Arterial access
FEMORAL ARTERY ACCESS
Retrograde approach
Describing the access site as the femoral artery should be considered somewhat of a misnomer as recent data has clearly demonstrated that the ideal site for puncture is the common femoral artery (CFA) [1212. Garrett PD, Eckart RE, Bauch TD, Thompson CM, Stajduhar KC. Fluoroscopic localization of the femoral head as a landmark for common femoral artery cannulation. Catheter Cardiovasc Interv. 2005;65(2):205-207.
Description of the radiographic anatomy of the femoral artery and its tributaries.]. The common femoral artery lies between the external iliac artery and the common femoral bifurcation into the superficial femoral and profunda femoris arteries ( Figure 1 ). This vessel also has a close relationship with the femoral vein and nerve ( Figure 2A and 2B ) For many years, operators have almost exclusively used external anatomical landmarks. This is sometimes termed traditional anatomical landmark guidance (TALG). The most important of these landmarks, the inguinal ligament, although invisible, is represented by a line that traverses from the clinically palpable superior anterior iliac spine to the pubic symphysis. Amongst interventional cardiologists it is commonly recommended to puncture 1-2cm below this invisible line where the femoral arterial pulse is palpable and compression to obtain haemostasis is against the femoral head. The puncture itself is made with an open needle (upwards-facing bevel) at an angle of 30-45 degrees. As the needle approaches the artery it will ‘dance’. If it is directly kitover the anterior wall of the artery this will be in a vertical plane, whereas if it is off-axis it will be inclined slightly in the horizontal plane. For safer femoral puncture only the anterior wall should be punctured ( Figure 3 ). Once the central lumen is entered, pulsatile blood under arterial pressure will flow out of the needle hub. At this point the angle of needle entry should be lowered carefully towards the skin in order to align the needle axis with the central axis of the CFA. A guidewire is then advanced gently through the needle and into the artery, the needle removed (with the guidewire in situ), and a vascular sheath assembly inserted into the artery ( Figure 4 ).
A common misconception, however, is to rely on the skin crease, which does not reflect the position of the ligament in patients who are overweight and is rarely over the centre-line of the femoral head. Equally as important, the inguinal ligament itself is not superior to the superior margin of the femoral head in all patients.
Complications of a low puncture are penetration or sheath insertion through the superficial femoral artery or profunda femoris artery, augmenting the risks of pseudoaneurysm formation, laceration, and thrombosis [1313. Kim D, Orron DE, Skillman JJ, Kent KC, Porter DH, Schlam BW, Carrozza J, Reis GJ, Baim DS. Role of superficial femoral artery puncture in the development of pseudoaneurysm and arteriovenous fistula complicating percutaneous transfemoral cardiac catheterization. Cathet Cardiovasc Diagn. 1992;25(2):91-97. ]. Indeed, obtaining adequate haemostasis is more difficult once the entry site is below the femoral head as there is no stable tissue support to buttress against during compression and vascular closure devices cannot be used below the femoral bifurcation. Furthermore both bifurcation vessels taper distally increasing the risk of obstructive complications. The femoral venous circulation runs parallel and frequently overlaps with femoral bifurcation vessels, hence predisposing to inadvertent puncture and the creation of an arteriovenous communication [1414. Altin RS, Flicker S, Naidech HJ. Pseudoaneurysm and arteriovenous fistula after femoral artery catheterization: association with low femoral punctures. Ajr. 1989;152(3):629-631. ]. A high puncture site may result in penetration or sheath insertion through the external iliac artery or the inferior epigastric artery, causing similarly challenging compression of the vessel to obtain haemostasis with a subsequent 18-fold increased predisposition to retroperitoneal bleeding [1515. Ellis SG,Bhatt D,Kapadia S, et al. Correlates and outcomes of retroperitoneal hemorrhage complicating percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 67: 541–545 ].
To ensure puncture of the CFA and to limit risks of vascular complications, two techniques to assist traditional manual palpation have been proposed. The first is to use fluoroscopy while the second relies on ultrasound to guide the puncture. Although using fluoroscopy was proposed as early as 1978 by Dr Charles Dotter, there have been surprisingly few randomised studies[1616. Dotter CT, Rosch J, Robinson M. Fluoroscopic guidance in femoral artery puncture. Radiology. 1978;127(1):266-267. ]. Several authors though have shown a consistent anatomical relationship between the femoral head and the common femoral artery Grier et al. surveyed practice patterns in the 1990’s and found that in spite of this finding most physicians still preferred to use the skin crease (39.2%) or the location of maximal femoral pulsation (24.7%) as their default puncture site [1717. Grier D, Hartnell G. Percutaneous femoral artery puncture: practice and anatomy. Br J Radiol. 1990;63(752):602-604. ]. They showed that the skin crease was distal to the CFA bifurcation in 71.9% whereas the site of maximal femoral pulsation was over the CFA in 92.7%. In a more recent study, CFA bifurcation was below the inguinal ligament and the centre-line across the femoral head in nearly all 126 angiograms studied ( Figure 5 ). The CFA bifurcation was located at a mean 7.5 cm below the inguinal ligament, 2.9 cm below the middle line crossing the femoral head, and 0.8 cm below the inferior border of the femoral head. In contrast, the inguinal skin crease was a mean of 1.8 cm (3.9-6.5 cm) below the CFA bifurcation (the weight of patients was not reported) [1212. Garrett PD, Eckart RE, Bauch TD, Thompson CM, Stajduhar KC. Fluoroscopic localization of the femoral head as a landmark for common femoral artery cannulation. Catheter Cardiovasc Interv. 2005;65(2):205-207.
Description of the radiographic anatomy of the femoral artery and its tributaries.]. The CFA pathway coursed over the medial femoral head in 92% of the cases.
Fitts and colleagues reviewed the impact of fluoroscopic guidance in 2,651 consecutive patients (mean weight ~85 kg undergoing PCIs from 2000 to 2003) [1818. Fitts J, Ver Lee P, Hofmaster P, Malenka D. Fluoroscopy-guided femoral artery puncture reduces the risk of PCI-related vascular complications. J Interv Cardiol. 2008;21(3):273-278.
Description of fluoroscopically-assisted femoral puncture.]. The use of fluoroscopy was categorised as < 20%, 35-50% and >70% among 8 operators. Overall, the use of fluoroscopy was associated with a lower incidence of pseudo-aneurysms (0.3% vs 1.1%, P = 0.017) and any arterial injury, defined by haematoma >8 cm, pseudo-aneurysm, A/V fistula, retroperitoneal bleeding or any vascular complication requiring intervention (0.7% vs 1.9%, P < 0.01) and a reduced length of stay (2.1 days vs 2.4 days, P < 0.01). Of note, there was no difference in bleeding requiring transfusion (1.6% vs 1.8%, P = 0.69). For each physician, there were fewer vascular injuries when fluoroscopy was used. To date there has been only a single randomised trial comparing fluoroscopy-guided puncture to puncturing below the inguinal ligament [1919. Abu-Fadel MS, Sparling JM, Zacharias SJ, Aston CE, Saucedo JF, Schechter E, Hennebry TA. Fluoroscopy vs. traditional guided femoral arterial access and the use of closure devices: a randomized controlled trial. Catheter Cardiovasc Interv. 2009;74(4):533-539.
A randomised study showing that fluoroscopic-guidance reduces the number of of low-femoral punctures.]. This was obtained by puncturing 2-3 cm below an imaginary line from the anterior superior iliac crest to the pubic symphysis with a needle at 30˚-45˚ angle. Overall, the mean time to successfully insert an arterial sheath in the CFA was similar in both groups. Furthermore in this study, involving a large majority of diagnostic procedures (~70%), the rate of vascular complications (albeit numerically lower in the fluoroscopy-guided group) did not reach statistical significance. Potential superiority for fluoroscopic guidance in avoiding a lower puncture site was suggested in women and obese patients. An accompanying editorial emphasised the existence of a learning curve to optimise results with fluoroscopic guidance [2020. Turi ZG. Fluoroscopy guided vascular access: asking the right question, but getting the wrong answer? Catheter Cardiovasc Interv. 2009;74(4):540-542. ]. Two other randomised studies comparing CFA puncture with and without fluoroscopic guidance found similar results, with a potential benefit in patients with a BMI > 30 kg/m2 [2121. Huggins CE, Gillespie MJ, Tan WA, Laundon RC, Costello FM, Darrah SB, Tate DA, Cohen MG, Stouffer GA. A prospective randomized clinical trial of the use of fluoroscopy in obtaining femoral arterial access. J Invasive Cardiol. 2009;21(3):105-109. , 2222. Jacobi JA, Schussler JM, Johnson KB. Routine femoral head fluoroscopy to reduce complications in coronary catheterization. Proc (Bayl Univ Med Cent). 2009;22(1):7-8. ]. Therefore, although the true clinical benefit of fluoroscopic guidance remains to be demonstrated, it is a technique worth applying to avoid the CFA bifurcation in instances where VCD use is planned [2323. Schnyder G, Sawhney N, Whisenant B, Tsimikas S, Turi ZG. Common femoral artery anatomy is influenced by demographics and comorbidity: implications for cardiac and peripheral invasive studies. Catheter Cardiovasc Interv. 2001;53(3):289-295.
Description of the radiographic anatomy of the common femoral artery.] ( Chapter 1.05 ). A randomised study compared the use of ultrasound guidance to femoral pulse to guide CFA puncture in 112 patients referred for diagnostic angiography [2424. Dudeck O, Teichgraeber U, Podrabsky P, Lopez Haenninen E, Soerensen R, Ricke J. A randomized trial assessing the value of ultrasound-guided puncture of the femoral artery for interventional investigations. Int J Cardiovasc Imaging. 2004;20(5):363-368. ]. In this population at low risk of vascular complications (mean weight of 74 kg), ultrasound guidance was helpful in successfully cannulating the CFA only in patients with weak femoral pulsation or with a leg circumference ≥ 60 cm. No benefit in reducing vascular complications was found.
Seto et al. reported the results of the large FAUST (Femoral Arterial Access with Ultrasound Trial) trial, which randomized 1,004 patients to ultrasound or fluoroscopic-guidance in several US centres [2525. Seto AH, Abu-Fadel MS, Sparling JM, Zacharias SJ, Daly TS, Harrison AT, Suh WM, Vera JA, Aston CE, Winters RJ, Patel PM, Hennebry TA, Kern MJ. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial). JACC Cardiovasc Interv. 2010;3(7):751-758.
Description of ultrasound-assisted femoral puncture]. Overall, ultrasound-guidance facilitated puncture of the CFA, especially in high CFA bifurcations (82.6% vs 69.8%, P < 0.01) and reduced the risk of venous puncture (2.4% vs 15.8%, P < 0.0001). Vascular complications (haematoma > 5cm, pseudo-aneurysm, retroperitoneal bleeding, arterial dissection or thrombosis or major bleeding as defined by PCI Linking Angiomax to Reduced Clini">REPLACE-2 criteria i.e., clinically overt blood loss resulting in a decrease in haemoglobin of > 3 g/dl, any decrease in haemoglobin of > 4 g/dl, or transfusion of ≥ 2 units of packed red blood cells or whole blood) were significantly reduced with ultrasound-guidance as compared with fluoroscopic-guidance (1.4% vs 3.4%, P = 0.04). Of note, while the success rate for CFA cannulation under fluoroscopic-guidance was 83.3%, the authors observed a significant learning curve with ultrasound-guidance as the success rate improved from 82.4% with < 6 ultrasound-guided procedures to 87.6% with ≥ 15 US-guided procedures.
A further important consideration is the course of the inferior epigastric artery, a retroperitoneal branch of the external iliac artery, which frequently makes and inferiorly directed loop over the superior margin of the femoral head. This means that any puncture should aim to avoid both the common femoral bifurcation as well as the external iliac artery (including the inferior epigastric artery).
Based upon the aforementioned imaging-assisted studies, a distinct benefit of using fluoroscopy or ultrasound-guidance in all patients cannot be recommended as mandatory, although it may be beneficial in selected high-risk populations, particularly obese patients. In patients where the inguinal ligament can be easily inferred from the line joining the superior anterior iliac crest to the pubic symphysis, it appears safe to puncture 2-3 cm below that line where the pulse is maximal with the important caveat that these landmarks have a variable relationship with the femoral head. In case of doubt or difficulties, a safe CFA landing zone can be inferred from fluoroscopy being between the centre-line of the femoral head and a 14 mm inferior limit. This target zone gives the highest chance of avoiding both the femoral bifurcation and retroperitoneal vessels ( Figure 6 ). Fluoroscopic guidance should be performed in the antero-posterior projection (as RAO or LAO angulation will make the femoral bifurcation appear more cranial or caudal respectively) and with screening of the needle and guidewire entry into the target zone to ensure optimal result.
Femoral sheath angiography before commencing an intervention can help inform decisions on intensity of antithrombotic therapy and even progression to elective intervention, particularly in cases of high puncture. Image acquisition of femoral anatomy also serves a useful purpose for future procedures, as knowledge of the position of the femoral bifurcation will alter the position of the optimal target zone.
More recently a micropuncture technique has been proposed as a further means to optimise femoral arterial puncture particularly when large calibre sheath insertion is being planned. The exact clinical impact remains unclear [2626. Cilingiroglu M, Feldman T, Salinger MH, Levisay J, Turi ZG. Fluoroscopically-guided micropuncture femoral artery access for large caliber sheath insertion. J Invasive Cardiol. 2011,4:157-61. , 2727. Ben-Dor I, Maluenda G, Mahmoudi M, Torguson R, Xue Z, Bernardo N, Lindsay J, Satler LF, Pichard AD, Waksman R. A novel, minimally invasive access technique versus standard 18-gauge needle set for femoral access. Catheter Cardiovasc Interv. 2012 Feb 14. doi: 10.1002/ccd.23330. [Epub ahead of print]. ].
Anterograde approach
For invasive diagnostic and interventional access to the lower limb arterial system either a contralateral retrograde femoral approach or an ipsilateral antegrade approach are used. Fluoroscopic or ultrasound guidance and are commonly practiced and sheaths are typically <6Fr.
Femoral artery access
- Puncture and sheath placement should be performed in the common femoral artery (CFA)
- The femoral bifurcation, external iliac and the inferior epigastric arteries must be avoided.
- The skin crease, maximum pulsation, and the inguinal ligament, can be misleading anatomical landmarks
- In cases of technical or anatomical difficulties, fluoroscopy or ultrasound guidance might be helpful to locate CFA
- Using fluoroscopy, the optimal landing zone for CFA is located between the centre line and the lower part of the femoral head
FEMORAL ARTERY ACCESS COMPLICATIONS
With the expansion of worldwide experience, several factors and independent predictors have been associated with an increased incidence of vascular complications after diagnostic and interventional procedures ( Table 2 ). Due to larger sheath sizes and a more potent antithrombotic milieu, the incidence of vascular complications is 2-3 times higher after PCI compared to diagnostic angiography [2828. Chandrasekar B, Doucet S, Bilodeau L, Crepeau J, deGuise P, Gregoire J, Gallo R, Cote G, Bonan R, Joyal M, Gosselin G, Tanguay JF, Dyrda I, Bois M, Pasternac A. Complications of cardiac catheterization in the current era: a single-center experience. Catheter Cardiovasc Interv. 2001;52(3):289-295. ]. Overall, there has been a reduced incidence of vascular complications with severe bleeding, especially in the last 5 years mainly due to a few technical improvements and lower peri-procedural levels of anticoagulation. The clinical impact of VCDs in reducing femoral complications remains controversial ( Chapter 1.05 ). Bed rest is usually recommended for up to 6-8 hours after manual compression depending on sheath size but can be shortened with closure devices [2929. Schwartz BG, Burstein S, Economides C, Kloner RA, Shavelle DM, Mayeda GS. Review of vascular closure devices. J Invasive Cardiol. 2010;22(12):599-607. ]. Accelerated ambulation protocols using 2-3 hours, however, have also been shown to be safe Studies in the early days of PTCA or other percutaneous devices reported femoral access site complications between 2% and >15% in patients undergoing diagnostic or interventional procedures. A single centre study in the late nineties reported an incidence of vascular complications of 1.8% for diagnostic and 4% for interventional procedures [2828. Chandrasekar B, Doucet S, Bilodeau L, Crepeau J, deGuise P, Gregoire J, Gallo R, Cote G, Bonan R, Joyal M, Gosselin G, Tanguay JF, Dyrda I, Bois M, Pasternac A. Complications of cardiac catheterization in the current era: a single-center experience. Catheter Cardiovasc Interv. 2001;52(3):289-295. ]. More recently, data from another high-volume centre reported an incidence of 0.9% for diagnostic procedures and 3.6% post-PCI [3030. Sherev DA, Shaw RE, Brent BN. Angiographic predictors of femoral access site complications: implication for planned percutaneous coronary intervention. Catheter Cardiovasc Interv. 2005;65(2):196-202. ]. A recent study from the Mayo Clinic in 300 patients with femoral angiography showed that in 13% the access site was located outside the optimal landing zone [3131. Pitta SR, Prasad A, Kumar G, Lennon R, Rihal CS, Holmes DR. Location of femoral artery access and correlation with vascular complications. Catheter Cardiovasc Interv. 2011. ]. Overall, vascular complications occurred in 5.7%, with a significantly higher incidence when the puncture site was outside the optimal landing zone (18% vs 4%, P<0.001).
The most common complication of percutaneous arterial puncture remains puncture site oozing, bruising and haematoma [3232. Cosman TL, Arthur HM, Natarajan MK. Prevalence of bruising at the vascular access site one week after elective cardiac catheterisation or percutaneous coronary intervention. J Clin Nurs. 2011;20(9-10):1349-1356. ] Although haematomas per se have not been correlated with impaired long-term outcomes, they remain a significant source of morbidity and costs often prolonging hospitalisation duration. As definitions have evolved, the true incidence of haematoma is difficult to evaluate throughout the different PCI eras [3333. Nikolsky E, Mehran R, Halkin A, Aymong ED, Mintz GS, Lasic Z, Negoita M, Fahy M, Krieger S, Moussa I, Moses JW, Stone GW, Leon MB, Pocock SJ, Dangas G. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta-analysis. J Am Coll Cardiol. 2004;44(6):1200-1209. ]. In the nineties, definitions encompassed large haematoma with a significant haematocrit drop, requirement of surgical repair or transfusion. Using those definitions, rates of 2-5% were reported. More recently, haematoma have been defined as > 5-10 cm and their prevalence in large series or in randomised trials evaluating closure devices or antithrombotic agents has dropped. Most important risk factors associated with haematoma formation are female gender, the type and duration of antithrombotic regimen, and the sheath size. The prevalence of femoral artery pseudo-aneurysm has ranged from 0.7% to 5.3% with recent studies suggesting a contemporary incidence of < 1%. It should be noted however, that in a recent study including 400 consecutive patients using systematic duplex ultrasound the incidence pseudoaneurysm was found to be 3.7% [3434. Kacila M, Vranic H, Hadzimehmedagic A, Sehovic S, Granov N. The frequency of complications of pseudo aneurysms after cardiac interventional diagnostic and therapeutic interventions. Med Arh. 2011;65(2):78-81. ]. As discussed previously, avoiding access site puncture below the CFA bifurcation and using single anterior wall puncture are techniques recommended to avoid pseudoaneurysm formation. Initially such complications often required surgical intervention following the failure of echo-guided compression. Pseudoaneurysms with necks >3mm are less likely to close spontaneously or with compression therapy and thus require interventional management. In current practice, direct thrombin injection is the preferred technique although this should be performed by expert operators as thrombin passage to the main circulation may have catastrophic consequences [3535. Gabrielli R, Rosati MS, Vitale S, Millarelli M, Chiappa R, Siani A, Irace L, Caselli G. Fatal Complication after Thrombin Injection for Post-Catheterization Femoral Pseudoaneurysm. Thorac Cardiovasc Surg. 2011.
Analysis of the risk factors associated with retroperitoneal haemorrhage from a cohort of 3,500 patients.].. Arterio-venous fistulae are rare complications (0.1-0.4%) and have been associated with the inadvertent puncture of the femoral vein while searching for the femoral artery, during the placement of a venous sheath or resulting from multiple vein punctures for electrophysiological studies. In most instances initial conservative management will lead to spontaneous resolution. If high flow rates persist surgical repair may be required. Femoral artery dissection using the retrograde approach is clinically benign as the centripetal blood flow will splint the endothelial dissection flap against its tunica media and promote healing. Dissection will, however, make it difficult to continue the procedure if further catheter exchanges are required and so frequently necessitates the use of an alternative access site. Femoral obstruction leading to thrombosis and limb ischaemia is a limb and potentially life-threatening event which has been reported in 0.2-0.4% of cases. It requires immediate recognition and urgent surgical or percutaenous intervention. Some types of vascular closure devices have been associated with reports of femoral artery thrombosis or limb ischemia due to embolisation of foreign material or in-situ thrombus formation on intravascular device components. Infection although described without the use of VCDs (0.6%) has more recently been associated predominantly with different types of VCDs. As with all invasive vascular procedures strict attention to aseptic technique is mandatory. Any persistent discomfort over a femoral puncture site following hospital discharge with or without signs of sepsis should prompt investigation to exclude both vascular complications and local sepsis.
One of the most feared complications of femoral artery access remains retroperitoneal bleeding (RPB). A high puncture site has been consistently identified as a predictor of RPB, hence avoiding sheath placement in the external iliac artery or injuring the inferior epigastric artery both of which lie in the retroperitoneal compartment. Other predictors are female gender and low body-surface-area. The incidence of RPB has ranged from 0.2% to 6%. In one large series involving 3,508 patients undergoing PCI and reported in 2005, its incidence was 0.74%. This was concurred in a very recent series involving 11,129 patients post-PCI where the incidence remained at 0.7% [3636. Farouque HM, Tremmel JA, Raissi Shabari F, Aggarwal M, Fearon WF, Ng MK, Rezaee M, Yeung AC, Lee DP. Risk factors for the development of retroperitoneal hematoma after percutaneous coronary intervention in the era of glycoprotein IIb/IIIa inhibitors and vascular closure devices. J Am Coll Cardiol. 2005;45(3):363-368. , 3737. Maluenda G, Delhaye C, Gonzalez M, Ben-Dor I, Gaglai M, Collins S, Wakabayashi K, Hanna N, Torguson R, Xue Z, Suddath W, Satler LF, Kent KM, Lindsay J, Pichard AD, Bernardo N, Waksman R. Conservative versus invasive management strategy for retroperitoneal hemorrhage after percutaneous coronary intervention. J Am Coll Cardiol. 2010;55(10A):A215:E2039 (abstract). ]. Mortality rates ranging from 4% to 12% have been reported. Nearly all patients suffer significant blood loss and require blood transfusions (73.5%-100%). The acquisition of a femoral angiogram before removal of the sheath may allow detection of active bleeding, or even extrinsic bladder compression by a haematoma and occasionally allow for urgent treatment with selective embolisation. Making this diagnosis early is, however, very rare [3838. Chan YC, Morales JP, Reidy JF, Taylor PR. Management of spontaneous and iatrogenic retroperitoneal haemorrhage: conservative management, endovascular intervention or open surgery? Int J Clin Pract. 2008;62(10):1604-1613.
Overview of suggested management options for retroperitoneal haemorrhage.]. Most commonly haemorrhagic complications manifest in the first 6 hours following the end of the procedure. It should be emphasised that in contrast to radial approach, a significant proportion of vascular complications involving femoral artery access require either blood transfusions or surgical repair.
Femoral Artery Access Complications
- Puncture in the CFA is associated with less vascular complications than higher (external iliac artery) or lower (superficial femoral artery/profunda femoris) puncture
- Major femoral bleeding have decreased over the last decade with better access site management
- Bed rest for > 2 hours after femoral artery access is required and limits the risks of access-site bleeding and complications
- Vascular complications often requires transfusion, urgent surgical repair or percutaneous intervention and prolonged hospitalisation
RADIAL ARTERY ACCESS
The major anatomical advantage of the radial artery as compared to femoral artery resides in its relative superficiality, which makes it more easily palpable and facilitates compression to obtain hemostasis ( Figure 7 ). The risks of access-site bleeding thus appear limited. In contrast to femoral or brachial arteries, there are no major nerves or veins in close proximity to the radial artery, hence preventing possible nerve injury at the time of arterial puncture. The radial artery is smaller than the CFA. Yoo et al. used two-dimensional ultrasound in 1191 cases and found a mean radial artery diameter of 2.69 ± 0.40 mm in men and 2.43 ± 0.38 mm in women (range 1.15 mm to 3.95 mm)[3939. Yoo BS, Yoon J, Ko JY, Kim JY, Lee SH, Hwang SO, Choe KH. Anatomical consideration of the radial artery for transradial coronary procedures: arterial diameter, branching anomaly and vessel tortuosity. Int J Cardiol. 2005;101(3):421-427. ]. This is in contrast to the mean diameter of the CFA, which is in the 6 mm range. The ulnar artery which is sometimes used as an alternate to the radial artery, has a similar diameter to the radial artery but it is deeper and the ulnar nerve lies in close proximity.
It remains highly debated in the literature and among radial operators whether dual hand circulation must be verified prior to radial artery puncture [4040. Greenwood MJ, Della-Siega AJ, Fretz EB, Kinloch D, Klinke P, Mildenberger R, Williams MB, Hilton D. Vascular communications of the hand in patients being considered for transradial coronary angiography: is the Allen’s test accurate? J Am Coll Cardiol. 2005;46(11):2013-2017. ]. The radial and ulnar artery are connected at the hand level by the superficial and deep palmar arches. However, a significant number of people have incomplete vascular interconnection at the hand level. To verify dual hand circulation prior to radial artery access, use of the Allen test has been recommended. The test was first described in 1929 to evaluate collateral circulation in the hands of patients with thromboangiitis obliterans [4141. Brzezinski M, Luisetti T, London MJ. Radial artery cannulation: a comprehensive review of recent anatomic and physiologic investigations. Anesth Analg. 2009;109(6):1763-1781.
Description of the arterial anatomy of the hand and management of complications related to arterial cannulation.]. It was then modified by Wright in the 1950s and has lead to the ‘modified Allen test’ as it is applied today ( Figure 8 ). While maintaining occlusive pressure on both the radial and ulnar arteries, the patient is asked to clinch his fist several times until the palmar skin of the hand appears ‘white or blanched’ due to the interruption of the arterial circulation to the hand. The patient is then asked to open his/her hand while avoiding hyperextension. The operator then releases the pressure on the ulnar artery and observes the hand recolouration, hence reperfusion while the radial artery remains occluded. The time for recolouration is noted and is highly variable from 3 to 15 seconds [4141. Brzezinski M, Luisetti T, London MJ. Radial artery cannulation: a comprehensive review of recent anatomic and physiologic investigations. Anesth Analg. 2009;109(6):1763-1781.
Description of the arterial anatomy of the hand and management of complications related to arterial cannulation.]. The test is then repeated while maintaining the ulnar artery occluded (inverse modified Allen test). The test is abnormal when no return to colouration is observed or if it takes excessively long time. The major limitation of the Allen test pertains to its subjectivity and the lack of evidence that it is a reliable predictor of hand ischemia after radial artery canulation or harvest [4141. Brzezinski M, Luisetti T, London MJ. Radial artery cannulation: a comprehensive review of recent anatomic and physiologic investigations. Anesth Analg. 2009;109(6):1763-1781.
Description of the arterial anatomy of the hand and management of complications related to arterial cannulation.]. Barbeau et al. proposed the use of oximetry-plethysmography as a more objective method to assess dual hand circulation prior to radial access ( Figure 9 ) [4242. Barbeau GR, Arsenault F, Dugas L, Simard S, Lariviere MM. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen’s test in 1010 patients. Am Heart J. 2004;147(3):489-493.
Excellent study outlining a sensitive method for determining whether there is sufficient ulnopalmar collateral circulation to the hand.]. Except for the presence of a ‘D’ curve which may indicate lack or poor collateral flow from the ulnar artery and constitutes a relative contra-indication to the use of radial artery access, oximetry-plethysmography testing permitted the use of the radial approach in > 98% of the cases. Importantly no cases of hand ischaemia were seen using this approach. Although the modified Allen test or the oximetry-plethysmography are both non-invasive and rapid techniques to evaluate dual hand circulation prior to radial artery access, they remain controversial given the lack of predictive value for hand ischaemia. Indeed, hand ischaemia remains extremely rare and may in some instances result from distal embolisation rather than radial artery occlusion (RAO) [4343. Valentine RJ, Modrall JG, Clagett GP. Hand ischemia after radial artery cannulation. J Am Coll Surg. 2005;201(1):18-22. ]. Further research using functional assessment of the hand is necessary to determine whether RAO has detrimental clinical consequences and if any test can be predictive of RAO or indicative of not using radial access for cardiac catheterisation.
Given the radial artery’s smaller calibre and its propensity for spasm, it is important that both the patient and the operator work in a quiet environment. Depending on individual preferences, the operator may prefer to puncture the radial artery while being seated or standing with the arm either abducted or alongside the patient (right radial approach). For a left radial approach, the arm can be abducted and then fixed over the abdomen to allow the operator to work from the right side. Catheter laboratory teams must be able to prepare either access site. In particular the use of a radial arm board ( Figure 10A ) and comfortable extension (dorsiflexion) of the wrist will facilitate the success of radial puncture. Dedicated ultrasound guidance is also possible for pulses that are weak to palpation. This may be particularly relevant in patients with cardiogenic shock where radial access may only be palpable in a limited proportion of cases.
Recent multivariate analyses have identified factors predictive of radial access failure in both stable and unstable patients, although these are based on the experience of a tertiary level long-standing radial programme they serve as a useful template. [4444. Abdelaal E, Brousseau-Provencher C, Montminy S, Plourde G, MacHaalany J, Bataille Y, Molin P, Déry J-P, Barbeau G, Roy L, Larose E, De Larochellière, R, Nguyen CM, Proulx, G, Costerousse O, Bertrand OF. Risk Score, Causes, and Clinical Impact of Failure of Transradial Approach for Percutaneous Coronary Interventions. J Am Coll Cardiol Intv. 2013;6(11):1129-1137. ] ( Figure 10B and Figure 10C )
The choice of left vs right radial approach is in most cases a matter of preference [4545. Bertrand OF, Rao SV, Mann T. Reply to: Facilitating radial conversion. JACC Cardiovasc Interv. 2011;4(4):468-469. , 4646. Bertrand OF, Rao SV, Pancholy S, Jolly SS, Rodes-Cabau J, Larose E, Costerousse O, Hamon M, Mann T. Transradial approach for coronary angiography and interventions: results of the first international transradial practice survey. JACC Cardiovasc Interv. 2010;3(10):1022-1031.
Cross-sectional survey of global and regional transradial practice.]. In the TALENT study, performed in normal weight patients, procedural success and radiation exposure both for patients and operators remained similar, although less radiation exposure was noted in procedures performed from the left side by fellows [4747. Sciahbasi A, Romagnoli E, Burzotta F, Trani C, Sarandrea A, Summaria F, Pendenza G, Tommasino A, Patrizi R, Mazzari M, Mongiardo R, Lioy E. Transradial approach (left vs right) and procedural times during percutaneous coronary procedures: TALENT study. Am Heart J. 2011;161(1):172-179. , 4848. Sciahbasi A, Romagnoli E, Trani C, Burzotta F, Sarandrea A, Sunmaria F, Patrizi R, Rao S, Lioy E. Operator radiation exposure during percutaneous coronary procedures through the left or right radial approach. The TALENT Dosimetric Substudy. Circ Cardiovasc Interv. 2011;(in press). ]. It is generally assumed that left radial access can be easier in older patients, women or patients with short stature due to less vessel tortuosities. Intubation of a left internal mammary graft also makes a left radial approach preferable.
Importantly access from the right upper limb may result in catheters being torqued against two pressure points (aortic wall and subclavian vessel wall) as opposed to one (femoral and left upper limb approaches) which makes manipulation more challenging particularly in the presence of anatomical variants.
Several micropuncture kits have been developed for radial (or ulnar) artery puncture. The radial puncture site is usually about 2 cm from the styloid. Radial artery puncture can be performed two ways. With the first technique, the operator can use a modified Seldinger technique with a single anterior wall puncture with 19-21 gauge needle and a short wire (0.018’’-0.035’’) is introduced once back flow is visible ( Figure 3 ). With the ‘angiocath’ technique (through-and-through or posterior wall puncture technique), once the operator observes back flow in the reservoir, (s)he advances the needle through the posterior wall until back flow stops. The needle is then removed and the cannula is slowly pulled back until back flow is observed ( Figure 11 ). At this stage, a small wire may safely be inserted as the cannula is positioned in the right lumen. There has been no study comparing the success rate with the 2 techniques. Therefore, the selection of either one remains a matter of preference. Similar technique can be used for ulnar artery access ( Figure 12 ). Anterior puncture requires a longer learning curve compared to the through-and-through technique and is associated with more guidewire access failure with a similar in haematoma and occlusion rate [4949. Pancholy SB, Sanghvi KA, Patel TM. Radial artery access technique evaluation trial: Randomized comparison of seldinger versus modified seldinger technique for arterial access for transradial catheterization. Catheter Cardiovasc Interv. 2012 Mar 14. doi: 10.1002/ccd.23445. [Epub ahead of print]. ]. Metallic hollow puncture needles facilitate latter technique, whereas cannula-style needles with a flashback chamber facilitate the former. Ultrasound guidance can help improve the success of first time puncture particularly in cases where difficult access is anticipated [5050. Seto AH, Roberts JS, Abu-Fadel MS, Czak SJ, Latif F, Jain SP, Raza JA, Mangla A, Panagopoulos G, Patel PM, Kern MJ, Lasic Z.Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery access with Ultrasound Trial). JACC Cardiovasc Interv. 2015;8:283-291. doi: 10.1016/j.jcin.2014.05.036. Epub 2015 Jan 14. ]. Sheath diameters from 4Fr to 8Fr have been used with radial access although most diagnostic cases can be performed in 4Fr-5Fr and most PCI are currently performed in 5 and 6Fr. More recently, sheathless techniques with home-made standard catheters or specially designed hydrophilic large lumen guiding catheters (Eucath, ASAHI, Japan) have been described with radial or ulnar access [5151. Mamas M, D’Souza S, Hendry C, Ali R, Iles-Smith H, Palmer K, El-Omar M, Fath-Ordoubadi F, Neyses L, Fraser DG. Use of the sheathless guide catheter during routine transradial percutaneous coronary intervention: a feasibility study. Catheter Cardiovasc Interv. 2010;75(4):596-602. ]. The true clinical benefit of this approach remains to be clarified. Of note, hydrophilic-coated sheaths have become popular with transradial techniques as they reduce the force required to remove them and prevent the occurrence of spasm, improving patient comfort. The impact of hydrophilic coating of sheaths or catheters on reducing the risks of RAO post-catheterisation also remains unproven. Long radial sheaths ie 23 cm were usually preferred in the early days of radial approach as a way to reduce radial artery spasm but they have been largely abandoned more recently and replaced by shorter ones with the use of a vasodilator cocktail.
Radial artery access
- The radial artery which is superficial with no adjacent nerve, allows easy compression with no need for bed rest
- Radial or ulnar artery access can be obtained by direct anterior wall puncture or through-and-through technique
- Anatomical variations of radial-brachial-subclavian axis may complicate or prevent catheter advancement or progression of the procedure.
- It is far easier to prevent radial artery spasm than treat it
RADIAL ARTERY ACCESS COMPLICATIONS
Like any other vessel, puncture, sheath insertion and catheter manipulation of the radial (or ulnar) artery may lead to injury and vascular complications [5252. Caputo RP, Tremmel JA, Rao S, Gilchrist IC, Pyne C, Pancholy S, Frasier D, Gulati R, Skelding K, Bertrand O, Patel T. Transradial arterial access for coronary and peripheral procedures: Executive summary by the transradial committee of the SCAI. Catheter Cardiovasc Interv. 2011.
Good overview of contemporary recommended transradial practice, 5353. Kanei Y, Kwan T, Nakra NC, Liou M, Huang Y, Vales LL, Fox JT, Chen JP, Saito S. Transradial cardiac catheterization: A review of access site complications. Catheter Cardiovasc Interv. 2011. ]. However, a small diameter combined with its superficial location, makes the incidence of vascular complications after transradial catheterisation quite limited compared with the femoral artery approach, and more importantly clinical consequences remain relatively benign. Even neointima formation after transradial catheterisation does not preclude repeat use of radial artery. Nevertheless, several vascular complications related to the small size of the radial artery and are specific to the transradial approach (TRA). For example, reduced endothelium-dependent and independent vasodilation has been reported following transradial catheterization [5454. Dawson EA, Rathore S, Cable NT, Wright DJ, Morris JL, Green DJ. Impact of introducer sheath coating on endothelial function in humans after transradial coronary procedures. Circ Cardiovasc Interv. 2010;3(2):148-156. ]. However, it remains reversible at 3 months and its clinical impact remains unknown. In the Table 3 vascular complications associated with femoral or transradial catheterisation as reported in the literature are presented.
A recent meta-analysis of randomised studies reported an incidence of major vascular complications of 1.4% (49/3507 cases) with TRA compared to 3.7% (131/3514 cases) with the femoral approach, a relative reduction of 63% (OR 0.37, 95% CI 0.27 to 0.52, P < 0.0001) [5555. Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, Budaj A, Niemela M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377(9775):1409-1420.
Largest randomised trial comparing femoral and radial approaches.]. Initial and more recent data have generally reported a frequency of radial access-site failure of less than 5% [5656. Guedes A, Dangoisse V, Gabriel L, Jamart J, Chenu P, Marchandise B, Schroeder E. Low rate of conversion to transfemoral approach when attempting both radial arteries for coronary angiography and percutaneous coronary intervention: a study of 1,826 consecutive procedures. J Invasive Cardiol. 2010;22(9):391-397. ]. Reasons associated with access site failure include puncture failure, spasm and anatomical variants (e.g., radial, brachio-cephalic trunk abnormalities or excessive loops), and can be additionally related to lack of experience, small artery size or transient or permanent reduction in vessel diameter. ( Table 4 )
The radial artery is a muscular artery with high reactivity to several vasoactive agents. Cardiac surgeons have long recognised its exquisite propensity of severe and sometimes longstanding spasm and several vasodilating agents have been tested both in vitro and in vivo. In particular, the abundance of alpha-adrenoreceptors in the adventitia makes the radial artery particularly sensitive to circulating agents and to local trauma that trigger spasm. Kiemeneij et al. found an incidence of 34% of radial artery spasm (RAS) defined as pain and maximum force > 1 kg to pull back a 23 cm 6Fr sheath when no vasodilating agent was used [5656. Guedes A, Dangoisse V, Gabriel L, Jamart J, Chenu P, Marchandise B, Schroeder E. Low rate of conversion to transfemoral approach when attempting both radial arteries for coronary angiography and percutaneous coronary intervention: a study of 1,826 consecutive procedures. J Invasive Cardiol. 2010;22(9):391-397. ]. This incidence was reduced to 14% when a combination of verapamil (5 mg) and nitroglycerine was injected intra-arterially. The optimal pharmacological agent or combination to minimize the risk of RAS remains to be determined, although in the randomized SPASM trial, Varenne et al. found an incidence of RAS of 4.9% with a combination of 2.5 mg of verapamil + 1 mg molsidomine compared to 13.3% with verapamil-only (5 mg) and 22.2% when no agent was used [5757. Kiemeneij F, Vajifdar BU, Eccleshall SC, Laarman G, Slagboom T, van der Wieken R. Evaluation of a spasmolytic cocktail to prevent radial artery spasm during coronary procedures. Catheter Cardiovasc Interv. 2003;58(3):281-284. ]. It has been reported that shorter sheaths provoke less RAS and additional factors such as pre-medication with anxiolytics and use of hydrophilic sheaths reduced the incidence of RAS by 60 to 100% [5252. Caputo RP, Tremmel JA, Rao S, Gilchrist IC, Pyne C, Pancholy S, Frasier D, Gulati R, Skelding K, Bertrand O, Patel T. Transradial arterial access for coronary and peripheral procedures: Executive summary by the transradial committee of the SCAI. Catheter Cardiovasc Interv. 2011.
Good overview of contemporary recommended transradial practice, 5959. Rathore S, Stables RH, Pauriah M, Hakeem A, Mills JD, Palmer ND, Perry RA, Morris JL. Impact of length and hydrophilic coating of the introducer sheath on radial artery spasm during transradial coronary intervention: a randomized study. JACC Cardiovasc Interv. 2010;3(5):475-483. ]. In cases of severe RAS occurring during the procedure, papaverine, a direct myorelaxant, or profound analgesia with intravenous midazolam/sufentanil or regional anaesthesia may be required. Very rare cases of partial or complete radial artery avulsion have also been described when operators forcefully removed catheter or sheath entrapped into diffuse and severe RAS [6060. Dieter RS, Akef A, Wolff M. Eversion endarterectomy complicating radial artery access for left heart catheterization. Catheter Cardiovasc Interv. 2003;58(4):478-480. ].
Access site failure may also result from anatomical variations, severe tortuosities or loops in radial or brachial arteries. The incidence of these variations in vessel anatomy is close to 5% [6161. Burzotta F, Trani C, De Vita M, Crea F. A new operative classification of both anatomic vascular variants and physiopathologic conditions affecting transradial cardiovascular procedures. Int J Cardiol. 2010;145(1):120-122. , 6262. Lo TS, Nolan J, Fountzopoulos E, Behan M, Butler R, Hetherington SL, Vijayalakshmi K, Rajagopal R, Fraser D, Zaman A, Hildick-Smith D. Radial artery anomaly and its influence on transradial coronary procedural outcome. Heart. 2009;95(5):410-415. ]. Most of the time, operators manage to negotiate them using hydrophilic wires, 0.014” angioplasty wires or hydrophilic catheters. It is advised to perform upper limb angiography of the arm in case of difficulty of wire or catheter advancement, as failure to identify the problem may lead to vessel perforation or dissection. Radial or brachial artery dissections can produce dramatic angiographic images but it should be emphasised that they represent retrograde dissections. Therefore, it is worth attempting to carefully recross them with a soft 0.014” angioplasty wire. If this attempt is successful, the catheter will usually seal the dissection or perforation, and there will be no clinical consequence for the patient. Failure to recognise these perforations might be dramatic as slow and delayed intra-muscular bleeding may potentially lead to compartment syndrome.
The incidence of compartment syndrome after intramuscular bleeding or vascular damage is extremely low but should be promptly recognized as it may lead to devastating consequences. One series reported acute compartment syndrome in 5 cases out of 250 patients treated (2%) while a more recent study reported 2 cases in 51,296 procedures (<0.01%) [6363. Tizon-Marcos H, Barbeau GR. Incidence of compartment syndrome of the arm in a large series of transradial approach for coronary procedures. J Interv Cardiol. 2008;21(5):380-384. ]. Open surgical fasciotomy can be avoided if effective preventative measures are employed as soon as local bleeding is suspected. These include:
1. stopping intravenous anticoagulant therapy,
2. control of pain and blood pressure,
3. use of transient external compression with a blood pressure cuff.
Two meta-analyses of randomised trials have found a relative reduction > 75% of bleeding with TRA compared with the femoral approach [6464. Agostoni P, Biondi-Zoccai GG, de Benedictis ML, Rigattieri S, Turri M, Anselmi M, Vassanelli C, Zardini P, Louvard Y, Hamon M. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44(2):349-356. , 6565. Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157(1):132-140. ]. The benefit for TRA remains even if closure devices are used with femoral access [6666. Bertrand OF, Larose E, Rodes-Cabau J, Gleeton O, Taillon I, Roy L, Poirier P, Costerousse O, Larochelliere RD. Incidence, predictors, and clinical impact of bleeding after transradial coronary stenting and maximal antiplatelet therapy. Am Heart J. 2009;157(1):164-169. ]. Access site bleeding represents 60-80% of bleeding episodes with the femoral approach and the major benefit of TRA is the dramatic reduction of major bleeding related to access site. Most initial studies comparing bleeding with radial and femoral approaches did not use standardized bleeding classifications. More recently, in the EASY trial, using radial access and heparin plus abciximab in all-comers, authors reported an incidence of 1.4% major bleeding using the PCI Linking Angiomax to Reduced Clini">REPLACE-2 criteria and 0.5% using the TIMI criteria [6767. Bertrand OF. Acute forearm muscle swelling post transradial catheterization and compartment syndrome: prevention is better than treatment! Catheter Cardiovasc Interv. 2010;75(3):366-368.
Useful practical guidance for the prevention and management of compartment syndrome following a transradial procedure.]. They also developed a hematoma classification scheme adapted to TRA and which can be compared to recent hematoma scale proposed with femoral approach [6868. Mina GS, Gobrial GF, Modi K, Dominic P.Combined Use of Bivalirudin and Radial Access in Acute Coronary Syndromes Is Not Superior to the Use of Either One Separately: Meta-Analysis of Randomized Controlled Trials. JACC Cardiovasc Interv. 2016 ;9:1523-31. doi: 10.1016/j.jcin.2016.05.023. ] ( Figure 13 ). In this trial performed with maximal antiplatelet therapy, the incidence of haematoma < 5 cm (grade I) was 5.3%, < 10 cm (grade II) was 2.5%, distal to the elbow (grade III) was 1.6%, and proximal to elbow (grade IV) was 0.1%. It should be noted that while grade I and II haematomas, are directly related to the puncture site, higher grades usually result from wire damages to vessels and small perforations. These may induce very unusual and rare hematomas such as haematoma of the pectoral muscle, of the neck or even of the mediastinum. It should be noted that the use of new anti-thrombotic agents such as bivalirudin reduces the incidence of major bleeding with transfemoral but not TRA [6969. Spaulding C, Lefevre T, Funck F, Thebault B, Chauveau M, Ben Hamda K, Chalet Y, Monsegu H, Tsocanakis O, Py A, Guillard N, Weber S. Left radial approach for coronary angiography: results of a prospective study. Cathet Cardiovasc Diagn. 1996;39(4):365-370. ].
Interestingly, anticoagulant therapy is necessary with TRA to reduce the risks of RAO after transradial catheterisation. Although asymptomatic most of the time, the incidence of RAO at hospital discharge varies from 0% to >10% depending on the technique used to obtain hemostasis, the anticoagulation regimen and the methods to assess radial artery patency. Spaulding et al. evaluated post-procedure RAO using echo-doppler, 4-5 hours after TRA with 5Fr sheaths and various doses of heparin. Without heparin, the incidence of asymptomatic RAO was 74%, which was reduced to 4.3% with 5,000 IU of heparin [7070. Bernat I, Bertrand OF, Rokyta R, Kacer M, Pesek J, Koza J, Smid M, Bruhova H, Sterbakova G, Stepankova L, Costerousse O. Efficacy and safety of transient ulnar artery compression to recanalize acute radial artery occlusion after transradial catheterization. Am J Cardiol. 2011;107(11):1698-1701. ]. A recent randomised study using 5Fr sheaths for diagnostic angiography confirmed that 5,000 IU heparin was more effective than 2,000 IU heparin dose [7171. Cubero JM, Lombardo J, Pedrosa C, Diaz-Bejarano D, Sanchez B, Fernandez V, Gomez C, Vazquez R, Molano FJ, Pastor LF. Radial compression guided by mean artery pressure versus standard compression with a pneumatic device (RACOMAP). Catheter Cardiovasc Interv. 2009;73(4):467-472. ]. Other factors have been implicated with radial artery patency post-catheterisation and include large artery-catheter mismatch, female gender, pre-treatment with clopidogrel, diabetes, and haemostasis technique. Indeed, it has been shown recently that maintaining radial artery patency during hemostasis could reduce the incidence of RAO by 50% [7272. Pancholy S, Coppola J, Patel T, Roke-Thomas M. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. 2008;72(3):335-340.
Description of the patent haemostasis technique to reduce the frequency of radial artery occlusion following a transradial procedure., 7373. Pancholy SB. Comparison of the effect of intra-arterial versus intravenous heparin on radial artery occlusion after transradial catheterization. Am J Cardiol. 2009;104(8):1083-1085. ]. Notably, the incidence of RAO does not seem to be influenced whether bivalirudin or low molecular weight heparin is used in place of heparin, or alternatively whether the drug is delivered intravenously or intra-arterially [7373. Pancholy SB. Comparison of the effect of intra-arterial versus intravenous heparin on radial artery occlusion after transradial catheterization. Am J Cardiol. 2009;104(8):1083-1085. , 7474. Plante S, Cantor WJ, Goldman L, Miner S, Quesnelle A, Ganapathy A, Popel A, Bertrand OF. Comparison of bivalirudin versus heparin on radial artery occlusion after transradial catheterization. Catheter Cardiovasc Interv. 2010;76(5):654-658. ]. At the present time, although the technique of patent haemostasis is beneficial in reducing RAO it remains undetermined whether it can be further modulated by the type of haemostastic device used. Overall there are 4 main types: the simple use of gauze pads, bracelet-type, ‘plastic’ and elastic straps and an ‘air-bag’ system [4646. Bertrand OF, Rao SV, Pancholy S, Jolly SS, Rodes-Cabau J, Larose E, Costerousse O, Hamon M, Mann T. Transradial approach for coronary angiography and interventions: results of the first international transradial practice survey. JACC Cardiovasc Interv. 2010;3(10):1022-1031.
Cross-sectional survey of global and regional transradial practice.].
Furthermore, it should be emphasised that considering the dual hand circulation, most occurrences of RAO remain asymptomatic and their incidence decreases with time post-procedure. Conversely, prolonged duration of radial artery compression can be associated with persisting RAO and delayed reflex sympathetic dystrophy.
Complications resulting from vessel trauma such as pseudo-aneurysm or arterio-venous fistula are rare, presumably due to the small calibre of these vessels. They are usually managed by repeat and prolonged compression, and do not require surgical intervention. The use of certain types of hydrophilic coated-sheaths (Cook Inc., USA) has been associated with severe granulomatous local reactions of the skin [7575. Zellner C, Ports TA, Yeghiazarians Y, Boyle AJ. Sterile radial artery granuloma after transradial procedures: a unique and avoidable complication. Catheter Cardiovasc Interv. 2010;76(5):673-676. ]. The practice of radial artery angiography or intervention will temporarily reduce digital perfusion during the procedure itself, but it is not associated with permanent hand dysfunction [7676. van Leeuwen MAH, van der Heijden DJ, Hollander MR, Mulder MJ, van de Ven PM, Ritt MJPF, Kiemeneij F, van Mieghem NM, van Royen N. ACRA Perfusion Study. Circ Cardiovasc Interv. 2019 ;12:e007641. ]
In conclusion, vascular access complications after TRA for diagnostic angiography or interventions are rare and, almost always can be managed conservatively. The reduction of these complications when compared to those resulting from the femoral approach is also associated with a 15% reduction in health-care costs [7777. Zellner C, Ports TA, Yeghiazarians Y, Boyle AJ. Sterile radial artery granuloma after transradial procedures: a unique and avoidable complication. Catheter Cardiovasc Interv. 2010;76(5):673-676. ]. It should be emphasised that experience accumulated over the last 15 years with TRA has demonstrated that rapid recognition of these complications is vital in order to prevent severe complications ie hand ischaemia or surgical repair.
Radial Artery Complications
- Access site bleeding is rare and easily managed
- Vascular complications are rare and do not require surgical repair
- Anticoagulation is required to reduce the rate of radial artery occlusion, even following diagnostic angiography
- Radial artery occlusion, although most of the time asymptomatic, can be associated with hand ischaemia, therefore its occurrence should be reduced by the use of heparin, smaller sheath sizes, and ‘patent-haemostasis’
- Compartment syndrome is a rare but limb-threatening complication of rapidly evolving forearm haematoma. This must be detected and managed aggressively to prevent the development of ischaemia
ALTERNATIVE ACCESS
Ulnar artery access
The ulnar artery typically has a larger diameter than the radial artery. It can be used as a feasible alternative access point even in the case of ipsilateral access leading to radial occlusion or when used immediately after an unsuccessful radial attempt. The first reported case series was by Terashima et al in the context of coronary angiography [7878. Terashima M, Meguro T, Takeda H, et al. Percutaneous ulnar artery approach for coronary angiography: a preliminary report in nine patients. Catheter Cardiovasc Interv 2001; 53:410-4. ]. Observational and randomised series have been published subsequently supporting transulnar access as a safe and efficient access approach. A small randomised study by experienced radial operators has suggested equivalent success rates for both radial and ulnar access for diagnostic and interventional coronary procedures without neurological injury to the ulnar nerve that lies in close proximity [7979. Aptecar E, Pernes JM, Chabane-Chaouch M, et al. Transulnar versus transradial artery approach for coronary angioplasty: the PCVI-CUBA study. Catheter Cardiovasc Interv 2006; 67:711-20. ]. A larger multicentre study randomising 902 patients to default radial or ulnar approach PCI found the composite primary endpoint of cross-over to another arterial access, major adverse cardiovascular events, and major vascular events of the arm at 60 days to be inferior with the ulnar approach as a result of a high frequency of crossover (32.3% vs 5.9%). Once ulnar access was obtained successfully, however, major complications were not significantly different between the approaches [8080. Hahalis G, Tsigkas G, Xanthopoulou I, Deftereos S, Ziakas A, Raisakis K, Pappas C, Sourgounis A, Grapsas N, Davlouros P, Galati A, Plakomyti TE, Mylona P, Styliadis I, Pyrgakis V, Alexopoulos D. Transulnar compared with transradial artery approach as a default strategy for coronary procedures: a randomized trial. The Transulnar or Transradial Instead of Coronary Transfemoral Angiographies Study (the AURA of ARTEMIS Study). Circ Cardiovasc Interv. 2013 Jun;6(3):252-61. ]. Systematic review and pairwise meta-analysis of 6 randomised trials (n=5267) comparing transulnar with transradial support, non-inferiority for access related endpoints [8181. Fernandez R, Zaky F, Ekmejian A, Curtis E, Lee A. Safety and efficacy of ulnar artery approach for percutaneous cardiac catheterization: Systematic review and meta-analysis. Catheter Cardiovasc Interv. 2018 ;91:1273-1280. doi: 10.1002/ccd.27479. Epub 2018 Feb 1. ]. However, the majority of procedures have been performed by experienced operators.
Further data are required before this approach is considered equivalent to radial access in the broader interventional community, and the use of ultrasound is considered mandatory in order to help minimise complications. Although the frequency of anatomical anomalies, in particular radio-ulnar loops, that preclude a transradial approach is low (<2%), the ability to utlise an alternate upper limb access point is a valuable skill [8282. Fujii T, Masuda N, Tamiya S, Shima M, Toda E, Ito D, et al. Angiographic evaluation of right upper-limb arterial anomalies: implications for transradial coronary interventions. J Invasive Cardiol. 2010;22:536–40. ].
Distal Transradial access
This technique involves puncture of the distal radial artery in the anatomical snuffbox. It was developed by Babunashvilli [8383. Sgueglia GA, Di Giorgio A, Gaspardone A, Babunashvili A. Anatomic Basis and Physiological Rationale of Distal Radial Artery Access for Percutaneous Coronary and Endovascular Procedures. JACC Cardiovasc Interv. 2018 22;11:2113-2119. doi: 10.1016/j.jcin.2018.04.045. ] and since then the advantage of left distal transradial artery (ldTRA) for coronary angiography and intervention has been promulgated by early adopters who advocate it as a default approach [8484. Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). EuroIntervention. 2017;13:851-857. doi: 10.4244/EIJ-D-17-00079. , 8585. Soydan E, Akın M. Coronary angiography using the left distal radial approach - An alternative site to conventional radial coronary angiography. Anatol J Cardiol. 2018;19:243-248. doi: 10.14744/AnatolJCardiol.2018.59932. Epub 2018 Mar 21. , 8686. Lee JW, Park SW, Son JW, Ahn SG, Lee SH.Real-world experience of the left distal transradial approach for coronary angiography and percutaneous coronary intervention: a prospective observational study (LeDRA). EuroIntervention. 2018;14:e995-e1003. doi: 10.4244/EIJ-D-18-00635. ]. The technique is also feasible for viscieral interventions [8787. Pua U, Sim JZT, Quek LHH, Kwan J, Lim GHT, Huang IKH.Feasibility Study of "Snuffbox" Radial Access for Visceral Interventions. J Vasc Interv Radiol. 2018 ;29:1276-1280. doi: 10.1016/j.jvir.2018.05.002. ].The knowledge of the technique has benefitted from social media on twitter (using the#ldtra).
Advantages include greater comfort for patients (by using the non-dominant hand) and by allowing freer movement (compared to conventional proximal radial access), more ergonomic support in the case of ldTRA, more rapid patient mobilisation/discharge [ref 88], and preservation of the proximal (conventional) radial access site. In addition, it can preserve the proximal radial for use as a conduit in surgical coronary revascularisation and for fistulous access in the case of potential renal replacement therapy. These benefits are of particular value in patients with significant upper limb arthritis or obese patients.
Randomised controlled clinical trials comparing ldTRI with conventional radial intervention are required to evaluate relative efficacy. Published data have thus far reported a low frequency of complications however randomised and also larger multicentre observational datasets are needed to confirm safety.
Current potential indications for left distal transradial access:
Right-handed patients
Left-handed patients with an:
Inaccessible proximal radial artery
Proximal radial vasospasm
Anatomically hostile right radial approach
The presence of an iatrogenic AV fistula in the right arm
Significant upper limb arthritis
Obesity
Requirement for LIMA graft angiography
Current contra-indications for left distal transradial access:
No palpable artery in the anatomical snuffbox
Distal radial artery minimum diameter <2mm
Emergency invasive coronary angiography/intervention during the learning curve
Left-sided hand venous cannula obstructing the snuffbox.
Tips and tricks for left distal transradial access
Ask the patient to flex the thumb under fully flexed fingers and apply mild ulnar flexion
Use the right femoral drape hole in the conventional fenestrated drape
Place support under the left arm to direct the pronated wrist as far toward the right groin as possible
Aim for an anterior wall puncture (to avoid injury to the bony floor of the snuffbox)
Use ultrasound to guide precise puncture.
Advance the guidewire under fluoroscopy (to avoid passage into the palmar arch)
All other aspects are identical to a conventional proximal radial approach to access
The aim is to puncture in the anatomical snuffbox, distal to the superficial palmar branch and proximal to the pollicis brevis artery. At this point the diameter is similar to that of the proximal radial. By being distal to the superficial palmar branch any occlusion does not compromise the arterial supply to the hand. The anatomical snuffbox is bounded at its ulnar border by the tendon of extensor pollicis longus, on its radial border by the tendons of abductor pollicis longus and extensor pollicis brevis. The floor includes the carpal bones, scaphoid and trapezium.
OTHER
Brachial artery access
Open brachial surgical cut-down was the standard access technique in the pioneer era of invasive cardiology. The skill set required for this technique is diminishing with time as less of these procedures are performed and contemporary radial and femoral techniques are nearly always possible. There may be instances where brachial access is required such as severe peripheral arterial disease or Takayasu’s arteritis. The close proximity of the median nerve and lack of collateral circulation mean that it should only be undertaken by operators already sufficiently experienced with the technique. Arterial thrombosis and also neurological injury being potentially devastating complications Percutaneous access although reported carries potentially higher risk of complications as superior haemostasis is possible with open surgical cut-down.
Translumbar access
This is of historical interest only. It involved a prone patient and fluoroscopically-guided puncture in the angle between the vertebral column and the 12th rib by ’walking’ the puncture needle across a vertebra until aortic pulsation is encountered.
Axillary access
This is frequently described as ‘subclavian’ access but anatomically refers to percutaneous (as opposed to open surgical ‘cutdown’) access to the axillary artery between the outer border of the first rib and the medial border of Teres major. There are limited data and anecdotal experience with this access point. Historically, invasive coronary procedures have been associated with a high frequency of complications including brachial plexus injury, but there has been resurgent interest in the era of percutaneous aortic valve replacement as an alternative large bore access site to the transfemoral approach. In this context, more contemporary series using fluoroscopic and ultrasound guidance as well as the presence of a ‘safety’ wire via the femoral approach, both to identify the artery and allow for covered stent deployment in the vent of unsuccessful vascular closure have made this approach more predictable. Extended datasets, however, in the wider interventional community are required to evaluate efficacy and safety.
Impact of access site and non-access site bleeding
It should be recognised that some blood loss and moderate bleeding has long been associated with femoral PCI. Indeed, in the 1980’s, patients after PTCA and heparin anticoagulation suffered haemoglobin (Hb) drops of 1-2 g/dl within 24h, with 6% of patients presenting with a fall in Hb of > 3g/dl [9292. Ellis SG, Roubin GS, Wilentz J, Douglas JS, Jr., King SB, 3rd. Effect of 18- to 24-hour heparin administration for prevention of restenosis after uncomplicated coronary angioplasty. Am Heart J. 1989;117(4):777-782. , 9393. Jauch W, Kurnik PB, Hanlon SJ, Siegel JE, Matthai WH, Jr. Expected hemoglobin decrease following percutaneous transluminal coronary angioplasty. Am J Cardiol. 1997;80(1):71-74. ]. In a recent study with transradial PCI and heparin plus abciximab, the mean Hb fall was 0.6 ± 1.0 g/dl with only 1.3% of patients presenting Hb drop > 3 g/dl within 24h [9494. Bertrand OF, Larose E, Rodes-Cabau J, Rinfret S, Dery JP, Bagur R, Gleeton O, Nguyen CM, Proulx G, De Larochelliere R, Poirier P, Costerousse O, Roy L. Incidence, range, and clinical effect of hemoglobin changes within 24 hours after transradial coronary stenting. Am J Cardiol. 2010;106(2):155-161. ]. Most vascular complications arising with femoral PCI i.e. large haematomas or retroperitoneal bleeding are significant, sometimes requiring transfusion, invasive surgical intervention and importantly premature discontinuation of antithrombotic therapy following stent deployment. Major bleeding, whichever definition or antithrombotic regimen used, has been identified as an independent predictor for mortality and major adverse events post-PCI performed by femoral or radial access [6767. Bertrand OF. Acute forearm muscle swelling post transradial catheterization and compartment syndrome: prevention is better than treatment! Catheter Cardiovasc Interv. 2010;75(3):366-368.
Useful practical guidance for the prevention and management of compartment syndrome following a transradial procedure., 9595. Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, Dangas GD, Lincoff AM, White HD, Moses JW, King SB, 3rd, Ohman EM, Stone GW. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49(12):1362-1368. ]. It should be emphasised that access site related bleeding was reported to represent > 50% of the major haemorrhagic events. More recently, non-access site bleeding after PCI has been reported as representing ~60% of major bleeding in patients presenting with acute coronary syndromes [5555. Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, Budaj A, Niemela M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377(9775):1409-1420.
Largest randomised trial comparing femoral and radial approaches.]. Doyle et al. reviewed the incidence of major femoral bleeding at the Mayo Clinic in 17,901 patients separated into 3 groups: group 1 (1994-95), group 2 (1996-99) and group 3 (2000 to 2005). Overall, the incidence of major femoral bleeding (haematoma > 4 cm or any femoral bleeding requiring transfusion, surgery or prolonged stay or retroperitoneal bleeding) decreased from 8.4% in group 1 to 5.3% in group 2, and 3.5% in group 3 [9696. Doyle BJ, Ting HH, Bell MR, Lennon RJ, Mathew V, Singh M, Holmes DR, Rihal CS. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994 to 2005. JACC Cardiovasc Interv. 2008;1(2):202-209.
Large registry evaluating the factors associated with femoral arterial access complications]. Reductions in sheath size, intensity and duration of anticoagulation with heparin and procedural time were identified as independent predictors of major femoral bleeding. A large observational study including 103,070 patients undergoing femoral PCI has also shown that larger calibre sheaths (7Fr and 8Fr) were associated with more vascular access complications, bleeding and transfusions, and also with mortality and major cardiac adverse events when compared with 6Fr [9797. Grossman PM, Gurm HS, McNamara R, Lalonde T, Changezi H, Share D, Smith DE, Chetcuti SJ, Moscucci M. Percutaneous coronary intervention complications and guide catheter size: bigger is not better. JACC Cardiovasc Interv. 2009;2(7):636-644. ]. In addition to sheath size reduction, the use of the direct antithrombin, bivalirudin has been associated with a significant reduction of access site and non-access site bleeding following femoral PCI [9898. Hamon M, Rasmussen LH, Manoukian SV, Cequier A, Lincoff MA, Rupprecht HJ, Gersh BJ, Mann T, Bertrand ME, Mehran R, Stone GW. Choice of arterial access site and outcomes in patients with acute coronary syndromes managed with an early invasive strategy: the ACUITY trial. EuroIntervention. 2009;5(1):115-120. ]. The impact of non-access site bleeding on outcomes appears greater than access site bleeding [9999. Verheugt FW, Steinhubl SR, Hamon M, Darius H, Steg PG, Valgimigli M, Marso SP, Rao SV, Gershlick AH, Lincoff AM, Mehran R, Stone GW. Incidence, prognostic impact, and influence of antithrombotic therapy on access and nonaccess site bleeding in percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4(2):191-197.
Study highlighting the importance of non-access related bleeding and the effect of different antithrombotic regimes.].
The principal benefit of the transradial approach is the virtual abolition of access-site related bleeding whichever antithrombotic regimen is employed [5555. Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, Budaj A, Niemela M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377(9775):1409-1420.
Largest randomised trial comparing femoral and radial approaches., 6767. Bertrand OF. Acute forearm muscle swelling post transradial catheterization and compartment syndrome: prevention is better than treatment! Catheter Cardiovasc Interv. 2010;75(3):366-368.
Useful practical guidance for the prevention and management of compartment syndrome following a transradial procedure., 100100. Pristipino C, Trani C, Nazzaro MS, Berni A, Patti G, Patrizi R, Pironi B, Mazzarotto P, Gioffre G, Biondi-Zoccai GG, Richichi G. Major improvement of percutaneous cardiovascular procedure outcomes with radial artery catheterisation: results from the PREVAIL study. Heart. 2009;95(6):476-482. ]. In the large MORTAL observational study, the authors evaluated the incidence and clinical impact of peri-procedural tranfusion in 32,822 patients after radial or femoral PCI [101101. Chase AJ, Fretz EB, Warburton WP, Klinke WP, Carere RG, Pi D, Berry B, Hilton JD. Association of the arterial access site at angioplasty with transfusion and mortality: the M.O.R.T.A.L study (Mortality benefit Of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg). Heart. 2008;94(8):1019-1025. ]. The rate of transfusion was halved with radial access and this was correlated with a reduction in 30-day and 1-year mortality after adjustment for confounding variables. The benefit of radial access compared to the femoral approach to reduce the rate of transfusion was also demonstrated in a meta-analysis of randomized studies with a rate of 0.8% (43/5424) after radial approach compared to 1.2% (67/5438) after femoral approach [5555. Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, Budaj A, Niemela M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377(9775):1409-1420.
Largest randomised trial comparing femoral and radial approaches.]. Given the relationship between peri-procedural bleeding, transfusion and mortality, it has been hypothesized that a radial approach could reduce the risks of mortality compared to the standard femoral approach. In the largest study to date, radial versus femoral trial (RIVAL), Jolly et al. randomised 7021 ACS patients referred for diagnostic angiography and possible PCI. At 30 days, the primary outcome combining death, MI, stroke or non-CABG related bleeding (cut-off value of 5 g for haemoglobin drop and overt bleeding) was 3.7% in the radial group and 4.0% in the femoral group (P = 0.50). Overall, the rate of major vascular complications was significantly reduced to 1.4% in the radial group compared to 3.7% in the femoral group (P < 0.0001). The rate of 30-day mortality after primary PCI for the STEMI pre-specified sub-group was significantly reduced to 1.3% with a radial approach compared to 3.2% after a femoral approach (HR 0.39, 95% CI 0.20-0.76, P = 0.006) [5555. Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, Budaj A, Niemela M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377(9775):1409-1420.
Largest randomised trial comparing femoral and radial approaches.]. Importantly, the authors observed that better results were obtained in centres with more experience (>300 procedures per annum) with the radial approach and in higher-risk patients (STEMI). Following the publication of RIVAL’s results, interventional societies have recommended better education and training in the radial approach, which is now a class IIa recommendation in the ACC/AHA/SCAI guidelines [5252. Caputo RP, Tremmel JA, Rao S, Gilchrist IC, Pyne C, Pancholy S, Frasier D, Gulati R, Skelding K, Bertrand O, Patel T. Transradial arterial access for coronary and peripheral procedures: Executive summary by the transradial committee of the SCAI. Catheter Cardiovasc Interv. 2011.
Good overview of contemporary recommended transradial practice, 102102. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. J Am Coll Cardiol. 2011. ]. More recently RIFLE-STEACS randomised 1001 patients to a radial or femoral approach in acute STEMI. Investigators found a benefit for the radial approach in terms of the primary endpoint of net adverse clinical events [NACE:MACE+ major bleeding complications] (21% vs 13.6% p=0.003). The 30 day MACE was favourable for the radial approach (7.2% vs 11.4% p=0.029), driven by a reduction in cardiac mortality [103103. Romagnoli E, Biondi-Zoccai G, Sciahbasi A, Politi L, Rigattieri S, Pendenza G, Summaria F, Patrizi R, Borghi A, Di Russo C, Moretti C, Agostoni P, Loschiavo P, Lioy E, Sheiban I, Sangiorgi G. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol. 2012 60(24):2481-9. ]. Haemorrhagic complications were also reduced, driven entirely by a 47% reduction in access site bleeding. STEMI-RADIAL randomised 707 patients to a radial or femoral approach in acute STEMI found similar dramatic reduction in access-related complications (1.4% vs 7.2% p=0.0001) but not in MACE between the groups [NACE 4.6%vs 11.0% p=0.0028, MACE:3.5% vs 4.2% p=0.7] [104104. Bernat I, Horak D, Stasek J, Mates M, Pesek J, Ostadal P, Hrabos V, Dusek J, Koza J, Sembera Z, Brtko M, Aschermann O, Smid M, Polansky P, Al Mawiri A, Vojacek J, Bis J, Costerousse O, Bertrand OF, Rokyta R. ST-segment elevation myocardial infarction treated by radial or femoral approach in a multicenter randomized clinical trial: the STEMI-RADIAL trial. J Am Coll Cardiol. 2014;63(10):964-72. ]. Notable in these studies were differences in antithrombotic therapy, the low frequency of Bivalirudin (<10%) use and femoral vascular closure device use (<30%). The former is being addressed specifically in the forthcoming SAFARI-STEMI and MATRIX randomised studies.
The SAFE-PCI registry-based randomised study has evaluated radial and femoral access in women undergoing PCI or coronary angiography (predominantly lower risk elective and NonSTEACS procedures). In 1787 patients investigators showed some evidence of reduction in bleeding according to the BARC classification in the total cohort (0.6% vs 1.7% p=0.03), but not in the subgroup of patients undergoing PCI (1.2% vs 2.9% p=0.12). Proportionate reduction on bleeding, however, was consistent with that seen in previous studies. Bivalirudin was used in 59.1% of radial and 65.8% of femoral cases. Vascular closure devices were used in 5.1% (radial crossover to femoral cases) and 65.5% of intention-to-treat femoral cases [Presented at TCT 2013 Rao S].
Thus patients with a higher risk profile appear to benefit the most. Even cardiogenic shock is not a contraindication to the radial approach in experienced centres but is more challenging due to the presence of a weak pulse [105105. Bernat I, Abdelaal E, Plourde G, Bataille Y, Cech J, Pesek J, Koza J, Jirous S, Machaalany J, Déry J-P, Costerousse O, Rokyta R, Bertrand OF. Early and late outcomes after primary percutaneous coronary intervention by radial or femoral approach in patients presenting in acute ST-elevation myocardial infarction and cardiogenic shock. Am Heart J. 2013; 165:338-43 ]. Furthermore door-device times in PPCI are not significantly increased compared to the femoral approach in large registry observations [106106. Baklanov DV, Kaltenbach LA, Marso SP, Subherwal SS, Feldman DN, Garratt KN, Curtis IP, Messenger JC, Rao SV. The prevalence and outcomes of transradial percutaneous coronary intervention for ST-segment elevation myocardial infarction: analysis from the National Cardiovascular Data Registry (2007 to 2011). J Am Coll Cardiol. 2013;61:420-426 ].
Nevertheless, despite the evidence of reduced access-site complications and potential utility in acute STEMI with the radial approach some recent observational data suggest conversely that default radial operators may encounter more frequent access-site complications when crossing-over to a femoral approach [107107. Rafie IM, Uddin MM, Ossei-Gerning N, Anderson RA, Kinnaird TD. Patients undergoing PCI from the femoral route by default radial operators are at high risk of vascular access-site complications. EuroIntervention. 2014 ;9(10):1189-94. ].
Given the increased uptake of the radial approach, updated consensus guidelines now exist from Europe and North America to give practitioners a contemporary framework for defining technique, optimal indications and operator training experience [108108. Hamon M, Pristipino C, Di Mario C, Nolan J, Ludwig J, Tubaro M, Sabate M, Mauri-Ferré J, Huber K, Niemelä K, Haude M, Wijns W, Dudek D, Fajadet J, Kiemeneij F. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care** and Thrombosis of the European Society of Cardiology European Association of Percutaneous Cardiovascular Interventions; Working Group on Acute Cardiac Care of the European Society of Cardiology; Working Group on Thrombosis on the European Society of Cardiology. EuroIntervention. 2013 ;8(11):1242-51. , 109109. Rao SV, Tremmel JA, Gilchrist IC, Shah PB, Gulati R, Shroff AR,Van Crisco, Woody W, Zoghbi G, Duffy PL, Sanghvi K, Krucoff MW, Pyne CT, Skelding KA, Patel T, Pancholy SB. Best Practices for Transradial Angiography and Intervention: A Consensus Statement From the Society for Cardiovascular Angiography and Intervention’s Transradial Working Group ]
In summary, although the femoral approach remains the most popular access site for cardiac catheterisation and interventions, the benefits of radial access in reducing vascular complications and peri-procedural bleeding may translate into increased uptake in the future. Whether, direct antithrombin agents such as bivalirudin or new anticoagulation regimens will further reduce non-access site bleeding with both radial and femoral approaches represent the next area to be tested in randomised trials. Importantly, there is a learning curve and operator volume required for any practical technique to be conducted safely and efficiently. This means that high volume transfemoral operators who occasionally use a transradial approach may encounter unacceptable procedural success rates or complications and vice-versa for radial operators. Under such circumstances no one access technique can be adopted universally. Instead a balanced approach based on clinical, anatomical, technical, and operator factors needs to be used.
Access site and non-access site bleeding
- Major bleeding after femoral or transradial PCI is an independent predictor of mortality
- Radial access reduces substantially access-site related bleeding and complications
- The clinical impact of non-access site bleeding is stronger than access-site related bleeding



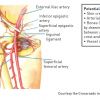


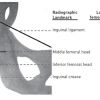


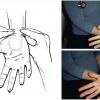





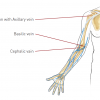




Very nice demonstration.Rotablation might have helped both the case and the viewers.
Use of Long 23 Cm Femoral sheaths & stiffer guidewires becomes necessary with very tortuous Iliac arteries prevent Torsion-kinking of diagnostic & guide catheters. Also when catheters initially fail to cross these iliac tortuosities use of stiff wires to straighten the iliac arteries thus facilitating placement of catheters.
Absolutely clear.
very clearly