Summary
The aorta is stratified into thoracic aorta (aorta ascending, aortic arch and aorta descending) and abdominal aorta.
Aortic disease includes occlusive disease (not covered in this chapter), aneurysmal dilatation, aorta dissection and trauma.
The pathogenesis of aortic aneurysm is not fully understood, but is believed to be multifactorial. Disturbance of the metabolic balance resulting in excessive extracellular matrix degradation may be the key to progressive wall deterioration with subsequent expansion or rupture.
Aortic dissections are classified according to the anatomical location using the Stanford and DeBakey classification. The fundamental distinction is whether the dissection is proximal or distal to the left subclavian artery origin.
Diagnostic modalities to identify and classify aortic disease include computed tomography and magnetic resonance imaging.
Asymptomatic aneurysms are initially managed medically, while an intervention is indicated for symptomatic and expanding aneurysms. Patients with an acute uncomplicated aortic dissection should be admitted to a monitoring unit and treated medically for pain and blood pressure control.
In general an intervention is indicated for Type A dissections and complicated Type B dissections.
The choice between a surgical or endovascular intervention for aorta disease will depend on the localisation and extent of disease. It is anticipated that there will be a shift from conventional open surgery to combinations of open and endovascular interventions (hybrid procedures) and eventually to full endovascular procedures with fenestrated and branched stent-grafts.
Aneurysms of the thoracic aorta
DEFINITION
The normal diameter of the ascending aorta has been defined as 2.1 cm/m2 [11. Aronberg DJ, Glazer HS, Madsen K, Sagel SS. Normal thoracic aortic diameters by computed tomography. J Comput Assist Tomogr. 1984;8:247–50. , 22. Hager A, Kaemmerer H, Rapp-Bernhardt U, Blücher S, Rapp K, Bernhardt TM, Galanski M, Hess J. Diameters of the thoracic aorta throughout life as measured with helical computed tomography. J Thorac Cardiovasc Surg. 2002;123:1060–6. ], and the value for the descending aorta is 1.6 cm/m2 [33. Erbel R, Alfonso F, Boileau C, Dirsch O, Eber B, Haverich A, Rakowski H, Struyven J, Radegran K, Sechtem U, Taylor J, Zollikofer C, Klein WW, Mulder B, Providencia LA; Task Force on Aortic Dissection, European Society of Cardiology. Task Force on Aortic Dissection of the European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22:1642–81.
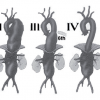
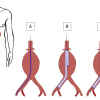
This manuscript describes the provisional guidelines prepared by the ESC taskforce on aortic dissection.]. Analysis of variance reveals no influence of weight, height or body surface area, but there is a correlation with sex and age [22. Hager A, Kaemmerer H, Rapp-Bernhardt U, Blücher S, Rapp K, Bernhardt TM, Galanski M, Hess J. Diameters of the thoracic aorta throughout life as measured with helical computed tomography. J Thorac Cardiovasc Surg. 2002;123:1060–6. ]. Regarding the latter, current opinion is consistent with the study of Aronberg et al, who showed that aortic diameters increase about 1mm per decade during adulthood [11. Aronberg DJ, Glazer HS, Madsen K, Sagel SS. Normal thoracic aortic diameters by computed tomography. J Comput Assist Tomogr. 1984;8:247–50. ]. Thus, dilatation of aortic segments should be defined as deviation of more than 2 SD from the normal value. Therefore, a localised aneurysm should continue to be defined as a greater than 50% dilatation compared to the diameter of the adjacent normal vessel [44. Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery. J Vasc Surg. 1991;13:452–8. ]. Aneurysms distal to the origin of the left subclavian artery are classified according to Crawford classification (type I to type IV), recently adapted by Safi(type V) [55. Crawford ES, Crawford JL, Safi HJ, Coselli JS, Hess KR, Brooks B, Norton HJ, Glaeser DH. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg. 1986;3:389-404. , 66. Safi HJ, Miller CC. Spinal cord protection in descending thoracic and thoracoabdominal aortic repair. Ann Thorac Surg. 1999;67:1937-9. ] ( Figure 1 ).
In contrast to abdominal aneurysms (AAA), which had a male predominance, up to one half of TAA were identified in women [77. Clouse WD, Hallett JW, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA. 1998;280:1926-9. , 88. Lilienfeld DE, Gunderson PD, Sprafka JM, Vargas C. Epidemiology of aortic aneurysms. Mortality trends in the United States, 1951 to 1981. Arteriosclerosis. 1987;7:637-43. ]. Moreover, a quarter of the patients with a TAA had concomitant infrarenal aneurysmal aortic disease and up to 13% had multiple aneurysms. When AAA was previously diagnosed the risk of having a TAA ranged from 3.5% to 12% [99. Gloviczki P, Pairolero P, Welch T, Cherry K, Hallett J, Toomey B, Naessens J, Orszulak T, Schaff H. Multiple aortic aneurysms: the results of surgical management. J Vasc Surg. 1990;11:19-27. ]. Twenty-two per cent of patients with aortic aneurysm and dissection did not reach hospital alive with the diagnosis made at autopsy, and aortic rupture occurred in 74% of all TAAs with a mortality rate of 94.3% [1010. Olsson C, Thelin S, Stähle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14 000 cases from 1987 to 2002. Circulation. 2006 ;114:2611-8. ].
AETIOLOGY
The pathogenesis of aortic aneurysms is not fully understood, but is believed to be multifactorial [1111. Panneton JM, Hollier LH. Nondissecting thoracoabdominal aortic aneurysms. Part I. Ann Vasc Surg. 1995;9:503-14. ].
The medial layer of the aorta is composed of both vascular smooth muscle cells and extracellular matrix (ECM) proteins, primarily elastin and collagen, and a balanced composition of those constituents is critical for preserving functional properties and mechanical compliance of the aorta. Disturbance of metabolic balance resulting in excessive ECM degradation may be central to progressive aortic wall deterioration with subsequent expansion or rupture. With increasing age, aortic wall stiffness increases as a result of structural changes induced by hypertension, hyperlipidaemia, diabetes mellitus and smoking, all of which promote atherosclerosis [22. Hager A, Kaemmerer H, Rapp-Bernhardt U, Blücher S, Rapp K, Bernhardt TM, Galanski M, Hess J. Diameters of the thoracic aorta throughout life as measured with helical computed tomography. J Thorac Cardiovasc Surg. 2002;123:1060–6. ]. Genetic defects are also involved in thoracic aneurysm formation. Three major inherited disorders are known to cause aortic diseases: Marfan syndrome, Ehlers-Danlos syndrome and other familial forms of connective tissue diseases. However, most cases represent so-called “overlap” syndromes reflecting the currently incomplete knowledge of genetic defects associated with aortic diseases. Dilatation of the aorta is found in 50% of childhood Marfan syndrome with progression over time. The greatest progression of aortic dilatation occurs in the aortic root at 0.2 cm/year [1212. Fenoglio J, McAllister H, DeCastro C, Davia JE, Cheitlin MD. Congenital bicuspid aortic valve after age 20. Am J Cardiol. 1977;39:164–9. , 1313. Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorax Surg. 2002;74:1877–80. ]. Weakening of the aortic wall can also be induced by inflammation resulting from microbiological diseases or multisystem inflammation disorders. Aortitis induced by syphilis and Staphylococcus aureus infection is well known. Kawasaki’s syndrome is characterised by more circumscriptive aneurysm formation, whereas syphilis can induce diffuse wall thickening and aneurysm formation of the ascending aorta. Behçet’s disease, like other forms of vasculitis, may lead to local aneurysm formation and perforation rather than dissection [1414. Tsui K, Lee K, Chan W, Chan HK, Hon SF, Leung TC, Lee KL, Tsoi TH, Li SK. Behçet’s aortitis and aortic regurgitation: a report of two cases. J Am Soc Echocardiogr. 2004;17:83–6. ]. In giant-cell arteritis, thoracic and abdominal aneurysm may develop [1515. Calvo-Romero JM. Giant cell arteritis. Postgrad Med J. 2003; 9:511–5. ]. The use of cocaine and amphetamines can also lead to aortic wall thinning and aneurysm formation. In aortic stenosis, post-stenotic aneurysm formation can occur, which may even be enhanced after aortic valve prosthesis implantation [1616. Lawrie GM, Earle N, DeBakey ME. Long-term fate of the aortic root and aortic valve after ascending aneurysm surgery. Ann Surg. 1993;217:711–20. ]. An important cause of aneurysm formation is related to trauma, particularly high-speed deceleration trauma, involving the aortic isthmus in 95% [1717. Parmley LF, Mattingly TW, Manion WC, Jahnke EJ Jr. Nonpenetrating traumatic injury of the aorta. Circulation. 1958;17:1086–101. ]. About 15%-20% of deaths are related to aortic trauma in these patients.
Distribution of thoracic aortic aneurysms [11. Aronberg DJ, Glazer HS, Madsen K, Sagel SS. Normal thoracic aortic diameters by computed tomography. J Comput Assist Tomogr. 1984;8:247–50. ]
- 51% ascending aorta
- 11% aortic arch
- 38% descending aorta
CLINICAL FEATURES
Patients with thoracic aortic aneurysms are often asymptomatic at the time of diagnosis. However, depending upon size and location, chest, back, flank, or abdominal pain may occur. Symptoms are usually attributed to unilateral compression, erosion or distortion either of adjacent vessels, with vascular consequences such as superior vena cava compression syndrome, aortic regurgitation or thromboembolic sequelae, or neighbouring structures leading to phrenic nerve dysfunction or hoarseness. Ascending aortic aneurysms may present first with clinical signs of aortic valve regurgitation or subsequent heart failure. Importantly, an aneurysm involving a sinus of Valsalva can rupture into right-sided cardiac chambers, leading to continuous shunting of blood and heart failure. Moreover, ascending and arch aneurysms can erode into the mediastinum, producing hoarseness from compression of vagus nerve or recurrent laryngeal nerve, or, from hemidiaphragmatic paralysis due to compression of the phrenic nerve, wheezing, cough, haemoptysis, dyspnoea, or, pneumonitis in the case of compression of the tracheobronchial tree, and dysphagia from oesophageal compression. At an advanced stage, compression of other intrathoracic structures or erosion of adjacent bony structures may even cause continuous chest or back pain. Occasionally, emboli from layered thrombus within the aneurysm may cause cerebral, renal, and mesenteric ischaemia or claudication.
NATURAL HISTORY
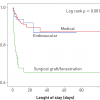
The natural history of thoracic aortic aneurysms is still somewhat unclear. One reason for this is that both aetiology and location of an aneurysm may affect its rate of growth and propensity for dissection or rupture. Longitudinal observation showed that the mean rate of growth for all thoracic aneurysms was significantly lower (0.1cm/y) than of abdominal aneurysms (0.2-0.5cm/y). However, the rate of growth was greater for aneurysms of the descending aorta versus ascending aorta, and greater for dissected versus non-dissected aneurysms, and finally most pronounced in Marfan syndrome [88. Lilienfeld DE, Gunderson PD, Sprafka JM, Vargas C. Epidemiology of aortic aneurysms. Mortality trends in the United States, 1951 to 1981. Arteriosclerosis. 1987;7:637-43. ]. Initial size can also be an important predictor of the rate growth. A study based on 721 patients supported the fact that TAA size had a profound impact on risk for rupture with an annual rate of 2% in aneurysms <5 cm, 3% in aneurysms 5-5.9 cm, and 7% for aneurysms beyond 6 cm in diameter. Therefore the risk appears to rise abruptly as thoracic aneurysms reach a size of 6 cm [1818. Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, Elefteriades JA. Yearly rupture or dissections rate for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17-27. ]. Similar results were reported over 5 years of follow-up in 133 patients with a rupture risk of 0% for ectasia less than 40 mm, compared to 16% and 31% for aneurysms of 40 to 59 and ≥ 65 mm, respectively [1313. Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorax Surg. 2002;74:1877–80. , 1919. Ellis PR, Cooley DA, Bakey ME. Clinical consideration and surgical treatment of annuloaortic ectasia. J Thorac Cardiovasc Surg. 1961;42:363–70. ] ( Figure 2). Beyond this dimensional view other variables with impact on expansion rate and risk of rupture are important. Older age and the history of COPD are independent risk factors for rupture of TAA in a multivariate regression analysis. In this context, symptomatic thoracic aortic aneurysms have a 27% five-year survival, compared to 58% in asymptomatic patients [1313. Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorax Surg. 2002;74:1877–80. , 1818. Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, Elefteriades JA. Yearly rupture or dissections rate for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17-27. , 1919. Ellis PR, Cooley DA, Bakey ME. Clinical consideration and surgical treatment of annuloaortic ectasia. J Thorac Cardiovasc Surg. 1961;42:363–70. ].
Chest radiographic findings in thoracic aortic aneurysm
- widening of the mediastinum
- left-sided enlargement of the ascending aorta
- change in aortic knob
- enlarged/elongated descending aorta
DIAGNOSTIC PROCEDURES
Various tomographic imaging techniques are useful to diagnose aortic aneurysm formation, which in many instances is an incidental finding. A chest x-ray for example is likely to fail to distinguish an aneurysm from a tortuous aorta and, thus, some early aneurysms are missed ( Figure 3 ). On plain chest film aortic abnormalities were detected in only 22 out of 36 patients (61%), even in the presence of recent chest or back pain [2020. von Kodolitsch Y, Nienaber CA, Dieckmann C, Schwartz AG, Hofmann T, Brekenfeld C, Nicolas V, Berger J, Meinertz T. Chest radiography for the diagnosis of acute aortic syndrome. Am J Med. 2004;116:73-7. ]. Conversely, computed tomography (CT) with contrast image enhancement, is instrumental in determining location, size and/or any complications. Aortic wall thickness, calcium deposits in the area of the coronary arteries and aortic wall, side-branch anatomy as well as potential complications can be visualised clearly ( Figure 4 ). Disadvantages are the use of potentially nephrotoxic contrast agents and the inability to visualise aortic regurgitation and cardiac wall motion abnormalities. Magnetic resonance imaging (MRI) produces unrestricted high-resolution views of the aorta in transverse, sagittal and coronal planes. Because of its higher quality images, MRI provides optimal delineation of the origin and extent of aneurysm formation ( Figure 5 ). A big advantage is the lack of radiation burden, which allows multiple follow-up studies particularly in young adults and women of childbearing age. Ultrasound can visualise the whole aorta using transthoracic, suprasternal, subcostal and abdominal investigation usually combined with colour duplex imaging. However, the ascending part of the aortic arch can never be imaged by transoesophageal ultrasound because of the interposition of the trachea and the right main bronchus between oesophagus and aorta [2121. Willens HJ, Kessler KM. Transesophageal echocardiography in the diagnosis of disease of the thoracic aorta: part 1. Aortic dissection, aortic intramural hematoma, and penetrating atherosclerotic ulcer of the aorta. Chest. 1999;116:1772–9. , 2222. Willens HJ, Kessler KM. Transesophageal echocardiography in the diagnosis of diseases of the thoracic aorta: part II. Atherosclerotic and traumatic disease of the aorta. Chest. 2000;117:233–43. ]. The invasive technique most widely used is aortography, which allows visualisation of aortic regurgitation, cardiac function, coronary artery disease, side-branch involvement and aneurysm location, size and extent. The disadvantage is the exposure to ionising radiation and the use of contrast agents [2323. Dake MD, Kato N, Mitchell RS, Semba CP, Razavi MK, Shimono T, Hirano T, Takeda K, Yada I, Miller DC. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340:1546–52. , 2424. Nienaber CA, Fattori R, Lund G, Dieckmann C, Wolf W, von Kodolitsch Y, Nicolas V, Pierangeli A. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539–45.
Preliminary results of endovascular treatment for thoracic aorta dissection compared with a matched group of patients treated by open surgical repair.]. Positron emission tomography (PET) allows detection of increased metabolic activity in thoracic and abdominal aneurysms with 18F-Fluorodeoxyglucose uptake as a strong predictor of aneurysm expansion and rupture [2525. Sakalihasan N, Van Damme P, Gomez P, Rigo P, Lapiere CM, Nusgens B, Limet R. Positron emission tomography (PET) evaluation of abdominal aortic aneurysm (AAA). Eur J Vasc Endovasc Surg. 2002;23:431–6. ]. The combination of PET and CT may potentially offer new insights, especially with regards to ongoing local inflammation or aortitis. Besides imaging techniques, laboratory tests may be helpful for identifying active inflammatory processes. Elevated levels of fibrinogen, a1-antitrypsin, haptoglobin and caeruloplasmin C-reactive protein and D-dimers are found frequently [2626. Nienaber CA, Eggebrecht H, Ince H. Biomarker bei Aortenerkranungen. Kardiologie Update 2005;1:18–20. , 2727. Lindholt JS, Vammen S, Fasting H, Henneberg EW, Heickendorff L. The plasma level of matrix metalloproteinase 9 may predict the natural history of small abdominal aortic aneurysms. A preliminary study. Eur J Vasc Endovasc Surg. 2000;20 281–5. ].
MEDICAL MANAGEMENT
Asymptomatic aneurysms are initially managed medically, while surgery is indicated for symptomatic and expanding aneurysms, and those beyond 55 mm in diameter in the ascending aorta, or 60 mm in the descending aorta regardless of site or symptoms. A novel predictor for rupture of thoracic aortic aneurysm, the aortic size index, may be useful to predict increasing rates of rupture, dissection or death. Individual body surface area information is utilised for the aortic size index (aortic diameter/m2) enabling improved and individualised selection for surgical repair. An aortic size index stratification ≥2.75 cm/m2 represents a low risk (approximately 4%/year), 2.75‑4.24 cm/m2 a moderate risk (approximately 8% per year), and >4.25 cm/m2 a high risk (approximately 20% per year), underlining the importance of relative aortic size for predicting complications [2828. Muhs BE, Vincken KL, van Prehn J, Stone MK, Bartels LW, Prokop M, Moll FL, Verhagen HJ. Dynamic Cine-CT Angiography for the Evaluation of the Thoracic Aorta; Insight in Dynamic Changes with Implications for Thoracic Endograft Treatment. Eur J Vasc Endovasc Surg. 2006;32:532-6. ]. In an asymptomatic patient, medical management includes aggressive blood pressure lowering and rate-control medication such as potent-adrenergic receptor blocking agents. The use of multiple antihypertensive drugs may be necessary, together with surveillance and serial tomographic imaging by CT or MRI to evaluate growth and anatomy of the aneurysm. Moreover, patients should be advised to avoid heavy lifting or any strain since straining isometric exercise may abruptly increase intrathoracic pressure and blood pressure.
SURGICAL AND ENDOVASCULAR MANAGEMENT
In addition to medical treatment with beta-blocking agents, surgical and/or endovascular management may become necessary. Aortic dissection and rupture are the most severe complication of aortic aneurysm formation, leading to high operative risk in urgent or emergency situations [33. Erbel R, Alfonso F, Boileau C, Dirsch O, Eber B, Haverich A, Rakowski H, Struyven J, Radegran K, Sechtem U, Taylor J, Zollikofer C, Klein WW, Mulder B, Providencia LA; Task Force on Aortic Dissection, European Society of Cardiology. Task Force on Aortic Dissection of the European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22:1642–81.
This manuscript describes the provisional guidelines prepared by the ESC taskforce on aortic dissection.]. Operative mortality has been reported at 1.5% for elective, 2.6% for emergency and 11.7% for urgent surgery. Consequently, elective surgery has been recommended for aneurysms of the ascending aorta > 5.5 cm diameter and for those > 4.5 cm in Marfan syndrome or other connective tissue disease [1313. Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorax Surg. 2002;74:1877–80. , 2929. Roman MJ, Devereux RB, Kramer-Fox R, O’Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989;64 507–12. ]. Composite mechanical valve conduits have been used since their introduction by Bentall and De Bono in 1968 [3030. Zehr KJ, Orszulak TA, Mullany CJ, Matloobi A, Daly RC, Dearani JA, Sundt TM 3rd, Puga FJ, Danielson GK, Schaff HV. Surgery for aneurysms of the aortic root. Circulation. 2004;110:134–7. , 3131. Bentall H, Bono A. A technique for complete replacement of the ascending aorta. Thorax. 1968;23:338–9. ]. Valve-preserving procedures would be ideal to avoid lifelong anticoagulation, but often the valve itself needs replacement [3232. Missirlis YF, Armeniades CD, Kennedy JH. Mechanical and histological study of aortic valve tissue from a patient with Marfan’s disease. Atherosclerosis. 1976;24:335–8. , 3333. Fleischer KJ, Nousari HC, Anhalt GJ, Stone CD, Laschinger JC. Immunohistochemical abnormalities of fibrillin in cardiovascular tissues in Marfan’s syndrome. Ann Thorac Surg. 1997;63:1012–7. ]. If the aortic root exceeds 6 cm, most of the cusps demonstrate tissue abnormalities [3434. Oliveira NC, David TE, Ivanov J, Armstrong S, Eriksson MJ, Rakowski H, Webb G. Results of surgery for aortic root aneurysm in patients with Marfan syndrome. J Thorac Cardiovasc Surg. 2003;125:789–96. ]. Therefore, it is not surprising that in 203 patients operated on in the Mayo Clinic, composite valve conduit reconstruction resulted in a more durable result during a follow-up period of 20 years [3030. Zehr KJ, Orszulak TA, Mullany CJ, Matloobi A, Daly RC, Dearani JA, Sundt TM 3rd, Puga FJ, Danielson GK, Schaff HV. Surgery for aneurysms of the aortic root. Circulation. 2004;110:134–7. ]. Sarsam and Yacoub [3535. Sarsam MA, Yacoub M. Remodeling of the aortic valve annulus. J Thorac Cardiovasc Surg. 1993;105:435–8. ], as well as David and Feindel [3636. David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg. 1992;103:617–22. ], have developed valve-preserving reconstruction techniques. The reoperation rate was reported to be 11% at 10 years [3737. Yacoub MH, Gehle P, Chandrasekaran V, Birks EJ, Child A, Radley-Smith R. Late results of a valve-preserving operation in patients with aneurysms of the ascending aorta and root. J Thorac Cardiovasc Surg. 1998;115:1080–90. ] and only 3% at 8 years, respectively [3838. David TE, Ivanov J, Armstrong S, Feindel CM, Webb GD. Aortic valve-sparing operations in patients with aneurysms of the aortic root or ascending aorta. Ann Thorac Surg. 2002;74:1758–61. ]. However, a high rate of residual aortic regurgitation developed in 25%-45% at 8-10 years [3434. Oliveira NC, David TE, Ivanov J, Armstrong S, Eriksson MJ, Rakowski H, Webb G. Results of surgery for aortic root aneurysm in patients with Marfan syndrome. J Thorac Cardiovasc Surg. 2003;125:789–96. , 3838. David TE, Ivanov J, Armstrong S, Feindel CM, Webb GD. Aortic valve-sparing operations in patients with aneurysms of the aortic root or ascending aorta. Ann Thorac Surg. 2002;74:1758–61. ]. For the aortic arch, surgical intervention is most likely to be the method of choice, which nowadays is more frequently combined with stent graft implantation in order to seal the distal aortic arch to the descending aorta. Special systems have been designed so that implantation can be performed via an antegrade strategy [3939. Coselli JS, Conklin LD, LeMaire SA. Thoracoabdominal aortic aneurysm repair: review and update of current strategies. Ann Thorac Surg.2002;74:1881-4. ]. For thoracic descending or thoracoabdominal aortic aneurysms, the current surgical strategy has been developed over the last 15 years to prevent ischaemic complications. The operation requires permissive hypothermia (32°–34°C nasopharyngeal), moderate heparinisation with 1 mg/kg, renal artery perfusion with 4°C crystalloid solution, aggressive reattachment of segmental arteries (especially between T8 and L1), sequential aortic clamping as well as cerebrospinal fluid drainage, left heart bypass during proximal anastomosis, and selective perfusion of coeliac and superior mesenteric arteries during intercostal, visceral and renal anastomosis [3939. Coselli JS, Conklin LD, LeMaire SA. Thoracoabdominal aortic aneurysm repair: review and update of current strategies. Ann Thorac Surg.2002;74:1881-4. ]. With these protective measures the rate of paraplegia was reduced from about 15 to less than 5% [4040. Coselli JS, LeMaire SA, Köksoy C, Schmittling ZC, Curling PE. Cerebrospinal fluid drainage reduces paraplegia following thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg. 2002;35:635–9. ]. Also renal failure (serum creatinine elevation > 50% above baseline value) could be reduced from about 60% to 20% [4141. Köksoy C, LeMaire SA, Curling PE, Raskin SA, Schmittling ZC, Conklin LD, Coselli JS. Renal perfusion during thoracoabdominal aortic operations: cold crystalloid is superior to normothermic blood. Ann Thorac Surg. 2002;73:730–8. ]. The 5-year survival of 1,773 patients reached nearly 75% compared to a historical figure of 20% [3939. Coselli JS, Conklin LD, LeMaire SA. Thoracoabdominal aortic aneurysm repair: review and update of current strategies. Ann Thorac Surg.2002;74:1881-4. , 4242. Crawford ES, DeNatale RW. Thoracoabdominal aortic aneuysms: observations regarding the natural course of the disease. J Vasc Surg. 1986;3:578-82. ].
For localised aortic pathologies, regardless of true or false aneurysms, endovascular techniques have become alternative treatment options ( Figure 6 ). Currently, there is ongoing controversy on which patients should be treated by endovascular means ( Table 1 ). The long-term durability of aortic stent grafts is promising, but not yet fully proven and careful patient selection is still recommended [4343. Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, Eggebrecht H, Elefteriades JA, Erbel R, Gleason TG, Lytle BW, Mitchell RS, Nienaber CA, Roselli EE, Safi HJ, Shemin RJ, Sicard GA, Sundt TM 3rd, Szeto WY, Wheatley GH 3rd; Society of Thoracic Surgeons Endovascular Surgery Task Force. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85:1-41.
This document reviews the natural history of aortic dissection, indications for repair, outcome after conventional open surgery, available devices and insights from outcomes of randomised studies using stent grafts.]. The suitability of a given patient for endovascular repair is based on both clinical and anatomical considerations. At present, stent grafts are routinely used to treat patients with thoracic aneurysms distal to the aortic arch, and at the infrarenal abdominal aorta ( Figure 7 ). Endovascular treatment of thoracic aortic aneurysms is achieved by transluminal placement of one or more stent graft devices across the longitudinal extent of the lesion. The prosthesis bridges the aneurysmal sac to exclude it from high-pressure aortic blood flow, thereby allowing for sac thrombosis around the endograft and possible remodelling of the aortic wall. Successful TAA exclusion requires normal segments of native aorta at both ends of the lesion of at least 15 mm to 25 mm to ensure adequate landing and contact between the stent graft and the aortic wall with a tight circumferential seal.
Devices are oversized by 10% to provide sufficient radial force for adequate fixation. The preferred and most common site (41%-58%) of vascular access is the common femoral artery. Less frequently, access to the iliac artery (9%-44%) via an extraperitoneal approach is required [4444. Czerny M, Zimpfer D, Fleck T, Hofmann W, Schoder M, Cejna M, Stampfl P, Lammer J, Wolner E, Grabenwoger M. Initial results after combined repair of aortic arch aneurysms by sequential transposition of the supra-aortic branches and consecutive endovascular stent-graft placement. Ann Thorac Surg. 2004;78:1256-60. ]. Thoracotomy, aortic cross clamping, left-heart bypass, and single-lung ventilation are all avoided with an endovascular procedure. Volodos et al first reported the use of stent grafts in TAAs in 1986 in a patient with posttraumatic pseudoaneurysm of the thoracic aorta, using a home-made device [4545. Volodos’ NL, Shekhanin VE, Karpovich IP, Troian VI, Gur’ev IuA. [A self-fixing synthetic blood vessel endoprosthesis] Vestn Khir Im I I Grek. 1986;137:123-5. ]. With rapidly evolving technology both custom-designed and commercially available stent grafts became available for treating thoracic aortic disease and other pathologies [4646. Dake MD, Miller DC, Mitchell RS, Semba CP, Moore KA, Sakai T. The “first generation” of endovascular stent-grafts for patients with aneurysms of the descending thoracic aorta. J Thorac Cardiovasc Surg. 1998;116:689-703. , 4747. Ehrlich M, Grabenwoeger M, Cartes-Zumelzu F, Grimm M, Petzl D, Lammer J, Thurnher S, Wolner E, Havel M. Endovascular stent graft repair for aneurysms on the descending thoracic aorta. Ann Thorac Surg. 1998;66:19-24. , 4848. Cartes-Zumelzu F, Lammer J, Kretschmer G, Hoelzenbein T, Grabenwöger M, Thurnher S. Endovascular repair of thoracic aortic aneurysms. Semin Interv Cardiol. 2000;5:53-7. , 4949. Grabenwöger M, Hutschala D, Ehrlich MP, Cartes-Zumelzu F, Thurnher S, Lammer J, Wolner E, Havel M. Thoracic aortic aneurysm: treatment with endovascular self-expandable stent grafts. Ann Thorac Surg. 2000;692:441-5. , 5050. Najibi S, Terramani TT, Weiss VJ, Mac Donald MJ, Lin PH, Redd DC, Martin LG, Chaikof EL, Lumsden AB. Endoluminal versus open treatment of descending thoracic aortic aneurysms. J Vasc Surg. 2002;36 :732-7. , 5151. Heijmen RH, Deblier IG, Moll FL, Dossche KM, van den Berg JC, Overtoom TT, Ernst SM, Schepens MA. Endovascular stent-grafting for descending thoracic aortic aneurysms. Eur J Cardiothorac Surg. 2002;21:5-9. , 5252. Schoder M, Cartes-Zumelzu F, Grabenwoger M, Cejna M, Funovics M, Krenn CG, Hutschala D, Wolf F, Thurnher S, Kretschmer G, Lammer J. Elective endovascular stent-graft repair of atherosclerotic thoracic aortic aneurysms: Clinical results and midterm follow-up. Am J Roentgenol. 2003;180:709-15. , 5353. Bell RE, Taylor PR, Aukett M, Sabharwal T, Reidy JF. Mid-term results for second-generation thoracic stent grafts. Br J Surg. 2003;90:811-7. , 5454. Lepore V, Lonn L, Delle M, Mellander S, Rådberg G, Risberg B. Treatment of descending thoracic aneurysms by endovascular stent grafting. J Card Surg. 2003;18:416-23. , 5555. Ouriel K, Greenberg RK. Endovascular treatment of thoracic aortic aneurysms. J Card Surg. 2003;18:455-63. , 5656. Czerny M, Cejna M, Hutschala D, Fleck T, Holzenbein T, Schoder M, Lammer J, Zimpfer D, Ehrlich M, Wolner E, Grabenwoger M. Stent-graft placement in atherosclerotic descending thoracic aortic aneurysms : Midterm results. J Endovasc Ther. 2004;11:26-32. , 5757. Makaroun MS, Dillavou ED, Kee ST, Sicard G, Chaikof E, Bavaria J, Williams D, Cambria RP, Mitchell RS. Endovascular treatment of thoracic aortic aneurysms: Results of the phase II multicenter trial of the GORE TAG thoracic endoprothesis. J Vasc Surg. 2005;41:1-9. , 5858. Leurs LJ, Bell R, Degrieck Y, Thomas S, Hobo R, Lundbom J; EUROSTAR; UK Thoracic Endograft Registry collaborators. Endovascular treatment of thoracic aortic disease : Combined experience from the EUROSTAR and United Kingdom Thoracic Endograft registries. J Vasc Surg. 2004;40:670-9. , 5959. Glade GJ, Vahl AC, Wisselink W, Linsen MA, Balm R. Mid-term survival and cost of tratment of patients with descending thoracic aortic aneurysms; endovascular versus open repair: a case-control study. Eur J Vasc Endovasc Surg. 2005;29:28-34. , 6060. Greenberg RK, O`Neill S, Walker E, Haddad F, Lyden SP, Svensson LG, Lytle B, Clair DG, Ouriel K. Endovascular repair of thoracic aortic lesions with the Zenith TX1 and TX2 thoracic grafts; intermediate-term results. J Vasc Surg. 2005;41:589-96. , 6161. Riesenman PJ, Farber MA, Mendes RR, Marston WA, Fulton JJ, Mauro M, Keagy BA. Endovascular repair of lesions involving the descending thoracic aorta. J Vasc Surg. 2005;42:1063-74. , 6262. Ricco JB, Cau J, Marchand C, Marty M, Rodde-Dunet MH, Fender P, Allemand H, Corsini A. Stent-graft repair for thoracic aortic disease : results of an independent nationwide study in France from 1999 to 2001. J Thorac Cardiovasc Surg. 2006;131:131-7. , 6363. Wheatley GH 3rd, Gurbuz AT, Rodriguez-Lopez JA, Ramaiah VG, Olsen D, Williams J, Diethrich EB. Midterm outcome in 158 consecutive Gore TAG thoracic endoprotheses: single center experience. Ann Thorac Surg. 2006;81:1570-7. , 6464. Bavaria JE, Appoo JJ, Makaroun MS, Verter J, Yu ZF, Mitchell RS; Gore TAG Investigators. Endovascular stent-grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients : a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007;1333:369-77. , 6565. Qu L, Raithel D. Two-year single-center experience with thoracic endovascular repair with the EndoFit thoracic stent-graft. J Endovasc Ther. 2008;15:530-8. , 6666. Ting AC, Cheng SW, Ho P, Chan YC, Poon JT, Cheung GC. Endovascular repair for thoracic aortic pathologies-early and midterm results. Asian J Surg. 2009;32:39-46. ] ( Table 2 ).
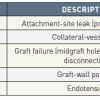
The aortic arch morphology is challenging because of angulation and the proximity of the supra-aortic branches that need to be preserved. Traditional open arch reconstruction using hypothermic cardiac arrest, extracorporeal circulation and selective cerebral perfusion is a classic surgical operation to manage aortic arch pathologies. However, this standard procedure for arch pathology carries significant mortality (2-9%) and risk of paraplegia and cerebral stroke in 4-13% of cases [6767. Kazui T, Washiyama N, Muhammad BA, Terada H, Yamashita K, Takinami M. Improved results of atherosclerotic arch aneurysm operations with a refined technique. J Thorac Cardiovasc Surg. 2001;121:491-9. , 6868. Nakai M, Shimamoto M, Yamazaki F, Fujita S, Aoyama A, Chin T, Nakata T, Yamada T. Long-term results after surgery for aortic nondissection aneurysm. Kyobu Geka. 2002;55:280-4. ]. Therefore, open repair is often reserved for low-risk patients. For comorbid and elderly patients hybrid arch procedures (HAP) with a combination of debranching bypass (supra-aortic vessel transposition) for cerebral perfusion and subsequent thoracic endografting are likely to offer patient-centred better and safer solutions for complex aortic arch lesions. HAP does not require hypothermic circulatory arrest or extracorporeal circulation and could expand the treatment group to high-risk patients and redo-surgery currently ineligible for open surgical intervention ( Figure 8 ). Endoleak is defined as perigraft leakage of contrast medium into a TAA sac as demonstrated by imaging either post-interventional or during follow-up examinations. If there is an endoleak from one of the attachment sites the TAA is not considered completely excluded ( Table 3 ).
Despite limited follow-up, endovascular techniques appear attractive in emergency situations, with high procedural and clinical success rates. A cooperative effort of cardiologists, radiologists, anaesthesiologists and cardiovascular surgeons appears necessary for optimal results. Several studies reported high success rates with 85% to 100% of procedures in successful deployment and functional exclusion of the aneurysm ( Table 2 ).
Dissection of the thoracic aorta
DEFINITION AND CLASSIFICATION
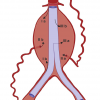
Aortic dissection diagnosed within 2 weeks of the onset of symptoms during the early phase of high mortality, is considered acute. Patients surviving 2 weeks are considered sub-acute or even chronic after 8 weeks [6969. Spittell PC, Spittell JA, Joyce JW, Tajik AJ, Edwards WD, Schaff HV, Stanson AW. Clinical features and differential diagnosis of aortic dissection: Experience with 236 cases. Mayo Clin Proc. 1993;68:642-51. ]. Aortic dissections are further classified according to their anatomical location using the Stanford and DeBakey classification. The fundamental distinction is whether the dissection is proximal (involving the aortic root or ascending aorta) or distal (beyond the left subclavian artery). The Stanford classification of aortic dissection distinguishes between type A and type B [7070. De Bakey ME, Beall AC, Cooley DA, Crawford ES, Morris GC Jr, Garrett HE. Dissecting aneurysms of the aorta. Surg Clin North Am. 1966;46:1045–55. , 7171. Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissection. Ann Thorac Surg. 1970;10:237-47. ] ( Figure 9 ). Type A involves the ascending aorta, while type B dissection does not involve the ascending aorta. The DeBakey classification subdivides the dissection process into type I dissection involving the entire aorta, type II dissection involving only the ascending aorta, and type III dissection sparing the ascending aorta and the arch. Various attempts to further subdivide both classification systems were not successful [7272. Lansman SL, McCullough JN, Nguyen KH, Spielvogel D, Klein JJ, Galla JD, Ergin MA, Griepp RB. Subtypes of acute aortic dissection. Ann Thorac Surg. 1999;67:1975–8. , 7373. Erbel R, Oelert H, Meyer J, Puth M, Mohr-Katoly S, Hausmann D, Daniel W, Maffei S, Caruso A, Covino FE, et al. Effect of medical and surgical therapy on aortic dissection evaluated by transesophageal echocardiography: implication for prognosis and therapy (The European Cooperative Study Group on Echocardiography). Circulation. 1993;83:1604–15. ], although the arch region deserves integration into a modern classification system. Recent observations highlight the importance of precursors of typical aortic dissection, such as intramural haematoma, penetrating aortic ulcers or localised intimal tears as variants of a wall-dissecting process [7474. Nienaber CA, von Kodolitsch Y, Petersen B, Loose R, Helmchen U, Haverich A, Spielmann RP. Intramural hemorrhage of the thoracic aorta. Diagnostic and therapeutic implications. Circulation. 1995;92:1465–72. , 7575. Vilacosta I, San Roman JA, Ferreiros J, Aragoncillo P, Méndez R, Castillo JA, Rollán MJ, Batlle E, Peral V, Sánchez-Harguindey L. Natural history and serial morphology of aortic intramural haematoma: a novel variant of aortic dissection. Am Heart J. 1997;134:495–507. , 7676. Svensson LG, Labib SB, Eisenhauer AC, Butterly JR. Intimal tear without haematoma. Circulation. 1999;99:1331-6. ].
Acute aortic syndrome
- Aortic dissection
- Intramural hematoma
- Penetrating aortic ulcer
Classic aortic dissection
Acute aortic dissection is characterised by the rapid development of an intimal flap separating the true and false lumen [7777. Pretre R, Segesser LK. Aortic dissection. Lancet. 1997;349:1461–4. , 7878. Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L, Szép L. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271–8. , 7979. Roberts CS, Roberts WC. Aortic dissection with the entrance tear in the descending thoracic aorta. Ann Surg. 1991;213:356–68. ]. In the majority of cases (~ 90%) intimal tears are identified as sites of communication between true and false lumen. The dissection can spread in an antegrade or retrograde fashion, involving side branches and causing complications such as malperfusion syndrome by dynamic or static obstruction (from coronary to iliac arteries), tamponade or aortic insufficiency. From a pathophysiological point of view, progression of dissection is difficult to predict once a patient with dissection has survived the initial 2 weeks after inception, although false lumen expansion is likely to develop over time. Several clinical features may be used to roughly estimate late risk, including evidence of persistent communication, patent false channel, and others [7373. Erbel R, Oelert H, Meyer J, Puth M, Mohr-Katoly S, Hausmann D, Daniel W, Maffei S, Caruso A, Covino FE, et al. Effect of medical and surgical therapy on aortic dissection evaluated by transesophageal echocardiography: implication for prognosis and therapy (The European Cooperative Study Group on Echocardiography). Circulation. 1993;83:1604–15. , 7777. Pretre R, Segesser LK. Aortic dissection. Lancet. 1997;349:1461–4. , 7878. Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L, Szép L. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271–8. ].
Intramural haematoma
Aortic intramural haematoma is considered a precursor of classic dissection, and originates from ruptured vasa vasorum in medial wall layers, eventually provoking a secondary communication with the aortic lumen [7575. Vilacosta I, San Roman JA, Ferreiros J, Aragoncillo P, Méndez R, Castillo JA, Rollán MJ, Batlle E, Peral V, Sánchez-Harguindey L. Natural history and serial morphology of aortic intramural haematoma: a novel variant of aortic dissection. Am Heart J. 1997;134:495–507. , 8080. Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management. Part I: from etiology to diagnostic strategies. Circulation. 2003;108:628–35. , 8181. von Kodolitsch Y, Csösz SK, Koschyk DH, Schalwat I, Loose R, Karck M, Dieckmann C, Fattori R, Haverich A, Berger J, Meinertz T, Nienaber CA. Intramural hematoma of the aorta: predictors of progression to dissection and rupture. Circulation. 2003;107:1158–63. ]: this process may be initiated by “aortic wall infarction”. Similar to classic dissection, intramural haematoma may extend along the aorta or progress, regress or reabsorb. The prevalence of intramural haematoma is 10%-30% and can lead to acute aortic dissection in 21%-47% of patients or to regression in about 10% [7575. Vilacosta I, San Roman JA, Ferreiros J, Aragoncillo P, Méndez R, Castillo JA, Rollán MJ, Batlle E, Peral V, Sánchez-Harguindey L. Natural history and serial morphology of aortic intramural haematoma: a novel variant of aortic dissection. Am Heart J. 1997;134:495–507. , 8181. von Kodolitsch Y, Csösz SK, Koschyk DH, Schalwat I, Loose R, Karck M, Dieckmann C, Fattori R, Haverich A, Berger J, Meinertz T, Nienaber CA. Intramural hematoma of the aorta: predictors of progression to dissection and rupture. Circulation. 2003;107:1158–63. , 8282. Evangelista A, Mukherjee D, Mehta RH, O’Gara PT, Fattori R, Cooper JV, Smith DE, Oh JK, Hutchison S, Sechtem U, Isselbacher EM, Nienaber CA, Pape LA, Eagle KA; International Registry of Aortic Dissection (IRAD) Investigators. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation. 2005;111:1063–70. , 8383. Ide K, Uchida H, Otsuji H, Nishimine K, Tsushima J, Ohishi H, Kitamura S. Acute aortic dissection with intramural hematoma: possibility of transition to classic dissection or aneurysm. J Thorac Imaging. 1996;11:46–52. ]. Involvement of the ascending aorta is considered an indication for expeditious surgery due to the inherent risk of rupture, tamponade or compression of coronary ostia. Distal intramural haematoma may warrant watchful waiting and, potentially, stent graft placement in case of local expansion [8181. von Kodolitsch Y, Csösz SK, Koschyk DH, Schalwat I, Loose R, Karck M, Dieckmann C, Fattori R, Haverich A, Berger J, Meinertz T, Nienaber CA. Intramural hematoma of the aorta: predictors of progression to dissection and rupture. Circulation. 2003;107:1158–63. , 8383. Ide K, Uchida H, Otsuji H, Nishimine K, Tsushima J, Ohishi H, Kitamura S. Acute aortic dissection with intramural hematoma: possibility of transition to classic dissection or aneurysm. J Thorac Imaging. 1996;11:46–52. , 8484. Kaji S, Akasaka T, Horibata Y, Nishigami K, Shono H, Katayama M, Yamamuro A, Morioka S, Morita I, Tanemoto K, Honda T, Yoshida K. Long-term prognosis of patients with type A aortic intramural hematoma. Circulation. 2002;106:248-52. , 8585. Song JK, Kim HS, Kang DH, Lim TH, Song MG, Park SW, Park SJ. Different clinical features of aortic intramural hematoma versus dissection involving the ascending aorta. J Am Coll Cardiol. 2001;37:1604–10. , 8686. Neri E, Capannini G, Carone E, Diciolla F, Sassi C. Evolution toward dissection of an intramural hematoma of the ascending aorta. Ann Thorac Surg. 1999; 68: 1855–1866. ].
Plaque rupture/ulceration
Ulceration of atherosclerotic aortic plaques can lead to aortic dissection or perforation [8787. Kodolitsch Y, Nienaber CA. Penetrating ulcer of the thoracic aorta: natural history, diagnostic and prognostic profiles. Z Kardiol. 1998;87:917–27. , 8888. Movsowitz HD, Lampert C, Jacobs LE, Kotler MN. Penetrating atherosclerotic aortic ulcers. Am Heart J. 1994;128:1210–7. , 8989. Braverman AC. Penetrating atherosclerotic ulcers of the aorta. Curr Opin Cardiol. 1994; 9:591–7. ]. Non-invasive imaging of aortic ulceration has been improved by tomographic scanning and has shed light on pathophysiology and aetiology. Aortic ulcers occur predominantly in the descending thoracic and abdominal aorta, penetrate intimal borders and appear as nipple-like projections with an adjacent haematoma [9090. Ganaha F, Miller DC, Sugimoto K, Do YS, Minamiguchi H, Saito H, Mitchell RS, Dake MD. The prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: a clinical and radiological analysis. Circulation. 2002;106:342–8. , 9191. Stanson AW, Kazmier FJ, Hollier LH, Edwards WD, Pairolero PC, Sheedy PF, Joyce JW, Johnson MC. Penetrating atherosclerotic ulcers of the thoracic aorta: natural history and clinicopathologic correlations. Ann Vasc Surg. 1986;1:15–23. ]: symptomatic ulcers and those with signs of deep erosion are more likely to rupture than others.
EPIDEMIOLOGY
Aortic dissection is a rare condition, with an incidence of approximately 2.6 to 3.5 cases per 100,000 person/year, and with high prevalence in Italy (4.04/100,000/year) [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. , 9393. Clouse WD, Hatlett JW, Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, Melton LJ 3rd. Acute aortic dissection: populationbased incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79:176-18. ]. Around 0.5% of patients presenting to an emergency department with chest or back pain suffer from aortic dissection [9494. Kodolitsch Y, Schwartz AG, Nienaber CA. Clinical prediction of acute aortic dissection. Arch Intern Med. 2000;160:2977–82. ]. Two-thirds of patients are male, with an average age at presentation of approximately 65 years. A history of systemic hypertension, found in up to 72% of patients, is by far the most common risk factor [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. , 9595. Larson E, Edwards W. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984;53:849–55. ]. The epidemiology of aortic dissection is substantially different in young patients (<40 years of age) with risk factors such as Marfan syndrome or other connective tissue diseases. Conditions for dissection are listed in Table 4.
Acquired conditions
Chronic hypertension affects arterial wall composition, causing intimal thickening, fibrosis and calcification and extracellular fatty acid deposition. In parallel, the extracellular matrix undergoes accelerated degradation, apoptosis and elastolysis with hyalinisation of collagen. Both mechanisms may eventually lead to intimal disruption. Moreover, adventitial fibrosis may obstruct nutrient vessels feeding the arterial wall as well as small intramural vasa vasorum. Both result in necrosis of smooth muscle cells and fibrosis of elastic structures, rendering the vessel wall vulnerable to pulsatile forces and creating a substrate for aneurysms and dissections [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. , 9696. von Kodolitsch Y, Aydin MA, Koschyk DH, Loose R, Schalwat I, Karck M, Cremer J, Haverich A, Berger J, Meinertz T, Nienaber CA. Predictors of aneurysm formation after surgery of aortic coarctation. J Am Coll Cardiol. 2002;39:617–24. , 9797. Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83:81–5. , 9898. Reed D, Reed C, Stemmermann G. Are aortic aneurysms caused by atherosclerosis? Circulation. 1992;85:205–11. , 9999. Stefanadis CI, Karayannacos PE, Boudoulas HK, Stratos CG, Vlachopoulos CV, Dontas IA, Toutouzas PK. Medial necrosis and acute alterations in aortic distensibility following removal of the vasa vasorum of canine ascending aorta. Cardiovasc Res. 1993;27:951–6. ]. fragilityIn addition to chronic hypertension, smoking, dyslipidaemia and, potentially, the use of crack cocaine are modulating risk factors. On rare occasions, inflammatory diseases destroy the media layers and cause weakening, expansion and dissection of the aortic wall. Iatrogenic aortic dissection may occur in association with invasive retrograde catheter interventions, or during or after valve or aortic surgery [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. , 100100. Januzzi J, Sabatine MS, Eagle KA, Evangelista A, Bruckman D, Fattori R, Oh JK, Moore AG, Sechtem U, Llovet A, Gilon D, Pape L, O’Gara PT, Mehta R, Cooper JV, Hagan PG, Armstrong WF, Deeb GM, Suzuki T, Nienaber CA, Isselbacher EM; International Registry of Aortic Dissection Investigators. Iatrogenic aortic dissection. Am J Cardiol. 2002;89:623–6. , 101101. von Kodolitsch Y, Simic O, Schwartz A, Dresler C, Loose R, Staudt M, Ostermeyer J, Haverich A, Nienaber CA. Predictors of proximal aortic dissection at the time of aortic valve replacement. Circulation. 1999;100:287-94. , 102102. Pieters FAA, Widdershoven JW, Gerardy AC, Geskes G, Cheriex EC, Wellens HJ. Risk of aortic dissection after aortic valve replacement. Am J Cardiol. 1993;72:1043–7. ].
Marfan syndrome
Among hereditary diseases, Marfan syndrome is the most prevalent connective tissue disorder, with an estimated incidence of 1 in 7,000 and autosomal dominant inheritance with variable penetrance. More than 150 mutations on the fibrillin-1 (FBN-1) gene have been identified encoding for a defective fibrillin in the extracellular matrix, which may affect the ocular, cardiovascular, skeletal and pulmonary systems, as well as skin and dura mater. The diagnosis of Marfan syndrome is currently based on the revised clinical criteria of the “Ghent nosology” [103103. Paepe A, Devereux R, Dietz H, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62:417–26. ]. The Ghent criteria pay particular attention to genetic information, for example the appearance of Marfan syndrome in the kindred of an unequivocally affected individual. Moreover, both skeletal and cardiovascular features are major (i.e., diagnostic) criteria if four of eight typical manifestations are present. However, borderline manifestations such as the MASS (mitral valve, aorta, skin, skeletal) phenotype or subtle phenotypic features, the molecular analysis of suspected Marfan syndrome and the delineation of criteria for differentiating other inherited conditions (genotypes) from the Marfan phenotype are attracting interest [104104. Collod G, Babron MC, Jondeau G, Coulon M, Weissenbach J, Dubourg O, Bourdarias JP, Bonaïti-Pellié C, Junien C, Boileau C. A second locus for Marfan syndrome maps to chromosome 3p24.2–p25. Nat Genet. 1994;8:264–8. , 105105. Milewicz DM, Pyeritz RE, Crawford ES, Byers PH. Marfan syndrome: defective synthesis, secretion and extracellular matrix formation of fibrillin by cultured dermal fibroblasts. J Clin Invest. 1992;89:79–86. , 106106. Aoyama T, Francke U, Dietz H, Furthmayr H. Quantitative differences in biosynthesis and extracellular deposition of fibrillin in cultured fibroblasts distinguish five groups of Marfan syndrome patients and suggest distinct pathogenetic mechanisms. J Clin Invest. 1994;94:130–7. ]. The clinical variety of Marfan syndrome is only partially explained by the number of mutations on the FBN-1 gene. Genetic heterogeneity and the involvement of a second gene (MFS2, Marfan syndrome type 2) may further add to the broad spectrum of symptoms [107107. Boileau C, Jondeau G, Babron MC, Coulon M, Alexandre JA, Sakai L, Melki J, Delorme G, Dubourg O, Bonaïti-Pellié C. Autosomal dominant Marfan-like connective-tissue disorder with aortic dilatation and skeletal anomalies not linked to the fibrillin genes. Am J Hum Genet. 1993;53:46–54. ].
Ehlers–Danlos syndrome
Ehlers-Danlos syndrome is a heterogeneous group of hereditable connective tissue disorders characterised by articular hypermobility, skin hyperextensibility and tissue fragility. Eleven types of Ehlers-Danlos syndrome have been characterised: the true prevalence is unknown. An aggregate incidence of 1 in 5,000 births is often cited, with no racial or ethnic predisposition. Aortic involvement is seen primarily in autosomal dominant Ehlers–Danlos syndrome type IV [108108. Steinmann B, Royce P, Superti-Furga A. The Ehlers–Danlos syndrome. In: Royce, PM & Steinmann, B. Connective Tissue and its Heritable Disorders, 1993. New York: Wiley-Liss, pp. 351–407. ].
Annuloaortic ectasia and familial aortic dissection
More than five mutations in the FBN-1 gene have now been identified in patients presenting with either sporadic or familial forms of thoracic aortic aneurysm and dissection [109109. Glesby M, Pyeritz R. Association of mitral valve prolapse and systemic abnormalities of connective tissue. A phenotypic continuum. JAMA. 1989;262:523–8. , 110110. Furthmayr H, Francke U. Ascending aortic aneurysm with or without features of Marfan syndrome and other fibrillinopathies: new insights. Semin Thorac Cardiovasc Surg. 1997;9:191–205. ]. Histological examination of the aortic wall reveals elastolysis or loss of elastic fibres, deposits of mucopolysaccharide-like material and cystic medial degeneration similar to that in Marfan syndrome. However, no abnormalities of types I and III collagen or any specific fibrillopathy have been found in fibroblast cultures.
CLINICAL FEATURES
The challenge in managing acute aortic syndrome, and especially dissection, is appropriate clinical suspicion and action in pursuing diagnosis and therapy [111111. Fuster V, Halperin JL. Aortic dissection: a medical perspective. J Cardiovasc Surg (Torino). 1994;9:713–28. , 112112. DeSanctis RW, Doroghazi RM, Austen WG, Buckley MJ. Aortic dissection. N Engl J Med. 1987;317:1060–7. ]. The differential diagnosis for acute aortic dissection includes acute coronary syndrome, pulmonary embolism, pneumothorax, pneumonia, musculoskeletal pain, acute cholecystitis, oesophageal spasm or rupture, acute pancreatitis and acute pericarditis. Typical features of dissection are the acute onset of chest and/or back pain of blunt, radiating and migrating nature. The pain could be sharp, ripping, tearing, or knife-like in nature but the abruptness is the most specific characteristic of the pain. According to a report on 464 patients from the International Registry of Acute Aortic Dissection (IRAD) 95% of patients reported pain, and 85% reported an abrupt onset [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. ].
Sharp pain was reported by 64% of patients, whereas the classic tearing or ripping type of pain was reported by 51% of patients. The most common site of pain was the chest (73%), with anterior location being more common than the posterior location (61% vs. 36%, respectively). Back pain was experienced in 53% of patients, and abdominal pain was experienced by 30% of patients. Extension of the pain down to the back, abdomen, hips, and legs indicates the extension of the dissection process distally. Chronic hypertension is common if obvious signs of connective tissue disorders are absent. Clinical manifestations of acute aortic dissection are often explained by specific malperfusion syndrome from dissection-related side-branch obstruction. More than one third of patients with aortic dissection demonstrate signs and symptoms related to the involved organ system [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. ]. Aortic regurgitation accompanies 18% to 50% of cases with proximal aortic dissection. Acute, severe aortic regurgitation is the second most common cause of death (after aortic rupture) in patients with aortic dissection. Patients with this condition usually present with acute cardiac decompensation and shock.
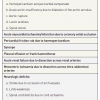
The mechanism of aortic regurgitation in aortic dissection include dilatation of the aortic root and annulus, tearing of the annulus or valve cusps, downward displacement of one cusp below the line of the valve closure, loss of support of the cusp, and physical interference in the closure of the aortic valve by an intimal flap. Although most patients with aortic dissections have hypertension at the time of presentation, an initial systolic BP <100 mmHg has been reported in about 25% of patients with aortic dissection. Hypotension and shock in patients with aortic dissection are caused by acute severe aortic regurgitation, aortic rupture, cardiac tamponade, or left ventricular systolic dysfunction or bleeding from rupture [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. ]. The presence of pulse differentials is the most specific physical sign of aortic dissection, and it has been reported in 38% of patients with aortic dissection [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. ]. Cerebrovascular manifestations, limb ischaemia or pulse deficits are caused by involvement of a side-branch orifice into the dissection or obliteration of the true lumen by an expanding false lumen [113113. Mehta RH, O’Gara PT, Bossone E, Nienaber CA, Myrmel T, Cooper JV, Smith DE, Armstrong WF, Isselbacher EM, Pape LA, Eagle KA, Gilon D; International Registry of Acute Aortic Dissection (IRAD) Investigators. Acute type A aortic dissection in the elderly: clinical characteristics, management, and outcomes in the current era. J Am Coll Cardiol. 2002;40:685–92. , 114114. Miller DC. The continuing dilemma concerning medical versus surgical management of patients with acute type B dissection. Semin Thorac Cardiovasc Surg. 1993;5:33–46. ] ( Table 5 ). Recurrent abdominal pain, elevation of acute-phase proteins and increase of lactate dehydrogenase are indicators of involvement of either the coeliac trunk (observed in ~ 8%) or superior mesenteric artery (in 8%-13%). Involvement of renal arteries may result in oliguria or anuria and propagation of dissection is heralded by repetitive bouts of pain or a deteriorating clinical picture [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. , 113113. Mehta RH, O’Gara PT, Bossone E, Nienaber CA, Myrmel T, Cooper JV, Smith DE, Armstrong WF, Isselbacher EM, Pape LA, Eagle KA, Gilon D; International Registry of Acute Aortic Dissection (IRAD) Investigators. Acute type A aortic dissection in the elderly: clinical characteristics, management, and outcomes in the current era. J Am Coll Cardiol. 2002;40:685–92. , 114114. Miller DC. The continuing dilemma concerning medical versus surgical management of patients with acute type B dissection. Semin Thorac Cardiovasc Surg. 1993;5:33–46. ].
NATURAL HISTORY
Despite major advances in the non-invasive diagnosis of aortic dissection and in therapy, up to 28%-55% of patients die without a correct antemortem diagnosis [6969. Spittell PC, Spittell JA, Joyce JW, Tajik AJ, Edwards WD, Schaff HV, Stanson AW. Clinical features and differential diagnosis of aortic dissection: Experience with 236 cases. Mayo Clin Proc. 1993;68:642-51. , 7878. Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L, Szép L. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271–8. , 9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. , 115115. Mehta RH, Suzuki T, Hagan PG, Bossone E, Gilon D, Llovet A, Maroto LC, Cooper JV, Smith DE, Armstrong WF, Nienaber CA, Eagle KA; International Registry of Acute Aortic Dissection (IRAD) Investigators. Predicting death in patients with acute type a aortic dissection. Circulation. 2002;105:200–6. , 116116. Nallamothu BK, Mehta RH, Saint S, Llovet A, Bossone E, Cooper JV, Sechtem U, Isselbacher EM, Nienaber CA, Eagle KA, Evangelista A. Syncope in acute aortic dissection: diagnostic, prognostic, and clinical implications. Am J Med. 2002;113:468–71. ].
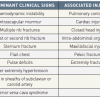
Predictors of increased mortality [115115. Mehta RH, Suzuki T, Hagan PG, Bossone E, Gilon D, Llovet A, Maroto LC, Cooper JV, Smith DE, Armstrong WF, Nienaber CA, Eagle KA; International Registry of Acute Aortic Dissection (IRAD) Investigators. Predicting death in patients with acute type a aortic dissection. Circulation. 2002;105:200–6. , 116116. Nallamothu BK, Mehta RH, Saint S, Llovet A, Bossone E, Cooper JV, Sechtem U, Isselbacher EM, Nienaber CA, Eagle KA, Evangelista A. Syncope in acute aortic dissection: diagnostic, prognostic, and clinical implications. Am J Med. 2002;113:468–71. ]
- Age >70 years
- Pericardial tamponade
- Involvement of the coronary arteries
- Malperfusion of brain/intestine
- Arterial hypotension
- Kidney failure
- Pulse differences
Less appreciated predisposing factors for type A dissection include prior cardiac and valvular surgery (15%) and iatrogenic dissection from cardiac surgery or catheterisation (5%). Iatrogenic aortic dissection carries slightly higher mortality than non-iatrogenic (35% versus 24%) [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. , 9595. Larson E, Edwards W. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984;53:849–55. ]. Data from the largest registry of acute aortic dissection showed that, in the absence of immediate surgical repair, medical management is associated with a mortality of nearly 24% at day 1, 29% at 48 hours, 44% at day 7, and 50% after 2 weeks [33. Erbel R, Alfonso F, Boileau C, Dirsch O, Eber B, Haverich A, Rakowski H, Struyven J, Radegran K, Sechtem U, Taylor J, Zollikofer C, Klein WW, Mulder B, Providencia LA; Task Force on Aortic Dissection, European Society of Cardiology. Task Force on Aortic Dissection of the European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22:1642–81.
This manuscript describes the provisional guidelines prepared by the ESC taskforce on aortic dissection., 9696. von Kodolitsch Y, Aydin MA, Koschyk DH, Loose R, Schalwat I, Karck M, Cremer J, Haverich A, Berger J, Meinertz T, Nienaber CA. Predictors of aneurysm formation after surgery of aortic coarctation. J Am Coll Cardiol. 2002;39:617–24. ]. Less than 10% of untreated patients with proximal aortic dissection live for 1 year, and almost all patients die within 10 years. Most of these deaths occur within the first 3 months. The risk of the fatal aortic rupture in patients with untreated proximal aortic dissection is around 90%, and 75% of these ruptures take place in the pericardium, the left pleural cavity and the mediastinum. Even with surgical repair, in-hospital mortality rates are 10% after 1 day, 12% at 2 days, and nearly 20% at 2 weeks with aortic rupture, stroke, visceral ischaemia, cardiac tamponade, and circulatory failure as the most common causes for death [33. Erbel R, Alfonso F, Boileau C, Dirsch O, Eber B, Haverich A, Rakowski H, Struyven J, Radegran K, Sechtem U, Taylor J, Zollikofer C, Klein WW, Mulder B, Providencia LA; Task Force on Aortic Dissection, European Society of Cardiology. Task Force on Aortic Dissection of the European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22:1642–81.
This manuscript describes the provisional guidelines prepared by the ESC taskforce on aortic dissection., 9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. , 9595. Larson E, Edwards W. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984;53:849–55. ]. Acute aortic dissection of the descending aorta is less frequently lethal. In the absence of treatment, survival rates are 89% at 1 month, 84% at 1 year, and 80% at 5 years [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. ]. However, patients with complications such as renal failure, visceral ischaemia, or contained rupture often require urgent repair, with a mortality of 20% at day 2 and 25% at 1 month. Similar to type A dissection, advanced age, rupture, shock, and malperfusion are important independent predictors of early mortality [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. ]. Proximal (type A) IMH is no longer related to early death when surgical repair is performed [117117. von Kodolitsch Y, Nienaber CA. Intramural hemorrhage of the thoracic aorta: diagnosis, therapy and prognosis of 209 in vivo diagnosed cases [in German]. Z Kardio. 1998;87:797– 807. ]. However, the high risk of “wait and see” (conservative management) in type A IMH is reflected in 55% early mortality with medical treatment compared to 8% with surgical repair (p= 0.004). Moreover, actuarial survival analysis showed better long-term survival on oral ß-blocker treatment with 95% versus 67% without ß-blockers (p=0.004). Beta-adrenergic blocking agents protect by reducing wall stress, systolic arterial pressure and the rate of pressure changes, and presumably stabilise the extracellular vascular matrix of the aorta [7474. Nienaber CA, von Kodolitsch Y, Petersen B, Loose R, Helmchen U, Haverich A, Spielmann RP. Intramural hemorrhage of the thoracic aorta. Diagnostic and therapeutic implications. Circulation. 1995;92:1465–72. , 117117. von Kodolitsch Y, Nienaber CA. Intramural hemorrhage of the thoracic aorta: diagnosis, therapy and prognosis of 209 in vivo diagnosed cases [in German]. Z Kardio. 1998;87:797– 807. ]. The observation that older age (> 55 years) at initial diagnosis of IMH has a better long-term prognosis may be explained by more focal microscars along the aortic wall inherently limiting the longitudinal progression of IMH [9090. Ganaha F, Miller DC, Sugimoto K, Do YS, Minamiguchi H, Saito H, Mitchell RS, Dake MD. The prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: a clinical and radiological analysis. Circulation. 2002;106:342–8. ]. Accordingly, favourable outcomes of IMH are consistently reported in patients beyond 65 years [9090. Ganaha F, Miller DC, Sugimoto K, Do YS, Minamiguchi H, Saito H, Mitchell RS, Dake MD. The prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: a clinical and radiological analysis. Circulation. 2002;106:342–8. ]. Thus, considering both advanced atherosclerosis with older age and the lower risk of progression, a conservative strategy (with ß-blockade and serial imaging) appears be justified in elderly multimorbid patients and in distal IMH [9090. Ganaha F, Miller DC, Sugimoto K, Do YS, Minamiguchi H, Saito H, Mitchell RS, Dake MD. The prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: a clinical and radiological analysis. Circulation. 2002;106:342–8. ]. Ulcer-like projections in aortic segments of IMH identify a subset of patients at high risk. Aortic ulcers are preferentially (> 90%) observed in IMH of the descending aorta, while IMH without PAU is more frequently present in the ascending aorta. Symptomatic PAU incurs complications such as formation of aneurysm, pseudoaneurysm, and dissection, or unpredictable rupture. Careful imaging is vital to identify both diameter and depth of ulcers with IMH, since width > 2 cm and depth > 1 cm may herald the need for interventional or surgical repair to avoid rupture and death [113113. Mehta RH, O’Gara PT, Bossone E, Nienaber CA, Myrmel T, Cooper JV, Smith DE, Armstrong WF, Isselbacher EM, Pape LA, Eagle KA, Gilon D; International Registry of Acute Aortic Dissection (IRAD) Investigators. Acute type A aortic dissection in the elderly: clinical characteristics, management, and outcomes in the current era. J Am Coll Cardiol. 2002;40:685–92. ].
DIAGNOSTIC PROCEDURES
The diagnosis of aortic dissection begins with clinical suspicion, as the most crucial step in work-up of this catastrophic condition.
Diagnostic modalities in aortic dissection
- Patient history
- Physical examination
- Laboratory findings
- Transthoracic / transoesophageal echocardiography
- Computer tomography
- Magnetic resonance imaging
- Aortography
Most importantly, any diagnostic study must confirm or refute the diagnosis of aortic dissection. Second, any diagnostic efforts must clarify whether the dissection involves the ascending aorta or is confined to the descending aorta or arch. Third, anatomical features of the dissection, including its extent, the sites of entry and re-entry, the presence of thrombus in the false lumen, branch vessel involvement by the dissection, presence or absence of pericardial effusion and any coronary involvement needs to be known. Unfortunately, no single imaging modality provides all the details. The diagnosis should be confirmed rapidly and accurately, preferable with an easily available non-invasive modality. Aortic dissection causes extensive damage to the smooth muscle cells of the media, leading to the release of structural proteins of the smooth muscle cells including smooth muscle myosin heavy chain into the circulation and generalised signs of inflammation. The most common electrocardiographic abnormality is left ventricular hypertrophy from systemic hypertension. Acute electrocardiographic changes occur in up to 55% of patients and include ST segment depression, T-wave changes, and ST segment elevation. Myocardial infarction occurs in 1% to 2% of patients due to compromise of the coronary ostium by the haematoma or intimal flap [118118. Suzuki T, Mehta RH, Ince H, Nagai R, Sakomura Y, Weber F, Sumiyoshi T, Bossone E, Trimarchi S, Cooper JV, Smith DE, Isselbacher EM, Eagle KA, Nienaber CA; International Registry of Aortic Dissection. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons learned from the International Registry of Aortic Dissection (IRAD). Circulation. 2003;108:312–7. ]. In the emergency department, chest radiography is often used but is abnormal in only 56% of cases of suspected aortic dissection [118118. Suzuki T, Mehta RH, Ince H, Nagai R, Sakomura Y, Weber F, Sumiyoshi T, Bossone E, Trimarchi S, Cooper JV, Smith DE, Isselbacher EM, Eagle KA, Nienaber CA; International Registry of Aortic Dissection. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons learned from the International Registry of Aortic Dissection (IRAD). Circulation. 2003;108:312–7. ].
The classic radiographic sign that is suggestive of aortic dissection is the widening of the mediastinal shadow. Other signs are altered configuration of the aorta, a localised hump on the aortic arch, a widening of the aortic knob past the origin of the left subclavian artery, aortic wall thickness indicated by the width of the aortic shadow beyond intimal calcification, and displacement of the calcification in the aortic knob.
Transthoracic echocardiography has a sensitivity of 60% and a specificity of 83% for type A dissection and also shows aortic regurgitation, pleural effusion and pericardial effusion/tamponade [119119. Erbel R, Engberding R, Daniel W, Roelandt J, Visser C, Rennollet H. Echocardiography in diagnosis of aortic dissection. Lancet. 1989;8:457–61. ]. Transoesophageal echocardiography (TOE) with colour Doppler interrogation overcomes the limitations of transthoracic echocardiography, with a sensitivity of 94%–100% for identifying an intimal flap and 77%–87% for identifying the site of entry: specificity ranges from 77% to 97% [120120. Nienaber CA, Kodolitsch Y, Nicolas V, Siglow V, Piepho A, Brockhoff C, Koschyk DH, Spielmann RP. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328:1–9. ]. In addition to providing excellent visualization of the thoracic aorta, TOE provides superb images of the pericardium and detailed assessment of aortic valve function at the bedside. For this reason, TOE is particularly useful in haemodynamically unstable patients.
Multislice CT is available in many hospitals and should be offered on an emergency basis in case of suspected dissection [121121. Moore AG, Eagle KA, Bruckman D, Moon BS, Malouf JF, Fattori R, Evangelista A, Isselbacher EM, Suzuki T, Nienaber CA, Gilon D, Oh JK. Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD). Am J Cardiol. 2002;89:1235–8. ]. Computer tomography provides complete anatomical information of the aorta including branch vessel involvement and enables visualisation of the ostium and proximal part of both coronary arteries, with a sensitivity of 83%-100% and a specificity of 90%-100% for aortic dissection [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. , 120120. Nienaber CA, Kodolitsch Y, Nicolas V, Siglow V, Piepho A, Brockhoff C, Koschyk DH, Spielmann RP. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328:1–9. , 121121. Moore AG, Eagle KA, Bruckman D, Moon BS, Malouf JF, Fattori R, Evangelista A, Isselbacher EM, Suzuki T, Nienaber CA, Gilon D, Oh JK. Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD). Am J Cardiol. 2002;89:1235–8. ]. In randomised trials, cardiac MRI was more precise than transoesophageal echocardiography and CT and nearly 100% specific for aortic dissection. For identifying the site of entry, sensitivity was 85% and specificity 100% [9494. Kodolitsch Y, Schwartz AG, Nienaber CA. Clinical prediction of acute aortic dissection. Arch Intern Med. 2000;160:2977–82. , 122122. Eagle KA, Quertermous T, Kritzer GA, Newell JB, Dinsmore R, Feldman L, DeSanctis RW. Spectrum of conditions initially suggesting acute aortic dissection but with negative aortograms. Am J Cardiol. 1986;57:322–6. ]. Aortography, an invasive procedure, is no longer required for diagnosing aortic dissection. The sensitivity and specificity of aortography are inferior to non-invasive imaging modalities. False negatives may occur if both the true and false lumen opacity equally with contrast, or if the false lumen is completely thrombosed. Coronary angiography adds little to the decision-making process and should generally be avoided in type A dissection [120120. Nienaber CA, Kodolitsch Y, Nicolas V, Siglow V, Piepho A, Brockhoff C, Koschyk DH, Spielmann RP. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328:1–9. ]. In the large International Registry of Aortic Dissection (IRAD), the first diagnostic step was transthoracic echocardiography or transoesophageal echocardiography in 33%, computed tomography in 61%, magnetic resonance imaging in 2% and angiography in 4%. Secondary techniques included 56% TTE or TOE, 18% CT, 9% MRI and 17% angiography. An average of 1.8 methods were utilised to diagnose aortic dissection [123123. Tsai TT, Nienaber CA, Eagle KA. Acute aortic syndromes. Circulation. 2005;112:3802-13.
This manuscript provides information on the risk factors, clinical characteristics, diagnosis and management of acute aortic dissections.].
MEDICAL MANAGEMENT
Patients with suspected acute aortic dissection should be admitted to an intensive care or monitoring unit and undergo diagnostic evaluation immediately. Pain and blood pressure control to a target systolic pressure of 110 mmHg can be achieved using morphine and intravenous beta-blockers (metoprolol, esmolol or labetalol) or in combination with vasodilating drugs such as sodium nitroprusside or angiotensin-converting enzyme inhibitors. Intravenous verapamil or diltiazem may also be used if beta-blockers are contraindicated. Monotherapy with beta-blocking agents may be adequate to control mild hypertension and, in concert with sodium nitroprusside at an initial dose of 0.3 μg/kg/min, is often effective in a severe hypertensive state ( Table 6 ). In normotensive or hypotensive patients, careful evaluation for loss of blood, pericardial effusion or heart failure (by cardiac echo) is mandatory before administering volume. Patients with profound haemodynamic instability often require intubation mechanical ventilation and urgent bedside transoesophageal echocardiography or rapid CT for confirmatory imaging. In rare cases, the external ultrasound diagnosis of cardiac tamponade may justify immediate sternotomy and surgical access to the ascending aorta to prevent circulatory arrest, shock and ischaemic brain damage. Percutaneous pericardiocentesis as a temporary step often fails, and can accelerate bleeding and shock, and so is contra-indicated [124124. Isselbacher EM, Cigarroa JE, Eagle KA. Cardiac tamponade complicating proximal aortic dissection: is pericardiocentesis harmful? Circulation. 1994;90:2375–9. ].
SURGICAL MANAGEMENT
The aim of surgical therapy in proximal type A (type I, II) aortic dissection is prevention of rupture or development of pericardial effusion, which may lead to cardiac tamponade and death. Similarly, sudden onset of aortic regurgitation and coronary flow obstruction require urgent surgical intervention, with the aim of resecting the region of intimal tear in dissection limited to the ascending aorta and replacement by a composite or interposition graft (if the aortic valves are intact or resuspendable). When the dissection extends to the aortic arch or the descending aorta, resection of the entire intimal flap may not be possible or the patient may require partial or total arch replacement [125125. Lai DT, Robbins RC, Mitchell RS, Moore KA, Oyer PE, Shumway NE, Reitz BA, Miller DC.. Does profound hypothermic circulatory arrest improve survival in patients with acute type A aortic dissection? Circulation. 2002;106:218–28. ]. A recent report highlights the problem of either resecting or leaving unrecognised intimal tears in the arch or descending thoracic aorta, which is seen in 20%-30% and predisposes to later distal aortic reoperation [126126. Schor JS, Yerlioglu ME, Galla JD, Lansman SL, Ergin MA, Griepp RB. Selective management of acute type B aortic dissection: long-term follow-up. Ann Thorac Surg. 1996;61:1339–41. ]. Even in centres of excellence, using adjunctive measures such as profound hypothermic circulatory arrest and selective retrograde perfusion of head vessels for surgical management of arch repair, operative mortality ranges between 15% and 35% [196196. Xenos ES, Abedi NN, Davenport DL, Minion DJ, Hamdallah O, Sorial EE, Endean ED. Meta-analysis of endovascular vs open repair for traumatic descending thoracic aortic rupture. J Vasc Surg. 2008;48:1343-51. ]. Although gaining growing acceptance for improved outcome (5-year survival of 73 ± 6%), profound hypothermic circulatory arrest has failed to improve early complications, survival and distal reoperation rates in patients with acute type A dissection; 30-day, 1-year and 5-year survival estimates are 81 ± 2%, 74 ± 3% and 63 ± 3%, and not different from other techniques based on propensity-matched retrospective analysis [125125. Lai DT, Robbins RC, Mitchell RS, Moore KA, Oyer PE, Shumway NE, Reitz BA, Miller DC.. Does profound hypothermic circulatory arrest improve survival in patients with acute type A aortic dissection? Circulation. 2002;106:218–28. ]. The key to success is rapid surgery prior to any haemodynamic instability or deterioration.
Aortic arch in acute type A (type I and II) dissection
Treatment of the acutely dissected aortic arch remains an unresolved issue. At present there is growing consensus that any dissected arch should be explored during hypothermic circulatory arrest [128128. Gott VL, Greene PS, Alejo DE, Cameron DE, Naftel DC, Miller DC, Gillinov AM, Laschinger JC, Pyeritz RE. Replacement of the aortic root in patients with Marfan’s syndrome. N Engl J Med. 1999;340:1307–13. , 129129. Ergin MA, O’Connor J, Guinto R, Griepp RB. Experience with profound hypothermia and circulatory arrest in the treatment of aneurysms of the aortic arch.Aortic arch replacement for acute aortic arch dissections. J Thorac Cardiovasc Surg. 1982;84:649–55. ]. In the absence of an arch tear, an open distal anastomosis of the graft and the conjoined aortic wall layers at the junction of the ascending and arch portions is justified. Arch tears occur in up to 30% of patients with acute dissection [129129. Ergin MA, O’Connor J, Guinto R, Griepp RB. Experience with profound hypothermia and circulatory arrest in the treatment of aneurysms of the aortic arch.Aortic arch replacement for acute aortic arch dissections. J Thorac Cardiovasc Surg. 1982;84:649–55. , 130130. Nguyen B, Muller M, Kipfer B, Berdat P, Walpoth B, Althaus U, Carrel T. Different techniques of distal aortic repair in acute type A dissection: impact on late aortic morphology and reoperation. Eur J Cardiothorac Surg. 1999;15:496–500. ]. Whenever extensive tears are found that continue beyond the junction of the transverse and descending aortic segments, or with acute dissection of a previously aneurysmal arch, subtotal or total arch replacement may be required, with reconnection of some or all supra-aortic vessels to the graft during hypothermic circulatory arrest and antegrade head perfusion [131131. Griepp RB, Ergin MA, Lansman SL, Galla JD, Pogo G. The physiology of hypothermic circulatory arrest. Semin Thorac Cardiovasc Surg. 1991;3:188–93. ]. In dissecting and non-dissecting aneurysms extending to the downstream aorta, an elephant trunk extension of the arch graft is an option described by Borst et al [132132. Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using ’elephant trunk’ prosthesis. Thorac Cardiovasc Surg. 1983;31:37–40. ]. This technique greatly facilitates later procedures on the downstream aorta. Instead of performing a conventional anastomosis between the end of the graft and the descending aorta, the graft is allowed to float freely in the aortic lumen. In a later procedure the elephant trunk section of the graft may be either connected surgically to the distal descending aorta directly or extended with another tubular prosthesis or interventionally by a customised endovascular stent graft that may then be anastomosed at any desired downstream level of the aorta.
Surgery in type B (type III) aortic dissection
In the current era, the indications for active management in patients with acute type B (type III) are limited to the prevention or relief of life-threatening complications such as malperfusion syndrome, intractable pain, rapidly expanding aortic diameter or signs of imminent aortic rupture that can all be managed by interventional stent graft placement. In particular signs of malperfusion or vital aortic side-branch occlusion warrant interventional therapy via stent grafting to improve distal true lumen flow or, in rare instances, via catheter-guided side-branch stenting. Conversely, uncomplicated type B (type III) aortic dissections are at present usually treated medically, since surgical repair has no proven superiority over medical or interventional treatment in stable patients. In complicated cases, the concept of interventional stent graft placement seems to be associated with higher survival rates than the open surgical approach [133133. Ince H, Nienaber CA. The concept of interventional therapy in acute aortic syndrome. J Cardiovasc Surg (Torino). 2002;17:135–42. , 134134. Koschyk D, Nienaber CA, Knap M, Hofmann T, Kodolitsch YV, Skriabina V, Ismail M, Franzen O, Rehders TC, Dieckmann C, Lund G, Reichenspurner H, Meinertz T. How to guide stent-graft implantation in type B aortic dissection? Comparison of angiography, transesophageal echocardiography, and intravascular ultrasound. Circulation. 2005; 112:260-4. ].
INTERVENTIONAL ENDOVASCULAR STRATEGY
Conventional treatment of Stanford type A (DeBakey type I, II) dissection consists of surgical reconstruction of the ascending aorta with complete or partial resection of the dissected aortic segment. Endovascular strategies have no clinical application except for relief of critical malperfusion prior to surgery of the ascending aorta by distal fenestration in cases of thoraco-abdominal extension (DeBakey type I) and peripheral ischaemic complications. In distal dissection, however, endovascular stent graft placement has the potential to repair the aorta by sealing one (or multiple) proximal entry tears with a prosthetic-covered scaffold, thus reconstructing the previously collapsed true lumen with re-establishment of side-branch flow, and finally initiation of thrombosis of the false lumen [2424. Nienaber CA, Fattori R, Lund G, Dieckmann C, Wolf W, von Kodolitsch Y, Nicolas V, Pierangeli A. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539–45.
Preliminary results of endovascular treatment for thoracic aorta dissection compared with a matched group of patients treated by open surgical repair., 133133. Ince H, Nienaber CA. The concept of interventional therapy in acute aortic syndrome. J Cardiovasc Surg (Torino). 2002;17:135–42. , 134134. Koschyk D, Nienaber CA, Knap M, Hofmann T, Kodolitsch YV, Skriabina V, Ismail M, Franzen O, Rehders TC, Dieckmann C, Lund G, Reichenspurner H, Meinertz T. How to guide stent-graft implantation in type B aortic dissection? Comparison of angiography, transesophageal echocardiography, and intravascular ultrasound. Circulation. 2005; 112:260-4. ] ( Table 7 ), ( Figure 10 ). Most scenarios of malperfusion syndrome are amenable to endovascular management, considering that surgical mortality rates in patients with acute peripheral vascular ischaemic complications are similar to those in patients with mesenteric ischaemia. [135135. Walkers PJ, Dake MD, Mitchell RS, Miller DC. The use of endovascular techniques for the treatment of complications of aortic dissection. J Vasc Surg. 1993;18:1042–51. , 136136. Fann JI, Sarris GE, Mitchell RS, Shumway NE, Stinson EB, Oyer PE, Miller DC. Treatment of patients with aortic dissection presenting with peripheral vascular complications. Ann Surg. 1990;212:705–13. ]. In the near future, combined surgical and interventional procedures, even for proximal dissection, are likely to emerge [137137. Yano H, Ishimaru S, Kawaguchi S, Obitsu Y. Endovascular stent-grafting of the descending thoracic aorta after arch repair in acute type A dissection. Ann Thorac Surg. 2002;73:288–91. , 138138. Iannelli G, Piscione F, Tommaso L, Monaco M, Chiariello M, Spampinato N. Thoracic aortic emergencies: impact of endovascular surgery. Ann Thorac Surg. 2004;77:591–6. ] considering that endovascular procedures have already been applied successfully to the ascending aorta ( Figure 11 ).
Indications for stent graft placement
The most effective method for excluding an enlarging and dilated false lumen is the sealing of proximal entry tears with a customised stent graft. The absence of a distal re-entry tear is desirable for optimal results but not a prerequisite. Depressurisation and shrinking of the false lumen is the most beneficial result to be gained, ideally followed by complete thrombosis of the false lumen and remodelling of the entire dissected aorta: on rare occasions, this even occurs in retrograde type A dissection [139139. Kato N, Shimono T, Hirano T, Ishida M, Yada I, Takeda K. Transluminal placement of endovascular stent-grafts for the treatment of type A aortic dissection with an entry tear in the descending thoracic aorta. J Vasc Surg. 2001;34:1023–8. ]. Similar to previously accepted indications for surgical intervention in type B dissection, scenarios such as intractable pain with descending dissection, rapidly expanding false lumen diameter, extra-aortic blood collection as a sign of imminent rupture or distal malperfusion syndrome are accepted indications for emergency stent graft placement [134134. Koschyk D, Nienaber CA, Knap M, Hofmann T, Kodolitsch YV, Skriabina V, Ismail M, Franzen O, Rehders TC, Dieckmann C, Lund G, Reichenspurner H, Meinertz T. How to guide stent-graft implantation in type B aortic dissection? Comparison of angiography, transesophageal echocardiography, and intravascular ultrasound. Circulation. 2005; 112:260-4. , 139139. Kato N, Shimono T, Hirano T, Ishida M, Yada I, Takeda K. Transluminal placement of endovascular stent-grafts for the treatment of type A aortic dissection with an entry tear in the descending thoracic aorta. J Vasc Surg. 2001;34:1023–8. , 140140. Nienaber CA, Ince H, Weber F, Rehders T, Petzsch M, Meinertz T, Koschyk DH. Emergency stent-graft placement in thoracic aortic dissection and evolving rupture. J Cardiovasc Surg (Torino). 2003;18:464–70. ]. Moreover, late onset of complications such as malperfusion of vital aortic side branches may justify endovascular stent grafting as a first option.
Interventional therapy in an elective setting
Medical therapy, as the first-line approach to uncomplicated type B aortic dissections, is justified because of a relatively good short-term prognosis with 85% of patients surviving their initial hospital stay. The reported 30-day mortality of uncomplicated type B dissections ranges at 11% versus 30% in complicated cases with limbs or visceral ischaemia, renal failure, or contained rupture [9292. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. ]. Unfortunately, the long-term outcome of medical therapy is suboptimal with a reported 50% 5-year survival rate and delayed expansion of the false lumen in 25% patients at 4 years. This expansion of the false lumen predisposes patients to aortic rupture or retrograde migration of the dissection plane with involvement of the ascending aorta and a consequent increased mortality rate [141141. Nienaber CA, Zannetti S, Barbieri B, Kische S, Schareck W, Rehders TC; INSTEAD study collaborators. Investigation of Stent grafts in patients with type B Aortic Dissection Design of the INSTEAD trial- a prospective, multicenter, European randomized trial. Am Heart J. 2005; 149:592-9. , 142142. Nienaber CA, Fattori R, Lund G, Dieckmann C, Wolf W, von Kodolitsch Y, Nicolas V, Pierangeli A. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539–45. , 143143. Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders TC, Kundt G, Scheinert D, Czerny M, Kleinfeldt T, Zipfel B, Labrousse L, Ince H; INSTEAD Trial. Randomized comparison of strategies for type B aortic dissection: the Investigation of STEnt grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120:2519-28.
Results from a randomised trial including 140 patients in a stable condition at least 2 weeks after aortic dissection. Randomisation between endovascular treatment and optimal medical therapy.]. Initial experience of the endovascular management of chronic aortic dissection versus open surgery was reported by Nienaber et al [142142. Nienaber CA, Fattori R, Lund G, Dieckmann C, Wolf W, von Kodolitsch Y, Nicolas V, Pierangeli A. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539–45. ]. Whether prophylactic use of thoracic endovascular aortic repair (TEVAR) in patients with chronic type B aortic dissections is superior to medical treatment alone was evaluated in the prospective, randomized, and controlled Investigation of STEnt grafts in Aortic Dissection (INSTEAD) trial [143143. Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders TC, Kundt G, Scheinert D, Czerny M, Kleinfeldt T, Zipfel B, Labrousse L, Ince H; INSTEAD Trial. Randomized comparison of strategies for type B aortic dissection: the Investigation of STEnt grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120:2519-28.
Results from a randomised trial including 140 patients in a stable condition at least 2 weeks after aortic dissection. Randomisation between endovascular treatment and optimal medical therapy.]. One hundred and forty patients in a stable clinical condition at least 2 weeks after index dissection were randomly subjected to elective stent graft placement in addition to optimal medical therapy (n=72) or to optimal medical therapy alone (n=68). There was no difference in all-cause deaths, with a 2-year cumulative survival rate of 95.5±2.5% with optimal medical therapy vs. 88.9±3.7% with TEVAR (p=0.15) Moreover, the aorta-related death rate was not different (2.9% vs. 5.6%; p=0.68), and the risk for the combined endpoint of aorta-related death and progression was similar (p=0.65). Aortic remodelling occurred in 91.3% of patients with TEVAR, but only in 19.4% of patients under medical treatment (p<0.001). Thus, data from INSTEAD trial suggest no prognostic advantage of TEVAR within 2 years compared with monitored medical therapy for uncomplicated chronic type B dissection, suggesting that TEVAR should be reserved for complicated cases of acute or chronic descending thoracic aortic dissection, or when medical management fails. Paraplegia may occur after use of multiple stent grafts but still appears to be a rare phenomenon, especially with a stented segment of less than 20 cm. Results of short-term follow-up are excellent, with a 1-year survival rate of > 90%; tears can be re-adapted and aortic diameters generally decrease with complete thrombosis of the false lumen. This suggests that stent placement may facilitate healing of the dissection, sometimes of the entire aorta, including abdominal segments. However, late reperfusion of the false lumen has been observed occasionally, underlining the need for stringent follow-up imaging.
Interventional therapy in an emergency setting
The concept of emergency stent graft placement for urgent endovascular aortic repair of dissection is attractive, and a growing number of acute type B aortic dissections undergo endovascular repair (with little evidence of periprocedural morbidity, aborted malperfusion or leakage) and reconstruction of the dissected aorta [142142. Nienaber CA, Fattori R, Lund G, Dieckmann C, Wolf W, von Kodolitsch Y, Nicolas V, Pierangeli A. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539–45. , 143143. Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders TC, Kundt G, Scheinert D, Czerny M, Kleinfeldt T, Zipfel B, Labrousse L, Ince H; INSTEAD Trial. Randomized comparison of strategies for type B aortic dissection: the Investigation of STEnt grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120:2519-28.
Results from a randomised trial including 140 patients in a stable condition at least 2 weeks after aortic dissection. Randomisation between endovascular treatment and optimal medical therapy., 144144. Bortone AS, De Cillis E, D’Agostino D, de Luca Tupputi Schinosa L. Endovascular treatment of thoracic aortic disease: four years of experience. Circulation. 2004;110:262-7. , 145145. Dialetto G, Cocino FE, Scognamiglio G, Manduca S, Della Corte A, Giannolo B, Scardone M, Cotrufo M. Treatment of type B aortic dissection: endoluminal repair or conventtional medical therapy? Eur J Cardiothorac Surg. 2005;27:826-30. , 146146. Eggebrecht H, Nienaber CA, Neuhäuser M, Baumgart D, Kische S, Schmermund A, Herold U, Rehders TC, Jakob HG, Erbel R. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J. 2006;27:489-98.
Meta-analysis on published data in respect of clinical success, complications and outcome of endovascular stent-graft placement in patients with descending aorta dissection., 147147. Xu S, Huang F, Yang J, Li ZZ, Wang XY, Zhang ZG, Du JH. Endovascular repair of acute type B aortic dissection: early and mid-term results. J Vasc Surg. 2006;43:1090-5. , 148148. Verhoye JP, Miller DC, Sze D, Dake MD, Mitchell RS. Complicated acute type B aortic dissection: midterm results of emergency stent-grafting. J Thorac Cardiovasc Surg. 2008;136:424-30. , 149149. Szeto WY, McGarvey M, Pochettino A, Moser GW, Hoboken A, Cornelius K, Woo EY, Carpenter JP, Fairman RM, Bavaria JE. Results of a new surgical paradigm: endovascular repsir for acute complicated type B aortic dissection. Ann Thorac Surg. 2008;86:87-94. , 150150. Khoynezhad A, Donayre CE, Omari BO, Kopchok GE, Walot I, White RA. Midterm results of endovascular treatment of complicated acute type B aortic dissection. J Thorac Cardiovasc Surg. 2009;138:625-31. , 151151. Alves CM, da Fonseca JH, de Souza JA, Kim HC, Esher G, Buffolo E. Endovascular treatment of type B aortic dissection: the challenge of late success. Ann Thorac Surg. 2009;87:1360-5. , 152152. Persa CJ, Schroder JN, Daneshmand MA, McCann RL, Hughes GC. Midterm results for endovascular repair of complicated acute and chronic type B aortic dissection. Ann Thorac Surg. 2010;89:97-104. , 153153. Kato N, Hirano T, Ishida M, Shimono T, Cheng SH, Yada I, Takeda K. Acute and contained rupture of the descending thoracic aorta: treatment with endovascular stent-grafts. J Vasc Surg. 2003;37:100-5. , 154154. Eggebrecht H, Herold U, Kuhnt O, Schmermund A, Bartel T, Martini S, Lind A, Naber CK, Kienbaum P, Kühl H, Peters J, Jakob H, Erbel R, Baumgart D. Endovascular stent-graft treatment of aortic dissection: determinants of postinterventional outcome. Eur Heart J. 2005;26:489-97. , 155155. Jing QM, Han YL, Wang XZ, Deng J, Luan B, Jin HX, Liu XJ, Li F. Endovascular stent-graft for acute and chronic type B aortic dissection: comparison of clinical outcomes. Chin Med J. 2008;121:2213-7. , 156156. Guangqi C, Xiaoxi L, Wei C, Songqi L, Chen Y, Zilun L, Shenming W. Endovascular repair of Stanford type B aortic dissection: early and midterm outcome of 121 cases. Eur J Vasc Endovasc Surg. 2009;38:422-6. , 157157. Kim U, Hong SJ, Kim J, Kim JS, Ko YG, Choi D, Lee do Y, Chang BC, Shim WH. Intermediate and long-term outcomes of endoluminal stent-graft repair in patients with cronic type B aortic dissection. J Endovasc Ther. 2009;16:42-7. , 158158. Fattori R, Tsai TT, Myrmel T, Evangelista A, Cooper JV, Trimarchi S, Li J, Lovato L, Kische S, Eagle KA, Isselbacher EM, Nienaber CA. Complicated acute type B dissection: is surgery still the best option?. A report from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol Intv. 2008;1:395-402.
Results from the registry on complicated acute Type B dissections. Comparison between medical treatment, open surgical treatment and endovascular treatment.] ( Table 8 ). Recent reports suggest that percutaneous stent graft placement in the dissected aorta is safer and produces better results than surgery for type B dissection. Data from the IRAD registry on 571 patients with acute type B aortic dissection provided better survival rates after endovascular treatment versus open surgical repair in patients with complicated type B dissection [158158. Fattori R, Tsai TT, Myrmel T, Evangelista A, Cooper JV, Trimarchi S, Li J, Lovato L, Kische S, Eagle KA, Isselbacher EM, Nienaber CA. Complicated acute type B dissection: is surgery still the best option?. A report from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol Intv. 2008;1:395-402.
Results from the registry on complicated acute Type B dissections. Comparison between medical treatment, open surgical treatment and endovascular treatment.] ( Figure 12 ).
Long-term therapy and follow-up
The long-term approach to patients with successful initial treatment of acute aortic dissection begins with the appreciation of a systemic illness. Systemic hypertension, advanced age, aortic size and presence of patent false lumen are all factors which identify higher risk, as does the entire spectrum of Marfan’s syndrome [143143. Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders TC, Kundt G, Scheinert D, Czerny M, Kleinfeldt T, Zipfel B, Labrousse L, Ince H; INSTEAD Trial. Randomized comparison of strategies for type B aortic dissection: the Investigation of STEnt grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120:2519-28.
Results from a randomised trial including 140 patients in a stable condition at least 2 weeks after aortic dissection. Randomisation between endovascular treatment and optimal medical therapy., 158158. Fattori R, Tsai TT, Myrmel T, Evangelista A, Cooper JV, Trimarchi S, Li J, Lovato L, Kische S, Eagle KA, Isselbacher EM, Nienaber CA. Complicated acute type B dissection: is surgery still the best option?. A report from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol Intv. 2008;1:395-402.
Results from the registry on complicated acute Type B dissections. Comparison between medical treatment, open surgical treatment and endovascular treatment., 159159. Pansini S, Gagliardotto PV, Pompei E, Parisi F, Bardi G, Castenetto E, Orzan F, di Summa M. Early and late risk factors in surgical treatment of acute type A aortic dissection. Ann Thorac Surg. 1998;66:779–84. , 160160. Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term beta adrenergic blockade in Marfan’s syndrome. N Engl J Med. 1994;330:1335–41. , 161161. Nienaber CA, Kodolitsch Y. Therapeutic management of patients with Marfan syndrome: focus on cardiovascular involvement. Cardiol Rev. 1999;7:332–41. , 162162. Pereira L, Levran O, Ramirez F, Lynch JR, Sykes B, Pyeritz RE, Dietz HC. A molecular approach to the stratification of cardiovascular risk in families with Marfan’s syndrome. N Engl J Med. 1994;331:148–53. ]. All patients merit aggressive medical therapy, follow-up visits and serial imaging. It has been estimated that one third of survivors of dissection will experience extension of dissection or rupture, or require surgery for aortic aneurysm formation within 5 years. Adjunctive ß-blockade is important by lowering both blood pressure and sheer wall stress (dp/dt). Blood pressure should be titrated to less than 130/80 mmHg regardless underlying disease [33. Erbel R, Alfonso F, Boileau C, Dirsch O, Eber B, Haverich A, Rakowski H, Struyven J, Radegran K, Sechtem U, Taylor J, Zollikofer C, Klein WW, Mulder B, Providencia LA; Task Force on Aortic Dissection, European Society of Cardiology. Task Force on Aortic Dissection of the European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22:1642–81.
This manuscript describes the provisional guidelines prepared by the ESC taskforce on aortic dissection., 163163. Finkbohner R, Johnston D, Crawford ES, Coselli J, Milewicz DM. Marfan syndrome. Long-term survival and complications after aortic aneurysm repair. Circulation. 1995;91:728–33. , 164164. Silverman DI, Burton KJ, Gray J, Bosner MS, Kouchoukos NT, Roman MJ, Boxer M, Devereux RB, Tsipouras P. Life expectancy in the Marfan syndrome. Am J Cardiol. 1995;75:157–60. ]. Additionally, heart rate should be controlled since a heart rate <60 bpm significantly decreases secondary adverse events (aortic expansion, recurrent aortic dissection, aortic rupture and/or need for aortic surgery) in type B aortic dissection [165165. Kodama K, Nishigami K, Sakamoto T, Sawamura T, Hirayama T, Misumi H, Nakao K. Tight heart rate control reduces secondary adverse events in patients with type B acute aortic dissection. Circulation. 2008;118 (suppl 1):167-70. ]. Choice of serial follow-up imaging is dependent on institutional availability and expertise. Previous recommendations suggest follow-up imaging at 1, 3, 6, 9 and 12 months following discharge, and annually thereafter [33. Erbel R, Alfonso F, Boileau C, Dirsch O, Eber B, Haverich A, Rakowski H, Struyven J, Radegran K, Sechtem U, Taylor J, Zollikofer C, Klein WW, Mulder B, Providencia LA; Task Force on Aortic Dissection, European Society of Cardiology. Task Force on Aortic Dissection of the European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22:1642–81.
This manuscript describes the provisional guidelines prepared by the ESC taskforce on aortic dissection.]. This aggressive strategy underlines the observation that both hypertension and aortic expansion/dissection are common and not easily predicted in the first months following hospital discharge. Imaging should not be confined to the region of initial involvement since both dissection and aneurysm formation may occur anywhere along the entire length of the aorta.
Traumatic rupture of the aorta
DEFINITION
Acute traumatic rupture of the aorta remained rare until the widespread introduction of high-speed motor vehicles in the middle of the 20th century. It has been estimated that around 8,000 cases of blunt thoracic injuries occur each year in the United States [166166. Nagy K, Fabian T, Rodman G, Fulda G, Rodriguez A, Mirvis S. Guidelines for the diagnosis and management of blunt aortic injury: an EAST Practice Management Guidelines Work Group. J Trauma. 2000;48:1128-43. ]. Up to 16% of persons who die from an automobile accident sustain an aortic rupture. Greendyke reported a 27% incidence of rupture for those who were ejected from their car and only 12% for those who remained seated [167167. Greendyke RM. Traumatic rupture of aorta; special reference to automobile accidents. JAMA. 1966;195:527-30. ].
It has been estimated that in patients who sustain a traumatic rupture of the aorta, 85% die at the accident scene from massive mediastinal and pleural haemorrhage. Therefore, major trauma centres see only 2.6 patients per year (range: 0.2-10.7) [168168. Bhaskar J, Foo J, Kumar Sharma A. Clamp-and-Sew Technique for Traumatic Injuries of the Aorta: 20-Year Experience. Asian Cardiovasc Thorac Ann. 2010;18:161–5.
This manuscript describes the guidelines for diagnosis and treatment of blunt aortic trauma.]. Among survivors, the mortality rate is 50% within 24 hours, and almost 75% will die within one week without definitive treatment [168168. Bhaskar J, Foo J, Kumar Sharma A. Clamp-and-Sew Technique for Traumatic Injuries of the Aorta: 20-Year Experience. Asian Cardiovasc Thorac Ann. 2010;18:161–5.
This manuscript describes the guidelines for diagnosis and treatment of blunt aortic trauma.].
The extent and morphology of traumatic aortic injuries range from intimal haemorrhage to complete transection. Aortic injury most commonly results from transverse tears and can be segmental (55%) or circumferential (45%) and can be further categorised in partial (65%) or transmural (35%) [169169. Steenburg SD, Ravenel JG, Ikonomidis JS, Schönholz C, Reeves S. Acute Traumatic Aortic Injury: Imaging Evaluation and Management. Radiology. 2008;248:248-62.
Landmark study on imaging management in patients with traumatic aortic injury. Multidetector CT-scan seems to be the preferred imaging modality.]. Spiral and irregular tears are uncommon. Acute traumatic aortic injuries are classified into 4 groups [170170. Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, Murad MH, Fairman RM. Endovascular repair of traumatic thoracic aortic injury: Clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187-92.
Clinical practice guidelines from the U.S. on endovascular management of traumatic thoracic injury based on a systematic review and meta-analysis of the literature., 171171. Azizzadeh A, Keyhani K, Miller CC 3rd, Coogan SM, Safi HJ, Estrera AL. Blunt traumatic aortic injury: initial experience with endovascular repair. J Vasc Surg. 2009;49:1403-8. ] ( Figure 13 ). Partial lacerations usually involve only the intima and media of the aortic vessel wall layers, resulting in a contained rupture. The adventitia may be injured in up to 40% of cases, and is almost universally fatal due to rapid exsanguination and subsequent death [172172. Holtzman SR, Yucel EK, Rybicki FJ, Baum RA, Desjardins B, Flamm SD, Foley WD, Koss SA, Mammen L, Mansour MA, Narra VR, Expert Panel on Vascular Imaging. ACR Appropriateness Criteria® blunt chest trauma - suspected aortic injury. Reston (VA): American College of Radiology. 2009. 5 p. ]. In traumatic aortic rupture survivors, the pseudoaneurysm is contained by the adventitia and occasionally the mediastinal structures.
Traumatic aortic rupture includes the aorta, the proximal section of the great vessels, and the sinuses of Valsalva. In 66% the location for aortic traumatic rupture is at the isthmus, just beyond the orifice of the great vessels [173173. Teixeira PG, Inaba K, Barmparas G, Georgiou C, Toms C, Noguchi TT, Rogers C, Sathyavagiswaran L, Demetriades D. Blunt thoracic aortic injuries: an autopsy study. J Trauma. 2011;70:197-202. ]. The proximal descending aorta, where the relatively mobile aortic arch can move in opposition to the fixed descending aorta (ligamentum arteriosum), is at greatest risk from the shearing forces of sudden deceleration. Other locations, in decreasing order of frequency, are the descending thoracic aorta, the ascending aorta, the aortic arch, and the abdominal aorta. Ruptures at the aortic hiatus are less common [173173. Teixeira PG, Inaba K, Barmparas G, Georgiou C, Toms C, Noguchi TT, Rogers C, Sathyavagiswaran L, Demetriades D. Blunt thoracic aortic injuries: an autopsy study. J Trauma. 2011;70:197-202. ] ( Figure 14 ).
AETIOLOGY AND PATHOGENESIS
Despite extensive research, the exact mechanism of traumatic aortic rupture remains unclear. The aorta is at greatest risk in frontal or side vehicle impacts, pedestrians struck by a car and falls from a height [173173. Teixeira PG, Inaba K, Barmparas G, Georgiou C, Toms C, Noguchi TT, Rogers C, Sathyavagiswaran L, Demetriades D. Blunt thoracic aortic injuries: an autopsy study. J Trauma. 2011;70:197-202. ]. The majority of all cause traumatic aortic rupture patients are generally young and otherwise healthy people with an average age of 43 years, and a male:female ratio of 7:3 [173173. Teixeira PG, Inaba K, Barmparas G, Georgiou C, Toms C, Noguchi TT, Rogers C, Sathyavagiswaran L, Demetriades D. Blunt thoracic aortic injuries: an autopsy study. J Trauma. 2011;70:197-202. ].
Postulated mechanisms for aortic injury are deceleration shear forces, compression between the sternum and the spine (osseous pinch), and sudden increases in intra-luminal aortic pressure at the moment of impact (water-hammer effect) [174174. Karmy-Jones R, Jackson N, Long W, Simeone A. Current Management of Traumatic Rupture of the Descending Thoracic Aorta. Current Cardiology Reviews. 2009;5:187-95. ]. It is estimated that 75%-80% of the thoracic aortic injuries are a result of high speed motor vehicle collisions, with most incidents occurring after rapid deceleration as a result of head-on or side impact collisions above 50 km/hr. For a long time, head-on collisions have been thought to play a predominant role in the trauma mechanism. Mounting evidence suggests that serious lateral impact crashes also have a substantial risk of a traumatic rupture of the aorta. The majority of the aortic lacerations sustained in automobile crashes were side impact collisions in 42% and head-on collisions in 58%. Seventy-three per cent and 67% of the victims in side impact and head-on collisions, respectively, had aortic lacerations at the isthmus [175175. Feczko JD, Lynch L, Pless JE, Clark MA, McClain J, Hawley DA. An autopsy case review of 142 nonpenetrating (blunt) injuries of the aorta. J Trauma. 1992;33:846-9. ]. In rapid deceleration accidents in the anterior-posterior and lateral directions, the heart, ascending aorta, and the transverse arch continue to move forward, while movement of the isthmus and the descending aorta is limited by their posterior attachments, mainly the ligamentum arteriosum, aortic root, and diaphragm. In addition, there is evidence that the isthmus is relatively weaker compared to other parts of the aorta [169169. Steenburg SD, Ravenel JG, Ikonomidis JS, Schönholz C, Reeves S. Acute Traumatic Aortic Injury: Imaging Evaluation and Management. Radiology. 2008;248:248-62.
Landmark study on imaging management in patients with traumatic aortic injury. Multidetector CT-scan seems to be the preferred imaging modality.].
A sternum trauma against the dashboard would result in an upward and backward displacement of the lower portion of the sternal fragment [172172. Holtzman SR, Yucel EK, Rybicki FJ, Baum RA, Desjardins B, Flamm SD, Foley WD, Koss SA, Mammen L, Mansour MA, Narra VR, Expert Panel on Vascular Imaging. ACR Appropriateness Criteria® blunt chest trauma - suspected aortic injury. Reston (VA): American College of Radiology. 2009. 5 p. ]. This would result in a cranial force on the mediastinum and ascending aorta. This cranial displacement would result in a stretch on the proximal descending aorta, causing rupture near the attachment of the ligamentum arteriosum. A combination of compression and upward thrust of the heart has been suggested [169169. Steenburg SD, Ravenel JG, Ikonomidis JS, Schönholz C, Reeves S. Acute Traumatic Aortic Injury: Imaging Evaluation and Management. Radiology. 2008;248:248-62.
Landmark study on imaging management in patients with traumatic aortic injury. Multidetector CT-scan seems to be the preferred imaging modality.]. Lateral compression can result in severe internal chest deformation, resulting in anterior displacement of the heart and thus shearing forces at the aortic isthmus. Increased intravascular pressure, following direct compression of the aorta has shown to result in mainly transverse tears at the level of the isthmus [169169. Steenburg SD, Ravenel JG, Ikonomidis JS, Schönholz C, Reeves S. Acute Traumatic Aortic Injury: Imaging Evaluation and Management. Radiology. 2008;248:248-62.
Landmark study on imaging management in patients with traumatic aortic injury. Multidetector CT-scan seems to be the preferred imaging modality.].
CLINICAL FEATURES
The clinical signs and symptoms associated with traumatic aortic rupture are nonspecific and mostly indirect. Patients may complain of chest pain, midscapular pain, or shortness of breath [172172. Holtzman SR, Yucel EK, Rybicki FJ, Baum RA, Desjardins B, Flamm SD, Foley WD, Koss SA, Mammen L, Mansour MA, Narra VR, Expert Panel on Vascular Imaging. ACR Appropriateness Criteria® blunt chest trauma - suspected aortic injury. Reston (VA): American College of Radiology. 2009. 5 p. ]. The most frequent clinical signs and associated injuries are depicted in ( Table 9). Almost half of all patients with aortic disruption have no external signs of chest trauma. The exception is the rapid onset of severe hypovolemic shock associated with a non-contained aortic rupture. The signs and symptoms of contained traumatic aortic rupture are often missed due to distracting associated injuries of polytrauma patients, such as fractures, and spinal cord and head injury. An autopsy study comparing traumatic deaths with and without thoracic aortic rupture showed significant more concomitant injuries in the aortic rupture group with cardiac injury 44% versus 25%, haemothorax 86% versus 56%, rib fractures 86% versus 72%, and intra-abdominal injury 74% versus 49% [173173. Teixeira PG, Inaba K, Barmparas G, Georgiou C, Toms C, Noguchi TT, Rogers C, Sathyavagiswaran L, Demetriades D. Blunt thoracic aortic injuries: an autopsy study. J Trauma. 2011;70:197-202. ].
The mortality rate of a missed thoracic aorta injury is high, with a 4-month survival of less than 2% [176176. Kuhlman JE, Pozniak MA, Collins J, Knisely BL. Radiographic and CT findings of blunt thoracic chest trauma : aortic injuries and looking beyond them. RadioGraphics. 1998;18:1085-1106. ]. Approximately 10% to 20% of all patients with an acute thoracic aortic rupture survive their initial trauma. If no treatment is initiated, 30% of these initial survivors will die within 6 hours, 50% within 24 hours, 75% within the first week, and more than 90% within 10 weeks.
DIAGNOSTIC MODALITIES
No ideal diagnostic algorithm is available for traumatic aortic rupture. A high index of suspicion combined with the clinical history and trauma mechanism can point in the direction of a likely traumatic thoracic aortic rupture. Plain radiography is usually the first imaging study [172172. Holtzman SR, Yucel EK, Rybicki FJ, Baum RA, Desjardins B, Flamm SD, Foley WD, Koss SA, Mammen L, Mansour MA, Narra VR, Expert Panel on Vascular Imaging. ACR Appropriateness Criteria® blunt chest trauma - suspected aortic injury. Reston (VA): American College of Radiology. 2009. 5 p. ]. The chest radiograph has been studied extensively as a screening test. The optimal upright posterior-anterior chest evaluation is often replaced with a portable examination with the patient still on the spine board [169169. Steenburg SD, Ravenel JG, Ikonomidis JS, Schönholz C, Reeves S. Acute Traumatic Aortic Injury: Imaging Evaluation and Management. Radiology. 2008;248:248-62.
Landmark study on imaging management in patients with traumatic aortic injury. Multidetector CT-scan seems to be the preferred imaging modality.]. The choice between computed tomography (CT) and angiography may depend on institutional preferences, the patient’s condition, and the likelihood of other injuries. Diagnostic imaging should be deferred in patients presenting with hypovolemic shock or cardiac arrest. Advanced (64 slice and greater) spiral CT can produce near-angiographic quality images, and when available, it should be considered as the diagnostic procedure of choice for traumatic aortic rupture [174174. Karmy-Jones R, Jackson N, Long W, Simeone A. Current Management of Traumatic Rupture of the Descending Thoracic Aorta. Current Cardiology Reviews. 2009;5:187-95. ]. Angiography is appropriate in cases where endovascular intervention (e.g., stent graft) is considered. Except for cases involving exsanguinating haemorrhage from a pelvic fracture, angiography has no role in the early treatment of patients in unstable condition. MRI generally has no role in the acute evaluation of traumatic thoracic aortic rupture, due to the relatively large amount of time required. Some centres advocate the early use of transoesophageal echography, which is a sensitive screening test, with a sensitivity of 56%-99%, and a specificity of 89%-99% [177177. Cinnella G, Dambrosio M, Brienza N, Tullo L, Fiore T. Transesophageal echocardiography for diagnosis of traumatic aortic injury: an appraisal of the evidence. J Trauma. 2004;57:1246-55. ]. However, an abnormal transoesophageal echocardiography is generally followed by other diagnostic modalities. Despite its sensitivity, transoesophageal echocardiography has some disadvantages. It requires specific training and expertise and may not be as readily available as a spiral CT or angiography. Transoesophageal echocardiography does not visualise the ascending aorta or the aortic branches well. Its usefulness may lie in the ability to follow small intimal injuries that are not visible on the angiography or for patients too unstable to transport [166166. Nagy K, Fabian T, Rodman G, Fulda G, Rodriguez A, Mirvis S. Guidelines for the diagnosis and management of blunt aortic injury: an EAST Practice Management Guidelines Work Group. J Trauma. 2000;48:1128-43. ]. The choice and timing of plain radiography, spiral CT, and/or angiography must be considered in context of the patient’s condition as a whole and on the local facilities [166166. Nagy K, Fabian T, Rodman G, Fulda G, Rodriguez A, Mirvis S. Guidelines for the diagnosis and management of blunt aortic injury: an EAST Practice Management Guidelines Work Group. J Trauma. 2000;48:1128-43. ].
Chest radiography
Chest radiography is still used as a primary screening test. It has a low sensitivity, but a 98% negative predictive value [172172. Holtzman SR, Yucel EK, Rybicki FJ, Baum RA, Desjardins B, Flamm SD, Foley WD, Koss SA, Mammen L, Mansour MA, Narra VR, Expert Panel on Vascular Imaging. ACR Appropriateness Criteria® blunt chest trauma - suspected aortic injury. Reston (VA): American College of Radiology. 2009. 5 p. ]. In trauma patients with negative chest radiograph findings, only 3% have occult mediastinal haemorrhage discovered at spiral CT, and only 0.4% have an aortic injury [176176. Kuhlman JE, Pozniak MA, Collins J, Knisely BL. Radiographic and CT findings of blunt thoracic chest trauma : aortic injuries and looking beyond them. RadioGraphics. 1998;18:1085-1106. ]. The anteroposterior chest radiograph is one of the standard adjuncts to the primary survey in blunt trauma patients. A wide variety of signs on the chest radiograph are suggestive of traumatic aortic injury, but none are pathognomonic. All are related to the identification of mediastinal haemorrhage and haematoma. This mediastinal haemorrhage is possibly due to tears of mediastinal venous structures and as such is an indirect indicator of possible aortic injury. It does not constitute the aortic injury itself. The majority of patients with mediastinal haemorrhage do not have an aortic injury. Additionally, mediastinal haemorrhage can result from injury to the spine and surrounding structures. Thus, the significance of radiographic signs for aortic injury is significantly decreased in the presence of vertebral trauma [176176. Kuhlman JE, Pozniak MA, Collins J, Knisely BL. Radiographic and CT findings of blunt thoracic chest trauma : aortic injuries and looking beyond them. RadioGraphics. 1998;18:1085-1106. ].
While many signs of aortic injury have been reported, the most sensitive indicator of a traumatic rupture remains a widened mediastinum. A mediastinal width of more than 8 cm at the level of the aortic arch, or a mediastinal/chest width ratio of >0.38, is considered abnormal and an indication for further imaging [166166. Nagy K, Fabian T, Rodman G, Fulda G, Rodriguez A, Mirvis S. Guidelines for the diagnosis and management of blunt aortic injury: an EAST Practice Management Guidelines Work Group. J Trauma. 2000;48:1128-43. ]. A widened mediastinum is reported as having a sensitivity of 90%, but only a specificity of 10% for traumatic aortic injury [172172. Holtzman SR, Yucel EK, Rybicki FJ, Baum RA, Desjardins B, Flamm SD, Foley WD, Koss SA, Mammen L, Mansour MA, Narra VR, Expert Panel on Vascular Imaging. ACR Appropriateness Criteria® blunt chest trauma - suspected aortic injury. Reston (VA): American College of Radiology. 2009. 5 p. ].
To maintain spinal precautions in blunt trauma patients, most anteroposterior chest radiographs are taken in the supine position. This will lead to fluid shifts that may cause a widened mediastinum. Repeating the chest radiograph with the patient erect, if the spine can be cleared prior to this, will show normalisation of the widened mediastinum in approximately 40% [169169. Steenburg SD, Ravenel JG, Ikonomidis JS, Schönholz C, Reeves S. Acute Traumatic Aortic Injury: Imaging Evaluation and Management. Radiology. 2008;248:248-62.
Landmark study on imaging management in patients with traumatic aortic injury. Multidetector CT-scan seems to be the preferred imaging modality.].
Other mediastinal abnormalities on the chest radiograph that are considered strongly suggestive of a traumatic thoracic aortic rupture are an obscure or indistinct aortic knob and an unclear aortic outline from the arch down to the diaphragm. A clear aortic knob outline has a 72% sensitivity, 47% specificity and 87% negative predictive value. When the aortic outline is not clearly seen, it is impossible to exclude aortic injury on the chest radiograph. Other less sensitive signs of mediastinal great vessel injury include depression of the left main-stem bronchus, deviation of the nasogastric tube to the right, apical pleural haematoma, and disruption of the calcium ring in the aortic knob [172172. Holtzman SR, Yucel EK, Rybicki FJ, Baum RA, Desjardins B, Flamm SD, Foley WD, Koss SA, Mammen L, Mansour MA, Narra VR, Expert Panel on Vascular Imaging. ACR Appropriateness Criteria® blunt chest trauma - suspected aortic injury. Reston (VA): American College of Radiology. 2009. 5 p. ]. Unfortunately, none of these classic signs contribute to any useful sensitivity to use them as a screening for traumatic thoracic aortic rupture.
Angiography
Angiography, contrast aortography, has been used as the gold standard diagnostic test for traumatic thoracic aortic rupture. It is the diagnostic modality to which all others are compared. There is a small incidence of false-positive angiograms resulting from anatomical abnormalities such as a ductus diverticulum. A ductus diverticulum is a developmental knob of the aorta which is usually seen at the anteromedial aspect of the aorta at the site of the previous ductus arteriosus. It appears as a focal bulge along with in inner curvature of the isthmus and may be confused with a pseudoaneurysm [178178. Pelberg R, Mazur W. Vascular CT angiography manual. Springer-Verlag London. 2011; p93-4. ]. With the rapid improvements in spiral CT technologies, an angiography may be reserved for those patients with an equivocal or indeterminate CT, because of more detailed images on the angiogram [170170. Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, Murad MH, Fairman RM. Endovascular repair of traumatic thoracic aortic injury: Clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187-92.
Clinical practice guidelines from the U.S. on endovascular management of traumatic thoracic injury based on a systematic review and meta-analysis of the literature., 172172. Holtzman SR, Yucel EK, Rybicki FJ, Baum RA, Desjardins B, Flamm SD, Foley WD, Koss SA, Mammen L, Mansour MA, Narra VR, Expert Panel on Vascular Imaging. ACR Appropriateness Criteria® blunt chest trauma - suspected aortic injury. Reston (VA): American College of Radiology. 2009. 5 p. ].
Computed tomography
A spiral CT scan is the best screening modality for traumatic aortic rupture. The sensitivity of modern CT scanners is 97%-100%, with a negative predictive value of 100% and specificity of 82% [176176. Kuhlman JE, Pozniak MA, Collins J, Knisely BL. Radiographic and CT findings of blunt thoracic chest trauma : aortic injuries and looking beyond them. RadioGraphics. 1998;18:1085-1106. ]. A CT finding of mediastinal haemorrhage alone is sensitive for traumatic aortic injury, but the visualisation of actual aortic injury is even more diagnostic. False positive impressions can be caused by thymus tissue, periaortic atelectasis, volume averaging and pleural effusions [166166. Nagy K, Fabian T, Rodman G, Fulda G, Rodriguez A, Mirvis S. Guidelines for the diagnosis and management of blunt aortic injury: an EAST Practice Management Guidelines Work Group. J Trauma. 2000;48:1128-43. , 169169. Steenburg SD, Ravenel JG, Ikonomidis JS, Schönholz C, Reeves S. Acute Traumatic Aortic Injury: Imaging Evaluation and Management. Radiology. 2008;248:248-62.
Landmark study on imaging management in patients with traumatic aortic injury. Multidetector CT-scan seems to be the preferred imaging modality., 172172. Holtzman SR, Yucel EK, Rybicki FJ, Baum RA, Desjardins B, Flamm SD, Foley WD, Koss SA, Mammen L, Mansour MA, Narra VR, Expert Panel on Vascular Imaging. ACR Appropriateness Criteria® blunt chest trauma - suspected aortic injury. Reston (VA): American College of Radiology. 2009. 5 p. ].
The introduction of the spiral CT has improved the overall sensitivity significantly, and is now comparable to contrast aortography. When encountering a large mediastinal haematoma, the most predominant injury location is at the isthmus and is most frequently visible along the medial curvature of the arch at the level of the left pulmonary artery and left mainstem bronchus. In case of a visible haematoma on the spiral CT around the great thoracic vessels, their origin, or the arch, but no definitive injury is seen, angiography is recommended [166166. Nagy K, Fabian T, Rodman G, Fulda G, Rodriguez A, Mirvis S. Guidelines for the diagnosis and management of blunt aortic injury: an EAST Practice Management Guidelines Work Group. J Trauma. 2000;48:1128-43. , 172172. Holtzman SR, Yucel EK, Rybicki FJ, Baum RA, Desjardins B, Flamm SD, Foley WD, Koss SA, Mammen L, Mansour MA, Narra VR, Expert Panel on Vascular Imaging. ACR Appropriateness Criteria® blunt chest trauma - suspected aortic injury. Reston (VA): American College of Radiology. 2009. 5 p. ].
The American College of Radiology guideline on suspected traumatic aortic injury supports the continued use of the chest radiograph as the initial screening examination in patients who have sustained a blunt chest trauma [172172. Holtzman SR, Yucel EK, Rybicki FJ, Baum RA, Desjardins B, Flamm SD, Foley WD, Koss SA, Mammen L, Mansour MA, Narra VR, Expert Panel on Vascular Imaging. ACR Appropriateness Criteria® blunt chest trauma - suspected aortic injury. Reston (VA): American College of Radiology. 2009. 5 p. ]. In case of a chest radiograph demonstrating mediastinal widening or other signs of mediastinal haemorrhage, thoracic aortography or spiral chest CT is indicated. As CTA has emerged as a very sensitive and specific examination for aortic injury, it has replaced aortography as the primary imaging tool in many trauma centres. With this expanding role for CTA, the role for transoesophageal echography and intravascular ultrasound is diminishing, but may be useful in selected patients [172172. Holtzman SR, Yucel EK, Rybicki FJ, Baum RA, Desjardins B, Flamm SD, Foley WD, Koss SA, Mammen L, Mansour MA, Narra VR, Expert Panel on Vascular Imaging. ACR Appropriateness Criteria® blunt chest trauma - suspected aortic injury. Reston (VA): American College of Radiology. 2009. 5 p. ].
TREATMENT
Most traumatic aortic ruptures reaching hospital alive are partial transections, and should be managed with blood pressure control until definitive repair. The priority in the management of haemodynamically unstable patients with potential aortic injury is to identify and control ongoing haemorrhage from other sites rapidly, and to avoid over-resuscitation. Sites of concealed haemorrhage are identified with chest and pelvis radiographs and focussed assessment with sonography in trauma (FAST) or diagnostic peritoneal lavage [179179. Kortbeek JB, Al Turki SA, Ali J, Antoine JA, Bouillon B, Brasel K, Brenneman F, Brink PR, Brohi K, Burris D, Burton RA, Chapleau W, Cioffi W, Collet e Silva Fde S, Cooper A, Cortes JA, Eskesen V, Fildes J, Gautam S, Gruen RL, Gross R, Hansen KS, Henny W, Hollands MJ, Hunt RC, Jover Navalon JM, Kaufmann CR, Knudson P, Koestner A, Kosir R, Larsen CF, Livaudais W, Luchette F, Mao P, McVicker JH, Meredith JW, Mock C, Mori ND, Morrow C, Parks SN, Pereira PM, Pogetti RS, Ravn J, Rhee P, Salomone JP, Schipper IB, Schoettker P, Schreiber MA, Smith RS, Svendsen LB, Taha W, van Wijngaarden-Stephens M, Varga E, Voiglio EJ, Williams D, Winchell RJ, Winter R. Advanced trauma life support, 8th edition, the evidence for change. J Trauma. 2008;64:1638-50. ]. Delaying the operative thoracic aorta repair to allow the patient to recover from associated injuries, combined with a strict antihypertensive regime, is associated with a 0% to 6.7% rate of free aortic rupture [180180. Durham CA, McNally MM, Parker FM, Bogey WM, Powell CS, Goettler CE, Rotondo MF, Stoner MC. A contemporary rural trauma center experience in blunt traumatic aortic injury. J Vasc Surg. 2010;52:884-90. ]. Challenging patients are those with a traumatic aortic tear with impending rupture. These patients classically present as relatively stable, respond to fluid resuscitation and then drop their blood pressure in a cyclical manner. These are the patients who should be recognised early to avoid aggressive fluid resuscitation, as this will ultimately lead to free rupture of the aorta.
Deliberate non-operative management of traumatic aortic rupture, has been described in a carefully selected population with stable aortic lesions [181181. Caffarelli AD, Mallidi HR, Maggio PM, Spain DA, Miller C, Mitchell RS. Early outcomes of deliberate nonoperative management for blunt thoracic aortic injury in trauma. J Thorac Cardiovasc Surg. 2010;140:598-605 ]. This non-operative management is performed at the intensive care unit with invasive cardiovascular monitoring. An aggressive antihypertensive regimen is necessary to target the systolic blood pressure between 100-120 mm Hg with ongoing assessment of end-organ perfusion. In this population, a CT angiography was performed at 24 hours and then every 48 to 72 hours until the aortic injury was unchanged for 7 days [181181. Caffarelli AD, Mallidi HR, Maggio PM, Spain DA, Miller C, Mitchell RS. Early outcomes of deliberate nonoperative management for blunt thoracic aortic injury in trauma. J Thorac Cardiovasc Surg. 2010;140:598-605 ].
Traditionally, the gold standard for traumatic aortic ruptures has been open surgical repair, but over the past decade, a shift has occurred in that these patients are being treated more commonly with endovascular stent grafts [170170. Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, Murad MH, Fairman RM. Endovascular repair of traumatic thoracic aortic injury: Clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187-92.
Clinical practice guidelines from the U.S. on endovascular management of traumatic thoracic injury based on a systematic review and meta-analysis of the literature., 182182. Balm R, Hoornweg LL. Traumatic aortic ruptures. J Cardiovasc Surg. 2005;46:101-5. , 183183. Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, Hemmila MR, O’Connor JV, McKenney MO, Moore FO, London J, Singh MJ, Lineen E, Spaniolas K, Keel M, Sugrue M, Wahl WL, Hill J, Wall MJ, Moore EE, Margulies D, Malka V, Chan LS; American Association for the Surgery of Trauma Thoracic Aortic Injury Study Group. Operative repair or endovascular stent graft in blunt traumatic thoracic aortic injuries: results of an American Association for the Surgery of Trauma Multicenter Study. J Trauma. 2008;64:561-70. ].
Treatment in paediatric patients
A traumatic rupture of the aorta in the paediatric population is an extremely rare finding and is accountable for only 2.1% of the traumatic paediatric deaths [184184. Pabon-Ramos WM, Williams DM, Strouse PJ. Radiologic Evaluation of Blunt Thoracic Aortic Injury in Pediatric Patients. AJR. 2010;194:1197–1203. ]. This might be due to the increased compliance of the chest wall and the elasticity of the tissues, lack of atherosclerotic disease, decreased body mass index, resulting in a lower magnitude of force on impact [184184. Pabon-Ramos WM, Williams DM, Strouse PJ. Radiologic Evaluation of Blunt Thoracic Aortic Injury in Pediatric Patients. AJR. 2010;194:1197–1203. ]. Children have an initial survival rate that is only half of the adult initial survival rate because of the smaller tolerance to massive haemorrhage.
Approximately 30%-50% of paediatric patients with traumatic aortic rupture have no external signs of direct chest trauma. They usually present with more evident neurological, skeletal, or abdominal injuries. The lack of visible external thoracic injuries, the presence of distracting injuries and the low a priori incidence of traumatic aortic rupture in the paediatric population all contribute to a low clinical index of suspicion. Thus, determining the mechanism of injury is important to raise the index of suspicion. If the mechanism of injury involves high-energy trauma to the torso including deceleration, thoracic compression, or thoracic crush, the possibility of traumatic aortic rupture should be considered [184184. Pabon-Ramos WM, Williams DM, Strouse PJ. Radiologic Evaluation of Blunt Thoracic Aortic Injury in Pediatric Patients. AJR. 2010;194:1197–1203. ].
A chest x-ray is still used as the primary screening test, but the signs of aorta rupture can be very subtle. Depending on the trauma mechanism and the clinical findings, the threshold for a CT should be low, despite radiation exposure [184184. Pabon-Ramos WM, Williams DM, Strouse PJ. Radiologic Evaluation of Blunt Thoracic Aortic Injury in Pediatric Patients. AJR. 2010;194:1197–1203. , 185185. Manson D, Babyn PS, Palder S, Bergman K. CT of blunt chest trauma in children. Pediatr Radiol. 1993;23:1-5. ]. Open repair in children is durable and feasible. Endovascular repair is an alternative in patients unfit for open repair. The very small diameters of the aorta have resulted in off-label use of aortic cuffs, iliac contralateral legs of abdominal endografts, and other emergency solutions [186186. Keyhani K, Estrera AL, Safi HJ, Azizzadeh A. Endovascular repair of traumatic aortic injury in a pediatric patient. J Vasc Surg. 2009;50:652-4. ].
Close follow-up must take place, especially taken the growing aorta diameter into account with increasing age. Replacement of the endograft with open surgery can be offered if and when the endograft repair fails or the patient outgrows the prosthesis and develops coarctation symptoms [186186. Keyhani K, Estrera AL, Safi HJ, Azizzadeh A. Endovascular repair of traumatic aortic injury in a pediatric patient. J Vasc Surg. 2009;50:652-4. ].
Open surgical treatment
In the acute setting, open surgical repair is often challenging, with up to 70% of the patients having severe associated injuries such as pulmonary contusion requiring high positive pressure ventilation, head injury, splenic injury, and pelvic fractures [187187. Mattox KL, Feliciano DV, Burch J, Beall AC Jr, Jordan GL Jr, De Bakey ME. Five thousand seven hundred sixty cardiovascular injuries in 4459 patients. Epidemiologic evolution 1958 to 1987. Ann Surg. 1989;209:698-705. ]. The elderly with coronary artery disease are also at higher risk as they might not tolerate aortic cross clamping well during open repair. Open surgical repair is performed with either a primary anastomosis or interposition graft. This can be accomplished with clamp-and-sew technique or with additional circulatory assistance [168168. Bhaskar J, Foo J, Kumar Sharma A. Clamp-and-Sew Technique for Traumatic Injuries of the Aorta: 20-Year Experience. Asian Cardiovasc Thorac Ann. 2010;18:161–5.
This manuscript describes the guidelines for diagnosis and treatment of blunt aortic trauma., 188188. Estrera AL, Gochnour DC, Azizzadeh A, Miller CC, Coogan S, Charlton-Ouw K, Holcomb JB, Safi HJ. Progress in the Treatment of Blunt Thoracic Aortic Injury: 12-Year Single-Institution Experience. Ann Thorac Surg. 2010;90:64 –71. ]. This clamp-and-sew technique is performed by a rapid exposure of the proximal aorta with minimal dissection of the intercostals and the injured area. After proximal and distal clamping, the damaged area is excised and replaced with an interposition graft [168168. Bhaskar J, Foo J, Kumar Sharma A. Clamp-and-Sew Technique for Traumatic Injuries of the Aorta: 20-Year Experience. Asian Cardiovasc Thorac Ann. 2010;18:161–5.
This manuscript describes the guidelines for diagnosis and treatment of blunt aortic trauma.].
Patients receive general anaesthesia and a double-lumen endotracheal tube or a selective left main bronchus blocker is used, enabling selective right lung ventilation. Access to the chest is predominantly preformed by a left posterolateral thoracotomy between the 4th and 5th intercostal space with minimal disruption of the intercostal arteries. The aortic arch between the left subclavian and carotid arteries and the mid part of the descending aorta is dissected without disturbing the haematoma and the intercostals. This is followed by proximal and distal clamp application. Fluctuations in blood pressure, especially during clamping, should be kept to a minimum. A primary anastomosis can be performed or an interposition graft can be placed and de-aired prior to clamp removal. During clamping and clamp removal, haemodynamic stability can be optimised by pharmacological and blood volume management in clear and essential communication between surgeon, anaesthetist and nursing staff [168168. Bhaskar J, Foo J, Kumar Sharma A. Clamp-and-Sew Technique for Traumatic Injuries of the Aorta: 20-Year Experience. Asian Cardiovasc Thorac Ann. 2010;18:161–5.
This manuscript describes the guidelines for diagnosis and treatment of blunt aortic trauma., 188188. Estrera AL, Gochnour DC, Azizzadeh A, Miller CC, Coogan S, Charlton-Ouw K, Holcomb JB, Safi HJ. Progress in the Treatment of Blunt Thoracic Aortic Injury: 12-Year Single-Institution Experience. Ann Thorac Surg. 2010;90:64 –71. , 189189. Verdant A. Contemporary results of standard open repair of acute traumatic rupture of the thoracic aorta. J Vasc Surg. 2010;51:294-8. ]. Depending on the location of the rupture, selective antegrade brain perfusion is used by means of catheters in the innominate and left carotid artery and connected to the extra-corporal circuit. The main area of controversy with the open technique is the management of the distal circulation during clamping. The major advantages of the clamp-and-sew technique are its relative simplicity and the lack of systemic heparinisation. The advantages of bypass techniques, for instance an arterio-femoral, or arterio-distal aorta bypass, with distal aortic perfusion are potentially lower paraplegia rates as well as reduction of reperfusion injury [188188. Estrera AL, Gochnour DC, Azizzadeh A, Miller CC, Coogan S, Charlton-Ouw K, Holcomb JB, Safi HJ. Progress in the Treatment of Blunt Thoracic Aortic Injury: 12-Year Single-Institution Experience. Ann Thorac Surg. 2010;90:64 –71. ]. Nevertheless, systemic heparinisation increases morbidity and mortality in patients with multi-organ injuries, especially of the lung and brain. Heparin-bonded shunts have been used to overcome this issue. However, the placement of shunts can be difficult due to patient position, periaortic haematoma, and time constraints dictated by expanding pulsatile and uncontrolled haematoma. Evidence suggests that if clamp time is below 30 minutes, there is little advantage to distal aortic perfusion [190190. Fabian TC, Richardson JD, Croce MA, Smith JS Jr, Rodman G Jr, Kearney PA, Flynn W, Ney AL, Cone JB, Luchette FA, Wisner DH, Scholten DJ, Beaver BL, Conn AK, Coscia R, Hoyt DB, Morris JA Jr, Harviel JD, Peitzman AB, Bynoe RP, Diamond DL, Wall M, Gates JD, Asensio JA, Enderson BL, et al. Prospective study of blunt aortic injury: Multicenter Trial of the American Association for the Surgery of Trauma. J Trauma. 1997;42:374-80. ]. After the procedure, protamine sulphate can be given in order to reverse systemic heparinisation.
Spinal cord management
Spinal cord protection
There is no evidence for routine spinal cord protection in an acute traumatic rupture of the aorta [170170. Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, Murad MH, Fairman RM. Endovascular repair of traumatic thoracic aortic injury: Clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187-92.
Clinical practice guidelines from the U.S. on endovascular management of traumatic thoracic injury based on a systematic review and meta-analysis of the literature.]. Spinal cord protection is highly recommended in case of prior abdominal aortic surgery (open or endovascular), concurrent planned coverage of >50% of the thoracic aorta, or planned coverage of the T7-L1 segment, where the artery of Adamkiewicz (arteria radicularis magnus) is presumed to arise [191191. Griepp RB, Griepp EB. Spinal Cord Perfusion and Protection During Descending Thoracic and Thoracoabdominal Aortic Surgery: The Collateral Network Concept. Ann Thorac Surg. 2007;83:S865–9. ]. Other indications are occlusion (planned or chronic) of one or both hypogastric arteries or planned coverage of the left subclavian artery [192192. Chung J, Corriere MA, Veeraswamy RK, Kasirajan K, Milner R, Dodson TF, Salam AA, Chaikof EL. Risk factors for late mortality after endovascular repair of the thoracic aorta. J Vasc Surg. 2010;52:549-55. ].
Spinal cord protection includes cerebrospinal fluid drainage, naloxone, corticosteroids or mannitol administration, and moderate hypothermia [193193. Tang GL, Tehrani HY, Usman ChB A, Katariya K, Otero C, Perez E, Eskandari MK. Reduced mortality, paraplegia, and stroke with stent graft repair of blunt aortic transections: A modern meta-analysis. J Thorac Cardiovasc Surg. 2009;138:768-9. ]. Cerebrospinal fluid drainage can be performed preprocedural, but can also take place postoperatively if the patient starts to present symptoms of spinal cord ischaemia. If no paraparesis develops after 24 hours, the drains can be capped for an additional 24 hours and removed thereafter. Careful evaluation of concomitant anticoagulation is necessary.
Moderate passive hypothermia (32-34°C) is clinically safe and effective to prolong tolerated ischaemic intervals of the spinal cord [191191. Griepp RB, Griepp EB. Spinal Cord Perfusion and Protection During Descending Thoracic and Thoracoabdominal Aortic Surgery: The Collateral Network Concept. Ann Thorac Surg. 2007;83:S865–9. ].
Postoperative arterial pressures in the high physiological range have been adopted to improve adequate blood flow to the spinal cord. In addition, reduction of the flow resistance in the spinal cord is optimised with drainage of spinal fluid to maintain an intrathecal pressure of 10 cm H2O or less [191191. Griepp RB, Griepp EB. Spinal Cord Perfusion and Protection During Descending Thoracic and Thoracoabdominal Aortic Surgery: The Collateral Network Concept. Ann Thorac Surg. 2007;83:S865–9. ]. The addition of bypass techniques, such as an arterio-femoral or arterio-distal aorta bypass appears to decrease overall paraplegia rates during open surgical approach [193193. Tang GL, Tehrani HY, Usman ChB A, Katariya K, Otero C, Perez E, Eskandari MK. Reduced mortality, paraplegia, and stroke with stent graft repair of blunt aortic transections: A modern meta-analysis. J Thorac Cardiovasc Surg. 2009;138:768-9. , 194194. Akowuah E, Angelini G, Bryan AJ. Open versus endovascular repair of traumatic aortic rupture: A systematic review. J Thorac Cardiovasc Surg. 2009;138:768-9. , 195195. Arthurs ZM, Starnes BW, Sohn VY, Singh N, Martin MJ, Andersen CA. Functional and survival outcomes in traumatic blunt thoracic aortic injuries: An analysis of the National Trauma Databank. J Vasc Surg . 2009;49:988-94. ].
Spinal cord monitoring
Although not standard practice, monitoring of the spinal cord function is believed to reduce the risk of spinal cord injury. Somatosensory evoked potentials (SSEP) monitoring registers the sensory pathways and not the motor pathways. The sensory pathways usually do not respond as rapidly to spinal cord ischaemia as motor evoked potentials (MEP), but can be used postoperatively until the patient is fully awake [191191. Griepp RB, Griepp EB. Spinal Cord Perfusion and Protection During Descending Thoracic and Thoracoabdominal Aortic Surgery: The Collateral Network Concept. Ann Thorac Surg. 2007;83:S865–9. ]. For a sensitive and rapid responsive system to monitor spinal cord ischaemia, motor evoked potentials can be used. Spinal cord ischaemia, detected with the aid of somatosensory or motor evoked potentials can be corrected in the majority of cases with manipulation of the haemodynamics, in the majority of cases by raising the arterial blood pressure. Postoperatively, maintaining a mean arterial blood pressure of at least 80 mmHg and with signs of spinal cord ischaemia as high as 90 to 100 mmHg is vital [191191. Griepp RB, Griepp EB. Spinal Cord Perfusion and Protection During Descending Thoracic and Thoracoabdominal Aortic Surgery: The Collateral Network Concept. Ann Thorac Surg. 2007;83:S865–9. ].
Endovascular management
The perceived advantages of endovascular traumatic aortic rupture repair are comprehensible. It is less invasive and does not require thoracotomy, single lung ventilation or aortic cross clamping [196196. Xenos ES, Abedi NN, Davenport DL, Minion DJ, Hamdallah O, Sorial EE, Endean ED. Meta-analysis of endovascular vs open repair for traumatic descending thoracic aortic rupture. J Vasc Surg. 2008;48:1343-51. ]. The procedure can be performed percutaneously and under local anaesthesia. On the other hand, endovascular repair of traumatic aortic rupture requires technical expertise in thoracic aortic stent grafting and large bore vascular access. There is no device currently commercially available with an on-label indication for repair of traumatic aortic rupture, this has led to the off-label use of endovascular devices intended for aneurysmal aorta diseases [197197. Tefera G. Traumatic thoracic aortic injury and ruptures. J Vasc Surg. 2010;52:41S-4S. ]. A summary of the Society for Vascular Surgery guideline on thoracic endovascular in traumatic thoracic aortic injuries is presented in Table 10 [170170. Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, Murad MH, Fairman RM. Endovascular repair of traumatic thoracic aortic injury: Clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187-92.
Clinical practice guidelines from the U.S. on endovascular management of traumatic thoracic injury based on a systematic review and meta-analysis of the literature.].
After local, regional or general anaesthesia, an introducer sheath is placed percutaneously or after groin cut down for placement of the endovascular stent device. No evidence is present on the indication for systemically anticoagulation with heparin [198198. Rahimi SA, Darling RC, Mehta M, Roddy SP, Taggert JB, Sternbach Y. Endovascular repair of thoracic aortic traumatic transections is a safe method in patients with complicated injuries. J Vasc Surg. 2010;52:891-6. ]. An arch aortogram is performed through a 5 Fr femoral access, to confirm the location of the injury.
Deployment of the stent may include coverage of the left subclavian artery orifice given that the most common location of the traumatic aortic rupture is at the isthmus just distal to the left subclavian artery. Prophylactic transposition or bypass to the left subclavian artery in selected patients with a dominant left vertebral artery to decrease neurologic complications is an option; others prefer a delayed transposition only in symptomatic patients [193193. Tang GL, Tehrani HY, Usman ChB A, Katariya K, Otero C, Perez E, Eskandari MK. Reduced mortality, paraplegia, and stroke with stent graft repair of blunt aortic transections: A modern meta-analysis. J Thorac Cardiovasc Surg. 2009;138:768-9. ]. In patients where no left subclavian artery revascularisation was performed, a significantly higher incidence of paraplegia was present [200200. Buth J, Harris PL, Hobo R, Eps van R, Cuypers Ph, Duijm L, Tielbeek X. Neurologic complications associated with endovascular repair of thoracic aortic pathology: Incidence and risk factors. A study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) Registry. J Vasc Surg. 2007;46:1103-11. ]. In patients with an intact left internal mammary artery after coronary bypass surgery a carotid-to-subclavian bypass is required to preserve coronary flow [193193. Tang GL, Tehrani HY, Usman ChB A, Katariya K, Otero C, Perez E, Eskandari MK. Reduced mortality, paraplegia, and stroke with stent graft repair of blunt aortic transections: A modern meta-analysis. J Thorac Cardiovasc Surg. 2009;138:768-9. ]. To ensure accurate deployment adenosine-induced cardiac asystole can be used. A second method is inducing controlled hypotension with sodium nitroprusside (3 microg/kg/min) [199199. Forbes TL, Harris JR, Lawlor DK, DeRose G. Aortic dilatation after endovascular repair of blunt traumatic thoracic aortic injuries. J Vasc Surg. 2010;52:45-8. , 201201. Nicolaou G, Forbes TL. Strategies for accurate endograft placement in the proximal thoracic aorta. Semin Cardiothorac Vasc Anesth. 2010;14:196-200. ]. Rapid ventricular pacing at 200 bpm using the femoral vein to place the pacing catheter in the right ventricle can also be used for a controlled decrease in aortic pressure and flow, facilitating an accurate deployment of the graft [201201. Nicolaou G, Forbes TL. Strategies for accurate endograft placement in the proximal thoracic aorta. Semin Cardiothorac Vasc Anesth. 2010;14:196-200. , 202202. Nienaber CA, Kische S, Rehders TC, Schneider H, Chatterjee T, Bünger CM, Höppner R, Ince H. Rapid pacing for better placing: comparison of techniques for precise deployment of endografts in the thoracic aorta. J Endovasc Ther. 2007;14:506-12. ].
Logistically, an inventory of appropriate sized devices must be available for emergency use. Thoracic stent grafting in the traumatic aortic rupture has several technical limitations. Trauma patients tend to be younger and have a smaller aortic diameter (16-20 mm) compared to older patients with aneurysmal disease for which the currently available thoracic stent grafts have been designed. The Talent™ thoracic device (Medtronic, Santa Rosa, CA, USA), is available in diameter from 22 to 46 mm. The TAG® device (W.L. Gore & Associates, Inc. (Gore), Flagstaff, AZ, USA) has recently become available in diameters ranging from 21 to 45 mm to treat aortas between 16 and 42 mm. With the approval of the smaller diameter devices, a wider range of patients can be treated [188188. Estrera AL, Gochnour DC, Azizzadeh A, Miller CC, Coogan S, Charlton-Ouw K, Holcomb JB, Safi HJ. Progress in the Treatment of Blunt Thoracic Aortic Injury: 12-Year Single-Institution Experience. Ann Thorac Surg. 2010;90:64 –71. ].
Collapse of devices oversized too generously, in combination with the more tightly angulated aortic arch in younger patients, has been reported [203203. Garcia-Toca M, Naughton PA, Matsumura JS, Morasch MD, Kibbe MR, Rodriguez HE, Pearce WH, Eskandari MK. Endovascular Repair of Blunt Traumatic Thoracic Aortic Injuries; Seven-Year Single-Center Experience. Arch Surg. 2010;145:679-83. , 204204. Idu MM, Reekers JA, Balm R, Ponsen KJ, de Mol BA, Legemate DA. Collapse of a stent-graft following treatment of a traumatic thoracic aortic rupture. J Endovasc Ther. 2005;12:503-7. , 205205. Bandorski D, Brück M, Günther HU, Manke C. Endograft Collapse After Endovascular Treatment for Thoracic Aortic Disease. Cardiovasc Intervent Radiol. 2010;33:492-7. ]. Endograft collapse can occur between 24 hours and 6 months after stent graft implantation [205205. Bandorski D, Brück M, Günther HU, Manke C. Endograft Collapse After Endovascular Treatment for Thoracic Aortic Disease. Cardiovasc Intervent Radiol. 2010;33:492-7. ]. The use of smaller diameter aortic cuffs or devices meant for infrarenal aortic aneurysms may be limited by short delivery systems that do not reach into the thoracic aorta from a femoral approach in taller patients, requiring an iliac artery or aortic cutdown. In addition, aortic cuffs come in short lengths requiring overlap of multiple cuffs for traumatic aortic rupture repair, predisposing patients to future development of type III endoleak [206206. Cambria RP, Crawford RS, Cho JS, Bavaria J, Farber M, Lee WA, Ramaiah V, Kwolek CJ; GORE TAG Investigators. A multicenter clinical trial of endovascular stent graft repair of acute catastrophes of the descending thoracic aorta. J Vasc Surg. 2009;50:1255-64. ]. Small diameter iliac arteries or the development of intense vasospasm, which is more common in younger patients, results in higher access complication rates compared to the endovascular treated population with aortic aneurysms. The relatively high occurrence of endoleak in this population seems to be due to several factors. The majority of ruptures are located at the distal isthmus and endografts conform poorly to the inner curvaABCture of the aortic arch, especially in young patients with even sharper aortic arch angulation. Another factor contributing to the development of endoleak appears to be to inadequate preoperative sizing of the endograft due to the influence of haemodynamic instability on the aortic diameter. The aortic diameter in haemodynamically unstable trauma patients is smaller from the ascending thoracic aorta to the infrarenal aorta compared to their actual aortic diameter. These differences in aortic diameter range from 4.2% in the ascending aorta, up to 12.8% in the proximal descending aorta [207207. Jonker FH, Verhagen HJ, Mojibian H, Davis KA, Moll FL, Muhs BE. Aortic endograft sizing in trauma patients with hemodynamic instability. J Vasc Surg. 2010;52:39-44. ]. There is no consensus regarding the optimal degree of oversizing, and the current options range from no oversizing up to 10% oversizing of the endograft [170170. Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, Murad MH, Fairman RM. Endovascular repair of traumatic thoracic aortic injury: Clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187-92.
Clinical practice guidelines from the U.S. on endovascular management of traumatic thoracic injury based on a systematic review and meta-analysis of the literature.]. The manufactures highlight the off-label use and advise maximal standard oversizing according to the device specific recommendations.
Factors associated with a higher incidence of paraplegia are: previous or simultaneous infrarenal aortic repair, a compromised hypogastric artery inflow, the number of stent grafts used corresponding with the covered length of the thoracic aorta, and renal failure [200200. Buth J, Harris PL, Hobo R, Eps van R, Cuypers Ph, Duijm L, Tielbeek X. Neurologic complications associated with endovascular repair of thoracic aortic pathology: Incidence and risk factors. A study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) Registry. J Vasc Surg. 2007;46:1103-11. ].
OUTCOME
Two large meta-analyses comparing open surgical repair and endovascular repair for traumatic aortic rupture have recently been published with equivalent results [193193. Tang GL, Tehrani HY, Usman ChB A, Katariya K, Otero C, Perez E, Eskandari MK. Reduced mortality, paraplegia, and stroke with stent graft repair of blunt aortic transections: A modern meta-analysis. J Thorac Cardiovasc Surg. 2009;138:768-9. , 196196. Xenos ES, Abedi NN, Davenport DL, Minion DJ, Hamdallah O, Sorial EE, Endean ED. Meta-analysis of endovascular vs open repair for traumatic descending thoracic aortic rupture. J Vasc Surg. 2008;48:1343-51. ]. A third extensive meta-analysis comparing open surgical repair, endovascular repair and non-operative management also had a similar outcome [208208. Murad MH, Rizvi AZ, Malgor R, Carey J, Alkatib AA, Erwin PJ, Lee WA, Fairman RM. Comparative effectiveness of the treatments for thoracic aortic transaction. J Vasc Surg. 2011;53:193-199. ]. In general it must be emphasised that as expected in a disease that is fairly rare and fatal, the available literature consists of small nonrandomised case series, and must be interpreted with care. In addition, evidence of a publication bias for the outcome of death, suggesting that case series with higher event rates may have been unpublished, is present [208208. Murad MH, Rizvi AZ, Malgor R, Carey J, Alkatib AA, Erwin PJ, Lee WA, Fairman RM. Comparative effectiveness of the treatments for thoracic aortic transaction. J Vasc Surg. 2011;53:193-199. ].
Non-operative management is associated with the least favourable outcome, with a mortality rate of 46%. Mortality, paraplegia and stroke rates were all significantly in favour of endovascular repair. The overall mortality rate between endovascular and open was 9% versus 19%. Spinal cord ischaemia was 3% after endovascular repair compared to 9% in the open group, notwithstanding the fact that stent grafts result in sudden complete occlusion of a large number of segmental spinal cord vessels, occurring at relatively normothermic temperatures [208208. Murad MH, Rizvi AZ, Malgor R, Carey J, Alkatib AA, Erwin PJ, Lee WA, Fairman RM. Comparative effectiveness of the treatments for thoracic aortic transaction. J Vasc Surg. 2011;53:193-199. ]. Stroke rates were also less in the endovascular group, with 0.8% versus 5.3% in the open group [193193. Tang GL, Tehrani HY, Usman ChB A, Katariya K, Otero C, Perez E, Eskandari MK. Reduced mortality, paraplegia, and stroke with stent graft repair of blunt aortic transections: A modern meta-analysis. J Thorac Cardiovasc Surg. 2009;138:768-9. , 196196. Xenos ES, Abedi NN, Davenport DL, Minion DJ, Hamdallah O, Sorial EE, Endean ED. Meta-analysis of endovascular vs open repair for traumatic descending thoracic aortic rupture. J Vasc Surg. 2008;48:1343-51. ]. Technical success rates were similar with endovascular repair 96.5% and open repair 98.5%. The predominant procedure specific complications for the endovascular group were endoleak (4.2%) and access complications (2.8%), with an overall procedure specific complication rate of 13.1%. In the open repair group, the overall complication rate was 17%, with laryngeal nerve palsy (14.4%) as the most common complication Open surgical repair was also associated with an increased risk of graft infections and systemic infections. Major morbidity, including acute respiratory distress syndrome and acute renal failure (8% versus 5%) are more common in the open surgical repair group [194194. Akowuah E, Angelini G, Bryan AJ. Open versus endovascular repair of traumatic aortic rupture: A systematic review. J Thorac Cardiovasc Surg. 2009;138:768-9. ].
Early outcome results for endovascular repair are encouraging; however, long-term studies are necessary to evaluate the effectiveness and the durability of this procedure. This is analagous with the endovascular procedures for non-traumatic injuries, however the younger traumatic population may show additional long-term device related complications in the future [209209. Pitton MB, Herber S, Schmiedt W, Neufang A, Dorweiler B, Düber C. Long-Term Follow-Up After Endovascular Treatment of Acute Aortic Emergencies. Cardiovasc Intervent Radiol. 2008;31:23–35. ]. A small study by Forbes showed (in a 2 year follow-up period) significant thoracic aortic dilatation occurs after endovascular repair for traumatic aorta injuries, with growth rates between 0.47 to 0.83 mm a year [199199. Forbes TL, Harris JR, Lawlor DK, DeRose G. Aortic dilatation after endovascular repair of blunt traumatic thoracic aortic injuries. J Vasc Surg. 2010;52:45-8. ]. Furthermore, there are concerns about the cumulative ionising radiation and iodinated contrast exposure. In addition, adherence to follow-up imaging protocols (essential in endovascular repair) is challenging in this young patient population [170170. Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, Murad MH, Fairman RM. Endovascular repair of traumatic thoracic aortic injury: Clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187-92.
Clinical practice guidelines from the U.S. on endovascular management of traumatic thoracic injury based on a systematic review and meta-analysis of the literature.].
The risks of death and spinal cord ischaemia are significantly lower after endovascular repair compared to open surgery and these early benefits outweigh the concerns of potential late complications. However, in surgically fit patients with poor anatomy for endovascular repair, conventional open repair should be considered.
An economic comparison between open surgical treatment and endovascular repair in traumatic thoracic aorta injuries in Canada was in favour of endovascular repair. The estimated total medical costs after 1 year were $70,442 for endovascular repair versus $72,833 for open surgical treatment [210210. Zhen-Yu Tong M, Koka P, Forbes TL. Economic evaluation of open vs endovascular repair of blunt traumatic thoracic aortic injuries. J Vasc Surg. 2010;52:31-8. ]. Again, long-term follow-up will be required to see if this cost-benefit is maintained over time.
Aneurysms of the abdominal aorta
DEFINITION
The first description of abdominal aortic aneurysm (AAA) was by the 16th century anatomist Vesalius. The term aneurysm originates from the Greek aneurysma, for dilatation. An aneurysm of the abdominal aorta (AAA) is considered a permanent localised dilatation involving all three layers of the vessel wall and defined by an increase of diameter of more than 50% compared with the diameter of the unaffected aorta at the level of the renal arteries [211211. Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991;13:452-8.8. ]. In other definitions, a diameter of the abdominal aorta of > 30 mm is considered an AAA.
AAA’s can be classified anatomically. Based on the upper level, an AAA is considered supra-renal if the renal arteries are involved in the aneurysmatic dilatation (if the upper level is above the coeliac axis and diaphragm the aneurysm is considered a thoraco-abdominal aneurysm). An AAA is considered juxta-renal if the aneurysm starts immediately at the distal level of the renal arteries, and infrarenal if there is a (short) segment of normal aorta below the renal arteries (infrarenal neck) ( Figure 15 ).
Infrarenal aneurysms, the most common type, are usually fusiform. In a fusiform aneurysm the entire circumference of the vessel wall is involved in the dilatation, whereas in a saccular aneurysm a limited part of the vessel wall is involved.
EPIDEMIOLOGY
AAA is largely a disease of elderly men with a reported incidence of 3-117 per 100,000 person-years when based on rates of repair. However, when screening programmes are in place, the incidence can be as much as 3.5 per 1,000 person-years, as reported in the UK Small Aneurysm Study [212212. Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants. Ann Surg. 1999; 230:289-96. ]. Prevalence may be more accurate than incidence, now that large ultrasound screening studies have been performed.
The prevalence of AAAs 2.9 to 4.9 cm in diameter ranges from 1.3% for men aged 45 to 54 years to up to 12.5 % for men 75 to 84 years of age. For women these prevalences are 0% and 5.2%, respectively [213213. Singh K, Bonaa KH, Jacobsen BK, Bjork L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study : The tromso study. Am J Epidemiol. 2001;154:236-44. ]. This prevalence is, however, based on an AAA being defined as having a diameter of >30 mm. Wanhainen recently demonstrated that by using the standard definition for AAA of >30 mm the prevalence for 65-75 year olds was 16.9% for men and 3.5% for women, whereas when using another definition, ≥ 1.5x normal infrarenal aortic diameter (predicted from a nomogram), the prevalence was 12.9% for men and 9.8% for women [214214. Wanhainen A. How to define an abdominal aortic aneurysm--influence on epidemiology and clinical practice. Scand J Surg. 2008;97:105-109; discussion 109. ].
The prevalence of AAA varies with a number of factors, including older age, male gender, white race, family history, hypertension, and tobacco use. These risk factors may not be independent predictors, and may be just markers rather than causes of AAA disease. Of these, age, gender and smoking seem to have the largest impact on AAA prevalence.
The risk of rupture also increases with age and ranges from 1-21 per 100,000 person-years
AETIOLOGY AND PATHOGENESIS
Vessel wall degeneration is the common aetiology for AAA.
Demographic risk factors associated with aortic aneurysm disease include ageing, male gender, hypertension, cigarette smoking, chronic obstructive pulmonary disease, atherosclerosis, and peripheral arterial disease [215215. Alcorn HG, Wolfson SK, Jr., Sutton-Tyrrell K, Kuller LH, O’Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler Thromb Vasc Bio. 1996;16:963-70. , 216216. Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, Krupski WC, Barone GW, Acher CW, Ballard DJ. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126:441-49. ]. Patients with degenerative AAAs often exhibit a familial pattern of disease, with 15-20% of patients having a positive family history [217217. van Vlijmen-van Keulen CJ, Pals G, Rauwerda JA. Familial abdominal aortic aneurysm: a systematic review of a genetic background. Eur J Vasc Endovasc Surg. 2002;24:105-16. ]. These observations have raised the possibility that AAAs might arise through an inherited genetic predisposition [218218. Armstrong PJ, Johanning JM, Calton WC Jr, Delatore JR, Franklin DP, Han DC, Carey DJ, Elmore JR. Differential gene expression in human abdominal aorta: aneurysmal versus occlusive disease. J Vasc Surg. 2002;35:346-55. ]. The fundamental genetic aspects of degenerative AAAs are under active investigation.
The histopathology of established AAAs is dominated by chronic inflammation, destruction of the elastic lamellae, and depletion of medial smooth muscle. These processes are closely interrelated, and together with redistribution of haemodynamic stresses on the vessel wall they account for the progressive tissue degeneration resulting in aortic dilatation.
Haemodynamic factors have long been implicated in the degenerative arterial remodelling associated with AAAs, particularly in their predilection for the infrarenal aorta. The infrarenal aorta is subject to greater turbulence and haemodynamic stresses, due to its peculiar location with respect to the mesenteric and renal artery branches and the reflection of pressure waves from the iliac bifurcation [219219. Moore JE Jr, Ku DN, Zarins CK, Glagov S. Pulsatile flow visualization in the abdominal aorta under differing physiologic conditions: implications for increased susceptibility to atherosclerosis. J Biochem Eng. 1992;114:391-97. ]. Clinical patterns of disease suggest that the infrarenal aorta is structurally predisposed toward aneurysmal degeneration. The elastin-to-collagen ratio decreases distally, translating into increased stiffness in this section of the aorta [220220. Peterson L, Jensen RE, Parnell J. Mechanical properties of arteries in vivo. Circ Res. 1960;8:622-633. ]. Furthermore, the infrarenal aorta is normally devoid of vasa vasorum [221221. Wolinsky H, Glagov S. Comparison of abdominal and thoracic aortic medial structure in mammals: deviation of man from the usual pattern. Circ Res. 1969;25:677-86. ]. Together with the presence of a thick intraluminal thrombus, this indicates that oxygen and nutrient supply to the tunica media is dependent on diffusion from the lumen which is compromised by atherosclerotic plaques and intima thickening [222222. Vorp DA, Lee PC, Wang DH, Makaroun MS, Nemoto EM, Ogawa S, Webster MW. Association of the intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg. 2001;34:291-99. ].
Histological examination of human AAA tissue demonstrates an inflammatory cell infiltrate consisting of T cells, B cells, dendritic cells, plasma cells and macrophages [223223. Beckman EN, Plasma cell infiltrates in atherosclerotic abdominal aortic aneurysm. Am J Clin Pathol. 1986;85:21-24. , 224224. Bobryshev YV, Lord RS, Parsson H. Immunophenotypic analysis of the aortic aneurysm wall suggests that vascular dendritic cells are involved in the immune responses. Cardiovasc Surg. 1998;6:240-49. , 225225. Koch AE, Haines GK, Rizzo RJ, Radosevich JA, Pope RM, Robinson PG, Pearce WH. Human abdominal aortic aneurysms. Immunephenotypic analysis suggesting an immune-mediated response. Am J Pathol. 1990;137:1199-1213. ]. Once inflammatory cells migrate into tissues, their subsequent course of action can be variable. The inflammatory cells are likely stimulated by proinflammatory mediators of chemotaxis and immune activation, including chemokines, cytokines, hypoxia, and the products of matrix degradation. Studies of homogenised AAA tissue and culture of AAA tissue explants have demonstrated overexpression of pro-inflammatory cytokines, such as tumour necrosis factor (TNF-α), and interleukins (IL-6 and I-1β). The cytokine interferon IFN-γ has also been implicated in human AAA and elevated circulating levels of IFN-γ appear to correlate with more rapid aneurysm expansion [226226. Szekanecz Z, Shah MR, Pearce WH, Koch AE. Human atherosclerotic abdominal aortic aneurysms produce intyerleukin (IL)-6 andinterferon-γ but not IL-2 and IL-4. The possible role for IL-6 and interferon-γ in vascular inflammation. Agents Actions. 1994;42:159-62. , 227227. Newman KM, Jean-Claude J, Li H, Ramey WG, Tilson MD. Cytokines that ativate proteolysis are increased in abdominal aortic aneurysms. Circulation. 1994;90:II224-27. ]. Circumstantial evidence indicates that infectious agents (i.e., Chlamydia pneumoniae) may possibly play a role, at least in some patients [228228. Juvonen J, Juvonen T, Laurila A, Alakärppä H, Lounatmaa K, Surcel HM, Leinonen M, Kairaluoma MI, Saikku P. Demonstration of Chlamydia Pneumoniae in the walls of abdominal aortic aneurysms. J Vasc Surg. 1997;25:499-505. ].
AAA’s are characterised microscopically by destruction of the medial elastic fibres and disordered compensatory remodelling of the extracellular matrix, which is likely mediated by a spectrum of proteolytic enzymes, particularly members of the matrix metalloproteinase (MMP) family [229229. Visee R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure , function, and biochemistry. Circ Res. 2003;92:827-39. , 230230. Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491-94. ]. MMP’s are family of structural related enzymes that are capable of digesting the various components of the extracellular matrix. They are produced by most cell types native to the aorta, such as endothelial cells, smooth muscle cells, and adventitial fibroblasts, and are produced by invading inflammatory cells such as macrophages. The normal function of these cells is thought to be primarily extracellular matrix remodelling, although participation in other functions, such as cell motility and cell signalling are also known. MMP-9, also known as gelatinase-ß, in one of the most extensively studied MMPs within AAA research. Immunohistochemical studies of human AAA tissue have demonstrated increased amounts of MMP-9 compared to normal tissue.
Smooth muscle cell depletion in the AAA wall probably plays a prominent role in progression of disease, by reducing the capacity for fibrous remodelling of the extracellular matrix in response to injury [231231. López-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. J Am Pathol. 1997;150:993-1007. ].
CLINICAL FEATURES
The majority of abdominal aortic aneurysms produce few if any symptoms and are often detected as an incidental finding on physical examination, ultrasonography, abdominal CT, or magnetic resonance imaging (MRI) performed for other purposes. Abdominal palpation is the oldest method of AAA detection. Few aneurysms are found at routine palpation of the abdomen, but careful evaluation of the aorta detects most clinically relevant AAAs. During the examination the patient should be in supine position, with the abdomen relaxed and the knees flexed to relax the anterior abdominal muscles. Findings of abdominal or femoral bruits or absent femoral pulses do not contribute to the diagnosis of an AAA. The sensitivity of abdominal palpation for detecting AAA depends on the diameter of both aneurysm and patient. Three quarters of AAAs that are 50 mm in diameter are detectable by abdominal palpation, whereas less than one quarter of AAAs 30-39 mm are palpable [232232. Lederle FA, Simel DA. The rational clinical examination: does this patient have abdominal aortic aneurysm. JAMA. 1999;281:77-82. ]. The sensitivity of palpation is also much lower in patients with an abdominal girth of more than 104 cm compared to thinner patients [233233. Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal artic aneurysm. Arch Intern Med. 2000;160:833-36. ].
Most aneurysms remain asymptomatic until rupture, although some are discovered during the course of evaluation for acute or chronic abdominal pain. Aneurysms that produce symptoms (symptomatic AAA) are at increased risk for rupture. The two main findings suggestive of recent aneurysm expansion are abdominal or back pain, and pain at aneurysm palpation. Acute thrombosis of an AAA rarely occurs, but can cause catastrophic ischaemia. Atheroembolism is much more common than acute thrombosis, and both combined occurs in less than 2-5% of patients with AAA [234234. Jaakkola P, Hippeläinen M, Farin P, Rytkönen H, Kainulainen S, Partanen K. Interobserver variability in measuring the dimensions of the abdominal aorta: comparison of ultrasound and computed tomography. Eur J Vasc Endovasc Surg. 1996;12:230-37. ].
Patients with a ruptured abdominal aortic aneurysm classically present with abdominal or back pain, hypotension, and a pulsatile abdominal mass. Aneurysm rupture typically causes exsanguinating haemorrhage and profound, unstable hypotension. If the patient’s blood pressure is relatively stable, the aneurysm rupture may be temporarily contained and tamponaded by the surrounding retroperitoneum. The overall survival rate of patients who experience a ruptured abdominal aortic aneurysm is approximately 25 per cent. About 50 per cent of patients reach the hospital alive and of those who reach the hospital, up to 50 per cent do not survive repair with only a gradual decline in mortality rates over the last 50 years.
DIAGNOSTIC PROCEDURES
Almost all imaging modalities have been used in the diagnostic work-up of a patient.
Ultrasound
Ultrasound is most commonly used to establish the diagnosis of an AAA. Abdominal B-mode ultrasound is the least expensive, least invasive, and most frequently used examination, particularly for confirmation of a suspected diagnosis of AAA and surveillance of small AAAs. Diameter measurement of AAA with ultrasound have an interobserver variability of approximately 5 mm in 85% of studies and are more accurate in anteroposterior than in lateral dimension [234234. Jaakkola P, Hippeläinen M, Farin P, Rytkönen H, Kainulainen S, Partanen K. Interobserver variability in measuring the dimensions of the abdominal aorta: comparison of ultrasound and computed tomography. Eur J Vasc Endovasc Surg. 1996;12:230-37. ]. Ultrasound cannot accurately determine the presence of rupture and often cannot determine the upper extent of an AAA [235235. Shuman WP, Hastrup W Jr, Kohler TR, Nyberg DA, Wang KY, Vincent LM, Mack LA. Suspected leaking abdominal aortic aneurysm: use of sonography in the emergency room. Radiology. 1988;168:117-19. , 236236. Pavone P, Di Cesare E, Di Renzi P, Marsili L, Ventura M, Spartera C, Passariello R. Abdominal aortic aneurysm evaluation: comparison of US, CT, MRI, and angiography. Magn Reson Imaging. 1990;8:199-204. ].
Spiral CTA
Computed tomographic angiography (CTA) is more expensive than ultrasound and involves the exposure of radiation and intravenous nephrotoxic contrast, but it provides more accurate measurement of diameter, with 91% of studies showing less than 5 mm interobserver variability [234234. Jaakkola P, Hippeläinen M, Farin P, Rytkönen H, Kainulainen S, Partanen K. Interobserver variability in measuring the dimensions of the abdominal aorta: comparison of ultrasound and computed tomography. Eur J Vasc Endovasc Surg. 1996;12:230-37. ]. Ultrasound and CTA can overestimate AAA diameter if an oblique rather than perpendicular section is obtained in a tortuous aneurysm. This results in an elliptical rather than a circular cross-section. Curved linear reformats (CLR) can be used for accurate assessment of the diameters and length of the potential attachment zones in the aorta and iliac arteries. Unlike axial CT images, CLR allow visualisation of the aortic and iliac lumen in a plane perpendicular to the central arterial axis. By avoiding oblique projection, the images represent the true cross-sectional shape of the aorta. CLRs perpendicular to the vessel axis can be acquired using central lumen lines, which can be created by positioning markers in the centre of the aorta and iliac arteries at several levels. Dedicated computer programmes are currently available to facilitate multiplanar reconstructions.
High-quality contrast-enhanced CTA is essential and is currently the imaging technique of choice for EVAR. It provides detailed information on vascular anatomy and thus helps in selecting suitable patients and the right stent graft. The scan must image from the celiac axis to the common femoral artery.
Arterial angiography
Arterial angiography can provide additional information. It is superior to CTA in the assessment of the grade of stenosis of major branches of the aorta. By itself, it is insufficient for treatment selection because thrombus in the aneurysm and arteriosclerotic disease of the arterial wall are not displayed. For many years arterial angiography was mandatory in the pre-operative work-up for endovascular procedures, especially to perform accurate length measurements. With currently available dedicated computer programmes, arterial angiography has become redundant.
Magnetic resonance angiography
Like spiral CTA, magnetic resonance angiography (MRA) can produce multiplanar two-dimensional and 3D images. Although MRA is currently inferior to spiral CTA regarding spatial resolution, and is technically demanding and time-consuming, it certainly has advantages worthy of mention. For example, the patient is not exposed to ionising radiation and no nephrotoxic contrast is used. MRA cannot be used in all patients. Approximately 10% to 15% of patients are claustrophobic or have metal implants that make it impossible to use MRA.
TREATMENT
Large clinical trials have demonstrated the safety of observation of AAAs with a maximum diameter of less than 55 mm [237237. The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352:1649-55. , 238238. Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, Ballard DJ, Messina LM, Gordon IL, Chute EP, Krupski WC, Busuttil SJ, Barone GW, Sparks S, Graham LM, Rapp JH, Makaroun MS, Moneta GL, Cambria RA, Makhoul RG, Eton D, Ansel HJ, Freischlag JA, Bandyk D; Aneurysm Detection and Management Veterans Affairs Cooperative Study Group. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-44. ]. In an effort to prevent rupture, these studies required frequent observation (mainly ultrasound) every 6 months, with indication for intervention for symptoms, rapid expansion, or growth to 55 mm.
Medical management
It has been postulated that beta-blockers decrease the rate of AAA expansion. This effect has been demonstrated in animal models. Subsequent retrospective analysis in humans and prospective randomised trials were in contradiction to show any reduction in AAA growth rate with beta-blockade [239239. Wilmink AB, Hubbard CS, Day NE, Quick CR: Effect of propranolol on the expansion of abdominal aortic aneurysms: a randomized study. Br J Surg. 2000;87:499. , 240240. Propanolol Aneurysm Trial Investigators. Propanolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg. 2002;35:72-79. ].
The evidence is mixed with regard to the effect of angiotensin-converting-enzyme inhibitors (ACEI).
Doxycycline has been shown to slow the rate of AAA expansion in two randomised trials, and Roxithromycin was shown to reduce expansion rate in a similar trial [241241. Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW Jr, Kent KC, Upchurch GR Jr, Chaikof EL, Mills JL, Fleckten B, Longo GM, Lee JK, Thompson RW. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. J Vasc Surg. 2002;36:1-12. , 242242. Vammen S, Lindholt JS, Ostergaard L, Fasting H, Henneberg EW. Randomized double-blind controlled trial of roxithromycin for prevention of abdominal aortic aneurysm expansion. Br J Surg. 2001;88:1066-72. ]. These antibiotics have an activity against Chlamydia Pneumoniae, which has been shown to be present in AAAs. It is not entirely certain that the medication effect is related to its antibiotic properties. In the Roxithromycin study there was no correlation between Chlamydia titres and the ability to prevent aneurysm expansion. Doxycycline has been shown to suppress expression of matrix metalloproteinase in human AAAs.
Recently at least 3 studies have suggested that statins are associated with reduced AAA expansion rates [243243. Schouten O, van Laanen JH, Boersma E, Vidakovic R, Feringa HH, Dunkelgrün M, Bax JJ, Koning J, van Urk H, Poldermans D. Statins are associated with a reduced infrarenal abdominal aortic aneurysm growth. Eur J Vasc Endovasc Surg. 2006;32:21-26. , 244244. Schlösser FJ, Tangelder MJ, Verhagen HJ, van der Heijden GJ, Muhs BE, van der Graaf Y, Moll FL; SMART study group. Growth predictors and prognosis of small abdominal aortic aneurysms. J Vasc Surg. 2008;47:1127-33. , 245245. Sukhija R, Aronow WS, Sandhu R, Kakar P, Babu S. Mortality and size of abdominal aortic aneurysm at long-term follow-up of patients not treated surgically and treated with and without statins. Am J Cardiol. 2006;97:279-80. ]. Although there is no clear association between cholesterol and aneurysm expansion rates, there is an association between cholesterol and AAA presence. Similar to Doxycycline, statins have been shown to decrease MMP-9 within the aneurysm wall, suggesting a mechanism unrelated to cholesterol levels [246246. Evans J, Powell JT, Schwalbe E, Loftus IM, Thompson MM. Simvastatin attenuates the activity of matrix metalloprotease-9 in aneurysmal aortic tissue. Eur J Vasc Endovasc Surg. 2007;34:302-3. , 247247. Abisi S, Burnand KG, Humphries J, Waltham M, Taylor P, Smith A. Effect of statins on proteolytic activity in the wall of abdominal aortic aneurysms. Br J Surg. 2008;95:333-37. ].
There is mounting evidence for the potential of medical therapy, but more investigation is warranted for definitive clinical guidelines to be issued.
Surgical management
Conventional open repair of an AAA was performed for the first time in 1951, replacing the abdominal aortic aneurysm by a homograft [248248. Dubost C, Allary M, Oeconomos N. Treatment of aortic aneurysms; removal of the aneurysm; re-establishment of continuity by grafts of preserved human aorta. Mem Acad Chir (Paris). 1951;77:381-83. ]. Two years later, open repair was performed using synthetic grafts [249249. DeBakey ME, Cooley DA. Successful resection of aneurysm of thoracic aorta and replacement by graft. JAMA. 1953;152:673-76. ]. Due to the remarkable progress in preoperative imaging techniques, the recognition and management of patient comorbidities, surgical and anaesthetic techniques, and postoperative management, this approach has nearly universal applicability for the treatment of even extensive aneurysmal and associated occlusive disease. Multiple reproducible studies have firmly established that, in experienced centres, low mortality and morbidity rates are consistently achievable with this approach.
In the 1990s the UK Small Aneurysm Trial (UKSAT) and the Veterans administration Aneurysm Detection and Management (ADAM) Trial compared early elective surgery versus ultrasound surveillance for small AAAs (40-55 mm in diameter) [250250. U.K Small Aneyrysm trial participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352:1649-55. , 251251. Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, Ballard DJ, Messina LM, Gordon IL, Chute EP, Krupski WC, Busuttil SJ, Barone GW, Sparks S, Graham LM, Rapp JH, Makaroun MS, Moneta GL, Cambria RA, Makhoul RG, Eton D, Ansel HJ, Freischlag JA, Bandyk D; Aneurysm Detection and Management Veterans Affairs Cooperative Study Group. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Eng J Med. 2002;346:1437-44. ]. Both randomised trials concluded that AAAs measuring 40-55 mm in diameter should be kept under surveillance. It was also concluded that patients with factors such as severe chronic obstructive pulmonary disease (COPD), multiple familial aneurysms, poorly controlled hypertension, saccular AAAs and rapid enlargement (>5 mm in 6 months) are associated with higher than average rupture rates and like patient age and gender may need to be balanced against the risk of elective repair for some AAAs that are smaller than 55 mm [252252. Brewster DC, Cronenwett JL, Hallett JW Jr, Johnston KW, Krupski WC, Matsumura JS; Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Guidelines fort he treatment of abdominal aortic aneurysms: report of a subcommittee oft he Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37:1106-17.
This manuscript provides a guide in the decision making with regards to elective repair of abdominal aortic aneurysms.].
There are 2 potential surgical approaches for open surgical repair: trans-abdominal and retroperitoneal. Other surgical techniques such as laparoscopic aneurysm repair and trans-abdominal repair via a mini-laparotomy incision have been advocated, but have not been widely adopted. The goal is to obtain adequate exposure of the aorta proximal and distal to the aneurysmal segment.
Intraoperative management of patients undergoing abdominal surgery is a dynamic, complex and challenging undertaking. The intraoperative course is remarkable for haemodynamic and metabolic demands related to the aortic clamping and de-clamping, and the consequences of organ ischaemia during clamping. Replacement of blood loss, usually by autotransfusion, and maintenance of normothermia is critical. The haemodynamic consequences of aortic cross clamping and de-clamping are profound and multifactorial [253253. Gelman S: The pathophysiology of aortic cross-clamping and unclamping. Anestthesiology. 1995;1026-60. ].
The level of aortic clamping is the principal factor influencing cardiac function during aortic surgery. Regardless of clamp location, aortic occlusion results in proximal arterial hypertension secondary to increased systemic vascular resistance. Clamping results in redistribution of blood from organs distal to the aortic clamp toward the central venous circulation and organs proximal to the clamp. The splanchnic circulation serves as a venous reservoir, which causes moderate changes in preload, filling pressure and cardiac output.
De-clamping of the aorta is associated with abrupt haemodynamic changes, with hypotension due to a sudden decrease is systematic vascular resistance. Other important contributing factors are the central hypovolaemia caused by vascular engorgement in distal reperfused tissues and the release of sequestered vasodilatory metabolites back into the systemic circulation.
Early mortality rates of elective open surgical repair vary considerable in the literature. Centres of excellence report 30-day mortality rates of 1-5%.
Many of the early complications that occur after AAA repair are common after any major surgical procedure.
Complications after open surgical repair of AAA
- Early complications
-Ischaemic cardiac complications
-Bleeding/ coagulopathy
-Colon ischaemia
-Pulmonary decompensation
-Renal dysfunction
-Lower extremity ischaemia
-Spinal cord ischaemia
- Late complications
-Incisional hernia
-Anastamotic aneurysm
-Graft infection
Because of the increased prevalence of pre-existing atherosclerotic arterial disease and underlying pulmonary dysfunction in patients with AAA, the frequency of early postoperative complications including myocardial infarction, pulmonary decompensation resulting in pneumonia and renal dysfunction is elevated.
Endovascular management
In the last 10 to 15 years, the interest in minimally invasive surgery has grown and the same trend can be observed in vascular surgery and interventional radiology, leading to what is commonly referred as “endovascular surgery”. In the early 1990s, Volodos et al and Parodi et al introduced endovascular treatment of abdominal aortic aneurysm (AAA) with a device composed of a Dacron graft and a balloon-expandable stent [254.255].
Since the first use of a stent graft for the endovascular exclusion of an AAA, the use of endovascular treatment of AAA has greatly expanded.
Patient selection
Not all patients with an AAA are suitable for endovascular repair. Preprocedural assessment must reject unsuitable patients, identify potential difficulties, and allow selection of an appropriate stent graft. The main criteria for patient selection apply to anatomic details of the vascular tree between the renal arteries and the external iliac artery. Endovascular repair of the aorta (EVAR) brings many new challenges for preoperative imaging. Unlike conventional surgery, in endovascular repair the anatomical configuration of the infrarenal aorta must be known to a high degree of accuracy. Regardless of which kind of stent graft is to be used, a similar series of measurements must be obtained for each patient. Every company, every study, and every registry has its own worksheet, which can be filled out to record the most relevant details. One of the most commonly used, is the worksheet from the EuroSTAR registry [256256. Harris PL, Buth J, Mialhe C, Myhre HO, Norgren L. The need for clinical trials of endovascular abdominal aortic repair. The EUROSTAR Project. EUROpean collaborators on Stent-graft techniques for abdominal aortic aneurysm repair. J Endovasc Surg. 1997;4:72–77; discussion 78-9. ] ( Figure 16 ).
The most common reasons to reject a patient, based on anatomical configurations are listed below.
Visceral and renal supply
Patency of the coeliac axis and superior mesenteric artery should be assessed before a patent inferior mesenteric artery can be over-stented. Renal arteries and accessory renal arteries should be identified.
Diameter of proximal neck
Depending on the device used, an infrarenal neck with a diameter of more than 30 mm is considered unsuitable. Newer generation stent grafts are capable in accommodating even larger infrarenal diameters. All stent grafts need to achieve a seal between the device and the aortic wall. Usually, the stent graft is oversized by 10% to 20% compared with the diameter of the proximal neck.
Length of proximal neck
To achieve a good seal between the stent graft and the aortic wall, a snug apposition over a length of 10 mm to 15 mm is required. Some of the limitations of the large diameter and short length of the infrarenal neck are overcome with the introduction of fenestrated and branched stent grafts.
Angulation of the proximal neck
Angulation of the proximal neck is not uncommon and is more often seen with larger aneurysms (>60 mm). Aortic neck angulation appears to be an important determinant of outcome after EVAR. Mild angulation (<40º) is associated with favourable outcome, whereas those with moderate (40°–59º) and severe (>60º) angulation have a higher risk of adverse events, especially type I endoleaks [257257. Sternbergh WC 3rd, Carter G, York JW, Yoselevitz M, Money SR. Aortic neck angulation predicts adverse outcome with endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2002;35:482–86. ] ( Figure 17 ).
Conical nature of the proximal neck
A contour change of the proximal neck (reversed tapered) of more than 10% is associated with a higher proximal endoleak rate, and therefore considered unfavourable [258258. Stanley BM, Semmens JB, Mai Q, Goodman MA, Hartley DE, Wilkinson C, Lawrence-Brown MD. Evaluation of patient selection guidelines for endoluminal AAA repair with the Zenith stent-graft: the Australian experience. J Endovasc Ther. 2001;8:457–64. ].
Calcification and mural thrombus in proximal neck
Calcification and mural thrombus in the proximal neck are expressed in degrees of circumference. Mural thrombus in the neck over more than 90º is considered a risk factor for endoleak, whereas extended calcification will increase the probability of stent graft migration.
Diameter of iliac artery
In general, the stent graft will end in the common iliac artery. Therefore, there is a size limitation toward the diameter of the common iliac artery, depending on the device used. If the diameter of the common iliac artery is too large, the stent graft can be extended to the external iliac artery. In such a case, it is advisable to coil-embolise the hypogastric artery, since retrograde flow from the hypogastric artery can cause an endoleak. Currently, newer generation stent grafts have the option of a hypogastric side-branch. In this situation the stent graft is extended to the external iliac artery. The patency of the hypogastric artery can thus be preserved with the covered side-branch
Length of distal sealing zone
In a similar way to the proximal sealing zone, a snug apposition over a length of 10mm to 15mm is required.
Tortuosity of iliac arteries
The introducer systems are large (14–22 Fr in diameter) and relatively inflexible therefore if the iliac arteries are small, calcified and tortuous, it will be difficult for the delivery system to pass without causing damage to the iliac tract. There are no strict criteria in this matter and a decision should be made based on diameter and flexibility of the delivery system and the surgeon’s experience.
Components of endovascular devices
Various materials have been tested, and all the major producers now use the two materials most frequently used in surgery, including polyester weaves and polytetrafluoroethylene (PTFE). Ideally, if stent grafts are to provide the advantages they potentially offer over conventional surgery, they must have certain characteristics. Each device must have as many of these characteristics as possible to warrant a safe implantation, high rates of immediate technical success, and excellent long-term clinical success.
Diagnostic modalities in aortic dissection
- Durability
- Biocompatibility
- Stent graft size range
- Lowest delivery device profile
- Delivery device flexibility
- Delivery device reliability
- Radial force stability
- Sealing capacity
- Excellent apposition of stent graft
- Radio-opacity
- Low/thrombogenicity
- Customisation
Delivery systems
Stent grafts are delivered in the vascular tree using introducer sheaths, delivery systems, deployment capsules, and retractable covers. The profile of the introducer sheaths must be small enough to pass through the iliac tract, without causing vascular damage. However, the introducer sheaths must be wide enough for easy passage of the stent graft, rigid enough to resist kinking, and flexible enough to follow the angulations in the iliac arteries. A haemostatic valve is mandatory.
Graft material
The graft material must be strong enough to resist late deterioration and damage due to friction with metallic parts of the stent and yet thin enough to be compressed in a small delivery catheter. In most stent grafts, conventional polyester has been preferred. Over time, companies have started using thinner polyester variations in order to downsize the profile of the delivery systems. PTFE is an alternative graft material that is currently used in several stent graft systems. Other materials such as polycarbonate, polyurethane, and other polymers are under investigation.
Graft attachment systems
Vascular stents can be constructed from stainless steel, elgiloy, tantalum, or nitinol. Friction with the vessel wall is the main mechanism of attachment, but hooks, anchors, and barbs are used in addition to secure a better fixation and seal inside the artery.
Deployment accessories
Regular interventional tools are essential accessories for a successful stent graft placement. Among these are floppy to super-stiff guidewires, angiographic and guide catheters, dilatation balloons, snares, and power injectors.
Technique of endovascular repair
Endovascular repair of an AAA can be performed in the operating theatre, radiology angio suite, or catheterisation laboratory under general, regional, and even local anaesthesia. The choice of one of these locations is subjective, and is influenced by the preference of the interventionist. If the procedure is not performed in an operating theatre, it should nonetheless meet the standards of hygiene and sterility associated with an operating theatre.
Radiological requirements
Minimum radiological requirements for fluoroscopy are a mobile C-arm image intensifier with possibilities for cine loop angiography, digital subtraction, roadmap, and frame-by-frame replay. Ceiling or floor mounted fluoroscopy equipment has several advantages over the portable image intensifier. Most important are better field size/spatial resolution ratio and a suitable radiolucent table that can be adjusted to the fluoroscopic field.
The typical aorta at the infrarenal level has a slight anterior angulation (10°–15º) due to lordosis of the lumbar vertebrae. This angulation is more pronounced in the majority of patients with AAA (the angle can vary from 10º to 60º), because the aneurysm raises the aorta from the vertebral column. Fluoroscopy with the C-arm in the AP position produces projection errors and this may lead to less optimal or inaccurate placement of the stent graft in the infrarenal aortic neck [259259. Broeders IAMJ, Blankensteijn JD. A simple technique to improve the accuracy of proximal AAA endograft deployment. J Endovasc Ther. 2000;7:389–93. ]. In addition to an angulation of the C-arm, sometimes a rotation of the C-arm is required for optimal projection of the orifice of the renal arteries. It is essential to have aortic angulation and rotation data available preoperatively. Only lateral angiograms or CTA reconstructions can give an indication of neck angulation.
Stent graft insertion
The common femoral artery in the ipsilateral groin is exposed through a surgical cut-down and intravenous heparin is given to the patient ( Figure 18 ). In some institutions there is extensive experience in percutaneous access. In general a closure device is “pre-loaded” before the introducer sheath is inserted. A 6-8 Fr introducer sheath is inserted and a flexible guidewire is positioned in the suprarenal aorta. With the use of a (straight) diagnostic catheter, the flexible guidewire is exchanged for a super-stiff guidewire, which is positioned in the descending aorta. At the same time, another introducer sheath (6-8 Fr) is inserted into the contralateral common femoral artery, either percutaneously or through a groin cut-down. With the use of a flexible guidewire, a pigtail catheter is positioned at the level of the renal arteries. The introducer sheath in the ipsilateral common femoral artery is removed and a transverse arteriotomy is performed in such a way that the super-stiff guidewire is situated within the arteriotomy. A large introducer sheath (depending on the device used) or the delivery device and stent graft within it are introduced under fluoroscopic control until the superior end of the prosthesis is at the assumed level of the renal arteries. If necessary, the image intensifier is angulated and rotated, and moved to place the superior end of the stent graft in the centre of the field, in order to eliminate errors caused by parallax. Magnification can be advantageous in identifying the superior end of the stent graft in relation to the orifice of the renal arteries. At this time, the first aortogram is performed with the use of a power injector. Rotation of the stent graft and repositioning can be performed.
Once it has been ascertained that the stent graft is in the optimal position, immediately distal from the renal arteries, the pigtail catheter is pulled distally. The trunk and ipsilateral limb of the stent graft can be deployed under fluoroscopic control. A guidewire from the contralateral femoral artery is directed into the short contralateral stump of the stent graft. this can usually be achieved with a “free-style” catheterisation using an angled guiding catheter. If difficulty is experienced, a guidewire can be passed from the ipsilateral side, through the ipsilateral limb of the stent graft, and, with the aid of a “cross-over” guiding catheter, over the bifurcation in the contralateral short limb into the aneurysm sac. With a snare catheter from the contralateral femoral artery, the guidewire can be retrieved. An approach from the brachial artery can also be used if the “free-style” catheterisation of the short contralateral stump fails. The brachial approach can also be used in the case of extreme tortuosity in the iliac tract. After retrieval of the brachial wire with the snare catheter, tension can be applied at the brachial and femoral ends. This technique termed ‘‘body-flossing’’ results in considerable straightening of the iliac arteries.
After cannulation of the contralateral short stump, the guidewire is exchanged for a super-stiff guidewire. The 6-8 Fr introducer sheath is exchanged for a larger sheath and the contralateral limb is directed under fluoroscopic control to a position within, and overlapping, the contralateral stump. The position of the hypogastric artery can be assessed by regular antegrade angiography or retrograde angiography through the introducer sheath. The contralateral limb is deployed under fluoroscopic control. The overlapping attachment site and distal attachment site can be dilated.
A pigtail catheter is reintroduced, and a post-intervention digital subtraction aortogram is performed for the detection of the presence of extravasation of contrast, which can indicate an endoleak. Stent graft limbs and iliac arteries are examined for kinking or twisting.
The technique for introduction and deployment differs for every individual stent graft, and discussion on these differences is beyond the scope of this chapter. For individual techniques of introduction and deployment, the reader is referred to the relevant specialised manuals and instructions for use.
Complications
Most of the complications of EVAR have no open surgical counterparts.
Compliactions of EVAR
- Access artery injury
- Peripheral micro-embolisation
- Post/implant fever
- Endoleak
- Endotension
- Graft limb thrombosis
- Stent graft infection
- Stent graft migration
- Device failure, stent graft deterioration
- Late rupture
Many studies have reported higher rates of late complications and reintervention in EVAR patients.
Endoleak is a condition associated with endovascular stent grafts, defined by persistent blood flow outside the lumen of the stent graft but within the aneurysm sac or adjacent vascular segment being treated by the stent graft [260260. White GH, May J, Waugh RC, Yu W. Type I and type II endoleak: a more useful classification for reporting results of endoluminal repair of AAA (Letter). J Endovasc Surg. 1998;5:189–91. ] ( Figure 19 ). An endoleak is evidence of incomplete exclusion of the aneurysm from the circulation. There is evidence that an endoleak may resolve spontaneously, but a proportion of those that do persist are associated with late aneurysm rupture [261261. Lumsden AB, Allen RC, Chaikof EL, Resnikoff M, Moritz MW, Gerhard H, Castronuovo JJ Jr. Delayed rupture of aortic aneurysms following endovascular stent grafting. Am J Surg. 1995;
170:174–78.].
Although intrasac pressure may approach systemic arterial pressure in the presence of an endoleak, some type II endoleaks have been associated with a decrease in aneurysm volume and intrasac pressures that are substantially less than systemic pressures [262262. Malina M, Ivancev K, Chuter TA, Lindh M, Länne T, Lindblad B, Brunkwall J, Risberg B. Changing aneurysmal morphology after endovascular grafting: relation to leakage or persistent perfusion. J Endovasc Surg. 1997;4:23–30. ].
An endoleak can be classified according to the time of occurrence [263263. White GH, Yu W, May J, Chaufour X, Stephen MS. Endoleaks as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis and management. J Endovasc Surg. 1997;4:152–68. ]. An endoleak first observed during the perioperative (<30 days) period is defined as a “primary endoleak”, and detection thereafter is termed a “secondary endoleak”. Further categorisation requires precise information regarding the course of the blood flow into the aneurysm sac [264264. Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, Matsumura JS, May J, Veith FJ, Fillinger MF, Rutherford RB, Kent KC; Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aneurysm repair. J Vasc Surg. 2002;35:1048–60. ].
A type I endoleak is indicative of a persistent perigraft channel of blood flow caused by inadequate seal at either the proximal (I-a) or distal (I-b) stent graft end. In the case of an aorto-mono-iliac prosthesis, a type I endoleak may also refer to blood flow around an iliac occluder plug (I-c).
A type II endoleak is attributed to retrograde flow from the inferior mesenteric artery (II-a), lumbar arteries (II-b), or other collateral vessels. Origin and outflow sources of a type II endoleak should be specified, such as lumbar-lumbar, lumbar-inferior mesenteric artery (IMA), accessory renal-lumbar/IMA, hypogastric-lumbar/IMA, or undefined. It should be emphasised that any connection with a proximal or distal attachment zone will classify the endoleak as a type I endoleak.
A type III endoleak is caused by component disconnection (III-a) or fabric tear, fabric disruption, or graft disintegration (III-b). Type III-b endoleak can be further stratified as minor (<2 mm) or major (>2 mm).
A type IV endoleak is caused by blood flow through an intact but otherwise porous fabric, observed during the first 30 days after stent graft implantation. This definition is not applicable to fabric-related endoleaks observed after the first 30-day period.
If an endoleak is visualised in imaging studies but the precise source cannot be determined, the endoleak is categorised as an endoleak of undefined origin.
Follow-up
As the long-term outcome of endovascular repair is unknown, careful and indefinite follow-up is required. Physical examination and CTA are recommended within 1 month of the procedure, at 6, 12, and 18 months after the intervention, and annually thereafter. Although conflicting studies exist, most investigations suggest that the sensitivity of CTA is superior to that of other non-invasive imaging techniques, such as duplex ultrasonography, for endoleak detection [265265. McWilliams RG, Martin J, White D, Gould DA, Rowlands PC, Haycox A, Brennan J, Gilling-Smith GL, Harris PL. Detection of endoleaks with enhanced ultrasound imaging: Comparison with biphasic computed tomography. J Endovasc Ther. 2002;9:170–9. , 266266. Sato DT, Goff CD, Gregory RT, Robinson KD, Carter KA, Herts BR, Vilsack HB, Gayle RG, Parent FN 3rd, DeMasi RJ, Meier GH. Endoleak after aortic stent graft repair: diagnosis by color duplex ultrasound versus computed tomography. J Vasc Surg. 1998;28:657–63. ]. Besides the search for endoleaks, changes in the dimension of the aneurysm sac may assist in defining the success or failure of aneurysm exclusion. Aneurysm growth after endovascular repair is an indicator of incomplete aneurysm exclusion and therefore a continued risk for late aneurysm rupture. Only a diameter change of 5 mm or more is considered significant.
A plain abdominal x-ray in four planes should be obtained at regular intervals to assess kinking and migration of the stent graft, and to assess the integrity of the support system.
OUTCOME
Prior to the development of modern surgical techniques, successful outcome of AAA treatment was rare. In 1951, it was Dubost, who began treating AAAs with aortic homografts. This treatment was associated with unacceptable rates of degeneration, calcification and poor patency rates. In 1967, DeBakey developed the first synthetic graft made out of a knitted elastic textile (velours). Together with Creech and colleagues he introduced the current surgical standard procedure “endoaneurysmorrhaphy with intraluminal graft placement”. This surgical technique remained essentially unchanged for nearly 40 years until Parodi and Volodos in 1991 introduced the clinical use of endovascular stent graft placement for AAA repair.
EVAR seems to be a reasonable alternative for open surgical repair of abdominal aortic aneurysm. But, it is not easy to compare both techniques for the following reasons. Both are prophylactic operations to prevent aneurysm rupture, an event that occurs unpredictably and infrequently. Perioperative death is an infrequent occurrence associated with both techniques. EVAR and surgery have different complications. Many of the complications (such as endoleak or migration) have no correlate in open surgery. The results of EVAR are dependent on the device and at the same time devices and results are improving. Because of the less invasive nature of EVAR it is not difficult to imagine that an endovascular procedure has less effect on pulmonary function, cardiac function, renal function, and mesenteric perfusion and catecholamine levels.
Much of the early literature consisted of case series from individual centres without surgical control. These studies showed that with EVAR there were fewer complications, shorter intensive care stay, a more rapid recovery and shorter hospital stay [267267. Moore WS, Rutherford RB. Transfemoral endovascular repair of abdominal aortic aneurysms: results of the North-American EVT phase 1 trial. J Vasc Surg. 1996; 23:543-53. , 268268. Makaroun MS, Chaikof EL, Naslund T, Matsumura JS. Efficacy of a bifurcated endograft versus open repair of abdominal aortic aneurysms: a reappraisal. J Vasc Surg. 2002; 35:203-10. , 269269. Maher MM, McNamara AM, MacEneaney PM, Sheehan SJ, Malone DE. Abdominal aortic aneurysms: elective endovascular repair versus conventional surgery—evaluation with evidence-based medicine techniques. Radiology. 2003; 228:647-58. , 270270. Moore WS, Kashyap VS, Vescera CL, Quinones-Baldrich WJ. Abdominal aortic aneurysm: six-year comparison of endovascular versus transabdominal repair. Ann Surg. 1999; 230:298-308. ].
European Collaborators on Stent/Graft Techniques (EuroSTAR) and the Lifeline Registry were 2 large registries including thousands of patients. The size and the heterogeneity of these registries made it a rich source of information of such variables as operator experience, AAA size, AAA extent, gender, type of anaesthesia and risk factors.
Three large randomised trials, EVAR-1 in UK, DREAM Trial in the Netherlands and the OVER Trial in the United States have yielded the only Level 1 evidence for comparison between open and endovascular repair of AAA [271271. United Kingdom EVAR Trial Investigators Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1863-71.
Landmark study (randomised controlled trial) from the United Kingdom on the comparison of open surgical repair and endovascular treatment in patients with an abdominal aortic aneurysm., 272272. De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW, van Sambeek MR, Balm R, Grobbee DE, Blankensteijn JD; DREAM Study Group. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1881-89.
Landmark study (randomised controlled trial) from The Netherlands on the comparison of open surgical repair and endovascular treatment in patients with an abdominal aortic aneurysm., 273273. Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT Jr, Matsumura JS, Kohler TR, Lin PH, Jean-Claude JM, Cikrit DF, Swanson KM, Peduzzi PN; Open Versus Endovascular Repair (OVER) Veterans Affairs Cooperative Study Group. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302:1535-42.
Landmark study (randomised controlled trial) from the United States of America on the comparison of open surgical repair and endovascular treatment in patients with an abdominal aortic aneurysm.].
The EVAR trial 1 included 1,082 patients with AAA who were healthy enough to be suitable candidates for surgery. The 30-day mortality in this randomised controlled trial was 1.7% in the endovascular group and 4.7% in the open surgical group (p<0.001). At 4 years, the aneurysm-related mortality rate in the endovascular group was 4% compared to 7% in the open surgical group (p<0.04). By the end of follow-up, there was no significant difference between the two groups in the rate of death from any cause (adjusted hazard ratio, 1.03; 95% CI, 0.86 to 1.23; p=0.72). The rates of graft-related complications and re-interventions were higher with endovascular repair, and new complications occurred up to 8 years after randomisation, contributing to higher overall costs. In this large, randomised trial, endovascular repair of abdominal aortic aneurysm was associated with a significantly lower operative mortality than open surgical repair. However, no differences were seen in total mortality or aneurysm-related mortality in the long term. Endovascular repair was associated with increased rates of graft-related complications and re-interventions and was more costly
The Dutch Randomised Endovascular Aneurysm Management (DREAM) trial had a similar design of the EVAR trial 1 but was much smaller (351 patients). Two years after randomisation, the cumulative survival rates were 89.6% for open repair, and 89.7% for endovascular repair (p=0.86). The cumulative rates for aneurysm-related death two years after randomisation were 5.7% in the open-repair group and 2.1% in the endovascular repair group (p=0.005). Two years after randomisation the rates of survival free of severe events were 80.6% for open repair and 83.1% for endovascular repair (p=0.39). Kaplan-Meier estimates of the likelihood of freedom from re-interventions were 95% for open repair and 87% for endovascular repair. Six years after randomisation, the cumulative survival rates were 69.9% for open repair and 68.9% for endovascular repair (difference, 1.0 percentage point; 95% confidence interval [CI], -8.8 to 10.8; p=0.97). The cumulative rates of freedom from secondary interventions were 81.9% for open repair and 70.4% for endovascular repair (difference, 11.5 percentage points; 95% CI, 2.0 to 21.0; p=0.03).
The OVER Trial included 881 patients (aged > or = 49 years) from 42 Veterans Affairs Medical Centres with eligible AAA who were candidates for both elective endovascular repair and open repair of AAA. Perioperative mortality (30 days or inpatient) was lower for endovascular repair (0.5% vs. 3.0%; p=0.004), but there was no significant difference in mortality at 2 years (7.0% vs. 9.8%, p=0.13). Patients in the endovascular repair group had reduced median procedure time (2.9 vs. 3.7 hours), blood loss (200 vs. 1,000 mL), transfusion requirement (0 vs. 1.0 units), duration of mechanical ventilation (3.6 vs. 5.0 hours), hospital stay (3 vs. 7 days), and intensive care unit stay (1 vs. 4 days), but required substantial exposure to fluoroscopy and contrast. There were no differences between the 2 groups in major morbidity, procedure failure, secondary therapeutic procedures, aneurysm-related hospitalisations, health-related quality of life, or erectile function. In the OVER Trial the early advantage of endovascular repair was not offset by increased morbidity or mortality in the first 2 years after repair.
Similar results were found in a large systematic review and meta-analysis including 28,862 patients [274274. Franks SC, Sutton AJ, Bown MJ, Sayers RD: Systematic review and meta-analysis of 12 years of endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2007;33:154-71.
This meta-analysis demonstrates a low mortality and gradual reduction in vascular morbidity and mortality associated with endovascular repair of AAA since introduction.]. 163 studies pertaining to 28,862 patients undergoing ER were identified as relevant for the review and meta-analysis. The pooled estimate for operative mortality was 3.3% (95% confidence interval 2.9 to 3.6%). The pooled estimate for type 1 endoleaks was 10.5% (95% confidence interval 9.0 to 12.1%), with an annual rate of 8.4% (95% confidence interval 5.7% to 12.2%). The pooled estimate of type 2,3 and 4 endoleaks was 13.7% (95% confidence interval 12.3 to 15.3%), with an annual rate of 10.2% (95% confidence interval 7.4% to 14.1%). The pooled estimate for primary conversion to open repair was 3.8% (95% confidence interval 3.2 to 4.4%), and for secondary conversion to open repair 3.4% (95% confidence interval 2.8 to 4.2%). The pooled estimate for postoperative rupture was 1.3% (95% confidence interval 1.1 to 1.7%), with an annual rupture rate of 0.6% (95% confidence interval 0.5% to 0.8%). Multivariate meta-regression analysis showed that rates of operative mortality, postoperative rupture and total number of endoleaks all fell significantly (p<0.05) over time. This review demonstrated a low mortality and a gradual reduction in vascular morbidity and mortality associated with endovascular repair since it was first introduced.
FUTURE DEVELOPMENTS
Commercially available stent grafts have limitations with respect to unfavourable anatomy which most often include a short (<15 mm) and angulated (>60) proximal neck, a reversed cone-shaped neck. Fenestrated and branched devices have been proposed as means to address the proximal neck limitations.
Internal iliac artery inflow obstruction can cause hip claudication and may predispose patients to higher incidence of colon, spinal cord or pelvic ischaemia. Endovascular internal iliac artery branch-grafts were designed to prevent these complications.
The experience with fenestrated and branched stent grafts is limited and only highly specialised centres are using these techniques. There are technical issues with respect to the complexity of these procedures. Nevertheless promising results have been reported. As these procedures mature, long-term results and randomised clinical trials will ultimately be required to determine the safety and efficacy of these procedures
Personal perspective – Marc R.H.M. Van Sambeek
The aorta is the largest artery in the human body. Like others arteries the aorta is affected by atherosclerotic disease. Due to the relative diameter obstructive disease is a lesser problem. More prominent are diseases like aneurysmal formation, dissection and laceration due to trauma.
Open surgical treatment of aortic disorders was for a long time the only possibility. Prominent surgeons have developed excellent techniques for aorta surgery with improved outcome every time. The pioneers in endovascular technique for aortic disease were Volodos and Parodi. They started the ongoing shift away from surgical procedures to minimally invasive endovascular techniques. Nowadays, the choice between a surgical or endovascular intervention for aorta disease depends on the localisation and extent of disease. The current commercially available stent grafts have limitations with respect to complex and unfavourable anatomy. Hybrid procedures (a combination of open surgery and an endovascular technique) afford the possibility to treat complex and unfavourable anatomy in a less invasive way. Yet, it is foreseeable that hybrid procedures may be a temporary step between routine surgical and routine endovascular management. Innovative technology will increase treatment options for aortic disease with the introduction of fenestrated and branched devices. The complexity of these procedures will require a dedicated and specialised interventionist. The complexity of the disease will require a multidisciplinary approach.
The future of treatment of aortic disease will be less invasive!