Summary
Arterial hypertension represents a significant global health burden. Despite lifestyle modification and the availability of effective antihypertensive drugs, blood pressure control remains suboptimal worldwide. Several factors account for that problem, among them non-compliance of physicians and patients, untreated secondary forms of hypertension and true treatment-resistant hypertension (i.e. blood pressure > 140/90 mmHg in spite of 3 antihypertensive drugs including a diuretic).
Both experimental and clinical studies have emphasised the important role of sympathetic nervous system activation in the development and progression of arterial hypertension. Catheter-based renal denervation is a new therapeutic option to reduce renal sympathetic nerve traffic in experimental and human hypertension. The data so far obtained in patients with drug-resistant hypertension have demonstrated that this procedure may reduce blood pressure effectively and safely, if renal nerve ablation has been performed properly.
Targeting the renal sympathetic nerves could also be an attractive future strategy for other diseases, where the activation of the sympathetic nervous system plays an important pathophysiological role such as in sleep apnoea syndrome, heart failure, chronic kidney disease or polycystic ovary syndrome.
Importance of hypertension
HISTORICAL PERSPECTIVE
High blood pressure (systemic arterial hypertension) was only recognised as a clinical entity in the 20th century. In fact, although the Reverend Stephen Hales had measured blood pressure for the first time in a living horse in 1740, using a glass cannula inserted into the carotid artery [11. Riva-Rocci S, Zanchetti A, Mancia G. A new sphygmomanometer. Sphygmomanometric technique by Riva Rocci. J Hypertens. 1996;14:1-12. ], it wasn’t until 1896, when Scipione Rive-Rocci invented the sphygmomanometer, that blood pressure could be easily and repeatedly determined in humans [22. Korotkoff NC. On the question of methods of determining the blood pressure. Reports of the Imperial Military Academy, St. Petersberg. 1905;365:1905. ]. Initially, only systolic blood pressure could be assessed using palpation of the radial pulse. Then Korotkoff (using the stethoscope introduced by René Laenec in 1828) discovered arterial sounds and described the method in 1904 that is still used today [33. Janeway TC. The Clinical Study of Blood-Pressure: A Guide to the Use of the Sphygmomanometer in Medical, Surgical, and Obstetrical Practice, with a Summary of the Experimental and Clinical Facts Relating to the Blood-Pressure in Health and in Disease, ed. D. A. a. Company1904, New York. (a) p. 173; (b) p.91-92 ].
Soon it became apparent that blood pressure levels varied among individuals and patient groups, particularly those with renal disease. At first, high blood pressure was not recognised as a condition responsible for disease of various organs, although Richard Bright had described in 1827 an association between ‘’shrunken kidneys and a thickened heart’’ during autopsy. Indeed, until the beginning of the 20th century, hypertension was considered a compensatory phenomenon required to maintain adequate perfusion of damaged kidneys (“Erfordernishochdruck”), although Theodor Caldwell Janeway had published „A clinical study of blood pressure“ in 1904 [33. Janeway TC. The Clinical Study of Blood-Pressure: A Guide to the Use of the Sphygmomanometer in Medical, Surgical, and Obstetrical Practice, with a Summary of the Experimental and Clinical Facts Relating to the Blood-Pressure in Health and in Disease, ed. D. A. a. Company1904, New York. (a) p. 173; (b) p.91-92 ], where he for the first time described endorgan damage induced by high blood pressure. Even when Franklin D. Roosevelt died in 1945 from a cerebral hemorrhage, his personal physician Dr. Ross McIntyre pretended that the fatal bleeding was unexpected, although his blood pressure values had reached levels as high as 310/190 mmHg [44. Messerli FH. This day 50 years ago. N Engl J Med. 1995;332:1038-9. ]. Similarly, the famous Harvard professor Paul Dudley White stated in 1937„ Hypertension may be an important compensatory mechanism which should not be tampered with, even were it is certain that we could control it.“
The relationship of blood pressure with stroke and myocardial infarction was established eventually in several epidemiological studies (among them the Framingham Cohort that begun in 1945) establishing hypertension as one of the most important cardiovascular risk factors [55. Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46-51. ].
EPIDEMIOLOGY
Arterial hypertension is highly prevalent in the overall population, but particularly in adults and the elderly ( Figure 1) [66. Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y; American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25-146. ]. Systolic blood pressure especially increases with age due to a loss in elasticity of the vasculature and hence a loss of the “Windkessel Effect”. In Western countries, around 20% of the adult population have elevated blood pressure values (above 140/90 mmHg) making it one of the most important cardiovascular risk factors.
CLINICAL CONSEQUENCES
Hypertension per se does not cause symptoms, but rather, it represents a major risk factor for myocardial infarction and stroke [77. Ruilope LM, Hypertension in 2010: Blood pressure and the kidney. Nat Rev Nephrol. 2011;7:73-4. ] ( Figure 2). Both major adverse cardiovascular events exhibit a linear relationship with the blood pressure [88. Neaton JD, Kuller L. Stamler J, Wentworth D. Impact of systolic and diastolic blood pressure on cardiovascular mortality, in Hypertension: Pathophysiology, Diagnosis and Management. 2nd ed, B. B. Laragh JH, Editor 1995, Raven Press Ltd: New York. p.127-144 ]. Although myocardial infarction is the more prevalent complication of hypertension, stroke is even more tightly linked to blood pressure, and in particular to age ( Figure 3). Certainly at any age, increasing blood pressure levels are associated with an increased risk of stroke [99. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-13. ]. Similarly, high blood pressure carries an increased risk of vascular dementia [1010. Nagai M, Hoshide S, Kario K. Hypertension and dementia. Am J Hypertens. 2010;23:116-24. ]. In addition, it is a major cause of chronic renal failure leading to haemodialysis.
Hypertension and cardiovascular diseaseHigh blood pressure is highly prevalent in the overall population and represents a major risk factor for cardiovascular diseases:
- myocardial infarction
- peripheral artery disease
- stroke
- dementia
- chronic renal failure
Causes of hypertension
In patients considered for renal sympathetic denervation, secondary forms of hypertension ( Table 1 ) must be excluded prior to the procedure [1111. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A; European Society of Hypertension. 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens. 2007;25:1751-62.
Contemporary guidelines for the diagnosis and treatment of systemic arterial hypertension].
ESSENTIAL HYPERTENSION
In over 95% of the patients, no apparent cause for the elevated blood pressure values can be found, a condition which is referred to as essential hypertension [1212. Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370:591-603. ]. It is likely that this condition has a hereditary basis, as it is seen to run in families. In fact, the risk of developing high blood pressure with advancing age increases three to five–fold if one or two parents respectively are hypertensive [1313. Tozawa M, Oshiro S, Iseki C, Sesoko S, Higashiuesato Y, Tana T, Ikemiya Y, Iseki K, Fukiyama K. Family history of hypertension and blood pressure in a screened cohort. Hypertens Res. 2001;24:93-8. ]. Recently, it has been identified that genes themselves account for small changes in blood pressure [1414. Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677-87. ]. Furthermore, the condition is more prevalent in certain populations over others (the so-called “low blood pressure populations”) [1515. Ilias I, Sahdev A, Reznek RH, Grossman AB, Pacak K. The optimal imaging of adrenal tumours: a comparison of different methods. Endocr Relat Cancer. 2007; 14:587-99. , 1616. Wolf-Maier K, Cooper RS, Kramer H, Banegas JR, Giampaoli S, Joffres MR, Poulter N, Primatesta P, Stegmayr B, Thamm M. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10-7. ].
Besides a genetic disposition, dietary factors such as sodium and potassium intake as well as obesity have been linked to essential hypertension. Of note, an over-activation of the sympathetic nervous system is often associated with essential hypertension.
RENOVASCULAR HYPERTENSION
Renovascular hypertension is the most common curable form of secondary hypertension. Its prevalence ranges from between 1-3% in the hypertensive population, but is more common in referral centres [1717. Elliott WJ. Secondary hypertension: renovascular hypertension, in Hypertension: a Companion to Braunwald’s Heart Disease, B. E. WG, Editor 2007, Saunders Elsevier. Philadelphia. p.93-105 ]. Two forms of renovascular hypertension can be distinguished: 1) Fibromuscular dysplasia and 2) atherosclerotic renal artery stenosis [1818. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-42. ]. Both are amenable to percutaneous renal angioplasty [1919. Luscher, T.F., Keller HM, Imhof HG, Greminger P, Kuhlmann U, Largiadèr F, Schneider E, Schneider J, Vetter W., Fibromuscular hyperplasia: extension of the disease and therapeutic outcome. Results of the University Hospital Zurich Cooperative Study on Fibromuscular Hyperplasia. Nephron, 1986; Suppl 1:109-14. , 2020. Luscher, T.F., Lie JT, Stanson AW, Houser OW, Hollier LH, Sheps SG. Arterial fibromuscular dysplasia. Mayo Clin Proc, 1987;62: 931-52. ], and in the case of atherosclerotic lesions, to stenting as well [2121. Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, Braam B, Huysmans FT, Schultze Kool LJ, Rutten MJ, Doorenbos CJ, Aarts JC, Rabelink TJ, Plouin PF, Raynaud A, van Montfrans GA, Reekers JA, van den Meiracker AH, Pattynama PM, van de Ven PJ, Vroegindeweij D, Kroon AA, de Haan MW, Postma CT, Beutler JJ. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med. 2009;150:840-8, W150-1. ].
RENAL-PARENCHYMATOUS HYPERTENSION
Most forms of chronic kidney disease involve both kidneys, and lead to a steady decline in renal function over years and decades. Hypertension is associated with all forms of chronic kidney disease, in particular diabetic glomerulosclerosis (characterised by Kimmelstiel-Wilson lesions on renal biopsy) and chronic glomerulonephritis [2222. Campos C, Segura J, Rodicio JL. Investigations in secondary hypertension: renal disease, in Hypertension, i. A. H. Zanchett, L. Rodicio, J. L. , Editor 2001, McGraw Hill International: London. p.119-126 ]. Specific treatment modalities are rarely available, although inhibitors of the renin-angiotensin system (i.e., ACE-inhibitors and AT1-receptor antagonists) reduce proteinuria and delay the decay in kidney function over time [1111. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A; European Society of Hypertension. 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens. 2007;25:1751-62.
Contemporary guidelines for the diagnosis and treatment of systemic arterial hypertension, 2323. Neumann J, Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int. 2004;65:1568-76. ].
ENDOCRINE HYPERTENSION
In 1955 Jerome Conn described patients with hypertension, hypokalaemia and adenomas of the adrenal cortex (the so-called “Conn’s syndrome”) [2424. Conn JW. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955;45:3-17. ].
Mineralocorticoid hypertension is characterised by elevated plasma aldosterone levels and suppressed plasma renin activity reflecting an autonomous aldosterone secretion by the adenoma [2525. Bravo EL. Secondary Hypertension: Mineralocorticoid excess states, in Hypertension: A Companion to Braunwald’s Heart diseases, E. W. Black HR, Editor 2007, Saunders-Elsevier: Amsterdam. p.106-118 ]. Localisation of the adenoma is best performed with computer tomography [1515. Ilias I, Sahdev A, Reznek RH, Grossman AB, Pacak K. The optimal imaging of adrenal tumours: a comparison of different methods. Endocr Relat Cancer. 2007; 14:587-99. ].
Patients with phaeochromocytoma typically experience palpitations, sweating and sometimes headaches due to sudden releases of catecholamines from the tumour. The diagnosis involves either computer tomography or magnetic resonance imaging [1111. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A; European Society of Hypertension. 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens. 2007;25:1751-62.
Contemporary guidelines for the diagnosis and treatment of systemic arterial hypertension, 2626. Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24:2331-9. , 2727. Goldstein DS, Eisenhofer G, Flynn JA, Wand G, Pacak K. Diagnosis and localization of pheochromocytoma. Hypertension. 2004;43:907-10. ]. Catecholamine levels in plasma or in urine (metanephrine, vanillinic acid) are typically elevated [2626. Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24:2331-9. , 2828. Kaplan, N.M., Current diagnosis and treatment of primary aldosteronism. Expert Rev Cardiovasc Ther. , 2010;. 8:1527-30. ].
Hypertension is one of the most distinguishing features of endogenous Cushing’s syndrome. The diagnosis is based on clinical observations and laboratory parameters (i.e., morning plasma cortisol, 24 hour cortisol metabolites in urine) [1111. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A; European Society of Hypertension. 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens. 2007;25:1751-62.
Contemporary guidelines for the diagnosis and treatment of systemic arterial hypertension]. The latter often requires a cortisol-suppression test to distinguish Cushing’s syndrome from elevated cortisol values in simple obesity.
True Cushing’s disease due to a pituitary adenoma producing ACTH should be distinguished from a cortisol-producing adrenal adenoma or bilateral adrenal hyperplasia, primarily by using imaging techniques such as MRI and/or adrenal scintigraphy. In the former clinical condition, removal of the tumour by transsphenoidal hypophysectomy is the treatment of choice, whereas adrenal tumours are managed by unilateral adrenalectomy.
Secondary hypertensionDetermining possible secondary causes of hypertension is an important part in diagnosing patients with elevated blood pressure. The following (including less common causes of hypertension) should always be excluded in severe hypertension, resistant hypertension or those aged <40 before starting or continuing long-term conventional pharmacological treatment:
- renal parenchymal disease
- renovascular disease
- phaeochromocytoma
- primary aldosteronism (Conn’s syndrome)
- Cushing’s syndrome
- coarctation of the aorta
- thyroid dysfunction (hypo or hyperthyroidism)
- primary hyperparathyroidism
- acromegaly
- obstructive sleep apnoea
- drug/toxin-induced
- monogenic renal tubular syndromes
- pre-eclampsia
The sympathetic nervous system and blood pressure regulation
The cardiovascular system is neurally governed by the sympathetic and parasympathetic (vagal) neuronal afferents and efferents, which act as opposing mechanisms to activate and deactivate the heart and blood vessels. While the former is used in “fight or flight” reactions, the latter is active post-prandially as well as during rest and sleep. Changes in blood pressure are closely linked to changes in sympathetic outflow as assessed by microneurography, heart rate variability and/or catecholamine levels [2929. Esler M. The 2010 Paton Lecture. The sympathetic nervous system through the ages: From Thomas Willis to resistant hypertension. Exp Physiol. 2011;96:611-22.
Updated review about the role of the sympathetic nervous system in hypertension, 3030. Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719-34. ].
The sympathetic nervous system originates in the cardiovascular centres of the brain stem and activates the organs of the body via cholinergic neurones which, within the paravertebral ganglia, synapse with efferent adrenergic neurones which reach out to blood vessels, the kidney and other organs ( Figure 4 ) [3131. Shepherd JT, Vanhoutte PM. Local modulation of adrenergic neurotransmission in blood vessels. J Cardiovasc Pharmacol. 1985;7:S167-78. ].
Of note, offspring of hypertensive parents who are normotensive exhibit a marked overactivity of muscle sympathetic nerve activity during episodes of mental stress ( Figure 5 ) [3232. Noll G, Wenzel RR, Schneider M, Oesch V, Binggeli C, Shaw S, Weidmann P, Lüscher TF. Increased activation of sympathetic nervous system and endothelin by mental stress in normotensive offspring of hypertensive parents. Circulation. 1996;93:866-9.
First study showing that sympathetic nervous activity is already altered in the offspring of hypertensive parents]. Similarly, particularly in young hypertensives, renal norepinephrine overflow, an index of renal sympathetic nerve activity, is markedly upregulated, an effect that becomes less pronounced in ageing hypertensives ( Figure 6 ) [3333. Esler M, Lambert G, Jennings G. Increased regional sympathetic nervous activity in human hypertension: causes and consequences. J Hypertens Suppl. 1990;8:S53-7. ].
The sympathetic nervous system
- Hyperactivity of the sympathetic nervous system is one of the pivotal ‘players’ in the pathogenesis of arterial hypertension
- The sympathetic nerve fibres are distributed ubiquitously within the heart, the blood vessels, the kidney, and major peripheral baroreceptor sites, a finding that suggests a direct effect on:
- fluid balance
- cardiac output
- peripheral vascular resistance
Renal nerves and blood pressure regulation
The kidneys play a key role in long-term pressure regulation. In fact, the degree of water and salt excretion (pressure diuresis) at any given level of blood pressure determines the long-term homeostasis of the circulation [3434. Guyton AC. Blood pressure control –special role of the kidneys and body fluids. Science. 1991;252:1813-6. ]. The sympathetic nervous system has: 1) direct effects on renal vascular resistance via efferent adrenergic nerve endings activating alpha-receptor on smooth muscle cells of the circulation of the kidneys; and 2) activates renin release in juxtaglomerular cells via beta-receptors ( Figure 7 ) [3535. Bomzon A. Sympathetic control of the renal circulation. J Auton Pharmacol. 1983:3:37-46. , 3636. DiBona GF. Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1517-24. ]. This in turn leads to the formation of angiotensin I and II and aldosterone, all important regulatory hormones for blood pressure which act upon both the resistance of the arteries as well as within the kidneys.
Sympathetic renal blood pressure regulation involves efferent and afferent nerves. The former transmit sympathetic outflow to the kidney, while the latter provide feed-back information from the kidney to the cardiovascular centres ( Figure 7 ) [3636. DiBona GF. Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1517-24. , 3737. DiBona GF. Functionally specific renal sympathetic nerve fibers: role in cardiovascular regulation. Am J Hypertens. 2001;14:163S-170S.
Useful review focused on renal sympathetic fibres and cardiovascular system control]. The neurones of both systems form a dense network within the adventitia of the main renal artery and its branches. The concept of ‘’detaching’’ the regulatory organs implicated in blood pressure, such as the kidneys and muscles from the influence of the sympathetic nervous system was based on a large series of animal experiments and already applied clinically in the 1950s. This was reported by Smithwick in 1953 ( Figure 8 ) [3838. Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc. 1953;152:1501-4. ]. Surgical sympathectomy, however, was not very selective and (although it reduced blood pressure and diminished the consequences of hypertension in those surviving the procedure) was associated with severe complications; in particular, orthostatic hypotension and an unacceptably high operative morbidity and mortality. Similarly, antihypertensive drugs such as the ganglion blocker guanethidine, introduced at the same time in the management of hypertension, were associated with marked side effects [3939. Doyle AE. The introduction of ganglion blocking drugs for the treatment of hypertension. Br J Clin Pharmacol. 1982;13:63-5. , 4040. Cowley AW Jr. Long-term control of arterial blood pressure. Physiol Rev. 1992;72:231-300. , 4141. Mathias CJ. Management of hypertension by reduction in sympathetic activity. Hypertension. 1991;17:III69-74. ].
Renal nerves and hypertension
- The kidneys play a key role in long-term pressure regulation through efferent and afferent nerves
- The surgical and pharmacological blockade of the renal nerves were found to be effective in reducing blood pressure, but were also associated with an increase in serious side effects
Interventional strategies for treating for therapy-resistant hypertension
Already in the fifties of the last century, surgical sympathectomy in experimental animals as well as in patients has been shown to reduce norepinephrine spillover ( Figure 9 ), to prevent or reverse hypertension and to reduce major cardiovascular events [4242. Allen EV. Sympathectomy for essential hypertension. Circulation. 1952:6:131-40. , 4343. Smithwick RH. Sympathectomy, splanchniecoctomy and vagotomy. Rev Surg. 1973;30:153-73. ] However, this technique was quite invasive and in patients with severe hypertension associated with considerable side-effects, among them orthostatic hypotension.
The current percutaneous renal nerve ablation technology is much more selective than surgical sympathectomy and thus appeared suitable for clinical development. [4242. Allen EV. Sympathectomy for essential hypertension. Circulation. 1952:6:131-40. , 4343. Smithwick RH. Sympathectomy, splanchniecoctomy and vagotomy. Rev Surg. 1973;30:153-73. ] The fact that both muscle sympathetic nerve activity as well as renal norepinephrine spillover are elevated in human hypertension ( Figure 5 ), [3333. Esler M, Lambert G, Jennings G. Increased regional sympathetic nervous activity in human hypertension: causes and consequences. J Hypertens Suppl. 1990;8:S53-7. ] led to the development of a catheter-based renal denervation procedure by Murray Esler and co-workers using radiofrequency energy similar to that employed for the ablation of arrhythmias[4444. Farré J, Wellens HJJ, Rubio JM, Benezet J. Supraventricular tachycardias, in the ESC Textbook of Cardiovascular Medicine. Camm JA, Luscher TF, Serruys PW, Editor. 2009. Oxford University Press; Oxford. p. 1013-68. ]. In a proof-of-concept study, renal nerve ablation reduced muscle sympathetic activity in the peroneal nerve ( Figure 10 ) and renal norepinephrine spillover in hypertensive patients from 72 and 79 ng/minute in the left and right kidney to 37 and 20 ng/min respectively and blunted total body norepinephrine spillover by 42%.[4545. Schlaich, MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932-4.
Interesting study showing catheter-based renal denervation was associated with a reduction in systemic sympathetic nervous activity in patients with resistant hypertension]
Baroceptor activation therapy (BAT) represents another, already known concept in the management of high blood pressure. The first publications about ist blood-pressure lowering effects date to more that fifty years back. Although effective in lowering blood pressure, the implantation of devices activation the baroreceptor is quite invasive. Thus, with the discovery of numerous and effective antihypertensive drugs, besides the unacceptable adverse effects related to the first generation-baroceptor pacemakers, made this approach unattractive for both patients and physicians. Further studies with the aim to overcome the safety concerns are on-going [4646. Erne P, Sudano I, Resink TJ, Lüscher TF. Interventional therapy for hypertension: Back on track again?. Crit Rev Clin Lab Sci. 2016 16:1-8. ].
The creation of a arteriovenous anastomosis using the ROX Coupler system (ROX Coupler®, ROX Medical, San Clemente, CA, USA) also lowers blood pressure in patients with uncontrolled hypertension as reported in the ROX Control HTN study that enrolled 83 patients with an avarage daytime ambulatory blood pressure ≥135/*85 mmHg, despite antihypertensive drugs, with satisfactory results (-14 mmHg vs -1 mmHg at 6 months). [4747. Gronda E, Seravalle G, Trevano FQ, Costantino G, Casini A, Alsheraei A, et al. Long-term chronic baroreflex activation: persistent efficacy in patients with heart failure and reduced ejection fraction. J Hypertens 2015;33:1704-8. ]. In a post-hoc analysis Ott and collegues stratified 42 patients in two groups, the first with combined hypertension (n=31) and the second with isolated systolic hypertension (n=11), with no differences in baseline blood pressure both for office (p=0.163) and ambulatory systolic blood pressure (p=0.463), with a significant reduction in office systolic blood pressure and 24-hours ambulatory systolic blood pressure, without significant differences between the two groups, after the creation of an arteriovenous anastomosis using the ROX coupler system [4848. Ott C, Lobo MD, Sobotka PA, Mahfoud F, Stanton A, Cockcroft J, Sulke N, Dolan E, van der Giet M, Hoyer J, Furniss SS, Foran JP, Witkowski A, Januszewicz A, Schoors D, Tsioufis K, Rensing BJ, Saxena M, Scott B, Ng GA, Achenbach S, Schmieder RE. Effect of Arteriovenous Anastomosis on Blood Pressure Reduction in Patients With Isolated Systolic Hypertension Compared With Combined Hypertension. J Am Heart Assoc. 2016;5. pii: e004234. doi: 10.1161/JAHA. 116. 004234. ]. The high incidence of venous stenosis after the procedure, however, is a serious concern and will require optimization oft he device and or procedure or stenting oft he venous part of the fistula. Furthermore, it remains unclear what consequences the marked increase in cardiac output after placement of the fistula will have in cardiovascular function and clinical outcome.
Renal denervation
Percutaneous renal ablation systems, devices and techniques
Systems: Initially, the most commonly used system for percutaneous renal nerve ablation has been developed by Ardian Inc., which was acquired by Medtronic Inc. in 2010 (Ardian Inc., a division of Medtronic, Mountain View, CA, USA). The system comprises a single electrode ablation catheter and a power source ( Figure 11 ).
Shortly thereafter, numerous systems have been developed. Indeed, currently five systems received a European CE-Mark, i.e. Medtronic’s Symplicity spyral catheter system, St. Jude’s EnligHTN basket, the Vessix’s V2 balloon, Cordis’ Renalane system, and Recor’s Paradise system (see Table 2 ). Most of these technologies use radiofrequency energy to target renal sympathetic nerves except for the ultrasound-based Recor’s Paradise balloon which uses ultrasound energy [4949. Sakakura K, Roth A, Ladich E, Shen K, Coleman L, Joner M, Virmani R. Controlled circumferential renal sympathetic denervation with preservation of the renal arterial wall using intraluminal ultrasound: a next-generation approach for treating sympathetic overactivity. EuroIntervention. 2015;10:1230-8. doi: 10. 4244/EIJY14M10_14. ]. Some other systems using radiofrequency, ultrasound or injection of guanetidine are under development. Although conventional ablation catheters used to treat arrhythmias might in principle be suitable as well, the energy used in these procedures is several times higher than that used to ablate renal sympathetic nerves (30-55 vs. 5-8 Watt).
Access Site: The first-generation system required an 8 F sheath and guiding catheter using a femoral approach. Alternatively, a radial or ulnar approach can now be chosen since smaller 6F catheter systems became available. A radial approach for renal denervation is safe and feasible and could also have potential benefits compared to the femoral access in case of anatomical variation (i.e. very steep angle of the take off of the renal artery), peripheral artery disease [5050. Honton B, Pathak A, Sauguet A, Fajadet J. First report of transradial renal denervation with the dedicated radiofrequency Iberis™ catheter. EuroIntervention 2014;9:1385-1388. ] or in those at high risk of bleeding [5151. Daemen J,Van Mieghem N. First-in-man radial access renal denervation with the ReCor Radiance™ catheter. EuroIntervention 2015;10:1209-1212. ]. Moreover, a left radial access could be particularly safe, as for coronary angiography it has been shown that this approach has a lower risk of brain embolism when compared to the right radial access [5252. Pacchioni A, Versaci F, Mugnolo A, Penzo C, Nikas D, Saccà S, Favero L, Agostoni PF, Garami Z, Prati F, Reimers B. Risk of brain injury during diagnostic coronary angiography: comparison between right and left radial approach. Int J Cardiol. 2013;167:3021-6. ]. Recently, also a brachial and left radial approach was used with the multi-electrode RF system [5353. Heradien MJ, Augustyn J, Saaiman A, Brink PA. First reported cases: renal denervation with secondgeneration multi-electrode catheter via brachial and radial access. Cardiovascular journal of Africa• Volume 27, No 1, January/February 2016. ].
Guiding Catheters: Depending on the anatomy, either a right coronary (in the presence of a 90° take off from the main renal artery) or an internal mammary artery catheter (with a downward directed angle of the main renal artery) is employed. Special catheters are available for difficult anatomy.
Ablation Technique with Different Systems: With the first generation Symplicity® system a 0.014 F guidewire was advanced, once the guiding catheter had been intubated into the renal artery ostium, deep into the renal circulation. Then a 6 F multipurpose straight tip catheter was advanced over the guidewire close to the bifurcation of the main renal artery. Finally, the ablation catheter was advanced through the 6F catheter and the multipurpose catheter then pulled back. Six ablations were then applied in a spiral fashion starting from the distal part of the renal artery up to its origin from the aorta with ablation spots distributed like an interrupted spiral in order to provide an effective nerve block – a technique that was particularly challanging for unexperienced operators. After a control angiograpy, the guidewire was removed and placed in the left renal artery where the same procedure was repeated in a similar fashion.
The second-generation Symplicity® system allows for direct advancement of the ablation catheter through a 6 F guiding catheter without the use of a guidewire and a straight tip guiding catheter. The ablation catheter can be flexed, pushing a lever towards the back or front of the handle and turned with a handle rotator at the tip of the body of the shaft of the catheter ( Figure 11 and Table 2 ). A power unit provides radiofrequency energy. It is important to turn the catheter in such a way as to obtain optimal wall contact. Extensive force, however, should be avoided, as should small side branches of the renal artery. Before and during the ablation procedure, the renal artery must be continuously flushed with saline solution using a pressure cuff around the solution package.
Indeed, renal artery spasm and/or oedema may occur during the ablation procedure, particularly in smaller vessels, and/or when flushing with cold saline is not performed adequately. Continuous temperature monitoring assures that the power unit detects any overheating of the arterial wall in which case the application of energy is interrupted immediately. Commonly, 5-8 watts are applied for 2 minutes at each of the at least six ablations sites. Impedance may be used to assure good wall contact (optimal range: 300 – 350 Ω).
With the third-generation Spyral system a multi-electrode is introduced over the wire catheter in the renal arteries over an extra stiff wire. By removing the wire the catheter automatically forms a helical pattern through which the 4 electrodes achieve vessel wall contact. All 4 electrodes can deliver radio-frequency energy simultaneously (60 s ablation time). It is recommended to perform at least 8 radio-frequency ablations per renal artery. Böhm and collegues invastigated safety and efficacy of RDN with the Symplicity SpyralTM multielectrode catheter in a limited cohort of the Global SYMPLICITY Registry, demonstrating no significant adverse events, particularly no renal artery stenosis was observed over 6 months of follow up [5454. Böhm M, Brilakis N, Mancia G, Narkiewicz K, Ruilope L, Schlaich M, Mahfoud F. TCT-762 Renal denervation treatment with the Symplicity Spyral multielectrode catheter: 6-month safety and blood pressure outcomes from the Global SYMPLICITY Registry. J Am Coll Cardiol. 2016;68(18S):B308. doi: 10. 1016/j. jacc. 2016. 09.792 ]. The SPYRAL HTN Global Clinical Trial Program aims to evaluate the potential efficacy of RDN without all the confounding factors which probably contributed to the failure of the SYMPLICITY HTN. The study consists of two branches, the SPYRAL HTN OFF-MED and the SPYRAL HTN ON-MED, both conducted in parallel, prospectively, multicenter, international, randomized, blinded sham-controlled trials of RDN for patients with uncontrolled hypertension. The titration of medical therapy suggested for the patients included in the ON-MED branch should avoid the confounding role of drug changes and variability of medical adherence, while the OFF-MED branch should isolate the response to RDN on hypertension without any confounding antihypertensive medications. Moreover, the study population is represented by combined systo-diystolic hypertensive patients and RDN will be performed by 4-quadrant treatments per artery, both to avoid potential limitation already showed in the SYMPLICITY HTN [5555. Kandzari DE, Kario K, Mahfoud F, Cohen SA, Pilcher G, Pocock S, Townsend R, Weber MA, Böhm M. The SPYRAL HTN Global Clinical Trial Program: Rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J. 2016 ;171:82-91. doi: 10.1016/j.ahj.2015.08. 021. Epub 2015 Sep 11. ].
With the St. Jude basket, an 8 F guiding catheter is mandatory to place the rather stiff ablation catheter in the renal artery without the help of a guide wire ( Table 2 ). With the second-generation system of St. Jude, the basket containing 4 electrodes is expanded distally in the renal artery before the bifurcation and 4 ablations are automatically applied for 60 seconds. In contrast to the Symplicity system, the St. Jude system is temperature driven. After the first ablation series, the basket is folded by turning the node at the steering end of the ablation catheter, slightly pulled towards the ostium of the renal artery, simultaneously turned by 45° and again expanded to allow for optimal contact of the 4 electrodes with the artery wall. Then 4 ablations are again done automatically in a serial fashion.
The Vessix balloon catheter (Boston Scientific Corporation Natick, MA; Table 2 ) is an over the wire system using bipolar energy. The system consists of a low pressure balloon (3 atm) available in 4, 5, 6, 7 mm diameter sizes with offset electrode pairs placed in helical pattern (gold electrodes for good thermal and electrical conductivity, and radiopacity of each electrode on angiogram); the temperature is precisely sensed and controlled at 68°C. With simple anatomy, the balloon can be easily advanced into the renal artery over a 0.014 F guidewire. Whether the rather stiff balloon with attached electrodes is suitable for tortuous arteries and steep take-off, needs to be seen.
The Cordis Renlane (Cordis, CA) catheter is a 7-F compatible, helically shaped multi-electrode catheter with 5 irrigated electrodes that are powered by an external multi-channel RF generator and features five irrigated electrodes. Irrigation technology is frequently used in electrophysiology and aims to prevent collateral tissue damage.
The Paradise Ultrasound Renal Denervation System is a new-generation catheter-based device which aims to investigate whether the target ablation area can be checked by changing ultrasound energy and duration to optimize nerve ablation and eventually prevente arterial wall injury. Preliminary results come from a swine model, where the authors showed that total ablation area and depth of ablation can be optimised by changing ultrasound power and duration. Particularly, according to these authors, it seems that a low power-long duration of ultrasound energy leads to a deeper ablation while a high power-ultra short duration lead to a more superficial one, sparing renal arterial tissue damage but allowing sufficient peri-arterial nerve damage [4949. Sakakura K, Roth A, Ladich E, Shen K, Coleman L, Joner M, Virmani R. Controlled circumferential renal sympathetic denervation with preservation of the renal arterial wall using intraluminal ultrasound: a next-generation approach for treating sympathetic overactivity. EuroIntervention. 2015;10:1230-8. doi: 10. 4244/EIJY14M10_14. ]. RADIANCE-HTN is a blinded, randomized and sham-controlled trial designed to evaluate the blood pressure lowering effect of the Paradise System in two patient populations, the first one, so called SOLO, will address subjects with essential hypertension on two or fewer antihypertensive medications, and the second one, so called TRIO, will evaluate subjects with treatment-resistant hypertension on a minimum of 3 antihypertensive medications. This trial will enroll 292 patients from up to 40 sites.
Another innnovative methodology for renal denervation is the Kona Medical Sorround Sound System providing externally delivered, completely non-invasive focused therapeutic ultrasound. Neuzil and collegues, investigated renal denervetion in an initial sample of 69 patients using the Kona System. All patients tolerated externally renal denervation well and achieved a reduction of office blood pressure by 23.8±24.1/10.3±13.1 mmHg after 1 year follow-up [5656. Neuzil P, Ormiston J, Brinton TJ, Starek Z, Esler M, Dawood O, Anderson TL, Gertner M, Whitbourne R, Schmieder RE. Externally Delivered Focused Ultrasound for Renal Denervation. JACC Cardiovasc Interv. 2016;9:1292-9. doi: 10.1016/j.jcin.2016.04. 013. Epub 2016 Jun 20. ].
Fischell and collegues recently presented a novel microneedle delivery catheter (PeregrinTM, Ablative Solutions, Inc. Menlo Park, CA) for chemical denervation using a very small amount of Ethanol (150-600 µL EtOH). The delivery of very low dose of ethanol in the adventia of the renal arteries seems to be a valid alternative tot he conventional energybased systems, with minimal injury tot he normal renal arterial wall and it could be potentially more confortable for the patients. This system has now been tested in pre-clinical studies and sussessfully in the porcine model [5757. Fischell TA, Vega F, Raju N, Johnson ET, Kent DJ, Ragland RR, Fischell DR, Almany SL, Ghazarossian VE. Ethanol-mediated perivascular renal sympathetic denervation: preclinical validation of safety and efficacy in a porcine model. EuroIntervention. 2013;9:140-7. ]. Furthermore, recently promising results at 6 months in a smal sample of 18 patients with refractory hypertension have been presented [5757. Fischell TA, Vega F, Raju N, Johnson ET, Kent DJ, Ragland RR, Fischell DR, Almany SL, Ghazarossian VE. Ethanol-mediated perivascular renal sympathetic denervation: preclinical validation of safety and efficacy in a porcine model. EuroIntervention. 2013;9:140-7. ].
Current indications for renal nerve ablation
Currently, renal nerve ablation is recommended in patients with treatment-resistant essential hypertension as defined by the recent ESC/ESH Guidelines[5959. Mahfoud F, Lüscher TF, Andersson B, Baumgartner I, Cifkova R, Dimario C, Doevendans P, Fagard R, Fajadet J, Komajda M, Lefèvre T, Lotan C, Sievert H, Volpe M, Widimsky P, Wijns W, Williams B, Windecker S, Witkowski A, Zeller T, Böhm M. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J. 2013; 34:2149-57. , 6060. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Rydén L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34: 2159-219. ], i.e., a blood pressure >140/100 mmHg despite the use of at least 3 antihypertensive drugs. Furthermore, patients must have a suitable renal anatomy, i.e., renal arteries >4 mm in diameter and >20 mm in length, and preferably only a single renal artery supplying each kidney, although this depends on the size of the arteries ( Table 3 ) [5959. Mahfoud F, Lüscher TF, Andersson B, Baumgartner I, Cifkova R, Dimario C, Doevendans P, Fagard R, Fajadet J, Komajda M, Lefèvre T, Lotan C, Sievert H, Volpe M, Widimsky P, Wijns W, Williams B, Windecker S, Witkowski A, Zeller T, Böhm M. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J. 2013; 34:2149-57. , 6161. Mahfoud F, Lüscher TF, Andersson B, Baumgartner I, Cifkova R, Dimario C, Doevendans P, Fagard R, Fajadet J, Komajda M, Lefèvre T, Lotan C, Sievert H, Volpe M, Widimsky P, Wijns W, Williams B, Windecker S, Witkowski A, Zeller T, Böhm M. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J. 2013;34:2149-57. , 6262. Moss JG, Belli AM, Coca A, Lee M, Mancia G, Peregrin JH, Redon J, Reekers JA, Tsioufis C, Vorwerk D, Schmieder RE. Executive Summary of the Joint Position Paper on Renal Denervation of the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) and the European Society of Hypertension(ESH). Cardiovasc Intervent Radiol. 2016;39:1681-1683. ]. Indeed, circumstantial evidence suggests that also somewhat smaller arteries may be suitable for ablation and that in patients with extremely difficult anatomy on one side, unilateral renal denervation might work as well.
Patient preparation
After informed consent, the patient is brought to the catheterisation laboratory and premedication is applied intravenously. As the ablation of the afferent nerves during application of the radiofrequency energy is very painful, premedication with midazolam (midazolam 2-6 mg IV) and morphine (4-10 mg IV) is mandatory. Alternatively, Remifentanil (0,025–0,2 μg/kg/min IV) might be used. The disadvantage of morphine is the long time required to take effect, as well as side effects such as nausea and vomiting, particularly with higher dosages. Ideally, an anaesthetist should assist during the procedure.
Unfractionated heparin is given intravenously at a dose aimed at reaching an activated clotting time (ACT) of > 250 seconds. The experience of most operators, however suggests that neither nitroglycerin nor calcium antagonists (e.g. verapamil 2-5 mg i.v.) are very effective as it appears that other mechanisms maintain the profound vascular contraction after the procedure [6363. Schmieder RE, Redon J, Grassi G, Kjeldsen SE, Mancia G, Narkiewicz K, Parati G, Ruilope L, van de Borne P, Tsioufis C. ESH position paper: renal denervation - an interventional therapy of resistant hypertension. J Hypertens. 2012.30:837-41 ].
Ablation-induced renal artery changes
Frequently after ablation, a characteristic notch is seen angiographically at the ablation site reflecting the oedema that formed in response to the energy and the local heating ( Figure 12 ). Two recent studies addressed renal arterial lesions after renal denervation with the Symplicity™ (Medtronic) or the EnligHTN™ (St Jude medical) system [6464. Templin C, Jaguszewski M, Ghadri JR, Sudano I, Gaehwiler R, Hellermann JP, Schoenenberger-Berzins R, Landmesser U, Erne P, Noll G, Lüscher TF. Vascular lesions induced by renal nerve ablation as assessed by optical coherence tomography: pre- and post-procedural comparison with the Simplicity catheter system and the EnligHTN multi-electrode renal denervation catheter. Eur Heart J. 2013. ;34:2141-8, 2148b. ] or the OneShot™ Renal Denervation System (Covidien, Campbell, CA, USA) [6565. Templin C, Jaguszewski M, Ghadri JR, Sudano I, Gaehwiler R, Hellermann JP, Schoenenberger-Berzins R, Landmesser U, Erne P, Noll G, Lüscher TF. Vascular lesions induced by renal nerve ablation as assessed by optical coherence tomography: pre- and post-procedural comparison with the Simplicity catheter system and the EnligHTN multi-electrode renal denervation catheter. Eur Heart J. 2013;34:2141-8, 2148b. ]. Particularly the RAPID Study showed the safety and efficacy of delivering RF energy by the OneShot Renal Denervation System for renal denervation, as demonstrated by a significant reduction in office and 24-hour ambulatory blood pressure measurement for six months (-11/-6 mmHg at six months compared to baseline (p=0.0085/p=0.037) [6666. Heeger C, Kaiser L, Brooks S, Kuck K-H, Bergmann M, New concepts for sympathetic renal artery denervation: Review of existing literature and case report. Eur Med J Interventional Cardiology. 2013;1:35-42. ]. The arterial wall was evaluated by intravascular optical coherence tomography (OCT) in both studies. This new technique allows the acquisition of detailed images at an axial resolution of 10-15 μm, enabling real-time visualisation of blood vessel wall microstructure in vivo. The first study reported data obtained in a series of 16 patients (32 renal artery) with therapy resistant hypertension [6565. Templin C, Jaguszewski M, Ghadri JR, Sudano I, Gaehwiler R, Hellermann JP, Schoenenberger-Berzins R, Landmesser U, Erne P, Noll G, Lüscher TF. Vascular lesions induced by renal nerve ablation as assessed by optical coherence tomography: pre- and post-procedural comparison with the Simplicity catheter system and the EnligHTN multi-electrode renal denervation catheter. Eur Heart J. 2013;34:2141-8, 2148b. ] and found that after renal nerve ablation vasospasm, oedema, thrombus formation and rarely arterial dissection and/or disruption occur. While the incidence of oedema was similar with the two systems, the EnligHTN™ catheter appeared to induce more commonly thrombus formation ( Figure 13 ). As platelet aggregates or even thrombi have been noted, it is recommended to pre-treat patients with oral aspirin (100 mg p.o. for several days) or to inject 250-500 mg of acetylsalicylic acid i.v. prior to the procedure. A case report [6767. Verheye S, Ormiston J, Bergmann MW, Sievert H, Schwindt A, Werner N, Vogel B, Colombo A. Twelve-month results of the rapid renal sympathetic denervation for resistant hypertension using the OneShotTM ablation system (RAPID) study. EuroIntervention. 2015;10:1221-9. doi: 10. 4244/EIJY14M12_02. ] suggested that the irrigated balloon of the OneShot™ renal denervation system may induce less endothelial damage after renal nerve ablation, but this needs to be confirmed in larger series. Moreover, the clinical significance of such renal artery lesions in terms of effective destruction of renal nerves and long-term safety is not known and it needs to be explored whether they are reversible and if the patients would benefit from an antithrombotic therapy after renal nerve ablation. Obviously, the number of patients studied is too small to draw any final conclusions at this point, and large registries will be required to further document the safety of this procedure.
Procedural complications
Complications of the procedure can rarely lead to renal artery dissection. In a series of around 100 patients in the HTN-1 [6060. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Rydén L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34: 2159-219. ] and HTN-2 [6262. Moss JG, Belli AM, Coca A, Lee M, Mancia G, Peregrin JH, Redon J, Reekers JA, Tsioufis C, Vorwerk D, Schmieder RE. Executive Summary of the Joint Position Paper on Renal Denervation of the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) and the European Society of Hypertension(ESH). Cardiovasc Intervent Radiol. 2016;39:1681-1683. ], renal artery dissection occurred in 1 patient, and femoral artery pseudoaneurysms were observed in 2 patients. However, intravascular imaging using OCT was not applied, therefore the true incidence of local vascular lesions may be underdiagnosed. With the current use of a 6 F system, both complications should further decrease in frequency.
Although solid data are missing, ablation-induced stenosis appears to be a very rare complication. In current registries and trials renal artery stenosis was assessed in most, but not all patients using either angiography follow-up or renal Duplex studies, and so far was not observed in the several hundred patients treated with an observation period of up to 24 months [6868. Krum H, Barman N, Schlaich M, Sobotka P, Esler M, Mahfoud F. Long-term Follow-up of Catheter-based Renal Denervation For Resistant Hypertension Confirms Durable Blood Pressure Reduction. J Am Coll Cardiol. 2012. 59:E1704. ]. However, a few cases of ablation-induced stenosis have been reported recently [6969. Kaltenbach B, Id D, Franke JC, Sievert H, Hennersdorf M, Maier J, Bertog SC. Renal artery stenosis after renal sympathetic denervation. J Am Coll Cardiol, 2012;60:2694-5. ]. As systematic Duplex examinations have not been performed in all patients, the true incidence is unknown. As with any interventional procedure, bleeding at the femoral puncture site is a common complication.
Other long-term complications could potentially involve persistent hypotension and orthostatic hypotension. Fortunately however, symptomatic hypotension, and in particular orthostatic hypotension, does not seem to occur after renal nerve ablation, at least in the series of currently treated patients with resistant hypertension in which antihypertensive drugs are commonly withdrawn in a stepwise manner when blood pressure falls.
Effects of renal nerve ablation on blood pressure
Registries
In the long-term follow-up of the patients included in the Symplicity HTN-1 registry, renal nerve ablation led to a long-lasting decrease in blood pressure in the range of 31/16 mmHg up to 36 months after the intervention [6565. Templin C, Jaguszewski M, Ghadri JR, Sudano I, Gaehwiler R, Hellermann JP, Schoenenberger-Berzins R, Landmesser U, Erne P, Noll G, Lüscher TF. Vascular lesions induced by renal nerve ablation as assessed by optical coherence tomography: pre- and post-procedural comparison with the Simplicity catheter system and the EnligHTN multi-electrode renal denervation catheter. Eur Heart J. 2013;34:2141-8, 2148b. , 6868. Krum H, Barman N, Schlaich M, Sobotka P, Esler M, Mahfoud F. Long-term Follow-up of Catheter-based Renal Denervation For Resistant Hypertension Confirms Durable Blood Pressure Reduction. J Am Coll Cardiol. 2012. 59:E1704. ]. Of note, the initial decrease in blood pressure immediately after the procedure is small, suggesting that partial denervation of the kidneys leads to a slowly developing re-setting of the neurohumoral control of water, salt excretion and, in turn, blood pressure in the kidney. In our own series of 40 patients treated mainly with the Symplicity catheter and partially with the St. Jude basket we were able to reproduce these findings [7070. Sudano I, Pourmand A, Shoenenberger-Berzins R, Suter PM, Roas S, Templin C, Landmesser U, Erne P, Luscher TF. Klinische Effekte der katheter-basierten Nierennervenablation bei therapieresistenter Hypertonie. Cardiovascular Medicine, 2014 17:8-13. ].
Meanwhile other systems were successfully used and the published results confirmed the safety and efficacy of renal denervation in lowering office blood pressure. Renal artery denervation, using the EnligHTN™ system similarly and significantly reduced office blood pressure from baseline up to 1, 3, and 6 months by -28/10, -27/10 and -26/10 mmHg, respectively [7171. Papademetriou V, Tsiofis C, Worthley S, Worthley M, Sinhal A, Chew D, Meredith I, Malaiapan Y. EnligHTN (TM) I, First-in-Man Multi-Center Study of a Novel Multi-Electrode Renal Denervation Catheter in Patients With Drug-Resistant Hypertension. Circulation. 2012;126:2788-2788. , 7272. Worthley SG, Tsioufis CP, Worthley MI, Sinhal A, Chew DP, Meredith IT, Malaiapan Y, Papademetriou V. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013;34:2132-40. ]. It appeared that this fall in blood pressure was reached faster, i.e. already at 3 months with the St. Jude compared to the Simplicity system. Furthermore, a mean reduction of around 30 mmHg systolic office blood pressure was reported in a small series of patients with therapy-resistant hypertension treated with the ballon-based OneShot™ Renal Denervation System (formerly Maya Medical now Covidien, Campbell, CA, USA) [7373. Ormiston JA, Watson T, van Pelt N, Stewart R, Haworth P, Stewart JT, Webster MW. First-in-human use of the OneShot renal denervation system from Covidien. EuroIntervention, 2013. 8: 1090-4. , 7474. Ormiston JA, Watson T, van Pelt N, Stewart R, Stewart JT, White JM, Doughty RN, Stewart F, Macdonald R, Webster MW. Renal denervation for resistant hypertension using an irrigated radiofrequency balloon: 12-month results from the Renal Hypertension Ablation System (RHAS) trial. EuroIntervention. 2013;9:70-4. ].
The REDUCE-HTN clinical program is evaluating the ability of the balloon-based Vessix™ V2 renal denervation system (now Boston Scientific Corporation) to reduce blood pressure in patients with therapy-resistant hypertension. The initial results were presented at the EuroPCR Meeting 2014. At 6 months, patients experienced a significant mean reduction in blood pressure of 24.5/10.3 mmHg (n=143). Long-term efficacy was demonstrated with a sustained 30.2/12.7 mmHg mean reduction in systolic/diastolic blood pressure in the subset of patients (n=51) for whom 18-month data were available [7575. Sievert H, Schofer J, Ormiston J, Hope UC, Meredith IT, Walters DL, Azizi M, Diaz-Cartelle J, Cohen-Mazor M. Renal denervation with a percutaneous bipolar radiofrequency balloon catheter in patients with hypertension: 6-month results from the REDUCE-HTN clinical study. Eurointervention 2015;10:1213-20. ].
A recent metanalysis [7676. Davis MI, Filion KB, Zhang D, Eisenberg MJ, Afilalo J, Schiffrin EL, Joyal D. Effectiveness of Renal Denervation Therapy for Resistant Hypertension: A Systematic Review and Meta-Analysis. J Am Coll Cardiol, 2013;62: 231-41. ] showed that 6-month after renal denervation the reduction in office blood pressure was similar (i.e. around 30 mmHg systolic and around 10 mmHg diastolic) irrespective of study design and catheter employed.The correlation between blood pressure and target organ damage, cardiovascular risk, and long-term prognosis is much greater for ABPM than office measurements. While an initial small series of 8 patients published in Hypertension by the group of Kjeldsen [7777. Fadl Elmula FE, Hoffmann P, Fossum E, Brekke M, Gjønnæss E, Hjørnholm U, Kjær VN, Rostrup M, Kjeldsen SE, Os I, Stenehjem AE, Høieggen A. Renal sympathetic denervation in patients with treatment-resistant hypertension after witnessed intake of medication before qualifying ambulatory blood pressure. Hypertension. 2013;62:526-32. ] claimed a lack of effect of renal denervation on 24h blood pressure, a very recent paper by Mahfoud et al. as well as a smaller study by our own group [7070. Sudano I, Pourmand A, Shoenenberger-Berzins R, Suter PM, Roas S, Templin C, Landmesser U, Erne P, Luscher TF. Klinische Effekte der katheter-basierten Nierennervenablation bei therapieresistenter Hypertonie. Cardiovascular Medicine, 2014 17:8-13. ] demonstrated that after renal denervation not only office blood pressure but also ambulatory blood pressure is significantly reduced. In 346 patients with resistant hypertension recruited in the Symplicity HTN-I and HTN-II a significant reduction in 24-hour systolic and diastolic blood pressure (SBP −10.1/−10.2/−11.7 mmHg; DBP −4.8/−4.9/−7.4 mmHg) was observed 3, 6, and 12 months after renal denervation. The changes were similar during daytime and night-time [7878. Mahfoud F, Ukena C, Schmieder RE, Cremers B, Rump LC, Vonend O, Weil J, Schmidt M, Hoppe UC, Zeller T, Bauer A, Ott C, Blessing E, Sobotka PA, Krum H, Schlaich M, Esler M, Böhm M. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128:132-40. ]. Moreover, a subanalysis of 54 patients with moderate treatment resistant hypertension published by Ott and colleagues showed as renal denervation reduced office and 24-h ambulatory blood pressure at 6-month follow up [7979. Ott C, Mahfoud F, Schmid A, Ditting T, Sobotka PA, Veelken R, Spies A, Ukena C, Laufs U, Uder M, Böhm M, Schmieder RE. Renal denervation in moderate treatment resistant hypertension. J Am Coll Cardiol. 2013;62:1880-6. ] as did our own series of 40 patients [7070. Sudano I, Pourmand A, Shoenenberger-Berzins R, Suter PM, Roas S, Templin C, Landmesser U, Erne P, Luscher TF. Klinische Effekte der katheter-basierten Nierennervenablation bei therapieresistenter Hypertonie. Cardiovascular Medicine, 2014 17:8-13. ].
Furthermore, an increasing number of smaller case series are available in the literature showing that the technique reduces blood pressure in a „real word experience“ as much as shown in clinical studies [8080. Schlaich MP, Schmieder RE, Bakris G, Blankestijn PJ, Böhm M, Campese VM, Francis DP, Grassi G, Hering D, Katholi R, Kjeldsen S, Krum H, Mahfoud F, Mancia G, Messerli FH, Narkiewicz K, Parati G, Rocha-Singh KJ, Ruilope LM, Rump LC, Sica DA, Sobotka PA, Tsioufis C, Vonend O, Weber MA, Williams B, Zeller T, Esler MD. International expert consensus statement: Percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol. 2013;2:2031-45. ]. Moreover, a significant blood pressure reduction was found in truly resistant hypertension patients [7878. Mahfoud F, Ukena C, Schmieder RE, Cremers B, Rump LC, Vonend O, Weil J, Schmidt M, Hoppe UC, Zeller T, Bauer A, Ott C, Blessing E, Sobotka PA, Krum H, Schlaich M, Esler M, Böhm M. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128:132-40. ] as well as in moderate hypertensives [7979. Ott C, Mahfoud F, Schmid A, Ditting T, Sobotka PA, Veelken R, Spies A, Ukena C, Laufs U, Uder M, Böhm M, Schmieder RE. Renal denervation in moderate treatment resistant hypertension. J Am Coll Cardiol. 2013;62:1880-6. ].
The Global SIMPLICITY Registry (NCT01534299) is a prospective, open-label, multicenter registry accounting for 998 patients, of which 323 with severe hypertension. The study aimed to assess the safety and the efficacy of renal denervation using the Simplicity system in patients with uncontrolled hypertension and showed a statistical significant decrease in office and 24-hours systolic blood pressure for all patients (-11.6±25.3 and −6.6±18.0 mm Hg, p<0.001 for both), particularly for those with severe hypertension (−20.3±22.8 and −8.9±16.9 mm Hg for those with severe hypertension (P<0.001 for both). In the real world patients renal denervation seems to lead to a significant reduction in office and 24-hour BPs with a favorable safety profile, with better results particularly for those with with higher baseline pressures [8181. Böhm M, Ukena C, Ewen S, Linz D, Zivanovic I, Hoppe U, Narkiewicz K, Ruilope L, Schlaich M, Negoita M, Schmieder R, Williams B, Zeymer U, Zirlik A, Mancia G, Mahfoud F; Global SYMPLICITY Registry Investigators. Renal denervation reduces office and ambulatory heart rate in patients with uncontrolled hypertension: 12-month outcomes from the global SYMPLICITY registry. J Hypertens. 2016;34:2480-2486. ]. Subjects with severe resistant hypertension seem to benefit more than the others from renal denervation. Desch and collegues, indeed, randomized 71 patients with resitant hypertension with only mildly elevated blood pressure (day-time systolic pressure, 135–149 and diastolic pressure, 90–94 mm Hg on 24-hour ambulatory measurement) 1:1 to to renal sympathetic denervation with the Symplicity Flex Catheter (Medtronic) or to an invasive sham procedure. In this specific population the renal denervation failed to show a significant reduction of 24-systolic blood pressure at 6 months [8282. Desch S, Okon T, Heinemann D, Kulle K, Röhnert K, Sonnabend M, Petzold M, Müller U, Schuler G, Eitel I, Thiele H, Lurz P. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension. 2015;65:1202-8. doi: 10.1161/HYPERTENSIONAHA. 115. 05283. ].
Mahfoud and collegues recently analyzed the data from 1103 patients from the SYMPLICIY HTN-3 and the Global SYMPLICITY Registry, comparing baseline characterisctics and systolic blood pressure changes at 6 months after renal denervation between patients with isolated systolic hypertension and combined systolic-diastolic hypertension. The patients with isolated systolic hypertension were generally more diabetic, significantly older and with lower estimated glomerular filtration rate in comparison to combined systolic-diastolic hypertensive patients. The reduction of blood pressure was less pronounced among patients with isolated systolic blood pressure than among combined systo-diastolic hypertensive patients (systolic 24h-blood pressure -8.8±16.2 mmHg vs -5.8±15.4 mmHg, -3.0 mmHg, CI -5.4, -0.6, p=0.015) [8383. Mahfoud F, Bakris G, Bhatt DL, Esler M, Ewen S, Fahy M, Kandzari D, Kario K, Mancia G, Weber M, Böhm M. Reduced blood pressure-lowering effect of catheter-based renal denervation in patients with isolated systolic hypertension: data from SYMPLICITY HTN-3 and the Global SYMPLICITY Registry. Eur Heart J. 2016 Jul 28. pii: ehw325.
[Epub ahead of print]]. A further effect of renal denervation is the reduction of sympathetic activity that leads to a decrease in heart rate, which mainly depends on baseline hear rate. This effect seems tob e durable up to one year and unchanged by β-blocker therapy, and could represent a target for RDN in patients with elevated heart rate at the baseline [8181. Böhm M, Ukena C, Ewen S, Linz D, Zivanovic I, Hoppe U, Narkiewicz K, Ruilope L, Schlaich M, Negoita M, Schmieder R, Williams B, Zeymer U, Zirlik A, Mancia G, Mahfoud F; Global SYMPLICITY Registry Investigators. Renal denervation reduces office and ambulatory heart rate in patients with uncontrolled hypertension: 12-month outcomes from the global SYMPLICITY registry. J Hypertens. 2016;34:2480-2486. ].
The same authors reported also the 3-years outcome from the Global Symplicity Registry, showing that a significant blood pressure reduction after renal denervation is sustained to 3 years, with constantly low long-term incidence of adverse events and decline of eGFR within the expected range [8484. Mahfoud F, Brilakis N, Böhm M, Narkiewicz K, Ruilope L, Schlaich M, Mancia G. TCT-761 Long-term (3-year) safety and effectiveness from the Global SYMPLICITY Registry of renal denervation in a real world patient population with uncontrolled hypertension. J Am Coll Cardiol. 2016;68(18S):B308. doi: 10. 1016/j. jacc. 2016. 09.791 ]. No changes on heart failure biomarkers such as NT-proBNP, ST-2, galectin-3 and hs-TnI was noted 6 months after renal denervation [8585. Neumann JT, Ewen S, Mortensen K, Nef H, Zeller T, Ojeda F, Sydow K, Mahfoud F, Böhm M, Hamm C, Dörr O, Blankenberg S. Effects of renal denervation on heart failure biomarkers and blood pressure in patients with resistant hypertension. Biomark Med. 2016;10:841-51. doi: 10. 2217/bmm-2016-0098. ].
Obstructive sleep apnea is very frequent condition in patients with hypertension and seems to contribute to the progression to resistant hypertension. A further analysis performed by Linz and collegues from the Global SYMPLICITY Registry showed that renal denervation leads to a significant reduction in blood pressure at 6 months in hypertensive patients with and without obstrucitve sleep apnea syndrome, treatedor not with continuous positive airway pressure usage [8686. Linz D, Mancia G, Mahfoud F, Narkiewicz K, Ruilope L, Schlaich M, Kindermann I, Schmieder RE, Ewen S, Williams B, Böhm M; Global SYMPLICITY Registry Investigators. Renal artery denervation for treatment of patients with self-reported obstructive sleep apnea and resistant hypertension: results from the Global SYMPLICITY Registry. J Hypertens. 2017;35:148-153. ].
The renal denervation seems to have also pleiotropic effects by interruption of sympathetic nerve activity. In this setting Ott anc collegues showed in a pool of 40 patients that fasting plasma glucose and HbA1c level was significantly reduced 6 month after renal denervation (both p < 0.01), with concomitant increasead levels of C-peptide and insulin after glucagon injection (both p < 0.05) [8787. Ott C, Kistner I, Schmid A, Friedrich S, Ditting T, Veelken R, Mahfoud F, Böhm M, Uder M, Schmieder R. Secretory capacity of pancreatic beta cells is enhanced 6 months after renal denervation in treatment resistant hypertensive patients. J Hypertens. 2016;34 Suppl 1 - ISH 2016 :e251. ].
Randomized Trials
In the Symplicity-HTN-2, Esler and collegues showed in a multicentre, prospective, randomised trial, that catheter-based renal denervation was safe and substantially reduced blood pressure in treatment-resistant hypertensive patients. In this trail authors enrolled 106 patients who had a baseline systolic blood pressure of 160 mm Hg or more (≥150 mm Hg for patients with type 2 diabetes), despite taking three or more antihypertensive drugs, and randomly assigned to renal denervation with previous treatment (three or more antihypertensive drugs) or to maintain previous treatment alone (control group) at 24 participating centres. Of these 106 patients, 52 were allocated to renal denervation and 54 to control with previous antihypertensive treatment. Office-based blood pressure measurements in the renal denervation group was reduced by 32/12 mm Hg (SD 23/11, baseline of 178/96 mm Hg, p<0.0001), whereas they did not differ from baseline in the control group (change of 1/0 mm Hg [8787. Ott C, Kistner I, Schmid A, Friedrich S, Ditting T, Veelken R, Mahfoud F, Böhm M, Uder M, Schmieder R. Secretory capacity of pancreatic beta cells is enhanced 6 months after renal denervation in treatment resistant hypertensive patients. J Hypertens. 2016;34 Suppl 1 - ISH 2016 :e251. ], baseline of 178/97 mm Hg, p=0.77 systolic and p=0.83 diastolic). At 6 months, 41 (84%) of 49 patients who underwent renal denervation had a reduction in systolic blood pressure of 10 mm Hg or more, compared with 18 (35%) of 51 controls (p<0.0001). No serious procedure-related or device-related complications and occurrence of adverse events presented a difference between groups [8888. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903-9. doi: 10. 1016/S0140-6736(10)62039-9. Epub 2010 Nov 17. ].
In 2015, the results of the first sham-controlled SYMPLICITY HTN-3 trial were published [8989. Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393-401. ]. SYMPLICITY HTN-3 included patients with a systolic blood pressure ≥160 mmHg in spite of 3 antihypertensive drugs including a diuretic at maximally tolerated dosages. A total of 1441 patients were assessed for eligibility, whereof 535 patients (37%) from 88 sites in the USA were enrolled. After initial screening, patients were observed for 2 weeks and eventually randomised to renal denervation using the Symplicity Flex catheter (Medtronic, MN, USA) or renal angiography only (sham control). Patients were unaware of group allocation. There were few side effects in the trial, amounting to 1.4% with an upper boundary of the one-sided 95% confidence interval of 2.9% in the treatment group and 0.6% in the sham group, with a difference of 0.8% (P=0.67). The primary efficacy endpoint was not statistically different between the groups; with a reduction of office BP by 14.1 ± 24 mmHg in the renal denervation arm and 11.7 ± 26 mmHg in the control arm (P=0.255). The pre-specified superiority margin was 5 mmHg. Similar results were observed in the secondary endpoint, which was a 24-h ambulatory systolic BP change from baseline to 6 months between the two groups (P=0.979). Moreover the same group performed a cross-over analysis on the 12-month SYMPLICITY HTN-3 results randomizing the elegible subjects 2:1 to denervation (cross-over group) or sham procedure (non-cross-over group) after the 6-month endpoint. Subjects were unblinded to their treatment group and change in the blood pressure at 1 year was analyzed. Unfortunately also these data did not support a further reduction in BP, both in office or ambulatory measurements, after 1 follow-up. Particularly even if in the denervation group the 12-months office systolic BP decreased more than that observed at 6 month (-15.5±24.1 mmHg vs -18.9±25.4 mmHg, p=0.025), these results did not obtained a statistical significancy in the 24-BP changes (p=0.229). The adherence to medication, finally, seems to play a fundamental role in mantaining BP reduction through the time as the non-crossover group analysis showed (p=0.01) [9090. Bakris GL, Townsend RR, Flack JM, Brar S, Cohen SA, D’Agostino R, Kandzari DE, Katzen BT, Leon MB, Mauri L, Negoita M, O’Neill WW, Oparil S, Rocha-Singh K, Bhatt DL; SYMPLICITY HTN-3 Investigators. 12-month blood pressure results of catheter-based renal artery denervation for resistant hypertension: the SYMPLICITY HTN-3 trial. J Am Coll Cardiol. 2015;65:1314-21. doi: 10. 1016/j. jacc. 2015. 01.037 ].
Of note, Kandzari and collegues showed in a post-hoc analysis oft he SYMPLICITY HTN Trial that a higher number of renal artery ablations leads to a significant and greater reduction in office and ambulatory blood pressure and heart rate. Particularly once control patients and renal denervation patients were propensity score matched according to baseline characteristics, the seconds presented significantly lower levels of office (P value for trend 0.01) and ambulatory SBP and heart rate (P value for trend <0.01) increased with increasing numbers of ablations delivered (if more than 10). Moreover the increase number of ablations did not lead to an increase in safety events (no MAEs occurred in patients receiving ≥13 ablations) [9191. Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, Flack JM, Katzen BT, Lea J, Lee DP, Leon MB, Ma A, Massaro J, Mauri L, Oparil S, O’Neill WW, Patel MR, Rocha-Singh K, Sobotka PA, Svetkey L, Townsend RR, Bakris GL. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial.Eur Heart J. 2015;36:219-27. doi: 10. 1093/eurheartj/ehu441. Epub 2014 Nov 16. ]. The diameter of renal arteries correlates with systolic blood pressure changes after renal denervation at 6 months, while lenght oft he renal arteries, presence of accessory renal arteries and renal arteries disease seem to play no relevant role in affecting systolic blood pressure. Interesting is the fact that the numbers of ablations delivered by a mono-electrode cathteter produced the same results [9292. Ewen S, Ukena C, Lüscher TF, Bergmann M, Blankestijn PJ, Blessing E, Cremers B, Dörr O, Hering D, Kaiser L, Nef H, Noory E, Schlaich M, Sharif F, Sudano I, Vogel B, Voskuil M, Zeller T, Tzafriri AR, Edelman ER, Lauder L, Scheller B, Böhm M, Mahfoud F. Anatomical and procedural determinants of catheter-based renal denervation. Cardiovasc Revasc Med. 2016;17:474-479. doi: 10.1016/j.carrev.2016.08. 004. Epub 2016 Aug 20. ].
There was widespread surprise and disappointment in the scientific community due to the positive results of the previously published HTN-1 registry and the HTN-2 randomized tiral, with their respective follow-up and several open label registries from around the world ( Figure 13 ). These studies have an increasingly more pronounced effect over time. The 36-month results from the SYMPLCITY HTN-2 Trial confirmed a sustained lowering of blood pressure after 3 years for both systolic and diastolic levels in a selected population of 40 subjects with severe, treatment resistant hypertension (for the initial renal denervation group -33 mmHg (p = 0.01) -14 mmHg (p=0.01) [9393. Esler MD, Böhm M, Sievert H, Rump CL, Schmieder RE, Krum H, Mahfoud F, Schlaich MP. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur Heart J. 2014;35:1752-9. doi: 10. 1093/eurheartj/ehu209. Epub 2014 Jun 4. ]. Several possibilities why SYMPLICITY HTN-3 did not confirm the results of previous published registries and randomised trials have been suggested:
- Renal nerve ablation using radiofrequency ablation may simply not work in humans, in contrast to animals.
- The statistical power of SYMPLICITY HTN-3 may not suffice to show differences between usual medical care and radiofrequency ablation, as the sample size calculation was based on previous studies with a potentially overestimated treatment effect. However, as HTN-3 enrolled 535 patients this is an unlikely explanation.
- Antihypertensive drugs have been maximized, but the patients may not have been stabilized appropriately before randomisation and indeed 40% of patients changed medication during the study period. This is a major concern because the patients were not titrated to a consistant therapeutic regimen in terms of drugs and wash-out period.
- The patient population may differ from Caucasians recruited in previous trials, mainly performed in centres of excellence in Europe and Australia as in the subgroup of Caucasian patients the primary efficacy endpoint was met, unlike in Afroamericans who may have a different, more volume-dependent rather than sympathetically driven hypertension.
- The results may just reflect the play of chance.
- The procedures may not have been performed effectively. It is notable that the 535 patients were recruited in 88 centres. Overall, 364 renal denervation procedures were performed by 111 operators. Thus, on average, operators performed around three procedures in the trial without previous experience, some even less. As the procedure is not approved in the USA and no roll-in phase was introduced, one has to assume that the learning curve of the operators fell within the period of the trial. This is especially important as there is still no test available allowing assessment of proper wall contact and effective destruction of renal nerves during or after the procedure. Notably, there was a significant correlation between number of ablation attempts, and blood pressure reduction as reported by Kanzari et al. (see above) [9494. Kandzari DE, Bhatt DL, Sobotka PA, O’Neill WW, Esler M, Flack JM, Katzen BT, Leon MB, Massaro JM, Negoita M, Oparil S, Rocha-Singh K, Straley C, Townsend RR, Bakris G. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol, 2012;35: 528-35. ].
A post-hoc analysis identified predictors of systolic blood pressure change in the subjects of the SIMPLICITY HTN-3, particularly severe baseline systolic hypertension (SBP > 180 mmHg), aldosterone antagonist use, non-use of vasodilators and, in the denervation group, the number of ablation. Moreover the delivery of ablation in a four quadrant-pattern and the number of ablations lead to greater reduction of office and ambulatory SBP and heart rate in this population [9595. Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, Flack JM, Katzen BT, Lea J, Lee DP, Leon MB, Ma A, Massaro J, Mauri L, Oparil S, O’Neill WW, Patel MR, Rocha-Singh K, Sobotka PA, Svetkey L, Townsend RR, Bakris GL. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015;36:219-27. doi: 10. 1093/eurheartj/ehu441. ].
Recently, the distribution and density of the renal sympathetic nervous system in human has been assessed, showing that although most of the nerves are located in the proximal and middle tract of the renal arteries’ wall, the nerves are less concentrated, but much closer to the lumen of the renal artery in its distal part (Figure 14). These data suggest that an effective renal denervation could be achieve only with more distal renal artery targeting of leasion formation, thereby allowing for a more effective full four-qadrant ablation on both renal arteries [9696. Mahfoud F, Lüscher TF. Renal denervation: symply trapped by complexity?. Eur Heart J. 2015 ;36:199-202. doi: 10. 1093/eurheartj/ehu450. Epub 2014 Nov 16. ]. In contrast, the application of radiofrequency energy to a part of the renal arteries where the nerves are at greatest distance from the arterial wall – as was common practice initially - produces suboptimal denervation that could lead to an unsatisfactory result as reported by Esler [9797. Esler M. Renal denervation: Not as easy as it looks. Sci Transl Med. 2015;7:285fs18. doi: 10. 1126/scitranslmed. aaa5457. ].
The Renal Denervation for Hypertension (DENERHTN) is a more recent prospective, randomized, open-label controlled-trial with blinded end-point evalutation conducted in 15 French centers specialized in hypertension management. This trial included 106 patients randomly assigned to a standardised stepped-care antihyperntensive treatment (SSAHT) plus renale denervation, after 4 weeks titration with indapamide 1-5 mg, ramipril 10 mg/irbesartan 300 mg and amlodipin 10 mg to confirm treatment resistence, versus standardised step-care antihypertensive treatment alone with progressive addition of spironolactone 25 mg, biosoprolol 10 mg, prazosin 5 mg and rimelnidine 1 mg pro day in the follow-up period if the home blood pressure was superior to 135/85 mmHg. Renal denervation plus SSAHT decresed singnificantly the ambulatory blood pressure at 6 months more than SSAHT alone (−15.8 mm Hg in the renal denervation group and −9.9 mm Hg in the SSAHT group, p=0.0329), and this can lead to a reduction in cardiovascular morbidity if mantained in the long term after renal denervation, but obviously further studies are needed [9898. Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, Midulla M, Mounier-Véhier C, Courand PY, Lantelme P, Denolle T, Dourmap-Collas C, Trillaud H, Pereira H, Plouin PF, Chatellier G; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957-65. doi: 10. 1016/S0140-6736(14)61942-5. Epub 2015 Jan 26. ].
Rosa and collegues investigated in a randomized, multicenter study the effect of spironolactone addition versus renal denervation in a cohort of 102 patients with true resistant hypertension. The study showed that over a period of 12 months the renal denervation is safe but not superior to intensified pharmacological treatment in the settings of true resistant hypertensive patients with confirmed compliance, and even that spironolactone’s addition (if tolerated) seems to be more effective in blood pressure control [9999. Rosa J, Widimský P, Waldauf P, Lambert L, Zelinka T, Táborský M, Branny M, Toušek P, Petrák O, Čurila K, Bednář F, Holaj R, Štrauch B, Václavík J, Nykl I, Krátká Z, Kociánová E, Jiravský O, Rappová G, Indra T, Widimský J Jr. Role of Adding Spironolactone and Renal Denervation in True Resistant Hypertension: One-Year Outcomes of Randomized PRAGUE-15Study.Hypertension.2016;67:397-403. doi:10.1161/HYPERTENSIONAHA.115. 06526. Epub 2015 Dec 22. ]. The same authors assessed also that spironolactone’s addition (if tolerated) seems to be of better and more efficent than renal denervation in blood pressure reduction over a period of 24 months, but, by contrast to the 12-month results, blood pressure changes were not significantly greater [100100. Rosa J, Widimský P, Waldauf P, Zelinka T, Petrák O, Táborský M, Branny M, Toušek P, Čurila K, Lambert L, Bednář F, Holaj R, Štrauch B, Václavík J, Kociánová E, Nykl I, Jiravský O, Rappová G, Indra T, Krátká Z, Widimský J Jr. Renal denervation in comparison with intensified pharmacotherapy in true resistant hypertension: 2-year outcomes of randomized PRAGUE-15 study. J Hypertens. 2017 Jan 23. doi: 10. 1097/HJH. 0000000000001257. ].
Renal denervation seems to be effective also in patients who are not on blood pressure lowering therapy for intolerance or documented allergy to medications or reluctance to take antihypertensive drugs [101101. De Jager RL, Sanders MF, Bots ML, Lobo MD, Ewen S, Beeftink MM, Böhm M, Daemen J, Dörr O, Hering D, Mahfoud F, Nef H, Ott C, Saxena M, Schmieder RE, Schlaich MP, Spiering W, Tonino PA, Verloop WL, Vink EE, Vonken EJ, Voskuil M, Worthley SG, Blankestijn PJ. Renal denervation in hypertensive patients not on blood pressure lowering drugs. Clin Res Cardiol. 2016;105:755-62. doi: 10. 1007/s00392-016-0984-y. Epub 2016 Apr 22. ]. In a small pilot study, 53 unmedicated hypertensive patients who were off therapy due to intolerance, non-compliance or research reasons, the mean change in 24h SBP at 6 months was -5.7 mmHg [95 % confidence interval (CI) -11.0 to -0.4; p = 0.04] while the mean change in office SBP was -13.1 mmHg (95 % CI -20.4 to -5.7; p = 0.001). These data suggest that at least in some subjects renal denervation lowers blood pressure independently from concomitant antihypertrensive medication.
Many other studies are ongoing: the RAPID Study (ClinicalTrials.gov Identifier: NCT01520506) with the OneShot™ Renal Denervation System (by Maya Medical now part of Covidien), the REALISE (NCT01529372) and ACHIEVE (NCT01789918) Study with the Paradise™ Renal Denervation System (ReCor Medical, Ronkonkoma, NY, USA), the EnligHTN II and III (NCT01705080 and NCT01836146) with the EnligHTN™ Multi Electrode Renal Denervation System (St. Jude Medical), a study with the Symplicity Spyral™ Renal Denervation System (Medtronic), with the Cordis renal denervation Catheter (Cordis and Biosense Webster, two subsidiaries of Johnson&Johnson) as well as the follow-up of the REDUCE-HTN Study (NCT01541865) with the V2 Renal Denervation System™ (Vessix Vascular, Boston Scientific).
Innovative and refined clinical trials should be conducted to investigate further the potential of renal nerve ablation in distinct hypertension populations. Larger outcome trials are required to establish the clinical relevance of renal nerve ablation for patients with resistant hypertension and particularly attention should be payd to the elegibility of patients for renal denervation. The ENCOReD study showed that only 40% of patients referred for renal denervation, mostly by specialists, were really elegible for the procedure. Most of the time, a treatment adjustment by a hypertension specialist is sufficent to reach blood pressure normalization [102102. Persu A, Jin Y, Baelen M, Vink E, Verloop WL, Schmidt B, Blicher MK, Severino F, Wuerzner G, Taylor A, Pechère-Bertschi A, Jokhaji F, Fadl Elmula FE, Rosa J, Czarnecka D, Ehret G, Kahan T, Renkin J, Widimsky J Jr, Jacobs L, Spiering W, Burnier M, Mark PB, Menne J, Olsen MH, Blankestijn PJ, Kjeldsen S, Bots ML, Staessen JA; European Network Coordinating research on REnal Denervation Consortium. Eligibility for renal denervation: experience at 11 European expert centers. Hypertension. 2014 Jun;63(6):1319-25. doi: 10.1161/HYPERTENSIONAHA.114. 03194. Epub 2014 Mar 24. ]. However, persistant blood pressure lowering by renal denervation may have the advantage to provide blood pressure control independently from patient's compliance and may reduce the number of tablets required to reach target levels.
The question remains whether renal denervation should be tested in a large outcome trial. However, even the Federal Drug Administration in the United States accepts blood pressure as a surrogate endpoint for the registration of antihypertensive drugs. Furthermore, a close correlation exists between blood pressure lowering and the reduction of cardiovascular events during pharmacological treatment of hypertension [6565. Templin C, Jaguszewski M, Ghadri JR, Sudano I, Gaehwiler R, Hellermann JP, Schoenenberger-Berzins R, Landmesser U, Erne P, Noll G, Lüscher TF. Vascular lesions induced by renal nerve ablation as assessed by optical coherence tomography: pre- and post-procedural comparison with the Simplicity catheter system and the EnligHTN multi-electrode renal denervation catheter. Eur Heart J. 2013;34:2141-8, 2148b. ]. Moreover blood pressure change after renal denervation is highly variable, and subjects can be divided in hyperresponders and no-respoenders, but this is mainly related to the relevation of office blood pressure rather than to ambulatory blood pressure [103103. Persu A, Azizi M, Jin Y, Volz S, Rosa J, Fadl Elmula FE, Pechere-Bertschi A, Burnier M, Mark PB, Elvan A, Renkin J, Sapoval M, Kahan T, Kjeldsen S, Staessen JA; European Network COordinating research on Renal Denervation (ENCOReD) consortium. Hyperresponders vs. nonresponder patients after renal denervation: do they differ?. J Hypertens. 2014;32:2422-7; discussion 2427. doi: 10. 1097/HJH. 0000000000000347. ]. According to this issue, recently, a European consensus conference for renal denervation suggested strongly the ambulatory blood pressure as primary measure of response to renal denervation [104104. Mahfoud F, Böhm M, Azizi M, Pathak A, Durand Zaleski I, Ewen S, Tsioufis K, Andersson B, Blankestijn PJ, Burnier M, Chatellier G, Gafoor S, Grassi G, Joner M, Kjeldsen SE, Lüscher TF, Lobo MD, Lotan C, Parati G, Redon J, Ruilope L, Sudano I, Ukena C, van Leeuwen E, Volpe M, Windecker S, Witkowski A, Wijns W, Zeller T, Schmieder RE. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. EUR Heart J. 2015;36:2219-27. doi: 10. 1093/eurheartj/ehv192. Epub 2015 May 18. ]. Thus, in summary, although a large outcome trial would be desirable, more consistant evidence is required at this point how renal denervation is best performed and in which patients.
Effects on renal function
Renal function remains unchanged in most patients after renal denervation despite the profound lowering of perfusion pressure [4949. Sakakura K, Roth A, Ladich E, Shen K, Coleman L, Joner M, Virmani R. Controlled circumferential renal sympathetic denervation with preservation of the renal arterial wall using intraluminal ultrasound: a next-generation approach for treating sympathetic overactivity. EuroIntervention. 2015;10:1230-8. doi: 10. 4244/EIJY14M10_14. ]. Particularly, results from two small studies suggest that renal denervation might be efficient and safe in patients with moderate to severe chronic kidney disease [105105. Hering, D, Esler MD, Schlaich MP, Chronic kidney disease: role of sympathetic nervous system activation and potential benefits of renal denervation. EuroIntervention. 2013;9 Suppl R:R127-35. ] and in patients with end-stage renal disease [106106. Schlaich, M.P., Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW. Sympathetic Activation in Chronic Renal Failure. J Am Soc Nephrol. 2009;20:933-939. ]. Moreover, in another small study, has been proved that in patients with chronic kidney disease stage III and IV the treatment of hypertension with renal denervation can slow or even alt the progression of renal dysfunction [107107. Ott C, Mahfoud F, Schmid A, Toennes SW, Ewen S, Ditting T, Veelken R, Ukena C, Uder M, Böhm M, Schmieder RE. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J Hypertens. 2015;33:1261-6. doi: 10. 1097/HJH. 0000000000000556. ]. Another small study accounting for 30 patients with chronic kidney disease II-IV reported the acute reduction of blood pressure after renal denervation and its sustained effect during a 12 months follow-up period and even an improvement oft he renal function from baseline [108108. Kiuchi MG, Chen S, Graciano ML, Carreira MA, Kiuchi T, Andrea BR, Lugon JR. Acute effect of renal sympathetic denervation on blood pressure in refractory hypertensive patients with chronic kidney disease. Int J Cardiol. 2015;190:29-31. doi: 10. 1016/j. ijcard. 2015. 04. 039. ].
Effects beyond blood pressure reduction
Of note, renal denervation not only reduces sympathetic nerve activity to the kidneys, but – as Donazzan and collegues demonstrated – also reduces cardiac sympathetic activity independently from its BP effects [109109. Donazzan L, Mahfoud F, Ewen S, Ukena C, Cremers B, Kirsch CM, Hellwig D, Eweiwi T, Ezziddin S, Esler M, Böhm M. Effects of catheter-based renal denervation on cardiac sympathetic activity and innervation in patients with resistant hypertension. Clin Res Cardiol. 2016;105:364-71. doi: 10. 1007/s00392-015-0930-4. Epub 2015 Oct 22. ]. This may have implications for patients with heart failure where sympathetic activation plays a major role. Indeed, renal denervation appears to be safe in patients with chronic heart failure and refractory ventricular arrhythmias, and is associated with a reduced arrhythmogenic burden [110110. Ukena C, Mahfoud F, Ewen S, Bollmann A, Hindricks G, Hoffmann BA, Linz D, Musat D, Pavlicek V, Scholz E, Thomas D, Willems S, Böhm M, Steinberg JS. Renal denervation for treatment of ventricular arrhythmias: data from an International Multicenter Registry. Clin Res Cardiol. 2016;105:873-9. doi: 10. 1007/s00392-016-1012-y. Epub 2016 Jun 30. ]
Besides blood pressure reduction, renal denervation improves glucose metabolism [111111. Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, Hoppe UC, Vonend O, Rump LC, Sobotka PA, Krum H, Esler M, Böhm M. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940-6. ], left ventricular hypertrophy and diastolic function [112112. Brandt, M.C., Mahfoud F, Reda S, Schirmer SH, Erdmann E, Böhm M, Hoppe UC. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012; 59:901-9. ] in patients with resistant hypertension [113113. Sudano I, Noll G, Lüscher TF. Potential new indications and future studies. Eurointervention. 2013; 9 Suppl R:R155-60. ].
Moreover, many small clinical studies or case reports suggest that renal denervation may also be useful in diseases other than hypertension such as metabolic syndrome, sleep-related breathing disorders, chronic kidney disease and renal failure, chronic heart failure and polycystic ovary syndrome [113113. Sudano I, Noll G, Lüscher TF. Potential new indications and future studies. Eurointervention. 2013; 9 Suppl R:R155-60. ]. On the contrary no significant effects on endothelial function or inflammatory markers associated to the onset and the progression of hypertension has been up to now demonstrated [114114. Eikelis N, Hering D, Marusic P, Sari C, Walton A, Phillips S, Lambert E, Duval J, Krum H, Lambert G, Esler M, Schlaich M. The effect of renal denervation on endothelial function and inflammatory markers in patients with resistant hypertension. Int J Cardiol. 2015;188:96-8. doi: 10. 1016/j. ijcard. 2015. 04.041 ]. Left ventricular hypertrophy and diastolic dysfunction are known predictors of long-term cardiovascular events and seem to be influenced by renal denervation. A small study accounting for 17 true resistan-hypertensive patients showed that multielectrode renal denervation can improve diastolic dysfunction and reduce LV mass after a follow-up of 24 months, even if these data, due tot he poor numerosity of the speciemen, did not reach a statistical significancy [115115. Long-term effects of multielectrode renal denervation on cardiac adaptations in resistant hypertensive patients with left ventricular hypertrophy. Tsioufis C, Papademetriou V, Dimitriadis K, Kasiakogias A, Kordalis A, Andrikou E, Milkas A, Liatakis I, Lau EO, Tousoulis D. J Hum Hypertens. 2016;30:714-719. doi: 10. 1038/jhh. 2015.127 ]. These data find a confirmation in a recent meta-analysis that reported a benefit in terms of left ventricular hypertrophy reduction and also of left atrium enlargement 6 and 12 months after renal denervation [116116. Lu D, Wang K, Liu Q, Wang S, Zhang Q, Shan Q. Reductions of left ventricular mass and atrial size following renal denervation: a meta-analysis. Clin Res Cardiol. 2016;105:648-56. ]. Another study conducted by Mahfoud and collegues demostrated that catheter-based renal denervation significantly reduced blood pressure, left ventricular mass index and improved EF in 72 patients with resistant hypertension detected by magnetic resonance [117117. Mahfoud F, Urban D, Teller D, Linz D, Stawowy P, Hassel JH, Fries P, Dreysse S, Wellnhofer E, Schneider G, Buecker A, Schneeweis C, Doltra A, Schlaich MP, Esler MD, Fleck E, Böhm M, Kelle S. Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multi-centre cardiovascular magnetic resonance imaging trial. Eur Heart J. 2014;35:2224-31b. ].
The renal denervation seems to be helpful and safe also in patients with chronic kidney disease which develop cardiac remodelling such as left ventricular hypertrophy and/or cardiac fibrosis. In a small cohort of patients, Kiuchi and collegues demonstrated for the first time the reduction of left ventricual mass with improvement of systolic function and correlation with improvement of renal function 6 months after renal denervation [118118. Kiuchi MG, Graciano ML, de Queiroz Carreira MA, Kiuchi T, Chen S, Andrea BR, Lugon JR. Effects of renal sympathetic denervation in left ventricular hypertrophy in CKD refractory hypertensive patients. Int J Cardiol. 2016;202:121-3. ].
On the contrary only a modest effect of renal denervation and also without a statistical singnificancy has been demonstrated on end organ damage 12 months after treatment [119119. Verloop WL, Vink EE, Spiering W, Blankestijn PJ, Doevendans PA, Bots ML, Vonken EJ, Voskuil M, Leiner T. Effects of renal denervation on end organ damage in hypertensive patients. Eur J Prev Cardiol. 2015;22:558-67. ].
The role of renal denervation in patients with heart failure, particularly those with preserved ejection fraction (HFpEF) is still controversial. Heart failure is in fact associated with increased sympathetic nervous system activity and theoretically attenuating the sympathetic nervous system with renal denervation might be helpful concerning the ventricular remodelling that interests this particular population of patients. A small study with 25 patients aimed to this issue but was prematurely terminated because of difficulties in recruiting patients and failed to demostrate an improvement in quality of life, biomarkers and heart remodelling, even if a statistical significance was reached concerning the improvement of VO2peak (p=0.025) [120120. Patel HC, Rosen SD, Hayward C, Vassiliou V, Smith GC, Wage RR, Bailey J, Rajani R, Lindsay AC, Pennell DJ, Underwood SR, Prasad SK, Mohiaddin R, Gibbs JS, Lyon AR, Di Mario C. Renal denervation in heart failure with preserved ejection fraction (RDT-PEF): a randomized controlled trial. Eur J Heart Fail. 2016;18:703-12. doi: 10.1002/ejhf. 502. Epub 2016 Mar 16. ].
Personal perspective – Felix Mahfoud and Thomas F. Lüscher
Renal denervation is safe and lowers office and ambulatory blood pressure in certain patients with resistant hypertension, if properly performed with at least 12-16 effective ablations. Overall, in spite of mixed results, it appears that such a conclusion is justified based on the overall data, the subanalysis of Symplicity HTN-3 and the experience in registries.
Besides blood pressure reduction, renal denervation may improve glucose metabolism, left ventricular hypertrophy and diastolic function in patients with resistant hypertension. Moreover, many small clinical studies and case reports suggest that renal denervation may also be useful in diseases other than hypertension such as metabolic syndrome, sleep-related breathing disorders, chronic kidney disease and renal failure, chronic heart failure and polycystic ovary syndrome.
Many questions still remain open therefore in this fascinating new scenario: (1) how to better select patients undergoing renal denervation? (2) should we expand the indication of this procedure to treat less severe hypertensive patients or even those off antihypertensive drugs? (3) how can we refine catheter systems to achive an effective nerve block? (4) how can we predict effective renal nerve ablation in the cathlab? (5) should we treat patients after renal denervation with antiaggregation/antithrombotic therapy? and if yes, (6) which drugs should we use and how long? Future studies will highlight these and many other aspect of this very promising new therapy for arterial hypertension.
Acknowledgments
The authors wish to report the following conflicts of interest: TFL has received honoraria and research grants from Medtronic, Tollachenaz, Switzerland and St. Jude Medical, Brussels, Belgium.
Felix Mahfoud is supported by Deutsche Hochdruckliga und Deutsche Gesellschaft für Kardiologie. Felix Mahfoud has received research grants, speaker honorarium and consultancy fees from Medtronic/Ardian, St. Jude, Boston Scientific, and/or Cordis.



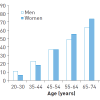
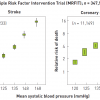
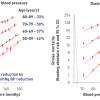

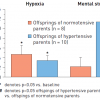
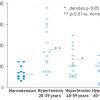
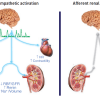
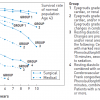
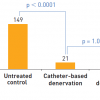
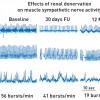
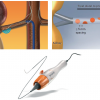




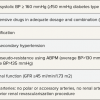
Last update 2017! Deserve an update by now!
Thank you for your comment. This chapter is due to benefit from an update in 2021. The editorial team