Renal artery intervention
INTRODUCTION
The first renal artery balloon angioplasties were performed by Felix Mahler in Berne and Andreas Grüntzig in Zurich in 1977 [11. Grüntzig A, Vetter W, Meier B, Kuhlmann U, Lütolf U, Siegenthaler W. Treatment of renovascular hypertension with percutaneous transluminal dilatation of a renal artery stenosis. Lancet. 1978;1:801-2. , 22. Mahler F, Krneta A, Haertel M. Treatment of renovascular hypertension by transluminal renal artery dilatation. Ann Intern Med. 1979;90:56-7. ]. Until the beginning of the 1990s, balloon angioplasty was the only method of percutaneous treatment of RAS with satisfactory acute and long-term results. For angioplasty of stenoses caused by fibromuscular dysplasia (FMD) and atherosclerotic RAS of the renal artery trunk, procedural success rates of 82% to 100% and 1-year restenosis rates of about 10% were achieved [33. Plouin PF, Chatellier G, Darne B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: A randomized trial. The EMMA-study group. Hypertension. 1998;31:823-9. , 44. Van Bockel JH, Weibull H. Fibrodysplastic disease of renal arteries. Eur J Vasc Surg. 1994;8:655-7. , 55. Baert AL, Wilms G, Amery A, Vermylen J, Suy R. Percutaneous transluminal renal angioplasty: Initial results and long-term follow-up in 202 patients. Cardiovasc Intervent Radiology. 1990;13:22-8. , 66. Bonelli FS, McKusick A, Textor SC, Kos PB, Stanson AW, Johnson CM, Sheedy PF 2nd, Welch TJ, Schirger A. Renal artery angioplasty: Technical results and clinical outcome in 320 patients. Mayo Clin Proc. 1995; 70:1041-52. , 77. Cluzel P, Raynaud A, Beyssen B, Pagny JY, Gaux JC. Stenoses of renal branch arteries in fibromuscular dysplasia: Results of percutaneous transluminal angioplasty. Radiology. 1994;193:227-32. ]. However, balloon angioplasty of ostial atherosclerotic lesions was limited, with a low acute technical success rate of 50% to 62% and a high long-term restenosis rate of up to 47% due to dissections, elastic recoil and rigidity of the mostly calcified lesion [33. Plouin PF, Chatellier G, Darne B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: A randomized trial. The EMMA-study group. Hypertension. 1998;31:823-9. , 88. Dorros G, Prince C, Mathiak L. Stenting of renal artery stenosis achieves better relief of the obstructive lesion than balloon angioplasty. Cath Cardiovasc Diagn. 1993;29:191-8. ].
The introduction of stenting in the late 1990s has revolutionised percutaneous renal revascularisation. Following promising single-centre reports [88. Dorros G, Prince C, Mathiak L. Stenting of renal artery stenosis achieves better relief of the obstructive lesion than balloon angioplasty. Cath Cardiovasc Diagn. 1993;29:191-8. , 99. Blum U, Krumme B, Flügel P, Gabelmann A, Lehnert T, Buitrago-Tellez C, Schollmeyer P, Langer M. Treatment of ostial renal-artery stenoses with vascular endoprotheses after unsuccessful balloon angioplasty. N Engl J Med. 1997;336:459-65. , 1010. Dorros G, Jaff M, Mathiak L, Dorros II, Lowe A, Murphy K, He T. 4-year follow-up of Palmaz-Schatz stent revascularisation as treatment for atherosclerotic renal artery stenosis. Circulation. 1998;98:642-7. , 1111. Henry M, Amor M, Henry I, Ethevenot G, Allaoui M, Tricoche O, Porte JM, Touchot N. Stent placement in the renal artery: Three-year experience with the Palmaz stent. J Vasc Interv Radiol. 1996;7:343-50. , 1212. White CJ, Ramee SR, Collins TJ, Jenkins JS. Renal artery stent placement: Utility in lesions difficult to treat with balloon angioplasty. J Am Coll Cardiol. 1999; 30:1445-50. , 1313. Zeller T, Müller C, Frank U, Bürgelin K, Horn B, Cook-Bruns N, Schwarzwälder U, Neumann FJ. Stent-angioplasty of severe atherosclerotic ostial renal artery stenosis in patients with diabetes mellitus and nephrosclerosis. Catheter Cardiovasc Interv. 2003;58:510-5. , 1414. Zeller T, Frank U, Müller C, Bürgelin K, Bestehorn HP, Cook-Bruns N, Schwarzwälder U, Neumann FJ. Predictors of improved renal function after primary stenting of severe atherosclerotic ostial renal artery stenosis. Circulation. 2003;108:2244-9. , 1515. Zeller T, Frank U, Müller C, Bürgelin K, Schwarzwälder U, Horn B, Sinn L, Roskamm H, Neumann FJ. Stent-supported angioplasty of severe atherosclerotic renal artery stenosis preserves renal function and improves blood pressure control: Long-term results of a prospective registry with 456 lesions. J Endovasc Ther. 2004; 11:95-106. ], two randomised studies proved the superiority of stenting over conventional balloon angioplasty [33. Plouin PF, Chatellier G, Darne B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: A randomized trial. The EMMA-study group. Hypertension. 1998;31:823-9. , 1616. van de Ven PJ, Kaatee R, Beutler JJ, Beek FJ, Woittiez AJ, Buskens E, Koomans HA, Mali WP. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomised trial. Lancet. 1999; 353:282-6. ] in the treatment of atherosclerotic ostial RAS, the most common manifestation of RAS. Nowadays, atherosclerotic RAS can be treated successfully in almost all cases with 1-year restenosis rates ranging from 0% to 23%, depending on the diameter of the renal artery, utilising pre-mounted low-profile stent devices [1616. van de Ven PJ, Kaatee R, Beutler JJ, Beek FJ, Woittiez AJ, Buskens E, Koomans HA, Mali WP. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomised trial. Lancet. 1999; 353:282-6. , 1717. Zeller T, Rastan A, Kliem M, Schwarzwälder U, Frank U, Bürgelin K, Schwarz T, Amantea P, Müller C, Neumann FJ. Impact of Carbon Coating on Restenosis Rate After Stenting of Atherosclerotic Renal Artery Stenosis. J Endovasc Ther. 2005;12:605-11. , 1818. Zeller T, Müller C, Frank U, Bürgelin K, Sinn L, Horn B, Roskamm H. Gold coating and restenosis after primary stenting of ostial renal artery stenosis. Catheter Cardiovasc Interv. 2003;60:1-6. , 1919. Sapoval M, Zähringer M, Pattynama P, Rabbia C, Vignali C, Maleux G, Boyer L, Szczerbo-Trojanowska M, Jaschke W, Hafsahl G, Downes M, Beregi JP, Veeger N, Talen A. Low-profile stent system for treatment of atherosclerotic renal artery stenosis: The GREAT Trial. J Vasc Interv Radiol. 2005;16:1195-202. , 2020. Lederman RJ, Mendelsohn FO, Santos R, Phillips HR, Stack RS, Crowley JJ. Primary renal artery stenting: characteristics and outcomes after 363 procedures. Am Heart J. 2001;142:314-23. , 2121. Jaff MR, Bates M, Sullivan T, Popma J, Gao X, Zaugg M, Verta P; HERCULES Investigators. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Catheter Cardiovasc Interv. 2012;80(3):343-50. ].
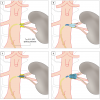
Atherosclerosis accounts for approximately 90% of the cases of RAS in developed nations with numbers increasing due to the ageing demographic of the population. Atherosclerotic RAS is most frequently caused by plaque formation in the aortic wall with progression into the renal artery origin resulting in the typical appearance of the eccentric ostial atherosclerotic RAS ( Figure 1 ). The prevalence of atherosclerotic RAS increases with age, particularly in patients with diabetes, hyperlipidaemia, diverse types of peripheral occlusive disease, coronary artery disease, or hypertension [2222. Harding MB, Smith LR, Himmelstein SI, Harrison K, Phillips HR, Schwab SJ, Hermiller JB, Davidson CJ, Bashore TM. Prevalence and associated risk factors in patients undergoing routine cardiac catheterization. J Am Soc Nephrol. 1992; 2:1608. , 2323. Missouris CG, Buckenham T, Cappuccio FP, MacGregor GA.Renal artery stenosis: A common and important problem in patients with peripheral vascular disease. Am J Med. 1994;96:10-4. ]. Atherosclerotic RAS is a progressive disease even in initially unaffected arteries with a wide range of 1-year occlusion rates from 1% to 18 % [2424. Zierler RE, Bergelin RO, Davidson RC, Cantwell-Gab K, Polissar NL, Strandness DE Jr. A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. Am J Hypertens. 1996; 9:1055-61. , 2525. van Jaarsveld BC, Krijnen P, Pieterman H, Derkx FH, Deinum J, Postma CT, Dees A, Woittiez AJ, Bartelink AK, Man in ‘t Veld AJ, Schalekamp MA. The Effect of Balloon Angioplasty on Hypertension in Atherosclerotic Renal-Artery Stenosis. Dutch Renal Artery Stenosis Interventional Cooperative Study Group. N Engl J Med. 2000;342:1007-14. , 2626. Tollefson DFJ, Emst CB. Natural history of atherosclerotic renal artery stenosis associated with aortic disease. J Vasc Surg. 1991;14:327-31. , 2727. Schreiber MJ, Pohl MA, Novick AC. The natural history of atherosclerotic and fibrous renal artery disease. Urol Clin North Am. 1984; 11:383-92. , 2828. Safak E, Wilke C, Derer W, Busjahn A, Gross M, Moeckel M, Mueller DN, Luft FC, Dechend R. Long-term follow-up of patients with atherosclerotic renal artery disease. J Am Soc Hypertens. 2013;7(1):24-31. ]. There is a close association between severity of RAS and kidney atrophy, which may lead to ischaemic nephropathy [2929. Rimmer JM, Gennari FJ. Atherosclerotic renovascular disease and progressive renal failure. Ann Intern Med. 1993; 118:712-9. , 3030. Caps MT, Zierler RE, Polissar NL, Bergelin RO, Beach KW, Cantwell-Gab K, Casadei A, Davidson RC, Strandness DE Jr. Risk of atrophy in kidneys with atherosclerotic renal artery stenosis. Kidney Int. 1998;53:735-42. ]. However, the loss of kidney filtration capacity in RAS is not only due to hypoperfusion, but also because of recurrent microembolism and possibly other mechanisms resulting in progressive kidney fibrosis. The exact aetiology of the development of ischaemic nephropathy is therefore still uncertain [3131. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-42. , 3232. Odudu A, Vassallo D, Kalra PA. From anatomy to function: diagnosis of atherosclerotic renal artery stenosis. Expert Rev Cardiovasc Ther. 2015 Dec;13(12):1357-75. ].
Renal artery stenosis
- Atherosclerosis is the most frequent aetiology of RAS (approximately 90%) with preferentially ostial location
- Stenting has become the therapy of choice with annual restenosis rates of about 10% with current devices
- Fibromuscular dysplasia, the second most frequent cause of RAS affecting mostly young female patients, is primarily treated with balloon angioplasty only
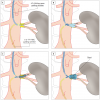
FMD, the second most frequent aetiology of RAS, is a collection of vascular diseases that affects the three layers of the arterial vessel wall: intima, media, and adventitia, and is of unknown aetiology. FMD accounts for less than 10% of cases of RAS. FMD tends to affect mainly younger women, is located at the distal half of the renal artery trunk or the side branches, and the most commonest medial dysplasia type is characterised by a beaded, aneurysm-like appearance on angiography ( Figure 2). Intimal fibroplasia, a less common type of FMD, is localized close to the renal artery origin and might mimic atherosclerotic RAS [3333. Mousa AY, Campbell JE, Stone PA, Broce M, Bates MC, AbuRahma AF. Short- and long-term outcomes of percutaneous transluminal angioplasty/stenting of renal fibromuscular dysplasia over a ten-year period. J Vasc Surg. 2012 Feb;55(2):421-7. ]. FMD rarely leads to vessel occlusion or ischaemic nephropathy [3131. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-42. , 3434. Sang CN, Whelton PK, Hamper UM, Connolly M, Kadir S, White RI, Sanders R, Liang KY, Bias W. Etiologic factors of renovascular fibromuscular dysplasia : a case-controlled study. Hypertension. 1989;14:472-9. ].
CLINICAL MANIFESTATIONS OF RENAL ARTERY STENOSIS
Arterial hypertension
Hypoperfusion of the kidney activates the renin-angiotensin- aldosterone system (the Goldblatt phenomenon) causing classic reno-vascular hypertension primarily in young patients with FMD [3131. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-42. , 3434. Sang CN, Whelton PK, Hamper UM, Connolly M, Kadir S, White RI, Sanders R, Liang KY, Bias W. Etiologic factors of renovascular fibromuscular dysplasia : a case-controlled study. Hypertension. 1989;14:472-9. , 3535. Border WA, Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31:161-88. ]. However, in patients with atherosclerosis, RAS induces an acceleration of a pre-existing essential hypertension, usually in bilateral stenotic kidney disease, occasionally leading to the development of the pathognomonic recurrent flash pulmonary oedema [3131. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-42. ]. Thus, in atherosclerotic RAS, renal artery revascularisation rarely cures hypertension but may improve blood pressure control. Uncontrolled hypertension may lead to organ dysfuction, such as heart failure, end-stage kidney disease, and aneurysms as well as acute decompensations with flash pulmonary oedema, hypertensive encephalopathy, and intracerebral haemorrhage [3131. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-42. , 3636. Zeller T. Renal artery stenosis: Epidemiology, clinical manifestation and percutaneous endovascular therapy. J Intervent Cardiol. 2005;18:497-506. , 3737. van den Berg DT, Deinum J, Postma CT, van der Wilt GJ, Riksen NP. The efficacy of renal angioplasty in patients with renal artery stenosis and flash oedema or congestive heart failure: a systematic review. Eur J Heart Fail. 2012;14(7):773-81. ].
Left ventricular dysfunction
Left ventricular hypertrophy has a substantial impact on morbidity and mortality [3838. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort: the Framingham Heart Study. Ann Intern Med. 1989;110:101-7. , 3939. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561-6. ]. The most common cause of left ventricular hypertrophy (and subsequent heart failure) is hypertension [4040. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557-62. , 4141. Vasan RS, Levy D. The role of hypertension in the pathogenesis of heart failure: a clinical mechanistic overview. Arch Intern Med. 1996;156:1789-96. ]. Hypoperfusion of the kidney activates the renin-angiotensin-aldosterone system [3131. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-42. , 3535. Border WA, Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31:161-88. ]. Aldosterone is not only a well-recognised promoter of hypertension [4242. Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, Benjamin EJ, Levy D. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:8-10. ] but is also associated with myocardial fibrosis and with left ventricular remodelling in heart failure patients leading to left ventricular hypertrophy [4343. Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, Murabito JM, Sam F, Colucci WS, Wilson PW. Relations of serum aldosterone to cardiac structure: gender-related differences in the Framingham Heart Study. Hypertension. 2004;43:957-62. ]. Left ventricular fibrosis and hypertrophy lead initially to diastolic dysfunction and in the long run to systolic dysfunction.
Renal insufficiency
Ischaemic kidney dysfunction may be caused by severe bilateral RAS or by unilateral stenosis in a functional single kidney [3131. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-42. ]. Renal function is also similarly impaired in the case of unilateral RAS with two preserved kidneys: here renal insufficiency is not only caused by hypoperfusion of the affected kidney but also by structural (mostly bilateral) kidney disease potentially induced by chronic hypertension and/or diabetes mellitus (nephrosclerosis) [3131. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-42. , 4444. Shanly PF. The pathology of chronic renal ischemia. Semin Nephrol. 1996;16:21-32. , 4545. Dean RH, Tribble RW, Hansen KJ, O’Neil E, Craven TE, Redding JF 2nd. Evolution of renal insufficiency in ischaemic nephropathy. Ann Surg. 1991;213:446-55. , 4646. Koivuviita N, Liukko K, Kudomi N, Oikonen V, Tertti R, Manner I, Vahlberg T, Nuutila P, Metsärinne K. The effect of revascularization of renal artery stenosis on renal perfusion in patients with atherosclerotic renovascular disease. Nephrol Dial Transplant. 2012;27(10):3843-8. , 4747. Eirin A, Ebrahimi B, Zhang X, Zhu XY, Tang H, Crane JA, Lerman A, Textor SC, Lerman LO. Changes in glomerular filtration rate after renal revascularization correlate with microvascular hemodynamics and inflammation in Swine renal artery stenosis. Circ Cardiovasc Interv. 2012;5(5):720-8. ].
Clinical consequences and aim of renovascular intervention
- Clinical consequences of atherosclerotic RAS include deterioration of pre-existing essential hypertension, impairment of myocardial function (both diastolic and systolic), and, in the presence of bilateral RAS or RAS of a single functional kidney, renal insufficiency and flash pulmonary oedema
- Revascularisation of significant RAS might result in improved blood pressure control, reduction of left ventricular hypertrophy and stabilised renal function
Pre-interventional diagnostic strategy
Prior to any renal intervention a proper patient evaluation is mandatory. This should include a complete cardiovascular history and physical examination, a comorbid diseases-based determination of potential life expectancy, a 24-hour blood pressure monitoring, a renal duplex scan and the measurement of renal function. A key diagnostic tool for determining the haemodynamic relevance of a given RAS is duplex ultrasound. Colour-coded duplex ultrasound is a non-invasive frequently repeatable bedside examination and is currently the only non-invasive diagnostic method to differentiate reliably between a haemodynamically relevant or irrelevant stenosis using the side-to-side difference of the intrarenal resistance index (RI). There is a highly specific correlation between a side difference of the RI of > 0.05 and an at least 70% angiographic diameter stenosis [4848. Zeller T, Frank U, Späth M. Detection of Renal Arteries and Classification of Haemodynamically Relevant Renal Artery Stenoses by Colour Coded Doppler Ultrasound. [article in German] Zeitschr Ultraschall Med. 2001;22:116-21. , 4949. Zeller T, Bonvini RF, Rastan A, Sixt S. Colour-Coded Duplex Ultrasound for Diagnosis of Renal Artery Stenosis and as Follow-up Examination after Revascularization. Catheter Cardiovasc Interv. 2008;71:995-9. , 5050. Noory E, Rastan A, Beschorner U, Macharzina R, Zeller T. Duplex derived intrarenal resistance index correlates with invasive pressure gradient measurements in detecting relevant unilateral renal artery stenosis. Vasa. 2016 Apr;45(2):175-80. ]. The same is true for an extended acceleration time of more than 70 ms. Other duplex parameters such as a peak systolic flow velocity > 200 cm/sec or a renal aortic flow velocity ratio (RAR) > 3.5 are correlated to a 50% or 60% angiographic diameter stenosis and therefore offer a high sensitivity (> 90%) in terms of detecting a RAS [5151. Strandness E. Duplex imaging for the detection of renal artery stenosis. Am J Kidney Dis. 1994;24:674-8. , 5252. Berland LL, Koslin DB, Routh WD, Keller FS. Renal artery stenosis: prospective evaluation of diagnosis with color duplex US compared with angiography. Radiology. 1990;174:421-3. ]. However, the specificity for detecting a haemodynamically relevant RAS is low (< 60%). Provided that duplex ultrasound is performed by an experienced physician with a suitable ultrasound machine, then it should be the preferred first-line imaging method. Alternatively, multislice CT may be used as it is the most accurate non-invasive structural imaging technique. A high RI at baseline (usually defined as > 0.8) is associated with a reduced probability of clinical improvement following RAS revascularisation but does not exclude treatment benefit in the presence of a significant RAS [4242. Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, Benjamin EJ, Levy D. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:8-10. , 5353. Li JC, Xu ZH, Yuan Y, Zhang YX, Wang L, Cai S, Wang YH, Jiang YX. Impact of atherosclerosis and age on Doppler sonographic parameters in the diagnosis of renal artery stenosis. J Ultrasound Med. 2012;31(5):747-55. , 5454. AbuRahma AF, Srivastava M, Mousa AY, Dearing DD, Hass SM, Campbell JR, Dean LS, Stone PA, Keiffer T. Critical analysis of renal duplex ultrasound parameters in detecting significant renal artery stenosis. J Vasc Surg. 2012;56(4):1052-9. , 5555. Radermacher J, Chavan A, Bleck J, Vitzthum A, Stoess B, Gebel M, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med 2001;44:410-417. ].
Although evaluation with conventional invasive contrast angiography is still considered to be the gold standard, duplex ultrasound, MR-angiography and CT-angiography are more frequently used for the assessment of renal arteries. The angiographic degree of diameter stenosis is measured as the ratio of the minimal lumen diameter (MLD) to the reference vessel diameter (REF): % diameter stenosis = (MLD/REF) x 100 [5656. Rees CR, Palmaz JC, Becker GJ, Ehrman KO, Richter GM, Noeldge G, Katzen BT, Dake MD, Schwarten DE. Palmaz stent in atherosclerotic stenoses involving the ostia of the renal arteries: preliminary report of a multicenter study. Radiology. 1991;181:507-14. ]. However, angiography is invasive and carries the potential risk of haematoma, pseudoaneurysm, contrast- induced nephropathy and atheromatous embolisation [5757. Zeller T, Frank U, Müller C, Bürgelin K, Schwarzwälder U, Sinn L, Horn B, Roskamm H, Neumann FJ. Technological Advances in the Design of Catheters and Devices Used in Renal Artery Interventions: Impact on complications. J Endovasc Ther. 2003;10:1006-14. ]. Moreover, the accuracy of this 2-dimensional diagnostic method remains questionable. CT-angiography is similarly limited by the potential for contrast-induced nephropathy and radiation exposure and is thus not the ideal method for serial follow-up examinations after revascularisation of RAS. MR-angiography has recently been implicated in causing nephrogenic systemic sclerosis, especially in patients with chronic kidney disease stage 3 and higher according to the K/DOQI classification [5858. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002; 39:S1-S266. ]. Furthermore, MR-angiography is an invalid tool for follow-up examinations after stent placement due to stent artefacts.
In recent years, measuring the translesional pressure gradient with a dedicated pressure wire has been described to identify a significant RAS. A distal-to-the-lesion to aortic pressure ratio (FFR) at rest of < 0.9 was linked to an up-regulation of renin production [5959. De Bruyne B, Manoharan G, Pijls NH, Verhamme K, Madaric J, Bartunek J, Vanderheyden M, Heyndrickx GR. Assessment of renal artery stenosis severity by pressure gradient measurements. J Am Coll Cardiol. 2006; 48:1851-5. ].This ratio correlates to a papaverine-induced hyperemic systolic pressure gradient of >21 mmHg [6060. Rimmer JM, Gennari Kapoor N, Fahsah I, Karim R, Jevans AJ, Leesar MA. Physiological assessment of renal artery stenosis: comparisons of resting with hyperemic renal pressure measurements. Catheter Cardiovasc Interv. 2010;76:726-32. ]. A dopamine-induced mean pressure gradient of >20 mmHg predicted a beneficial blood pressure response to renal stenting [6161. Mangiacapra F, Trana C, Sarno G, Davidavicius G, Protasiewicz M, Muller O, Ntalianis A, Misonis N, Van Vlem B, Heyndrickx GR, De Bruyne B. Translesional pressure gradients to predict blood pressure response after renal artery stenting in patients with renovascular hypertension. Circ Cardiovasc Interv. 2010;3:537-42. ]. ]. In another recent study, a FFR < 0.9 was significantly correlated with a side difference of intrarenal RI of < 0.05 for the affected kidney [5050. Noory E, Rastan A, Beschorner U, Macharzina R, Zeller T. Duplex derived intrarenal resistance index correlates with invasive pressure gradient measurements in detecting relevant unilateral renal artery stenosis. Vasa. 2016 Apr;45(2):175-80. ].
Another invasive parameter for diagnosing a significant RAS is the „renal frame count, RFC“ which is defined as the number of angiographic images during an angiographic run until the complete filling of the kidney vessel tree [6262. Mahmud E, Smith TW, Palakodeti V, Zaidi O, Ang L, Mitchell CR, Zafar N, Bromberg-Marin G, Keramati S, Tsimikas S. Renal frame count and renal blush grade: quantitative measures that predict the success of renal stenting in hypertensive patients with renal artery stenosis. JACC Cardiovasc Interv. 2008;1:286-92. ]. A RFC of >30 was predictive for a significant reduction of blood pressure and patient condition in the presence of an angiographic > 70% diameter stenosis [6363. Naghi J, Palakodeti S, Ang L, Reeves R, Patel M, Mahmud E. Renal frame count: a measure of renal flow that predicts success of renal artery stenting in hypertensive patients. Catheter Cardiovasc Interv. 2015 Aug;86(2):304-9. ].
Imaging modalities and renal artery stenosis
- The key to clinical success in endovascular treatment of RAS is the appropriate patient selection in terms of correctly identifying a haemodynamically relevant RAS
- Duplex ultrasound as a non-invasive modality is the most reliable functional diagnostic tool to detect severe RAS
- The measurement of pressure gradients at rest and after pharmacological stress with drugs such as adenosine and dopamine (FFR) is reserved as a preprocedural diagnostic technique in angiographically equivocal lesions
TECHNICAL ASPECTS OF RENOVASCULAR STENTING [6464. Zeller T. Endovascular treatment of renal artery stenosis. In: Marco J, Serruys P, Biamino G, Fajadet J, de Feyter P, Morice MC: The Paris Course on Revascularisation. 2004:387-415. ]
Arterial access
Femoral approach
The femoral access site is the standard approach for renovascular interventions with a few exceptions, such as untreated severe stenosis/occlusion of the pelvic arteries or abdominal aorta, severe kinking of the pelvic arteries or an acute angle of the renal artery. Using the right femoral approach for right RAS and the left femoral approach for left RAS is often helpful, resulting in a good adaptation of the guiding catheter to the anatomy of the pelvic artery and aorta ( Figure 3 ).
The “guide catheter technique” is recommended ( Figure 3 ) requiring a 6 Fr or 7 Fr sheath with a haemostatic valve and a 55 cm guide catheter. Usually a standard sheath with a length of 11 cm is used (various manufacturers, e.g., Avanti™; J&J Cordis Corp., Miami, FL, USA, or Terumo Corp., Leuven, Belgium). In tortuous iliac arteries the use of a longer sheath (23 cm, various manufacturers) for better manipulation of catheters and wires can be helpful. Alternatively, a pre-shaped sheathless guide catheter can be used. Hereby the device diameter is downsized by 1 Fr (5 Fr instead of 6 Fr) and the risk of access site complications may be reduce.
Brachial approach
Both a left and a right brachial approach can be used. However, the left brachial access is safer because only one brain-perfusing artery (the left vertebral artery) has to be passed with a wire or catheter. Most frequently a 6 Fr, or less frequently a 7 Fr sheath, is introduced into the brachial artery via the antecubital fossa using a micro puncture set. As for the femoral approach, the use of a guiding catheter (90 cm length) is recommended. The most used catheter types are the “right Judkins 4”, the “right Amplatz 1”, and the “multipurpose” ( Figure 4 [A] and Figure 4 [C]). Alternatively, a 90 cm long straight 6 Fr sheath or a multipurpose shaped guiding sheath can be positioned proximal to the origin of the renal artery.
Arterial access for renovascular intervention
The femoral access for renal artery intervention is most frequently used. In variant take-off anatomies a brachial or even a radial access has to be used
Renal artery angiography
In a non-tortuous aorta, the origins of right and left renal arteries are often best identified in a 20° left anterior oblique (LAO) projection since the right renal artery take-off is slightly anterior (10 to 11 o’clock) and that of the left renal artery is slight posterior (four to five o’clock) ( Figure 5 ). However, in up to 15% there might exist variations. Semi-selective and selective baseline angiographic acquisition should be performed to determine the optimal projection for the intervention. Conventional angiography sometimes allows a better visualisation of the lesion and the anatomy compared to the digital subtracted technique (DSA) due to potential movement artefacts of DSA. Prior to the first selective renal angiography, the guiding catheter should be cleaned from debris by passive back bleeding of the catheter or aspiration of 10 cc of blood via a Y-connector (“proximal protection”) ( Figure 6 ). This technique reduces the risk of renal embolism.
Renal ostial intervention
For ostial lesions the appropriate angulated view of the C-arm is essential to identify correctly the most severe lesion area and the exact position of the take-off of the renal artery to guarantee a correct stent position
Technique of renal artery stenting
For the femoral approach a variety of guiding catheter configurations such as “renal double curve, RDC”, “hockey stick”, “right Amplatz, AR-1”, “right Judkins, JR-4” or “internal mammary, IMA” are available to guarantee a stable position proximal to the renal artery origin. The guide catheter technique ( Figure 7 [A] and Figure 7 [D]) is the fastest technique with the lowest intervention and radiation exposure time. After placing the guide catheter close to the renal artery origin, a steerable extra support 0.014 inch guidewire with a flexible tip should be used to cross the lesion. Some authors recommend a so-called “no-touch technique” ( Figure 8 [A] and Figure 8 [D]) [6565. Feldman RL, Wargovich TJ, Bittl JA. No-touch technique for reducing aortic wall trauma during renal artery stenting. Catheter Cardiovasc Interv. 1999;46:245-8. ]. This technique requires a 1 Fr upsized guide catheter diameter (7 Fr) to allow the placement of one 0.014 inch guidewire in the renal artery and a second 0.014 inch guidewire in the aorta that keeps the tip of the guiding catheter away from direct contact with the aortic wall or renal artery origin respectively.
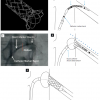
When using the femoral access it can be difficult to introduce a guidewire in renal arteries with an acutely angled off-take. The telescoping technique can solve this access problem. The renal artery orifice can be cannulated with a 5 Fr sidewinder configured diagnostic catheter advanced through the lumen of a 7 Fr guiding catheter. After introduction of the guidewire through the SOS catheter into the renal artery, the guiding catheter is advanced over the diagnostic catheter close to the orifice of the renal artery and the diagnostic catheter is pulled out thereafter ( Figure 9 [A], Figure 9 [D], Figure 10 [A] and Figure 10 [D]).
For renal artery revascularisation via the brachial approach, various shapes of 6 Fr guiding catheters such as “JR-4”, “AR-1” or “multipurpose” are available ( Figure 1 and Figure 4 ). As an alternative to a guiding catheter, a 90 cm 6 Fr sheath can be positioned near the origin of the renal artery. Engagement of the renal artery has to be performed with a 4 Fr or 5 Fr diagnostic catheter of various shapes (“multipurpose” or others) to introduce a long 0.014 inch guidewire (300 cm) through the lesion into the renal artery. After removal of the 5 Fr diagnostic catheter the stent can be placed.
For RAS located in renal arteries with either a short main trunk or a common origin of two renal arteries without a common trunk ( Figure 11 and Figure 12 ) the use of a kissing balloon technique is recommended. There are two options: using a single guiding catheter technique with one 8 Fr guiding catheter coming via a femoral access ( Figure 11 ) or using two 6 Fr guiding catheters, one introduced through a femoral access and the second guiding catheter introduced via a brachial access ( Figure 12 ).
In the presence of a short main trunk, a stent of appropriate length could be placed, just covering the main trunk, which is then secondarily flared with two balloons into the two branches ( Figure 11). Alternatively, the smaller side branch could be jailed with the stent secondarily opening the stent struts with a kissing balloon angioplasty as shown in Figure 12 .
Usually an extra-support 0.014 inch guidewire with soft tip (various manufacturers) is used. In the case of highly calcified tight lesions, predilatation should be performed starting with a 3 mm followed by a 5 mm monorail balloon. If the 5 mm balloon does not open up completely, a cutting balloon (Boston Scientific) or a scoring balloon (Angiosculpt™; Biotronik, Bülach, Switzerland) may be used for plaque preparation to ensure that full stent expansion is possible. Pre-interventional pressure gradient measurement can be performed in equivocal cases, but is not recommended as routine procedure because a reliable determination of the gradient requires a pressure wire, which adds to cost and can be time-consuming. However, while the measurement of resting pressure gradients was not predictive for the blood pressure response [6666. Mitchell J, Subramanian R, White C, Soukas P, Almagor Y, Stewart R, Rosenfield K. Predicting blood pressure improvement in hypertensive patients after renal artery stent placement. Catheter Cardiovasc Interv. 2007;69:685-9. ], Leesar et al have found that the best correlation with blood pressure response after successful revascularisation is the baseline hyperaemic translesional pressure gradient [6767. Leesar MA, Varma J, Shapira A, Fahsah I, Raza ST, Elghoul Z, Leonard AC, Meganathan K, Ikram S. Prediction of hypertension improvement after stenting of renal artery stenosis: comparative accuracy of translesional pressure gradients, intravascular ultrasound, and angiography. J Am Coll Cardiol. 2009;53:2363-71. ]. In 62 patients with RAS who also had angiographic and intravascular ultrasound (IVUS) measurements performed, they found that a hyperaemic systolic gradient (HSG) of > 21 mmHg measured with a 0.014 inch pressure wire following intra-renal injection of 30 mg of papaverine was the best predictor of improved blood pressure response after revascularisation. Hypertension improvement at one year occurred in 84% of those with HSG ≥ 21 mmHg compared to 36% with HSG < 21 mmHg (p < 0.01). These results need to be confirmed in a larger series of patients including clinical endpoints. De Bruyne et al [5959. De Bruyne B, Manoharan G, Pijls NH, Verhamme K, Madaric J, Bartunek J, Vanderheyden M, Heyndrickx GR. Assessment of renal artery stenosis severity by pressure gradient measurements. J Am Coll Cardiol. 2006; 48:1851-5. ] found an increase in ipsilateral renal vein renin release starting with a distal-to-the-lesion to aortic systolic pressure ratio of 0.9 and below.
Mahmud et al reported that hypertensive patients with RAS have decreased renal perfusion as measured by renal frame counts (RFC) and renal blush grade (RBG) [6262. Mahmud E, Smith TW, Palakodeti V, Zaidi O, Ang L, Mitchell CR, Zafar N, Bromberg-Marin G, Keramati S, Tsimikas S. Renal frame count and renal blush grade: quantitative measures that predict the success of renal stenting in hypertensive patients with renal artery stenosis. JACC Cardiovasc Interv. 2008;1:286-92. ]. In a series of patients they were able to demonstrate separation between hypertensive patients without RAS (RFC = 20.1 ± 5.4) from hypertensive patients with RAS (RFC = 26.6 ± 9.1) that returned towards the control group values following stent placement (RFC = 21.4 ± 6.7). Clinical responders (systolic blood pressure fell by ≥ 15 mmHg after revascularisation) tended to have higher baseline RFC’s as compared to non-responders and had significantly greater improvement in their RFC values following stenting. Three quarters of the blood pressure responders had a baseline RFC ≥ 25 and, of those patients with an improvement in the RFC > 4, 79% were responders to revascularisation. RFC adds a functional assessment to the morphologic assessment of RAS. The application of RBG as an indicator of microvascular flow will be helpful for evaluating the efficacy of embolic protection devices.
For ostial lesions, balloon-expandable stents are standard. Several low-profile renal stent devices allow direct stenting in the vast majority of cases. For post-ostial lesions short self-expanding nitinol stents can be used as an alternative (4 Fr shaft devices can be introduced via a 5 Fr or 6 Fr guiding catheter). The balloon or stent to vessel ratio should be 1.1:1 for balloon-expandable stents and in general 1 mm oversized for self-expanding stents. Overexpansion of the stent compared to the vessel diameter should not exceed more than 0.5 mm due to the potential risk of vessel perforation or dissection. For full stent expansion dilatation time should be at least 20 to 30 seconds. Stent length should be kept as short as possible to cover the entire lesion but not more. Of note, especially in renal arteries supplying “hyper-mobile” kidneys, stent fractures with consecutive acute thrombotic vessel occlusions have recently been reported.
A stent device dedicated to ostial stenting is available ( Figure 13 and Figure 14 ). This dual-balloon delivery system enables rapid, precise placement of the stent with the initially inflated locator balloon, which physically stops at the ostium and visually confirms the right position. Inflating the second balloon results in stent placement and, further increasing the pressure in the locator balloon, results in a flaring of the proximal stent end. The flared stent conforms anatomically to the aorto-ostial junction resulting in increased proximal scaffolding and ostial coverage. Thus the aorto-renal plaque is completely attached to the vessel wall and recrossing the stent in case of restenosis can easily be performed. A pilot study showed a 100% acute technical success rate.
The role of both drug-eluting and covered stents is still uncertain. Excellent outcome results have been reported for in-stent restenoses [6868. Zeller T, Rastan A, Schwarzwälder U, Müller C, Schwarz T, Frank U, Bürgelin K, Sixt S, Noory E, Beschorner U, Hauswald K, Branzan D, Neumann FJ. Treatment of instent restenosis following stent-supported renal artery angioplasty. Catheter Cardiovasc Interv. 2007;70:454-9. , 6969. Zeller T, Sixt S, Rastan A, Schwarzwälder U, Müller C, Noory E, Bürgelin K, Schwarz T, Hauswald K, Brantner R, Neumann FJ. Treatment of Reoccurring Instent Restenosis Following Reintervention after Stent-supported Renal Artery Angioplasty. Catheter Cardiovasc Interv. 2007;70:296-300. , 7070. Boateng FK, Greco BA. Renal artery stenosis: prevalence of, risk factors for, and management of in-stent stenosis. Am J Kidney Dis. 2013;61(1):147-60. ]. Another possible indication for both devices, in particular for drug-eluting stents, might be small renal artery diameters (< 5 mm) due to the higher risk of restenosis development in smaller renal arteries [1717. Zeller T, Rastan A, Kliem M, Schwarzwälder U, Frank U, Bürgelin K, Schwarz T, Amantea P, Müller C, Neumann FJ. Impact of Carbon Coating on Restenosis Rate After Stenting of Atherosclerotic Renal Artery Stenosis. J Endovasc Ther. 2005;12:605-11. , 1818. Zeller T, Müller C, Frank U, Bürgelin K, Sinn L, Horn B, Roskamm H. Gold coating and restenosis after primary stenting of ostial renal artery stenosis. Catheter Cardiovasc Interv. 2003;60:1-6. , 2020. Lederman RJ, Mendelsohn FO, Santos R, Phillips HR, Stack RS, Crowley JJ. Primary renal artery stenting: characteristics and outcomes after 363 procedures. Am Heart J. 2001;142:314-23. ]. The Formula Renal PTX trial by Cook Medical Corp., Bloomington, IN, USA, is investigating the performance of the paclitaxel-eluting Formula PTX™ stent compared to the bare metal Formula™ stent, data release is expected for 2018.
The role of distal protection devices for renal interventions is still a matter of debate. So far, no dedicated protection device for renal application is available [7171. Henry M, Klonaris C, Henry I, Tzetanov K, Le Borgne E, Foliguet B, Hugel M. Protected renal stenting with the PercuSurge GuardWire device: a pilot study. J Endovasc Ther. 2001;8:227-37. , 7272. Khosla A, Misra S, Greene EL, Pflueger A, Textor SC, Bjarnason H, McKusick MA. Clinical outcomes in patients with renal artery stenosis treated with stent placement with embolic protection compared with those treated with stent alone. Vasc Endovascular Surg. 2012;46(6):447-54. ]. The FiberNet® (Invatec) might be a safe and effective device for this indication; however, larger trials need to confirm its potential benefits. A small randomised trial using the Angioguard™ device (J&J Cordis) did result in better outcome of distal protected renal angioplasty in terms of improved renal function when combining the filter with a GPIIb/IIIa receptor antagonist (Reopro) [7373. Cooper CJ, Haller ST, Colyer W, Steffes M, Burket MW, Thomas WJ, Safian R, Reddy B, Brewster P, Ankenbrandt MA, Virmani R, Dippel E, Rocha-Singh K, Murphy TP, Kennedy DJ, Shapiro JI, D‘Agostino RD, Pencina MJ, Khuder S. Embolic protection and platelet inhibition during renal artery stenting. Circulation. 2008;117:2752-60. ].
Renovascular intervention
- In atherosclerotic RAS the guiding catheter technique is the safest, fastest and most effective interventional technique
- The key to the clinical success of the intervention is avoiding distal embolisation by using dedicated techniques such as “no-touch techniques”
- The role of distal protection devices and the use of GPIIb/IIIa receptor antagonists is still uncertain
- Measurement of resting pressure gradients and hyperaemic pressure gradients and the renal frame count might be helpful in determining significance of an angiographically borderline RAS
- A stent system dedicated for ostial lesions flaring the proximal end of the stent to the aortic wall improves re-engagement of the stent in case of in-stent restenosis
- Considering the acceptable low restenosis rates of bare metal stents, the role of drug-eluting stents and covered stents is still uncertain, randomised controlled trials with these devices are still on-going
Treatment of FMD
FMD lesions are most frequently located in the mid to distal segments of the renal artery trunk and in the side branches ( Figure 2 ) in some cases resulting in renal artery aneurysms ( medial dysplasia, Figure 15 ). The endovascular technique of choice is still plain old balloon angioplasty (POBA). However, recurrence rates after POBA might be higher than reported in the literature. Thus, cutting balloon angioplasty is becoming more and more popular. It is likely that the cutting balloon is more effective in disrupting the intraluminal membranes associated with FMD. However, use of this technology carries an increased risk of perforation. Thus, it is recommended strictly to avoid balloon oversizing. Using a cutting balloon undersized by 1 mm followed by post-dilatation with a standard balloon of a diameter matching the reference vessel diameter leads to excellent acute results ( Figure 2 ). Longer-term follow-up data has not, as yet, been published for this treatment technique. Cutting balloon angioplasty is the appropriate method for the ostial located intimal fibrodysplasia which is characterized by a shrunken hypoplastic vessel lumen which is frequently resistant to POBA. Direct stenting is contraindicated because of the risk of incomplete stent expansion without vessel preparation with a cutting or scoring balloon.
Endovascular treatment of renal artery aneurysms consists of coil embolisation, covered stent implantation and multilayer stent angioplasty, with the last being a new treatment modality under investigation.
Renovascular intervention for fibromuscular dysplasia
Balloon angioplasty is still considered the treatment of choice for FMD of the renal arteries. However, suboptimal acute results and restenosis might be more frequent as reported in the literature. Thus, the use of focal force balloons such as the cutting balloon is under investigation in order to improve further the technical results of the endovascular treatment of renal FMD
Post-interventional result documentation
After each intervention, selective and semi-selective angiograms of the target lesion and the distal (intra-renal) arteries should be performed without a guidewire in place to document the final result and exclude peripheral embolism, dissection or perforation. Intravascular ultrasound is only indicated for study purposes. Treatment success is achieved if the residual diameter stenosis after angioplasty is less than 30%.
Post-interventional care
Antiplatelet medication after intervention is adapted from the coronary routine and consists of 100 mg of acetylsalicylic acid (ASA) once a day for life and 75 mg clopidogrel once a day (alternatively 250 mg ticlopidine twice daily) for four weeks [7474. Mousa AY, Broce M, Campbell J, Nanjundappa A, Stone PA, Abu-Halimah S, Srivastava M, Bates MC, Aburahma AF. Clopidogrel use before renal artery angioplasty with/without stent placement resulted in tertiary procedure risk reduction. J Vasc Surg. 2012;56(2):416-23. ]. Of note, there is no comparative controlled data for this regimen. No additional heparin has to be given after intervention. Short-term critical care monitoring may be indicated, especially in younger patients with an aetiology of RAS other than atherosclerosis, because of the risk of a sudden unpredictable fall in blood pressure following the renal artery revascularisation.
Sufficient volume replacement with iso-osmolar saline infusion, depending on the patient’s cardiac function, is recommended, particularly in patients with pre-existing impaired renal function to help prevent contrast-induced nephropathy. Monitoring of renal function and blood pressure is mandatory before discharge. The length of time for bed rest after the intervention depends on the sheath size used, the access location, and the method of vascular closure.
A pre-discharge duplex examination including the calculation of the RAR and the intrarenal RI as a baseline for further follow-up examinations is mandatory. A pre-discharge ambulatory 24-hour blood pressure assessment is recommended.
Renovascular post-intervention management
- Careful documentation of the final interventional results after wire retrieval (‘’wire out shot’’) is essential for the detection of more distally located complications such as perforations or embolism
- Post-interventional drug therapy is adapted from coronary guidelines for bare metal stent deployment, recommending dual antiplatelet therapy for at least 4 weeks
- Pre-discharge diagnostic evaluation should consist of duplex ultrasound to confirm vessel patency, measurement of serum creatinine ideally earliest 48 hours after the procedure in order to detect deterioration of renal function as a result of embolism or contrast-induced nephropathy. Moreover, 24-hour ambulatory measurement of the blood pressure should be performed
- Outpatient follow-up procedures should be recommended 6 and 12 months after the procedure and annually thereafter
- About 80% of in-stent restenoses develop during the first 6 months following the intervention
SUMMARY
Even if randomised controlled trials [7575. Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, Braam B, Huysmans FT, Schultze Kool LJ, Rutten MJ, Doorenbos CJ, Aarts JC, Rabelink TJ, Plouin PF, Raynaud A, van Montfrans GA, Reekers JA, van den Meiracker AH, Pattynama PM, van de Ven PJ, Vroegindeweij D, Kroon AA, de Haan MW, Postma CT, Beutler JJ. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function. Ann Intern Med. 2009;150:840-8. , 7676. ASTRAL Investigators, Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J. Revascularization versus Medical Therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953-62. , 7777. Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB, Dworkin LD, for the CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370: 13–22. ] have not shown any clinical benefit for RAS stenting over medical treatment, there remain accepted indications for renal stenting in specific patient subsets. These studiesincluded a significant number (more than 50%) of patients without severe stenoses likely causing inconclusive results in overall underpowered studies, in particular the STAR trial. In ASTRAL, the patient selection criterion was “patients should be randomised if it is uncertain whether to revascularise”. Moreover, 49% of this study population had a < 70% angiographic diameter stenosis and a high selection bias was present. Thus, the only firm conclusion of ASTRAL is that patients with an equivical clinical indication might not profit from revascularisation. Very similar limitations characterise the CORAL trial with a majority of patients enrolled without having been diagnosed with a > 70% diameter stenosis or well controlled hypertension. . A recent Cochrane analysis of all randomized controlled trials of balloon angioplasty with and without stenting compared to best medical therapy identified the reduction of diastolic blood pressure as the only significant benefit of revascularization of RAS [7878. Jenks S, Yeoh SE, Conway BR. Balloon angioplasty, with and without stenting, versus medical therapy for hypertensive patients with renal artery stenosis. Cochrane Database Syst Rev. 2014;12:CD002944. ].The unanswered question that remains is what would be the interventional outcome of patients excluded from the trial? The answer could have been provided by the RADAR trial [7979. Schwarzwälder U, Hauk M, Zeller T. RADAR - A randomised, multi-centre, prospective study comparing best medical treatment versus best medical treatment plus renal artery stenting in patients with haemodynamically relevant atherosclerotic renal artery stenosis. Trials. 2009;10:60. ] which only selected patients with haemodynamically relevant stenosis based on duplex ultrasound examination (either showing a side difference in RI > 0.05 in unilateral RAS or a bilateral increase in acceleration time > 100 msec in bilateral RAS). However, unfortunately this trial was terminated, after having enrolled one third of the patients, due to lack of financial support and slow enrolment rates..
Proper patient selection and a safe interventional technique are the key elements of a clinically successful outcome following a renovascular procedure. In particular, patients with a short history of deterioration of renal function might have the greatest benefit from intervention. In patients with an advanced stage of renal failure the indication for stenting of atherosclerotic RAS must be determined individually for each patient considering the overall prognosis. In a recent prospective dual-centre analysis of real world registries, including 908 patients with significant RAS either treated conservatively or with angioplasty, a substantial improvement of renal function was observed in patients with CKD stage 2 to 5 and a significantly reduced 1-year mortality (by 45%) in those patients who underwent revascularisation [8080. Kalra PA, Chrysochou C, Green D, Cheung CM, Khavandi K, Sixt S, Rastan A, Zeller T. The benefit of renal artery stenting in patients with atheromatous renovascular disease and advanced chronic kidney disease. Catheter Cardiovasc Interv. 2010;75:1-10. ]. Moreover, a recent prospective cohort study identified patients suffering from heart failure in the presence of a RAS benefiting from renal artery revascularization in terms of a significantly increased survival rate [8181. Green D, Ritchie JP, Chrysochou C, Kalra PA. Revascularisation of renal artery stenosis as a therapy for heart failure: an observational cohort study. Lancet. 2015 Feb 26;385 Suppl 1:S11. ]. One underlying pathological mechanism for improved survival could be the regression of left ventricular mass following renal stenting [8282. Marcantoni C, Zanoli L, Rastelli S, Tripepi G, Matalone M, Mangiafico S, Capodanno D, Scandura S, Di Landro D, Tamburino C, Zoccali C, Castellino P. Effect of renal artery stenting on left ventricular mass: a randomized clinical trial. Am J Kidney Dis. 2012;60(1):39-46. ].
Evidence base for renovascular intervention
- Recent randomised controlled trials suggest no benefit from stenting of RAS over medical therapy only. However, these trials had substantial limitations in study design and patient selection and cannot be applied to any patient presenting with RAS
- In patients with RAS of aetiology other than atherosclerosis, endovascular treatment is still the treatment of choice with a high probability of curing hypertension
- In selected patients with severe atherosclerotic RAS, clinical benefits have been demonstrated in terms of improved renal function, blood pressure control and regression of left ventricular hypertrophy
- Proper patient selection and safe interventional techniques are key issues for the clinical success of the endovascular treatment of RAS
Mesenteric artery intervention
INTRODUCTION
Symptomatic chronic mesenteric ischaemia (CMI) is an uncommon, potentially under-diagnosed condition caused by fixed stenoses or occlusions of in most cases at least two visceral arteries. CMI is, independent of the aetiology, characterised by frequently unrecognised nonspecific symptoms such as diffuse postprandial abdominal pain, diarrhoea induced by ischaemic enteritis, and unintended weight loss [8383. Jarvinen O, Laurikka J, Sisto T, Salenius JP, Tarkka MR. Atherosclerosis of the visceral arteries. VASA. 1995;24:9-14. , 8484. Thomas JH, Blake K, Pierce GE, Hermreck AS, Seigel E. The clinical course of asymptomatic mesenteric arterial stenosis. J Vasc Surg. 1998;27:840-4. , 8585. van Bockel JH, Geelkerken RH, Wasser MN. Chronic splanchnic ischemia. Best Pract Res Clin Gastroenterol. 2001;15:99-119. , 8686. Babu SC, Shah PM. Celiac territory ischaemic syndrome in visceral artery occlusion. Am J Surg. 1993;166:227-30. ]. Due to the nonspecific symptoms it often takes years until the correct diagnosis is established [8787. Liberski SM, Koch KL, Atnip RG, Stern RM. Ischaemic gastroparesis: resolution after revascularisation. Gastroenterology. 1990;99:252-7. ].
Stenosis of one or even two visceral vessels is usually well tolerated because of the abundant collateral circulation between the coeliac trunk, the superior mesenteric artery, and the inferior mesenteric artery (the last connected via branches of the internal iliac arteries).
Atherosclerosis is the predominant cause of CMI (95%). Typically, patients affected by CMI have diffuse atherosclerotic vascular disease including coronary artery disease. Non-atherosclerotic causes of CMI such as fibromuscular disease, coeliac artery compression syndrome (compression of the coeliac trunk by the arcuate ligament called Dunbar Syndrome) and inflammation will not be discussed in detail in this chapter.
Mesenteric vascular disease
- Whereas acute mesenteric ischaemia can be diagnosed relatively easily and is still a major indication for surgical repair, chronic mesenteric disease remains a diagnostic challenge. Even if there are typical clinical signs that should lead to the right diagnosis, this is frequently delayed due to a wide variety of more frequent differential diagnoses
- Atherosclerosis is the leading cause of the disease and at least 2 out of the 3 mesenteric arteries must be affected to result in clinical symptoms
Clinical presentation
Patients with CMI usually present with abdominal angina, a clinical syndrome characterised by painful abdominal cramps (“abdominal angina”) and colic occurring typically during the postprandial phase [8383. Jarvinen O, Laurikka J, Sisto T, Salenius JP, Tarkka MR. Atherosclerosis of the visceral arteries. VASA. 1995;24:9-14. , 8484. Thomas JH, Blake K, Pierce GE, Hermreck AS, Seigel E. The clinical course of asymptomatic mesenteric arterial stenosis. J Vasc Surg. 1998;27:840-4. , 8585. van Bockel JH, Geelkerken RH, Wasser MN. Chronic splanchnic ischemia. Best Pract Res Clin Gastroenterol. 2001;15:99-119. ]. Symptoms usually start about 20 minutes following ingestion and may last up to 3 hours. Patients may suffer from ischaemic gastropathy and enteropathy, a condition characterised by the fear of food, nausea, vomiting, diarrhoea, symptoms of malabsorption, and unintended progressive weight loss [8686. Babu SC, Shah PM. Celiac territory ischaemic syndrome in visceral artery occlusion. Am J Surg. 1993;166:227-30. , 8787. Liberski SM, Koch KL, Atnip RG, Stern RM. Ischaemic gastroparesis: resolution after revascularisation. Gastroenterology. 1990;99:252-7. ]. Even if rarely seen, CMI can develop into acute mesenteric ischaemia over time (“acute upon chronic visceral ischaemia“) [8484. Thomas JH, Blake K, Pierce GE, Hermreck AS, Seigel E. The clinical course of asymptomatic mesenteric arterial stenosis. J Vasc Surg. 1998;27:840-4. ].
Clinical manifestation of chronic mesenteric ischaemia
Depending on the collateral circulation and lesion location, CMI can mimic different kinds of gastric and enteric disorders. Typically, symptoms are related to food intake associated with unintended weight loss
Natural history
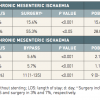
The incidence of CMI in the general population is approximately 1/100,000 per year [8888. Taylor LM, Moneta GM. Intestinal ischemia. Ann Vasc Surg. 1991;5:403-6. ]. In patients with known atherosclerotic disease, the prevalence of asymptomatic and symptomatic CMI may range from 8% to 70% (based on data from clinical studies and autopsy series respectively) and a greater than 50% stenosis of more than one splanchnic artery may be detected in up to 15% of cases [8989. Kihara TK, Blebea J, Anderson KM, Friedman D, Atnip RG. Risk factors and outcomes following revascularization for chronic mesenteric ischemia. Ann Vasc Surg. 1999;13:37-44. ]. In patients with abdominal aortic aneurysm, aorto-iliac occlusive disease and lower extremity vascular disease, a significant stenosis of at least one of the three visceral arteries may be found in 40%, 29%, and 25% of the cases, respectively. Predisposing conditions for the development of CMI include arterial hypertension, diabetes mellitus, and hypercholesterolaemia. Untreated, symptomatic CMI may lead to starvation, bowel infarction, and death. Even in the absence of symptoms, CMI is associated with a cardiovascular mortality rate of up to 40% within 6 years depending on the severity of the disease [8989. Kihara TK, Blebea J, Anderson KM, Friedman D, Atnip RG. Risk factors and outcomes following revascularization for chronic mesenteric ischemia. Ann Vasc Surg. 1999;13:37-44. , 9090. Mensink PB, van Petersen AS, Geelkerken RH, Otte JA, Huisman AB, Kolkman JJ. Clinical significance of splanchnic artery stenosis. Br J Surg. 2006;93:1377-82. , 9191. Moawad J, Gewertz BL. Chronic mesenteric ischemia. Clinical presentation and diagnosis. Surg Clin North Am. 1997;77:357-69. , 9292. Wilson DB, Mostafavi K, Craven TE, Ayerdi J, Edwards MS, Hansen KJ. Clinical course of mesenteric artery stenosis in elderly Americans. Arch Intern Med. 2006;166:2095-100. ]. The likely explanation for this finding is that those patients invariably have concomitant advanced systemic atherosclerotic disease. A subgroup of 553 participants of the Cardiovascular Health Study underwent visceral duplex ultrasonography: 97 (17.5%) had disease of the coeliac trunk or superior mesenteric artery. At a mean follow-up of six and a half years, 20 participants with CMI (20.6%) and 93 without CMI (20.4%) had died (relative risk, 1.01; 95% confidence interval, 0.66-1.55). No deaths were attributed to intestinal infarction. No association existed between the presence of CMI and prevalent cardiovascular disease, all-cause mortality, or adverse cardiovascular events. Interestingly, no participant reported symptoms or weight loss consistent with CMI. This finding might be explained by the results of another report of the same study group [9393. Hansen KJ, Wilson DB, Craven TE, Pearce JD, English WP, Edwards MS, Ayerdi J, Burke GL. Mesenteric artery disease in the elderly. J Vasc Surg. 2004;40:45-52. ]. Most of these study participants had isolated coeliac trunk stenosis. It is very likely that in a significant proportion of these individuals identified with isolated coeliac trunk disease the lesion was caused by external compression (Dunbar Syndrome), which is not associated with an increased risk of cardiovascular mortality. Superior mesenteric artery disease was present in only 2.5% of the population, being associated with renal artery stenosis and weight loss. Lesions of the superior mesenteric artery are more likely to represent malperfusion due to atherosclerotic disease and may have more clinical relevance.
Atherosclerotic mesenteric ischaemia
CMI of atherosclerotic origin is frequently associated with atherosclerosis in other vascular beds such as the coronary arteries. This results in an increased overall cardiovascular mortality as in patients suffering from atherosclerotic RAS. CMI can convert to acute mesenteric ischaemia in cases of acute local thrombosis
PRE-INTERVENTIONAL DIAGNOSTIC STRATEGY
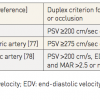
Colour duplex ultrasound has become the screening method of choice for CMI. In the majority of patients, sufficient visibility of the arteries is possible despite frequently seen trapped air in the frame of the colon. Table 1 lists the duplex criteria for significant stenosis or occlusion for each affected vessel. The most commonly used duplex criteria for significant visceral disease are, for the coeliac trunk, a peak systolic velocity greater than 200 cm/s or, for a superior mesenteric artery, a peak systolic velocity greater than 275 cm/s [9494. Armstrong PA. Visceral duplex scanning: evaluation before and after artery intervention for chronic mesenteric ischemia. Perspect Vasc Surg Endovasc Ther. 2007;19:386-92. , 9595. Dietrich CF, Jedrzejczyk M, Ignee A. Sonographic assessment of splanchnic arteries and the bowel wall. Eur J Radiol. 2007;64:202-12. , 9696. Moneta GL, Lee RW, Yeager RA, Taylor LM Jr, Porter JM. Mesenteric duplex scanning: a blinded prospective study. J Vasc Surg. 1993;17:79-84. , 9797. Pellerito JS, Revzin MV, Tsang JC, Greben CR, Naidich JB. Doppler sonographic criteria for the diagnosis of inferior mesenteric artery stenosis. J Ultrasound Med. 2009;28:641-50. , 9898. Zwolak RM. Can duplex ultrasound replace arteriography in screening for mesenteric ischemia?. Semin Vasc Surg. 1999;12:252-60. ].
CT-angiography (CTA) and gadolinium-enhanced MR-angiography are useful initial tests for supporting the clinical diagnosis of symptomatic CMI if duplex ultrasound is inconclusive (approximately 10% of cases are inconclusive due to bowel gas shadowing effects) [9999. Cademartiri F, Palumbo A, Maffei E, Martini C, Malagò R, Belgrano M, La Grutta L, Bartolotta TV, Luccichenti G, Midiri M, Raaijmakers R, Mollet N, Zompatori M, Crisi G. Noninvasive evaluation of the celiac trunk and superior mesenteric artery with multislice CT in patients with chronic mesenteric ischaemia. Radiol Med. 2008;113:1135-42. , 100100. Hellinger JC. Evaluating mesenteric ischemia with multidetector-row CT angiography. Tech Vasc Interv Radiol. 2004;7:160-6. , 101101. Horton KM, Fishman EK. Multidetector CT angiography in the diagnosis of mesenteric ischemia. Radiol Clin North Am. 2007;45:275-88. , 102102. Laghi A, Iannaccone R, Catalano C, Passariello R. Multislice spiral computed tomography angiography of mesenteric arteries. Lancet. 2001;358:638-9. , 103103. Laissy JP, Trillaud H, Douek P. MR angiography: noninvasive vascular imaging of the abdomen. Abdom Imaging. 2002;27:488-506. ]. CMI can be diagnosed by a test for actual ischaemia. A recent study investigated the role of CTA as a follow-up tool of mesenteric bypass surgery for the treatment of CMI [104104. Lopera JE, Trimmer CK, Lamba R, Suri R, Cura MA, El-Merhi FM, Kroma G. MDCT angiography of mesenteric bypass surgery for the treatment of chronic mesenteric ischemia. AJR Am J Roentgenol. 2009;193:1439-45. ]. CTA has been shown to play an important role for the postoperative evaluation of patients with CMI. However, knowledge of the type of surgical procedure performed by the radiologist is essential for the accurate interpretation of the examination. CTA can detect early and late complications after surgery and guide additional surgical or endovascular interventions.
Recently, gastrointestinal 24-hour tonometry has been validated as a diagnostic test to detect splanchnic ischaemia and to guide management [105105. Otte JA, Huisman AB, Geelkerken RH, Kolkman JJ. Jejunal tonometry for the diagnosis of gastrointestinal ischemia. Feasibility, normal values and comparison of jejunal with gastric tonometry exercise testing. Eur J Gastroenterol Hepatol. 2008;20:62-7. ].
Ischaemic colitis is frequently diagnosed by histology following biopsy during bowel endoscopy [106106. Cleveland TJ, Nawaz S, Gaines PA. Mesenteric arterial ischaemia: diagnosis and therapeutic options. Vasc Med. 2002;7:311-21. ]. Intra-arterial digital subtracted angiography is still considered the diagnostic gold standard even if multislice CT reconstructions offer a better three-dimensional understanding of the vessel anatomy especially for planning the interventional access [107107. Cognet F, Ben Salem D, Dranssart M, Cercueil JP, Weiller M, Tatou E, Boyer L, Krausé D. Chronic mesenteric ischemia: imaging and percutaneous treatment. Radiographics. 2002;22:863-79. ].
Imaging modalities and mesenteric artery disease
- Duplex ultrasound is the diagnostic measure of choice for detection of proximal occlusive mesenteric disease
- Additional three-dimensional diagnostic procedures such as MR- and CT-angiography facilitate the planning of an endovascular interventional procedure
- Intra-arterial angiography is no longer indicated as diagnostic tool
TREATMENT OF CHRONIC MESENTERIC ISCHAEMIA
Current literature recommends conservative treatment in patients with asymptomatic disease even though data from Thomas et al [8484. Thomas JH, Blake K, Pierce GE, Hermreck AS, Seigel E. The clinical course of asymptomatic mesenteric arterial stenosis. J Vasc Surg. 1998;27:840-4. ] reported a high progression rate to symptomatic disease and a high mortality rate particularly in patients with at least two significantly affected mesenteric arteries. Contemporary medical treatment includes all aspects of secondary preventive drug treatment including statins, antiplatelet therapy, hypertension control, lowering HbA1c below 7% and smoking cessation. However, thus far, no controlled data exist to justify these recommendations. Patients with symptoms that can be attributed to CMI should be revascularised. During the last decade endovascular therapy has replaced surgery as first line revascularisation strategy.
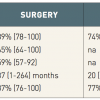
Recent reports have suggested that POBA, with (POBA/S) and without stenting, may have a lower perioperative mortality rate compared to open surgery for revascularisation of CMI [107107. Cognet F, Ben Salem D, Dranssart M, Cercueil JP, Weiller M, Tatou E, Boyer L, Krausé D. Chronic mesenteric ischemia: imaging and percutaneous treatment. Radiographics. 2002;22:863-79. , 108108. Atkins MD, Kwolek CJ, LaMuraglia GM, Brewster DC, Chung TK, Cambria RP. Surgical revascularization versus endovascular therapy for chronic mesenteric ischemia: a comparative experience. J Vasc Surg. 2007;45:1162-71. , 109109. Davies RS, Wall ML, Silverman SH, Simms MH, Vohra RK, Bradbury AW, Adam DJ. Surgical versus endovascular reconstruction for chronic mesenteric ischemia: a contemporary UK series. Vasc Endovascular Surg. 2009;43:157-64. , 110110. Fioole B, van de Rest HJ, Meijer JR, van Leersum M, van Koeverden S, Moll FL, van den Berg JC, de Vries JP. Percutaneous transluminal angioplasty and stenting as first-choice treatment in patients with chronic mesenteric ischemia. J Vasc Surg. 2010;51:386-91. , 111111. Mell MW, Acher CW, Hoch JR, Tefera G, Turnipseed WD. Outcomes after endarterectomy for chronic mesenteric ischemia. J Vasc Surg. 2008;48:1132-8. , 112112. Oderich GS, Malgor RD, Ricotta II JJ. Open and endovascular revascularization for chronic mesenteric ischemia: tabular review of the literature. Ann Vasc Surg. 2009;23:700-12. , 113113. Schermerhorn ML, Giles KA, Hamdan AD, Wyers MC, Pomposelli FB. Mesenteric revascularization: management and outcomes in the United States, 1988-2006. J Vasc Surg. 2009;50:341-8. ]. Retrospective data from a US nationwide inpatient sample analysis extending from 1988 to 2006, including 22,413 individuals treated for CMI, suggested a lower mortality rate associated with POBA/S than with surgical bypass (3.7% vs. 13%, p < 0.01, Table 2 ) [114114. Zerbib P, Lebuffe G, Sergent-Baudson G, Chamatan A, Massouille D, Lions C, Chambon JP. Endovascular versus open revascularization for chronic mesenteric ischemia: a comparative study. Langenbecks Arch Surg. 2008;393:865-70. ]. In addition, bowel resection was less frequently encountered in the endovascular group than in the surgical group treated for CMI (3% vs. 7%, p < 0.01, Table 2 ). This subgroup of patients showed a high in-hospital mortality rate for both strategies (25% and 54%, respectively). Overall, these results have to be interpreted with caution since CMI patients undergoing surgery may have had an advanced stage of the visceral and/or systemic atherosclerotic disease. Nevertheless, the lower in-hospital mortality rate documented in patients treated with POBA/S supports POBA/S as an appropriate therapy for selected patients with CMI. In addition, the high prevalence of coronary disease in this patient population confers high attractiveness to a minimal invasive endovascular approach.
Accordingly, POBA/S is being used with increasing frequency for revascularisation of patients with CMI. Longitudinal data is needed to determine the durability of this benefit. The greater proportion of patients undergoing bowel resection with bypass for acute mesenteric disease suggests a more advanced level of ischaemia in this group, rendering a comparison with POBA/S difficult.
The technical success of endovascular revascularisation of mesenteric lesions is 81% to 100% and, even in the presence of total occlusions, the success rate exceeds 80% [115115. AbuRahma AF, Stone PA, Bates MC, Welch CA. Angioplasty/stenting of the superior mesenteric artery and celiac trunk: early and late outcomes. J Endovasc Ther. 2003;10:1046-53. , 116116. Lee RW, Bakken AM, Palchik E, Saad WE, Davies MG. Long-term outcomes of endoluminal therapy for chronic atherosclerotic occlusive mesenteric disease. Ann Vasc Surg. 2008;22:541-6. , 117117. Peck MA, Conrad MF, Kwolek CJ, Lamuraglia GM, Paruchuri V, Cambria RP. Intermediate-term outcomes of endovascular treatment for symptomatic chronic mesenteric ischemia. J Vasc Surg. 2010;51:140-7. , 118118. Schaefer PJ, Schaefer FK, Hinrichsen H, Jahnke T, Charalambous N, Heller M, Mueller-Huelsbeck S. Stent placement with the monorail technique for treatment of mesenteric artery stenosis. J Vasc Interv Radiol. 2006;17:637-43. , 119119. Zeller T, Rastan A, Schwarzwälder U, Schwarz T, Frank U, Bürgelin K, Sixt S, Müller C, Rothenpieler U, Flügel PC, Pochert V, Neumann FJ. Endovascular therapy of chronic mesenteric ischemia. EuroIntervention. 2007;2:444-51. ]. Due to the aorto-ostial location ( Figure 13 ) of most lesions, stenting has become the endovascular procedure of choice. Symptom relief following revascularisation is reported up to 100% with recurrence in case of restenosis, which is much more frequent as compared to renal stenting (29% to 65% after 1 year) [115115. AbuRahma AF, Stone PA, Bates MC, Welch CA. Angioplasty/stenting of the superior mesenteric artery and celiac trunk: early and late outcomes. J Endovasc Ther. 2003;10:1046-53. , 116116. Lee RW, Bakken AM, Palchik E, Saad WE, Davies MG. Long-term outcomes of endoluminal therapy for chronic atherosclerotic occlusive mesenteric disease. Ann Vasc Surg. 2008;22:541-6. , 117117. Peck MA, Conrad MF, Kwolek CJ, Lamuraglia GM, Paruchuri V, Cambria RP. Intermediate-term outcomes of endovascular treatment for symptomatic chronic mesenteric ischemia. J Vasc Surg. 2010;51:140-7. , 118118. Schaefer PJ, Schaefer FK, Hinrichsen H, Jahnke T, Charalambous N, Heller M, Mueller-Huelsbeck S. Stent placement with the monorail technique for treatment of mesenteric artery stenosis. J Vasc Interv Radiol. 2006;17:637-43. , 119119. Zeller T, Rastan A, Schwarzwälder U, Schwarz T, Frank U, Bürgelin K, Sixt S, Müller C, Rothenpieler U, Flügel PC, Pochert V, Neumann FJ. Endovascular therapy of chronic mesenteric ischemia. EuroIntervention. 2007;2:444-51. ] ( Table 3 ). As already known from surgical revascularisation data using univariate analysis, 2-vessel treatment was protective against symptom recurrence and reintervention [117117. Peck MA, Conrad MF, Kwolek CJ, Lamuraglia GM, Paruchuri V, Cambria RP. Intermediate-term outcomes of endovascular treatment for symptomatic chronic mesenteric ischemia. J Vasc Surg. 2010;51:140-7. ].
Besides individual patient preferences, anatomic considerations might guide the choice between surgical reconstruction and POBA/S. Patients with aneurysmal disease or presence of total occlusions without a visible stump in conjunction with CMI should undergo surgical reconstruction. Symptomatic coeliac artery compression syndrome indicates surgical lysis of the diaphragmatic crura and reconstruction of the coeliac trunk if appropriate. In all other cases, due to its less invasive nature, endovascular revascularisation should be the first line treatment strategy of choice. Considering the high likelihood of restenosis, especially after endovascular therapy, revascularisation of at least 2 vessels should be the revascularisation strategy ( Figure 13 ).
Revascularisation strategy in mesenteric disease
- Asymptomatic CMI has to be treated conservatively with focus on secondary prevention of atherosclerosis
- Endovascular stent-based angioplasty has widely replaced open surgical reconstruction in symptomatic patients due to less invasiveness and lower complication rates at the expense of higher restenosis rates even of stent placement compared to surgery in atherosclerotic disease
- Revascularisation of two affected vessels might result in more durable freedom from recurrent symptoms compared to a single vessel revascularisation
TECHNICAL ASPECTS OF MESENTERIC STENTING
Arterial access
For anatomical reasons the brachial or radial access should be the preferred access site. Both the coeliac trunk and the superior mesenteric artery usually present with caudal take-offs from the abdominal aorta ( Figure 16 ). Thus, access from the upper extremity facilitates support and, in particular, stent placement compared to femoral access. The inferior mesenteric artery is only a rare target for endovascular revascularisation due to the tight collateral network originating from both internal iliac arteries. However, if indicated, the right femoral arterial access should be preferred due to the left latero-dorsal take-off coming from the distal abdominal aorta.
Vascular access for mesenteric intervention
For anatomical reasons access from the upper extremity is preferred for the endovascular treatment of CMI
Mesenteric artery angiography
The coeliac trunk and the superior mesenteric artery arise ventrally from the proximal abdominal aorta. Most lesions are located at the ostium. Thus, a lateral view is most appropriate to visualise properly the proximal course of both vessels. For the coeliac trunk the most appropriate angulations are a right lateral 60° to 90° view whereas for the superior mesenteric artery origin the most appropriate view is a left lateral 60° to 90° angulation. If lateral angulation is not performed then the lesion cannot be visualised properly and the exact analysis of lesion severity and stent placement can be challenging or even impossible ( Figure 17 ). Of note, anatomical variations are frequent. The lateral views can be achieved either by rotating the C-arm into the appropriate position or if the patient is positioned in a lateral location. After some test injections verifying the appropriate angulations a non-selective angiogram with 30 to 40 mls of contrast medium has to be performed using a pigtail catheter.
Invasive angiographic imaging of the coeliac axis vessels
A strict lateral angulation (90 degree projection) of the C-arm is appropriate for a proper opacification of the origin of the coeliac trunk and the superior mesenteric artery
Technique of mesenteric artery angioplasty and stenting
Following lesion location the diagnostic catheter is replaced by a 6 Fr guiding catheter, if coming from the brachial approach, this being most likely in a “multipurpose” configuration ( Figure 18 [A]); in cases where more back-up is needed such as in total occlusions, an “Amplatz right” ( Figure 18 [B]) or in larger aortas even an “Amplatz left” configuration might be more appropriate to reinforce support from the catheter at the contralateral wall of the aorta. For femoral access the most appropriate guiding catheter configurations are the “IMA” or “hockey stick”.
The standard guidewire size is 0.014 inches. For stenosis intervention uncoated extra-support wires offer sufficient support for POBA and stent placement. For the treatment of chronic total occlusions, dedicated coronary guidewires, either hydrophilic-coated or with progressive tapered tip design could be used. Predilatation using dedicated coronary low-profile balloons is only indicated in very tight and calcified lesions, otherwise direct stenting can be performed. Vessel size ranges from 6 mm to 8 mm. Thus dedicated renal stent devices can be used but need to be upsized in larger vessels because of their limited diameter size of a maximum 7 mm. For lesions located in the main trunk of the superior mesenteric artery, self-expanding nitinol stents serve as an alternative, in particular for lesions of embolic origin ( Figure 19A and Figure 19B ) or in long diffuse atherosclerotic lesions ( Figure 20 ). A special indication for stenting is mesenteric ischaemia due to stenosis in type B aortic dissection ( Figure 21 ).
The technique of stenting of ostial mesenteric lesions is similar to renal artery stenting. However, mesenteric arteries are high flow vessels resulting in a need for more imaging of the vessel origin in particular after predilatation. Thus, exact stent placement can become a challenge. This challenge could be overcome by using a dedicated ostial stent device (Archstent™) ( Figure 22 ).
The role of drug-eluting stents, covered stents or drug-eluting balloons has not yet been evaluated in larger series and cannot yet be recommended outside of controlled protocols. Their major indication might become the treatment of in-stent restenosis, being more frequent in the mesenteric as compared to the renal arteries.
Mesenteric percutaneous intervention
- The guiding catheter technique is the preferred technique for mesenteric artery revascularisation
- Stenting is strongly recommended for atherosclerotic ostial lesions, whereas for lesions affecting the trunk balloon angioplasty might be sufficient
- A dedicated ostial stent device facilitates the proper stent positioning
Post-interventional assessment
After each intervention, selective and semi-selective angiograms of the target lesion and the distal artery segments, including the side branches, should be performed without a guidewire in place to document the final result and exclude peripheral embolism, dissection or wire-induced perforation. This should be done preferably using the DSA technique. Treatment success is achieved if the residual diameter stenosis after angioplasty with or without stent is less than 30%.
Post-interventional care
Without the benefit of randomised data to help guide practice, post-interventional drug regimen usually consists of dual antiplatelet therapy after endovascular therapy. In the case of bare metal stents this consists of lifelong aspirin 100 mg per day as well as clopidogrel 75 mg per day for four weeks. Following drug-eluting stent or covered stent placement, dual antiplatelet therapy should be recommended at least for six months. After surgical reconstruction, single daily administration of aspirin is considered to be sufficient for prevention of graft thrombosis.
A pre-discharge duplex examination under fasting conditions should be performed to document baseline flow velocities in the treated vessel segments as reference values for later follow-up procedures. Due to the relatively high likelihood of restenosis, particularly after endovascular revascularisation, patients should be included in a regular surveillance programme, initially at least every six months, including duplex ultrasound. Moreover, these patients should be enrolled in a surveillance programme for cardiovascular diseases considering that myocardial infarction is the main cause of mortality in this patient cohort.
Post-intervention management of mesenteric intervention
- Post-procedural angiography, including the peripheral branches to exclude wire-induced complications and distal embolisation, is mandatory using DSA technique
- Post-interventional medication consists of dual antiplatelet therapy for four weeks followed by ASS only for life in adaption of the coronary practice
- Follow-up is based on duplex ultrasound
SUMMARY
The prevalence of CMI is likely to have been underestimated with a potential increase expected during the next decade due to an ageing population demographic. Advanced stages of CMI disease, with significant two-vessel or three-vessel disease, are associated with an increased cardiovascular and intestinal-related mortality and are thus an indication for revascularisation, probably even if asymptomatic. Duplex ultrasonography has become the primary screening method for CMI; if inconclusive, MR-angiography or CT-angiography are appropriate alternative diagnostic tools.
In patients anatomically suited for endovascular revascularisation, percutaneous revascularisation has replaced surgical revascularisation as first-line therapeutic strategy even if patency rates are in favour of surgical revascularisation ( Table 3 ). However, surgery should still be indicated in all cases of acute-on-chronic mesenteric ischaemia or acute mesenteric ischaemia if there is a potential need for partial bowel resection.