Specifics of revascularisation in diabetics
ANTITHROMBOTIC REGIMEN IN DIABETICS
Diabetes mellitus: a prothombotic state
DM is linked to an increased atherothrombotic risk. Patients with DM and coronary artery disease suffer a high rate of recurrences following their index MI [88. Angiolillo DJ. Antiplatelet therapy in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2007; 14:124-31. ]. Atherothrombotic disease is accelerated in subjects with both type 1 and type 2 diabetes, with diverse underlying mechanisms, despite the common characteristic of hyperglycaemia. The main feature of type 2 DM is insulin resistance, which precedes the development of hyperglycaemia [1111. Reaven G. Role of insulin resistance in human disease. Diabetes. 1988;37:1595-607. ] ( Figure 2 ). By contrast, in type 1 diabetes, hyperglycaemia is the dominant abnormality with insulin resistance appearing in longer-standing patients who develop renal disease [1212. Grant PJ. Diabetes mellitus as a prothrombotic condition. J Inter Med. 2007;262:157-72. ]. Insulin resistance on the one hand and hyperglycaemia on the other exert several important effects by altering coagulation and platelet function, and thus contributing to a prothrombotic status.
Insulin resistance determines increased levels of the fibrinolytic inhibitor Plasminogen Activator Inhibitor-1 (PAI-1), and provides the link between type 2 DM and fibrinolysis suppression [1313. Juhan-Vague I, Roul C, Alessi M, Ardissone J, Heim M, Vague P. Increased plasminogen activator inhibitor activity in non insulin dependent diabetic patients; Relationship with plasma insulin. Thromb Haemost. 1989;61:370-3. ]. Insulin resistance also affects the cellular phases of haemostasis, by impairing both endothelial and platelet function. In insulin resistant subjects endothelial-dependent vasodilatation is impaired [1414. Taylor A. Pathophysiology of hypertension and endothelial dysfunction in patients with diabetes mellitus. Endocrinol Metab Clin North Am. 2001;30:983-97. ]. Platelet function is also regulated by insulin which under normal conditions antagonises the effect of a number of agonists, while in the setting of insulin resistance platelet aggregation is up-regulated [99. Vinik A, Erbas T, Sun Park T, Nolan R, Pittenger G. Platelet dysfunction and type II diabetes. Diabetes Care. 2001;24:1476-85. , 1515. Westerbacka J, Yki-Jarvinen H, Rissanen A, Vehkavaara S, Syrjälä M, R. L. Inhibition of platelet-collagen interaction - An in vivo action of insulin is abolished by insulin resistance in obesity. Arterioscler Thromb Vasc Biol. 2002;22:167-72. ].
Insulin resistance effects on platelet function are related to intra-cytosolic calcium levels, a mediator of platelet activation ( Figure 3 ): whilst insulin decreases the intra-cellular concentration of calcium in platelets from insulin-sensitive subjects in vivo and in vitro, it appears to increase the intra-platelet calcium concentrations in the insulin-resistant state, promoting platelet aggregation and activation [1616. Baldi S, Natali A, Buzzigoli G, Gavlan A, Sironi A, Ferrannini E. In vitro effect of insulin on intracellular calcium concentrations: relation to insulin resistance. Metabolism. 1996;45:1402-7. ].
Hyperglycaemia in turn affects platelet and endothelial function by participating in the prothrombotic status of these patients. Protein glycation and Advanced Glycation End (AGE) products formation seem to be the underlying mechanisms [1717. Khechai F, Ollivier V, Bridey F. Effect of advanced glycation end product-modified albumin on tissue factor expression by monocytes. Arterioscler Vasc Biol. 1997;17:2885-90. ]. Endothelial alterations result in both an increased production of tissue factor, a strong pro-coagulant, and also in soluble coagulation and fibrinolytic factors [1717. Khechai F, Ollivier V, Bridey F. Effect of advanced glycation end product-modified albumin on tissue factor expression by monocytes. Arterioscler Vasc Biol. 1997;17:2885-90. ]. Hyperglycaemia provokes platelet hyper-reactivity and enhanced thromboxane biosynthesis. Moreover, glycation of platelet membrane proteins may cause the enhanced expression of receptors like P-selectin and glycoprotein IIb/IIIa, facilitating platelet interactions. Additionally, hyperglycaemia provokes non-enzymatic glycation of LDL and VLDL which in turn may induce platelet dysfunction.
The overall picture of platelet abnormalities in DM results in hypersensitivity of diabetic platelets to agonists ( Figure 3 ). In fact, platelets in diabetic subjects appear to be in an activated state even in the absence of vascular injury, and they respond more frequently even to subthreshold stimuli, as evidenced by greater expression of the fibrinogen-binding glycoprotein IIb/IIIa receptor, which constitutes the final common pathway of platelet activation and allows for cross-linking of individual platelets by fibrinogen molecules and formation of thrombus [1818. Ferroni P, Basili S, Falco A, Davì G. Platelet activation in type 2 diabetes mellitus. J Thromb Haemost. 2004;2:1282-91. ].
Pathophysiological substrate in type 2 diabetes mellitus
- Insulin resistance and hyperglycaemia provide a substrate for both metabolic dysfunction and a pro-thrombogenic state in type 2 DM
Current antiplatelet therapy options
Overall, three classes of platelet-inhibiting drugs, aspirin, thienopyridines and glycoprotein (GP) IIb/IIIa inhibitors (with different mechanisms of action) are most commonly used for the prevention and treatment of arterial vascular thrombosis. Aspirin (ASA) inhibits thromboxane-A2 (TXA2) production; and, GPIIb/IIIa antagonists (abciximab, tirofiban, and eptifibatide) prevent platelet fibrinogen binding. Thienopyridines are orally-active antagonists of the platelet ADP (P2Y12) receptor. Clopidogrel, ticagrelor and prasugrel are currently the thienopyridines of choice in different clinical scenarios The following description reviews the role of these agents in diabetic patients and highlights the data regarding new pharmacological approaches.
Aspirin
Aspirin therapy has been used for over 100 years and provides marked benefits in the primary and secondary prevention of coronary, cerebral and peripheral vascular disease [1919. AntithromboticTrialistsCollaboration. Collaborative meta-analyses of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71-86. ]. Aspirin inhibits TXA2 production by acetylating a serine residue at position 529 within the active site of the enzyme cyclo-oxygenase [2020. Smith JB, Willis AL. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol. 1971;231:235-7. ]. Inhibition is irreversible and lasts for the lifespan of the platelet (7 to 10 days). It is the first antiplatelet agent of choice for secondary prevention of ischaemic events in patients with atherothrombotic disease, including those with DM. The American Diabetes Association (ADA) recommends the use of aspirin as a secondary prevention measure in diabetic patients with atherosclerotic disease [2121. Colwell J. American Diabetes Association. Aspirin therapy in diabetes (Position Statement). Diabetes Care. 2004;27:S72-S73. ]. This recommendation is supported by data from two large meta-analyses of major secondary prevention trials by the Antithrombotic Trialists’ Collaboration (ATC) [1919. AntithromboticTrialistsCollaboration. Collaborative meta-analyses of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71-86. , 2222. Antiplatelet Trialists Collaboration. Collaborative overview of randomized trials of antiplatelets therapy. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81-106. ]. In more than 4,500 diabetic patients studied in the ATC, the incidence of vascular events was reduced from 23.5% in the control group to 19.3% in the group treated with antiplatelet therapy (p<0.01), leading to 42 vascular events prevented for every 1,000 diabetic patients.
The use of ASA in primary prevention in the general population remains somewhat controversial, but there is an overall expert consensus on the appropriateness of its use for primary prevention in certain patients with DM. The ADA recommends that a dosage of 81 mg to 325 mg of enteric-coated aspirin be used as a preventive strategy in high-risk diabetic individuals, defined on the basis of the following risk factors [2121. Colwell J. American Diabetes Association. Aspirin therapy in diabetes (Position Statement). Diabetes Care. 2004;27:S72-S73. ]: family history of coronary artery disease, cigarette smoking, hypertension, weight >120% of ideal body weight, micro- or macro-albuminuria, total cholesterol >200 mg/dl (LDL cholesterol >100, HDL cholesterol <55 in women and <45 in men, and triglycerides >200).
ASA resistance
The definition of the aspirin resistance phenomenon is still controversial. Strictly speaking, “resistance” is defined as the failure of a specific antiplatelet agent to inhibit its target. Therefore, aspirin resistance should be defined as the failure of aspirin to block arachidonic acid-induced platelet aggregation, inhibiting the platelet thromboxane A2 production [2323. Pamukcu B. A review of aspirin resistance: definition, possible mechanisms, detection with platelet function tests, and its clinical outcomes. J Thromb Thrombolysis. 2007;23:213-22. ]. While in scientific literature the term “resistance” has been applied to failure to prevent occurrence of atherothrombotic vascular events in patients taking aspirin (or other antiplatelet agents), this phenomenon should be defined more appropriately as “therapeutic failure” [2424. Barnes GD, Li J, Kline-Rogers E, Vedre A, Armstrong DF, Froehlich JB, Eagle KA, Gurm HS. Dual antiplatelet agent failure: a new syndrome or clinical nonentity?. Am Heart J. 2007;154:732-5. ].
The reported frequency of aspirin resistance varies widely, from 5% to 40%, depending on the assay used for identification and the population studied [2525. Michelson AD. Platelet function testing in cardiovascular diseases. Circulation. 2004;110:e489-e493. ]. When responsiveness to aspirin is assessed using COX-1 specific assays, resistance to aspirin is virtually absent and such resistance is a phenomenon primarily linked to non-compliance to treatment [2626. Tantry US, Blinden KP, Gurbel PA. Overstimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulation. J Am Coll Cardiol. 2005;46:1705-9. ].The redundancy of platelet activation pathways and receptors, not inhibited by aspirin, contributes to the presence of variable aspirin-induced antiplatelet effects when using non-COX-1 specific assays. More specifically, pathways involving non-TXA2-dependent activators such as thrombin, ADP, epinephrine and collagen can bypass the aspirin-mediated inhibitory effect leading to platelet activation and thrombosis [2727. Mason PJ, Jacobs AK, Freedman JE. Aspirin resistance and atherothrombotic disease. J Am Coll Cardiol. 2005;46:986-93. ]. The concomitant administration of commonly used analgesics may modulate the effect of low-dose aspirin. A clinical dosing regimen of ibuprofen may competitively inhibit the sustained inhibitory COX-1 effects on platelets [2828. Catella-Lawson F, Reilly M, Kapoor S, Cucchiara AJ, DeMarco S, Tournier B, Vyas SN, FizGerald GA. Cyclooxygenase inhibitors and the platelet effects of aspirin. N Engl J Med. 2001;345:1809-17. ]. In diabetics the potential mechanisms involving ASA resistance include: hyperglycaemia, as augmented protein glycation may be associated with decreased aspirin-mediated protein acetylation; increased TXA2 synthesis associated with a poor metabolic control; and accelerated platelet turnover, due to the fact that introduction into the bloodstream of newly generated platelets not exposed to ASA may continue to generate TXA2, thereby activating the thromboxane/prostaglandin receptor despite COX-1 inhibition.
All the above-mentioned reasons may explain the poor outcomes that patients with DM exhibited in clinical trials. The Heart Outcomes Prevention Evaluation trial, for example, demonstrated a 50% higher rate of cardiovascular events in those with, compared to those without, diabetes despite aspirin therapy [2929. Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulacion. 2002;105:1650-5. ]. In the Primary Prevention Project, aspirin use was not associated with cardiovascular protection in those with diabetes, but there was a 40% decrease in cardiovascular death in those without diabetes [3030. Sacco M, Pellegrini F, Roncaglioni MC, Avanzini F, Tognoni G, Nicolucci A, Group. PC. Primary prevention of cardiovascular events with low-dose aspirin and vitamin E in type 2 diabetic patients: results of the Primary Prevention Project (PPP) trial. Diab Care. 2003;26:3264-72. ]. Cubbon et al recently observed a significant reduction in mortality rate among 2,499 patients admitted to 11 coronary care units in the UK in those who were non-diabetic but not in diabetic patients receiving ASA [3131. Cubbon RM, Gale CP, Rajwani A, Abbas A, Morrell C, Das R, Barth JH, Grant PJ, Kearney MT, Hall AS. Aspirin and mortality in patients with diabetes sustaining acute coronary syndrome. Diabetes Care. 2008; 31:363-5. ].
Clopidogrel
The benefit of clopidogrel (alone or in combination with ASA) in diabetic patients has been demonstrated in several trials [3232. Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH, Investigators. C. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS). Circulation. 2000;102:624-9. ]. The CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial was a randomised, blinded trial, involving more than 19,000 patients, designed to assess the relative efficacy of clopidogrel and ASA in reducing the risk of a composite outcome cluster of ischaemic stroke, myocardial infarction or vascular death [3333. CAPRIE Steering Committee. A randomized blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-39. ]. A retrospective analysis of the CAPRIE study showed for the first time a superiority of clopidogrel compared to aspirin in the diabetic subgroup. This was attributable to its more potent antiplatelet effect with more efficient inhibition of the hyper-reactive diabetic platelet. The CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) trial evaluated the efficacy and safety of the clopidogrel when given with aspirin to patients who suffered from acute coronary syndromes without ST-segment elevation for 3 to 12 months (n = 12,562). The use of clopidogrel in this subgroup reduced the rate of the combination of death, MI or stroke (14.2 % rate of primary endpoint in the diabetic cohort on clopidogrel vs. 16.7 % in the diabetic cohort on placebo) without it reaching statistical significance [3434. Yusuf S, Zhao F, Metha SR; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494-502. ].
Clopidogrel response variability
Variability in antiplatelet effects following clopidogrel therapy is present in both the acute and the chronic phases of therapy [3535. Angiolillo DJ, Bernardo E, Sabaté M, Jimenez-Quevedo P, Costa MA, Palazuelos J, Hernández-Antolin R, Moreno R, Escaned J, Alfonso F, Bañuelos C, Guzman LA, Bass TA, Macaya C, Fernandez-Ortiz A. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2007;50:1541-7.
A description of the effects of diabetes mellitus on platelet reactivity and its relationship to cardiovascular outcomes]. Importantly, increased rates of coronary stent thrombosis and recurrent ischaemic events after PCI have been noted in poor clopidogrel responders. Clopidogrel response variability is a multifactorial process, in which clinical, cellular, and genetic factors are involved. Among the clinical factors, diabetes mellitus has been associated with a greater prevalence of poor responsiveness [88. Angiolillo DJ. Antiplatelet therapy in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2007; 14:124-31. ]. In particular, diabetic patients have shown to have poor clopidogrel response in both the acute and the chronic phases of therapy. Of note, diabetics requiring insulin are those who persist with highest platelet reactivity despite dual antiplatelet therapy [3636. Angiolillo DJ, Bernardo E, Ramirez C, Costa MA, Sabaté M, Jimenez-Quevedo P, Hernandez R, Moreno R, Escaned J, Alfonso F, Bañuelos C, Bass TA, Macaya C, Fernandez-Ortiz A. Insulin therapy is associated with platelet dysfunction in patients with type 2 diabetes mellitus on dual oral antiplatelet treatment. J Am Coll Cardiol. 2006;48:298-304. ].
Overall, the persistence of elevated platelet reactivity and reduced response to aspirin and clopidogrel therapy enhances the atherothrombotic risk of DM patients. Multiple causes have been implicated in these observations. Poor glycaemic control is an important cause of increased platelet reactivity. Hyperglycaemia leads to non-enzymatic glycation of platelet glycoproteins, causing changes in their structure and conformation, as well as alterations of membrane lipid dynamics. This may explain why platelet reactivity can be reduced with tight control of glucose levels [3737. Davì G, Averna M, Catalano I, Barbagallo CM, Giovenco E, Carroccio A, Notarbartolo A, Strano A. Platelet function in patients with type 2 diabetes mellitus: the effect of glycemic control. Diabetes Res Clin Pract. 1989;10:7-12. ].
GP IIB/IIIa inhibitors
Three GP IIb/IIIa inhibitors are available for clinical use: abciximab, eptifibatide and tirofiban. These are used mainly as an adjunctive therapy on top of ASA and a thienopyridine for the acute phase of a high-risk acute coronary syndrome due to their intravenous administration. The efficacy of this therapy was demonstrated in a meta-analysis of six large-scale trials [3838. Roffi M, Chew DP, Mukherjee D, Bhatt DL, White JA, Heeschen C, Hamm CW, Moliterno DJ, Califf RM, White HD, Kleiman NS, Théroux P, Topol EJ. Platelet glycoprotein IIb/IIIa inhibitors reduce mortality in diabetic patients with non-ST-segment-elevation acute coronary syndromes. Circulation. 2001;104:2767-71. ]. This analysis included 6,458 diabetic patients presenting with an acute coronary syndrome. Abciximab was associated with a significant reduction in the 30-day mortality rate (6.2% vs. 4.6%; p=0.007). Therefore, the survival benefit appeared to be of a greater magnitude in diabetic patients receiving IIb/IIIa inhibitors during PCI. However, these trials were performed before current clopidogrel regimen was administered (pre-treatment, high loading dose, etc). Two more recent trials, evaluated the use of GP IIb/IIIa inhibitors on top of dual antiplatelet therapy. The Intracoronary Stenting and Antithrombotic Regimen: Is Abciximab a Superior Way to Eliminate Elevated Thrombotic Risk in Diabetics (ISAR-SWEET) trial did not show any benefit from abciximab in diabetic patients undergoing elective PCI after pre-treatment with clopidogrel 600 mg loading dose at least 2 hours before the intervention [3939. Mehilli J, Kastrati A, Schühlen H, Dibra A, Dotzer F, von Beckerath N, Bollwein H, Pache J, Dirschinger J, Berger PP, Schömig A. Intracoronary Stenting and Antithrombotic Regimen: Is Abciximab a Superior Way to Eliminate Elevated Thrombotic Risk in Diabetics (ISAR-SWEET) Study Investigators Randomized clinical trial of abciximab in diabetic patients undergoing elective percutaneous coronary interventions after treatment with a high loading dose of clopidogrel. Circulation. 2004;110:3627-35. ]. Conversely, in the Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) trial, abciximab showed a 25% reduction of the risk of adverse events (death, MI or urgent target vessel revascularisation at 30 days) as compared to placebo in high-risk acute coronary syndromes undergoing PCI after pre-treatment with 600 mg of clopidogrel. This benefit was restricted to those patients with elevated troponin levels and was observed across all subgroups including DM [4040. Ndrepepa G, Kastrati A, Mehilli J, Neumann FJ, ten Berg J, Bruskina O, Dotzer F, Seyfarth M, Pache J, Dirschinger J, Berger PB, Schömig A. One-year clinical outcomes with abciximab vs. placebo in patients with non-ST-segment elevation acute coronary syndromes undergoing percutaneous coronary intervention after pre-treatment with clopidogrel: results of the ISAR-REACT 2 randomized trial. Eur Heart J. 2008;29:455-61. ]. A meta-regression of randomised trials evaluating the effect of any GP IIb/IIIa inhibitors on top of dual antiplatelet therapy in patients with ST elevation MI undergoing primary PCI showed a relationship in benefit in terms of mortality [4141. De Luca G, Navarese E, Marino P. Risk profile and benefits from Gp IIb-IIIa inhibitors among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: a meta-regression analysis of randomized trials. Eur Heart J. 2009;30:2705-13. ] and a patient’s risk profile. Recently, GP IIb/IIIa inhibitors have been tested against bivalirudin, a direct thrombin inhibitor, in the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial [4242. Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht HJ, Hoekstra J, Mehran R, Ohman EM; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203-16. ]. Bivalirudin showed similar efficacy in terms of ischaemic events but lower rates of major bleeding. In the DM subgroup (n=3,852), bivalirudin produced a reduction in the major bleeding rates (3.7% vs. 7.1%; p<0.001) with similar efficacy in ischaemic outcomes (7.9% vs. 8.9%;p=0.39) when compared to GP IIb/IIIa inhibitors plus heparin in moderate-risk non-ST-elevation MI patients.
Treating ASA and clopidogrel “resistance”
The treatment for failed antiplatelet therapy, in particular amongst diabetic patients, is as yet undefined. Initially, physicians should enquire about patient compliance, as well as seek to minimise drug-drug interactions and polypharmacy. Moreover, an optimal control of glucose levels, cholesterol levels, and blood pressure, all of which minimises platelet reactivity, must be emphasised.
Theoretically, there are 3 potential approaches to treat “true” ASA or clopidogrel resistance ( Table 1 ): increasing the dose of current antiplatelet regimen, adding a third antiplatelet drug or incorporating newer antiplatelet agents.
Increasing the dose of antiplatelet therapy
The optimal dose to use is controversial. There is no good evidence to date that increasing the ASA dose would be more efficacious, and may entail an increased risk of bleeding [4343. Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl. J Med. 2005;353:2373-83. ]. In support of this view, increasing the dose of aspirin is not associated with further inhibition of COX-1. Increasing loading or maintenance doses of clopidogrel, however, may represent an option [4343. Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl. J Med. 2005;353:2373-83. , 4444. Angiolillo DJ, Shoemaker SB, Desai B, Yuan H, Charlton RK, Bernardo E, Zanni MM, Guzman LA, Bass TA, Costa MA. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease. Circulation. 2007; 115:708-16. ]. Indeed, increasing the loading dose increases drug responsiveness and has been associated with improved clinical outcomes [4545. Lotrionte M, Biondi-Zoccai GG, Agostoni P, Abbate A, Angiolillo DJ, Valgimigli M, Moretti C, Meliga E, Cuisset T, Alessi MC, Montalescot G, Collet JP, Di Sciascio G, Waksman R, Testa L, Sangiorgi G, Laudito A, Trevi GP, Sheiban I. Meta-analysis appraising high clopidogrel loading in patientes undergoing percuntaneous coronary intervention. Am J Cardiol. 2007;100:1199-206. ]. However, this approach is valid only for the acute phase of treatment as patients must rely on their daily maintenance therapy for their long-term prevention of ischaemic events. The Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study evaluated the functional impact of a 150 mg maintenance dose of clopidogrel compared to a standard 75 mg dose selectively in type 2 DM patients with suboptimal response to standard dose therapy. High maintenance therapy was associated with enhanced antiplatelet effects compared with 75 mg [4444. Angiolillo DJ, Shoemaker SB, Desai B, Yuan H, Charlton RK, Bernardo E, Zanni MM, Guzman LA, Bass TA, Costa MA. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease. Circulation. 2007; 115:708-16. ]. Recently, the Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent EveNTs/Optimal Antiplatelet Strategy for InterventionS (CURRENT/OASIS 7) trial randomised in a 2x2 factorial design to high or standard dose of clopidogrel for a month to high (300 mg to 325 mg daily) versus low dose (75 mg to 100 mg daily) of aspirin [4646. CURRENT-OASIS 7 Investigators, Mehta SR, Bassand JP, Chrolavicius S, Diaz R, Eikelboom JW, Fox KA, Granger CB, Jolly S, Joyner CD, Rupprecht HJ, Widimsky P, Afzal R, Pogue J, Yusuf S. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med. 2010;363:930-42. ]. Although in the overall population (n=25,087) no benefit was derived from the high-dose regimen in the subgroup of patients undergoing PCI (n=17,232), the high clopidogrel dose strategy diminished the rates of ischaemic events [3.9% versus 4.5%: hazard ratio (HR) =0.85; p=0.036] and the risk of stent thrombosis by 30%. However, there was an increase in study-defined major bleedings. There was no difference in efficacy among the subset of patients with DM undergoing PCI [4.9% vs. 5.6%; HR=0.87 (0.66-1.15)] [4747. Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, Faxon DP, Rupprecht HJ, Budaj A, Avezum A, Widimsky P, Steg PG, Bassand JP, Montalescot G, Macaya C, Di Pasquale G, Niemela K, Ajani AE, White HD, Chrolavicius S, Gao P, Fox KA, Yusuf S; CURRENT-OASIS 7 trial investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233-43. ].
Adding a third antiplatelet agent
The addition of a third antiplatelet agent in DM patients has been at the forefront of recent interest. Specifically, cilostazol has been tested in addition to aspirin and clopidogrel, and it was associated with a reduced risk of stent thrombosis [4848. Lee SW, Park SW, Hong MK, Kim YH, Lee BK, Song JM, Han KH, Lee CW, Kang DH, Song JK, Kim JJ, Park SJ. Triple versus dual antiplatelet therapy after coronary stenting: impact on stent thrombosis. J Am Coll Cardiol. 2005;46:1833-7. , 4949. Biondi-Zoccai GG, Lotrionte M, Anselmino M, Moretti C, Agostoni P, Testa L, Abbate A, Cosgrave J, laudito A, G.P. T, Sheiban I. Systematic review and meta-analysis of randomized clinical trials appraising the impact of cilostazol after percutaneous coronary intervention. Am Heart J. 2008;155:1081-9. ]. In the bare metal stent (BMS) era, triple therapy was shown to be beneficial in high-risk patients, including diabetics, in reducing restenosis rates [4848. Lee SW, Park SW, Hong MK, Kim YH, Lee BK, Song JM, Han KH, Lee CW, Kang DH, Song JK, Kim JJ, Park SJ. Triple versus dual antiplatelet therapy after coronary stenting: impact on stent thrombosis. J Am Coll Cardiol. 2005;46:1833-7. ]. In the DES era, recent findings from the DECLARE-DIABETES study have shown triple therapy to be associated with reduced TLR and MACE (major adverse cardiac events) at 9 months [5050. Lee SW, Park SW, kim YH, Yun SC, Park DW, Lee CW, Hong MK, Kim HS, Ko JK, Park JH, Lee JH, Choi SW, Seong IW, Cho YH, Lee NH, Kim JH, Chun KJ, Park SJ. Drug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with diabetes mellitus the DECLARE-DIABETES trial (A Randomized Comparison of Triple Antiplatelet Therapy with Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation in Diabetic Patients). J Am Coll Cardiol. 2008;51:1181-7. ]. The mechanisms underlying such benefit may reside in the greater number of antiplatelet effects achieved, in addition to effects of cilostazol on endothelial cells and smooth vascular muscle cells. The OPTIMUS-2 study evaluated the functional impact of adding cilostazol to aspirin and clopidogrel therapy in type 2 DM patients. This study showed that cilostazol compared to placebo was associated with marked inhibition of P2Y12 signalling [5151. Angiolillo DJ, Capranzano P, Goto S, Aslam M, Desai B, Charlton RK, Suzuki Y, Box LC, Shoemaker SB, Zenni MM, Guzman LA, Bass TA. A randomized study assessing the impact of cilostazol on platelet function profiles in patients with diabetes mellitus and coronary artery disease on dual antiplatelet therapy: results of the OPTIMUS-2 study. Eur Heart J. 2008;29:2202-11. ]. The addition of a IIb/IIIa inhibitor, such as tirofiban, in aspirin or clopidogrel poor responders has recently been tested in the Tailoring Treatment with Tirofiban in Patients Showing Resistance to Aspirin and/or Resistance to Clopidogrel study (3T/2R) [5252. Valgimigli M, Campo G, de Cesare N, Meliga E, Vranckx P, Furgieri A, Angiolillo DJ, Sabaté M, Hamon M, Repetto A, Colangelo S, Brugaletta S, Parrinello G, Percoco G, Ferrari R; Tailoring Treatment With Tirofiban in Patients Showing Resistance to Aspirin and/or Resistance to Clopidogrel (3T/2R) Investigators. Intensifying platelet inhibition with tirofiban in poor responders to aspirin, clopidogrel, or both agents undergoing elective coronary intervention: results from the double-blind, prospective, randomized Tailoring Treatment with Tirofiban in Patients Showing Resistance to Aspirin and/or Resistance to Clopidogrel study. Circulation. 2009;119:3215-22. ]. Among 1,277 screened patients, 93 aspirin, 147 clopidogrel, and 23 dual poor responders, based on a point-of-care assay, who underwent elective PCI at 10 European sites for stable or low-risk unstable coronary artery disease, were enrolled. Patients were randomly assigned in a double-blind manner to receive either tirofiban (n=132) or placebo (n=131) on top of standard aspirin and clopidogrel therapy. The primary endpoint, consisting of troponin I/T elevation at least 3 times the normal upper limit, was attained in 20.4% (n=27) in the tirofiban group compared with 35.1% (n=46) in the placebo group (relative risk, 0.58; 95% confidence interval, 0.39 to 0.88; P=0.009). The rate of MACE within 30 days in the tirofiban group was also reduced (3.8% versus 10.7%; P=0.031). The efficacy of tirofiban was observed across multiple pre-specified subgroups including diabetics. This benefit was extended up to 1 year follow-up [5353. Campo G, Fileti L, de Cesare N, Meliga E, Furgieri A, Russo F, Colangelo S, Brugaletta S, Ferrari R, Valgimigli M; 3T/2R Investigators Long-term clinical outcome based on aspirin and clopidogrel responsiveness status after elective percutaneous coronary intervention: a 3T/2R (tailoring treatment with tirofiban in patients showing resistance to aspirin and/or resistance to clopidogrel) trial substudy. J Am Coll Cardiol. 2010;56:1447-55. ].
Prasugrel/Ticagrelor
New P2Y12 inhibitors with a more uniform and potent effect have recently been evaluated. Prasugrel is a P2Y12 inhibitor of the third generation, with more potent and less variable antiplatelet effects compared to clopidogrel [5454. Wallentin L, Varenhorst C, James S, Erlinge D, Braun OO, Jakubowski JA, Sugidachi A, Winters KJ, Siegbahn A. Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J. 2008;29:21-30. ]. The TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis in Myocardial Infarction) trial showed significantly reduced rates of ischaemic events, including stent thrombosis, in patients presenting with acute coronary syndromes undergoing PCI treated with prasugrel compared to clopidogrel [5555. Wiviott SD, Brunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM, Investigators ftT-T. Prasugrel versus clopidogrel in patients with acute coronary syndromes. New Engl J Med. 2007;357:2001-2015. ]. The net clinical benefit achieved with prasugrel in the overall study population was however diminished by the increased risk of bleeding occurring with prasugrel, but remaining still statistically significant for better clinical outcomes with prasugrel. Of note, in this trial the greatest risk reduction (rate of primary endpoint, defined as death from cardiovascular causes, non-fatal MI or non-fatal stroke, in diabetic patients on prasugrel 12.2% vs. diabetic patients on clopidogrel 17.0 % with 30% relative risk reduction) was observed in the diabetic population (n= 3,146). Importantly, in these patients prasugrel was not associated with an increased risk of major bleedings compared to clopidogrel [5656. Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, Goodman SG, Corbalan R, Purdy DA, Murphy SA, McCabe CH, Antman EM; TRITON-TIMI 38 Investigators. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation. 2008;118:1626-36.
This substudy from TRITON-TIMI 38 trial assessed the benefit of prasugrel in diabetic patients with acute coronary syndromes]. The functional impact of prasugrel versus clopidogrel specifically in diabetic patients is currently being evaluated in the OPTIMUS-3 study. Ticagrelor, a novel P2Y12 receptor inhibitor, has a faster onset and offset of action and achieves higher inhibition of platelet aggregation than clopidogrel. The phase III Study of Platelet Inhibition and Patient Outcomes (PLATO) trial randomised acute coronary syndrome patients (n=18,624) to receive either ticagrelor (180 mg loading dose followed by 90 mg twice daily) or clopidogrel (300 mg to 600 mg loading dose followed by 75 mg daily). The primary endpoint (death from vascular causes, MI or stroke) at 12 months was significantly decreased in the ticagrelor arm (10.2% vs. 12.3%; HR=0.84; p<0.0001) in the overall population [5757. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-57. ]. In a predefined subgroup analysis of diabetic patients (n=4,662) there was a non-significant reduction of the primary endpoint [14.1% vs. 16.2%; HR 0.88 (0.76-1.03)], while no difference in major bleeding rates was found [14.1% vs. 14.8%; HR=0.95 (0.81-1.12)] [5858. James S, Angiolillo DJ, Cornel JH, Erlinge D, Husted S, Kontny F, Maya J, Nicolau JC, Spinar J, Storey RF, Stevens SR, Wallentin L; PLATO Study Group. Ticagrelor vs clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2010;31:3006-16.
A substudy from the PLATO trial demonstrating the benefit of ticagrelor in diabetic patients presenting with acute coronary syndromes]. Current guidelines recommend the use of aspirin and clopidogrel in stable coronary artery disease as first option and prasugrel or ticagrelor in specific high risk patients. Conversely, in acute coronary syndromes, prasugrel or ticagrelor on top of aspirin are preferred to clopidogrel which is only recommended when prasugrel or ticagrelor are not available or are contraindicated [5959. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018 Jan 14;39(3):213-260. ].
Antiplatelet therapy in diabetes mellitus
- ASA and clopidogrel have been the cornerstone antiplatelet treatment in diabetic patients during the last two decades
- In addition, GP IIb/IIIa inhibitors have also been considered specifically in acute coronary syndromes
- Despite this regimen, clinical events are persistently higher in diabetics as compared to non-diabetics
- Aspirin resistance and clopidogrel response variability may play a role in this regard
- New antiplatelet agents such as prasugrel and ticagrelor are currently the first-line treatment in diabetic with acute coronary syndromes.
METABOLIC CONTROL IN DIABETICS
As noted above, hyperglycaemia has arisen as the main factor involving pathophysiological disarrangements in DM leading to a prothrombotic state and impaired responsiveness to current antiplatelet therapy. Thus, metabolic control has become the cornerstone to improve outcomes in diabetic patients. In the BMS era, optimal glycaemic control was associated with a lower rate of target vessel revascularisation in treated type 2 diabetic patients undergoing elective PCI. In a case-control study diabetic patients with HbA1c>7% during follow-up exhibited a higher incidence of target vessel revascularisation as compared to non-diabetic or diabetics with HbA1c<7% [6060. Corpus RA, George PB, House JA, Dixon SR, Ajluni SC, Devlin WH, Timmis GC, Balasubramaniam M, O’Neill WW. Optimal glycemic control is associated with a lower rate of target vessel revascularization in treated type II diabetic patients undergoing elective percutaneous coronary intervention. J Am Coll Cardiol. 2004;43:8-14. ]. Similarly, in the current DES era the addition of cilostazol to dual antiplatelet therapy improved the restenosis rate in diabetic patients in a randomised controlled trial [6161. Psaty BM, Furberg CD. Rosiglitazone and cardiovascular risk. N Engl J Med. 2007 356:2522-4. ]. Relatively new hypoglycaemic agents, like rosiglitazone, were pointed out as a strategy for the prevention of restenosis in diabetic patients. Rosiglitazone is a member of the class of drugs known as thiazolidinediones. These drugs are peroxisome proliferators-activated receptor (PPAR)- χ agonists, potentially able to increase insulin sensitivity, decrease inflammation, endogenous fibrinolysis and glycaemic control, as well as reduce neointimal proliferation after PCI in type 2 diabetes. However, a recent report of a meta-analysis of treatment trials of rosiglitazone, as compared either with other therapies for type 2 diabetes or with placebo, showed that rosiglitazone was associated with a significant increase in the risk of MI (HR 1.43; 95% CI; P=0.03) and a borderline-significant finding for increased death from cardiovascular causes (HR 1.64; 95% CI; P=0.06) [6060. Corpus RA, George PB, House JA, Dixon SR, Ajluni SC, Devlin WH, Timmis GC, Balasubramaniam M, O’Neill WW. Optimal glycemic control is associated with a lower rate of target vessel revascularization in treated type II diabetic patients undergoing elective percutaneous coronary intervention. J Am Coll Cardiol. 2004;43:8-14. ]. The role of these agents as adjunctive therapy for diabetics undergoing percutaneous revascularisation has to be revised.
More controversial remains glycaemic control during the acute phase of the admission. Glycaemic control may be especially relevant for the outcomes in diabetic patients undergoing CABG. The use of a continuous insulin infusion has been correlated with reduced perioperative mortality compared with subcutaneous insulin. A recent clinical trial compared continuous glucose-insulin-potassium (GIK) infusion to achieve a target serum glucose level of 125 to 200 mg/dL against standard therapy (serum glucose < 250 mg/dL). Patients treated with GIK infusion developed fewer perioperative infections and atrial fibrillation and experienced shorter hospital stays [6262. Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109:1497-502. ]. Over 2 years, patients treated with GIK infusion also had a significantly improved survival rate and fewer recurrent ischaemic events. However, the intensive glucose control during hospitalisation has recently been questioned. The NICE-SUGAR trial [6363. NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283-97. ] randomised patients admitted to the Intensive Care Unit (ICU) to intensive glucose control (81 to 108 mg/dl; n = 3,054) vs. conventional glucose control (<180 mg/dl; n = 3,050). Insulin was given intravenously and nutrition was given enterally. All-cause mortality at 90 days was significantly higher in the intensive treatment arm (27.5% vs. 24.9%; p=0.02). Severe hypoglycaemia was observed more often in the rigid control arm (6.5% vs. 0.5%; p<0.001). Glycaemic levels around 140 mg/dl may be sufficient to prevent complications during hospitalisation. In the same way, a recent meta-analysis regarding intensive insulin therapy in ICUs [6464. Griesdale Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821-7. ] revealed no benefit in terms of mortality rate in both medical and mixed ICUs. Only surgical ICUs benefited from this intensive control. Interestingly, hypoglycaemic events, closely related to mortality, were observed significantly more often following rigid glucose control.For these reasons, systematic use of GIK in diabetics undergoing revascularisation is not indicated( Table 2 ).
Adequate metabolic control in diabetic patients may also decelerate disease progression. Recently, inhibitors of sodium-glucose cotransporter 2 improved clinical outcomes and reduced mortality in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease [147147. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28.PMID:26378978 ] and also reduced the risk of worsening heart failure or death from cardiovascular causes in patients with heart failure and a reduced ejection fraction, regardless of the presence or absence of diabetes [148148. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381: 1995–2008. PMID:31535829 ]. The mechanisms of action of those drugs are not completely understood but they surely go beyond glycemic control.
Metabolic control in diabetes mellitus
- Glycaemic control is essential to improve long-term outcomes in diabetic patients, especially after revascularisation
- During hospitalisation, refined glucose control is desirable to avoid ominous hypoglycaemia
- New inhibitors of sodium-glucose cotransporter 2 (empagliflozin and dapagliflozin) may play a role in decelerating the progression of atherosclerosis in diabetic patients
SPECIFIC COMPLICATIONS DURING PCI IN DIABETIC PATIENTS
Contrast-induced nephropathy (CIN)
One of the main complications seen after coronary angiography is CIN. This potentially severe complication is associated with adverse outcomes. It is defined as an increase of 0.5 mg per decilitre or a 25% increase of creatinine levels from baseline levels following contrast use [6565. Jabara R, Gadesam RR, Pendyala LK, Knopf WD, Chronos N, Chen JP, Viel K, King SB 3rd, Manoukian SV. Impact of the definition utilized on the rate of contrast-induced nephropathy in percutaneous coronary intervention. Am J Cardiol. 2009;103:1657-62. ]. The main determinant of this renal impairment is the presence of previous renal insufficiency. Even in the absence of renal insufficiency, the presence of diabetes increases the risk of CIN as compared to non-diabetics. In this regard, guidelines for PCI recommend monitoring levels of creatinine in both diabetics and patients with renal insufficiency [6666. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, Morrison DA, O’neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; American College of Cardiology/American Heart Association Task Force onPractice Guidelines; ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous CoronaryIntervention. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention – summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (ACC/AHA/ SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2006;47:216-35. ]. Furthermore, nephrotoxic agents such as antibiotics, non-steroidal anti-inflammatory agents, cyclosporine or metformin should be withdrawn 24 to 48 hours before the procedure in patients with renal impairment. These should be restarted 48 hours later after ruling out the development of CIN [6767. Barrett BJ, Parfrey PS. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379-86. ]. Hydration is the main therapeutic agent to prevent the development of CIN. More controversial is the administration of N-acetyl cysteine or sodium bicarbonate.
Metformin-induced acidosis
Metformin-induced lactic acidosis following contrast use is also controversial. There is no conclusive evidence indicating that the intravascular use of contrast media precipitates the development of metformin-induced lactic acidosis in patients with normal S-creatinine. The complication has almost always been observed in non-insulin-dependent diabetic patients with abnormal renal function before injection of contrast media [6868. Thomsen HS, Morcos SK. Contrast media and the kidney. European Society of Urogenital Radiology (ESUR) guidelines. Br J Radiol. 2003;76:513-8. ]. A recent single centre study paper analysed the factors related to lactic acidosis. Diabetes rather than metformin therapy was associated with the development of lactic acidosis. A synergistic effect occurred between diabetes, age and acute illness (acute renal impairment and sepsis) [6969. Scale T, Harvey N. Diabetes, metformin and lactic acidosis. Clinical Endocrinol. 2011;74:191-6. ].
The CIN Consensus Working Panel [7070. Stacul F, Adam A, Becker CR, Davidson C, Lameire N, McCullough PA, Tumlin J; CIN Consensus Working Panel. Strategies to reduce the risk of contrast induced nephropathy. Am J Cardiol. 2006;98;59K-77K. ] agreed that, provided renal function is within the normal range, metformin should be stopped at the time of the procedure and resumed 48 hours afterwards. If the patient’s baseline renal function is abnormal, however, metformin should be stopped 48 hours before the study and should only be restarted 48 hours afterwards if renal function is unchanged. In emergency situations, clinical judgement should be used and the patient should be monitored closely, with particular attention to hydration. This is in line with the recommendations of the European Society for Urogenital Radiology and the Society for Cardiac Angiography and Interventions and has been reflected in the ESC guidelines on coronary revascularisation [7171. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019 Jan 7;40(2):87-165.
Recent guidelines on coronary revascularisation. These were elaborated under the auspices of both the European Society of Cardiology and the European Association of Cardio-Thoracic Surgery] ( Table 2 ).
Renal complication after contrast administration in diabetic patients
- Diabetes mellitus per se represents a high risk of contrast-induced nephropathy
- Some of the comorbidities commonly seen in diabetics (i.e., previous renal dysfunction) may have a synergistic and detrimental effect on renal function following diagnostic or therapeutic interventions
- Close monitoring of renal parameters and the avoidance of nephrotoxic agents are essential to prevent and treat such complications. In the event of nephropathy, metformin has to be stopped during catheterisation or PCI
OUTCOMES AFTER REVASCULARISATION
From balloon angioplasty to bare metal stent
As compared to non-diabetics, diabetics show less favourable long-term clinical outcomes after percutaneous transluminal balloon angioplasty (BA) despite a procedural success rate of 90%. Diabetes mellitus has been identified as an independent predictor of restenosis which has been the major limiting factor for the long-term success of BA. The restenosis rate following BA in diabetics ranges between 35% and 71%, much higher than that of the general population (30% to 35%) [7272. Rensing BJ, Hermans WR, Vos J, Tijssen JG, Rutch W, Danchin N, Heyndrickx GR, Mast EG, Wijns W, PW. S. Luminal narrowing after percutaneous transluminal coronary angioplasty: A study of clinical, procedural, and lesional factors related to long-term angiographic outcome - Coronary Artery Restenosis Prevention on Repeated Thromboxane Antagonism (CARPORT) Study Group. Circulation. 1993;88:975-85. ]. Beside these data, the pattern of restenosis is more severe as these patients typically show more proliferative and occlusive types of restenosis ( Figure 4 ). The latter has been associated with an increased long-term mortality and impaired left ventricular ejection fraction [7373. Van Belle E, Ketelers R, Bauters C, Périé M, Abolmaali K, Richard F, Lablanche JM, McFadden EP, Bertrand ME. Patency of percutaneous transluminal coronary angioplasty sites at 6-month angiographic follow-up: A key determinant of survival in diabetics after coronary balloon angioplasty. Circulation. 2001;103:1218-24. ]. Four mechanisms are involved in the restenosis process following plain BA: elastic recoil, negative remodelling, platelet–mediated thrombus formation and proliferation of smooth muscle cells. Amongst these, negative remodelling (i.e., vessel shrinkage) is the main contributor [7474. Kimura T, Kaburagi S, Tamura T, Yokoi H, Nakagawa Y, Yokoi H, Hamasaki N, Nosaka H, Nobuyoshi M, Mintz GS, Popma JJ, Leon MB. Remodeling of human coronary arteries undergoing coronary angioplasty or atherectomy. Circulation. 1997;96:475-83. ]. Positive remodelling consists of vessel wall dilation in response to plaque development, to preserve arterial lumen [7575. Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987; 316:1371-5. ]. In diabetic patients this adaptative response of the arterial wall is impaired, and a negative remodelling appears at lesion sites, which is even more evident following balloon inflation. Negative remodelling in diabetics accounts for 73% of lumen reduction after balloon angioplasty, while plaque burden only affects 27% [7676. Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Wong C, Hong MK, Kovach JA, MB. L. Arterial remodeling after coronary angioplasty: a serial intravascular ultrasound study. Circulation. 1996;94:35-43. ].
Coronary stenting was able to reduce restenosis due to its scaffolding properties which were able to prevent elastic recoil and late arterial wall constrictive remodelling [7777. Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, Detre K, Veltri L, Ricci D, Nobuyoshi M, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331:496-501. , 7878. Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331:489-95. ]. Two pivotal randomised controlled trials demonstrated the beneficial effects of stenting as compared to BA, the STRESS and the BENESTENT trials [7777. Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, Detre K, Veltri L, Ricci D, Nobuyoshi M, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331:496-501. , 7878. Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331:489-95. ]. The analysis of diabetic patients also revealed a significant reduction in restenosis rate (STRESS: stent 32%, balloon 42%; p= 0.046; BENESTENT: stent 22%, balloon 42%; p= 0.02) and clinical outcomes improvement at 6 months and at 4 years follow-up (including cardiac death, non-fatal MI and the need for repeat revascularisation) [7979. Van Belle E, Périé M, Braune D, Chmaït A, Meurice T, Abolmaali K, McFadden EP, Bauters C, Lablanche JM, Bertrand ME. Effects of coronary stenting on vessel patency and long-term clinical outcome after percutaneous coronary revascularization in diabetic patients. J Am Coll Cardiol. 2002;40:410-7. ]. Despite these results, restenosis rate remained higher in diabetics compared to non-diabetics. In a meta-analysis [8080. West NE, Ruygrok PN, Disco CM, Webster MW, Lindeboom WK, O’Neill WW, Mercado NF, Serruys PW. Clinical and angiographic predictors of restenosis after stent deployment in diabetic patients. Circulation. 2004;109:867-73. ] of 16 studies, after stent implantation angiographic restenosis (defined as ≥50% diameter stenosis at follow-up) occurred in 550 of 2,672 (20.6%) of non-diabetics as compared to 130 of 418 (31.1%) of diabetic patients (p<0.001). The authors identified, among others, insulin treatment in type 2 diabetes, a marker of disease duration and severity, as an independent predictor of restenosis. The prevailing mechanism of restenosis after stenting is accelerated intimal hyperplasia which is especially exaggerated in diabetic patients [8181. Takagi T, Akasaka T, Yamamuro A, Honda Y, Hozumi T, Morioka S, Yoshida K. Troglitazone reduces neointimal tissue proliferation after coronary stent implantation in patients with non-insulin dependent diabetes mellitus: a serial intravascular ultrasound study. J Am Coll Cardiol. 2000;36:1529-35. ] ( Figure 3). Thus, the recent development of stents with the ability to elute medication with anti-proliferative properties (drug-eluting stent; DES) to tackle directly this mechanism of restenosis appears to be the new revolution in the field.
Drug-eluting stents vs. bare metal stents and drug-eluting stents vs. drug-eluting stents ( Table 3 and Table 4 )
Efficacy of DES in diabetics
The pivotal randomised controlled trial which evaluated the efficacy of the sirolimus-eluting stent (SES, Cypher® stent; Cordis, Johnson & Johnson, Warren, NJ, USA) was the SIRIUS trial (Sirolimus-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) [8282. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, William DO, Teirstein PA, al e. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery; The SIRIUS Investigators. N Engl J Med. 2003;349:1315-23. ]. In this trial, a total of 1,058 patients were randomised to either SES or BMS for the treatment of de novo coronary stenosis. The primary endpoint was target vessel failure (cardiac death, myocardial infarction and target vessel revascularisation [TVR]) at 9-month follow-up. Patients allocated to receive SES showed a significant reduction in the primary endpoint rate as well as in angiographic parameters of restenosis. Subgroup analysis of the SIRIUS trial included 279 diabetics, 131 receiving SES and 148 receiving BMS [8383. Moussa I, Leon MB, Baim DS, O’Neill WW, Popma JJ, Buchbinder M, Midwall J, Simonton CA, Keim E, Wang P, Kuntz RE, Moses JW. Impact of sirolimus-eluting stents on outcome in diabetic patients - A SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation. 2004;109:2273-8. ]. In this subgroup of patients, SES implantation demonstrated favourable results with significant reductions in restenosis rates (in-lesion 50% for BMS vs. 17% for SES), and in MACE (25% for BMS vs. 9.2% for SES).
The use of the paclitaxel-eluting stent (PES; Taxus® stent; Boston Scientific, Natick, MA, USA) has been assessed in the pivotal multicentre, randomised, large-scale, controlled trial, TAXUS IV trial [8484. Hermiller JB, Raizner A, Cannon L, Gurbel PA, Kutcher MA, Wong SC, Russell ME, Ellis SG, Mehran R, Stone GW; TAXUS-IV Investigators. Outcomes with the polymer-based paclitaxel-eluting TAXUS stent in patients with diabetes mellitus: The TAXUS-IV trial. J Am Coll Cardiol. 2005;45:1172-9. ]. In this trial, 1,326 patients were randomised to PES or BMS for the treatment of de novo coronary stenosis. The primary endpoint was ischaemia driven TVR and the incidence of cardiac death, and MI at one year. Overall, the PES group showed a significant reduction in the occurrence of the primary endpoint (TVR 7.4% vs. 20.9%, p = 0.0008). The study included 155 diabetic patients (32% of the total population) and 33% of the diabetics were insulin-dependent DM. In this subgroup, the use of PES significantly reduced the risk of binary restenosis (70% reduction of in-segment restenosis). This reduction was also observed in insulin-dependent DM subjects (42.9% for BMS vs. 7.7% for PES; p = 0.007).
The DIABETES (Diabetes and Sirolimus-Eluting Stent) trial [8585. Sabaté M, Jiménez-Quevedo P, Angiolillo DJ, Gómez-Hospital JA, Alfonso F, Hernández-Antolín R, Goicolea J, Bañuelos C, Escaned J, Moreno R, Fernández C, Fernández-Avilés F, Macaya C; DIABETES Investigators. Randomized comparison of sirolimus-eluting stent versus standard stent for percutaneous coronary revascularization in diabetic patients: the diabetes and sirolimus-eluting stent (DIABETES) trial. Circulation. 2005;112:2175-83.
The first trial specifically designed for patients with diabetes aimed at assessing the efficacy of the sirolimus-eluting stent versus a bare metal stent platform] was the first randomised multicentre controlled trial specifically designed to assess the efficacy of SES vs. BMS in diabetics. This study included 160 diabetic patients, 80 of whom received BMS, while 80 were treated with SES. Late lumen loss assessed by QCA at 9-month follow-up was the primary endpoint. The SES treated group showed a significant reduction of late lumen loss (relative reduction 87%). The study considered a sub-randomisation according to the type of anti-diabetic treatment and the SES benefit was independent from diabetic status. This benefit was maintained up to 5-year follow-up [8686. Jiménez-Quevedo P, Hernando L, Gómez-Hospital JA, Iñiguez A, Sanroman A, Alfonso F, Hernández-Antolín R, Angiolillo DJ, Bañuelos C, Escaned J, Gonzalo N, Fernández C, Macaya C, Sabaté M. Sirolimus-eluting stent versus bare metal stent in diabetic patients: the final five-year follow-up of the DIABETES trial. EuroIntervention. 2013 Jul 22;9(3):328-35. ]. Subsequently, 3 other randomised trials also designed for diabetic patients (SCORPIUS [8787. Baumgart D, Klauss V, Baer F, Hartmann F, Drexler H, Motz W, Klues H, Hofmann S, Völker W, Pfannebecker T, Stoll HP, Nickenig G; SCORPIUS Study Investigators. One-year results of the SCORPIUS study: a German multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patients. J Am Coll Cardiol. 2007;50:1627-34. ], DESSERT [8888. Maresta A, Varani E, Balducelli M, Varbella F, Lettieri C, Uguccioni L, Sangiorgio P, Zoccai GB; DESSERT Investigators. Comparison of effectiveness and safety of sirolimus-eluting stents versus bare-metal stents in patients with diabetes mellitus (from the Italian Multicenter Randomized DESSERT Study). Am J Cardiol. 2008;101:1560-6. ] and DECODE [8989. Chan C, Zambahari R, Kaul U, Lau CP, Whitworth H, Cohen S, Buchbinder M; DECODE Investigators. A randomized comparison of sirolimus-eluting versus bare metal stents in the treatment of diabetic patients with native coronary artery lesions: the DECODE study. Catheter Cardiovasc Interv. 2008;72:591-600. ]) have corroborated the same positive results of SES in reducing neointimal proliferation ( Table 3 ).
A recent meta-analysis of all available data in diabetics treated with PCI [9090. Stettler C, Allemann S, Wandel S, Kastrati A, Morice MC, Schömig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabaté M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, De Carlo M, Erglis A, Chechi T, Ortolani P, Schalij MJ, Diem P, Meier B, Windecker S, Jüni P. Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis. BMJ. 2008;29:337:a1331.
Meta-analysis aimed at comparing the outcomes between drug-eluting stents and bare metal stents in diabetics and in non-diabetics] demonstrated the benefit of DES in terms of restenosis and target lesion revascularisation.
Finally, other studies compared both DES against each other in terms of efficacy ( Table 4 ). The SIRTAX (SIRolimus versus pacliTAXel-eluting stents) trial [9191. Windecker S, Remondino A, Eberli FR, Jüni P, Räber L, Wenaweser P, Togni M, Billinger M, Tüller D, Seiler C, Roffi M, Corti R, Sütsch G, Maier W, Lüscher T,Hess OM, Egger M, Meier B. Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N Engl J Med. 2005;353:653-62. ], was a single-centre, controlled, single-blind trial comparing SES with PES in 1,012 patients undergoing PCI. The primary endpoint was a composite of MACE (death from cardiac causes, myocardial infarction, and ischaemia-driven revascularisation of the target lesion) at 9 months. MACE rate was reduced in the SES group as compared to the PES group. This difference was more pronounced in diabetics, analysed as a subgroup. The ISAR (In-Stent Angiographic Restenosis)-DIABETES trial [9292. Dibra A, Kastrati A, Mehilli J, Pache J, Schühlen H, von Beckerath N, Ulm K, Wessely R, Dirschinger J, Schömig A. Paclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patients. ISAR-DIABETES Study N Engl J Med. 2005;353:663-70. ], was a prospective non-inferiority trial which included 250 diabetic patients who received SES (n = 150) or PES (n = 150). The use of SES in diabetics was associated with a decrease in late lumen loss. However, the trial was not powered to detect a reduction in clinical restenosis (i.e., target lesion revascularisation).
The efficacy of new generation DES has also been evaluated. The everolimus-eluting stent (EES) has been tested against PES in the SPIRIT V diabetic randomized controlled trial [9393. Grube E, Chevalier B, Guagliumi G, Smits PC, Stuteville M, Dorange C, Papeleu P, Kaul U, Džavík V. The SPIRIT V diabetic study: a randomized clinical evaluation of the XIENCE V everolimus-eluting stent vs the TAXUS Liberté paclitaxel-eluting stent in diabetic patients with de novo coronary artery lesions. Am Heart J. 2012 May;163(5):867-875. ] and against SES in the ESSENCE DIABETES [9494. Kim WJ, Lee SW, Park SW, Kim YH, Yun SC, Lee JY, Park DW, Kang SJ, Lee CW, Lee JH, Choi SW, Seong IW, Lee BK, Lee NH, Cho YH, Shin WY, Lee SJ, Lee SW, Hyon MS, Bang DW, Park WJ, Kim HS, Chae JK, Lee K, Park HK, Park CB, Lee SG, Kim MK, Park KH, Choi YJ, Cheong SS, Yang TH, Jang JS, Her SH, Park SJ; ESSENCE-DIABETES Study Investigators. "Randomized comparison of everolimus-eluting stent versus sirolimus-eluting stent implantation for de novo coronary artery disease in patients with diabetes mellitus (ESSENCE-DIABETES): results from the ESSENCE-DIABETES trial". Circulation. 2011 Aug 23;124(8):886-92. ]. EES was superior to PES for in-stent late loss at 9 months (0.19 mm vs 0.39 mm, respectively; P (superiority) = 0.0001 and non-inferior to SES for in-segment late loss at 8 months. (0.23 ± 0.27 versus 0.37 ± 0.52 mm; P (non-inferiority) <0.001). Recently, the TUXEDO-INDIA trial randomised 1830 patients to either EES (n=916) or PES (n=914). The primary endpoint, target vessel failure at 1 year, was significantly reduced by EES (5.9% vs. 3.2%; psup=0.005) mainly driven by a reduction in target vessel myocardial infarction and ischaemia driven-target vessel revascularisation whereas cardiac death rate was comparable between groups [9595. Kaul U, Bangalore S, Seth A, Arambam P, Abhaychand RK, Patel TM, Banker D, Abhyankar A, Mullasari AS, Shah S, Jain R, Kumar PR, Bahuleyan CG; TUXEDO–India Investigators. Paclitaxel-Eluting versus Everolimus-Eluting Coronary Stents in Diabetes. N Engl J Med. 2015;373:1709-19. ]. At 2 years, treatment with EES had a lower rate of TVF (4.3% vs. 6.6%, p=0.03). Of the secondary endpoints, EES significantly reduced any MI (1.6% vs. 3.5%, p=0.01), TV-MI (0.7% vs. 3.1%, p=0.0001), ST (0.4% vs. 2.2%, p=0.001), cardiac death or target vessel MI (2.9% vs. 4.8%, p=0.04) and TLR (1.9% vs. 3.7%, p=0.02), compared with PES [9696. Kaul U, Bhagwat A, Pinto B, Goel PK, Jagtap P, Sathe S, Wander GS, Arambam P, Bangalore S. Paclitaxel-eluting stents versus everolimus-eluting coronary stents in a diabetic population: two-year follow-up of the TUXEDO-India trial. EuroIntervention. 2017 Nov 20;13(10):1194-1201. ].
The efficacy of the zotarolimus-eluting stent (ZES) has been assessed in the Endeavor IV trial against PES [9797. Leon MB, Mauri L, Popma JJ, Cutlip DE, Nikolsky E, O’Shaughnessy C, Overlie PA, McLaurin BT, Solomon SL, Douglas JS Jr, Ball MW, Caputo RP, Jain A, Tolleson TR, Reen BM 3rd, Kirtane AJ, Fitzgerald PJ, Thompson K, Kandzari DE; ENDEAVOR IV Investigators. A randomized comparison of the ENDEAVOR zotarolimus-eluting stent versus the TAXUS paclitaxel-eluting stent in de novo native coronary lesions 12-month outcomes from the ENDEAVOR IV trial. J Am Coll Cardiol. 2010;55:543-54. ]. The cohort of diabetic patients (n=477) demonstrated a trend towards higher in-stent late loss with ZES as compared to PES, but with comparable clinical outcomes at 1-year follow-up. The Resolute™ stent (Medtronic, Minneapolis, MN, USA), a new generation ZES, has been tested against EES in a multicentre randomised non-inferiority trial [9898. Serruys PW, Silber S, Garg S, van Geuns RJ, Richardt G, Buszman PE, Kelbaek H, van Boven AJ, Hofma SH, Linke A, Klauss V, Wijns W, Macaya C, Garot P, DiMario C, Manoharan G, Kornowski R, Ischinger T, Bartorelli A, Ronden J, Bressers M, Gobbens P, Negoita M, van Leeuwen F, Windecker S. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med. 2010;363:136-46. ]. The primary endpoint was target-lesion failure within 12 months and ZES was demonstrated to be non-inferior to EES. Among the 2,292 patients included, 538 were diabetics. In this subgroup of patients non-inferiority of ZES was also achieved (odds ratio 1.45 [0.82, 2.58] p=0.25).
The biolimus-eluting stent (BES) has been compared to SES in the LEADERS all-comer trial. BES appeared to be non-inferior to SES with regard to the primary endpoint (composite of cardiac death, MI, or clinically-indicated TVR within 9 months). This trial randomised 1,707 patients. Among them, 223 patients allocated to BES and 191 patients allocated to SES were diabetics. The primary endpoint was also comparable in this subgroup of patients (HR: 1.03 [0.61-1.75]) [9999. Windecker S, Serruys PW, Wandel S, Buszman P, Trznadel S, Linke A, Lenk K, Ischinger T, Klauss V, Eberli F, Corti R, Wijns W, Morice MC, di Mario C, Davies S, van Geuns RJ, Eerdmans P, van Es GA, Meier B, Jüni P. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008;372:1163-73. ].
Finally, the amphilimus-eluting stent (AES), a reservoir-based polymer-free stent,,was compared against EES in the RESERVOIR trial [100100. Romaguera R, Brugaletta S, Gomez-Lara J, Pinar E, Jiménez-Quevedo P, Gracida M, Roura G, Ferreiro JL, Teruel L, Gómez-Hospital JA, Montanya E, Alfonso F, Valgimigli M, Sabate M, Cequier A. Rationale and study design of the RESERVOIR trial: a randomized trial comparing reservoir-based polymer-free amphilimus-eluting stents versus everolimus-eluting stents with durable polymer in patients with diabetes mellitus. Catheter Cardiovasc Interv. 2015;85:E116-22. ]. The primary endpoint, which was neointimal volume obstruction as assessed by optical coherence tomography at 9-month follow-up, was comparable between AES and EES (11.9% vs 16.1%, respectively; p
non-inferiority=0.0003) [101101. Romaguera R, Gómez-Hospital JA, Gomez-Lara J, Brugaletta S, Pinar E, Jiménez-Quededo P, Gracida M, Roura G, Ferreiro JL, Teruel L, Montanya E, Fernández-Ortiz A, Alfonso F, Valgimigli M, Sabate M, Cequier A. A Randomized Comparison of Reservoir-Based Polymer-Free Amphilimus-Eluting Stents Versus Everolimus-Eluting Stents With Durable Polymer in Patients With Diabetes Mellitus: The RESERVOIR Clinical Trial. J Am Coll Cardiol Intv. 2016;9:42-50. ]. A multicenter randomised trial powered for clinically-oriented endpoint (target vessel failure at 1 year) is currently ongoing ( Second-generation drUg-elutinG Stents in diAbetes: a Randomized Trial: the SUGAR trial- NCT03321032).
The effectiveness of different DES platforms has been addressed in the Swedish Angiography and Angioplasty Registry (SCAAR) [102102. Fröbert O, Lagerqvist B, Carlsson J, Lindbäck J, Stenestrand U, James SK. Differences in Restenosis Rate With Different Drug-Eluting Stents in Patients With and Without Diabetes Mellitus: A Report From the SCAAR (Swedish Angiography and Angioplasty Registry). J Am Coll Cardiol. 2009;53:1660-7. ]. Data on restenosis from 2004 and 2008 was collected. Four DES types qualified for inclusion. In total, 35,478 DES were implanted at 22,962 procedures in 19,004 patients and 1,807 restenoses were reported over a mean 29-month follow-up. In the entire population, the restenosis rate per stent was 3.5% after 1 year and 4.9% after 2 years. The adjusted risk of restenosis was higher in patients with DM compared with that in patients without DM (relative risk [RR]: 1.23, 95% confidence interval [CI]: 1.10 to 1.37). In patients with DM, restenosis was twice as frequent with the ZES stent compared with that in SES and PES types.
Safety of DES in diabetics
Safety of DES mainly refers to the incidence of ST, MI or death during follow-up. Diabetes has been identified as an independent predictor of ST in many registries with the use of first generation DES (SES and PES) [1010. Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126-30. , 103103. Urban P, Gershlick AH, Guagliumi G, Guyon P, Lotan C, Schofer J, Seth A, Sousa JE, Wijns W, Berge C, Deme M, Stoll HP, Investigators; e-Cypher investigators. Safety of coronary sirolimus-eluting stents in daily clinical practice: one-year follow-up of the e-Cypher registry. Circulation. 2006;113:1434-41. ]. In a large multicentre registry [103103. Urban P, Gershlick AH, Guagliumi G, Guyon P, Lotan C, Schofer J, Seth A, Sousa JE, Wijns W, Berge C, Deme M, Stoll HP, Investigators; e-Cypher investigators. Safety of coronary sirolimus-eluting stents in daily clinical practice: one-year follow-up of the e-Cypher registry. Circulation. 2006;113:1434-41. ] of more than 15,000 patients treated with SES, the overall incidence of stent thrombosis at 1 year was 0.87% and the most potent independent predictor of thrombosis was the insulin-dependent DM. Diabetic patients, as mentioned previously, exhibit specific pathophysiological factors as well as unfavourable angiographic parameters which confer upon them an especially high risk for thrombosis ( Figure 1).
Recent network collaborative meta-analyses of main randomised controlled trials demonstrated the safe profile of first generation DES (SES and PES) in diabetic patients (n=3,239) at 4-year follow-up [9090. Stettler C, Allemann S, Wandel S, Kastrati A, Morice MC, Schömig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabaté M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, De Carlo M, Erglis A, Chechi T, Ortolani P, Schalij MJ, Diem P, Meier B, Windecker S, Jüni P. Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis. BMJ. 2008;29:337:a1331.
Meta-analysis aimed at comparing the outcomes between drug-eluting stents and bare metal stents in diabetics and in non-diabetics] as compared to BMS. Similar results were observed in a meta-analysis of individual patient data from four randomised trials reporting on SES in diabetics [104104. de Waha A, Dibra A, Kufner S, Baumgart D, Sabaté M, Maresta A, Schömig A, Kastrati A. Long-term outcome after sirolimus-eluting stents versus bare metal stents in patients with Diabetes mellitus: a patient-level meta-analysis of randomized trials. Clin Res Cardiol. 2011;100:561-70. ]. This meta-analysis included 583 patients (SES vs. BMS; median follow-up of 4.2 years). There was a significant reduction in the overall hazard of MACE (hazard ratio, [HR] 0.48, 95% confidence interval [CI] 0.36-0.63, P<0.001) with SES. The overall hazard of death (HR 0.91, 95% CI 0.59-1.41, P=0.68) as well as death or MI (HR 0.77, 95% CI 0.54-1.09, P=0.14) were not significantly different between the groups. No significant differences were observed regarding ST (HR 0.50, 95% CI 0.15-1.69, P=0.26) [104104. de Waha A, Dibra A, Kufner S, Baumgart D, Sabaté M, Maresta A, Schömig A, Kastrati A. Long-term outcome after sirolimus-eluting stents versus bare metal stents in patients with Diabetes mellitus: a patient-level meta-analysis of randomized trials. Clin Res Cardiol. 2011;100:561-70. ].
Reassuring data also comes from the Massachusetts Data Analysis Registry which included 6,008 diabetics treated between April 2003 and September 2004. After propensity score-matched risk analysis, the use of DES was associated with a significantly lower rate of death, MI and TVR [105105. Garg P, Normand SL, Silbaugh TS, Wolf RE, Zelevinsky K, Lovett A, Varma MR, Zhou Z, Mauri L. Drug-eluting or bare-metal stenting in patients with diabetes mellitus: results from the Massachusetts Data Analysis Center Registry. Circulation. 2008;118:2277-92.
Clinical results of a large registry comparing drug-eluting stents and bare metal stents in patients with diabetes mellitus].
New generation EES stent showed a safety benefit as compared to PES in the Spirit V- diabetic randomised trial [9393. Grube E, Chevalier B, Guagliumi G, Smits PC, Stuteville M, Dorange C, Papeleu P, Kaul U, Džavík V. The SPIRIT V diabetic study: a randomized clinical evaluation of the XIENCE V everolimus-eluting stent vs the TAXUS Liberté paclitaxel-eluting stent in diabetic patients with de novo coronary artery lesions. Am Heart J. 2012 May;163(5):867-875. ] (Grube E; EuroPCR 2010). At 1 year, the composite of death and MI was reduced by EES (9.6% vs. 3.7%; p=0.04) as well as the thrombosis rate (1.9% vs. 0%; p=ns). In the TUXEDO-INDIA trial, definite or probable stent thrombosis was significantly reduced at 12 month in the EES arm (0.5% vs. 2.2% in the PES arm; p=0.001) [9595. Kaul U, Bangalore S, Seth A, Arambam P, Abhaychand RK, Patel TM, Banker D, Abhyankar A, Mullasari AS, Shah S, Jain R, Kumar PR, Bahuleyan CG; TUXEDO–India Investigators. Paclitaxel-Eluting versus Everolimus-Eluting Coronary Stents in Diabetes. N Engl J Med. 2015;373:1709-19. ]. Data concerning safety of BES in diabetics comes from a sub-study from the LEADERS trial [106106. Linke A, Lenk K, Serruys PW, van Es GA, Buszman P, Ischinger T, Klauss V, Eberli F, Corti R, Wijns W, Morice MC, di Mario C, Juni P, Schuler G, Windecker S. Impact of Diabetes on Angiographic And Clinical Outcomes of Revascularization With Biolimus-eluting Stent With Biodegradable Polymer and Sirolimus-eluting Stent With Durable Polymer - LEADERS Trial Substudy. Am J Cardiol. 2009;104:S138D. ]. Amongst insulin-dependent diabetics, the rate of all-cause death and cardiac death was 0% after BES implantation as compared to 9.1% and 6.5% respectively, after SES implantation at 12 months follow-up (p<0.01). Finally, the Resolute™ stent showed a higher incidence of definite ST at 1-year follow-up as compared to EES (1.2% vs. 0.3%; <0.01) in the all-comer RESOLUTE trial [9898. Serruys PW, Silber S, Garg S, van Geuns RJ, Richardt G, Buszman PE, Kelbaek H, van Boven AJ, Hofma SH, Linke A, Klauss V, Wijns W, Macaya C, Garot P, DiMario C, Manoharan G, Kornowski R, Ischinger T, Bartorelli A, Ronden J, Bressers M, Gobbens P, Negoita M, van Leeuwen F, Windecker S. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med. 2010;363:136-46. ]. Data on diabetics has not yet been published.
Bioresorbable vacular scaffolds in diabetic patients
Bioresorbable vascular scaffolds (BVSs) are a novel approach to the treatment of coronary artery disease. They are able to provide transient vessel support and drug delivery to the vessel wall. As diabetics often present with diffuse disease, scaffolding of coronary arteries are an appealing therapeutic option. To date, only few reports have tested the feasibility of BVS in patients with diabetes mellitus. Outcomes of 136 diabetic patients from ABSORB cohort B and ABSORB Extend trials were compared with 882 diabetic patients treated with EES from SPIRIT First, II, III and IV trials [107107. Muramatsu T, Onuma Y, van Geuns RJ, Chevalier B, Patel TM, Seth A, Diletti R, García-García HM, Dorange CC, Veldhof S, Cheong WF, Ozaki Y, Whitbourn R, Bartorelli A, Stone GW, Abizaid A, Serruys PW; ABSORB Cohort B Investigators; ABSORB EXTEND Investigators; SPIRIT FIRST Investigators; SPIRIT II Investigators; SPIRIT III Investigators; SPIRIT IV Investigators. 1-year clinical outcomes of diabetic patients treated with everolimus-eluting bioresorbable vascular scaffolds: a pooled analysis of the ABSORB and the SPIRIT trials. JACC Cardiovasc Interv. 2014;7:482-93. ]. At 1 year, in a propensity matched population (n=102 treated with BVS vs n=172 treated with EES), outcomes were similar between groups in terms of device-oriented endpoint (3.9% in the BVS arm vs 6.4% in the EES arm; p=0.38) and definite or probable device thrombosis rate (1.0% in the BVS arm vs 1.7% in the EES arm; p=1.0) [142142. Klein R, Klein BEK, Moss SE, DeMets D, Davis MD. Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA. 1988;260: 2864-71. ]. Another registry of 120 diabetic patients treated with BVS showed a MACE rate of 8.4% and a scaffold thrombosis rate of 2.7% at 6-month follow-up. [108108. Wiebe J, Gilbert F, Dörr O, Liebetrau C, Wilkens E, Bauer T, Elsässer A, Möllmann H, Hamm CW, Nef HM. Implantation of everolimus-eluting bioresorbable scaffolds in a diabetic all-comers population. Catheter Cardiovasc Interv. 2015;86:975-81. ] In 2017, the Absorb-BVS was restricted to be used only in centers already participating in clinical trials and registries and ultimately, it was removed from the market.
PCI vs. coronary artery bypass grafting (CABG)
Currently, CABG is the treatment of choice for diabetic patients with multivessel disease [7171. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019 Jan 7;40(2):87-165.
Recent guidelines on coronary revascularisation. These were elaborated under the auspices of both the European Society of Cardiology and the European Association of Cardio-Thoracic Surgery]. Historically, numerous trials have been designed to evaluate the efficacy of PCI versus CABG. We will summarise the main results of these trials using a chronological perspective.
Trials comparing CABG and BA ( Table 5 )
Four trials designed to compare the efficacy of CABG versus BA have reported data on the subgroup of patients with diabetes mellitus.
The EAST study (Emory Angioplasty versus Surgery Trial) was a single-centre randomised comparison of a strategy of initial BA (n =198; 24.7% DM) or CABG (n=194; 21.2% DM) for patients with multivessel disease. The 8-year survival was 79.3% in the BA group and 82.7% in the surgical group (p =0.40); however, survival tended to be greater in diabetic patients who underwent CABG (75.5%) compared with those who underwent PTCA (60.1%; p = 0.23) [109109. King SB 3rd, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eight-year mortality in the Emory Angioplasty versus Surgery Trial (EAST) J Am Coll Cardiol. 2000;35:1116-21. ].
The BARI study (Bypass Angioplasty Revascularization Investigation Study) was a trial designed to compare long-term survival in patients with multivessel disease randomised to BA or CABG. Overall, the 10-year survival was 71.0% for BA and 73.5% for CABG (p = 0.18). Although the BA group had substantially higher subsequent revascularisation rates than the CABG group (76.8% vs. 20.3%, p < 0.001), angina rates for the 2 groups were similar. In the subgroup of patients with diabetes, CABG offered a higher survival rate than BA (CABG 57.8%, BA 45.5%; p = 0.025) [110110. BARI Investigators. The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol. 2007;49:1600-6. ].
The CABRI trial (Coronary Angioplasty versus Bypass Revascularization Investigation) randomised 1,054 patients in Europe. At five years, survival rates were similar between CABG or BA. However, in the diabetic subgroup, mortality was lower (although not significantly so) in the CABG arm (12.5% vs. 22.6% in the BA arm) [111111. Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF. Difference in the mortality of the CABRI diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J Cardiol. 2001;87:947-50. ].
The RITA trial (Randomised Intervention Treatment of Angina Trial) randomised 1,011 patients. At a median of 6.5 years, the predefined primary endpoint of death or non-fatal myocardial infarction was similar in both arms [112112. Henderson RA, Pocock SJ, Sharp SJ, Nanchahal K, Sculpher MJ, Buxton MJ, Hampton JR. Long-term results of RITA-1 trial: clinical and cost comparisons of coronary angioplasty and coronary-artery bypass grafting; Randomised Intervention Treatment of Angina. Lancet. 1998;352:1419-25. ]. Conversely, in the subgroup of diabetic patients, survival showed a trend towards being better with BA.
Trials comparing CABG versus PCI with bare metal stents ( Table 6 )
The ARTS (Arterial Revascularization Therapy Study) trial compared outcomes from bypass surgery versus coronary stenting in patients with multivessel disease. This study reported a reduced event-free survival at 1 year in diabetics treated with stenting as compared with those treated with CABG (63.4% vs. 84.4%, p = 0.001) [113113. Abizaid A, Costa MA, Centemero M, Abizaid AS, Legrand VM, Limet RV, Schuler G, Mohr FW, Lindeboom W, Sousa AG, Sousa JE, van Hout B, Hugenholtz PG, Unger F, Serruys PW; Arterial Revascularization Therapy Study Group. Clinical and economic impact of diabetes mellitus on percutaneous and surgical treatment of multivessel coronary disease patients: insights from the Arterial Revascularization Therapy Study (ARTS) trial. Circulation. 2001;104:533-8. ]. This difference was largely due to a significantly lower rate of complete revascularisation in patients who underwent PCI (70.5%) compared with those who had CABG (84.1%). Conversely, diabetic and non-diabetic patients experienced similar 1-year event-free survival rates when treated with CABG (84.4% and 88.4%). At 5 years, the mortality rate of diabetic patients was greater in the stent group (13.4%) compared with that of the surgical group (8.3%; RR 1.61, 95% CI 0.71 to 3.63). Within the stent group, the mortality of diabetic patients remained higher than that of non-diabetic patients (13.4% vs. 6.8%; p = 0.03) whereas, in the surgical group, mortality rate was comparable given the presence or absence of diabetes (8.3% in diabetics vs. 7.5% in non-diabetics; p = 0.8).
The AWESOME trial (Angina With Extremely Serious Operative Mortality Evaluation Trial) randomised 454 patients with multivessel disease to either CABG or stenting. Among diabetics, the respective CABG and PCI 36-month survival rates were comparable (72% for CABG vs. 81% for PCI) [114114. Sedlis SP, Morrison DA, Lorin JD, Esposito R, Sethi G, Sacks J, Henderson W, Grover F, Ramanathan KB, Weiman D, Saucedo J, Antakli T, Paramesh V, Pett S,Vernon S, Birjiniuk V, Welt F, Krucoff M, Wolfe W, Lucke JC, Mediratta S, Booth D, Murphy E, Ward H, Miller L, Kiesz S, Barbiere C, Lewis D; Investigators of the Dept of Veterans Affairs Cooperative Study #385, the Angina With Extremely Serious Operative Mortality Evaluation (AWESOME). Percutaneous coronary intervention versus coronary bypass graft surgery for diabetic patients with unstable angina and risk factors for adverse outcomes with bypass: outcome of diabetic patients in the AWESOME randomized trial and registry. J Am Coll Cardiol. 2002;40:1555-66. ].
The SOS (Stent Or Surgery) trial showed less repeat revascularisation with CABG than with PCI among the whole cohort at 2 years, but the diabetic subgroup was not analysed separately [115115. Sigwart U, Stables RH, Booth J, et al. Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): a randomised controlled trial. Lancet. 2002;360:965-70. ].
In the ERACI II trial (ArgentinE RAndomized Study: Coronary Angioplasty with Stenting vs. Coronary Bypass Surgery In Patients with Multiple Vessel Disease) [116116. Rodriguez AE, Baldi J, Fernández Pereira C, Navia J, Rodriguez Alemparte M, Delacasa A, Vigo F, Vogel D, O’Neill W, Palacios IF. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II); ERACI II Investigators. J Am Coll Cardiol. 2005;46:589-91. ] PCI-treated subjects had an increased incidence of repeat revascularisation at follow-up compared with CABG. However, a separated analysis of diabetic subjects was not performed.
In summary, the systematic use of stents reduced the need for subsequent revascularisation following PCI as compared to the era of plain BA. However, it still remained significantly higher than that seen after CABG in the diabetic population with multivessel disease. Moreover, the overall mortality in diabetics may be higher at long-term follow-up with the use of stents as compared to CABG. Thus, we have to consider that in the BMS era, revascularisation of multivessel diseased diabetics is still a niche for CABG unless major comorbidities preclude this kind of treatment.
Trials comparing CABG and DES
The main data available in the current era of DES come from multicentre registries: the ERACI-3 and the ARTS 2 registries. Both registries compared a current cohort of patients with multivessel disease treated with drug-eluting stents with the cohort of patients from the historical trial (ERACI 2 and ARTS 1 trial respectively) treated with either CABG or conventional BM stenting.
The ARTS 2 registry demonstrated a comparable event-free survival rate to that of the historical CABG arm (80.6% versus 83.8% respectively) and better than the stent arm of the ARTS-1 (66%) at 3-year follow-up. In diabetic patients, MACCE rate was comparable at 1 year. However, recent data at 3 years [117117. Daemen J, Kuck KH, Macaya C, LeGrand V, Vrolix M, Carrie D, Sheiban I, Suttorp MJ, Vranckx P, Rademaker T, Goedhart D, Schuijer M, Wittebols K, Macours N, Stoll HP, Serruys PW; ARTS-II Investigators. Multivessel coronary revascularization in patients with and without diabetes mellitus: 3-year follow-up of the ARTS-II (Arterial Revascularization Therapies Study-Part II) trial. J Am Coll Cardiol. 2008;52:1957-67. ] evidenced a late increase rate of repeat revascularisation in the drug-eluting stent arm that led to a significant increase in the major adverse cardiovascular and cerebrovascular event (MACCE) rate as compared to the historical CABG arm of the ARTS-1 trial (27.7% vs. 17.7%).
In the ERACI-3 registry [118118. Rodriguez AE, Maree AO, Mieres J, Berrocal D, Grinfeld L, Fernandez-Pereira C, Curotto V, Rodriguez-Granillo A, O’neill W, Palacios IF. Late loss of early benefit from drug-eluting stents when compared with bare-metal stents and coronary artery bypass surgery: 3 years follow-up of the ERACI III registry. Eur Heart J. 2007;17:2118-25. ], MACCE rates at 3 years were similar in drug-eluting stent and CABG-treated patients (22.7%, p=1.0), in contrast to results at 1 year which favoured DES (12% vs. 19.6%, p=0.038). When stratified by treatment modality, MACCE rates among diabetics at 3 years were 36.2% in the DES arm, 43.6% in the BMS arm, and 30.8% in the CABG group (p = 0.49). Of the components of MACCE, TVR was the only one that differed significantly across the three groups: drug-eluting stent (21.3%), bare metal stent (38.5%), and CABG (15.4%); p=0.048. There was a non-significant trend towards more death and non-fatal MI among diabetics in the ERACI-3 drug-eluting stent cohort (19.1%) than in the bare metal stent (12.8%) or CABG (15.4%) arms of ERACI-2.
Another registry [119119. Briguori C, Condorelli C, Airoldi F, Focaccio A, D’Andrea D, Cannavale M, Abarghouei AA, Giordano S, De Vivo F, Ricciardelli B, Colombo A. Comparison of Coronary Drug-Eluting Stents Versus Coronary Artery Bypass Grafting in Patients With Diabetes Mellitus. Am J Cardiol. 2007;99:779-84. ] compared DES implantation with off-pump CABG. This study addresses the effect of DES versus off-pump CABG on 1-year outcome of diabetic patients with multivessel disease and critical stenosis involving the proximal left anterior descending coronary artery who underwent elective myocardial revascularisation. After propensity score analysis, adjusting for baseline differences between the 2 cohorts, DES increased the risk of 12-month MACCE (HR 1.88, 95% CI, p = 0.020). This was due to the higher rate for repeat revascularisation in the DES group (19% vs. 5%, HR 2.05, 95% CI, p = 0.001). In contrast, there was no difference in the rate of the composite endpoints of death, MI, and stroke (DES group 13%, CABG group 12%; adjusted analysis, HR 0.80, 95% CI, p = 0.40).
Data on most important randomized trials ( Table 7) comparing DES and CABG is now complete. The FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease)Trial of 1900 patients with multivessel disease (triple vessel disease in 87%) demonstrated a significant reduction on the primary outcome of death, nonfatal myocardial infarction, and nonfatal stroke at 5 years in patients treated with CABG versus PCI (18.7% versus 26.6% P=0.005) [120120. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S, 3rd, Bertrand M, Fuster V, Investigators FT. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375-84.
This is the largest trial comparing CABG and first generation DES in diabetics]. This was primarily driven by a reduction in the rates of myocardial infarction and all cause death (P=0.049) with a higher rate of stroke in the CABG group (5 year rates of 5.2% versus 2.4 % P=0.03). The CARDIA trial (Coronary Artery Revascularisation in Diabetes) [121121. Kapur A, Hall RJ, Malik IS, Qureshi AC, Butts J, de Belder M, Baumbach A, Angelini G, de Belder A, Oldroyd KG, Flather M, Roughton M, Nihoyannopoulos P, Bagger JP, Morgan K, Beatt KJ. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients; 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol. 2010;55:432-40. ] is a non-inferiority trial comparing optimal PCI with modern CABG as a revascularisation strategy for patients with diabetes who have multivessel or complex single-vessel coronary disease. The primary endpoint is a composite of death, non-fatal MI, and cerebrovascular accident at 1 year, with long-term follow-up (3 to 5 years). At 1 year, the primary endpoint (composite of death, non-fatal MI and non-fatal stroke) was comparable between arms (10.5% in CABG vs. 13.0% in PCI arm; p=0.39). However, further revascularisation was significantly higher in the PCI arm (2% vs. 11.8%; p<0.001). The SYNTAX (Synergy between percutaneous coronary intervention with TAXus and cardiac surgery) trial randomly allocated 1,800 patients with left main and/or 3-vessel coronary artery disease to PES implantation or CABG. In the subgroup of patients with DM (n=452), 5-year rates were significantly higher for PCI vs CABG for MACCE (PCI: 46.5% vs CABG: 29.0%; P < 0.001) and repeat revascularization (PCI: 35.3% vs CABG: 14.6%; P < 0.001). There was no difference in the composite of all-cause death/stroke/MI (PCI: 23.9% vs CABG: 19.1%; P = 0.26) or individual components all-cause death (PCI: 19.5% vs CABG: 12.9%; P = 0.065), stroke (PCI: 3.0% vs CABG: 4.7%; P = 0.34) or MI (PCI: 9.0% vs CABG: 5.4%; P = 0.20) [122122. Kappetein AP, Head SJ, Morice MC, Banning AP, Serruys PW, Mohr FW, Dawkins KD, Mack MJ; SYNTAX Investigators. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg. 2013 May;43(5):1006-13. ]. Another randomized trial specifically designed in diabetic patients was prematurely stopped due to low recruitment rate [123123. Kamalesh M, Sharp TG, Tang XC, Shunk K, Ward HB, Walsh J, King S III, Colling C, Moritz T, Stroupe K, Reda D; CARDS Investigators VA. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol. 2013;61:808–816.
]] With less than 200 patients included the VA-CARDS (Veterans Affairs-Coronary Artery Revascularization in Diabetes) trial had no power to demonstrate differences in death or MI between groups.
In the Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease (BEST) trial the subgroup of patients with diabetes treated with PCI with second generation DES had a higher rate of the primary endpoint of death, MI, or TVR compared with CABG (EES: n=177; CABG: n=186) (19.2 vs. 9.1%, P = 0.007) [124124. Park SJ, Ahn JM, Kim YH, Park DW, Yun SC, Lee JY, Kang SJ, Lee SW, Lee CW, Park SW, Choo SJ, Chung CH, Lee JW, Cohen DJ, Yeung AC, Hur SH, Seung KB, Ahn TH, Kwon HM, Lim DS, Rha SW, Jeong MH, Lee BK, Tresukosol D, Fu GS, Ong TK; BEST Trial Investigators. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med. 2015;372:1204–1212. ]
Besides randomised controlled trials, registry data, such as the New York registry [125125. Hannan EL, Wu C, Walford G, Culliford AT, Gold JP, Smith CR, Higgins RS, Carlson RE, Jones RH. Drug-eluting stents vs. coronary-artery bypass grafting in multivessel coronary disease. N Engl J Med. 2008;358:331-41.
A large registry comparing clinical results of coronary artery bypass grafting and drug-eluting stents in patients with multivessel disease], showed a trend towards improved outcomes in diabetic patients treated with CABG compared with DES (OR for death or MI at 18 months 0.84, 95% CI 0.69–1.01; p=0.07).
The BARI 2 Diabetes (BARI 2D) [126126. BARI 2D Study Group, Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503-15. ] is a randomised, open, controlled, multicentre trial that compared optimal medical management with prompt revascularisation (PCI or CABG) in patients with type 2 DM and stable coronary disease. The primary endpoint was death from any cause. At 5-year follow-up, survival rate was comparable between groups (88%) with no difference in MACE or death. Patients treated with CABG showed much greater atherosclerotic burden and more lesions than the PCI stratum. Prompt revascularisation significantly reduced the MACE rate in those patients treated with CABG largely because of a reduction in MI events, but not among those selected to undergo PCI as compared to optimal medical treatment. However, up to 42% of the patients allocated to optimal medical therapy required coronary revascularisation with PCI during the 5 years of follow-up [126126. BARI 2D Study Group, Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503-15. ].
The decision to use either PCI or CABG as preferred mode of revascularization in diabetics should be based on anatomical factors (Syntax score), together with clinical factors, and other logistic or local factors. As a rule PCI with second generation DES may be recommended in diabetics with single vessel disease. Conversely, CABG should be performed in diabetics with complex multivessel disease but both strategies may be performed always after discussion in a heart team meeting.
CABG in diabetic patients - potential complications
Early morbidity and mortality are higher in diabetic patients undergoing CABG, compared with non-diabetics [127127. Herlitz J, Wognsen GB, Karlson BW, Sjöland H, Karlsson T, Caidahl K, Hartford M, Haglid M. Mortality, mode of death and risk indicators for death during 5 years after coronary artery bypass grafting among patients with and without a history of diabetes mellitus. Coron Artery Dis. 2000;11:339-46. , 128128. Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, Guyton RA. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999;67:1045-52. ]. In terms of postoperative morbidity after CABG, diabetics exhibit a 3.5-fold risk of neurological complications and a 5-fold risk of renal complications; on the other hand, perioperative MI was not increased [128128. Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, Guyton RA. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999;67:1045-52. ]. Following CABG, stroke occurs more commonly in diabetic patients and, at long-term follow-up, quality of life appeared to be particularly reduced in diabetics [127127. Herlitz J, Wognsen GB, Karlson BW, Sjöland H, Karlsson T, Caidahl K, Hartford M, Haglid M. Mortality, mode of death and risk indicators for death during 5 years after coronary artery bypass grafting among patients with and without a history of diabetes mellitus. Coron Artery Dis. 2000;11:339-46. ]. There is also a higher rate of sternal wound infections [129129. Cohen Y, Raz I, Merin G, Mozes B. Comparison of factors associated with 30-day mortality after coronary artery bypass grafting in patients with versus without diabetes melitus - Israely Coronary Artery Bypass (ISCAB) Study Consortium. Am J Cardiol. 1998;81:7-11. ] as well as at artery harvest site [130130. Trick WE, Scheckler WE, Tokars JI, Jones KC, Smith EM, Reppen ML, Jarvis WR. Risk factors for radial artery harvest site infection following coronary artery bypass graft surgery. Clin Infect Dis. 2000;30:270-75. ], due to microvascular complications and impaired healing response typical of this systemic metabolic disease.
The benefit of CABG in diabetic patients is greater in those receiving an internal mammary artery graft [131131. Stevensb LM, Carriera M, Perraulta LP, Hébert Y, Cartiera R, Bouchard D, Fortiera A, Pellerin M. Influence of diabetes and bilateral internal thoracic artery grafts on long-term outcome for multivessel coronary artery bypass grafting. Eur J Cardthorac Surg. 2005;27:281-8. ]. After CABG, diabetic subjects show an increasing burden of vein graft disease. A recent CABG study, however, reported a similarly high 12-month incidence of death or SVG stenosis >75% in diabetic (48.3%) and non-diabetic (44.2%) subjects [132132. Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TBJ, Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D, Califf RM, Kouchoukos NT, Investigators; PI. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446-54. ].
Registry reports indicate that the prevalence of diabetics undergoing PCI with prior CABG is rising. In diabetic patients with recurrent ischaemia, despite surgical revascularisation and maximum medical therapy, repeat CABG presents a much higher risk than PCI. Thus, in this patient group PCI should be preferred.
Current recommendations for revascularisation in diabetic patients
Contemporary guidelines place emphasis on the long-term survival benefit conferred by CABG for treatment of diabetics with multivessel disease. A clinician’s judgement on the revascularisation strategy remains an important factor. Although PCI with DES has narrowed the gap with surgery, in CABG-eligible diabetic patients with multivessel disease, CABG remains the gold standard treatment [7171. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019 Jan 7;40(2):87-165.
Recent guidelines on coronary revascularisation. These were elaborated under the auspices of both the European Society of Cardiology and the European Association of Cardio-Thoracic Surgery].
Revascularisation in diabetic patients
- Percutaneous coronary intervention in DM has significantly improved with the advent of DES
- New generation DES has been demonstrated to be safer than previous generation DES in diabetics
- Despite these advances, diabetics with high-risk multivessel disease may benefit more from CABG than from DES
Specific clinical conditions during revascularisation
Acute coronary syndromes
Primary PCI is the treatment of choice in diabetics presenting with acute myocardial infarction. Cardiac surgery in the setting of ST-elevation myocardial infarction (STEMI) is only indicated when the coronary anatomy is not suitable for a percutaneous intervention [7171. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019 Jan 7;40(2):87-165.
Recent guidelines on coronary revascularisation. These were elaborated under the auspices of both the European Society of Cardiology and the European Association of Cardio-Thoracic Surgery].
Diabetics with unstable angina (UA)/Non-STEMI or STEMI face a much higher risk of mortality within one year of their acute coronary syndrome than non-diabetics [133133. Kishore J, Harjai KJ, Stone GW, Boura J, Mattos L, Chandra H, Cox D, Grines L, O’Neill W, Grines C, Investigators - PAMI. Comparison of outcomes of diabetic and nondiabetic patients undergoing primary angioplasty for acute myocardial infarction. Am J Cardiol. 2003;91:1041-5. , 134134. Donahoe SM, Stewart GC, McCabe CH, Mohanavelu M, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes JAMA. 2007;298:765-75. ]. The risk of dying in the first year after an ACS event for a diabetic patient with UA/Non-STEMI is almost the same as that for a non-diabetic patient with STEMI. This data first became apparent in the analysis comparing diabetics and non-diabetics enrolled in the Primary Angioplasty in Myocardial Infarction (PAMI) studies [133133. Kishore J, Harjai KJ, Stone GW, Boura J, Mattos L, Chandra H, Cox D, Grines L, O’Neill W, Grines C, Investigators - PAMI. Comparison of outcomes of diabetic and nondiabetic patients undergoing primary angioplasty for acute myocardial infarction. Am J Cardiol. 2003;91:1041-5. ]. This analysis set out to determine whether DM independently conferred a poor prognosis in myocardial infarction patients undergoing primary PCI. In-hospital and 6-month mortality rates as well as the 6-month MACE rate of the 626 patients with, and the 3,116 patients without, diabetes were compared. Diabetics had worse baseline clinical characteristics, such as older age and later presentation, and had a higher atherosclerotic burden. Despite similar acute angiographic results of PCI, diabetics had a higher incidence of morbid events (pulmonary oedema, dialysis, sustained hypotension) and mortality (4.6% vs. 2.6%) during hospitalisation. The difference in in-hospital mortality between diabetics and non-diabetics was completely explained by the difference in baseline characteristics. However, diabetes was found to be an independent predictor of 6-month mortality. Furthermore, after adjustment for baseline clinical and angiographic differences, a history of diabetes still remained an independent correlate of 6-month mortality.
More recently Donahoe S.M. and colleagues reported the results of their analysis of 11 Thrombolysis in Myocardial Infarction (TIMI) clinical trials conducted between 1997 and 2006 [134134. Donahoe SM, Stewart GC, McCabe CH, Mohanavelu M, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes JAMA. 2007;298:765-75. ]. This analysis was performed to evaluate the influence of diabetes on mortality following ACS in a robust sample size of diabetic patients (a full 10,613 diabetic patients out of the 62,036 total patients). Mortality at 30 days was significantly higher among patients with diabetes mellitus than non-diabetics presenting with UA/Non-STEMI (2.1% vs. 1.1%, P= 0.001) and STEMI (8.5% vs. 5.4%, P= 0.001). After adjustment, diabetes was independently associated with higher 30-day mortality after UA/Non-STEMI (OR 1.78; 95% CI) or STEMI (HR 1.40; 95% CI). One year after an ACS, diabetic patients presenting with UA/Non-STEMI had a similar risk of death as non-diabetics presenting with STEMI (7.2% vs. 8.1%). Previous observations on the adverse effect of diabetes on UA/ Non-STEMI and STEMI, such as GUSTO-1, OASIS registry, the GRACE multinational registry [135135. Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, Avezum A, Goodman SG, Flather MD, Anderson FAJ, Granger CB. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE) BMJ. 2006;333:1091. ] also identified diabetes mellitus as being a significant contributor to in-hospital and 6-month out-of-hospital mortality. In this regard, diabetes status was included in the TIMI risk scores for both UA/Non-STEMI and STEMI [136136. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835-42. , 137137. Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, Giugliano RP, McCabe CH, Braunwald E. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031-7. ].
Revascularisation in diabetic patients with systemic complications
Diabetes may affect cardiac structure and function in the absence of changes in blood pressure and coronary artery disease, a condition called diabetic cardiomyopathy. Metabolic perturbations in the myocardial cell are the most probable cause of myocardial dysfunction in these patients with diabetes. On the other hand, autonomic neuropathy is a common complication of diabetes, and 20% of asymptomatic diabetic patients have abnormal cardiovascular autonomic function. Besides, atherosclerotic complications may involve coronary arteries as well as other territories, such as carotids, aorta, and femoral arteries. Finally, diabetes provokes microangiopathy, the physiopathological mechanism of retinopathy and nephropathy.
Diabetic nephropathy is a clinical syndrome of albuminuria, declining glomerular filtration rate, hypertension, and an increased cardiovascular risk that is 2 to 4 times higher than in the general population. In general, reduced glomerular filtration rate is associated with worse outcomes in patients undergoing coronary revascularisation, either by CABG or PCI, by increasing MACCE rate and long term mortality [138138. Sajja LR, Mannam G, Chakravarthi RM, Sompalli S, Naidu SK, Somaraju B, Penumatsa RR. Coronary artery bypass grafting with or without cardiopulmonary bypass in patients with preoperative non-dialysis dependent renal insufficiency: a randomized study. J Thorac Cardiovasc Surg. 2007;133:378-88. , 139139. Solomon RJ, Natarajan MK, Doucet S, Sharma SK, Staniloae CS, Katholi RE, Gelormini JL, Labinaz M, Moreyra AE, Study IotC. Cardiac Angiography in Renally Impaired Patients (CARE) study: a randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation. 2007;115:3189-96. ]. In diabetics this association becomes especially true [140140. Mehran R, Nikolsky E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl. 2006;100:S11-15. , 141141. D’Alessandro C, Leprince P, Golmard JL, Ouattara A, Aubert S, Pavie A, Gandjbakhch I, Bonnet N. Strict glycemic control reduces EuroSCORE expected mortality in diabetic patients undergoing myocardial revascularization. J Thorac Cardiovasc Surg. 2007;134:29-37.
A paper outlining the importance of metabolic control in diabetic patients undergoing myocardial revascularisation].
Diabetic retinopathy is a frequent and early sign of microvascular complication of diabetes, and is directly related to the degree and duration of hyperglycaemia [142142. Klein R, Klein BEK, Moss SE, DeMets D, Davis MD. Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA. 1988;260: 2864-71. ]. Retinal examination represents a unique opportunity to visualise directly and to grade the progression of the disease and extent of microangiopathy. Several studies show that the presence of diabetic retinopathy is a predictor of all-cause mortality following CABG and PCI [143143. Ono T, Kobayashi J, Sasako Y, Bando K, Tagurasi O, Niwaya K, Imanaka H, Nakatani T, Kitamura S. The impact of diabetic retinopathy on long-term outcome following coronary artery bypass graft surgery. J Am Coll Cardiol. 2002;40:428-36. , 144144. Kim YH, Hong MK, Song JM, Han KH, Kang DH, Song JK, Kim JJ, Park SW, Park SJ. Diabetic retinopathy as a predictor of late clinical events following percutaneous coronary intervention. J Invas Cardiol. 2002;14:599-602. ]. Ono T and colleagues identified 223 consecutive diabetics with multivessel CAD and different severity of retinopathy prior to CABG, and followed them up to a mean of 11 years [143143. Ono T, Kobayashi J, Sasako Y, Bando K, Tagurasi O, Niwaya K, Imanaka H, Nakatani T, Kitamura S. The impact of diabetic retinopathy on long-term outcome following coronary artery bypass graft surgery. J Am Coll Cardiol. 2002;40:428-36. ]. Diabetic retinopathy was a strong independent predictor of overall mortality and repeat revascularisation. In separate analyses of diabetics with and without retinopathy, predictors of mortality differed significantly between the two groups. Among those with retinopathy, the presence of either preoperative renal (RR, 2.5) or ventricular (RR, 2.0) dysfunction had unfavourable effects on mortality, but the survival curves did not differ significantly according to the presence or absence of internal thoracic artery (ITA) grafting. By comparison, among diabetics without retinopathy, ITA grafting (RR, 0.34) had a beneficial effect on mortality, and the survival curves did vary somewhat according to the presence or absence of renal or ventricular dysfunction. The authors concluded that diabetics with retinopathy had a distinct post-CABG course with a worse long-term prognosis, as compared with diabetics without retinopathy. Retina evaluation, as an index of the presence and burden of microangiopathy, is useful for prediction of long-term prognosis and management of diabetics who require CABG.
In an interesting study from Kim YH et al [144144. Kim YH, Hong MK, Song JM, Han KH, Kang DH, Song JK, Kim JJ, Park SW, Park SJ. Diabetic retinopathy as a predictor of late clinical events following percutaneous coronary intervention. J Invas Cardiol. 2002;14:599-602. ], diabetic retinopathy has been shown to be a predictor of mortality in diabetic patients after PCI. The author divided 365 non-insulin dependent DM patients who underwent PCI and fundoscopic examination into 2 groups: 115 patients with retinopathy and 250 patients without retinopathy. Patients with retinopathy had longer duration of DM and more insulin dependency. The 2-year cumulative survival rate was 96.3% and 99.6% respectively for patients with retinopathy and without retinopathy (p = 0.02). In the same direction, Briguori et al [145145. Briguori C, Condorelli G, Airoldi F, Manganelli F, Violante A, Focaccio A, Ricciardelli B, Colombo A. Impact of microvascular complications on outcome after coronary stent implantations in patients with diabetes. J Am Coll Cardiol. 2005;45:464-6. ] identified diabetic patients with microvascular complications treated with conventional stenting as having poorer outcomes as compared to those without.
Recently, Ohno T [146146. Ohno T, Ando J, Ono M, Morita T, Motomura N, Hirata Y, Takamoto S. The beneficial effect of coronary-artery-bypass surgery on survival in patients with diabetic retinopathy. Eur J Cardiothorac Surg. 2006;30:881-6. ] et al compared mortality rate after revascularisation by CABG versus PCI in diabetics with (n=153) or without (n= 166) retinopathy. The average follow-up from the time of initial revascularisation was 48.2 ± 28.6 months. In 153 diabetics with retinopathy, 59 underwent CABG. During the entire follow-up period, there were 2 (3.4%) deaths in diabetics with retinopathy who underwent CABG and 14 (14.9%) deaths in those who did not have CABG. Mortality curves differed significantly between the two groups (P = 0.007). After adjustment for risk factors, the relative risk of death was 0.13 afforded by CABG (95% CI; P = 0.011). In 166 diabetics without retinopathy, mortality curves were similar between the patients who underwent CABG and those who did not have CABG (P = 0.94). As a result, CABG may confer a survival advantage in diabetics with retinopathy.
Impact of baseline clinical condition on outcomes after revascularisation
- The occurrence of an acute coronary syndrome in a diabetic patient represents a worse prognosis as compared to that in a non-diabetic
- Anatomical and pathophysiological factors confer on the diabetic patient an especially high risk for events during follow-up
- The presence of micro- and macro-vascular complications has a profound and negative impact in DM during revascularisation




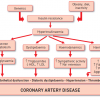









Thank You for this chapter and the personal perspective. 1. OPTIMUS-3 {Angiolillo et al., 20.01.2011, European Heart Journal, null-null}- prasugrel in diabetic patients? 1. FREEDOM? - when will this part be revised? {Farkouh ME et al. N Engl J Med 2012;367:2375-2384} DM, MVD, CAD --> PCI/CABG Death, MI, stroke n=2.400; PCI vs CABG 16,8 vs. 11,8%, p=0,004? 2. I think reference 123 should be: {Harjai et al., 2003, Am J Cardiol, 91, 1041-5}? Alessandro Cuneo