NATURAL HISTORY OF CARDIAC MEDICAL DEVICE VALIDATION IN LARGE ANIMALS
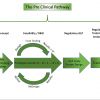
The creation, development and validation of a novel medical device from the initial idea through to commercialisation and human use requires managing many aspects including engineering, legal, preclinical bench and animal evaluation, human clinical evaluation, regulatory reporting and publication, market access planning and commercialisation strategy. The preclinical stage is pivotal as, without robust experimental animal results demonstrating the efficacy and safety of the device, the project might end up at a dead end, lacking financing or regulatory approval. This stage includes different phases: proof-of-concept studies, feasibility and R&D studies, regulatory studies and finally the training of the end-users before performing the first-in-human (FIH) implantation or intervention ( Figure 1).
When developing an implantable medical device, central areas of focus naturally include the implant itself and its delivery system. However, in the process, these are not the only two components to be developed and validated. Indeed, when developing an innovative device, the techniques of implantation (surgical procedure, interventional procedure, imaging procedure, any unique anaesthesia requirements…) also have to be developed and stringently documented, as they will be part of the final instructions for use (IFU) and will serve as the basis of training for the end-users. With the development of new imaging systems and tools, these procedures are constantly evolving. The preclinical animal testing facility has to provide the latest equipment and trained team to enable development and repeatability of these procedures. Finally, the animal studies should also serve for the development and validation of algorithms and tables ensuring optimal matching criteria, defining what size or version of an implant should be used for what size range or unique features of a patient’s anatomy. Preoperative screening/planning with echocardiographic or preferably computed tomography (CT) imaging should be performed for each animal to be implanted in order to document the native anatomy precisely, guide selection of the size of implant to be used, and develop and perfect the matching process.
proof of concept
This is the phase when the project moves from concept to a physical product that will be implanted in an animal. The proof of concept is the time when the device will first encounter the hostile conditions of the in vivo environment. It is a feverish exercise, as the device needs to demonstrate its potential, albeit while it is a very early stage prototype design that is not yet optimised for implantation. At this stage, the first in vivo implantation series can fail, and the project can be shut down if the imperfect device is not handled properly by a team not only with appropriate experience and expertise but also with creativity to problem-solve in real time to overcome all the added pitfalls and limitations of the animal model. The proof-of-concept stage usually requires a small series of animals (5 to 10 typically) and does not provide definitive results but is intended to provide enough data to give confidence to all stakeholders that the device has a promising future.
feasibility/R&D
The second phase of the preclinical testing pathway is composed of feasibility/R&D studies, during which all problems identified on the device design have to be solved. This phase includes the repetition of rounds of implants in the animal model. Each round of implantations will enable testing of a prototype version, at the end of which remaining design problems should be identified and addressed for the next round of implantations. Each round of implantation should include several animals using the same prototype because cases can fail for multiple reasons, including product design flaws, poor manufacturing of the implants or delivery systems, and/or procedure-related issues, and therefore conclusions cannot be drawn from single animal experience. Each round requires approximately 4-6 animals. The animal testing is of high value at this stage as it permits improvement of the device design until a version of the product is achieved that proves efficient and safe, allowing the design to be frozen. This phase can be the longest portion of the preclinical process, potentially requiring evaluation of many iterations of a device design. The more complex the device and its implant procedure, the more likely they will require multiple rounds of studies. An innovative complex device such as a transcatheter mitral valve may require approximately 100 implants before the prototype approaches its efficient and safe version. One should not be discouraged by such a long-distance run. Many teams that have developed breakthrough technology and innovative medical devices that have revolutionised interventional cardiology have travelled this road.
regulatory/GLP/histoPATHOLOGY
It is the frozen version of the product design that should enter the regulatory study. Indeed, at this stage there should not be any modification of the design as the regulatory authorities require the device to be tested in animals in a form identical to that in which it will be used in humans.
Competent authorities (government agencies) in each country have structured their requirements for evaluating and validating medical devices according to EU and country-level regulations.
Medical devices are classified into Class I, II, and III (with variations according to countries or continents). Regulatory control increases from Class I to Class III with potential higher risk to the patient. The device classification defines the regulatory testing requirements for all device types. Most of the interventional cardiology devices are higher risk Class II and III, requiring animal testing data to be validated.
In Europe, for harmonisation purposes, on 25 May 2016, the European Commission released a draft for the proposed European Medical Device Regulation (MDR). The text was ratified by the European Parliament in 2017 for full application after a 3-year transition period (May 2020). Due to the onset of the COVID pandemic, the transition period was prolonged to May 2021. By the end of the transition period, all medical devices will have to comply with EU MDR 2017/745, this new set of directives. Notified bodies are authorised entities that can evaluate and certify the conformity of medical devices to the essential requirements and award the products the CE mark (Conformité Européenne), thereby allowing their commercial use across the EU. These organisations may be private or public, there may be a specific notified body designated within a given country, or in some instances there may be more than one in the same country. Companies often have flexibility in choosing which notified body to work with to obtain the CE mark.
In the USA, the Food and Drug Administration is the central regulatory authority that manages all requests for regulatory clearance (Class I and II) or pre-market approval (Class III) of medical devices. The FDA's Center for Devices and Radiological Health (CDRH) is responsible for regulating medical devices and all associated legal aspects (initial approval, approvals for repackaging, relabelling, manufacturing changes, product design enhancements and/or import of medical devices for sale in the USA).
In China, it is the National Medical Products Administration (NMPA) that has the responsibility for approving medical devices. The NMPA (formerly Chinese FDA [CFDA]) is rapidly evolving towards a highly organsed, structured approval process and to standards equivalent to the EU and the USA.
The guidelines of each country to be followed for medical device approvals, including guidelines for animal preclinical testing, are not easy to navigate; the maze of information can be overwhelming. In addition, medical devices are becoming more complex and more sophisticated, and requirements for testing and documentation for validation are also evolving. International scientific guidelines have been created by the International Standards Organization (ISO). For example, biocompatibility testing is the process of evaluating materials comprising medical devices. It consists of a number of tests designed to provide assurance that the materials within the final product will not harm patients. When a material is being considered for use in a final product, the appropriateness of its use should be assessed. Some materials are known and have been extensively tested, but other new materials and chemical components or a new combination of these have not. Even variations in the manufacturing process of known materials need to be scrutinised. The type of testing needed for any given material/device is determined by the intended patient contact and the duration of that contact. The ISO series of guidance documents for biocompatibility (ISO 10993) offers the direction needed to determine which test methods may be most appropriate. Part of the ISO 10993 testing is done in small animal models; these are very standardised, reproducible batteries of tests. However, the functional safety and efficacy part of the animal testing needs to be performed on large animals, as these require the implantation of the device in its final form, thus requiring an animal model that resembles the human anatomy and size. Typically, the ISO 10993 part 6 (evaluation of local effects) and part 11 (evaluation of systemic effects) tests can be optimally performed only in a large animal model.
The design of the regulatory study should be carefully elaborated and include several levels of evaluation: 1) clinical level of evaluation of animals with real-time, in-life monitoring of all adverse events, 2) biological level of evaluation via fluid samplings such as blood (full clinical pathology including full biochemistry and complete blood count) or urine, 3) functional level of evaluation focusing on the function of the device in the target implanted organ (for example, full haemodynamic cardiovascular evaluation of a cardiac valve device including ultrasound, angiography and CT scan), 4) tissue level evaluation including macroscopic evaluation during full necropsy and then mandatory complete histological evaluation. The study design should include a control arm using a predicate article, which is a device similar to the investigational device that is already available commercially. The predicate will serve as a comparison item, to ensure that the new device achieves the same or better performance according to all safety and effectiveness acceptance criteria. Most of the time, the regulatory studies are performed in healthy animal models. However, authorities require that efforts should be made to develop and test the new devices in diseased large animal models to mimic better the pathology in humans for a more relevant extrapolation of the preclinical results to the sick patient environment. Therefore, a regulatory study could be composed of an arm with a healthy animal model and an arm with a diseased animal model, including ad hoc predicate device implantations. When designing the groups comprising each arm, one should keep in mind that the definition of a group is stringent: a group is composed of similar animal models, implanted with the exact same device, followed up for the same duration with the same methods of evaluation. It is commonly accepted that in large animal studies a group is composed of at least 5-6 animals. Back-up animals should be included at the initiation of the study so that, if an adverse event occurs, the final group will not be truncated. It is mandatory that the performance and safety are evaluated acutely, subacutely and chronically. Theoretically, the design of the study should ensure that animals are attributed to all groups: acute (1-3 days), subacute (7-15 days) and chronic (3-6 months) follow-up duration. However, for ethical reasons, in order to diminish the number of animals used in the study, the acute and subacute evaluation can be performed via interim control investigations such as ultrasound, angiography and CT scan to gather information at all stages of the study without sacrificing the animals. The time points of the interim investigations should respect the acute and subacute phase and be repeated at close intervals so as not to miss crucial interim findings. All results including adverse events must be reported in the regulatory study report and all target and systemic organs analysed histologically.
For interventional cardiovascular evaluation, special attention should be focused on the thrombogenicity test as it is a key part of the submission package to the notified bodies. In vitro tests can be performed as an initial process, but they cannot replace in vivo thrombogenicity tests, invaluable in that they are the only tests that can substantially inform safety for use in human patients. In a nutshell, the in vivo thrombogenicity tests should be performed in a large animal model that can accept the size of an implanted device and its delivery system (mainly ovine, bovine, porcine and canine). Indeed, it is not only the nature of the material in contact with the blood that participates in the activation of coagulation, but also the extent of surface area and the overall geometry inducing contact of the device and delivery system with cardiovascular structures (and large surfaces of endothelium) and modifying the blood flow inducing turbulence and stasis that contribute to activation of the coagulation cascade. The thrombogenicity test should include testing of the implant itself at acute, subacute and chronic time points. As explained above, the acute and subacute thrombogenicity evaluation can be performed on the occasion of interim control investigations such as full ultrasound evaluation of thrombus formation on the device. The chronic thrombogenicity of the implant will be evaluated on the occasion of the final investigation, repeating the ultrasound examination and performing full necropsy and histological evaluation of the device and downstream organs. The device and downstream organs should be stringently dissected and screened for thrombosis macroscopically and a “bread loaf” slicing technique should be used when preparing the histological slides. All major organs downstream from the implanted device should be submitted to such an evaluation. For a device implanted in the left heart, for example, this includes the heart, the brain and its rete mirabile, the liver and the kidneys. Bread loaf slicing consists of several (10) samples for histology analysis for relevant downstream organs that are filtering blood. Kidneys are known to be a remarkable witness of thromboembolism events originating from cardiovascular devices upstream, as, with their terminal vascularisation anatomy, all obstructed vessels in the kidney will induce an infarction that can be observed at the surface of the organ. Of note, the delivery system should be evaluated in a separate acute study. As the delivery system is only used for the time of the implantation procedure, experiments dedicated to assessing the behaviour of the delivery system without the implant being loaded inside are recommended. It should expose the delivery system to blood contact in an orthotopic position (as indicated for use) for a duration that is twice the approximate expected duration of implantation in humans. In this way it will ensure that, if the procedure is longer than expected in the human implants, the delivery system can still be used safely. At the end of the acute thrombogenicity of the delivery system experiment, the animal should be sacrificed, the delivery system left in place and all vessels on the pathway of the system should be open and evaluated in situ. Indeed, removal of the delivery systems through the vessels and introducers can swipe out the thrombi at the surface of the system and mask potential thrombus formation. During these thrombogenicity tests, the activated clotting time (ACT) should be carefully monitored (kinetics of ACT performed by blood sampling every 15 minutes approximately), ensuring that ACT values remain within representative values of clinical practice (ACT value after anticoagulation during the procedure should be approximately 2 to 3 times the basal ACT value).
The large animal studies are complex, custom made, and tailored to the needs of the innovative device. Internationally recognised quality management systems exist that can be applied to medical device preclinical testing, such as the Good Laboratory Practices. It is highly recommended to follow these guidelines to perform the preclinical animal testing to ensure acceptance by regulatory authorities of the quality of the preclinical data that are generated and submitted.
physician training
The final act of the preclinical animal phase is the training of the medical team that will first implant the new innovative device in patients. It is a regulatory requirement that training is performed, with structured training courses and documented processes and validation records for each trainee. The training courses should include didactic courses, bench demonstration (when possible), and animal training implantations. These implantations serve as a full rehearsal session for all aspects of the implant and implantation technique. Typically, four animal implantations are needed to train a medical team properly for the implantation of an innovative device. The list of trainees should include a representative of all Heart Team members, the interventionalist, the surgeon (cardiac, cardiovascular, cardiothoracic or vascular surgeon), the imager (mastering all imaging modalities used for the implantation procedure such as ultrasound, angiography and CT scan) and the scrub nurse who will assist in the procedure. It goes without saying that the training phase should not be neglected as it will maximise the safety of the patient, ensuring that the final curtain will rise on the clinical use of the new medical device and never fall.
WHICH ANIMAL MODEL SHOULD BE USED?
Interventional cardiologists are mostly involved in animal studies as regards medical device R&D and training. Such devices have to be tested in an anatomic environment that is as representative as possible of human anatomy. The first aspect is sizing. Even if non-human primates are theoretically the best animal models considering that they share a large portion of their genetic inheritance with humans, using primates in animal research is ethically, technically and financially fraught with difficulties. Further, only very large primates such as great apes would be appropriate as far as the sheer size of the heart is concerned. It is practically impossible to use such animals for cardiology research. Therefore, farm animals such as pigs and sheep, sometimes goats or calves, are the most common animals used for cardiac research. They are readily available, relatively inexpensive, are not considered as pets in most countries and are raised as food animals so their use raises fewer ethical questions. Canines have also been studied frequently for cardiac R&D in the past but, because of ethical dilemmas associated with their use as well as anatomy that is frequently impractically small, they are nowadays mostly utilised for selected technologies where they have specific attributes that dictate their use. Such examples include electrophysiology studies, because of their cardiac conduction properties, and left atrial appendage (LAA) device studies as they are the only species of the three to have a true LAA resembling that of a human ( Figure 2, Figure 3). Young bovines (calves) can also be appropriate for certain studies: given their large thoracic cavity, they have been used extensively in ventricular assist device studies. Otherwise, pigs and sheep are the most common species used for actual size cardiac device studies. Please note that, in the current article, pig or porcine or swine may be used indiscriminately, as well as sheep or ovine, dog or canine and man or human or humans.
CARDIAC valves
Our laboratory saw the birth of what was going to become the Melody™ valve (Medtronic, Minneapolis, MN, USA), the SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) and the CoreValve® (Medtronic), at the turn of the 20th century. Being very much specialised in structural heart devices ever since, and considering the level of attention both physicians and the industry have focused on the mitral and the tricuspid valves, we have performed comparative anatomy studies to assess which species is the most appropriate for mitral or tricuspid devices. These will be published in detail separately and are reviewed briefly here as examples of the anatomic considerations across animal models which, despite their limitations, remain invaluable.
Pros and cons of different animal models: examples of the mitral valve
The more relevant animal model should share the same anatomy as the human to obtain more predictive value of the implantability of the device, valvular function and potential modes of failure.
Many parameters we measured were similar in all four species, but we highlighted critical variations between species that have to be considered in terms of ease of implantation and adaptation of the mitral devices to the mitral valve and subvalvular apparatus.
A first major difference is the presence of a true aorto-mitral “curtain” along the human anterior leaflet, below the mitral annulus, defined as a space between the mitral and aortic valves. This curtain is poorly defined in sheep and pigs, leading to a more restricted space between the two valves ( Figure 4). This anatomical proximity often explains possible damage to the adjacent aortic valve when a mitral device is implanted in animals. It also explains the risks of impinging the anterior leaflet of the mitral valve when performing transcatheter aortic valve implantation (TAVI) in sheep and, to a lesser extent, in pigs. We also observed a continuous band of tissue all along and on part of the posterior leaflet just below the mitral annulus in the human mitral valve, but not in the ovine, porcine and canine valves where the cleft reached the posterior annulus. This clearly shows that the tissue of the human mitral valve is more extended than in the other species we studied.
Of note, the annulus is more compressed along the anteroposterior axis in sheep. Conversely, the mitral valve is rounder in humans, dogs and pigs. Therefore, a round-shaped stent sustains more mechanical forces in this specific orientation in sheep and is at higher risk of paravalvular leakage at the commissural level. We observed that the human anterior leaflet is much longer than the posterior leaflet with an anterior to posterior leaflet height ratio close to 2 whereas this ratio was near 1 in the porcine, ovine and canine leaflets.
Our results substantiate the relevance of the porcine as a better animal model based on anatomical measurements. The porcine mitral valve is indeed closer to the human counterpart for many parameters: symmetry of the valve indicated by the ratio of the anterior to posterior leaflet, number of chordae tendineae from or to the anterior and posterior leaflets, numbers of heads of the papillary muscles and, even though thinner than in man, the thickest leaflets among the three animal species we studied ( Figure 5).
Obviously, other pros and cons have to be considered in the choice of the preclinical large animal model. The ovine heart better resembles a dilated human heart from a patient suffering from cardiac insufficiency. The sheep is also a greater and more challenging model for studying the ageing of an implanted cardiac device [11. Frink RJ, Merrick B. The sheep heart: coronary and conduction system anatomy with special reference to the presence of an os cordis. Anat Rec 1974 179:189-200. PMID: 4829081. ]. In particular, adolescent sheep are more prone to calcification than other large animal species used in preclinical valve testing ( Figure 6) [22. Ozaki S, Van Nooten G, Herijgers P, Van Belleghem Y, Flameng W. Modified stentless porcine valve enhances accelerated cuspal calcification in the juvenile sheep model. Jpn J Thorac Cardiovasc Surg 2003 51:420-426. PMID: 14529157. ]. In contrast, the rapid growth rate of pigs prevents the use of this species for long-term studies, but they can be used for feasibility or short-term studies. Sheep are also more robust animals than pigs and less prone to infection; they recover faster after anaesthesia and major surgery. Contractility of the left ventricle is less marked in sheep than in pigs and is therefore a more favourable environment for stented technologies (fewer fractures). More generally, sheep and pigs are easy to procure and to be justified as a model whereas ethical and size reasons constrain the use of dogs.
Pros and cons of different animal models: example of the tricuspid valve
To aid the preclinical development of new tricuspid devices, this study compared key points in the choice of animal model, such as size, landmarks during implantation, anchoring points and possible obstacles to proper deployment.
Our study compared humans to three species used in preclinical cardiac medical device research - pigs, sheep and dogs - so as to identify the best preclinical models for validation of novel tricuspid surgical and transcatheter devices. Particular attention was given to tricuspid annulus length, leaflet shape and size, commissural indentation, chordae tendineae, papillary muscle location and conformation, and the presence, number, size and shape of any moderator bands.
Study animals are most often healthy while devices are developed for diseased human patients with a dilated right ventricle. Valvular structures such as leaflets, papillary muscles and moderator bands could deform a device and/or interfere with its proper deployment and function. Accurate knowledge of tricuspid valve structures and how they relate to the corresponding diseased human anatomy are therefore of paramount importance to predict successful implantation and function of prosthetic valves.
Our study allowed us to confirm that sheep and pigs are appropriate animal models in the preclinical phase of tricuspid device development. However, the optimal choice will depend on the intended intervention and treatment period. Seventy to 90 kg pigs may be more suitable for annuloplasty devices, due to a wide range of tricuspid annulus sizes available, but at this size they are not adults. The fast-growing potential of this species could be an issue after several months of implantation. Consequently, younger pigs would be most appropriate for acute or short-term studies. Slow-growing swine (so called mini-pigs), such as the Yucatan or Göttingen breeds, can be interesting alternatives. However, they are harder to source, less cost-effective, and potentially more fragile animals. At any rate, these animals are rarely of a sufficient size to host a large tricuspid device.
Knowledge of the subvalvular apparatus is important for surgical planning as it can help with landmarks, such as the systematic presence of the anterior papillary muscle, or affect the deployment of a prosthetic valve, such as by a thick moderator band. Dogs do not appear to be a good model: we observed that the depth of indentation was not sufficient to characterise a commissure, and more precisely the anteroposterior one of humans. Sheep anatomy is close to human anatomy and tricuspid annulus size is comparable to that in a normal human right heart. Sheep are a suitable model for long-term study. Rams could be a good alternative larger model (90-120 kg); we use them regularly in our laboratory. We certainly encounter a larger tricuspid annulus. Nevertheless, healthy animals remain an imperfect model because the atrial and ventricular cavities are not dilated as in diseased patients, and so tissue interaction with devices is different. To that end, we developed a model of functional regurgitation with severely dilated ventricles and atria. See the section entitled PATHOLOGIC MODELS below.
Beyond length and anatomy of mitral and tricuspid valves, other elements have to be taken into account regarding the definition of the pros and cons of each species for mitral and tricuspid devices. Not least among these are tissue thickness and frailty. Even in large adult sheep, the leaflets, chordae and annulus are somewhat thinner and more fragile than in humans, and this in turn can become an issue when assessing the anchoring strategy of a novel device. Width and height of the ventricle and atrium are paramount for some large devices, especially when the devices involve the subannular apparatus for the former and a large delivery device for the latter. Indeed, farm pigs have a very muscular heart, hypertrophic by human standards; their use is not warranted when the implanted device requires a large amount of real estate in the left ventricle or enough space behind the chordae. Likewise, the height of the left atrium, for instance in a healthy 70 kg sheep or pig, can be less than half that of a diseased human ( Figure 7). This can sometimes make the use of a large and non-pliable transseptal delivery catheter challenging, with a very steep angle once the fossa ovalis has been crossed. Further, the angle of the vena cava, relative to the tricuspid or mitral planes, and the small height of the fossa with regard to the mitral plane can make it even more challenging. Finally, the vessels taper down very quickly as they course distally in four-legged animals. This makes peripheral vascular access difficult for large-bore catheters and sheaths. It is not unusual to need intra-abdominal access into iliac vessels in order to use such large delivery systems.
coronary DEVICES
The association between coronary artery obstruction and angina was known at the end of the 19th century. The surgical treatment of coronary artery disease began decades later and, in the search for the best techniques, animal models have played a key role. In 1910, Alexis Carrel anastomosed an innominate (brachiocephalic) artery of a dog to the coronary artery of a second dog. In 1952, Demikhov used an internal mammary artery graft to perform the first direct coronary artery bypass grafting (CABG) in dogs [33. Mack MJ. Advances in the treatment of coronary artery disease. Ann Thorac Surg 2003 76:S2240-5. PMID: 14667693. ].
The introduction of cardiopulmonary bypass (CPB) facilitated the CABG procedure by fostering a motionless and bloodless field: coronary artery bypass surgery could be performed widely with generally good results. However, significant adverse events can occur with the use of CPB, including an inflammatory response that can affect every organ and tissue, and the requirement of an open chest approach. The need for a less invasive procedure with lower procedural mortality and morbidity emerged. Nowadays, catheter-based, percutaneous coronary interventions (PCI, introduced in the 1970s) and off-pump CABG (introduced in the late 1990s) have become frequent revascularisation procedures.
Preclinical research in animal models has been a crucial step in the development of PCI techniques and is still a mandatory stage in the assessment of coronary devices. Several animal models have been used to assess the effects of endovascular procedures and devices, including rodents, rabbits, dogs, sheep, swine, and non-human primates.
Murine models and rabbits are widely used because of low cost and easy handling, but they show limitations: blood flow is different from that in human coronary arteries, and the elastic nature of their arteries (carotid for the murine model, iliac for the rabbit) could limit otherwise device-induced vascular injuries, which could prevent neointimal proliferation. Moreover, potential end-organ damage in the heart (toxicity, embolisation…) is missed [44. de Prado AP, Perez-Martinez C, Cuellas C, Gonzalo-Orden JM, Diego A, Regueiro M, Martinez-Fernandez B, Altonaga JR, Marin Francisco JG, Fernandez-Vazquez F. Preclinical evaluation of coronary stents: focus on safety issues. Curr Vasc Pharmacol 2013 11:74-99. PMID: 22724463. ]. Dogs and non-human primate models suffer from different limitations (mainly ethical issues) and offer no unique advantages. Furthermore, because of an abundance of collateral coronary arteries, the canine model is not representative of humans and makes direct extrapolation of experimental results difficult.
The swine coronary artery model has been and is still essential to develop coronary therapies and interventions. This is related to the close similarities of the anatomy and physiology of the pig and human hearts. The porcine coronary arterial circulation is remarkably similar to that of man: the coronary arteries arise from their respective aortic sinus, the right coronary artery passing directly into the right coronary groove, and the left coursing only a short distance before dividing into the anterior interventricular and the circumflex branches. In approximately 80% of porcine hearts, the right coronary artery is dominant, encircling the tricuspid valve and giving rise to the posterior interventricular branch ( Figure 8, Figure 9) [55. Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat 1998 193:105-19. PMID: 9758141. ]. Most human hearts (90%) also display right coronary arterial dominance [55. Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat 1998 193:105-19. PMID: 9758141. ]. Moreover, multiple sites to implant single or overlapping stents are available, as in human procedures. Cardiac catheterisation techniques in the pig are similar to those used in humans. Standard human diagnostic and interventional equipment can also be used.
However, as with all animal models, the porcine model has limitations: the costs of housing are higher than in rodents or rabbits, and the arteries of farm pigs grow substantially over time, a factor that must be considered for long-term testing. Miniature pigs such as Yucatan swine can resolve this issue, but are more expensive and come with another set of problems of their own. Furthermore, the anatomy of swine coronary venous return is slightly different from that of the human heart, mainly because of the presence of a prominent left azygos vein [44. de Prado AP, Perez-Martinez C, Cuellas C, Gonzalo-Orden JM, Diego A, Regueiro M, Martinez-Fernandez B, Altonaga JR, Marin Francisco JG, Fernandez-Vazquez F. Preclinical evaluation of coronary stents: focus on safety issues. Curr Vasc Pharmacol 2013 11:74-99. PMID: 22724463. , 66. Genain MA, Morlet A, Herrtage M, Muresian H, Anselme F, Latremouille C, Laborde F, Behr L, Borenstein N. Comparative anatomy and angiography of the cardiac coronary venous system in four species: human, ovine, porcine, and canine. J Vet Cardiol 2018 20:33-44. PMID: 29191414. ].
The porcine model has largely been used as a myocardial infarction and ischaemia/reperfusion model. To induce myocardial ischaemia, open-chest occlusions of the left anterior descending coronary artery (LAD) and left circumflex coronary artery (LCx) have been performed. Nowadays, to follow the development of PCI, cardiac catheterisation is preferred: through the use of an intracoronary balloon inflation technique, both the location and duration of coronary artery occlusion are well controlled. However, this model can be difficult to manage because of its predisposition to arrhythmogenesis (ventricular fibrillation or ventricular tachycardia after induction of myocardial infarction). It is crucial to prepare for and implement aggressive critical care procedures to overcome this issue [77. Suzuki Y, Yeung AC, Ikeno F. The representative porcine model for human cardiovascular disease. J Biomed Biotechnol 2011 2011:195483. PMID: 21253493. ]. A model of myocardial infarction has also been described in sheep: an anteroapical aneurysm has been induced percutaneously by coil embolisation of the LAD [88. Cheng Y, Aboodi MS, Wechsler AS, Kaluza GL, Granada JF, Van Bladel K, Annest LS, Yi GH. Epicardial catheter-based ventricular reconstruction: a novel therapy for ischaemic heart failure with anteroapical aneurysm. Interact Cardiovasc Thorac Surg 2013 17:915-22. PMID: 23985410. ]. We have extensive experience with this model. It can be very successful if adequate resuscitation measures are available and applied promptly when needed.
Porcine coronary restenosis model
PCI has evolved from balloon angioplasty to drug-eluting stents (DES), and the restenosis rate has been reduced, but not fully eliminated. Optimal preclinical models of restenosis are needed to allow appropriate testing of new therapies and medical devices. Balloon angioplasty and stent implantation in animal coronary arteries are both standard methods of inuring the vessels and engendering neointimal hyperplasia. As mentioned, the porcine coronary artery restenosis model seems to be the best adapted. When porcine coronary arteries are injured, thick neointima is seen within 28 days and is identical to human restenotic neointima [44. de Prado AP, Perez-Martinez C, Cuellas C, Gonzalo-Orden JM, Diego A, Regueiro M, Martinez-Fernandez B, Altonaga JR, Marin Francisco JG, Fernandez-Vazquez F. Preclinical evaluation of coronary stents: focus on safety issues. Curr Vasc Pharmacol 2013 11:74-99. PMID: 22724463. , 99. Iqbal J, Chamberlain J, Francis SE, Gunn J. Role of Animal Models in Coronary Stenting. Ann Biomed Eng 2016 44:453-65. PMID: 26259974. ].
In order to determine which technique is best suited for restenosis, Mitsutake et al have compared the restenotic responses after balloon or stent overstretch injury in a porcine coronary artery, at various balloon-to-artery (B:A) ratios. They concluded that both techniques produced restenosis, but oversized stents produced a significantly higher degree of neointimal proliferation than balloon overstretching. In both techniques, it appeared that higher B:A ratios induced better results (greater lumen area stenosis, larger plaque burden, greater negative remodelling) [1010. Mitsutake Y, Reifart J, Pyun WB, Lyons JK, Deuse T, Schrepfer S, Ikeno F. Differences in Vascular Response between Balloon Overstretch and Stent Overexpansion in Nonatherosclerotic Porcine Coronary Arteries. Comp Med 2017 67:350-355. PMID: 28830582. ].
However, the “stent-induced” model presents drawbacks: as foreign material, the stent can produce artefacts and make imaging follow-up (such as CT, magnetic resonance imaging, intravascular ultrasound and optical coherence tomography) difficult to read.
Suzuki et al have investigated a model of porcine heat-injury restenosis, using radiofrequency thermal balloon angioplasty. This model might be useful for the evaluation of bifurcation and bioabsorbable stents, coronary imaging studies and training for complex PCI [77. Suzuki Y, Yeung AC, Ikeno F. The representative porcine model for human cardiovascular disease. J Biomed Biotechnol 2011 2011:195483. PMID: 21253493. ].
In summary, animal models of stent implantation provide a practical test bed for feasibility and safety of deployment of new devices, and insight into their efficacy. The porcine model has contributed and still contributes to understanding the biology of vascular interventions, healing and developing new interventional devices.
Evaluation of stent technologies and angioplasty
The first approach to transluminal treatment of coronary artery disease was described by Dotter and Judkins in 1964 [44. de Prado AP, Perez-Martinez C, Cuellas C, Gonzalo-Orden JM, Diego A, Regueiro M, Martinez-Fernandez B, Altonaga JR, Marin Francisco JG, Fernandez-Vazquez F. Preclinical evaluation of coronary stents: focus on safety issues. Curr Vasc Pharmacol 2013 11:74-99. PMID: 22724463. ], and a detailed technique of balloon angioplasty was provided by Grüntzig in the 1970s [99. Iqbal J, Chamberlain J, Francis SE, Gunn J. Role of Animal Models in Coronary Stenting. Ann Biomed Eng 2016 44:453-65. PMID: 26259974. ]. The balloon angioplasty technique was progressively replaced by coronary stenting because of major drawbacks, such as elastic recoil leading to restenosis and high risk of abrupt closure of the artery. Today, balloon angioplasty is still used in preclinical research to create models of coronary restenosis.
Bare metal stents
Studies have confirmed the clinical superiority of coronary stents over balloon angioplasty, but rates of restenosis (caused by excessive neointimal proliferation) using bare metal stents were still quite high. The swine coronary artery model has been, by far, the most commonly used model in evaluation of the effects of stents on arterial injury, restenosis and vascular healing. The vascular healing processes and stages in this animal model and in humans are well documented, and quite similar, although the time courses are different [44. de Prado AP, Perez-Martinez C, Cuellas C, Gonzalo-Orden JM, Diego A, Regueiro M, Martinez-Fernandez B, Altonaga JR, Marin Francisco JG, Fernandez-Vazquez F. Preclinical evaluation of coronary stents: focus on safety issues. Curr Vasc Pharmacol 2013 11:74-99. PMID: 22724463. ].
Drug-eluting stents (DES)
The solution to the in-stent restenosis problem appeared almost twenty years ago, namely drug-eluting stents (DES). These are based on the concept of local delivery of antirestenotic drugs, mostly -limus and -taxol families of drugs, and animal models were used in their development.
Preclinical assessment of the safety and efficacy of DES relies on well-defined methods. First, a drug or bioactive substance released from the proposed surface should be characterised both in vitro and in vivo. Studies directed to assessing drug kinetics are often performed in rabbits, because that model has been used historically and allows comparison with other technologies. The porcine model of choice is the normolipaemic domestic crossbreed (farm pigs) or mini-swine. Regarding the stent itself, recommendations suggest that the stent should be appropriately sized using a stent:artery ratio of between 1.0 and 1.2 (using a higher ratio could induce severe arterial injury and considerable stenosis), and implanted into naïve arteries with no prior injury. The rabbit iliac model is an accepted and validated method to assess the feasibility, safety, and biocompatibility of DES. However, this model is suboptimal for survival endpoints designed to monitor thrombotic or other clinical complications (peripheral implants do not allow evaluation of coronary complications such as arrhythmias) [77. Suzuki Y, Yeung AC, Ikeno F. The representative porcine model for human cardiovascular disease. J Biomed Biotechnol 2011 2011:195483. PMID: 21253493. , 1111. Schwartz RS, Edelman E, Virmani R, Carter A, Granada JF, Kaluza GL, Chronos NA, Robinson KA, Waksman R, Weinberger J, Wilson GJ, Wilensky RL. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv 2008 1:143-53. PMID: 20031669. ]. Only one tested stent should be used per artery except when study objectives or scientific hypotheses necessitate treatment overlap or multiple dosing data. Most study designs incorporate a stent implanted in 2 or 3 major epicardial arteries. Stent overlap is also good to evaluate stent fracture as it provides a hinge point for the distal stent. Stent fractures should be screened by appropriate methods, i.e., high-resolution magnified X-ray from several views, in vivo or ex vivo computed tomographic angiography, or rotational angiography or micro CT. When testing a new polymer-coated DES, it is relevant to have two control groups, the equivalent bare metal stent and the polymer-coated stent without the drug [99. Iqbal J, Chamberlain J, Francis SE, Gunn J. Role of Animal Models in Coronary Stenting. Ann Biomed Eng 2016 44:453-65. PMID: 26259974. ]. Clinical and histopathologic data should be obtained at an early time point (3 to 7 days) to determine thrombotic risk, and also at short-term endpoints (14 to 28 days: evaluation of endothelial toxicity and quantitation of neointimal hyperplasia). At least two late time points should be tested to examine long-term effects (usually 90 days, but longer-term data should also be tested to examine late effects, e.g., 180 or 360 days). All animals should receive antiplatelet therapy daily, beginning one day before procedure until sacrifice [1212. Schwarz ER, Pollick C, Dow J, Patterson M, Birnbaum Y, Kloner RA. A small animal model of non-ischemic cardiomyopathy and its evaluation by transthoracic echocardiography. Cardiovasc Res 1998 39:216-23. PMID: 9764201. ].
The success of DES demonstrates the utility of the animal model, especially of the porcine restenosis artery model [1313. Li Y, Tellez A, Rousselle SD, Dillon KN, Garza JA, Barry C, Granada JF. Biological effect on drug distribution and vascular healing via paclitaxel-coated balloon technology in drug eluting stent restenosis swine model. Catheter Cardiovasc Interv 2016 88:89-98. PMID: 26613810. , 1414. Lim KS, Jeong MH, Bae IH, Park DS, Kim JM, Kim JH, Cho DL, Sim DS, Park KH, Hong YJ, Ahn Y. Histopathological Comparison among Biolimus, Zotarolimus and Everolimus-Eluting Stents in Porcine Coronary Restenosis Model. Korean Circ J 2013 43:744-51. PMID: 24363750. , 1515. Lemos PA, Farooq V, Takimura CK, Gutierrez PS, Virmani R, Kolodgie F, Christians U, Kharlamov A, Doshi M, Sojitra P, van Beusekom HM, Serruys PW. Emerging technologies: polymer-free phospholipid encapsulated sirolimus nanocarriers for the controlled release of drug from a stent-plus-balloon or a stand-alone balloon catheter. EuroIntervention 2013 9:148-56. PMID: 23685303. ].
Drug-eluting balloon (DEB)
Different DEBs based on distinct antiproliferative drugs have demonstrated their efficacy in preclinical research [1313. Li Y, Tellez A, Rousselle SD, Dillon KN, Garza JA, Barry C, Granada JF. Biological effect on drug distribution and vascular healing via paclitaxel-coated balloon technology in drug eluting stent restenosis swine model. Catheter Cardiovasc Interv 2016 88:89-98. PMID: 26613810. ], but seem to show a delayed re-endothelialisation and abnormal vasomotor response of treated vessels [44. de Prado AP, Perez-Martinez C, Cuellas C, Gonzalo-Orden JM, Diego A, Regueiro M, Martinez-Fernandez B, Altonaga JR, Marin Francisco JG, Fernandez-Vazquez F. Preclinical evaluation of coronary stents: focus on safety issues. Curr Vasc Pharmacol 2013 11:74-99. PMID: 22724463. ]. Development of biodegradable or polymer-free drug carriers seems to be promising and may result in an expansion of the technological possibilities for other intravascular drug-delivery systems, such as metal-free or even implant-free alternatives [44. de Prado AP, Perez-Martinez C, Cuellas C, Gonzalo-Orden JM, Diego A, Regueiro M, Martinez-Fernandez B, Altonaga JR, Marin Francisco JG, Fernandez-Vazquez F. Preclinical evaluation of coronary stents: focus on safety issues. Curr Vasc Pharmacol 2013 11:74-99. PMID: 22724463. , 1515. Lemos PA, Farooq V, Takimura CK, Gutierrez PS, Virmani R, Kolodgie F, Christians U, Kharlamov A, Doshi M, Sojitra P, van Beusekom HM, Serruys PW. Emerging technologies: polymer-free phospholipid encapsulated sirolimus nanocarriers for the controlled release of drug from a stent-plus-balloon or a stand-alone balloon catheter. EuroIntervention 2013 9:148-56. PMID: 23685303. ]. Animal models continue to provide invaluable information in further developments to minimise thrombogenicity and promote healing - new antiproliferative agents, biocompatible and bioabsorbable polymer coatings and, recently, fully bioresorbable scaffolds (BRS) [1616. Onuma Y, Serruys PW, Perkins LE, Okamura T, Gonzalo N, Garcia-Garcia HM, Regar E, Kamberi M, Powers JC, Rapoza R, van Beusekom H, van der Giessen W, Virmani R. Intracoronary optical coherence tomography and histology at 1 month and 2, 3, and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: an attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation 2010 122:2288-300. PMID: 20975003. ].
Key to understanding device performance and compatibility is healing. Histopathology remains the gold standard in the assessment of performance of these devices. Nevertheless, in vivo imaging techniques may enhance evaluation at different time points: angiography allows measurements, evaluation of stent thrombosis and functional assessment (using different vasomotor drugs). Intravascular ultrasound (IVUS) is also a common tool in preclinical research, as angioscopy and optical coherence tomography (OCT) could be useful to assess re-endothelialisation of stents. It is relevant to note that any “invasive” imaging procedure, as with the placing of an angioplasty wire, can induce significant damage in the endothelial lining that could persist up to 5 days in the animal model [44. de Prado AP, Perez-Martinez C, Cuellas C, Gonzalo-Orden JM, Diego A, Regueiro M, Martinez-Fernandez B, Altonaga JR, Marin Francisco JG, Fernandez-Vazquez F. Preclinical evaluation of coronary stents: focus on safety issues. Curr Vasc Pharmacol 2013 11:74-99. PMID: 22724463. , 1212. Schwarz ER, Pollick C, Dow J, Patterson M, Birnbaum Y, Kloner RA. A small animal model of non-ischemic cardiomyopathy and its evaluation by transthoracic echocardiography. Cardiovasc Res 1998 39:216-23. PMID: 9764201. ].
Safety, toxicity and technical feasibility can be evaluated thanks to large animal models. Efficacy evaluation is more limited by the lack of a realistic model of atherosclerosis. This issue seemed to be resolved by using pigs with metabolic syndrome (Yucatan swine with a high cholesterol diet) or genetically modified models (PCSK9 mini-pigs) [99. Iqbal J, Chamberlain J, Francis SE, Gunn J. Role of Animal Models in Coronary Stenting. Ann Biomed Eng 2016 44:453-65. PMID: 26259974. ], but the atherosclerotic swine model is time-consuming and the survival rate of these animals can be quite low.
Coronary arteries in the sheep are also suitable for the assessment of catheter-based interventional devices that may be used in humans. The sheep coronary model is indeed an interesting model, because it is quite similar to the swine coronary model in terms of vascular response, and to human physiology in terms of coagulation and fibrinolytic activity. It also offers a wide availability of large vessel bifurcations to test dedicated devices: the sheep coronary model seems to be an adequate anatomic model to test bifurcated stents [1111. Schwartz RS, Edelman E, Virmani R, Carter A, Granada JF, Kaluza GL, Chronos NA, Robinson KA, Waksman R, Weinberger J, Wilson GJ, Wilensky RL. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv 2008 1:143-53. PMID: 20031669. ]. The sheep model is commonly perceived as less prone to arrhythmogenesis after catheterisation or intervention on coronary arteries. Moreover, sheep growth appears to be more compatible with long-term studies. Nevertheless, as opposed to human hearts, in the majority of sheep hearts, the left coronary artery is dominant: the circumflex arterial branch encircles the entirety of the mural leaflet of the mitral valve and gives rise to the posterior interventricular branch at the crux ( Figure 10, Figure 11). The left azygos vein is also found in ruminants. Recognition of these anatomical difference is important for the cardiologist, but they do not contraindicate the utilisation of the sheep model. The normal coronary sheep model has been used for stent testing [1717. Ribeiro PA, Gallo R, Antonius J, Mimish L, Sriram R, Bianchi S, Duran CG. A new expandable intracoronary tantalum (Strecker) stent: early experimental results and follow-up to twelve months. Am Heart J 1993 125:501-10. PMID: 8427147. ] and for the validation of a remote control percutaneous coronary procedure [1818. Beyar R, Wenderow T, Lindner D, Kumar G, Shofti R. Concept, design and pre-clinical studies for remote control percutaneous coronary interventions. EuroIntervention 2005 1:340-5. PMID: 19758927. ]. The coronary overstretch sheep model has been used to confirm the relation between vessel injury and restenosis, and allows the discovery of the relation between vessel strain/symmetry and re-narrowing [1919. Schulz C, Herrmann RA, Beilharz C, Pasquantonio J, Alt E. Coronary stent symmetry and vascular injury determine experimental restenosis. Heart 2000 83:462-7. PMID: 10722552. ]. In our lab, we can readily adapt to anatomical specificities in different species; sheep is our preferred animal model. We also largely use sheep for the creation of secondary mitral or tricuspid insufficiency (see the section entitled PATHOLOGIC MODELS below).
With the emerging new treatment options for various cardiovascular diseases (such as cardiac resynchronisation therapy or percutaneous transvenous mitral valve annuloplasty), preclinical research focusing on the coronary sinus has increased. Anatomy of the cardiac venous system is quite similar in sheep, dogs and humans. However, differences among these animal species and humans have been identified: the number and position of venous valves are different, the animals do not have a vein of Marshall, and pigs and sheep have a left azygos vein that enters the coronary sinus. This last difference can make catheterisation of the coronary sinus challenging in pigs and sheep: the ostium of the left azygos vein being larger than that of the great cardiac vein, guidewires are more prone to enter the azygos vein [66. Genain MA, Morlet A, Herrtage M, Muresian H, Anselme F, Latremouille C, Laborde F, Behr L, Borenstein N. Comparative anatomy and angiography of the cardiac coronary venous system in four species: human, ovine, porcine, and canine. J Vet Cardiol 2018 20:33-44. PMID: 29191414. ]. Nevertheless, in our experience, after a learning curve, catheterisation of the coronary sinus is perfectly feasible in these species.
Large animal models have proven their utility at the early stages of almost every technical and biological development in stenting. Safety remains the primary concern. They can also be valuable to address clinical issues that arise.
pathologic models
In theory, diseased animal models should be the best platform for assessing the safety and efficacy of novel devices or medical strategies. Nevertheless, achieving a reliable pathologic model in large animals is extremely challenging. Creating lesions is never an issue but the challenge is to have enough dysfunction to make it relevant (and not too much such that it becomes cost-prohibitive) and, more importantly, ethically acceptable. Depending on the type and severity of the disease induced, and the experience of the laboratory in providing appropriate supportive veterinary care, survival rates in disease models can be unacceptably low.
Heart failure (HF) or valvular insufficiency are two good examples. Heart failure models in large animals have been achieved through many different modalities and in different species. Rapid pacing does provide reproducible left ventricular (LV) dilation and signs of dilated cardiomyopathy but is known to be reversible and is therefore unfit for long-term studies [77. Suzuki Y, Yeung AC, Ikeno F. The representative porcine model for human cardiovascular disease. J Biomed Biotechnol 2011 2011:195483. PMID: 21253493. , 88. Cheng Y, Aboodi MS, Wechsler AS, Kaluza GL, Granada JF, Van Bladel K, Annest LS, Yi GH. Epicardial catheter-based ventricular reconstruction: a novel therapy for ischaemic heart failure with anteroapical aneurysm. Interact Cardiovasc Thorac Surg 2013 17:915-22. PMID: 23985410. ]. Volume or pressure overload has commonly been used in the past but these methods are limited by the difficulty of controlling disease severity [77. Suzuki Y, Yeung AC, Ikeno F. The representative porcine model for human cardiovascular disease. J Biomed Biotechnol 2011 2011:195483. PMID: 21253493. ]. Heart failure has been obtained through acute or chronic, complete or partial, surgical or interventional occlusion of the coronary arteries [2020. Devlin G, Matthews K, McCracken G, Stuart S, Jensen J, Conaglen J, Bass J. An ovine model of chronic stable heart failure. J Card Fail 2000 6:140-3. PMID: 10908088. , 2121. Ikeda Y, Yutani C, Huang Y, Masuda K, Yuasa T, Kawaguchi O, Hunyor SN. Histological remodeling in an ovine heart failure model resembles human ischemic cardiomyopathy. Cardiovasc Pathol 2001 10:19-27. PMID: 11343991. , 2222. Kim DH, Morris B, Guerrero JL, Sullivan SM, Hung J, Levine RA. Ovine Model of Ischemic Mitral Regurgitation. Methods Mol Biol 2018 1816:295-308. PMID: 29987829. , 2323. Moainie SL, Gorman JH 3rd, Guy TS, Bowen FW 3rd, Jackson BM, Plappert T, Narula N, St John-Sutton MG, Narula J, Edmunds LH Jr, Gorman RC. An ovine model of postinfarction dilated cardiomyopathy. Ann Thorac Surg 2002 74:753-60. PMID: 12238835. , 2424. O’Konski MS, White FC, Longhurst J, Roth D, Bloor CM. Ameroid constriction of the proximal left circumflex coronary artery in swine. A model of limited coronary collateral circulation. Am J Cardiovasc Pathol 1987 1:69-77. PMID: 3455237. , 2525. Ruel MA, Sellke FW, Bianchi C, Khan TA, Faro R, Zhang JP, Cohn WE. Endogenous myocardial angiogenesis and revascularization using a gastric submucosal patch. Ann Thorac Surg 2003 75:1443-9. PMID: 12735560. , 2626. Sakaguchi G, Sakakibara Y, Tambara K, Lu F, Premaratne G, Nishimura K, Komeda M. A pig model of chronic heart failure by intracoronary embolization with gelatin sponge. Ann Thorac Surg 2003 75:1942-7. PMID: 12822640. ].
Serial microbead injection into the coronary vessels allows controlled, progressive and well-tolerated onset of left and/or right ventricular (RV) dysfunction with symptoms of HF. This has been our preferred technique and has allowed us to fine-tune LV or RV dysfunction, tailored to the needs of studies (location of the dysfunction, amount of dilatation) [2727. Squara P, Borenstein N, Daniel P. Hemodynamic exercise testing and hormonal status in a sheep model of congestive heart failure. Minerva Anestesiol 2011 77:283-91. PMID: 21441883. ]. Such models have shown stable recruitment of myocardial remodelling mechanisms that involve an interaction among haemodynamic load, contractile efficiency/energetics, neurohormonal activation, response of the extracellular matrix, wall stress, and the myocyte apoptotic pathway [2828. Huang Y, Hunyor SN, Jiang L, Kawaguchi O, Shirota K, Ikeda Y, Yuasa T, Gallagher G, Zeng B, Zheng X. Remodeling of the chronic severely failing ischemic sheep heart after coronary microembolization: functional, energetic, structural, and cellular responses. Am J Physiol Heart Circ Physiol 2004 286:H2141-50. PMID: 15148056. ].
As regards non-ischaemic disease, a spontaneous model of dilated cardiomyopathy (DCM) is well known in the Syrian hamster but the size of the animals makes them unsuitable for the study of novel surgical modalities such as neuromodulation or mechanical assist devices. DCM is also a well-recognised cause of spontaneous HF in large and giant breed dogs. However, canine DCM is a relatively rare condition and there is no readily available colony for HF studies.
Doxorubicin has been widely used to induce HF in several animal models. Doxorubicin is one of the most widely prescribed and effective cytotoxic drugs used in oncology. It is a potent, broad spectrum chemotherapeutic agent effective against solid tumours and malignant haematological disease [1919. Schulz C, Herrmann RA, Beilharz C, Pasquantonio J, Alt E. Coronary stent symmetry and vascular injury determine experimental restenosis. Heart 2000 83:462-7. PMID: 10722552. ]. However, the use of doxorubicin is limited by cumulative, dose-related, progressive myocardial damage that may lead to irreversible HF. Rodents, rabbits, non-human primates, dogs and ruminants have been used in doxorubicin-induced HF studies [1212. Schwarz ER, Pollick C, Dow J, Patterson M, Birnbaum Y, Kloner RA. A small animal model of non-ischemic cardiomyopathy and its evaluation by transthoracic echocardiography. Cardiovasc Res 1998 39:216-23. PMID: 9764201. , 2929. Borenstein N, Bruneval P, Behr L, Laborde F, Montarras D, Daures JP, Derumeaux G, Pouchelon JL, Chetboul V. An ovine model of chronic heart failure: echocardiographic and tissue Doppler imaging characterization. J Card Surg 2006 21:50-6. PMID: 16426348. , 3030. Chekanov VS. A stable model of chronic bilateral ventricular insufficiency (dilated cardiomyopathy) induced by arteriovenous anastomosis and doxorubicin administration in sheep [letter comment]. J Thorac Cardiovasc Surg 1999 117:198-9. PMID: 9869781. , 3131. Gralla EJ, Fleischman RW, Luthra YK, Stadnicki SW. The dosing schedule dependent toxicities of adriamycin in beagle dogs and rhesus monkeys. Toxicology 1979 13:263-73. PMID: 118548. , 3232. Hanai K, Takaba K, Manabe S, Nakano M, Kohda A, Matsuo M. Evaluation of cardiac function by echocardiography in dogs treated with doxorubicin. J Toxicol Sci 1996 21:1-10. PMID: 8852283. , 3333. Klimtova I, Simunek T, Mazurova Y, Hrdina R, Gersl V, Adamcova M. Comparative study of chronic toxic effects of daunorubicin and doxorubicin in rabbits. Hum Exp Toxicol 2002 21:649-57. PMID: 12540035. , 3434. Magovern JA, Christlieb IY, Badylak SF, Lantz GC, Kao RL. A model of left ventricular dysfunction caused by intracoronary adriamycin. Ann Thorac Surg 1992 53:861-3. PMID: 1570984. , 3535. Monnet E, Orton EC. Myocardial oxygen consumption is affected by dynamic cardiomyoplasty in dogs with adriamycin-induced cardiomyopathy. J Card Surg 1998 13:475-83. PMID: 10543463. , 3636. Monnet E, Orton EC. A canine model of heart failure by intracoronary adriamycin injection: hemodynamic and energetic results. J Card Fail 1999 5:255-64. PMID: 10496198. , 3737. Shah HR, Vaynblat M, Ramdev G, Cunningham JN Jr, Chiavarelli M. Experimental cardiomyopathy as a model of chronic heart failure. J Invest Surg 1997 10:387-96. PMID: 9654396. , 3838. Tessier D, Lajos P, Braunberger E, Pouchelon JL, Carpentier A, Chachques JC, Chetboul V. Induction of chronic cardiac insufficiency by arteriovenous fistula and doxorubicin administration. J Card Surg 2003 18:307-11. PMID: 12869175. , 3939. Toyoda Y, Okada M, Kashem MA. A canine model of dilated cardiomyopathy induced by repetitive intracoronary doxorubicin administration. J Thorac Cardiovasc Surg 1998 115:1367-73. PMID: 9628680. ]. There are pros and cons for each of these ischaemic or non-ischaemic HF models with regard to technical ease, cost, reliability, and outcome.
As to valvular dysfunction and valvular replacement, the need for a diseased model might be less critical. Indeed, the sheer fact that during replacement the valve is either removed or crushed under a stent causes massive insufficiency, which itself is treated by the prosthetic valve. So, efficacy is necessarily tested. However, for valvular repair or pharmacological treatments, there might be a real need for a valvular dysfunction model. Likewise, enlarged, round annuli might be warranted for specific stented valves.
It is not difficult to create valvular dysfunction through direct insult to the different components of the valve. For the mitral and the tricuspid valves, severing via a surgical or interventional approach the annulus [4040. Walter EM, Vasilyev NV, Sill B, Padala M, Jimenez J, Yoganathan AP, Hetzer R, del Nido PJ. Creation of a tricuspid valve regurgitation model from tricuspid annular dilatation using the cardioport video-assisted imaging system. J Heart Valve Dis 2011 20:184-8. PMID: 21560820. ], the leaflets [4141. Bai Y, Chen HY, Zong GJ, Jiang HB, Li WP, Wu H, Zhao XX, Qin YW. Percutaneous establishment of tricuspid regurgitation: an experimental model for transcatheter tricuspid valve replacement. Chin Med J (Engl) 2010 123:806-9. PMID: 20497668. ], the chordae [4242. Hoppe H, Pavcnik D, Chuter TA, Tseng E, Kim MD, Bernat I, Uchida B, Keller FS, Rosch J. Percutaneous technique for creation of tricuspid regurgitation in an ovine model. J Vasc Interv Radiol 2007 18:133-6. PMID: 17296714. ] or the papillary muscle is an option [4343. Lauten A, Figulla HR, Willich C, Laube A, Rademacher W, Schubert H, Bischoff S, Ferrari M. Percutaneous caval stent valve implantation: investigation of an interventional approach for treatment of tricuspid regurgitation. Eur Heart J 2010 31:1274-81. PMID: 19933224. ]. Endomyocardial biopsy forceps-induced lesions [4141. Bai Y, Chen HY, Zong GJ, Jiang HB, Li WP, Wu H, Zhao XX, Qin YW. Percutaneous establishment of tricuspid regurgitation: an experimental model for transcatheter tricuspid valve replacement. Chin Med J (Engl) 2010 123:806-9. PMID: 20497668. ] and chordae rupture via chordal snaring [4242. Hoppe H, Pavcnik D, Chuter TA, Tseng E, Kim MD, Bernat I, Uchida B, Keller FS, Rosch J. Percutaneous technique for creation of tricuspid regurgitation in an ovine model. J Vasc Interv Radiol 2007 18:133-6. PMID: 17296714. ] or cutting [4343. Lauten A, Figulla HR, Willich C, Laube A, Rademacher W, Schubert H, Bischoff S, Ferrari M. Percutaneous caval stent valve implantation: investigation of an interventional approach for treatment of tricuspid regurgitation. Eur Heart J 2010 31:1274-81. PMID: 19933224. ] are potential options. As regards the aortic valve, percutaneous [4444. Griffin BP, Flachskampf FA, Reimold SC, Lee RT, Thomas JD. Relationship of aortic regurgitant velocity slope and pressure half-time to severity of aortic regurgitation under changing haemodynamic conditions. Eur Heart J 1994 15:681-5. PMID: 8056010. ] tearing with biopsy forceps or controlled perforation and balloon dilatation to the desired diameter to induce more or less regurgitation are acceptable and efficient techniques [4545. Boudjemline Y, Agnoletti G, Bonnet D, Behr L, Borenstein N, Sidi D, Bonhoeffer P. Steps toward the percutaneous replacement of atrioventricular valves an experimental study. J Am Coll Cardiol 2005 46:360-5. PMID: 16022968. ].
We have developed an interventional approach to the creation of a secondary (ischaemic) ovine model of either mitral or tricuspid insufficiency. As for ventricular dysfunction, it is based on segmental, targeted embolisation of microbeads such that there is sufficient dilatation of the ventricle to induce regurgitation and displacement of the papillary muscles. As an example, sequential silicone microbead embolisations were carried out in the circumflex artery in order to target the posteromedial papillary muscle and cause inadequate coaptation. It takes on average 1 to 3 embolisations (exceptionally 4) to reach the necessary amount of mitral regurgitation (from mild to moderate) ( Figure 12, Figure 13, Figure 14, Video 1 and Video 2). The limitations of such an approach are, on the one hand, that it might prove costly whenever the coaptation resists dilatation or, on the other hand, that, once mitral regurgitation is established, the vicious circle of HF and progressive dilatation might make the animals fragile and unstable to withstand invasive procedures.