engineered CD34 antibody coated stent (GenousStent) designed to promote vascular healing by capture of circulating endothelial progenitor cells in CAD patients. Atherosclerosis. 2011;219(1):245-252. ] Data from the TRIAS HR study, which is the only randomized trial published so far, reported a late loss as high as 1.14±0.64 mm, and an overall higher target vessel failure with the Genous stent compared to the TAXUS PES.[347347. Beijk MA, Klomp M, Verouden N, et al. Genous Endothelial progenitor cell capturing stent versus the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomised, single-centre, pilot study. Eur Heart J. 2009;31(9):1055-1064. ] Encouragingly, preliminary data at two-year follow-up demonstrated a lower absolute increase in TLR between 1 and 2 years in those treated with EPC stent compared to PES.[365365. Beijk M. Two year follow-up of the endothelial progenitor cell capturing stent versus a Paclitaxel-eluting stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: the single-centre randomised TRIAS study. Presented at EuroPCR 2009 19th-22nd May 2009. ] This may reflect regression of late loss with the EPC stent, as was previously observed in the HEALING II study where late loss fell by 16.9% between 6- and 18-months, and/or late catch-up with PES.[325325. Aoki J, Abizaid AC, Ong AT, et al. Serial assessment of tissue growth inside and outside the stent after implantation of drug-eluting stent in clinical trials. - Does delayed neointimal growth exist?. EuroIntervention. 2005;1(3):235-255. , 363363. Duckers HJ, Silber S, de Winter R, et al. Circulating endothelial progenitor cells predict angiographic and intravascular ultrasound outcome following percutaneous coronary interventions in the HEALING-II trial: evaluation of an endothelial progenitor cell capturing stent. EuroIntervention. 2007;3(1):67-75. ] Additional data comes from the 5000 patients enrolled in e-HEALING registry, which reported rates of MACE, MI and ST at 1-year follow-up of 7.7%, 1.7% and 1.0% respectively.[366366. Silber S, Damman P, Klomp M, et al. Clinical results after coronary stenting with the Genous Bio-engineered R stent: 12-month outcomes of the e-HEALING (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth) worldwide registry. EuroIntervention. 2011;6(7):819-825. ]
A new application of the EPC capture technology has been to combine it with DES technology in a Combo Stent, as described previously.
Stents with novel coatings
Bare metal stents with novel coatings such as polyzene F (Catania and Cobra stent), titanium oxide (TiTAN stent) and endothelial progenitor cell receptors (Genous stent) have been developed to improve stent endothelialisation and reduce the risk of ST. Clinical evaluation of these stents continues, however they have been associated with higher late loss and reduced clinical efficacy compared with conventional DES.
Safety and efficacy of coronary artery stents
IN-HOSPITAL OUTCOME
The advent of coronary artery stents has transformed PCI into a safe and reproducible revascularization procedure. The most important benefit of stents was the effective treatment of abrupt or threatened vessel closure following balloon angioplasty. Whereas abrupt closure in the balloon angioplasty era was associated with an increased risk of death (5%), MI (40%) and need of emergency CABG (40%), Schömig et al. reported a significant reduction of these adverse events with the use of stents (death: 1.3%, MI: 4.0%, emergency coronary artery bypass grafting: 1.0%) ( Figure 25). The ACC/National Cardiovascular Data Registry analyzed data from 558,273 percutaneous coronary interventions performed between 2001 and 2004 and observed an in-hospital mortality of 0.7%, a rate of MI of 1.1%, and the need of emergency CABG in 0.6% of patients. Mortality is not only related to the procedure but also the clinical condition of the patient at baseline. Accordingly, several clinical and angiographic variables have been identified which are associated with an increased mortality risk including advanced age, female gender, diabetes, renal dysfunction, cardiogenic shock, a large area of myocardium at risk, impaired left ventricular function and an urgent procedure. In addition, PCI performed in the setting of STEMI (primary PCI) is associated with a higher death rate than is observed among patients undergoing elective PCI. The risk of stroke is low ranging from 0.1% to 0.3% in the ACC/NCDR registry during the in-hospital period.
PERI-PROCEDURAL MYOCARDIAL INJURY
Balloon inflation during a procedure almost always results in ischemia even if not accompanied by ST-T wave changes. The aetiology of peri-procedural myonecrosis is multifactorial and includes among others side branch occlusion and distal embolisation. Any increase in CK-MB after PCI is associated with a small, but statistically and clinically significant increase in the subsequent risk of death.11 An analysis of 6,755 SES treated patients demonstrated that irrespective of lesion complexity, mortality at 6 month follow-up was highest among those who incurred a peri-procedural MI (4.3%), followed by those who developed any troponin elevation (2.5%) and was significantly lower among those who remained free of these events (1.3%). In a subgroup analysis of the TAXUS V trial among patients requiring multiple overlapping stents13, PES use was associated with an increased incidence of MI at 30 days (8.3% vs. 3.3%; P = 0.047) compared with the otherwise identical BMS, most of which were non–Q-wave infarctions. The rate of MI was numerically increased with both planned (6.3% vs. 2.9%) and unplanned (14.0% vs. 4.2%) use of multiple PES. Blinded core laboratory angiographic analysis in the multiple stent cohort demonstrated more frequent occurrence of progressive side-branch narrowing to more than 70% diameter stenosis or to total occlusion with PES (42.6% vs. 30.6%; P = 0.03) than BMS and a greater likelihood of reduced side branch TIMI flow (41.9% vs. 28.6%; P = 0.02). No differences were present in main vessel TIMI flow, acute occlusion, ST, distal embolization, or other angiographic complications compared to the BMS counter-part. Reductions in rates of clinical and angiographic restenosis were present at 9 months in patients who received multiple stents and who had been assigned to PES rather than BMS, with similar rates of cardiac death, MI, and ST.
In the ENDEAVOR IV Trial14, ZES resulted in a significantly lower rate of MI at 30 days compared to PES (0.8% vs. 2.3%; P = 0.02). A subgroup analysis, which focused specifically on the incidence of side branch occlusion immediately after stent implantation as a possible explanation for the higher rates of post-procedural MI, demonstrated a significantly higher rate of side branch occlusion with PES than ZES (4.0% vs. 2.2%; P = 0.03). Similar findings were evidenced in the SPIRIT III trial15 comparing EES with PES: at 30 days there tended to be fewer MI among the patients randomized to receive EES compared with PES (7/667 patients [1.0%] vs. 9/330 [2.7%], respectively; relative risk, 0.38 [95% CI, 0.14 to 1.02]; P=0.06), with comparable rates of cardiac death (0% in both groups) and TLR (3/667 patients [0.4%] vs. 1/330 [0.3%], respectively; relative risk, 1.48 [95% CI, 0.15 to 14.21]; P>0.99). These results underline the central role of the distinct mechanical features of each individual device on clinical outcome. Newer generation DES appear to be associated with a lower rate of peri-procedural myonecrosis most likely related to improved stent design with less side branch compromise.
LONG-TERM MORTALITY AND MYOCARDIAL INFARCTION
In balancing the risks and benefits of PCI with the use of different technologies from a patient’s perspective, the assessment of safety end points such as overall mortality and MI are most important. A meta-analysis of 29 trials in 9,918 patients comparing balloon angioplasty with BMS observed a similar risk of death and MI during follow-up (5.4% versus 4.8%, OR=0.9, CI 0.72-1.11). After their extensive use, concerns were raised that improved efficacy with early generation DES was offset by a higher rate of very late adverse events such as ST,[419419. Nordmann AJ, Briel M, Bucher HC. Mortality in randomized controlled trials comparing drug-eluting vs. bare metal stents in coronary artery disease: a meta-analysis. Eur Heart J. 2006;27(23):2784-2814. , 420420. Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115(11):1440-1455; discussion 1455. , 421421. Pfisterer M, Brunner-La Rocca HP, Buser PT, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48(12):2584-2591. ] resulting in the need for long-term DAPT.[420420. Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115(11):1440-1455; discussion 1455. ] In the aftermath of these studies, which caused widespread concern, several patient based meta-analysis were performed which reassuringly demonstrated the overall comparable outcomes between DES and BMS in terms of death and MI, at both short and long term follow-up ( Table 4). The largest of these studies by Stettler et al reported a similar risk of death between patients treated with SES, PES and BMS; the risk of MI although comparable between PES and BMS (p=0.99), was significantly lower with SES compared to BMS (p=0.03).[9090. Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937-948. ] Additional meta-analyses performed at a similar time by Stone et al, Spaulding et al, Kastrati et al, Mauri et al and Kirtane et al all reiterated the safety of DES, by demonstrating the absence of any significant increased risk of death and/or MI with the use of early generation DES compared to BMS. [9090. Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937-948. , 9090. Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937-948. , 9090. Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937-948. , 9090. Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937-948. , 9090. Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937-948. ]
More contemporary meta-analyses have also dispelled the earlier concerns by consistently showing no increased risk of mortality with the use of DES compared to BMS.[144144. Valgimigli M, Sabate M, Kaiser C, et al. Effects of cobalt-chromium everolimus eluting stents or bare metal stent on fatal and non-fatal cardiovascular events: patient level meta-analysis. Bmj. 2014;349:g6427. ], [425425. Kang SH, Park KW, Kang DY, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: a systematic review and Bayesian approach network meta-analysis. Eur Heart J. 2014;35(17):1147-1158. , 426426. Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. Bmj. 2013;347:f6625. , 427427. Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2014;63(4):299-307. , 428428. Sabate M, Raber L, Heg D, et al. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. JACC Cardiovasc Interv. 2014;7(1):55-63. ] A meta-analysis by Kang et al,[425425. Kang SH, Park KW, Kang DY, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: a systematic review and Bayesian approach network meta-analysis. Eur Heart J. 2014;35(17):1147-1158. ] which included 90,584 patients from 113 trials, reported comparable mortality between DES (SES, PES, E-ZES, R-ZES, EES, Promus EES and BES) and BMS. Furthermore, all DES, apart from PES, were shown to significantly reduce the risk of MI compared to BMS with hazard ratios between 0.42 and 0.85. A larger network meta-analysis by Bangalore et al,[426426. Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. Bmj. 2013;347:f6625. ] which included data from 126 trials and 258,544 patient years of follow-up, showed similar performance in terms of comparable all-cause mortality for all DES apart from EES, which was shown to significantly reduce mortality (HR 0.72, 95% CI 0.58-0.90). Rates of MI were significantly lower compared to BMS for all DES apart from PES.
A smaller meta-analysis by Valgimigli et al[144144. Valgimigli M, Sabate M, Kaiser C, et al. Effects of cobalt-chromium everolimus eluting stents or bare metal stent on fatal and non-fatal cardiovascular events: patient level meta-analysis. Bmj. 2014;349:g6427. ] which only included patients receiving EES or BMS in 4896 patients, followed up for 720 days reported findings consistent with the previous mega studies, with significant reductions in cardiovascular mortality (HR 0.67, p=0.01) and MI (HR 0.71, p<0.01) with EES. Amongst patients receiving BES, Palmerini et al[427427. Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2014;63(4):299-307. ] showed significant reductions compared with BMS in cardiac death and MI at 1-year follow-up amongst 85,490 patients, with similar results seen at longer follow-up in over 50,000 patients. In STEMI patients, Sabate et al[428428. Sabate M, Raber L, Heg D, et al. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. JACC Cardiovasc Interv. 2014;7(1):55-63. ] showed no adverse risk of all-cause mortality (HR 0.98, 95% CI 0.60-1.35) with the use of EES or BES compared to BMS at 1-year and a significant reduction in target vessel re-infarction (HR 0.36, 95% CI 0.14-0.91).
RESTENOSIS AND REPEAT REVASCULARIsATION ( Refer to  View chapter )
View chapter )
Restenosis, defined as greater than 50% diameter stenosis at follow-up angiography, was the most important limitation of balloon angioplasty with an incidence of 30-50% and a need for target vessel revascularisation in 20-30% of patients. The mechanism of restenosis following balloon angioplasty is multifactorial, nevertheless roughly two thirds is mediated by pathologic arterial remodelling with shrinkage of the dilated segment, whilst one third is mediated by neointimal proliferation. Coronary artery stents have been found to be beneficial as anti-restenosis devices by eliminating arterial shrinkage due their radial force.
The STRESS[1111. Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. The New England journal of medicine. 1994;331(8):496-501. ] and the BENESTENT[1010. Serruys PW, De Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. New Engl J Med. 1994;331(8):489-495. ] trials compared elective stenting with angioplasty alone in native coronary arteries with short (<15 mm in length) de novo lesions and showed a 25-30% reduction in restenosis rate. Subsequent stent trials confirmed reduced rates of restenosis and TLR in patients undergoing stent implantation of SVG,[429429. Abizaid AS, Mintz GS, Abizaid A, et al. One-year follow-up after intravascular ultrasound assessment of moderate left main coronary artery disease in patients with ambiguous angiograms. J Am Coll Cardiol. 1999;34(3):707-715. ] lesions located in the proximal left anterior descending artery,[430430. Kastrati A, Mehilli J, Dirschinger J, et al. Intracoronary Stenting and Angiographic Results : Strut Thickness Effect on Restenosis Outcome (ISAR-STEREO) Trial. Circulation. 2001;103(23):2816-2821. ] restenotic lesions[431431. Erbel R, Haude M, Hopp HW, et al. Coronary-artery stenting compared with balloon angioplasty for restenosis after initial balloon angioplasty. Restenosis Stent Study Group. The New England journal of medicine. 1998;339(23):1672-1678. ] and patients presenting with acute STEMI.[432432. Grines CL, Cox DA, Stone GW, et al. Coronary Angioplasty with or without Stent Implantation for Acute Myocardial Infarction. The New England journal of medicine. 1999;341(26):1949-1956. ] A meta-analysis of 29 trials in 9,918 patients comparing balloon angioplasty with BMS implantation confirmed a significant reduction in restenosis (odds ratio: 0.52. 95% CI [0.37 to 0.69]) and the need of repeat PCI (odd ratio: 0.59. 95% CI [0.50 to 0.68]).[433433. National Institute for Health and Clinical Excellence. Final appraisal determination: Drug-eluting stents for the treatment of coronary artery disease. 2008. ]
The clinical consequences of restenosis depend on the degree of restenosis, area at risk subtended by the restenosed target lesion, amount of recruitable collaterals, and lesion location. Restenosis impacts on clinical outcome in different ways: first, repeat revascularization procedures influence patient’s quality of life. In the Optimal Angioplasty versus Primary Stenting (OPUS) I trial, a randomized comparison of routine and provisional BMS implantation that prospectively assessed quality of life parameters, patients without restenosis had less frequent angina (17% vs 22%, P = 0.03), fewer limitations in physical activities (21% vs 27%, P < 0.004), and better quality of life (25% vs 21%, P = 0.003).[434434. Weaver WD, Reisman MA, Griffin JJ, et al. Optimum percutaneous transluminal coronary angioplasty compared with routine stent strategy trial (OPUS-1): a randomised trial. Lancet. 2000;355(9222):2199-2203. ] Secondly, restenosis is associated with a risk of MI; several studies report a 2-19% rate of restenosis-related MI.[435435. Walters DL, Harding SA, Walsh CR, et al. Acute coronary syndrome is a common clinical presentation of in-stent restenosis. Am J Cardiol. 2002;89(5):491-494. , 436436. Chen MS, John JM, Chew DP, et al. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006;151(6):1260-1264. , 437437. Doyle B, Rihal CS, O’Sullivan CJ, et al. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation. 2007;116(21):2391-2398. Epub 2007 Nov 2395. ] In addition, the treatment of restenosis itself is also associated with a small but finite procedural risk of mortality or MI.[438438. Stone GW, Ellis SG, Colombo A, et al. Offsetting Impact of Thrombosis and Restenosis on the Occurrence of Death and Myocardial Infarction After Paclitaxel-Eluting and Bare Metal Stent Implantation. Circulation. 2007;115(22):2842-2847. ] Finally, observational studies suggest a potential negative impact of restenosis on prognosis. The outcome of 3,363 patients treated with balloon angioplasty and repeat angiography was analysed according to the presence (1,570 patients) or absence (1,793 patients) of restenosis.[439439. Weintraub WS, Ghazzal ZM, Douglas JS, Jr., et al. Long-term clinical follow-up in patients with angiographic restudy after successful angioplasty. Circulation. 1993;87(3):831-840. ] Although survival at 6 years was similar in both groups (95% vs 93%, P = 0.16), patients with restenosis had an increased rate of MI (15% vs 12%, P = 0.0001). A study of 603 patients with diabetes who underwent balloon angioplasty observed a higher 10-year mortality in patients with, as opposed to without, restenosis (24% without restenosis, 35% with non-occlusive restenosis, and 59% with occlusive restenosis) ( Figure 26). Notably, occlusive restenosis was an independent predictor of mortality in multivariate analysis.[440440. Van Belle E, Ketelers R, Bauters C, et al. Patency of percutaneous transluminal coronary angioplasty sites at 6-month angiographic follow-up: A key determinant of survival in diabetics after coronary balloon angioplasty. Circulation. 2001;103(9):1218-1224. ] A series of 2,272 consecutive patients treated with BMS between 1992 and 1996 reported a mortality rate of 6.0% at 4 years in patients without and 8.8% in patients with restenosis (P = 0.02) ( Figure 27).[441441. Schuhlen H, Kastrati A, Mehilli J, et al. Restenosis detected by routine angiographic follow-up and late mortality after coronary stent placement. Am Heart J. 2004;147(2):317-322. ] Another large analysis including 10,004 patients with 15,004 treated patients found that restenosis at angiographic follow-up was associated with a small but significant increase in the risk of mortality at 4-year follow-up (adjusted HR 1.23, 95%CI 1.03-1.46).[442442. Cassese S, Byrne RA, Schulz S, et al. Prognostic role of restenosis in 10 004 patients undergoing routine control angiography after coronary stenting. Eur Heart J. 2015;36(2):94-99. ] While these data raise the question as whether surveillance angiography should be part of the routine follow-up after PCI, recent randomized evidence does not support a benefit from follow-up angiography in terms of clinical outcomes.[443443. Shiomi H, Morimoto T, Kitaguchi S, et al. The ReACT Trial: Randomized Evaluation of Routine Follow-up Coronary Angiography After Percutaneous Coronary Intervention Trial. JACC Cardiovasc Interv. 2017;10(2):109-117. ]
Numerous randomized clinical trials and meta-analyses attest that DES reduce rates of restenosis and repeat TLR compared with BMS.[66. Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346(23):1773-1780. , 5454. Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315-1323. ], [100100. Stone GW, Ellis SG, Cannon L, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. Jama. 2005;294(10):1215-1223. ], [144144. Valgimigli M, Sabate M, Kaiser C, et al. Effects of cobalt-chromium everolimus eluting stents or bare metal stent on fatal and non-fatal cardiovascular events: patient level meta-analysis. Bmj. 2014;349:g6427. ], [425425. Kang SH, Park KW, Kang DY, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: a systematic review and Bayesian approach network meta-analysis. Eur Heart J. 2014;35(17):1147-1158. , 426426. Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. Bmj. 2013;347:f6625. , 427427. Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2014;63(4):299-307. , 428428. Sabate M, Raber L, Heg D, et al. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. JACC Cardiovasc Interv. 2014;7(1):55-63. ], [444444. Babapulle MN, Joseph L, Belisle P, et al. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet. 2004;364(9434):583-591. , 445445. Serruys PW, Kutryk MJ, Ong AT. Coronary-artery stents. N Engl J Med. 2006;354(5):483-495. ] In a collaborative network meta-analysis of 38 randomized trials involving 18,023 patients the rate of TLR was reduced by 70% with the use of SES and by 58% with the use of PES compared to BMS at 4 years. For the comparison of SES versus BMS, this benefit translated into a number needed to treat to prevent one revascularization event of 7 patients; for PES versus BMS the figure was 8 patients. More contemporary meta-analyses show a consistent marked reduction in TLR with DES compared to BMS.[144144. Valgimigli M, Sabate M, Kaiser C, et al. Effects of cobalt-chromium everolimus eluting stents or bare metal stent on fatal and non-fatal cardiovascular events: patient level meta-analysis. Bmj. 2014;349:g6427. ], [425425. Kang SH, Park KW, Kang DY, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: a systematic review and Bayesian approach network meta-analysis. Eur Heart J. 2014;35(17):1147-1158. , 426426. Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. Bmj. 2013;347:f6625. , 427427. Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2014;63(4):299-307. , 428428. Sabate M, Raber L, Heg D, et al. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. JACC Cardiovasc Interv. 2014;7(1):55-63. ] Kang et al reported reductions in TLR of 81% with BES versus BMS (HR 0.19, 95% CI 0.12-0.29) and 80% with EES versus BMS (HR 0.20, 95% CI 0.14-0.28) at 1-year.[425425. Kang SH, Park KW, Kang DY, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: a systematic review and Bayesian approach network meta-analysis. Eur Heart J. 2014;35(17):1147-1158. ] Bangalore et al reported slightly lower reductions in TLR compared to BMS, however hazard ratios still ranged from 0.30 with EES to 0.57 with E-ZES.[426426. Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. Bmj. 2013;347:f6625. ] Of note, DES are superior to BMS in all lesion and patient subsets studied to date including small vessels, large vessels, long lesions, diabetic patients and those with acute MI. Although the greatest benefits of DES over BMS regarding TLR reduction are seen during the first year after implantation, all long-term analyses indicate that the benefit is sustained long-term.
STENT THROMBOSIS ( refer to  View chapter )
View chapter )
ST is a rare but devastating adverse event following PCI, which results in abrupt closure of the treated artery with the incumbent risk of sudden death or MI ( Figure 26).[3434. Baim DS, Cutlip DE, O’Shaughnessy CD, et al. Final results of a randomized trial comparing the NIR stent to the Palmaz-Schatz stent for narrowings in native coronary arteries. Am J Cardiol. 2001;87(2):152-156. , 446446. Windecker S, Meier B. Late coronary stent thrombosis. Circulation. 2007;116(17):1952-1965. ] The impact of ST is influenced by the myocardial area at risk, its viability, the degree of instantly recruitable collaterals, and the speed of reperfusion therapy. Mortality rates of ST vary across studies depending on its definition even though the Academic Research Consortium[447447. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351. ] criteria have been used in most contemporary trials. In an analysis from the ADAPT-DES study, which included 8,582 patients treated with a DES, 1 in 5 non-procedural MIs were due to a ST event during 2 years follow-up.[448448. Dohi T, Maehara A, Witzenbichler B, et al. Etiology, Frequency, and Clinical Outcomes of Myocardial Infarction After Successful Drug-Eluting Stent Implantation: Two-Year Follow-Up From the ADAPT-DES Study. Circ Cardiovasc Interv. 2015;8(12):e002447. ] Similarly, very late ST was the underlying cause of MI in a fifth of the 4,184 patients with SCAD included in the CORONOR registry and, importantly, the mortality rate in those patients was substantially higher than that related to MIs due to other aetiologies (18% per year vs. 7% per year).[449449. Lemesle G, Tricot O, Meurice T, et al. Incident Myocardial Infarction and Very Late Stent Thrombosis in Outpatients With Stable Coronary Artery Disease. J Am Coll Cardiol. 2017;69(17):2149-2156. ]
In light of the potential devastating consequences of ST, risk stratification and identification of patients at highest risk for ST is of great importance. Multiple factors have been identified to portend an increased risk for ST following DES implantation and include: 1. patient related factors, 2. procedural characteristics, 3. lesion properties, 4. factors related to the implanted device ( Figure 28).[450450. Holmes DR, Jr., Kereiakes DJ, Garg S, et al. Stent thrombosis. J Am Coll Cardiol. 2010;56(17):1357-1365. ] Studies using OCT have shown that uncovered struts, malapposition, under-expansion, and edge dissection are dominant findings of early ST, whereas malapposition, neoatherosclerosis, uncovered struts, and under-expansion are principal correlates of very late ST.[451451. Adriaenssens T, Joner M, Godschalk TC, et al. Optical Coherence Tomography Findings in Patients With Coronary Stent Thrombosis: A Report of the PRESTIGE Consortium (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort). Circulation. 2017;136(11):1007-1021. , 452452. Souteyrand G, Amabile N, Mangin L, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J. 2016;37(15):1208-1216. , 453453. Taniwaki M, Radu MD, Zaugg S, et al. Mechanisms of Very Late Drug-Eluting Stent Thrombosis Assessed by Optical Coherence Tomography. Circulation. 2016;133(7):650-660. ]
Meta-analyses have shown that the thin-strut, fluoropolymer coated EES is associated with lower rates of definite ST than other DES, and unexpectedly, even lower than BMS.[453453. Taniwaki M, Radu MD, Zaugg S, et al. Mechanisms of Very Late Drug-Eluting Stent Thrombosis Assessed by Optical Coherence Tomography. Circulation. 2016;133(7):650-660. , 453453. Taniwaki M, Radu MD, Zaugg S, et al. Mechanisms of Very Late Drug-Eluting Stent Thrombosis Assessed by Optical Coherence Tomography. Circulation. 2016;133(7):650-660. , 453453. Taniwaki M, Radu MD, Zaugg S, et al. Mechanisms of Very Late Drug-Eluting Stent Thrombosis Assessed by Optical Coherence Tomography. Circulation. 2016;133(7):650-660. , 453453. Taniwaki M, Radu MD, Zaugg S, et al. Mechanisms of Very Late Drug-Eluting Stent Thrombosis Assessed by Optical Coherence Tomography. Circulation. 2016;133(7):650-660. ] Furthermore, data from the large SCAAR (Swedish Coronary Angiography and Angioplasty Registry) registry, showed a similar risk of very late ST in patients undergoing primary PCI treated with new generation DES and BMS. [455455. Sarno G, Lagerqvist B, Nilsson J, et al. Stent thrombosis in new-generation drug-eluting stents in patients with STEMI undergoing primary PCI: a report from SCAAR. J Am Coll Cardiol. 2014;64(1):16-24. ]In a larger network meta-analysis including 147 randomised trials in 126,526 patients, the risk of definite or probable ST at 1-year was significantly lower with PtCr-EES, R-ZES, and Orsiro SES when compared against BMS.[304304. Kang SH, Chae IH, Park JJ, et al. Stent Thrombosis With Drug-Eluting Stents and Bioresorbable Scaffolds: Evidence From a Network Meta-Analysis of 147 Trials. JACC Cardiovasc Interv. 2016;9(12):1203-1212. ] The notion of new-generation DES being safer than BMS represents a paradigm shift in the evolution of PCI.
STENT FRACTURE
Stent fracture remains an uncommon late complication of DES implantation,[456456. Sianos G, Hofma S, Ligthart JM, et al. Stent fracture and restenosis in the drug-eluting stent era. Catheter Cardiovasc Interv. 2004;61(1):111-116. , 457457. Surmely JF, Kinoshita Y, Dash D, et al. Stent strut fracture-induced restenosis in a bifurcation lesion treated with the crush stenting technique. Circ J. 2006;70(7):936-938. ] whose true incidence amongst early generation DES remains unknown, however rates of 1-2%, 1-8% and up to 29% have been reported in randomised, observational and autopsy studies, respectively. [458458. Lee MS, Jurewitz D, Aragon J, et al. Stent fracture associated with drug-eluting stents: clinical characteristics and implications. Catheter Cardiovasc Interv. 2007;69(3):387-394. , 459459. Aoki J, Nakazawa G, Tanabe K, et al. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2007;69(3):380-386. , 460460. Lee SH, Park JS, Shin DG, et al. Frequency of stent fracture as a cause of coronary restenosis after sirolimus-eluting stent implantation. Am J Cardiol. 2007;100(4):627-630. , 461461. Nakazawa G, Finn AV, Vorpahl M, et al. Incidence and predictors of drug-eluting stent fracture in human coronary artery a pathologic analysis. J Am Coll Cardiol. 2009;54(21):1924-1931. ] Notably the majority of stent fractures have been reported with the Cypher SES, whereas stent fractures with TAXUS PES and BMS have been seen very rarely. This difference may be related to the increased radio-opacity of the Cypher SES, its closed cell design, and/or the greater neointimal coverage seen with the PES and BMS that may strengthen and stabilize the struts to withstand the mechanical forces that result in stent fracture. Overall stent fractures can range from a single strut fracture (Grade I) to multiple strut fractures (Grade V) ( Figure 29).
There are several causes for stent fractures which include both mechanical and lesion based factors: a) Mechanical factors: Stent fracture may be the consequence of an excessive mechanical vessel wall stress that occurs from extreme repetitive contraction and flexion of the vessel.[459459. Aoki J, Nakazawa G, Tanabe K, et al. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2007;69(3):380-386. ] Of note this may actually be a protective mechanism for stress relief within the vessel. b) Lesion factors: Predictors for stent fractures have included lesions located in the right coronary artery, and/or lesions in very tortuous/severely calcified vessels. Additional factors increasing the risk of stent fracture include implantation of long and/or overlapping stents, underlying diffuse disease, saphenous vein graphs, and treatment of chronic total occlusions.[456456. Sianos G, Hofma S, Ligthart JM, et al. Stent fracture and restenosis in the drug-eluting stent era. Catheter Cardiovasc Interv. 2004;61(1):111-116. ], [461461. Nakazawa G, Finn AV, Vorpahl M, et al. Incidence and predictors of drug-eluting stent fracture in human coronary artery a pathologic analysis. J Am Coll Cardiol. 2009;54(21):1924-1931. , 462462. Popma JJ, Tiroch K, Almonacid A, et al. A qualitative and quantitative angiographic analysis of stent fracture late following sirolimus-eluting stent implantation. Am J Cardiol. 2009;103(7):923-929. , 463463. Doi H, Maehara A, Mintz GS, et al. Classification and potential mechanisms of intravascular ultrasound patterns of stent fracture. Am J Cardiol. 2009;103(6):818-823. ] In a recent autopsy study longer stent length, use of the Cypher stent and longer stent duration were all identified as independent predictors of stent fracture.[461461. Nakazawa G, Finn AV, Vorpahl M, et al. Incidence and predictors of drug-eluting stent fracture in human coronary artery a pathologic analysis. J Am Coll Cardiol. 2009;54(21):1924-1931. ]
Patients with stent fractures may remain asymptomatic, however they may present with ACS, ST, or recurrent angina due to clinical restenosis; overall 70-80% of those patients with a stent fracture will present with in-stent restenosis or ST.[456456. Sianos G, Hofma S, Ligthart JM, et al. Stent fracture and restenosis in the drug-eluting stent era. Catheter Cardiovasc Interv. 2004;61(1):111-116. , 457457. Surmely JF, Kinoshita Y, Dash D, et al. Stent strut fracture-induced restenosis in a bifurcation lesion treated with the crush stenting technique. Circ J. 2006;70(7):936-938. , 459459. Aoki J, Nakazawa G, Tanabe K, et al. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2007;69(3):380-386. , 464464. Brilakis ES, Maniu C, Wahl M, et al. Unstable angina due to stent fracture. J Invasive Cardiol. 2004;16(9):545. ] The extent of symptoms appears to be related to the grade of the stent fracture with few symptoms occurring as a result of Grade I-IV stent factures, whilst Grade V stent fractures are associated with the most adverse clinical events.[461461. Nakazawa G, Finn AV, Vorpahl M, et al. Incidence and predictors of drug-eluting stent fracture in human coronary artery a pathologic analysis. J Am Coll Cardiol. 2009;54(21):1924-1931. ]
There are no data on definitive treatment, however repeat PCI, which is the current preferred strategy, appears to provide prompt symptom relief.[456456. Sianos G, Hofma S, Ligthart JM, et al. Stent fracture and restenosis in the drug-eluting stent era. Catheter Cardiovasc Interv. 2004;61(1):111-116. , 457457. Surmely JF, Kinoshita Y, Dash D, et al. Stent strut fracture-induced restenosis in a bifurcation lesion treated with the crush stenting technique. Circ J. 2006;70(7):936-938. ] Some suggest treatment using a short stent, together with extending DAPT beyond 12-months.[465465. Carter AJ. Drug-eluting stent fracture promise and performance. J Am Coll Cardiol. 2009;54(21):1932-1934. ]
ANEURYSMS
Coronary artery aneurysms are a rare complication of stenting, whose true incidence, clinical course and treatment are largely unknown ( Figure 30). Nevertheless, studies report an incidence of between 0.3-6.0% after DES and BMS implantation.[466466. Aoki J, Kirtane A, Leon MB, et al. Coronary artery aneurysms after drug-eluting stent implantation. JACC Cardiovasc Interv. 2008;1(1):14-21. ] There are a number of postulated causes for these coronary artery aneurysms, some of which are specific for DES. In general, mechanical causes include the use of oversized balloons or stents, high-pressure balloon inflations and atherectomy all of which can cause residual dissection and deep arterial wall injury eventually leading to aneurysm formation.[467467. Slota PA, Fischman DL, Savage MP, et al. Frequency and outcome of development of coronary artery aneurysm after intracoronary stent placement and angioplasty. STRESS Trial Investigators. Am J Cardiol. 1997;79(8):1104-1106. , 468468. Baumbach A, Bittl JA, Fleck E, et al. Acute complications of excimer laser coronary angioplasty: a detailed analysis of multicenter results. Coinvestigators of the U.S. and European Percutaneous Excimer Laser Coronary Angioplasty (PELCA) Registries. J Am Coll Cardiol. 1994;23(6):1305-1313. , 469469. Bell MR, Garratt KN, Bresnahan JF, et al. Relation of deep arterial resection and coronary artery aneurysms after directional coronary atherectomy. J Am Coll Cardiol. 1992;20(7):1474-1481. ] Of note for DES, the elution of anti-proliferative drugs and/or presence of a polymer can lead to delayed re-endothelialization, inflammatory changes in the medial wall, ISA, and hypersensitivity reactions all of which can result in coronary artery aneurysm formation.[466466. Aoki J, Kirtane A, Leon MB, et al. Coronary artery aneurysms after drug-eluting stent implantation. JACC Cardiovasc Interv. 2008;1(1):14-21. ] Data on coronary aneurysms are mainly derived from case reports, which indicate a variable clinical course, which is similar irrespective of whether the aneurysm is post BMS or DES implantation. In particular aneurysms have been detected from as earlier as 3-days post DES implantation,[470470. Gupta RK, Sapra R, Kaul U. Early aneurysm formation after drug-eluting stent implantation: an unusual life-threatening complication. J Invasive Cardiol. 2006;18(4):E140-142. ] and as late as 9-year following BMS implantation.[471471. Porto I, MacDonald S, Banning AP. Intravascular ultrasound as a significant tool for diagnosis and management of coronary aneurysms. Cardiovasc Intervent Radiol. 2004;27(6):666-668. ]
Two post-stenting coronary phenomena linked to coronary aneurysms have been recently described: a) Coronary evaginations detected by means of OCT and suspected whenever the luminal vessel contour extends in a pouchlike fashion beyond the line connecting all stent struts (stent contour). Theses were more common among SES- than PES-treated lesions (17 vs. 7 per 100 cross sections, p = 0.003) in the SIRTAX Late Study. Combined with a high degree of malapposition and protrusion, they were associated to 2 cases of very late ST during a extended clinical follow-up of the aforementioned trial.[472472. Raber L, Baumgartner S, Garcia-Garcia HM, et al. Long-term vascular healing in response to sirolimus- and paclitaxel-eluting stents: an optical coherence tomography study. JACC Cardiovasc Interv. 2012;5(9):946-957. , 473473. Radu MD, Raber L, Kalesan B, et al. Coronary evaginations are associated with positive vessel remodelling and are nearly absent following implantation of newer-generation drug-eluting stents: an optical coherence tomography and intravascular ultrasound study. Eur Heart J. 2014;35(12):795-807. ] b) Peri-stent contrast staining (PSS) which is defined as contrast staining outside the stent contour extending to ≥ 20% of the stent diameter, measured by quantitative coronary angiography. Severe PSS is defined as contrast staining outside the stent contour extending to ≥ 50% of the stent diameter. Lesions with PSS within 12 months after SES implantation were associated with higher rates of subsequent TLR as well as very late ST compared with lesions without PSS.[474474. Imai M, Kadota K, Goto T, et al. Incidence, risk factors, and clinical sequelae of angiographic peri-stent contrast staining after sirolimus-eluting stent implantation. Circulation. 2011;123(21):2382-2391. ]
Coronary artery aneurysms can be associated with restenosis,[475475. Alfonso F, Perez-Vizcayno MJ, Ruiz M, et al. Coronary aneurysms after drug-eluting stent implantation: clinical, angiographic, and intravascular ultrasound findings. J Am Coll Cardiol. 2009;53(22):2053-2060. ] whilst turbulent and sluggish blood flow in the area of the aneurysm, coupled with a metallic stent can predispose patients to the risk of ST and/or distal embolization.[476476. Feres F, Costa JR, Jr., Abizaid A. Very late thrombosis after drug-eluting stents. Catheter Cardiovasc Interv. 2006;68(1):83-88. , 477477. Aziz S, Morris JL, Perry RA. Late stent thrombosis associated with coronary aneurysm formation after sirolimus-eluting stent implantation. J Invasive Cardiol. 2007;19(4):E96-98. ] Currently there are no definitive data on the best management of patients with coronary artery aneurysms, treatment of which is complicated by some aneurysms resolving spontaneously[478478. Sano K, Mintz GS, Carlier SG, et al. Volumetric intravascular ultrasound assessment of neointimal hyperplasia and nonuniform stent strut distribution in sirolimus-eluting stent restenosis. The American journal of cardiology. 2006;98(12):1559-1562. ], whilst others lead to life-threatening complications. Besides the use of long-term DAPT to minimise the risks of ST, therapeutic options that can be considered include the use of coils, and cardiac surgery.
Benefits and risks of stents
- Coronary stenting has dramatically reduced the immediate risks of PCI when compared with balloon angioplasty including abrupt or threatened vessel closure and the need for emergent coronary artery bypass grafting.
- Newer generation DES are associated with a lower rate of peri-procedural myonecrosis, which is most likely to be related to improved stent design resulting in less side branch compromise.
- Data suggest that the use DESs for ‘off-label’ indications is associated with poorer clinical outcomes in terms of death, MI and repeat revascularization when compared to DES use for ‘on-label’ indications. However, the relative risk reduction as compared with outcomes achieved with BMS remains maintained.
- Restenosis is not a benign phenomenon and can present with MI, or can be associated with morbidity and mortality.
- ST is an undesired complication of coronary stenting, and is associated with a significant morbidity and mortality.
- The cause of ST is multi-factorial and includes patient related factors, procedural characteristics, lesion properties and device factors. One of the most prominent causes remains premature discontinuation of DAPT and poor procedural result.
- Newer generation stents are associated with lower rates of ST, and the associated risk of MI.
CORONARY ARTERY STENTS IN SELECTED SUBSETS OF PATIENTS AND LESIONS
Coronary stenting is the standard of care for flow-limiting atherosclerotic disease in a wide variety of lesions and patients subsets. Complexity of both has been associated with worse long-term results. Notwithstanding, continued research and technological advances have contributed to improved outcomes. Such subsets include
- interventions for stable coronary artery disease ( refer to
 View chapter ),
View chapter ),
- interventions for STEMI and NSTEMI ( refer to
 View chapter and
View chapter and  View chapter ),
View chapter ),
- interventions for patients with diabetes mellitus ( refer to
 View chapter ),
View chapter ),
- interventions for patients with chronic renal disease ( refer to
 View chapter ),
View chapter ),
- left main coronary artery disease ( refer to
 View chapter ),
View chapter ),
- chronic total occlusion ( refer to
 View chapter ), and
View chapter ), and
- saphenous vein graft disease ( refer to
 View chapter ) among others.
View chapter ) among others.
Small vessels, long lesions
A small, randomized trial (n = 500) comparing SES with thin-strut BMS showed significantly lower angiographic restenosis (8.3% vs 25.5%, respectively; RR 0.33, 95% CI 0.19-0.56, P <0.001) and TVR (7.2% vs 18.8%; RR 0.38, 95% CI 0.22-0.66, P <0.001) with the DES.[7272. Pache J, Dibra A, Mehilli J, et al. Drug-eluting stents compared with thin-strut bare stents for the reduction of restenosis: a prospective, randomized trial. Eur Heart J. 2005;26(13):1262-1268. ] The benefit was, however, largely restricted to patients with small vessel disease (reference vessel diameter <2.8 mm; restenosis of SES 7.0% vs 34.2% for BMS; P <0.001). In contrast, binary restenosis was similar in vessels with a reference vessel diameter of 2.8 mm or more (mean RVD 3.1 mm; SES 10% vs BMS 13%; P = 0.52).[7272. Pache J, Dibra A, Mehilli J, et al. Drug-eluting stents compared with thin-strut bare stents for the reduction of restenosis: a prospective, randomized trial. Eur Heart J. 2005;26(13):1262-1268. ] Another randomized trial comparing newer generation DES with BMS in 826 patients without routine angiographic follow-up observed lower rates of TVR at 18 months (7.5% vs 11.6%, respectively; P = 0.05). A stratified subgroup analysis of this trial indicated that the benefit was limited to patients who received at least one small stent (<3.0 mm diameter; TVR HR 0.44 in favour of DES; P = 0.02), whereas no difference was observed in patients who received only large stents (≥3 mm).[479479. Rocca HP, Kaiser C, Pfisterer M. Targeted stent use in clinical practice based on evidence from the BAsel Stent Cost Effectiveness Trial (BASKET). Eur Heart J. 2007;28(6):719-725. ] The Ontario propensity score matched comparison of DES and BMS showed that DES were particularly beneficial in patients with small vessels (<3 mm) and long lesions (≥20 mm).[480480. Tu JV, Bowen J, Chiu M, et al. Effectiveness and safety of drug-eluting stents in Ontario. New Engl J Med. 2007;357(14):1393-1402. ] The number needed to treat in patients with small vessels ranged from 0 to 12 in patients with diabetes, to 27 to 83 in non-diabetic patients. Similarly, the number needed to treat in patients with long lesions ranged from 10 to 23 in patients with diabetes, to 27 to 53 in non-diabetic patients. Accordingly, DES seem particularly useful in vessels with a reference vessel diameter of 3 mm or less and long lesions.
Data from studies of newer generation DES have failed to show any influence of vessel diameter on stent performance. One-year data from the XIENCE V USA study showed comparable performance of EES in terms of MACE, TLR and ST amongst patients receiving a single EES <=2.5mm (n=838) or >2.5mm (n=2015) in diameter.[481481. Hermiller JB, Rutledge DR, Mao VW, et al. Clinical outcomes in real-world patients with small vessel disease treated with XIENCE V(R) everolimus-eluting stents: one year results from the XIENCE V(R) USA condition of approval post-market study. Catheter Cardiovasc Interv. 2014;84(1):7-16. ] Two-year outcomes of patients enrolled in five R-ZES studies demonstrated comparable performance in terms of TLF (10.1% vs. 8.7%, P=0.54) when R-ZES was used in vessels with a reference vessel diameter below (n=1956) and above 2.5mm (n=3174).[482482. Caputo R, Leon M, Serruys P, et al. Performance of the resolute zotarolimus-eluting stent in small vessels. Catheter Cardiovasc Interv. 2014;84(1):17-23. ] In contrast, in the LEADERS study TLR (9.6% vs. 2.6%, p=0.001) and MACE (12.1% vs. 7.1%, p=0.04) were significantly higher in patients treated with BES for vessels less than- or more than 2.75mm in diameter.[483483. Wykrzykowska JJ, Serruys PW, Onuma Y, et al. Impact of vessel size on angiographic and clinical outcomes of revascularization with biolimus-eluting stent with biodegradable polymer and sirolimus-eluting stent with durable polymer the LEADERS trial substudy. JACC Cardiovasc Interv. 2009;2(9):861-870. ] Amongst those patients with a small vessel diameter, there was no difference in performance of SES versus BES. Similarly no between-stent difference was observed in a propensity matched analysis of 1302 patients treated for acute MI in the KAMIR registry who had vessels <=2.75mm in diameter and received either EES to R-ZES.[484484. Cho SC, Jeong MH, Kim W, et al. Clinical outcomes of everolimus- and zotarolimus-eluting stents in patients with acute myocardial infarction for small coronary artery disease. J Cardiol. 2014;63(6):409-417. ] In the PLATINUM SV study, PROMUS EES had significantly lower rates of TLF (2.4% vs. 21.1%, P<0.001) when compared to historical controls receiving TAXUS PES in vessels between 2.25 and 2.5mm in diameter.[217217. Teirstein PS, Meredith IT, Feldman RL, et al. Two-year safety and effectiveness of the platinum chromium everolimus-eluting stent for the treatment of small vessels and longer lesions. Catheter Cardiovasc Interv. 2015;85(2):207-215. ]
Likewise, in the PLATINUM LL, the PROMUS EES was used to treat lesions between 24 and 34 mm with outcomes compared to historical controls receiving TAXUS PES.[217217. Teirstein PS, Meredith IT, Feldman RL, et al. Two-year safety and effectiveness of the platinum chromium everolimus-eluting stent for the treatment of small vessels and longer lesions. Catheter Cardiovasc Interv. 2015;85(2):207-215. ] At 1-year TLF was significantly lower with PROMUS EES (3.2% vs. 19.4%, P<0.001). Non-inferior angiographic and clinical outcomes have been reported in studies comparing EES to SES, R-ZES to SES and Nobori BES to Promus EES in patients with lesions longer than 24mm.[172172. Park DW, Kim YH, Song HG, et al. Comparison of everolimus- and sirolimus-eluting stents in patients with long coronary artery lesions: a randomized LONG-DES-III (Percutaneous Treatment of LONG Native Coronary Lesions With Drug-Eluting Stent-III) Trial. JACC Cardiovasc Interv. 2011;4(10):1096-1103. , 213213. Ahn JM, Park DW, Kim YH, et al. Comparison of resolute zotarolimus-eluting stents and sirolimus-eluting stents in patients with de novo long coronary artery lesions: a randomized LONG-DES IV trial. Circ Cardiovasc Interv. 2012;5(5):633-640. , 268268. Lee JY, Park DW, Kim YH, et al. Comparison of biolimus A9-eluting (Nobori) and everolimus-eluting (Promus Element) stents in patients with de novo native long coronary artery lesions: a randomized Long Drug-Eluting Stent V trial. Circ Cardiovasc Interv. 2014;7(3):322-329. ]
Large vessels
BASKET PROVE prospectively addressed the question whether DES are beneficial among patients with large vessels as compared to BMS and randomly assigned treatment of 774 patients with EES, 775 patient with SES and 765 patients with BMS. During a median follow-up of 745 days, the investigators noted no difference in terms of death (EES 3.2%, SES 3.6%, BMS 4.4%), MI (EES 1.7%, SES 0.9%, BMS 2.6%) and definite or probable ST (EES 0.6%, SES 0.6%, BMS 1.2%). However, rates of target revascularization were significantly lower for both EES (3.7%, PEES vs BMS = 0.002) and SES (4.3%, PSES vs BMS = 0.005) compared with BMS suggesting that the use of DES is safe and more effective than BMS in patients undergoing PCI for lesions in large vessels.
The role of biodegradable-polymer DES in large vessel stenting has been assessed by the randomized BASKET-PROVE II trial that showed similar performance of a BP BES compared with the durable- polymer EES.[267267. Kaiser C, Galatius S, Jeger R, et al. Long-Term Efficacy and Safety of Biodegradable-Polymer Biolimus-Eluting Stents: Main Results of the Basel Stent Kosten-Effektivitats Trial-PROspective Validation Examination II (BASKET-PROVE II), A Randomized, Controlled Noninferiority 2-Year Outcome Trial. Circulation. 2015;131(1):74-81. ] Both DES showed a superior efficacy profile over BMS but without evidence for better safety.
Overlapping stents
Stent overlap occurs in up to 30% of patients undergoing PCI in contemporary clinical practice for a variety of reasons including excessive target lesion length, incomplete lesion coverage, edge dissections and residual thrombus.[485485. O’Sullivan CJ, Stefanini GG, Raber L, et al. Impact of stent overlap on long-term clinical outcomes in patients treated with newer-generation drug-eluting stents. Eurointervention. 2014;9(9):1076-1084. ] The use of overlapping BMS is associated with inferior clinical outcomes compared to patients treated with a single BMS or overlapping DES, mainly due to higher rates of TLR.[485485. O’Sullivan CJ, Stefanini GG, Raber L, et al. Impact of stent overlap on long-term clinical outcomes in patients treated with newer-generation drug-eluting stents. Eurointervention. 2014;9(9):1076-1084. ]
In the largest study to date addressing long-term clinical outcomes among patients treated with the unrestricted use of early-generation overlapping DES, patients with DES overlap had a higher incidence of MACE (cardiac death, MI, ischaemia-driven TLR) and impaired angiographic results as compared with non-overlapping or single DES control groups.[398398. Raber L, Juni P, Loffel L, et al. Impact of stent overlap on angiographic and long-term clinical outcome in patients undergoing drug-eluting stent implantation. J Am Coll Cardiol. 2010;55(12):1178-1188. ]
Contemporary data from a prospective, unrestricted DES registry,[177177. Raber L, Juni P, Nuesch E, et al. Long-term comparison of everolimus-eluting and sirolimus-eluting stents for coronary revascularization. J Am Coll Cardiol. 2011;57(21):2143-2151. ] continued to show that patients with DES overlap have a higher incidence of ischemic events as compared with non-overlapping DES controls during three-year follow-up. However, this finding was driven primarily by a higher incidence of such events in the early-generation SES overlap patient cohort when compared with non-overlapping SES controls. Conversely, among patients treated with the newer-generation EES, rates of the composite primary endpoint were similar among patients with and without overlap.[485485. O’Sullivan CJ, Stefanini GG, Raber L, et al. Impact of stent overlap on long-term clinical outcomes in patients treated with newer-generation drug-eluting stents. Eurointervention. 2014;9(9):1076-1084. ]