Summary
This chapter reviews the different stages a patient should go through once a coronary angiogram has been scheduled. It is important to clearly define up front what to expect from a coronary angiography and in which way the information gathered from the examination will help the operator for further therapeutic decisions making.
The pre-procedure evaluation as well as the post-procedure care of any patient is as important as the procedure itself. Besides the standard screening tests, special attention is focused on the group of patients for whom the examination carries a higher than normal risk for complications such as in the presence of diabetes, heart failure, chronic kidney disease, bleeding disorders.
Although watching a routine coronary angiogram performed by an experienced operator may look easy and straight forward, the fact is that it takes skills, knowledge of the coronary anatomy, insight into cardiac physiology and haemodynamic profiles, catheter material, action of drugs and above all experience.
A detailed description of each step of the procedure is discussed focusing in particular on catheter selection and the choice of optimal projections for each coronary segment.
The acquired data during the procedure, both anatomic and physiologic should be processed, if needed by computer assisted software, and integrated to extract as much information as possible to optimize the final diagnosis and conclusion.
It stands to reason that any invasive procedure including routine diagnostic coronary angiography is bound to encounter some complications. The most frequent, but often avoidable complications are described and illustrated in this chapter. In the event of complications the outcome will heavily depend on the team’s experience and level of training to cope with the emergency situations.
Introduction
Coronary angiography is an integral part of the haemodynamic workup of patients with heart disease and a key element in the evaluation of patients with coronary artery disease.
The main goals of this investigation are 1) to confirm the presence and the nature of, or in some cases dismiss, a clinical diagnosis of coronary artery disease, 2) to assess the location and extent of a luminal stenosis and 3) to decide upon a therapeutic strategy (optimal medical therapy (OMT) versus percutaneous coronary intervention (PCI) +OMT versus coronary artery bypass graft surgery (CABG) + OMT.
Coronary angiography has evolved over the last 40 years or so, from being a “hit and run” procedure to a sophisticated imaging modality which is routinely coupled with tools that accurately evaluate the lesion-specific anatomical and potentially physiological impact. Today the borders between diagnostic coronary angiogram, functional evaluation by means of intracoronary pressure measurements, anatomical evaluation using imaging and the subsequent interventional procedure are no longer distinct, since more and more PCI’s are being performed in a single session. However, it is worth bearing in mind that this should only be undertaken in those laboratories where the appropriate equipment and expertise are available.
Although coronary angiography is a relatively safe procedure in experienced hands (with a mortality rate of 0.098%, reported in the registry of the Society for Cardiac Angiography and Intervention (SCAI) in more than 200,000 patients over a period of 42 months) [11. Lozner EC, Johnson LW, Johnson S, Krone R, Pichard AD, Vetrovec GW, Noto TJ. Coronary arteriography 1984-1987: a report of the Registry of the Society for Cardiac Angiography and Interventions. II. An analysis of 218 deaths related to coronary arteriography. Cathet Cardiovasc Diagn. 1989;17:11-4. ], one must remember that it can, on occasion, be potentially harmful [22. Johnson LW, Krone R. Cardiac catheterization 1991: a report of the Registry of the Society for Cardiac Angiography and Interventions (SCA&I). Cathet Cardiovasc Diagn. 1993;28:219-20. ]. We must remember that angiography is not without its difficulties and risks, for example the arterial puncture for vascular access, the radiation exposure for both operator and patient, the nephrotoxicity of the contrast agents, the procedural risk of stroke or myocardial infarction and the bleeding risks following of anticoagulation With this in mind, the risks and benefits of the angiography should always be taken into account when ordering the test. This is even more valid when we consider that the proportion of high-risk patients referred for coronary angiography is steadily increasing. Besides the above mentioned risks, these authors strongly believe that the greatest risk to patients results from an incomplete examination, arising from poor image quality, and non-selective injections. This in turn leads to suboptimal interpretation leading to wrong therapeutic decisions.
It is often overlooked that coronary angiography is not just a simple confidential interaction between the operator and the patient but requires not only a harmonious interaction between nurses assisting the procedure, technicians running the hardware and the operator performing the procedure but depends also on a disciplined, well-oiled organisation, and a well-defined departmental structure to run a smooth patient flow. If one of these elements fails the operator will feel the firm ground under his feet change into quicksand. It is highly recommended that before starting a program in invasive cardiology every trainee should invest time in reading the basics as explained in several standard works on heart catheterisation including the current guidelines [33. Baim DS, Grossman W. Chapter 12 Coronary Angiography and Intravascular Ultrasonography; Grossman’s cardiac catheterization, angiography, and intervention. Philadelphia: Lippincott Williams & Wilkins. 2006.
A standard reference for all beginners which covers concisely the general principals of cardiac catheterisation, major technical aspects, evaluation of left ventricular function and haemodynamic and angiographic profiles in specific disorders., 44. Braunwald E, Zipes DP, Libby P. Heart disease : a textbook of cardiovascular medicine. Philadelphia: Saunders, 2001. , 55. Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, Legako RD, Leon DF, Murray JA, Nissen SE, Pepine CJ, Watson RM, Ritchie JL, Gibbons RJ, Cheitlin MD, Gardner TJ, Garson A Jr, Russell RO Jr, Ryan TJ, Smith SC Jr. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation.1999;99:2345-57. , 66. Pepine CJ, Hill JA, Lambert CR. Diagnostic and therapeutic cardiac catheterization. Baltimore: Williams & Wilkins. 1998. , 77. Kern MJ. The cardiac catheterization handbook. Philadelphia, PA: Mosby. 2003. , 88. Gensini GG, Coronary arteriography. Mount Kisco N.Y. 1975.
A detailed work for everyone, especially the section covering the anatomy of normal coronary arteries as well as the anatomy of coronary arteries in disease is highly recommended. This monograph may no longer be available but is integrated in Braunwald’s Heart Disease. A textbook of Cardiovascular Medicine, 1980.].
Aim of the examination
The ultimate goals of the coronary angiogram are to make a correct diagnosis in order to understand the symptoms of the patient, to stratify the risk profile and to tailor the appropriate therapeutic strategy. In the era of PCI more detailed information is required from the diagnostic coronary angiogram than a mere list and location of coronary lesions.
A more detailed description of lesions apart from the % stenosis is required, such as length, eccentricity, relationship with side branches, presence of dissection, calcium or thrombus as well as the presence and extent of collateral circulation.
The challenge of the diagnostic and therapeutic use of angiography is the necessary translation from the acquired two-dimensional image to the three-dimensional target. To address this problem successfully, a detailed anatomical knowledge is mandatory as well as an appropriate imaging technique with the use of different angiographic views to picture realistically the target region with no (or only little) bias caused by the image acquisition itself. In particular, in complex anatomical structures e.g., the coronary artery tree, complete visualisation requires care and rigour to ensure complete anatomical documentation. Often bifurcations, vessel foreshortening and overlap cause errors in stenosis estimation. Nevertheless, there are techniques in development for online 3D acquisition which will allow the operator to appreciate quickly the true 3-dimensional nature of the coronary artery.
In addition, more and more pressure is put on the operator to evaluate the physiological significance of coronary lesions during the diagnostic procedure. Finally, it is crucial to report accurately any anatomical variations as they may well influence the PCI procedure, as well as any procedural problems which may be useful to know in case a subsequent angiography or PCI has to be performed by a different operator.
LEFT VENTRICULOGRAPHY
The left ventriculogram should be considered as an integral part of the coronary angiography and is probably best performed at the start of the examination. This also applies in the case of patients presenting with acute coronary syndromes, with the exception of patients presenting with haemodynamic instability. The left ventriculogram yields important information regarding the functional repercussion of the underlying coronary atherosclerosis, starting with measurement of the left ventricular end diastolic pressure, analysis of global and regional function and evaluation of mitral valve regurgitation. In patients with acute coronary syndromes it is not uncommon that the ECG does not aid the clinician in the identification of the culprit vessel. Consequently, regional wall motion abnormalities seen on left ventriculography can help to direct the attention of the operator to the culprit artery.
DEFINING CORONARY ANATOMY
Coronary anatomy develops on a general blueprint based on the presence of a left and a right coronary artery originating from the left and right sinus of Valsalva, respectively, but with a rich phenotypic variability ( Figure 1 ).
The left coronary artery usually consists of a short main trunk known as the left main stem, which divides into the left anterior descending (LAD) artery which follows the anterior interventricular groove to the apex of the heart and the circumflex artery (LCx) which runs along the left part of the atrioventricular groove, parallel to the coronary sinus. Alternatively the LAD and LCx may have separate origins meaning that the left main stem is absent. The LAD may be classified according to its length into type I, II, and III. A type I LAD reaches only two thirds of the distance between the base and the apex. A type II LAD by definition reaches the apex of the heart. A type III LAD runs over the apex around the diaphragmatic section of the left ventricle. From the LAD, diagonal branches lead to the anterolateral wall and the septal branches lead into the interventricular septum. From the LCx, marginal branches lead to the lateral wall of the left ventricle. Sometimes there is a third artery originating from the left main artery which can be viewed, either as a diagonal or marginal branch, with a very early origin. However, this is labelled as the intermediate or angular branch.
The right coronary artery (RCA) has a rather long main stem and originates from the right sinus of Valsalva. The RCA then traverses the right part of the atrioventricular groove until it separates at its crux into the right posterolateral (RPL) artery and right posterior descending (PDA) artery [88. Gensini GG, Coronary arteriography. Mount Kisco N.Y. 1975.
A detailed work for everyone, especially the section covering the anatomy of normal coronary arteries as well as the anatomy of coronary arteries in disease is highly recommended. This monograph may no longer be available but is integrated in Braunwald’s Heart Disease. A textbook of Cardiovascular Medicine, 1980.].
CORONARY DOMINANCE
The individual importance of the LAD, LCx and RCA varies considerably and is the basis for the description of the coronary predominance. The coronary artery responsible for perfusing the inferior septum is referred to as the posterior descending artery and is known as the dominant artery. In 90% of patients the right coronary artery is the dominant artery on the basis of this anatomical definition. When both the right coronary artery and the left coronary artery provide a posterior descending branch, the system is said to be “balanced”. However, the fact that the left coronary artery gives rise to the greatest number of ramifications towards the left ventricular myocardial mass is evidence that the left coronary artery is by far the predominant artery in man.
Within each category important individual variations can exist. Of note is that these variations are complementary. A short LAD (type I) is usually compensated for by a longer posterior descending artery, running over the apex. Conversely, a longer LAD (type III) running over the apex will be associated with a rather short posterior descending artery. In all other cases the LAD is considered to be type II. Similarly, in the case of a right dominant coronary anatomy the importance and number of the posterolateral branches of the distal right coronary artery will vary inversely with the importance and number of the lateral branches from the left circumflex coronary artery.
Variability of the origin and size of smaller side branches may be of importance
The sinus node artery most often (55%-66%) originates from the RCA, more often from the proximal segment but can also arise from the mid or distal RCA segment. Alternatively the sinus node artery can also originate from either the proximal or distal LCx (34%-45%). Septal branches may occasionally originate from the proximal RCA.
ANOMALOUS CORONARY CIRCULATION
Anatomical variants of the classical blueprint should be acknowledged especially since some coronary anomalies may cause sudden death in young athletes in the absence of additional heart abnormalities. CT angiography and/or cardiac MRI are excellent tools for identifying coronary artery anomalies and defining their course and relationship to the great vessels and surrounding structures; their value is incremental to conventional angiography. Below we will outline briefly some of the better known coronary anomalies.
- Single coronary artery either originating from the left aortic sinus or from the right aortic sinus ( Moving image 1 , Moving image 2 )
- Anomalous origin of the left main coronary artery originating from the right aortic sinus. Four different trajectories of the left main coronary artery are known: anterior or posterior of the pulmonary artery, inter-arterial between the aorta and the pulmonary trunk and an intraseptal course. The trajectory between the aorta and the right ventricular outflow tract (RVOT) has been associated with different coronary syndromes due to compression of the left main stem in case of dilatation of either the aorta or RVOT ( Figure 2 )
- Anomalous origin of the left circumflex coronary artery from the right aortic sinus or directly from the proximal RCA. (Figure 3) ( Moving image 3). This anomaly can already be suspected from the ventriculogram (obtained in RAO) showing the contour of the anomalous circumflex artery passing behind the right coronary sinus; this is known as the “aortic root sign or Page’s sign” [168168. Page HL, Engel HJ, Campbell WB. Anomalous origin of the left circumflex coronary artery. Circulation. 1974;50:768-72. ] ( Figure 3a ), ( Moving image 37 ).
- Anomalous origin of the right coronary artery from the left aortic sinus ( Figure 4 )
- More rarely the anomalous origin of the left coronary artery arising from the pulmonary artery (ALCAPA or Bland-White-Garland syndrome). It is usually the cause of severe myocardial ischaemia and infarction during the first months after birth. Only 25% of patients will reach adult age ( Moving image 4 , Moving image 5 ).
Coronary fistulas are frequent and may vary from very small, multiple to very extensive fistulas ( Figure 5 ) ( Moving image 6, Moving image 7). Fistulas can cause significant under-perfusion. Approximately 50% of the fistulas arise from the RCA, and 50% from the LCA. In 50% of cases the fistula drains into the right ventricle, 25% into the right atrium, followed by the pulmonary artery, the right atrium, left atrium and finally the left ventricle.
Myocardial bridges are not infrequently seen in asymptomatic patients and occur when a segment of an epicardial coronary artery tunnels through the myocardium. The typical location is the mid segment of the LAD, although other locations have been reported, such as diagonal and marginal branches, the PDA and even the left main. On angiography one sees typically a systolic compression with diameter reduction which varies from mild (less than 50% diameter reduction) to severe (more than 75% diameter reduction) ( Figure 6 ) ( Moving image 8 ).
Several interventions may either augment or attenuate the degree of systolic compression. Typically, interventions that will decrease LV dimensions and/or increase contractility will accentuate the systolic compression, such as atrial pacing, administration of isoproterenol or nitrates [99. Arbogast R, Bourassa MG. Myocardial function during atrial pacing in patients with angina pectoris and normal coronary arteriograms. Comparison with patients having significant coronary artery disease. Am J Cardiol. 1973;32:257-63. , 1010. Bourassa MG, Butnaru A, Lesperance J, Tardif JC. Symptomatic myocardial bridges: overview of ischemic mechanisms and current diagnostic and treatment strategies. J Am Coll Cardiol. 2003;41:351-9. ]. Conversely, administration of beta-blockers will attenuate the systolic compression, confirming the dynamic character of these muscular bridges. One would expect that these bridges would not interfere with normal coronary perfusion on the basis that coronary perfusion is mainly a diastolic event. However, detailed anatomical (IVUS) and functional (Doppler flow) evaluation have shown that in some cases what appears as normal coronary perfusion is indeed abnormal. In addition, several cases have been described where myocardial bridges were responsible for acute coronary syndromes and needed to be treated surgically.
PRESENCE, LOCATION AND EXTENT OF CORONARY PATHOLOGY
Coronary angiography should enable the operator to answer the question - is coronary artery disease present and if so, where and how extensive is it? Therefore it stands to reason that high quality images from selective coronary injections in multiple orthogonal views are of paramount importance. The choice of the optimal catheter i.e., size and curvature, will influence the quality of the coronary injection and subsequently the diagnostic accuracy of the angiogram.
With PCI as a therapeutic option, coronary angiograms should be carefully screened for details relevant to the potential intervention. Details such as take-off of the target vessel, excessive tortuosity proximally or distally to a lesion, presence of side branches in relation to lesions are crucial features that must be acknowledged. Special attention should be given to record the lesion type, lesion length, lesion characteristics and to avoid overlapping pictures which can lead to misinterpretation.
IDENTIFYING VENOUS AND ARTERIAL GRAFTS
Angiography in patients who have already undergone CABG poses specific challenges. It is vital that all grafts, even the occluded ones, are selectively engaged and visualised ( Moving image 9 , Moving image 10 ). It is worth remembering that a graft which cannot be found is not necessarily occluded. Therefore it is mandatory that a detailed report of the CABG procedure is at hand in the catheterisation laboratory at the time of the procedure, detailing the exact number of grafts, type of conduits, presence of Y-graft or other unconventional surgical constructions.
The proximal anastomosis of a venous conduit or free IMA or free radial artery is not always marked by a metal clip, making the search sometimes time consuming and leading to excessive use of contrast. Performing an aortogram in the LAO projection is sometimes needed to ascertain the location and patency of the grafts.
Both the LIMA and RIMA are sometimes difficult to engage selectively from the femoral approach due to a tortuous ascending aorta and/or iliac arteries resulting in non-selective injections with poor distal opacification of the recipient vessel ( Moving image 11, Moving image 12). In this case the transradial approach from the right and/or left can be used to selectively inject the IMA.
PRESENCE AND EXTENT OF COLLATERAL CIRCULATION
Collateral circulation develops in response to ischaemia in the presence of severe stenoses or chronic total occlusions. The presence or absence of collateral circulation will determine the degree of residual perfusion and therefore the clinical symptoms as well as the regional myocardial function [1111. Levin DC. Pathways and functional significance of the coronary collateral Circulation. Circulation. 1974;50:831-7. , 1212. Gensini GG, Bruto da Costa BC. The coronary collateral Circulation in living man. Am J Cardiol. 1969;24:393-400. ]. Collaterals can be anterograde or retrograde, bridging between segments of an occluded coronary artery, they can be intracoronary, connecting different segments of the same coronary artery, or intercoronary, connecting different segments of different coronary arteries [44. Braunwald E, Zipes DP, Libby P. Heart disease : a textbook of cardiovascular medicine. Philadelphia: Saunders, 2001. ] ( Figure 7 , Figure 8 , Figure 9 ) ( Moving image 13 , Moving image 14 , Moving image 15 ).
The variability and extent of collateral vessels differs considerably from one patient to another. Visualisation of collateral vessels varies from faint to fully developed epicardial connections. They are usually classified according to the Rentrop classification. ( Table 1 ) [1313. Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587-92. ]. Several specific collateral anatomical patterns are well known e.g., the Kügel artery ( Figure 8 ) but less well frequent collateral pathways may be observed. Werner et al proposed an angiographic grading of collateral connections (CC) : CC0, no continuous connection between donor and recipient artery, CC1, continuous threadlike connection and CC2, continuous small side branch-like size of the collateral throughout its course [157157. Werner GS, Ferrari M, Heinke S, Kuethe F, Surber R, Richartz BM, Figulla HR. Angiographic Assessment of Collateral Connections in Comparison With Invasively Determined Collateral Function in Chronic Total Occlusions. Circulation. 2003; 107:1972-1977 ]. Detailed knowledge of collateral pathways, including the donor artery as well as the recipient vessels, is important when PCI is planned especially in the case of chronic total occlusion (CTO). Damaging a donor artery, may result in ischaemia when the collateral vessel is compromised. In order to carefully visualise collaterals, long film sequences are needed. In order to minimise radiation and contrast, injection may be started well before hitting the cine pedal.
In some circumstances, simultaneous injection of the RCA and LCA may be warranted to provide a more accurate estimation of the true extent of the collateral circulation. By using this technique in the diagnostic setting you are not only opacifying the anterograde and retrograde circulation but you are obtaining more accurate information on the length of an occlusion.
ASSESSMENT OF CORONARY VASOMOTION
Coronary arteries, including the large epicardial conduits, are equipped with smooth muscles cells in which tension varies thereby determining the lumen diameter and thus the vascular resistance to flow. In contrast to the arteriolar vessels, the larger epicardial vessels only contribute to a minor degree in overall vascular resistance. In the absence of atherosclerosis, vasomotion of epicardial vessels barely interferes with coronary flow even during maximal stress. Abnormal vasomotion may occur either focally, and is then referred to as coronary spasm, or it may affect the coronary vessels globally. It is not infrequent that a non-significant atheroma plaque becomes significant when vasoconstriction occurs on top of it during exercise or stress conditions. Coronary artery spasm has been shown to be the principal mechanism triggering ischaemia in patients with variant angina, the so called Prinzmetal’s Angina [99. Arbogast R, Bourassa MG. Myocardial function during atrial pacing in patients with angina pectoris and normal coronary arteriograms. Comparison with patients having significant coronary artery disease. Am J Cardiol. 1973;32:257-63. , 1414. Meller J, Pichard A, Dack S. Coronary arterial spasm in Prinzmetal’s angina: a proved hypothesis. Am J Cardiol. 1976;37:938-40. , 1515. Maseri A, L’Abbate A, Baroldi G, Chierchia S, Marzilli M, Ballestra AM, Severi S, Parodi O, Biagini A, Distante A, Pesola A. Coronary vasospasm as a possible cause of myocardial infarction. A conclusion derived from the study of preinfarction angina. N Engl J Med. 1978;299:1271-7. , 1616. Chahine RA. Prinzmetal’s variant angina. A syndrome apart or another clinical presentation of atheromatous heart disease. Arch Intern Med 1979;139:26-7. ]. Bertrand et al. demonstrated that the incidence of provoked coronary artery spasm after IV methyl-ergonovine was 20% in patients with a previous coronary event and was frequently superimposed on organic lesions [1717. Bertrand ME, LaBlanche JM, Tilmant PY, Thieuleux FA, Delforge MR, Carre AG, Asseman P, Berzin B, Libersa C, Laurent JM. Frequency of provoked coronary arterial spasm in 1089 consecutive patients undergoing coronary arteriography. Circulation. 1982;65:1299-306. ].
Both conditions, if suspected clinically, should be investigated using specific stress tests such as ergonovine challenge, acetylcholine, cold pressure test, and hyperventilation test.
ASSESSING THE FUNCTIONAL SIGNIFICANCE OF A LESION
Times are gone when lesion severity was routinely assessed by eyeballing. Most modern angiographic equipment allows rapid online quantitative coronary angiography (QCA) analysis to be carried out to determine the degree of diameter stenosis and lesion length. 3D vessel reconstruction can produce some spectacular images useful for detailed lesion description and quantification. While this technology is promising as with 2D QCA, it is only as good as the images that have been acquired during coronary angiography. Even this approach lacks the accuracy to quantify correctly all lesions in particular intermediate coronary lesions. Therefore, additional intracoronary trans-gradient pressure measurements have now become the gold standard for the analysis of lesion severity [1818. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 31:2501-55.
Guidelines (including ref 5 Scanlon et al.) in general have a role in framing the practice of angiography to help the operators in daily practice. The above mentioned reference is vital in terms of highlighting the fact that coronary angiography alone is not a sufficient modality to assess the severity of a coronary lesion.]. Today this technique should be available in all laboratories and is becoming an integral part of the diagnostic procedure. Operators who have not been trained in interventional cardiology should strongly consider learning how to measure intracoronary pressures properly to assess objectively the severity of coronary stenoses. This topic will be covered in much more detail in  View chapter .
View chapter .
Aim of the coronary angiography
- Coronary angiography is a team effort
- LV angiogram is an integral part of a diagnostic coronary angiography procedure
- Anatomical variations and coronary anomalies in part determine prognosis
- Coronary lesions should be described in detail with the eyes of an interventional cardiologist
- Detailed description of the collateral circulation is of importance to the interventional cardiologist
- Detection of resting vasomotor tone will influence therapeutic decisions
- Imaging is only half the picture, physiology the other half
Indications
ELECTIVE PROCEDURES
Any patient in whom a diagnosis of coronary artery disease is suspected or made on clinical grounds, or on the basis of additional non-invasive stress tests, should be scheduled for a diagnostic coronary angiography for the purpose of confirming the diagnosis as well as for defining the optimal therapeutic strategy. In addition, patients (especially younger patients with a low cardiovascular risk) in whom the diagnosis is only suspected and in whom one or more non-invasive tests, including a CT scan of the coronary arteries, remain inconclusive should undergo a diagnostic coronary angiogram.
Elective procedures may be scheduled in stable patients with known coronary artery disease, to evaluate the medium and/or long term results after a particular type of PCI procedure, such as left main stenting or recanalisation of a chronic total occlusion. Diagnostic coronary angiography may be considered as a preoperative work-up in patients with known coronary artery disease or severe peripheral vascular disease in whom major non-cardiac surgery is planned.
SEMI-URGENT PROCEDURES
Coronary angiography is indicated on a semi-urgent basis in all patients presenting with unstable angina pectoris/NSTEMI, defined as recent (<3 months) onset of exercise induced angina, angina at rest or accelerating angina pattern in naïve patients or in patient with previous stable angina on the basis that, when treated only medically, more than 40% of these patients will subsequently need some form of revascularisation [1919. Fulton M, Lutz W, Donald KW, Kirby BJ, Duncan B, Morrison SL, Kerr F, Julian DG, Oliver MF. Natural history of unstable angina. Lancet. 1972;1:860-5. , 2020. Braunwald E. Unstable angina. A classification. Circulation. 1989;80:410-4. ]. In this respect, several studies have shown that the Braunwald classification has been useful in identifying high-risk patients in whom coronary angiography is warranted. Similarly the GRACE risk score may be helpful to guide clinicians when it comes to deciding which NSTEMI patient needs angiography within 24 hours from those that can be safely done within 72 hours of diagnosis.[158158. Tang EW, Wong CK, Herbison P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J. 2007;153:29-35. ]
EMERGENCY PROCEDURES
All patients presenting with acute ST elevation myocardial infarction are entitled to an urgent (< 90 min after admission to the hospital) diagnostic coronary angiogram followed by a primary PCI if suitable. It stands to reason that patients developing serious haemodynamic deterioration (cardiogenic shock due to VSD, mitral regurgitation, pericardial tamponade) should be catheterised immediately.
CONTRAINDICATIONS
In general terms, there are no absolute contraindications for a diagnostic coronary angiography. Relative contraindications are ongoing infections, acute kidney injury or failure, severe anaemia, active bleeding, and severe electrolyte imbalance. However, the indication for each individual patient should be the result of a balanced analysis of the risk-benefit, by considering such factors as advanced age, presence of comorbidity, life expectancy as well as patient preference.
PATIENTS AT INCREASED RISK OF COMPLICATIONS AFTER CORONARY ANGIOGRAPHY
Several conditions and medical factors have been associated with an increased risk of complications, such as patients with advanced age (>80 years), morbid obesity, diabetes, severe COPD with hypoxia or hypoxaemia, renal insufficiency, anaemia, NYHA class IV, low ejection fraction and high filling pressures, pulmonary hypertension, severe peripheral vascular disease, uncontrolled arterial hypertension, recent myocardial infarction and recent stroke.
Pre - procedure
This section deals with the issues which need to be addressed in order to prepare the patient to safely undergo a cardiac catheterisation. The quotation “By failing to prepare, you are preparing to fail” is particularly apt in this instance.
Later in this chapter we will outline some of the potential pitfalls associated with coronary angiography; however, many of these adverse reactions can be avoided if we devote the same attention to detail to the before-care as we do during the patient’s procedure in the catheterisation laboratory. There are several general principles which must be applied to every patient, scheduled for coronary angiography. In addition to these basic set of requirements, there are special cases that warrant extra attention. In the first instance we will cover the basic principles and then we will cover the special cases in subsequent sections.
THE STANDARD PATIENT
This section deals with the basic checklist required for every patient attending the cardiac catheterisation laboratory. In every case, the cardiologist must carefully establish the indication from the referral letter and then confirm or refute this on the basis of his own history and clinical examination and carefully assess the possible contraindications. Moreover, in the time between the clinic visit and the coronary angiography, clinical symptoms may have changed. This is important to establish since it may alter the way in which the patient is then managed. Outlined in Table 2 are the issues which must be addressed in every case.
There is no doubt that a written informed consent should be obtained in each and every patient following a clear and full description, in layperson’s terms, about his/her illness, treatment options and necessary explanation regarding the procedure itself so that they can decide to proceed with, restrict, or decline the proposed intervention. Legislation in many countries dictates that informed consent can only be sought by the operator themselves or at least by someone who is experienced at coronary angiography. To obtain informed consent the cardiologist must cover, preferably in laypersons terms, the following:
- The nature of the procedure explained in a step by step fashion; i.e., what will happen to them.
- The major risks they face, including stroke, heart attack, and death.
- The more common minor risks, including pain, blood vessel damage, allergic reactions to the contrast or medications, bleeding from the access site and possible infection,
- Briefly touch on what actions will be taken should problems arise.
While many of us assume that patients completely understand the procedure from the information leaflet or the explanation given by their cardiologist, it can never be understated how important it is to ensure that this is indeed the case. The act of re-explaining to the patient the process on a second occasion can be extremely comforting to the already nervous patient and will enhance his confidence, especially when the patient is given ample opportunity to ask questions.
History and examination
As mentioned previously a detailed history and a careful clinical examination must be undertaken to re-establish that there are sufficient grounds to perform coronary angiography and to help decide if additional investigation(s) should be done during the procedure. It is important to emphasise this since in many laboratories around the world a habit of skipping the left ventriculography or of not crossing a diseased aortic valve is becoming routine under the pretext that echocardiography reveals the same information.
Blood testing
A routine set of blood samples taken within the last week are necessary to ensure patient safety. The necessary blood samples are outlined in Table 2. From the haematology blood results there are several values that are pertinent to performing coronary angiography, of which the following 3 main parameters are of major interest:
- The haemoglobin – since it is well known that a value of haemoglobin below 11g/dl is associated with increased morbidity and mortality especially if the patient proceeds to ad hoc PCI [2121. Maluenda G, Lemesle G, Collins SD, Ben-Dor I, Syed AI, Torguson R, Kaneshige K, Xue Z, Pakala R, Suddath WO, Satler LF, Kent KM, Lindsay J, Pichard AD, Waksman R. The clinical significance of hematocrit values before and after percutaneous coronary intervention. Am Heart J . 2009;158:1024-30. , 2222. McKechnie RS, Smith D, Montoye C, Kline-Rogers E, O’Donnell MJ, DeFranco AC, Meengs WL, McNamara R, McGinnity JG, Patel K, Share D, Riba A, Khanal S, Moscucci M; Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). Prognostic implication of anemia on in-hospital outcomes after percutaneous coronary intervention. Circulation. 2004;110:271-7.
This study raises awareness of the importance of avoiding anaemia before coronary angiography which not infrequently converts to ad hoc PCI.].
- The white cell count – since this is a surrogate marker of underlying infection which is obviously a relative contraindication to invasive testing
- The platelet count – since a low platelet count i.e., <100,000/ml is associated with increased risk of bleeding, particularly if femoral access is chosen. It is also important to recognise a high platelet count, known as thrombocytosis, since this may be associated with an increased thrombotic potential.
From the biochemistry profile there are two sets of results to focus on, the first is blood urea and creatinine and the second liver indices. The renal indices, urea and creatinine are rough indicators of renal dysfunction. What is clear is that the higher the creatinine the higher the risk of contrast-induced nephropathy [2323. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393-9. ]. This will be discussed in more detail in the renal failure section. The liver profile is equally important because it may reveal the presence of infection such as hepatitis B or C which will trigger our awareness for needles and other sharps during the procedure. In more extreme cases the presence of liver dysfunction may increase the risk of bleeding because of an inability of the liver to manufacture important clotting factors.
Bleeding following coronary angiography is always a concern. It is vital to know therefore if there is any history of bleeding, and from the blood samples evidence of an elevated INR or aPTT. The consensus recommends that coronary angiography should not be performed if the INR is above 1.8 [55. Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, Legako RD, Leon DF, Murray JA, Nissen SE, Pepine CJ, Watson RM, Ritchie JL, Gibbons RJ, Cheitlin MD, Gardner TJ, Garson A Jr, Russell RO Jr, Ryan TJ, Smith SC Jr. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation.1999;99:2345-57. ]; however in a time where the transradial approach is becoming increasingly popular, particularly in Europe, this threshold may not necessarily apply. Indeed there is evidence that coronary angiography can be done in relative safety without interrupting anticoagulation using either the transradial or transfemoral access [2424. Karjalainen PP, Vikman S, Niemelä M, Porela P, Ylitalo A, Vaittinen MA, Puurunen M, Airaksinen TJ, Nyman K, Vahlberg T, Airaksinen KE. Safety of percutaneous coronary intervention during uninterrupted oral anticoagulant treatment. Eur Heart J. 2008;29:1001-10. , 2525. Ziakas AG, Koskinas KC, Gavrilidis S, Giannoglou GD, Hadjimiltiades S, Gourassas I, Theofilogiannakos E, Economou F, Styliadis I. Radial versus femoral access for orally anticoagulated patients. Catheter Cardiovasc Interv. 2010;76:493-9. ]. If a haematoma does arise around the radial artery, the superficial nature of the radial artery and its remoteness from any vital organs lends itself to simple direct compression, which invariably arrests any bleeding. While the same applies for the transfemoral approach, a sizeable proportion of patients are overweight, making effective compression to achieve haemostasis more difficult. In this case a closure device should be considered.
There is a growing body of evidence that the new oral anticoagulants (NOACs) er antithrombotic agents, such as dabigatran, apixaban and rivaroxaban, and edoxaban are equally efficacious when compared to warfarin in terms of reducing the risk of stroke in patients with atrial fibrillation (AF) but with a lower risk of bleeding [2626. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-51. , 2727. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O’Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S; AVERROES Steering Committee and Investigators. Apixaban in Patients with Atrial Fibrillation. New England Journal of Medicine. 364:806-817. ]. The current
guidelines on stroke prophylaxis in AF suggest that aspirin may actually be harmful for patients, [159159. Lip GYH, Laroche C, Dan GA, Santini M, Kalarus Z, Rasmussen LH, Popescu MI, Tica O, Boriani G, Cimaglia P, Hellum CF, Mortensen B, Maggioni AP. Antithrombotic treatment in 'real-world' patients with atrial fibrillation: A report from the Euro Observational Research Programme Pilot survey on Atrial Fibrillation. American Journal of Medicine. 2014. doi 10.1016/j.amjmed.2013.12.022 ] therefore the number of patients on warfarin or NOACs appears to be steadily increasing. Historically, anticoagulation would have been stopped to avoid bleeding complications associated with coronary angiography especially with femoral access. In recent times the number of transradial procedures has dramatically increased worldwide and with that the confidence to do transradial angiography without interruption to a patient‘s warfarin. This has been largely driven by significantly lower bleeding rates in those patients undergoing transradial angiography. While this may be true for warfarin the safety profile of NOACs remains unknown.
Clearly there are a number of other blood results to be noted, however the only other blood test that needs attention, and needs to be corrected if abnormal before coronary angiography, is blood glucose. It is not uncommon for the blood work-up pre-coronary angiography to identify diabetes for the first time. Every operator is duty bound to check the results of every test and to act upon the result if abnormal, however the bloods mentioned above are the bare minimum required to be checked before coronary angiography can proceed safely.
Chest x-ray and other radiological investigations
Today, virtually every patient admitted into hospital will have a chest x-ray. A huge amount of information is available from a simple chest x-ray which can be very important for the cardiologist before coronary angiography. The chest x-ray can help guide the operator in terms of catheter selection and help in deciding whether additional diagnostic evaluation is needed apart from simply injecting contrast into the coronaries.
Obviously, recognising features such as cardiomegaly, bilateral pleural effusions and diffuse alveolar shadowing point towards the diagnosis of heart failure, which warrants measurement of left and right sided pressures. Apart from this, the appearance of the aorta is very important. The finding of a large unfolded aorta (which is usually the case in patients over 65) and calcification at the site of the aortic position may suggest aortic stenosis in which case haemodynamic assessment may be justified. Furthermore, unfolding of the aorta or aortic dilatation, regardless of aetiology, seen on chest x-ray will help guide the choice of diagnostic catheter. This will be discussed in more detail in the section dealing with catheter selection and manipulation. Evidence of pericardial calcification is useful as it may indicate the presence of constrictive pericarditis. Other diagnoses such as pneumonia, pulmonary fibrosis or tumours can all be seen and may explain the vague or atypical symptoms that the patient complains of as opposed to coronary disease.
While not entirely necessary CT angiography and cardiac MRI may have been done and should be carefully interpreted before angiography.
Echocardiography
Ideally, everyone coming to the catheterisation laboratory should have already had a transthoracic echocardiogram; however, this is not always feasible in many countries because of economic constraints or long waiting lists. Again it is not uncommon that in many centres left ventriculography is not any longer done, which is all the more reason for echocardiography to be carried out. In many cases the echo identifies cardiomegaly, valvular disease or regional wall motion abnormalities which may prompt the operator to consider doing a right heart catheterisation as well. In addition, the presence and severity of the valvular disease can be confirmed during the heart catheterisation. Obviously assessment of mitral insufficiency in the catheterisation laboratory is often less reliable than echocardiography but this may not be the case in the assessment of aortic stenosis, although crossing a severely stenosed aortic valve is not without its risks, these are extremely low. Echocardiography is also useful when it identifies left ventricular thrombus. Refraining from placing a pigtail catheter into the left ventricle in this case is probably wise.
Pre-hydration
There is unequivocal evidence to support the use of hydration before administration of contrast medium, particularly in patients at risk of contrast induced nephropathy (CIN); however, the modalities of fluid administration remain uncertain [2828. Mueller C, Buerkle G, Buettner HJ, Petersen J, Perruchoud AP, Eriksson U, Marsch S, Roskamm H. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329-36. ]. With the vogue of day-case angiography, the length of stay for some patients is getting even shorter, particularly with the advent of closure devices and transradial angiography. Historically patients included in studies exploring the optimal pre-hydration regime were scheduled to stay overnight and so virtually all protocols tested cannot be directly applied to the patient attending for day case diagnostic angiography.
Assessing the hydration status prior the procedure is of paramount importance for several reasons. First and foremost, hydration helps to prevent CIN, which in itself is associated with significant morbidity and mortality [2929. Gruberg L, Mintz GS, Mehran R, Gangas G, Lansky AJ, Kent KM, Pichard AD, Satler LF, Leon MB. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542-8. ]. Secondly, under-utilisation of fluids in combination to overnight fasting prior to coronary angiography can more easily precipitate profound vasovagal reactions, which are particularly hazardous in the presence of significant aortic stenosis since a relative reduction of intravascular volume leads to a decrease in coronary perfusion which may be already compromised, potentially leading to lethal arrhythmias. Thirdly, those patients that are already hypervolaemic from congestive heart failure will not respond well to over judicious prescribing of IV fluids.
It is worth remembering that contrast injections, left ventriculography and supplemental fluid during the procedure will all expand the intravascular volume to some extent. This is an even more relevant statement when we consider that ad hoc PCI frequently occurs, meaning additional volume expansion. Over enthusiastic use of fluids, in particular, in the presence of incipient heart failure is likely to increase the risk of volume overload leading to pulmonary oedema, and hypoxaemia which in the presence of coexisting significant coronary disease may induce a vicious circle when ischaemia precipitates acute systolic and diastolic dysfunction. Finally the patient will not be able to lie flat from breathlessness which may prematurely end the investigation.
The ultimate aim of pre-hydration before coronary angiography is to have the patient eu- or even slightly hypervolaemic to preserve water and electrolyte balance, provide nutrition and avoid CIN. In the vast majority of patients this can be achieved by simply asking the patient to eat and drink up until midnight of the previous night and then supplementing them with 1,000 ml of intravenous fluid overnight at a minimum of 2 hours before they receive contrast medium.
While hydration before exposure to contrast medium generally only occurs in patients with evidence of renal insufficiency, it is probably reasonable to routinely pre-hydrate all patients regardless of renal function although this is not proven. For most patients 1,000 ml of 0.9 % Saline infused overnight is sufficient. Although this whole area has not been explored in any great detail, one previous randomised study has shown that 0.9% saline is more effective than 0.45% saline at preventing contrast nephropathy in patients undergoing coronary angioplasty [2828. Mueller C, Buerkle G, Buettner HJ, Petersen J, Perruchoud AP, Eriksson U, Marsch S, Roskamm H. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329-36. ]. The volume and type of fluid to be given, the infusion duration and infusion rate are all parameters which should have clear recommendations, although the evidence to date has varied for all of these parameters suggesting that there is a notable lack of standardisation in this area [3030. Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379-86. ]. Based on the observation that initiating and maintaining a high urine output is beneficial to prevent kidney damage, the RenalGuard® system has been developed in order to achieve precise real-time high volume fluid balance using a closed loop hydration monitoring and infusion which was shown to reduce the need for dialysis in patients with chronic kidney disease after catheterisation procedures. [160160. Briguori C, Visconti G, Focaccio A, Airoldi F, Valgimigli M, Sangiorgi GM, Golia B, Ricciardelli B, Condorelli G, for the REMEDIAL II Investigators. Renal Insufficiency After Contrast Media Administration Trial II (REMEDIAL II) RenalGuard System in High-Risk Patients for Contrast-Induced Acute Kidney Injury. Circulation. 2011;124:1260-9 ]. To complicate the issue further, there are several special settings in which the prescription of IV fluids needs to be modified even more, such as patients with diabetes, renal disease, severe valvular disease, or who are very large (>100kg) or very small (<60kg) or in patients with reduced left ventricular function (EF<40%). In Table 3 there is a recommended schedule since the optimal hydration schedule has not yet been determined. Further information will be found  View chapter .
View chapter .
Check list prior angiography
- Find the referral letter and read it
- Obtain a detailed history and examination
- Obtain informed consent
- Look at the ECG
- Check blood results
- Check for a stress test result
- Check for an ECHO report
- Check for a chest x-ray report
- Check for CT angiogram report
- Check for cardiac MRI report
- Are they pre-hydrated?
THE DIABETIC PATIENT
 View chapter
View chapter
The number of patients diagnosed with diabetes mellitus has significantly increased over the last 10 years as a result, on the one hand, of lower diagnostic thresholds and, on the other hand, of altered dietary habits as well as an increasingly aged population [3131. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 87:4-14. ]. Currently around 30% of the patients attending for coronary angiography are diabetics. Failure to manage the diabetic patient before coronary angiography may pose several problems, predominantly from denying oral intake and exposure to contrast.
Several issues should be addressed in diabetic patients scheduled for coronary angiography. First, subclinical diabetic nephropathy is frequently present. It is worth bearing in mind that renal indices, if normal, are fairly inaccurate markers of renal function since there needs to be approximately a 50% reduction in glomerular filtration rate (GFR) before the blood creatinine begins to rise [3232. Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933-1953. ].
Even with the creatinine in the normal range, the risk of contrast induced nephropathy is higher and so it is reasonable to suggest that in all patients with diabetes we should be even more vigilant when trying to avoid renal dysfunction.
Obviously, the ultimate aim when preparing the diabetic for coronary angiography is to maintain the patient within a euglycaemic range, in order to avoid significant morbidity and mortality associated with hypoglycaemia and hyperglycaemia. The preprocedural diabetic regime may vary slightly from institution to institution; however, within each centre this protocol should be strictly followed. It goes without saying that in difficult cases the local endocrinologist should be consulted.
Essentially all diabetic patients should be approached in the same way where they are to undergo minor surgery, defined as a patient who is expected to be awake during the procedure, eating and drinking by the next meal and in whom the total period of starvation post-procedure amounts to less than 4 hours. There are several practical issues to consider pre-catheterisation. Firstly, the diabetic should be scheduled, if possible at the beginning of the list although if this is not possible the patient need not be rescheduled. Secondly, medications such as metformin must be stopped 48 hours before catheterisation to avoid lactic acidosis. Thirdly, blood glucose must be more regularly monitored throughout the hospital stay. Below is a more detailed scheme to help manage the patient with diabetes undergoing coronary angiography based on whether they are a Type I or Type II diabetic ( Figure 10).
CONTRAST reaction
 View chapter
View chapter  View chapter
View chapter
One of the crucial ingredients used to perform coronary angiography is contrast media. Serious reactions to contrast media are relatively uncommon, in the largest series from the Mayo Clinic there were 4 for every 100,000 patients exposed to contrast [3333. Collins MS, Hunt CH, Hartman RP. Use of IV Epinephrine for Treatment of Patients with Contrast Reactions: Lessons Learned from a 5-Year Experience. Am. J. Roentgenol. 2009;192:455-461. ]. Nevertheless, the consequences are potentially devastating and even life threatening in a few instances. The following elements are vital:
- Know how to prevent contrast related reactions,
- Know and recognise the features of contrast allergic reactions
- Remember to document the events accurately
- Know how to treat the consequences, particularly when symptoms of dyspnoea and wheeze occur.
Even though some patients experience life threatening reactions to contrast media this is eminently treatable as we will discuss later.
“Prevention is better than cure” we are told, although when dealing with someone who has already had a documented reaction to contrast, premedication to prevent further reactions is not always effective. Furthermore, a person may react to a contrast agent on the first exposure and then not react to the same contrast on the next exposure, or vice versa. The reason for this is that reactions to radio-contrast are anaphylactoid, and not immunoglobin E-mediated therefore the risk of escalation with repeated exposure does not occur. In fact, there is virtually no evidence to support premedication in patients at risk of contrast related adverse reaction using either antihistamines or steroids. However despite a relative paucity of literature to support the practice of premedication, an expert consensus seems to suggest that it is sensible to pre-medicate those individuals who have had an adverse reaction to contrast.
The presence of a previous allergy to seafood which was believed to be a particularly important risk factor for experiencing an allergy to contrast does not appear to be the case. Several factors that increase the risk of experiencing an adverse response to contrast are outlined in Table 4 .
In all cases it is vital to establish whether the patient has received a contrast-based agent in the past and to ascertain if any adverse reaction was associated with the previous exposure. Documentation and history, including symptoms and signs as well as the contrast agent used i.e., osmolarity and the brand of the contrast needs to be carefully recorded. Reactions to contrast are of varying severity and our ability to predict the occurrence or severity of an event is poor. Below is a table that helps us define the severity of the reaction based on the guidelines set down by the American College of Radiologists. ( Table 5 ). (please refer to the American College of Radiology Manual on Contrast Media.)
ANTICOAGULATION AND BLEEDING RISK
 View chapter
View chapter
Before considering anyone for cardiac catheterisation it is essential to evaluate bleeding risk since bleeding is an independent predictor of adverse outcomes in patients undergoing PCI [3434. Doyle BJ, Rihal CS, Gastineau DA, Holmes DR, Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol. 2009;53:2019-27.
Pivotal paper highlighting the prognostic impact of bleeding during coronary intervention.]. This is particularly relevant when one considers that it is frequently the case that coronary angiography proceeds towards ad hoc PCI.
One of the most important and easily modifiable issues to consider is whether or not the patient is taking anticoagulants such as warfarin. This is even more relevant today since more elderly patients (i.e, over 75 years where the incidence of atrial fibrillation increases together with the need for warfarin use) are referred for coronary angiography. This more aggressive therapeutic approach towards elderly patients combined with warfarin treatment brings with it a higher chance of complications thus warranting an even greater vigilance.
Interrupting anticoagulation before an elective procedure appears to cause significant confusion as evidenced by considerable practice variations even among experienced physicians. It is therefore vital to reiterate the most recent published guidelines so that best practice is achieved. Once the physician has established that the patient is taking warfarin and found out the reason why, he then needs to assess the potential risk of interrupting therapy and the need for substituting therapy, before diagnostic coronary angiography.
Stratifying risk in patients taking warfarin
Table 6 lists the risk categories in patients taking warfarin from low to high. Once the patient has been stratified into the appropriate risk category then the appropriate management can be followed as illustrated in Figure 11 . However it is worth mentioning that all of these recommendations have a relatively low level of supporting evidence. Therefore, this is certainly an area that warrants further research.
The transradial approach to coronary angiography is increasingly being used as the access route of choice. One of the major advantages of this technique is the safety profile in terms of local access bleeding. In contrast to femoral access, radial access is appealing because of the lack of important adjacent structures. Therefore the clinical impact of bleeding around the radial artery is low. While it has not been proven, it may be reasonable to approach anticoagulation in patients undergoing transradial angiography as you would in patients identified to be at a low risk of bleeding ( Figure 12 ).
Currently, a standard recommendation for these patients has been the discontinuation of warfarin before invasive cardiac procedures to be replaced by unfractionated or low molecular weight heparin as a “bridging therapy” in patients at moderate to high risk of thromboembolism. However recent data is emerging indicating that bridge therapy offers no real advantage over the simple strategy of performing cardiac interventions during uninterrupted therapeutic oral anticoagulation therapy [161161. Airaksinen JKE, Schlitt A, Rubboli A, Karjalainen P, Lip GYH. How to manage antithrombotic treatment during percutaneous coronary interventions in patients receiving long-term oral anticoagulation: to “bridge” or not to “bridge”. Eurointervention. 2010;6:520-526 ].
Reversing the effects of warfarin
Warfarin exerts its anticoagulant effect by acting as a vitamin K antagonist and inhibiting the biosynthesis of vitamin K-dependent pro-coagulant factors II, VII, IX, and X. In addition to knowing how to manage patients taking warfarin in the elective setting, managing patients on warfarin who require urgent diagnostic coronary angiography warrants mentioning. The goal of urgent warfarin reversal is to elevate or replace vitamin K-dependent clotting factors and this can be achieved in three different ways:
- Administration of vitamin K (IV or PO)
- Administration of fresh frozen plasma (FFP)
- Pro-thrombin complex concentrate (PCC)
All of these antidotes to warfarin are effective; however, there are several caveats that one must be aware of and certain clinical situations where one is preferred over the other. When considering the appropriate antidote, the issues of how quickly the INR needs to be reversed, the status of the patient's left ventricular function and the reasons why the patient is taking warfarin in the first place, need to be carefully considered.
Vitamin K is attractive in that it is readily available and easier to administer than FFP. Its onset of action is relatively rapid at around 1-3 hours after intravenous administration and 4-6 hours after oral doses and so it is desirable in the setting of patients who need a diagnostic procedure in a semi-acute setting, such as in the next 4-24 hours. However, the major drawback of vitamin K is that it has an enduring effect leading to some resistance to re-anticoagulation of patients using warfarin. This is particularly the case when high doses of vitamin K are given. While this is not a problem in patients who will no longer need to receive warfarin, it is important in high risk patients, i.e., patients with a history of recurrent strokes or those with a metallic prosthetic valve in the mitral position.
If more urgent reversal of warfarin is necessary then FFP and PCC are more effective than Vitamin K. FFP is by far the most common product used for urgent reversal of warfarin. One of the potential drawbacks of using FFP is the risk of overloading patients, particularly those with reduced left ventricular function, thereby precipitating pulmonary oedema which can be difficult to treat with diuretic therapy. In addition FFP contains isohaemagglutinins and must be blood group-specific. It must also be thawed before use, which can delay treatment, and infection transmission is a potential risk. In patients with very high INRs who have profound decreases in vitamin K dependent factors, replacement of haemostatic levels of these factors cannot be achieved with tolerable doses of FFP. Furthermore, the administration of FFP in recommended doses is often insufficient to normalise factor IX levels.
An alternative to FFP is PCC, and by administering it one can avoid some of the problems associated with FFP. PCCs provide more rapid and complete factor replacement, are infused in lower volume and have enhanced safety because of viral inactivation. Unfortunately PCC is not as widely available and is more expensive than FFP. In places where it is available its use is regulated and can generally only be sanctioned for use by a haematologist. Below is a summary algorithm to aid decision making when urgent reversal of warfarin is necessary ( Figure 13 ).
New alternatives to warfarin and the antidotes
Warfarin has been around since 1954, is a very effective therapy and is increasingly being prescribed because of the increasingly older population who have atrial fibrillation; however, it comes with its problems. Specifically patients taking warfarin need their INR to be carefully monitored to remain in the therapeutic window. Routine monitoring takes time for the patient and the physician and is expensive, particularly in a time where the numbers of patients with atrial fibrillation is at epidemic proportions. Therefore the introduction of newer agents which are as effective as warfarin is very attractive. The main advantages of the new agents are rapid onset of anticoagulant effect, more predictable pharmacokinetics and a lower potential for clinically important interactions with food, lifestyle and other drugs. There is no requirement for routine monitoring and dose adjustment as required with warfarin.
There are several newer anticoagulants targeting various points in the coagulation cascade, such as factor Xa or thrombin. These include dabigatran, rivaroxaban, and apixaban and edoxaban. Several recent studies show that each of these four new anticoagulants are as effective and possibly better than warfarin in preventing strokes without any increase in bleeding events in patients with non-valvular atrial fibrillation. The first of these to be reported was the Re-Ly study in which dabigatran (a competitive reversible non-peptide antagonist of thrombin) was compared to warfarin for non-valvular atrial fibrillation and this was shown to be non-inferior to warfarin [2626. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-51. ]. The next study to be published was ARISTOTLE [3535. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-92. ] quickly followed by ROCKET AF [3636. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-91. ] where again apixaban and rivaroxaban (competitive reversible antagonist of activated factor X (Xa)) were shown to be as effective as warfarin (like dabigatran). In the light of these results it seems inevitable that the use of newer anticoagulants will become increasingly common. Therefore we need to know how to deal appropriately with these agents before performing a procedure.
At present, guidance does exist as to how one should manage NOACs in the peri-procedural period however, this is primarily in the setting of surgical procedures. In the case of high risk surgical procedures, NOACs should be discontinued 48 hours before the procedure in contrast to low risk procedures, where the recommendation is 24 hours. Intuitively the latter applies to coronary angiography and PCI therefore 24 hours should suffice. However a cautionary note is made for femoral access as the data, albeit limited, seems to suggest a significantly higher bleeding risk in those patients who continue on NOACs. This appears to be especially true for those undergoing transfemoral PCI versus transradial PCI. Some operators suggest that it may be legitimate to let patients continue on NOACs and perform coronary angiography, however this has not been formally studied. The only data to suggest that it may indeed be safe to do this comes from registry data where it says that performing coronary angiography is safe in patients where warfarin has not been interrupted. [161161. Airaksinen JKE, Schlitt A, Rubboli A, Karjalainen P, Lip GYH. How to manage antithrombotic treatment during percutaneous coronary interventions in patients receiving long-term oral anticoagulation: to “bridge” or not to “bridge”. Eurointervention. 2010;6:520-526 ] Therefore until the research is published, it may be more sensible to apply the practice of holding NOACs for 24 hours where possible. Adopting the 24 hour rule may reduce the bleeding complications which may become even more important in those where transradial fails and transfemoral is necessary especially in an elective setting.
As with its predecessor these newer antithrombotics also have their fair share of bleeding; however, unlike warfarin there are currently no official approved therapies to reverse the effect of any of these new therapies. Some investigators have suggested that prothrombin complex concentrate (PCC) may be effective at reversing the anticoagulant effect of rivaroxaban; however, this has not yet been rigorously tested.
PRESENCE OF ANAEMIA
The presence of severe anaemia is a poor prognostic indicator for patients undergoing coronary angiography and is considered a relative contraindication [3737. Voeltz MD, Patel AD, Feit F, Fazel R, Lincoff AM, Manoukian SV. Effect of anemia on hemorrhagic complications and mortality following percutaneous coronary intervention. Am J Cardiol. 2007;99:1513-7. ]. In addition, anaemia is often a trigger for myocardial ischaemia in otherwise asymptomatic patients thereby unmasking the presence of underlying coronary artery disease in particular in elderly patients. Correcting the anaemia will often cure the symptoms and sometimes make the coronary angiogram superfluous.
It is not clear, if the occurrence of contrast-induced nephropathy (CIN) is directly associated with poor outcomes, despite the facts that episodes of acute kidney injury predispose patients to long-term loss of kidney function [3838. Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009;76:1089-97. ]. It is suggested that the adverse outcome of these patients may simply reflect a greater burden of co-morbidity; however, recent trials have shown that the reduction of CIN did also improve patients long-term-outcome [3939. Solomon RJ, Mehran R, Natarajan MK, Doucet S, Katholi RE, Staniloae CS, Sharma SK, Labinaz M, Gelormini JL, Barrett BJ. Contrast-induced nephropathy and long-term adverse events: cause and effect? Clin J Am Soc Nephrol. 2009;4:1162-9. , 4040. Marenzi G, Lauri G, Campodonico J, Marana I, Assanelli E, De Metrio M, Grazi M, Veglia F, Fabbiocchi F, Montorsi P, Bartorelli AL. Comparison of two hemofiltration protocols for prevention of contrast-induced nephropathy in high-risk patients. Am J Med. 2006;119:155-62. ]
The most common definition of CIN is a rise of serum creatinine of 0.5 mg/dL or a 25% relative rise in creatinine at 48 hours after contrast exposure. The incidence of CIN ranges from 2% to 25% of patients undergoing coronary intervention [2323. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393-9. ]. Patients are at risk of the development of CIN when they have a glomerular filtration rate of below 60 mL/min, diabetes mellitus, congestive heart failure, if the procedural contrast volume is high or an intra-aortic balloon pump is required [2323. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393-9. , 4141. Brown JR, DeVries JT, Piper WD, Robb JF, Hearne MJ, Ver Lee PM, Kellet MA, Watkins MW, Ryan TJ, Silver MT, Ross CS, MacKenzie TA, O’Connor GT, Malenka DJ; Northern New England Cardiovascular Disease Study Group. Serious renal dysfunction after percutaneous coronary interventions can be predicted. Am Heart J. 2008;155:260-6. ].
One widely used concept for prevention of CIN is the administration of antioxidants such as N-acetylcysteine (NAC), ascorbic acid, or sodium bicarbonate to protect the tubule cell from reactive oxygen species. However, meta-analyses of multiple clinical trials have documented that the current data are not consistent, and, it is currently not possible to recommend the use of antioxidants for the prevention of CIN unambiguously [4242. Trivedi H, Daram S, Szabo A, Bartorelli AL, Marenzi G. High-dose N-acetylcysteine for the prevention of contrast-induced nephropathy. Am J Med. 2009;122:874 e9-15. , 4343. Kshirsagar AV, Poole C, Mottl A, Shoham D, Franceschini N, Tudor G, Agrawal M, Denu-Ciocca C, Magnus Ohman E, Finn WF. N-acetylcysteine for the prevention of radiocontrast induced nephropathy: a meta-analysis of prospective controlled trials. J Am Soc Nephrol. 2004;15:761-9. , 4444. Joannidis M, Schmid M, Wiedermann CJ. Prevention of contrast media-induced nephropathy by isotonic sodium bicarbonate: a meta-analysis. Wien Klin Wochenschr. 2008;120:742-8. ]. Similarly, it is unclear if contrast media can be discriminated as being more or less nephrotoxic by their chemical characteristics. Recent meta-analysis of clinical trials [4545. Reed M, Meier P, Tamhane UU, Welch KB, Moscucci M, Gurm HS. The relative renal safety of iodixanol compared with low-osmolar contrast media: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2009;2:645-54. , 4646. Heinrich MC, Haberle L, Muller V, Bautz W, Uder M. Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast media: meta-analysis of randomized controlled trials. Radiology. 2009;250:68-86. ] supports equivalent safety of iso-osmolar and low-osmolar contrast, with the possible exceptions of ioxaglate and iohexol.
Clinically, prevention of CIN requires the identification of patients at risk, minimising the amount of contrast agent and the meticulous hydration of the patient. Adequate hydration is recommended to consist of an intravenous volume expansion with isotonic crystalloid (1.0–1.5 mL/kg per hr) for 3–12 hrs before and continuing for 6–24 hrs after the procedure [4747. Caixeta A, Mehran R. Evidence-based management of patients undergoing PCI: contrast-induced acute kidney injury. Catheter Cardiovasc Interv. 2010;75 Suppl 1:S15-20. ]. To avoid CIN contrast volume should be limited to the minimum quantity necessary and should never exceed 350 ml, or ideally 4 mg/kg in patients with mild to moderate chronic kidney disease. An accurate formula to calculate the maximum safe dose of contrast media is: contrast volume/Cl creatinine. The risk of CIN increases significantly when the ratio of total contrast volume to GRF exceeds 3.7:1 according to the ESC guidelines.[4848. Laskey WK, Jenkins C, Selzer F, Marroquin OC, Wilensky RL, Glaser R, Cohen HA, Holmes DR Jr; NHLBI Dynamic Registry Investigators. Volume-to-creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:584-90.
Study in 3,179 unselected consecutive patients indicating a contrast volume to creatinine clearance ratio > 3.7 appears to be a significant and independent predictor of an early increase in serum creatinine after PCI. Together with reference 23 (Mehran et al.) this information may help us avoiding contrast nephropathy.] ]. Similarly, the use of the ratio of grams of iodine to glomerular filtration rate (I-dose/GFR) of <1 indicates a low risk of CIN. It is worth noting that automated contrast injection devices are associated with an average of 45 ml less contrast per procedure compared to manual injection, resulting in 15% less CIN [162162. Minsinger KD, Kassis HM, Block CA, Sidhu M, Brown JR. (2014). Meta-analysis of the effect of automated contrast injection devices versus manual injection and contrast volume on risk of contrast-induced nephropathy. Am J Cardiol. 2014;113:49-53. ].
Patients at risk of CIN should not have multiple successive doses of iodinated contrast in close proximity. The operator should calculate a target contrast dose based on one of these formulae and if possible terminate a procedure (and stage the procedure if necessary) before reaching the predetermined maximal contrast dose. In patients at risk for CIN it is important to monitor the renal function parameters at least at 48 h and probably at 1 week after the procedure. With the current short hospital stays this responsibility should be clearly communicated to the patient’s GP.
Peri - procedure
 View chapter
View chapter
ACCESS
Invasive assessment of cardiac haemodynamics, coronary imaging and physiology requires temporary vascular access. Today, arterial puncture, either through the femoral, brachial, radial, or in rare circumstances, axillary/subclavian artery to access the left heart and coronary arteries, has mostly replaced arterial denudation techniques. The transradial approach is still gaining popularity and findings from the RIVAL study suggest that transradial access is should be preferred over transfemoral access to reduce vascular bleeding complications after PCI [4949. Jolly SS, Yusuf S, Cairns J, Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimsky P, Budaj A, Niemelä M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR; RIVAL trial group. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. The Lancet. 2011;377:1409-1420.
With the current shift from femoral to radial approach this trial is important in demonstrating a significant reduction in bleeding complications as well as a trend for reduction in MACE. The down side of the radial approach is sometimes the less than perfect intubation of the target vessel resulting in inadequate opacifying the coronary arteries and inadequate support during PCI.].
We recommend the reader to go to  View chapter and
View chapter and  View chapter where vascular access, in particular transradial access as well as vascular closure are described in greater detail.
View chapter where vascular access, in particular transradial access as well as vascular closure are described in greater detail.
All catheters should be introduced via an introducer sheath and over a guidewire, typically with a distal J-curve, which allows smooth passage even in severely diseased aortas. Whenever the J-guidewire encounters resistance as it travels 'upstream' a hydrophilic wire can be used. The common femoral artery is punctured as follows: the three middle fingers of the left hand palpate the pulse and the skin is pierced with the needle three finger-breadths below the inguinal ligament. An important point to remember before penetrating the skin is to ensure that the tip of the needle known as the bevel must point upwards to aid the passage of the wire ( Figure 14 ).
After puncture of the anterior arterial wall of the artery, avoiding transfixing the artery, a 0.035-0.038 inch J-guidewire should be advanced carefully into the needle. It should move freely up the aorta and be placed at the level of the diaphragm. Problems that can be encountered in advancing the guidewire include severe arterial tortuosity, stenosis, occlusion or dissection. When there is difficulty exiting from the needle, either slightly advancing or retracting or changing the angulation of the needle will allow the guidewire to enter successfully. In this situation it is always advisable that efforts to advance the guidewire up the iliac artery or the abdominal aorta is done under fluoroscopic control. If these manoeuvers are unsuccessful, the next step is to withdraw the wire to ascertain that forceful arterial flow is still present. It is now worth considering exchanging the standard guidewire for a hydrophilic guidewire (e.g., Terumo®) which proves successful in many cases. Injecting gently a small amount of contrast through the needle can be performed in order to confirm the correct position into the radial/femoral artery. If all of these tactics fail then the needle should be removed and the groin compressed for 5 min. However if the operator feels that he or she has advanced the wire a sufficient distance to introduce a standard 15 cm sheath safely, and that the wire is indeed in the correct lumen, then it should be placed and 10 ml of contrast injected to obtain more information as to why the wire cannot be passed. Puncturing the femoral artery under fluoroscopic guidance, aiming at the middle of the femoral head, may be necessary in case of cardiac arrest, cardiogenic shock or any other condition in which the peripheral pulse is weak or absent.
In the case of severely tortuous calcified iliac arteries it can be almost impossible either to advance or to manipulate the wire or catheters past the tortuous segment. As mentioned above, crossing the obstacle normally requires a hydrophilic wire such as a Terumo® wire (Terumo Corp., Tokyo, Japan) or in extreme circumstances a coronary guidewire may be useful. To improve catheter manipulation it is generally helpful to swap the standard sheath for a long 40-75 cm sheath. This usually resolves any problems with catheter manipulation and aids the passage of any shape of coronary catheter. If there are difficulties in passing the long sheath over the softer Terumo wire then a Judkins right coronary catheter should then be passed. Once the catheter has successfully crossed the tortuous segment with the tip left at the level of the diaphragm the Terumo wire should be exchanged for a Amplatz Super Stiff™ guidewire (Boston Scientific, Natick, MA, USA). With this extra support the passage of the long sheath is almost invariably successful. In even more extreme cases a plastic sheath armoured with metal rings, such as an Arrow® sheath (Arrow International Inc., Reading, PA, USA) can help to straighten the tortuous segment. The stiffness of this type of sheath not only straightens the segment but also prevents kinking of both the sheath itself and any catheters used. If all of these measures fail, then an alternative access route such as the transradial route should be considered.
Left heart catheterisation via the femoral approach is performed using an appropriately sized vascular sheath (5-6Fr for diagnostic coronary angiography). The radial approach for standard procedures has become the access of choice in a number of institutions for several reasons: easy access, better haemostasis, early mobilisation and increased patient comfort [2525. Ziakas AG, Koskinas KC, Gavrilidis S, Giannoglou GD, Hadjimiltiades S, Gourassas I, Theofilogiannakos E, Economou F, Styliadis I. Radial versus femoral access for orally anticoagulated patients. Catheter Cardiovasc Interv. 2010;76:493-9. , 5050. Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157:132-40. ]. As with the transfemoral access transradial procedures should involve a vascular sheath as well. Intra-arterial administration of verapamil, or nitrates via the sheath will significantly limit the occurrence of vascular spasm, an issue not encountered with transfemoral access.
DRUGS USED DURING CORONARY ANGIOGRAPHY
 View chapter
View chapter
Sedation
There is no standard practice in relation to the routine use of sedation for coronary angiography. While many institutions routinely give sedation, e.g., Midazolam® (Dormicum™, Hypnovel™) at the beginning of the procedure before local anaesthetic many other institutions only consider sedation case by case. Sedation is aimed at relieving patient anxiety as they face the unknown, and to help alleviate any discomfort or pain resulting from the procedure. In many instances, correct patient information, effective counselling before the procedure, reassurance and simply talking to the patient during the examination often render the administration of sedatives superfluous.
In case sedation is needed, special preprocedural assessment of physical condition is needed with special attention for pre-existing medical conditions. Depending on the type of sedation, close monitoring of vital signs during and after the procedures are required.
Anticoagulation
Unfractionated and low molecular weight heparin
For routine diagnostic coronary angiography, an intravenous bolus of 2,500 units of unfractionated heparin (UFH) is adequate for optimal short term anticoagulation, while longer diagnostic procedures may require 5,000 units or more, guided, ideally, by onsite ACT monitoring. UFH is unique in the sense that it can be quickly neutralized by iv protamine sulfate. In the case of transradial coronary angiography it seems now that patients as a matter of course should receive 5,000 units of heparin to avoid radial artery occlusion an important and underestimated complication of transradial access.
In the case of low molecular weight heparin the patient should have received their last dose on the previous morning and all other doses withheld until after the diagnostic angiogram has been completed.
Heparin-induced thrombocytopaenia and allergy to heparin
In the vast majority of centres heparin is given to prevent thrombus formation during coronary angiography. Non-immune heparin-induced thrombocytopaenia is not infrequent, occurring in some 10% to 30% of cases; however, immune type heparin-induced thrombocytopaenia (HIT) is much less common, occurring in around 1% to 3 % of cases exposed to unfractionated heparin and 0 to 0.8% of patients receiving low molecular weight heparin [5151. Shantsila E, Lip GY, Chong BH. Heparin-induced thrombocytopenia. A contemporary clinical approach to diagnosis and management. Chest. 2009;135:1651-64. ]. HIT can be classified into Type 1 or 2, is an immune-mediated disorder, and is characterised by the formation of antibodies against the heparin-platelet factor 4 complex. In these patients heparin must be avoided, as even the smallest amount used to flush the sheath, catheters, etc, can precipitate HIT. In this case, there are several alternatives such as Lepirudin (Refludan®), a recombinant hirudin, fondaparinux, argatroban, danaparoid and bivalirudin. Obviously the same management applies to any documented allergy to heparin
Bivalirudin
Bivalirudin is a reversible direct thrombin inhibitor which is the most commonly used anticoagulant in the US in patients requiring PCI. However, it can be used in the setting of diagnostic coronary angiography at a dose of 0.15-0.2 mg/kg/hour, adjusted to an aPTT of 1.5-2.5 times baseline value [5252. Warkentin TE. Fondaparinux versus direct thrombin inhibitor therapy for the management of heparin-induced thrombocytopenia (HIT)--bridging the River Coumarin. Thromb Haemost. 2008;99:2-3. ]. While Bivalirudin originally appeared to be associated with lower rates of bleeding in the setting of PCI, these findings have now been challenged by the HEAT PPCI study.[163163. Shahzad A, Kemp I, Mars C, Wilson K, Roome C, Cooper R, Andron M, Appleby C, Fisher M, Khand A, Kunadian B, Mills JD, Morris JL, Morrison WL, Munir S, Palmer ND, Perry RA, Ramsdale DR, Velavan P, Stables RH, for the HEAT-PPCI trial investigators. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. The Lancet. 2014;384:1849-1858 ] Its benefits in the setting of only doing a diagnostic procedure are less clear and at greater expense than UFH or LMWH.
Fondaparinux
Fondaparinux has been studied in the setting of acute coronary syndromes; however, it is important to realise that catheter thrombosis has been reported. Therefore supplementary UFH should be given during the procedure.
Vasodilators
It is general practice to administer nitrates (sublingual, intravenous or intracoronary) before starting the coronary angiographic injections. The aim is to obtain maximal dilatation of the coronary arteries and prevent potential catheter spasm at the time of catheter manipulation. Nitrates, however, should be withheld when vasospasm is suspected and needless to say in patients with severe hypotension. When nitrates are only given after the first coronary injection – which is highly recommended – information regarding the presence of resting vasomotion, which can occasionally be very impressive, will be documented by the increase in vessel diameter at the time of the second coronary injection ( Figure 15 ) ( Moving image 16 , Moving image 17 ).
CONTRAST AGENTS
 View chapter
View chapter
Contrast agents are either ionic or non-ionic and are of variable osmolalities. The first agents used tended to be ionic and were generally highly hyperosmolar (1,400 to 1,800 mosmol/kg) compared to the osmolality of plasma. The second generation of agents were non-ionic and even though the osmolality was significantly lower (500 to 850 mosmol/kg; e.g., iohexol) than their predecessors the osmolality remained higher than that of plasma. Now available to us are non-ionic iso-osmolar agents (e.g., iodixanol) which have an osmolality in the region of 290 mosmol/kg. The osmolality is important because high osmolality is associated with a higher risk of contrast-induced nephropathy (CIN). With the introduction of lower osmolality agents the risk of CIN has decreased although surprisingly several studies comparing the nephrotoxicity of low osmolality contrast media to iso-osmolality contrast media have reported no additional reduction in the risk of CIN [4545. Reed M, Meier P, Tamhane UU, Welch KB, Moscucci M, Gurm HS. The relative renal safety of iodixanol compared with low-osmolar contrast media: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2009;2:645-54. ].
RADIATION DURING CATHETERISATION
 View chapter
View chapter
Radiation protection is of particular importance due to the rapidly increasing number of interventional cardiovascular procedures [5353. Rotter M, Pfiffner D, Maier W, Zeiher AM, Meier B. Interventional cardiology in Europe 1999. Eur Heart J. 2003;24:1164-70. ]. Injuries secondary to ionising radiation may be a consequence of dose-dependent deterministic effects which follow the excess of a specific threshold dose, such as radiation-induced skin lesions in patients [5454. Vañó E, Arranz L, Sastre JM, Moro C, Ledo A, Gárate MT, Minguez I. Dosimetric and radiation protection considerations based on some cases of patient skin injuries in interventional cardiology. Br J Radiol. 1998;71:510-6. ], as well as cataracts in operators [5555. Vano E, Gonzalez L, Beneytez F, Moreno F. Lens injuries induced by occupational exposure in non-optimized interventional radiology laboratories. Br J Radiol. 1998; 71:728-33. ]. The absolute local entrance dose is related to these deterministic effects. On the other hand, the often discussed increased risk for cancer, especially the risk of left sided brain tumor [164164. Roguin A, Goldstein J, Bar O, Goldstein JA. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol. 2013 111:1368-72. ] in interventional cardiologists is independent from any threshold dose since radiation induced cancer occurs secondary to stochastic effects [5656. Efstathopoulos EP, Makrygiannis SS, Kottou S, Karvouni E, Giazitzoglou E, Korovesis S, Tzanalaridou E, Raptou PD, Katritsis DG. Medical personnel and patient dosimetry during coronary angiography and intervention. Phys Med Biol . 2003;48:3059-68. , 5757. Folkerts KH, Munz A, Jung S. (Estimation of radiation exposure and radiation risk for employees of a heart catheterization laboratory). Z Kardiol. 1997;86:258-63. ]. The effective patient dose, which is related to this stochastic risk, is a weighted sum of equivalent doses delivered to various organs and ranges from 5–20 mSv per coronary angiography. Since the effective dose is difficult to assess, the so called dose-area-product (Gy/cm²) is widely used as substitute.
Patient protection
For the patient, radiation protection starts with the correct indication for any radiation exposure and continues with the ALARA principle (“As Low As Reasonably Achievable”) [5858. Balter S. An overview of radiation safety regulatory recommendations and requirements. Catheter Cardiovasc Interv. 1999;47:469-74. ]. If radiation exposure is medically indicated, every effort should be made to reduce the dose-area product (DAP), and, thus, the effective patient dose.
The highest radiation exposure for the patient is at the entry site. Part of the radiation dose is transferred into energy within the patient’s body by photoelectric interactions, part is scattered in different directions by the so called Compton Effect, and less than 1% of the entry dose reaches the image receptor ( Figure 16 ). Consequently, due to the inverse square law, stating that the level of radiation is inversely proportional to the distance squared, a high table position dramatically reduces the effective dose to the patients skin ( Figure 17 ). Another critical point is the position of the image receptor: all other conditions unchanged, moving the image receptor toward the patient lowers radiation output rate and scatter radiation dose in automated systems significantly since this measure increases the radiation dose received by the image receptor.
Other factors influencing the DAP are the use of low-level and lower pulse fluoroscopy (12.5 rather than 50 frames/sec), the reduction of fluoroscopy time and cinegraphic runs, as well as the complexity of the procedure, and the skills of the interventionalist [5959. Clark AL, Brennan AG, Robertson LJ, McArthur JD. Factors affecting patient radiation exposure during routine coronary angiography in a tertiary referral centre. Br J Radiol. 2000;73:184-9. ]. The latter are of importance since blind positioning of devices, the choice of the most adequate position with optimal visualisation, as well as the avoidance of oblique positions, do significantly contribute to the reduction of the dose-area-product. The frequently cited correlation between the dose-area-product and patients body mass index is rather weak [6060. Kuon E, Schmitt M, Dahm JB. Significant reduction of radiation exposure to operator and staff during cardiac interventions by analysis of radiation leakage and improved lead shielding. Am J Cardiol. 2002;89:44-9. , 6161. Kuon E, Glaser C, Dahm JB. Effective techniques for reduction of radiation dosage to patients undergoing invasive cardiac procedures. Br J Radiol. 2003;76:406-13. ]; however, since generally thicker tissue masses absorb more radiation and thus lead to the upregulation of intensity in automated systems, the risk for the skin is greater with steeper beam angles.
Whether the choice of access site (e.g., radial vs. femoral) does influence the dose-area-product needs further investigation and is most likely also strongly dependent on operator experience [6262. Neill J, Douglas H, Richardson G, Chew EW, Walsh S, Hanratty C, Herity N. Comparison of radiation dose and the effect of operator experience in femoral and radial arterial access for coronary procedures. Am J Cardiol. 2010;106:936-40. ]. Recently published data comparing the dose-area-product levels in six centres from different European countries led to the recommendations of the “European Research Cardiology Group for Measures for Optimizing Radiological Information and Dose in Digital Imaging and Interventional Radiology” which proposed temporary rference levels of 45 Gy/cm² and 75 Gy/cm² for coronary diagnostics and PCI respectively [6363. Neofotistou V, Vano E, Padovani R, Kotre J, Dowling A, Toivonen M, Kottou S, Tsapaki V, Willis S, Bernardi G, Faulkner K. Preliminary reference levels in interventional cardiology. Eur Radiol. 2003;13:2259-63. ]. This will be covered in greater detail in  View chapter.
View chapter.
Staff protection
The interventionist receives the highest radiation doses of the medical staff. He/she is not directly exposed to the ionising radiation beam, but to a considerable amount of scatter radiation emitted from the patient’s body and from the laboratory walls. Thus, patient radiation protection is closely related to operator radiation protection.
The scatter radiation dose is highest at the site of the highest radiation dose which is the entry site of the patient. Moreover, the scatter radiation dose is directly related to the mass the radiation beam has to penetrate. In left anterior oblique projections angulated to a cranial position the penetrated body mass is high and the radiation source is close to an operator standing on the right side of the patient, thus it is evident why for the operator, the radiation dose is 5-6 time higher in these projections than in non-angulated left or right anterior oblique, or even in angulated posterior-anterior projections [6464. Cusma JT, Bell MR, Wondrow MA, Taubel JP, Holmes DR, Jr. Real-time measurement of radiation exposure to patients during diagnostic coronary angiography and percutaneous interventional procedures. J Am Coll Cardiol. 1999;33:427-35. ]. In addition, the use of collimators wherever possible also reduces the penetrated mass and consequently the amount of scatter radiation ( Figure 18 ); however, collimation may also lead to the loss of relevant information (e.g., distal wire perforations).
Similarly the consequent use of wedges ( Figure 19 ) does not only improve the image quality, but also reduces patient’s radiation dose and thus the amount of scatter radiation. Usually wedges are positioned on the right side of the image in right anterior oblique projections and on the left side of the image in left anterior oblique projections. Finally magnification is a significant factor. The higher the magnification, the higher the dose applied to the patient’s skin and subsequently the scatter radiation dose ( Figure 20 ).
Due to the use of a lead apron the annual effective dose for the operator will in almost all cases not exceed the annual dose limit of 20 mSv. However, this does not reflect the exposure of unprotected parts of the body, such as the hands, the thyroid and the eyes. The dose to the left hand or the left eye of a right-handed operator standing on the right side of a patient exceeds the dose to his right hand or his right eye by far and may range between 120 and 510 mSv per procedure [6565. Kuon E, Dahm JB, Empen K, Robinson DM, Reuter G, Wucherer M. Identification of less-irradiating tube angulations in invasive cardiology. J Am Coll Cardiol. 2004;44:1420-8.
Systematic study of radiation angulations on a phantom-model yielding that PA views and RAO views of >or=40 degrees, should be favored over steep LAO projections >or=40 degrees whenever possible to protect the operator from excessive radiation. Tube angulations that are radiation intensive to the patient exponentially increase the operator’s radiation risk. In addition ref 59 (Clark et al.) highlights the factors affecting patient radiation exposure during coronary angiography.]. Depending on the caseload, it is possible that an operator may reach the recommended limits of 150 mSv for the eye and 500 mSv for the hands. Consequently the use of additional shielding such as a lead glass screen, lead shield under the table, lead glasses and a thyroid-shield are strongly recommended. However, these measures do not sufficiently protect the hands. Awareness of the operator is currently still the best protection to avoid any unnecessary fluoroscopy to the hands.
The most important protection for medical staff is distance from the radiation source and the patient. Again, due to the inverse square law, a person standing 2m from the radiation source receives more than double the dose than somebody standing 3m away, and fourfold the dose of somebody standing 4m from the radiation source. Therefore, just learning to hit the cine pedal with the left foot, instead of the right, will distance the operator at least another 20cm from the radiation beam.
RaySafe® is an active dosimetry system that gives real-time insight about personal radiation exposure, as well as access to time stamped dose data. By providing easily accessible information about radiation exposure, this system allows medical staff to immediately change their behaviour in order to minimize their radiation dose.
CATHETER SELECTION, MANIPULATION AND ANGIOGRAPHIC VIEWS
Improvements in catheter technology have allowed the flow rate obtained with old 8 Fr (1 Fr = 0.33 mm) diagnostic catheters to be achieved with 6 Fr thin-walled catheters and satisfactory coronary opacification even with 5 Fr and 4 Fr diagnostic catheters. Newly developed automatic injectors with adjustable increases in injection pressure have the potential to allow more consistent homogeneous opacification of large left coronary arteries through 4 Fr-5 Fr catheters. The drawback of using smaller size catheters is lesser torque control and stability with catheter recoil during forceful injections resulting in sub-selective contrast injections, less contrast flow with inadequate visualisation of the coronary arteries. Judkins catheters are used in many laboratories over the world for both the right and the left coronary artery. Their ease of use stems from the fact that both catheters are preshaped. The left Judkins catheter typically consists of two distal curves and comes in different curve lengths [3, 3.5, 4, 5 and 6]. Other pre-shaped catheters (e.g., Amplatz) can be used for injecting both coronary vessels ( Figure 21 ). While initially the same type of preformed coronary catheters (Judkins, Amplatz) were used for the radial/brachial approach with somewhat less ease of manipulation, more dedicated catheters are now available for radial procedures such as the Kimny (Boston Scientific®), Optitorque Tiger, Jacky and Sarah (Terumo®), Sones (Cordis®) and PaPa (Medtronic®) catheters.
While it may seem obvious it is mandatory to prepare catheters before they can be used. Specifically, all catheters need to be flushed with heparinised saline to avoid development of thrombus during the procedure.
Angiographic views are labelled according to the position of the x-ray machine in relation to the patient ( Figure 22 ). Since the acquired images are only 2-dimensional, two orthogonal views are generally required for a correct diagnosis and adequate treatment guidance.
Unless indicated otherwise radiographic images should be acquired in deep inspiration to minimise the radiation and to dissociate the shadow of the diaphragm from the heart silhouette. The number and length of cine runs should be balanced between the necessity for obtaining maximal diagnostic information and the concern with radiation and contrast exposure.
Left ventriculogram
Catheter selection and manipulation
In case of transradial access when universal catheters are used for right and left coronary angiography it is recommended not to use this catheter for the left ventriculography for reasons of risks involved of damaging the left ventricle by the increased force of injecting a large volume through a single side-hole cathter. Exchanging the universal catheter over a guidewire to a pigtail catheter is therefore recommended.
As mentioned earlier, left ventricular angiography is an essential part of the examination with pigtail catheters being the first choice. Crossing the aortic valve in the absence of aortic valve disease should be done without force or extra manipulation. Firstly the pigtail catheter is gently pushed against the aortic valve until the catheter curves upward almost in the shape of the number 6. Then the catheter is gently pulled back while simultaneously applying a clockwise rotation allowing the catheter to jump across the valve into the ventricle. Once the pigtail catheter enters the ventricle an anticlockwise rotation will bring the catheter across the inflow of the mitral valve ( Figure 23 ). Positioning the catheter in the apex of the left ventricle will invariably result in a burst of premature ventricular contractions (PVC’s) during the angiogram with pseudo-mitral regurgitation and regional abnormal contraction patterns ( Moving image 18 , Moving image 19 , Moving image 20 , Moving image 21 ). In the presence of aortic valve stenosis the J curved guidewire needs to be exchanged for a straight tip guidewire. The difficulty of crossing the stenotic valve has less to do with the severity of the gradient than with the changing plane of the valve. In this case, due to its tip orientation, the use of a left Amplatz or a right Judkins catheter will facilitate crossing the valve.
After entering the left ventricle cavity, a correct measurement of the left ventricular end diastolic pressure is the first and foremost measurements to evaluate global LV function. Optimal visualisation of the LV requires the injection of an adequate volume of contrast (0.7 ml/kg not exceeding 50 ml except in large dilated ventricles) at an optimal flow rate (15 ml/sec) and is performed using a power injector. The amount of total contrast volume and flow rate needs to be adjusted according to the LV volume, stroke volume, and LV end diastolic filling pressures. Volume may therefore vary from 30-60 ml and flow rate from 10-20ml/sec. In the presence of severe heart failure with elevated filling pressures total volume needs to be reduced (30ml) and flow rate increased (20ml/sec). One must be aware, that injecting 45-50 ml in a short time equals an acute volume loading and may worsen an already compromised function. In case the LVEDP is elevated, nitrates should be administered before performing the angiogram. If the high EDP reflects ongoing myocardial ischaemia, nitrates will decrease filling pressures. If, on the other hand the high EDP is the result of a chronic condition, nitrates will only marginally decrease EDP and angiography will be well tolerated.
Myocardial staining can occur as a consequence of improper guidewire or catheter handling or positioning in relation to the free LV wall. Usually this is of no clinical concern. Forced drainage of contrast through the network of thebesian veins into the coronary sinus venous sytem can be observed ( Moving image 22 ).
Optimal angiographic views
Usually, the left ventricular angiogram is obtained in two orthogonal views: 30˚ RAO and 40°-60° LAO. With plain fluoroscopy, cardiac implants and cardiovascular calcifications can be seen and analysed ( Moving image 23 , Moving image 24 , Moving image 25 , Moving image 26 , Moving image 27 ).
The RAO projection ( Figure 24 ) shows the longitudinal axis of the left ventricle, and thus allows the assessment of the high anterolateral, anterior wall, the apex, and the inferior and inferoposterior wall. In this projection the mitral valve separates clearly the left ventricle and the left atrium and allows diagnosing even mild regurgitation. The LAO projection is an optimal addition to the RAO projection ( Figure 24 ). It allows assessment of the interventricular septum, (in particular with an additional cranial angulation of 15°-20°), the left ventricular outflow tract (e.g., in the presence of hypertrophic obstructive cardiomyopathy), the high lateral and the posterolateral wall. While the RAO projection allows assessing the movement of the posterior mitral leaflet only, the movement of the anterior mitral leaflet can be judged very well in the LAO projection, just as well as the left and right coronary leaflet of the aortic valve.
In the presence of significant mitral regurgitation a 90° LAO projection is sometimes helpful, while a ventricular septal defect is optimally visualised in a cranially angulated LAO view (e.g., 15°-20° CRAN, 40-60° LAO).
If case biplane angiography is available, more information is obtained with less contrast compared to two separate injections in monoplane angiography. In a monoplane setting, the RAO projection is the optimal view to analyse left ventricular wall motion, volumes, and mitral regurgitation.
Left coronary artery
Catheter selection and manipulation
In case of transradial access left coronary angiography can usually be performed using the same femoral catheter curves taking into account that a shorter JL curve will often result in a proper fit into the ostium. However, there is a tendency of using more often universal or multipurpose catheters for left and right coronary angiography, a possibility that also exists in case of femoral access but never gained the same popularity for obvious reasons. The main benefit of using this universal catheter seems to be the one-pass technique with less catheter exchange, less spasm, presumed less fluoroscopy times and cost compared to multi catheter procedure. The drawbacks of using universal catheters are, difficulty in manipulation, less optimal coaxial engagement, risk of deep intubation, and notwithstanding these , in the case of PCI, the notorious suboptimal back up provided.
Intubation of the left main coronary artery should be done with the possibility of left main disease in mind. Retrieving the guidewire when the catheter tip lies before the ostium of the left main should be done slowly as sudden recoil of the catheter tip may injure the left main by entering the vessel forcefully. The operator should focus on the way the catheter tip is engaged in the left main coronary artery.
Although this seems like a detail, it is of paramount importance that the catheter tip is coaxial to the left main stem to avoid potential injury to the vessel wall, especially in the presence of plaque. This is more often a challenge in case of the transradial approach. Any reduction in arterial pressure and/or change in the morphology of the arterial waveform, such as that seen with ventricularisation (low diastolic values) of the pressure waveform, should alert the operator that the catheter is obstructing flow. This may be caused by the presence of a true ostial stenosis, by the small size of the coronary ostium or by deep engagement of the catheter beyond the left main. Injection of intracoronary nitrates and gentle test injections with careful withdrawal of the catheter can identify and solve these various problems. It is very important not to start the injection before the angiographic image acquisition in order to identify calcifications or late staining of contrast. Contrast injection should be sufficient to fully replace the epicardial vascular volume and avoid the phenomenon of streaming or incomplete visualisation. When the proximal coronary segments are fully opacified, the prolongation of injection offers no diagnostic advantage, increases the contrast volume used and carries potential risks in vessels with a small epicardial volume and/or slow flow. On the other hand, angiographic image acquisition should be prolonged to allow visualisation of the distal vessels, identification of flow characteristics and characterisation of type of dissection (with/without persistence of contrast at the end of the injection). An important determinant of injection duration is the need to visualise collaterals for occluded vessels, which also means adjustment of the view to include the recipient vessel in the image.
Selection of coronary catheters should aim at an optimal coaxial atraumatic intubation of the coronary artery and should, therefore, in the first place be based on the patient size and the dimension of the aortic root, which can be easily appreciated from a routine chest x-ray ( Figure 25 ). Rather than opting for “one curve fits all” (4.0 Judkins left is still the standard catheter in many laboratories) the choice of the catheter should be guided by the dimension of the ascending aorta ( Figure 26 ). In the absence of a chest x-ray a rule of thumb is to start with a 5.0 Judkins in patients older than 60 years or when arterial hypertension or aortic valve disease is present, situations invariably associated with elongation of the aorta and/or dilated aortic root. Smaller sizes, 3.5 or 3 Judkins can be a first choice in small female patients, while large sizes (6 Judkins or Amplatz left 3) may be needed in the presence of a very dilated ascending aorta.
The optimal view for engaging the left coronary artery is the left anterior oblique view where the ostium is not covered by the aorta. The left coronary artery requires only minimal catheter manipulation: the J-tipped 0.89 mm (0.035 inch) wire is carefully advanced to the level of the aortic valve. Following this, the Judkins catheter is advanced into the left coronary cusp while gently retracting the wire. This leaves the catheter below the left main stem orifice and now gentle retraction of the catheter along with clockwise (or in some cases anticlockwise) rotation should lead to gentle selective intubation of the left main. Incidentally, it is vital not to remove the guidewire too forcefully after positioning the Judkins catheter in the cusp since this may precipitate a rapid upward spring-like motion of the catheter tip into the left main coronary artery, leading to injury to the left main stem which only becomes apparent as a dissection following the first injection of contrast. Before engaging the left main or any vessel it is important to recognize two things; (1) that blood is coming freely from the catheter ensuring that the catheter has been purged of air and (2) to obtain a satisfactory arterial pressure trace before making any injection. Once retrograde bleeding is seen, the pressure line can be safely connected and a test injection performed. By injecting a puff of contrast we then know the location of the catheter tip, that is, either selectively engaged or located immediately above, below or in front of the ostium. In the latter case, gentle withdrawal of the catheter tip (helped by asking the patient to take a deep breath) will allow engagement of the catheter tip in most cases. If the tip of the catheter immediately closes in the ascending aorta, prolonged attempts with the same catheter should be avoided and rapid switching to a larger catheter is probably more advantageous in terms of time lost and contrast used. When it is already known that the coronary ostia are of an unusual size or position, such as that seen in aortic valve disease, Marfan syndrome or congenital heart disease, it is probably worthwhile performing an aortic angiogram in the left anterior oblique view in order to guide catheter selection, since this may require unusual shapes (e.g., left Judkins 6.0 or left Amplatz 2.0 and 3.0). In the case of a separate origin of the LAD and LCx, referred to by some as the “double-barrelled ostia” it is important to recognise that two catheters such as a JL 4.0 and 3.5 will invariably be necessary to satisfactorily visualise both arteries. In general, in order to selectively intubate the LAD when it has its own ostium a smaller sized catheter is required.
Optimal angiographic views ( Figure 27 ) ( Table 7 ).
Although individual preferences between operators exist as to which view to select to intubate the LCA, it is advised to intubate the LCA in LAO 50°-55° for the simple reason that if the catheter does not spontaneous enter the left main artery the operator will be able to judge more easily the reason why (abnormal high origin, wrong size of the catheter).
The main advantage of the so-called spider view (left 60°-70°, caudal 25°-40°) is to delineate the branching of the left main coronary artery from the aorta and its bifurcation into the left anterior descending (LAD) and left circumflex (LCx) arteries (or trifurcation if an intermediate artery is present). For this reason it is better to acquire this view first in order to exclude this most fearsome lesion location at risk of plaque disruption during catheter injection and which may require an urgent revascularisation ( Moving image 28 ). The LAD artery is greatly foreshortened in the mid and distal segments in this view but stenosis of the proximal segment or of the ostium of the first diagonal branch is often optimally shown. The LCx artery, on the other hand, is optimally opened in its proximal and mid segments. The classical 30°-40° RAO view is limited by the frequent superimposition of the proximal LAD and LCx and should be replaced by shallow right caudal views, which opens up the LAD/LCx bifurcation and helps minimise radiation ( Figure 28 ). A smaller angulation to the right (10°-20°) avoids superimposition of the catheter and the spine and, combined with relatively skewed caudal angulations (30°-40°), opens the angle between LAD and LCx (and intermediate branch, if present) sufficiently to obtain optimal images of the proximal and mid segment of the LCx and bifurcation of its marginal branches. The image is of more limited interest for the proximal and mid LAD because of the frequent superimposition of diagonal and septal branches but remains the most important view for the distal LAD. Skewed cranial views (40° or more), with 5°-10° angulation to the right to avoid superimposition of the catheter and the vertebral spine, elongate the proximal and mid LAD and open the bifurcation of the proximal diagonal branches which run to the right, clearly distinguished from the septal branches which run to the left of the screen. The segments that are foreshortened in this view are the distal LAD and the proximal LCx but this view is also ideal for visualising the distal posterolateral branches of the LCx and, in the 15%-20% of cases with left dominance or codominance, the left posterior descending artery (PDA). In the left cranial view (30°-45° left, 25°-40° cranial) the LAD is further elongated by asking the patient to take a deep breath and maintain breath-holding during injection. The cranial view also offers optimal views of the mid and distal segments of the LCx, and is especially useful in the presence of a dominant LCx ( Figure 29 ). The lateral view is less often used because of technical issues in case of radial approach. The only additional value of this view is limited, only providing excellent visualisation of the mid/distal LAD around the apex, information which is at most, complementary to right caudal views ( Figure 30 ). Occasionally however, eccentric short lesions of the proximal and mid segment of the LAD might be covered by septal or diagonal branches in all the conventional previously reported views and can be visualised better in the lateral projection, sometimes using variable cranial or caudal angulations to ensure no superimposition from the diagnostic catheter and side branches.
Right coronary artery
In case of transradial access right coronary angiography can usually be performed using the same femoral catheter curves taking into account that a longer JR curve will often result in a better fit into the ostium. As for the left coronary angiography there is this tendency of using more often universal or multipurpose catheters for both left and right coronary angiography for reasons mentioned higher.
Catheter selection for cannulation of the right coronary artery should be based on the same principle as for the left coronary artery taking into account the size of the aortic root. Intubation of the RCA should be performed in the LAO projection if transradial access has been used and in either LAO or lateral if the transfemoral access route has been chosen. However it is arguably easier to engage the right coronary ostium in the lateral projection than the LAO projection. After positioning the catheter in the right coronary sinus, the catheter must be rotated towards the left of the screen to enable selective intubation of the right coronary orifice. This is achieved better when the rotation is accompanied by a slow pull-back motion. Breath-holding after a deep inspiration may facilitate this manoeuvre. In 10%-15% of cases a high origin of the RCA complicates the search for the right coronary ostium. Even in the presence of a hypoplastic non-dominant RCA, selective injection is still required because small proximal branches can be an important source of collaterals for occluded vessels. It is often possible to obtain a semi-selective injection with the Judkins catheter that will further guide catheter selection. A multipurpose catheter should be used for downward-looking RCAs, and Amplatz right 2 or Amplatz left 1 or 2 are required in patients with a high take-off and/or with dilatation of the coronary sinus and ascending aorta. Not infrequently the intubation of the RCA will elicit catheter spasm due to direct contact of the catheter tip with the first curve of the right coronary artery. It is mandatory to administer intracoronary nitrates to differentiate this angiographic artifact from a genuine atherosclerotic lesion.
Careful review of the images should be performed before finishing the examination in order to avoid missing a separate origin from the aorta or an abnormal origin of the LCx from the proximal RCA (the most frequent coronary anomaly) or a separate origin of a conus branch that provides important collaterals to occluded arteries. Not infrequently the catheter tip will enter a conus branch where the origin is separate from the RCA or very proximal in the RCA, resulting in pressure damping ( Moving image 29 ). In this case, an alternative catheter such as a right Amplatz can be used. A less orthodox but very useful way to solve the problem of always intubating the conus branch is to shorten the right Judkins catheter by cutting the tip with a sharp blade. This will easily overcome the problem, and was first demonstrated by Andreas Grüntzig himself.
Optimal angiographic views ( Figure 27 ) ( Table 8 ).
The RCA has few branches in the first, second and third segments (from the ostium to the crux cordis) and often two views (left anterior and right anterior oblique views) are sufficient to identify all stenoses, including eccentric stenosis ( Figure 31 , Figure 32 ). The lateral view is more helpful for intubating the RCA and might be ideal for assessment of the mid segment of the artery and may occasionally be used as a working projection for occlusions in this segment or stent positioning. The problems with these standard views lie in the difficulties of interpretation in the presence of stenoses at the crux cordis or at the ostia of the PDA and posterolateral branches. Cranial angulation (30°) of the left anterior view is often sufficient to solve diagnostic questions but many operators prefer to use as a routine view a cranial (30°) posteroanterior view of the RCA, which clearly delineates the region of the crux cordis and possible lesions of the PDA or posterolateral branches.
Venous bypass grafts, free arterial grafts and internal mammary artery
The targets for visualisation of venous and arterial aorto-coronary bypass grafts are: i) the proximal graft anastomosis with the aorta, ii) the main body of the bypass graft, iii) the distal graft anastomosis, and iv) the periphery of the native vessel distal to the anastomosis.
Aortic anastomoses of venous grafts and free arterial grafts are rarely indicated by radio-opaque markers positioned at the time of operation (a neglected practice in cardiac surgery), but can often be visualised in the left anterior oblique view a few centimetres above the ostium of the RCA by dragging the right Judkins catheter along the right profile of the ascending aorta. Although the position and direction of aortic anastomoses is influenced by the surgical technique, in general the anastomosis for the RCA tends to be the lowest and to have a more vertical origin from the aorta, so that selective cannulation may require the use of a multipurpose catheter. Grafts for the marginal or diagonal branches often require catheter rotation and occasionally catheters with a longer tip (right or left Amplatz 1 and 2, left coronary bypass catheters, multipurpose catheters or dedicated graft finders) are required. Instead of wasting a large amount of contrast in locating the ostium, it can be cost-effective to perform an aortogram with the pigtail catheter slightly above the usual supravalvular position, immediately above the level of the RCA, in order to ascertain at least the number of open grafts. A limitation is obviously the inability to detect grafts with extremely slow flow; however, these are often identified because of the presence of contrast staining in cases of recent occlusion.
For the left mammary artery, the origin of the subclavian arteries can be more easily engaged in a left anterior oblique view (40°-60°). Complete visualisation of the internal mammary is of paramount importance because it is crucial not only to know whether the left internal mammary artery is patent but also to exclude the presence of distal stenoses (anastomotic or in the distal native vessels) and to visualise collaterals for other occluded vessels. The selective visualisation of the mammary artery is more easily achieved with a specially designed catheter, which has a longer tip than the classical right Judkins catheter. In case of failure, other types of modified left internal mammary catheter with a hook-like shape can be tested. Selective engagement is often made difficult by the presence of severe tortuosity of the subclavian artery, which makes manipulation of the internal mammary catheter very difficult. The problems are greater for the right internal mammary artery because of its more tortuous track from the ascending aorta. A power injection through 6 Fr large-lumen diagnostic or guide catheters can occasionally avoid the troubles and risks of a true super selective injection of the internal mammary arteries in very tortuous and frail subclavian vessels, reducing brachial flow with a pressure cuff inflated around the arm. Alternatively, the transradial approach (more often left as the left mammary is most frequently used) can be the safest and quickest solution especially if multiple attempts from the groin remain unsuccessful. Selective intubation of the contralateral IMA (LIMA from the right radial or RIMA from the left radial is also feasible but technically more demanding.In some instances, selective instead of sub-selective visualisation is important for diagnostic reasons. The use of a standard coronary guidewire is then worth trying. This wire will often easily slip into the RIMA or LIMA, despite the fact that the tip of the catheter is centimetres away from the ostium of the vessel, and guide the catheter selectively into the ostium.
The distal anastomosis of the mammary artery is often optimally opened in the lateral view, which is also ideal for excluding adhesion of the left internal mammary to the sternum, a condition which may increase the risk of surgical re-intervention with median sternotomy.
Selective injection of the gastroepiploic artery- a graft only rarely used today- requires knowledge of the anatomy of the coeliac trunk. Selective catheterisation of the coeliac trunk is usually performed using a Cobra or SIM 1 or 2 catheter (all from Cook, Bloomington, IN, USA), mounted on a Terumo® guidewire. After selective catheterisation of the common hepatic artery, which departs from the coeliac trunk, repeated contrast injections will help visualise the gastro-duodenal artery. After selective catheterisation of the gastro-duodenal artery further contrast injections will visualise the gastroepiploic artery and its anastomosis to the RCA ( Figure 33 ).
Renal – iliac – carotid arteries.
Taking advantage of the transfemoral vascular access, it may be useful in cases of arterial hypertension or suspected renovascular disease to inject both renal arteries using the right coronary artery catheter on the way out ( Figure 34 ) ( Moving image 30 ). Similarly, selective injections of the carotid arteries at the end of the diagnostic angiography in case of suspected carotid artery disease may increase the diagnostic yield of the examination. In the presence of known peripheral vascular disease or in case mounting the diagnostic catheters was difficult or if port access surgery or transcatheter aortic valve implantation (TAVI) is planned, information regarding the iliac arteries could easily be obtained by local contrast injection at the end of the procedure through a pigtail catheter.
PROVOCATION TESTS
In patients undergoing a coronary angiography, provocation tests should be performed whenever the clinical history is suggestive of vasospastic angina (angina at rest, nocturnal angina) and in particular when only minimal angiographic lesion(s) are present. The test is of little or no value in patients who experience angina only during exercise.
Ergonovine
Ergonovine is an ergot alkaloid, stimulates alpha-adrenergic and serotonergic receptors and therefore exerts a direct constrictive effect on vascular smooth muscle. Systemic injections of incremental doses of methylergonovine maleate (50,100,300 μg (administered at 3-5 min interval will exert its vasoconstrictor effect via the alpha-adrenergic receptors. Minimal diffuse (< 20%) reduction in coronary diameter is a normal pharmacological response [6666. Bertrand ME, Lablanche JM, Tilmant PY. Treatment of Prinzmetal’s variant angina. Role of medical treatment with nifedipine and surgical coronary revascularization combined with plexectomy. Am J Cardiol. 1981;47:174-8. ]. Focal narrowing of a coronary artery associated with clinical symptoms and/or ECG changes and relieved with IC nitrates or calcium blockers is considered to be a positive test.( Figure 35 ) ( Moving image 31 , Moving image 32 ) The similarity of ergonovine-induced and spontaneous attacks of variant angina was demonstrated by Curry et al. [6767. Curry RC, Jr., Pepine CJ, Sabom MB, Conti CR. Similarities of ergonovine-induced and spontaneous attacks of variant angina. Circulation. 1979;59:307-12. ]. Although the test can also be performed non-invasively, for example in the coronary care unit, we highly recommend that the test be performed in the cathlab for two reasons; 1) performing the test in association with the visualisation of the coronary arteries offers the advantage to visualise the location, extent and severity of coronary spasm and 2) immediately relieve the coronary spasm through intracoronary injections of nitrates or calcium channel blockers. Be aware however that the vasodilatory effect of the calcium channel blockers is significantly slower than that of nitrates in this case.
Acetylcholine
The vascular endothelium plays an important role in modulating blood vessel tone by releasing different dilator and constrictor substances. Nitric oxide (NO) is probably the most potent endothelium-derived relaxing factor and is released in response to a variety of receptor-dependent and receptor-independent factors as well as to rheological stimuli such as increased shear stress. Acetylcholine (ACH) causes endothelium-dependent vasodilatation due to release of NO via endothelium muscarinic receptors. Several clinical studies have shown that there is a progressive impairment in endothelium-mediated vasodilatation with ACH with different stages of atherosclerosis and thus of with endothelium dysfunction [6868. Zeiher AM, Drexler H, Wollschlager H, Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391-401. , 6969. Quyyumi AA, Dakak N, Andrews NP, Husain S, Arora S, Gilligan DM, Panza JA, Cannon RO 3rd. Nitric oxide activity in the human coronary Circulation. Impact of risk factors for coronary atherosclerosis. J Clin Invest. 1995;95:1747-55. , 7070. Egashira K, Inou T, Hirooka Y, Yamada A, Maruoka Y, Kai H, Sugimachi M, Suzuki S, Takeshita A. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. J Clin Invest. 1993;91:29-37. ]. Therefore, the response to ACH has been advocated as a surrogate for the evaluation of endothelium integrity.
Acetylcholine is infused directly into a coronary artery using a microcatheter, mounted in a guiding catheter. If this microcatheter is equipped with a Doppler sensor, flow velocity measurements are available. ACH is infused over a 1- minute period in incremental doses of 10, 30, 100 and 300 μg and doses should be separated by 5-minute intervals. Coronary diameter (by QCA) and flow velocity measurements are performed at rest and at each dose level of ACH. It is of note that in the absence of an intact endothelium ACH elicits vasoconstriction through direct stimulation of its specific muscarinic receptors on vascular smooth muscle cells [7171. Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046-51. ].
Cold pressure test
Immersion of the hand and forearm in ice will elicit an α-adrenergic response, characterised by an increase in heart rate, systolic and mean arterial blood pressure and cardiac output. While in normal individuals this stress will elicit an increase in coronary flow and decrease in coronary vascular resistance, in patients with underlying coronary atherosclerosis it may cause coronary vasoconstriction, decrease in regional coronary flow resulting in changes in regional LV function [7272. Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation. 1988;77:43-52. ].
Atrial pacing test
Rapid atrial pacing (110, 130, 150 beats/min) has been shown to dilate epicardial arteries and increase coronary blood flow in angiographic normal coronary arteries but failed to do so in arteries with minimal atherosclerosis and elicit paradoxically vasoconstriction in patients with advanced obstructive atherosclerosis [7373. Nabel EG, Selwyn AP, Ganz P. Paradoxical narrowing of atherosclerotic coronary arteries induced by increases in heart rate. Circulation. 1990;81:850-9. ].
Vasodilatation of epicardial coronary arteries in response to augmentation in coronary blood flow is mainly mediated through increased shear stress and depends on the integrity of the endothelium function. Response to atrial pacing is therefore a valuable surrogate to assess endothelium integrity.
Hyperventilation
Hyperventilation is also a known spasmogenic stimulus and requires the full cooperation of the patient. Hyperventilation is performed with the patient breathing as deeply as possible 30 times a minute as long as 5 minutes whereby an increase in the arterial pH (7.6-7.7) is targeted. The sensitivity of this test is somewhat lower than the pharmacological tests [7474. Nakao K, Ohgushi M, Yoshimura M, Morooka K, Okumura K, Ogawa H, Kugiyama K, Oike Y, Fujimoto K, Yasue H. Hyperventilation as a specific test for diagnosis of coronary artery spasm. Am J Cardiol. 1997;80:545-9. ].
Recommendations during coronary angiography
- Nitrates at the start of the procedure will often mask the presence of resting vasomotor tone. Delay nitrates until after the first coronary injection.
- Limiting radiation dose and radiation protection are as important as antiseptic measures
- Correct catheter selections will speed up the procedure, guarantee optimal contrast visualisation and limit catheter-induced complications
- High quality images will enhance the diagnostic accuracy and allow for optimal therapeutic decision
- Provocation tests should be considered whenever vasospastic angina (angina at rest, nocturnal angina) is suspected, in particular when only minimal angiographic lesions are present
Post - procedure
SHEATH REMOVAL AND POST-PROCEDURAL PATIENT CARE
Despite an increased use of radial access, the femoral approach is still used in many cathlabs. Standard manual compression after sheath removal followed by an external compressive bandage for several hours, especially with smaller sheath sizes (4-6 Fr) and after reversing systemic heparinisation with protamine is normally not an issue. However, in obese patients and/or with intensive anticoagulant and antiplatelet therapy haematomas, even large ones, can occasionally occur. This approach may be time consuming but at the same time allows some reassuring conversation with the patient.
Mechanical passive closure devices are available, such as the Femstop® device (RADI Medical Systems AB, Uppsala, Sweden), and are substitutes for manual compression; however, their clinical value is unclear.
In case of transradial access manual compression after removing the sheath can often stop the bleeding. A number of devices have been designed to provide haemostasis, from a simple bandage or haemodialysis band compressing a gauze bullet over the radial arterial puncture to specifically designed compression bands. Regardless the chosen device a number of steps need to be taken into account as described in  View chapter .
View chapter .
VASCULAR CLOSURE DEVICES
Vascular closure devices will be covered in greater detail in  View chapter, although it is worth mentioning that they have been introduced to alleviate potential complications from manual sheath compression in particular, the occurrence of haematomas. The use of vascular closure devices is a procedure in its own right and should be mastered like any other procedure. Operators should be aware of contraindications for applying closing devices such as small (< 5 mm) artery size, presence of peripheral vascular disease at the puncture site, anomalous branches or calcifications.
View chapter, although it is worth mentioning that they have been introduced to alleviate potential complications from manual sheath compression in particular, the occurrence of haematomas. The use of vascular closure devices is a procedure in its own right and should be mastered like any other procedure. Operators should be aware of contraindications for applying closing devices such as small (< 5 mm) artery size, presence of peripheral vascular disease at the puncture site, anomalous branches or calcifications.
Several different types of devices are available: Suture-based, collagen-based, non-collagen based (e.g., polyglycolic acid [PGA]) and clip closure. Their major advantage comes when trying to obtain haemostasis when larger calibre sheaths are being used which is seldom the case for diagnostic procedures. However it is worth bearing in mind that the use of vascular closure devices does not mean that significant haematomas will never occur.  View chapter.
View chapter.
DATA ANALYSIS, DIAGNOSIS AND PROTOCOL
Analysis of global and regional left ventricle function
Global left ventricular function and regional wall motion analyses are clinically important since therapeutic strategies as well as prognostic information can be derived. LV ejection fraction as well as regional function is measured either visually or by automated contour analysis ( Figure 36 ). Regional function analysis may be helpful to distinguish between normal functioning, possibly ischaemic, and stunned / hibernating myocardium or myocardial necrosis, respectively. Figure 37 illustrates the different segments to be analysed both in RAO and LAO views. Table 9 summarises the qualitative description of regional function abnormalities. LV angiography will help delineate and locate aneurysms ( Moving image 33) as well as occasionally identify the typical Tako-Tsubo pattern in the absence of significant coronary lesions. ( Moving image 34 , Moving image 35 ).
There are two fundamentally different methods for estimating left ventricular volume: Simpson's Rule and the Dodge-Sandler Area-Length Method ( Figure 38 ). However, both methods also have much in common. Both depend on the accurate determination of the LV border. Both methods also require the determination of the long axis of the LV. The long axis is typically defined as a line between the mid-point of the aortic valve and the left ventricular apex. Because the apex can be defined as the point farthest from the mid-point of the aortic valve, the long axis can be determined automatically.
Simpson's Rule is a fundamental mathematical principle [7575. Schiller NB, Acquatella H, Ports TA, Drew D, Goerke J, Ringertz H, Silverman NH, Brundage B, Botvinick EH, Boswell R, Carlsson E, Parmley WW. Left ventricular volume from paired biplane two-dimensional echocardiography. Circulation. 1979;60:547-55. ]. It is based on the idea that the volume of an object can be determined by "cutting" the object into thin "slices", measuring the volume of each slice and summing the volumes of all slices. Simpson's Rule is applied to the left ventricle by slicing the left ventricle into "discs" along the long axis. If a single-plane angiogram is used, each disc is assumed to be circular, since only one diameter is known. If biplane angiography is used, each disc is assumed to be an ellipse, with a major axis determined from one plane and the minor axis determined from the other plane. The area of each disc is calculated and multiplied by the disc's thickness to determine its volume.
The Dodge-Sandler Area-Length Method is dependent upon the assumption of an elliptical shape of the left ventricle [7676. Dodge HT, Sandler H, Ballew DW, Lord JD, Jr. The use of biplane angiocardigraphy for the measurement of left ventricular volume in man. Am Heart J. 1960;60:762-76. ]. The calculation of the volume of an ellipsoid of revolution requires knowledge of the length of the axis and the area of the ellipse. The length of the long axis of the left ventricle is taken as the length of the axis of revolution and the area of the left ventricle is used as area of the ellipse.
Assessment of mitral insufficiency from left ventriculography
Mitral insufficiency can only be assessed semi-quantitatively from observing the retrograde filling of the left atrium during LV angiography. First and foremost attention should be directed to avoid catheter induced premature beats during contrast injection since these may induce several degrees of mitral insufficiency. The correct position of the pigtail catheter has been described earlier. In addition, the volume of the contrast injected should be adequate to fill the left ventricle homogeneous. This is particularly important in the presence of LV dilatation. Besides the LV dimensions, other anatomical and haemodynamic factors such as filling pressures, left atrial dimensions, presence of atrial fibrillation, the acute versus chronic regurgitation, rate and injected volume will affect the degree of regurgitation.
Mitral insufficiency is generally graded 1-4 according to the degree of opacification of the left atrium by the regurgitant flow ( Table 10 ). Regarding the limitation for accurate assessment angiographic evaluation should always be compared with echocardiographic findings. Bear in mind that mitral regurgitation is often a dynamic process and therefore very sensitive to the filling pressures at the time of angiography. Volume loading in patients who are hypovolemic, often seen after a prolonged diuretic treatment, may be necessary to unmask severe regurgitation.
Analysis of the coronary angiogram
Degree of stenosis
In clinical practice, the severity of a coronary lesion is routinely estimated by online visual screening by the operator. This method is often an approximation of the diameter reduction and is prone to error, particularly in eccentric lesions, bifurcation lesions and in the presence of diffuse disease where the reference diameter may not reflect a truly normal arterial segment. The conventional angiographic classification is based on visual estimation of the diameter reduction of the stenosis compared to a normal segment and is calculated in the projection where the most severe narrowing is observed. The early lesion severity classification ranged from normal (<25%), to low grade stenoses (25-49%), to intermediate grade stenoses (50-74%), high grade stenoses (75-90%), subtotal (91-99%) and total occlusion (100%) [88. Gensini GG, Coronary arteriography. Mount Kisco N.Y. 1975.
A detailed work for everyone, especially the section covering the anatomy of normal coronary arteries as well as the anatomy of coronary arteries in disease is highly recommended. This monograph may no longer be available but is integrated in Braunwald’s Heart Disease. A textbook of Cardiovascular Medicine, 1980.].
It is crucial to obtain at least two unforeshortened orthogonal views of each vessel segment with no overlaps and sufficient contrast filling to allow for a proper diagnosis. Errors can also derive from misclassification due to coronary spasm, misdiagnosed coronary anomalies, deep intubation of ostial stenoses, poststenotic dilatation, and from inadequate judgement by the operator. However it is misleading to talk about a stenosis of 90% in the presence of normal TIMI 3 flow since this would require an unphysiologically high transstenotic pressure gradient. The limitations of eyeballing a stenosis are well recognised and have led to the development of less subjective measurements such as quantitative coronary analysis, intravascular ultrasound and fractional flow reserve.
Alternative methods - Quantitative coronary angiography (QCA) is an automated, computer based method for the analysis of two dimensional coronary angiographic images with automated contour detection. It is available for immediate online or post hoc offline analysis and can overcome operator related errors, but requires rigorous standardisation of image acquisition to be reliable ( Figure 39 ).
Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are intracoronary imaging methods which can be used in addition to the two-dimensional angiography and allow for true three-dimensional quantification of coronary dimensions.
Morphology of coronary stenoses
Several different systems exist to describe the morphology of a coronary stenosis. Parameters to describe coronary lesions, in addition to the percentage of lumen obstruction are, the length of the stenosis, the eccentricity, tortuosity, degree of calcification, and relation to bifurcation.
The classification system that is mostly accepted is the AHA/ACC classification system ( Table 11 ). In addition, specific classifications for e.g., in-stent restenosis [7777. Mehran R, Mintz GS, Hong MK, Tio FO, Bramwell O, Brahimi A, Kent KM, Pichard AD, Satler LF, Popma JJ, Leon MB. Validation of the in vivo intravascular ultrasound measurement of in-stent neointimal hyperplasia volumes. J Am Coll Cardiol. 1998;32:794-9. , 7878. Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW, Leon MB. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100:1872-8. ] and bifurcation lesions are available [7979. Medina A, Suarez de Lezo J, Pan M. (A new classification of coronary bifurcation lesions). Rev Esp Cardiol. 2006;59:183. ].
Myocardial perfusion
The Thrombosis in Myocardial Infarction (TIMI) study group proposed angiographic criteria to assess anterograde blood flow in patients with acute myocardial infarction as a surrogate for myocardial perfusion measurements. ( Table 12 ) These criteria can also be applied in patients with NSTEMI and in patients with stable conditions. The blush grade (0, 1, 2 and 3) is a visual estimate of anterograde perfusion ( Table 13 ) [8080. Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, McCabe CH, Van De Werf F, Braunwald E. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000;101:125-30. ]. The TIMI frame count was subsequently proposed as a semi-quantitative method to quantify myocardial perfusion. It is defined as the contrast density in the region perfused by the target vessel and in particular the infarct related area. Blush should be assessed distal to the culprit lesion. Angiographic views should be chosen to minimise superimposition of non-infarcted territories in the assessment of the culprit artery’s TIMI Grade. The duration of cine filming should exceed 3 cardiac cycles in the washout phase to assess the washout of the myocardial blush. This would require filming for approximately 5 to 6 cardiac cycles. Care should be taken not to mistake filling of the venous system, such as the great cardiac vein, as blush. This appears as a linear vascular structure rather than as a diffuse or round collection of dye. Blush should be assessed during the same phase of the cardiac cycle as it may be different during diastole [8080. Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, McCabe CH, Van De Werf F, Braunwald E. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000;101:125-30. ].
Scoring systems
Over the years a number of scoring systems have been proposed to serve particular needs. The AHA classification was initially proposed as a reporting system based on the definition of the coronary tree system. (AHA) This classification was later modified and used in ARTS I and II trials [8181. Serruys PW, Ong AT, van Herwerden LA, Sousa JE, Jatene A, Bonnier JJ, Schönberger JP, Buller N, Bonser R, Disco C, Backx B, Hugenholtz PG, Firth BG, Unger F. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol. 2005;46:575-81. , 8282. Serruys PW, Onuma Y, Garg S, Vranckx P, De Bruyne B, Morice MC, Colombo A, Macaya C, Richardt G, Fajadet J, Hamm C, Schuijer M, Rademaker T, Wittebols K, Stoll HP; ARTS II Investigators. 5-year clinical outcomes of the ARTS II (Arterial Revascularization Therapies Study II) of the sirolimus-eluting stent in the treatment of patients with multivessel de novo coronary artery lesions. J Am Coll Cardiol. 2010;55:1093-101. ].
Leaman score
The ‘Leaman score’ is based on the severity of lumen diameter narrowing (a lesion is defined as significant when 50% diameter reduction is present by visual assessment) and weighed according to the usual blood flow to the myocardium in each vessel or vessel segment. According to this scoring system, in the presence of a right dominant system 84% of the blood flow to the left ventricle is derived from the left coronary artery and 16 % from the right coronary artery. In this case 64% of the blood flow to the left ventricle is derived from the left anterior descending coronary artery and 33% from the left circumflex coronary artery. In other words the relative contribution of the left main coronary artery, the left anterior descending coronary artery and the left circumflex coronary artery to the perfusion of the left ventricle is respectively: 5, 3.5 and 1.5 times the contribution of the right coronary artery. The distribution varies in the case of a left dominant system where the right coronary artery does not supply blood to the left ventricle [8383. Leaman DM, Brower RW, Meester GT, Serruys P, van den Brand M. Coronary artery atherosclerosis: severity of the disease, severity of angina pectoris and compromised left ventricular function. Circulation. 1981;63:285-99. ].
ACC/AHA Classification
The ACC/AHA lesion classification system focuses on overall lesion characteristics in view of anticipated procedural difficulty and success rate ( Table 11). The major characteristics include: lesion length, eccentricity, angulation, presence of calcification and/or thrombus, involvement of side branches, and severity of stenosis. Typically, lesions are categorised into Type A (high success rate and low risk, Type B (moderate success and moderate risk or Type C (low success and high risk) [55. Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, Legako RD, Leon DF, Murray JA, Nissen SE, Pepine CJ, Watson RM, Ritchie JL, Gibbons RJ, Cheitlin MD, Gardner TJ, Garson A Jr, Russell RO Jr, Ryan TJ, Smith SC Jr. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation.1999;99:2345-57. , 8484. Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB 3rd, Loop FD, Peterson KL, Reeves TJ, Williams DO, Winters WL Jr, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation. 1988;78:486-502. ].
Classification of bifurcations
Several classification systems for bifurcation and trifurcation lesions have been proposed, such as Duke and ICPS bifurcation lesion classification [126126. Louvard Y, Thomas M, Dzavik V, Hildick-Smith D, Galassi AR, Pan M, Burzotta F, Zelizko M, Dudek D, Ludman P, Sheiban I, Lassen JF, Darremont O, Kastrati A, Ludwig J, Iakovou I, Brunel P, Lansky A, Meerkin D, Legrand V, Medina A, Lefèvre T. Classification of coronary artery bifurcation lesions and treatments: time for a consensus! Catheter Cardiovasc Interv. 2008;71:175-83. ] Lefèvre et al. [8585. Lefevre T, Louvard Y, Morice MC, Dumas P, Loubeyre C, Benslimane A, Premchand RK, Guillard N, Piéchaud JF. Stenting of bifurcation lesions: classification, treatments, and results. Catheter Cardiovasc Interv. 2000;49:274-83. ], Medina et al. [7979. Medina A, Suarez de Lezo J, Pan M. (A new classification of coronary bifurcation lesions). Rev Esp Cardiol. 2006;59:183. ], of which the latter classification appeals through its simplicity. These classifications will be dealt with in  View chapter.
View chapter.
Syntax Score
As a preamble to the SYNTAX (SYNergy between PCI with TAXUS™ and Cardiac Surgery) trial the investigators designed the SYNTAX score to characterise the coronary anatomy prospectively with respect to the number of lesions and their functional impact, location and complexity. This scoring system combines the elements from the AHA system, the Leaman score and the ACC/AHA lesion classification system as well as elements from the bifurcation and trifurcation lesion classification, and the CTO classification system. An important characteristic of the SYNTAX score is that it is lesion based. For each lesion a separate score is calculated. (see Figure 40 ).
The final SYNTAX score is calculated by a computer program consisting of sequential and interactive self-guided questions. The higher the SYNTAX score the higher the complexity of the condition, therefore representing a bigger therapeutic challenge with a potentially worse prognosis [8686. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, Serruys PW. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219-27.
This reference is important to understand this scoring system and why it helps to better determine the most appropriate revascularization strategy.]. The SYNTAX score calculator is accessible through the SYNTAX score website (www.syntaxscore.com). Combining the SYNTAX score with a set of clinical factors ( age, female gender, COPD, PVD, creatine clearance, LVEF, presence of unprotected left main) resulted into the SYNTAX score II which turned out to be a significantly better predictor for the 4-year mortality comparing patients undergoing CABG with those undergoing PCI. [165165. Farooq V, van Klaveren D, Steyerberg EW, Meliga E, Vergouwe Y, Chieffo A, Kappetein AP,Colombo A, Holmes Jr DR, Mack M, Feldman T, Morice M-C, Stahle E, Onuma Y, Morel M-A, Garcia-Garcia HM, van Es GA, Dawkins KD, Mohr F, Serruys PW. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet 2013; 381:639-650 ]
QUANTITATIVE CORONARY ANGIOGRAPHY
Visual assessment of stenosis severity is still being employed in everyday clinical practice and in the inclusion criteria of randomised trials. However, this “eyeballing” practice has been associated with considerable intraobserver and interobserver variability, usually resulting in overestimation of severe stenosis and vice versa [8787. Detre KM, Wright E, Murphy ML, Takaro T. Observer agreement in evaluating coronary angiograms. Circulation. 1975;52:979-86. , 8888. DeRouen TA, Murray JA, Owen W. Variability in the analysis of coronary arteriograms. Circulation. 1977;55:324-8. , 8989. Gensini GG, Kelly AE, Da Costa BC, Huntington PP. Quantitative angiography: the measurement of coronary vasomobility in the intact animal and man. Chest. 1971;60:522-30. , 9090. Toth GG, Toth B, Johnson NP, De Vroey F, Di Serafino L, Pyxaras S, Rusinaru D, Di Gioia G, Pellicano M, Barbato E, Van Mieghem C, Heyndrickx GR, De Bruyne B, Wijns W. Revascularization decisions in patients with stable angina and intermediate lesions: results of the international survey on interventional strategy. Circ Cardiovasc Interv. 2014;7:751-759. ]. Quantitative coronary angiography (QCA) has evolved from the era of digital calipers into highly sophisticated software algorithms [9191. Serruys PW, Reiber JH, Wijns W, van den Brand M, Kooijman CJ, ten Katen HJ, Hugenholtz PG. Assessment of percutaneous transluminal coronary angioplasty by quantitative coronary angiography: diameter versus densitometric area measurements. Am J Cardiol. 1984;54:482-8. , 9292. Reiber JH, Serruys PW, Kooijman CJ, Wijns W, Slager CJ, Gerbrands JJ, Schuurbiers JC, den Boer A, Hugenholtz PG. Assessment of short-, medium-, and long-term variations in arterial dimensions from computer-assisted quantitation of coronary cineangiograms. Circulation. 1985;71:280-8. , 9393. Gronenschild E, Janssen J, Tijdens F. CAAS. II: A second generation system for off-line and on-line quantitative coronary angiography. Cathet Cardiovasc Diagn. 1994;33:61-75. , 9494. Van der Zwet PM, Reiber JH. A new approach for the quantification of complex lesion morphology: the gradient field transform; basic principles and validation results. J Am Coll Cardiol 1994;24:216-24. , 9595. Dvir D, Marom H, Guetta V, Kornowski R. Three-dimensional coronary reconstruction from routine single-plane coronary angiograms: in vivo quantitative validation. Int J Cardiovasc Intervent 2005. 7:141-145. , 9696. Ramcharitar S, Onuma Y, Aben JP, Consten C, Weijers B, Morel MA, Serruys PW. A novel dedicated quantitative coronary analysis methodology for bifurcation lesions. EuroIntervention. 2008; 3:553-557. , 9797. Schuurbiers JC, Lopez NG, Ligthart J, Gijsen FJ, Dijkstra J, Serruys PW, Van der Steen AF, Wentzel JJ. In vivo validation of CAAS QCA-3D coronary reconstruction using fusion of angiography and intravascular ultrasound (ANGUS). Catheter Cardiovasc Interv. 2009;73:620-6. , 9898. Tu S, Koning G, Jukema W, Reiber JH. Assessment of obstruction length and optimal viewing angle from biplane X-ray angiograms. Int J Cardiovasc Imaging. 2010;26:5-17. , 9999. Tuinenburg JC, Koning G, Rares A, Janssen JP, Lansky AJ, Reiber JH. Dedicated bifurcation analysis: basic principles. Int J Cardiovasc Imaging. 2011; 27:167-174. , 100100. Girasis C, Schuurbiers JC, Muramatsu T, Aben JP, Onuma Y, Soekhradj S, Morel MA, van Geuns RJ, Wentzel JJ, Serruys PW. Advanced three-dimensional quantitative coronary angiographic assessment of bifurcation lesions: methodology and phantom validation. EuroIntervention. 2013; 8:1451-1460. ] providing us with accurate and precise results on diameter, area, length and angulation measurements from target segments in the coronary artery tree ( Figure 39). Being based on the analysis of a “luminogram”, QCA derived measurements have a modest correlation with invasive imaging [101101. Ozaki Y, Violaris AG, Kobayashi T, Keane D, Camenzind E, Di Mario C, de Feyter P, Roelandt JR, Serruys PW. Comparison of coronary luminal quantification obtained from intracoronary ultrasound and both geometric and videodensitometric quantitative angiography before and after balloon angioplasty and directional atherectomy. Circulation . 1997;96:491-9. , 102102. Abizaid AS, Mintz GS, Abizaid A, Mehran R, Lansky AJ, Pichard AD, Satler LF, Wu H, Kent KM, Leon MB. One-year follow-up after intravascular ultrasound assessment of moderate left main coronary artery disease in patients with ambiguous angiograms. J Am Coll Cardiol. 1999;34:707-15. , 103103. Bruining N, Tanimoto S, Otsuka M, Weustink A, Ligthart J, de Winter S, van Mieghem C, Nieman K, de Feyter PJ, van Domburg RT, Serruys PW. Quantitative multi-modality imaging analysis of a bioabsorbable poly-L-lactic acid stent design in the acute phase: a comparison between 2- and 3D-QCA, QCU and QMSCT-CA. EuroIntervention. 2008;4:285-91. , 104104. Gutierrez-Chico JL, Serruys PW, Girasis C, Garg S, Onuma Y, Brugaletta S, Garcia-Garcia H, van Es GA, Regar E. Quantitative multi-modality imaging analysis of a fully bioresorbable stent: a head-to-head comparison between QCA, IVUS and OCT. Int J Cardiovasc Imaging. 2012; 28:467-478 ] and fractional flow reserve [105105. Koo BK, Kang HJ, Youn TJ, Chae IH, Choi DJ, Kim HS, Sohn DW, Oh BH, Lee MM, Park YB, Choi YS, Tahk SJ. Physiologic assessment of jailed side branch lesions using fractional flow reserve. J Am Coll Cardiol. 2005;46:633-7. , 106106. Yong AS, Ng AC, Brieger D, Lowe HC, Ng MK, Kritharides L. Three-dimensional and two-dimensional quantitative coronary angiography, and their prediction of reduced fractional flow reserve. Eur Heart J. 2010;32:345-53. , 107107. Naganuma T, Latib A, Costopoulos C, Takagi K, Naim C, Sato K, Miyazaki T, Kawaguchi M, Panoulas VF, Basavarajaiah S, Figini F, Chieffo A, Montorfano M, Carlino M, Colombo A. The role of intravascular ultrasound and quantitative angiography in the functional assessment of intermediate coronary lesions: correlation with fractional flow reserve. Cardiovasc Revasc Med. 2014;15:3-7. , 108108. Voros S, Rinehart S, Vazquez-Figueroa JG, Kalynych A, Karmpaliotis D, Qian Z, Joshi PH, Anderson H, Murrieta L, Wilmer C, Carlson H, Ballard W, Brown C. Prospective, head-to-head comparison of quantitative coronary angiography, quantitative computed tomography angiography, and intravascular ultrasound for the prediction of hemodynamic significance in intermediate and severe lesions, using fractional flow reserve as reference standard (from the ATLANTA I and II Study). Am J Cardiol. 2014;113:23-29. ]. Even though QCA has not yet been shown to impact on clinical outcomes or cost effectiveness, it does offer the potential for a reliable diagnosis and has been the generally accepted standard in the follow-up of studies evaluating intracoronary devices such as stents [109109. Serruys PW, Kay IP, Disco C, Deshpande NV, de Feyter PJ. Periprocedural quantitative coronary angiography after Palmaz-Schatz stent implantation predicts the restenosis rate at six months: results of a meta-analysis of the BElgian NEtherlands Stent study (BENESTENT) I, BENESTENT II Pilot, BENESTENT II and MUSIC trials. Multicenter Ultrasound Stent In Coronaries. J Am Coll Cardiol. 1999;34:1067-74. , 110110. Pocock SJ, Lansky AJ, Mehran R, Popma JJ, Fahy MP, Na Y, Dangas G, Moses JW, Pucelikova T, Kandzari DE, Ellis SG, Leon MB, Stone GW. Angiographic surrogate end points in drug-eluting stent trials: a systematic evaluation based on individual patient data from 11 randomized, controlled trials. J Am Coll Cardiol. 2008;51:23-32. ] and the progression-regression of atherosclerosis [111111. De Feyter PJ, Serruys PW, Davies MJ, Richardson P, Lubsen J, Oliver MF. Quantitative coronary angiography to measure progression and regression of coronary atherosclerosis. Value, limitations, and implications for clinical trials. Circulation. 1991;84:412-23. ].
In the interest of consistency and reproducibility a standard operator procedure for acquisition and analysis has to be followed. From each digital angiogram that clearly displays the coronary artery segment to be analysed, a homogeneously filled end-diastolic frame is selected, usually out of the second or third cardiac cycle. In this way motion blur is minimised and bias of frame selection is avoided. Calibration can be performed against an object of known dimensions, conventionally the catheter. The contours of the contrast-filled arteries can be traced via either edge-detection or videodensitometric algorithms [112112. Di Mario C, Haase J, den Boer A, Reiber JH, Serruys PW. Edge detection versus densitometry in the quantitative assessment of stenosis phantoms: an in vivo comparison in porcine coronary arteries. Am Heart J. 1992;124:1181-9. , 113113. Haase J, Escaned J, van Swijndregt EM, Ozaki Y, Gronenschild E, Slager CJ, Serruys PW. Experimental validation of geometric and densitometric coronary measurements on the new generation Cardiovascular Angiography Analysis System (CAAS II). Cathet Cardiovasc Diagn. 1993;30:104-14. ]. Based on the final lumen contours, the diameter function is determined as the distance between the corresponding left and right contour positions for every step along the artery centreline; the lowest value of the diameter function for the arterial segment analyzed is defined as the minimal luminal diameter (MLD). Owing to the much higher image quality and contrast resolution of digital flat panels [114114. Van Herck PL, Gavit L, Gorissen P, Wuyts FL, Claeys MJ, Bosmans JM, Benali K, Vrints CJ. Quantitative coronary arteriography on digital flat-panel system. Catheter Cardiovasc Interv. 2004;63:192-200. , 115115. Tuinenburg JC, Koning G, Seppenwoolde Y, Reiber JH. Is there an effect of flat-panel-based imaging systems on quantitative coronary and vascular angiography? Catheter Cardiovasc Interv. 2006;68:561-6. ] and the evolution of detection algorithms, current QCA software can provide accurate and precise measurements even for diameter values considerably smaller than 1.00mm [116116. Girasis C, Schuurbiers JC, Onuma Y, Aben JP, Weijers B, Morel MA, Wentzel JJ, Serruys PW. Advances in two-dimensional quantitative coronary angiographic assessment of bifurcation lesions: improved small lumen diameter detection and automatic reference vessel diameter derivation. EuroIntervention. 2012; 7:1326-1335. ].
MLD is the single most important parameter derived from QCA, having the best possible correlation with fractional flow reserve as opposed to the per cent diameter stenosis (DS) [117117. Beatt KJ, Luijten HE, de Feyter PJ, van den Brand M, Reiber JH, Serruys PW. Change in diameter of coronary artery segments adjacent to stenosis after percutaneous transluminal coronary angioplasty: failure of percent diameter stenosis measurement to reflect morphologic changes induced by balloon dilation. J Am Coll Cardiol. 1988;12:315-23. , 118118. Serruys PW, Foley DP, de Feyter PJ. Restenosis after coronary angioplasty: a proposal of new comparative approaches based on quantitative angiography. Br Heart J. 1992;68:417-24. ]. The latter is calculated at the site of the MLD, according to the equation DS= 1-MLD/RVD, RVD being the reference vessel diameter. In order to determine RVD, a “user-defined” or an “interpolated” reference can be selected [9191. Serruys PW, Reiber JH, Wijns W, van den Brand M, Kooijman CJ, ten Katen HJ, Hugenholtz PG. Assessment of percutaneous transluminal coronary angioplasty by quantitative coronary angiography: diameter versus densitometric area measurements. Am J Cardiol. 1984;54:482-8. , 119119. Lansky A, Tuinenburg J, Costa M, Maeng M, Koning G, Popma J, Cristea E, Gavit L, Costa R, Rares A, Van Es GA, Lefevre T, Reiber H, Louvard Y, Morice MC; European Bifurcation Angiographic Sub-Committee. Quantitative angiographic methods for bifurcation lesions: a consensus statement from the European Bifurcation Group. Catheter Cardiovasc Interv. 2009;73:258-66. ]. The former is usually the average of the mean diameters of apparently normal segments proximal and distal to the target lesion, resulting in a horizontal RVD function; alternatively this user-defined value could be the single largest diameter value (Dmax) proximal or distal to the MLD position [120120. Gomez-Lara J, Diletti R, Brugaletta S, Onuma Y, Farooq V, Thuesen L, McClean D, Koolen J, Ormiston JA, Windecker S, Whitbourn R, Dudek D, Dorange C, Veldhof S, Rapoza R, Regar E, Garcia-Garcia HM, Serruys PW. Angiographic maximal luminal diameter and appropriate deployment of the everolimus-eluting Bioresorbable Vascular Scaffold as assessed by optical coherence tomography. EuroIntervention. 2011 Oct 28. pii: 2010;1124-01. [Epub ahead of print]. ]. In the interpolation method, the reference diameter function is determined by a regression technique resulting in a straight tapering line, which approximates the perceived normal or disease-free arterial contour over the obstruction site [9191. Serruys PW, Reiber JH, Wijns W, van den Brand M, Kooijman CJ, ten Katen HJ, Hugenholtz PG. Assessment of percutaneous transluminal coronary angioplasty by quantitative coronary angiography: diameter versus densitometric area measurements. Am J Cardiol. 1984;54:482-8. ]. Because of the angiogram’s inability to show the full extent of atherosclerotic burden, presumed healthy reference segments are frequently diseased impacting on the validity of RVD and DS measurements. Nevertheless, RVD determination has to be based on the diameter function, in order to provide the operator with a reliable estimate of the true reference size of the vessel. Appropriate sizing of intracoronary devices and careful selection of deployment pressure has been shown to ensure optimal apposition to the vessel wall, at the same time avoiding edge dissections and/or vessel perforations [120120. Gomez-Lara J, Diletti R, Brugaletta S, Onuma Y, Farooq V, Thuesen L, McClean D, Koolen J, Ormiston JA, Windecker S, Whitbourn R, Dudek D, Dorange C, Veldhof S, Rapoza R, Regar E, Garcia-Garcia HM, Serruys PW. Angiographic maximal luminal diameter and appropriate deployment of the everolimus-eluting Bioresorbable Vascular Scaffold as assessed by optical coherence tomography. EuroIntervention. 2011 Oct 28. pii: 2010;1124-01. [Epub ahead of print]. , 121121. De Jaegere P, Mudra H, Figulla H, Almagor Y, Doucet S, Penn I, Colombo A, Hamm C, Bartorelli A, Rothman M, Nobuyoshi M, Yamaguchi T, Voudris V, DiMario C, Makovski S, Hausmann D, Rowe S, Rabinovich S, Sunamura M, van Es GA. Intravascular ultrasound-guided optimized stent deployment. Immediate and 6 months clinical and angiographic results from the Multicenter Ultrasound Stenting in Coronaries Study (MUSIC Study). Eur Heart J. 1998;19:1214-23. , 122122. Russo RJ, Silva PD, Teirstein PS, Attubato MJ, Davidson CJ, DeFranco AC, Fitzgerald PJ, Goldberg SL, Hermiller JB, Leon MB, Ling FS, Lucisano JE, Schatz RA, Wong SC, Weissman NJ, Zientek DM; AVID Investigators. A randomized controlled trial of angiography versus intravascular ultrasound-directed bare-metal coronary stent placement (the AVID Trial). Circ Cardiovasc Interv. 2009;2:113-23. ].
The derivation of the true reference size is even more difficult around a bifurcation lesion, especially at the ostium of a side branch [119119. Lansky A, Tuinenburg J, Costa M, Maeng M, Koning G, Popma J, Cristea E, Gavit L, Costa R, Rares A, Van Es GA, Lefevre T, Reiber H, Louvard Y, Morice MC; European Bifurcation Angiographic Sub-Committee. Quantitative angiographic methods for bifurcation lesions: a consensus statement from the European Bifurcation Group. Catheter Cardiovasc Interv. 2009;73:258-66. ]. The vessel segments distal to the bifurcation carina taper in size, their reference diameter being dictated by laws of fractal geometry [123123. Finet G, Gilard M, Perrenot B, Rioufol G, Motreff P, Gavit L, Prost R. Fractal geometry of arterial coronary bifurcations: a quantitative coronary angiography and intravascular ultrasound analysis. EuroIntervention. 2008;3:490-8. ]. However, the intuitive way in which even experienced operators interpret stenosis severity at or close to the ostium of distal bifurcation branches, is by referring to the proximal vessel, thus resulting in overestimated DS values [8989. Gensini GG, Kelly AE, Da Costa BC, Huntington PP. Quantitative angiography: the measurement of coronary vasomobility in the intact animal and man. Chest. 1971;60:522-30. ]. Conversely, though to a lesser extent, lesions in the proximal vessel may be underestimated. This effect is also reproduced when conventional QCA algorithms are applied to bifurcation lesions [124124. Ishibashi Y, Grundeken MJ, Nakatani S, Iqbal J, Morel MA, Genereux P, Girasis C, Wentzel JJ, Garcia-Garcia HM, Onuma Y, Serruys PW. In vitro validation and comparison of different software packages or algorithms for coronary bifurcation analysis using calibrated phantoms: Implications for clinical practice and research of bifurcation stenting. Catheter Cardiovasc Interv. 2015;85:554-63 ]. In order to overcome these shortcomings, dedicated bifurcation QCA algorithms have been developed that report MLD, RVD and DS separately for all three vessel segments around the bifurcation region. The bifurcation region itself is treated in a different way compared to single vessel segments; luminal diameters, MLD and RVD function for this region are derived with methodology specific to software developer [9999. Tuinenburg JC, Koning G, Rares A, Janssen JP, Lansky AJ, Reiber JH. Dedicated bifurcation analysis: basic principles. Int J Cardiovasc Imaging. 2011; 27:167-174. , 116116. Girasis C, Schuurbiers JC, Onuma Y, Aben JP, Weijers B, Morel MA, Wentzel JJ, Serruys PW. Advances in two-dimensional quantitative coronary angiographic assessment of bifurcation lesions: improved small lumen diameter detection and automatic reference vessel diameter derivation. EuroIntervention. 2012; 7:1326-1335. , 125125. Girasis C, Schuurbiers JC, Onuma Y, Aben JP, Weijers B, Boersma E, Wentzel JJ, Serruys PW. Two-dimensional quantitative coronary angiographic models for bifurcation segmental analysis: in vitro validation of CAAS against precision manufactured plexiglas phantoms. Catheter Cardiovasc Interv. 77(6):830-839. ]. In addition to MLD, RVD and DS, QCA analysis of bifurcation lesions can also quantify the angulation between bifurcation branches. According to the European Bifurcation Club definition, a proximal and a distal bifurcation angle are delineated between the proximal main vessel and the side branch, and between the distal main vessel and the side branch, respectively [126126. Louvard Y, Thomas M, Dzavik V, Hildick-Smith D, Galassi AR, Pan M, Burzotta F, Zelizko M, Dudek D, Ludman P, Sheiban I, Lassen JF, Darremont O, Kastrati A, Ludwig J, Iakovou I, Brunel P, Lansky A, Meerkin D, Legrand V, Medina A, Lefèvre T. Classification of coronary artery bifurcation lesions and treatments: time for a consensus! Catheter Cardiovasc Interv. 2008;71:175-83. ]. Bifurcation angulation is the single most important piece of information next to diameter derived data when planning bifurcation PCI, as it has been shown to impact both treatment strategy, as well as angiographic and clinical outcome [127127. Dzavik V, Kharbanda R, Ivanov J, Ing DJ, Bui S, Mackie K, Ramsamujh R, Barolet A, Schwartz L, Seidelin PH. Predictors of long-term outcome after crush stenting of coronary bifurcation lesions: importance of the bifurcation angle. Am Heart J. 2006;152:762-9. , 128128. Adriaenssens T, Byrne RA, Dibra A, Iijima R, Mehilli J, Bruskina O, Schomig A, Kastrati A. Culotte stenting technique in coronary bifurcation disease: angiographic follow-up using dedicated quantitative coronary angiographic analysis and 12-month clinical outcomes. Eur Heart J. 2008;29:2868-2876. , 129129. Girasis C, Farooq V, Diletti R, Muramatsu T, Bourantas CV, Onuma Y, Holmes DR, Feldman TE, Morel MA, van Es GA, Dawkins KD, Morice MC, Serruys PW. Impact of 3-dimensional bifurcation angle on 5-year outcome of patients after percutaneous coronary intervention for left main coronary artery disease: a substudy of the SYNTAX trial (synergy between percutaneous coronary intervention with taxus and cardiac surgery). JACC Cardiovasc Interv. 2013;6:1250-1260. ].
Due to the eccentric nature of coronary lesions, diameter/area measurements derived from a single two-dimensional (2D) image should be interpreted with caution [9191. Serruys PW, Reiber JH, Wijns W, van den Brand M, Kooijman CJ, ten Katen HJ, Hugenholtz PG. Assessment of percutaneous transluminal coronary angioplasty by quantitative coronary angiography: diameter versus densitometric area measurements. Am J Cardiol. 1984;54:482-8. ]. Moreover, notwithstanding the expertise of even highly experienced operators, foreshortening of the target vessel segments in selected views could be unappreciated, resulting in inadequate length measurements [130130. Green NE, Chen SY, Hansgen AR, Messenger JC, Groves BM, Carroll JD. Angiographic views used for percutaneous coronary interventions: a three-dimensional analysis of physician-determined vs. computer-generated views. Catheter Cardiovasc Interv. 2005;64:451-9. ]. Overlap of adjacent branches, especially when filming the left coronary artery, could hamper our analysis, especially in the case of bifurcation lesions, where it is important to clearly visualise the ostium of the side branch. Lastly, a further geometric error can be introduced, if the calibration object and analysed vessel segment are not in the same plane, resulting in a difference in magnification (out-of plane magnification) [131131. Green NE, Chen SY, Messenger JC, Groves BM, Carroll JD. Three-dimensional vascular angiography. Curr Probl Cardiol. 2004;29:104-42. ]. All these well-known pitfalls of 2D angiography and QCA analysis may result in erroneous sizing and deployment of stents; this could translate into either incomplete lesion coverage and need for additional stents, or excessive stent length, jailing of a side branch and increased restenosis rates [9898. Tu S, Koning G, Jukema W, Reiber JH. Assessment of obstruction length and optimal viewing angle from biplane X-ray angiograms. Int J Cardiovasc Imaging. 2010;26:5-17. , 132132. Gollapudi RR, Valencia R, Lee SS, Wong GB, Teirstein PS, Price MJ. Utility of three-dimensional reconstruction of coronary angiography to guide percutaneous coronary intervention. Catheter Cardiovasc Interv . 2007;69:479-82. , 133133. Sadamatsu K, Sagara S, Yamawaki T, Tashiro H. Three-dimensional coronary imaging for the ostium of the left anterior descending artery. Int J Cardiovasc Imaging 2009;25:223-8. ].
In the last 10 years 3D QCA algorithms have been shown to be accurate, precise and reproducible [9595. Dvir D, Marom H, Guetta V, Kornowski R. Three-dimensional coronary reconstruction from routine single-plane coronary angiograms: in vivo quantitative validation. Int J Cardiovasc Intervent 2005. 7:141-145. , 9797. Schuurbiers JC, Lopez NG, Ligthart J, Gijsen FJ, Dijkstra J, Serruys PW, Van der Steen AF, Wentzel JJ. In vivo validation of CAAS QCA-3D coronary reconstruction using fusion of angiography and intravascular ultrasound (ANGUS). Catheter Cardiovasc Interv. 2009;73:620-6. , 9898. Tu S, Koning G, Jukema W, Reiber JH. Assessment of obstruction length and optimal viewing angle from biplane X-ray angiograms. Int J Cardiovasc Imaging. 2010;26:5-17. , 100100. Girasis C, Schuurbiers JC, Muramatsu T, Aben JP, Onuma Y, Soekhradj S, Morel MA, van Geuns RJ, Wentzel JJ, Serruys PW. Advanced three-dimensional quantitative coronary angiographic assessment of bifurcation lesions: methodology and phantom validation. EuroIntervention. 2013; 8:1451-1460. ]. In order to facilitate on-line analysis, most algorithms do not create a volumetric reconstruction of the true morphology of the vessel lumen, which can be computationally demanding, but rather a 3D model which is based on the reconstruction of 3D centreline data [131131. Green NE, Chen SY, Messenger JC, Groves BM, Carroll JD. Three-dimensional vascular angiography. Curr Probl Cardiol. 2004;29:104-42. ]. Such a reconstruction requires at least two 2D angiographic images separated by at least 30° in viewing angle, having at the same time the least amount of vessel overlap and foreshortening; images acquired by either a biplane, monoplane or a rotational angiographic system can be used. Calibration can be performed against the catheter in the first selected 2D image, if necessary [132132. Gollapudi RR, Valencia R, Lee SS, Wong GB, Teirstein PS, Price MJ. Utility of three-dimensional reconstruction of coronary angiography to guide percutaneous coronary intervention. Catheter Cardiovasc Interv . 2007;69:479-82. ], however full automatic calibration is the default (and only) choice in other algorithms [9898. Tu S, Koning G, Jukema W, Reiber JH. Assessment of obstruction length and optimal viewing angle from biplane X-ray angiograms. Int J Cardiovasc Imaging. 2010;26:5-17. , 100100. Girasis C, Schuurbiers JC, Muramatsu T, Aben JP, Onuma Y, Soekhradj S, Morel MA, van Geuns RJ, Wentzel JJ, Serruys PW. Advanced three-dimensional quantitative coronary angiographic assessment of bifurcation lesions: methodology and phantom validation. EuroIntervention. 2013; 8:1451-1460. ].
Analysis is initiated by demarcating the target vessel segment (possibly a bifurcation) on the first selected 2D image and subsequently running automatic contour detection. Automatically the region of interest is indicated in the other selected 2D image(s), in order to assist the analyst in demarcating the correct vessel segment, which is then traced with the same contour detection algorithm. Reference points representing corresponding anatomical landmarks (e.g. bifurcation points) are identified in the selected 2D images, in order to correct for the isocentre offset, that is the geometric distortion of the angiographic system [9898. Tu S, Koning G, Jukema W, Reiber JH. Assessment of obstruction length and optimal viewing angle from biplane X-ray angiograms. Int J Cardiovasc Imaging. 2010;26:5-17. , 100100. Girasis C, Schuurbiers JC, Muramatsu T, Aben JP, Onuma Y, Soekhradj S, Morel MA, van Geuns RJ, Wentzel JJ, Serruys PW. Advanced three-dimensional quantitative coronary angiographic assessment of bifurcation lesions: methodology and phantom validation. EuroIntervention. 2013; 8:1451-1460. ]; automatically determined reference points are verified or relocated, as appropriate. Finally, the 3D reconstruction of the target vessel segment can be performed within seconds rendering a 3D image and data on area/diameter derived parameters, lesion length and, when applicable, bifurcation angulation [100100. Girasis C, Schuurbiers JC, Muramatsu T, Aben JP, Onuma Y, Soekhradj S, Morel MA, van Geuns RJ, Wentzel JJ, Serruys PW. Advanced three-dimensional quantitative coronary angiographic assessment of bifurcation lesions: methodology and phantom validation. EuroIntervention. 2013; 8:1451-1460. , 134134. Girasis C, Serruys PW, Onuma Y, Colombo A, Holmes DR Jr, Feldman TE, Bass EJ, Leadley K, Dawkins KD, Morice MC. 3-Dimensional Bifurcation Angle Analysis in Patients With Left Main Disease A Substudy of the SYNTAX Trial (SYNergy Between Percutaneous Coronary Intervention With TAXus and Cardiac Surgery). JACC Cardiovasc Interv. 2010;3:41-48. , 135135. Tu S, Jing J, Holm NR, Onsea K, Zhang T, Adriaenssens T, Dubois C, Desmet W, Thuesen L, Chen Y, Reiber JH. In vivo assessment of bifurcation optimal viewing angles and bifurcation angles by three-dimensional (3D) quantitative coronary angiography. Int J Cardiovasc Imaging. 2012;28:1617-1625. ].
The benefits of 3D QCA are probably even more pronounced in the analysis of bifurcation lesions, because of the inherently three-dimensional curvilinear course of bifurcating vessels. A dedicated bifurcation 3D QCA algorithm has been recently developed, adopting the 2D algorithms regarding the derivation of MLD and RVD within the bifurcation region [100100. Girasis C, Schuurbiers JC, Muramatsu T, Aben JP, Onuma Y, Soekhradj S, Morel MA, van Geuns RJ, Wentzel JJ, Serruys PW. Advanced three-dimensional quantitative coronary angiographic assessment of bifurcation lesions: methodology and phantom validation. EuroIntervention. 2013; 8:1451-1460. ]; by applying this methodology to a 3D model, results of 2D and 3D bifurcation analysis became directly comparable. Preliminary in-vivo data suggest that the dedicated bifurcation 3D QCA algorithm is expected to provide more accurate information regarding vessel size, degree and location of stenosis severity within or adjacent to the bifurcation region.
Another relative merit of most 3D QCA algorithms, especially important for the assessment and treatment of bifurcation lesions, is the determination of the optimal viewing angle [100100. Girasis C, Schuurbiers JC, Muramatsu T, Aben JP, Onuma Y, Soekhradj S, Morel MA, van Geuns RJ, Wentzel JJ, Serruys PW. Advanced three-dimensional quantitative coronary angiographic assessment of bifurcation lesions: methodology and phantom validation. EuroIntervention. 2013; 8:1451-1460. , 133133. Sadamatsu K, Sagara S, Yamawaki T, Tashiro H. Three-dimensional coronary imaging for the ostium of the left anterior descending artery. Int J Cardiovasc Imaging 2009;25:223-8. , 135135. Tu S, Jing J, Holm NR, Onsea K, Zhang T, Adriaenssens T, Dubois C, Desmet W, Thuesen L, Chen Y, Reiber JH. In vivo assessment of bifurcation optimal viewing angles and bifurcation angles by three-dimensional (3D) quantitative coronary angiography. Int J Cardiovasc Imaging. 2012;28:1617-1625. , 136136. Tu S, Hao P, Koning G, Wei X, Song X, Chen A, Reiber JH. In vivo assessment of optimal viewing angles from X-ray coronary angiography. EuroIntervention. 2011;7:112-120. ]. Although there are multiple slightly variant definitions, optimal visualization of a given bifurcation lesion can be found in one single best view, the one with minimal mean foreshortening of bifurcated vessels, absence of vessel overlap, and widest possible opening of the bifurcation [119119. Lansky A, Tuinenburg J, Costa M, Maeng M, Koning G, Popma J, Cristea E, Gavit L, Costa R, Rares A, Van Es GA, Lefevre T, Reiber H, Louvard Y, Morice MC; European Bifurcation Angiographic Sub-Committee. Quantitative angiographic methods for bifurcation lesions: a consensus statement from the European Bifurcation Group. Catheter Cardiovasc Interv. 2009;73:258-66. , 135135. Tu S, Jing J, Holm NR, Onsea K, Zhang T, Adriaenssens T, Dubois C, Desmet W, Thuesen L, Chen Y, Reiber JH. In vivo assessment of bifurcation optimal viewing angles and bifurcation angles by three-dimensional (3D) quantitative coronary angiography. Int J Cardiovasc Imaging. 2012;28:1617-1625. ]. It follows that in any other projection certain parts of the anatomy, usually the ostia of the distal branches, are obscured due to overlap. 3D bifurcation QCA algorithms can retrieve missing information even from a combination of suboptimal views; nevertheless, a reconstruction including the optimal 2D projection is expected to be even more robust. Even if the optimal view is not among the images initially acquired, it can be suggested by a 3D reconstruction based on two suboptimal views; time requirements (<10 sec for reconstruction of two images, <60 sec for three images) should not be an issue [100100. Girasis C, Schuurbiers JC, Muramatsu T, Aben JP, Onuma Y, Soekhradj S, Morel MA, van Geuns RJ, Wentzel JJ, Serruys PW. Advanced three-dimensional quantitative coronary angiographic assessment of bifurcation lesions: methodology and phantom validation. EuroIntervention. 2013; 8:1451-1460. ]. Furthermore, the optimal working view could facilitate stent positioning, at the same time keeping radiation and contrast usage to a minimum. However, limitations imposed by patients’ anatomy (vessel overlap), constraints in gantry and patient positioning may make it difficult to acquire the optimal view exactly as suggested. In that respect an algorithm was developed that can accurately and quickly determine the optimal viewing angle at the same time predicting and thereby avoiding untoward vessel overlap with major branches which could limit the visibility of the target vessel [135135. Tu S, Jing J, Holm NR, Onsea K, Zhang T, Adriaenssens T, Dubois C, Desmet W, Thuesen L, Chen Y, Reiber JH. In vivo assessment of bifurcation optimal viewing angles and bifurcation angles by three-dimensional (3D) quantitative coronary angiography. Int J Cardiovasc Imaging. 2012;28:1617-1625. , 136136. Tu S, Hao P, Koning G, Wei X, Song X, Chen A, Reiber JH. In vivo assessment of optimal viewing angles from X-ray coronary angiography. EuroIntervention. 2011;7:112-120. ].
Finally, 3D QCA-derived models have been recently used for a computational based assessment of the FFR in intermediate lesions. Several approaches have been proposed that rely on the 3D reconstruction of the coronary anatomy from the QCA data, blood flow simulation in the 3D models, and implementation of computational fluid dynamic techniques to estimate the FFR in intermediate lesions [137137. Morris PD, Ryan D, Morton AC, Lycett R, Lawford PV, Hose DR, Gunn JP. Virtual fractional flow reserve from coronary angiography: modeling the significance of coronary lesions: results from the VIRTU-1 (VIRTUal Fractional Flow Reserve From Coronary Angiography) study. JACC Cardiovasc Interv. 2013;6:149-157. , 138138. Tu S, Barbato E, Koszegi Z, Yang J, Sun Z, Holm NR, Tar B, Li Y, Rusinaru D, Wijns W, Reiber JH. Fractional flow reserve calculation from 3-dimensional quantitative coronary angiography and TIMI frame count: a fast computer model to quantify the functional significance of moderately obstructed coronary arteries. JACC Cardiovasc Interv. 2014;7:768-777. , 139139. Papafaklis MI, Muramatsu T, Ishibashi Y, Lakkas LS, Nakatani S, Bourantas CV, Ligthart J, Onuma Y, Echavarria-Pinto M, Tsirka G, Kotsia A, Nikas DN, Mogabgab O, van Geuns RJ, Naka KK, Fotiadis DI, Brilakis ES, Garcia-Garcia HM, Escaned J, Zijlstra F, Michalis LK, Serruys PW. Fast virtual functional assessment of intermediate coronary lesions using routine angiographic data and blood flow simulation in humans: comparison with pressure wire - fractional flow reserve. EuroIntervention. 2014 ;10:574-583. ]. Each one of these methodologies introduced significant assumptions in the boundary conditions applied for blood flow simulation (e.g., Morris et al. utilize an Windkessel model to estimate microvascular resistance in the outflow of the model, Papafaklis et al. assumed a fixed flow in all the 3D models and did not include side branches in the reconstructions, while Tu et al. utilized TIMI frame count to calculate flow in hyperemic conditions during adenosine infusion); however preliminary validation studies have shown that they can provide reliable estimations of the FFR and can differentiate with a high accuracy flow-limiting from non-flow limiting stenosis (accuracy that varied between: 0.92-0.97). A computational assessment of the FFR is highly desirable as it is anticipated to reduce the cost, allow evaluation of lesions with a complex anatomy where the FFR-wire cannot be advanced and permit the functional assessment of lesions in patients who cannot tolerate adenosine. However further research is required in order to reduce the time needed for the computational assessment of the FFR so as these techniques to have application in clinical setting while the patient is on the catheterization table, develop more sophisticated approaches that will be able to overcome the methodological limitations of the first methods, and finally validate more extensively in a larger populations (i.e., diabetic patients with microvascular dysfunction) with complex coronary artery disease (i.e., chronic total occlusions, ostial lesions, long diffuse lesions involving coronary bifurcations) the new methodologies so as to have a better understanding about their performance and their potential value in clinical arena.
Analysis and reporting results from the investigation
- The procedure is not terminated with the last coronary injection
- Eyeballing should be replaced by quantitative measurements
- Look at the coronary angiogram with the eyes of the interventionalist
- Correlate regional function abnormalities with angiographic coronary lesions
- Provide a detailed report of the procedure, including any complications
- Enter all information into a database for quality control and research purposes
Non-atherosclerotic coronary artery disease
The angiographic finding of normal epicardial coronary vessels during diagnostic coronary angiography in patients with exercise induced angina pectoris should not immediately dismiss patient’s symptoms. It is well established that several conditions, such as primary and secondary myocardial hypertrophy as well as syndrome X, may be associated with a decrease in myocardial coronary flow reserve. Several mechanisms are responsible for the decrease in coronary flow reserve, such as a decrease in capillary density, increase in myocardial vascular resistance and extravascular compressive forces. The presence of extravascular compressive forces are often visible during coronary injection, especially when the injection starts at the onset of systole. The contrast column seems to stop momentarily before progressing down the coronary during the subsequent phase of diastole. In addition a typically ‘milking’ effect can be observed at the level of the septal branches ( Moving image 36 ). Myocardial ischaemia can be demonstrated in these patients during atrial pacing by a reduction in lactate consumption in the presence of symptoms, ST changes and increase in left ventricular end diastolic pressure [140140. Cannon RO 3rd, Dilsizian V, O’Gara PT, Udelson JE, Schenke WH, Quyyumi A, Fananapazir L, Bonow RO. Myocardial metabolic, hemodynamic, and electrocardiographic significance of reversible thallium-201 abnormalities in hypertrophic cardiomyopathy. Circulation. 1991;83:1660-7. ]. This reduced hyperaemic response during stress can be documented by quantitative measurements of coronary flow reserve using PET [141141. Cannon RO, 3rd, Schenke WH, Quyyumi A, Bonow RO, Epstein SE. Comparison of exercise testing with studies of coronary flow reserve in patients with microvascular angina. Circulation. 1991;83:III77-81. ].
More exceptional causes for ischaemic complications in the absence of coronary atherosclerosis are coronary embolic events secondary to infective endocarditis, mitral valve prolapse, prosthetic valves, mural thrombi, calcium emboli, and Takayasu’s arteritis to name only a few.
Complications
 View chapter
View chapter
As mentioned earlier coronary angiography is not harmless and carries a certain mortality and morbidity, albeit very low. Therefore the risk-benefit should be analysed every time the examination is ordered. This is especially true for the elderly patient and the patient with severe co-morbidity.
DEATH
The frequency of serious complications, such as death, myocardial infarction or cerebro-vascular accident with permanent damage, is very low (0.1–0.2%) and is usually attributed to high risk patients and not the least to the operator’s experience and credibility. Death as a procedural complication, in the absence of onsite PCI facilities, is often due to myocardial infarction secondary to catheter-induced ostial damage due to pre-existing severe pathology or the presence of unstable plaques at risk of embolisation. Left main dissection even in patients with normal coronary arteries has been reported. Death as a late result from relative minor or not-life- threatening complications such as access site bleeding, renal failure, pulmonary oedema, infections leading to prolonged hospitalization multi organ failures is a frustrating and sobering experience from which lessons should be drawn, since in principle avoidable.
STROKE
Transient ischaemic attacks or stroke can occasionally occur and arise as a consequence of thromboembolic events due to thrombi dislodgement from the access sheath, the guidewire or the catheter. In addition, dislodgement of plaques from the aorta, calcium from the aortic valve or thrombi in the left ventricle may be another source. Meticulous attention to catheter flushing and atraumatic wire-lead insertion can reduce but not eliminate the risk. There is no clear evidence that systemic heparinisation is required for diagnostic catheterisation. A clear explanation of other more frequent albeit minor and promptly controlled adverse effects helps the patient to accept them without unnecessary stress.
ACCESS SITE COMPLICATIONS
The most frequent complications of angiography occur at the catheter entry site in case of femoral access. The 2%-5% incidence currently quoted is based on series using a femoral approach before the use of 4 Fr and 5 Fr catheters. Closure devices have reduced the time to ambulation, increased patient comfort and shortened the hospital stay but do not appear to have modified the bleeding risk and have added some rare specific new complications (infection, embolisation or arterial stenoses due to components of the closure device, procoagulant factors injected into the bloodstream or local inflammation due to nickel allergy in case of the Perclose® system (Abbot Vascular, Redwood City, CA, USA).
Large haematomas requiring drainage, blood transfusions and prolonged bed rest, and hospitalisation are rare and often consequent to inability to comply with bed rest or clinical need for prolonged anticoagulation. Other more serious vascular complications include pseudo-aneurysm, fortunately often closed with ultrasound-guided compression and/or selective thrombin injection, arterio-venous fistulae, arterial thrombosis and distal embolisation. For radial procedures a negative Allen test was deemed necessary exclude critical hand ischaemia, even in cases of total radial occlusion (1%-4% of patients),.This test is not any longer performed is many centres around the world. large haematomas after removal of the radial sheath are rare. Although this all sounds reassuring severe complications with permanent functional deficit of the hand after radial procedures, albeit very rare, have been reported.The potentially serious complications of the brachial approach, percutaneous or surgical, can be obviated by not using this route, as a radial approach can almost always be substituted.
REACTIONS TO THE CONTRAST MEDIUM
With the newer generation of contrast agents, side effects, such as nausea, vomiting, and rash are becoming less frequent.
More severe reactions, including prolonged hypotension, bronchospasm, laryngeal oedema, and severe anaphylactic shock, although rare, require an aggressive response. Patients with a previous history of anaphylactic reaction to contrast or with a history of atopy or asthma are at a higher risk and should be pre-treated accordingly.
An anaphylactoid reaction with marked hypotension requires prompt administration of large volumes of fluid, IV epinephrine, antihistaminic agents, hydrocortisone and H2- receptor blockers.
HYPOTENSION
Vasovagal reactions
Vasovagal reactions resulting in bradycardia and hypotension are not infrequent and occur most often in stressed patients during painful arterial/venous puncture or sheath removal with groin compression. Exceptionally, excessive catheter manipulation at the level of the aortic arch may stimulate the aortic bodies, home to stretch receptors, and elicit a depressor response mediated by the depressor nerve (X).
Early recognition of this reaction (yawning is often an early premonitory sign even before hypotension and bradycardia occurs) as well as prompt and adequate treatment with leg rising, IV atropine and administration of fluid will rapidly normalise the haemodynamics. Refractory cases may need phenylephrine (alpha-receptor agonist: slower onset, long acting). Vasovagal reactions may be prevented or minimised by generous sedation, liberal local anaesthesia, reassurance and appropriate filling with intravenous fluids.
Drug-induced hypotension
Drug induced hypotension may occur after intracoronary injection of nitrates, especially in the presence of pre-existing hypovolaemia. Rapid volume expansion with intravenous administration of normal saline may normalize the arterial pressure.
Occasionally, narcotic induced hypotension will need the administration of the antidote naloxone
Cardiac tamponade
Cardiac tamponade during routine angiography is very rare and may occur abruptly or more insidiously.Perforation of the left ventricle free wall by means of a straight guidewire while crossing the aortic valve with subsequently misguided positioning of the pig catheter partially into the LV free wall has been reported. ( Moving image 38 ) Right ventricle perforation due to a temporary pacemaker catheter or at the time of myocardial biopsy is another possible cause of cardiac tamponade. .The diagnosis is confirmed by echocardiography: in emergency situations plain x-ray can be helpful to visualise pericardial effusion. Pressure measurements may be misleading early during perforation since their results strongly depend on the position of the pressure probe/catheter. The treatment consists of neutralising heparin and immediate pericardial puncture and, if necessary, surgical intervention.
Retroperitoneal bleeding
Spontaneous retroperitoneal bleeding is a most dreadful but fortunately rare complication, mostly managed conservatively. Iliac or aortic dissections tend to seal spontaneously if anterograde flow is preserved. This complication should always be suspected in case of insidious and prolonged refractory hypotension post procedure. Computed tomography scan should help in confirming the diagnosis when suspected.
Anaphylactic Shock
Anaphylactic shock, a potentially life-threatening condition, is caused by an intense anaphylactoid reaction to contrast media. The dramatic haemodynamic reaction with fall in arterial pressure is due to a sudden increase of the vascular capillary volume as a consequence of a general vasodilatation with redistribution of blood volume towards the acutely increased vascular space resulting in a condition of relative severe hypovolaemia and cardiovascular collapse.
Treatment in the case of severe anaphylactic reaction should be immediately directed at reducing vascular capillary volume by giving IV epinephrine and correcting the relative hypovolaemia with large volumes of normal saline until blood pressure increases to normal levels. High doses of hydrocortisone, as well as antihistamines e.g., diphenhydramine hydrochloride, H2- receptor blockers e.g., ranitidine® and dopamine infusions are invariably needed. In the case of bronchospasm and /or laryngeal oedema, pulmonary oedema and cardiopulmonary resuscitation intubation is often required.
CARDIAC ARRHYTHMIAS
Catheter manipulation inside the heart will invariably trigger cardiac arrhythmias, most often without consequences. In case of depressed left ventricular function and /or pre-existing arrhythmias, ventricular tachycardia and even ventricular fibrillation may occur. Similarly during left ventricular contrast injection, especially when the pigtail catheter is positioned in the apex rather than in the mitral inflow tract of the left ventricle runs of ventricular extra systoles may occur. It is worth noting that prolonged contrast injection into a small non-dominant RCA or into a conus branch will sometimes result in ventricular fibrillation, even in patients with normal coronary arteries.
Atrial fibrillation may also occur during a diagnostic procedure and will usually respond to medical treatment.
PULMONARY OEDEMA
Pulmonary oedema can occur during a procedure, especially in those patients with an already decreased left ventricular function and elevated filling pressures. Prolonged periods of recumbency during long procedures may result in fluid shifts towards the lungs due to altered gravity. Contrast and volume loading with or without transient myocardial ischaemia during coronary injection in the presence of pre-existing cardiac failure, severe valvular heart disease (aortic stenosis), critical left main stenosis or severe three vessel disease may trigger pulmonary oedema. It is important to recognise and intercept this evolving condition as soon as possible. Onset of restlessness, coughing, tachycardia, hypertension should alert the operator as well as the staff.
A series of measurements, such as the administration of oxygen, morphine, nitrates (depending on arterial pressure) together with placing the patient in a more comfortable upright position, may interrupt the vicious circle, lest more drastic measures like IABP, vasopressors, artificial ventilation and temporary pacemaker are needed. If caused by occlusion of a major coronary vessel, urgent revascularisation may be required (PCI or CABG).
MYOCARDIAL ISCHAEMIA
Short lasting transient myocardial ischaemia which occurs with every coronary injection of contrast due to replacement of blood by contrast material is usually harmless.
Accidental injection of air bubbles, in contrast can result in severe transient ischaemia with severe angina, ST segment changes and hypotension. In most instances these episodes may resolve spontaneously with time. Exceptionally supportive measures are needed, such as injection of vasopressors.
Air embolism can and should be avoided by frequently checking catheter's connections and manifold for air bubbles and by observing the pressure signal before each injection and refraining from injecting in case of damped pressure signal. Closed contrast injection systems like the ACIST® system can prevent this complication.
Thrombus dislodgement and subsequent embolisation may also induce myocardial ischaemia. Formation of clot is always a risk with prolonged procedures, excessive catheters exchanges, lack of adequate catheter flushing.
VASCULAR DISSECTION
Dissection of the access vessel and of the aorta
Forced manipulation of a guidewire against resistances in case of peripheral arterial disease may result in retrograde dissection of the iliac artery extending, in some cases into the descending aorta.
Dissection of a coronary artery or of the ascending aorta
Contrast injection when the catheter is too deeply engaged or lies against the vessel wall in case of a non-coaxial position may result in an acute coronary dissection of the left main stem. Excessive manipulation of catheters in the coronary ostium of the right coronary artery can cause coronary dissection with retrograde dissection into the aorta ascending.
NEEDLESTICK INJURIES IN THE CATHETERISATION LABORATORY
While the vast majority of complications that occur in the catheterisation laboratory happen to the patient, complications can occur which involve the operator or the other members of staff in the catheterisation laboratory. Arguably, the most serious injury that can happen for the staff is a needlestick injury where contamination with blood is a possibility.
For the purpose of convenience all types of injury will be referred to as a needlestick injury in the following text. Although we are mainly focusing on events that happen in the catheterisation laboratory the following information is applicable to any clinical setting or otherwise where a person may be at increased risk of needlestick injury. The main reason why we have to be extremely cautious when handling sharp instrumentation, in the presence of blood and other body fluids, is cross contamination through needlestick injuries which can result in the acquisition of serious life threatening blood borne diseases. More specifically health workers are at risk of getting infections such as
- HIV
- Hepatitis B
- Hepatitis C
Fortunately, even after needlestick injury, the number of healthcare workers who finally develop any of these infections is relatively low. The main reason for this is that most healthcare facilities have already implemented universal precautions i.e., education and implementation of guidelines for the prevention of needlestick injuries.
In Table 14 we have outlined the simple precautions that must be applied to ensure a safe system of work. Aside from these recommendations it is important for those at risk of blood borne infections to receive immunisation.
If a possible contamination has occurred the steps outlined in Table 15 should be rigorously adhered to:
In general, after the incident has occurred and the above recommendations have been carried out then it is vital that the details of the incident are accurately reported.
Normally hospital policy dictates that the emergency or occupational health department is attended in order to ascertain the infectious status of the injured person at baseline. Moreover, consent must be obtained from the patient or person (if known) whose blood it is that was involved in the needlestick. Following this, the status of the patient must be ascertained. Finally after all of these measures have been carried out then the responsible physician must decide on whether prophylactic therapy for HIV is necessary and counselling services made available for the injured party since they may be develop a serious blood borne infection.
Beware of complications
- Complications can and should be avoided whenever possible
- Anticipation and quick action is essential whenever a complication occurs
- Have your team trained for emergency situationsthe patient must be ascertained. Finally after all these measures have been carried out then the responsible physician must decide on whether prophylactic therapy for HIV is necessary and counselling services made available for the injured party since they may be develop a serious blood borne infection.
Mistakes/Pitfalls in interpretation
As mentioned earlier, the greatest risk of a coronary angiogram for the patient is an incomplete or non-diagnostic examination, notwithstanding the misinterpretation of the angiogram, because wrong therapeutic conclusions may be drawn.
- Poor and inhomogeneous filling of the coronary vessels due to inadequate force of manual injection will result in laminar flow, incomplete mixing and layering of contrast agent and blood into the injected vessel which may then give rise to pseudo- pathological appearance.
- Standard routine protocol (fixed number of injections and projection) should be overruled each time there is doubt regarding some segments due to superimposition, foreshortening, angulation or looping of vessels and also to avoid superimposition of the diaphragm which may obscure visualisation of the corresponding overlying myocardium.
- Catheter spasm is often seen after intubation of the RCA and more rarely with intubation of the left main stem.
- Anomalous origin of the left circumflex coronary artery from the right sinus of Valsalva or from the RCA may occasional be missed. The operators attention should be alerted in this case by the paradoxical observation when injecting the left coronary artery of a normal contracting lateral wall with apparent no perfusion.
- Total amputation of a coronary branch typically in case of occlusion of the mid LAD in the absence of collateral may occasionally be missed, particularly when a large septal branch or diagonal branch runs parallel to the course of missing distal LAD.
Current advances and future perspectives on coronary angiography
The diagnostic accuracy of standard coronary angiography relies on a limited number of subjectively selected 2D acquisitions, notwithstanding adherence with acquisition guidelines and individual expertise. On the other hand, rotational angiography can, potentially, provide us with significantly more information from multiple viewing planes [142142. Garcia JA, Agostoni P, Green NE, Maddux JT, Chen SY, Messenger JC, Casserly IP, Hansgen A, Wink O, Movassaghi B, Groves BM, Van Den Heuvel P, Verheye S, Van Langenhove G, Vermeersch P, Van den Branden F, Yeghiazarians Y, Michaels AD, Carroll JD. Rotational vs. standard coronary angiography: an image content analysis. Catheter Cardiovasc Interv. 2009;73:753-61. ], at the same time minimising contrast volume, radiation exposure and procedural time [143143. Maddux JT, Wink O, Messenger JC, Groves BM, Liao R, Strzelczyk J, Chen SY, Carroll JD. Randomized study of the safety and clinical utility of rotational angiography versus standard angiography in the diagnosis of coronary artery disease. Catheter Cardiovasc Interv. 2004;62:167-74. , 144144. Akhtar M, Vakharia KT, Mishell J, Gera A, Ports TA, Yeghiazarians Y, Michaels AD. Randomized study of the safety and clinical utility of rotational vs. standard coronary angiography using a flat-panel detector. Catheter Cardiovasc Interv. 2005;66:43-9. ]. New gantries allow rapid isocentric automatic rotation of the imaging camera over arcs of ~180° within a few seconds; moreover, dual motion coronary angiography allows rotation in several axes rather than in a simple arc [145145. Garcia JA, Chen SY, Messenger JC, Casserly IP, Hansgen A, Wink O, Movassaghi B, Klein AJ, Carroll JD. Initial clinical experience of selective coronary angiography using one prolonged injection and a 180 degrees rotational trajectory. Catheter Cardiovasc Interv. 2007;70:190-6. ]. Standardised acquisition protocols have to be followed utilising a single but slightly longer (~5 sec) continuous contrast injection per coronary artery/graft studied.
In addition, using the data from rotational angiography acquisitions a 3D reconstruction could be created (Dyna-CT) [146146. Eide KR, Odegard A, Myhre HO, Haraldseth O. Initial observations of endovascular aneurysm repair using Dyna-CT. J Endovasc Ther. 2007;14:50-3. ]; the principle is similar to that used for conventional CT-scans rather than to coronary (ECG-triggered) multislice CT. The 3D models so obtained are particularly useful for the evaluation of 3D complex structures, for example during aortic graft-stent placement or during structural heart disease interventions. Dyna-CT has been also been useful to reconstruct aortic valve and annulus anatomy and plan transcatheter aortic valve implantation [166166. Van Leer-Greenberg B, Martinez CA, Heldman AW. Multimodality image guidance with Dyna-CT for transcatheter treatment of paravalvular leak of a stentless valve. Catheter Cardiovasc Interv. 2014 Nov 22 doi: 10.1002/ccd.25756. [Epub ahead of print] , 167167. Maeda K, Kuratani T, Torikai K, Shimamura K, Sawa Y. Transcatheter aortic valve replacement using DynaCT. J Card Surg. 2012;27:551-553 ]. However, whereas this approach has been successfully established for large vascular structures with relatively little motion, the evaluation of the coronary tree is challenging due to the low temporal resolution of rotational angiography (5 sec per half rotation as opposed to around 180 ms per half rotation on a modern MSCT scanner). Retrospective ECG gating is not very effective in improving the quality of 3D reconstructions of rotational angiography images. However, image reconstruction methods based on complex mathematical models for the detection of motion fields can dramatically improve the appearance of 3D coronary models [147147. Rohkohl C, Lauritsch G, Prummer M, Hornegger J. Interventional 4-D motion estimation and reconstruction of cardiac vasculature without motion periodicity assumption. Med Image Comput Comput Assist Interv. 2009;12:132-9. ]. ]. Evaluation of the accuracy of these reconstruction methods and clinical applications of these approaches in guiding PCI are pending.
Angiography cannot provide us with information on vessel wall composition and the true vessel size, at least to the extent that this can be derived from other imaging techniques such as MSCT or IVUS/OCT. Since fluoroscopy is currently the only imaging modality available in real time during percutaneous coronary intervention, the operator has no other option than to mentally co-register the analysed MSCT/IVUS/OCT data with the angiographic image. This process can be inaccurate, especially in diffusely diseased vessels without apparent landmarks (e.g., side branches). To overcome this limitation several systems have been recently developed that are able to reconstruct coronary anatomy from MSCT data and co-register MSCT and coronary angiography [148148. Metz CT, Schaap M, Klein S, Neefjes LA, Capuano E, Schultz C, van Geuns RJ, Serruys PW, van Walsum T, Niessen WJ. Patient specific 4D coronary models from ECG-gated CTA data for intra-operative dynamic alignment of CTA with X-ray images. Med Image Comput Comput Assist Interv. 2009;12:369-76.
Together with [150] these references give us a glimpse into the near future on how co-registration of 3D angiography and other imaging modalities (IVUS/OCT/MSCT) will increase the diagnostic accuracy of coronary angiograph, 149149. Roguin A, Abadi S, Engel A, Beyar R. Novel method for real-time hybrid cardiac CT and coronary angiography image registration: visualising beyond luminology, proof-of-concept. EuroIntervention. 2009 ;4:648-53. ]. Feasibility studies have shown that these tools provide comprehensive representation of coronary anatomy that can be useful for guiding treatment in patients with complex coronary artery disease (e.g., chronic total occlusion lesions).
Several software have also been recently developed that permit online co-registration of intravascular imaging and X-ray angiographic data ( Figure 41 ) [150150. Tu S, Holm NR, Koning G, Huang Z, Reiber JH. Fusion of 3D QCA and IVUS/OCT. Int J Cardiovasc Imaging. 2011;27:197-207. , 151151. Carlier S, Didday R, Slots T, Kayaert P, Sonck J, El-Mourad M, Preumont N, Schoors D, Van Camp G. A new method for real-time co-registration of 3D coronary angiography and intravascular ultrasound or optical coherence tomography. Cardiovasc Revasc Med. 2014;15:226-32. ]. These systems provide complete and comprehensive representation of coronary pathology as X-ray angiography gives information about the vessel geometry while intravascular imaging (IVUS/OCT) gives details about lesion morphology and the composition of the plaque. A unique advantage of these software is the fact allow online co-registration and operate in a easy to use environment, however they do not provide reliable coronary reconstruction as they are unable to estimate the absolute orientation of the intravascular imaging data onto the luminal centerline. To overcome these limitations several other methodologies have been developed which implement efficient algorithms and take advantage of the information provided by anatomical landmarks seen at both X-ray and intravascular images to accurately estimate the absolute orientation of IVUS/OCT images ( Figure 42 ) [152152. Bourantas CV, Kalatzis FG, Papafaklis MI, Fotiadis DI, Tweddel AC, Kourtis IC, Katsouras CS, Michalis LK. ANGIOCARE: an automated system for fast three-dimensional coronary reconstruction by integrating angiographic and intracoronary ultrasound data. Catheter Cardiovasc Interv. 2008 ;72:166-75. , 153153. Bourantas CV, Papafaklis MI, Athanasiou L, Kalatzis FG, Naka KK, Siogkas PK, Takahashi S, Saito S, Fotiadis DI, Feldman CL, Stone PH, Michalis LK. A new methodology for accurate 3-dimensional coronary artery reconstruction using routine intravascular ultrasound and angiographic data: implications for widespread assessment of endothelial shear stress in humans. EuroIntervention. 2013 ;9:582-93. , 154154. Papafaklis MI, Bourantas CV, Yonetsu T, Vergallo R, Kotsia A, Nakatani S, Lakkas LS, Athanasiou LS, Naka KK, Fotiadis DI, Feldman CL, Stone PH, Serruys PW, Jang IK, Michalis LK. Anatomically correct three-dimensional coronary artery reconstruction using frequency domain optical coherence tomographic and angiographic data: head-to-head comparison with intravascular ultrasound for endothelial shear stress assessment in humans. EuroIntervention. 2014 Jun 30. pii: 20140207-04. doi: 10.4244/EIJY14M06_11. [Epub ahead of print] ]. These approaches have considerable applications in research arena and shed light into the role of the local hemodynamic forces on neointima proliferation and atherosclerotic evolution, but they have limited value in clinical practice as they require increased time for coronary reconstruction and thus they cannot be used for on-line applications [155155. Vergallo R, Papafaklis MI, Yonetsu T, Bourantas CV, Andreou I, Wang Z, Fujimoto JG, McNulty I, Lee H, Biasucci LM, Crea F, Feldman CL, Michalis LK, Stone PH, Jang IK. Endothelial shear stress and coronary plaque characteristics in humans: combined frequency-domain optical coherence tomography and computational fluid dynamics study. Circ Cardiovasc Imaging. 2014 ;7:905-11. , 156156. Bourantas CV1, Papafaklis MI, Lakkas L, Sakellarios A, Onuma Y, Zhang YJ, Muramatsu T, Diletti R, Bizopoulos P, Kalatzis F, Naka KK,Fotiadis DI, Wang J, Garcia Garcia HM, Kimura T, Michalis LK, Serruys PW. Fusion of optical coherence tomographic and angiographic data for more accurate evaluation of the endothelial shear stress patterns and neointimal distribution after bioresorbable scaffold implantation: comparison with intravascular ultrasound-derived reconstructions. Int J Cardiovasc Imaging. 2014;30:485-94. ].
Personal perspective – Guy R. Heyndrickx
Diagnostic catheterisation started when Forssmann, in 1929, introduced a kind of hollow tube in his own ante cubital vein and under fluoroscopic guidance advanced it into his own right atrium with the sole purpose of testing the feasibility of injecting drugs directly into the heart in order to quicken the action of cardiac stimulating drugs. What should have been viewed at that time as a potential major technological breakthrough in medicine was disregarded with disdain. Subsequently in 1948, Cournand and collaborators realised the immense opportunity this technique could offer in the field of cardiology. First, catheters were used to measure intracardiac pressures and sample blood for diagnostic purposes. Since then, extensive studies of cardiac pressures, recorded in all 4 chambers of the heart, combined with the careful analysis of the morphology of pressure tracings in normal and disease states have vastly increased or understanding of cardiac physiology and pathophysiology. Further developments led to the introduction of angiography and dynamic cardiac imaging. This, added to the physiologic pressure data completed our diagnostic view. Coronary angiography, performed almost by accident in 1967 by Sones, allowed cardiologists at last to diagnose coronary artery disease. Since then there have been major advances in catheter technology, data acquisition methods, and x-ray equipment as well as in the procedure itself.
POSITIVE DEVELOPMENTS
These include the gradual shift from the initial brachial cutdown, to access both artery and vein, to a percutaneous access, first via femoral and more recently via radial artery, increasing patient comfort and decreasing bleeding complication. More excitingly, the development of computer assisted analysis of angiographic images, the emergence of a multitude of newer intravascular imaging modalities such as IVUS, virtual histology, OCT and last but not least the introduction of the pressure wire, have upgraded 2D luminology to 3D real time vascular images, which in combination with FFR measurements has given us a far greater insight into pathohysiology of coronary artery disease thereby refining our decision making.
POTENTIAL NEGATIVE DEVELOPMENTS
There appears to be a trend to rely more on imaging than on physiology for routine diagnostic procedures. Indeed we are living more and more in a world where ‘image driven medicine’ has become the rule. It is important to remember that the heart is not a static but a complex dynamic organ.
Moreover, a tendency exists to deviate from the concept “unité de temps et de lieu” where all relevant information, needed for an accurate diagnosis, is gathered at the same time and in the same place. Instead, a habit is observed in many catheterisation laboratories to combine fragmented information recorded at a different time point e.g., volumes derived from echocardiography and pressure data obtained during catheterisation, which are then pieced together. Following on from that, it seems to be the case that an increasing number of catheterisation laboratories are no longer routinely catheterising the left ventricle to measure end diastolic pressure, let alone crossing the aortic valve for measuring the gradient or even perform a routine LV angiogram. The main explanation for this appears to be that this information can be readily obtained by other non-invasive means.
Increasing use of the transradial approach is possibly being used as a pretext for no longer performing a left ventriculogram or right heart catheterisation when indicated.
Although the shift from femoral access to radial access has proven beneficial for the patient we have noticed this paradox in centres, where radial access is near100%, that younger colleagues no longer know how to puncture and close the femoral artery. A consummate interventional cardiologist should remain familiar with both procedures and therefore should keep practicing the femoral approach in a certain percentage of his procedures, in case one day the femoral access turns out to be the only possible access.
IS ROUTINE DIAGNOSTIC CORONARY ANGIOGRAPHY A PROCEDURE WITHOUT A FUTURE?
Millions of image pixels generated by CT scans are currently being interrogated and extensively manipulated so as to reveal hidden information that may result in translating anatomical images into functional information making the coronary angiographic procedure, including FFR measurements, superfluous as some proponents believe. The answer to the question, whether non-invasive FFR-CT technology may potentially be disruptive with a promise of dethroning invasive coronary angiography or whether this highflying mathematical wizardry will distract us from the essence that is: a carefully performed coronary angiography eventually complemented with intravascular imaging and/or FFR measurements, is awaiting in the wings of history. Meanwhile as long as invasive haemodynamic evaluation continues to be necessary, cardiologist should adhere to a strict pre- per- and post-procedural protocol combining cardiac imaging with physiology.




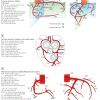
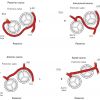

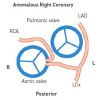


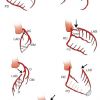
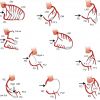
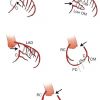

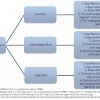


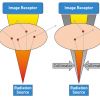






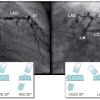
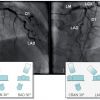


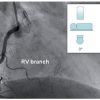
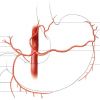




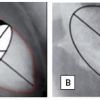

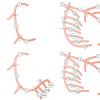





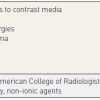

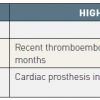
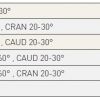

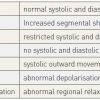

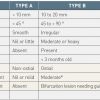
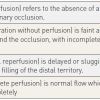


Reversal of NOACs section desperately needs an update.
In the part that is writen "cases the fistula drains into the right ventricle, 25% into the right atrium, followed by the pulmonary artery, the right atrium, left atrium and finally the left ventricle"..... Its repeated "right atrium"