Summary
A clinical quality registry systematically collects clinical data for the assessment of how medical care is provided and to what extent international or local guidelines are followed in order to improve quality of care. Collection of outcome variables is of utmost importance to evaluate temporal trends and the effects of changes of treatment alternatives. Of particular interest and importance are the collection of complication rates and side effects of novel pharmaceutical agents, medical devices or new treatment strategies used in clinical practice, often beyond the original indications studied in inceptional randomised studies. While prospective randomised trials provide the highest level of scientific evidence, they are often limited by including selected populations and are expensive to perform. Observational studies from well-developed registries are inexpensive and relatively simple to perform. Therefore, observational studies from large-scale registries are valuable complements to prospective randomised trials. However, an inherent disadvantage is the limited ability to independently adjudicate endpoints. Hence total mortality is the most reliable hard endpoint for these studies. Despite appropriate statistical methodologies to adjust for differences in baseline characteristics, there are always known and/or hidden confounders that cannot be completely compensated. Therefore, it is important to be cautious in the interpretation of observational studies when comparing outcome between different treatment alternatives and always consider the results as non-definitive and hypothesis generating. By including a randomisation module in a clinical quality registry, it is possible to combine some of the most important features of a prospective randomised trial with the key strengths of a large-scale clinical registry using unselected consecutive enrolment. Such a trial has been described as a prospective randomised clinical registry trial (RCRT) and may represent a powerful new tool for quality assurance and outcomes-based research.
Healthcare registries for descriptive measures and quality assurance
BACKGROUND
Patient management should be based on the best available evidence of its safety and efficacy, and cost-effectiveness. The proof of the quality and efficacy of clinical management is derived preferably from systematic patient-oriented scientific research. Based upon data that are obtained from cohorts of patients, associations are studied between the incidence and prognosis of diseases and their determinants. The impetus for quality assurance comes from variation in practice. The implication that such variation may indicate sub-optimal quality led the WHO to publish an international overview of quality assurance in healthcare in 1982 [11. Vouri H. Quality assurance of health services. Copenhagen: WHO, Regional office for Europe, 198 ]. Despite this, well-organised large-scale registries of clinical practice and outcome are still largely absent in many hospitals, healthcare settings and countries. Patient clinical files are seldom structured in a way that allows for easy data evaluation. These files are typically not searchable and cannot be used for systematic collection of clinical variables. Furthermore, for legal reasons, patient file data, even in anonymised form, are rarely extractable outside of hospitals. It is encouraging that these practices are slowly changing and as a result registries are sprouting in many settings. But as we shall see in this chapter, the historic absence of systematic patient data collection within major cardiovascular areas has had huge impact on pharmacological and procedural treatments and negatively affected patient care and outcome. Political will and government resources are needed to establish, utilise and assure quality, and to maintain health registries.
REQUIREMENTS AND CLINICAL USE OF QUALITY REGISTRIES
Clinical quality registries, however, provide an opportunity to systematically collect clinical and laboratory data for the assessment of how medical care is being provided, the resources used and to what extent guidelines are being followed. Collection of data for quality registries should ideally be an integrated part of any healthcare system. All patients with a given diagnosis or receiving a given treatment should be registered consecutively. Thereby, it can then be estimated what proportion of a certain population of patients with a specific disease entity have been treated, and complications and outcome can be monitored. Selective registration will confound the data and the ability to describe the patient population of interest will be limited. Furthermore, a quality registry should cover an entire region or country to improve the clinical practice and effectiveness of care, and similarly highlight possible regional differences in the availability of treatment alternatives and standards. Regional differences in demographics and disease prevalence may to some extent be adjusted for by statistical case-mix analysis, which allows comparisons despite baseline differences. In addition to completeness of data, a number of factors must be considered when collecting healthcare quality data and this can be summarised in the “ten commandments” as depicted in Table 1 .
With the introduction of new techniques and methodologies it may be tempting for clinicians to use these treatment options beyond their clinical indication and in patient subsets where they have not been formally tested or approved. In this situation a registry may be able to identify overutilisation of such devices or strategies. Novel therapies may also be underutilised due to financial barriers, insufficient training or lack of confidence amongst clinicians and/ or administrators. In this case, a registry can show the actual utilisation of the specific therapy and so may provide a stronger argument for additional resources or training. For clinicians in training the use of a registry can be of great importance to verify whether the training has been adequate. The data can be used by the individual physician to support an argument for more diverse training, greater patient volume or more educational programmes, but can also be used to document the number of procedures performed and their outcomes.
The introduction of new therapies also makes it challenging for an individual doctor or department to identify possible rare complications. With collection of outcome data from large cohorts of patients, however, linking complication patterns to a new therapy becomes easier. There are several examples of how registries have contributed to the identification of rarely occurring complications, which has led to a development of new devices, strategies and techniques. A recent illustration in this genre was the debate on drug-eluting coronary stents (DES) which arose after the publication of data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR).
Clinical quality registry
- Provides an opportunity to systematically collect all clinical data about a certain diagnosis or medical procedure in order to describe and improve the quality of care
- Needs to have consecutive enrolment and ideally include all patients within a region
- Requires management and political support to ensure uptake and adequate resources
- Users must be trained and motivated professionals
- All levels of relevant healthcare providers must be engaged
- Stable and long-term financial support
THE SCAAR REGISTRY DATA AND DRUG-ELUTING STENTS
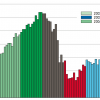
Following the introduction of coronary artery bare metal stenting, the major limitation recognised was in-stent restenosis secondary to intimal hyperplasia. In 2002-2004 encouraging results from small randomised controlled trials of DES with sirolimus or paclitaxel coating demonstrated significant reductions of in-stent restenosis and even improved clinical outcome (although the follow-up periods were short in most studies). Regulatory approval soon followed and the use of DES became widespread. Early on, however, the use of DES was extended broadly to those who were perceived to potentially benefit the most, such as patients with diabetes, renal failure, lesions in small coronary arteries, very long lesions, chronic total occlusions, bifurcations, left main coronary artery disease, in-stent restenosis, multivessel disease, acute myocardial infarction, or saphenous vein graft disease. Nevertheless, most of these indications had not been investigated in randomised trials and were considered off-label indications. Warning safety signals soon began to appear as DES emerged to be more prone than bare metal stents to late stent thrombosis. The debate on the then postulated danger of DES use was fierce. Proponents argued that the adverse effects associated with DES use were outweighed by the advantageous outcome on restenosis and reduced need for repeat revascularisation. The debate exploded in early 2007 when data were published from the SCAAR on 19,771 patients treated with one or more stents [22. Public health in Europe, 1992. p. 16. 2. Lagerqvist B, James SK, Stenestrand U, Lindbäck J, Nilsson T, Wallentin L; SCAAR Study Group. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009-19. ]. Not only was this the largest group of patients treated with DES ever published, the data also represented full coverage of the practice of stent use in an entire country with 100% follow-up of nationals (only emigrants were not included). The propensity-score–adjusted cumulative event rates demonstrated that patients treated with a DES had a 32% higher risk of death after 6 months and onwards compared with patients treated with a bare metal stent. Subsequent reports described how several adverse effect data were not adequately accounted for in initial publications of randomised controlled trials for stents. The effect on clinical use and DES sales was dramatic as numbers plummeted in the following years ( Figure 1 ). This resulted in billion dollar losses for the leading stent manufactures (and corresponding savings for the healthcare providers) but also in reduced death rates among patients with coronary artery disease thanks to the identification of several risk factors (e.g., undersized stents deployed at inappropriately low pressures, stent polymer design and poor compliance with 12 months of dual antiplatelet therapy, amongst others) [33. James SK, Wallentin L, Lagerqvist B, Swedish Coronary Angiography and Angioplasty Registry (SCAAR) study group. The SCAAR-scare in perspective. EuroIntervention. 2009;5:501-4.
The following reference gives examples of how observational studies can be used as a complement to randomised trials.]. New generation DES with thinner struts, absence of or new polymers, improved stent deployment techniques, adherence to guidelines and compliance with antiplatelet therapy has reduced risk of stent thrombosis and stent-related deaths. Recent investigations, primarily based on registry data, now report of improved survival with DES compared to bare metal stents. The DES “SCAAR scare” is an excellent example of initial overutilisation (when the first DES became commercially available) followed by underutilisation, when even DES use for bona fide indications came to a halt after publication of the SCAAR data [22. Public health in Europe, 1992. p. 16. 2. Lagerqvist B, James SK, Stenestrand U, Lindbäck J, Nilsson T, Wallentin L; SCAAR Study Group. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009-19. ]. Without registry information the caveats associated with DES deployment would have reached the clinical community at a much later stage, they may have resulted in additional stent thromboses, myocardial infarctions and deaths.
Registry data for research
OPPORTUNITIES AND LIMITATIONS WITH OBSERVATIONAL STUDIES
The highest levels of scientific clinical evidence stem from prospective randomised clinical trials. The American Food and Drug Administration generally requires more than one adequate and well-controlled investigation to establish treatment effectiveness thus reflecting the need for independent substantiation of experimental results. A single clinical experimental finding of efficacy, unsupported by other independent evidence, has not usually been considered adequate scientific support for a conclusion of efficacy.
In the medical field and particularly within cardiology, an increasing number of pharmaceutical agents, medical devices and clinical procedures are tested in appropriately designed prospective randomised trials. Despite this, only about half of the recommendations given in international guidelines are based on prospective randomised trials [44. Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA. 2009;301:831-41.
Description of the mechanism of guideline formulation.]. Such trials are limited by including selected populations through narrow inclusion criteria and multiple exclusion criteria thus limiting their applicability. Furthermore, they are expensive and cumbersome to perform. By contrast, observational registry studies are inexpensive and relatively simple to perform and more often reflect the true clinical situation. Therefore, observational studies from large-scale registries provide valuable complementary data to prospective randomised trials and can enhance clinical guidance.
Retrospective analyses of prospectively collected data are ideally used for descriptive studies, and for outcome analyses for a given therapy, but have limited value for comparisons between therapeutic options. However, for various reasons in many areas of medicine, randomised trials are highly unlikely to be performed. For these patient subsets and clinical scenarios observational studies provide the second best and often the only available alternative to evaluate outcome.
A prominent example of applied registry knowledge within the field of interventional cardiology is the suspected pharmacological interaction between clopidogrel and proton pump inhibitors (PPI), which are widely prescribed for gastro-oesophageal reflux disease. Clopidogrel is activated by cytochrome P450 2C19, which also metabolises PPIs. Whether or not the supposed interaction between the two types of drugs, which experimentally results in reduced platelet inhibition, adversely influences clinical efficacy is highly controversial and the subject of debate. Based mostly on anecdotal post hoc analyses, the U.S. Federal Drug Administration’s and the European Medicines Agency discourage PPI use (particularly omeprazole) in patients treated with clopidogrel. Although the jury is still out on this controversy, observational data and randomised studies indicate that the clinical impact of the interaction is absent and that PPIs may be associated without increased risk for adverse cardiovascular outcomes after discharge, regardless of clopidogrel use [55. Benson, K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342:1878-86.
Useful resource descrining the strengths and limitations of the two major study designs in contemporary medicine.].
STATISTICAL CONSIDERATIONS
A clinician always chooses a specific treatment alternative for a specific reason which gives rise to selection. This selection bias makes direct comparisons of different therapeutic options challenging. Despite appropriate statistical methodologies to adjust for differences in baseline characteristics there are always known and/or hidden confounders that cannot be completely compensated. Therefore, it is important to be cautious in the interpretation of observational studies comparing outcomes between different treatment alternatives and to always consider the results as non-definitive and hypothesis generating. Yet is has been shown that randomised and large observational trials frequently give consistent results [55. Benson, K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342:1878-86.
Useful resource descrining the strengths and limitations of the two major study designs in contemporary medicine.]. In order to try to compensate for differences in baseline characteristics and risk profile between the groups of interest for comparison, multivariable adjustments are usually performed. With sizeable patient numbers a large number of co-variates can be entered into a statistical model. One method frequently employed is to include the calculated propensity for a patient to receive the studied treatment in the model. A propensity score adjusted model usually improves the performance of the statistical analyses moderately.
Observational studies
- Drawn from large-scale registries, observational studies are complementary to prospective randomised trials
- Despite appropriate statistical adjustments there are always confounders that cannot be completely compensated
- It is thus important to be cautious in the interpretation of observational studies comparing outcomes between different treatment alternatives and always consider the results as non-definitive and hypothesis generating
Follow-up on hard endpoints
Collection of outcome variables is of outmost importance to be able to evaluate temporal trends of outcome and the effects of changes of treatment alternatives. Of particular interest and importance is the collection of complication rates and side effects of novel pharmaceutical agents, medical devices or new treatment strategies. In-hospital events should always be collected as part of the registry. It is generally a challenge to be able to collect complete data on complication rates and other hard endpoints at discharge in a continuous registry. A key for success may be to make the collection mandatory and make it impossible to close the case until all possible complications have been entered. Collection of long-term outcome is of great importance and in order to make the collection automated it is crucial to have an automated linkage to national mortality and diagnosis registries or claims databases. Variables of importance in the field of interventional cardiology are repeat revascularisation procedures, recurrent myocardial infarction, restenosis, stent thrombosis and bleeding complications. In a modern world with patients moving between different healthcare providers and regions or countries this may become especially challenging. In order to collect complete information the quality registry needs to cover a large geographical area, preferably nationwide.
Collection of outcome variables
- Crucially important for the evaluation of quality of healthcare
- Must be systematic to ensure complete patient capture
- Must cover as large a geographical region as possible
- Must be linked to national mortality registers
- Total mortality, both short and long term, is the most reliable hard endpoint
Post-marketing surveillance
In Europe, devices receive a CE mark and approval for use based on relatively limited clinical documentation or evidence. Since prospective randomised trials typically include selected populations it is important to evaluate the performance in real world clinical populations. The regulating authorities therefore, sometimes mandate post-marketing surveillance studies. The European Medical Association (EMA) and national medical products agencies will most likely in the future give conditional approval more often for commercial use of medical devices and pharmaceutical agents with additional requests to prove safety and efficacy in less selective populations. Moreover, this form of surveillance is beneficial for pharmaceutical companies and manufacturers of medical devices who have an interest in proving safety and efficacy beyond what has been demonstrated in small randomised trials. Large-scale post-marketing studies, however, are costly to perform and require specially designed web-based clinical report forms. Hence they are often funded and driven by industry. Since different post-marketing surveillance studies will enrol different patient populations they cannot be used for direct comparisons between different products. Ideally post-marketing studies should be performed within national quality registries enrolling consecutive patients in order to ensure studies of broad unselected populations and to avoid competition between randomised trials and registries. This would also stimulate input from the academia rather than industry, and enhance the support and development of large-scale continuous quality registries. In comparison to “all-inclusive” registries, patients participating in post-marketing studies typically have diverse clinical and procedural characteristics and different mortality rates compared with non-participants. Thus, there is a possibility that the results from post-marketing and registry studies will differ and one could therefore anticipate legal liability issues when registry data are published. There is, therefore, a need for formal regulation within the grey area that exists between registries and post-marketing surveillance in order to assure that registries can collect data and publish results freely on the one hand, and on the other that the pharmaceutical industry can expect a certain degree of data quality and a reasonable objection period before results are published.
Randomisation within clinical registries
Large-scale randomised clinical trials are expensive to undertake, and economic revenue is typically the primary incentive to inaugurate such trials on the part of commercial interests. It cannot be expected that this model can address many of the important clinical issues or issues with the evaluation of medical devices. Furthermore, the very requirements for documentation in randomised clinical trials, developed to protect patient anonymity, ensure reproducibility and avoid fraud, impede realisation of simple yet relevant trials because of the economic barriers required to overcome them. A Catch-22 situation may therefore exist and, in order to protect patient interests, several relevant clinical problems are not addressed and thus evidence is never established. By including a randomisation module in a clinical quality registry, however, it is possible to combine some of the finest attributes of a prospective randomised trial ( Figure 2 ) with the best features of a large-scale clinical registry ( Figure 3 ) including the key strength of unselected consecutive enrolment. Such a trial has been described as a registry based prospective randomised clinical trial (RRCT) by way of analogy with a prospective randomised clinical trial (RCT) ( Figure 4 ). This concept is currently developed within the Swedeheart registry in which manual thrombus aspiration as adjunctive treatment for primary PCI was being tested prospectively in a large number of patients adequately powered for the evaluation of mortality as the primary endpoint [66. Fröbert O, Lagerqvist B, Gudnason T, Thuesen L, Svensson R, Olivecrona GK, James SK. Thrombus Aspiration in ST-Elevation myocardial infarction in Scandinavia (TASTE trial). A multicenter, prospective, randomized, controlled clinical registry trial based on the Swedish angiography and angioplasty registry (SCAAR) platform. Study design and rationale. Am Heart J. 2010;160:1042-8.
Example of a randomised clinical registry trial.]. The registry helped identifying patients suitable for inclusion, proposed enrolment and the treating physician only had to check a few exclusion criteria and get verbal consent from the patient. Randomisation was performed directly in the registry clinical report form. There was no additional study related activities for staff or patients. All endpoints were automatically collected from national registries. No study specific monitoring of data or adjudication of events was performed for the trial. Therefore all-cause mortality collected from the personal registry was chosen as the primary endpoint of the trial. Secondary end points were collected from the SCAAR/SWEDEHEART registry and from the mandatory national hospital admission registry [77. Fröbert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angerås O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Kåregren A, Nilsson J, Robertson L, Sandhall L, Sjögren I, Ostlund O, Harnek J, James SK. Thrombus Aspiration during ST-Segment Elevation Myocardial Infarction. N Engl J Med. 2013;369:1587-97 , 88. Lagerqvist B, Fröbert O, Olivecrona GK, Gudnason T, Maeng M, Alström P, Andersson J, Calais F, Carlsson J, Collste O, Götberg M, Hårdhammar P, Ioanes D, Kallryd A, Linder R, Lundin A, Odenstedt J, Omerovic E, Puskar V, Tödt T, Zelleroth E, Ostlund O, James SK. Outcomes 1 Year after Thrombus Aspiration for Myocardial Infarction. Engl J Med. 2014;371:1111-20. ].
In the Cath/PCI Registry of the National Cardiovascular Data Registry (NCDR, http://www.ncdr.com) in the United States this concept was used for the evaluation of a radial vs. femoral access for PCI in women [99. Rao, S.V., Krucoff, M.W. Transradial PCI in women: problem solved or clinical equipoise? J Invasive Cardio. l2011 23, 4 p preceding 101. ]. The study was a prospective, randomised clinical trial comparing radial with femoral arterial access in women undergoing PCI. The primary efficacy end point was a composite of bleeding or vascular complication requiring intervention. The trial design had major advantages over a traditional design; identifying trial sites and investigators and leveraging trial data. Patient demographics, medical history, concomitant medications, procedural details, and index hospitalisation clinical outcomes were routinely coded into the registry's data collection form using standardised data elements and electronically captured without study site coordinator effort. For the trial, additional information specific to vascular access and bleeding definitions not in CathPCI Registry was obtained using additional electronic case report form (CRF) pages.
The first registry based randomized trial evaluating a pharmaceutical agent using the national SWEDEHEART registry as a basis for randomisation, collection of baseline variables, and detection of primary outcome data is the DETermination of the role of OXygen in suspected Acute Myocardial Infarction (DETOX AMI) trial (ClinicalTrials.gov Identifier: NCT02290080) [1010. Hofmann, R., James SK, Svensson L, Witt N, Frick M, Lindahl B, Östlund O, Ekelund U, Erlinge D, Herlitz J, Jernberg T.. DETermination of the role of OXygen in suspected Acute Myocardial Infarction trial. Am Heart J. 2014;167:322-328. ]. This trial is evaluating if oxygen as compared to air reduces mortality in 6600 patients with suspected or confirmed myocardial infarction.
The randomised clinical registry trial
- Large-scale RCTs are expensive and logistically challenging to undertake
- In RCTs important clinical questions are frequently not addressed due to commercially driven and economic imperatives
- Randomising patients into a clinical quality registry combines the features of a prospective randomised trial with a large-scale clinical registry
- Unselected enrolment is a key strength of this form of study
Examples from different countries
National databases exist in several European countries but are still missing in many others.
The Swedish SCAAR registry is nationwide and 100% complete in terms of patient enrolment; every patient undergoing coronary angiography or angioplasty in Sweden can be found in the database. In 2010 SCAAR was merged with the Swedish coronary care unit registry (RIKS-HIA), the thoracic surgery registry, the Transcatheter Aortic Valve Implantation (TAVI) registry and the secondary prevention registry into a nationwide registry called the SWEDEHEART [77. Fröbert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angerås O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Kåregren A, Nilsson J, Robertson L, Sandhall L, Sjögren I, Ostlund O, Harnek J, James SK. Thrombus Aspiration during ST-Segment Elevation Myocardial Infarction. N Engl J Med. 2013;369:1587-97 ]. All data in SWEDEHEART are collected in a common web-based entry page and follow-up is automatically merged with other national databases.
The British Cardiac society’s Myocardial Ischaemia National Audit Project (MINAP) registry (http://www.ucl.ac.uk/nicor/audits/minap) is also a near-complete national CCU registry which has been successful in enrolling patients consecutively and is also collecting follow-up on hard endpoints [77. Fröbert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angerås O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Kåregren A, Nilsson J, Robertson L, Sandhall L, Sjögren I, Ostlund O, Harnek J, James SK. Thrombus Aspiration during ST-Segment Elevation Myocardial Infarction. N Engl J Med. 2013;369:1587-97 ]. Data completeness in MINAP is >80% in most variables entered but as low as 31% in others. Furthermore, the British Cardiovascular Intervention Society (BCIS) has collected PCI audit data since 1991 including more than 81 angiography and 105 PCI sites. These data can be linked with national databases to collect long-term outcome. Denmark has two regional well-developed PCI registries with consecutive enrolment of patients undergoing angiography or PCI. However, merging of data in the shared Danish Heart Register database (http://www.dhreg.dk/uk/default.shtml) is currently suspended because of database conversion problems, thus demonstrating some of the challenges of completing registries to a high standard.
As an illustration of the prospects of healthcare quality registries the ongoing recruitment into the world’s largest person identification registry, the Indian Aadhaar unique identification project (http://uidai.gov.in/) which aims to include 1.2 billion people, deserves mentioning. Although Aadhaar is in an early phase of development and currently focuses on enrolment, one of the future aims is to use the registry also as a platform for healthcare service evaluation and delivery.
Conclusions
Over the last few years, registries in cardiovascular medicine in general, and within interventional cardiology in particular, have gained more attention as well as an increasingly prominent profile in medical journals. By consecutive enrolment of complete patient populations, the methodology is a powerful tool for describing the healthcare that is actually being provided, including the complications and benefits of different therapies. When reviewing reports and papers originating from registries, it is very important to be cautious in the interpretation of the comparison of outcomes between different treatment alternatives. Observational studies should always be considered non-definitive and hypothesis generating. In order to avoid selection bias, randomisation of patients may be included within a clinical registry, combining some of the most important features of a prospective randomised trial with the key strengths of a large-scale clinical registry.
For devices and pharmaceutical agents with inadequate proof of clinical efficacy and safety, less selective populations in post-marketing surveillance studies may provide only a conditional commercial approval from the regulatory bodies, with further requests to prove safety and efficacy. Ideally, these should be performed within large-scale clinical registries driven by academic institutions, rather than those of industry.
Personal perspective - Stefan James
The future of evidence-based medicine belongs to large healthcare quality registries. The impact of registries on treatment and excellence of care will depend on data quality, completeness and coverage. Prospective use of quality registries could potentially revolutionise clinical trials by the fast inclusion of large patient numbers, focus on hard endpoints and complete follow-up and at a fraction of the costs of today’s RCTS. With the organisation of quality registries in large European countries and initiatives such as Aadhaar in India, the future could soon be here.