Pulmonary embolism
SUMMARY
In contrast to the systemic circulation, the pulmonary circulation is a high-flow low-resistance system. The right ventricle responds to an increase in resistance within the pulmonary vascular bed by increasing right ventricular pressure to preserve cardiac output.
Pulmonary embolism (PE) is a relatively common cardiovascular emergency, yet it is a rare but significant complication of percutaneous coronary intervention (PCI). Data about the incidence of PE in association with PCI is limited. A small study described that PE was the reason for death in 1.7% of patients, who died during their acute hospitalisation for PTCA [44. Malenka DJ, O’Rourke D, Miller MA, Hearne MJ, Shubrooks S, Kellett MA, Jr., Robb JF, O’Meara JR, VerLee P, Bradley WA, Wennberg D, Ryan T, Jr., Vaitkus PT, Hettleman B, Watkins MW, McGrath PD and O’Connor GT. Cause of in-hospital death in 12,232 consecutive patients undergoing percutaneous transluminal coronary angioplasty. The Northern New England Cardiovascular Disease Study Group. Am Heart J. 1999. ]. Venous thromboembolism (VTE) shares risk factors with atherothrombosis [55. Pesavento R, Piovella C and Prandoni P. Heart disease in patients with pulmonary embolism. Curr Opin Pulm Med. 2010. ]. Prevention of arterial vascular events with rosuvastatin in the Jupiter trial [66. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT and Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008. ] prevented arterial as much as venous thrombotic events [77. Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT and Ridker PM. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009. ]. Within two years, treatment with rosuvastatin prevented 99 acute myocardial infarctions, 97 strokes, but also 94 symptomatic cases of deep venous thrombosis/pulmonary embolism.
PE is a challenging diagnosis which may be missed because of variable symptoms. Early diagnosis is needed because immediate treatment is highly effective.
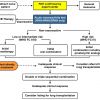
Depending on the clinical presentation, initial therapy is primarily aimed either at life-saving restoration of flow through occluded pulmonary arteries or at the prevention of early recurrences. Immediate risk stratification is essential ( Figure 1 ).
Both initial treatment and long-term anticoagulation must be justified in each patient by a validated diagnostic strategy.
Failure to resolve acute pulmonary thromboemboli is a potential explanation for chronic thromboembolic pulmonary hypertension (CTEPH). This condition appears to be one of the more common subsets of pulmonary hypertension [88. Lang IM. Chronic thromboembolic pulmonary hypertension--not so rare after all. N Engl J Med. 2004. ], yet hard to diagnose. Valuable epidemiological data will be derived from the ongoing European CTEPH Registry (https://www.cteph-association.org/).
EPIDEMIOLOGY
Pulmonary embolism (PE) and deep venous thrombosis (DVT) are two closely related clinical presentations of venous thromboembolism (VTE). About 50% of patients with proximal DVT subsequently develop mostly asymptomatic PE [99. Moser KM, Fedullo PF, LitteJohn JK and Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994. ]. Conversely, the complication of a DVT of the lower limbs accounts for approximately 70% of PEs [1010. Dalen JE. Pulmonary embolism: what have we learned since Virchow? Natural history, pathophysiology, and diagnosis. Chest. 2002. , 1111. Kearon C. Natural history of venous thromboembolism. Circulation. 2003. ]. VTE is the third most common cardiovascular disease after acute ischaemic syndromes and stroke and is associated with substantial morbidity and mortality [66. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT and Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008. ]. In the USA, the overall age and sex-adjusted incidence of VTE is 77.6 per 100,000 [1212. Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006. ]. The incidence of PE among hospitalised patients is about 0.4% [1313. Stein PD, Beemath A and Olson RE. Trends in the incidence of pulmonary embolism and deep venous thrombosis in hospitalized patients. Am J Cardiol. 2005. ]. VTE is predominantly a disease of older age. The incidence rates increase exponentially with age in both men and women [11. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM and Melton LJ, 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998. ] [1414. Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P and Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004. ].
The crude mortality rate for acute PE at three months is about 15%, which is higher than that for myocardial infarction [1515. Goldhaber SZ, Visani L and De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999. ]. One-year mortality after a VTE is 17% to 22%[1616. Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR and Hammerstrom J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007. , 1717. Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E and Prins MH. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996. ] , while two-year mortality is 20% to 25% [1717. Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E and Prins MH. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996. , 1818. Anderson FA, Jr., Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, Forcier A and Dalen JE. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991. ] . Murin et al reported a 6-month fatality rate of 10.5% among patients with DVT and 14.7% among those with PE [1919. Murin S, Romano PS and White RH. Comparison of outcomes after hospitalization for deep venous thrombosis or pulmonary embolism. Thromb Haemost. 2002. ]. Sudden death occurs in almost 25% of patients with PE [1212. Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006. ]. Right heart failure resulting in cardiovascular collapse is the most common reason for death [1212. Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006. ]. It has been shown that patients diagnosed with PE during hospitalisation for another condition had a higher case-fatality rate (32.5%) than those admitted for PE. 11.6% of patients died during their initial hospitalisation, 5% of those with DVT and 23% of those with PE [1818. Anderson FA, Jr., Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, Forcier A and Dalen JE. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991. ] .
Increased mortality risk in patients with PE is associated with systolic arterial hypotension, congestive heart failure, cancer, tachypnoea, poor right-ventricular function by echocardiography, chronic obstructive pulmonary disease, and age >70 years [1515. Goldhaber SZ, Visani L and De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999. ].
A large epidemiological study reported that 59% of incident VTE cases could be attributed to immobilisation or nursing home residence, 18% to cancer and 12% to trauma. Congestive heart failure and prior central venous catheter or pacemaker placement were responsible for 10% and 9% of VTE cases respectively. In summary, recognised clinical risk factors are responsible for ~ 75% of VTE cases, while ~ 25% are idiopathic [2020. Heit JA, O’Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN and Melton LJ, 3rd. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002. ].
PE has a relatively high recurrence rate. Analyses of the California Patients Discharge Data Set [1919. Murin S, Romano PS and White RH. Comparison of outcomes after hospitalization for deep venous thrombosis or pulmonary embolism. Thromb Haemost. 2002. ] revealed that the 6-month recurrence rate of VTE was 6.4% in the cohort of patients hospitalised for DVT and 5.8% in the cohort initially hospitalised for PE. In a prospective cohort study of 738 patients with DVT, reported recurrence rates of VTE were 7.0% at 1 year and 22.0% at 5 years [2121. Hansson PO, Sorbo J and Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Arch Intern Med. 2000. ]. Survivors of acute PE have an increased risk of CTEPH [2222. Fanikos J, Piazza G, Zayaruzny M and Goldhaber SZ. Long-term complications of medical patients with hospital-acquired venous thromboembolism. Thromb Haemost. 2009. ].
PATHOPHYSIOLOGICAL MECHANISMS OF PULMONARY EMBOLISM
The clinical manifestation of VTE can be subdivided into DVT and PE [2323. Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008. ]. In most cases, PE is a severe complication of DVT of the legs, and ranges from asymptomatic, incidentally discovered thromboemboli to massive embolism that results in obstruction of the pulmonary vasculature. Thrombi in the leg may form at any point along the vein wall and any venous bed can be involved. The vast majority of venous thrombi are asymptomatic, and form on the valve pockets of deep veins in the calf [2424. Kakkar VV, Howe CT, Flanc C and Clarke MB. Natural history of postoperative deep-vein thrombosis. Lancet. 1969. ]. However, these thrombi can extend into the proximal veins, including and above the popliteal veins [2525. Cotton LT and Clark C. Anatomical Localization of Venous Thrombosis. Ann R Coll Surg Engl. 1965. ], become symptomatic and prone to embolisation. In about 80% of patients with PE, signs of a DVT are documented [2626. Sandler DA and Martin JF. Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis?. J R Soc Med. 1989. ]. Conversely, up to 50% of patients will develop a PE in the setting of DVT. If embolisation does not occur, the thrombus within the vein can be partially or completely resolved via recanalisation, organisation, and lysis.
PE as a complication of percutaneous coronary intervention
Percutaneous coronary intervention (PCI) is associated with various in-hospital complications including death, myocardial infarction, emergency coronary artery bypass grafting, stroke, contrast-induced nephropathy, and vascular access-site complications [2727. Smith SC, Jr., Dove JT, Jacobs AK, Kennedy JW, Kereiakes D, Kern MJ, Kuntz RE, Popma JJ, Schaff HV, Williams DO, Gibbons RJ, Alpert JP, Eagle KA, Faxon DP, Fuster V, Gardner TJ, Gregoratos G and Russell RO. ACC/AHA guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines)-executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) endorsed by the Society for Cardiac Angiography and Interventions. Circulation. 2001. ]. PE is a relatively common cardiovascular emergency, yet it is a rare but significant complication of PCI, occurring in 1.7% of cases [44. Malenka DJ, O’Rourke D, Miller MA, Hearne MJ, Shubrooks S, Kellett MA, Jr., Robb JF, O’Meara JR, VerLee P, Bradley WA, Wennberg D, Ryan T, Jr., Vaitkus PT, Hettleman B, Watkins MW, McGrath PD and O’Connor GT. Cause of in-hospital death in 12,232 consecutive patients undergoing percutaneous transluminal coronary angioplasty. The Northern New England Cardiovascular Disease Study Group. Am Heart J. 1999. ]. The mortality related to PE in this PCI population is 0.016% [44. Malenka DJ, O’Rourke D, Miller MA, Hearne MJ, Shubrooks S, Kellett MA, Jr., Robb JF, O’Meara JR, VerLee P, Bradley WA, Wennberg D, Ryan T, Jr., Vaitkus PT, Hettleman B, Watkins MW, McGrath PD and O’Connor GT. Cause of in-hospital death in 12,232 consecutive patients undergoing percutaneous transluminal coronary angioplasty. The Northern New England Cardiovascular Disease Study Group. Am Heart J. 1999. ]. No data exists regarding more recent experience after introduction of femoral vascular closure devices, which have reduced the time to ambulation.
Under dual antiplatelet therapy with aspirin and clopidogrel, PE was observed in only 0.026% of patients within 6 months [2828. Serebruany V, Pokov I, Kuliczkowski W, Vahabi J and Atar D. Incidence and causes of new-onset dyspnea in 3,719 patients treated with clopidogrel and aspirin combination after coronary stenting. Thromb Haemost. 2008. ]. Data from the PEP study and from the Anti-Platelet Trialists’ systematic review shows that aspirin significantly reduces the risk of VTE in surgical patients. However, there is no general evidence that aspirin is a drug that is useful for the prevention of VTE in any patient group [2929. O’Brien J, Duncan H, Kirsh G, Allen V, King P, Hargraves R, Mendes L, Perera T, Catto P, Schofield S, Ploschke H, Hefner T, Churchland M, Woolnough S, Wuttke R, Manning M, Jeffries T, Hensley L, Bath P, Bainbridge D, Guinane F, McMahon L, Zavattaro D, Wilson D, Blake K, Morton J, Sharman D, Locke R, Ghabrial J, McNeil S, Rehfisch P, van der Merwe S, Einoder B, Douglas D, Ashwell J, Morrissey A, Brown A, Simm R, Fisch G, Crawford W, Everding T, Tanham D, Hooper J, Aldana C, Goldwasser M, Mibus M, Rowden N, Mills J, Johnson P, Stilwell J, Williams R, Stevenson T, Zwar J, Bauze R, Nyunt B, Russell R, Day G, Cameron M, Clements P, Beck T, Ellis A, Phipps S, Quaill W, Skirving A, McGuiness M, Gray P, Rankin J, Wood D, Senior J, Courtenay B, Green M, Harris I, Rush J, Kilgour M, Davies J, Newcombe J, Lewis C, Leitl S, Chilcott R, Pianta R, Aguila A, Hawe C, Nade S, Smith M, Robertson P, Rothwell A, May S, Williams S, Rothwell A, Shamy S, Martin G, Steele V, Jeffery K, Kelman I, McKillan J, Dayaram P, Culling J, Lawson D, Winfield P, Kuzel R, Milburn C, Palmer N, Pitts A, Lamb G, Prodan L, Gray H, Walker K, Smith K, Newton C, Atkinson D, Fail B, Panting A, Menzies J, Watkiss A, Robertson P, Outhred J, Clay D, Lander R, Fou R, Clarke DS, Murrell T, Rolleston B, Clarke DS, Keyworth S, Medlicott P, Woods T, Dawe C, Waldron R, Taine W, Le Roy L, Smith A, Cowley G, Campbell C, Maxwell R, Elvy M, Thurston A, Newth S, Cousins L, Sanderson M, MacMillan C, Golele R, Walters J, Kruger S, Ramsden T, Joseph H, Gantz D, Steyn S, Hadjichristofis S, Nojoko L, Snowdowne R, Botha M, Knebel R, Sernbo I, Ferris B, Tello E, Nevelos A, Dunn C, Warwick D, Sudhakar J, Tuite JD, Francis J, Wallace ME, Jordan A, Baird P, Fowler W, Ainscow DAP, Harrison A, Butler-Manuel A, Palmer N, Broome G, Swailes P, Stahl TJ, Randall AM, Forester AJ, Hucker J, Redden J, Logan L, Howard PW, Brownlee D, Angus P, Boyce A, Porter BB, Arbid M, Youll J, Ogden AS, MacDowell C, Beverly M, Ramdeholl P, Nelson I, Howlett I, Warwick D, Weeber A, Peacock A, Hubbard MJS, Flanagan M, Crawshaw C, Kinninmonth A, Campbell T, Tibrewal S, Lennox CME, Clifford I, Gillham N, Hunt M, Bulstrode C, Handley R, Iyer V, Lawton J, Robbins J, Harper WM, Davison J, Hope P, Smallbones K, Jones W, Ratnam KR, Upton J, Hirst P, Knox S, Sadique T, Khan M, Umar M, Anderson J, Frank J, Briggs P, Sanderson P, McBride D, Leese K, Bayliss NC, El-Deen M, Banan H, Williams S, Parker MJ, Minhas H, Best A, Blakeway C, Salter T, Foy MA, Pollitt A, Blayney JDM, Davis S, Parnell E, Ko C, Grover ML, Howells S, Williams D, Saunders C, Brenkel I, Mani G, Grizzle M, Henderson A, Board T, Chadwick CJ, Overton M, Hodkinson S, Macdonald DA, Lewis V, McDonald RJM, Adamson J, Bradley J, Hodgson E, Plewes JL, Clarke D, Hullin M, Smith J, Lemon GJ, Greatrex K, Miles C, Bannister G, Dare J, Carden DG, Aughton J, Stirrat A, Fishburn J, Ebizie AO, Mason M, Irvine G, Facey T, Spratt C, Briggs P, Kanagavel N, Gerrard T, Reissis N, Tzanetos P, Stefanotti M, Greiss M, Wright V, Rahmaty A, Archer J, Bedford A, Taylor D, Griffith M, Evans J, Heron K, Livingstone B, O’Dwyer K, Wheatley D, Lemon GJ, Miles C, Campbell P, Dixon P, McSweeney L, Shanahan MDG, Griffith I, MacMahon S, Rodgers A, Collins R, Prentice C, Gray H, MacMahon S, Norton R, Ockleford P, Rodgers A, Rutland M, Collins R, Prentice C, Dickinson J, Gregg P, Macdonald D, Mollan RAB, Douglas J, Beaumont D, Broad J, Clark T, Henderson M, McCulloch A, Neal B, Prasad R, Walker N, Wood M, Beighton A, Bell P, Farrell B, Murch K, Sharpe N, Gordon G, Doughty R, Ratnasabapathy Y, Danesh J, Sleight P, Peto R, Simes J, Keech A, Rodgers A, MacMahon S, Collins R, Prentice C and Tri PEP. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000. , 3030. Watson HG and Chee YL. Aspirin and other antiplatelet drugs in the prevention of venous thromboembolism. Blood Rev. 2008. ].
Venous thromboembolism and atherothrombosis
Atherothrombosis and VTE are associated [3131. Becattini C, Vedovati MC, Ageno W, Dentali F and Agnelli G. Incidence of arterial cardiovascular events after venous thromboembolism: a systematic review and a meta-analysis. J Thromb Haemost. 2010. ], and share risk factors such as obesity, hypertension, dyslipidaemia, diabetes, and smoking [3232. Ageno W, Becattini C, Brighton T, Selby R and Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008. , 3333. Goon PK and Lip GY. Arterial disease and venous thromboembolism: a modern paradigm?. Thromb Haemost. 2006. , 3434. . Holst AG, Jensen G and Prescott E. Risk factors for venous thromboembolism: results from the Copenhagen City Heart Study.
Circulation]. Inflammation, systemic and local hypercoagulability, and endothelial injury play crucial roles in the development of both atherothrombosis and VTE [3333. Goon PK and Lip GY. Arterial disease and venous thromboembolism: a modern paradigm?. Thromb Haemost. 2006. , 3535. Piazza G and Goldhaber SZ. Venous thromboembolism and atherothrombosis: an integrated approach. Circulation. 2010. ]]. Patients with acute coronary syndromes or stroke have an increased susceptibility of VTE [3636. Piazza G, Fanikos J, Zayaruzny M and Goldhaber SZ. Venous thromboembolic events in hospitalised medical patients. Thromb Haemost. 2009. , 3737. Sorensen HT, Horvath-Puho E, Lash TL, Christiansen CF, Pesavento R, Pedersen L, Baron JA and Prandoni P. Heart disease may be a risk factor for pulmonary embolism without peripheral deep venous thrombosis. Circulation. 2011. ]. Additionally, an association between atherosclerotic disease and spontaneous venous thrombosis has been demonstrated [3838. Prandoni P, Bilora F, Marchiori A, Bernardi E, Petrobelli F, Lensing AW, Prins MH and Girolami A. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003. ]. Subsequent studies detected a drastically increased cardiovascular risk in patients with a prior history of VTE compared with those without such a history [3939. Becattini C, Agnelli G, Prandoni P, Silingardi M, Salvi R, Taliani MR, Poggio R, Imberti D, Ageno W, Pogliani E, Porro F and Casazza F. A prospective study on cardiovascular events after acute pulmonary embolism. Eur Heart J. 2005. , 4040. Klok FA, Mos IC, Broek L, Tamsma JT, Rosendaal FR, de Roos A and Huisman MV. Risk of arterial cardiovascular events in patients after pulmonary embolism. Blood. 2009. , 4141. Sorensen HT, Horvath-Puho E, Pedersen L, Baron JA and Prandoni P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet. 2007. , 4242. Spencer FA, Ginsberg JS, Chong A and Alter DA. The relationship between unprovoked venous thromboembolism, age, and acute myocardial infarction. J Thromb Haemost. 2008. ].
The prognostic value of C-reactive protein, a sensitive systemic marker of inflammation for cardiovascular events [4343. Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, Hutchinson WL and Pepys MB. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999. , 4444. Ridker PM, Cushman M, Stampfer MJ, Tracy RP and Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997. ] and VTE [4545. Folsom AR, Lutsey PL, Astor BC and Cushman M. C-reactive protein and venous thromboembolism. A prospective investigation in the ARIC cohort. Thromb Haemost. 2009. ], has been investigated. Rosuvastatin prevented arterial events in a population with elevated high-sensitivity C-reactive protein levels [4646. Albert MA, Danielson E, Rifai N and Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001. ] in the Jupiter trial (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) [66. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT and Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008. ], and simultaneously prevented symptomatic venous thrombotic events [77. Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT and Ridker PM. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009. ].
Hypercoagulability plays an important role in the development of VTE as well as in atherothrombosis. An increased risk of both conditions has been described in women on oestrogen and progesterone therapy and in patients with lupus anticoagulant [4747. Rosendaal FR, Helmerhorst FM and Vandenbroucke JP. Female hormones and thrombosis. Arterioscler Thromb Vasc Biol. 2002. ]. Endothelial injury as a trigger for atherothrombosis and DVT has been well established [4848. Joffe HV, Kucher N, Tapson VF and Goldhaber SZ. Upper-extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004. , 4949. Libby P, Ridker PM and Maseri A. Inflammation and atherosclerosis. Circulation. 2002. , 5050. van Stralen KJ, Rosendaal FR and Doggen CJ. Minor injuries as a risk factor for venous thrombosis. Arch Intern Med. 2008. ].
RISK FACTORS FOR PE
Although PE can occur in patients without any known risk factor, certain predisposing factors are associated with an increased risk of VTE. The International Cooperative Pulmonary Embolism Registry (ICOPER) showed that less than a quarter of PE were idiopathic or unprovoked [1515. Goldhaber SZ, Visani L and De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999. ]. In 1856, Virchow first proposed a triad of factors leading to intravascular coagulation, including stasis of blood flow, vascular endothelial damage, and hypercoagulability of blood [5151. Virchow R. Phlogose und Thrombose im Gefässystem. Gesammelte Abhandlungen zur Wissenschaftlichen Medicin. Frankfurt, Germany: Meidinger. 1856. ]. Risk factors for VTE are outlined Table 1. [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism, 5353. Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet. 1999. ]. The incidence of VTE correlates with increasing age for both idiopathic and secondary PE [5454. Oger E. Incidence of venous thromboembolism: a community-based study in Western France. EPI-GETBP Study Group. Groupe d’Etude de la Thrombose de Bretagne Occidentale. Thromb Haemost. 2000. ]. Approximately 65% of patients with PE are aged 60 years or older. The incidence of PE is eight times higher in patients over 80 compared with those under 50 years of age [5555. Hansson PO, Welin L, Tibblin G and Eriksson H. Deep vein thrombosis and pulmonary embolism in the general population. ’The Study of Men Born in 1913’. Arch Intern Med. 1997. ]. Total risk depends on the number of predisposing factors [5656. Anderson FA, Jr. and Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003. ].
Two large meta-analyses have investigated vascular access-related complications in patients undergoing percutaneous transfemoral coronary procedures [5757. Koreny M, Riedmuller E, Nikfardjam M, Siostrzonek P and Mullner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004. , 5858. Nikolsky E, Mehran R, Halkin A, Aymong ED, Mintz GS, Lasic Z, Negoita M, Fahy M, Krieger S, Moussa I, Moses JW, Stone GW, Leon MB, Pocock SJ and Dangas G. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta-analysis. J Am Coll Cardiol. 2004. ]. In both studies, venous thromboembolic events were not included in the reporting of vascular access-related complications. Whether the condition is under-reported, or VTE as a complication of coronary angiography has in fact decreased since the general implementation of arterial puncture site closing devices shortening bed rest after coronary angiography, is unknown. In addition, the improvement of patient care and the implementation of dual antiplatelet therapy and continued anticoagulation might be responsible for the apparent reduction of VTE after PCI.
RISK STRATIFICATION
PE is a potentially life-threatening disorder. The rate of recurrent and potentially fatal events may be significantly reduced by treatment with either unfractionated heparin (UFH), low-molecular weight heparin (LMWH), or fondaparinux [2323. Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008. ]. Risk stratification of patients with PE helps to distinguish between those candidates at low risk who are appropriate for a therapy outside the hospital setting and those individuals at higher risk who require hospitalisation.
According to the ESC guidelines, the severity of PE is stratified into high (>15%), intermediate (3% to 15%) and low risk (<1%) of PE-related early death (in-hospital or 30-day mortality) as depicted in Figure 1A [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism]. The presence of hypotension or shock at the time of PE diagnosis is the most powerful predictor of early death, independent of further risk markers [1515. Goldhaber SZ, Visani L and De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999. , 5959. Kucher N, Rossi E, De Rosa M and Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006. ]. Consequently, haemodynamically unstable individuals with suspected PE should immediately be classified as high-risk patients and require an emergency diagnostics and treatment. All other patients are thus automatically identified as non-high-risk patients and should be further stratified into intermediate and low risk patients after PE has been confirmed. This stratification may critically influence treatment and the duration of hospitalisation and should be based on the Pulmonary Embolism Severity Index (PESI) [6060. Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, Roy PM and Fine MJ. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005. ] or simplified PESI (sPESI) [6161. Jimenez D, Aujesky D, Moores L, Gomez V, Lobo JL, Uresandi F, Otero R, Monreal M, Muriel A and Yusen RD. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010. ] ( Figure 1 [B] ). The PESI, comprising 11 routinely available clinical parameters, represents a well-established approach to estimate 30-day mortality for patients with acute PE. This model can be used to give clinicians a beside risk assessment tool for patients with PE, without any need for imaging studies such as echocardiography or laboratory tests. To reduce the technical complexity of the original prediction rule, a simplified version of the PESI score has been developed, which is easily calculated at bedside ( Figure 1 [B] ) [6161. Jimenez D, Aujesky D, Moores L, Gomez V, Lobo JL, Uresandi F, Otero R, Monreal M, Muriel A and Yusen RD. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010. ]. Recent data suggest that both PESI and the sPESI predict 30-day mortality after acute symptomatic PE.
Patients at intermediate risk are further classified into intermediate-high and intermediate-low risk patients according to signs of right ventricular (RV) dysfunction based on imaging and/or biomarkers. Defined markers of RV dysfunction are RV dilatation, hypokinesis or pressure overload on echocardiography, RV dilatation on spiral computed tomography, and elevated right heart pressures at right-heart catheterisation. N-terminal (NT)-proBNP represents the best validated biomarker for severity of haemodynamic compromise and RV dysfunction in the setting of PE [6262. Henzler T, Roeger S, Meyer M, Schoepf UJ, Nance JW, Jr., Haghi D, Kaminski WE, Neumaier M, Schoenberg SO and Fink C. Pulmonary embolism: CT signs and cardiac biomarkers for predicting right ventricular dysfunction. Eur Respir J. 2012. ]. NT-proBNP plasma levels of 600 pg/mL were found as the optimal cut-off value for identifying patients at increased risk [6363. Lankeit M, Jimenez D, Kostrubiec M, Dellas C, Kuhnert K, Hasenfuss G, Pruszczyk P and Konstantinides S. Validation of N-terminal pro-brain natriuretic peptide cut-off values for risk stratification of pulmonary embolism. Eur Respir J. 2014. ]. Troponin T or I testing can detect myocardial injury and positive results identify patients at higher risk. The development of high-sensitive assays has further improved the prognostic value of troponin T [6464. Lankeit M, Friesen D, Aschoff J, Dellas C, Hasenfuss G, Katus H, Konstantinides S and Giannitsis E. Highly sensitive troponin T assay in normotensive patients with acute pulmonary embolism. Eur Heart J. 2010. ].
The presence of concomitant DVT in patients with acute symptomatic PE is an independent predictor of death in the three months following the diagnosis [6161. Jimenez D, Aujesky D, Moores L, Gomez V, Lobo JL, Uresandi F, Otero R, Monreal M, Muriel A and Yusen RD. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010. ]. Therefore, bilateral lower extremity compression ultrasonography should assist with risk stratification of patients with acute PE. Venous ultrasound imaging of the entire deep vein system is highly specific (about 95%) and sensitive (over 90%) for the diagnosis of DVT [6565. Kearon C, Ginsberg JS and Hirsh J. The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism. Ann Intern Med. 1998. , 6666. Perrier A and Bounameaux H. Ultrasonography of leg veins in patients suspected of having pulmonary embolism. Ann Intern Med. 1998. , 6767. Wells PS. Integrated strategies for the diagnosis of venous thromboembolism. J Thromb Haemost. 2007. ].
DIAGNOSTIC STRATEGIES
Due to the large variety and low specificity of symptoms, the diagnosis of PE is challenging. Chest computed tomography (CT) is regarded as highly sensitive and specific test for PE [6868. Stein PD, Beemath A, Kayali F, Skaf E, Sanchez J and Olson RE. Multidetector computed tomography for the diagnosis of coronary artery disease: a systematic review. Am J Med. 2006. ]. The key to the diagnosis of PE is a high index of clinical suspicion. In order to avoid unnecessary exposure to radiation and contrast agent, without overlooking the condition, a sophisticated diagnostic algorithm has been established [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism].
Clinical signs and symptoms
The clinical presentation of patients with PE is highly variable. Frequently reported symptoms include dyspnoea, pleuritic and substernal chest pain, palpitations, cough, seizures, faints and haemoptysis. Patients with high thrombus burden can present with circulatory collapse, mental status changes, syncope and arrhythmias [6969. Aujesky D, Roy PM, Le Manach CP, Verschuren F, Meyer G, Obrosky DS, Stone RA, Cornuz J and Fine MJ. Validation of a model to predict adverse outcomes in patients with pulmonary embolism. Eur Heart J. 2006. , 7070. Stein PD and Henry JW. Clinical characteristics of patients with acute pulmonary embolism stratified according to their presenting syndromes. Chest. 1997. ] . Clinical signs include tachypnoea, tachycardia, signs of DVT (leg pain, warmth, or swelling), fever and cyanosis.
Differential diagnosis of chest pain
Acute chest pain represents one of the most common diagnostic challenges in emergency medicine, accounting for approximately 8% to 10% of the 119 million emergency department visits yearly in the USA [7171. Pitts SR, Niska RW, Xu J and Burt CW. National hospital ambulatory medical care survey: 2006 emergency department summary. In: Services UDoHaH, editor. ; 2006. ]. The differential diagnosis ranges from non-serious musculoskeletal aetiologies to life-threatening cardiac disease. In one large study [7272. Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, Griffith JL and Selker HP. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000. ] of patients attending the emergency department with chest pain, 8% were diagnosed with AMI, 9% with unstable angina, 6% with stable angina, 21% had non-ischaemic cardiac problems, and at least 2% of patients with AMI were discharged in error. However, more than 50% of patients with acute chest pain had non-cardiac problems, such as aortic dissection or PE, which may mimic coronary syndromes [7373. Erhardt L, Herlitz J, Bossaert L, Halinen M, Keltai M, Koster R, Marcassa C, Quinn T and van Weert H. Task force on the management of chest pain. Eur Heart J. 2002. ]. Approximately 0.5% of patients who attend emergency departments with suspicion of acute coronary syndrome have a PE [7373. Erhardt L, Herlitz J, Bossaert L, Halinen M, Keltai M, Koster R, Marcassa C, Quinn T and van Weert H. Task force on the management of chest pain. Eur Heart J. 2002. ]. The underlying cause of chest pain varies depending on whether a patient is seen by a general practitioner or at the emergency department. In general practices nearly 50% of patients with chest pain have musculoskeletal pain, whereas in the emergency department 45% have cardiac problems [7373. Erhardt L, Herlitz J, Bossaert L, Halinen M, Keltai M, Koster R, Marcassa C, Quinn T and van Weert H. Task force on the management of chest pain. Eur Heart J. 2002. , 7474. Herlitz J, Bang A, Isaksson L and Karlsson T. Outcome for patients who call for an ambulance for chest pain in relation to the dispatcher’s initial suspicion of acute myocardial infarction. Eur J Emerg Med. 1995. , 7575. Klinkman MS, Stevens D and Gorenflo DW. Episodes of care for chest pain: a preliminary report from MIRNET. Michigan Research Network. J Fam Pract. 1994. , 7676. Svavarsdóttir A, Jónasson M, Gudmundsson G and Fjeldsted K. Chest pain in family practice. Diagnosis and long-term outcome in a community setting. Can Fam Physician 1996. ] . However, even in general practices, PE was identified in 3% of patients with thoracic pain [7676. Svavarsdóttir A, Jónasson M, Gudmundsson G and Fjeldsted K. Chest pain in family practice. Diagnosis and long-term outcome in a community setting. Can Fam Physician 1996. ]. About 3% of patients admitted to a coronary care unit with acute chest pain suffered from PE [7676. Svavarsdóttir A, Jónasson M, Gudmundsson G and Fjeldsted K. Chest pain in family practice. Diagnosis and long-term outcome in a community setting. Can Fam Physician 1996. ]. The enhancement of patient care by rapid diagnoses is an important health care issue.
Physical examination
The diagnosis for PE begins with a careful clinical examination and determination of risk factors. Decreased breath sounds, wheezing, respiratory crackles, accessory muscle use, increased jugular venous pressure, and a right ventricular heave may be detected by physical examination.
Electrocardiogram
An electrocardiogram (ECG) is routinely performed in patients with chest pain attending the emergency department. The ECG is, however, a poor diagnostic tool for PE. Though the ECG is often abnormal, the findings are neither sensitive nor specific. The greatest use of the ECG in patients with suspected PE is to rule out other potential life-threatening diagnoses that can be more readily diagnosed such as myocardial infarction.
The S1Q3T3 pattern is a typical ECG manifestation of acute right ventricular pressure and volume overload. An S wave in lead I indicates a complete or more often incomplete right bundle-branch block. A Q-wave, mild ST-elevation and an inverted T wave in lead III are repolarisation abnormalities possibly triggered by subendocardial ischaemia of the right ventricle.
Further ECG signs of RV strain are T-wave inversion in leads V1–V4, a QR pattern in lead V1, and incomplete or complete right bundle-branch block [7777. Geibel A, Zehender M, Kasper W, Olschewski M, Klima C and Konstantinides SV. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J. 2005. , 7878. Rodger M, Makropoulos D, Turek M, Quevillon J, Raymond F, Rasuli P and Wells PS. Diagnostic value of the electrocardiogram in suspected pulmonary embolism. Am J Cardiol. 2000. ]. These findings may be helpful, particularly when of new onset. Nevertheless, such changes are generally associated with the more severe forms of PE and may be found in right ventricular strain of any cause such as acute bronchospasm, pneumothorax and other acute lung disorders.
Implicit and explicit (prediction) rules
Clinical signs, symptoms and routine laboratory tests do not allow the exclusion or confirmation of acute PE but help to estimate a likelihood of PE [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism]. The assessment of clinical probability of PE is based on a combination of individual symptoms, signs and common tests, either implicitly by the clinician or by the use of prediction rules. Implicit clinical judgement combines the knowledge and experience of the clinician to estimate the likelihood of PE. The value of this assessment has been shown in several large series [7979. Musset D, Parent F, Meyer G, Maitre S, Girard P, Leroyer C, Revel MP, Carette MF, Laurent M, Charbonnier B, Laurent F, Mal H, Nonent M, Lancar R, Grenier P and Simonneau G. Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study. Lancet. 2002. , 8080. Perrier A. Noninvasive diagnosis of pulmonary embolism. Hosp Pract (Minneap). 1998. ]. The main limitations of implicit judgement are lack of standardisation and the challenge of acquiring sufficient experience.
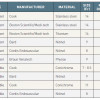
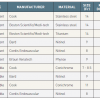
Therefore, prediction rules have been developed for calculating the probability of clinically suspected PE which are independent of physicians’ implicit judgement and which have demonstrated similar accuracy [8181. Chagnon I, Bounameaux H, Aujesky D, Roy PM, Gourdier AL, Cornuz J, Perneger T and Perrier A. Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism. Am J Med. 2002. ]. Prediction rules calculate the probability of suspected PE from a combination of symptoms and clinical signs. The revised Geneva score [8282. Le Gal G, Righini M, Roy PM, Sanchez O, Aujesky D, Bounameaux H and Perrier A. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006. ] and Wells score [8383. Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, Turpie AG, Bormanis J, Weitz J, Chamberlain M, Bowie D, Barnes D and Hirsh J. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000. ] represent two established and well validated prediction rules that were further simplified to increase their usefulness in clinical practice ( Table 2 and Table 3 [8484. Gibson NS, Sohne M, Kruip MJ, Tick LW, Gerdes VE, Bossuyt PM, Wells PS, Buller HR and Christopher study i. Further validation and simplification of the Wells clinical decision rule in pulmonary embolism. Thromb Haemost. 2008. , 8585. Klok FA, Mos IC, Nijkeuter M, Righini M, Perrier A, Le Gal G and Huisman MV. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008. ]).
D-dimer
Plasma D-dimer, a degradation product of cross-linked fibrin, is a very sensitive but non-specific marker for VTE. A negative test result is valid to rule out PE in patients with a low or moderate clinical probability [6767. Wells PS. Integrated strategies for the diagnosis of venous thromboembolism. J Thromb Haemost. 2007. ]. However, positive D-dimer tests should be considered with caution because this parameter is susceptible to false positive results. Levels of D-dimer are elevated in many other clinical conditions, such as cancer, inflammation, infection, necrosis, bleeding, dissection of the aorta, pregnancy and hospitalisation per se [8686. Bruinstroop E, van de Ree MA and Huisman MV. The use of D-dimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009. ]. For this reason, D-dimer is not a useful parameter for confirming PE. The number of patients with suspected PE in whom D-dimer needs to be determined to exclude one PE is between three in the emergency department and ten or above in other conditions [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism]. Physical examination should be performed as the first diagnostic step, prior to considering D-dimer [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism]. It has become evident that the specificity of D-dimer in individuals with suspected PE declines with age [8787. Righini M, Goehring C, Bounameaux H and Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med. 2000. ]. Therefore, an age-adjusted cut-off (patient's age in years × 10 µg/L) has been developed to improve the specificity of D-dimer testing for excluding PE [8888. Douma RA, le Gal G, Sohne M, Righini M, Kamphuisen PW, Perrier A, Kruip MJ, Bounameaux H, Buller HR and Roy PM. Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: a retrospective analysis of three large cohorts. BMJ. 2010. ]. The combination of pretest clinical probability assessment with an age-adjusted D-dimer cutoff increased the specificity of D-dimer as diagnostic biomarker significantly without any loss in sensitivity [8989. Righini M, Kamphuisen PW and Le Gal G. Age-adjusted D-dimer cutoff levels and pulmonary embolism--reply. JAMA. 2014. ].
Echocardiography
Echocardiography is non-invasive, can immediately be performed at the bedside, provides rapid results, and circumvents radiographic contrast and radiation exposure. Tricuspid insufficiency jet velocity, RV dimensions, disturbed RV ejection pattern or depressed contractility of the RV free wall are accepted as indirect signs of PE. Due to the reportedly low sensitivity of around 60% to70%, echocardiography cannot exclude PE [9090. Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, Conti A, Agnelli G and Berni G. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000. , 9191. Miniati M, Monti S, Pratali L, Di Ricco G, Marini C, Formichi B, Prediletto R, Michelassi C, Di Lorenzo M, Tonelli L and Pistolesi M. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med. 2001. , 9292. Roy PM, Colombet I, Durieux P, Chatellier G, Sors H and Meyer G. Systematic review and meta-analysis of strategies for the diagnosis of suspected pulmonary embolism. BMJ. 2005. ]. Hence, echocardiographic examination is reserved for haemodynamically unstable, hypotensive patients. Direct visualisation of right heart enlargement and of right heart thrombi, as in 4% to18% of patients with acute PE, justifies the initiation of specific treatment [9393. Casazza F, Bongarzoni A, Centonze F and Morpurgo M. Prevalence and prognostic significance of right-sided cardiac mobile thrombi in acute massive pulmonary embolism. Am J Cardiol. 1997. , 9494. Torbicki A, Galie N, Covezzoli A, Rossi E, De Rosa M and Goldhaber SZ. Right heart thrombi in pulmonary embolism: results from the International Cooperative Pulmonary Embolism Registry. J Am Coll Cardiol. 2003. ].
Transoesophageal echocardiography for searching emboli in main pulmonary arteries may be considered for immediate decision-making in patients with severe haemodynamic compromise [9393. Casazza F, Bongarzoni A, Centonze F and Morpurgo M. Prevalence and prognostic significance of right-sided cardiac mobile thrombi in acute massive pulmonary embolism. Am J Cardiol. 1997. , 9494. Torbicki A, Galie N, Covezzoli A, Rossi E, De Rosa M and Goldhaber SZ. Right heart thrombi in pulmonary embolism: results from the International Cooperative Pulmonary Embolism Registry. J Am Coll Cardiol. 2003. ], visualising thrombus in the proximal pulmonary arteries [9595. Pruszczyk P, Torbicki A, Kuch-Wocial A, Chlebus M, Miskiewicz ZC and Jedrusik P. Transoesophageal echocardiography for definitive diagnosis of haemodynamically significant pulmonary embolism. Eur Heart J. 1995. , 9696. Rittoo D, Sutherland GR, Samuel L, Flapan AD and Shaw TR. Role of transesophageal echocardiography in diagnosis and management of central pulmonary artery thromboembolism. Am J Cardiol. 1993. ], and in systemic veins and the right heart. However, the proximal part of the left pulmonary artery can be assessed in only 47% of patients [9797. Pruszczyk P, Torbicki A, Kuch-Wocial A, Szulc M, Styczynski G, Bochowicz A and Kostrubiec M. Visualization of the central pulmonary arteries by biplane transesophageal echocardiography. Exp Clin Cardiol. 2001. ].
Computed tomography
CT angiography has replaced pulmonary angiography as the method of choice for imaging the pulmonary vasculature for suspected PE, particularly since the implementation of multidetector CT [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism]. Conventional pulmonary angiography leads to an increased bleeding risk during thrombolysis [9898. Agnelli G, Becattini C and Kirschstein T. Thrombolysis vs heparin in the treatment of pulmonary embolism: a clinical outcome-based meta-analysis. Arch Intern Med. 2002. , 9999. Wan S, Quinlan DJ, Agnelli G and Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004. ], and is associated with higher mortality in unstable patients [100100. Stein PD, Athanasoulis C, Alavi A, Greenspan RH, Hales CA, Saltzman HA, Vreim CE, Terrin ML and Weg JG. Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation. 1992. ] . Multidetector CT is at least as accurate as invasive pulmonary angiography [101101. Piazza G and Goldhaber SZ. Acute pulmonary embolism: part I: epidemiology and diagnosis. Circulation. 2006. , 102102. Quiroz R, Kucher N, Zou KH, Kipfmueller F, Costello P, Goldhaber SZ and Schoepf UJ. Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review. JAMA. 2005. ] and allows the adequate visualisation of the pulmonary arteries up to at least the segmental level [103103. Ghaye B, Szapiro D, Mastora I, Delannoy V, Duhamel A, Remy J and Remy-Jardin M. Peripheral pulmonary arteries: how far in the lung does multi-detector row spiral CT allow analysis?. Radiology. 2001. , 104104. Patel S, Kazerooni EA and Cascade PN. Pulmonary embolism: optimization of small pulmonary artery visualization at multi-detector row CT. Radiology. 2003. ] .
A positive CT has a high positive predictive value (92% to 96%) in patients with intermediate or high clinical probability of PE [105105. Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, Leeper KV, Jr., Popovich J, Jr., Quinn DA, Sos TA, Sostman HD, Tapson VF, Wakefield TW, Weg JG and Woodard PK. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006. ]. Large clinical trials have established Multidetector CT as a reliable method to exclude PE [106106. Elias A, Cazanave A, Elias M, Chabbert V, Juchet H, Paradis H, Carriere P, Nguyen F, Didier A, Galinier M, Colin C, Lauque D, Joffre F and Rousseau H. Diagnostic management of pulmonary embolism using clinical assessment, plasma D-dimer assay, complete lower limb venous ultrasound and helical computed tomography of pulmonary arteries. A multicentre clinical outcome study. Thromb Haemost. 2005. , 107107. van Belle A, Buller HR, Huisman MV, Huisman PM, Kaasjager K, Kamphuisen PW, Kramer MH, Kruip MJ, Kwakkel-van Erp JM, Leebeek FW, Nijkeuter M, Prins MH, Sohne M and Tick LW. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006. ]. However, whether patients with a negative CT should be further examined by compression ultrasonography (CUS) and/or ventilation-perfusion scintigraphy (V/Q scan) or pulmonary angiography is still controversial [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism].
Ventilation–perfusion scintigraphy
Ventilation–perfusion scintigraphy (V/Q scan) is a validated option for patients with contraindications to CT, such as allergy to iodine contrast dye or renal failure, despite a high proportion of inconclusive results [108108. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). The PIOPED Investigators. JAMA. 1990. ].
Lung scan results usually indicate the level of probability of PE according to criteria established in the North American PIOPED trial [108108. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). The PIOPED Investigators. JAMA. 1990. ]. In general, V/Q scan results have to be verified by further tests. Only high-probability V/Q scan results in patients with high degree of probability are accepted as diagnosis without further clarification [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism].
Diagnostic algorithm
According to the ESC guidelines [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
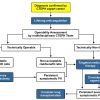
Latest European guidelines on acute pulmonary embolism], the diagnostic algorithm differs substantially between suspected high-risk and non-high-risk PE. Therefore, prompt and accurate risk stratification to guide appropriate diagnostic steps is of major importance. Both diagnostic algorithms are illustrated in Figure 2 .
Suspected high-risk PE
High-risk PE, characterised by the presence of shock or arterial hypotension, accounts for 5% of all cases of PE and has a short-term mortality of at least 15% [109109. Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD, Rauber K, Iversen S, Redecker M and Kienast J. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997. ]. This underlines the potential life-threatening nature of high-risk PE, and the need for emergency treatment. Therefore, a simple and rapid diagnostic algorithm for the diagnosis of PE is of great practical benefit. Crucially, emergency CT or bedside echocardiography is recommended for diagnostic purposes.
Due to its availability in most emergency rooms, bedside transthoracic echocardiography is the most useful initial examination for the diagnosis of right ventricular dysfunction. In the absence of echocardiographic signs of RV overload or dysfunction, massive PE is excluded and the search for other causes is indicated. In the case of positive echocardiographic findings, CT should confirm the diagnosis of PE. In highly unstable patients, or if other tests are not available, echocardiography alone is sufficient to justify rapid treatment. If the patient is stabilised and CT is available, a CT should be performed immediately. After confirming the diagnosis, PE-specific treatment is justified.
Suspected non-high-risk pulmonary embolism
In the majority of patients admitted to the emergency department with suspected PE, the diagnosis can be excluded by careful evaluation. The assessment of clinical probability by the revised Geneva score [8282. Le Gal G, Righini M, Roy PM, Sanchez O, Aujesky D, Bounameaux H and Perrier A. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006. ] and the Wells score [8383. Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, Turpie AG, Bormanis J, Weitz J, Chamberlain M, Bowie D, Barnes D and Hirsh J. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000. ] is recommended as a first step ( Table 2 and Table 3 ). If the likelihood of PE is low or intermediate, D-dimer measurements as the next diagnostic step that should be performed for ruling out PE. D-dimer determination combined with clinical probability assessment allows PE to be ruled out in around 30% of this patient group [107107. van Belle A, Buller HR, Huisman MV, Huisman PM, Kaasjager K, Kamphuisen PW, Kramer MH, Kruip MJ, Kwakkel-van Erp JM, Leebeek FW, Nijkeuter M, Prins MH, Sohne M and Tick LW. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006. , 110110. Perrier A, Desmarais S, Miron MJ, de Moerloose P, Lepage R, Slosman D, Didier D, Unger PF, Patenaude JV and Bounameaux H. Non-invasive diagnosis of venous thromboembolism in outpatients. Lancet. 1999. , 111111. Kruip MJ, Slob MJ, Schijen JH, van der Heul C and Buller HR. Use of a clinical decision rule in combination with D-dimer concentration in diagnostic workup of patients with suspected pulmonary embolism: a prospective management study. Arch Intern Med. 2002. , 112112. Perrier A, Roy PM, Aujesky D, Chagnon I, Howarth N, Gourdier AL, Leftheriotis G, Barghouth G, Cornuz J, Hayoz D and Bounameaux H. Diagnosing pulmonary embolism in outpatients with clinical assessment, D-dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study. Am J Med. 2004. , 113113. Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, Forgie M, Kovacs G, Ward J and Kovacs MJ. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001. ]. In patients with high clinical probability of PE, the measurement of D-Dimer is frequently unhelpful due to its low negative predictive value [114114. Righini M, Aujesky D, Roy PM, Cornuz J, de Moerloose P, Bounameaux H and Perrier A. Clinical usefulness of D-dimer depending on clinical probability and cutoff value in outpatients with suspected pulmonary embolism. Arch Intern Med. 2004. ].
If the likelihood of PE is high, or if the D-dimer is elevated, then CT angiography is indicated. This has become the main thoracic imaging modality for suspected PE [115115. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax. 2003. , 116116. Schoepf UJ, Savino G, Lake DR, Ravenel JG and Costello P. The Age of CT Pulmonary Angiography. J Thorac Imaging. 2005. ]. Hence, Multidetector CT is the second-line test in patients with an elevated D-dimer level and the first-line test in patients with a high clinical probability [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism].
THERAPEUTIC STRATEGIES
The treatment of choice depends on the calculated risk of PE-related early mortality. Therefore, accurate risk stratification is crucial for the selection of treatment. The initial risk stratification of suspected and/or confirmed PE based on the presence of shock and hypotension is needed to differentiate between high-risk and non-high-risk patients for selecting appropriate therapeutic strategies.
In non-high-risk PE patients, further stratification to intermediate or low-risk PE patients on the presence of imaging or biochemical markers of RV dysfunction and myocardial injury is recommended. However, anticoagulation with unfractionated heparin should be initiated without delay in high-risk as well as non-high-risk PE patients [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism]. A therapeutic algorithm in accordance with the ESC guidelines is depicted in Figure 3 .
New oral anticoagulants (NOACs)
Rivaroxaban, abixaban and edoxaban are oral factor Xa inhibitors. Dabigatran is an orally administered direct thrombin inhibitor. NOACs do not require laboratory monitoring and have no food interactions and only a few drug interactions [117117. Kubitza D, Becka M, Voith B, Zuehlsdorf M and Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005. , 118118. Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008. ]. Over the last decades physicians were well trained in the use of conventional anticoagulant treatments with heparin and vitamin K antagonists. Since the new oral anticoagulants (NOACs) received regulatory approval for the acute and continued treatment of PE, physicians are challenged to optimally implement their use in clinical practice. Table 4 helps identify patients that are suitable for treatment with a NOACs. The use of NOACs is not recommended in patients with severe renal impairment. Table 5 gives an overview about dosing of NOACs. A recently published review [119119. Yeh CH, Gross PL and Weitz JI. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood. 2014.
Broad review of contemporary NOACs] summarises in more detail NOACs for the treatment and prevention of thromboembolic diseases.
High-risk pulmonary embolism
High-risk PE patients have a high mortality rate and complication risk [5959. Kucher N, Rossi E, De Rosa M and Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006. , 109109. Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD, Rauber K, Iversen S, Redecker M and Kienast J. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997. , 120120. Stein PD and Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest. 1995. ]. Acute RV failure with resulting low systemic output is the leading cause of death in patients with high-risk PE. The first few hours after admission to the emergency department are associated with an increased risk of in-hospital death[120120. Stein PD and Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest. 1995. ]. Therefore, rapid haemodynamic and respiratory support is of vital importance. The infusion of saline solution is useful to maintain adequate systemic pressure. If the systemic arterial pressure remains below 90 mmHg or if tissue perfusion is not sufficient, the administration of vasopressors or catecholamines is recommended [5959. Kucher N, Rossi E, De Rosa M and Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006. , 109109. Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD, Rauber K, Iversen S, Redecker M and Kienast J. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997. ]. Immediate pharmacological or mechanical reopening of the occluded pulmonary arteries is indicated.
Anticoagulation
A parenteral anticoagulation should be administered without delay in haemodynamically unstable patients with suspected PE. Intravenous weight-adjusted unfractionated heparin is the treatment of choice. Subcutaneous LMWH or fondaparinux have not been tested in the setting of hypotension and shock. The anticoagulant effect of unfractionated heparin can be easily monitored and if necessary reversed rapidly by protamine. High-risk PE patients should not receive NOACs in the acute phase as these drugs have not been evaluated in conjunction with primary reperfusion therapies.
Thrombolytic therapy
Systemic thrombolysis improves RV function and haemodynamic status in patients with acute PE [121121. Goldhaber SZ, Haire WD, Feldstein ML, Miller M, Toltzis R, Smith JL, Taveira da Silva AM, Come PC, Lee RT, Parker JA and et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet. 1993. ]. Therefore, thrombolytic therapy is the first-line treatment that should be immediately administrated in high-risk patients as soon as PE is confirmed. However, the beneficial effects of thrombolysis are limited to the first few days; in survivors, differences disappear one week after administration [122122. Becattini C, Agnelli G, Salvi A, Grifoni S, Pancaldi LG, Enea I, Balsemin F, Campanini M, Ghirarduzzi A, Casazza F and Group TS. Bolus tenecteplase for right ventricle dysfunction in hemodynamically stable patients with pulmonary embolism. Thromb Res. 2010. , 123123. Konstantinides S, Tiede N, Geibel A, Olschewski M, Just H and Kasper W. Comparison of alteplase versus heparin for resolution of major pulmonary embolism. Am J Cardiol. 1998. ].
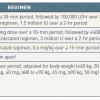
Because direct local infusion of the thrombolytic agent has not been shown to be advantageous [124124. Verstraete M, Miller GA, Bounameaux H, Charbonnier B, Colle JP, Lecorf G, Marbet GA, Mombaerts P and Olsson CG. Intravenous and intrapulmonary recombinant tissue-type plasminogen activator in the treatment of acute massive pulmonary embolism. Circulation. 1988. ], systemic intravenous administration is recommended. Approved thrombolytic agents, regimens, and contraindications are summarised in Table 6 [125125. Konstantinides S. Clinical practice. Acute pulmonary embolism. N Engl J Med. 2008. ]. Accelerated thrombolytic regimens are preferable to prolonged infusions of thrombolytic drugs over 12-24 hours [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism].
In high-risk PE patients, thrombolysis is associated with a critical reduction of mortality of approximately 22% compared to heparin alone. Despite these impressive outcome data, thrombolysis is withheld in more than two-thirds of patients with high-risk PE according to real world registry data [126126. Lin BW, Schreiber DH, Liu G, Briese B, Hiestand B, Slattery D, Kline JA, Goldhaber SZ and Pollack CV, Jr. Therapy and outcomes in massive pulmonary embolism from the Emergency Medicine Pulmonary Embolism in the Real World Registry. Am J Emerg Med. 2012. , 127127. Stein PD and Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: saves lives but underused. Am J Med. 2012. ]. The reasons for underuse of systemic thrombolysis is unclear and cannot be completely explained by rising catheter-based or surgical revascularisation rates [127127. Stein PD and Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: saves lives but underused. Am J Med. 2012. ]. Challenging the validity of relative contraindications to thrombolysis may help to increase the use of thrombolysis in unstable patients with life-threatening PE.
The main complication of thrombolytic therapy is bleeding. The Pulmonary Embolism Thrombolysis (PEITHO) trial revealed a 2% incidence of haemorrhagic stroke and 6.3% incidence of major non-intracranial bleeding in patients treated with tenecteplase [128128. Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, Bluhmki E, Bouvaist H, Brenner B, Couturaud F, Dellas C, Empen K, Franca A, Galie N, Geibel A, Goldhaber SZ, Jimenez D, Kozak M, Kupatt C, Kucher N, Lang IM, Lankeit M, Meneveau N, Pacouret G, Palazzini M, Petris A, Pruszczyk P, Rugolotto M, Salvi A, Schellong S, Sebbane M, Sobkowicz B, Stefanovic BS, Thiele H, Torbicki A, Verschuren F, Konstantinides SV and Investigators P. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014.
RCT examining the role of fibrinolysis in patients with pulmonary embolism and markers of intermediate risk], which has led to the recommendation that routine thrombolysis is not recommended in patients who are not in shock.
Interventional treatment
In patients with absolute contraindications and in those whose unstable conditions do not allow sufficient time for systemic thrombolysis to be effective, surgical embolectomy or catheter-directed intervention are alternative reperfusion therapies if appropriate expertise is available [129129. Yalamanchili K, Fleisher AG, Lehrman SG, Axelrod HI, Lafaro RJ, Sarabu MR, Zias EA and Moggio RA. Open pulmonary embolectomy for treatment of major pulmonary embolism. Ann Thorac Surg. 2004. ]. Approximately one third of patients with an acute major PE are not eligible for thrombolysis because of contraindications such as recent surgery, trauma, stroke, advanced cancer or concomitant active bleeding [109109. Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD, Rauber K, Iversen S, Redecker M and Kienast J. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997. ]. Furthermore, interventional management should be considered as adjunctive therapy when thrombolysis has failed. Critical clinical conditions which warrant interventional strategies have not been clearly defined. However, several criteria, such as shock with large thrombus burden, severe RV failure with large saddle embolism or moderate RV failure with proximal embolism, justify an interventional approach in a patient with acute PE.
Surgical embolectomy
Historically, surgical embolectomy was restricted to clinically futile circumstances in moribund patients who required cardiopulmonary resuscitation. Over recent years, however, pulmonary embolectomy has become a routine operation with significantly reduced operative risk in centres with established cardiac surgery programmes [129129. Yalamanchili K, Fleisher AG, Lehrman SG, Axelrod HI, Lafaro RJ, Sarabu MR, Zias EA and Moggio RA. Open pulmonary embolectomy for treatment of major pulmonary embolism. Ann Thorac Surg. 2004. , 130130. Leacche M, Unic D, Goldhaber SZ, Rawn JD, Aranki SF, Couper GS, Mihaljevic T, Rizzo RJ, Cohn LH, Aklog L and Byrne JG. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg. 2005. ]. This operation requires rapid induction of anaesthesia, a median sternotomy, incision of the main pulmonary artery, and institution of cardiopulmonary bypass. Emboli can be removed from both pulmonary arteries using forceps under direct vision. Generally, this procedure is performed under normothermia without cardioplegic cardiac arrest [130130. Leacche M, Unic D, Goldhaber SZ, Rawn JD, Aranki SF, Couper GS, Mihaljevic T, Rizzo RJ, Cohn LH, Aklog L and Byrne JG. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg. 2005. ]. The first successful surgical pulmonary embolectomy, called Trendelenburg’s operation, was performed in 1924 [131131. Kirschner M. Ein durch die Trendelenburgsche Operation geheilter Fall von Embolie der Arteria pulmonalis. Arch Klin Chir. 1924. ].
Current guidelines recommend surgical embolectomy in patients with failed thrombolysis and in those with contraindications to thrombolysis. As surgical embolectomy represents a relatively simple operation and outcome is not critically affected by the site of surgical care, delays in treatment by hospital transfer should be avoided as long as an experienced surgeon and cardiopulmonary bypass are available [132132. Kilic A, Shah AS, Conte JV and Yuh DD. Nationwide outcomes of surgical embolectomy for acute pulmonary embolism. J Thorac Cardiovasc Surg. 2013. ]. Peripheral extracorporeal membrane oxygenation support is a rapid, effective option in critical situations for ensuring circulation and oxygenation until surgical embolectomy can be performed [133133. Malekan R, Saunders PC, Yu CJ, Brown KA, Gass AL, Spielvogel D and Lansman SL. Peripheral extracorporeal membrane oxygenation: comprehensive therapy for high-risk massive pulmonary embolism. Ann Thorac Surg. 2012. ].
Surgical embolectomy represents a valuable treatment option in high-risk PE patients with comparable in-hospital mortality rates and significantly less bleeding complications than thrombolysis [134134. Aymard T, Kadner A, Widmer A, Basciani R, Tevaearai H, Weber A, Schmidli J and Carrel T. Massive pulmonary embolism: surgical embolectomy versus thrombolytic therapy--should surgical indications be revisited?. Eur J Cardiothorac Surg. 2013. ]. In patients with failed thrombolysis, surgical embolectomy significantly improves the haemodynamic status but is associated with increased bleeding rates [135135. Doerge H, Schoendube FA, Voss M, Seipelt R and Messmer BJ. Surgical therapy of fulminant pulmonary embolism: early and late results. Thorac Cardiovasc Surg. 1999. , 136136. Meneveau N, Seronde MF, Blonde MC, Legalery P, Didier-Petit K, Briand F, Caulfield F, Schiele F, Bernard Y and Bassand JP. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest. 2006. ].
Pulmonary embolectomy has also shown promising results in patients with PE and RV dysfunction without persistent hypotension or shock [137137. Aklog L, Williams CS, Byrne JG and Goldhaber SZ. Acute pulmonary embolectomy: a contemporary approach. Circulation. 2002. ]. Initially, pulmonary embolectomy was associated with high early mortality rates [135135. Doerge H, Schoendube FA, Voss M, Seipelt R and Messmer BJ. Surgical therapy of fulminant pulmonary embolism: early and late results. Thorac Cardiovasc Surg. 1999. , 138138. Gray HH, Miller GA and Paneth M. Pulmonary embolectomy: its place in the management of pulmonary embolism. Lancet. 1988. , 139139. Meyer G, Tamisier D, Sors H, Stern M, Vouhe P, Makowski S, Neveux JY, Leca F and Even P. Pulmonary embolectomy: a 20-year experience at one center. Ann Thorac Surg. 1991. ]. With expansion of indications for surgical embolectomy in patients with RVD but no severe shock, early mortality rates of 6% to 8% have been reported [135135. Doerge H, Schoendube FA, Voss M, Seipelt R and Messmer BJ. Surgical therapy of fulminant pulmonary embolism: early and late results. Thorac Cardiovasc Surg. 1999. , 138138. Gray HH, Miller GA and Paneth M. Pulmonary embolectomy: its place in the management of pulmonary embolism. Lancet. 1988. , 139139. Meyer G, Tamisier D, Sors H, Stern M, Vouhe P, Makowski S, Neveux JY, Leca F and Even P. Pulmonary embolectomy: a 20-year experience at one center. Ann Thorac Surg. 1991. ]. In patients with CTEPH a dedicated pulmonary endarterectomy (PEA) (which differs from thrombectomy as it targets the intimal-medial layer of the vessel as the surgical dissection plane rather than the thrombus surface) has to be performed by an experienced surgeon [140140. Hoeper MM, Mayer E, Simonneau G and Rubin LJ. Chronic thromboembolic pulmonary hypertension. Circulation. 2006. ].
Catheter-based revascularisation
The removal of obstructing thrombi by percutaneous catheter intervention is a promising treatment alternative to surgical embolectomy for patients with high-risk PE to reverse RV failure and cardiogenic shock [141141. Kucher N. Catheter embolectomy for acute pulmonary embolism. Chest. 2007. , 142142. Todoran TM and Sobieszczyk P. Catheter-based therapies for massive pulmonary embolism. Prog Cardiovasc Dis. 2010. , 143143. Uflacker R. Interventional therapy for pulmonary embolism. J Vasc Interv Radiol. 2001. ]. Catheter-based revascularization techniques comprise approaches with local thrombolysis and those without thrombolysis. Catheter-techniques without thrombolysis, including thrombus fragmentation, rheolytic or rotational thrombectomy, or suction thrombectomy, are indicated when contraindications for thrombolysis exist [144144. Engelberger RP and Kucher N. Catheter-based reperfusion treatment of pulmonary embolism. Circulation. 2011. ]. For patients without absolute contraindications to thrombolysis, catheter-based thrombolysis might be a promising therapeutic option. A summary of available devices and techniques for catheter-based revascularization of PE is given in Table 7 .
A “clean” angiographic result is not the goal of catheter embolectomy. Instead, the aim of this procedure is instantaneously to reduce pulmonary vascular resistance and right ventricular afterload, and to increase cardiac output and systemic arterial pressure. The intervention is finished as soon as haemodynamic improvement is obtained, regardless of the extent of residual thrombi in the pulmonary vasculature [145145. Kucher N and Goldhaber SZ. Management of massive pulmonary embolism. Circulation. 2005. , 146146. Kucher N and Goldhaber SZ. Mechanical catheter intervention in massive pulmonary embolism: proof of concept. Chest. 2008. ]. Substantial improvement in pulmonary blood flow may result from a visually modest angiographic result.
During pulmonary angiography or catheter thrombectomy, continuous monitoring of haemodynamic parameters and ECG is required. The intervention necessitates a vascular approach with selective catheterisation of the pulmonary arteries and injection of a contrast agent. The common femoral veins are the preferred venous access sites using a 6 French introducer sheath for patients who are scheduled for unilateral catheter placement or a 10 French double-lumen introducer sheath for those who are scheduled for bilateral catheter insertion [146146. Kucher N and Goldhaber SZ. Mechanical catheter intervention in massive pulmonary embolism: proof of concept. Chest. 2008. ]. In the case of ilio-femoral deep vein thrombosis, the contralateral common femoral vein may be considered for venous access.
One practical approach is the use of a “pharmaco-mechanical” strategy, using local low-dose thrombolytics (if lytic eligible) along with the EKOS ultrasonic catheters ( Figure 4 ). Typically 10-15mg tissue plasminogen activator (tPA) are used per pulmonary artery, infused over 12 hours. There is bench data to suggest that tPA in conjunction with the EKOS infusion catheter is much more effective than local tPA alone [147147. Braaten JV, Goss RA and Francis CW. Ultrasound reversibly disaggregates fibrin fibers. Thromb Haemost. 1997. ]. Retrospective analyses revealed better thrombus removal and less treatment-related complications for ultrasound-assisted thrombolysis compared to conventional catheter-directed thrombolysis [148148. Lin PH, Annambhotla S, Bechara CF, Athamneh H, Weakley SM, Kobayashi K and Kougias P. Comparison of percutaneous ultrasound-accelerated thrombolysis versus catheter-directed thrombolysis in patients with acute massive pulmonary embolism. Vascular. 2009. ].
Current experience is limited [149149. Kuo WT, van den Bosch MA, Hofmann LV, Louie JD, Kothary N and Sze DY. Catheter-directed embolectomy, fragmentation, and thrombolysis for the treatment of massive pulmonary embolism after failure of systemic thrombolysis. Chest. 2008. ] because no randomised controlled trial has compared catheter embolectomy with surgical embolectomy or thrombolytic therapy in this setting. Promising results of a small non-randomised observational cohort study [150150. Skaf E, Beemath A, Siddiqui T, Janjua M, Patel NR and Stein PD. Catheter-tip embolectomy in the management of acute massive pulmonary embolism. Am J Cardiol. 2007. ] demonstrated a similar clinical outcome for high-risk PE after percutaneous catheter intervention compared to surgical embolectomy. Because catheter interventions were commonly combined with pharmacological thrombolysis, the efficacy of the mechanical intervention alone remains unclear [149149. Kuo WT, van den Bosch MA, Hofmann LV, Louie JD, Kothary N and Sze DY. Catheter-directed embolectomy, fragmentation, and thrombolysis for the treatment of massive pulmonary embolism after failure of systemic thrombolysis. Chest. 2008. , 150150. Skaf E, Beemath A, Siddiqui T, Janjua M, Patel NR and Stein PD. Catheter-tip embolectomy in the management of acute massive pulmonary embolism. Am J Cardiol. 2007. ]. A small study [151151. Eid-Lidt G, Gaspar J, Sandoval J, de los Santos FD, Pulido T, Gonzalez Pacheco H and Martinez-Sanchez C. Combined clot fragmentation and aspiration in patients with acute pulmonary embolism. Chest. 2008. ] suggests that mechanical catheter intervention alone without concomitant thrombolysis can lead to immediate improvement in systemic arterial pressure. An analysis of studies evaluating the safety and efficacy of percutaneous catheter interventions showed an overall clinical success rate, defined as immediate haemodynamic improvement, of > 80%. The reported mortality rates range from 0 to 25% [141141. Kucher N. Catheter embolectomy for acute pulmonary embolism. Chest. 2007. ]. The recently published ULTIMA trial showed for the first time in a randomized fashion that an ultrasound-assisted catheter-directed thrombolysis in patients with acute PE was superior to anticoagulation with heparin alone in improving RV dysfunction at 24 hours [152152. Kucher N, Boekstegers P, Muller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, Tebbe U, Horstkotte J, Muller R, Blessing E, Greif M, Lange P, Hoffmann RT, Werth S, Barmeyer A, Hartel D, Grunwald H, Empen K and Baumgartner I. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014. ].
Complications of percutaneous catheter interventions
General complications of percutaneous catheter interventions are associated with the passage of the pulmonary artery catheter and then thrombectomy itself. The most serious complication is the perforation or dissection of a branch of the pulmonary artery, which may lead to massive pulmonary haemorrhage or immediate death. The risk of perforation increases when the diameter of treated pulmonary vessels is < 6 mm [153153. Biederer J, Charalambous N, Paulsen F, Heller M and Muller-Hulsbeck S. Treatment of acute pulmonary embolism: local effects of three hydrodynamic thrombectomy devices in an ex vivo porcine model. J Endovasc Ther. 2006. ]. To reduce the risk of perforation and dissection, thrombectomy is exclusively recommended in the main and lobar pulmonary arteries, and not in the segmental pulmonary arteries [141141. Kucher N. Catheter embolectomy for acute pulmonary embolism. Chest. 2007. , 143143. Uflacker R. Interventional therapy for pulmonary embolism. J Vasc Interv Radiol. 2001. ]. Pericardial tamponade is a further hazard In particular, the right ventricular outflow tract is thin and fragile, and caution is warranted when pushing any device into the pulmonary trunk. An additional risk involves the distal embolisation of proximal thrombi that can exacerbate the patient’s haemodynamic instability during thrombectomy [145145. Kucher N and Goldhaber SZ. Management of massive pulmonary embolism. Circulation. 2005. ]. In order to avoid perforation and damage of susceptible structures, catheter interventions should only be performed by interventionists specialised in high-risk PE and percutaneous catheter techniques. Furthermore, the operator should be able to handle emergent pericardiocentesis in case of a perforation and should be familiar with measures to achieve rapid reversal of anticoagulation. Device-related complications also include substantial blood loss in the case of prolonged aspiration, or even mechanical haemolysis. Mechanical haemolysis may lead in turn to hypotension and acute pancreatitis [154154. Danetz JS, McLafferty RB, Ayerdi J, Rolando LA, Schmittling ZC, Ramsey DE and Hodgson KJ. Pancreatitis caused by rheolytic thrombolysis: an unexpected complication. J Vasc Interv Radiol. 2004. ]. Arrhythmia may occur during catheter passage through the right heart. Further complications are bleeding caused by heparin anticoagulation, contrast-induced nephropathy, anaphylactic reaction to iodine contrast, and vascular access complications, such as haematoma, pseudoaneurysm, or arteriovenous fistula [144144. Engelberger RP and Kucher N. Catheter-based reperfusion treatment of pulmonary embolism. Circulation. 2011. ].
Non-high-risk pulmonary embolism
Non-high-risk PE patients have a favorable short-term prognosis. The subcutaneous administration of weight-adjusted doses of LMWH or fondaparinux is the recommended treatment for normotensive patients with PE [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism]. As alternative to the combination of parenteral anticoagulation with a vitamin K antagonist, NOACs represents a valuable treatment option in those patients in whom no primary reperfusion therapy is planned. Table 4 may help to identify patients that are suitable for anticoagulant therapy with NOACs. Further treatment decisions should be based on further risk stratification into low-risk or intermediate risk patients.
Low-risk pulmonary embolism
If patients are haemodynamically stable, have no signs of RV dysfunction and negative biomarkers, anticoagulation alone is the treatment of choice. Thrombolytic therapy has demonstrated no clinical benefit for this patient population [9999. Wan S, Quinlan DJ, Agnelli G and Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004. ]. If proper outpatient care and anticoagulant treatment can be provided, early hospital discharge should be considered. A prospective study showed that outpatient management of PE is feasible and safe for the majority of these patients [155155. Kovacs MJ, Anderson D, Morrow B, Gray L, Touchie D and Wells PS. Outpatient treatment of pulmonary embolism with dalteparin. Thromb Haemost. 2000. ].
Intermediate-risk pulmonary embolism
Haemodynamically stable patients with evidence of RV dysfunction or myocardial injury are classified as intermediate-risk patients. Whereas thrombolysis is accepted as gold standard treatment for unstable patients, the benefits of thrombolytic therapy in intermediate-risk patients are controversial. Despite normal systemic arterial blood pressure, patients with RV dysfunction have a higher mortality than those with normal RV function [1515. Goldhaber SZ, Visani L and De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999. , 9090. Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, Conti A, Agnelli G and Berni G. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000. ]. Therefore, intermediate risk patients will be further stratified into intermediate-high and intermediate-low risk patients ( Figure 1 ) to identify the appropriate therapy.
Intermediate-high risk patients
There is growing evidence that intermediate-high risk patients may benefit from a primary reperfusion therapy. The PEITHO trial [128128. Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, Bluhmki E, Bouvaist H, Brenner B, Couturaud F, Dellas C, Empen K, Franca A, Galie N, Geibel A, Goldhaber SZ, Jimenez D, Kozak M, Kupatt C, Kucher N, Lang IM, Lankeit M, Meneveau N, Pacouret G, Palazzini M, Petris A, Pruszczyk P, Rugolotto M, Salvi A, Schellong S, Sebbane M, Sobkowicz B, Stefanovic BS, Thiele H, Torbicki A, Verschuren F, Konstantinides SV and Investigators P. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014.
RCT examining the role of fibrinolysis in patients with pulmonary embolism and markers of intermediate risk] revealed systemic thrombolysis as beneficial therapy in intermediate-high risk patients to prevent life-threatening haemodynamic decompensation or collapse. However, this benefit is counterbalanced by an increased risk of haemorrhagic stroke or major non-intracranial bleeding. Based on these results, thrombolytic therapy may be favourable in selected patients with intermediate-high risk PE, particularly in those with low bleeding risk. This observation emphasizes the need for rapid and reliable risk stratification in all patients with PE. In critical patients with high bleeding risk surgical pulmonary embolectomy or percutaneous catheter-directed treatment may be considered as revascularization option. Particularly, catheter-directed ultrasound-accelerated thrombolysis has been identified to be superior in reversing RV dilatation at 24 hours, without an increase in bleeding complications compared to anticoagulation with heparin alone [152152. Kucher N, Boekstegers P, Muller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, Tebbe U, Horstkotte J, Muller R, Blessing E, Greif M, Lange P, Hoffmann RT, Werth S, Barmeyer A, Hartel D, Grunwald H, Empen K and Baumgartner I. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014. ].
Intermediate-low risk patients
Anticoagulation alone is the treatment of choice. There is no evidence to recommend primary reperfusion therapy as well as bed rest.
Incidental clinically unsuspected pulmonary embolism
The widespread use of CT scans has led to an increased number of incidentally diagnosed, clinical unsuspected PE during diagnostic work-up for other diseases [156156. Farrell C, Jones M, Girvin F, Ritchie G and Murchison JT. Unsuspected pulmonary embolism identified using multidetector computed tomography in hospital outpatients. Clin Radiol. 2010. ]. Incidental discovery of PE was most frequently observed in patients with cancer, but also in patients with paroxysmal atrial fibrillation and heart failure [157157. Jia CF, Li YX, Yang ZQ, Zhang ZH, Sun XX and Wang ZQ. Prospective evaluation of unsuspected pulmonary embolism on coronary computed tomographic angiography. J Comput Assist Tomogr. 2012. , 158158. Sahut D’Izarn M, Caumont Prim A, Planquette B, Revel MP, Avillach P, Chatellier G, Sanchez O and Meyer G. Risk factors and clinical outcome of unsuspected pulmonary embolism in cancer patients: a case-control study. J Thromb Haemost. 2012. ]. Despite a lack of clear evidence, the guidelines carefully recommend that patients with cancer and those with emboli at the lobar or more proximal level should be managed in the same way as symptomatic PE [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism].
Long-term anticoagulation and secondary prophylaxis
VTE recurs in approximately one fourth of patients within five years after the initial venous thromboembolic event [159159. Eichinger S, Heinze G, Jandeck LM and Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. 2010. ], which justifies long-term anticoagulant treatment of patients with PE to prevent fatal and non-fatal recurrent VTE events. Anticoagulant therapy of VTE is classified into three stages. Following the initial therapy, the long-term treatment, and if necessary extended anticoagulation have to be established.
The transition from initial therapy to long-term treatment occurs traditionally in two overlapping steps. Immediate full anticoagulation with a parenteral anticoagulant as initial therapy. Simultaneously with parenteral anticoagulation, oral anticoagulation with vitamin K antagonists is initiated, which remains the mainstay of secondary prophylaxis. Heparin and vitamin K antagonist therapy should initially overlap because vitamin K antagonists also reduce the activity of anticoagulant proteins C and S. Full therapeutic efficacy at an international normalised ratio of 2.0 to 3.0 is achieved after five days of therapy [160160. Piazza G and Goldhaber SZ. Acute pulmonary embolism: part II: treatment and prophylaxis. Circulation. 2006. ]. Heparin treatment can be discontinued when the INR has been in the therapeutic range on two measurements at least 24 hours apart. Since treatment with a vitamin K antagonist requires laboratory monitoring and dose adjustment and may be complicated by drug and food interactions, outpatient therapy remains challenging. Whether oral anticoagulation can be stopped or must be taken indefinitely depends on the underlying disease and the risk of recurrence [160160. Piazza G and Goldhaber SZ. Acute pulmonary embolism: part II: treatment and prophylaxis. Circulation. 2006. ]. The guidelines [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism] recommend anticoagulation for at least three months in patients with unprovoked PE or with PE secondary to a transient (reversible) risk factor. For patients with PE and cancer, weight-adjusted LMWH anticoagulant therapy should be considered for the first 3 to 6 months. Before stopping treatment clinicians must balance the long-term risks of recurrent VTE if anticoagulation is stopped against the burden and risks of ongoing therapy [161161. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE and Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008. ]. In those patients at high-risk of recurrent VTE, an extended anticoagulation therapy should be considered as long as the bleeding risk is not excessive. Extended anticoagulation should regularly be reevaluated, based on changes in the balance between the risks of recurrence and bleeding. Lifelong treatment anticoagulation is recommended for patients with a second episode of unprovoked PE [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism]. A risk score has been proposed for the estimation of the risk of recurrence after a first symptomatic PE, based on thrombus location, gender and D-Dimer at three months [159159. Eichinger S, Heinze G, Jandeck LM and Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. 2010. ].
Over the past years the new oral anticoagulants, including dabigatran, rivaroxaban, and apixaban, have been intensively evaluated as treatment option for long-term as well as extended therapy of patients with VTE [162162. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Porcari A, Raskob GE, Weitz JI and Investigators P-E. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013. , 163163. Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F and Schellong S. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010. , 164164. Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, Kvamme AM, Friedman J, Mismetti P, Goldhaber SZ, Investigators R-MT and Investigators R-ST. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013. ]. In contrast to VKA, treatment initiation with rivaroxaban and apixaban are started in the acute phase without initial parenteral coagulation and an overlapping switch. Administration of dabigatran is recommended following acute phase parenteral anticoagulation. For long-term anticoagulation NOACs were found noninferior to conventional therapy with vitamin K antagonist for VTE treatment and are associated with less bleeding [165165. Prins MH, Lensing AW, Bauersachs R, van Bellen B, Bounameaux H, Brighton TA, Cohen AT, Davidson BL, Decousus H, Raskob GE, Berkowitz SD, Wells PS and Investigators E. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thrombosis journal. 2013. , 166166. van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA and Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014. ]. In the setting of extended therapy, dabigatran, rivaroxaban, and apixaban are superior to placebo for the prevention of recurrent VTE and are associated with low rates of major bleeding. There is growing evidence that the dose of NOACs can be lowered for extended VTE treatment to reduce the bleeding risk without compromising efficacy [162162. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Porcari A, Raskob GE, Weitz JI and Investigators P-E. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013. ]. On the contrary, a lower dose of vitamin K antagonist for extended VTE therapy was associated with reduced efficacy without evidence of less bleeding [167167. Kearon C, Ginsberg JS, Kovacs MJ, Anderson DR, Wells P, Julian JA, MacKinnon B, Weitz JI, Crowther MA, Dolan S, Turpie AG, Geerts W, Solymoss S, van Nguyen P, Demers C, Kahn SR, Kassis J, Rodger M, Hambleton J, Gent M and Extended Low-Intensity Anticoagulation for Thrombo-Embolism I. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003. ].
Inferior vena cava filters
Inferior vena cava (IVC) filters were originally developed for selected patients with absolute contraindications to anticoagulation, failure of anticoagulation or complication whilst on anticoagulation, who have a venous thromboembolic event, or prophylactically for patients with a high risk of PE. It is important to emphasise that the use of an IVC filter does not obviate the need for anticoagulation, unless a major complication has ensued [161161. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE and Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008. ]. In particular, after implanting an IVC filter as a temporary alternative, anticoagulation should be started as soon as the risk of bleeding has resolved.
IVC filters are used in the indication of DVT or PE to reduce the risk of recurrent PE. Filters are implanted percutaneously just below the junction of the IVC and the lowest renal vein. In cases of renal vein thrombosis or where thrombus exists at the level of the renal veins, filters can be placed in a suprarenal position. The right internal jugular vein or the right femoral vein is the preferred point of vascular access, but left-sided venous approaches or approaches using arm veins can be used depending on patient anatomy. Bedside ultrasound-guided placement and bedside intravascular ultrasound-guided placement may improve the safety and efficacy of this procedure [168168. Chiou AC. Intravascular ultrasound-guided bedside placement of inferior vena cava filters. Semin Vasc Surg. 2006. , 169169. Uppal B, Flinn WR and Benjamin ME. The bedside insertion of inferior vena cava filters using ultrasound guidance. Perspect Vasc Surg Endovasc Ther. 2007. ].
Filters span the luminal diameter of the IVC and are designed to mechanically trap thrombus from the lower half of the body, reducing the risk of emboli from reaching the pulmonary circulation. Filters per se do not have any anticoagulant effects or prevent the recurrence of DVT and emboli [170170. Streiff MB. Vena caval filters: a review for intensive care specialists. J Intensive Care Med. 2003. ].
There are numerous IVC filters currently available ( Table 8 , Figure 5 ) which are principally of two types: permanent or retrievable. Additionally, filters differ in terms of material, maximum radial diameter, and MRI compatibility.
The implantation of permanent IVC filters may provide lifelong protection against recurrent PE, but not against the sequelae of lower limb venous disease. The latter include recurrent DVT (in 20% of implantations) and post-thrombotic syndrome (in 40% of patients) [171171. Hann CL and Streiff MB. The role of vena caval filters in the management of venous thromboembolism. Blood Rev. 2005. ]. Procedural complications include access site haematomas (2.4% to 4.2% of cases), access site thrombosis (3.8% to 4.2%) and filter displacement (1.1% to 4.6%) [172172. Chung J and Owen RJ. Using inferior vena cava filters to prevent pulmonary embolism. Can Fam Physician. 2008. ].
Retrievable filters were designed to reduce the long-term complications associated with permanent filters, in particular the increased risk of DVT [173173. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005. , 174174. Decousus H, Leizorovicz A, Parent F, Page Y, Tardy B, Girard P, Laporte S, Faivre R, Charbonnier B, Barral FG, Huet Y and Simonneau G. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998. , 175175. Imberti D and Prisco D. Retrievable vena cava filters: key considerations. Thromb Res. 2008. ]. It is recommended that retrievable devices should be removed within 2 weeks of implantation (an example of the retrieval technique is illustrated in ( Figure 6 ). The availability of retrievable filters led to an increased use of IVC filters with lower threshold for filter placement [176176. Rutherford RB. Prophylactic indications for vena cava filters: critical appraisal. Semin Vasc Surg. 2005. , 177177. Van Ha TG, Chien AS, Funaki BS, Lorenz J, Piano G, Shen M and Leef J. Use of retrievable compared to permanent inferior vena cava filters: a single-institution experience. Cardiovasc Intervent Radiol. 2008. ], although up to 70% of retrievable filters were not removed [178178. Grande WJ, Trerotola SO, Reilly PM, Clark TW, Soulen MC, Patel A, Shlansky-Goldberg RD, Tuite CM, Solomon JA, Mondschein JI, Fitzpatrick MK and Stavropoulos SW. Experience with the recovery filter as a retrievable inferior vena cava filter. J Vasc Interv Radiol. 2005. ]. Reasons for this may be that removal of these filters requires a second interventional procedure leading to additional costs and human resources, radiation exposure, and procedural risks. Furthermore, some retrievable filters cannot be removed for technical reasons such as angulation or thrombus [179179. Berczi V, Bottomley JR, Thomas SM, Taneja S, Gaines PA and Cleveland TJ. Long-term retrievability of IVC filters: should we abandon permanent devices?. Cardiovasc Intervent Radiol. 2007. , 180180. Seshadri T, Tran H, Lau KK, Tan B and Gan TE. Ins and outs of inferior vena cava filters in patients with venous thromboembolism: the experience at Monash Medical Centre and review of the published reports. Intern Med J. 2008. ].
The risk/benefit ratio of IVC filters is unclear. A large randomised study[173173. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005. ] demonstrated a reduced risk of recurrent PE at the cost of an increased risk of recurrent DVT, with no effect on overall survival. Current guidelines do not recommend the routine use of IVC filters in patients with PE (Class III, Level B). Even the recommendation for IVC filter implantation in patients with absolute contraindications to anticoagulation and a high risk of VTE recurrence is cautious (Class IIb, Level B) [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism].
Primary prophylaxis
Patients at risk, including nearly all hospitalised patients, should receive mechanical or pharmacological prophylaxis for VTE to reduce the incidence of symptomatic DVT and/or PE. Especially in post-operative patients, the risk of VTE persists several weeks after hospital discharge [173173. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005. ]. Mechanical strategies include the use of compression stockings and intermittent pneumatic compression, which can reduce the risk of DVT by 60% [181181. Urbankova J, Quiroz R, Kucher N and Goldhaber SZ. Intermittent pneumatic compression and deep vein thrombosis prevention. A meta-analysis in postoperative patients. Thromb Haemost. 2005. ]. Unfractionated heparin, LMWH, fondaparinux, and warfarin are recommended as pharmacological prophylaxis for VTE, in combination with mechanical devices in high-risk patients [160160. Piazza G and Goldhaber SZ. Acute pulmonary embolism: part II: treatment and prophylaxis. Circulation. 2006. ]. Furthermore, the effectiveness of NOACs in the prevention of VTE after orthopaedic surgery has been demonstrated [182182. Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, Schnee JM and Friedman RJ. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost. 2011. , 183183. Eriksson BI, Kakkar AK, Turpie AG, Gent M, Bandel TJ, Homering M, Misselwitz F and Lassen MR. Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. J Bone Joint Surg Br. 2009. , 184184. Friedman RJ, Dahl OE, Rosencher N, Caprini JA, Kurth AA, Francis CW, Clemens A, Hantel S, Schnee JM and Eriksson BI. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res. 2010. , 185185. Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel TJ, Benson A, Misselwitz F and Fisher WD. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009. ].
In addition, subanalyses of the Jupiter trial [77. Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT and Ridker PM. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009. ] have demonstrated that treatment with rosuvastatin prevents venous thrombotic events in apparently healthy persons with elevated C-reactive protein.
Prevention of recurrent pulmonary embolism
- The new acute PE risk stratification is utilising right ventricular function, the PESI score and biomarkers to predict outcome and guide treatment strategies [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism]
- Annual risk of recurrence after symptomatic pulmonary embolism 8.70% [186186. Douketis J, Tosetto A, Marcucci M, Baglin T, Cushman M, Eichinger S, Palareti G, Poli D, Tait RC and Iorio A. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med. 2010. ]
- Annual risk of bleeding with anticoagulation 3.36% [187187. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD and Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009. ]
- Anticoagulation for at least 3 months in patients with unprovoked PE or with PE secondary to a transient (reversible) risk factor [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism]
- The new oral anticoagulants (Dabigatran, Rivaroxaban, Apixaban and Edoxaban) have diminished bleeding rates in the presence of at least equal efficacy, and selected NOACs are efficacious for long-term prevention of recurrence at reduced doses [119119. Yeh CH, Gross PL and Weitz JI. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood. 2014.
Broad review of contemporary NOACs]
Pulmonary hypertension
SUMMARY
“The end is where we start from” refers to the vast loss of functional pulmonary vessels which has already taken place in >80% of patients with pulmonary arterial hypertension (PAH) at the point when clinical symptoms of pulmonary hypertension (PH) occur for the first time.
According to the current guidelines for the diagnosis and treatment of pulmonary hypertension [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension] the diagnostic classification separates precapillary pulmonary hypertension, i.e., pulmonary hypertension due to pulmonary vascular disease mainly affecting the precapillary arteriolar compartment (i.e., groups 1, 3, 4 and 5), from postcapillary disease which originates distal to the capillaries, and entails morphological changes in the precapillary compartment only after a significant pressure increase in the venous compartment (i.e., group 2). Currently, a single haemodynamic parameter, i.e., wedge pressure [189189. Lipman J. Pitfalls in the interpretation of pulmonary capillary wedge pressure. S Afr Med J. 1985. ] which is commonly flawed by methodological errors [190190. Tonelli AR, Mubarak KK, Li N, Carrie R and Alnuaimat H. Effect of balloon inflation volume on pulmonary artery occlusion pressure in patients with and without pulmonary hypertension. Chest. 2011. ] is used to distinguish between pre and postcapillary disease. This distinction is key because treatments are completely different for the two classes of disease [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. For example, epoprostenol, the life-saving treatment for PAH [191191. McLaughlin VV, Shillington A and Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002. ] , was shown to be detrimental in congestive heart failure [192192. Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE, Darius H, Schulman K, Zannad F, Handberg-Thurmond E, Harrell FE, Jr., Wheeler W, Soler-Soler J and Swedberg K. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1997. ].
INTRODUCTION
Major advances have taken place in the area of pulmonary hypertension (PH) over the past decade. Our understanding of the pathophysiological mechanisms underlying the condition has improved significantly, has led to new treatment strategies [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension].
DEFINITION
PH is a haemodynamic and pathophysiological state that can be found in multiple clinical conditions, and is defined by an invasively measured mean pulmonary arterial pressure (PAP) ≥25 mmHg at rest [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. The normal resting mean PAP is 14 ± 3 mmHg with an upper limit of normal of approximately 20 mmHg. No standardised definition exists of PH on exercise. Borderline PH with mean PAP values in the upper normal should be carefully followed when further risk factors for PAH are present. Preliminary data suggests that borderline PH with resting mean PAP greater than 17 mmHg may be associated with adverse events and reduced survival [193193. Kovacs G, Maier R, Aberer E, Brodmann M, Scheidl S, Troster N, Hesse C, Salmhofer W, Graninger W, Gruenig E, Rubin LJ and Olschewski H. Borderline pulmonary arterial pressure is associated with decreased exercise capacity in scleroderma. Am J Respir Crit Care Med. 2009. ].
Hemodynamically, PH is classified into pre-capillary PH, characterized by a mean pulmonary arterial wedge pressure (PAWP) ≤15 mm Hg, and post-capillary PH, as indicated by a PAWP >15 mm Hg. This distinction is important because medications that have been approved for pre-capillary disease do not work in post-capillary disease. PAH represents a rare form of pre-capillary PH, defined by a pulmonary PAWP ≤15 mmHg and additionally a PVR > 3 Wood units (WU) in the absence of other causes of precapillary PH such as lung diseases, chronic thromboembolism or other rare diseases [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. However, evidence exists that a PAWP of 12mmHg may be a better cutoff to safely classify pre-capillary PH [194194. Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, Maurer G and Lang IM. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in "out-of-proportion" pulmonary hypertension. Chest. 2013. , 195195. Naeije R, Vachiery JL, Yerly P and Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J. 2013. ]
Post-capillary PH related to left heart and valve disease may be further stratified into isolated post-capillary PH, classified by a diastolic pressure gradient (DPG) < 7 mmHg and/or a PVR ≤ 3 WU, or a combined post-capillary PH with a pre-capillary component (DPG ≥ 7 mmHg and/or a PVR > 3 WU) [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension, 194194. Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, Maurer G and Lang IM. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in "out-of-proportion" pulmonary hypertension. Chest. 2013. ].
CLINICAL CLASSIFICATION OF PULMONARY HYPERTENSION
The early clinical signs and symptoms of PH are scarce, and >80% of patients have advanced disease with World Health Organization functional classes >III at first presentation [22. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E and Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006. ]. Pulmonary arterial hypertension (PAH) should be considered in the differential diagnosis of unexplained fatigue, exertional dyspnoea, syncope, angina and progressive limitation of exercise capacity, particularly in patients without signs of common cardiovascular or respiratory disorders.
Recently published guidelines for the diagnosis and treatment of pulmonary hypertension have provided an updated classification [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension], and recommendations for contemporary diagnosis and treatment of PH [196196. Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM and Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013. ]. PH is classified into five groups according to pathological and pathophysiological characteristics, with several new genetic entities that were added, e.g. group 1’ which is veno-occlusive disease and/or pulmonary capillary haemangiomatosis as a consequence of mutations in the EIF2AK4 gene (eukaryotic translation initiation factor 2 alpha kinase 4) ( Table 9 ) Group 1 has been extended to include idiopathic, heritable, drug-, toxin- and radiation-induced and associated forms. Group 2 has been amended by congenital/acquired left heart inflow and outflow tract obstruction. Group 3 has taken on Developmental lung diseases. Hemolytic anemia associated PH was eliminated from group 1.
Due to subset-specific treatments, an exact diagnosis and group assignment are of major importance. Patients from group 1 have a benefit from specific (“targeted”) vasodilator therapy, whereas in patients from groups 2, 3 and 5 treatment of the underlying cardiac and pulmonary disease is indicated.
EPIDEMIOLOGY
The incidence and prevalence of group 1 PAH, which includes idiopathic, heritable, drug-induced PAH, as well as PAH associated with a variety of conditions and diseases, are estimated at 2.4 cases/million annually and 15 cases/million in France [22. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E and Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006. ]] and 7.6 cases/million annually and 26 cases/million in Scotland [197197. Peacock AJ, Murphy NF, McMurray JJ, Caballero L and Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007. ]. Idiopathic PAH (IPAH, formerly primary pulmonary hypertension, PPH) is the prototype of PAH. In the subgroup of associated PAH conditions, the leading disease is systemic sclerosis. The prevalence of PAH in the developing world is probably greater because risk factors for PAH, such as HIV, schistosomiasis, and sickle cell disease, are more prevalent in these countries [198198. Butrous G, Ghofrani HA and Grimminger F. Pulmonary vascular disease in the developing world. Circulation. 2008. ]. The mortality at 1 year for PAH is 15% [199199. Thenappan T, Shah SJ, Rich S and Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982-2006. Eur Respir J. 2007. ]
The prevalence of group 2 PH, which is caused by left heart disease, is very high in patients with left ventricular (LV) dysfunction. About 60% of patients with severe LV systolic dysfunction and up to 70% of patients with isolated LV diastolic dysfunction develop PH, which is a prognostic factor [200200. Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F and Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001. ]. In left-sided valvular heart diseases the prevalence of PH depends on the severity of the defect and of the symptoms. Nearly all patients with severe symptomatic mitral valve disease and up to 65% of those with symptomatic aortic stenosis have concomitant PH [201201. Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ and Torbicki A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009. , 202202. Oudiz RJ. Pulmonary hypertension associated with left-sided heart disease. Clin Chest Med. 2007. , 203203. Joint Task Force on the Management of Valvular Heart Disease of the European Society of C, European Association for Cardio-Thoracic S, Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL and Zembala M. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012. ].
Group 3 PH due to lung diseases and/or hypoxaemia as occurring in patients with chronic obstructive pulmonary disease (COPD) carries a prevalence of 20% and increases >50% in advanced COPD [204204. Chaouat A, Bugnet AS, Kadaoui N, Schott R, Enache I, Ducolone A, Ehrhart M, Kessler R and Weitzenblum E. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005. , 205205. Thabut G, Dauriat G, Stern JB, Logeart D, Levy A, Marrash-Chahla R and Mal H. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005. ]. Interstitial lung disease is associated with PH in between 32% and 39% [206206. Lettieri CJ, Nathan SD, Barnett SD, Ahmad S and Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006. ].
The incidence of CTEPH is estimated at 5 per million and year [33. Pepke-Zaba J, Jansa P, Kim NH, Naeije R and Simonneau G. Chronic thromboembolic pulmonary hypertension: role of medical therapy. Eur Respir J. 2013. ]. CTEPH is derived from pulmonary embolism, but may also occur in the absence of symptomatic PE [207207. Guerin L, Couturaud F, Parent F, Revel MP, Gillaizeau F, Planquette B, Pontal D, Guegan M, Simonneau G, Meyer G and Sanchez O. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost. 2014. ].
Due to the heterogeneity of group 5 PH with unclear and/or multifactorial mechanisms, epidemiological data are scarce.
DIAGNOSIS
A series of examinations is necessary in patients with suspected PH. Assignment to a PH subset is of major importance for optimal therapy. An integrated diagnostic algorithm [208208. Lang IM and Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation. 2014. ] as shown in Figure 7 summarises recommended diagnostic steps.
Clinical presentation
Pulmonary hypertension commonly goes clinically silent until advanced stages of the disease. More than 75% of patients first present in NYHA classes III and IV. Cardinal symptom is breathlessness on exertion. Other common symptoms are fatigue, weakness and angina pectoris as a correlate for RV ischaemia. Syncope, pre-syncope, vertigo, peripheral oedema and haemoptyses may occur in advanced stages [209209. Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK and et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987. ], as well as symptoms at rest (WHO class IV). Physical examination reveals a palpable parasternal RV impulse, an accentuated pulmonary component of second heart sound, a right-sided fourth heart sound, and tricuspid regurgitation. Signs of right heart failure, including increased jugular venous pressure, hepatomegaly, peripheral oedema, ascites, and peripheral cyanosis [210210. Gaine SP and Rubin LJ. Primary pulmonary hypertension. Lancet. 1998. ]. Lung auscultation and oxygen saturation are usually normal in idiopathic PAH.
Any of the above symptoms can be caused by many other diseases, such as coronary artery disease, emphysema, pneumonia, congestive heart failure and asthma. Therefore, the diagnosis of PH is often delayed until symptoms become severe and extensive vascular damage has occurred [211211. McGoon M, Gutterman D, Steen V, Barst R, McCrory DC, Fortin TA and Loyd JE. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004. ]. However, early diagnosis and treatment are believed to be crucial [212212. McLaughlin VV, Presberg KW, Doyle RL, Abman SH, McCrory DC, Fortin T and Ahearn G. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004. ]. Various tests can be used for the initial evaluation of patients suspected of having PH. Chest x-rays and ECG are diagnostic tools with low accuracy for the diagnosis of PH, however, due to their broad availability and low cost, they can be employed as first diagnostic steps.
Diagnostic algorithm/Imaging
PAH should be considered in the differential diagnosis of dyspnoea, syncope, angina, and/or progressive limitation of exercise capacity. In particular, patients with associated conditions and/or risk factors for development of PAH, such as family history, connective tissue disease, congenital heart disease, HIV infection, portal hypertension, haemolytic anaemia, or a history of intake of drugs and toxins, should be systematically examined. ECG, chest radiograph, transthoracic echocardiogram, pulmonary function tests and high-resolution chest CT are recommended to identify potential patients of group 2 with left heart disease or group 3 with lung diseases. In patients with diagnosed scleroderma, annual screening (echocardiogram, DLCO, and BNP) is recommended [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. According to a recent algorithm ( Figure 7 ), a V/Q-scan is indicated as a first step to rule out CTEPH [213213. Lang IM, Plank C, Sadushi-Kolici R, Jakowitsch J, Klepetko W and Maurer G. Imaging in pulmonary hypertension. JACC Cardiovasc Imaging. 2010. ]. If elevated pressure in the pulmonary circulation is suspected, an RHC is necessary. After a diagnosis of PH has been confirmed, additional specific diagnostic tests are to be performed to determine the specific aetiology of PH ( Table 10 ) [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. Since PAH, and particularly IPAH, is a diagnosis of exclusion, it is essential to follow this algorithm to facilitate the diagnostic investigation.
Electrocardiogram
Typical signs of PH in the ECG are RV hypertrophy and strain, right axis deviation and right atrial dilatation. Supraventricular arrhythmias may be present in advanced stages and are associated with clinical deterioration [214214. Tongers J, Schwerdtfeger B, Klein G, Kempf T, Schaefer A, Knapp JM, Niehaus M, Korte T and Hoeper MM. Incidence and clinical relevance of supraventricular tachyarrhythmias in pulmonary hypertension. Am Heart J. 2007. ]. The absence of any abnormalities in the ECG does not exclude PH. About 13% of patients with a confirmed diagnosis of PH have initially been shown to have normal ECGs [215215. Ahearn GS, Tapson VF, Rebeiz A and Greenfield JC, Jr. Electrocardiography to define clinical status in primary pulmonary hypertension and pulmonary arterial hypertension secondary to collagen vascular disease. Chest. 2002. ]. Due to its low sensitivity (55%) and specificity (70%), ECG may provide only suggestive or supportive evidence of PH [215215. Ahearn GS, Tapson VF, Rebeiz A and Greenfield JC, Jr. Electrocardiography to define clinical status in primary pulmonary hypertension and pulmonary arterial hypertension secondary to collagen vascular disease. Chest. 2002. ]. However, ECG criteria in combination with biomarkers and echocardiography have been shown to improve specificity substantially [216216. Bonderman D, Wexberg P, Martischnig AM, Heinzl H, Lang MB, Sadushi R, Skoro-Sajer N and Lang IM. A noninvasive algorithm to exclude pre-capillary pulmonary hypertension. Eur Respir J. 2011. ]. These tools help avoid one of ten invasive haemodynamic assessments, which are performed for suspicion of precapillary disease.
Pulmonary function tests
Diffusion capacity of the lung for carbon monoxide (DLCO) is an important diagnostic and prognostic variable in PH patients [217217. Trip P, Nossent EJ, de Man FS, van den Berk IA, Boonstra A, Groepenhoff H, Leter EM, Westerhof N, Grunberg K, Bogaard HJ and Vonk-Noordegraaf A. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J. 2013. , 218218. Sun XG, Hansen JE, Oudiz RJ and Wasserman K. Pulmonary function in primary pulmonary hypertension. J Am Coll Cardiol. 2003. ]. The differential diagnosis for PH patients with abnormal DLCO < 45% includes PVOD, PAH associated with scleroderma and parenchymal lung disease [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension].
Chest X-ray
The chest x-ray is abnormal in 90% of patients with IPAH at the time of diagnosis [209209. Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK and et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987. ]. The chest x-ray may reveal hilar enlargement, which reflects pulmonary artery dilation. Right atrium and RV enlargement may be detected in more advanced cases. The chest x-ray also plays an essential role in the exclusion of lung diseases that form an important part of the differential diagnosis.
Echocardiography
Transthoracic Doppler echocardiography is the predominant screening modality in early stages of diagnosis. Importantly, echocardiography should be used to determine the probability of PH, but echocardiography alone is not sufficient to support a treatment decision [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. Three levels of probability of PH (low, medium, and high) are established based on peak tricuspid regurgitation velocity (TRV) at rest and, as mandated in the guidelines, on the presence of additional echocardiographic variables, including PH signs in ventricles (eccentricity index), the pulmonary artery diameter and the inferior vena cava and right atrium (collapsibility upon inspiration). Grading of the probability of pulmonary hypertension in symptomatic patients with a suspected diagnosis based on echocardiographic parameters is outlined in Table 11.
In patients with a high probability of PH, it is recommended to perform an invasive diagnostic evaluation [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. In patients with low probability of PH, an alternative diagnosis should be considered. In patients with intermediate probability of PH and risk factors or associated conditions for PAH or CTEPH, further investigations including RHC are recommended.
Surrogates of right ventricular function e.g. tricuspid annular plane systolic excursion, global longitudinal strain of the RV free wall or color tissue Doppler S wave of tricuspid annulus may be useful to follow up right ventricular function over time [219219. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W and Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015. ]. Furthermore, significant progress has been made in use of knowledge-based reconstruction of 3D RV structure and function from 2D images [220220. Bhave NM, Patel AR, Weinert L, Yamat M, Freed BH, Mor-Avi V, Gomberg-Maitland M and Lang RM. Three-dimensional modeling of the right ventricle from two-dimensional transthoracic echocardiographic images: utility of knowledge-based reconstruction in pulmonary arterial hypertension. J Am Soc Echocardiogr. 2013. ]. Studies have suggested that 3D echo imaging of the RV is feasible, and its results compare well with magnetic resonance imaging (MRI) [220220. Bhave NM, Patel AR, Weinert L, Yamat M, Freed BH, Mor-Avi V, Gomberg-Maitland M and Lang RM. Three-dimensional modeling of the right ventricle from two-dimensional transthoracic echocardiographic images: utility of knowledge-based reconstruction in pulmonary arterial hypertension. J Am Soc Echocardiogr. 2013. ].
Right heart catheterisation and vasoreactivity testing
The diagnosis of pulmonary hypertension is based on right heart catheterisation [219219. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W and Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015. ]. Right heart catheterisation (RHC) is associated with low rates of morbidity (1.1%) and mortality (0.055%) when performed in experienced centres [221221. Hoeper MM, Lee SH, Voswinckel R, Palazzini M, Jais X, Marinelli A, Barst RJ, Ghofrani HA, Jing ZC, Opitz C, Seyfarth HJ, Halank M, McLaughlin V, Oudiz RJ, Ewert R, Wilkens H, Kluge S, Bremer HC, Baroke E and Rubin LJ. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006. ]. The following parameters should be assessed during RHC: pulmonary artery pressure (systolic, diastolic and mean), right atrial pressure, pulmonary artery occlusion pressure (PCOP), and RV pressure. Cardiac output should be measured in quadruplicate by thermodilution or by the Fick method which is obligatory when systemic-to-pulmonary shunt is present). The measurement of superior vena cava, pulmonary artery, and systemic arterial blood oxygen saturations and pulmonary vascular resistance is recommended. The assessment of PAWP is essential to discriminate between pre and postcapillary PH. A PAWP >15 mmHg suggests elevated LV filling pressures, and generally excludes the diagnosis of precapillary PAH, except for extremely rare cases where pre and postcapillary PH coexist. During RHC the vasoreactivity of the pulmonary circulation is assessed in patients with precapillary PH. Recommendations for RHC and vasoreactivity test are summarised in Table 12. The use of nitric oxide is recommended for vasoreactivity testing. A positive acute response is defined as a decrease of the mean PAP ≥10 mmHg to reach an absolute value of mean PAP ≤40 mmHg with an increased or unchanged cardiac output [222222. Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Herve P and Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005. ]. A positive acute test result predicts a high likelihood of a clinical and haemodynamic response to calcium channel blockers, but does not guarantee a response. The simultaneous performance of a coronary angiography is indicated in patients with a high-risk profile for coronary artery disease, or in case of listing for double lung transplantation or pulmonary endarterectomy (PEA) in patients with CTEPH.
Ventilation/perfusion lung scan
A V/Q-scan should be performed in patients with PH to exclude CTEPH ( Figure 7). V/Q-scan has a higher sensitivity than CT and remains the screening method of choice for CTEPH [223223. Tunariu N, Gibbs SJ, Win Z, Gin-Sing W, Graham A, Gishen P and Al-Nahhas A. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007. ].
Contrast-enhanced computed tomography/High-resolution computed tomography
These examinations provide detailed information about the pulmonary artery trunk, proximal and distal pulmonary arteries, and morphology of the heart and lung parenchyma. In patients with PH, the diameter of the pulmonary artery trunk is significantly enlarged and correlates well with pulmonary artery pressure measurements [224224. Karazincir S, Balci A, Seyfeli E, Akoglu S, Babayigit C, Akgul F, Yalcin F and Egilmez E. CT assessment of main pulmonary artery diameter. Diagn Interv Radiol. 2008. ]. For identifying CTEPH, contrast CT angiography is an important diagnostic tool, although less sensitive than pulmonary angiography.
Cardiac magnetic resonance imaging
Cardiac magnetic resonance imaging has been shown to be highly accurate and reproducible in the measurement of RV morphology and function, and allows non-invasive assessment of RV mass. In patients with PAH, cardiac magnetic resonance provides useful prognostic information at baseline and at follow-up [225225. van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, Postmus PE and Vonk-Noordegraaf A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007. , 226226. van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM, Westerhof N and Vonk-Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011. ].
Pulmonary angiography
Pulmonary angiography is important to examine the pulmonary vasculature for identifying patients who may benefit from PEA or percutaneous balloon pulmonary angioplasty in the setting of CTEPH [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension].
Genetic testing and counseling
Genetic testing for IPAH, heritable pulmonary arterial hypertension and veno-occlusive PH are recommended upon diagnosis of the disease [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. Patients with IPAH should be screened for BMPR2, ACVRL1 and ENG mutations. Heterozygous BMPR2 mutations represent the most common genetic disorders and account for approximately 75% of familial PAH. In patients with sporadic or familial pulmonary veno-occlusive disease and/or pulmonary capillary haemangiomatosis screening for EIF2AK4-mutations (eukaryotic translation initiation factor 2 alpha kinase 4) is recommended. No underlying genetic basis has been detected in patients with PH in groups 2 to 5.
Genetic counseling should be offered to selected PAH patients by a specialized multidisciplinary team, involving PH specialists, genetic counsellors, geneticists, psychologists and nurses in line with local legislation [227227. Tenorio J, Navas P, Barrios E, Fernandez L, Nevado J, Quezada CA, Lopez-Meseguer M, Arias P, Mena R, Lobo JL, Alvarez C, Heath K, Escribano-Subias P and Lapunzina P. A founder EIF2AK4 mutation causes an aggressive form of pulmonary arterial hypertension in Iberian Gypsies. Clin Genet. 2015. ].
PROGNOSIS AND TREATMENT
Comprehensive prognostic evaluation and risk assessment is critical for further therapeutic strategies and therefore highlighted by current guidelines [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. It has become apparent that a single variable does not provide sufficient diagnostic and prognostic information. Therefore, the severity of PAH should be regularly assessed in expert PH centres at baseline and every 3-6 months based on a multidimensional approach, including clinical assessment, exercise tests, biochemical markers and echocardiographic and haemodynamic evaluations. PAH patients should be stratified into low- (< 5%), intermediate- (5%-10%), and high-risk (> 10%) patients according to their calculated risk of 1 year mortality as followed:
Low risk: No signs of right heart failure, no progression of symptoms, New York Heart Association (NYHA)/WHO functional class (FC) I, II, 6MWD >440 m, peak VO2 >15 ml/min/kg, NT-proBNP <300 ng/L, no pericardial effusion, normal right atrial (RA) size, RA pressure <8 mm Hg, CI ≥2.5 L/min/m2, and SvO2 >65%.
Intermediate risk: No signs of right heart failure, occasional syncope, slow progression of symptoms, WHO FC III, 6MWD 165-440 m, peak VO2 11-15 ml/min/kg, NT-proBNP 300-1400 ng/L, no or minimal pericardial effusion, RA area >26 cm², RA pressure >14 mm Hg, CI <2.0 L/min/m2, and SvO2 <60%.
High risk: Clinical signs of right heart failure, rapid progression, repeated syncope, WHO FC IV, 6MWD <165 m, peak VO2 <11 ml/min/kg, NT-proBNP >1400 ng/L, pericardial effusion, right arterial pressure >14 mm Hg, cardiac index (CI) <2.0 L/m/m2, and mixed venous oxygen saturation (SvO2) <60%.
A major objective in the treatment of patients with PAH is to maintain a low-risk profile and thus prolong life [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. Recent data suggest that a morbidity event up to month 3 after treatment escalation carried a greater than 4-fold increased risk of death within the next 20 months. Early intervention, regular monitoring, and escalation of treatment are key factors that influence survival, and evaluation of risk plays a driving role at each of these key stages.
TREATMENT
The first approach of PH therapy includes referral to an expert centre and general therapeutic measures including avoidance of pregnancy, immunization against influenza and pneumococcal infection as well as psychosocial support. When arterial blood oxygen pressure is consistently <8 kPa, continuous long-term oxygen therapy should be started.
Treatment strategies for PAH are depicted in Figure 8. High doses of calcium antagonists are indicated as first-line medical therapy in those patients with IPAH, HPAH, and drug/toxin-associated PH that respond to initial acute vasodilator testing. Achievement/maintenance of a low-risk profile (see chapter Prognosis) is considered as adequate treatment response. Reevaluation of therapy should be performed 3-4 months after initiation of therapy by RHC, including vasoreactivity testing. PAH treatment is recommended in patients with WHO-FC I/II and significant haemodynamic improvement. In those patients with FC III/IV or no significant haemodynamic stabilization, the initiation of PAH specific therapies, primarily targeting a relief of right ventricular afterload by vasodilation via stimulation of soluble guanylate cyclase, prostacyclins and analogues, and inhibitors of phosphodiesterases and endothelin receptors, is recommended.
The most recent treatment algorithm for PAH patients recommends the initiation of a mono- or combination therapy depending on calculated individual risk, rather than on WHO class alone. In high-risk patients, combination therapy including parenteral prostacyclins should be started immediately. Treatment has to be continuously upscaled untila low risk state is achieved. Low-and intermediate risk patients should receive upfront combination therapy, preferentially with ambrisentan and tadalafil [228228. Galie N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiery JL, Grunig E, Oudiz RJ, Vonk-Noordegraaf A, White RJ, Blair C, Gillies H, Miller KL, Harris JH, Langley J, Rubin LJ and Investigators A. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N Engl J Med. 2015. ], but given the appropriate setting, monotherapy is acceptable. When treatment goals are not met with monotherapy, double therapy, and finally triple sequential therapy should be started. Recommendations for specific drug mono- and combination therapies for PAH are comprehensively outlined in current guidelines for the diagnosis and treatment of pulmonary hypertension [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. For patients with PH in groups 2 and 3, PAH-specific drugs are not recommended.
Interventional treatments of PH have been limited to interventions for pediatric pulmonary vascular disease, and to graded balloon atrial septostomy for selected cases of severe pulmonary arterial hypertension [229229. Galie N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, Rubin LJ, Sandoval J, Seeger W and Keogh A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013. ]. Recently, goal-orientated therapeutic approach using sequential combinations of bosentan, sildenafil, and inhaled iloprost [230230. Hoeper MM, Markevych I, Spiekerkoetter E, Welte T and Niedermeyer J. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J. 2005. ] are giving way to more aggressive upfront combination regimens [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. Evidence is accumulating that a “hit-hard-and-early strategy with multiple agents” for the treatment of severe PAH may be associated with better survival [231231. Kemp K, Savale L, O’Callaghan DS, Jais X, Montani D, Humbert M, Simonneau G and Sitbon O. Usefulness of first-line combination therapy with epoprostenol and bosentan in pulmonary arterial hypertension: an observational study. J Heart Lung Transplant. 2012. , 232232. Sitbon O, Jais X, Savale L, Cottin V, Bergot E, Macari EA, Bouvaist H, Dauphin C, Picard F, Bulifon S, Montani D, Humbert M and Simonneau G. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J. 2014. ].
In patients with inadequate response to treatments, end-of-life decisions, and consideration of referral for lung transplantation should be discussed.
Any treatment-resistant pulmonary hypertensive condition may be considered for balloon atrial septostomy (BAS). The creation of an inter-atrial right-to-left shunt can decompress the right heart chambers, and increase LV preload and CO [233233. Sandoval J, Gaspar J, Pulido T, Bautista E, Martinez-Guerra ML, Zeballos M, Palomar A and Gomez A. Graded balloon dilation atrial septostomy in severe primary pulmonary hypertension. A therapeutic alternative for patients nonresponsive to vasodilator treatment. J Am Coll Cardiol. 1998. ]. Systemic O2 transport is improved despite arterial O2 desaturation and decreases sympathetic hyperactivity. The recommended technique is graded balloon dilation atrial septostomy, which carries a reduced risk compared with the original blade technique. Recently, modified atrial septal occluders [234234. Althoff TF, Knebel F, Panda A, McArdle J, Gliech V, Franke I, Witt C, Baumann G and Borges AC. Long-term follow-up of a fenestrated Amplatzer atrial septal occluder in pulmonary arterial hypertension. Chest. 2008. ], or interatrial stents have been used to prevent the need for repeated procedures which pose an unacceptable risk to these vulnerable patients. BAS should be avoided in end-stage patients showing a baseline mean RAP of >20 mmHg and O2 saturation at rest of <80% on room air. Patients should be on optimal medical therapy, which may include pre-conditioning with IV inotropic drugs, prior to BAS. Evidence shows improvements in CI and decreases in right atrial pressure with improvement in 6MWT. The impact of BAS on long-term survival has not been established in RCTs [235235. Kurzyna M, Dabrowski M, Bielecki D, Fijalkowska A, Pruszczyk P, Opolski G, Burakowski J, Florczyk M, Tomkowski WZ, Wawrzynska L, Szturmowicz M and Torbicki A. Atrial septostomy in treatment of end-stage right heart failure in patients with pulmonary hypertension. Chest. 2007. ]. To perform BAS, it is not sufficient to be experienced in structural heart disease interventions in general. Clinicians must be trained in the management of patients with severe pulmonary vascular disease, as they pose additional challenges to catheter laboratory procedures [233233. Sandoval J, Gaspar J, Pulido T, Bautista E, Martinez-Guerra ML, Zeballos M, Palomar A and Gomez A. Graded balloon dilation atrial septostomy in severe primary pulmonary hypertension. A therapeutic alternative for patients nonresponsive to vasodilator treatment. J Am Coll Cardiol. 1998. ]. In the current world BAS is hardly performed any more for PAH.
PAH is a life-threatening condition that requires both regular monitoring and timely escalation of treatment in order to limit or ideally reverse progressive vascular remodelling and right-heart failure. Recent predictive algorithms from large national registries [236236. Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG and McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010. , 237237. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jais X, Montani D, Souza R and Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010. ] suggest gender, PAH subtype, functional class, haemodynamics, biomarkers, 6MWD, renal function, and parameters such as pericardial effusion and carbon monoxide lung diffusion capacity as being prognostic indicators, and they are currently being tested as on-treatment predictors. Additionally, new research is leading to evolution in diagnosis and treatments. One of these may be advanced imaging of pulmonary vascular pruning, which may lead to treatment being started significantly earlier [238238. Haitao S, Ning L, Lijun G, Fei G and Cheng L. Fractal dimension analysis of MDCT images for quantifying the morphological changes of the pulmonary artery tree in patients with pulmonary hypertension. Korean journal of radiology : official journal of the Korean Radiological Society. 2011. ].
A recent algorithm [208208. Lang IM and Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation. 2014. ] for the treatment of CTEPH is depicted in Figure 9. All patients with newly diagnosed CTEPH should be seen by an expert referral center that is defined as high-volume centre treating at least 50 patients with PAH or CTEPH and receiving at least two new referrals per month with documented PAH or CTEPH [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. Generally, CTEPH is a surgical disease curable by PEA. In expert referral centers, PEA is associated with low in-hospital mortality rate of 4.7% [239239. Mayer E, Jenkins D, Lindner J, D’Armini A, Kloek J, Meyns B, Ilkjaer LB, Klepetko W, Delcroix M, Lang I, Pepke-Zaba J, Simonneau G and Dartevelle P. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011. ]. A multidisciplinary team approach should guide the assessment of operability and decisions regarding other potential therapeutic strategies. Optimal medical therapy for CTEPH includes anticoagulants, diuretics, and oxygen. Lifelong anticoagulation is recommended in all CTEPH patients, even after PEA [239239. Mayer E, Jenkins D, Lindner J, D’Armini A, Kloek J, Meyns B, Ilkjaer LB, Klepetko W, Delcroix M, Lang I, Pepke-Zaba J, Simonneau G and Dartevelle P. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011. ], while the routine use of IVC filters is not recommended [5252. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D and Management of Acute Pulmonary Embolism of the European Society of C. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014.
Latest European guidelines on acute pulmonary embolism]. For patients with non-operable CTEPH, due to distal surgically inaccessible disease or unfavorable risk:benefit ratio for PEA, or patients who have persistent or recurrent PH after PEA, percutaneous balloon pulmonary angioplasty (BPA) may offer a valuable treatment option.
BPA represents a classical angioplasty technique that aims to improve pulmonary blood flow by dilation of occluded or stenotic pulmonary arteries.Staged interventions with repeat catheterisations and dilatations (3-10 sessions at intervals of ≥1 week) are generally needed to achieve optimal pulmonary perfusion and haemodynamic results. BPA utilizes 0.014-inch guidewires and compatible semi-compliant balloons sized from 1.2 to 8.0mm. An 8F sheath is inserted into the internal jugular, subclavian or femoral vein [n=2]). After a standard right heart catheterization, the Swan-Ganz catheter is replaced by an 8F short guiding catheter or a sheath with an internal 6F (Mach 1 peripheral MP; Boston Scientific, Natick, MA) guiding catheter. Heparin (2000 U) is administered when the sheath is inserted, and 1000 U of heparin are added every hour during the procedure. The guide is advanced to the pulmonary artery (from the groin primarily to the left PA, from the jugular approach primarily to the right PA) over a 0.035-inch wire (Radifocus Guide Wire M; Terumo, Tokyo, Japan). In general, the lobe with the worst perfusion, identified by lung perfusion scintigraphy, is primarily addressed. The right lower lobe represents a major target for BPA because of its size and physiological blood flow compartment size. In severely compromised patients, the primary goal is to reduce pulmonary pressure by targeting simpler lesions first. After passing the lesion with the guide wire, its correct placement must be proven before angioplasty. The branch is selected by the 6F guiding catheter and a hand-injected angiography is performed in an anterior-posterior and lateral projection. Typical pulmonary arterial vascular lesions are ring-like stenoses, fibrous webs and complete occlusions appearing as break-offs ( Figure 10) or pouches. A 0.014-inch wire (SionBlue Asahi Intecc, Tokyo, Japan) is advanced across the lesion and the lumen size of the vessel is imaged with IVUS (Eagle Eye Platinum; Volcano, San Diego, CA). Because organized thrombi are isoechoic, ChromaFlo (Volcano, San Diego, CA) computer software is employed to clearly visualize and distinguish lumen and thrombi, and identify the distal reference diameter. To minimize the risk for vessel injury and reperfusion pulmonary oedema, undersized balloon catheters should be initially used followed by larger balloons according to lesion size and residual pressure (2 to 4 mm, TREK, Boston Scientific, Natick, MA, and Aviator Plus, Cordis/Johnson & Johnson, New Brunswick, NJ;). The maximal balloon size is 50% of the original size of the vessel diameter in cases with a mean pulmonary artery pressure ≥40mmHg, and 70% of the original size of the vessel diameter in all other cases. The balloon is inflated by hand until an indentation disappeares or the balloon is fully expanded. The mechanism of BPA is “breaking, compressing and dissecting the intravascular obstructions” ( Figure 11 ) rather than expanding and dissecting the medial and adventitial vessel layers as in coronary intervention [240240. Kitani M, Ogawa A, Sarashina T, Yamadori I and Matsubara H. Histological changes of pulmonary arteries treated by balloon pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2014. ]. The procedure is stopped when 4-8 targets has been addressed, or when oxygen desaturation >4% or hemoptysis occurs.BPA can significantly reduce pulmonary artery pressure in patients with CTEPH as shown in an international multicentre study [241241. Feinstein JA, Goldhaber SZ, Lock JE, Ferndandes SM and Landzberg MJ. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001. ], and in more recent reports of the refined technique [242242. Mizoguchi H, Ogawa A, Munemasa M, Mikouchi H, Ito H and Matsubara H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012. , 243243. Sugimura K, Fukumoto Y, Satoh K, Nochioka K, Miura Y, Aoki T, Tatebe S, Miyamichi-Yamamoto S and Shimokawa H. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J. 2012. , 244244. Kataoka M, Inami T, Hayashida K, Shimura N, Ishiguro H, Abe T, Tamura Y, Ando M, Fukuda K, Yoshino H and Satoh T. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012. ]. Improvement in New York Heart Association functional class, six-minute walk capacity [241241. Feinstein JA, Goldhaber SZ, Lock JE, Ferndandes SM and Landzberg MJ. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001. ], respiratory efficiency [245245. Fukui S, Ogo T, Goto Y, Ueda J, Tsuji A, Sanda Y, Kumasaka R, Arakawa T, Nakanishi M, Fukuda T, Takaki H, Yasuda S, Ogawa H and Nakanishi N. Exercise intolerance and ventilatory inefficiency improve early after balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2015. ], and improvement of right ventricular function [246246. Fukui S, Ogo T, Morita Y, Tsuji A, Tateishi E, Ozaki K, Sanda Y, Fukuda T, Yasuda S, Ogawa H and Nakanishi N. Right ventricular reverse remodelling after balloon pulmonary angioplasty. Eur Respir J. 2014. ] have been observed after successful balloon pulmonary angioplasty. Given the increasing age of the population, the 36.6% of non-operable patients in Europe [247247. Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, Treacy C, D’Armini AM, Morsolini M, Snijder R, Bresser P, Torbicki A, Kristensen B, Lewczuk J, Simkova I, Barbera JA, de Perrot M, Hoeper MM, Gaine S, Speich R, Gomez-Sanchez MA, Kovacs G, Hamid AM, Jais X and Simonneau G. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011. ], and the availability of drug-eluting balloons, BPA is an emerging technique in Europe.
Despite their wide off-label use in Europe [247247. Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, Treacy C, D’Armini AM, Morsolini M, Snijder R, Bresser P, Torbicki A, Kristensen B, Lewczuk J, Simkova I, Barbera JA, de Perrot M, Hoeper MM, Gaine S, Speich R, Gomez-Sanchez MA, Kovacs G, Hamid AM, Jais X and Simonneau G. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011. ], drugs approved for PAH are not recommended for CTEPH outside clinical trials, except in specific situations [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension].
Riociguat, an oral stimulator of the NO receptor soluble guanylate cyclase is the first approved medication that is recommended by current guidelines to treat non-operable CTEPH as well as patients with persistent or recurrent pulmonary hypertension after PEA [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]. Riociguat has been found to significantly improve exercise capacity and hemodynamic parameters [248248. Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C and Group C-S. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013. , 249249. Simonneau G, D’Armini AM, Ghofrani HA, Grimminger F, Hoeper MM, Jansa P, Kim NH, Wang C, Wilkins M, Fritsch A, Davie N, Colorado P and Mayer E. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J. 2014. ]. The MERIT trial has recently demonstrated efficacy and safety of macitentan in non-operable CTEPH (NCT02060721).
Pulmonary hypertension
- Pulmonary hypertension is defined by an invasively measured mean pulmonary arterial pressure ≥25 mmHg at rest [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension]
- In general, right heart catheterisation can be performed with low risk (overall mortality 0.055%) in this very ill patient group [221221. Hoeper MM, Lee SH, Voswinckel R, Palazzini M, Jais X, Marinelli A, Barst RJ, Ghofrani HA, Jing ZC, Opitz C, Seyfarth HJ, Halank M, McLaughlin V, Oudiz RJ, Ewert R, Wilkens H, Kluge S, Bremer HC, Baroke E and Rubin LJ. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006. ]
- Pulmonary arterial wedge pressure (PAWP) is used to distinguish between pre and postcapillary disease [188188. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H and Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016.
Latest European guidelines on pulmonary hypertension].
- “Pulmonary revasculariation” has gained new attention for PE
- Acute PE: thromboysis (PEITHO trial illustrating the risk and benefits of intravenous thrombolysis with tenecteplase) and mechanical thrombectomy (ULTIMATE trial) [128128. Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, Bluhmki E, Bouvaist H, Brenner B, Couturaud F, Dellas C, Empen K, Franca A, Galie N, Geibel A, Goldhaber SZ, Jimenez D, Kozak M, Kupatt C, Kucher N, Lang IM, Lankeit M, Meneveau N, Pacouret G, Palazzini M, Petris A, Pruszczyk P, Rugolotto M, Salvi A, Schellong S, Sebbane M, Sobkowicz B, Stefanovic BS, Thiele H, Torbicki A, Verschuren F, Konstantinides SV and Investigators P. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014.
RCT examining the role of fibrinolysis in patients with pulmonary embolism and markers of intermediate risk].
- CTEPH: Balloon pulmonary angioplasty is evolving as an alternative for patients who are no candidates for surgical pulmonary endarterectomy