Overview of chapter
The treatment of ISR and the determinant factors involved in the development of late stent thrombosis (LST) are well described elsewhere and are outside the scope of this chapter [4242. Garg S, Serruys PW. Coronary stents: looking forward. J Am Coll Cardiol. 2010;56:S43-78. , 4343. Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol 2010;56:S1-42. , 4444. Alfonso F. Treatment of drug-eluting stent restenosis the new pilgrimage: quo vadis? J Am Coll Cardiol. 2010;55:2717-20. ]. The underlying mechanisms of restenosis with DES can broadly be divided into 4 main causes ( Table 1 ), namely biological, arterial, stent and implantation factors, accepting that this classification is somewhat arbitrary with mechanisms of restenosis being attributable to more than one factor. In this chapter we explore these 4 main mechanisms and identify the potentially controllable and non-controllable factors from the perspective of the interventional cardiologist intending to implant a DES.
BIOLOGICAL FACTORS
Resistance to antiproliferative drugs
The underlying mechanisms of action and causes of resistance to paclitaxel or sirolimus are well documented in the cancer literature and can either be present in genetically predetermined individuals or be acquired, following cytotoxic exposure to the drug [4545. Richardson A, Kaye SB. Drug resistance in ovarian cancer: the emerging importance of gene transcription and spatio-temporal regulation of resistance. Drug Resist Updat. 2005;8:311-21. , 4646. Huang S, Houghton PJ. Mechanisms of resistance to rapamycins. Drug Resist Updat. 2001;4:378-91. ].
The so-called “drug resistance gene expression programme,” described for paclitaxel resistance from the cancer literature, best exemplifies the complex pathways involved in the aetiology of drug resistance [4545. Richardson A, Kaye SB. Drug resistance in ovarian cancer: the emerging importance of gene transcription and spatio-temporal regulation of resistance. Drug Resist Updat. 2005;8:311-21. ]. Essentially, the cellular context determines the genes that are expressed which contribute to drug resistance either in genetically predetermined cells or primed for expression following the cytotoxic insult after exposure to the drug. These genes may operate in conventional pathways that are well known (drug delivery and metabolism, apoptosis regulation, DNA repair), but the temporal (i.e., pro- and anti-apoptotic gene activity) and spatial regulation (i.e., cell survival signalling pathways) of these gene products after exposure to the drug also appear to be important.
As examples, polymorphisms in the genes that encode mTOR or proteins involved in paclitaxel or sirolimus metabolism have been shown to confer drug resistance both in vitro and in vivo [1010. van Der Giessen WJ, Regar E, Harteveld MS, Coen VL, Bhagwandien R, Au A, Levendag PC, Ligthart J, Serruys PW, den Boer A, Verdouw PD, Boersma E, Hu T, van Beusekom HM. “Edge Effect” of (32)p radioactive stents is caused by the combination of chronic stent injury and radioactive dose falloff. Circulation. 2001;104:2236-41. , 1111. Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr., Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85-151. ] : decreased binding of sirolimus to mTOR due to mutations in FK-B12 and mTOR and mutations of downstream effector molecules of mTOR may all cause resistance to sirolimus [1111. Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr., Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85-151. ].
- Drug-eluting stents (DES) were conceived as the next step - after balloon angioplasty and bare metal stents (BMS) - in tackling the iatrogenic entity of neointimal hyperplasia
- The distribution of late lumen loss (LLL) after drug-eluting stent implantation has been shown to follow a bimodal pattern of distribution with both paclitaxel (PES) and sirolimus (SES) eluting stents
- “DES failure” equates to approximately 5-10% of cases, with one estimate suggesting approximately 200,000 repeat revascularisations in the US alone [1515. Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480-6. ]
- The pattern of restenosis seen with DES is usually focal. By contrast, the pattern of restenosis with BMS is primarily diffuse
- Persistence of the inflammatory response beyond 90 days after arterial injury is strongly associated with an increased level of neointimal thickness and consequent restenosis
- In-stent restenosis (ISR) may not be as “benign” as once originally thought with 30-60% of ISR cases presenting with an acute coronary syndrome (ACS)
Potentially overcoming drug resistance through the delivery of higher doses of antiproliferative agent to the implantation site
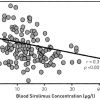
Given the possibility that drug resistance is one potential mechanism of restenosis, attempts have been made to give much higher doses of oral sirolimus to patients with refractory ISR in the theoretical attempt of overcoming drug resistance and delivering increased amounts of drug to the implantation site. The OSIRIS study [4747. Hausleiter J, Kastrati A, Mehilli J, Vogeser M, Zohlnhofer D, Schuhlen H, Goos C, Pache J, Dotzer F, Pogatsa-Murray G, Dirschinger J, Heemann U, Schomig A. Randomized, double-blind, placebo-controlled trial of oral sirolimus for restenosis prevention in patients with in-stent restenosis: the Oral Sirolimus to Inhibit Recurrent In-stent Stenosis (OSIRIS) trial. Circulation. 2004;110:790-5. ] investigated the administration of higher doses of oral sirolimus to patients with refractory ISR and demonstrated a significant correlation between the level of sirolimus concentration in the bloodstream and rates of further late lumen loss ( Figure 4 ). Given that the patients received a short duration of oral sirolimus (7 days), it was unclear if these findings would be maintained at longer-term follow-up. It has been anecdotally reported that courses of sirolimus given for 30 days after POBA to the restenotic lesion, in the theoretical attempt to cover the injury period following POBA, can reduce restenosis in refractory restenosis cases [4848. Teirstein PS. Drug-eluting stent restenosis: an uncommon yet pervasive problem. Circulation. 2010;122:5-7. ].
Furthermore, evidence has suggested that the concomitant administration of steroids to patients implanted with BMS, particularly in patients with a persistent inflammatory state, as indicated by elevated C-reactive protein, may reduce the incidence of ISR [4949. Ribichini F, Tomai F, De Luca G, Boccuzzi G, Presbitero P, Pesarini G, Ferrero V, Ghini AS, Abukaresh R, Aurigemma C, De Luca L, Zavalloni D, Soregaroli D, Marino P, Garbo R, Zanolla L, Vassanelli C. Immunosuppressive therapy with oral prednisone to prevent restenosis after PCI. A multicenter randomized trial. The American journal of medicine. 2011;124:434-43. , 5050. Ribichini F, Tomai F, Ferrero V, Versaci F, Boccuzzi G, Proietti I, Prati F, Crea F, Vassanelli C. Immunosuppressive oral prednisone after percutaneous interventions in patients with multi-vessel coronary artery disease. The IMPRESS-2/MVD study. EuroIntervention. 2005;1:173-80. , 5151. Ribichini F, Ferrero V, Rognoni A, Marino P, Brunelleschi S, Vassanelli C. Percutaneous treatment of coronary bifurcations: lesion preparation before provisional bare metal stenting and subsequent immunosuppression with oral prednisone. The IMPRESS-Y study. Journal of interventional cardiology. 2007;20:114-21. , 5252. Versaci F, Gaspardone A, Tomai F, Ribichini F, Russo P, Proietti I, Ghini AS, Ferrero V, Chiariello L, Gioffre PA, Romeo F, Crea F. Immunosuppressive Therapy for the Prevention of Restenosis after Coronary Artery Stent Implantation (IMPRESS Study). JACC. 2002;40:1935-42. , 5353. Ferrero V, Tomai F, Versaci F, Feola M, Proietti I, Rognoni A, Ghini AS, Gaspardone A, Vacca G, De Luca L, Vassanelli C, Ribichini F. Long-term results of immunosuppressive oral prednisone after coronary angioplasty in non-diabetic patients with elevated C-reactive protein levels. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2009;5:250-4. ].
A patient-level meta-analysis of seven randomised trials (n=1246) investigating oral administration of oral immunosuppressive therapy (oral prednisone or sirolimus) to prevent in-stent restenosis (RAMSES cooperation) after BMS or DES has recently been published. [242242. Cassese S, De Luca G, Ribichini F, Cernigliaro C, Sansa M5 Versaci F, Proietti I, Stankovic G, Stojkovic S, Fernandez-Pereira C, Tomai F, Vassanelli C, Antoniucci D, Serruys PW, Kastrati A, Rodriguez AE. ORAl iMmunosuppressive therapy to prevent in-Stent rEstenosiS (RAMSES) ] PCI with the use of BMS and oral immunosuppressive therapy was shown to reduce the risk of revascularisation compared to BMS alone, findings that were not apparent with DES alone. Findings that support the concept that anti-proliferative drug dose and release kinetics are much more important in the prevention of ISR, than simply high dose anti-proliferative drug administration, as discussed in the subsequent section on stent factors.
Hypersensitivity reactions (the polymer)
Polymer layers in DES are used as both drug reservoirs and non-drug-coated external films to allow optimal drug release kinetics, as described in Stent Factors. As examples with the first-generation DES, the Cypher® (SES) stent (Cordis Corporation, Johnson & Johnson, Warren, NJ, USA) consists of a stainless steel platform covered with a basecoat formulation (67%) consisting of the polymers PEVA (polyethylene vinyl acetate) and PBMA (poly n-butyl methacrylate) mixed with sirolimus (33%); a drug-free PBMA topcoat is also applied over the polymer drug mixture to control drug release kinetics. The Taxus® (PES) stent (Boston Scientific, Natick, MA, USA) consists of a stainless steel platform with Translute™ (poly [styrene-b-isobutylene-b-styrene]) polymer combined with paclitaxel without a primer or topcoat layer.
The inflammatory reaction that occurs after arterial injury is a critical factor which influences the extent of neointimal response, with the persistence of this inflammatory response beyond 90 days being strongly associated with delayed healing and implicated in an increased risk of LST and restenosis long term [1313. Mauri L, Orav EJ, Kuntz RE. Late loss in lumen diameter and binary restenosis for drug-eluting stent comparison. Circulation. 2005;111:3435-42. , 1414. Byrne RA, Eberle S, Kastrati A, Dibra A, Ndrepepa G, Iijima R, Mehilli J, Schomig A. Distribution of angiographic measures of restenosis after drug-eluting stent implantation. Heart. 2009;95:1572-8.
The distribution of late lumen loss after first generation DES implantation - associating it with bimodal neointimal response.].
The inflammatory and potential hypersensitivity response
Both PES and SES have been demonstrated to provoke distinctive inflammatory responses in animal models beyond 90 days, with SES triggering giant cell infiltrations and PES causing eosinophilic reactions around stent struts [2626. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193-202.
One of the first reports of delayed arterial healing associated with first generation (Cypher and Taxus) DES., 2727. Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, Skorija K, Weber DK, Gold HK, Virmani R. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005;112:270-8. , 2828. Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500-10. , 2929. Finn AV, Nakazawa G, Kolodgie FD, Virmani R. Temporal course of neointimal formation after drug-eluting stent placement: is our understanding of restenosis changing? JACC Cardiovasc Interv. 2009;2(4):300-2. , 3030. Farb A, Burke AP, Kolodgie FD, Virmani R. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation. 2003;108:1701-6. ]. The inflammatory responses associated with SES have been shown to persist beyond 180 days and up to 2 years ( Figure 5 ). This phenomenon has also been shown to be potentially further exacerbated at sites of overlapping DES [2828. Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500-10. ]. This is in contrast to BMS and the new-generation everolimus-eluting stent (EES; Xience V; Abbott Vascular, Santa Clara, CA, USA) with a more biocompatible polymer, where the inflammatory responses have been demonstrated to be limited to a period of 90 days and 12 months, respectively ( Figure 5 ) [5454. Nakazawa G, Finn AV, Ladich E, Ribichini F, Coleman L, Kolodgie FD, Virmani R. Drug-eluting stent safety: findings from preclinical studies. Expert Rev Cardiovasc Ther. 2008;6:1379-91. ].
Evidence of persistent inflammatory responses in humans have also been reported both in autopsy cases, with one case reported to involve up to one third of struts in first-generation DES at 3 months, and demonstrating signs of persistent inflammation characterised by granuloma formation and extensive eosinophilic infiltration as seen in the animal models. Furthermore, evidence of persistent inflammation has been demonstrated from thrombus aspirates taken at the time of emergency PCI in patients presenting with very LST [5555. Cook S, Ladich E, Nakazawa G, Eshtehardi P, Neidhart M, Vogel R, Togni M, Wenaweser P, Billinger M, Seiler C, Gay S, Meier B, Pichler WJ, Juni P, Virmani R, Windecker S. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120:391-9. ].
Further details related to this “late restenosis” phenomenon are described in Arterial factors.
Hypersensitivity reactions (metallic stent platform)
Koster et al [5656. Koster R, Vieluf D, Kiehn M, Sommerauer M, Kahler J, Baldus S, Meinertz T, Hamm CW. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet. 2000;356:1895-7. ] first reported an apparent association between the risk of restenosis and metal allergy, namely nickel and molybdenum, with BMS. This study has been controversial, however, and the research methodology subjected to criticism, in particular the methodology of identifying nickel allergy [5757. Keane FM, Morris SD, Smith HR, Rycroft RJ. Allergy in coronary in-stent restenosis. Lancet. 2001;357:1205-6; author reply 1206-7. , 5858. Ha T, Lalla S. Allergy in coronary in-stent restenosis. Lancet 2001;357(9263):1206; author reply 1206-7. , 5959. Mimouni D, Trattner A, David M. Allergy in coronary in-stent restenosis. Lancet. 2001;357:1206-7; author reply 1206-7. ]. Small-scale, predominantly retrospective studies have failed to show an association between metal allergy in BMS and restenosis [6060. Norgaz T, Hobikoglu G, Serdar ZA, Aksu H, Alper AT, Ozer O, Narin A. Is there a link between nickel allergy and coronary stent restenosis? Tohoku J Exp Med. 2005;206:243-6. , 6161. Hillen U, Haude M, Erbel R, Goos M. Evaluation of metal allergies in patients with coronary stents. Contact Dermatitis. 2002;47:353-6. ]. Saito et al [6262. Saito T, Hokimoto S, Oshima S, Noda K, Kojyo Y, Matsunaga K. Metal allergic reaction in chronic refractory in-stent restenosis. Cardiovasc Revasc Med. 2009;10:17-22. ] did, however, report nickel allergy as being an independent predictor for refractory ISR in BMS (odds ratio 5.1, p=0.0033), with almost one fifth of patients with refractory ISR having a documented true allergy to nickel (24 of 128 patients). Of note is the fact that the nickel allergy assessment was performed by an independent dermatologist blinded to the study results. Conversely, Lijima et al [6363. Iijima R, Ikari Y, Amiya E, Tanimoto S, Nakazawa G, Kyono H, Hatori M, Miyazawa A, Nakayama T, Aoki J, Nakajima H, Hara K. The impact of metallic allergy on stent implantation: metal allergy and recurrence of in-stent restenosis. Int J Cardiol. 2005;104:319-25. ] suggested an association between nickel allergy by patch test and the recurrence of ISR, in patients treated with POBA for ISR after BMS implantation. Within their study no association was found with BMS implantation and first presentation of ISR.
The issue of ISR has also been linked to gold-coated stents, where several studies have associated these with contact allergy and a considerable increase in the risk of ISR [6464. Pache J, Dibra A, Schaut C, Schuhlen H, Dirschinger J, Mehilli J, Kastrati A, Schomig A. Sustained increased risk of adverse cardiac events over 5 years after implantation of gold-coated coronary stents. Catheterization and cardiovascular interventions. 2006;68:690-5. , 6565. Ekqvist S, Svedman C, Moller H, Kehler M, Pripp CM, Bjork J, Gruvberger B, Holmstrom E, Gustavsson CG, Bruze M. High frequency of contact allergy to gold in patients with endovascular coronary stents. The British journal of dermatology. 2007;157:730-8. , 6666. Kastrati A, Schomig A, Dirschinger J, Mehilli J, von Welser N, Pache J, Schuhlen H, Schilling T, Schmitt C, Neumann FJ. Increased risk of restenosis after placement of gold-coated stents: results of a randomized trial comparing gold-coated with uncoated steel stents in patients with coronary artery disease. Circulation. 2000;101:2478-83. , 6767. Svedman C, Moller H, Gustavsson CG, Bruze M. Coronary restenosis and contact allergy to stent material. The Journal of invasive cardiology. 2011;23:3 p following E94. , 6868. Svedman C, Ekqvist S, Moller H, Bjork J, Pripp CM, Gruvberger B, Holmstrom E, Gustavsson CG, Bruze M. A correlation found between contact allergy to stent material and restenosis of the coronary arteries. Contact Dermatitis. 2009;60:158-64. ].Consequently, the use of gold in coronary stents has been abandoned.
Whether the issue of nickel hypersensitivity is a potential issue with DES is both speculative and theoretical. To date, only one small study (Nakazawa et al [6969. Nakazawa G, Tanabe K, Aoki J, Onuma Y, Higashikuni Y, Yamamoto H, Ohtsuki S, Yachi S, Yagishita A, Nakajima H, Hara K. Sirolimus-eluting stents suppress neointimal formation irrespective of metallic allergy. Circ J. 2008;72:893-6. ]) has examined this issue and found no association between the risk of ISR and SES implantation. A very recent single autopsy case report has however reported hypersensitivity reactions with newer generation DES. With both the cobalt chromium everolimus-eluting stent (EES) and Resolute zotarolimus eluting stents (R-ZES) hypersensitivity reactions were seen that were speculated to be attributable to either the “-limus” anti-proliferative drug or the fluorinated copolymer [poly n-butyl methacrylate (PBMA) which was present in both stents ( Figure 15 and Figure 16 ) and has previously been implicated in hypersensitivity reactions with first generation SES. [28629]
Inflammatory biomarkers and genetics
Inflammatory biomarkers
The inflammatory status, as assessed by C-reactive protein levels, has consistently failed to demonstrate any association with ISR after DES implantation, despite being associated with ISR after BMS implantation; C-reactive protein levels have, however, been implicated in the risk of stent thrombosis [7070. Niccoli G, Montone RA, Ferrante G, Crea F. The evolving role of inflammatory biomarkers in risk assessment after stent implantation. JACC. 2010;56:1783-93. , 7171. Park DW, Lee CW, Yun SC, Kim YH, Hong MK, Kim JJ, Park SW, Park SJ. Prognostic impact of preprocedural C reactive protein levels on 6-month angiographic and 1-year clinical outcomes after drug-eluting stent implantation. Heart. 2007;93:1087-92. ].
Circulating matrix metalloproteinases (MMP) have been shown to be potentially useful in identifying patients at a greater risk of developing ISR following DES implantation [7272. Katsaros KM, Kastl SP, Zorn G, Maurer G, Wojta J, Huber K, Christ G, Speidl WS. Increased restenosis rate after implantation of drug-eluting stents in patients with elevated serum activity of matrix metalloproteinase-2 and -9. JACC Cardiovasc Interv. 2010;3:90-7. ]. It is well established that both MMP-2 and MMP-9 play fundamental roles in the migration of vascular SMCs and matrix remodelling during wound healing and are produced by vascular SMCs, endothelial cells, macrophages, lymphocytes and mast cells in response to mechanical injury [7373. Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539-45. , 7474. Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251-62. , 7575. Southgate KM, Fisher M, Banning AP, Thurston VJ, Baker AH, Fabunmi RP, Groves PH, Davies M, Newby AC. Upregulation of basement membrane-degrading metalloproteinase secretion after balloon injury of pig carotid arteries. Circ Res. 1996;79:1177-87. ]. Significant elevations in MMP-9 levels at baseline and 24 hours post PCI, and MMP-2 levels 24 hours post PCI, have all proven to be strongly associated with the development of ISR following DES implantation [7272. Katsaros KM, Kastl SP, Zorn G, Maurer G, Wojta J, Huber K, Christ G, Speidl WS. Increased restenosis rate after implantation of drug-eluting stents in patients with elevated serum activity of matrix metalloproteinase-2 and -9. JACC Cardiovasc Interv. 2010;3:90-7. ].Conversely, in the same study, low and near-normal MMP-2 and MMP-9 levels were strongly associated with a lack of a significant restenotic response.
Furthermore, other inflammatory biomarkers such as serum levels of PAI-1 [7676. Katsaros KM, Speidl WS, Kastl SP, Zorn G, Huber K, Maurer G, Glogar D, Wojta J, Christ G. Plasminogen activator inhibitor-1 predicts coronary in-stent restenosis of drug-eluting stents. Journal of thrombosis and haemostasis : JTH. 2008;6:508-13. ] and complement components (C3a and C5a) [7777. Speidl WS, Katsaros KM, Kastl SP, Zorn G, Huber K, Maurer G, Wojta J, Christ G. Coronary late lumen loss of drug eluting stents is associated with increased serum levels of the complement components C3a and C5a. Atherosclerosis. 2010;208:285-9. ] have also been implicated with ISR after DES implantation.
Genetics
It would also appear that the effects of ISR are perhaps not immune from genetics. As to whether this is due to the resistance (predetermined or acquired) to the drug as previously described, or due to biological mechanisms, in particular the inflammatory response of the restenosis process itself, is presently unclear. Inflammatory gene polymorphisms in 4 differing genes have been previously demonstrated to be associated with ISR [7878. Monraats PS, Pires NM, Agema WR, Zwinderman AH, Schepers A, de Maat MP, Doevendans PA, de Winter RJ, Tio RA, Waltenberger J, Frants RR, Quax PH, van Vlijmen BJ, Atsma DE, van der Laarse A, van der Wall EE, Jukema JW. Genetic inflammatory factors predict restenosis after percutaneous coronary interventions. Circulation. 2005;112:2417-25.
Study associating polymorphisms in genes considered to be involved in the inflammatory reaction after vessel injury, and shown to be associated with repeat revascularisation after PCI.]. For example, homozygosity of the 16/glycine variant in the beta2-adrenergic receptor (ADRB2), a mediator of nitrous oxide synthetase, has been associated with ADRB2 receptor down-regulation and an increased risk of restenosis [7878. Monraats PS, Pires NM, Agema WR, Zwinderman AH, Schepers A, de Maat MP, Doevendans PA, de Winter RJ, Tio RA, Waltenberger J, Frants RR, Quax PH, van Vlijmen BJ, Atsma DE, van der Laarse A, van der Wall EE, Jukema JW. Genetic inflammatory factors predict restenosis after percutaneous coronary interventions. Circulation. 2005;112:2417-25.
Study associating polymorphisms in genes considered to be involved in the inflammatory reaction after vessel injury, and shown to be associated with repeat revascularisation after PCI.].Vogiatzi et al [7979. Vogiatzi K, Apostolakis S, Voudris V, Thomopoulou S, Kochiadakis GE, Spandidos DA. Interleukin 8 gene polymorphisms and susceptibility to restenosis after percutaneous coronary intervention. J Thromb Thrombolysis. 2010;29:134-40. ] have previously described a powerful association, by a factor of over 15-fold, between two functional polymorphisms of interleukin-8 (a strong mediator of inflammation) and the subsequent risk of restenosis. These latter gene polymorphisms were relatively rare, which subsequently limited any clinical application. Other gene mutations have also previously been described as being associated with restenosis [8080. Kastrati A, Koch W, Berger PB, Mehilli J, Stephenson K, Neumann FJ, von Beckerath N, Bottiger C, Duff GW, Schomig A. Protective role against restenosis from an interleukin-1 receptor antagonist gene polymorphism in patients treated with coronary stenting. J Am Coll Cardiol. 2000;36:2168-73. , 8181. de Maat MP, Jukema JW, Ye S, Zwinderman AH, Moghaddam PH, Beekman M, Kastelein JJ, van Boven AJ, Bruschke AV, Humphries SE, Kluft C, Henney AM. Effect of the stromelysin-1 promoter on efficacy of pravastatin in coronary atherosclerosis and restenosis. Am J Cardiol. 1999;83:852-6. ]. Conversely, genetic markers such as angiotensin-converting enzyme (ACE), despite showing initial promise, have failed to demonstrate a clinical role – perhaps due to the multifactorial nature of ISR [8282. Kitsios G, Zintzaras E. ACE (I/D) polymorphism and response to treatment in coronary artery disease: a comprehensive database and meta-analysis involving study quality evaluation. BMC medical genetics 2009;10:50. ].
Potential clinical application
The prospect of potentially being able to identify patients with a greater propensity to develop ISR after DES implantation may perhaps allow a more “personalised revascularisation” with, for example, DES which deliver higher drug concentrations to the vessel or even the prospect of considering surgical revascularisation in this cohort of patients. This individualised approach to revascularisation based on individual genetic risk factor profiling is still in its infancy and extensive preclinical and clinical investigations are required before this can even be considered to enter conventional clinical practice.
- Biological factors related to in-stent restenosis (ISR) can be secondary to resistance to antiproliferative drugs, hypersensitivity reactions to the polymer or metallic stent platform, and inflammatory biomarkers and genetics
- ISR secondary to antiproliferative drugs can either be present in genetically predetermined individuals or be acquired following cytotoxic exposure to the drug. Limited evidence exists for using oral sirolimus to overcome drug resistance – further trials are required to establish if this is feasible or practical
- Hypersensitivity to the polymer is well documented and has been associated with late restenosis (see Arterial factors) and stent thrombosis
- Hypersensitivity to the metallic platform of drug-eluting stents remains hypothetical and unproven with nickel. Gold-coated stents have a proven association with contact allergy and have been linked to restenosis: consequently, coronary stents are no longer manufactured from gold. Hypersensitivity reactions of newer generation DES have been anecdotally reported.
- The identification of biomarkers (e.g., MMP) and genes associated with ISR is in its infancy – at the time of writing the clinical application is awaiting to be defined
ARTERIAL FACTORS
Wall shear stress
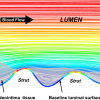
Wall shear stress refers to the principle that fluid dynamics and vessel geometry may play a potential role in the cause of focal plaque or neointimal formation [8383. Shaaban AM, Duerinckx AJ. Wall Shear Stress and Early Atherosclerosis: A Review. Am. J. Roentgenol. 2000;174:1657-1665. ]. The concept of wall shear stress is that fluid (i.e., blood) does not move at the same velocity at every point within the vessel, with blood flowing fastest in the vessel centre (i.e., a high shear stress area) and slowest when closest to the vessel wall (i.e., a low shear stress area) due to frictional forces exerted by the vessel endothelium. For example, in coronary bifurcations this phenomenon becomes more notable with a lower shear stress occurring at the ostium of a side branch [8383. Shaaban AM, Duerinckx AJ. Wall Shear Stress and Early Atherosclerosis: A Review. Am. J. Roentgenol. 2000;174:1657-1665. , 8484. Wentzel JJ, Gijsen FJ, Schuurbiers JC, van der Steen AF, Serruys PW. The influence of shear stress on in-stent restenosis and thrombosis. EuroIntervention. 2008;4 Suppl C:C27-32. , 8585. Wentzel JJ, Gijsen FJ, Stergiopulos N, Serruys PW, Slager CJ, Krams R. Shear stress, vascular remodeling and neointimal formation. J Biomech. 2003;36:681-8. ]. This may subsequently lead to the accumulation of growth factors, mitogenic cytokines and platelets, which may promote either atherosclerosis or neointimal formation if the side branch undergoes vessel injury, such as after angioplasty or stenting [8383. Shaaban AM, Duerinckx AJ. Wall Shear Stress and Early Atherosclerosis: A Review. Am. J. Roentgenol. 2000;174:1657-1665. , 8585. Wentzel JJ, Gijsen FJ, Stergiopulos N, Serruys PW, Slager CJ, Krams R. Shear stress, vascular remodeling and neointimal formation. J Biomech. 2003;36:681-8. , 8686. Suzuki N, Nanda H, Angiolillo DJ, Bezerra H, Sabate M, Jimenez-Quevedo P, Alfonso F, Macaya C, Bass TA, Ilegbusi OJ, Costa MA. Assessment of potential relationship between wall shear stress and arterial wall response after bare metal stent and sirolimus-eluting stent implantation in patients with diabetes mellitus. Int J Cardiovasc Imaging. 2008;24:357-64. , 8787. Bassiouny HS, Song RH, Hong XF, Singh A, Kocharyan H, Glagov S. Flow regulation of 72-kD collagenase IV (MMP-2) after experimental arterial injury. Circulation. 1998;98:157-63. , 8888. Bassiouny HS, Song RH, Kocharyan H, Kins E, Glagov S. Low flow enhances platelet activation after acute experimental arterial injury. J Vasc Surg. 1998;27:910-8. , 8989. Gijsen FJ, Oortman RM, Wentzel JJ, Schuurbiers JC, Tanabe K, Degertekin M, Ligthart JM, Thury A, de Feyter PJ, Serruys PW, Slager CJ. Usefulness of shear stress pattern in predicting neointima distribution in sirolimus-eluting stents in coronary arteries. JACC. 2003;92:1325-8. ]. Conversely, the carina of the side branch is a high shear stress area and atherosclerosis or restenosis rarely occurs here: indeed, animal models have shown that high shear stress areas can potentially directly inhibit SMC proliferation [9090. Sterpetti AV, Cucina A, D’Angelo LS, Cardillo B, Cavallaro A. Shear stress modulates the proliferation rate, protein synthesis, and mitogenic activity of arterial smooth muscle cells. Surgery. 1993;113:691-9. ]. Other examples include differences in the shear stress in the inner and outer curvatures of a stented vessel ( Figure 6 ) [8989. Gijsen FJ, Oortman RM, Wentzel JJ, Schuurbiers JC, Tanabe K, Degertekin M, Ligthart JM, Thury A, de Feyter PJ, Serruys PW, Slager CJ. Usefulness of shear stress pattern in predicting neointima distribution in sirolimus-eluting stents in coronary arteries. JACC. 2003;92:1325-8. ].
Clinical implications of wall shear stress
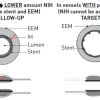
In a novel experiment in an animal model, Carlier et al [9191. Carlier SG, van Damme LCA, Blommerde CP, Wentzel JJ, van Langehove G, Verheye S, Kockx MM, Knaapen MWM, Cheng C, Gijsen F, Duncker DJ, Stergiopulos N, Slager CJ, Serruys PW, Krams R. Augmentation of Wall Shear Stress Inhibits Neointimal Hyperplasia After Stent Implantation: Inhibition Through Reduction of Inflammation? Circulation. 2003;107:2741-2746. ] demonstrated that, through the implantation of a “flow divider” into the centre of a stent implanted in the iliac arteries, they were able to modulate the local wall shear stress and the subsequent growth of NIH. The flow divider significantly increased the local wall shear stress and was consequently found to lead to a local reduction in inflammation and injury, with reduced NIH growth and subsequent late lumen loss. The closest human model of this example has been the use of the principle of simultaneous V-stenting (so-called “shotgun stenting”) with the formation of a metallic neo-carina in the left main stem or other suitably sized vessels [9292. Kim YH, Park DW, Suh IW, Jang JS, Hwang ES, Jeong YH, Lee SW, Lee CW, Hong MK, Kim JJ, Park SW, Park SJ. Long-term outcome of simultaneous kissing stenting technique with sirolimus-eluting stent for large bifurcation coronary lesions. Catheter Cardiovasc Interv .2007;70:840-6. , 9393. Morton AC, Siotia A, Arnold ND, Korgul P, Bowles J, Heppenstall J, Gunn J. Simultaneous kissing stent technique to treat left main stem bifurcation disease. Catheter Cardiovasc Interv. 2007;69:209-15. ].Kim et al [9292. Kim YH, Park DW, Suh IW, Jang JS, Hwang ES, Jeong YH, Lee SW, Lee CW, Hong MK, Kim JJ, Park SW, Park SJ. Long-term outcome of simultaneous kissing stenting technique with sirolimus-eluting stent for large bifurcation coronary lesions. Catheter Cardiovasc Interv .2007;70:840-6. ] demonstrated that in 36 consecutive patients (29 with left main stem interventions) using this technique with SES implantation, a 14% (5 patients) restenosis rate occurred over an average follow-up period of over 2 years. Interestingly, a “membranous diaphragm” at the carina was identified in 14 patients (47%) with restenosis occurring in just one of these patients. Conversely, Stinis et al [9494. Stinis CT, Hu SP, Price MJ, Teirstein PS. Three-year outcome of drug-eluting stent implantation for coronary artery bifurcation lesions. Catheter Cardiovasc Interv. 2010;75:309-14. ] showed that, in 74 consecutive patients with predominantly left anterior descending-diagonal lesions, the target lesion revascularisation rate was more than twice as high in the simultaneous V-stenting group (14 patients, 40%), compared with the crush group (5 patients, 12.8%) at a follow-up of >3 years. Whether lesion location played a role in the disparity of these results remains unclear. Robust, randomised controlled trials are therefore required to evaluate the feasibility of this technique.
The issue as to whether the actual presence of the stent in the vessel wall negatively alters the wall shear stress sufficiently to promote restenosis has proven to be controversial, with conflicting evidence existing in the literature. In a more recent, larger, well-designed trial, Papafaklis et al [9595. Papafaklis MI, Bourantas CV, Theodorakis PE, Katsouras CS, Naka KK, Fotiadis DI, Michalis LK. The Effect of Shear Stress on Neointimal Response Following Sirolimus- and Paclitaxel-Eluting Stent Implantation Compared With Bare-Metal Stents in Humans. J Am Coll Cardiol Intv. 2010;3:1181-1189.
The role of shear stress and how its clinical effects can be modulated with certain DES.] demonstrated the presence of significant numbers of pockets of low shear stress within stented segments, secondary to local geometric factors such as angulation or curvature, and showed that these pockets were significantly associated with NIH formation at 6-month follow-up with BMS and PES. Interestingly, this was not seen with SES, suggesting that sirolimus significantly attenuated the neointimal response to low shear stress. Paclitaxel was unable to do this, perhaps because of its differing pharmacological mode of action or even its shorter drug-release kinetics as discussed in Stent factors [33. Henricus J Duckers EGN, Patrick W Serruys. Essentials of Restenosis For the Interventional Cardiologist: Humana Press; 2007. ].
"Thromborestenosis" phenomenon
“Thromborestenosis” is a term first described by Oikawa et al [9696. Oikawa Y YJ, Costa M, Matsuno S, Akabane M, Funada R, Inaba T, Nakagawa Y, Nakamura M, Nagashima K, Kirigaya H, Ogasawara K, Sawada H, Aizawa T. Intravascular ultrasound, angioscopic and histopathological characterisation of heterogeneous patterns of restenosis after sirolimus-eluting stent implantation: insights into potential “thromborestenosis” phenomenon. EuroInterv. 2010;6:380-387. ] to describe a novel theory in which chronic thrombus formation may play an integral part in the development of ISR following DES implantation. The uniqueness of this study was the combined use of intravascular ultrasound (IVUS), coronary angioscopy and histopathological analyses (taken by direct coronary atherectomy) in all patients who had presented with ISR following SES implantation. The major findings of this study were that, in patients presenting with ISR, the incidence of thrombus and fibrin deposition were substantially more frequently observed within ISR lesions associated with SES implantation (12 of 13 cases), as compared to BMS (2 of 8 cases), and that the thrombus seen was not only located at uncovered stent strut sites (if present) but also, more importantly, on covered stent strut sites ( Figure 7 ). A theory to explain the presence of neointimal thrombus put forward by the authors was that the neointima covering a SES strut site was potentially more thrombogenic.
Joner et al [2626. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193-202.
One of the first reports of delayed arterial healing associated with first generation (Cypher and Taxus) DES.] have previously described evidence to support the concept of "thromborestenosis." In 2 of 14 autopsy cases of patients who died of LST, evidence of ISR with superimposed thrombus was seen [2626. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193-202.
One of the first reports of delayed arterial healing associated with first generation (Cypher and Taxus) DES.]. Further support comes from, Cook et al [5555. Cook S, Ladich E, Nakazawa G, Eshtehardi P, Neidhart M, Vogel R, Togni M, Wenaweser P, Billinger M, Seiler C, Gay S, Meier B, Pichler WJ, Juni P, Virmani R, Windecker S. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120:391-9. ] who demonstrated evidence of the widespread presence of chronic thrombi, as evidenced by the presence of a chronic inflammatory response, within all thrombus aspirates taken at the time of emergency percutaneous coronary intervention (PCI) in patients presenting with very LST. This was in addition to the acute thrombus seen in all samples and hypereosinophilia (likely to be secondary to polymeric hypersensitivity) observed in a proportion of aspirates [5555. Cook S, Ladich E, Nakazawa G, Eshtehardi P, Neidhart M, Vogel R, Togni M, Wenaweser P, Billinger M, Seiler C, Gay S, Meier B, Pichler WJ, Juni P, Virmani R, Windecker S. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120:391-9. ].
Conversely, Rittersma et al [9797. Rittersma SZH, van der Wal AC, Koch KT, Piek JJ, Henriques JPS, Mulder KJ, Ploegmakers JPHM, Meesterman M, de Winter RJ. Plaque Instability Frequently Occurs Days or Weeks Before Occlusive Coronary Thrombosis: A Pathological Thrombectomy Study in Primary Percutaneous Coronary Intervention. Circulation. 2005;111:1160-1165. ] also showed evidence of chronic thrombi which was days to weeks old in at least 50% of 211 consecutive STEMI patients who had thrombus aspirates taken within 6 hours of onset of symptoms. Only 4 patients (2%) within the study group had prior PCI to the infarct-related artery, with the theory for the presence of older thrombi being speculated to be related to “clinically silent non-occlusive atherothrombotic events” in the preceding days to weeks prior to the clinical presentation of occlusive thrombosis.
As to whether "clinically silent non-occlusive atherothrombotic events" is also an explanation for the presence of chronic thrombi seen with ISR, or if this is related to “thromborestenosis”, is presently unclear.
Vessel remodelling
Implantation of DES in vessels that have previously undergone positive remodelling (the Glagov phenomenon [9898. Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med .1988;112:1018-31. ]) secondary to a large plaque burden have been shown to be a significant predictor of restenosis ( Figure 8 ) [9898. Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med .1988;112:1018-31. , 9999. Okura H, Morino Y, Oshima A, Hayase M, Ward MR, Popma JJ, Kuntz RE, Bonneau HN, Yock PG, Fitzgerald PJ. Preintervention arterial remodeling affects clinical outcome following stenting: an intravascular ultrasound study. J Am Coll Cardiol. 2001;37:1031-5. , 100100. Nakamura M, Yock PG, Bonneau HN, Kitamura K, Aizawa T, Tamai H, Fitzgerald PJ, Honda Y. Impact of peri-stent remodeling on restenosis: a volumetric intravascular ultrasound study. Circulation. 2001;103:2130-2. ]. Theoretically, the level of NIH formation would be the same between a non-remodelled and a remodelled vessel following stent implantation; however, the phenomenon of where the NIH would potentially grow post stent implantation would be significantly different between the two vessels. In vessels with limited positive remodelling, the NIH can be partially accommodated between the stent and the external elastic membrane (EEM), thereby limiting neointimal growth within the vessel lumen. Conversely, in a fully remodelled vessel, this process cannot occur to the same extent, and the bulk of the NIH growth would therefore preferentially occur within the stented lumen with a subsequent greater likelihood of restenosis.
Small vessels
This is discussed in Stent factors with strut thickness.
- Wall shear stress refers to the principle that blood flow is fastest (high shear stress) in the vessel centre and slowest (low shear stress) when closest to the vessel wall due to frictional forces exerted by the vessel endothelium. Low shear stress areas lead to the local accumulation of growth factors, mitogenic cytokines and platelets which may promote either atherosclerosis or neointimal formation after vessel injury
- SES, but not PES, are able to effectively inhibit the neointimal response in low shear stress areas
- "Thromborestenosis" describes the theory in which chronic thrombus formation may play a role in the development of ISR
Late restenosis
Whereas parallel neointimal proliferation and healing with BMS have been shown to be complete after 3 to 6 months [101101. Farb A, Sangiorgi G, Carter AJ, Walley VM, Edwards WD, Schwartz RS, Virmani R. Pathology of acute and chronic coronary stenting in humans. Circulation. 1999;99:44-52. ], potentially followed by a late lumen enlargement beyond one year, a different pattern of healing has emerged with early-generation DES. This has been characterised by delayed healing with an ongoing neointimal growth beyond 30 days in experimental studies [102102. Carter AJ, Aggarwal M, Kopia GA, Tio F, Tsao PS, Kolata R, Yeung AC, Llanos G, Dooley J, Falotico R. Long-term effects of polymer-based, slow-release, sirolimus-eluting stents in a porcine coronary model. Cardiovasc Res. 2004;63:617-24. ] as previously described ( Figure 5 ), and beyond 6 months in clinical studies [103103. Raber L, Wohlwend L, Wigger M, Togni M, Wandel S, Wenaweser P, Cook S, Moschovitis A, Vogel R, Kalesan B, Seiler C, Eberli F, Luscher TF, Meier B, Juni P, Windecker S. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization LATE trial. Circulation. 2011;123:2819-28, 6 p following 2828.
The association of late restenosis with the implantation of certain DES].
Different mechanisms have been identified in the mechanisms of delayed neointimal growth and these are elaborated in the following paragraphs.
Decreasing drug dose
The antiproliferative drug concentration diminishes over time according to the individual elution profile of the different DES (see Stent factors): with decreasing drug dose, the antiproliferative inhibitive effect progressively declines. If the arterial healing is not terminated at the point in time when the drug elution has ceased, neointimal growth may continue to accrue ( Figure 9 ).
Chronic inflammation
Chronic inflammation is a trigger for late neointimal growth. Animal studies have suggested that the inflammatory response among different DES is clearly distinct in terms of the proportion of giant cells, granulomas, eosinophils, lymphocytes and fibrin deposition as previously described [2626. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193-202.
One of the first reports of delayed arterial healing associated with first generation (Cypher and Taxus) DES., 2727. Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, Skorija K, Weber DK, Gold HK, Virmani R. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005;112:270-8. , 2828. Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500-10. , 2929. Finn AV, Nakazawa G, Kolodgie FD, Virmani R. Temporal course of neointimal formation after drug-eluting stent placement: is our understanding of restenosis changing? JACC Cardiovasc Interv. 2009;2(4):300-2. , 3030. Farb A, Burke AP, Kolodgie FD, Virmani R. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation. 2003;108:1701-6. ]. Carter et al compared SES and BMS in a porcine coronary artery model and found late neointimal formation between 30 and 90 days, which resulted in a similar amount of neointimal area at 90 days between SES and BMS, thus mitigating the initial suppression achieved with SES at 30 days [102102. Carter AJ, Aggarwal M, Kopia GA, Tio F, Tsao PS, Kolata R, Yeung AC, Llanos G, Dooley J, Falotico R. Long-term effects of polymer-based, slow-release, sirolimus-eluting stents in a porcine coronary model. Cardiovasc Res. 2004;63:617-24. ]. Histological data documented a progressive increase in injury and inflammation scores between 30 and 180 days, probably representative of a chronic inflammatory response with a predominantly lymphocytic reaction with giant cells.
The presence of inflammatory reactions during the long-term time course following SES implantation was further corroborated by Virmani et al [104104. Wilson GJ, Nakazawa G, Schwartz RS, Huibregtse B, Poff B, Herbst TJ, Baim DS, Virmani R. Comparison of inflammatory response after implantation of sirolimus- and paclitaxel-eluting stents in porcine coronary arteries. Circulation. 2009;120:141-9, 1-2. ]. Histological evaluation of stented porcine coronary arteries demonstrated an intense circumferential granulomatous, eosinophil-rich inflammatory response during long-term follow-up (90 and 180 days) in SES, and to a lesser extent PES; conversely, inflammation was absent with BMS. PES as opposed to SES was further characterised by an increase in fibrin deposition. The presence of fibrin - which has been described in the vicinity of stent struts in experimental [104104. Wilson GJ, Nakazawa G, Schwartz RS, Huibregtse B, Poff B, Herbst TJ, Baim DS, Virmani R. Comparison of inflammatory response after implantation of sirolimus- and paclitaxel-eluting stents in porcine coronary arteries. Circulation. 2009;120:141-9, 1-2. ] and autopsy studies [105105. Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol. 2011;57:390-8. ] is an initiator of smooth muscle cell migration and proliferation [106106. Naito M, Stirk CM, Smith EB, Thompson WD. Smooth muscle cell outgrowth stimulated by fibrin degradation products. The potential role of fibrin fragment E in restenosis and atherogenesis. Thromb Res. 2000;98:165-74. ]. Porcine coronary models have demonstrated an increasing amount of fibrin in the long-term course (90 days), [104104. Wilson GJ, Nakazawa G, Schwartz RS, Huibregtse B, Poff B, Herbst TJ, Baim DS, Virmani R. Comparison of inflammatory response after implantation of sirolimus- and paclitaxel-eluting stents in porcine coronary arteries. Circulation. 2009;120:141-9, 1-2. ] which is analogous to delayed wound healing and excessive scarring. Delayed fibrinolysis is a stimulus to smooth muscle cell proliferation and excessive collagenous matrix deposition, leading to late restenosis.
The most likely culprits for the prolonged inflammatory reactions of the vessel wall are hypersensitivity reactions to the durable polymer. Durable polymers serve as a standard component of early-generation DES and are of importance as they facilitate drug delivery over a certain time (see Stent factors) [107107. Liistro F, Stankovic G, Di Mario C, Takagi T, Chieffo A, Moshiri S, Montorfano M, Carlino M, Briguori C, Pagnotta P, Albiero R, Corvaja N, Colombo A. First clinical experience with a paclitaxel derivate-eluting polymer stent system implantation for in-stent restenosis: immediate and long-term clinical and angiographic outcome. Circulation. 2002;105:1883-6. ]. Animal data have demonstrated that a peak in hypersensitivity reactions occurs only after the complete release of the drug (>60 days), supporting the notion that the durable polymer may be the more important cause [2828. Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500-10. ].
Taken together, early-generation SES and PES showed distinct long-term vessel responses, which have not been described in BMS. Whereas SES may cause a granulomatous and eosinophilic reaction, PES is mainly characterised by fibrin deposition. Both of these inflammatory reactions may trigger a continued neointimal proliferation and thereby potentially cause late restenosis ( Figure 9 ). Inflammatory reactions are most likely caused by the durable polymers which have made them the consequent targets for improving stent designs.
The strategy of more biocompatible polymers or biodegradable polymers have been shown to result in a lower inflammatory response and consequent improved longer term clinical outcomes. [259259. Serruys PW, Farooq V, Kalesan B, de Vries T, Buszman P, Linke A, Ischinger T, Klauss V, Eberli F, Wijns W, Morice MC, Di Mario C, Corti R, Antoni D, Sohn HY, Eerdmans P, Rademaker-Havinga T, van Es GA, Meier B, Jüni P, Windecker S. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC Cardiovasc Interv. 2013;6:777-89 , 260260. Serruys P.W., Windecker S., Silber S. Final five-year report of the RESOLUTE all-comers randomised study. EuroPCR 2014, Paris, France. , 261261. Gada H, Kirtane AJ, Newman W, Sanz M, Hermiller JB, Mahaffey KW, Cutlip DE, Sudhir K, Hou L, Koo K, Stone GW. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6:1263-6. ]. For example, 5 year follow up of the randomised LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating Trial), investigating biodegradable polymer biolimus-eluting stents (BES) (n = 857) against the durable polymer sirolimus-eluting stents (SES) (n = 850), demonstrated improved long term safety and efficacy outcomes. At 5 years, the BES was associated with a significant reduction in the patient-orientated composite endpoint of all-cause death, any MI, and all-cause revascularization (297 [35.1%] vs. 339 [40.4%], RR: 0.84 [95% CI: 0.71 to 0.98], p for superiority = 0.023) ( Figure 17 [A] ). An effect that predominantly secondary to reduced all cause revascularisation (206 [24.0%] vs. 241 [28.4%], RR: 0.81 [95% CI: 0.67 to 0.98], p for superiority = 0.029). Moreover, although the trial was underpowered to assess outcomes by anatomical complexity, the reduction in clinical events was shown to be potentially secondary to improved outcomes in patients with more anatomically complex disease ( Figure 17 [B] ).
Neoatherosclerosis
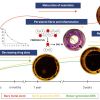
Neoatherosclerosis is defined as the presence of atherosclerotic disease within the neointima of a stented segment, ranging from pathological intimal thickening with presence of intercellular lipid accumulations to ruptured/unruptured thin-cap fibroatheroma. It has been speculated that the presence of neoatherosclerosis, namely ruptured thin-cap fibroatheromas, may be responsible for the acute clinical presentation of patients suffering from late restenosis.
Nakazawa et al described in a pathology registry the incidence of neoatherosclerosis within 142 BMS, 81 SES and 76 PES post-mortem specimens [108108. Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57:1314-22.
The pathogenesis underlying neoatherosclerosis in bare and drug eluting stents - an essential read.]. The incidence of neoatherosclerosis was higher in DES (31%) as compared to BMS (16%, p<0.001), whereas no meaningful differences were observed between the two early-generation DES. An important difference between BMS and early-generation DES was that the first occurrence of neoatherosclerosis occurred earlier with first-generation DES compared to BMS. Vulnerable plaques, namely thin-cap fibroatheroma (TCFA), were found in 1-4% of lesions without differences among stent types, but with a delayed occurrence in BMS as compared to early-generation DES.
The underlying reason for the difference in the occurrence of neoatherosclerosis is currently speculative. The mechanisms of neoatherosclerosis in DES are likely to be different from the ones observed in BMS. DES have been associated with impaired re-endothelialisation and with a disturbed functionality of the neoendothelium. This potentially leads to the initialisation of the atherosclerotic cascade commencing with monocyte adhesion and migration into the neointima and an increased permeability of the endothelium for circulatory lipid migrating into the subendothelial matrix. Conversely, re-endothelialisation is faster and the endothelium exhibits a preserved functionality in BMS-treated segments, suggesting different mechanisms involved in the genesis of neoatherosclerosis. Nakazawa et al [108108. Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57:1314-22.
The pathogenesis underlying neoatherosclerosis in bare and drug eluting stents - an essential read.] speculated whether shear stress may be a major contributing explanatory factor, as evidenced by some differences in the longitudinal distribution of neoatherosclerosis, in particular an increased occurrence in the proximal part of BMS-treated segments.
Illustrative examples of histological cross-sections depicting neoatherosclerosis are provided in Figure 10 and two examples of OCT cross-sections displaying neoatherosclerotic lesions are shown in Figure 11 .
Two in vivo studies have corroborated the aforementioned histopathological findings. Takano et al compared the appearances of neointimal tissue in BMS-treated lesions at 6 months and separate BMS-treated lesions more than 5 years previously [109109. Takano M, Yamamoto M, Inami S, Murakami D, Ohba T, Seino Y, Mizuno K. Appearance of lipid-laden intima and neovascularization after implantation of bare-metal stents extended late-phase observation by intracoronary optical coherence tomography. J Am Coll Cardiol. 2009;55:26-32. ]. Whereas neoatherosclerosis was absent in the early group, a transformation of the neointima during long-term follow-up was noted with lipid-rich intima (68%), calcifications (10%), intimal disruptions (38%), thrombi, and neovascularisation (52%). These results were confirmed in another OCT study by Habara et al who evaluated the neointimal composition in BMS-treated patients presenting with early (<1 year) versus late (>1 year) clinical restenosis [110110. Habara M, Terashima M, Nasu K, Kaneda H, Inoue K, Ito T, Kamikawa S, Kurita T, Tanaka N, Kimura M, Kinoshita Y, Tsuchikane E, Matsuo H, Ueno K, Katoh O, Suzuki T. Difference of tissue characteristics between early and very late restenosis lesions after bare-metal stent implantation: an optical coherence tomography study. Circ Cardiovasc Interv. 2010;4:232-8. ]. Whilst the neointima appeared to be relatively normal in the early restenosis group, a significant proportion of late restenosis lesions presented with atherosclerotic changes, thrombi or neointimal tears. An extension of these findings to DES was obtained by Kang et al [111111. Kang SJ, Mintz GS, Akasaka T, Park DW, Lee JY, Kim WJ, Lee SW, Kim YH, Whan Lee C, Park SW, Park SJ. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation. 2011;123:2954-63. ] in 50 patients presenting with clinical restenosis who underwent optical coherence tomography (OCT), intravascular ultrasound (IVUS) and IVUS-VH (IVUS-virtual histology) after a mean duration of 32 months post DES implantation. The main finding was that 90% of all patients exhibited a lipid-rich intima, indicating that atherosclerotic transformation was indeed highly predominant in delayed restenotic lesions. Whilst a majority of patients with stable angina presented with a structurally intact intima, more than 50% of patients presenting with unstable clinical symptoms showed evidence of a ruptured and thrombosed thin-cap fibroatheroma.
Taken together, the evidence derived from histology and in vivo imaging studies suggests that neoatherosclerosis occurs during the long-term time course after both BMS and DES implantation and can contribute to late restenosis.
Furthermore, neoatherosclerosis seems to be more prevalent in lesions causing symptomatic restenosis. The neoatherosclerotic transformation of the neointima potentially leads to the formation of (neo) thin-cap fibroatheromas, which can rupture and trigger an unstable clinical presentation. Therefore, in-stent neoatherosclerosis should also be considered as a differential diagnosis in patients presenting with stent thrombosis.
Angiographic and intravascular imaging data on late neointimal growth and restenosis in humans
Longitudinal angiographic and angioscopic follow-up series in patients implanted with BMS have observed late improvements in lumen diameter and an increased transparency (defined according to the visibility of the majority of the stent) respectively at three years of follow-up, suggesting late lumen remodelling on the basis of fibrotic maturation and regression of the neointima. Kimura et al reported a significant improvement in MLD from 1.94±0.48mm at 6 months to 2.09±0.48 (p≤0.001) at 3 years [112112. Kimura T, Yokoi H, Nakagawa Y, Tamura T, Kaburagi S, Sawada Y, Sato Y, Hamasaki N, Nosaka H, et al. Three-year follow-up after implantation of metallic coronary-artery stents. N Engl J Med. 1996;334:561-6. ]. A longitudinal angioscopic evaluation in 12 patients following BMS implantation exhibited a change in neointimal appearance from 6 months to 3 years characterised by an increase in transparency [113113. Asakura M, Ueda Y, Nanto S, Hirayama A, Adachi T, Kitakaze M, Hori M, Kodama K. Remodeling of in-stent neointima, which became thinner and transparent over 3 years: serial angiographic and angioscopic follow-up. Circulation. 1998;97:2003-6. ]. A prolongation of the angiographic follow-up of the above-mentioned study by Kimura et al demonstrated late re-narrowing beyond 4 years, [114114. Kimura T, Abe K, Shizuta S, Odashiro K, Yoshida Y, Sakai K, Kaitani K, Inoue K, Nakagawa Y, Yokoi H, Iwabuchi M, Hamasaki N, Nosaka H, Nobuyoshi M. Long-term clinical and angiographic follow-up after coronary stent placement in native coronary arteries. Circulation. 2002;105:2986-91. ] suggesting a triphasic pattern following BMS implantation: 1) pronounced neointimal proliferation within the first 6 months, followed by 2) a lumen enlargement with maturation of the neointima, and finally 3) leading to a re-narrowing, probably paralleled by an atherosclerotic transformation as previously described ( Figure 9 ).
In contrast to BMS, angiographic and IVUS studies of early-generation DES documented a continued increase in neointimal formation beyond the time point at which neointimal proliferation is halted in BMS. Table 2 provides an overview of IVUS studies that assessed serial changes in neointimal volumes among different stent types. Whereas in BMS no increase in neointimal volume was observed, early-generation DES have been associated with an on-going growth up to 4 years. Notably, all of these studies have only provided a snapshot within the first year after implantation, and at a single time point beyond one year (e.g., at 2, 3, or 4 years). In view of this methodological limitation, there is no definite answer in respect to the exact dynamics of late neointimal growth, and presently it remains unclear whether the continued growth is halted at 2, 3, or 4 years, or whether it continues beyond 4 years.
Angiographic data
Table 3 provides an overview of angiographic long-term studies investigating delayed late loss, namely the difference in late loss between 6-12 months and at long-term follow-up beyond one year.
In SIRTAX LATE, 293 patients underwent serial angiography at baseline, 8 months and 5 years (SES=142, PES=151) [103103. Raber L, Wohlwend L, Wigger M, Togni M, Wandel S, Wenaweser P, Cook S, Moschovitis A, Vogel R, Kalesan B, Seiler C, Eberli F, Luscher TF, Meier B, Juni P, Windecker S. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization LATE trial. Circulation. 2011;123:2819-28, 6 p following 2828.
The association of late restenosis with the implantation of certain DES]. Overall, an ongoing reduction of the minimal lumen diameter was noted between 8 months and 5 years, resulting in a late loss of 0.33±0.66 mm. Whilst SES was superior in terms of late loss at 8 months, differences between PES and SES were balanced at five years. This was explained by a late catch-up observed with SES, namely a numerically higher delayed late loss with SES (SES 0.37±0.73 mm, PES 0.29.59 mm, p=ns). In keeping with the findings from SIRTAX LATE, Byrne et al demonstrated in a large, unpaired angiographic patient cohort that the late loss at 6-8 months further accrued with first-generation DES (PES and SES) [115115. Byrne RA, Iijima R, Mehilli J, Pinieck S, Bruskina O, Schomig A, Kastrati A. Durability of antirestenotic efficacy in drug-eluting stents with and without permanent polymer. JACC Cardiovasc Interv. 2009;2:291-9. ]. A numerically higher increase of late loss was consistently shown with SES (0.17±0.50 mm) compared to PES (0.13±0.50 mm). As the absolute increase in late loss from 8 months to 2 years observed by Byrne et al was numerically lower than the increase from 8 months to 5 years in SIRTAX LATE, one may speculate that neointimal growth continued beyond 2 years. Interestingly, a third group in the study of Byrne et al, composed of polymer-free DES, exhibited only a minimal delayed late loss of 0.01±0.42 mm, suggesting that polymer-free stents may be less affected.
Newer-generation drug-eluting stents
Notably newer generation DES do not appear to be more protective against neoatherosclerosis compared with the first-generation DES, with cardiovascular risk factors and stent age noted to be the main determinant of the frequency of neoatherosclerosis. [262262. Lee SY, Hur SH, Lee SG, Kim SW, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Hong MK. Optical coherence tomographic observation of in-stent neoatherosclerosis in lesions with more than 50% neointimal area stenosis after second-generation drug-eluting stent implantation. Circ Cardiovasc Interv. 2015;8:e001878. , 263263. Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, Kutys R, Ladich E, Finn AV, Kolodgie FD, Virmani R. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation. 2014;129:211-23 , 264264. Taniwaki M, Windecker S, Räber L. Neoatherosclerosis as reason for stent failures beyond 5 years after drug-eluting stent implantation. Eur Heart J. 2014;35:1980 ] Histological data comparing the long-term inflammatory responses of newer-generation DES using durable polymer are relatively scarce. As they relate to devices using biodegradable polymer technology for drug delivery, it will be of interest to investigate how the bio-absorption process, which is a known trigger for at least a transient inflammation, may enhance neointimal proliferation during the long-term follow-up [116116. van der Giessen WJ, Lincoff AM, Schwartz RS, van Beusekom HM, Serruys PW, Holmes DR, Jr., Ellis SG, Topol EJ. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation. 1996;94(7):1690-7. ]. With polymer-free DES, few animal studies have reported a decrease in both inflammatory reactions and fibrin deposition up to 180 days, with a subsequent lower extent of angiographically defined delayed late loss [115115. Byrne RA, Iijima R, Mehilli J, Pinieck S, Bruskina O, Schomig A, Kastrati A. Durability of antirestenotic efficacy in drug-eluting stents with and without permanent polymer. JACC Cardiovasc Interv. 2009;2:291-9. ].
Clinical significance of late catch-up
The most relevant question emerging from the angiographic and intravascular imaging data is whether delayed neointimal proliferation translates into a clinically meaningful need for target lesion revascularisation (TLR) during long-term follow-up, reducing the early efficacy benefit of DES. Long-term results from randomised controlled trials of early and newer-generation DES consistently show a yearly TLR rate of less than 2% beyond one year, without meaningful differences compared to BMS ( Table 4 ). After subtraction of stent-thrombosis-related TLR - which are at least in part not related to restenosis - the annual TLR rate is as low as 1–1.5%. This relatively low frequency of late TLR is explainable by the magnitude of the delayed late loss (between 1 and 5 years = 0.30-0.40 mm), which is below the threshold that usually causes clinically significant restenosis. Against this backdrop it is reasonable to conclude that early-generation DES delay neointimal formation and healing during the long-term course, but without significantly compromising the early benefit in efficacy. Prolonged neointimal proliferation, however, may be a useful marker to assess the delay in healing. The presence of delayed healing may contribute to a mechanistic explanation of the ongoing risk of very late stent thrombosis as it has been identified as the principal pathological finding in an autopsy study distinguishing late thrombosed from patent early-generation DES [117117. Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435-41. ].
Newer-generation DES, such as an everolimus-eluting stent, have shown superior clinical safety and efficacy outcomes when compared to PES up to 2 years [118118. Smits PC, Kedhi E, Royaards KJ, Joesoef KS, Wassing J, Rademaker-Havinga TA, McFadden E. 2-year follow-up of a randomized controlled trial of everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice. COMPARE (Comparison of the everolimus eluting XIENCE-V stent with the paclitaxel eluting TAXUS LIBERTE stent in all-comers: a randomized open label trial). JACC. 2011;58:11-8. , 119119. Stone GW, Rizvi A, Sudhir K, Newman W, Applegate RJ, Cannon LA, Maddux JT, Cutlip DE, Simonton CA, Sood P, Kereiakes DJ. Randomized comparison of everolimus- and paclitaxel-eluting stents. 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV trial. JACC. 2011;58:19-25. ]. The annual incidence of late (>1 year) TLR in patients included in SPIRIT IV amounted to 2.1% in EES and 2.9% in PES. Patients included in the allcomers study COMPARE showed 0.9% late TLR in the EES and 1.5% in the PES-treated patient group, respectively. Although there was no significant statistical difference in late TLR in both studies, it is important to note the continued separation of the TLR curves beyond 1 year. This continued separation suggests a potential decrease in late TLR with the use of newer-generation DES as compared to the early-generation PES, potentially due to a less extensive inflammatory reaction.
Functional stent coverage by endothelium and vasomotor response
As a consequence of stent implantation, there is a substantial reduction in the integrity of the vessel endothelium within the treated vessel segment. Furthermore, histological studies have confirmed that recovery of the endothelial cellular layer is significantly delayed following DES compared to BMS implantation [120120. Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, Wilson PS, Skorija K, Cheng Q, Xu X, Gold HK, Kolodgie FD, Virmani R. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333-42. ]. It is commonly accepted that the delay in the recovery of the endothelial cellular layer following DES implantation is a consequence of the applied antiproliferative drug, which non-selectively inhibits mitosis of smooth muscle cells, fibroblasts and endothelial cells. The endothelial cell layer of the vessel wall has, however, a vital role in mediating vasomotion of the vessel wall by the excretion of nitric oxide (NO).
Notably, several studies have associated a lower vasoreactivity in the vessel segments adjacent to implanted early-generation DES compared to BMS [121121. Togni M, Windecker S, Cocchia R, Wenaweser P, Cook S, Billinger M, Meier B, Hess OM. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J Am Coll Cardiol. 2005;46:231-6. , 122122. Togni M, Raber L, Cocchia R, Wenaweser P, Cook S, Windecker S, Meier B, Hess OM. Local vascular dysfunction after coronary paclitaxel-eluting stent implantation. Int J Cardiol. 2007; 21;120:212-20. , 123123. Hofma SH, van der Giessen WJ, van Dalen BM, Lemos PA, McFadden EP, Sianos G, Ligthart JM, van Essen D, de Feyter PJ, Serruys PW. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:166-70. ]. Within these studies different methodologies have been used to induce vasomotion of the vessel segment edges, namely physical stress (e.g., bicycle stress test), rapid atrial pacing, and high dose acetylcholine infusion. Newer-generation DES, integrating features such as reduced strut thickness, lower drug dose and a more biocompatible polymer, have allowed for less traumatic delivery and improved biocompatibility of the stent. These features may be the reasons why further studies have shown an improvement in the vasoreactivity of the adjacent vessel segments in implanted newer-generation DES, compared to earlier-generation DES [124124. Hamilos MI, Ostojic M, Beleslin B, Sagic D, Mangovski L, Stojkovic S, Nedeljkovic M, Orlic D, Milosavljevic B, Topic D, Karanovic N, Wijns W. Differential effects of drug-eluting stents on local endothelium-dependent coronary vasomotion. J Am Coll Cardiol. 2008;51:2123-9. , 125125. Hamilos M, Sarma J, Ostojic M, Cuisset T, Sarno G, Melikian N, Ntalianis A, Muller O, Barbato E, Beleslin B, Sagic D, De Bruyne B, Bartunek J, Wijns W. Interference of drug-eluting stents with endothelium-dependent coronary vasomotion: evidence for device-specific responses. Circ Cardiovasc Interv. 2008;1:193-200. ].
To date, the magnitude of the correlation between endothelial restoration within the stented segment of DES and the vasomotor response in the adjacent vessel segment remains unclear.
The introduction of intravascular optical coherence tomography (OCT) technology has permitted the assessment of vessel stent strut coverage, as an indicator of endothelial integrity. Fuji et al [126126. Fujii K, Kawasaki D, Oka K, Akahori H, Fukunaga M, Sawada H, Masutani M, Lee-Kawabata M, Tsujino T, Ohyanagi M, Masuyama T. Endothelium-dependent coronary vasomotor response and neointimal coverage of zotarolimus-eluting stents 3 months after implantation. Heart. 2011;97:977-82. ] assessed strut coverage 3 months after zotarolimus-eluting stent implantation and demonstrated a correlation between the degree of stent coverage and the vasomotor response assessed by acetylcholine infusion. Furthermore, an inverse correlation of the rate of uncovered struts with the vasomotor capacity of the vessel wall was shown, thus supporting the hypothesis of a relationship between restoration of the stent vessel endothelialisation and vasomotor response following DES implantation.
- Three principal factors may be responsible for the formation of late restenosis: 1) decreasing drug dose; 2) chronic inflammatory reactions and persistent fibrin deposition; 3) neoatherosclerosis
- Early-generation SES and PES, but not BMS, have been associated with chronic inflammation and fibrin deposition during the long-term time course
- Neoatherosclerosis occurs earlier and more frequently in DES compared to BMS
- Incomplete re-endothelialisation and impaired functionality of the endothelium may be the source of neoatherosclerosis in DES, whereas shear stress has been suggested to be a relevant contributor in BMS
- Lipidic transformation of the neointima can lead to the formation of thin-cap fibroatheroma, which can be responsible for unstable presentations of restenosis
- Early-generation SES and PES have been associated with a delayed late loss of 0.3-0.4 mm between 1-5 years in angiographic long-term studies without significant differences between the two devices.
- The small magnitude of delayed late loss observed with early-generation DES does not translate into clinically significant late TLR rates (incidence <1.5% per annum) and does not compromise the early benefit in efficacy achieved with the introduction of DES
- Angiographic and clinical data suggest that some of the newer-generation DES (e.g., polymer-free DES) may be less vulnerable to late restenosis
- Newer generation DES do not appear to be more protective against neoatherosclerosis compared with the first-generation DES, with cardiovascular risk factors and stent age noted to be the main determinant of the frequency of neoatherosclerosis.
STENT FACTORS
Polymer release kinetics
Polymer release kinetics play a key and fundamental role in the prevention of restenosis with the suggestion that it is not necessarily the total dosage of the antiproliferative drug delivered to the vessel wall that is important but more the kinetics of the release of the drug. The PISCES trial [127127. Serruys PW, Sianos G, Abizaid A, Aoki J, den Heijer P, Bonnier H, Smits P, McClean D, Verheye S, Belardi J, Condado J, Pieper M, Gambone L, Bressers M, Symons J, Sousa E, Litvack F. The effect of variable dose and release kinetics on neointimal hyperplasia using a novel paclitaxel-eluting stent platform: the Paclitaxel In-Stent Controlled Elution Study (PISCES). J Am Coll Cardiol. 2005;46:253-60.
The first human study to demonstrate the principle of polymer release kinetics with six different polymer-drug release formulations. The main finding of the trial was that the duration of the anti-proliferative drug release had a far greater impact on the neointimal inhibition than the dose of the drug delivered.] was the first human study to demonstrate this principle involving the use of the Conor stent and six different polymer-drug release formulations. The main finding of the trial was that the duration of the drug release had a far greater impact on the inhibition of NIH than the dose of the drug delivered ( Figure 12 ).
The polymer-free biolimus A9-eluting stent, with 2 differing doses of biolimus, has been investigated in animal models [128128. Tada N, Virmani R, Grant G, Bartlett L, Black A, Clavijo C, Christians U, Betts R, Savage D, Su S-H, Shulze J, Kar S. Polymer-Free Biolimus A9-Coated Stent Demonstrates More Sustained Intimal Inhibition, Improved Healing, and Reduced Inflammation Compared With a Polymer-Coated Sirolimus-Eluting Cypher Stent in a Porcine Model. Circ Cardiovasc Interv. 2010;3:174-183. ] and the first-in-human BIOFREEDOM study [129129. Grube E. 12-Month angiographic follow-up of the novel non-polymeric Biolimus A9-coated stent for the treatment of de novo coronary lesions: results from the prospective, multicenter, randomized BIOFREEDOM Clinical Trial. Paper presented at: Transcatheter Cardiovascular Therapeutics; September 21 to 25, 2010; Washington DC. ]. Initial studies in animals indicated that the lower (112 μg/per 14 mm of stent length) and higher dose (225 μg/per 14 mm of stent length) biolimus A9-eluting stents have equivalent effects on the NIH response and both have a superior late reduction in NIH as compared to SES at 180 days [128128. Tada N, Virmani R, Grant G, Bartlett L, Black A, Clavijo C, Christians U, Betts R, Savage D, Su S-H, Shulze J, Kar S. Polymer-Free Biolimus A9-Coated Stent Demonstrates More Sustained Intimal Inhibition, Improved Healing, and Reduced Inflammation Compared With a Polymer-Coated Sirolimus-Eluting Cypher Stent in a Porcine Model. Circ Cardiovasc Interv. 2010;3:174-183. ]. In the subsequent BIOFREEDOM study [129129. Grube E. 12-Month angiographic follow-up of the novel non-polymeric Biolimus A9-coated stent for the treatment of de novo coronary lesions: results from the prospective, multicenter, randomized BIOFREEDOM Clinical Trial. Paper presented at: Transcatheter Cardiovascular Therapeutics; September 21 to 25, 2010; Washington DC. ] in humans, powered to test for non-inferiority, statistical equivalency was achieved in all 3 groups with an in-stent late lumen loss reported as 0.17 mm (“standard-dose biolimus” group - 15.6 μg/per mm of stent length), 0.22 mm (“low-dose biolimus” group - 7.8 μg/per mm of stent length) and 0.35 mm for the Taxus stent with no differences in MACE or reported cases of stent thrombosis in all 3 groups.
It would therefore appear that a certain threshold of drug needs to be delivered to the vessel wall over a sustained, prolonged period of time, during the process of endothelialisation of the DES, in order to “dampen” down the inflammatory response and limit NIH formation. This is supported by molecular biology studies which have suggested that genes responsible for the proliferative response potentially remain active for a period of up to 21 days after vessel injury [130130. Tanner FC, Yang ZY, Duckers E, Gordon D, Nabel GJ, Nabel EG. Expression of cyclin-dependent kinase inhibitors in vascular disease. Circ Res. 1998;82:396-403. ]. Achieving the fine balance between the drug type, dosage and delivery over the appropriate time are therefore crucial factors in DES design.
In addition, an early peak in drug release may theoretically be of importance to inhibit the early inflammatory reaction of the vessel wall caused by the traumatic vessel wall injury following stent implantation. The early suppression of injury-induced inflammation may result in an antithrombotic effect, which may explain the decreased risk of acute stent thrombosis observed in a recent study comparing the newer generation everolimus-eluting stent with a bare metal stent for primary PCI [131131. Sabate M.. A randomised comparison between everolimus-eluting stents and cobalt-chromium bare-metal stents in ST-elevation myocardial infarction. Presented at European Society of Cardiology Congress, August 30 , 2011; Paris, France 2011. ].
Type of DES? Type of drug?
Differences relating to first-generation DES are discussed in the Late restenosis section of Arterial factors. Data from the SCAAR registry, involving >35,000 patients implanted with four different types of DES (ZES, SES, Taxus® Express® [Boston Scientific] and Liberté® [Boston Scientific]) in real-world practice at 2-year follow-up, showed that the rates of restenosis with DES implantation were significantly higher in diabetics and that important differences existed in the efficacy of differing brands of DES to reduce restenosis [2121. Frobert O, Lagerqvist B, Carlsson J, Lindback J, Stenestrand U, James SK. Differences in restenosis rate with different drug-eluting stents in patients with and without diabetes mellitus: a report from the SCAAR (Swedish Angiography and Angioplasty Registry). J Am Coll Cardiol. 2009;53:1660-7.
The different restenosis rates with different types of DES.]. In particular, the restenosis rates with Endeavor® (Medtronic, Inc., Minneapolis, MN, USA) were twice as high in diabetics as compared to other DES types. Higher restenosis rates were also evident in diabetics with Endeavor (RR: 1.77, 95% CI: 1.29 to 2.43) and SES (RR: 1.25, 95% CI: 1.04 to 1.51) when compared to non-diabetics. Five-year unpublished follow-up data from the SCAAR registry continued to demonstrate differences in the efficacy of the first and second-generation DES in reducing rates of stenosis, with a trend for better outcomes seen after nearly 2 years’ use of the everolimus-eluting stent.
The EES releases 80% of the drug within 30 days and nearly all the drug within 4 months. In the Spirit I, II, and III trials, a LLL of 0.10, 0.16, and 0.33 mm and TVR rates of 3.8%, 3.4%, and 4.6% were observed at 6, 12, and 24 months, respectively [4343. Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol 2010;56:S1-42. ]. Conversely, the Endeavor reported a LLL of 0.60 mm and 0.67 mm and TVR of 6.3% and 4.5%, respectively, in the Endeavor III and IV trials at 12 months. The Endeavor, however, elutes 95% of its drug very rapidly (within 14 days): this is highly likely to be the main reason for the poorer results seen.
The next-generation Endeavor® Resolute stent (Medtronic, Inc.), consisting of the same cobalt chromium metallic platform (Driver BMS; Medtronic, Inc.) and the same drug (zotarolimus) as the Endeavor stent, but incorporating the BioLinx™ polymer - an enhanced triple polymer combining a hydrophobic coating covering the stent, a hydrophilic, more biocompatible polymer on the abluminal surface with a third polymer binding the previous two polymers – allowed for substantially longer polymer drug release kinetics (180 days), compared to 14 days with the Endeavor stent. The Endeavor Resolute stent reported an in-stent LLL of 0.12, 0.22, and 0.27 mm at 4, 9, and 13 months, respectively, with angiographic equivalency (LLL 0.19 mm) in terms of meeting the criteria for non-inferiority being met when compared with EES. Equivalency in the 12-month primary endpoint of target lesion failure (a composite of cardiac death, target vessel MI, and clinically driven target lesion revascularisation [8.2% versus 8.3%]) and a slight increase in the rate of definite stent thrombosis (1.2% versus 0.3%, p=0.01) were also seen [132132. Serruys PW, Silber S, Garg S, van Geuns RJ, Richardt G, Buszman PE, KelbÃ?k H, van Boven AJ, Hofma SH, Linke A, Klauss V, Wijns W, Macaya C, Garot P, DiMario C, Manoharan G, Kornowski R, Ischinger T, Bartorelli A, Ronden J, Bressers M, Gobbens P, Negoita M, van Leeuwen F, Windecker S. Comparison of Zotarolimus-Eluting and Everolimus-Eluting Coronary Stents. New England Journal of Medicine. 2010;363:136-146. ].
Type of drug
Based on the vast experimental and clinical evidence associating inflammation with restenosis as previously discussed, multiple immunosuppressive and antiproliferative drugs – such as dexamethasone, actinomycin D, cytochalasin D, 17-beta-estradiol, mycophenolic acid, and angiopeptin – have been investigated for their effect in inhibiting the pathway of NIH [133133. Papafaklis MI, Chatzizisis YS, Naka KK, Giannoglou GD, Michalis LK. Drug-eluting stent restenosis: Effect of drug type, release kinetics, hemodynamics and coating strategy. Pharmacol Ther. 2011 Dec 22. 2012;134:43-53. , 134134. Giordano A, Romano A. Inhibition of human in-stent restenosis: a molecular view. Current opinion in pharmacology. 2011;11:372-7. ]. As an example, methylprednisolone, although shown to be promising in a porcine model [135135. de Scheerder I, Wang K, Wilczek K, van Dorpe J, Verbeken E, Desmet W, Schacht E, Piessens J. Local methylprednisolone inhibition of foreign body response to coated intracoronary stents. Coronary artery disease. 1996;7:161-6. ], demonstrated a restenosis (>50% diameter stenosis at follow-up) rate of 13.3% and a LLL of 0.45 mm at follow-up in the STRIDE (Study of antirestenosis with BiodivYsio dexamethasone-eluting stent) European Study [136136. Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111:2257-73. ]. The very short release profile of the drug – almost completely eluted in the first 24 hours after deployment – no doubt had a strong influence on the clinical effect of the drug.
The drugs that have been demonstrated to have superior performance in a consistent and reproducible fashion both in preclinical investigations and in clinical trials are sirolimus (rapamycin) and paclitaxel in first-generation stents, and the limus family of drugs (which includes sirolimus) in second-generation DES [133133. Papafaklis MI, Chatzizisis YS, Naka KK, Giannoglou GD, Michalis LK. Drug-eluting stent restenosis: Effect of drug type, release kinetics, hemodynamics and coating strategy. Pharmacol Ther. 2011 Dec 22. 2012;134:43-53. , 134134. Giordano A, Romano A. Inhibition of human in-stent restenosis: a molecular view. Current opinion in pharmacology. 2011;11:372-7. ]. Although polymer release kinetics are crucial to the antiproliferative effects of these drugs as previously described, because of the differing and potentially more potent mechanisms of action of the limus family of drugs compared to paclitaxel, the limus family of drugs are thought to deliver a more sustained antiproliferative NIH effect [133133. Papafaklis MI, Chatzizisis YS, Naka KK, Giannoglou GD, Michalis LK. Drug-eluting stent restenosis: Effect of drug type, release kinetics, hemodynamics and coating strategy. Pharmacol Ther. 2011 Dec 22. 2012;134:43-53. , 134134. Giordano A, Romano A. Inhibition of human in-stent restenosis: a molecular view. Current opinion in pharmacology. 2011;11:372-7. ]. Furthermore, in the randomised PAINT trial, Lemos et al. [137137. Lemos PA, Moulin B, Perin MA, Oliveira LA, Arruda JA, Lima VC, Lima AA, Caramori PR, Medeiros CR, Barbosa MR, Brito FS, Jr., Ribeiro EE, Martinez EE. Randomized evaluation of two drug-eluting stents with identical metallic platform and biodegradable polymer but different agents (paclitaxel or sirolimus) compared against bare stents: 1-year results of the PAINT trial. Catheterization and cardiovascular interventions. 2009;74:665-73.
Head to head comparison of paclitaxel vs. sirolimus anti-proliferative drugs in 2 otherwise identical metallic stent and polymer platforms, demonstrating the superiority of sirolimus over paclitaxel in neointima suppression.] conducted a head-to-head comparison of 2 DES with the same metallic stent platform and biodegradable-polymer carrier but releasing either sirolimus or paclitaxel, and a BMS of the same metallic platform. Nine-month in-stent late loss was significantly more favourable towards sirolimus (paclitaxel: 0.54-0.44 mm, sirolimus: 0.32-0.43 mm, vs. BMS: 0.90-0.45 mm, respectively, p< 0.01) in the 274 patients studied.
New-generation DES, such as the everolimus-eluting stent (XIENCE; Abbott Vascular) has shown superiority compared to the Taxus PES and shown itself to be a powerful independent predictor of 2-year freedom from ischaemia-driven target lesion revascularisation (hazard ratio: 0.59 [95% CI: 0.47–0.74], p=0.0001), ischaemia-driven target vessel revascularisation (0.70 [0.58,0.84]; p=0.0002), myocardial infarction (hazard ratio: 0.54 [95% CI: 0.41–0.71]; p=0.0001) and MACE (hazard ratio: 0.64 [0.54, 0.77]; p<0.0001) [138138. Kereiakes DJ, Smits PC, Kedhi E, Parise H, Fahy M, Serruys PW, Stone GW. Predictors of death or myocardial infarction, ischaemic-driven revascularisation, and major adverse cardiovascular events following everolimus-eluting or paclitaxel-eluting stent deployment: pooled analysis from the SPIRIT II, III, IV and COMPARE trials. EuroIntervention. 2011;7:74-83. ]. Furthermore, the biolimus-eluting stent with a biodegradable polymer has demonstrated non-inferiority to the SES (Cypher) at up to 4 years follow-up [139139. Windecker S, Serruys PW, Wandel S, Buszman P, Trznadel S, Linke A, Lenk K, Ischinger T, Klauss V, Eberli F, Corti R, Wijns W, Morice MC, di Mario C, Davies S, van Geuns RJ, Eerdmans P, van Es GA, Meier B, Juni P. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008;372:1163-73. , 140140. Stefanini GG, Kalesan B, Serruys PW, Heg D, Buszman P, Linke A, Ischinger T, Klauss V, Eberli F, Wijns W, Morice MC, Di Mario C, Corti R, Antoni D, Sohn HY, Eerdmans P, van Es GA, Meier B, Windecker S, Juni P. Long-term clinical outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer sirolimus-eluting stents in patients with coronary artery disease (LEADERS): 4 year follow-up of a randomised non-inferiority trial. Lancet. 2011;378:1940-8. ]. It is likely that a combination of favourable polymer release kinetics and the limus-based drug are reasons for the more advantageous clinical effects seen. Conversely, the Endeavor DES, eluting the limus-based zotarolimus drug, led to unfavourable clinical outcomes due to the very short polymer release kinetics (14 days) as previously discussed.
Stent gap, non-uniform strut distribution and drug deposition
Takebayashi et al [141141. Takebayashi H, Mintz GS, Carlier SG, Kobayashi Y, Fujii K, Yasuda T, Costa RA, Moussa I, Dangas GD, Mehran R, Lansky AJ, Kreps E, Collins MB, Colombo A, Stone GW, Leon MB, Moses JW. Nonuniform strut distribution correlates with more neointimal hyperplasia after sirolimus-eluting stent implantation. Circulation. 2004;110:3430-4. ] classically described the number and distribution of DES struts, as identified by IVUS, as being independent significant risk factors (fewer struts and non-uniform stent strut distribution) for NIH formation and the subsequent risk of restenosis. Non-uniform DES strut distribution has been suggested as being attributable to features such as stent design (e.g., open versus closed cell), stent gap, vessel curvature, coronary bifurcations, ostial lesions, stent underexpansion or overexpansion, polymer peeling, and stent fracture.
Small vessels and strut thickness
Small coronary artery disease is a recognised challenging subset within the field of coronary artery intervention with significant and unacceptable risks of restenosis seen with both POBA and BMS [142142. Elezi S, Kastrati A, Neumann FJ, Hadamitzky M, Dirschinger J, Schomig A. Vessel size and long-term outcome after coronary stent placement. Circulation. 1998;98:1875-80. , 143143. Kasaoka S, Tobis JM, Akiyama T, Reimers B, Di Mario C, Wong ND, Colombo A. Angiographic and intravascular ultrasound predictors of in-stent restenosis. J Am Coll Cardiol. 1998;32:1630-5. , 144144. Akiyama T, Moussa I, Reimers B, Ferraro M, Kobayashi Y, Blengino S, Di Francesco L, Finci L, Di Mario C, Colombo A. Angiographic and clinical outcome following coronary stenting of small vessels: a comparison with coronary stenting of large vessels. J Am Coll Cardiol. 1998;32:1610-8. , 145145. Agostoni P, Biondi-Zoccai GG, Gasparini GL, Anselmi M, Morando G, Turri M, Abbate A, McFadden EP, Vassanelli C, Zardini P, Colombo A, Serruys PW. Is bare-metal stenting superior to balloon angioplasty for small vessel coronary artery disease? Evidence from a meta-analysis of randomized trials. Eur Heart J. 2005;26:881-9. ]. A meta-analysis [146146. Biondi-Zoccai G, Moretti C, Abbate A, Sheiban I. Percutaneous coronary intervention for small vessel coronary artery disease. Cardiovasc Revasc Med. 2010;11:189-98.
Meta-analyses on small vessel disease demonstrating the disparity of clinical outcomes with differing DES, due to the complex interaction with metallic stent platform, polymer type, polymer release kinetics and type of anti-proliferative drug.] of the use of DES in small vessel disease demonstrated that both late loss and binary restenosis were largely dependent on the type of DES implanted ( Figure 13 ).
Mechanisms suggested to explain the poorer outcomes associated with small vessels include: (1) a high degree of vessel stretch and injury, (2) a smaller post-procedural lumen area, and (3) a higher metal density [147147. Briguori C, Sarais C, Pagnotta P, Liistro F, Montorfano M, Chieffo A, Sgura F, Corvaja N, Albiero R, Stankovic G, Toutoutzas C, Bonizzoni E, Di Mario C, Colombo A. In-stent restenosis in small coronary arteries: impact of strut thickness. J Am Coll Cardiol. 2002;40:403-9. ]. The overstretch theory is, however, controversial, with evidence suggesting a possible adverse effect with increased NIH [119119. Stone GW, Rizvi A, Sudhir K, Newman W, Applegate RJ, Cannon LA, Maddux JT, Cutlip DE, Simonton CA, Sood P, Kereiakes DJ. Randomized comparison of everolimus- and paclitaxel-eluting stents. 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV trial. JACC. 2011;58:19-25. , 120120. Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, Wilson PS, Skorija K, Cheng Q, Xu X, Gold HK, Kolodgie FD, Virmani R. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333-42. ], no significant effect [148148. Eshtehardi P, Cook S, Wandel S, Raber L, Wenaweser P, Togni M, Vogel R, Garachemani A, Eberli FR, Luscher TF, Juni P, Hess OM, Meier B, Windecker S. Impact of arterial injury on neointimal hyperplasia after implantation of drug-eluting stents in coronary arteries: an intravascular ultrasound study. EuroIntervention. 2010;6:467-74. ], or even potential benefit [142142. Elezi S, Kastrati A, Neumann FJ, Hadamitzky M, Dirschinger J, Schomig A. Vessel size and long-term outcome after coronary stent placement. Circulation. 1998;98:1875-80. , 148148. Eshtehardi P, Cook S, Wandel S, Raber L, Wenaweser P, Togni M, Vogel R, Garachemani A, Eberli FR, Luscher TF, Juni P, Hess OM, Meier B, Windecker S. Impact of arterial injury on neointimal hyperplasia after implantation of drug-eluting stents in coronary arteries: an intravascular ultrasound study. EuroIntervention. 2010;6:467-74. ]. The latter beneficial effects have been proposed to be related to a higher balloon-to-artery ratio, the so-called bigger is better paradigm (see Implantation factors), leading to appropriate apposition of the stent to the vessel wall.
Thicker stent struts have been linked to an increased risk of restenosis with BMS [149149. Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schuhlen H, Neumann FJ, Fleckenstein M, Pfafferott C, Seyfarth M, Schomig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103:2816-21. ] and small vessels [147147. Briguori C, Sarais C, Pagnotta P, Liistro F, Montorfano M, Chieffo A, Sgura F, Corvaja N, Albiero R, Stankovic G, Toutoutzas C, Bonizzoni E, Di Mario C, Colombo A. In-stent restenosis in small coronary arteries: impact of strut thickness. J Am Coll Cardiol. 2002;40:403-9. , 150150. Raisuke I, Yuji I, Akiyoshi M, Hiroyoshi N, Kazuhiro H. Predictors of restenosis after implantation of 2.5 mm stents in small coronary arteries. Circ J. 2004;68:236-40. , 151151. Pache J, Kastrati A, Mehilli J, Schuhlen H, Dotzer F, Hausleiter J, Fleckenstein M, Neumann FJ, Sattelberger U, Schmitt C, Muller M, Dirschinger J, Schomig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41:1283-8. ]. The underlying rationale is that a thinner stent strut would have less of a “footprint” on the vessel wall with a consequential reduced inflammatory response. With DES, however, a complex relationship exists between the strut material and characteristics, stent design, polymer type, and drug release kinetics. Both Cypher and XIENCE appear to have the lowest risk of binary restenosis in small vessels, despite a large disparity in stent strut thicknesses (approximately 150 μm versus 90 μm); moreover, Endeavor had the worst outcomes despite its strut thickness being approximately 10 microns more than the XIENCE V® [146146. Biondi-Zoccai G, Moretti C, Abbate A, Sheiban I. Percutaneous coronary intervention for small vessel coronary artery disease. Cardiovasc Revasc Med. 2010;11:189-98.
Meta-analyses on small vessel disease demonstrating the disparity of clinical outcomes with differing DES, due to the complex interaction with metallic stent platform, polymer type, polymer release kinetics and type of anti-proliferative drug.]. A fairer comparison perhaps would be between the Taxus Liberté and Express as both contain identical metallic platform materials, polymer coatings and drug concentrations (1 μg/mm2 of paclitaxel), except that the Taxus Liberté contains thinner struts, more flexible cell geometry, and uniform cell distribution. In the SCAAR registry, the Taxus Express was shown to have a mild but significantly higher adjusted risk of restenosis compared to Taxus Liberté (RR: 1.32, 95% CI: 1.10-1.60).21
"On" and "off" label use of DES
The Strategic Transcatheter Evaluation of New Therapies (STENT) Group is the largest, multicentre, prospective registry involving >15,000 patients to have evaluated the late outcomes associated with DES implantation in the United States [152152. Brodie BR, Stuckey T, Downey W, Humphrey A, Bradshaw B, Metzger C, Hermiller J, Krainin F, Juk S, Cheek B, Duffy P, Smith H, Edmunds J, Varanasi J, Simonton CA, STENT Group. Outcomes and Complications With Off-Label Use of Drug-Eluting Stents: Results From the STENT (Strategic Transcatheter Evaluation of New Therapies) Group. J Am Coll Cardiol Intv. 2008;1:405-414. ]. This compared on-label (short de novo lesions in coronary arteries measuring >2.5 mm and <3.5 mm for SES or <3.75 mm PES) and off-label (ostial, left main stem, chronic total occlusion, saphenous vein graft, small or large vessels/multivessel, STEMI, ISR lesions) indications for DES implantation. A near doubling in the TVR rate was seen in the off-label group at 9 months (5.7% versus 3.2%, p<0.0001) and 2 years (11.8% versus 6.5%, p<0.0001) [153153. Brodie BR, Stuckey T, Downey W, Humphrey A, Bradshaw B, Metzger C, Hermiller J, Krainin F, Juk S, Cheek B, Duffy P, Smith H, Edmunds J, Varanasi J, Simonton CA. Outcomes and complications with off-label use of drug-eluting stents: results from the STENT (Strategic Transcatheter Evaluation of New Therapies) group. JACC Cardiovasc Interv. 2008;1:405-14. ].
Data from the Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) study, reflecting a population of patients with highly complex off-label use of DES in 3-vessel or left main stem disease, have reported even higher rates of TVR at 1, 2, 3 and 4 years at 11.6%, 17.4%, 19.7% and 23%, respectively [154154. Kappetein AP, Feldman TE, Mack MJ, Morice MC, Holmes DR, Stahle E, Dawkins KD, Mohr FW, Serruys PW, Colombo A. Comparison of coronary bypass surgery with drug-eluting stenting for the treatment of left main and/or three-vessel disease: 3-year follow-up of the SYNTAX trial. European Heart Journal. 2011;32:2125-34. ]. Furthermore, recent evidence has suggested that one of the main determinants of future clinical events, including revascularisation, is the clinical risk profile of the patient. High EuroSCORE patients from the SYNTAX trial - in particular in the 3VD population - have been associated with more adverse clinical outcomes [155155. Serruys PW, Farooq V, Vrancx P, Brugaletta S, Holmes DR, Kappetein AP, Mack M, Feldman T, Morice MC, Ståhle E, Colombo A, Pereda P, Huang J, Morel MA, Van Es GA, Dawkins KD, Mohr FW, Steyerberg EW. The SYNTAX Trial at 3 Years: a Global Risk approach to identify patients with 3-vessel and/or left main stem disease who could safely and efficaciously be treated with percutaneous coronary intervention. Part 1: the randomised population. Presented at: TCT, San Francisco, USA, 8 Nov 2011. , 156156. Farooq V, Serruys PW, Vrancx P, Brugaletta S, Holmes DR, Kappetein AP, Mack M, Feldman T, Morice MC, Ståhle E, Colombo A, Pereda P, Huang J, Morel MA, Van Es GA, Dawkins KD, Mohr FW, Steyerberg EW. The SYNTAX trial at 3 years: a Global Risk approach to identify patients with 3-vessel and/or left main stem disease who could safely and efficaciously be treated with percutaneous coronary intervention. Part 2: The ‘All-Comers’ population. Presented at: TCT, San Francisco, USA, 8 Nov 2011. ]. Registries have also suggested these observations in patients with left main disease [157157. Capodanno D, Caggegi A, Miano M, Cincotta G, Dipasqua F, Giacchi G, Capranzano P, Ussia G, Di Salvo ME, La Manna A, Tamburino C. Global Risk Classification and Clinical SYNTAX (Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery) Score in Patients Undergoing Percutaneous or Surgical Left Main Revascularization. J Am Coll Cardiol Intv. 2011;4:287-297. , 158158. Capodanno D, Miano M, Cincotta G, Caggegi A, Ruperto C, Bucalo R, Sanfilippo A, Capranzano P, Tamburino C. EuroSCORE refines the predictive ability of SYNTAX score in patients undergoing left main percutaneous coronary intervention. American heart journal. 2010;159:103-9. ].
With newer generation DES, a post hoc analysis of the randomised TWENTE trial (n=1387) comparing the everolimus-eluting XIENCE V to the zotarolimus-eluting Resolute stents, demonstrated off-label DES to not significantly differ from patients with on-label DES use in 2-year clinical endpoints (cardiac death, MI, and target vessel revascularisation [TVR]), except for an increase in periprocedural MI (≤48 hrs) (5.0% vs. 1.4%; p=0.003). These largely positive findings perhaps underpin the increased safety and efficacy profile of newer generation DES. [245245. Sen H, Lam MK, Tandjung K, Basalus MW, de Man FH, Louwerenburg JH, Stoel MG, van Houwelingen GK, Löwik MM, Linssen GC, Saïd SA, Nienhuis MB, Verhorst PM, van der Palen J, von Birgelen C. Clinical outcome following second-generation drug-eluting stent use for off-label versus on-label indications: insights from the two-year outcome of the TWENTE trial. EuroIntervention. 2014 Oct;10:664-71. ]
Polymer disruption, peeling and cracking
Polymer disruption, peeling and cracking have been demonstrated to occur in bench studies involving both first [159159. Otsuka Y, Chronos NA, Apkarian RP, Robinson KA. Scanning electron microscopic analysis of defects in polymer coatings of three commercially available stents: comparison of BiodivYsio, Taxus and Cypher stents. J Invasive Cardiol. 2007;19:71-6. ] ( Figure 14 ) and newer ( Figure 15 ) generation DES, [160160. Basalus MW, Ankone MJ, van Houwelingen GK, de Man FH, von Birgelen C. Coating irregularities of durable polymer-based drug-eluting stents as assessed by scanning electron microscopy. EuroIntervention. 2009;5:157-65. , 161161. Basalus MW, Tandjung K, van Westen T, Sen H, van der Jagt PK, Grijpma DW, van Apeldoorn AA, von Birgelen C. Scanning electron microscopic assessment of coating irregularities and their precursors in Unexpanded durable polymer-based drug-eluting stents. Catheter Cardiovasc Interv. 2011 Jul 29. 2012 1;79:644-53. ] using light or scanning electron microscopy.
Although there is no direct evidence to suggest that the integrity of the polymer coating is a direct cause of restenosis, there are sufficient theoretical concerns to warrant concern through non-uniform local drug distribution or the disrupted polymer potentially acting as a nidus for an ongoing inflammatory response with the subsequent risk of restenosis [2727. Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, Skorija K, Weber DK, Gold HK, Virmani R. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005;112:270-8. , 2828. Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500-10. , 2929. Finn AV, Nakazawa G, Kolodgie FD, Virmani R. Temporal course of neointimal formation after drug-eluting stent placement: is our understanding of restenosis changing? JACC Cardiovasc Interv. 2009;2(4):300-2. , 5454. Nakazawa G, Finn AV, Ladich E, Ribichini F, Coleman L, Kolodgie FD, Virmani R. Drug-eluting stent safety: findings from preclinical studies. Expert Rev Cardiovasc Ther. 2008;6:1379-91. ].
Other concerns with regard to the potential for polymer disruption involve the percutaneous coronary intervention procedure itself. Wiemer et al. [162162. Wiemer M, Butz T, Mahmood K, Horstkotte D. Major polymer damage of drug-eluting stents. Circ Cardiovasc Interv. 2008;1:154. , 163163. Wiemer M, Butz T, Schmidt W, Schmitz KP, Horstkotte D, Langer C. Scanning electron microscopic analysis of different drug eluting stents after failed implantation: from nearly undamaged to major damaged polymers. Catheter Cardiovasc Interv. 2010;75:905-11. ] demonstrated that, in DES that had failed to be delivered to the intended implantation site in tortuous calcified lesions, significant damage and cracking of the polymer had occurred to varying extents with multiple types of second-generation DES. Scanning electron microscopy revealed many cases of deep damage to the polymer with exposure of the bare metal: in particular, the Endeavor RX stents showed up to 20% damage to the surface area ( Figure 16 ). With polymer-free DES, a large proportion of the surface area was shown to be without any layer of drug ( Figure 16 ).
Bifurcation stenting, especially if very complex, has been hypothesised as possibly leading to polymer disruption, peeling or even polymer void [159159. Otsuka Y, Chronos NA, Apkarian RP, Robinson KA. Scanning electron microscopic analysis of defects in polymer coatings of three commercially available stents: comparison of BiodivYsio, Taxus and Cypher stents. J Invasive Cardiol. 2007;19:71-6. ], with the consequent risk of non-uniform drug distribution and focal stenosis. In bench work utilising scanning electron microscopy of the polymer integrity of 5 different types of DES (Cypher, Cypher Select, Endeavor, Taxus Express, and Taxus Liberté) after undergoing kissing balloon post-dilatation, Guerin et al. [164164. Guerin P, Pilet P, Finet G, Goueffic Y, N’Guyen JM, Crochet D, Tijou I, Pacaud P, Loirand G. Drug-eluting stents in bifurcations: bench study of strut deformation and coating lesions. Circ Cardiovasc Interv. 2010;3:120-6. ] demonstrated significantly greater coating damage to the ostial struts, especially along the overstretched segments, with cracking of the polymer seen in all cases and even exposure of bare metal. Of note is that the Endeavor stent showed a subtotal destruction of its coating on the luminal surface in all segments, whereas the other DES demonstrated more focal localised abnormalities.
Stent fractures
Stent fracture related to DES implantation in coronary arteries was first reported in 2004 ( Figure 17 ) [165165. Sianos G, Hofma S, Ligthart JM, Saia F, Hoye A, Lemos PA, Serruys PW. Stent fracture and restenosis in the drug-eluting stent era. Catheter Cardiovasc Interv. 2004;61:111-6. ]. Subsequent retrospective and prospective registries have quoted restenosis rates ranging from 15% to 100% in patients identified as having stent fractures [166166. Canan T, Lee MS. Drug-eluting stent fracture: incidence, contributing factors, and clinical implications. Catheter Cardiovasc Interv. 2010;75:237-45. ]. In the only randomised controlled trial reporting the incidence of stent fracture and outcomes after DES implantation and subsequent mandatory angiographic follow-up (LONG-DES-II study), a 14% incidence of restenosis was observed [167167. Kim HS, Kim YH, Lee SW, Park DW, Lee CW, Hong MK, Park SW, Ko JK, Park JH, Lee JH, Choi SW, Seong IW, Cho YH, Lee NH, Kim JH, Chun KJ, Park SJ. Incidence and predictors of drug-eluting stent fractures in long coronary disease. Int J Cardiol. 2009;133:354-8. ].
The pattern of restenosis associated with DES fractures appears to be focal, reflecting the local trauma sustained by the vessel at the fracture site once this has occurred. Due to the underlying mechanism of DES fracture (as explained below), restenosis tends to occur fairly late, and invariably after most if not all of the antiproliferative drug has been eluted.
The subsequent healing response therefore occurs without any drug to suppress the NIH response, which in itself is exacerbated by further exposure of the vessel to the disrupted polymer. The aetiology of the DES fractures also appears to be relatively well understood and is related to two principal factors.
The first is the location of the implantation site of the DES. Mechanical fatigue of the metallic stent can occur due to excessive movement during cardiac contraction, especially at a “hinge point” where the potential for 2 opposing forces may occur at the same site [168168. Aoki J, Nakazawa G, Tanabe K, Hoye A, Yamamoto H, Nakayama T, Onuma Y, Higashikuni Y, Otsuki S, Yagishita A, Yachi S, Nakajima H, Hara K. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2007;69:380-6. ]. In these situations, excessive movements can occur to the DES implanted in one part of a vessel during cardiac contraction, in particular in the right coronary artery or a saphenous vein graft, because of their greater propensity for angulation and tortuosity [166166. Canan T, Lee MS. Drug-eluting stent fracture: incidence, contributing factors, and clinical implications. Catheter Cardiovasc Interv. 2010;75:237-45. , 168168. Aoki J, Nakazawa G, Tanabe K, Hoye A, Yamamoto H, Nakayama T, Onuma Y, Higashikuni Y, Otsuki S, Yagishita A, Yachi S, Nakajima H, Hara K. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2007;69:380-6. ].
Secondly, the design of the DES itself has been strongly incriminated with causing DES fracture. A closed-cell design, such as occurs with SES, is less likely to be able to withstand the pressures related to excessive movements compared with the open-cell design of a PES. In a recent meta-analysis, [166166. Canan T, Lee MS. Drug-eluting stent fracture: incidence, contributing factors, and clinical implications. Catheter Cardiovasc Interv. 2010;75:237-45. ] the incidence of stent fracture was reported to be less than 0.1% with the open-cell PES and approximately 2.3% with the closed-cell SES.
Long stents, overlapping stents, tight lesions that have been “vigorously post-dilated and expanded,” [167167. Kim HS, Kim YH, Lee SW, Park DW, Lee CW, Hong MK, Park SW, Ko JK, Park JH, Lee JH, Choi SW, Seong IW, Cho YH, Lee NH, Kim JH, Chun KJ, Park SJ. Incidence and predictors of drug-eluting stent fractures in long coronary disease. Int J Cardiol. 2009;133:354-8. ] myocardial bridge sites, areas of significant curvature (due to the lack of conformability of certain DES, especially of closed-cell design) are all other factors which may predispose to DES fracture [165165. Sianos G, Hofma S, Ligthart JM, Saia F, Hoye A, Lemos PA, Serruys PW. Stent fracture and restenosis in the drug-eluting stent era. Catheter Cardiovasc Interv. 2004;61:111-6. , 166166. Canan T, Lee MS. Drug-eluting stent fracture: incidence, contributing factors, and clinical implications. Catheter Cardiovasc Interv. 2010;75:237-45. , 167167. Kim HS, Kim YH, Lee SW, Park DW, Lee CW, Hong MK, Park SW, Ko JK, Park JH, Lee JH, Choi SW, Seong IW, Cho YH, Lee NH, Kim JH, Chun KJ, Park SJ. Incidence and predictors of drug-eluting stent fractures in long coronary disease. Int J Cardiol. 2009;133:354-8. , 168168. Aoki J, Nakazawa G, Tanabe K, Hoye A, Yamamoto H, Nakayama T, Onuma Y, Higashikuni Y, Otsuki S, Yagishita A, Yachi S, Nakajima H, Hara K. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2007;69:380-6. , 169169. Yamada KP, Koizumi T, Yamaguchi H, Kaneda H, Bonneau HN, Honda Y, Fitzgerald PJ. Serial angiographic and intravascular ultrasound analysis of late stent strut fracture of sirolimus-eluting stents in native coronary arteries. Int J Cardiol. 2008;130:255-9. , 170170. Lemos PA, Saia F, Ligthart JMR, Arampatzis CA, Sianos G, Tanabe K, Hoye A, Degertekin M, Daemen J, McFadden E, Hofma S, Smits PC, de Feyter P, van der Giessen WJ, van Domburg RT, Serruys PW. Coronary Restenosis After Sirolimus-Eluting Stent Implantation: Morphological Description and Mechanistic Analysis From a Consecutive Series of Cases. Circulation. 2003;108:257-260. , 171171. Umeda H, Gochi T, Iwase M, Izawa H, Shimizu T, Ishiki R, Inagaki H, Toyama J, Yokota M, Murohara T. Frequency, predictors and outcome of stent fracture after sirolimus-eluting stent implantation. Int J Cardiol. 2009;133:321-6. , 172172. Okumura M, Ozaki Y, Ishii J, Kan S, Naruse H, Matsui S, Ishikawa M, Hattori K, Gochi T, Nakano T, Yamada A, Kato S, Motoyama S, Sarai M, Takagi Y, Ismail TF, Nomura M, Hishida H. Restenosis and stent fracture following sirolimus-eluting stent (SES) implantation. Circ J. 2007;71:1669-77. , 173173. Lee SH, Park JS, Shin DG, Kim YJ, Hong GR, Kim W, Shim BS. Frequency of stent fracture as a cause of coronary restenosis after sirolimus-eluting stent implantation. Am J Cardiol. 2007;100:627-30. ].
Newer-generation DES have consequently aimed to maintain the open-cell design and have thinner struts by using new metallic alloys. Although the radial strength has been preserved with next-generation DES - recent case reports have suggested that, with certain types of next-generation DES, the longitudinal strength of the device may potentially be affected, increasing the likelihood for stent deformation (longitudinal elongation or compression) requiring post-dilation or further stent placement - episodes of late stent thrombosis have also been reported [174174. Hanratty CG, Walsh SJ. Longitudinal compression: a “new” complication with modern coronary stent platforms - time to think beyond deliverability? EuroIntervention. 2011;7:872-7. , 175175. Mortier P, De Beule M. Stent design back in the picture: an engineering perspective on longitudinal stent compression. EuroIntervention. 2011;7:773, 775. , 176176. Williams PD, Mamas MA, Morgan KP, El-Omar M, Clarke B, Bainbridge A, Fath-Ordoubadi F, Fraser DG. Longitudinal stent deformation: a retrospective analysis of frequency and mechanisms. EuroIntervention. 2011 Nov 4. ]. Higher incidences of restenosis have, at the time of writing, not yet been reported. Notably in the multicentre DUTCH PEERS randomised trial, [246246. von Birgelen C, Sen H, Lam MK, Danse PW, Jessurun GA, Hautvast RW, van Houwelingen GK, Schramm AR, Gin RM, Louwerenburg JW, de Man FH, Stoel MG, Löwik MM, Linssen GC, Saïd SA, Nienhuis MB, Verhorst PM, Basalus MW, Doggen CJ, Tandjung K. Third-generation zotarolimus-eluting and everolimus-eluting stents in all-comer patients requiring a percutaneous coronary intervention (DUTCH PEERS): a randomised, single-blind, multicentre, non-inferiority trial. Lancet. 2014 Feb 1;383:413-23. ] comparing cobalt-chromium-based zotarolimus-eluting stents (Resolute Integrity, Medtronic) to platinum-chromium-based everolimus-eluting stents (Promus Element, Boston Scientific), longitudinal stent deformation was reported to be rarely observed (9/1591 [0·6%]). Despite all reported longitudinal stent deformation being associated with the Promus Element stent (9/905 [1·0%]), this was not linked to any adverse clinical outcomes at 12 month follow up.
Bioresorbable Scaffolds
Bioresorbable scaffolds represent a relatively new technology introduced to overcome the limitations of the metallic stents, namely the potential risk of late stent thrombosis, neoatherosclerosis, and the local inflammation caused by the presence of a foreign body. These devices have the unique ability to provide a temporal scaffolding that is necessary to maintain the patency of the vessel and then are gradually resorbed liberating the vessel from its cage and allowing restoration of vessel physiologic function [235235. Zhang Y, Bourantas CV, Farooq V, Muramatsu T, Diletti R, Onuma Y, Garcia-Garcia HM, Serruys PW. Bioresorbable scaffolds in the treatment of coronary artery disease. Med Devices (Auckl). 2013;6:37-48. , 236236. Iqbal J, Onuma Y, Ormiston J, Abizaid A, Waksman R, Serruys P. Bioresorbable scaffolds: rationale, current status, challenges, and future. Eur Heart J. 2014;35:765-76. , 237237. García-García HM, Schultz C, Duckers E, Regar E, Ligthart J, Serruys PW, van Geuns RJ. Five-year follow-up of the ABSORB bioresorbable everolimus-eluting vascular scaffold system: multimodality imaging assessment. EuroIntervention. 2013;8:1126-7. , 238238. Karanasos A, Simsek C, Serruys P, Ligthart J, Witberg K, van Geuns RJ, Sianos G, Zijlstra F, Regar E. Five-year optical coherence tomography follow-up of an everolimus-eluting bioresorbable vascular scaffold: changing the paradigm of coronary stenting? Circulation. 2012;126:e89-91. , 254254. Bourantas CV, Onuma Y, Farooq V, Zhang Y, Garcia-Garcia HM, Serruys PW. Bioresorbable scaffolds: current knowledge, potentialities and limitations experienced during their first clinical applications. Int J Cardiol. 2013 Jul 15;167(1):11-21. , 255255. Serruys PW, Chevalier B, Dudek D, Cequier A, Carrié D, Iniguez A, Dominici M, van der Schaaf RJ, Haude M, Wasungu L,Veldhof S, Peng L, Staehr P, Grundeken MJ, Ishibashi Y, Garcia-Garcia HM, Onuma Y. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2015 Jan 3;385(9962):43-54. , 256256. Bourantas CV, Serruys PW, Nakatani S, Zhang YJ, Farooq V, Diletti R, Ligthart J, Sheehy A, van Geuns RJ, McClean D, Chevalier B, Windecker S, Koolen J, Ormiston J, Whitbourn R, Rapoza R, Veldhof S, Onuma Y, Garcia-Garcia HM. Bioresorbable vascular scaffold treatment induces the formation of neointimal cap that seals the underlying plaque without compromising the luminal dimensions: a concept based on serial optical coherence tomography data. EuroIntervention. 2014 Oct 14. , 257257. Bourantas CV, Papafaklis MI, Kotsia A, Farooq V, Muramatsu T, Gomez-Lara J, Zhang YJ, Iqbal J, Kalatzis FG, Naka KK, Fotiadis DI, Dorange C, Wang J, Rapoza R, Garcia-Garcia HM, Onuma Y, Michalis LK, Serruys PW. Effect of the endothelial shear stress patterns on neointimal proliferation following drug-eluting bioresorbable vascular scaffold implantation: an optical coherence tomography study. JACC Cardiovasc Interv. 2014 Mar;7(3):315-24 ]. The potential advantages of bioresorbable scaffolds have attracted substantial interest and today several devices have been developed, some of which are available in clinical setting whilst others are undergoing clinical evaluation ( Figure 18 ).
The first clinical studies validating the efficacy and safety of bioresorbable devices demonstrated previously unknown causes of scaffold failure and restenosis, such as late recoil caused by the too rapid absorption of the scaffold, focal mechanical failure or difficulties in optimising the anti-proliferative drug release kinetics. [254254. Bourantas CV, Onuma Y, Farooq V, Zhang Y, Garcia-Garcia HM, Serruys PW. Bioresorbable scaffolds: current knowledge, potentialities and limitations experienced during their first clinical applications. Int J Cardiol. 2013 Jul 15;167(1):11-21. ] Consequently further research and optimisation of the scaffold material, design and drug release kinetics, and extensive validation of the new revisions in human clinical trials were required before bioresorbable scaffolds were able to reach the clinical arena. [235235. Zhang Y, Bourantas CV, Farooq V, Muramatsu T, Diletti R, Onuma Y, Garcia-Garcia HM, Serruys PW. Bioresorbable scaffolds in the treatment of coronary artery disease. Med Devices (Auckl). 2013;6:37-48. , 236236. Iqbal J, Onuma Y, Ormiston J, Abizaid A, Waksman R, Serruys P. Bioresorbable scaffolds: rationale, current status, challenges, and future. Eur Heart J. 2014;35:765-76. , 237237. García-García HM, Schultz C, Duckers E, Regar E, Ligthart J, Serruys PW, van Geuns RJ. Five-year follow-up of the ABSORB bioresorbable everolimus-eluting vascular scaffold system: multimodality imaging assessment. EuroIntervention. 2013;8:1126-7. , 238238. Karanasos A, Simsek C, Serruys P, Ligthart J, Witberg K, van Geuns RJ, Sianos G, Zijlstra F, Regar E. Five-year optical coherence tomography follow-up of an everolimus-eluting bioresorbable vascular scaffold: changing the paradigm of coronary stenting? Circulation. 2012;126:e89-91. , 254254. Bourantas CV, Onuma Y, Farooq V, Zhang Y, Garcia-Garcia HM, Serruys PW. Bioresorbable scaffolds: current knowledge, potentialities and limitations experienced during their first clinical applications. Int J Cardiol. 2013 Jul 15;167(1):11-21. , 255255. Serruys PW, Chevalier B, Dudek D, Cequier A, Carrié D, Iniguez A, Dominici M, van der Schaaf RJ, Haude M, Wasungu L,Veldhof S, Peng L, Staehr P, Grundeken MJ, Ishibashi Y, Garcia-Garcia HM, Onuma Y. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2015 Jan 3;385(9962):43-54. , 256256. Bourantas CV, Serruys PW, Nakatani S, Zhang YJ, Farooq V, Diletti R, Ligthart J, Sheehy A, van Geuns RJ, McClean D, Chevalier B, Windecker S, Koolen J, Ormiston J, Whitbourn R, Rapoza R, Veldhof S, Onuma Y, Garcia-Garcia HM. Bioresorbable vascular scaffold treatment induces the formation of neointimal cap that seals the underlying plaque without compromising the luminal dimensions: a concept based on serial optical coherence tomography data. EuroIntervention. 2014 Oct 14. , 257257. Bourantas CV, Papafaklis MI, Kotsia A, Farooq V, Muramatsu T, Gomez-Lara J, Zhang YJ, Iqbal J, Kalatzis FG, Naka KK, Fotiadis DI, Dorange C, Wang J, Rapoza R, Garcia-Garcia HM, Onuma Y, Michalis LK, Serruys PW. Effect of the endothelial shear stress patterns on neointimal proliferation following drug-eluting bioresorbable vascular scaffold implantation: an optical coherence tomography study. JACC Cardiovasc Interv. 2014 Mar;7(3):315-24 ].
Recently the ABSORB II study was reported. [255255. Serruys PW, Chevalier B, Dudek D, Cequier A, Carrié D, Iniguez A, Dominici M, van der Schaaf RJ, Haude M, Wasungu L,Veldhof S, Peng L, Staehr P, Grundeken MJ, Ishibashi Y, Garcia-Garcia HM, Onuma Y. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2015 Jan 3;385(9962):43-54. ] Absorb II constitutes the first randomised controlled trial comparing the efficacy and safety of a 2nd generation bioresorbable scaffold with a contemporary DES. The ABSORB II trial had a 2:1 single-blind design, recruiting 501 patients with stable and unstable angina symptoms to treatment with an everolimus eluting bioresorbable scaffold or a contemporary everolimus eluting metallic DES. The co-primary endpoints of nitrate-induced vasomotion and changes in minimum lumen diameter (in-stent late loss) are to be reported at 3 years. Secondary outcomes recently reported at 1 year demonstrated no difference in major adverse cardiovascular events (defined as death, myocardial infarction or target lesion revascularisation) between patients treated with a bioresorbable or a contemporary metallic DES (5% vs. 3%, P=0.35). In addition, cumulative rates of first new or worsening angina were reported to be lower with the bioresorbable scaffold group compared to contemporary metallic DES (22% vs. 30%, p=0.04), whereas the performance during maximum exercise and angina status by Seattle Angina Questionnaire were reported to be similar ( Figure 19 ).
In vivo computational based studies have utilised optical coherence tomographic data (due to its superior resolution compared to intravascular ultrasound data [ Figure 20 ]) to reconstruct coronary anatomy and estimate the local haemodynamic forces in segments implanted with a 2nd generation everolimus eluting bioresorbable scaffolds. These studies have demonstrated that scaffold implantation alters the local haemodynamic milieu secondary to the protruded struts obstructing flow and creating flow disturbances that result in a low endothelial shear stress environment which promotes vessel wall healing and neointimal proliferation ( Figure 21 and Figure 22 ). Notably the composition of the plaque underlying the struts does not appear to affect neointimal distribution. [256256. Bourantas CV, Serruys PW, Nakatani S, Zhang YJ, Farooq V, Diletti R, Ligthart J, Sheehy A, van Geuns RJ, McClean D, Chevalier B, Windecker S, Koolen J, Ormiston J, Whitbourn R, Rapoza R, Veldhof S, Onuma Y, Garcia-Garcia HM. Bioresorbable vascular scaffold treatment induces the formation of neointimal cap that seals the underlying plaque without compromising the luminal dimensions: a concept based on serial optical coherence tomography data. EuroIntervention. 2014 Oct 14. , 257257. Bourantas CV, Papafaklis MI, Kotsia A, Farooq V, Muramatsu T, Gomez-Lara J, Zhang YJ, Iqbal J, Kalatzis FG, Naka KK, Fotiadis DI, Dorange C, Wang J, Rapoza R, Garcia-Garcia HM, Onuma Y, Michalis LK, Serruys PW. Effect of the endothelial shear stress patterns on neointimal proliferation following drug-eluting bioresorbable vascular scaffold implantation: an optical coherence tomography study. JACC Cardiovasc Interv. 2014 Mar;7(3):315-24 ]. Currently, there are no data with regards to the effect of the bioresorption process, vessel wall inflammation, the underlying plaque burden, and vessel wall injury with respect to the potential effects these may have on neointimal growth. Further research is required to investigate the mechanisms that regulate neointimal proliferation in different scaffolds with a different polymer and strut architecture.
IMPLANTATION FACTORS
Incomplete stent expansion
A smaller post-procedural minimal lumen diameter (MLD) and a greater residual stenosis ( Figure 17 ) have been associated with long-term DES patency and clinical outcomes [177177. Castagna MT, Mintz GS, Leiboff BO, Ahmed JM, Mehran R, Satler LF, Kent KM, Pichard AD, Weissman NJ. The contribution of “mechanical” problems to in-stent restenosis: An intravascular ultrasonographic analysis of 1090 consecutive in-stent restenosis lesions. Am Heart J. 2001;142:970-4. , 178178. Bertrand OF, De Larochelliere R, Joyal M, Bonan R, Mongrain R, Tardif JC. Incidence of stent under-deployment as a cause of in-stent restenosis in long stents. Int J Cardiovasc Imaging. 2004;20:279-84. , 179179. Fujii K, Mintz GS, Kobayashi Y, Carlier SG, Takebayashi H, Yasuda T, Moussa I, Dangas G, Mehran R, Lansky AJ, Reyes A, Kreps E, Collins M, Colombo A, Stone GW, Teirstein PS, Leon MB, Moses JW. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation. 2004;109:1085-8. , 180180. Escolar E, Mintz GS, Canos D, Cheneau E, Pichard AD, Satler LF, Kent KM, Waksman R, Weissman NJ. Serial intravascular ultrasound comparison of the extent and distribution of intimal hyperplasia six months after stent implantation for de novo versus in-stent restenosis lesions. Am J Cardiol. 2005;96:897-900. , 181181. Doi H, Maehara A, Mintz GS, Yu A, Wang H, Mandinov L, Popma JJ, Ellis SG, Grube E, Dawkins KD, Weissman NJ, Turco MA, Ormiston JA, Stone GW. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: an integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc Interv. 2009;2:1269-75. , 182182. de Ribamar Costa J, Jr., Mintz GS, Carlier SG, Fujii K, Sano K, Kimura M, Tanaka K, Costa RA, Lui J, Na Y, Castellanos C, Biro S, Moussa I, Stone GW, Moses JW, Leon MB. Intravascular ultrasound assessment of drug-eluting stent expansion. Am Heart J. 2007;153:297-303. , 183183. Yoon SC, Laskey WK, Assadourian A, Kelly D, Gellman J, Herzog W, Stafford JL. Assessment of contemporary stent deployment using intravascular ultrasound. Catheter Cardiovasc Interv. 2002;57:150-4. , 184184. Hanekamp CE, Koolen JJ, Pijls NH, Michels HR, Bonnier HJ. Comparison of quantitative coronary angiography, intravascular ultrasound, and coronary pressure measurement to assess optimum stent deployment. Circulation. 1999;99:1015-21. , 185185. Sonoda S, Morino Y, Ako J, Terashima M, Hassan AH, Bonneau HN, Leon MB, Moses JW, Yock PG, Honda Y, Kuntz RE, Fitzgerald PJ. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: serial intravascular ultrasound analysis from the sirius trial. J Am Coll Cardiol. 2004;43:1959-63. , 186186. Takebayashi H, Kobayashi Y, Mintz GS, Carlier SG, Fujii K, Yasuda T, Moussa I, Mehran R, Dangas GD, Collins MB, Kreps E, Lansky AJ, Stone GW, Leon MB, Moses JW. Intravascular ultrasound assessment of lesions with target vessel failure after sirolimus-eluting stent implantation. Am J Cardiol. 2005;95:498-502. , 187187. Hong MK, Mintz GS, Lee CW, Park DW, Choi BR, Park KH, Kim YH, Cheong SS, Song JK, Kim JJ, Park SW, Park SJ. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305-10. ]. Evidence of stent underexpansion has been reported in 20-40% of cases when assessed by quantitative coronary angiography (QCA) and 38-70% of cases when assessed by IVUS [182182. de Ribamar Costa J, Jr., Mintz GS, Carlier SG, Fujii K, Sano K, Kimura M, Tanaka K, Costa RA, Lui J, Na Y, Castellanos C, Biro S, Moussa I, Stone GW, Moses JW, Leon MB. Intravascular ultrasound assessment of drug-eluting stent expansion. Am Heart J. 2007;153:297-303. , 183183. Yoon SC, Laskey WK, Assadourian A, Kelly D, Gellman J, Herzog W, Stafford JL. Assessment of contemporary stent deployment using intravascular ultrasound. Catheter Cardiovasc Interv. 2002;57:150-4. , 184184. Hanekamp CE, Koolen JJ, Pijls NH, Michels HR, Bonnier HJ. Comparison of quantitative coronary angiography, intravascular ultrasound, and coronary pressure measurement to assess optimum stent deployment. Circulation. 1999;99:1015-21. ]. However, what proportion of these cases with stent underexpansion are clinically relevant in causing restenosis remains unclear. Serial IVUS analyses from the SIRIUS trial [185185. Sonoda S, Morino Y, Ako J, Terashima M, Hassan AH, Bonneau HN, Leon MB, Moses JW, Yock PG, Honda Y, Kuntz RE, Fitzgerald PJ. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: serial intravascular ultrasound analysis from the sirius trial. J Am Coll Cardiol. 2004;43:1959-63. ] and other studies [186186. Takebayashi H, Kobayashi Y, Mintz GS, Carlier SG, Fujii K, Yasuda T, Moussa I, Mehran R, Dangas GD, Collins MB, Kreps E, Lansky AJ, Stone GW, Leon MB, Moses JW. Intravascular ultrasound assessment of lesions with target vessel failure after sirolimus-eluting stent implantation. Am J Cardiol. 2005;95:498-502. , 187187. Hong MK, Mintz GS, Lee CW, Park DW, Choi BR, Park KH, Kim YH, Cheong SS, Song JK, Kim JJ, Park SW, Park SJ. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305-10. ] investigating SES use, have demonstrated a cut-off of a post-procedural MSA of between 5.0-5.5 mm2 as being the optimal area to reduce the likelihood of restenosis.
In an important meta-analysis, Casella et al [188188. Casella G, Klauss V, Ottani F, Siebert U, Sangiorgio P, Bracchetti D. Impact of intravascular ultrasound-guided stenting on long-term clinical outcome: a meta-analysis of available studies comparing intravascular ultrasound-guided and angiographically guided stenting. Catheter Cardiovasc Interv. 2003;59:314-21. ] compared IVUS against angiographic-guided BMS implantation (n=2,972 patients) and demonstrated that, at 6 months follow-up, there was reduced TVR (OR 0.62; 95% CI: 0.49-0.78; p=0.00003), binary restenosis (OR 0.75; 95% CI: 0.60-0.94; p=0.01) and MACE (OR 0.79; 95% CI: 0.64-0.98; p=0.03) when an IVUS approach to BMS implantation was used. Since then, the only new published randomised controlled trials of IVUS versus angiographic-guided stent implantation were the AVID trial [189189. Russo RJ, Silva PD, Teirstein PS, Attubato MJ, Davidson CJ, DeFranco AC, Fitzgerald PJ, Goldberg SL, Hermiller JB, Leon MB, Ling FS, Lucisano JE, Schatz RA, Wong SC, Weissman NJ, Zientek DM. A randomized controlled trial of angiography versus intravascular ultrasound-directed bare-metal coronary stent placement (the AVID Trial). Circ Cardiovasc Interv. 2009;2:113-23. ] for BMS and a small trial (Jakabcin et al [190190. Jakabcin J, Spacek R, Bystron M, Kvasnak M, Jager J, Veselka J, Kala P, Cervinka P. Long-term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance. Randomized control trial. HOME DES IVUS. Catheter Cardiovasc Interv. 2010;75:578-83. ]) for DES.
The AVID trial was published 10 years after the data collection and had mixed results, with no benefit in the incidence of 12-month TLR, a larger minimum stent area post BMS implantation and, on subgroup analysis, a lower 12-month TLR rate for vessels ≥2.5 mm or vessels with a high-grade pre-stent stenosis. With DES, Jakabcin et al failed to show any differences in the clinical endpoints of MACE (death, myocardial infarction and reintervention) at 18 months.
- Polymer release kinetics – namely antiproliferative drug dose and time course of delivery – play a crucial role in the prevention of restenosis
- The genes responsible for the proliferative response potentially remain active for a period of up to 21 days after vessel injury – a critical time period for antiproliferative drug delivery from DES
- DES with shorter drug release kinetics, such as Endeavor (14 days), lead to greater restenosis compared to DES with longer drug release kinetics
- Newer-generation DES with thinner struts, longer drug release kinetics, more biocompatible polymers have led to more effective neointima inhibition
- Off-label use of DES, such as in ostial, left main stem, chronic total occlusion, saphenous vein graft, small or large vessels/multivessel, STEMI, ISR lesions, are associated with higher restenosis and revascularisation rates
- Polymer disruption, peeling and cracking, either on the DES at baseline or induced by delivery or by interventional procedure, have been demonstrated with first and second-generation DES. As to whether this is implicated in restenosis is theoretical
- Stent fractures are more likely to occur with the closed-cell design of first-gener
Furthermore, results of the AVIO (Angiographic Versus IVUS Optimisation) randomised, multicentre trial assessing IVUS-guided DES implantation were presented in 2010 [191191. Rogacka R, Latib A, Colombo A. IVUS-Guided Stent Implantation to Improve Outcome: A Promise Waiting to be Fulfilled. Curr Cardiol Rev. 2009;5:78-86. , 192192. Colombo A. AVIO: a prospective randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions. Presented at: Transcatheter Cardiovascular Therapeutics; September 21 to 25, 2010; Washington, DC. ]. In 142 patients with complex lesions implanted with IVUS-guided DES, no clinical benefit was demonstrated compared to controls, with comparable clinical outcomes in the combined endpoint of MI, target lesion revascularisation (TLR), TVR or cardiac death at 30 days and 9 months (85.9% vs. 83.1%, p=0.47). The primary endpoint of a higher MLD was seen in the IVUS-guided DES implantation group (2.70 mm vs. 2.51 mm, p=0.0002). As only 39% of patients had QCA at 9 months, no comments could be made as to whether this approach would potentially lead to a reduction in restenosis rates. Until large-scale trials unequivocally demonstrate the clinical efficacy of an IVUS-guided DES implantation approach, IVUS will remain to be used at the operator’s discretion.
Data from the largest study of IVUS guided DES implantation to date (ADAPT-DES: Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents, n=8583) demonstrated IVUS guidance to be associated with a reduction in stent thrombosis, myocardial infarction, and major adverse cardiac events within 1 year following DES implantation when compared to angiography alone. ADAPT-DES was a prospective, multicentre, non-randomised "all-comers" trial. Based on propensity-adjusted analyses, a significant reduction in a composite of cardiac death, myocardial infarction, or stent thrombosis was shown (3.1% versus 4.7%; adjusted hazard radio, 0.70; 95% confidence interval, 0.55-0.88; P=0.002), a benefit that was especially evident in acute coronary syndromes and more complex lesions. [248248. Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Brodie BR, Stuckey TD, Mazzaferri EL Jr, Xu K, Parise H, Mehran R, Mintz GS, Stone GW. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129:463-70 ]
More recently data from multiple meta-analyses investigating almost 20 000 subjects, have supported the findings of ADAPT-DES and shown IVUS to improve clinical outcomes and reduce repeat revascularisation rates, even when analyses were restricted to propensity matched studies. [248248. Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Brodie BR, Stuckey TD, Mazzaferri EL Jr, Xu K, Parise H, Mehran R, Mintz GS, Stone GW. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129:463-70 , 249249. Zhang Y, Farooq V, Garcia-Garcia HM, Bourantas CV, Tian N, Dong S, Li M, Yang S, Serruys PW, Chen SL. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. 2012;8:855-65. , 250250. Zhang YJ, Garcia-Garcia HM, Farooq V, Bourantas CV, Serruys PW, Chen SL. Revisiting: ’Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients’. EuroIntervention. 2013;9:891-2. , 251251. Ahn JM, Kang SJ, Yoon SH, Park HW, Kang SM, Lee JY, Lee SW, Kim YH, Lee CW, Park SW, Mintz GS, Park SJ. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014;113:1338-47. , 252252. Jang JS, Song YJ, Kang W, Jin HY, Seo JS, Yang TH, Kim DK, Cho KI, Kim BH, Park YH, Je HG, Kim DS. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. JACC Cardiovasc Interv. 2014;7:233-43. , 253253. Klersy C, Ferlini M, Raisaro A, Scotti V, Balduini A, Curti M, Bramucci E, De Silvestri A. Use of IVUS guided coronary stenting with drug eluting stent: a systematic review and meta-analysis of randomized controlled clinical trials and high quality observational studies. Int J Cardiol. 2013;170:54-63. ]
The most plausible and the strongest theory to explain the underlying mechanism relating stent underexpansion to restenosis is the so-called “bigger-is-better” paradigm [193193. Honda Y, Fitzgerald PJ. Stent expansion as a mechanical parameter to predict late stent patency: back to the basics. JACC Cardiovasc Interv. 2009;2:1276-8. ]. Effectively, if the minimum stent area (MSA) is smaller at baseline, then the expected NIH formation post DES implantation would be more likely to be of significance in leading to a flow-limiting lesion. Conversely, if the MSA was larger, then the growth of the same amount of NIH would be clinically less relevant in causing binary restenosis [193193. Honda Y, Fitzgerald PJ. Stent expansion as a mechanical parameter to predict late stent patency: back to the basics. JACC Cardiovasc Interv. 2009;2:1276-8. ]. Other suggestions of DES underexpansion as a cause of ISR have been related to possible asymmetrical stent expansion, which may affect the pattern of neointimal growth through possible uneven drug delivery [194194. Hwang C-W, Wu D, Edelman ER. Physiological Transport Forces Govern Drug Distribution for Stent-Based Delivery. Circulation. 2001;104:600-605. , 195195. Lambert T, Dev V, Rechavia E, Forrester J, Litvack F, Eigler N. Localized arterial wall drug delivery from a polymer-coated removable metallic stent. Kinetics, distribution, and bioactivity of forskolin. Circulation. 1994;90:1003-1011. ]. Numerous factors, including “off-label” indications for DES implantation, as previously described, are involved in increasing the risk of suboptimal stent deployment ( Table 5 ).
Geographical miss/barotrauma to unstented segments
Geographical miss ( Figure 18 ), as the name suggests, is essentially a failure to cover appropriately an injured vessel or atherosclerotic plaque. This may be a consequence of lesion predilatation, balloon-associated vessel barotrauma and the subsequent failure to cover the entire injured site with a DES, incomplete coverage of the diseased segment of the vessel with significant plaque remaining at the stent margins, or failure adequately to overlap DES in long segments of disease.
Geographical miss has more accurately been described as longitudinal geographical miss (LGM: injured or diseased stenotic segment not fully covered by DES) or axial geographical miss (AGM: balloon-artery size ratio <0.9 or >1.3 mismatch) ( Figure 19 ) [196196. Costa MA, Angiolillo DJ, Tannenbaum M, Driesman M, Chu A, Patterson J, Kuehl W, Battaglia J, Dabbons S, Shamoon F, Flieshman B, Niederman A, Bass TA. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial). Am J Cardiol. 2008;101:1704-11.
One of the first reported associations of geographical miss with restensois.]. Geographical miss, associated with the implantation of SES, was investigated in the STLLR study [196196. Costa MA, Angiolillo DJ, Tannenbaum M, Driesman M, Chu A, Patterson J, Kuehl W, Battaglia J, Dabbons S, Shamoon F, Flieshman B, Niederman A, Bass TA. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial). Am J Cardiol. 2008;101:1704-11.
One of the first reported associations of geographical miss with restensois.]. As a whole, geographical miss was observed in nearly two thirds of the study group (66.5%), with almost half the patients experiencing LGM (47.6%), over one third AGM (35.2%), and 16.5% a combination of the two. At 1-year follow-up, there was more than a 2-fold increase in TVR (5.1% vs. 2.5%; p=0.025) and a 3-fold increase in MI (2.4% vs. 0.8%; p=0.04) in patients with geographical miss. Subgroup analyses indicated that these findings were almost exclusively related to LGM (6.1% vs. 2.6%; p=0.001), with two thirds of cases being secondary to balloon injury outside the stent margins, and AGM not seeming to be an important factor (4.2% vs. 4.3%; p non-significant). This latter finding has been corroborated, where it was shown that the balloon-to-artery ratio [148148. Eshtehardi P, Cook S, Wandel S, Raber L, Wenaweser P, Togni M, Vogel R, Garachemani A, Eberli FR, Luscher TF, Juni P, Hess OM, Meier B, Windecker S. Impact of arterial injury on neointimal hyperplasia after implantation of drug-eluting stents in coronary arteries: an intravascular ultrasound study. EuroIntervention. 2010;6:467-74. ] or the occurrence of edge dissections (potentially associated with AGM) [197197. Liu X, Tsujita K, Maehara A, Mintz GS, Weisz G, Dangas GD, Lansky AJ, Kreps EM, Rabbani LE, Collins M, Stone GW, Moses JW, Mehran R, Leon MB. Intravascular ultrasound assessment of the incidence and predictors of edge dissections after drug-eluting stent implantation. JACC Cardiovasc Interv. 2009;2:997-1004. ] did not have a significant impact on the risk of restenosis: this does perhaps argue against the practice of IVUS-guided DES implantation. The occurrence of edge dissections has, however, been linked to an increased periprocedural and 1-month MACE rate, driven primarily by an elevated risk of stent thrombosis and subsequent TVR [198198. Biondi-Zoccai GG, Agostoni P, Sangiorgi GM, Airoldi F, Cosgrave J, Chieffo A, Barbagallo R, Tamburino C, Vittori G, Falchetti E, Margheri M, Briguori C, Remigi E, Iakovou I, Colombo A. Incidence, predictors, and outcomes of coronary dissections left untreated after drug-eluting stent implantation. Eur Heart J. 2006;27:540-6. ].
A substudy of the STLLR study demonstrated that the clinical effects of balloon injury secondary to LGM were more pronounced in diabetics [199199. Tahara S, Bezerra HG, Kyono H, Carrigan T, Mehanna E, Wang W, Costa MA. Impact of acute gain on clinical outcomes of patients treated with sirolimus-eluting stent. - A sub-analysis study from the STLLR trial. Circulation journal. 2011;75:2113-9. ]. More than a four-fold increase (8%) in the need for target lesion revascularisation in diabetics, and almost a two-fold increase in non-diabetics (3.8%), in the presence of LGM were reported compared to no LGM being present.
The vascular response at the stent edges has been evaluated with first-generation DES. It appears to be dependent on the implanted device and the periprocedural-induced vascular trauma, as a consequence of the geographical miss phenomenon, as described. [196196. Costa MA, Angiolillo DJ, Tannenbaum M, Driesman M, Chu A, Patterson J, Kuehl W, Battaglia J, Dabbons S, Shamoon F, Flieshman B, Niederman A, Bass TA. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial). Am J Cardiol. 2008;101:1704-11.
One of the first reported associations of geographical miss with restensois.] Within the IVUS substudies of the E-SIRIUS and SIRIUS trials, both AGM and LGM were shown to be potentially reduced when periprocedural implantation parameters such as conservative pre-dilatation, less forceful stent implantation (~16 atm) and selective post-dilatation with balloons shorter than the stent were undertaken [200200. Hoffmann R, Guagliumi G, Musumeci G, Reimers B, Petronio AS, Disco C, Amoroso G, Moses JW, Fitzgerald PJ, Schofer J, Leon MB, Breithardt G. Vascular response to sirolimus-eluting stents delivered with a nonaggressive implantation technique: comparison of intravascular ultrasound results from the multicenter, randomized E-SIRIUS, and SIRIUS trials. Catheter Cardiovasc Interv. 2005;66:499-506. ].
The landing zone of the implanted device may be a potential mechanism for restenosis. Failure to cover fully a stenotic lipid-core lesion due to LGM as described has potentially been shown to increase the likelihood of DES failure due to plaque progression at the DES edge and subsequent edge restenosis ( Figure 20 ) [1717. Corbett SJ, Cosgrave J, Melzi G, Babic R, Biondi-Zoccai GG, Godino C, Morici N, Airoldi F, Michev I, Montorfano M, Sangiorgi GM, Bonizzoni E, Colombo A. Patterns of restenosis after drug-eluting stent implantation: Insights from a contemporary and comparative analysis of sirolimus- and paclitaxel-eluting stents. Eur Heart J. 2006;27:2330-7. , 170170. Lemos PA, Saia F, Ligthart JMR, Arampatzis CA, Sianos G, Tanabe K, Hoye A, Degertekin M, Daemen J, McFadden E, Hofma S, Smits PC, de Feyter P, van der Giessen WJ, van Domburg RT, Serruys PW. Coronary Restenosis After Sirolimus-Eluting Stent Implantation: Morphological Description and Mechanistic Analysis From a Consecutive Series of Cases. Circulation. 2003;108:257-260. , 196196. Costa MA, Angiolillo DJ, Tannenbaum M, Driesman M, Chu A, Patterson J, Kuehl W, Battaglia J, Dabbons S, Shamoon F, Flieshman B, Niederman A, Bass TA. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial). Am J Cardiol. 2008;101:1704-11.
One of the first reported associations of geographical miss with restensois., 201201. Waxman S, Freilich MI, Suter MJ, Shishkov M, Bilazarian S, Virmani R, Bouma BE, Tearney GJ. A case of lipid core plaque progression and rupture at the edge of a coronary stent: elucidating the mechanisms of drug-eluting stent failure. Circ Cardiovasc Interv. 2010;3:193-6. , 202202. Sarno G, Garg S, Gomez-Lara J, Garcia Garcia HM, Ligthart J, Bruining N, Onuma Y, Witberg K, van Geuns RJ, de Boer S, Wykrzykowska J, Schultz C, Duckers HJ, Regar E, de Jaegere P, de Feyter P, van Es GA, Boersma E, van der Giessen W, Serruys PW. Intravascular ultrasound radiofrequency analysis after optimal coronary stenting with initial quantitative coronary angiography guidance: an ATHEROREMO sub-study. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 2011;6:977-84. ]. Further studies are required to assess if the use of intracoronary imaging to ensure full lesion/plaque coverage is undertaken and to assess whether this leads to improved clinical outcomes.
Deployment of a DES in a clot-laden arterial segment
Deployment of a DES in a clot-laden arterial segment has been shown in an ex vivo model to lead to significant variability in arterial drug distribution [203203. Hwang CW, Levin AD, Jonas M, Li PH, Edelman ER. Thrombosis modulates arterial drug distribution for drug-eluting stents. Circulation. 2005;111:1619-26. ] which may potentially affect clinical outcomes ( Figure 21 ). Essentially in this study it was shown that thrombus between the stent strut and vessel wall lead to a reduction in drug penetration into the vessel wall by a factor of up to 10-fold. Despite these theoretical concerns, multiple studies [204204. Spaulding C, Henry P, Teiger E, Beatt K, Bramucci E, Carrie D, Slama MS, Merkely B, Erglis A, Margheri M, Varenne O, Cebrian A, Stoll HP, Snead DB, Bode C. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006;355:1093-104. , 205205. Valgimigli M, Percoco G, Malagutti P, Campo G, Ferrari F, Barbieri D, Cicchitelli G, McFadden EP, Merlini F, Ansani L, Guardigli G, Bettini A, Parrinello G, Boersma E, Ferrari R. Tirofiban and sirolimus-eluting stent vs abciximab and bare-metal stent for acute myocardial infarction: a randomized trial. JAMA. 2005;293:2109-17. , 206206. Valgimigli M, Campo G, Percoco G, Bolognese L, Vassanelli C, Colangelo S, de Cesare N, Rodriguez AE, Ferrario M, Moreno R, Piva T, Sheiban I, Pasquetto G, Prati F, Nazzaro MS, Parrinello G, Ferrari R. Comparison of angioplasty with infusion of tirofiban or abciximab and with implantation of sirolimus-eluting or uncoated stents for acute myocardial infarction: the MULTISTRATEGY randomized trial. JAMA. 2008;299:1788-99. , 207207. Laarman GJ, Suttorp MJ, Dirksen MT, van Heerebeek L, Kiemeneij F, Slagboom T, van der Wieken LR, Tijssen JG, Rensing BJ, Patterson M. Paclitaxel-eluting versus uncoated stents in primary percutaneous coronary intervention. N Engl J Med. 2006;355:1105-13. , 208208. Stone GW, Lansky AJ, Pocock SJ, Gersh BJ, Dangas G, Wong SC, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Mockel M, Ochala A, Kellock A, Parise H, Mehran R. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009;360:1946-59. , 209209. Menichelli M, Parma A, Pucci E, Fiorilli R, De Felice F, Nazzaro M, Giulivi A, Alborino D, Azzellino A, Violini R. Randomized trial of Sirolimus-Eluting Stent Versus Bare-Metal Stent in Acute Myocardial Infarction (SESAMI). J Am Coll Cardiol. 2007;49:1924-30. , 210210. Diaz de la Llera LS, Ballesteros S, Nevado J, Fernandez M, Villa M, Sanchez A, Retegui G, Garcia D, Martinez A. Sirolimus-eluting stents compared with standard stents in the treatment of patients with primary angioplasty. Am Heart J. 2007;154:164 e1-6. , 211211. van der Hoeven BL, Liem SS, Jukema JW, Suraphakdee N, Putter H, Dijkstra J, Atsma DE, Bootsma M, Zeppenfeld K, Oemrawsingh PV, van der Wall EE, Schalij MJ. Sirolimus-eluting stents versus bare-metal stents in patients with ST-segment elevation myocardial infarction: 9-month angiographic and intravascular ultrasound results and 12-month clinical outcome results from the MISSION! Intervention Study. J Am Coll Cardiol. 2008;51:618-26. , 212212. Kelbaek H, Thuesen L, Helqvist S, Clemmensen P, Klovgaard L, Kaltoft A, Andersen B, Thuesen H, Engstrom T, Botker HE, Saunamaki K, Krusell LR, Jorgensen E, Hansen HH, Christiansen EH, Ravkilde J, Kober L, Kofoed KF, Terkelsen CJ, Lassen JF. Drug-eluting versus bare metal stents in patients with st-segment-elevation myocardial infarction: eight-month follow-up in the Drug Elution and Distal Protection in Acute Myocardial Infarction (DEDICATION) trial. Circulation. 2008;118:1155-62. ] and a meta-analysis of 13 trials (n=7,244) [213213. Piscione F, Piccolo R, Cassese S, Galasso G, De Rosa R, D’Andrea C, Chiariello M. Effect of drug-eluting stents in patients with acute ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: a meta-analysis of randomised trials and an adjusted indirect comparison. EuroIntervention. 2010;5:853-60. ] have shown the significant short-term benefits of DES over BMS. Specifically in the meta-analysis by Piscione et al, [213213. Piscione F, Piccolo R, Cassese S, Galasso G, De Rosa R, D’Andrea C, Chiariello M. Effect of drug-eluting stents in patients with acute ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: a meta-analysis of randomised trials and an adjusted indirect comparison. EuroIntervention. 2010;5:853-60. ] a reduction of TVR (5.11% versus 11.19%, p<0.00001) and recurrent MI (3.03% versus 3.70%, p=0.02) in patients with STEMI were demonstrated up to 1 year. The widespread use of glycoprotein-IIb/IIIa inhibitors and aspiration thrombectomy may be the reasons why these concerns have not materialised in clinical trials in the short term.
Concerns over the long-term safety of DES in STEMI do persist, however, because of the potential risk of late-acquired stent malapposition and consequent LST [214214. Hassan AK, Bergheanu SC, Stijnen T, van der Hoeven BL, Snoep JD, Plevier JW, Schalij MJ, Wouter Jukema J. Late stent malapposition risk is higher after drug-eluting stent compared with bare-metal stent implantation and associates with late stent thrombosis. European Heart Journal. 2010;31:1172-80. , 215215. Alfonso F, Cruz A, Garcia J. Late stent malapposition: innocent phenomenon or major risk marker? European Heart Journal 2010;31:260; author reply 260-1. ]. The concerns about reduced absorption of the drug from DES should be borne in mind in a thrombus-laden vessel, especially when there has been inadequate resolution of thrombus and DES implantation is to be considered.
- Implantation factors are the most controllable factors for potentially reducing restenosis
- A smaller post-procedural minimal lumen diameter (MLD) and a greater residual stenosis have been strongly associated with long-term DES patency and clinical outcomes
- IVUS-guided BMS implantation has been associated with a reduced TVR and binary restenosis in classical meta-analyses
- To date, no randomised controlled trial has demonstrated the clinical superiority of IVUS-guided DES implantation despite being associated with a post-procedural higher MLD
- Data from a large scale registry and meta-analyses of almost 20 000 subjects (even when analyses were restricted to propensity matched studies) have shown IVUS guided DES implantation to improve clinical outcomes when compared to angiography guidance alone.
- Longitudinal geographical miss has been associated with greater TVR and MI, an effect that may be pronounced in diabetics
- Reductions in TVR and MI have been associated with DES implanted after STEMI at up to 1 year, despite theoretical concerns that thrombus may interfere with local drug absorption. Concerns over the long-term safety of DES in STEMI do persist