Summary
Valvar pulmonary stenosis was first described in 1761 by Morgagni in his classic text ‘De Sedibus et Causis Morborum’. He reported a patient with pulmonary stenosis and and atrial septal defect who had been cyanosed during life [11. Morgagni GB. De Sedibus et Causis Morborum [The Seats and Causes of Diseases]. Vol. 1. Venice: Remondini, 1761:154. ]. Just over two hundred years late Kan and colleagues reported the first case of percutaneous balloon pulmonary valvuloplasty in the New England Journal of Medicine revolutionising the management of this common congenital condition [22. Kan JS, White RI Jr, Mitchell SE, Gardner TJ. Percutaneous balloon valvuloplasty: a new method for treating congenital pulmonary-valve stenosis. N Engl J Med. 1982;307:540-2.
Report of the first balloon valvulolasty in pulmonary stenosis].
Valvar pulmonary stenosis
THE SCOPE OF THE PROBLEM
Valvar pulmonary stenosis is a relatively common condition occurring in around 5 out of every 10000 livebirths [33. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890-900. ]. The reported incidence however is widely variable and complicated by the fact that the condition may develop or retreat with age. Nevertheless, lone pulmonary stenosis is second only to ventricular septal defect in its prevelance and can also occur in up to 50% of patients with other congenital cardiac defects. There are no convincing frequency differences reported between males and females or between different racial groups.
Although most cases are sporadic, a recurrence rate of up to 3% has been described in siblings and autosomal dominant pedigrees have also been reported [44. Driscoll DJ, Michels VJ, Gersony WM, Hayes CT: Occurrence risk for congenital heart defect in relatives of patients with aortic stenosis, pulmonary stenosis or ventricular septal defect. Circulation 1993;87:114. , 55. Udwadia AD, Khambadkone S, Bharucha BA, Lokhandwala Y, Irani SF. Pediatr Cardiol. 1996;17:407-9.
Familial congenital valvar pulmonary stenosis: autosomal dominant inheritance.]. The condition, typically occurring in the context of a dysplastic valve, is the characteristic cardiac finding in Noonan syndrome [66. Burch M, Sharland M, Shinebourne E, Smith G, Patton M, McKenna W. Cardiologic abnormalities in Noonan syndrome: phenotypic diagnosis and echocardiographic assessment of 118 patients. J Am Coll Cardiol. 1993;22:1189-92. ]. Additionally, valvar pulmonary stenosis is seen in several rarer conditions that share a phenotypic overlap with the Noonan syndrome including the LEOPARD syndrome and neurofibromatosis type 1. Pulmonary stenosis may also occur in patients with congenital rubella, the Alagille syndrome and Williams syndrome, however these cases are rarely confined to the valve.
EMBRYOLOGY, PATHOLOGY AND PATHOPHYSIOLOGY
The origins of pulmonary stenosis are poorly understood; maldevelopment of the distaL bulbus cordis has been proposed, as has the possibility of fetal endocarditis [77. Keith A. Malformation of the heart. Lancet. 1909;2:359. , 88. Oka M, Angrist A. Mechanism of cardiac valvular fusion and stenosis. Am Heart J. 1967;74(1):37-47. ]. In some cases, as discussed above, there are clearly genetic elements.
The condition may be broadly divided into two categories, dome-shaped and dysplastic. Successful balloon pulmonary valvuloplasty can be achieved in most cases irrespective of the nature of the stenosis, however dysplastic valves may show variable results. In the case of the dome-shaped valve, the valvular tissue is not thickened and the arterial walls are essentially normal. The commissures, however, are fused with the three resultant fibrous raphes extending from the level of the sinutubular junction, over the surface of the valve to a central orifice. Occasionally, this process occurs in a bicuspid valve (two raphes) or an assymetrical tricuspid valve leading to an eccentric orifice. In contrast, the rarer dysplastic valve is characterized by severely thickened valve leaflets with cauliflower-like changes affecting the distal tips.
The zones of apposition between the dysplastic leaflets are not necessarily fused [99. Stamm C, Anderson RH, Ho SY. Clinical anatomy of the normal pulmonary root compared with that in isolated pulmonary valvular stenosis. J Am Coll Cardiol. 1998;31:1420-5. ]. A third subtype has been described, in which the thickened pulmonary trunk forms an hourglass narrowing at the level of the sinutubular junction [1010. Milo S, Fiegel A, Shem-Tov A, Neufeld HN, Goor DA. Hour-glass deformity of the pulmonary valve: a third type of pulmonary valve stenosis. Br Heart J. 1988;60:128–133. ].
The pulmonary artery frequently demonstrates post-stenotic dilatation, especially in older patients. The dilatation often extends to the left pulmonary artery, which receives the “jet” originating from the stenotic orifice. Elevated right ventricular pressures result in generalized wall hypertrophy often more marked in the infundibular region, where dynamic obstruction may occur. Antenatally, compensatory wall hyperplasia is also seen [1111. Rudolph AM. Myocardial growth before and after birth: clinical implications. Acta Paediatr. 2000;89(2):129-33. ]. In severe obstruction, there may be a small right ventricular cavity with reduced compliance leading to so-called restrictive physiology. The right atrium may also dilate and thicken as right ventricular end diastolic pressure increases. In the presence of atrial septal communication, right to left shunting will occur with a resultant reduction in pulmonary blood flow. On rare occasions where right ventricular pressures are supra-systemic, myocardial ischemia may ensue, leading to myocardial fibrosis and eventually congestive cardiac failure [1212. Francioso RA, Blanc WA. Myocardial infarct in infants and children. I. A necropsy study in infants and children. J Pediatr. 1968;73:309. ]. Appreciation of these morphological changes will guide successful percutaneous intervention.
CLINICAL FEATURES
The neonate with critical pulmonary stensosis typically presents in congestive heart failure or with cyanosis due to right-to-left shunting at the atrial level. As the pulmonary resistance falls in the first few days of life, the measured gradient across the valve increases. Rapid deterioration may occur when the arterial duct closes. Beyond the neonatal period, most cases of valvar pulmonary stenosis are assymptomatic as the right ventricle and atrium compensate to maintain resting cardiac output. Typically symptoms, when they do emerge, are exertional and include breathlessness and fatigue. With advancing age, right ventricular compliance falls and cyanosis due to shunting may once again feature. Rarely chest pain, syncope, and sudden death occur.
Unlike other congenital cardiac anomalies, auscultation is predictive of lesion severity [1313. Leatham A, Weitzman D. Auscultatory and phonocardiographic signs of pulmonic stenosis. Br Heart J 1957;19:303. , 1414. Vogelpoel L, Schrire V. Auscultatory and phonocardiographic assessment of pulmonary stenosis with intact ventricular septum. Circulation. 1960;22:55. ]. In mild to moderate stenosis, there is a normal first heart sound followed by an early systolic click that can be accentuated with expiration. The click is absent in patients with severe stenosis or a dysplastic valve. An ejection systolic murmur, heard best at the upper left sternal edge, follows, typically radiating to the left upper back. As the severity of the stenosis increases, the frequency of the murmur increases and the murmur lengthens, peaking later in systole. The second heart sound becomes widely split and increasingly fixed with respiration. A fourth heart sound, a palpable thrill and a right ventricular heave may also be found.
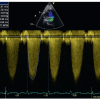
Electrocardiographic changes reflect right atrial enlargement and right ventricular hypertrophy [1515. Bassingthwaighte JB, Parkin TW, Dushane JW, Wood EH, Burchell HB. The electrocardiographic and hemodynamic findings in pulmonary stenosis with intact ventricular septum. Circulation. 1963;28:893. ]. The electrocardiogram is often normal in mild valvar stenosis, but rarely normal in severe cases. On chest radiography, post-stenotic dilatation of the main and left pulmonary artery may be detected. In the older patient, the stenosed valve may be calcified. Echocardiography permits prior visualization of the nature of the valvar stenosis and assessment of the peak instantaneous systolic gradient ( Figure 1 and Figure 2). 40 mmHg or less is considered mild, 40 to 70 mmHg moderate, and greater than 70 mmHg severe. In neonates there may be a patent arterial duct with left to right shunt. In infants with severe valvar stenosis, a right-to-left shunt across the foramen ovale may also occur. Magnetic resonance imaging and computed tomography can offer supplementary information on outflow tract anatomy but is not performed routinely. Invasively, the severity of the stenosis is determined by the peak to peak systolic pressure difference across the pulmonary valve. Resting right ventricular systolic pressure less than 50 mmHg with a gradient is less than 30 mmHg is considered mild. Systemic right ventricular systolic pressures with a gradient between 30 and 50 mmHg is moderate and suprasystemic right ventricular pressure with a gradient exceeding 50 mmHg is severe. The systolic pressure gradient is influenced by heart rate.
The role of percutaneous pulmonary valvuloplasty
Critical pulmonary stenosis in the neonate requires emergency intervention with balloon valvuloplasty to reduce mortality [1616. Hanley FL, Sade RM, Freedom RM, Blackstone EH, Kirklin JW. Outcomes in critically ill neonates with pulmonary stenosis and intact ventricular septum: a multiinstitutional study. Congenital Heart Surgeons Society. J Am Coll Cardiol. 1993;22:183-92. ]. A transvalvular gradient less than 40mmHg may be acceptable if the arterial duct is patent and there is a right to left shunt. A prostaglandin infusion should be commenced as early as possible to maintain ductal patency until treatment is available. Surgery is only considered if valvuloplasty has failed, the lesion is complex, for example the pulmonary annulus is hypoplastic, or there is a right ventricular dependant coronary circulation
In older children and adults, percutaneous pulmonary valvuloplasty is the first line treatment for valvar pulmonary stenosis in all those with an invasive gradient across the pulmonary valve exceeding 40mmHg, irrespective of symptoms [1717. Nugent EW, Freedom RM, Nora JJ, Ellison RC, Rowe RD, Nadas AS. Clinical course in pulmonary stenosis. Circulation. 1977;56:I38-47.
Excellent report on the clinical presentationg and outcome in patietns with pulmonary stenosis]. Asymptomatic infants with severe valvar pulmonic stenosis should be treated electively, usually around the age of 9 to 12 months. The presence of fixed subpulmonary obstruction or additional lesions requiring treatment may tip the balance in favour of surgery. In mild cases of pulmonary valvar stenosis (<40mmHg), no intervention should be undertaken and the patient, though monitored, should not be restricted in daily activities [1818. Wennevold A, Jacobsen JR. Natural history of valvular pulmonary stenosis in children below the age of two years: Long-term follow-up with serial heart catheterization. Eur J Cardiol. 1978;83:371. , 1919. Hayes CJ, Gersony WM, Driscoll DJ: Second natural history study of congenital heart defects: Results of treatment of patients with pulmonary valvar stenosis. Circulation. 1993;87:28. ].
Indications for balloon valvuloplasty and surgery are summarised in Focus Box 1 and 2 and are based on the recommendations of the ESC grown up congenital heart disease task force [2020. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke-Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas P, Widimsky P, Swan L, Andreotti F, Beghetti M, Borggrefe M, Bozio A, Brecker S, Budts W, Hess J, Hirsch R, Jondeau G, Kokkonen J, Kozelj M, Kucukoglu S, Laan M, Lionis C, Metreveli I, Moons P, Pieper PG, Pilossoff V, Popelova J, Price S, Roos-Hesselink J, Uva MS, Tornos P, Trindade PT, Ukkonen H, Walker H, Webb GD, Westby J. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010): The Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC). Eur Heart J. 2010.
These very recently updated guidelines for the management of patietns with congenital heart disease summarize the considerations and commence sence about timing and indications for RVOT interventions in the context of pulmonary stenosis or regurgitation.].
Indications for balloon pulmonary valvuloplasty in neonates and children
- Neonates : Symptomatic valvar pulmonary stenosis
- Transvalvular gradient <40mmHg acceptable in presence of low cardiac output or with patent arterial duct and a right to left shunt
- Children : Moderate or severe valvar pulmonary stenosis irrespective of symptoms
- Transvalvular gradient>40mmHg with normal resting cardiac output
- Elective at 9-12months if asymptomatic
Indications for balloon pulmonary valvuloplasty and surgery in adults
- Valvar pulmonary stenosis with an echocardiographically assessed transvalvar gradient of > 64 mmHg
- If balloon valvulopalsty is ineffective, surgery should be considered in asymptomatic patients with a transvalvar gradient gradient of >80mmHg
- Balloon valvuloplasty can be considered in patients with a transvalvar gradient of > 64 mmHg and
- symptoms related to valvar pulmonary stenosis
- impaired right ventricular function
- double-chambered right ventricle
- relevant arrhythmias
- right to left intracardiac shunting
Procedural technique
Percutaneous pulmonary valvuloplasty is conventionally performed under general anaesthesia from a transfemoral approach. 50 IU/kg heparin, or a standard dose of 5000 IU in adults, is administered routinely at the beginning of the procedure and repeated hourly thereafter as required. A full aseptic technique is used, broad-spectrum intravenous antibiotics is not given routinely for isolated balloon valvuloplasty.
Standard right heart catheterisation techniques are used to assess pulmonary artery and right ventricular pressures, to perform a pullback gradient and to characterize the outflow tract anatomy. A right ventricular angiogram is acquired in a lateral projection to visualise the thickened, doming leaflets of the pulmonary valve and to confirm the nature and site of stenosis. Careful measurement of the valve annulus is made at this stage to inform subsequent catheter selection. In neonates, a small right ventricular cavity may be seen as a consequence of severe wall hypertrophy. An end-hole catheter is advanced into a distal pulmonary artery ; a branch of the left pulmonary artery usually offers better wire and balloon stability. An exchange wire (0.018-0.035 inch) is passed through and the end-hole catheter is removed. In the neonate with a patent arterial duct, the guidewire can be place across this and positioned in the descending aorta to achieve optimal manoevrability of the balloon catheter.
The diameter of the balloon catheter chosen should be approximately 25% larger than the measured annulus diameter. Whilst balloon oversizing improves effectiveness of pulmonary valvuloplasty, injury to the annulus is minimised by using balloons no bigger than 140% of annular diameter [2121. Radtke W, Keane JF, Fellows KE, Lang P, Lock JE. Percutaneous balloon valvotomy of congenital pulmonary stenosis using oversized balloons. J Am Coll Cardiol. 1986;8:909-15.
Description of the experience with balloon valvuloplasty using oversized balloons, which represents and important technical alteration for successful relief of stenosis, 2222. Ring JC, Kulik TJ, Burke BA, Lock JE. Morphologic changes induced by dilation of the pulmonary valve anulus with overlarge balloons in normal newborn lambs. Am J Cardiol. 1985;55:210-4. ]. For infants, young children and adolescents or adults, the length of the balloon catheter is 2 cm, 3 cm and 4 cm respectively. The chosen balloon catheter is flushed with dilute contrast and advanced over the guidewire. The balloon is positioned such that the stenotic pulmonary valve rests at the mid point. Partial inflation with contrast is useful to confirm correct positioning prior to subsequent complete inflation and waist obliteration. To avoid prolonged outflow tract obstruction and consequent hypotension, the period of inflation is kept to a minimum, with the entire cycle of inflation and deflation usually taking no longer than 10 to 15 seconds. The balloon catheter is deflated and retrieved, leaving the guidewire in situ. Repeat angiography is performed and further balloon repositioning and inflation repeated as required. Typically, three to four inflations with minor adjustments are required to achieve adequate relief of severe stenoses. The pressure gradient is remeasured with the end hole catheter or more accurately with a Multi-Track™ catheter [NuMed Inc., Hopkinton, NY] which permits precise simultaneous haemodynamic and angiographic assessment. Reactive dynamic infundibular stenosis is a common complication, especially in those with severe pulmonary stenosis, and may be difficult to distinguish from residual valvular stenosis. Right ventricular angiography is helpful and further precise assessment with the Multi-Track™ catheter can help delineate the exact level of obstruction. The condition is rarely fatal and usually disappears spontaneously after a few hours ; reversal, however, can be expedited with fluid boluses or beta blocker administration ( ILLUSTRATIVE CASE 1).
In adults or those whose pulmonary valve annulus exceeds 18-19 mm, a double-balloon technique (either using a second exchange wire or a Multi-Track™ catheter) is preferred, although this does vary between operators. Advantages of this approach include the persistence of the lumen throughout the inflation period, a shorter duration of balloon deployment and less resulting trauma to the vessel wall. In selecting catheters for this procedure, two similar size balloons should be chosen whose sum diameter is approximately 60% greater than the annulus diameter [2323. Beekman RH, Lloyd TR, Hirsch R. Transcatheter Therapies for Congenital Heart Disease. In: Textbook of Interventional Cardiology (5th Edition). Editor: Topol E. Publishers: Saunders WB Co, Philadelphia. ].
Balloons and materials for balloon pulmonary valvuloplasty
- Ideal balloon diameter for effective dilatation of valvar stenosis is 110-120% of the annular dimeter measured in echo
- Balloon length should be chosen to facilitate stability during inflation. Length should be at least 1.5 times of the diameter
- Valvar balloon dilatation does not require high-pressure balloons and can be successfully achieved with low pressure inflation
- The most commonly used balloons are NuMed and Balt because these companies offer a large range of available balloon types and sizes.
- In children and neonates, an effort needs to be made to use balloons with low profile to insure an acceptable size of vascular access
- In bigger children and adults, a stiff exchange support wire of 0.035-0.038” is required. In smaller children and neonates, smaller wires tpically of 0.018” are used to support low profile balloons
Acute results
Since, the pioneering case reported by Kan and colleagues, multiple groups have confirmed the efficacy with which percutaneous pulmonary valvuloplasty achieves significant gradient reduction across all age groups [22. Kan JS, White RI Jr, Mitchell SE, Gardner TJ. Percutaneous balloon valvuloplasty: a new method for treating congenital pulmonary-valve stenosis. N Engl J Med. 1982;307:540-2.
Report of the first balloon valvulolasty in pulmonary stenosis, 2424. Zeevi B, Keane JF, Fellows KE, Lock JE. Balloon dilation of critical pulmonary stenosis in the first week of life. J Am Coll Cardiol. 1988;11:821-4. , 2525. Hanley FL, Sade RM, Freedom RM, Blackstone EH, Kirklin JW.Outcomes in critically ill neonates with pulmonary stenosis and intact ventricular septum: a multiinstitutional study. Congenital Heart Surgeons Society. J Am Coll Cardiol. 1993;22:183-92.
This multi-center study summarizes the results for balloon valvuloplasty in newborns with critical pulmonary stenosis and intact ventricular septum., 2626. Ladusans EJ, Qureshi SA, Parsons JM, Arab S, Baker EJ, Tynan M. Balloon dilatation of critical stenosis of the pulmonary valve in neonates. Br Heart J. 1990;63:362-7. , 2727. Colli AM, Perry SB, Lock JE, Keane JF. Balloon dilation of critical valvar pulmonary stenosis in the first month of life. Cathet Cardiovasc Diagn. 1995;34:23-8. , 2828. Ali Khan MA, al-Yousef S, Huhta JC, Bricker JT, Mullins CE, Sawyer W. Critical pulmonary valve stenosis in patients less than 1 year of age: treatment with percutaneous gradational balloon pulmonary valvuloplasty. Am Heart J. 1989;117:1008-14. , 2929. Rey C, Marache P, Francart C, Dupuis C. Percutaneous transluminal balloon valvuloplasty of congenital pulmonary valve stenosis, with a special report on infants and neonates. J Am Coll Cardiol. 1988;11:815-20. , 3030. Rocchini AP, Kveselis DA, Crowley D, Dick M, Rosenthal A. Percutaneous balloon valvuloplasty for treatment of congenital pulmonary valvular stenosis in children. J Am Coll Cardiol. 1984;3:1005-12. , 3131. Stanger P, Cassidy SC, Girod DA, Kan JS, Lababidi Z, Shapiro SR. Balloon pulmonary valvuloplasty: results of the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am J Cardiol. 1990;65:775-83.
This paper gives insight into the outcome of pulmonary valvuloplasty in patietns with dysplastic pulmonary valve, which is reportd to be inferiror as compared to non-dysplastic valves. The results of the study described are important for patients selection and consenting prior to the procedure., 3232. Pepine CJ, Gessner IH, Feldman RL. Percutaneous balloon valvuloplasty for pulmonic valve stenosis in the adult. Am J Cardiol. 1982;50:1442-5. , 3333. Al Kasab S, Ribeiro PA, al Zaibag M, Halim M, Habbab MA, Shahid M. Percutaneous double balloon pulmonary valvotomy in adults: one- to two-year follow-up. Am J Cardiol. 1988;62:822-4. , 3434. Chen CR, Cheng TO, Huang T, Zhou YL, Chen JY, Huang YG, Li HJ. Percutaneous balloon valvuloplasty for pulmonic stenosis in adolescents and adults. N Engl J Med. 1996;335:21-5.
Important report on outcome of pulmonary valvuloplasty in the adult population, 3535. Kaul UA, Singh B, Tyagi S, Bhargava M, Arora R, Khalilullah M. Long-term results after balloon pulmonary valvuloplasty in adults. Am Heart J. 1993;126:1152. , 3636. Herrmann HC, Hill JA, Krol J, Kleaveland JP, Pepine CJ. Effectiveness of percutaneous balloon valvuloplasty in adults with pulmonic valve stenosis. Am J Cardiol. 1991;68:1111. , 3737. Tentolouris CA, Kyriakidis MK, Gavaliatsis IP, Kourouklis CB, Toutouzas PC. Percutaneous pulmonary valvuloplasty in an octogenarian with calcific pulmonary stenosis. Chest. 1992;101:1456-8. ]. A final resting gradient of less than 30mmHg across the pulmonary valve should be expected irrespective of age. The presence of a dysplastic pulmonary valve is, however, associated with higher residual gradients and less consistent outcomes [3030. Rocchini AP, Kveselis DA, Crowley D, Dick M, Rosenthal A. Percutaneous balloon valvuloplasty for treatment of congenital pulmonary valvular stenosis in children. J Am Coll Cardiol. 1984;3:1005-12. ].
NEONATES AND INFANTS
The neonate or infant with severe pulmonary stenosis is typically critically ill and more likely to have a diminuitive right ventricle with a hypoplastic pulmonary annulus. Catheterization is technically more challenging and complications more frequent [1616. Hanley FL, Sade RM, Freedom RM, Blackstone EH, Kirklin JW. Outcomes in critically ill neonates with pulmonary stenosis and intact ventricular septum: a multiinstitutional study. Congenital Heart Surgeons Society. J Am Coll Cardiol. 1993;22:183-92. , 2323. Beekman RH, Lloyd TR, Hirsch R. Transcatheter Therapies for Congenital Heart Disease. In: Textbook of Interventional Cardiology (5th Edition). Editor: Topol E. Publishers: Saunders WB Co, Philadelphia. , 2424. Zeevi B, Keane JF, Fellows KE, Lock JE. Balloon dilation of critical pulmonary stenosis in the first week of life. J Am Coll Cardiol. 1988;11:821-4. , 2525. Hanley FL, Sade RM, Freedom RM, Blackstone EH, Kirklin JW.Outcomes in critically ill neonates with pulmonary stenosis and intact ventricular septum: a multiinstitutional study. Congenital Heart Surgeons Society. J Am Coll Cardiol. 1993;22:183-92.
This multi-center study summarizes the results for balloon valvuloplasty in newborns with critical pulmonary stenosis and intact ventricular septum., 2626. Ladusans EJ, Qureshi SA, Parsons JM, Arab S, Baker EJ, Tynan M. Balloon dilatation of critical stenosis of the pulmonary valve in neonates. Br Heart J. 1990;63:362-7. ]. Initiation and maintenance of a Prostaglandin E1 infusion prior to catheterization improves haemodynamic stability as well as maintaining ductal patency; this is useful both for catheter positioning and preservation of pulmonary blood flow during balloon occlusion of the right ventricular outflow tract. Despite these considerations, successful dilatation can still be achieved in up to 95% of neonates and infants ( ILLUSTRATIVE CASE 2). In the few cases where cyanosis persists, stabilisation on prostaglandin is slow or the therapy is not tolerated, ductal stenting or a systemic-to-pulmonary artery shunt should be considered.
ADULTS
Percutaneous pulmonary valvuloplasty is of comparable efficacy in adults, even into late age when valve calcification may be present [3131. Stanger P, Cassidy SC, Girod DA, Kan JS, Lababidi Z, Shapiro SR. Balloon pulmonary valvuloplasty: results of the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am J Cardiol. 1990;65:775-83.
This paper gives insight into the outcome of pulmonary valvuloplasty in patietns with dysplastic pulmonary valve, which is reportd to be inferiror as compared to non-dysplastic valves. The results of the study described are important for patients selection and consenting prior to the procedure., 3232. Pepine CJ, Gessner IH, Feldman RL. Percutaneous balloon valvuloplasty for pulmonic valve stenosis in the adult. Am J Cardiol. 1982;50:1442-5. , 3333. Al Kasab S, Ribeiro PA, al Zaibag M, Halim M, Habbab MA, Shahid M. Percutaneous double balloon pulmonary valvotomy in adults: one- to two-year follow-up. Am J Cardiol. 1988;62:822-4. , 3434. Chen CR, Cheng TO, Huang T, Zhou YL, Chen JY, Huang YG, Li HJ. Percutaneous balloon valvuloplasty for pulmonic stenosis in adolescents and adults. N Engl J Med. 1996;335:21-5.
Important report on outcome of pulmonary valvuloplasty in the adult population, 3535. Kaul UA, Singh B, Tyagi S, Bhargava M, Arora R, Khalilullah M. Long-term results after balloon pulmonary valvuloplasty in adults. Am Heart J. 1993;126:1152. , 3636. Herrmann HC, Hill JA, Krol J, Kleaveland JP, Pepine CJ. Effectiveness of percutaneous balloon valvuloplasty in adults with pulmonic valve stenosis. Am J Cardiol. 1991;68:1111. ]. Whilst the majority of procedures can be accomplished with a single balloon technique ( ILLUSTRATIVE CASE 1), the double balloon technique has comparable outcomes [3838. Fawzy ME, Mercer EN, Dunn B. Late Results of Pulmonary Balloon Valvuloplasty in Adults Using Double Balloon Technique. J Intervent Cardiol. 1988;1:35–42. ].
Late follow-up
Percutaneous balloon valvuloplasty compares favorably with surgical valvotomy, providing equivalent long-term gradient relief with only trivial to mild pulmonary insufficiency in the vast majority of patients [2424. Zeevi B, Keane JF, Fellows KE, Lock JE. Balloon dilation of critical pulmonary stenosis in the first week of life. J Am Coll Cardiol. 1988;11:821-4. , 3030. Rocchini AP, Kveselis DA, Crowley D, Dick M, Rosenthal A. Percutaneous balloon valvuloplasty for treatment of congenital pulmonary valvular stenosis in children. J Am Coll Cardiol. 1984;3:1005-12. , 3939. McCrindle B, Kan SJ: Long-term results after balloon pulmonary valvuloplasty. Circulation. 1991;83:1915.
This work focused on long-term outcome after pulmonary valvuloplasty, which serve as a reference for consenting and decicion making in these patients., 4040. O’Connor BK, Beekman RH, Lindauer A, Rocchini A: Intermediate-term outcome after pulmonary balloon valvuloplasty. Comparison with a matched surgical control group. J Am Coll Cardiol. 1992;20:169. , 4141. Masura J, Burch M, Deanfield JE, Sullivan ID: Five-year follow-up after balloon pulmonary valvuloplasty. J Am Coll Cardiol. 1993;21:132. ]. With up to 9 years follow-up, between 10 and 15% of patients require re-intervention, either repeated balloon dilatation or surgery, typically for infundibular stenosis or a dysplastic valve. Independent predictors of poor long-term outcome are younger age at initial valvoplasty, small valve annulus diameter, smaller balloon to annulus diameter ratio and high residual gradient post valvuloplasty [4242. Melgares R, Prieto JA, Azpitarte J: Success determining factors in percutaneous transluminal balloon valvuloplasty of pulmonary valve stenosis. Eur Heart J. 1991;12:15. , 4343. McCrindle BW. Independent predictors of long-term results after balloon pulmonary valvuloplasty. Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry Investigators. Circulation. 1994;89:1751-9.
This registry informs on the determinates of long-term results after pulmonary valvuloplasty]. Some investigators have suggested that intervention when the systolic outflow tract gradient lies between 40 and 60 mm Hg achieves the best hameodynamic and symptomatic outcome [4444. Mendelson AM, Banerjee A, Meyer RA, Schwarz DC: Predictors of successful pulmonary balloon valvuloplasty: 10-year experience. Cathet Cardiovasc Diagn. 1996;39:236. ]. Successful relief of obstruction is associated with improved clinical outcome [1717. Nugent EW, Freedom RM, Nora JJ, Ellison RC, Rowe RD, Nadas AS. Clinical course in pulmonary stenosis. Circulation. 1977;56:I38-47.
Excellent report on the clinical presentationg and outcome in patietns with pulmonary stenosis, 4545. Mody MR: The natural history of uncomplicated valvular pulmonic stenosis. Am Heart J 1975;90:317. , 4646. Tinker J, Howitt G, Markman P, Wade EG: The natural history of isolated pulmonary stenosis. Br Heart J. 1965;27:151. , 4747. Johnson LW, Grossman W, Dalen JE, Dexter L: Pulmonic stenosis in the adult. Long-term follow-up results. N Engl J Med. 1972;287:1159. , 4848. Engle MA, Ito T, Goldberg HP: The fate of the patient with pulmonic stenosis. Circulation. 1964;30:554. ].
Complications
Major complications include rupture of the right ventricular outflow tract and cardiac tamponade and injury to the tricuspid valve leaving significant regurgitation. The majority of patients will only have minimal residual pulmonary regurgitation following the procedure, however moderate or severe insufficiency can occur.
In this situation, a percutaneous pulmonary valve implantation could be considered [4949. Lurz P, Coats L, Khambadkone S, Nordmeyer J, Boudjemline Y, Schievano S, Muthurangu V, Lee TY, Parenzan G, Derrick G, Cullen S, Walker F, Tsang V, Deanfield J, Taylor AM, Bonhoeffer P. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117:1964-72. ]. Minor complications include femoral venous thrombosis, haemmorhage and transient arrhythmia and as with any invasive angiographic procedure there is a small risk of infection or reaction to the contrast solution. Dynamic infundibular obstruction with resulting hypotension and hypoxia is a particular risk in the young patient with critical stensosis. If it occurs, fluid boluses and a beta blocker infusion can stabilize the situation.
Complications are more common in neonates than older patients, in part due to the relative size of the catheters with the respect to the cardiac chambers but due to the clinical status of the patients.
Conclusion
Percutaneous pulmonary valvuloplasty is the first line treatment for all patients with more than moderate isolated pulmonary stenosis, from the critically ill neonate to the octogenarian with a calcified valve. Over 85% of those treated are free from reintervention up to 9 years later. Outcomes in patients with dysplastic valves are variable due to heterogenous pathology, but the procedure should in most cases be attempted.
Personal perspective – Philipp Bonhoeffer
The treatment of isolated pulmonary valve stenosis is one of the most rewarding procedures in interventional cardiology. Even in critical neonatal pulmonary stenosis, simple efficacious balloon dilatation can provide a definitive solution and avoid the need for future medical intervention. Excellent results can be obtained if the procedure is performed with care by the interventionalist. Whilst, balloon oversizing and rough handling of the tricuspid valve may not lead to problems in every patient, they can lead to disastrous results in some. When an easy intervention is performed incorrectly it can lead to a lifetime of trouble for the patient, creating a complex problem from a relatively simple condition.
Online data supplement
Video 1 :
Valvar pulmonary stenosis with infundibular obstruction in an adult (Lateral view).
Video 2 :
Valvar pulmonary stenosis with infundibular obstruction in an adult (Anteroposterior view)
Video 3 :
Percutaneous pulmonary valvuloplasty (single balloon) in an adult (Lateral view).
Video 4 :
Percutaneous pulmonary valvuloplasty (single balloon) in an adult (Anteroposterior view).
Video 5 :
Reactive dynamic infundibular stenosis followingpercutaneous pulmonary valvuloplasty (single balloon) in an adult (Lateral view).
Video 6 :
Reactive dynamic infundibular stenosis following percutaneous pulmonary valvuloplasty (single balloon) in an adult (Lateral view).
Valvular heart disease guidelines 2012
http://www.escardio.org/GUIDELINES-SURVEYS/ESC-GUIDELINES/Pages/valvular-heart-disease.aspx