Introduction
Stent thrombosis (ST) is a medical emergency often associated with death (20%-40%), MI (50%-70%) or the requirement for urgent repeat revascularisation [11. Holmes DR Jr, Kereiakes DJ, Garg S, Serruys PW, Dehmer GJ, Ellis SG, Williams DO, Kimura T, Moliterno DJ. Stent Thrombosis. J Am Coll Cardiol. 2010;56:1357-1365.
This manuscript is a comprehensive review of the issue, also offering an international perspective., 22. Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michey I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126-30. , 33. Doyle B, Rihal CS, O’Sullivan CJ, Lennon RJ, Wiste HJ, Bell M, Bresnahan J, Holmes DR Jr. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation. 2007;116:2391-8. , 44. Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Jünj P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667-78. ]. The clinical consequences of ST are dependent upon the volume and viability of myocardium at risk, the degree of collateral vessel recruitment and the timeliness of emergency reperfusion therapy. Although drug-eluting stents (DES) reduce angiographic and clinical restenosis compared with bare metal stents (BMS), the incidence of ST has been variable, depending on DES stent type and the time course for ST occurrence may differ as well [55. Roukoz H, Bavry AA, Sarkees ML, Mood GR, Kumbhani DJ, Rabbat MG, Bhatt DL. Comprehensive meta-analysis on drug-eluting stents versus bare-metal stents during extended follow-up. Am J Med. 2009;122:581.e1-10. , 66. Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, Brodie B, Hannan E, Harjai K, Jensen LO, Park SJ, Perry R, Racz M, Saia F, Tu JV, Waksman R, Lansky AJ, Mehran R, Stone GW. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198-206.
Very large meta-analysis that is pooling the results of a very large number of studies, including regsitries., 77. Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020-9. , 88. Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, Colombo A, Schampaert E, Grube E, Kirtane AJ, Cutlip DE, Fahy M, Pocock SJ, Mehran R, Leon MB. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998-1008. , 99. Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabaté M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, Petronio AS, Nordmann AJ, Diem P, Meier B, Zwahlen M, Reichenbach S, Trelle S, Windecker S, Jüni P. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937-48. ].
The development of standardised definitions for both the time course and probabilistic likelihood of thrombosis following stent deployment by the Academic Research Consortium (ARC) has facilitated comparative analyses across clinical studies and other data sets ( Table 1 ) [1010. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-51. ]. Although the ARC definitions add uniformity, they are still an imperfect balance of sensitivity and specificity. “Definite” ST is highly specific but probably underestimates the true frequency of ST, whereas “possible” ST is more sensitive but lacks diagnostic certainty. Most contemporary analyses combine the categories of “definite” and “probable” to provide a balance of specificity and sensitivity. The time course for ST occurrence is categorised as early (either acute or subacute), if within 30 days of stent deployment, and either late (>30 days to one year) or very late (>one year).
Multiple risk factors have been identified which contribute to the genesis of ST ( Figure 1 ) and may vary in importance as a function of time course following stent deployment [1111. Kereiakes DJ. Safety of drug-eluting stents. Rev Cardiovasc Med. 2010;11:187-200. , 1212. van Werkum JW, Heestermans AA, Zomer AC, Kelder JC, Suttorp MJ, Rensing BJ, Koolen JJ, Brueren BR, Dambrink JH, Hautvast RW, Verheugt FW, ten Berg JM. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol. 2009;53:1399-409. ]. Early events may be related to residual target lesion thrombus, plaque prolapse or medial tear, stent malapposition, undersizing and/or underexpansion as well as dual antiplatelet therapy (DAPT) noncompliance or a combination of these factors [22. Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michey I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126-30. , 1212. van Werkum JW, Heestermans AA, Zomer AC, Kelder JC, Suttorp MJ, Rensing BJ, Koolen JJ, Brueren BR, Dambrink JH, Hautvast RW, Verheugt FW, ten Berg JM. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol. 2009;53:1399-409. , 1313. Fujii K, Carlier SG, Mintz GS, Yang YM, Moussa I, Weisz G, Dangas G, Mehran R, Lansky AJ, Kreps EM, Collins M, Stone GW, Moses JW, Leon MB. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2005;45:995-8. , 1414. Cook S, Wenaweser P, Togni M, Billinger M, Morger C, Seiler C, Vogel R, Hess O, Meier B, Windecker S. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implementation. Circulation. 2007;115:2426-34. , 1515. Nakano M, Yahagi K, Otsuka F, Sakakura K, Finn AV, Kutys R, Ladich E, Fowler DR, Joner M, Virmani R. Causes of early stent thrombosis in patients presenting with acute coronary syndrome: an ex vivo human autopsy study. J Am Coll Cardiol. 2014;63:2510-2520. ]. Other factors incriminated in the etiology of early and late ST include longer target lesion and/or stent length, overlapping stents, smaller target vessel diameter, depressed left ventricular function and the acuity of the clinical syndrome at presentation [1212. van Werkum JW, Heestermans AA, Zomer AC, Kelder JC, Suttorp MJ, Rensing BJ, Koolen JJ, Brueren BR, Dambrink JH, Hautvast RW, Verheugt FW, ten Berg JM. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol. 2009;53:1399-409. , 1515. Nakano M, Yahagi K, Otsuka F, Sakakura K, Finn AV, Kutys R, Ladich E, Fowler DR, Joner M, Virmani R. Causes of early stent thrombosis in patients presenting with acute coronary syndrome: an ex vivo human autopsy study. J Am Coll Cardiol. 2014;63:2510-2520. ]. Additional correlates of late/very late ST include younger age, smoking, multivessel disease and vein graft target lesion [1616. Waksman R, Kirtane AJ, Torguson R, Cohen DJ, Ryan T, Räber L, Applegate R, Waxman S, Gordon P, Kaneshige K, Leon MB on behalf of the DESERT Investigators. Correlates and outcomes of late and very late drug-eluting stent thrombosis: Results from DESERT (International Drug-Eluting Stent Event Registry of Thrombosis). JACC Cardiovasc Interv. 2014;7:1093-1102. ]. Importantly, the antiproliferative properties of DES which are responsible for reducing restenosis (compared with BMS) have also been incriminated in the occurrence of late and/or very late ST following DES particularly in the absence of prolonged (>one year) DAPT therapy. Studies of both animal models and humans have demonstrated delayed endothelialisation and incomplete stent healing with consequent stent strut exposure following DES versus BMS [1717. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193-202.
Essential description of the various potential pathologic substrates of the clinical syndrome., 1818. Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435-41. , 1919. Gonzalo N, Barlis P, Serruys PW, Garcia-Garcia HM, Onuma Y, Ligthart J, Regar E. Incomplete stent apposition and delayed tissue coverage are more frequent in drug-eluting stents implanted during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction than in drug-eluting stents implanted for stable/unstable angina: insights from optical coherence tomography. JACC Cardiovasc Interv. 2009;2:445-52. ]. Although serial angioscopic and optimal coherence tomography (OCT) evaluations as well as autopsy studies have demonstrated a relationship between uncovered DES struts and ST, endoluminal mural thrombus may be present despite neointimal coverage due to inflammation related to the drug delivery polymer [1919. Gonzalo N, Barlis P, Serruys PW, Garcia-Garcia HM, Onuma Y, Ligthart J, Regar E. Incomplete stent apposition and delayed tissue coverage are more frequent in drug-eluting stents implanted during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction than in drug-eluting stents implanted for stable/unstable angina: insights from optical coherence tomography. JACC Cardiovasc Interv. 2009;2:445-52. , 2020. Guagliumi G, Musumeci G, Sirbu V, Bezerra HG, Suzuki N, Fiocca L, Matiashvili A, Lortkipanidze N, Trivisonno A, Valsecchi O, Biondi-Zoccai G, Costa MA; ODESSA trial investigators. Optical coherence tomography assessment of in vivo vascular response after implantation of overlapping bare-metal and drug-eluting stents. JACC Cardiovasc Interv. 2010;3:531-9. , 2121. Awata M, Kotani J, Uematsu M, Morozumi T, Watanabe T, Onishi T, Sera F, Nanto S, Hori M, Nagata S. Serial angioscopic evidence of incomplete neointimal coverage after sirolimus-eluting stent implantation: comparison with bare-metal stents. Circulation. 2007;116:910-6. ]. Indeed, differing grades of neointimal stent coverage and luminal thrombus scores have been reported among currently available DES platforms and have been associated with differences in the prevalence of inflammatory cells, including eosinophils, giant cells and fibrin [2222. Hara M, Nishino M, Tanijke M, Makino N, Kato H, Egami Y, Shutta R, Yamaguchi H, Tanouchi J, Yamada Y. High incidence of thrombus formation at 18 months after paclitaxel-eluting stent implantation: angioscopic comparison with sirolimus-eluting stent. Am Heart J. 2010;159:905-10. , 2323. Takano M, Yamamoto M, Murakami D, Inami S, Okamatsu K, Seimiya K, Ohba T, Seino Y, Mizuno K. Lack of association between large angiographic late loss and low risk of in-stent thrombus: angioscopic comparison between paclitaxel- and sirolimus-eluting stents. Circ Cardiovasc Interv. 2008;1:20-7. , 2424. Cook S, Ladich E, Nakazawa G, Eshtehardi P, Neidhart M, Vogel R, Togni M, Wenaweser P, Billinger M, Seiler C, Gay S, Meier B, Pichler WJ, Jüni P, Virmani R, Windecker S. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120:391-9. ]. Non-erodible polymers may precipitate mural thrombus formation by inciting localised inflammation/hypersensitivity reactions and apoptosis of vascular smooth muscle cells [1717. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193-202.
Essential description of the various potential pathologic substrates of the clinical syndrome., 1818. Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435-41. ]. Polymer-related inflammation may also contribute to vessel remodelling and the development of late-acquired incomplete stent apposition (ISA). Late ISA observed by intravascular ultrasound (IVUS) may also be due to gradual dissolution of thrombus or positive vessel remodelling [1414. Cook S, Wenaweser P, Togni M, Billinger M, Morger C, Seiler C, Vogel R, Hess O, Meier B, Windecker S. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implementation. Circulation. 2007;115:2426-34. , 2424. Cook S, Ladich E, Nakazawa G, Eshtehardi P, Neidhart M, Vogel R, Togni M, Wenaweser P, Billinger M, Seiler C, Gay S, Meier B, Pichler WJ, Jüni P, Virmani R, Windecker S. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120:391-9. ].
Although some reports have failed to identify a relationship between late stent malapposition and adverse clinical events, others have documented a direct association between the extent of stent malapposition and the degree of eosinophilic infiltration within thrombus aspirated during percutaneous coronary intervention (PCI) for very late stent thrombosis [2424. Cook S, Ladich E, Nakazawa G, Eshtehardi P, Neidhart M, Vogel R, Togni M, Wenaweser P, Billinger M, Seiler C, Gay S, Meier B, Pichler WJ, Jüni P, Virmani R, Windecker S. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120:391-9. ]. This association suggests the presence of a pathogenetic link between a local (Type IVb) hypersensitivity reaction to the polymer utilised in the CYPHER® (Cordis, Miami Lakes, FL, USA), sirolimus-eluting stent (SES) and excessive positive vessel remodelling with stent malapposition and stent thrombosis [2424. Cook S, Ladich E, Nakazawa G, Eshtehardi P, Neidhart M, Vogel R, Togni M, Wenaweser P, Billinger M, Seiler C, Gay S, Meier B, Pichler WJ, Jüni P, Virmani R, Windecker S. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120:391-9. ]. Finally, very late stent thrombosis may be a consequence of neoatherosclerosis – the development of yellow plaque and plaque rupture – within a previously deployed stent [2525. Nakano M, Vorpahl M, Otsuka F, Taniwaki M, Yazdani SK, Finn AV, Ladich ER, Kolodgie FD, Virmani R. Ex vivo assessment of vascular response to coronary stents by optical frequency domain imaging. JACC Cardiovasc Imaging. 2012;5:71-82. , 2626. Takano M, Yamamoto M, Inami S, Murakami D, Ohba T, Seino Y, Mizuno K. Appearance of lipid-laden intima and neovascularization after implantation of bare-metal stents extended late-phase observation by intracoronary optical coherence tomography. J Am Coll Cardiol. 2009;55:26-32. , 2727. Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, Kitys R, Ladich E, Finn AV, Kolodgie FD, Virmani R. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation. 2014;129:211-223. ]. Interestingly, neoatherosclerosis appears to occur more frequently and with an accelerated time course following DES compared with BMS. Clinical evidence supports a relationship between the degree of underlying vascular inflammation as reflected in the acuity of the presenting clinical syndrome and the subsequent propensity for risk of ST [2828. Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK, Gold HK, Burke AP, Kolodgie FD, Virmani R. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation. 2008;118:1138-45.
Another pathology study focusing on post-mortem observations from patients initially intervened upon during acute myocardial infarction.]. Patients who present with an acute coronary syndrome (ACS) and have coronary stent deployment may have a protracted risk for ST regardless of stent type (DES or BMS). However, beyond six to 12 months following stent deployment for ACS, the risk of ST appears to be greatest following either CYPHER® SES or TAXUS® paclitaxel-eluting (PES) DES versus BMS [3030. Leibundgut G, Nietlispach F, Pittl U, Brunner-La Rocca H, Kaiser CA, Pfisterer ME. Stent thrombosis up to 3 years after stenting for ST-segment elevation myocardial infarction versus for stable angina –comparison of the effects of drug-eluting versus bare-metal stents. Am Heart J. 2009;158:271-6. , 3131. Brodie BR, Stuckey T, Downey W, Humphrey A, Bradshaw B, Metzger C, Hermiller J, Krainin F, Juk S, Cheek B, Duffy P, Smith H, Edmunds J, Varanasi J, Simonton CA; STENT (Strategic Transcatheter Evaluation of New Therapies) Group. Outcomes and complications with off-label use of drug-eluting stents: results from the STENT (Strategic Transcatheter Evaluation of New Therapies) group. JACC Cardiovasc Interv. 2008;1:405-14. , 3232. Wenaweser P, Daemen J, Zwahlen M, van Domburg R, Jüni P, Vaina S, Hellige G, Tsuchida K, Morger C, Boersma E, Kukreja N, Meier B, Serruys PW, Windecker S. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J Am Coll Cardiol. 2008;52:1134-40. ] and even less frequent following newer-generation everolimus-eluting stents (EES). Indeed, lower rates of ST have been observed following EES compared with BMS in both randomised trial [3434. Sabaté M, Räber L, Heg D, Brugaletta S, Kelbaek H, Cequier A, Ostojic M, Iñiguez A, Tüller D, Serra A, Baumbach A, von Birgelen C, Hernandez-Antolin R, Roffi M, Mainar V, Valgimigli M, Serruys PW, Jüni P, Windecker S. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. JACC Cardiovasc Interv. 2014;7:55-63. ] and meta-analysis of trials [3535. Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Sabaté M, Valgimigli M, Frati G, Kedhi E, Smits PC, Kaiser C, Genereux P, Galatius S, Kirtane AJ, Stone GW. Clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2013;62:496-504. ]. These observations may reflect the fact that first generation (PES and SES) DES struts embedded into the necrotic lipid core of unstable plaque demonstrate incomplete healing and often lack neointimal coverage compared with struts of the same DES embedded in adjacent fibro-calcific plaque [2828. Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK, Gold HK, Burke AP, Kolodgie FD, Virmani R. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation. 2008;118:1138-45.
Another pathology study focusing on post-mortem observations from patients initially intervened upon during acute myocardial infarction.]. Conversely, the concept that the degree of neointimal thickness as reflected by in-stent late lumen loss may be protective against ST has been questioned by data from randomised clinical trials which demonstrate little or no relationship between angiographic late lumen loss and ST [3434. Sabaté M, Räber L, Heg D, Brugaletta S, Kelbaek H, Cequier A, Ostojic M, Iñiguez A, Tüller D, Serra A, Baumbach A, von Birgelen C, Hernandez-Antolin R, Roffi M, Mainar V, Valgimigli M, Serruys PW, Jüni P, Windecker S. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. JACC Cardiovasc Interv. 2014;7:55-63. , 3636. Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, Doostzadeh J, Cao S, Simonton CA, Sudhir K, Lansky AJ, Cutlip DE, Kereiakes DJ for the SPIRIT IV investigators.Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. New Engl J Med. 2010;362:1663-74. ]. Indeed, some DES types with the lowest late lumen loss were also associated with the lowest incidence of ST [3737. Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, Bhatt DL, Slater J. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117,762 patient-years of follow-up from randomized trials. Circulation. 2012;125:2873-2891. ]. These observations suggest that the physical presence and degree of neointimal thickness may not confer functional integrity and that chronic endothelial dysfunction and/or residual inflammatory changes may contribute to very late ST [3838. van Beusekom HM, Serruys PW. Drug-eluting stent endothelium. J Am Coll Cardiol Intv. 2010;3:76-7. ].
Predictors of stent thrombosis
Multivariable regression analyses from registries and clinical trials have identified multiple risk factors for ST which may be ascribed to being stent, patient, lesion and/or procedural related ( Figure 1 ).
BMS VERSUS DES
Meta-analyses of randomised controlled clinical trials (RCCT) have demonstrated no differences between BMS and DES with respect to the occurrences of death and/or MI at follow-up [55. Roukoz H, Bavry AA, Sarkees ML, Mood GR, Kumbhani DJ, Rabbat MG, Bhatt DL. Comprehensive meta-analysis on drug-eluting stents versus bare-metal stents during extended follow-up. Am J Med. 2009;122:581.e1-10. , 66. Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, Brodie B, Hannan E, Harjai K, Jensen LO, Park SJ, Perry R, Racz M, Saia F, Tu JV, Waksman R, Lansky AJ, Mehran R, Stone GW. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198-206.
Very large meta-analysis that is pooling the results of a very large number of studies, including regsitries.]. A substantial benefit of DES (versus BMS) for reduction in target lesion and/or vessel revascularisation has been demonstrated in both randomised trials as well as clinical registry analyses, particularly when stents are placed for “off-label” indications [66. Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, Brodie B, Hannan E, Harjai K, Jensen LO, Park SJ, Perry R, Racz M, Saia F, Tu JV, Waksman R, Lansky AJ, Mehran R, Stone GW. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198-206.
Very large meta-analysis that is pooling the results of a very large number of studies, including regsitries., 3939. Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol. 2010;56:S1-42.
Comprehensive review of all device-relators factors that may influence procedural results as well as efficay and safety outcome., 4040. Douglas PS, Brennan JM, Anstrom KJ, Sedrakyan A, Eisenstein EL, Hague G, Dai D, Kong DF, Hammill B, Curtis L, Matchar D, Brindis R, Peterson ED. Clinical effectiveness of coronary stents in elderly persons: results from 262,700 Medicare patients in the American College of Cardiology-National Cardiovascular Data Registry. J Am Coll Cardiol. 2009;53:1629-41. , 4141. Ko DT, Chiu M, Guo H, Austin PC, Goeree R, Cohen E, Labinaz M, Tu JV. Safety and effectiveness of drug-eluting and bare-metal stents for patients with off- and on-label indications. J Am Coll Cardiol. 2009;53:1773-82. , 4242. Applegate RJ, Sacrinty MT, Kutcher MA, Santos RM, Gandhi SK, Baki TT, Little WC. “Off-label” stent therapy 2-year comparison of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2008;51:607-14. ]. Although randomised trials demonstrate similar incidences of death and MI for both stent types, observational (real world) registry experiences demonstrate a consistent 20-25% reduction in mortality favouring DES ( Figure 2 ). The mortality reduction in these non-randomised studies may reflect the effect of confounding due to covariate imbalance (both measured and unmeasured) despite attempts at adjustment [66. Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, Brodie B, Hannan E, Harjai K, Jensen LO, Park SJ, Perry R, Racz M, Saia F, Tu JV, Waksman R, Lansky AJ, Mehran R, Stone GW. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198-206.
Very large meta-analysis that is pooling the results of a very large number of studies, including regsitries., 4141. Ko DT, Chiu M, Guo H, Austin PC, Goeree R, Cohen E, Labinaz M, Tu JV. Safety and effectiveness of drug-eluting and bare-metal stents for patients with off- and on-label indications. J Am Coll Cardiol. 2009;53:1773-82. , 4242. Applegate RJ, Sacrinty MT, Kutcher MA, Santos RM, Gandhi SK, Baki TT, Little WC. “Off-label” stent therapy 2-year comparison of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2008;51:607-14. ]. Although prior meta-analyses of RCCT demonstrated similar rates of ST for DES and BMS following either primary (ST-segment elevation myocardial infarction [STEMI]) or elective PCI, [66. Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, Brodie B, Hannan E, Harjai K, Jensen LO, Park SJ, Perry R, Racz M, Saia F, Tu JV, Waksman R, Lansky AJ, Mehran R, Stone GW. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198-206.
Very large meta-analysis that is pooling the results of a very large number of studies, including regsitries.] subsequent randomised trials, large scale observational studies and network meta-analyses have demonstrated lower rates of ST through two years follow-up for newer DES, particularly EES with a durable fluorocopolymer (PVDF) [3333. Sarno G, Lagerqvist B, Nilsson J, Frobert O, Hambraeus K, Varenhorst C, Jensen UJ, Tödt T, Götberg M, James SK. Stent thrombosis in new-generation drug-eluting stents in patients with STEMI undergoing primary PCI: A report from SCAAR. J Am Coll Cardiol. 2014;64:16-24. , 3434. Sabaté M, Räber L, Heg D, Brugaletta S, Kelbaek H, Cequier A, Ostojic M, Iñiguez A, Tüller D, Serra A, Baumbach A, von Birgelen C, Hernandez-Antolin R, Roffi M, Mainar V, Valgimigli M, Serruys PW, Jüni P, Windecker S. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. JACC Cardiovasc Interv. 2014;7:55-63. , 3737. Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, Bhatt DL, Slater J. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117,762 patient-years of follow-up from randomized trials. Circulation. 2012;125:2873-2891. , 4343. Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D’Ascenzo F, Kimura T, Briguori C, Sabaté M, Kim HS, De Waha A, Kedhi E, Smits PC, Kaiser C, Sardella G, Marullo A, Kirtane AJ, Leon MB, Stone GW. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393-1402. , 4444. Tada T, Byrne RA, Simunovic I, King LA, Cassese S. Joner M, Fusaro M, Schneider S, Schulz S, Ibrahim T, Ott I, Massberg S, Laugwitz KL, Kastrati A. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv. 2013;6:1267-1274. ]. A prospective propensity match (55 variables with perfect match on STEMI) comparison in 10,026 subjects enrolled into the Dual Antiplatelet Therapy study demonstrated an increased rate of ST following BMS (2.6%) versus DES (1.7%, p=0.01) through 0-33 months follow-up and a treatment effect (benefit) with longer (30 months) versus shorter (12 months) DAPT therapy that was consistent for both DES and BMS (HR [95% CI] 0.29 [0.17,0.48] and 0.49 [0.15, 1.65] respectively; p interaction=0.42) [4444. Tada T, Byrne RA, Simunovic I, King LA, Cassese S. Joner M, Fusaro M, Schneider S, Schulz S, Ibrahim T, Ott I, Massberg S, Laugwitz KL, Kastrati A. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv. 2013;6:1267-1274. ]. The greatest portion of risk difference favoring DES (versus BMS) for ST was observed during the first 12 months of follow-up in the propensity match comparison. The incidence of ST is increased following PCI for “off-label” indications or in patients with diabetes mellitus regardless of stent type. [4646. Patti G, Nusca A, Di Sciascio G. Meta-analysis comparison (nine trials) of outcomes with drug-eluting stents versus bare metal stents in patients with diabetes mellitus. Am J Cardiol. 2008;102:1328-34. , 4747. Kirtane AJ, Ellis SG, Dawkins KD, Colombo A, Grube E, Popma JJ, Fahy M, Leon MB, Moses JW, Mehran R, Stone GW. Paclitaxel-eluting coronary stents in patients with diabetes mellitus: pooled analysis from 5 randomized trials. J Am Coll Cardiol. 2008;51:708-15 ]. Beyond one-year follow-up, a small but definite increase in risk for very late ST accompanies first generation CYPHER® SES and TAXUS® PES, particularly when DAPT is discontinued. Indeed, a landmark analysis of pooled patient-level data from the TAXUS® I, II, III, IV and V randomised trials of PES versus BMS with follow-up through five years demonstrates an increased incidence of cardiac death or MI (6.7 vs. 4.5%; p=0.01) and protocol defined ST (0.9 vs. 0.2%; p=0.007) following PES compared with BMS respectively between one and five years of follow-up [4848. Stone GW, Ellis SG, Colombo A, Grube E, Popma JJ, Uchida T, Bleuit JS, Dawkins KD, Russell ME. Long-term safety and efficacy of paclitaxel-eluting stents final 5-year analysis from the TAXUS clinical trial program. JACC Cardiovasc Interv. 2011;4:530-42. ].
BMS versus DES
- Randomised controlled trials, observation studies and meta-analyses have demonstrated increased rates of ST through two to three years follow-up for BMS (versus newer generation DES) with the greatest portion of risk difference favoring DES observed during the first year of follow-up.
- The incidence of ST is increased following PCI for both “off label” indications or in patients with diabetes mellitus regardless of stent type
- The treatment effect (benefit) of longer (30 months) versus shorter (12 months) duration DAPT therapy for ST reduction appears to be consistent for DES and BMS.
DES VERSUS DES
Rates of ST may differ among currently available DES platforms. In a network meta-analysis, ST was more frequent following TAXUS® PES versus CYPHER® SES [99. Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabaté M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, Petronio AS, Nordmann AJ, Diem P, Meier B, Zwahlen M, Reichenbach S, Trelle S, Windecker S, Jüni P. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937-48. ]. Similar observations have been made from other data sets as well. For example, late ST was observed more frequently following TAXUS® PES than CYPHER® SES treatment through three years follow-up in the non-randomised, cumulative Bern-Rotterdam experience including 8,146 patients [44. Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Jünj P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667-78. ]. Conversely, the five-year follow-up of the randomised SIRolimus-eluting stent compared with pacliTAXel-eluting stent for coronary revascularisation (SIRTAX) trial demonstrated ST in 4.1% of TAXUS® PES versus 4.6% of CYPHER® SES-treated patients [4949. Räber L, Wohlwend L, Wigger M, Togni M, Wandel S, Wenaweser P, Cook S, Moschovitis A, Vogel R, Kalesan B, Seiler C, Eberli F, Lüscher TF, Meier B, Jüni P, Windecker S. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization LATE trial. Circulation. 2011;123:2819-28. ]. Similarly, the Bern-Rotterdam registry demonstrated that ST-event rates steadily increase for CYPHER® SES at 0.6% per year [44. Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Jünj P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667-78. , 3232. Wenaweser P, Daemen J, Zwahlen M, van Domburg R, Jüni P, Vaina S, Hellige G, Tsuchida K, Morger C, Boersma E, Kukreja N, Meier B, Serruys PW, Windecker S. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J Am Coll Cardiol. 2008;52:1134-40. ]. In both the SPIRIT IV and the Comparison of the everolimus-eluting XIENCE V® (Abbott Vascular, Santa Clara, CA, USA) stent with the paclitaxel-eluting TAXUS® Liberté® (Boston Scientific Corporation, Natick, MA, USA) stent in all-comers: a randomised open label (COMPARE) trials, the XIENCE V® EES was compared in a randomised fashion to either the TAXUS® Express® (Boston Scientific Corporation, Natick, MA, USA) or TAXUS® Liberté® PES respectively [3636. Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, Doostzadeh J, Cao S, Simonton CA, Sudhir K, Lansky AJ, Cutlip DE, Kereiakes DJ for the SPIRIT IV investigators.Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. New Engl J Med. 2010;362:1663-74. , 5050. Kedhi E, Joesoef KS, McFadden E, Wassing J, van Mieghem C, Goedhart D, Smits PC. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375:201-9. ]. In each of these large-scale randomised trials, EES was associated with a significantly lower incidence of ST up to three-year follow-up compared with TAXUS® PES [5151. Kedhi E, Stone GW, Kereiakes DJ, Serruys PW, Parise H, Fahy M, Smits PC. Stent thrombosis after everolimus-eluting versus paclitaxel-eluting coronary stents: insights from the SPIRIT II, SPIRIT III and SPIRIT IV and COMPARE pooled database. Circulation. 2010;122:A14593. ]. Each component of ST (early, late, very late) using the ARC definitions was significantly reduced by EES (versus PES). Furthermore, the relative benefit of XIENCE®/PROMUS® EES (versus TAXUS® PES) for reduction in ST is supported by a pooled, patient level analysis of the SPIRIT II, III, IV and COMPARE randomised trials, which compared the XIENCE®/PROMUS® EES with the TAXUS® Express® or Liberté® PES [5151. Kedhi E, Stone GW, Kereiakes DJ, Serruys PW, Parise H, Fahy M, Smits PC. Stent thrombosis after everolimus-eluting versus paclitaxel-eluting coronary stents: insights from the SPIRIT II, SPIRIT III and SPIRIT IV and COMPARE pooled database. Circulation. 2010;122:A14593. , 5252. Kirtane A, Sood P, Applegate RJ, Yaqub M, Onuma Y, Wang JC, Su X, Miguel-Hebert K, Lansky AJ, Kereiakes DJ, Simonton CA, Sudhir K, Serruys PW, Stone GW. Predictors of stent thrombosis after everolimus-eluting and paclitaxel-eluting stents: the pooled SPIRIT randomized trial experience at two years of follow-up. Circulation. 2010;122:A17174. ]. Multivariable regression analysis of the pooled 6,789 patient cohort (EES, n=4,247; PES, n=2,542) demonstrates that randomly assigned stent type (EES) is an independent predictor of freedom from ST (versus PES) [5252. Kirtane A, Sood P, Applegate RJ, Yaqub M, Onuma Y, Wang JC, Su X, Miguel-Hebert K, Lansky AJ, Kereiakes DJ, Simonton CA, Sudhir K, Serruys PW, Stone GW. Predictors of stent thrombosis after everolimus-eluting and paclitaxel-eluting stents: the pooled SPIRIT randomized trial experience at two years of follow-up. Circulation. 2010;122:A17174. ]. Subgroup analyses from this pooled data set suggests that the relative benefit of EES (versus PES) for reducing ST may be even more marked in patients treated for ACS (versus stable coronary artery disease [CAD]) [5353. Planer D, Smits PC, Kereiakes DJ, Kedhi E, Fahy M, Xu K, Serruys PW, Stone GW. Comparison of everolimus- and paclitaxel-eluting stents in patients with acute and stable coronary syndromes: pooled results from the SPIRIT (A Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (A Trial of Everolimus-Eluting Stents and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice) trials. JACC Cardiovasc Interv. 2011;4:1104-15. ]. A separate random effects meta-analysis of these four trials also demonstrates a significant reduction in MI (risk ratio [RR] 0.57; 95% confidence intervals [0.45, 0.73]), cardiac death or MI (RR 0.67 [0.54, 0.83]), and stent thrombosis (RR 0.35 [0.21, 0.60]) [5454. Alfonso F, Fernandez C. Second-generation drug-eluting stents. Moving the field forward. J Am Coll Cardiol. 2011;58:26-9. ].
These observations are extended by a meta-analysis of 13 randomised trials of various DES involving 17,097 patients which demonstrated a lower incidence of ST following XIENCE®/PROMUS® EES versus other DES types (TAXUS® PES, CYPHER® SES or ENDEAVOR®/Resolute zotarolimus-eluting stent [ZES]; RR 0.55 [0.38, 0.76]) ( Figure 3 ) [5555. Baber U, Mehran R, Sharma SK, Brar S, Yu J, Suh JW, Kim HS, Park SJ, Kastrati A, De Waha A, Krishnan P, Moreno P, Sweeny J, Kim MC, Suleman J, Pyo R, Wiley J, Kovacic J, Kini AS, Dangas GD. Impact of the everolimus-eluting stent on stent thrombosis: a meta-analysis of 13 randomized trials. J Am Coll Cardiol. 2011;58:1569-1577. ]. This large scale meta-analysis demonstrated relative benefit of EES (versus other DES) for reduction in target vessel revascularisation (RR 0.73 [0.61, 0.87]) and MI (RR 0.79 [0.63, 0.97]) as well. The benefit of EES (versus other DES) for reducing ST was evident regardless of statistical model used for analysis (random or fixed effects), duration of clopidogrel therapy (six vs. 12 months) or duration of clinical follow-up (≤ one year vs. ≥ one year). In addition, a separate landmark analysis at one to three years from the ENDEAVOR® IV (Randomized Comparison of Zotarolimus- and Paclitaxel-Eluting Stents in Patients With Coronary Artery Disease) randomised trial of the ENDEAVOR® (Medtronic, Minneapolis, MN, USA) ZES versus the TAXUS® Express® PES demonstrated a lower incidence of very late (>one year) ST following ENDEAVOR® ZES (0.1 vs. 1.5% respectively; p=0.004) [5656. Leon MB, Nikolsky E, Cutlip DE, Mauri L, Liberman H, Wilson H, Patterson J, Moses J, Kandzari DE; ENDEAVOR IV Investigators. Improved late clinical safety with zotarolimus-eluting stents compared with paclitaxel-eluting stents in patients with de novo coronary lesions: 3-year follow-up from the ENDEAVOR IV (Randomized Comparison of Zotarolimus- and Paclitaxel-Eluting Stents in Patients With Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2010;3:1043-50. ]. Although these trials suggest a relative increase in risk for ST associated with the TAXUS® Express® or Liberté® PES in comparison with either of the EES or ZES, each was underpowered for ST as a primary endpoint. Furthermore, in the propensity match analysis of patients enrolled into the Dual Antiplatelet Platelet Therapy trial, the weighted risk difference favoring DES for ST through 0-33 months follow-up was evident for EES, SES and ZES but not for PES ( Figure 4 ).Finally, the incidences of ARC definite and definite/probable ST were relatively increased in the RESOLUTE All-Comers (AC) randomised comparative trial of the ENDEAVOR® RESOLUTE ZES versus the XIENCE®/Promus® (Boston Scientific, Natick, MA, USA) EES following ZES deployment (1.2 vs. 0.3%; p=0.01 and 1.6 vs. 0.7%; p=0.05 respectively) at one-year follow-up [5757. Serruys PW, Silber S. Garg S, van Geuns RJ, Richardt G, Buszman PE, Kelbæk H, van Boven AJ, Hofma SH, Linke A, Klauss V, Wijns W, Macaya C, Garot P, DiMario C, Manoharan G, Kornowski R, Ischinger T, Bartorelli A, Ronden J, Bressers M, Gobbens P, Negoita M, van Leeuwen F, Windecker S. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med. 2010;363:136-46. ]. At two-year follow-up of RESOLUTE AC, the difference in ARC definite/probable ST was slightly less marked (1.9 ZES vs. 1.0% EES; p=0.073) [5858. Silber S, Windecker S, Vranckx P, Serruys PW; RESOLUTE All Comers investigators. Unrestricted randomized use of two new generation drug-eluting coronary stents: 2-year patient-related versus stent-related outcomes from the RESOLUTE All Comers trial. Lancet. 2011;377:1241-7. ]. Similarly, in the Dutch PEERS TWENTE randomised comparison of EES and RESOLUTE ZES, definite ST trended higher through two years follow-up (0.1 vs. 0.9% respectively; p=0.12). In aggregate, these data suggest that ST may be less frequent following XIENCE®/PROMUS® EES than other DES platforms although current studies lack the statistical power to be conclusive. The durable fluorocopolymer used in EES (XIENCE®/Promus®) may be more inert and biocompatible than the polymers utilized in either TAXUS® PES or CYPHER® SES. Measures of endothelial and microvascular function may be improved and associated with more rapid and complete endothelial coverage following XIENCE®/Promus® EES. Aggregate data from multiple sources suggests that BMS have a continued low risk for very late ST of approximately 0.15% to 0.2% per year [33. Doyle B, Rihal CS, O’Sullivan CJ, Lennon RJ, Wiste HJ, Bell M, Bresnahan J, Holmes DR Jr. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation. 2007;116:2391-8. , 3030. Leibundgut G, Nietlispach F, Pittl U, Brunner-La Rocca H, Kaiser CA, Pfisterer ME. Stent thrombosis up to 3 years after stenting for ST-segment elevation myocardial infarction versus for stable angina –comparison of the effects of drug-eluting versus bare-metal stents. Am Heart J. 2009;158:271-6. , 3939. Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol. 2010;56:S1-42.
Comprehensive review of all device-relators factors that may influence procedural results as well as efficay and safety outcome., 5959. Windecker S, Meier B. Late coronary stent thrombosis. Circulation. 2007;116:1952-65. ]. Late follow-up of almost 10,000 EES-treated patients from the SPIRIT II, III, IV and COMPARE RCCT as well as the XIENCE V® USA registry suggests that the incidence of late or very late ST is between 0.13% and 0.4% and is influenced by the complexity/acuity of the patients treated [6060. Krucoff MW, Rutledge DR, Gruberg L, Jonnavithula L, Katopodis JN, Lombardi W, Mao VW, Sharma SK, Simonton CA, Tamboli HP, Wang J, Wilburn O, Zhao W, Sudhir K, Hermiller JB. A new era of prospective real-world safety evaluation. Primary report of XIENCE V USA (XIENCE V everolimus eluting coronary stent system condition-of-approval post-market study). JACC Cardiovasc Interv Coll Cardiol Intv. 2011;4:1298-309. ]. The relative thrombo-resistant effects of the XIENCE®/PROMUS® EES fluorocopolymer were also evident in the Evaluation of Xience-VTM stent in Acute Myocardial INfArcTION (EXAMINATION) trial which compared XIENCE®/Promus® EES to the corresponding BMS platform (MULTILINK-VISION™) during primary PCI for STEMI. Freedom from both definite and definite/probable ST was enhanced through two years follow-up following XIENCE®/PROMUS®EES (versus BMS) [6161. Sabaté M, Brugaletta S, Cequier A, Iñiguez A, Serra A, Hernández-Antolin R, Mainar V, Valgimigli M, Tespili M, den Heijer P, Bethencourt A, Vázquez N, Backx B, Serruys PW. The EXAMINATION trial (Everolimus-eluting stents versus bare-metal stents in ST-segment elevation myocardial infarction): 2-year results from a multicenter randomized controlled trial. JACC Cardiovasc Interv. 2014;7:64-71. ]. Finally, the five year follow-up of the randomised “all-comers” LEADERS trial of a biodegradable polymer biolimus-eluting stent (BES) versus permanent polymer SES (CYPHER®) offers important insights into potential advantages associated with polymer bioresorption. Although the BES demonstrated equivalent safety and efficacy to the SES in this trial, BES performance was superior in ST-segment elevation MI patients and ST rates for BES (versus SES) were lower with divergence of outcomes over five years [6262. Serruys PW, Farooq V, Kalesan B, de Vries T, Buszman P, Linke A, Ischinger T, Klauss V, Eberli F, Wijns W, Morice MC, Di Mario C, Corti R, Antoni D, Sohn HY, Eerdmans P, Rademaker-Havinga T, van Es GA, Meier B, Jüni P, Windecker S. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC Cardiovasc Interv. 2013;6:777-789. ]. Specifically, BES had a lower ST rate than SES through five years that was highly statistically significant in a one to five year landmark analysis (beyond one year). These results are supported by prior OCT analyses at nine months following stent deployment, which demonstrate better stent strut coverage following BES compared with SES [6363. Barlis P, Regar E, Serruys PW, Dimopoulos K, van der Giessen WJ, van Geuns RJ, Ferrante G, Wandel S, Windecker S, Van Es GA, Eerdmans P, Jüni P, Di Mario C. An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur Heart J. 2010;31:165-76. ]. Recent randomised trials and meta-analyses have suggested that although BES have lower rates of late/very late ST than first generation DES (PES, SES) [6363. Barlis P, Regar E, Serruys PW, Dimopoulos K, van der Giessen WJ, van Geuns RJ, Ferrante G, Wandel S, Windecker S, Van Es GA, Eerdmans P, Jüni P, Di Mario C. An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur Heart J. 2010;31:165-76. ], similar or even slightly higher rates are evident in comparison with the newer fluorocopolymer EES platforms [6363. Barlis P, Regar E, Serruys PW, Dimopoulos K, van der Giessen WJ, van Geuns RJ, Ferrante G, Wandel S, Windecker S, Van Es GA, Eerdmans P, Jüni P, Di Mario C. An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur Heart J. 2010;31:165-76. ]. In aggregate, these data suggest that bioresorbable polymer DES have lower risk for late/very late ST than first generation durable polymer DES and BMS and appear comparable to new generation fluorocopolymer EES.
DES versus DES
- Rates of ST may differ between currently available DES platforms and are generally higher following deployment of PES
- Large scale randomised trials and meta-analyses of multiple randomised trials have demonstrated lower rates of early, late and very late ST following deployment of XIENCE®/PROMUS® EES, ENDEAVOR®/Resolute ZES and bioresorbable polymer DES
- Data from multiple sources suggest that BMS have a continued low risk for very late ST
DUAL ANTIPLATELET THERAPY (DAPT)
Dual antiplatelet therapy with a P2Y12 receptor inhibitor in combination with aspirin is critically important for the prevention of coronary stent thrombosis and is recommended by clinical practice guidelines for six to 12 months after DES and at least one month following BMS implantation in stable ischaemic heart disease (SIHD) [6666. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Lauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574-651. , 6767. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uya M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society fo Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. ]. Patients with ACS benefit from 12 months of DAPT therapy whether or not PCI with stent is performed and regardless of stent type (DES or BMS) [6868. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494-502. ]. These guidelines and U.S. FDA Advisory Panel recommendations [6969. Grines CL, Bonow RO, Casey DE Jr, Gardner TJ, Lockhart PB, Moliterno DJ, O’Gara P, Whitlow P; American Heart Association; American College of Cardiology; Society for Cardiovascular Angiography and Interventions; American College of Surgeons; American Dental Association; American College of Physicians. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation. 2007;115:813-8. ] are for ≥ six to 12 months of DAPT following DES in all patients without specific contraindications or bleeding risks. Newer generation, more potent P2Y12 receptor inhibitors (prasugrel, ticagrelor) in combination with aspirin further reduce the risk of stent thrombosis through 12-15 months following ACS (compared with clopidogrel plus aspirin) and are associated with a relative increase in major bleeding event risk [7070. Wiviott SD, Braunwald E, McCabe CH, Horvath I, Heitai M, Herrman JP, Van de Werf F, Downey WE, Scirica BM, Murphy SA, Antman EM; TRITON-TIMI 38 Investigators. Intensive oral antiplatelet therapy for reduction of ischemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomized trial. Lancet. 2008;371:1353-1363. , 7171. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husten S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA for the PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057. ]. Although observational data suggest that the relative risk of DAPT discontinuation for ischaemic events is greatest during the first six months following PCI [7272. Airoldi F, Colombo A, Morici N, Latib A, Cosgrave J, Buellesfeld L, Bonizzoni E, Carlino M, Gerckens U, Godino C, Melzi G, Michev I, Montorfano M, Sangiorgi GM, Qasim A, Chieffo A, Briguori C, Grube E. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation. 2007;116:745-54. , 7373. Eisenstein EL, Anstrom KJ, Kong DF, Shaw LK, Tuttle RH, Mark DB, Kramer JM, Harrington RA, Matchar DB, Kandzari DE, Peterson ED, Schulman KA, Califf RM. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297:159-68. ], these observations are complicated by the facts that <1% of persons who discontinue DAPT incur ST and ~80% of ST occur in patients who are DAPT adherent. Using population attributable risk methodology it has been estimated that 68-85% of ST are not ascribed to clopidogrel noncompliance [22. Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michey I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126-30. , 7474. Kuchulakanti PK, Chu WW, Torguson R, Ohlmann P, Rha SW, Clavijo LC, Kim SW, Bui A, Gevorkian N, Xue Z, Smith K, Fournadjieva J, Suddath WO, Satler LF, Pichard AD, Kent KM, Waksman R. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation. 2006;113:1108-13. , 7575. Tsai TT, Nallamothu BK, Bates ER. Letter by Tsai et al regarding article “Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents”. Circulation. 2006;114:e362. ]. This suggests that “other factors” such as aspirin and/or clopidogrel resistance, polymer hypersensitivity and/or neoatherosclerosis may be important pathophysiological mechanisms as well. Although non-randomised registry experiences have suggested that discontinuation of clopidogrel within six months following stent deployment, but not thereafter, is a strong predictor of ST, these observations have lacked power to be definitive and extended duration DAPT may also influence non-target-site-related (systemic) ischaemic events as well.
Multiple randomised trials have compared different durations of DAPT therapy following coronary stenting ( Table 2 ). Although these trials differ in design, populations enrolled and durations of therapy compared, they have often combined both safety (bleeding) and efficacy (ischaemia) measures into a single composite primary endpoint in an attempt to accrue power for the assessment of non-inferiority between DAPT treatment durations. This tactic, which obscures directionally divergent changes in measures of different relative value, may confound the accuracy of conclusions. Furthermore, the post-hoc aggregation of underpowered trials with variable study populations, protocols, methodologies and endpoints into meta-analysis often provides results that are proven incorrect by subsequent, adequately powered RCCT [8383. LeLorier J, Gregoire G, Benhaddad A, Lapierre J, Derderian F. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536-542. ]. Collectively, these trials compared shorter (three to six months) with longer duration (12-24 months) DAPT therapy on an unblinded basis in relatively small numbers of randomised subjects in context of the specific endpoints being evaluated. In general, the limitations shared by these trials include: (1) lack of power, often due to early study termination and poor enrollment; (2) unbalanced (between unblinded assigned treatment arms) study medication compliance; (3) lower than expected event rates often making the chosen non-inferiority margin inappropriate. For example, in OPTIMIZE, which compared three vs. 12 months of DAPT treatment, the four-fold relative increase in ST (ARC definite/probable definition) associated with shorter duration treatment was counterbalanced by a two-fold increase in major bleeding events associated with longer DAPT treatment [7979. Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, King SB 3rd, Negoita M, Liu M, de Paula JE, Mangione JA, Meireles GX, Castello HJ Jr, Nicolela EL Jr, Perin MA, Devito FS, Labrunie A, Salvadori D Jr, Gusmão M, Staico R, Costa JR Jr, de Castro JP, Abizaid AS, Bhatt DL; OPTIMIZE Trial Investigators. Three vs. twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310:2510-2522. ]. However, the upper bound of the 95% confidence interval surrounding the hazard ratio (HR) for ST associated with three months’ DAPT therapy was 35.5, thus allowing for as much as a 35-fold increase in ST. Furthermore, OPTIMIZE excluded subjects with biomarker positive ACS (greatest risk for ST) from enrollment and evaluated only the ENDEAVOR-ZES DES. Similarly, in ITALIC, which was designed to compare six months versus 24 months of DAPT therapy, very few events were observed (only three [0.16%] ST and 10 [0.45%] MI) which suggests the study population was very low risk and not representative of routine clinical practice [8282. Gilard M, Barragan P, Noryani AA, Noor HA, Majwal T, Hovasse T, Castellant P, Schneeberger M, Maillard L, Bressolette EE, Wojcik J, Delarche N, Blanchard D, Joute B, Ormezzano O, Paganelli F, Levy G, Sainsous J, Carrie D, Furber A, Berland J, Darremont O, Le Breton H, Lyuycx-Bore A, Gommeaux A, Cassat C, Kermarrec A, Cazaux P, Druelles P, Dauphin R, Armengaud J, Dupouy P, Champagnac D, Ohlmann P, Endresen KK, Benamer H, Kiss RG, Ungi I, Boschat JJ, Morice MC. Six-month versus 24-month dual antiplatelet therapy after implantation of drug eluting stents in patients non-resistant to aspirin: ITALIC, a randomized multicenter trial. J Am Coll Cardiol. 2014;Nov 16 [E-pub ahead of print]. ]. Only the XIENCE V EES DES was evaluated in ITALIC and the study composite primary endpoint of death, MI, urgent target vessel revascularisation, stroke or major bleeding event was assessed at 12 months and was observed in only 1.6 versus 1.5% of the six versus 12 months treatment groups respectively. Finally, the low event rates and sample size in the primary analysis of ITALIC make subgroup analysis (acute coronary syndromes) grossly underpowered to examine potential treatment interactions. Conversely, the Dual Antiplatelet Therapy study was designed in response to a request from the U.S. FDA to manufacturers of coronary stents and was conducted under an investigational device exemption through a public-private collaboration involving the FDA, eight stent and pharmaceutical manufacturers who funded the study and the Harvard Clinical Research Institute (HCRI) [8484. Mauri L, Kereiakes DJ, Normand SL, Wiviott SD, Cohen DJ, Holmes DR, Bangalore S, Cutlip DE, Pencina M, Massaro JM. Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am Heart J. 2010;160:1035-41,1041.e1.
This methodology manuscript describes the design of the large FDA-mandated international evaluation of the impact of longer term dual antiplatelet therapy. Patients having undergone stent placement (BMS or various brands of DES) are eligible for enrolment and eventually randomised to stop or continue for another 18 months.]. The DAPT study enrolled a broad spectrum of “real world” subjects in 452 sites across 11 countries. Subjects who required chronic anticoagulation or who were poor candidates for DAPT therapy of at least one year duration were excluded. Eligible subjects were treated with an FDA approved BMS or DES (CYPHER SES, ENDEAVOR ZES, TAXUS PES, XIENCE/PROMUS EES) and clopidogrel or prasugrel in labeled approved doses (ticagrelor was not yet FDA approved during trial enrollment). All subjects were enrolled within 72 hours of stent placement and received open-label DAPT for 12 months at which time those subjects who had not incurred a major adverse cardiovascular or cerebrovascular event (MACCE; composite occurrence of death, MI, stroke), repeat revascularisation, or moderate/severe (GUSTO scale) bleeding event and were adherent to thienopyridine therapy were eligible for randomisation. Eligible patients continued taking aspirin and were randomised 1:1 to either continued thienopyridine therapy or placebo (blinded) for an additional 18 months. Randomisation was stratified by stent type (BMS or DES), hospital site, type of thienopyridine drug and present or absence of at least one prespecified clinical or lesion related risk factor for ST. After the end of the randomised treatment period, all patients were followed for an additional three months (months 30 to 33 after enrollment) to assess the impact of thienopyridine discontinuation on the rates of endpoint events. The powered efficacy co-primary endpoint events for the DAPT study included both a stent-related endpoint (ARC definite/probable ST) [1010. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-51. ] and a patient-related endpoint (MACCE). The powered safety endpoint is the composite of severe/moderate (GUSTO definition) bleeding events [8585. The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673-682. ]. Bleeding was also evaluated according to the Bleeding Academic Research Consortium (BARC) criteria [8686. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Meno V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747. ]. The DAPT study enrolled 25,682 patients of whom 22.866 received a DES and 2,816 received a BMS. From these enrolled subjects, 9,961 DES and 1,687 BMS treated patients were randomised at one year to either extended duration (30 months) DAPT therapy or aspirin plus placebo beyond 12 months [8686. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Meno V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747. ]. By the end of the randomised treatment period (30 months), there was a 71% relative (1% absolute; p<0.001) reduction in ST driven largely by a 74% relative reduction in definite ST ( Table 3A) and a 29% relative (1.6% absolute; p<0.001) reduction in MACCE driven by a 53% relative (2% absolute; p<0.001) reduction in MI in favor of longer duration DAPT therapy. Furthermore, 55% of the MI prevented by longer duration therapy were not related to the stent but occurred in non-stented coronary distributions. The benefit of extended therapy was accompanied by a 0.5% absolute increase in mortality (2.0 vs. 1.5%; p=0.052) and a 61% relative (0.9% absolute; p=0.001) increase in severe/moderate bleeding ( Table 3B). Severe bleeding was not different (0.8 versus 0.6%; p=0.15) and fatal (BARC type 5) bleeding events were rare and not different between the randomly assigned treatment groups. The unexpected finding of an increased number of deaths from any cause during the treatment period in the group continuing to receive thienopyridine was driven by an increase in death from non-cardiovascular causes largely explained by increases in death due to cancer or trauma. Although the rate of cancer diagnosis did not differ significantly after randomisation, the increased relative incidence of death due to cancer in patients treated with continued thienopyridine may reflect a chance imbalance in patients with known cancer before enrollment. In addition, adjusted analyses demonstrated that the magnitude of benefit associated with longer (30 months) duration thienopyridine for reduction in ST or MI was consistent across the four DES types studied including the 4703 (47.2%) subjects treated with EES ( Figure 5 ). Indeed, the adjusted hazard ratios (95% confidence intervals) favoring longer therapy in EES treated patients were 0.38 (0.15-0.97) for ST and 0.63 (0.44-0.91) for MI. Similarly, consistent treatment benefit for ST and MI reduction was observed for both clopidogrel and prasugrel without evidence for a treatment duration by drug type interaction ( Figure 5 ). Subgroup analyses demonstrated consistency of treatment benefit without interaction for most subgroups with the exception of gender and diabetes. Although relative benefit (hazard ratio <1.0) was observed for women (versus men) and for patients with diabetes (versus those without) for both ST and MI, the relative magnitude of treatment benefit associated with prolonged DAPT therapy was greater for men (P interaction 0.04 for ST and 0.03 for MI) and among non-diabetic patients (P interaction 0.08 for ST and 0.004 for MI) ( Figure 6 ). Interestingly, treatment benefit was similar in magnitude whether or not prespecified clinical or lesion risk factors for ST were present. Importantly, prespecified clinical risk factors for ST included biomarker positive ACS (STEMI or non-STEMI).
Despite the magnitude of treatment benefit for ischaemic event reduction favoring longer therapy, a potential limitation of the DAPT study is that only those patients who were adherent to therapy and who did not experience MACCE, revascularisation or moderate/severe bleeding event in the first year underwent randomisation. This study design may have selected for subjects at lower risk for late adverse events. In addition, although the DAPT study did not quantify the net relative effect of ischaemic and bleeding events, a recent decision analysis model suggests that small absolute differences in rates of cardiovascular events may be sufficient to counterbalance bleeding risks [8888. Garg P, Galper BZ, Cohen DJ, Yeh RW, Mauri L. Balancing the risks of bleeding and stent thrombosis: A decision analytic model to compare durations of dual antiplatelet therapy after drug-eluting stents. Am Heart J. 2014;0:1-12.e5. ]. Based on a meta-analysis of published studies, this model predicts a 1.7-fold relative increase in major bleeding with 30 versus 12 months of DAPT therapy (similar to the 1.6-fold increase observed in the DAPT study). This bleeding hazard is counterbalanced by a 78% reduction in ST or a 5% reduction in MACCE for patients with SIHD (compared with a 44% and 2% reduction respectively for patients with ACS). These data suggest that despite the potential limitation of a lower risk population being randomised, the magnitude of benefit associated with prolonged thienopyridine therapy in the DAPT study is adequate to counterbalance the observed bleeding risk. Finally, the DAPT study was prospectively designed to include three month observation periods following thienopyridine discontinuation at both 12 (for subjects randomised to placebo) and 30 months (for subjects randomised at 12 months to extended thienopyridine). Adverse ischaemic events (ST and MI) were observed with increased frequency in the three months following thienopyridine discontinuation in both treatment arms ( Figure 7 ). This observation suggests that the preventative benefit of thienopyridine therapy is realised early and that treatment discontinuation may be associated with ischemic hazard even months to years after coronary stenting.
BARE METAL STENTS
BMS are a commonly used alternative to DES, particularly for patients presenting with ACS or in whom DAPT has perceived increased bleeding risk [8989. Badheka AO, Arora S, Panaich SS, Patel NJ, Chothani A, Mehta K, Deshmukh A, Singh V, Savani GT, Agnihotri K, Grover P, Lahewala S, Patel A, Bambhroliya C, Kondur A, Brown M, Elder M, Kaki A, Mohammad T, Grines C, Schreiber T. Impact on in-hospital outcomes with drug-eluting stents versus bare-metal stents (from 665,804 procedures). Am J Cardiol. 2014;114:1629-1637. ]. Furthermore, BMS are widely perceived to be “safer” and to require a shorter duration of DAPT therapy. The DAPT study was designed to provide insights as to whether the risks of ST and MACCE differ for BMS and DES and whether the optimal duration of DAPT therapy differs for BMS and DES. A prospective propensity match comparison of BMS and DES was incorporated into the DAPT study design and included all enrolled subjects with at least 29 months of follow-up or a ST/MACCE event. The BMS to DES match was 1:many (maximum eight) with subjects matched perfectly with respect to STEMI and for all other variables (total of 55) with a caliper of 0.10. Matching was established on baseline variables without knowledge of subsequent ST/MACCE events which were adjudicated by a central, independent clinical events committee. The hypothesis was that DES are not inferior with respect to ST and MACCE (versus BMS) through 0-33 months follow-up after stent deployment. In addition, the treatment effect of 30 (versus 12) months of DAPT therapy was evaluated in 1687 BMS treated patients who were randomised after one year of treatment (randomisation in the DAPT study was stratified by stent type [DES vs. BMS] at the index PCI). Finally, the consistency of prolonged thienopyridine treatment effect (30 versus 12 months) was compared for randomised DES or BMS treated patients. Among 10,026 propensity match DES (n=8308) or BMS (n=1718) treated patients, ST was more common following BMS ( Table 4 , Figure 8A ) with the greatest portion of benefit (risk difference) accrued during the first year post-PCI. Similarly, MACCE trended less frequent for DES (p=0.053 for difference 0-33 months) with the greatest portion of risk difference evident within the first year (p=0.01 for difference 0-12 months) ( Figure 8B ). Recent randomised trials and meta-analyses of trials have suggested lower rates of ST following new DES when compared to older DES or BMS [3737. Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, Bhatt DL, Slater J. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117,762 patient-years of follow-up from randomized trials. Circulation. 2012;125:2873-2891. , 4343. Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D’Ascenzo F, Kimura T, Briguori C, Sabaté M, Kim HS, De Waha A, Kedhi E, Smits PC, Kaiser C, Sardella G, Marullo A, Kirtane AJ, Leon MB, Stone GW. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393-1402. , 9090. Sabate M, Raber L, Heg D, Brugaletta S, Kelbaek H, Cequier A, Ostojic M, Iñiguez A, Tüller D, Serra A, Baumbach A, von Birgelen C, Hernandez-Antolin R, Roffi M, Mainar V, Valgimigli M, Serruys PW, Jüni P, Windecker S. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: A pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. J Am Coll Cardiol Interv. 2014;7:55-63. ]. In this regard, the risk difference favoring DES (versus BMS) for ST was evident for three types of DES included in the propensity match but not for PES ( Figure 4 ). The risk difference favoring DES (versus BMS) for MACCE was evident across all four DES subtypes. Among 1687 BMS treated patients who were randomised to 30 versus 12 months of DAPT therapy, a 51% relative reduction in definite ST was observed favouring longer treatment. Bleeding risk was increased with longer duration DAPT (severe/moderate bleeding event rate 2.03 versus 0.9%; p=0.07 for 30 versus 12 months respectively) ( Table 5 ). Finally, the magnitude of randomised treatment benefit for reduction in ST among BMS treated subjects was consistent with results observed among DES-treated randomised subjects with no evidence for a treatment duration by stent type interaction (pinteraction 0.42). Thus, the DAPT study results suggest that DES treated subjects have lower rates of ST (0-33 months) and MACCE (0-12 months) compared with BMS treated subjects with the greatest benefit for DES evident during the first year following stent deployment. In BMS treated patients, continued thienopyridine therapy beyond 12 months appears to provide ischaemic benefit in addition to increasing bleeding risk and requires further study.
Although these new data provide support for longer duration thienopyridine therapy for reduction in ST regardless of stent type, considerable data suggest that the vast majority of ST events occur in patients who are adherent to DAPT [7575. Tsai TT, Nallamothu BK, Bates ER. Letter by Tsai et al regarding article “Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents”. Circulation. 2006;114:e362. ]. Indeed, very late ST may have multiple pathophysiological mechanisms including uncovered stent struts (lack of healing), as well as neoatherosclerosis, stent fracture and restenosis.
PLATELET INHIBITORY RESPONSE
Roughly one quarter of patients undergoing stenting may be relatively “resistant” to the platelet-inhibiting effects of clopidogrel [9191. Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908-13.
Nice review of the issues involved with efficacy of clopidogrel treatment.]. Furthermore, patients who are poorly responsive to one agent (either aspirin or clopidogrel) are more likely to be hyporesponsive to the other agent and the risk of ischaemic events appears greatest in patients with “dual” resistance [9292. Gori AM, Marcucci R, Migliorini A, Valenti R, Moschi G, Paniccia R, Buonamici P, Gensini GF, Vergara R, Abbate R, Antoniucci D. Incident and clinical impact of dual nonresponsiveness to aspirin and clopidogrel in patients with drug-eluting stents. J Am Coll Cardiol. 2008;52:734-9. ].
The mechanisms underlying variability in clopidogrel responsiveness (distinct from noncompliance) include genetic variation in specific enzymes involved in clopidogrel absorption and metabolic conversion from pro-drug to active metabolite [9393. Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, Bhatt DL, Cattaneo M, Collet JP, Cuisset T, Gachet C, Montalescot G, Jennings LK, Kereiakes DJ, Sibbing D, Trenk D, Van Werkum JW, Paganelli F, Price MJ, Waksman R, Gurbel PA; Working Group on High On-Treatment Platelet Reactivity. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919-33. ] as well as phenotypic variables such as body mass index, the presence of diabetes, level of glucose control and renal function [9393. Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, Bhatt DL, Cattaneo M, Collet JP, Cuisset T, Gachet C, Montalescot G, Jennings LK, Kereiakes DJ, Sibbing D, Trenk D, Van Werkum JW, Paganelli F, Price MJ, Waksman R, Gurbel PA; Working Group on High On-Treatment Platelet Reactivity. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919-33. ].
Specific genotypes have been demonstrated to be associated with either reduced clopidogrel absorption (TT variant of the ABCB1 or MDR1 gene) or diminished conversion of clopidogrel pro-drug to active metabolite (CYP2C19*2, 3, 4 and 5 alleles) [9494. Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, Steg PG, Ferrières J, Danchin N, Becquemont L; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363-75. ]. Active metabolite generation is maximally reduced in CYP2C19*2 homozygotes (*2/*2) and intermediate in heterozygote carriers (*1/*2) of the *2 loss of function (LOF) allele [9595. Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354-62. , 9696. Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849-57. ]. This gene-dose response relationship for active metabolite generation has been correlated with a gene-dose response for both measured platelet inhibition as well as prevalence of high on-treatment residual platelet reactivity (HPR) and for the occurrence of clinical events such as ST ( Figure 10 ) [9797. Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, Horne BD, Hulot JS, Kastrati A, Montalescot G, Neumann FJ, Shen L, Sibbing D, Steg PG, Trenk D, Wiviott SD, Sabatine MS. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821-30. ]. This genetically determined limitation in clopidogrel responsiveness may not be overcome by increasing clopidogrel dose. Recent data suggests that the clopidogrel hyporesponsiveness observed in CYP2C19* heterozygotes (but not homozygotes) with respect to the magnitude of platelet inhibition and prevalence of HPR may be “overridden” by progressively increasing the dose of clopidogrel to ≥225mg daily [9898. Mega JL, Hochholzer W, Frelinger AL, Kluk MJ, Angiolillo DJ, Kereiakes DJ, Isserman S, Rogers WJ, Ruff CT, Contant C, Pencina MJ, Scirica BM, Longtine JA, Michelson AD, Sabatine MS. Dosing Clopidogrel Based on CYP2C19 Genotype and the Effect on Platelet Reactivity in Patients With Stable Cardiovascular Disease. JAMA. 2011;306;2221-2228. ]. Whether or not such large daily doses of clopidogrel are safe and effective in mitigating the increased clinical risk for ischaemic events observed in this cohort (25%-30% of the general population) remains to be determined by adequately powered randomised trials.
Recent clinical practice guideline updates [6767. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uya M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society fo Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. , 9999. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:2574-609. ] recommend an increase in the oral loading dose of clopidogrel from 300mg to 600mg prior to or immediately following either primary or non-primary PCI for STEMI or for elective PCI. Increasing the loading dose of clopidogrel from 300mg to 600mg accelerates the time course and increases the magnitude of platelet inhibition and also reduces the prevalence of clopidogrel resistance [100100. Montalescot G, Sideris G, Meuleman C, Bal-dit-Sollier C, Lellouche N, Steg PG, Slama M, Milleron O, Collet JP, Henry P, Beygui F, Drouet L; ALBION Trial Investigators. A randomized comparison of high clopidogrel loading doses in patients with non-ST-segment elevation acute coronary syndromes: the ALBION (Assessment of the Best Loading Dose of Clopidogrel to Blunt Platelet Activation, Inflammation and Ongoing Necrosis) trial. J Am Coll Cardiol. 2006;48:931-8.
This trial confirms the importance of adequate platelet inhibition and explores the added value of prescribing higher loading doses of clopidogrel in patients with acute presentations of coronary artery disease.]. Clinical data in support of the 600mg (versus 300mg) oral loading dose of clopidogrel comes from an analysis of the HORIZONS-AMI trial in which 62% of subjects received a 600mg oral clopidogrel load on a non-randomised basis. Those subjects who received a 600mg load (versus 300mg) experienced a reduction in major adverse cardiovascular events (MACE), especially those who had been randomly assigned to receive bivalirudin, and a reduction in subacute ST to 30 days (1.9% in the 600mg versus 3.2% in the 300mg clopidogrel load; hazard ratio [HR] 0.61 [0.38, 0.96]; p=0.033) [101101. Dangas G, Mehran R, Guagliumi G, Caixeta A, Witzenbichler B, Aoki J, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Rabbani LE, Parise H, Stone GW; HORIZONS-AMI Trial Investigators. Role of clopidogrel loading dose in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty: results from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J Am Coll Cardiol. 2009;54:1438-46. , 102102. Dangas GD, Caixeta A, Mehran R, Parise H, Lansky AJ, Cristea E, Brodie BR, Witzenbichler B, Guagliumi G, Peruga JZ, Dudek D, Möeckel M, Stone GW; Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) Trial Investigators. Frequency and predictors of stent thrombosis after percutaneous coronary intervention in acute myocardial infarction. Circulation. 2011;123:1745-56. ]. Randomised trial data in support of the 600mg load (versus 300mg) comes from the Atorvastatin for Reduction of MYocardial Damage during Angioplasty (ARMYDA)-6 trial of patients undergoing primary PCI for STEMI [103103. Patti G, Bárczi G, Orlic D, Mangiacapra F, Colonna G, Pasceri V, Barbato E, Merkely B, Edes I, Ostojic M, Wijns W, Di Sciascio G. Outcome comparison of 600- and 300-mg loading doses of clopidogrel in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: results from the ARMYDA-6 MI (Antiplatelet therapy for Reduction of Myocardial Damage during Angioplasty-Myocardial Infarction) randomized study. J Am Coll Cardiol. 2011;58:1592-9. ]. Patients randomly assigned to receive a 600mg load (versus 300mg) enjoyed an event-free survival advantage free from death or MI (p=0.029) and MACE (p=0.027). Finally, in the prespecified but non-randomised PCI subgroup analysis of the CURRENT OASIS 7 trial, subjects randomly assigned to receive a 600mg oral load followed by 150mg daily for seven days and then 75mg daily thereafter (versus 300mg load and 75mg daily thereafter) experienced a marked reduction in the incidence of definite ST [104104. Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, Faxon DP, Rupprecht HJ, Budaj A, Avezum A, Widimsky P, Steg PG, Bassand JP, Montalescot G, Macaya C, Di Pasquale G, Niemela K, Ajani AE, White HD, Chrolavicius S, Gao P, Fox KA, Yusuf S for CURRENT-OASIS 7 trial investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233-43. ]. The benefit of increased clopidogrel dosing with respect to reduction in ischaemic events was counterbalanced by an increase in major bleeding events (HR 1.41 [1.09, 1.83]; p=0.009). A meta-analysis of nine studies involving 18,263 patients undergoing primary PCI for STEMI confirmed a reduction in major adverse cardiovascular events (HR [95% CI] = 0.75 [0.63, 0.91]) following 600mg clopidogrel load (compared with 300mg) [105105. Vyas A, El Accaoui R, Blevins A, Karrowni W. Comparison of 600mg versus 300mg loading dose of clopidogrel for patients with STEMI: A meta-analysis. J Am Coll Cardiol. 2013;61:A10 [abstract]. ]. Finally, the ischaemic event benefit of 600mg clopidogrel loading dose (versus 300mg) prior to elective PCI in SIHD may be less apparent relative to the increased risk of bleeding [106106. Bellemain-Appaix A, O’Connor SA, Silvain J, Cucherat M, Collet J-P, Bernasconi F, Montalescot G. No benefit of clopidogrel pretreatment in stable patients undergoing elective PCI. J Am Coll Cardiol. 2013;62:B44 [abstract]. ]. In aggregate, these data suggest that the optimal oral dosing regimen of clopidogrel for primary or non-primary PCI for STEMI appears to be a 600mg load followed by maintenance therapy of 75mg daily. The choice of 600mg (versus 300mg) clopidogrel load prior to elective PCI in SIHD is based more on pharmacodynamics profile than on demonstrated differences in clinical events.
Finally, the novel third-generation thienopyridine, prasugrel, and the non-thienopyridine P2Y12 receptor antagonist, ticagrelor, are not influenced by the genetic polymorphisms which affect clopidogrel metabolism [106106. Bellemain-Appaix A, O’Connor SA, Silvain J, Cucherat M, Collet J-P, Bernasconi F, Montalescot G. No benefit of clopidogrel pretreatment in stable patients undergoing elective PCI. J Am Coll Cardiol. 2013;62:B44 [abstract]. , 106106. Bellemain-Appaix A, O’Connor SA, Silvain J, Cucherat M, Collet J-P, Bernasconi F, Montalescot G. No benefit of clopidogrel pretreatment in stable patients undergoing elective PCI. J Am Coll Cardiol. 2013;62:B44 [abstract]. ]. Neither prasugrel nor ticagrelor efficacy, with respect to primary endpoint events or the relative suppression of ST (compared with clopidogrel) in large-scale randomised trials (TRITON TIMI 38, PLATO), was influenced by CYP2C19 LOF allele carrier status [107107. Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias WL, Braunwald E, Sabatine MS. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119:2553-60. , 108108. Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, Husted S, Katus H, Steg PG, Shah SH, Becker RC; PLATO investigators. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320-8. ]. Indeed, the relative benefit of either prasugrel or ticagrelor for reduction in ST (compared with clopidogrel) was evident and consistent regardless of ACS type (STEMI, non-STEMI), stent type (DES, BMS), diabetic status, creatinine clearance, age (≥ versus <75 years), gender or CYP2C19 LOF allele status [109109. Wiviott SD, Braunwald E, McCabe CH, Horvath I, Keltai M, Herrman JP, Van de Werf F, Downey WE, Scirica BM, Murphy SA, Antman EM; TRITON-TIMI 38 Investigators. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet. 2008;371:1353-63. ]. Analysis of ST (ARC definite/probable) in the STEMI cohorts of PLATO (n=7,544) and TRITON TIMI 38 (n=3,534) demonstrates a 26% relative reduction in ST by ticagrelor (HR 0.74 [0.55, 1.00]; p=0.05) and a 42% relative reduction by prasugrel (HR 0.58 [0.36, 0.93]; p=0.02) through the course of the respective trial (12 months PLATO; 15 months TRITON TIMI 38) when compared with clopidogrel [110110. Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, Antman EM; TRITON-TIMI 38 investigators. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373:723-31. , 111111. Steg PG, James S, Harrington RA, Ardissino D, Becker RC, Cannon CP, Emanuelsson H, Finkelstein A, Husted S, Katus H, Kilhamn J, Olofsson S, Storey RF, Weaver WD, Wallentin L; PLATO Study Group. Ticagrelor versus clopidogrel in patient with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: A Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122:2131-41. ]. Recent studies suggest a greater degree of inter-individual variability in the magnitude of platelet inhibition during maintenance dose therapy with prasugrel (10mg daily) compared with ticagrelor (90mg twice daily) which may be influenced by CYP2C19 allele status in addition to phenotypic variables. In comparative studies, a more reliable, predictable higher level of platelet inhibition was achieved across a population of patients during maintenance therapy with ticagrelor (versus prasugrel) [112112. Angiolillo DJ, Curzen N, Gurbel P, Vaitkus P, Lipkin F, Li W, Jakubowski JA, Zettler M, Effron MB, Trenk D. Pharmacodynamic evaluation of switching from ticagrelor to prasugrel in patients with stable coronary artery disease: Results of the SWAP-2 study (Switching AntiPlatelet-2). J Am Coll Cardiol. 2014;63:1500-1509. , 113113. Perl L, Zemer-Wassercug N, Rechavia E, Vaduganathan M, Orvin K, Lerman-Shivek H, Kornowski R, Lev E. Variability in response to prasugrel and ticagrelor over time in patients with acute coronary syndrome. J Am Coll Cardiol. 2014;64(12_S)[abstract]; doi:10.1016/S0735-1097(14)60023-1. ]. Interestingly, pre-treatment with prasugrel in patients undergoing primary PCI for STEMI did not reduce the composite occurrence of cardiovascular death, MI or stroke and increased non-coronary artery bypass graft (CABG) related TIMI major or minor bleeding when compared with a prasugrel loading dose administered at the time of primary PCI [114114. Montalescot G, Collet JP, Ecollan P, Bolognese L, Ten Berg J, Dudek D, Hamm C, Widimsky P, Tanguay JF, Goldstein P, Brown E, Miller DL, LeNarz L, Vicaut E: ACCOAST Investigators. Effect of prasugrel pre-treatment strategy in patients undergoing percutaneous coronary intervention for NSTEMI: The ACCOAST-PCI Study. J Am Coll Cardiol. 2014;64:2563-2571. ]. Conversely, pre-hospital (versus in-hospital at the time of PCI) ticagrelor loading was associated with a significant reduction in ST (odds ratio 0.19 [95% CI] 0.04-0.86; p=0.06) to 30 days following PCI for non-ST elevation ACS [115115. Montalescot G, van ‘t Hof AW, Lapostolle F, Silvain J, Lassen JF, Bolognese L, Cantor WJ, Cequier A, Chettibi M, Goodman SG, Hammett CJ, Huber K, Janzon M, Merkely B, Storey RF, Zeymer U, Stibbe O, Ecollan P, Heutz WM, Swahn E, Collet JP, Willems FF, Baradat C, Licour M, Tsatsaris A, Vicaut E, Hamm CW: ATLANTIC Investigators. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. 2014;371:1016-1027. ].
DAPT
- The relative risk of DAPT discontinuation is greatest during the first six months following PCI but hazard for both ST and non-ST related MI persists following thienopyridine discontinuation after 30 months of therapy.
- Although prior small randomised trials demonstrated no apparent advantage of longer (>six months) DAPT duration following stenting, a recent adequately powered, placebo controlled, blinded randomised trial demonstrated highly significant reductions in ST and MACCE with 30 months of DAPT compared with 12 months.
- Prolonged DAPT (30 months) is associated with increased severe/moderate bleeding events (versus 12 months) but fatal bleeding is rare and not different between treatment groups (30 vs. 12 months).
- BMS have greater risk for ST (compared with DES) through 33 months follow-up and the treatment effect (benefit) of 30 months DAPT (versus 12 months) for reduction in ST is consistent between stent types (BMS or DES).
DAPT INTERRUPTION/DISCONTINUATION
Observational data suggests that the risk of late and very late ST is greatest in those individuals who discontinue both aspirin and thienopyridine, or in those who discontinue aspirin and have previously discontinued thienopyridine therapy [116116. Eisenberg MJ, Richard PR Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation. 2009;119:1634-42. ]. In addition, the time course for ST occurrence appears to be more abrupt when both aspirin and thienopyridine are discontinued simultaneously. For example, in the RESTART registry, the median time duration between DAPT (both agents) discontinuation and ST was 13 days (interquartile range [IQR] six-61 days) versus 314 days (IQR 79-711 days) following discontinuation of thienopyridine alone [117117. Kimura T, Morimoto T, Kozuma K, Honda Y, Kume T, Aizawa T, Mitsudo K, Miyazaki S, Yamaguchi T, Hiyoshi E, Nishimura E, Isshiki T; for the RESTART investigators. Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART). Circulation. 2010;122:52-61. ]. Additional observational data suggests that both the time from stent deployment to DAPT discontinuation for a surgical procedure, as well as the clinical syndrome which prompted stent deployment, influenced the likelihood for occurrence of death and/or ischaemic cardiac events. Those patients who had stent deployment for an ACS and who underwent non-cardiac surgery within 42 days of stent deployment had the greatest risk of adverse events, regardless of stent type (DES or BMS) [118118. Cruden NL, Harding SA, Flapan AD, Graham C, Wild SH, Slack R, Pell JP, Newby DE; Scottish Coronary Revascularisation Register Steering Committee. Previous coronary stent implantation and cardiac events in patients undergoing noncardiac surgery. Circ Cardiovasc Interv. 2010;3:236-42. ]. Furthermore, approximately 80% of ST occurred within ten days of performance of the non-cardiac surgical procedure [117117. Kimura T, Morimoto T, Kozuma K, Honda Y, Kume T, Aizawa T, Mitsudo K, Miyazaki S, Yamaguchi T, Hiyoshi E, Nishimura E, Isshiki T; for the RESTART investigators. Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART). Circulation. 2010;122:52-61. ]. The risk of DAPT cessation for surgical procedures may differ for DES or BMS. For example, the risk of MACE was greatest following BMS deployment when surgery was performed <30 days (odds ratio [OR] 4.0; p<0.001) but remained high when surgery was performed at 31-90 days (OR 1.4; p<0.001) when compared with ≥91 days [119119. Nuttall GA, Brown MJ, Stombaugh JW, Michon PB, Hathaway MF, Lindeen KC, Hanson AC, Schroeder DR, Oliver WC, Holmes DR, Rihal CS. Time and cardiac risk of surgery after bare-metal stent percutaneous coronary intervention. Anesthesiology 2008;109:588-95. ]. Recent large observational series with propensity matching have demonstrated an increased incidence of major adverse cardiovascular events when DAPT is discontinued between six weeks and six months following PCI with a BMS when compared with newer generation DES [120120. Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA. 2013;310:1462-1472. , 121121. Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv. 2014 Jul 24. doi:10.1002/ccd.25617 [E-pub ahead of print]. ] ( Figure 9 ). This relative hazard of adverse events when DAPT is discontinued for non-cardiac surgical procedures in BMS (versus DES) treated patients is counter to conventional perceptions and consensus guideline recommendations. In aggregate, these data suggests that non-cardiac surgery should be deferred at least six weeks to 90 days following stent deployment, especially in patients treated for ACS, regardless of stent type (DES or BMS). These data also support the practice of abbreviated and partial DAPT discontinuation, if possible, as has been recommended [122122. Kereiakes DJ. Does clopidogrel each day keep stent thrombosis away? JAMA. 2007;297:209-11. ]. This practice involves stopping P2Y12 receptor antagonist (clopidogrel or ticagrelor at five days, prasugrel at seven days) before surgery and restarting these agents within 48-72 hours postoperatively. Aspirin (81mg daily) therapy should not be discontinued if at all possible. Consideration may be given to reloading patients at greatest risk for ST (history of ACS, prior bifurcation stenting or vascular brachytherapy) with the P2Y12 receptor inhibitor following the non-cardiac surgical procedure.
DAPT interruption/discontinuation
- The risk of late and very late ST is greatest among individuals who discontinue both aspirin and platelet P2Y12 receptor inhibitor
- Both time from stent deployment to DAPT discontinuation for a surgical procedure and the clinical syndrome which prompted stent deployment influence the likelihood of ST or ischemic events
- The risk of DAPT cessation for surgical procedures may be less for newer DES (EES, ZES) than for BMS and non-cardiac surgery should be deferred for at least 90 days following stent deployment
- Abbreviated and partial DAPT discontinuation (if possible) is recommended for surgical procedures
TREATMENT AND PROGNOSIS FOLLOWING ST
The estimate that 68%-85% of ST is not ascribable to clopidogrel noncompliance [7575. Tsai TT, Nallamothu BK, Bates ER. Letter by Tsai et al regarding article “Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents”. Circulation. 2006;114:e362. ] and suggests the relative importance of “other factors” such as aspirin and/or clopidogrel non-responsiveness, polymer hypersensitivity, and/or neoatherosclerosis. Alternatively, novel receptors (protease activated receptor [PAR-1]) and pathways may be involved in the pathogenesis of stent thrombosis late following deployment [123123. Bonaca MP, Scirica BM, Braunwald E, Wiviott SD, O’Donoghue ML, Murphy SA, Morrow DA. Coronary stent thrombosis with vorapaxar versus placebo. J Am Coll Cardiol. 2014;64:2309-2317. ]. Observational data suggests that baseline patient demographics may vary significantly according to the timing of ST [117117. Kimura T, Morimoto T, Kozuma K, Honda Y, Kume T, Aizawa T, Mitsudo K, Miyazaki S, Yamaguchi T, Hiyoshi E, Nishimura E, Isshiki T; for the RESTART investigators. Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART). Circulation. 2010;122:52-61. ]. Demographic characteristics for late and very late ST (versus early ST) include haemodialysis, end-stage renal disease not on dialysis, chronic total occlusion target vessel and age <65 years. Furthermore, patients with late ST had higher rates of TIMI grade 2/3 flow at the time of ST than did those with early ST (36% vs. 13%; p<0.0001) and very late ST (17%; p<0.0001) while mortality at one year following ST was lower in patients with very late ST (10.5%) compared with those having either early (22.4%; p=0.003) or late ST (23.5%; p=0.009) [117117. Kimura T, Morimoto T, Kozuma K, Honda Y, Kume T, Aizawa T, Mitsudo K, Miyazaki S, Yamaguchi T, Hiyoshi E, Nishimura E, Isshiki T; for the RESTART investigators. Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART). Circulation. 2010;122:52-61. ]. Recent data suggest that patients who present with late/very late ST are younger, current smokers, more often have multivessel disease and Type C (modified ACC/AHA criteria) target lesion morphology with longer total stent length deployed and overlapping stents [1616. Waksman R, Kirtane AJ, Torguson R, Cohen DJ, Ryan T, Räber L, Applegate R, Waxman S, Gordon P, Kaneshige K, Leon MB on behalf of the DESERT Investigators. Correlates and outcomes of late and very late drug-eluting stent thrombosis: Results from DESERT (International Drug-Eluting Stent Event Registry of Thrombosis). JACC Cardiovasc Interv. 2014;7:1093-1102. ]. Interestingly, despite the mortality observed following acute/subacute ST, a relatively lower in-hospital mortality has been consistently associated with late/very late ST (3.8-11.0%) and suggests an predominant underlying pathogenesis of late restenosis/neoatherosclerosis with the potential for development of preconditioning ischaemia and collaterals[1616. Waksman R, Kirtane AJ, Torguson R, Cohen DJ, Ryan T, Räber L, Applegate R, Waxman S, Gordon P, Kaneshige K, Leon MB on behalf of the DESERT Investigators. Correlates and outcomes of late and very late drug-eluting stent thrombosis: Results from DESERT (International Drug-Eluting Stent Event Registry of Thrombosis). JACC Cardiovasc Interv. 2014;7:1093-1102. , 117117. Kimura T, Morimoto T, Kozuma K, Honda Y, Kume T, Aizawa T, Mitsudo K, Miyazaki S, Yamaguchi T, Hiyoshi E, Nishimura E, Isshiki T; for the RESTART investigators. Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART). Circulation. 2010;122:52-61. , 124124. Secemsky EA, Steg PG, Camenzind E, Wijns W, McFadden E, Mauri L on behalf of the PROTECT trial investigators. Early and late stent thrombosis are associated with long-term cardiac mortality: results from pooled PROTECT trials. Circulation. 2014;130:A17162 [abstract]. ]. Finally, patients who experience ST remain at high risk for recurrence, especially those who have underlying diabetes, depressed left ventricular function, longer total stent length, reduced TIMI flow following ST reperfusion and/or who require additional stent(s) deployment during the emergency PCI procedure [125125. Kramer MC, van der Wal AC, Koch KT, Ploegmakers JP, van der Schaaf RJ, Henriques JP, Baan J Jr, Rittersma SZ, Vis MM, Piek JJ, Tijssen JG, de Winter RJ. Presence of older thrombus is an independent predictor of long-term mortality in patients with ST-elevation myocardial infarction treated with thrombus aspiration during primary percutaneous coronary intervention. Circulation. 2008;118:1810-6. , 126126. van Werkum JW, Heestermans AA, de Korte FI, Kelder JC, Suttorp MJ, Ransing MG, Zwart B, Brueren G, Koolen JJ, Dambrink JH, van’t Hof AW, Verheugt FW, ten Berg JM. Long-term clinical outcome after a first angiographically confirmed coronary stent thrombosis: an analysis of 431 cases. Circulation. 2009;119:828-34. ]. Diagnostic assessment during catheter intervention for ST should include intravascular ultrasound evaluation to assess adequate stent sizing, expansion and apposition, as well as high-pressure re-dilatation with an optimal-sized non-compliant balloon catheter if indicated and re-stenting only for complex/occlusive type marginal endoluminal dissection as necessary. Patients who experience ST despite being compliant with clopidogrel therapy should be switched to either prasugrel or ticagrelor in the absence of specific absolute (history of prior stroke, transient ischaemic attack [TIA]) or relative (age >75 years) contraindications. Elderly or small (<60 kg) patients may be treated with the 5mg daily dose of prasugrel although clinical experience with this dose is relatively limited. The role of clopidogrel dose titration guided by serial platelet function assessment or treatment with triple antiplatelet therapy (aspirin, thienopyridine, cilostazol) in these patients has not been determined. Finally, a recent observation that the PAR-1 receptor antagonist Vorapaxar reduced ST in stable patients when added to aspirin or dual antiplatelet therapy with aspirin and a thienopyridine suggests the need for further study to better define safety and efficacy of alternative strategies for a long term ST prevention [123123. Bonaca MP, Scirica BM, Braunwald E, Wiviott SD, O’Donoghue ML, Murphy SA, Morrow DA. Coronary stent thrombosis with vorapaxar versus placebo. J Am Coll Cardiol. 2014;64:2309-2317. ].






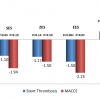






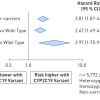
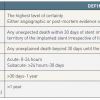


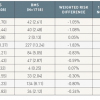

Do you think we should use prasugrel in general except in patients witj contraindications? Thank you Fantastic review,
Although the recent ISAR-REACT 5 concluded that prasugrel was superior to ticagrelor in reducing ischemic events after acute coronary syndromes, this was an open-label randomised study with several limitations. Thus, there is no clear and definitive evidence to support a superior antithrombotic efficacy of prasugrel vs. ticagrelor. Moreover, in patients presenting with NSTE-ACS prasugrel should be given after early coronary angiography whereas ticagrelor can be given before. Many healthcare systems cannot provide access to early angiography, and medical therapy alone, is appropriate for selected patients. Nevertheless, prasugrel may be preferred by physicians due to the once daily administration and lower rate of dyspnea-related discontinuation. Thus, compliance to prasugrel could be greater allowing for an enhanced antithrombotic protection that could be particularly relevant when risk factors and mechanisms of stent thrombosis (i.e. procedural issues), are present. Thank you very much for your comment. The editorial team
I totally agree, I think ISAR-REACT 5 has investigated the difference between two strategies (time of intervention) in addition to Prasugrel vs. Ticagrelor. This might also be responsible for reducing ischemic events, especially in NSTE-ACS.
I agree with you, the different administration strategies (prior vs. after coronary angiography) may also have impacted on overall results. Also, this comparison would suggest no potential benefit of routine pre-treatment in NSTE-ACS, supporting current European guidelines recommendations. Thank you very much for your comment. The Editorial Team.