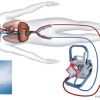
Percutaneous ventricular assist device
INTRA-AORTIC BALLOON PUMP
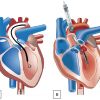
The intra-aortic balloon pump (IABP) has been the classic method for mechanical circulatory support. The first successful clinical use in a patient with cardiogenic shock following a myocardial infarction was reported in 1968 by Kantrowitz [2020. Kantrowitz A, Tjonneland S, Freed PS, Phillips SJ, Butner AN, Sherman JL, Jr. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA. 1968;203:113-118. ]. Since the introduction of a percutaneous IABP in 1980 [2121. Bregman D, Casarella WJ. Percutaneous intraaortic balloon pumping: initial clinical experience. Ann Thorac Surg. 1980;29:153-155. ], intra-aortic balloon counterpulsation has become the most frequently used mechanical circulatory assist in the world with more than 22,000 insertions worldwide between 1996 and 2001 [2222. Stone GW, Ohman EM, Miller MF, et al. Contemporary utilization and outcomes of intra-aortic balloon counterpulsation in acute myocardial infarction: the benchmark registry. J Am Coll Cardiol. 2003;41:1940-1945. ]. Left ventricular (LV) systolic unloading and diastolic augmentation with resultant improvements in coronary flow ( Figure 2 ) are the generally accepted mechanisms of action. IABP counterpulsation is traditionally used in a variety of surgical and nonsurgical patients with cardiogenic shock, either as a perioperative circulatory assist device, or in the setting of an acute coronary syndrome. Despite the widespread use of IABP in cardiogenic shock, the IABP-SHOCK II trial, the largest randomized trial evaluating patients undergoing early revascularization for myocardial infarction complicated by cardiogenic shock, demonstrated that the use of IABP did not reduce 30 day or 12 month mortality [2323. Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287-1296.
The IABP-SHOCK II trial is the largest randomized control trial that has evaluated the use of IABP in patients undergoing early revascularization for myocardial infarction complicated by cardiogenic shock, 2424. Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382:1638-1645. ]. This was later confirmed by a recent meta-analysis [2525. Unverzagt S, Buerke M, de Waha A, Haerting J, Pietzner D, Seyfarth M, Thiele H, Werdan K, Zeymer U, Prondzinsky R. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev. 2015; 3:CD007398. ]. Currently, the American and European guidelines for the management of acute myocardial infarction have downgraded the use IABP for cardiogenic shock from class I to class IIa and III recommendation [2626. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362-425. , 2727. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87‐165. doi:10. 1093/eurheartj/ehy394. ].
Physiology and haemodynamic effects
Intra-aortic balloon counterpulsation increases coronary blood flow by inflating during ventricular diastole and decreases myocardial oxygen demand by deflating during systole [2828. Nanas JN, Moulopoulos SD. Counterpulsation: historical background, technical improvements, hemodynamic and metabolic effects. Cardiology. 1994;84:156-167. , 2929. Schreuder JJ, Maisano F, Donelli A, et al. Beat-to-beat effects of intraaortic balloon pump timing on left ventricular performance in patients with low ejection fraction. Ann Thorac Surg. 2005;79:872-880. , 3030. Trost JC, Hillis LD. Intra-aortic balloon counterpulsation. Am J Cardiol. 2006;97:1391-1398. ]. These effects result in decreased myocardial wall stress and increased cardiac output. Myocardial oxygen consumption may decrease by half and stroke volume increases as much as 30% [3030. Trost JC, Hillis LD. Intra-aortic balloon counterpulsation. Am J Cardiol. 2006;97:1391-1398. ]. However, for optimal performance, the balloon must be appropriately positioned within the descending aorta, the displacement volume must be adequate, and the timing of inflation/deflation must be optimal within the cardiac cycle ( Figure 2 ). The balloon volume is proportional to the volume of blood displaced and the magnitude of haemodynamic augmentation decreases with tachycardia due to reduced filling time.
Multifactorial anaemia and thrombocytopenia are often seen in patients under IABP circulatory support, partly due to mechanical damages to erythrocytes and platelets as well as heparin administration [2828. Nanas JN, Moulopoulos SD. Counterpulsation: historical background, technical improvements, hemodynamic and metabolic effects. Cardiology. 1994;84:156-167. ]. Serum haemoglobin and platelet count should be monitored daily during this therapy.
Indications
- Cardiogenic shock
- Associated with acute myocardial infarction (AMI)
- Post-cardiotomy
- Weaning from cardiopulmonary bypass
- Refractory unstable angina
- Refractory ischaemic ventricular arrhythmias
- Stabilisation of ischaemic left main coronary artery disease
- Mechanical complications of AMI
- Ventricular septal defect
- Acute ischaemic mitral valve regurgitation
- High-risk PCI
- Complex coronary artery disease
- Severe left ventricular dysfunction
- Myocardial contusion with associated ventricular failure
- Right ventricular failure
- Septic shock with severe myocardial dysfunction (no human data) [3131. Engoren M, Habib RH. Effects of intraaortic balloon augmentation in a porcine model of endotoxemic shock. Resuscitation. 2004;60:319-326. ]
- Preoperative stabilisation for high-risk cardiac surgery
- Severe coronary artery disease
- Severe left ventricular dysfunction
IABP insertion
- percutaneous femoral arterial access
- passive cardiac support and increase in ischaemic threshold
- balloon sizing according to height:
- <160 cm:34 ml balloon,
- 160-182 cm:40 ml balloon,
- <182 cm:50 ml balloon
- balloon tip should be placed distal to the left subclavian artery at the level of the carina
Device description and implantation
The IABP is composed of a double-lumen 7.5 Fr catheter with a 25-50 ml balloon at the distal end and a console with the pump. After appropriate size selection, the IABP is inserted percutaneously via the femoral artery (less commonly the brachial artery). Once vascular access is obtained, the balloon is advanced and positioned 2-3 cm distal to the origin of the left subclavian artery (at the level of the carina). Final positioning is confirmed during placement with fluoroscopy or TOE/chest radiograph (if placed outside the catheterisation laboratory). Blind placement can be performed in emergency but must be followed immediately by imaging confirmation. The inner lumen is used to monitor arterial pressure and the outer lumen is used for gas delivery from the console. Helium is the most commonly used gas due to its low density and favourable physical properties allowing rapid transit from gas cylinder to balloon; however CO2 is used in some institutions as well. Complete balloon expansion should be confirmed upon initiation of counterpulsation and peripheral pulses should be assessed to ensure adequate limb perfusion (including lower limb and left radial pulses).
The console is programmed to a specific trigger for balloon inflation and deflation usually determined by the electrocardiogram (ECG), pacer or the direct arterial pressure waveform. When triggered by ECG, the peak of the R wave corresponds to the beginning of LV systole at which time balloon is deflated. Balloon inflation should begin at the onset of diastole which corresponds to the mid portion of the T wave. Cardiac arrhythmias, electrical interference from electrocautery or poor ECG quality may result in suboptimal aortic counterpulsation. In these cases, the triggering can be done by the arterial pressure waveform. Nevertheless, there are times in which all of the above triggering mechanisms are suboptimal, leading to further evaluation of technology, such as fibre-optic sensing of haemodynamic events [3232. Reesink KD, van der Nagel T, Bovelander J, Jansen JR, van der Veen FH, Schreuder JJ. Feasibility study of a fiber-optic system for invasive blood pressure measurements. Catheter Cardiovasc Interv. 2002;57:272-276. ]. The fibre-optic sensing IABPs include the AutoCAT 2 WAVE IABP System with AutoPilot™ operation (Teleflex Incorporated, Arrow International, Limerick, PA, USA) and the Sensation® 7FR & CS300™ IABP System (MAQUET, Getinge Group, Hirrlingen, Germany). These sophisticated balloons have the theoretical advantage of producing high fidelity individualised triggering regardless of electrocautery and arrhythmias.
The balloon should inflate after aortic valve closure (corresponding to the dicrotic notch on the arterial pressure waveform) and deflate immediately before aortic valve opening (corresponding to the nadir prior to the systolic upstroke). The frequency of balloon counterpulsation can be timed to augment every beat (1:1) to every second, third or fourth beat (1:2-4).
Maintenance and removal
Low dose heparin may be used to avoid thrombus formation in the balloon catheter and ischaemic limb complications. However, this practice is institution dependent and there are no data to support the routine use [3030. Trost JC, Hillis LD. Intra-aortic balloon counterpulsation. Am J Cardiol. 2006;97:1391-1398. ]. On the other hand, an immobile or non-functioning balloon can thrombose within minutes and should be removed promptly. Usually the balloon support is weaned by adjusting the frequency of augmentation. The coagulation parameters should be normalised prior to balloon removal. Once haemodynamic stability is achieved without circulatory support the balloon inflation is discontinued, and the balloon and sheath are removed with pressure applied for at least 30 minutes.
Contraindications
IABP counterpulsation is absolutely contraindicated in patients with significant aortic regurgitation (moderate or severe) or aortic dissection. Relative contraindications for femoral percutaneous insertion include the presence of severe peripheral arterial disease, coagulopathy, abdominal or thoracoabdominal aortic aneurysms and the presence of femoral-popliteal bypass grafts. However, its use has been described in the presence of aorto-bifemoral grafts.
Complications
Intra-aortic balloon counterpulsation can be associated with vascular complications including femoral arterial dissection, systemic embolisation (including stroke), limb ischaemia, bleeding and infection. The development of these complications may result in amputation and rarely death. Rarely aortic dissection or rupture can develop as a result of IABP insertion [3434. Subramaniam AV, Barsness GW, Vallabhajosyula S, Vallabhajosyula S. Complications of Temporary Percutaneous Mechanical Circulatory Support for Cardiogenic Shock: An Appraisal of Contemporary Literature. Cardiol Ther. 2019;8(2):211‐228. doi:10. 1007/s40119-019-00152-8. ].
It is estimated that approximately 7% of patients who require aortic counterpulsation experience a catheter-related complication [3333. Ferguson JJ, 3rd, Cohen M, Freedman RJ, Jr. , et al. The current practice of intra-aortic balloon counterpulsation: results from the Benchmark Registry. J Am Coll Cardiol. 2001;38:1456-1462. This study presents clinical data from the first large registry of aortic counterpulsation, a computerised database that incorporates prospectively gathered data on indications for intra-aortic balloon counterpulsation (IABP) use, patient demographics, concomitant medication and in-hospital outcomes and complications.
It describes the most frequent indications and complications]. In this particular study, 2.6% of 16,909 patients developed a major complication; major bleeding 0.8%, limb-threatening ischaemia in 0.9%, limb amputation in 0.1%, IABP-related death 0.05% [3333. Ferguson JJ, 3rd, Cohen M, Freedman RJ, Jr. , et al. The current practice of intra-aortic balloon counterpulsation: results from the Benchmark Registry. J Am Coll Cardiol. 2001;38:1456-1462. This study presents clinical data from the first large registry of aortic counterpulsation, a computerised database that incorporates prospectively gathered data on indications for intra-aortic balloon counterpulsation (IABP) use, patient demographics, concomitant medication and in-hospital outcomes and complications.
It describes the most frequent indications and complications]. Multiple risk factors for major IABP related complications have been identified and include age (>75 years), peripheral arterial disease, diabetes mellitus, female gender and small body surface area [3535. Cohen M, Dawson MS, Kopistansky C, McBride R. Sex and other predictors of intra-aortic balloon counterpulsation-related complications: prospective study of 1119 consecutive patients. Am Heart J. 2000;139:282-287. ].
Due to the catastrophic nature of complications related to this device, frequent vigilance is mandatory and includes assessment of infection, coagulopathy, and limb perfusion.
THE TANDEMHEART™ PLATFORM
The classic TandemHeart™ (Cardiac Assist Technologies, Inc., Pittsburg, PA, USA; LivaNova PLC – London, UK) is a transseptal-left atrial-to-femoral arterial PVAD that provides left heart bypass designed for short-term circulatory support ( Figure 3 ). The concept of left heart bypass was initially designed for post-cardiotomy shock in patients unable to be weaned from cardiopulmonary bypass (CPB) [3636. Pavie A, Leger P, Nzomvuama A, et al. Left centrifugal pump cardiac assist with transseptal percutaneous left atrial cannula. Artif Organs. 1998;22:502-507. ]. This technique was first described by Dennis in 1962 [3737. Dennis C, Carlens E, Senning A, et al. Clinical use of a cannula for left heart bypass without thoracotomy: experimental protection against fibrillation by left heart bypass. Ann Surg. 1962;156:623-637. ] with venous access achieved from the jugular approach. This system was initially limited and lacked widespread use due to unavailability of an adequate transseptal cannula for full circulatory support and high rate of haemolysis and thrombus formation with the high speed centrifugal pumps utilised. It wasn’t until the 1990s when the transfemoral percutaneous approach of this type of circulatory support for patients undergoing high risk coronary interventions was described for short term use [3838. Glassman E, Chinitz LA, Levite HA, Slater J, Winer H. Percutaneous left atrial to femoral arterial bypass pumping for circulatory support in high-risk coronary angioplasty. Cathet Cardiovasc Diagn. 1993;29:210-216. ].
The TandemHeart™ PVAD (Cardiac Assist Technologies, Inc., Pittsburg, PA, USA; LivaNova PLC – London, UK) is a low speed centrifugal continuous flow pump that has been approved by the FDA for short term (6 hours) circulatory support [1717. Thiele H, Smalling RW, Schuler GC. Percutaneous left ventricular assist devices in acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2007;28:2057-2063. , 1818. Naidu SS. Novel percutaneous cardiac assist devices: the science of and indications for hemodynamic support. Circulation. 123:533-543.
This recent review describes the common percutaneous ventricular assist devices including a thorough section on physiological mechanisms as well as the main differences between the TandemHeart™ and Impella® recover system, including a section on developing indications and choosing between the different available options] and CE mark for use up to 30 days [99. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, Tu T; Society for Cardiovascular Angiography and Interventions (SCAI); Heart Failure Society of America (HFSA); Society of Thoracic Surgeons (STS); American Heart Association (AHA), and American College of Cardiology (ACC). J Am Coll Cardiol. 2015;19:65: e7-e26. ]. These characteristics result in a theoretical reduction of haemolysis and thrombus formation. The system is capable of delivering up to 5 L/min of flow at 7500 rpm when used percutaneously and up to 8 L/min if utilised with a direct surgical cannulation method. The system is designed to provide circulatory assistance regardless of the patient’s rhythm [3939. Lee MS, Makkar RR. Percutaneous left ventricular support devices. Cardiol Clin. 2006;24:265-275, vii. ]. In a prospective randomised trial comparing IABP and TandemHeart™ PVAD in patients with revascularised myocardial infarction complicated with cardiogenic shock, the group supported with PVAD had more significant improvement of haemodynamics and metabolic variables compared with IABP, however, mortality was similar and there were more complications associated with PVAD [4040. Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26:1276-1283.
This is one of the first randomised controlled trials comparing intra-aortic balloon counterpulsation with percutaneous left ventricular assistance using the TandemHeart™. Despite improved haemodynamic and metabolic parameters with the TandemHeart™, there were more complications encountered in the VAD group]. These results were replicated in another small randomised trial [4141. Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152:469 e461-468.
Multicentre randomised clinical trial demonstrating that TandemHeart™ assist device improves haemodynamic variables including cardiac output, mean arterial blood pressure and reduction of pulmonary capillary wedge pressure when compared to intra-aortic balloon pump]. The TandemHeart™ has been successfully used as a bridge to procedure in patients undergoing high risk PCI, in particular in patients with unprotected left main disease and severe left ventricular dysfunction [4242. Aragon J, Lee MS, Kar S, Makkar RR. Percutaneous left ventricular assist device: "TandemHeart" for high-risk coronary intervention. Catheter Cardiovasc Interv. 2005;65:346-352.
Case series reporting the use of the TandemHeart assist device in 8 patients undergoing percutaneous coronary intervention (PCI) with severely compromised left ventricular systolic function and complex coronary lesions, including multivessel disease, left main disease, or bypass graft disease. There was 100% procedural success, adding to the potential indications of percutaneous left ventricular assistance as a bridge to procedure in high risk, critically ill patients., 4343. Alli OO, Singh IM, Holmes DR, Jr., Pulido JN, Park SJ, Rihal CS. Percutaneous left ventricular assist device with TandemHeart for high-risk percutaneous coronary intervention: The Mayo Clinic experience. Catheter Cardiovasc Interv. 2012;80:728-734. ]. The most important limitation of the left atrial-to-femoral arterial bypass is the requirement of large arterial and venous cannulas to achieve adequate circulatory support, and the need of transseptal puncture and dilation. Nevertheless, no significant residual left-to-right shunt has been observed after this procedure [4040. Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26:1276-1283.
This is one of the first randomised controlled trials comparing intra-aortic balloon counterpulsation with percutaneous left ventricular assistance using the TandemHeart™. Despite improved haemodynamic and metabolic parameters with the TandemHeart™, there were more complications encountered in the VAD group].
TandemHeart™
- Venous, arterial and transseptal access are required
- Provides active cardiac support with increased flow up to 5 L/min achievable
- Systemic heparinisation necessary to prevent thromboembolic complications
- Function not dependent on stable cardiac rhythm
- Insertion requires experience and multidisciplinary co-operation
Physiology and haemodynamic effects
The TandemHeart™ PVAD in the classic configuration actively augments cardiac output and has the capability to completely replace left ventricular function achieving flows up to 5 L/min, while unloading the ventricle and reducing ventricular work ( Table 3 ). The device functions as a left heart bypass with in-parallel circulatory support that drains fully oxygenated blood from the left atrium (provided normal lung function) to pump it retrogradely via the femoral artery, reducing cardiac work load, oxygen demand and LV filling pressure [1818. Naidu SS. Novel percutaneous cardiac assist devices: the science of and indications for hemodynamic support. Circulation. 123:533-543.
This recent review describes the common percutaneous ventricular assist devices including a thorough section on physiological mechanisms as well as the main differences between the TandemHeart™ and Impella® recover system, including a section on developing indications and choosing between the different available options].
Indications
The TandemHeart PVAD may be used as temporary support for patients with cardiogenic shock or to support patients during High risk PCI, particularly in the presence of severe LV dysfunction ( Table 1 and Figure 1 ). In general, these patients are poor surgical candidates due to severe comorbidities or inaccessible disease as well as patients in which the IABP support alone will not suffice to maintain physiological homeostasis.
Device description and insertion
The TandemHeart™ is usually inserted in the catheterisation laboratory and requires a transseptal puncture with the Brockenbrough needle in to the left atrium via femoral vein access. The inflow – transseptal cannula is a 21 Fr polyurethane catheter with a large end hole and 14 side holes to facilitate left atrium decompression. The centrifugal flow pump is a low prime volume device (10 ml) that includes a six blade rotating propeller which is powered by a direct current microprocessor-controlled electromagnetic rotary motor. The system includes two controllers, a primary and a back-up, which are constantly ready for use and have extensive self-diagnostics with alarm features to ensure continuous support. Built in batteries allow uninterrupted circulatory assistance for one hour to facilitate transport or in case of outage. Furthermore, a pressure transducer is used to monitor the infusion pressure and alerts for potential blockages in the infusion line. The outflow cannula is 15-17 Fr and is inserted in the common femoral artery. Since the outflow cannula does not reach the abdominal aorta, it does not preclude the concomitant use of IABP.
After percutaneous puncture of the femoral vein and standard transseptal puncture and pre-dilation of the fossa ovalis, the venous inflow cannula is inserted in the left atrium and position is confirmed with contrast injection. Right-to-left shunting can potentially occur if not all the side holes of the transseptal cannula are located in the left atrium and can be suspected with sudden desaturation of the arterial blood. Subsequently, the arterial inflow cannula is inserted in the ipsilateral or contralateral common femoral artery using the Seldinger technique and advanced until the tip is in the common iliac artery. After adequate air removal, the cannulae are connected to the centrifugal pump by standard heparin-coated Tygon® tubing. Oxygenated blood is retrieved from the left atrium and pumped into the abdominal aorta via the femoral inflow cannula. The entire assembly and institution of mechanical circulatory support can be achieved within 30 minutes in hands of experienced operators, and usage up to 14 days has been reported [4242. Aragon J, Lee MS, Kar S, Makkar RR. Percutaneous left ventricular assist device: "TandemHeart" for high-risk coronary intervention. Catheter Cardiovasc Interv. 2005;65:346-352.
Case series reporting the use of the TandemHeart assist device in 8 patients undergoing percutaneous coronary intervention (PCI) with severely compromised left ventricular systolic function and complex coronary lesions, including multivessel disease, left main disease, or bypass graft disease. There was 100% procedural success, adding to the potential indications of percutaneous left ventricular assistance as a bridge to procedure in high risk, critically ill patients., 4343. Alli OO, Singh IM, Holmes DR, Jr., Pulido JN, Park SJ, Rihal CS. Percutaneous left ventricular assist device with TandemHeart for high-risk percutaneous coronary intervention: The Mayo Clinic experience. Catheter Cardiovasc Interv. 2012;80:728-734. , 4444. Vranckx P, Meliga E, De Jaegere PP, Van den Ent M, Regar ES, Serruys PW. The TandemHeart, percutaneous transseptal left ventricular assist device: a safeguard in high-risk percutaneous coronary interventions. The six-year Rotterdam experience. EuroIntervention. 2008;4:331-337. ].
Maintenance and explantation
Adequate systemic anticoagulation is necessary to reduce the risk of thromboembolic complications. This is achieved with administration of intravenous heparin (100 units/kg) aiming for an initial activated clotting time (ACT) above 300 seconds prior to cannulation. Following successful weaning of circulatory support, due to myocardial recovery or after a critical revascularisation procedure, the device can be easily explanted percutaneously. After discontinuation of heparin, the arterial cannula is removed and manual compression or suture closure of the puncture site is performed until adequate haemostasis is achieved. After explantation of the venous transseptal cannula, there is a small residual atrial septal defect that usually closes within 4-6 weeks [4242. Aragon J, Lee MS, Kar S, Makkar RR. Percutaneous left ventricular assist device: "TandemHeart" for high-risk coronary intervention. Catheter Cardiovasc Interv. 2005;65:346-352.
Case series reporting the use of the TandemHeart assist device in 8 patients undergoing percutaneous coronary intervention (PCI) with severely compromised left ventricular systolic function and complex coronary lesions, including multivessel disease, left main disease, or bypass graft disease. There was 100% procedural success, adding to the potential indications of percutaneous left ventricular assistance as a bridge to procedure in high risk, critically ill patients.].
Contraindications
The TandemHeart PVAD used as classic left heart bypass configuration, depends on adequate right ventricular function for optimal circulatory assistance. Therefore, the presence of right ventricular failure or predominantly right acute myocardial infarction is a relative contraindication. However, The TandemHeart Platform includes a system that can be configured as a right ventricular assist device [4545. Atiemo AD, Conte JV, Heldman AW. Resuscitation and recovery from acute right ventricular failure using a percutaneous right ventricular assist device. Catheter Cardiovasc Interv. 2006;68:78-82. , 4646. Prutkin JM, Strote JA, Stout KK. Percutaneous right ventricular assist device as support for cardiogenic shock due to right ventricular infarction. J Invasive Cardiol. 2008;20:E215-216. ], or two systems can theoretically be used in combination for biventricular support ( Figure 1 ). Due to advances in PVADs using only arterial access, the TandemHeart platform has expanded to ECMO and RV support with the addition of the TandemLife Kit (addition of an oxygenator, and femoral venous-arterial cannulation) and TandemLung Kit with the ProtekDuo cannula used as RA-PA RVAD and also used as VV ECMO support for respiratory failure [7171. Vetrovec GW, Anderson M, Schreiber T, et al. The cVAD registry for percutaneous temporary hemodynamic support: A prospective registry of Impella mechanical circulatory support use in high-risk PCI, cardiogenic shock, and decompensated heart failure. Am Heart J. 2018;199:115‐121. ] Another contraindication for its use as LA-FA bypass is the presence of a ventricular septal defect (VSD) due to the risk of right-to-left shunting and subsequent hypoxaemia. As with the IABP, severe aortic insufficiency poses the risk of left ventricular overdistension and subendocardial ischaemia. Moreover, just like other PVADs that require percutaneous insertion of large cannulas in the femoral artery, the presence of severe peripheral arterial disease and femoral grafts may preclude the use of this device as well as any contraindication for systemic anticoagulation.
Complications and limitations
Complications in patients receiving PVADs can be divided in vascular, mechanical, infectious, neurologic and hematologic categories [3434. Subramaniam AV, Barsness GW, Vallabhajosyula S, Vallabhajosyula S. Complications of Temporary Percutaneous Mechanical Circulatory Support for Cardiogenic Shock: An Appraisal of Contemporary Literature. Cardiol Ther. 2019;8(2):211‐228. doi:10. 1007/s40119-019-00152-8. ]. Specific to TandemHeart, the use of large bore cannulae and transseptal puncture is not devoid of risk. General complications related to the femoral vessel cannulation include bleeding, infection, limb ischaemia [4242. Aragon J, Lee MS, Kar S, Makkar RR. Percutaneous left ventricular assist device: "TandemHeart" for high-risk coronary intervention. Catheter Cardiovasc Interv. 2005;65:346-352.
Case series reporting the use of the TandemHeart assist device in 8 patients undergoing percutaneous coronary intervention (PCI) with severely compromised left ventricular systolic function and complex coronary lesions, including multivessel disease, left main disease, or bypass graft disease. There was 100% procedural success, adding to the potential indications of percutaneous left ventricular assistance as a bridge to procedure in high risk, critically ill patients.], and poses relative contraindication in patients with inferior vena cava (IVC) filter. Nevertheless, successful use of this device has been described in this setting, without IVC filter displacement [4747. Chiam PT, Ruiz CE, Cohen HA. Placement of a large transseptal cannula through an inferior vena cava filter for TandemHeart percutaneous left ventricular assist. J Invasive Cardiol. 2008;20:E197-199. ]. Unique complications to this device include paradoxical embolism due to the transseptal atrial cannulation, accidental coronary sinus or posterior right atrial wall puncture with cardiac tamponade. Cannula dislodgement can be catastrophic in the setting of profound shock or during a critical portion of a complex PCI [4848. Thiele H, Lauer B, Hambrecht R, Boudriot E, Cohen HA, Schuler G. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation. 2001;104:2917-2922. ]. This can happen during CPR and reposition with fluoroscopic or echocardiographic guidance should be obtained immediately if suspected.
Although the beneficial physiological effects of this device have been demonstrated, there have been limited studies in humans to date and none have had the appropriate power to prove a substantial mortality benefit [4040. Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26:1276-1283.
This is one of the first randomised controlled trials comparing intra-aortic balloon counterpulsation with percutaneous left ventricular assistance using the TandemHeart™. Despite improved haemodynamic and metabolic parameters with the TandemHeart™, there were more complications encountered in the VAD group, 4141. Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152:469 e461-468.
Multicentre randomised clinical trial demonstrating that TandemHeart™ assist device improves haemodynamic variables including cardiac output, mean arterial blood pressure and reduction of pulmonary capillary wedge pressure when compared to intra-aortic balloon pump]. Another potential limitation is the added theoretical propagation of inflammatory response associated with extracorporeal bypass circuits [4949. Hall RI, Smith MS, Rocker G. The systemic inflammatory response to cardiopulmonary bypass: pathophysiological, therapeutic, and pharmacological considerations. Anesth Analg. 1997;85:766-782. ].
Procedural management
Considering the complexity of patients and procedure, the majority of these cases are performed under general anaesthesia despite the fact that the patient’s physiological status will be supported during the intervention. Nevertheless, as expertise is gained, they can be safely performed under expert sedation care.
The rate of complications and ease of cannula placement depends on the institution and expertise of the interventional cardiologist and team. Initial trials should be performed under general anaesthesia, with adequate preparation for emergent surgical support due to complications during transseptal placement or cannulation. Blood should be ready in the room, and a surgical scrub team should be notified and ideally a back-up plan for acute emergencies should be in place for possible emergent transfer to the operating room. Appropriate venous access includes peripheral as well as central venous access. Monitors should include direct radial arterial pressure monitoring, central venous and with or without a pulmonary artery catheter. Initially cardiac output is high with the circulatory support (7-8 L/min), but usually drops during critical procedures as the LV may stop contracting. In severely compromised patients, the use of a transoesophageal echocardiography, continuous cardiac output monitoring using a pulmonary artery catheter or mixed venous oxygen monitor can help tailor the haemodynamic support prior, during and post circulatory support by the PVAD.
Prior to insertion of the cannulae, the patient needs to be anticoagulated with a goal of ACT > 300 sec. Heparin is usually the anticoagulant of choice, however, there are protocols using direct thrombin inhibitors, like bivalirudin, to be used in case of contraindication to heparin therapy (heparin induced thrombocytopenia, etc.).
High vigilance for complications during cannulae placement is vital for effective and timely response. Complications as described above, including hypoxaemia due to right to left shunt, hypotension, cardiac tamponade, bleeding can be catastrophic. Prior to starting the centrifugal pump and adequate de-airing, a fluid bolus load of one litre isotonic crystalloid solution or 500 ml colloid is administered to fill the heart at the time of pump start, The centrifugal pump offers an advantage by being a low prime volume device with less haemodilution. Vigilance for air emboli is critical since a new atrial septal defect is created and all IV lines should be inspected for air to avoid this complication.
Inotropic support should be available and ready for use. When appropriate and ready for support discontinuation, a multidisciplinary approach led by the interventional cardiologist will wean the patient from the partial bypass by reducing the rpm and assisted flow to achieve an approximate mean arterial blood pressure of 70-75 mmHg. At the end of the procedure, the cardiologist will remove the cannulae and repair the vessels, usually with a vascular closure device after reversal of anticoagulation with protamine (if heparin used).
In patients with atrioventricular conduction abnormalities, consideration should be made to place ventricular pacing wires in case of complete heart block or asystole prior to circulatory support. If wires are present at the time of cannula insertion, they should be withdrawn prior to transseptal cannulation or the cardiologist should be notified. In institutions with extensive expertise, the TandemHeart™ can be readily placed with minimal complications, in these cases; consideration to perform the procedure under sedation can be made. However, close communication and constant vigilance by the anaesthetic personnel still is advocated.
THE IMPELLA® SYSTEM
The Impella® circulatory support system (Abiomed, Danvers, MA, USA) is another type of PVAD that utilises an Archimedes screw mechanism for left ventricular unloading and circulatory assist. It consists of a miniature axial flow rotary blood pump that is positioned across the aortic valve ( Figure 4). The system actively unloads the ventricle by drawing blood through the distal port within the ventricular cavity and pumping it into the ascending aorta through the proximal port of the device. The inflow cannula-pump is inserted via the femoral artery and advanced past the aortic valve under fluoroscopic guidance ( Figure 4A). This device is designed to provide short-term ventricular support for several hours to days and comes in two main sizes: The CP-14 Fr (3-4 L/min) which is inserted percutaneously (LP Figure 4A), and the LD-21Fr (5 L/min) which requires surgical cut-down. There are now newer models that include SmartAssist ® technology that allows for repositioning without the need for imaging and is guided by an optical sensor that detects the differential pressures between LVEDP and aorta. These newer pumps include the percutaneous Impella CP SmartAssist, which is capable of peak flows 4.3 lts/min and the surgically implanted Impalla 5.5 with SmartAssist. Both systems are mounted on a 9 Fr flexible pigtail catheter. Although the femoral artery is the most common access for left ventricular support, there are also direct ascending aorta insertion (Impella® LD – Figure 4B), and right axillary approach [5050. Sassard T, Scalabre A, Bonnefoy E, Sanchez I, Farhat F, Jegaden O. The right axillary artery approach for the Impella Recover LP 5.0 microaxial pump. Ann Thorac Surg. 2008;85:1468-1470. ]. The system (Impella® -RP) can also be used for percutaneous right ventricular support via femoral vein insertion ( Figure 5)[5151. Margey R, Chamakura S, Siddiqi S, et al. First experience with implantation of a percutaneous right ventricular impella right side percutaneous support device as a bridge to recovery in acute right ventricular infarction complicated by cardiogenic shock in the United States. Circ Cardiovasc Interv. 2013;6:e37-38. ].
The concept of axial flow pumps across the aortic valve for circulatory support was first described by Wampler in 1988 [5252. Wampler RK, Moise JC, Frazier OH, Olsen DB. In vivo evaluation of a peripheral vascular access axial flow blood pump. ASAIO Trans. 1988;34:450-454. ], however, despite initial optimism, the success of the Hemopump™ (Medtronic, Inc, Minneapolis, MN, USA) was hampered by a high rate of complications and technical difficulties [1717. Thiele H, Smalling RW, Schuler GC. Percutaneous left ventricular assist devices in acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2007;28:2057-2063. , 5353. Scholz KH, Hering JP, Schroder T, et al. Protective effects of the Hemopump left ventricular assist device in experimental cardiogenic shock. Eur J Cardiothorac Surg. 1992;6:209-214. ]. A resurgence of this concept with improved technology led to the development of the newer axial flow systems described above. At this time, the entire Impella® platform (CP, 5.0/LD and RP) is approved for management of cardiogenic shock (post MI, post-cardiotomy, post partum, myocarditis, acute decompensated heart failure), high-risk PCI and right ventricular failure as short-term haemodynamic support up to 14 days. At least one long-term follow-up study has demonstrated safe use of this device when used for three days, with no reported damage to the aortic valve after three years [5454. Engstrom AE, Sjauw KD, Baan J, et al. Long-term safety and sustained left ventricular recovery: long-term results of percutaneous left ventricular support with Impella LP2.5 in ST-elevation myocardial infarction. EuroIntervention. 2011;6:860-865. ]. Although, there are some case reports with successful bridge to recovery [5555. Colombo T, Garatti A, Bruschi G, et al. First successful bridge to recovery with the Impella Recover 100 left ventricular assist device for fulminant acute myocarditis. Ital Heart J. 2003;4:642-645. ], the most common use of this device to date is for complex patients undergoing high-risk PCI [5656. Henriques JP, Remmelink M, Baan J, Jr. , et al. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover LP 2. 5. Am J Cardiol. 2006;97:990-992.
This study evaluated the feasibility and safety of LV support with the percutaneous implantable Impella Recover LP 2. 5 system in 19 high-risk patients undergoing percutaneous coronary intervention. All patients were very poor candidates for surgery and procedural success was 100%. There were no procedural deaths, and 2 device-unrelated in-hospital late deaths.]. The use of the Impella® 2.5 in patients with cardiogenic shock caused by myocardial infarction has been studied in a randomised control trial led by Seyfarth [5757. Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52:1584-1588.
Randomised clinical trial evaluating the safety and efficacy of the Impella LP2. 5 in comparison to intra-aortic balloon pumping for treatment of cardiogenic shock after myocardial infarction. This study included 26 patients and demonstrated that the use of the Impella LP 2. 5 LVAD is feasible, safe and provides superior haemodynamic support when compared with intra-aortic balloon pump.] which demonstrated safety, feasibility and superiority compared to standard management with IABP. Unfortunately, a meta-analysis of controlled trials comparing PVADs to IABP by Cheng et al failed to show a 30-day mortality benefit [5858. Cheng JM, den Uil CA, Hoeks SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009;30:2102-2108.
First meta-analysis of randomised controlled trials comparing intra-aortic balloon pump counterpulsation with percutaneous left ventricular assist devices (Impella LP 2. 5 and TandemHeart). The potential benefits on haemodynamic parameters as well as on 30 day mortality were evaluated. Although percutaneous LVAD provided superior haemodynamic support in patients with cardiogenic shock as compared with IABP, the use of these more powerful devices did not improve early survival.]. The Impella 5.0 in patients with cardiogenic shock after ST elevation myocardial infarction suggests improved survival compared with the Impella 2.5 [5959. Engstrom AE, Cocchieri R, Driessen AH, et al. The Impella 2.5 and 5.0 devices for ST-elevation myocardial infarction patients presenting with severe and profound cardiogenic shock: The Academic Medical Center intensive care unit experience. Crit Care Med. 2011;39:2072-2079. , 6060. Lemaire A, Anderson MB, Lee LY, Scholz P, Prendergast T, Goodman A, Lozano AM, Spotnitz A, Batsides G. The Impella device for acute mechanical circulatory support in patients in cardiogenic shock. Ann Thorac Surg. 2014;97:133-8. ]. The USpella registry is a prospective registry for patients receiving hemodynamic support with the Impella 2.5. [6161. Maini B, Naidu SS, Mulukutla S, et al. Real-world use of the Impella 2.5 circulatory support system in complex high-risk percutaneous coronary intervention: the USpella Registry. Catheter Cardiovasc Interv. 2012;80:717-725. ] Results from this large registry reported the contrasting strategies of pre- and post-PCI Impella 2.5 placement in acute myocardial infarction patients complicated by cardiogenic shock [1010. Lee JM, Park J, Kang J, Jeon KH, Jung JH, Lee SE, Han JK, Kim HL, Yang HM, Park KW, Kang HJ, Koo BK, Kim SH, Kim HS. The efficacy and safety of mechanical hemodynamic support in patients undergoing high-risk percutaneous coronary intervention with or without cardiogenic shock: Bayesian approach network meta-analysis of 13 randomized controlled trials. Int J Cardiol. 2015;184:36-46. ]. Early circulatory support (pre-PCI) improved hospital survival (65.1% vs 40.7%; P = 0.003) and remained an independent predictor after adjustment for confounding variables (odds ratio: 0.37; P = 0.01). The early initiation of haemodynamic support also allowed more complete revascularisation (1.57 ± 0.67 vs 1.30 ± 0.57 vessels treated; P = 0.01. The USpella registry is the predecessor of the cVAD registry, which has enrolled 3339 patients through 2016 and will be used to assess the outcomes of Impella therapy across the entire platform of support (2.5, CP, 5.0/LD and RP). [7171. Vetrovec GW, Anderson M, Schreiber T, et al. The cVAD registry for percutaneous temporary hemodynamic support: A prospective registry of Impella mechanical circulatory support use in high-risk PCI, cardiogenic shock, and decompensated heart failure. Am Heart J. 2018;199:115‐121. ]) The cVAD registry is now a global initiative with participation of United States, Canada, Germany, Great Britain, Spain, France, Netherlands, Switzerland, and anticipated future international sites including Japan, Denmark and Italy. Similar to the INTERMACS registry for implantable LVADs, the purpose of the cVAD registry is to guide future efforts in optimal timing and circulatory support providing “real world” outcome and quality data. The widespread availability of the Impella CP, providing greater cardiac and circulatory support than the Impella 2.5, and also available by percutaneous approach, may be expected to produce similarly favourable or even better results, and has largely replaced the 2.5 for management of cardiogenic shock [7272. Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, de Winter RJ, Piek JJ, Lagrand WK, de Mol BA, Tijssen JG, Henriques JP. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2017;69:278-287. ]The PROTECT II study, a multicenter, prospective randomised trial evaluated patients requiring haemodynamic support during high risk PCI, comparing the effectiveness and outcomes between the IABP and Impella 2.5. The primary endpoint was the 30 day incidence of major adverse events (mortality, acute MI, stroke, repeat revascularisation, acute kidney injury, cardiopulmonary resuscitation, angiographic failure and aortic insufficiency). Whilst there was no difference in major adverse events between the groups at 30 days, there was trend towards superior clinical outcomes for the intention-to-treat population at 90 days. Moreover, the treatment effect of Impella® compared with IABP improved over the course of the study, suggesting a learning curve. [6262. O’Neill WW, Kleiman NS, Moses J, et al. A Prospective, Randomized Clinical Trial of Hemodynamic Support With Impella 2.5 Versus Intra-Aortic Balloon Pump in Patients Undergoing High-Risk Percutaneous Coronary Intervention: The PROTECT II Study. Circulation. 2012;126:1717-1727. ] As for shock, the Impella-EUROSHOCK registry, a study evaluating the safety and efficacy of Impella 2.5 support in patients with cardiogenic shock after myocardial infarction, demonstrated that the use of these pLVAD is feasible, and improves organ perfusion, however, mortality remains high [6363. Lauten A, Engstrom AE, Jung C, et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail. 2013;6:23-30. ]. When it comes to management of cardiogenic shock, the IMPRESS in severe shock trial compared Impella CP to IABP in a randomized control trial of 48 mechanical ventilated patients with severe cardiogenic shock and failed to demonstrate mortality benefit [7272. Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, de Winter RJ, Piek JJ, Lagrand WK, de Mol BA, Tijssen JG, Henriques JP. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2017;69:278-287. ]). Moreover, a recent meta-analysis of the 4 RCT to date comparing PVADs to IABP did not support the unselected use of these devices in cardiogenic shock, compared to IABP [1111. Thiele H Jobs A, Ouweneel DM, Henriques JPS, Seyfarth M, Desch S, Eitel I, Pöss J, Fuernau G, de Waha S. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J. 2017 ;38:3523-3531. ]). The PVADs used in this meta-analysis however included TandemHeart™ (2 studies), Impella 2.5 in another one, and only one study with Impella CP. Most recently, a recent large propensity matched registry based retrospective study of over 3300 patients with MI complicated with cardiogenic shock treated with PVAD compared with IABP was associated with higher adjusted risk of in-hospital mortality and major bleeding complications [1313. Dhruva SS, Ross JS, Mortazavi BJ, Hurley NC, Krumholz HM, Curtis JP, Berkowitz A, Masoudi FA, Messenger JC, Parzynski CS, Ngufor C, Girotra S, Amin AP, Shah ND, Desai NR. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump With In-Hospital Mortality and Major Bleeding Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA. 2020 Feb 323(8):734-74510. ].
When it comes to cardiogenic shock, it seems that timing and rationale of use is what makes the difference when using these devices. The National Cardiogenic Shock Initiative [7373. Basir MB, Kapur NK, Patel K, Salam MA, Schreiber T, Kaki A, Hanson I, Almany S, Timmis S, Dixon S, Kolski B, Todd J, Senter S, Marso S, Lasorda D, Wilkins C, Lalonde T, Attallah A, Larkin T, Dupont A, Marshall J, Patel N, Overly T, Green M, Tehrani B, Truesdell AG, Sharma R, Akhtar Y, McRae T 3rd, O'Neill B, Finley J, Rahman A, Foster M, Askari R, Goldsweig A, Martin S, Bharadwaj A, Khuddus M, Caputo C, Korpas D, Cawich I, McAllister D, Blank N, Alraies MC, Fisher R, Khandelwal A, Alaswad K, Lemor A, Johnson T, Hacala M, O'Neill WW; National Cardiogenic Shock Initiative Investigators. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2019 Jun 1;93(7):1173-1183. ] has demonstrated for the first time a tangible survival benefit by creating a protocol for timing of Impella use in a goal directed approach. The survival discharge was 72%. Introducing the concept of “door to support” time guided by objective evidence of elevated LV filling pressure and placing the PVAD support prior to PCI intervention as well as continuous assessment of need for escalation of support did make an important difference. This initiative is continuing to be assessed at a national in the US with over 30 centers and will likely yield promising results to further guide our understanding of PVAD support in cardiogenic shock. However, this single-arm registry lacks a control group and therefore, comparisons cannot be drawn to the contemporary standard of care.
Physiology and haemodynamic effects
The transvalvular axial flow pumps provide non-pulsatile circulatory support and LV unloading with increased cardiac output without the need of concomitant venous access and transseptal puncture. This is the main difference with the TandemHeart™ PVAD ( Table 3 ). The Impella® system provides in-series circulatory support (left ventricular to aortic), instead of in-parallel assistance (left atrial to aortic) [1818. Naidu SS. Novel percutaneous cardiac assist devices: the science of and indications for hemodynamic support. Circulation. 123:533-543.
This recent review describes the common percutaneous ventricular assist devices including a thorough section on physiological mechanisms as well as the main differences between the TandemHeart™ and Impella® recover system, including a section on developing indications and choosing between the different available options].
Indications
Similar to the TandemHeart™, the indications for the Impella® system include the need for partial circulatory support in patients with cardiogenic shock as a bridge to recovery or a bridge to procedure in complex, high-risk PCI ( Table 1 and Figure 1 ).
Implantation and device description
The Impella® LP 2.5 and CP models are suited for percutaneous implantation, whereas the LP 5.0 model requires surgical cutdown of the target artery (femoral vs. axillary) for insertion. When the percutaneous system is used, a 13 or 14 Fr sheath is placed into the femoral artery using the Seldinger technique. Subsequently, a 5 Fr pigtail catheter is used to access the ventricle in a retrograde fashion. This catheter is then exchanged over a wire for the catheter-pump assembly (12 Fr or 14 Fr) and, once confirmed position in the left ventricular cavity, circulatory support is initiated and titrated using the nine different performance levels (P1-9, maximal flow of 2.5 L/min or 4 L/min respectively). P1 and P9 performance levels are designed as starting/weaning and boost performance. Therefore should only be used for a few minutes. Usually the Impella operates between P2 and P8 performance levels during an episode of haemodynamic support. Proper placement should be confirmed prior to initiation and after every time the patient is transported for diagnostic tests or procedures. For correct catheter position, either TTE (parasternal long axis view or apical 5 chamber view) or TEE (mid esophageal long axis view 120-135o) can be used. Further manipulation now has become easier as newer pumps come with SmartAssist technology that includes an optical placement sensor. The catheter inlet area (not J tip) should be 4-4.5 cm below the aortic valve, the outlet area well above the aortic valve and the catheter should be angled toward the LV apex away from the heart wall and not curled up or blocking the mitral valve. For the Impella RP, the catheter inlet area should be in the IVC and the outlet opening in the pulmonary artery.
The Impella® system consists of the following components:
- Impella catheter with built-in pump
- Impella console with portable battery
- Power supply and cable
- Braun Vista basic infusion pump
Maintenance and explantation
As with other PVADs, systemic anticoagulation with intravenous heparin is necessary to reduce the risk of thromboembolic complications. The ACT is used to monitor this during the procedure. After an ACT of 250-500 seconds is achieved, the device is inserted as described above. The ACT should be maintained at 160-180 seconds after device insertion and throughout circulatory support. A unique feature of this device is the need for a purge system that delivers rinsing fluid to the Impella pump. This is achieved with the Braun Vista pump which uses 20% dextrose plus heparin 50 IU/ml solution. Anticoagulation management of these devices has become an important topic of discussion as the use of PVADs has increased and the length of therapy pushed to the limits. Using aPTT or anti-Xa based anticoagulation is being evaluated. [7272. Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, de Winter RJ, Piek JJ, Lagrand WK, de Mol BA, Tijssen JG, Henriques JP. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2017;69:278-287. ] Use of institution specific standard protocols is advised.
Two weaning protocols have been described by the manufacturer; however, physician discretion and institutional driven protocols may be used. The rapid weaning protocol consists of decreasing the performance level in 2-level steps every several minutes until P2 level is reached. The circulatory support should be maintained at this level for at least 10 minutes prior to discontinuing the device. After this interval, if the patient remains stable, the performance level is decreased to P1, the catheter is pulled back into the aortic root and the pump is stopped. Subsequently, the catheter-pump is explanted and a percutaneous arterial closer is usually needed.
When supporting sicker patients with cardiogenic shock, the slow weaning protocol may be more appropriate. This is achieved by decreasing the pump performance level 2-levels every 2-3 hour intervals with careful assessment of hemodynamics and cardiac output. Caution is made to not decrease the pump performance level below P2 while the catheter is in the ventricle since retrograde flow may occur. Once the patient is stable on performance level P2 for at least 2 hours, the performance level is reduce to P1 and the catheter is pulled back to the aorta to subsequently stop the pump. After discontinuation of heparin and ACT less than 150 seconds, the cannula is removed as previously described.
Contraindications
Due to the transvalvular nature of this device, it is contraindicated in the presence of aortic valve prosthesis, severely calcified aortic valve with or without aortic stenosis, significant aortic regurgitation (grade 2 or more), presence of LV thrombus, VSD and severe peripheral arterial disease. Other relative contraindications include abdominal or thoracoabdominal aortic aneurysms, aortic dissection, and the presence of femoral-popliteal bypass grafts. The use in the presence of aorto-bifemoral grafts has never been described and should be consulted with a vascular surgeon if used for elective circulatory support. However, axillary or subclavian artery approach has been used and could be considered in this patient population.
Complications and limitations
Similar to other PVADs, complications can be divided in vascular, mechanical, infectious, neurologic and hematologic categories [3434. Subramaniam AV, Barsness GW, Vallabhajosyula S, Vallabhajosyula S. Complications of Temporary Percutaneous Mechanical Circulatory Support for Cardiogenic Shock: An Appraisal of Contemporary Literature. Cardiol Ther. 2019;8(2):211‐228. doi:10. 1007/s40119-019-00152-8. ]. Potential complications of the Impella® PVAD include: cerebrovascular accident, aortic valve injury with resultant aortic insufficiency, arrhythmia (atrial and ventricular), cardiac tamponade, infection, vascular injury, limb ischaemia, bleeding and coagulopathy.
This device, like all centrifugal pumps can provoke haemolysis [6161. Maini B, Naidu SS, Mulukutla S, et al. Real-world use of the Impella 2.5 circulatory support system in complex high-risk percutaneous coronary intervention: the USpella Registry. Catheter Cardiovasc Interv. 2012;80:717-725. ] and thrombocytopenia. Therefore, complete blood count and markers for hemolysis (free Hb, haptoglobin, LDH) should be followed at least daily.
Although the use of this device has gained significant popularity for temporary circulatory support for cardiogenic shock and high risk PCI, due to the obviation of concomitant venous cannulation and transseptal puncture, the haemodynamic response to the device is variable and occasionally can cause LV volume overload [6565. Valgimigli M, Steendijk P, Serruys PW, et al. Use of Impella Recover(R) LP 2.5 left ventricular assist device during high-risk percutaneous coronary interventions; clinical, haemodynamic and biochemical findings. EuroIntervention. 2006;2:91-100. , 6666. Thomopoulou S, Manginas A, Cokkinos DV. Initial experience with the Impella Recover LP 2.5 micro-axial pump in patients undergoing high-risk coronary angioplasty. Hellenic J Cardiol. 2008;49:382-387. ]. This phenomenon could be related to malpositioning of the pump (too deep) or a peri-device leak limiting proper coaptation of the aortic valve leaflets. Echocardiography to monitor LV size and function is useful and new SmartAssist technology should reduce and improve the practical use and manipulation of the device.
Impella®
- Can be used for left or right ventricular assistance
- Active cardiac support provided with flow rates of 2.5-5 L/min
- Functions independent of a stable cardiac rhythm
- Impella LP 2.5 and CP can be inserted percutaneously without a surgical approach
OTHER PVADs
There are a few other pumps under development or in study phase that may be part of the armamentarium in the future. The PVADs already discussed are the main devices available for use at this time and the ones with more data to support their use. Nevertheless, it is worthwhile to introduce a few devices that may be available in the future.
The HeartMate PHPTM (Abbott, Minneapolis, MN, USA) is a next generation 14 Fr compatible axial flow pump that expands to 24 Fr at the level of the aortic valve and capable of generating up to 5 L/min flow. ( [7575. Van Mieghem NM, Daemen J, den Uil C, Dur O, Joziasse L, Maugenest AM, Fitzgerald K, Parker C, Muller P, van Geuns RJ.Design and principle of operation of the HeartMate PHP (percutaneous heart pump). EuroIntervention. 2018 ;13:1662-1666. ] ) It operates with a similar concept of Impella but smaller profile, percutaneous insertion, outperforming by flow standards the current Impella CP. Studies evaluating safety and suitability for cardiogenic shock and high risk PCI were underway but halted in 2018.
The PulseCath iVAC 2L (PulseCath, Amsterdam, The Netherlands) is a next generation pulsatile support system driven by any standard IABP console and generating pulsatile blood flow for up to 2 L, outperforming standard IABP and with the theoretical benefit of pulsatile support for coronary perfusion during high risk PCI [7676. den Uil CA, Daemen J, Lenzen MJ, Maugenest AM, Joziasse L, van Geuns RJ, Van Mieghem NM. Pulsatile iVAC 2L circulatory support in high-risk percutaneous coronary intervention. EuroIntervention. 2017 ;12:1689-1696. ]. The iVAC insertion kit consists of a 17 Fr 100 cm thin wall insertion catheter, a membrane pump and a catheter protector. The transparent membrane pump consists of a blood chamber and an air chamber. The blood chamber is connected to the catheter and while the air chamber is connected to a pneumatic filled helium filled IABP driver. The iVAC actively aspirates blood from the left ventricle during systole and ejects this blood into the ascending aorta in diastole creating a counter pulsation and an addition to circulatory assist of up to 2 Lts/min providing unique type of support.
EXTRACORPOREAL MEMBRANE OXYGENATION
The previously described PVADs depend on adequate lung function in order to provide adequate circulatory support. Extracorporeal membrane oxygenation (ECMO) consists of a blood pump and a circuit with a built-in oxygenator, with the capability to provide partial to near full cardiopulmonary support. The term “extracorporeal life support” (ECLS) refers to the use of ECMO as temporary support in cardiopulmonary collapse refractory to appropriate cardiopulmonary resuscitation (CPR) under medical direction. Although there are different cannulation routes, the classic access for adults in cardiopulmonary collapse is achieved via femoral vessels to provide venous-arterial bypass. Veno-venous ECMO via the trans-jugular approach with a dual lumen single cannula can be used for respiratory failure alone. In adults, a 15-23 Fr arterial cannula is inserted via femoral artery into the descending aorta and a 15-29 Fr venous cannula inserted via femoral vein into the right atrium (depending on manufacturer, vessel diameter and patients size). After appropriate de-airing, these cannulas are connected to the external pump and membrane oxygenator. The pump is primed with a crystalloid-colloid solution, and subsequently, blood is withdrawn from the right atrium, pumped through the heat exchanger, membrane oxygenator and returned via femoral artery to the aorta. The circulatory support is retrograde continuous flow, but usually pulsatile arterial pressure is maintained, unless ECMO is providing complete cardiopulmonary support ( Figure 1 ). Limitations of ECMO in the catheterisation laboratory include lack of direct LV unloading, increased LV afterload and requirement of more personnel (ECMO specialists, perfusionists). Furthermore, it produces a more pronounced systemic inflammatory response [4949. Hall RI, Smith MS, Rocker G. The systemic inflammatory response to cardiopulmonary bypass: pathophysiological, therapeutic, and pharmacological considerations. Anesth Analg. 1997;85:766-782. ]. Due to increased LV afterload, and potential for LV distention, the IABP [7777. Vallabhajosyula S, O’Horo JC, Antharam P, et al. Concomitant Intra-Aortic Balloon Pump Use in Cardiogenic Shock Requiring Veno-Arterial Extracorporeal Membrane Oxygenation. Circ Cardiovasc Interv. 2018;11(9):e006930. ] in the context of myocardial ischemia, or the Impella has increasingly been used from the contralateral femoral artery to provide LV venting, or kept in place when LV assistance has required upgrading to ECMO cardiopulmonary support. [7878. Vallabhajosyula S, O’Horo JC, Antharam P, et al. Venoarterial Extracorporeal Membrane Oxygenation With Concomitant Impella Versus Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. ASAIO J. 2020;66(5):497‐503. , 7979. Tepper S, Masood MF, Baltazar Garcia M, Pisani M, Ewald GA, Lasala JM, Bach RG, Singh J Balsara KR, Itoh A. Left Ventricular Unloading by Impella Device Versus Surgical Vent During Extracorporeal Life Support. Ann Thorac Surg. 2017;104:861-867. ]) . ECMO requires the slightly reduced level of anticoagulation achieved with heparin 50-100 units/kg , ACT 200-250 or antiXa level 0.3-0.5. In the era of partial circulatory support, the role of standard ECMO in the catheterisation laboratory is limited to refractory cardiopulmonary failure in which percutaneous ventricular assistance alone is not sufficient due to concomitant respiratory failure. Moreover, this mode of circulatory support can be used to maintain homeostasis as bridge to emergent cardiac surgery. Nevertheless, the role of ECMO in cardiac arrest (ECLS) and post arrest cardiogenic shock may improve survival and neurological outcome [8080. Ouweneel DM, Schotborgh JV, Limpens J, Sjauw KD, Engström AE, Lagrand WK, Cherpanath TGV, Driessen AHG, de Mol BAJM, Henriques JPS. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. 2016 ;42:1922-1934. ]. In a large national database of over 9 million patients admitted with AMI from 2000 to 2014, a retrospective review of over 2900 patients that received ECMO, remonstrated that the use has increased significantly over the past decade, with a mortality of 59%. Of the survivors, durable LVAD and cardiac transplantation was performed in 11.2% and 40% were discharged to skilled nursing facilities [8181. Vallabhajosyula S, Prasad A, Bell MR, Sandhu GS, Eleid MF, Dunlay SM, Schears GJ, Stulak JM, Singh M, Gersh BJ, Jaffe AS, Holmes DR Jr, Rihal CS, Barsness GW. Extracorporeal Membrane Oxygenation Use in Acute Myocardial Infarction in the United States, 2000 to 2014. Circ Heart Fail. 2019 Dec;12(12):e005929. doi: 10.1161/CIRCHEARTFAILURE.119.005929. Epub 2019 Dec 12. PMID: 31826642. ]
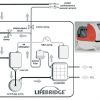
Three of the current, easy to use devices, designed for rapid availability of cardiopulmonary support include the LIFEBRIDGE® B2T (Medizintechnik GmbH, Ampfing, Germany) and the CARDIOHELP (MAQUET Getinge Group, Hirrlingen, Germany ) and the expanded TandemLife systems. LIFEBRIDGE® B2T system is a portable, modular, rapidly available mechanical circulatory support that can be used for short-term peripheral cardiopulmonary bypass for acute cardiopulmonary failure, as a bridge to procedure for high-risk PCI or emergent cardiac surgical procedures ( Figure 6 ) [6767. Krane M, Mazzitelli D, Schreiber U, et al. LIFEBRIDGE B2T--a new portable cardiopulmonary bypass system. ASAIO J. , 6868. Mehlhorn U, Brieske M, Fischer UM, et al. LIFEBRIDGE: a portable, modular, rapidly available "plug-and-play" mechanical circulatory support system. Ann Thorac Surg. 2005;80:1887-1892. ]. The system has achieved CE certification and FDA clearance, and the introduction of this system in clinical practice is well established in Europe. The LIFEBRIDGE® B2T system introduces numerous theoretical and practical advantages over all other classic ECMO systems. It is simple to use, safe and practically a “plug-and-play” device that can provide full cardiopulmonary support. The size, weight and semi-automatic priming and de-airing functions make it ideal for the emergency setting and for use in the catheterisation laboratory or any emergency setting outside the operating room. Furthermore, the state-of-the-art safety features, such as, active intra-aortic air emboli detection and management, flow and level monitoring, makes this device unique. For venous drainage a 22-24 Fr cannula is inserted in via femoral vein and for arterial cannulation a 16-20 Fr cannula can be used. The device is capable of providing full cardiopulmonary support with flows up to 6 L/min (4.1 L/min if peripheral cannulation) and adequate gas exchange [6868. Mehlhorn U, Brieske M, Fischer UM, et al. LIFEBRIDGE: a portable, modular, rapidly available "plug-and-play" mechanical circulatory support system. Ann Thorac Surg. 2005;80:1887-1892. ]. These unique characteristics of this device make it ideal for full cardiopulmonary support in the catheterisation laboratory for cardiogenic shock (transport or diagnosis or therapy) and for ECLS. Other possible indications include circulatory support in high-risk PCI, back-up assistance for transcatheter aortic valve replacement [8282. Vallabhajosyula S, Patlolla SH, Sandhyavenu H, et al. Periprocedural Cardiopulmonary Bypass or Venoarterial Extracorporeal Membrane Oxygenation During Transcatheter Aortic Valve Replacement: A Systematic Review.J Am Heart Assoc. 2018;7(14):e009608. Published 2018 Jul 9. doi:10.1161/JAHA.118. 009608. ], ventricular tachycardia ablation [88. Vallabhajosyula S, Vallabhajosyula S, Vaidya VR, et al. Venoarterial Extracorporeal Membrane Oxygenation Support for Ventricular Tachycardia Ablation: A Systematic Review [published online ahead of print, 2020 Jan 22]. ASAIO J. 2020;10. ], massive pulmonary embolism, as an active rewarming device in hypothermia and even as an alternative to the standard cardiopulmonary bypass machines used for cardiac surgery.
The CARDIOHELP SYSTEM ( Figure 7) is also a portable, compact, lightweight ECMO that is characterised by its user friendly operation, management and short preparation time [6969. Arlt M, Philipp A, Voelkel S, Camboni D, Rupprecht L, Graf BM, Schmid C, Hilker M. Hand-held minimised extracorporeal membrane oxygenation: a new bridge to recovery in patients with out-of-centre cardiogenic shock. Eur J Cardiothorac Surg. 2011;40:689-94. ]. It weighs approximately 10 kg and can be carried by one person. The heart-lung support (HLS) system includes the control unit or module (CARDIOHELP) and the disposable cannulae designed to support the patient for up to thirty days. It can provide up to 5 or 7 lt/min blood flow.
The TandemLife is an extension of the TanderHeart technology with the addition of the oxygenator. Centers with the TandemHeart platform can easily convert to ECMO with the addition of oxygenator and venous-arterial cannula configuration. We will be seeing more of this technology, stretching the limits of minimally invasive procedures and haemodynamic support.