Before embarking on PCI
PATIENT EVALUATION
Any medical intervention, particularly invasive ones, should have a clear indication that justifies the risk no matter how “small”. There are few data on the appropriateness of these decisions. Studies that have demonstrated the importance of the amount of ischaemia rather than the visual impression of a coronary stenosis when deciding to perform PCI the value of a clear indication. Is the procedure justified? Avoiding an unnecessary procedure may in turn avoid a complication [1616. Patel MR, Dehmer GJ, Hershfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009. Appropriateness Criteria for Coronary Revascularization. NEJM. 2009;360:213-24. , 1717. Torino PAL, De Bruyne B, Pijls NHJ, Siebert U, Ikeno F, van”t veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF ;FAME Study Investigators. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Intervention. NEJM. 2009;360:213-24. , 1818. Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates Er, Spertus JA, Berman DS, Mancini GB, Weintraub WS;COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. NEJM. 2007;356:1503-16. ].
The assessment that takes place in order to judge the feasibility for PCI is essentially one based on a combination of individualised risk/benefit analysis, lesion/technical and patient/co-morbid characteristics. The aim of such evaluations is to identify those at highest risk of an adverse procedural outcome and, either avoid the intervention by choosing an alternative therapeutic option, or prepare for a potential complication in order to mitigate it’s effect. Often these evaluations are subjective, and in the case of acute coronary syndromes are made without all the possible information available. The key point is to plan a strategy as much as possible before starting the procedure.
Preparative and preventive measures are predicated on an adequate history of presenting illness, drug history, cardiovascular risk factors, allergies and physical examination of the patient. Some areas of particular importance are highlighted below. A full consideration of these measures, however, is only possible for elective indications for PCI.
Patient clinical characteristics
These include factors such as advanced age, urgent or unscheduled intervention, haemodynamic instability, heart failure, renal insufficiency, diabetes mellitus, electrolyte disturbance and arrhythmia.
- Decompensated heart failure is a contraindication to coronary catheterisation unless the patient is intubated and ventilated. Without this advanced respiratory support, acute pulmonary oedema on-table or shortly afterwards is likely and carries significant morbidity and mortality.
- In patients with diabetes mellitus, blood glucose management should aim to prevent hypo or hyperglycaemia during the procedure. Metformin therapy, especially in the presence of concomitant renal impairment, should be stopped due to the risk of lactic acidosis after intervention
- Anaemia is not a contraindication to diagnostic catheterisation. The contribution of anaemia to symptoms should, however, be considered. Elective PCI with stents should be avoided if there is unexplained iron-deficiency.
- Additional steroid cover must be given for steroid dependent patients, and those with a history of prior contrast allergy.
- Baseline electrolytes, and coagulation/platelet profiles should be within acceptable limits to help prevent arrhythmia and haemorrhagic complications respectively. For the former, sodium and potassium within the normal range; and for the latter, an INR <1.8 (when using the femoral approach) and platelets >50x10 are considered sufficient for the safe conduct of a procedure.
- All invasive diagnostic and interventional procedures must be supported by a defibrillator and resuscitation trolley in close proximity It is good practice to have defibrillator pads attached to a patient suffering from acute STEMI.
- Rapid access to anaesthetic support, echocardiography and temporary pacing should be available. The opinion of an interventional cardiology colleague may be invaluable at the time of a procedure.
Radiocontrast
Renal insufficiency (estimated GFR<60ml/min) is associated with the development of nephropathy and acute renal failure after PCI. In particular the following features indicate high–risk patients: use of an Intra Aortic Balloon Pump (IABP), unscheduled PCI, chronic heart failure, diabetes mellitus, peripheral vascular disease, age>75 and high contrast volumes. In those with CKD or diabetes mellitus low osmolar contrast media should be used. A maximal allowable contrast dose (defined as 5ml x weight [Kg]/serum creatinine [mg/ml]) was the first to be validated in a large registry [1919. Brown JR, Robb JF, Block CA, Schoolwerth AC, Kaplan AV, O’Connor GT, Solomon RJ, and Malenka DJ. Does Safe Dosing of Iodinated Contrast Prevent Contrast-Induced Acute Kidney Injury? Circulation: Cardiovascular Interventions. 2010;3:346-350. , 2323. Freeman RV, O’Donnell M, Share D, Meengs WL, Kline-Rogers E, Clark Vl, DeFranco AC, Eagle KA, McGinty JG, Patel K, Maxwell-Eward A, Bondie D, Moscucci M; Blue Cross-Blue Shield of Michigan Cardiovascular Consortium (BMC-2). Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of adjusted contrast dose. Am J Cardiol. 2002;90:1068-73. ] as a potentially useful decision limit Recommendations for allowable contrast load, however, are variable and considered in more detail in  View chapter and
View chapter and  View chapter . The important point being that whichever of the available formulae is used, it should be integrated into local catheter laboratory practice. Automatic injection systems may play a role in limiting contrast exposure particularly in diagnostic coronary angiography [2020. Khoulaz S, Kern MJ, Bitar, Arzak E, Eisenhauer M, Wolford T, El-Shafei A. Coronary angiography using 4 Fr catheters with Acisted power injection: A randomized comparison to 6Fr manual technique and early ambulation. Catheter Cardiovasc Interven. 2001;52:393-98. , 2121. Goldstein JA, Kern M, Wilson R. A novel automated injection system for angiography. J Intervent Cardiol. 2001;14:147-52. , 2222. Goss JE, Ramo BW, Raff GL, Maddoux GL, Shadoff N, Leatherman GF. Power injection of contrast medium during percutaneous transluminal coronary artery angioplasty. Catheter Cardiovasc Diagn. 1989;16:195-98. ] but have not been practical for PCI.
View chapter . The important point being that whichever of the available formulae is used, it should be integrated into local catheter laboratory practice. Automatic injection systems may play a role in limiting contrast exposure particularly in diagnostic coronary angiography [2020. Khoulaz S, Kern MJ, Bitar, Arzak E, Eisenhauer M, Wolford T, El-Shafei A. Coronary angiography using 4 Fr catheters with Acisted power injection: A randomized comparison to 6Fr manual technique and early ambulation. Catheter Cardiovasc Interven. 2001;52:393-98. , 2121. Goldstein JA, Kern M, Wilson R. A novel automated injection system for angiography. J Intervent Cardiol. 2001;14:147-52. , 2222. Goss JE, Ramo BW, Raff GL, Maddoux GL, Shadoff N, Leatherman GF. Power injection of contrast medium during percutaneous transluminal coronary artery angioplasty. Catheter Cardiovasc Diagn. 1989;16:195-98. ] but have not been practical for PCI.
Anaphylactoid reactions to iodinated radiocontrast agents are rare events but can occur unexpectedly. They are caused by direct histamine release from mast cells and are not IgE-mediated allergic reactions. The risk is elevated in those who have either had a prior severe reaction, (whether to contrast agents or any unrelated substance), requiring medical intervention or actively treated asthma [2424. Morcos SK. Acute serious and fatal reactions to iodinated contrast media : our current understanding. Br. J. Radiol. 2005;78:686-93. ]. Seafood allergy is related to tropomyosin content rather than the presence marine iodininated biochemicals and so is not associated with an increased risk of a radiocontrast reaction.
Although the risk of anaphylactoid has fallen with the introduction of non-ionic agents [2525. Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. Radiology. 1990;175:621-8. ], operators should anticipate this possibility if unexplained hypotension occurs during the procedure. A patient under a drape is not easily identified as suffering the early dermatological signs of a reaction. Only repeated steroid premedication has an observable effect in reducing the respiratory manifestations of these reactions [2626. Tramer MR, von Elm E, Loubeyre P, Hauser C. Pharmacalogical prevention of serious anaphylactic reactions due to iodinated contrast media:systematic review. BMJ. 2006;333:675. ]. Therefore preventive measures will not be efficacious in the setting of PPCI. The emergency management of anaphylactoid managements should be known by all catheter lab personnel.
Vascular access
With either femoral or radial approaches the modified Seldinger technique [2727. Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953;39:368-76. ] is used. An adequate flow of blood on puncture indicates proper alignment of the needle shaft with a vessel of adequate calibre to admit a guidewire and introducer sheath. Attempts to introduce equipment into a vessel without these characteristics are likely to lead to access vessel dissection and the abandonment of the access site. Access site dissection when it occurs is not limb threatening on account of the direction of blood flow, which splints the dissection flap open and facilitates healing. A key difference between the Seldinger technique used for femoral as opposed to radial access is that for the former an anterior puncture is ideal whereas for the latter an anterior and posterior wall puncture is commonly performed.
Femoral artery
Arterial vascular access for PCI is still most commonly acquired via the femoral artery and may be associated with increased complications compared to the radial approach [3434. Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011; 377: 1409-1420. ].
Complications from femoral access for PCI
- Retroperitoneal haemorrhage
- Pseudoaneurysm
- AV fistula
- Infection
- Haematoma
- Neuropraxia
- Lower limb ischaemia (thrombosis or embolism)
- Dissection
Prevention of complications
To reduce these complications attention to puncture technique is fundamental. The use of the groin skin crease as an anatomical landmark should be avoided. In cases where surface anatomical landmarks are difficult to ascertain fluoroscopic assisted bony landmarks or portable ultrasound can be used. The aim should be to puncture the common femoral artery within a target zone 5 mm to 14 mm inferior to the centre of the femoral head [2929. Schnyder G, Sawhney N, Whisenant B, Tsimikas S, Turi ZG. Common Femoral Artery Anatomy Is Influenced By Demographics and Comorbidity:Implications for Cardiac and Peripheral Invasive Studies. Catheter Cardiovasc Interv. 2001;53:289-95. ] ( Figure 3 ), as this gives the best chance of avoiding both the femoral bifurcation and the inferior sweep of the inferior epigastric artery. In addition means that effective manual compression can be performed. The safety of vascular closure devices deployment can also be assessed by femoral angiography. Their use should probably be avoided if the diameter of the CFA is <5mm, the presence of a puncture site below the common femoral bifurcation or in the presence of significant atherosclerosis. Femoral angiography can be performed before starting or at the end of the coronary procedure. If femoral angiography is performed before instrumentation of the coronaries in elective patients then this gives the opportunity for postponement if the risk of vascular complications is deemed high.
Predictors of complications relating to vascular access
- Female gender
- Advanced age
- Small body surface area
- Prior instrumentation
- Anticoagulation
- Peripheral vascular disease
- Diabetes Mellitus
- Suboptimal technique (puncture and closure)
Management of complications
Acute ischaemia
Arterial thrombosis or embolism of the femoral artery is a limb-threatening complication. Any sign of acute limb ischaemia (pulseless, pale, painful, paraesthetic foot) should prompt emergency referral to a vascular surgeon for assessment.
Haematoma
The most common complication encountered is that of a haematoma. This is a palpable mass over the puncture site and must be distinguished from an ecchymosis, which is discolouration of skin tissue by extravasated blood. Management consists of manual or mechanical compression. Ultrasound examination is mandatory if there is any persistent discomfort or the presence of a bruit on auscultation.
Retroperitoneal haemorrhage
A large (>110,000 patients) contemporary retrospective analysis informs us that retroperitoneal haemorrhage is uncommon (<1%) after PCI. Although associated with high femoral puncture (superior to the centreline of the femoral head), it can occur with ideally situated punctures owing to movement of blood along fascial planes [2929. Schnyder G, Sawhney N, Whisenant B, Tsimikas S, Turi ZG. Common Femoral Artery Anatomy Is Influenced By Demographics and Comorbidity:Implications for Cardiac and Peripheral Invasive Studies. Catheter Cardiovasc Interv. 2001;53:289-95. , 3030. Trimarchi, Santi, Smith, Dean E., Share, David, Jani, Sandeep M., O’Donnell, Michael, McNamara, Richard, Riba, Arthur, Kline-Rogers, Eva, Gurm, Hitinder S., Moscucci, Mauro, on behalf of the BMC2 Registry, Retroperitoneal Hematoma After Percutaneous Coronary Intervention: Prevalence, Risk Factors, Management, Outcomes, and Predictors of Mortality: A Report From the BMC2 (Blue Cross Blue Shield of Michigan Cardiovascular Consortium) Registry. J Am Coll Cardiol Intv. 2010;3: 845-850. ]. They typically present within 3hrs of the index procedure and should be suspected if there is recurrent hypotension (in association with bradycardia or tachycardia), flank or abdominal pain ipsilateral to the puncture site following PCI. Initial management is with fluid resuscitation and only once a patient is stable should definitive diagnosis in the form of a CT pelvis/abdomen be undertaken. The vast majority (>90%) of RPH is managed conservatively, however, there is a significant mortality (6%) compared with those without this complication [3131. Farouque, H.M. Omar, Tremmel, Jennifer A., Shabari, Farshad Raissi, Aggarwal, Meenakshi, Fearon, William F., Ng, Martin K.C., Rezaee, Mehrdad, Yeung, Alan C., Lee, David P. Risk factors for the development of retroperitoneal hematoma after percutaneous coronary intervention in the era of glycoprotein IIb/IIIa inhibitors and vascular closure devices. J Am Coll Cardiol. 2005;45:363-36. ]. Although medical management is common, recurrent clinical signs of hypovolaemia and/or radiographic signs of active extravasation merit surgical consultation with a view to open exploration or interventional therapy, including the deployment of a covered stent via the contralateral femoral artery.
Pseudoaneurysm
Pseuoaneurysms are the result of punctures that fail to heal completely leaving a cap of haematoma over the puncture site. These frequently involve punctures below the femoral bifurcation as they can be difficult to compress effectively post procedure. They are typically tender, pulsatile and expansile and have an audible bruit. However, the latter depends on the position of the defect, with posterior or lateral pseudoaneurysms frequently being silent. Management consists of manual compression with an ultrasound and administration of opiate analgesia. If unsuccessful and an accessible track can be located to the neck of the aneurysm then ultrasound fibrin injection to thrombose the neck is commonly undertaken.
AV fistulae
AV fistulae are uncommon iatrogenic complications comprising <1% of transfemoral cardiac catheterisations. Prospective large-scale cohort analysis (n=10,272) identifies left femoral puncture (with right-handed operators), female gender, intensity of anticoagulation (warfarin and heparin) and hypertension as predictive factors [3232. Incidence and clinical outcome of iatrogenic femoral arteriovenous fistulas: implications for risk stratification and treatment. Kelm M, Perings SM, Jax T, Lauer T, Schoebel FC, Heintzen MP, Perings C, Strauer BE. J Am Coll Cardiol. 2002;40:291-7. ]. Puncture below the common femoral bifurcation may provide an anatomical substrate as at this level the femoral vein lie posterior to the superficial femoral artery rather than medial. These complications are not associated with limb injury, symptoms or right heart failure (owing to universally low associated shunt volumes [<500ml/min]). More than one third close spontaneously within a year, and so despite a number of invasive solutions (e.g., covered stent deployment and open surgery), an initial conservative approach with ultrasound-guided compression is supported by the observational data Further descriptions of interventional management are dealt with in  View chapter .
View chapter .
Neuralgic complications involving the femoral nerve whilst not life-threatening can be very symptomatic but have a good prognosis. They are always neuropraxias rather than transections and so will heal, however, the time course is variable and can be as long as 6 months.
How to reduce transfemoral access complications
- Access using fluoroscopy (or ultrasound)
- Puncture site at or below centreline of the femoral head
- Femoral angiogram (RAO 30 and LAO 60 degrees) prior to closure
- PCI and anticoagulate only if in the ‘safe zone’ (elective patients)
Radial artery
Radial arterial access reduces haemorrhagic complications, its advantage being especially evident in certain subgroups (those with anticoagulation, severe obesity, chronic respiratory disease, haemostasis disorders and PPCI) [3333. Kiemeneij F, Laarman GJ, Oderkerken D, Slagboom T, van der Wieken R. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial, and femoral approaches: the Access study. JACC. 1997;29:1269-75. ]. Importantly the results from the RIVAL randomised controlled trial [not included in contemporary meta-analyses comparing femoral and radial access] demonstrate that a clear mortality advantage for an all-comer radial approach could not be identified in those undergoing PCI, however, the sub-group undergoing primary PCI did demonstrate a mortality benefit [3434. Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011; 377: 1409-1420. ].
Prevention of complications
Although access site complications with the radial approach are dramatically reduced they are not entirely eliminated (vasospasm preventing the completion of the procedure, compartment syndrome, arterial dissection, radial avulsion, perforation or occlusion), and the technique requires a significant period of learning. Hand ischaemia has not been reported after PCI, and ischaemia relating to compartment syndrome or radial arterial cannulae (in the field of critical care) is rare [3535. Wallach SG. Cannulation Injury of the Radial Artery: Diagnosis and Treatment Algorithm. Am J Critical Care . 2004;13:315-319. , 3636. Tizón-Marcos H, Barbeau G. Incidence of Compartment Syndrome of the Arm in a Large Series of Transradial Approach for Coronary Procedures. Journal Interven Cardiol. 2008;21:380-384. ]. Radial artery occlusion rates of 3-5% can be reduced by procedural heparin, decreasing sheath size and the technique of perfused haemostasis following the end of the procedure [3737. Pancholy S, Coppola J, Tejas P, Marie Roke-Thomas M. Prevention of radial artery occlusion. Patent hemostasis evaluation trial (PROPHET study): A randomized comparison of traditional versus patency docu- mented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. 2008;72:335-340. ]. Overall the benefit clearly identified in studies carried out in pioneering or large volume centres needs to be weighed up against the experience of the operator; a high volume femoral operator may have fewer complications than a low volume radial operator. Radial and other contiguous forearm arteries are particularly prone to vasospasm due to the presence of a high density of vasoconstrictor alpha receptors (predominantly alpha-1 with some alpha-2). The prevention of this complication with a pharmacological “cocktail”, gentle handling of equipment and avoiding numerous exchanges is much more effective than attempts to reverse it. Except perhaps in high volume “radial” centres the femoral access site should always be prepared in case radial access needs to be abandoned, or where emergency placement of an IABP is needed or anticipated.
Reported complications from transradial access for PCI
- Vasospasm
- Pseudoaneurysm
- Puncture site granuloma (one brand of hydrophilic sheath use only)
- Dissection
- Perforation/forearm or chest wall haematoma
- Compartment syndrome
Management of complications
Compartment syndrome
The upper limb should always be evaluated for this in the immediate post-procedural phase. This syndrome is caused by raised pressure within a closed osseo-facial anatomical space. Pressure greater than 30 mmHg or within 30mmHg of diastolic pressure is sufficient to trigger ischaemia of the forearm muscles. The cardinal sign is pain on passive stretch of these muscles by hand dorsiflexion. Prompt application of proximal compression for any haematoma will help mitigate against this complication ( Figure 4 and Figure 5 ). As with the femoral artery, any sign of distal neurovascular deficit should prompt an emergency referral to a vascular surgeon.
Sterile abcess
This granulomatous reaction at the puncture site occurred with a specific introducer sheath, which is no longer available now.
Pseudoaneurysm
Although very uncommon, inadequate radial artery compression post-procedure can lead to a pseudoaneurym. Treatment is along the same lines as femoral pseudoaneurysms.
Vessel perforation or injury
Dissection and perforation of forearm vessels, manifest as sudden discomfort during the procedure Typically perforation occurs in the small branches of forearm or axillary vessels. Long sheaths and guidewire access to the aortic root may allow the procedure to continue but frequently the access site has to be abandoned. Close observations are required for compartment syndrome in the case of forearm vessel perforation.
Severe vasospasm
Vasospasm can occur anywhere along the arterial course of the radial, ulnar or axillary arteries with equipment still in place. Once fully developed it can be challenging to overcome. Sedation, analgesia, and additional nitrate and calcium channel antagonist may alleviate the spasm. On rare occasions, however, an anaesthetist may be required to administer a regional anaesthetic block or deep sedation in order to allow the equipment and sheath to be removed without risk of avulsion or severe discomfort.
Venous
Venous vascular access must not be overlooked in patients scheduled for PCI. The management of all life-threatening complications will require fluid and/or drug resuscitation In order to avoid delays, whilst central venous access is obtained, a good quality peripheral cannula, (if possible antecubital fossa and 21 Gauge or larger), can deliver fluid at rates of >125ml/min and therefore it is good practice to have this secured before the start of the procedure. Central venous access, typically 7Fr, via the common femoral vein, however, remains optimal practice in PCI emergencies and is worthwhile in high-risk PCI if peripheral access cannot be secured pre-procedure. When both femoral venous and femoral arterial access are required, then obtaining venous access first will help prevent inadvertent arterial injury. Furthermore, if venous access is used for temporary heart rate support via a pacing wire and access for fluid or drug administration in required, then a second ipsilateral or contralateral femoral venous puncture can be performed.
How to reduce transradial access complications
- Hydration and anxiolysis
- Vessel “cocktail” (usually calcium channel antagonist and/or nitrate)
- Heparin (at least 3-5000 units)
- Avoid puncture through the flexor retinaculum
- Perfusion haemostasis
LESION (ANGIOGRAPHIC) CHARACTERISTICS
These have been defined since the early days of angioplasty, and owing to the less invasive nature of coronary intervention compared with open surgery assume a greater importance in determining adverse outcome than patient characteristics alone. Calcification, tortuosity (angulation >45 degrees), left main, bifurcation subsets, degenerated saphenous vein graft, chronic total occlusion and multivessel disease all feature prominently in the diminished technical success of a procedure and the ability to be associated with complications [3838. Ellis SG, Vandourel MG, Cowley MJ, DiSciascio G, Deligonul U, Topol EJ, Bulle TM. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation. 1990;82:1193-1202. ]. This textbook illustrates technologies that have been devised to improve the anatomical success in these lesion subsets making the relationship between lesion type and success a dynamic one. Despite this progress in technology, angiographic features are still relevant. In patients with acute coronary syndromes [3939. Goto K, Lansky AJ, Fahy M, Cristea E, Feit F, Ohman EM, White HD, Alexander KP, Bertrand ME, Desmet W, Hamon M, Mehran R, and Stone GW. Predictors of Outcomes in Medically Treated Patients With Acute Coronary Syndromes Intervention Triage Strategy (ACUITY) Substudy. Circulation. 2010;121:853-62. ] they provide greater predictive value for ischaemic outcomes than traditional patient clinical characteristics alone.
RISK SCORES
(  View chapter ).
View chapter ).
The global prediction of cardiovascular complications is a potentially useful adjunct (Tables). The ACC/AHA lesion based score updated in 1990 [3838. Ellis SG, Vandourel MG, Cowley MJ, DiSciascio G, Deligonul U, Topol EJ, Bulle TM. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation. 1990;82:1193-1202. ] is perhaps the most widely known. It consists of angiographic variables to predict anatomical success but not complications. The SCAI modification [4040. Krone RJ, Shaw RE, Klein LW Block PC, Anderson HV, Weintraub WS, Brindis RG, Mckay CR. Evaluation of the American College of Cardiolgy/American Heart Association and the Society for Coronary Angiography and Interventions lesion classification system in the current “stent era” of coronary interventions (from the ACC-National Cardiovascular Data Registry). Am J Cardiol. 2003;92:389. ] is an improvement and has been validated against the ACC-National Cardiovascular Data Registry. These schemes, however, have only a modest ability to predict cardiovascular complications per se (c-statistic of 0.624 and 0.665 respectively). In part this may be due to the subjective nature of lesion assessment in clinical practice. Of all the lesion classifications only Type C contributes to predictive ability and may not be relevant in the contemporary DES era..
Risk stratification tools derived via multivariate analysis using a combination of patient and lesion characteristics improve on this (c-statistic 0.77) ( Table 5A and Table 5B ) [4141. Singh M, Lennon RJ, Holmes DR Jr, Bell MR, Rihal CS. Correlates of procedural complications and a simple integer score for percutaneous coronary intervention. JACC. 2002;40:387-93. ]. Patient characteristics and routine noninvasive measurements appear to have the greater discriminatory ability (c-statistic 0.89). The Mayo risk score focussing on the risk of procedural complications uses a 7 point simplified integer scoring scheme [4242. Singh M, Peterson ED, Milford-Beland MS, Rumsfeld JS, Spertus JA. Validation of the Mayo Clinic Risk Score for In-HospitalMortality After Percutaneous Coronary Interventions Using the National Cardiovascular Data Registry. Circ Cardiovasc Intervent. 2008;1:36-44. ]. These “safety” tools as well as the Syntax “PCI efficacy” score for multi-vessel disease are useful in helping to plan a strategy. More recently, the Euroheart PCI score has been developed providing the most contemporary risk scoring system currently available which has been validated in a large population. Interestingly, clinical factors appeared to have the greatest discriminatory power in predicting procedural mortality: age over 80, primary PCI for STEMI and haemodynamic instability [4343. de Mulder M, Gitt A, van Domburg R, Hochadel M, Seabra-Gomes R, Serruys PW, Silber S, Weidinger F, Wijns W, Zeymer U, Hamm C, Boersma E. EuroHeart score for the evaluation of in-hospital mortality in patients undergoing percutaneous coronary intervention. Eur Heart J. 2011;32:1398-1408.
Useful risk score system validated in a large cohort of European patients with high predictive value.]. Just as important as the predictive power of any score, however, is its ease of use and inter-observer reproducibility ( Figure 6 ).
During PCI
OVERVIEW
The three cardinal and potential life-threatening complications specific to PCI that can occur during the procedure are:
- unexpected acute impairment of myocardial perfusion
- coronary perforation, and
- retained equipment (including material loss, fracture or embolisation).
These complications are emergencies and require a systematic approach to their diagnosis and management. In particular, the necessary tools and drugs to deal with these situations as well as their location in the catheter laboratory must always be known to the operating team before a procedure is started ( Table 6 ). This is even more pertinent for those practitioners who practice at more than one catheter lab, or where a patient is identified a priori at high-risk of complications undergoes PCI.
As always in the case of emergencies in Medicine, the primary concern is to stabilise the airway, breathing and circulation (haemodynamic state) of the patient. To this end, advanced airway and respiratory skills (anaesthetic support), intravenous fluid, inotropic/pressor drugs, a temporary pacemaker and/or intra-aortic balloon pump may all be needed. The need for emergency cardiac surgery has reduced considerably but has not been eliminated [4444. Reinecke H, Fetsch T, Roeder N, Schmid C, Winter A, Ribbing M, Berendes E, Block M, Scheld HH, Breithardt G, Kerber S. Emergency coronary artery bypass grafting after failed coronary angioplasty:what has changed in a decade? Ann Thorac Surg. 2000;70:1997-2003. , 4545. Seshradi N, Whitlow PL, Acharya N, Houghtaling P, Blackstone EH, Ellis SG. Emergency coronary artery bypass surgery in the contemporary percutaneous coronary intervention era. Circulation. 2002;106:2346-2350. ], thus clear formalised channels of communication for obtaining assistance should exist, especially if surgical expertise is not immediately available on-site ( Figure 7 ).
Indications for emergency cardiac surgery following PCI [
4545. Seshradi N, Whitlow PL, Acharya N, Houghtaling P, Blackstone EH, Ellis SG. Emergency coronary artery bypass surgery in the contemporary percutaneous coronary intervention era. Circulation. 2002;106:2346-2350. ]
- Extensive coronary dissection (54%)
- Perforation/tamponade (20%)
- Recurrent acute vessel closure (20%)
- Haemodynamic instability (3%)
- Aortic dissection (2%)
- Guidewire fracture (1%)
n=18,593 procedures 1992-2000
Key to the success of any resuscitative effort in this context is the rapid restoration of anterograde coronary perfusion and/or the relief of cardiac tamponade. Attempts to secure this will need to occur simultaneously with cardiac massage and respiratory support and thus requires a high degree of team coordination. The use of automatic mechanical compression devices) ( Figure 8A and Figure 8B ) [4646. Steen S, Liao Q, Pierre L, Paskevicius A, Sjoberg T. Evaluation of LUCAS, a newdevice for automatic mechanical compression and active decompression resuscitation. Resuscitation. 2002;55:285–99. , 4747. Wagner H, Terkelsen CJ, Friberg H, Harnek J, Kern H, Lassen JF. Olivecrona GK. Cardiac arrest in the catheterisation laboratory; A 5-year experience of using mechanical chest compressions to facilitate PCI during prolonged resuscitation efforts. Resuscitation. 2010;81:383-387. ] may be of benefit in allowing operators to concentrate more on re-establishing coronary flow and/or decompressing tamponade. In accidents and complications occurring within the left coronary circulation, the main stem and proximal left anterior descending artery (LAD) must be considered as the privileged conduit at all times. The LAD alone can supply up to 60% of left ventricular myocardium and so loss of anterograde flow is associated with the highest morbidity and mortality. Pre-existing LV dysfunction is a further consideration in both the identification of a priori risk and the extent of support that may be required in the event of an ensuing complication. A NHBLI registry analysis reveals a higher in-hospital mortality for elective PCI in patients (N=1,458) with an EF <40% compared with >50% (3.0 % vs 0.1 %p<0.001) [4848. Keelan PC, Johnston JM, Koru-Sengul T Detre KM, Williams DO, Slater J, Block PC, Holmes DR Jr. Comparison of in-hospital and one year outcomes in patients with left ventricular ejection fractions 50% having percutaneous coronary revascularization. Am J Cardiol. 2003;91:1168-1172. ].
Physiological parameters that should prompt patient re-evaluation
- Decreased level of consciousness
- Respiratory rate <8 or >30min-1
- SaO2 <90% on room air
- Central systolic BP<90 mmHg or MAP<70 mmHg
- Change in cardiac rhythm
UNEXPECTED ACUTE IMPAIRMENT OF MYOCARDIAL PERFUSION
Epicardial Vessel closure
Abrupt vessel closure is the most common major complication during PCI. Its frequency has been reduced dramatically since the advent of routine stenting (11% pre-stent era vs 2% post-stent era) allowing practice to extend safely to sites without on-site cardiac surgery. Main vessel occlusion is the most dramatic manifestation, but more than 50% of PCI procedures involve a risk of sidebranch occlusion [4949. Meier B, Gruentzig A, King SB III, Douglas JS Jr, Hollman JJ, Ischinger T, Aueron F, Galan K. Risk of sidebranch occlusion during coronary angioplasty. Am J Cardiol. 1984;53:10-14. ]. The latter if involving a small calibre vessel is frequently asymptomatic and without prognostic significance.
Treatment of acute vessel closure is reserved for events that cause haemodynamic disturbance or electrocardiographic signs of persistent ischaemia and consists of stent deployment at the point of occlusion. Of note, the only randomised trial comparing stenting to balloon angioplasty for abrupt or threatened vessel closure, conducted in 1994 [5050. Keane DK, Roubin GS, Marco J, Fearnot N, Serruys PA. GRACE Gianturco-Robin stent Acute Closure Evaluation: substrate, challenges and design of a randomized trial of bailout management. J Interven Cardiol. 1994;7:333-9. ] was never completed. “Evidence” came by practice.
Acute impairment of epicardial perfusion or closure can be caused by dissection, iatrogenic thrombus formation on intracoronary equipment, embolisation of native thrombus or atheroma (the latter particularly during vein graft intervention), inadvertent injection of air, or rarely spasm.
Dissection overview
Any equipment that contacts vascular endothelium can cause traumatic injury. Mechanical dilatation of the artery with a balloon or a stent as well as the use of atheroablative devices, guidecatheters and guidewires, are associated with plaque fracture, intimal splitting and localised medial dissection. In the case of angioplasty it is part of its normal mechanism of action.
These tears (dissections) may separate the endothelial layer from the media. Dependent on the extent of injury they may extend into the media for varying distances, creating intraplaque haematoma and tissue flaps which may in turn compromise coronary blood flow through mechanical obstruction. Diminished coronary flow may then lead to platelet activation and thrombus formation. Procedural dissections are typically limited to the coronary circulation distal from the point of endothelial injury but can involve or extend retrogradely to a coronary ostium , the aorta or in the left coronary system both the LAD and Cx. Dissection planes large enough to impair anterograde flow typically consist of a complex spiral architecture making distal coronary access via a true lumen challenging. For this reason maintaining a secure wire position in the distal coronary bed that is undergoing intervention is one of the fundamental interventional technical skills.
Dissections may also occur in the aorta below the diaphragm or at the peripheral arterial access. These are not life or limb threatening per se as the centripetal flow of blood towards the periphery is sufficient to splint the vessel open until healing occurs. Nevertheless they need to be recognised so that an alternative access point is sought in order to continue with PCI.
• Coronary Dissection
Angiographically evident traumatic dissection may occur in as many as 40% of angioplasties. They can be classified into different morphologies with different risks of progression to proximal vessel closure [5151. Ferguson JJ, Barash E, Wilson JM Strony J, Wolfe MW, Schweiger MJ, Leya F, Bonan R, Isner JM, Roubin GS. The relation of clinical outcome to dissection and thrombus formation during coronary angioplasty. J Invasive Cardiol. 1995;7:2-10. , 5252. Klein LW. Coronary complications of percutaneous coronary intervention: a practical approach to the management of abrupt closure. Catheter. Cardiovasc. Interven. (2005) 64:395-401
Review of the management of abrupt vessel clossure.] ( Table 7). In general, type A and B dissections are considered clinically benign and do not adversely affect procedural outcome. However, types C through F are considered major dissections and carry a significant increase in morbidity and mortality if left untreated. Whilst the mechanism of angioplasty involves dissection and is inevitable, guidewire or guidecatheter injury remote from the target lesion can be minimised. Several elements are key: namely, selection of the optimal guiding catheter with adequate co-axial alignment of this catheter within the coronary ostium, and good tactile and visual feedback during guidewire manipulation. At the level of the left coronary artery, iatrogenic dissection of the roof of the left main stem is traditionally observed in patients with a large aortic root when using a “too short” classical JL 4 or 3.5 back-up type guiding catheter. Engaging catheters “on pressure” (connected to continuous pressure monitoring), whenever possible, and ensuring there is no pressure damping or ventricularisation can help avoid inadvertent dissection induced by contrast injection. Gradual ramping of the injection can further minimise the risk of this occurring. Importantly, all dissections may propagate with repetitive injections if not recognised by the operator, therefore vigilance is required during all phases of an intervention In the absence of distal guide wire position security, contrast injections should be avoided. If dissection is suspected but cannot be confirmed by angiographic projections, the use of intravascular ultrasound (IVUS) can be used as a diagnostic tool.
Type A and B dissections are probably best treated conservatively and the intervention postponed if elective. Type C or higher will need stabilisation by stenting. Following higher grades of coronary injury during PCI of the proximal LAD, “retrograde dissection” up to the level of the left main may be observed. This complication typically occurs after balloon perforation at high pressure and/or balloon oversizing. Treatment principles should be similar to that of anterograde dissection.
• Ostial dissection
Dissection of a coronary ostium is an emergency as the entire territory downstream of the injury is potentially threatened In addition retrograde extension into the ascending aorta is possible. An LAO 30 degree or caudal (“spider”) view can be used to assess adequate co-axial alignment of a left coronary intubation. Iatrogenic dissection of the ostium of the left main stem is a rare but critical condition that can occur at different stages from diagnostic to therapeutic catheterisation. The diagnosis can be missed as an ostial dissection flap may be supported by the guide catheter tip A clinical scenario of symptomatic ST elevation and/or haemodynamic disturbance that doesn’t fit with the angiographic appearance should prompt the exclusion of this complication ( Illustrative case 1 ). Exceptionally, total left main occlusion can be observed, an occurrence associated with rapid haemodynamic deterioration and cardiac arrest. This condition carries a high mortality, and will require advanced life support in the catheter laboratory with a brief attempt to find the true lumen. Prompt transfer to the operating theatre should be considered for emergency coronary bypass surgery if the situation cannot be recovered percutaneously.
Ostial dissection of the right coronary artery is more common than left main dissection, ( Illustrative case 2 ). It is observed after inadequate alignment of a diagnostic or guiding catheter combined with forceful injection of contrast medium. Anecdotally this occurs more frequently from the radial approach when using an AL1 catheter, Using an AL 0.75 for right coronary intubation should be considered. Proper alignment cannot be assessed from a classical left anterior oblique projection but should be checked from a sight left anterior oblique cranial view (10/10 degrees) and alignment can easily be obtained by further clockwise rotation.. If a coronary guide wire is in place to secure distal access, stenting of the ostial entry part of the dissection should be performed followed by the distal segment of the dissection. A single stent at each site will often be sufficient to stabilise this complication as distal subintimal bleeding will heal spontaneously. More stents, however, will be needed if normal anterograde flow is not immediately obtained. In the event that no guidewire is across the dissection or in case of inadvertent removal of the wire by the operator (on occasion because the operator has not recognised the dissection with collapse of the true lumen on wire retrieval) the situation is more delicate. The surgical team should be advised immediately, and attempts should be made to find the true lumen. Practically, a classical JR 4 guiding catheter should be kept at some distance from the ostium and a guidewire should be used to find the true lumen. Changing to a soft hydrophilic wire will often be successful in combination with multiple orthogonal views to seek out entry into the true lumen. This soft wire can be exchanged over a microcatheter for a more supportive wire prior to stenting. A microcatheter will also allow discrete distal contrast injections to assure an adequate position of the wire has been attained. A few alternative techniques have been described to facilitate access to the true lumen. Firstly, if there is a conal branch, this can be wired, and a small anchoring balloon inflated in order to stabilise the guiding catheter position immediately out of the coronary ostium. This approach will avoid repeated access to the “often” larger false lumen. Secondly, in case of persistent access to the false lumen, a microcatheter can be introduced in the false lumen and the subintimal bleeding site aspirated in order to facilitate access with a second soft wire into the true lumen. If there is difficulty in locating the true lumen then an IVUS catheter with Chroma function can be used.
Finally, in case of persistent failure and ongoing transmural ischaemia urgent bypass surgery should be considered.
• Aortic dissection
These dissections can be massive, accompanied by significant aortic regurgitation and/or cardiac tamponade. There is scarce data on their management. One early (1993-1996) retrospective review of 43,143 PCIs and cardiac catheterisation utilised a tripartite angiographic classification scheme ( Table 8 ). Class I dissections being limited to the sinus of valsalva, class II extending to the aorta <40 mm and class III extending >40 mm into the aorta. It found a frequency of aortic dissection to be 0.07 and 0.02% for PCI and diagnostic catheterisation respectively. There was a significant association with PCI for MI. Furthermore it suggested cardiac surgery for dissections extending 40 mm or more above the aortic root [5353. Dunning DW, Kahn JK, Hawkins ET, O’Neill WW. Iatrogenic Coronary Artery Dissections Extending Into and Involving the Aortic Root. Catheter Cardiovasc Interven. 2000;51:387-393. ] A more recent (1996-2005) prospective registry [5454. Gomez-Moreno S, Sabaté M, Jiménez-Quevedo P, Vazquez P, Alfonso F, Angiolillo DJ, Hérnandez-Antolin R, Moreno R, Banuelos C, Escaned J, Macaya C. Iatrogenic dissection of the ascending aorta following heart catheterisation :incidence, management and outcome. EuroInterv. 2006;2:197-202. ] of over 12,000 PCI and 26,000 catheterisations, suggests an incidence of 0.12% and 0.01% respectively. Class III dissections were more often associated with exclusive retrograde propagation. It also provides contrary evidence that virtually all iatrogenic dissections can be treated successfully, either by ostial placement of a covered stent or conservative management, with good prognosis. Other authors have advocated the use of a single or several non–covered stents. From a practical point of view, placement of a covered stent should be attempted if technically feasible to seal the entry of the dissection and stop its propagation ( Illustrative case 3 ). On rare occasions, surgical repair will be required if stenting fails ( Illustrative case 4). Bearing in mind the high mortality of unscheduled cardiac surgical intervention, this option should only be considered in extensive dissection with haemodynamic compromise.
Iatrogenic aortic dissection can also occur independent of a dissection extending from the coronary circulation. Manipulation of catheter equipment without a guidewire is the predominant cause. Analysis of the IRAD registry [5555. Januzzi JL, Sabatine MS, Eagle KA, Evangelista A, Brukman D, Fattori R, Moore A, Sechtern U, Llovet A, Gilon D, Pape L, O’Gara P, Mehta R, Cooper J, Hagan P, Armstrong W, Deeb M, Suzuki T, Nienaber C, Isselbacher E. Iatrogenic aortic dissection. Am J Cardiol. 2002;89(5):623-6. ] indicates that mortality from iatrogenic dissection originating from the ascending aorta (Stanford Type A) and descending aorta (Stanford Type B) are both substantial, with the latter being higher than that of spontaneous dissection.
Preventive pharmacotherapy
• Overview
Adequate anticoagulation and antiplatelet therapy are key determinants of complication rates and a variety of acceptable options exist [5656. Silber S, Albertsson P, Avilés FF, Camici PG, Colombo A, Hamm C, Jørgensen E, Marco J, Nordrehaug J-E, Ruzyllo W, Urban P, Stone GW, Wijns W. Guidelines for Percutaneous Coronary Interventions. Eur Heart J. 2005;26:804-847. ]. These therapies alone will not, however, always compensate for suboptimal technique [5757. Judkins MP, Gander MP. Prevention of complications of coronary arteriography. Circulation. 1974;49:599.
Original paper on complications related to coronary angiography.]. Any foreign material introduced into the vascular system is thrombogenic. Clot forming in introducer sheaths and equipment when outside the patient are a potential is an embolic source and also a potent catalyst for thrombosis when reintroduced. All material exchanges must therefore be frequently and methodically wiped down with saline, introducer sheaths regularly flushed and equipment dwell times kept to a minimum. A damped pressure trace with poor bleed-back is an early sign of a partially occluded catheter and warrants removal without injection. Catheters with multiple sideholes (e.g, pigtail, guide catheter with side-holes) will, however, demonstrate a normal arterial trace unless sufficient obstruction occurs and so requires particular attention. Iatrogenic coronary thrombosis is a life threatening complication during PCI and often directly related to mismanagement of this part of the therapy In a large North American registry [99. Stathopoulos I, Jimenez M, Panagopoulos G, Kwak EJ, Losquadro M, Cohen H, Iyer S, Ruiz C, Roubin G Garratt K. The Decline in PCI Complication Rate: 2003-2006 Versus 1999-2002. Hellenic J Cardiol . 2009;50:379-387. ] nearly three-quarters (73%) of deaths on the table in the catheter laboratory as a result of complications of PCI were associated with the de novo formation of intracoronary thrombus.
Pre-procedure Aspirin and thienopyridine therapy are mandatory for all PCI (unless known previous hypersensitivity) and in acute coronary syndromes they are mandated prior to diagnostic coronary angiography In acute situations or if vomiting has occurred there is a role for intravenous administration of aspirin and repeat oral/naso-gastirc loading with a fast-acting thienopyridine (prasugrel) or intravenous cangrelor (a non-thienopyridine P2Y12 inhibitor). By contrast, routine systematic pre- procedure thienopyridine is not necessary in patients undergoing elective diagnostic coronary angiography. If disease warranting ad hoc PCI is found then thienopyridine can be safely administered in between procedures [5858. Widimsky P, Motovská Z, Simek S, Kala P, Pudil R, Holm F, Petr R, Bílková D, Skalická H, Kuchynka P, Poloczek M, Miklík R, Maly M, Aschermann M. Clopidogrel pre-treatment in stable angina for all patients >6h before elective coronary angiography or only for angiographically selected patients a few minutes before PCI? A randomized multicentre trial PRAGUE-8. Eur Heart J. 2008;12:1495-1503. ].
Avoiding iatrogenic coronary thrombus
- Keep equipment dwell times to a minimum
- Wipe down all exteriorised equipment before reintroduction
- Flush introducer and catheters frequently and thoroughly
- Load unfractionated heparin before PCI equipment is used
- Keep ACT>250-400 seconds with regular checks
- Use weight-adjusted heparin dosing
In complex lesion and high-risk acute coronary syndrome subsets, particularly where there is a thrombus burden suspected angiographically, the use of additional glycoprotein 2b3a antagonists reduces the frequency of vessel closure.
Anticoagulation with unfractionated heparin is most commonly utilised but attention to dosing [5959. Boccara A, Benamer H, Juliard Jm et al. A randomized trial of a fixed high dose versus a weight-adjusted low dose of intravenous heparin during coronary angioplasty. Eur Heart J. 1997;18:631-5. ] and ACT levels is required. This is particularly the case when concomitant glycoprotein 2b3a antagonists have been used. Guidelines recommend a heparin dose 70-100U/Kg sufficient to reach an ACT of 250-300 (when using the HemoTec analyser) or 300-350 seconds (when using the HemoChron analyser) in glycoprotein 2b3a naïve patients. In the presence of glycoprotein 2b3a, heparin at 50-70U/Kg and an ACT >200 seconds is regarded as sufficient. The association of ACT levels and PCI outcomes whilst concordant in the balloon angioplasty era (where an ACT>250 seconds was associated with the least risk of abrupt vessel closure) have yielded conflicting data in contemporary practice. Analysis of six large randomised trials suggests that an ACT of 350-375 seconds may be the optimal target. Ethnic differences may exist with Asian patients requiring 10untis/kg less than standard doses to achieve the same ACT. Heparin administration is best given centrally to assure delivery of the correct dose [6060. Chew DP, Bhatt DL Lincoff MA, Moliterno DJ, Brener SJ, Wolski KE, Topol EJ, Clinical Investigation and Reports. Defining the Optimal Activated Clotting Time During Percutaneous Coronary Intervention.Aggregate Results From 6 Randomized, Controlled Trials. Circulation. 2001;103:961-966. ]. ACT levels must also be checked regularly. Empirically this should be within an hour of administration and at 30 minutes intervals thereafter
( Figure 9 ). More frequent checks are sometimes employed in longer procedures such as CTO PCI. The use of a timer for this task may be better than relying on unsynchronised time checks or the perception of the passage of time. Intravenous low-molecular weight enoxaparin is an acceptable alternative but cannot be monitored acutely [6161. Borentain M, Montalescot G, Bouzamondo A, Choussat R, Hulot JS, Lechat P. Low-molecular-weight heparin vs. unfractionated heparin in percutaneous coronary intervention: a combined analysis. Cath Cardiovasc.Interv. 2005;65:212-221. ]. The use of the latest factor Xa inhibitor fondaparinux prior to intervention should always be accompanied by standard unfractionated heparinisation during PCI [6262. Mehta SR, Granger CB, Eikelboom JW, Bassand JP, Wallentin L, Faxon DP, Peters RJ, Budaj A, Afzal R, Chrolavicius S, Fox KA, Yusuf S. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Results from the OASIS-5 trial. JACC. 2007;50:1742-1751. ]. The direct thrombin inhibitor bivalirudin is yet a third acceptable agent and is the agent of choice if there is a history of heaprin-induced thrombocytopaenia. More important than the agent chosen is a protocol takes into account the risk of haemorrhage. Large retrospective analysis [6363. Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, Pinnow EE, Kent KM, Pichard AD, Satler LF, Weissman NJ, Lindsay J, Fuchs S. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930-935.
Large registry analysing the predictors for haemorrhagic complications.] (n=12,029) of interventions reveals that periprocedural haemorrhage is a potent predictor of both procedural complications and in-hospital mortality. Hypotension, use of IABP and age>80 appear to have the strongest association. Tailoring therapy is therefore to be recommended. Moreover, catheter laboratories must ensure that harmonisation exists for the region they serve to avoid errors and unnecessary duplication of therapy. Post PCI instructions over antiplatelet and anticoagulation therapy are the responsibility of the operator and must be made explicit.
Predictors of periprocedural bleeding [
6363. Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, Pinnow EE, Kent KM, Pichard AD, Satler LF, Weissman NJ, Lindsay J, Fuchs S. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930-935.
Large registry analysing the predictors for haemorrhagic complications.]
- IABP OR 3 (2.2-4.1) p<0.001
- Procedural hypotension OR 2.9 (2.0-4.3) p<0.001
- Age>80 OR 1.9 (1.4-2.7) p<0.001
- Age 70-80 OR 1.6 (1.2-2.0) p 0.002
- Abciximab OR 1.8 (1.2-2.6) p 0.003
- Chronic renal insufficiency OR 1.5 (1.1-1.9) p 0.002
- History of hypertension OR 1.3 (1.0-1.6) p 0.032
Iatrogenic thrombotic vessel occlusion
De novo intracoronary thrombus formation or embolism of thrombus from instruments (introduced into the arterial vascular system ) should also be considered if coronary blood flow does not return despite administration of coronary vasodilatators. Anticoagulation level (ACT) should be checked and adjusted as soon as possible, even in the absence of visible thrombus at angiography. In the presence of visible thrombus and adequate level of anticoagulation, administration of intracoronary GP 2b3a antagonists and the use of aspiration devices should be considered. If anticoagulation levels are sub-optimal, central arterial (or intracoronary) administration of unfractionated heparin is the fastest means to achieve this.
In the presence of an ovoid filling defect or contrast staining consistent with a small and localised thrombus, placement of a coronary stent may be undertaken if flow is impaired. However, medical management with a glycoprotein 2b3a inhibitor will suffice if flow is not impaired,. In the presence of large burden of thrombus, balloon inflations alone should be avoided because of the risk of thrombus fragmentation, distal microembolisation, and development of angiographic no-reflow. In such a scenario, thrombus aspiration using dedicated devices is recommended followed by placement of a coronary stent.
Thrombotic occlusion of the left main stem
Rarely, thrombotic occlusion of the proximal left coronary artery can occur ( Illustrative case 5 ). The cause is often a combination of the following:
a. active and multiple instrumentation of the left coronary artery during PCI combined with insufficient flushing of the guiding catheter,
b. insufficient anticoagulation,
c. instrumentation of the left coronary artery by manual thrombectomy devices with fragmentation and loss of thrombus during retrieval of the aspiration catheter into the left main or proximal vessels, Particular attention is required when withdrawing an aspiration catheter so that suction is maintained until complete removal of the device from the patient. Placement of a distal protection device eg EZ Filterwire in the non infarct related artery has been described. This requires the use of a small calibre aspiration device eg Thrombuster II or an 8Fr guide catheter.
d. injection of thrombus contained in the guiding catheter following insufficient flushing.
Emergency treatment will depend on the haemodynamic status but independent of this, all actions should focus on prompt restoration of flow. ACT levels should be checked immediately and anticoagulation adapted if needed. Immediately administration of 2b/3a antagonists should be considered on top. Urgent thrombectomy by manual or motorised devices should be performed. Surgical bypass should be sought if the clinical situation deteriorates. Intracoronary injection of epinephrine together with ventricular assistance (IABP or other assist devices) should be considered as a temporising measure in case of haemodynamic compromise.
Coronary Spasm
Coronary artery vasospam in the context of PCI is a transient decrease in the lumen of a normal or diseased arterial segment >50% that is reversible and responds to nitrate administration [6464. Ozaki Y, Keane D, Serruys PW. Progression and regression of coronary stenosis in the long-term follow-up of vasospastic angina. Circulation. 1995;92:2446-56. ] ( Figure 10 ). It is most frequently observed in patients with acute coronary syndromes and Prinzmetal’s angina but can occur, in angiographically normal arteries [6565. Bertrand ME, LaBlanche JM, Tilmant PY, Thieuleux FA, Delforge MR, Carre AG, Asseman P, Berzin B, Libersa C, Laurent JM. Frequency of provoked coronary arterial spasm in1089 patients undergoing coronary angiography. Circulation. 1982;65:1299-1306. , 6666. Uren NG, Crake T, Lefroy DC, de Silva R, Davies GJ, Maseri A. Reduced coronary vasodilator function in infracted and normal myocardium after myocardial infarction. NEJM. 1994;331:222-227. ]. There may be ethnic differences is vasoconstrictor responses with Asian patients being more prone than Europeans [6767. Pristipino C, Beltrame JF, Finocchiaro ML, Hattori R, Fujita M, Mongiardo R, Cianflone D, Sanna T, Sasayama S, Maseri A. Major racial differences in coronary constrictor response between Japanese and Caucasians with recent myocardial infarction. Circulation. 2000;101:1102-1108. ]. The mechanism is likely to include endothelial dysfunction and tunica media smooth muscle hyper-responsiveness to vasoconstrictor stimuli. Typically this will occur in arterial segments with atherosclerosis especially if thrombus is present however impaired vasodilatation remote from the IRA is also possible [6868. Versaci F, Gaspardone A, Proietti I. Left anterior descending and circumflex coronary artery spasm after right coronary artery stent implantation. Heart. 2002; 88: 520. ]. Local mechanical force exerted by balloon angioplasty, stenting or the jet of a contrast injection can induce this response too. Coronary spasm severe enough to cause impaired TIMI flow, if present, may indicate associated vessel injury (dissection, thrombus, perforation). Nevertheless, as an isolated phenomenon during PCI it can be ruled out by intracoronary nitroglycerine administration. This drug provides endothelial-independent vasodilatation. Higher doses of nitroglycerine or calcium channel antagonists could be used for refractory spasm, taking the haemodynamic and rhythm status into account Importantly when bolus intracoronary nitrate is administered intravenous fluid should be running continuously. Calcium channel antagonists may need to be commenced in the catheter laboratory where spasm has been associated with ST segment elevation or cardiac arrest.
Air embolism
Air embolism is an ever present danger during percutaneous intervention Retrospective data suggest that inadequate technique is the predominant cause with junior operators, particularly in the first months of training, more prone than senior; and events more common during PCI than diagnostic catheterisation [6969. Dib J, Boyle AJ, Chan M, Resar JR. Coronary air embolism: A Case Report and Review of the Literature. Cathet Cardiovasc Diagn. 2006;68:897-900. ]. Conventional manual injections using a stopcock-manifold, equipment exchanges that can entrain air (venturi effect) via the Tuohey-Borst connector (Y connector), inadequate aspiration of catheters, and balloon rupture are all potential risks for the inadvertent introduction of air into the coronary circulation. More than half of these embolic events are asymptomatic. They can, however, precipitate unanticipated cardiac arrest and circumstances in which prolonged resuscitation may be necessary until the air is reabsorbed into the venous system. There are no controlled studies to guide decisions on therapy. The amount of gas required to cause clinical symptoms may be remarkably small (in porcine models as little as 0.02 ml/Kg) [7070. Kahn JK, Hartzler GO. The spectrum of symptomatic coronary air embolism during balloon angioplasty: Causes, consequences and management. Am Heart J. 2010;119:1374-1377. ]. Symptoms of chest pain and transient ST elevation may be accompanied by rhythm disturbances culminating in asystole or VF. Diagnosis is straightforward if captured using fluoroscopy and cineangiography, with radiolucent bubbles evident traversing the coronary arteries and leading to a reduction in TIMI flow ( Figure 11 ). The clinical response to embolism depends on the volume injected and the presence of an air lock. The latter may require arterial pressures of up to 200 mmHg to dislodge into the venous system [7171. Khan M, Schmidt DH, Bajwa T, Shaley Y. Coronary air embolism: Incidence, severity, and suggested approaches to treatment. Cathet Cardiovasc Diagn. 1995;36:313-318. ]. Prevention requires assiduous attention to PCI technique. Treatment consists of advanced life support if a rhythm disturbance occurs and increasing the concentration and partial pressure of oxygen in blood using a non-rebreathing mask If refractory, increasing mean arterial pressure by pressors and/or IABP may be used. Although oxygen in the air bubble is absorbed quickly into tissue, nitrogen, which composes more than 70% by volume, is not. The increased FiO2 creates a gradient in arterial blood that allows nitrogen to dissolve and the increased pressure assists with driving remaining obstruction through the circulation [7272. Stegmann T, Daniel W, Bellmann L, Trenkler G, Oelert H, Borst HG. Experimental coronary air embolism. Assessment of time course of myocardial ischemia and the protective effect of cardiopulmonary bypass. Thorac Cardiovasc Surg. 1980;28:141-149. , 7373. Van Blankenstein JH, Slager CJ, Soei LK, et al. Effect of arterial blood pressure and ventilation gases on cardiac depression induced by coronary air embolism? J Appl Physiol. 1994;77:1896-1902. ]. Anecdotal reports demonstrate that guidewire bougie or device aspiration to disperse or extract bubbles can also be used with success. Resuscitation can be prolonged if large volumes of air have been injected and so persistence with advanced life support is required as a return of spontaneous circulation is nearly always assured.
Although air is the predominant cause of gas embolism, helium escaping from a ruptured IABP balloon has also been reported [7474. Cruz-Flores S, Diamond A, Leira A. Cerebral embolism secondary to intra-aortic balloon pumping. Neurocrit Care. 2005;2:49-50. ].
Coronary air embolism underlies the importance of ensuring that all catheters and devices inserted into the vascular system are saline or contrast-filled and competent at all times. Furthermore any exchange of equipment should be followed by a visual check that back-bleeding through the Y connector has occurred. The latter is especially relevant during aspiration catheter exchanges in primary PCI.
No-reflow
Overview
The no-reflow phenomenon is defined as inadequate myocardial perfusion in a territory subtended by given epicardial coronary artery without angiographic evidence of mechanical vessel obstruction [7575. Eeckhout E, Kern M. The coronary no-reflow phenomenon: a review of mechanisms and therapies. Eur Heart J. 2001;22:729-739. ]. It typically accompanies primary PCI and complex lesion intervention, particularly vein graft intervention or when rotational atherectomy devices are used. This manifestation of microvascular injury must be distinguished from several mimics (thrombus, air embolism, dissection and severe spasm). Importantly whilst impaired TIMI flow (0-2) should raise the possibility of no-reflow it is not a sine qua non. Continued chest pain, lack of ST segment resolution in the setting of MI, new ST segment elevation in the setting of elective PCI or haemodynamic compromise all occurring despite TIMI 3 flow should also alert the operator to assess for myocardial perfusion grade (blush) followed by a systematic exclusion of mimics ( Figure 12A and 12B). The mechanism of no reflow is likely multifactorial with distal embolisation of thrombus burden, prolonged ischaemia, reperfusion injury, and pre-existing susceptibility each individually or in combination able to account for this complication [7676. Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial No-Reflow in Humans. JACC. 2009;54:281-292.
Contemporary review on the pathlogy, diagnosis and management of the no-reflow phenomenon.]. The evidence base for therapies has been inconsistent as a result of small sample sizes and inadequate selection of patients at high risk of no reflow. Small randomised studies have tended to produce positive clinical results. Validated predictors of no reflow can be used to guide management, especially in those undergoing PPCI [7777. Yip HK, Chen MC, Chang HW, Hang CL, Hsieh YK, Fang CY, Wu CJ. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction:predictors of slow-flow and no-reflow phenomenon. Chest. 2002;122:1322-32. ]. Preventive and therapeutic strategies can be divided into pharmacological and mechanical.
Predictors of the no-reflow phenomenon [
7676. Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial No-Reflow in Humans. JACC. 2009;54:281-292.
Contemporary review on the pathlogy, diagnosis and management of the no-reflow phenomenon.]
- Distal embolisation
− Thrombus burden
- Ischaemic injury
− Contact to balloon time
− Extent of ischaemia
- Reperfusion injury
− Neutrophil count
− Endothelin-1 level
− Thomboxane A2 level
− Mean platelet volume and reactivity
- Individual susceptibility
− Diabetes
− Acute hyperglycaemia
− Hypercholesterolaemia
− Absence of pre-conditioning
Predictors of distal embolisation during PPCI [
7777. Yip HK, Chen MC, Chang HW, Hang CL, Hsieh YK, Fang CY, Wu CJ. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction:predictors of slow-flow and no-reflow phenomenon. Chest. 2002;122:1322-32. ]
- Angiographic thrombus >3x the reference lumen diameter
- Abrupt cut-off without taper before the occlusion
- Presence of accumulated thrombus proximal to the occlusion
- Presence of floating thrombus proximal to the occlusion
- Persistent contrast medium distal to the obstruction
- Reference vessel diameter >4mm
Prognostic value of practical methods for diagnosing no-reflow [
7676. Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial No-Reflow in Humans. JACC. 2009;54:281-292.
Contemporary review on the pathlogy, diagnosis and management of the no-reflow phenomenon.]
Odds ratios for cardiac or all cause mortality for different parameters
- Impaired TIMI flow (OR 3.2)
- Impaired myocardial blush grade (OR 4.2)
- Impaired TIMI myocardial perfusion grade (OR 1.9)
- Absence of ST segment resolution (>70%) (OR 2.5)
- Impaired Myocardial blush grade+lack of ST segment resolution (OR 8.2)
- Impaired perfusion on myocardial contrast echocardiography (OR 10.7)
Pharmacological prevention and treatment
Prevention [7777. Yip HK, Chen MC, Chang HW, Hang CL, Hsieh YK, Fang CY, Wu CJ. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction:predictors of slow-flow and no-reflow phenomenon. Chest. 2002;122:1322-32. , 7878. Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW; AMISTAD-II Investigators. A randomized, double-blind, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). JACC (2005) 45:1775-80. , 7979. Ito H, Taniyama Y, Iwakura K et al. Intravenous nicorandil can preserve microvascular integrity and myocardial viability in patients with reperfused anterior wall myocardial infarction. JACC. 1999;33:654-60. , 8080. Tsubokawa A, Ueda K, Sakamoto H, Iwase T, Tamaki S. Effect of intracoronary nicorandil administration on preventing no-reflow/slow flow phenomenon during rotational atherectomy. Circulation. 2002;66:1119-23. ]
Chemical antidotes, of which the best known and studied is adenosine, have had mixed results in the prevention of this syndrome but have efficacy in treatment once no –reflow has occurred. Abciximab, however, has an important role and features in the ESC recommendations. An alternative agent, nicorandil given intravenously is also promising in this regard ( Figure 13).
Treatment
Intracoronary adenosine, verapamil, papaverine, sodium nitroprusside, abciximab, cyclosporine, epinephrine and streptokinase have all been used as treatment after the development of no-reflow with success. Clinical and haemodynamic factors may favour the use of one drug over another. Importantly, any drug chosen should be given as distal as possible into the coronary bed in order to specifically act on the microcirculation. Catheter laboratory teams should be familiar with at least one agent in order that swift institution of therapy is possible. Locally agreed protocols are the most practical means of ensuring prompt action.
Adenosine is a purine receptor antagonist. It is considered to be a first line drug due to its strong vasoactive properties, ease of application, familiarity of use in fractional flow reserve measurements and short half-life. Caffeine intake (<24hrs) and chronic aminophylline therapy may blunt the effect. Although asthma is stated as a manufacturer’s contraindication, it is not a clinical contraindication with bolus doses under the strict monitoring conditions of a catheter laboratory.
Verapamil is an L-type calcium channel antagonist that has action on both vascular smooth muscle and conduction tissue. On account of this back-up temporary pacing should be at hand when delivering this drug.
Papaverine can be used but has QT prolongation properties that make it undesirable in the setting of acute infarction.
Limited but randomised evidence exists for intra-coronary streptokinase in the setting of PPCI [8181. Sezer, M, Oflaz, H, Taner G., Okçular I, Umman B, Nişanci Y, Bilge AK, Şanli Y, Meriç, M and Umman S, Intracoronary Streptokinase after Primary Percutaneous Coronary Intervention. N Engl J Med . 2007;356:1823-34. ].
Intracoronary epinephrine has been used with success in patients suffering refractory no-reflow following elective or acute coronary interventions possible.
Mechanical prevention
Mechanical approaches are in essence preventive. They are centred on stopping the distal embolisation of atheromatous debris and thrombus.
• Distal protection
In vein graft PCI the rate of adverse outcomes (MACCE) when employing standard techniques approaches 20%. Distal protection devices can reduce significantly the incidence of periprocedural myocardial infarction and no-reflow (46% and 66% respectively) [8282. Burzotta F, Trani C, Romagnoli E, Mazzari MA, Rebuzzi AG, De Vita M, Garramone B, Giannico F, Niccoli G, Biondi-Zoccai GG, Schiavoni G, Mongiardo R, Crea F. Maunal thrombus aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus-aspiration in primary and rescue angioplasty (REMEDIA) trial. JACC. 2005;46:371-6. ] Thus for vein graft interventions both mechanical and chemical protection should be considered routine practice.
Despite the beneficial effect of mechanical protection seen in vein graft intervention distal protection has not been helpful in PPCI of native vessels [8383. Galiuto L, Garramone B, Burzotta F, Lombardo A, Barchetta S, Rebuzzi AG, Crea F; REMEDIA Investigators. Thrombus aspiration reduces microvascular obstruction after primary coronary intervention: a myocardial contrast echocardiography substudy of the REMEDIA trial. JACC. 2006;48:1355-60. , 8686. Stone GW, Webb J, Cox DA, Brodie BR, Qureshi M, Kalynych A, Turco M, Schultheiss HP, Dulas D, Rutherford BD, Antoniucci D, Krucoff MW, Gibbons RJ, Jones D, Lansky AJ, Mehran R; Enhanced Myocardial Efficacy and Recovery by Aspiration of Liberated Debris (EMERALD) Investigators. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA. 2005;293:1063–1072. ].
• Thrombus aspiration
In the latter systematic mechanical thrombus aspiration appears currently to be the most beneficial means of preventing distal embolisation. In the Thrombus Aspiration during Percutaneous Coronary Intervention in Acute Myocardial Infarction (TAPAS) Study, 1071 patients undergoing primary PCI for AMI were randomized to thrombus-aspiration followed by stenting or “conventional-PCI”. In this study, thrombus aspiration resulted in better reperfusion and clinical outcomes than conventional PCI. A myocardial blush grade of 0 or 1 occurred in 17.1% of the patients in the thrombus- aspiration group and in 26.3% of those in the conventional-PCI group (P<0.001). Complete ST-segment resolution occurred in 56.6% and 44.2% of patients, respectively (P<0.001). At one year, the rate of cardiac death at was 3.6% in the manual thrombus aspiration group and 6.7% in the conventional PCI group (p=0.020) [8787. Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F. Thrombus aspiration during primary percutaneous coronary intervention. NEJM. 2008;358(6):557-67. ]. Importantly, current evidence only supports manual as opposed to automated mechanical aspiration.
• Direct stenting
Direct stenting of a well-visualised lesion underlying a thrombotic occlusion to entrap potentially embolic material has been suggested but has limited evidence.
• Conditioning
This refers to the activation of intracellular myocardial protective mechanisms by inducing ischaemia in a vascular territory in the setting of MI. It represents an additional potential strategy to prevent reperfusion injury associated with no-reflow.
Ischaemic postconditioning in this context means repeated balloon occlusions of an infarct related artery. Evidence in support of this technique is strong in animal models of infarction and recent randomised controlled trial data in selected patients with STEMI is encouraging [8888. Lønborg J, Kelbaek H, Vejlstrup N, Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P, Holmvang L, Treiman M, Jensen JS, Engstrøm T. Cardioprotective Effects of Ischemic Postconditioning in Patients Treated With Primary Percutaneous Coronary Intervention, Evaluated by Magnetic Resonance. Circ Cardiovasc Interv. 2010;3:34-41. ]. Cyclosporin (intravenously) also has shown promise in mitigating against no-reflow, however, there are only preliminary data available. A mechanism of action based on the activation of mitochondrial intracellular pathways that facilitate preconditioning are postulated [8989. Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krussel LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sørensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomized trial. Lancet. 2010.375:727-734. ]. Figure 14 provides a suggested algorithm for the prevention, diagnosis and management of the no-reflow phenomenon.
CORONARY PERFORATION
Overview
Perforation is an anatomical breach in the integrity of the tunica adventitia of an epicardial artery leading to extravasation of blood, either into the myocardium, pericardium or a cardiac chamber. It can be caused by oversized balloons or stents, balloon rupture, agressive stent post-dilation, laser therapy, excessive rotablation or guidewire exit. High risk clinical and PCI-morphological predictors, which are likely inter-related, include females, the elderly, previous CABG, PCI for unstable syndromes, tortuous, calcified and small arteries, IVUS use and CTO intervention [9090. Cohen BM, Weber VJ, Reisman M, Casale A, Dorros G. Coronary perforation complicating rotational ablation: the U.S. multicenter experience. Cathet Cardiovasc Diagn. 1996;3:55-59. , 9191. Ellis SG, Ajluni S, Arnold AZ, Popma JJ, Bittl JA, Eigler NL, Cowley MJ, Raymond RE, Safian RD, Whitlow PL. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. 1994;90:2725–2730.
Original paper describing the classification and management of coronary perforation.]. The latter is the area of intervention with the most prominent association. This relates to the use of guidewires with the highest penetration power, strategies designed to cross the subintimal space and retrograde access via an epicardial vessel. Conversely, although retrograde access to a CTO via septal perforators can result in intramyocardial perforation and haematoma, this is not typically associated with adverse clinical consequences. Numerous studies have reported a low overall incidence of coronary perforation ranging from 0.1% to 3.0% of lesions treated [9292. Ajluni SC, Glazier S, Blankenship L, O’Neil WW, Safian RD. Perforations after percutaneous coronary interventions: clinical, angiographic, and therapeutic observations. Cathet Cardiovasc Diagn. 1994.32:206–212. , 9393. Bittl JA, Ryan TJ Jr, Keaney JF Jr, Tcheng JE, Ellis SG, Isner JM, Sanborn TA. Coronary artery perforation during excimer laser coronary angioplasty. The percutaneous Excimer Laser Coronary Angioplasty Registry. JACC. 1993;21:1158–1165. , 9494. Holmes DR, Jr., Reeder GS, Ghazzal ZM, Bresnahan JF, King SB III, Leon MB, Livtack F. Coronary perforation after excimer laser coronary angioplasty: the Excimer Laser Coronary Angioplasty Registry experience. JACC. 1994;23:330–335. , 9595. Gruberg L, Pinnow E, Flood R, Bonnet Y, Tebeica M, Waksman R, Satler LF, Pichard AD, Kent KM, Leon MB, Lindsay Jr. Incidence, management, and outcome of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2000;86:680–682. , 9696. Dippel EJ, Kereiakes DJ, Tramuta DA, Broderick TM, Shimshak TM, Roth EM, Hatterner CR, Runyon JP, Whang DD, Schneider JF, Abbottsmith CW. Coronary perforation during percutaneous coronary intervention in the era of abciximab platelet glycoprotein IIb/IIIa blockade: an algorithm for percutaneous management. Cathet Cardiovasc Interv. 2001;52:279–286. , 9797. Gunning MG, Williams IL, Jewitt DE, Shah AM, Wainwright RJ, Thomas M. Coronary artery perforation during percutaneous intervention: incidence and outcome. Heart. 2002;88:495–498. , 9898. Stankovic G, Orlic D, Corvaja N, Airoldi F, Chieffo A, Spanos V, Montorfano M, Carlino M, Finci L, Sangiorgi G, Colombo A. Incidence, predictors, in-hospital, and late outcomes of coronary artery perforations. Am J Cardiol. 2004;93:213–216. , 9999. Witzke CF, Martin-Herrero F, Clarke SC, Pomerantzev E, Palacios IF. The changing pattern of coronary perforation during percutaneous coronary intervention in the new device era. J Invasive Cardiol. 2004;16:257-301. ]. The decrease in use of debulking devices (rotablation and directional atherectomy) means a greater proportion of perforations are caused by guidewires nowadays in particular in the setting of PPCI and CTO intervention.
In the setting of CTO intervention, the increasing use of stiff wires for penetrating the proximal and distal caps of the total occlusion leads to an increased rate of perforation. Indeed, in a study of 7443 procedures, the incidence of perforation was 0.93% (69 of 7443) but 64% of these (44 of 69) occurred when treating a CTO [100100. Fukutomi T, Suzuki T, Popma JJ, Hosokawa H, Yokoya K, Inada T, Hayase M, Kondo H, Ito S, Suzuki S, Itoh M. Early and late clinical outcomes following coronary perforation in patients undergoing percutaneous coronary intervention. Circ J (2002) 66:349-356. ]. Data from a large North American, multicentre rotablation registry (n=2953) indicates that with this device perforation is more frequent in the RCA (1.1%) and circumflex (1.2%) than the LAD (0.06%). The use of IVUS is also associated with perforation probably reflecting more complex intervention or incorrect image interpretation and balloon oversizing.
Rapid haemodynamic compromise due to tamponade is associated with grade III perforation. Guidewire perforation is more forgiving unless in the presence of a glycoprotein 2b3a antagonist ( Illustrative case 6 ). Tamponade is unusual in vein graft intervention owing to pericardial surgical fibrous tissue but has been reported [101101. Lowe R, Hammond C, Perry RA. Prior CABG does not prevent pericardial tamponade following saphenous vein graft perforation associated with angioplasty. Heart (2005) 91:1052. ].
Prevention
Prevention can be enhanced by avoiding balloon oversizing (>1.1:1 balloon:artery ratio), and the careful manipulation of guidewires. The use of hydrophilic or penetrative guidewires, especially in occluded segments where there is no distal flow, are particularly vunerable situations. As a golden rule, angioplasty should not be performed as long as the distal vessel is not visualised. During PPCI the distal vessel trajectory may be highlighted by using a microcatheter or an over-the-wire balloon. During CTO interventions contralateral contrast injections are preferred as homolateral injections may damage and dissect the distal bed. Temporary transvenous pacemakers in support of transient bradyarrhythmia should be avoided if possible as this can cause right ventricular perforation with tamponade particularly in inferior MI and where a glycoprotein 2b3a antagonist has been employed. LAD perforations may be better tolerated compared to those of the Cx and RCA owing to the higher prevalence of muscular bridges in the LAD.
Diagnosis and management
Perforation can be asymptomatic or associated with pain (chest, neck, throat), vagal symptoms, hypotension (with or without tachycardia), ventricular ectopy and hypotension. Radiographic signs include extravasation of contrast, an errant wire position, and in massive tamponade, the “dead heart’ sign An angiographic classification serves as a useful template to guide clinical management [9191. Ellis SG, Ajluni S, Arnold AZ, Popma JJ, Bittl JA, Eigler NL, Cowley MJ, Raymond RE, Safian RD, Whitlow PL. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. 1994;90:2725–2730.
Original paper describing the classification and management of coronary perforation.] ( Table 9 ). Type I-IV refer to the proximal and mid segments of epicardial arteries, whereas Type V refers specifically to perforation in a distal segment or sidebranch. Sealing a persistent leak and treating any associated haemodynamic compromise due to tamponade are the key elements. The first response to such a perforation should be the inflation of an angioplasty balloon at the point of injury or proximal to the perforation for at least 10 minutes if clinically tolerated by the patient, in order to prevent tamponade developing. Some operators even advocate leaving the stent delivery balloon readily in the guiding catheter during high risk (for perforation) PCI procedures. Delay may also be minimised by using a second guide catheter pre-loaded wtih a stent-on-a-wire system (eg., SvelteTM), advanced from the contralateral femoral artery.
Type I perforations (extraluminal crater without contrast jet extavasation) Types II (pericardial/myocardial blushing without contrast jet extravasation) are generally benign and seal after balloon inflation or may occasionally be left untreated. In this respect, it is particularly important to recognise intramural myocardial hematoma that is mostly a “benign” condition. Patients should of course be monitored carefully on the ward, heparin and 2b3a antagonists should be stopped and if possible reverted. A type III (active extravasation through a frank [1mm] perforation) will usually need pericardiocentesis and drain insertion for haemodynamic stabilisation followed by stenting with a polytetrafluoroethylene or pericardium covered stent. Type IV (originally referred to as Type III cavity filling) often mandates conservative management as it involves extravasation into a cardiac chamber and seals spontaneously without treatment. Extravasation into a low pressure cardiac chamber in association with ischaemia does, however, benefit from a covered stent [7878. Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW; AMISTAD-II Investigators. A randomized, double-blind, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). JACC (2005) 45:1775-80. ]. Type V perforation may require distal embolisation using one of a variety of interventional radiological techniques. Thrombogenic vascular coils, gelfoam, polyvinyl alcohol, pre-clotted autologous blood clot, autologous fat, glue, and thombin have all been used ( Illustrative case 7 ). If the operator is not experienced in the use of any of these techniques, early assistance from an interventional radiologist should be sought. A further key difference with this form of perforation is that a portion of the vessel may have to be sacrificed and so an assessment of the territory at risk is vital. In particular, it is imperative that proximal migration of the embolic material is avoided. This can be ensured by prolonged inflation (20 minutes) of an angioplasty balloon just proximal to the deployment point. Deflation should occur only once the embolic material has had sufficient time to take anchor in the distal segment.
In all cases portable transthoracic echocardiography is a very useful adjunct to assist the safe management of this complication. Imaging both in the catheter laboratory and later on the ward are advised in order to capture persistent leaks. This typically occurs with distal perforations and can reappear several hours after the initial emergency. There are conflicting opinions on the benefits of reversing anticoagulation with protamine sulphate and the risks of inducing de novo or stent thrombosis. A pragmatic approach involves avoiding protamine if the patient responds to initial therapy in the catheter laboratory and the remaining PCI procedure time is anticipated to be prolonged. In the event of continued extravasation with life-threatening haemodynamic compromise unresponsive to initial mechanical bail-out measures or a short PCI procedure time, then protamine can be considered (1mg per 1000 units unfractionated heparin administered). This has potential for causing stent thrombosis therefore the decision to reverse heparin needs to be individualised. Further prolonged mechanical occlusion (20 minutes or more), however, is more commonly employed whilst a surgical opinion is sought In such a critical scenario, if abciximab has also been administered then a platelet transfusion may be warranted for reversal. There is no antidote for the small molecule glycoprotein 2b3a antagonists (tirofiban and eptifibatide) but their half-life is short (<4hrs).
Chamber perforation
Left ventricular perforation or myocardial staining is very rare during PCI but has been reported in association with left ventriculography. Incorrect positioning of a pigtail catheter (even with multiple holes) against the ventricular wall with a power injector are causal factors. More frequently encountered is right ventricular perforation associated with cardiac tamponade. Temporary transvenous pacemakers, in support of transient bradyarrhythmia should be avoided if possible, particularly during inferior MI where a glycoprotein 2b3a antagonist has been employed. Perforation can occur both during PCI and at the during temporary wire removal. When possible this manoeuvre should be carried out in a clinical environment where a pericardial drain can be introduced swiftly.
Cardiac tamponade
Coronary perforation including extensive coronary dissection (particularly ostial dissection) and can lead to haemorrhage into the pericardial sac. The anatomy and physiology of this structure are such that it does not tolerate acute accumulations of fluid well. As little as 80-200ml additional fluid can cause pressure to rise and exert a constraining force on the myocardium sufficient to reduce cardiac output. Relief of this pressure can restore a satisfactory systolic perfusion pressure, whilst the primary injury is being dealt with, and hence be life-saving. Tamponade has been reported as occurring in up to 17% (n=8,932) of patients with perforation. Although vein graft perforation is said to be protective on account of surgical adhesions, pericardial tamponade has been reported.
Diagnosis can be made with transthoracic echocardiography. If, however, echocardiography is unavailable to confirm the diagnosis or imaging windows are poor then clinical and haemodynamic parameters are required Following a major perforation, an obtunded patient (decreased level of consciousness) who is hypotensive (<100mmHg)) with a respiratory variation in central aortic pressure can be clinically diagnosed as being in tamponade. In the setting of chronic total occlusion PCI or patients with poor LV function, however, hypotension with or without tachycardia should also raise the suspicion of tamponade.
Aspiration and placement of a pericardial drain in an emergency can be technically challenging. Autologous return of tamponade blood drained from the pericardial sac to the venous circulation can be used to rectify hypovolaemia. If attempts are unsuccessful either in terms of locating the pericardium or in restoring haemodynamic stability, then a surgeon is required to salvage the situation.
A simplified algorithm for the management of coronary perforation is provided in Figure 15 .
When to suspect coronary perforation
- Extraluminal angiographic staining or frank extravasation
- Errant wire position especially in the presence of glycoprotein 2b3a
- Use of hydrophilic or stiff wires
- Unexplained hypotension or tachycardia
- Dead heart sign (see video online)
RETAINED EQUIPMENT
Overview
PCI equipment retained within the circulation is rare and includes stents, guidewire fragments, catheter fragments, misplaced intravascular coils and any diagnostic equipment used for PCI. Whilst intrinsic device structural failure is always a possible, inadvertent error or sub-optimal technique is frequently the underlying cause.
Stent loss and embolisation although infrequent (<1% of PCI) since factory crimping was introduced has not been eliminated entirely. The most vulnerable stage for this event occurs when the stent-balloon assembly is pulled back into the guiding catheter following failure to reach or pass the target lesion. There is therefore an association with poor guiding support, lack of coaxial guide catheter position, vessel tortuosity, calcification and the absence of pre-dilatation. A stent can be stripped off its delivery balloon at the lesion or, more commonly, a deformed stent strut can prevent re-entry into the guide catheter. If not anticpated inadvertent pulling on the stent catheter will cause the mouth of the guide to displace the stent off the delivery balloon. Retrospective analysis [102102. Eggbrecht H, Haude M, Von Birgelen C, von Birgelen C, Oldenburg O, Baumgart D, Herrmann J, Welge D, Bartel T, Dagres N, Erbel R. Nonsurgical retrieval of embolized coronary stents. Cath Cardiovasc Intervent. 2000;51:432-440. ] of over 4000 stent implantations has revealed higher embolisation rate for manually crimped stents (1% versus 0.3% (p<0.01) than factory crimped devices. There appears to be no association with different target vessels. Systemic embolisation may cause cerebrovascular events and vascular erosion, while intracoronary embolisation is associated with a high risk of coronary thrombosis and subsequent myocardial infarction.
Guidewire fragment embolisation is even rarer and is related to inadequate handling technique. Equipment knots and wire fractures occur as a result of pushing the structural integrity of equipment beyond its limit. Improved proficiency and training are the only means to prevent these occurrences. Care should be taken with newer mother in daughter devices when deploying stents.
Nonsurgical device retrieval
Stent or material loss can occur either within a coronary/graft conduit or outside of the coronary circulation. Importantly, stent loss need not automatically be subjected to retrieval. If managed by deployment within the coronary circulation or by removal to a non-critical circulation is not associated with adverse outcomes. Nevertheless retrospective analysis of 11,773 PCI with 38 instances of stent loss suggests that retrieval is attempted in the majority of cases (86%) [103103. Brilakis ES, Best PJM, Elesber AA, Barsness GW, Lennon RJ, Holmes DR Jr, Rihal CS, Garratt KN. Incidence, retrieval methods, and outcomes of stent loss during percutaneous coronary intervention: A large single-center experience. Cath Cardiovasc Interv. 2005;66:333-340.
Overview of the different methods for retrieving lost material during PCI.].
Retrieval techniques are technically challenging and evidence for their success is limited to case reports and case series from experienced operators. The key principle for retrieval is to bring the foreign body down into the iliac artery as this removes the risk of embolisation to the cerebrovascular system and or thrombosis in a coronary artery. If the primary arterial vascular access is radial then a second introducer sheath should be placed in the femoral artery to effect retrieval. Removal of material via the radial approach is possible but less frequently performed on account of the limit on sheath size that can be used.
The position of the guidewire will be crucial in the choice of the technique. Indeed, some techniques, are not possible in the case of inadvertent removal of the guidewire. Thus, taking great care to keep the wire position inside the stent and across the lesion is of major importance. For stent loss within the coronary circulation the key principle for successful retrieval is to keep the guidewire position secure at all times.
Guidewire within stent ( Illustrative case 8 )
• Balloon recovery and retrieval technique [109109. Cishek MB, Laslett L, Gershony G. Balloon catheter retrieval of dislodged coronary stents: a novel technique. Cath Cardiovasc Diagn. 1995;34:350-52. ]
Retrieval of the stent by using a low-profile angioplasty balloon is a technique used as a first attempt in the majority of patients because it does not require specially designed kit. A low-profile angioplasty balloon (1.5-2.0 mm diameter, 20 mm length) will suffice in the majority of patients. It is advanced on the guidewire and passed through the unexpanded stent. This is possible as the uninflated diameter of the tip is <0.6mm.The balloon is then inflated distally to the stent (at 1-2 atmospheres) and can then be used to gently pull back the stent into the guiding catheter. Once the stent is secured between the balloon and the guiding catheter, it can be brought to the iliac artery and removed through the arterial sheath en bloc. The technique can only be implemented without any resistance or damage or foreshortening of the stent. If the stent does not come back then the technique should be abandoned.
• Balloon recovery and deployment technique
An alternative to retrieval of the stent with the balloon catheter is to inflate the balloon inside the stent. That will allow the operator to advance the stent directly and deploy it at the target lesion or in a non-consequential location. Displaced stents in anatomically critical positions, however, are best retrieved.
• Stiff wire technique with snare [104104. Bogart D B, Jung SJ. Dislodged stent: A Simple Retrieval Technique. Cath Cardiovasc Interv. 1999;47:323-324. ]
This technique uses a single guide wire inside the dislodged or embolised stent. A second stiff PTCA-guidewire (300 cm) is placed parallel to the first one with the tip passing through the dislodged stent. Then an exchange catheter (“the wanderer”, 2.3 F, enclosed in the microsnare kit) may be inserted over the second guidewire which is removed, and the microsnare can now be advanced into the exchange catheter. After removal of the exchange catheter, the loop of the snare is gradually retracted in such a way, that the tip of the first guidewire is passed through the loop. Under fluoroscopic guidance, the loop of the snare is carefully placed at the distal end of the stent. Once the loop is in the right position, the exchange catheter is advanced to tighten the loop around the stent. If there is difficulty in positioning the snare, the use of a parallel stiff angioplasty guidewire adjacent to the wire of the stent may provide a better platform to move and position the snare without moving the dislodged stent.
Free stent (no guidewire through the central axis of the stent)
Snare technique [105105. Watson LE. Snare loop technique for removal of broken steerable PTCA wire. Cath Cardiovasc Diagn. 1987;13:44-49. ]
Retrieval of the stent by a snare is an approach which will be favoured in the case of inadvertent loss of guidewire position. Commercially available snares, sometimes referred to as “gooseneck” snares, have shaft diameters of 0.018 inch and are tipped by a closed wire loop of 2 mm diameter, angulated 90° to the shaft axis ( Figure 16 ). This right angle of the loop facilitates the grasping of the target object. Successful capture will depend on a correct alignment of the loop to the free end of the stent. There are two main commercially available snares: the Amplatz Goose neck snare (ev3, Plymouth, Minnesota) and the En Snare (Merit Medical Systems Inc, South Jordan, Utah). The former consists of a single loop and the latter has 3 loops, which provides a larger area for capture. Alternatively a “bespoke” snare can be fashioned using an 0.014 inch angioplasty wire threaded through a 5Fr diagnostic catheter looped back on itself so that both proximal and distal ends exit the hub.
The following technical tips are recommended:
- Careful localisation under fluoroscopy of the free end of the stent
- The plane of the snare must be held at right angles to the estimated plane of the stent. For that purpose, the full length of the stent should be seen under fluoroscopy. To confirm the perpendicular position of the snare loop in relation to the stent, it must appear as a straight line or a close loop. Only this position allows correct ensnarement of the free stent.
- Securing the free stent. The operator must be sure that the snare has correctly encircled the stent. The exchange catheter is then advanced and the embolised stent will bend when the snare is engaged. Withdrawing first the snare loop to capture the stent is not recommended because it can cause disengagement.
When the stent is secured by the snare, the whole assembly is pulled back and extracted through the femoral sheath. Sometimes, even an undeployed stent cannot be retrieved through a 6 Fr femoral sheath, which may therefore need to be upsized to 9Fr. A potential caveat when snaring the distal part of the stent concerns the potential for the stent to be bent at the proximal part when it is withdrawn into the femoral sheath. At that level, the force is applied to the distal end and if the proximal end catches on the edge of the sheath, stent deformation may result. If this occurs, the stent may not be withdrawn even into a large sheath. This can be avoided by snaring the proximal end of the stent so that is crushed. The resulting reduced profile will then be the first element to contact the distal end of the sheath. Cutting or flaring the distal end of the sheath can also be performed in order to facilitate material extraction.
• Double or multiple wire technique
This requires a second wire to be carefully advanced through a strut of the stent, the first wire being still in place ( Figure 17 ). The two wires outside the guiding catheter are bound together by a torquing device which is rotated about 15-20 times, until the wires are observed twisting together near the proximal end of the stent Then, the twisted wires are pulled back together and the guiding catheter advanced. The whole assembly consisting of the stent trapped between the twisted wires and the guiding catheter is pulled out gently. If the stent has been captured properly the guiding catheter should engage deeper into the ostium during initial removal. Once the stent is brought to the level of the common iliac artery, it can be removed by conventional methods after the wires are untwisted. The major step in this technique is to ensure that the second wire is placed through the struts of the stent and not through the central lumen. Once the stent is brought to the level of the introducer it can be pulled out gently through the introducer sheath.
• Stent crush technique
If a dislodged unexpanded stent cannot be retrieved, one alternative is to deploy a second stent at the same location and compress the dislodged stent against the coronary wall. Very often this is the easiest and only solution for stent loss within the coronary circulation, especially when a wire cannot be passed across the stent. There is, however, a potential thrombosis risk with this approach.
• Forceps removal
Retrieval using this is feasible if the stent is present within the most proximal part of the left main or right coronary arteries but cannot be recommended as a first line option as it is aggressive. If employed then use of small French (paediatric sized) myocardial bioptomes is best (3Fr-1.3mm). Alternatively a biliary forceps can be employed. Forceps can also be used from the homolateral or contralateral femoral sheath once the retained material is brought down to the level of the iliac artery.
Percutaneous material extraction
If the lost stent or material is partially within the tip of the guiding catheter, a standard (at least) 2.5 mm balloon can be put at the tip of the guiding catheter in order to secure the proximal part of the stent.
Once the stent is brought to the iliac artery, the main challenge is to retrieve the material through the vascular sheath without the need for surgical vascular cut-down. Percutaneous extraction through the common femoral artery requires an upgrading of the introducer sheath to greater than 8Fr and flaring the distal end of the sheath.
Retraction of the stent into the guiding catheter is feasible if there is favourable alignment between the stent and the guiding catheter. This will allow for straightforward extraction. A second technique involves grasping the material by a myocardial biopsy forceps [108108. Eeckhout E, Stauffer JC, Goy JJ. Retrieval of a migrated coronary stent by means of an alligator forceps. Cath Cardiovasc Diagn. 1993;30: 166-168. ]. If aligned favourably the stent can be removed through the sheath. More often a snare is needed via a contralateral femoral artery.
If these techniques are unsuccessful and the material cannot be deployed safely within the iliac artery, then open surgical intervention will provide definitive treatment. However, conservative management for a stent left below the common femoral artery is a commonly practiced option. Historically during the era of manually crimped stents, loss was not infrequent without successful. Although it was considered benign and treated conservatively there is no systematic data available on the sequelae of this complication.
On account of the multitude of techniques available a concerted effort should be made to effect percutaneous removal as open surgery may entail a greater risk of morbidity and mortality.
Wire entrapment or fracture
Retained fractured wire fragments are a rare complication of PCI with a reported frequency of 0.1%. Standard PCI guidewires can be fractured and hydrophilic guidewires both fractured and stripped of their coating if they are trapped (“jailed”) behind a deployed stent, or even the side-hole of a catheter.
Entrapment can occur during the treatment of bifurcation lesions where two guidewires (main branch and sidebranch) are used or when a “buddy wire” has been used on account of excessive tortuosity. In the former the mechanism is typically undue anterograde tension during deployment of the main branch stent. This tension allows a significant loop (>2700) to form at the bifurcation junction Upon deployment the side branch guidewire is wrapped around the lateral and medial walls of the stent. The increased area and friction results in entrapment. By contrast, retrograde tension during deployment will allow the side branch wire to “hug the corner” and expose only a minimum of wire to “jailing” [107107. Prasan, A, Brieger D, Adams MR, Bailey B. Stent Deployment Within a Guide Catheter Aids Removal of a Fractured Buddy Wire. Cath Cardiovasc Intervent. 2002;56:212-214. ].
Post-dilatation to high pressure (or stent deployment to greater than nominal pressure) before side branch or “buddy” guidewire removal can also lead to entrapment. Secondary PCI guidewires must therefore be removed before this manoeuvre.
Once entrapment has occurred management depends on whether the wire has fractured or is intact. A small fractured fragment retained behind a stent can be left as endothelialisation of the stent will cover the fragment. Similarly if a small section of wire is uncovered a second stent can be deployed to ensure no long-term exposure to the coronary circulation. Snaring long fractured segments of wire is technically challenging and has a much lower success rate when compared to stent snaring. If, however, the fractured but entrapped segment extends into the guide catheter, then a stent can be deployed to high pressure within the shaft of the catheter and all intracoronary equipment removed enbloc [107107. Prasan, A, Brieger D, Adams MR, Bailey B. Stent Deployment Within a Guide Catheter Aids Removal of a Fractured Buddy Wire. Cath Cardiovasc Intervent. 2002;56:212-214. ]. If an intact wire is trapped behind a stent following a 'jailed' manoeuvre, then an small balloonloaded on the trapped wire can be inflated behind the proximal stent margin to aid disentanglement. Following successful wire removal a second wire is placed distally through the central axis of the stent and the stent re-expanded with post-dilatation. Passage of a second wire by forming a loop in the vessel proximal to the stent ensures that it cannot pass behind the stent struts. In situations where a concerted effort at percutaneous retrieval has been unsuccessful open surgery can provide definitive treatment, but as with all unplanned cardiac surgery, is associated with significant morbidity and mortality. In contrast to retained stents there are no reports of thrombosis associated with retained wire fragments. However, due to the potential risk of thrombosis, especially if antiplatelet therapy is not feasible, a surgical solution may be warranted. In the presence of long-term dual antiplatelet therapy there is an argument for leaving small retained fragments in situ.
Equipment knot
This complication can occur especially with guide catheters when using a transfemoral approach and on attempted engagement of the right coronary artery. The mechanism of knotting is excessive torque movement being applied to a catheter shaft outside the patient. Tortuous peripheral arterial anatomy is the substrate for this complication. Friction at sites of catheter contact with the peripheral arterial wall will prevent transmission of energy distally. A lack of movement of the catheter tip under fluoroscopy combined with a damping of arterial pressure waveform or inability to inject contrast should alert the operator to this possibility. Typically the knot starts forming in the iliac artery. The key is to recognise this process before the structural limit of the catheter shaft is exceeded and fails. By applying torque in the opposite direction to that which caused the knot, under direct fluoroscopic guidance and with the aid of a guidewire in the lumen of the proximal catheter shaft, the situation can be recovered.
If unable to unite the knot, then a second access point is require (radial for femoral or vice-versa). The distal tip of the catheter can be grasped by a snare loop system and opposite rotation applied to that which caused the knot to form (i.e., anticlockwise rotation if the knot was formed with clockwise rotation and vice-versa). Anti clockwise rotation applied to disentangle the knot while traction is applied in both directions ( Figure 18A ).
As a preventive manoeuvre, whenever extreme tortuosity or incipient catheter knotting occurs, engagement of coronary ostia should be performed with a guidewire in order to stiffen the shaft of the catheter. The use of a long sheath or a reinforced (armoured) sheath (Arrow ™) is a further precautionary measure.
A simplified algorithm for the management of retained equipment is provided in Figure 18B .
Human performance and limitations
OPERATOR PERFORMANCE
Overview
Studies have demonstrated that volumes (both operator and institutional) rather than years of experience impact on the complication rate for PCI in both elective and non-elective settings. Recommendations on optimal operator volumes are based in part on data from 107,000 procedures showing a lower incidence of CABG in those centres with >400 cases per annum. Further retrospective data suggests that mortality may be affected in high risk PCI. Low volume centres performing PPCI appear to have higher mortality rates compared to more experienced centres. Guidance for minimum annual volumes for continued practice has therefore evolved. For example to perform elective PCI >75 cases per operator in an institution performing >400 cases with on-site cardiac surgery is a class 1 recommendation from the US [139139. Ellis SG, Weintraub W, Holmes D, Shaw R, Block PC, Spencer King III. Relation of Operator Volume and Experience to Procedural Outcome of Percutaneous Coronary Revascularization at Hospitals with High Interventional Volumes. Circulation. 1997;95:2479-2484.
Relationship between operator volume and outcome., 140140. Srinivas VS, Hailpern SM, Koss E, Monrad ES, Alderman MH. Effect of physician volume on the relationship between hospital volume and mortality during primary angioplasty. JACC. 2009;53:574-9. , 141141. Hannan EL, Wu C, Walford G, et al. Volume-outcome relationships for percutaneous coronary interventions in the stent era. Circulation. 2005;112:1171-1179. ].
These data, mainly based on risk-adjusted registries, overlook case selection, appropriateness of intervention, and variability thus possibly oversimplifying the association. Furthermore, although the operator is an integral part of any PCI procedure, these analyses take no account of other members of the team or system failures beyond the control of a single individual. Such a person-centred approach has some shortcomings but has been adopted as it is more suited to management processes.
The main assumption underlying the interpretation of these data is that with more experience and practice the greater the performance. Such logic whilst seemingly evident does not explain all facets of a complication “event”. It does however provide a useful measurement with which to analyse the complex challenge of operator performance.
The “learning curve”
These shortcomings are reinforced by the oft-quoted phrase “the learning curve”. There probably is no “learning curve”, as suggested by an idealised gradual, continuous process. Any “curve” will necessarily consist of irregular steps as many parts of a procedure are either performed well or not in an all-or-none fashion Furthermore it cannot explain variance. The most experienced of operators can make errors leading to a complication. The composite of operator skills and judgement probably cannot be measured satisfactorily or easily in a mathematical sense. Professions where dexterity and judgement are just as important to performance, often display unexplained variations despite high levels of skill and experience. The learning curve can “disappear” through case selection and supervision only to reappear when faced with more challenging cases.
Performance analysis
Florence Nightingale (1820-1910) ( Figure 21 ) and Ernest Codman (1869-1940) both gave original insights on the pitfalls of statistics used to assess performance and outcome which are still relevant today [142142. Spiegelhalter DJ. Surgical Audit : Statistical Lessons from Nightingale and Codman. Journal of the Royal Statistical Society. 1999;162:45-58. ]. The former identified inadequate control for the type of patient, data manipulation, and the singular use of mortality as major obstacles to the validity and usefulness of this type of information. The latter underlined that the key aims of any performance analysis should be to find out actively the clinical outcomes of patients, compare with other hospitals (units) and analyse the data to discover not just the “weak“ but also the “strong” aspects of care in order to improve future performance. In essence these thoughts emphasise that statistics alone without insight, especially if data quality is poor, risks being both useless and misleading. Crucially a distinction needs to be made between the detection of underperformance and the use of this information for disciplinary or regulatory action. The former can be helpful within a culture of self-improvement whereas the latter requires scientific certainty, which, is challenging if not impossible to achieve.
In an attempt to overcome these challenges contemporary innovations in performance surveillance have drawn from quality control processes designed for the manufacture of weapons ordnance during the Second World War. They fall into several forms, but two have been used to monitor cardiothoracic surgeons. Value Life Adjusted Display plots (VLAD) and Cumulative Summation graphs (CUSUM) utilise risk adjustment to account for case-mix differences in order to overcome the penalty that comes with taking on challenging disease but are only useful in a very broad sense as they focus on a very crude (in mathematical terms) output, namely “dead or alive”. For statistical relevance to an individual operator these methods would require a vast number of data points which may preclude their practical day-to-day relevance. They are nevertheless a significant advance that can be used to enhance performance monitoring and may give early warning indications of divergent performance in a unit. Importantly, however, they do not measure error.
ERRORS
Overview
( Figure 20 )
Professionals working in a hazardous environment are prone to errors and these are the predominant cause of adverse events. It is estimated that these account for thousands of deaths each year in Medicine. Errors occur for a variety of reasons that often have more to do with non-technical and communication skills than with technical proficiency itself. Furthermore, it is the combination of human errors and latent conditions within a system of care that can produce a complication.
Human errors
These are a normal feature of all human endeavour and all operators are vulnerable. They cannot be avoided completely but can be minimised and mitigated against. Traditional factors such as physical fatigue, emotion, and stress can combine to make an error more likely. There are also limits on how an individual in a critical situation can perform. , particularly in the absence of continued education and training. Operator fatigue in long cases such CTO, multivessel PCI or performing PPCI during the night are scenarios where errors may be more likely.
Only a fixed amount of stimuli can be dealt with by an operator at any one time. Beyond a threshold it becomes easy for an individual to become overloaded. Once this occurs a series of strategies are often adopted, influenced by culture and personality, which then impinge on the function of both the operator and the operating team leading to error. One such strategy is 'plan continuation' error. The operator when faced by a challenging situation fails to consider all the available options, persisting with the original plan despite changing circumstances The more difficult and unexpected the situation becomes, the more solid the fixed reasoning. In this environment violations of protocols or accepted practice become frequent. Effective team leadership is also crucial both in terms of managing people and equipment, but also information. During a critical phase of a procedure or in the midst of an acute or evolving complication, team roles must be assigned with non-essential personnel dismissed and instructions made clear to those who remain. Without this attention to detail haphazard decision making can prevail leading to or exacerbating a complication.
Latent conditions
By invoking a systems based approach, rather than a person-based approach to errors, it becomes evident that a combination of human and error prone conditions (“latent”) within an organisation conspire to produce a complication. Operators and organisations will have developed in-built mechanisms to protect against these ranging from individual operator habits or “tips and tricks” to statutory limits on duty periods and rest. The threats are, however, dynamic and so complications can still happen despite these measures. The “Swiss cheese” model is often used by industry to explain the relationship between the two. When the holes is consecutive moving layers align, a complication will result but any one hole in itself is not enough to allow the complication to occur [154154. Reason J. Human error: models and management. BMJ. 2000;320:768-70. ].
Effect of complications on operators
General
The traditional way of evaluating complications, particularly within interventional specialties has been adversarial, focussing on the individual operator rather than the system within which (s)he works. This culture has likely led to under-reporting of complications. When major sentinel events do occur the individual “punishment” of practitioners or use of crude performance indicators may serve to reinforce the traditional culture and foster defensive medical practice. Publication of league tables of individual cardiothoracic surgeons” “performance” has led to the promotion of risk averse practice [144144. Schneider EC, Epstein AM. Influence of cardiac-surgery performance reports on referral practices and access to care. NEJM. 1996;335 :251-256. ]. This highlights the importance of using these data in a constructive way for the benefit of not just those linked with the immediate case but also for future patients and practitioners [147147. Sexton JB, Thomas EJ, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ. 2000;320:745-749. ].
Individual
The most extreme form of complication, albeit rare, is death in the catheter laboratory. Given the volume of procedures and current adverse event rates it is likely that an interventionist will encounter this scenario more than once in their career. There is no formal guidance and few data on how best to respond. A pragmatic approach involving all team members is thus probably warranted. A questionnaire survey (n=371) of cardiac surgeons and anaesthetists, however, begins to shed some light on this topic. In this study 53% of surgeons and 22% of anaesthetists who had experienced an intra-procedural death reported that they had stopped working for the rest of the list. Less than a third considered a death to impact on subsequent performance. Of those that continued there were no further adverse events in the ensuing 48hrs but the length of stay of their patients (n=1135) was significantly more prolonged suggesting that subsequent practice may be affected in the short-term [143143. Goldstone AR, Callaghan CJ, Mackay J et al. Should surgeons take a break after an intraoperative death ? Attitude survey and outcome evaluation. BMJ. 2004;328:379. ].
Education and training
Education and training are often used interchangeably but have important differences. Education is concerned with the transfer of knowledge whereas the training is concerned with the transfer of skills. As techniques have progressively improved an inadvertent consequence is that only the most experienced operators are likely to have been exposed sufficiently to the full range of possible complications. Despite this, opportunities for learning how to prevent and deal with PCI complications are limited and almost completely restricted to knowledge-based meetings. The predominant “training model” continues to be live patients, making the ethical and efficient transfer of skills in this domain currently problematic.
The current published evidence base for acute complications and how best to prevent and manage them are often influenced by positive publication bias, small cohort sizes and retrospective design restricting their potential to influence or inform practitioners of best practice. Case conferences, both within institutions and at international congresses, are dominated by the old construct of operator, patient and lesion characteristics with angiographic images. While this is important, in isolation it avoids illustrating the systematic and multi-factorial nature of errors and subsequent complications. Departmental or in-hospital “morbidity and mortality” meetings are also useful but must be the manifestation of a catheter laboratory confidential incident reporting system focussing on error rather than just an opportunity to highlight dramatic sentinel cases. By isolating the “complication case” from the system within which it has occurred recurrent “error traps” and root causes are easily overlooked.
Dedicated complication courses are perhaps the most sophisticated contemporary form of educational programme available. They are unique way for sharing techniques and information acquired by serendipity and experience in a non-threatening atmosphere. However, they are rare and do not form part of current mandatory requirements.
The most important educational and training approach for an organisation is one where active “learning” can take place [146146. An organisation with a memory. Report of an expert group on learning from adverse events in the NHS. Crown Copyright 2000.
Useful overview describing the different factors that contribute to errors in healthcare .]. This is a culture where errors and complications are not considered “personal” failures with a risk of penalties attached but opportunities for all members of a service and hence the service itself to improve.
Towards a new paradigm
Safety critical industries
The aviation industry is frequently referred to as the best model for understanding the “anatomy” of complications [146146. An organisation with a memory. Report of an expert group on learning from adverse events in the NHS. Crown Copyright 2000.
Useful overview describing the different factors that contribute to errors in healthcare .] ( Figure 22 ). The high profile of accidents and loss of life in the early years of commercial jet travel led to an unprecedented level of investment by the industry to record accurately, and evaluate systematically the root causes of these adverse events. The realisation that two-thirds of accidents were primarily caused by human error led to a major change in the culture and training of aircrews. The result has been a fall in accident and mortality rates. The average fatality rate for commercial aviation (1997-2007) was 0.5 per million flight departures [148148. Boeing corporation. Statistical Summary of Commercial Jet Airplane Accidents: Worldwide Operations 1958-2007 (2009). ]. The average mortality associated with elective PCI procedures is of the order of 1 per 100 to 1 per 1,000.
Medical errors
- Procedural failures due to unantipated events
- Non complicance with protocols
- Breakdown in communication
- Insufficient proficiency
- Decision-making or strategy errors
Notwithstanding the difficulties in comparison, the aviation experience may have relevance to PCI [145145. Brennan TA, Leape LL, Laird NM et al. Incidence of adverse events and negligence in hospitalized patients. NEJM. 1991;324:370-376. ].
Checklists
There is perhaps a need for a new paradigm and new approach to the study of complications and how to prevent or prepare for them in interventional cardiology. Indeed some of these principles have already reached Medicine via the efforts of anaesthetists and surgeons. The World Health Organisation published a 19 point check-list to be applied globally to surgical patients in order to prevent complications. This check-list was subjected to trial scrutiny and revealed an impressive 40% fall in overall mortality from 1.5% to 0.8% [149149. http://www.who.int/patientsafety/education/curriculum/en/index.html. , 150150. Haynes AB,Weiser TG,Berry WR. Et al. for the Safe Surgery Saves Lives Study Group. A Surgical Safety Checklist to Reduce Morbidity and Mortality in a Global Population. NEJM. 2009;360:491-499. ]. Although a Hawthorne effect cannot be discounted in this form of study, the feasibility of its introduction across international centres is encouraging. Within interventional cardiology a similar check-list approach has been advocated although not tested [151151. Van De Walle S, Lerman A, Chevalier B, Sabate M, Aminian A, Roguelov C, Eeckhout E. Constructing a checklist for the prevention of complications during percutaneous coronary intervention. Eurointervention. 2008;4:189-190. ] ( Figure 23 ). Nevertheless it is a useful starting point. The checklist aims to assist with repetitive tasks, which are typically poorly performed, and guard against protocol violations. Indeed the latest iteration of PCI guidelines from the AHA /ACC recommend a “time-out” checklist process before starting a case in order to establish the patient particulars and clarify the nature of the procedure [22. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011 Dec 6;124(23):e574-651.
Updated North American guidelines for PCI.].
Ten causes of apparent hypotension during PCI
- Starvation/dehydration hypovolaemia
- Unnoticed scale change on haemodynamic monitor
- Catheter kinked or obstructed
- Tachy or bradyarrythmia (including vagal response)
- Tamponade
- Ischaemia (abrupt vessel closure)
- Retroperitoneal haemorrhage
- O ring haemorrhage during long procedure
- Drug-induced (especially nitroglycerine)
- Anaphylaxis or anaphylactoid reaction
The prevention of complications is clearly more desirable than having to manage them. We know much about lesion and patient variables to help us in this task. The systematic application of risk assessment would likely yield important benefits. The combination of a high risk patient and high risk lesion variables results in a greater chance of complications than either alone. Importantly as techniques and materials improve, patient variables are more likely to influence procedural outcome than the lesion variables.
New training methods, again, based on errors encountered in hazardous working environments may be needed in order to progress further, particularly as emergency workloads increase and the scope of PCI extends. A suggested framework for minimising complications are summarised ( Figure 24 ).
Crew Resource Management
Six areas that are of particular importance are:
1. the planning of a procedure using risk evaluation in a multidisciplinary team
2. improving information processing for repetitive tasks,
3. improving technical proficiency (without putting patients at risk),
4. robust data systems,
5. non-threatening case review, and
6. crew resource management. This latter training method (originally termed cockpit resource management) was developed by NASA in collaboration with the airline industry in order to reduce the aviation accident rate. The principles include maintaining team structure and communication during a crisis, problem-solving strategies, executing plans under pressure, and managing workload [152152. Helmreich RL, Merritt AC, Wilhelm JA. The evolution of crew resource management in commercial aviation. Int J Aviation Psychol. 1999;919-32. ].
Local institutional, operator and team patterns of complications and attitudes are central to informing such a process. Valid, confidential, and electronically collated data a prerequisite. Major complications represent the tip of an iceberg of errors. These can occur without adverse clinical events because they are treated promptly, the patient recovers from the complication or simply due to chance. Confidential incident reporting can serve as a useful indicator of “near-miss” events ( Figure 25 , Figure 26 ). To supplement this form of data collection, systematic direct observation of procedures and surveys of attitudes may yield additional valuable information.
Once attitudes to complications and rates of errors can be
established then locally adapted strategies can be developed to prevent them. Integral to this is formal training both in the systematic nature of error, simulation [153153. Gallagher AG, Ritter EM, Champion H, Higgins G, Fied MP, Moses G, Smith DG, Satava RM. Virtual reality simulation for the operating room: proficiency-based training as a paradigm shift in surgical skills training. Ann. Surg. 2005;241:364-372.
A “look into the future” with this description of simulation training for surgeons.] and scenario training for improving technical deficiencies and improving teamwork especially in critical situations. These areas of training are in their infancy of development within Medicine as are their evaluation.












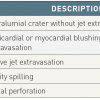


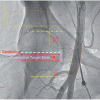








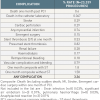



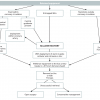


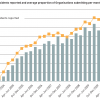

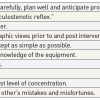




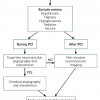









I have doubts when calculating the maximum dose of contrast using the formula that you provide. (defined as 5ml x weight [Kg]/serum creatinine [mg/ml]), if i use it for example with a real patient like this: 5 ml x 63 kg/ 0,0076 mg/ml the result is 41.447,3684 ml This is using a serum creatinine of 0,76 mg/dL. If you convert it to mg/ml, then the creatinine is 0,0076 mg/ml. I think there must be an error in that formula. Probably we should divide with the creatinine in mg/dl? In that case the result would be 5 x 63 kg/0,76 mg/dl= 414,47 ml. I look forward to receive your answer. Siro Buendía CCRN. [email protected] Juan Carlos Rubio CCRN Hospital Universitario Son Espases Palma de Mallorca. Spain
Data on stent-edge dissections can be viewed at TCT 2012, abstact# 247 &297, with MGH-OCT Registry & SORT OUT V OCT substudy, small sample sizes, but possibly a 'benign' problem ?
Small non-flow limiting dissections at edge of deployed stents -how should they be managed ? linear (parallel to coronaries) vs radial dissections (perpendicular to coronary vessel) at the Coronary ostium, or at side- branches would affect management strategy ? Will a second stent always be necessary to address this issue ? If left unattended these small dissections would they pose any long term complications ?
Very useful chapter! Unfortunately, the images in the illustrative case 7 are the same as in case 6, not showing the use of intravsacular coils. Hoperfully that can be corrected! Thank you very much, Leonhard Bruch, MD Berlin, Germany
Thank you for your comment. The problem has been corrected. Do not hesitate to comment if you have any question.