Summary
Early reperfusion therapy is a life-saving treatment for patients with ST-segment elevation myocardial infarction. Primary PCI is the preferred method of reperfusion. However, the advantages of the invasive approach over fibrinolytic therapy may be reduced by late initiation of mechanical reperfusion due to logistical problems related to transportation delay to a hospital with a 24/7 invasive service. To overcome this limitation, regional programmes for coordination of the treatment of acute coronary syndromes are being introduced based on the cooperation of a PCI-capable hospital (hub) with ambulances and non-PCI-capable hospitals (spokes). With appropriate planning, only in a small minority of patients the anticipated delay to primay PCI will be so long to require pre- or in-hospital fibrinolytic therapy as a bridge to invasive therapy. PCI technique, as well as adjunctive pharmacotherapy in ST-segment elevation myocardial infarction treatment, differ from standard PCI for stable angina due to the need for rapid intervention on thrombus containing lesions, which increases the risk of complications including distal embolisation or no-reflow. Modern aggressive antiplatelet therapyand new stent designs have improved the immediate and long-term results of primary PCI.
Networking of acute myocardial infarction treatment
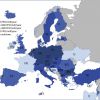
The annual incidence of hospital admission for ST-segment elevation myocardial infarction (STEMI) varies between 44-142/100,000 individuals per year [11. Widimsky P, Wijns W, Fajadet J, de Belder M, Knot J, Aaberge L, Andrikopoulos G, Baz JA, Betriu A, Claeys M, Danchin N, Djambazov S, Erne P, Hartikainen J, Huber K, Kala P, Klinceva M, Kristensen SD, Ludman P, Ferre JM, Merkely B, Milicic D, Morais J, Noc M, Opolski G, Ostojic M, Radovanovic D, De Servi S, Stenestrand U, Studencan M, Tubaro M, Vasiljevic Z, Weidinger F, Witkowski A, Zeymer U. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur Heart J. 2010;31:943-957. ]. Reperfusion therapy is the most effective method of STEMI treatment. Primary percutaneous coronary intervention (PCI) and thrombolysis represent the main strategies of reperfusion. The relative use of these two strategies is different across European countries. Importantly, in countries using mainly thrombolysis as a reperfusion strategy, the overall rate of reperfusion therapy in STEMI patients is lower than in countries with a dominance of primary PCI [11. Widimsky P, Wijns W, Fajadet J, de Belder M, Knot J, Aaberge L, Andrikopoulos G, Baz JA, Betriu A, Claeys M, Danchin N, Djambazov S, Erne P, Hartikainen J, Huber K, Kala P, Klinceva M, Kristensen SD, Ludman P, Ferre JM, Merkely B, Milicic D, Morais J, Noc M, Opolski G, Ostojic M, Radovanovic D, De Servi S, Stenestrand U, Studencan M, Tubaro M, Vasiljevic Z, Weidinger F, Witkowski A, Zeymer U. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur Heart J. 2010;31:943-957. ]. A major increase in primary PCI utilization was observed in Europe during the last years. According to registry data from years 2010/2011 most countries has primary PCI rate around 400-600 procedures per million inhabitants (with the lowest number of 23 and highest number of 884 PCIs) [22. Kristensen SD, Laut KG, Fajadet J, Kaifoszova Z, Kala P, Di Mario C, Wijns W, Clemmensen P, Agladze V, Antoniades L, Alhabib KF, de Boer MJ, Claeys MJ, Deleanu D, Dudek D, Erglis A, Gilard M, Goktekin O, Guagliumi G, Gudnason T, Hansen KW, Huber K, James S, Janota T, Jennings S, Kajander O, Kanakakis J, Karamfiloff KK, Kedev S, Kornowski R, Ludman PF, Merkely B, Milicic D, Najafov R, Nicolini FA, Noc M, Ostojic M, Pereira H, Radovanovic D, Sabate M, Sobhy M, Sokolov M, Studencan M, Terzic I, Wahler S, Widimsky P. Reperfusion therapy for ST elevation acute myocardial infarction 2010/2011: current status in 37 ESC countries. Eur Heart J. 2014. ] - Figure 1.
According to the European Society of Cardiology (ESC) Guidelines for STEMI treatment, primary PCI is the preferred method of reperfusion, but only when it is performed in a timely fashion by an experienced team [33. Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van ’t Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619. , 44. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa UM, Taggart D, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Kolh P, Alfieri O, Dunning J, Elia S, Kappetein P, Lockowandt U, Sarris G, Vouhe P, Kearney P, von Segesser L, Agewall S, Aladashvili A, Alexopoulos D, Antunes MJ, Atalar E, Brutel de la Riviere A, Doganov A, Eha J, Fajadet J, Ferreira R, Garot J, Halcox J, Hasin Y, Janssens S, Kervinen K, Laufer G, Legrand V, Nashef SA, Neumann FJ, Niemela K, Nihoyannopoulos P, Noc M, Piek JJ, Pirk J, Rozenman Y, Sabate M, Starc R, Thielmann M, Wheatley DJ, Windecker S, Zembala M. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2010;31:2501-2555. , 55. Kushner FG, Hand M, Smith SC, Jr., King SB, III, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr., Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205-2241. , 168168. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. , 211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. Data from national registries show that the recommended time delay from first medical contact (FMC) to primary PCI procedure is difficult to achieve, mainly due to the presentation of a large number of patients with STEMI in centres without an on-site primary PCI service [168168. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. , 211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. This is an important limitation of mechanical reperfusion (see also the paragraph on timing issues). To overcome this limitation, the concept of networking was born which, by optimising the organisation of STEMI care, allowed an increase in the number of patients receiving mechanical reperfusion within the recommended time window. Well organised regional networks usually cover an area of about 0.3 to 0.5 million inhabitants and consist of a PCI centre (hub), non-PCI hospitals (spokes) and the emergency medical systems. Networks covering larger populations also exist, especially when located in large metropolitan areas. In Europe, the average population for network is approximately 0.5 million inhabitants [88. Knot J, Widimsky P, Wijns W, Stenestrand U, Kristensen SD, van’t Hof AW, Weidinger F, Janzon M, Norgaard BL, Soerensen JT, van de Wetering H, Thygesen K, Bergsten PA, Digerfeldt C, Potgieter A, Tomer N, Fajadet J. How to set up an effective national primary angioplasty network: lessons learned from five European countries. EuroIntervention. 2009;5:299-309. ]. A smaller area provides a lower number of STEMI patients which decreases network effectiveness, whereas in larger areas transportation, catheterisation facilities and coronary care unit beds may be overloaded by the high number of patients. Typically, networks are centrally coordinated and have predefined transportation and treatment protocols which are important for reducing the time delay. Ideally, the coordinating centre should provide training programmes and quality control with evaluation of outcome via local audits compared with the general experience collected in national registries). These are important tools to improve the system. Only experienced, high-volume centres providing a 24/7 service should be part of a network because both operator volume and hospital volume influence primary PCI results [99. Srinivas VS, Hailpern SM, Koss E, Monrad ES, Alderman MH. Effect of physician volume on the relationship between hospital volume and mortality during primary angioplasty. J Am Coll Cardiol. 2009;53:574-579. , 1010. Kumbhani DJ, Cannon CP, Fonarow GC, Liang L, Askari AT, Peacock WF, Peterson ED, Bhatt DL. Association of hospital primary angioplasty volume in ST-segment elevation myocardial infarction with quality and outcomes. JAMA. 2009;302:2207-2213. ]. This is very important for network planning in order to obtain optimal balance between the number of primary PCI centres and the number of inhabitants in the area of a particular network (number of STEMI) because both will influence the operator’s experience and time delay, which is important for treatment results.
Pre-hospital diagnosis of STEMI allows bypassing the emergency room and transferring the patients directly to the cath lab, which reduces the time to reperfusion [1111. Terkelsen CJ, Lassen JF, Norgaard BL, Gerdes JC, Poulsen SH, Bendix K, Ankersen JP, Gotzsche LB, Romer FK, Nielsen TT, Andersen HR. Reduction of treatment delay in patients with ST-elevation myocardial infarction: impact of pre-hospital diagnosis and direct referral to primary percutanous coronary intervention. Eur Heart J. 2005;26:770-777. , 1212. Bang A, Grip L, Herlitz J, Kihlgren S, Karlsson T, Caidahl K, Hartford M. Lower mortality after prehospital recognition and treatment followed by fast tracking to coronary care compared with admittance via emergency department in patients with ST-elevation myocardial infarction. Int J Cardiol. 2008;129:325-332. ]. Electrocardiogram (ECG) interpretation can be done by the ambulance staff or via transmission from the ambulance to the PCI hospital or a central coordinating centre. This last modality can be helpful when the ECG interpretation is difficult, especially in paramedic-based ambulance systems. In the many regions worldwide where ECG teletrasmission has been implemented, this system leads to a reduced time to mechanical reperfusion and facilitates the activation of cath lab staff, especially during off-duty hours [1313. Rokos IC, French WJ, Koenig WJ, Stratton SJ, Nighswonger B, Strunk B, Jewell J, Mahmud E, Dunford JV, Hokanson J, Smith SW, Baran KW, Swor R, Berman A, Wilson BH, Aluko AO, Gross BW, Rostykus PS, Salvucci A, Dev V, McNally B, Manoukian SV, King SB, III. Integration of pre-hospital electrocardiograms and ST-elevation myocardial infarction receiving center (SRC) networks: impact on Door-to-Balloon times across 10 independent regions. JACC Cardiovasc Interv. 2009;2:339-346. , 1414. Rao A, Kardouh Y, Darda S, Desai D, Devireddy L, Lalonde T, Rosman H, David S. Impact of the prehospital ECG on door-to-balloon time in ST elevation myocardial infarction. Catheter Cardiovasc Interv. 2010;75:174-178. ]. Regional protocols for STEMI networks differ within European countries according to specific local constraints [88. Knot J, Widimsky P, Wijns W, Stenestrand U, Kristensen SD, van’t Hof AW, Weidinger F, Janzon M, Norgaard BL, Soerensen JT, van de Wetering H, Thygesen K, Bergsten PA, Digerfeldt C, Potgieter A, Tomer N, Fajadet J. How to set up an effective national primary angioplasty network: lessons learned from five European countries. EuroIntervention. 2009;5:299-309. , 1515. Danchin N. Systems of care for ST-segment elevation myocardial infarction: impact of different models on clinical outcomes. JACC Cardiovasc Interv. 2009;2:901-908. ]. In some of them, pre-hospital fibrinolysis was introduced as a bridge to mechanical reperfusion.
The STEMI networking concept was actively promoted by the European “Stent for Life” initiative (2008-2016), in which the main goal was to promote mechanical reperfusion in Europe by reducing the time delay to reperfusion and increasing the number of patients with STEMI undergoing mechanical reperfusion. During the last few years, many European countries have improved dramatically their rates of primary angioplasty implementing the plans advised by the “Stent for Life” group, inspired by the experience of the best-performing countries. The survey on reperfusion strategies promoted as part of the “Stent for Life” initiative has shown that most countries have reached the target penetration and a full supplement of EuroIntervention has been dedicated to an individual analysis of the situation in individual countries and regions. ( > Eurointervention Volume 8 Supplement P ). Currently the “Stent - Save a Life” programme is ongoing following the success of Stent for Life initiative. The primary intention of “Stent - Save a Life” is to extend this idea globally, according to the increasing needs, and to adapt it to the specific demands of the various regions of the world (www.stentsavealife.com).
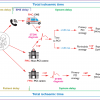
These principles have been adopted by the ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation myocardial infarction (MI) as well as the most recent ESC/EACTS Guidelines on myocardial revascularization. They recommend that the pre-hospital management of STEMI patients must be based on regional networks designed to deliver reperfusion therapy expeditiously and effectively, with efforts made to offer primary PCI to as many patients as possible (recommendation Class I; level of evidence B). Primary PCI-capable centres must deliver a 24/7 service and be able to start primary PCI as soon as possible but always within 60 min from the initial call (recommendation Class I; level of evidence B). It is recommended that the emergency medical system transfers STEMI patients to a PCI-capable centre, bypassing non-PCI centres (recommendation Class I; level of evidence C). Patients transferred to a PCI-capable centre for primary PCI should bypass the emergency department, coronary care unit/intensive cardiac care unit and be transferred directly to the cath lab (recommendation Class I; level of evidence B) [168168. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. , 211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. ( Figure 2).
Timing issues
There are a number of factors that may influence progression of myocardial necrosis during MI: completeness of coronary occlusion, presence of collateral circulation, pre- and post-conditioning, individual myocardial oxygen demand. Despite the variability of these factors, duration of ischaemia remains the most important determinant of infarct size and myocardial damage. It is widely accepted and recommended by current ESC guidelines that reperfusion therapy is indicated in all STEMI patients with symptoms of ischemia <12 hours (recommendation Class I; level of evidence A). Also, a primary PCI strategy is indicated in the presence of ongoing symptoms suggestive of ischaemia, haemodynamic instability, or life-threatening arrhythmias.(recommendation Class I.; level of evidence C). A routine primary PCI strategy should be considered in patients presenting late (12–48 h) after symptom onset (recommendation Class IIa; level of evidence B). [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. Primary PCI, which is the preferred reperfusion therapy, should be started in specific timeframes. This might be difficult, due to logistical problems, when prolonged transfer to the centre with 24/7 invasive facilities is necessary.
TIME DELAYS IN MYOCARDIAL INFARCTION
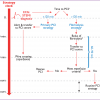
When analysing the timing of events in STEMI treatment, several delay times can be determined. The moment when the patient meets the medical system for the first time is called first medical contact (FMC). The ischaemic time before FMC is called delay between symptoms onset and FMC and this is mainly a patient-related delay, with improvements possible only via education to recognise symptoms and timely call to a unified number for the emergency service. At the FMC time point the initial diagnosis should be established and any further time delay is related to the medical system. According to recent ESC STEMI Guidelines, the delay from FMC to STEMI diagnosis should be reduced to less than 10 min. STEMI diagnosis time is the time zero to guide appropriate therapy. Another important moment is the start of reperfusion therapy, traditionally defined by first balloon inflation or first thrombectomy catheter pass but now identified from the wire passage distal to the occluded segment. Door-to-needle time represents its equivalent when fibrinolytic therapy is given. These three time points (symptoms onset, STEMI diagnosis, first wire passage with primary PCI and needle time with fibrinolysis) are the most important time-points in the diagnosis and treatment process in STEMI patients. System-related delay times may be calculated from FMC to hospital admission, cath lab admission, sheath insertion etc, but the most important is the time from STEMI diagnosis to first wire crossing since thrombectomy of direct stent implantation are often used instead of balloon dilatation during PCI. For patients treated with fibrinolysis, the most important parameter is the time from STEMI diagnosis to fibrinolytic therapy administration. The above-mentioned time delays are also called door-to-balloon time and door-to-needle time. However, the “door” moment may not always be defined as “first door” (which equals FMC), but can be hospital door or cath lab door that gives a different time relation. Especially when analysing primary PCI compared to fibrinolysis, there is controversy that the clock for primary PCI starts at the time of FMC but that for fibrinolysis the clock starts ticking after arrival at the hospital, which does not include any transportation delay. That is why it is better to use the FMC and STEMI diagnosis time points for description of delay times. Details concerning delay times are described in Table 1. Additionally, for primary PCI, wire crossing time point equates to the moment of reperfusion, whereas needle time equates to lysis delivery but precedes effective reperfusion.
IMPORTANCE OF DELAY TO MECHANICAL REPERFUSION
The relationship between reperfusion treatment delay and mortality has been studied extensively, mainly in post hoc analyses based on observational data from randomised trials and registries. Early studies suggested that the time dependency of mechanical reperfusion exists but it is less pronounced than in fibrinolysis. Zijlstra et al demonstrated the relationship between time delay and mortality in fibrinolysis, but not in primary PCI-treated patients [1616. Zijlstra F, Patel A, Jones M, Grines CL, Ellis S, Garcia E, Grinfeld L, Gibbons RJ, Ribeiro EE, Ribichini F, Granger C, Akhras F, Weaver WD, Simes RJ. Clinical characteristics and outcome of patients with early (4 h) presentation treated by primary coronary angioplasty or thrombolytic therapy for acute myocardial infarction. Eur Heart J. 2002;23:550-557. ]. Cannon et al found that mortality after primary PCI is not related to symptom onset-to-balloon time [1717. Cannon CP, Gibson CM, Lambrew CT, Shoultz DA, Levy D, French WJ, Gore JM, Weaver WD, Rogers WJ, Tiefenbrunn AJ. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA. 2000;283:2941-2947. ]. In a series of more than 1,300 patients Antoniucci et al showed a positive correlation between time to treatment and mortality in high-risk patients but not in low-risk patients [1818. Antoniucci D, Valenti R, Migliorini A, Moschi G, Trapani M, Buonamici P, Cerisano G, Bolognese L, Santoro GM. Relation of time to treatment and mortality in patients with acute myocardial infarction undergoing primary coronary angioplasty. Am J Cardiol. 2002;89:1248-1252. ]. Similarly, Brodie et al [1919. Brodie BR, Stuckey TD, Muncy DB, Hansen CJ, Wall TC, Pulsipher M, Gupta N. Importance of time-to-reperfusion in patients with acute myocardial infarction with and without cardiogenic shock treated with primary percutaneous coronary intervention. Am Heart J. 2003;145:708-715. ] demonstrated a significant relationship between time to treatment and mortality only in high-risk patients with cardiogenic shock. However, De Luca et al [2020. De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223-1225. ], analysing long-term outcome, found that there was a definite relationship between time delay to PCI treatment and 1-year mortality also in a general primary PCI population. Each 30 minutes of delay was associated with a relative risk increase of 7.5% at 1-year follow-up [2020. De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223-1225. ]. Similarly, Nallamothu et al [2121. Nallamothu B, Fox KA, Kennelly BM, Van de Werf F, Gore JM, Steg PG, Granger CB, Dabbous OH, Kline-Rogers E, Eagle KA. Relationship of treatment delays and mortality in patients undergoing fibrinolysis and primary percutaneous coronary intervention. The Global Registry of Acute Coronary Events. Heart. 2007;93:1552-1555. ], based on GRACE registry data, showed that longer treatment delays were associated with higher 6-month mortality in both fibrinolysis and primary PCI patients. For primary PCI patients, 6-month mortality increased by 0.18% per 10 minutes delay in door-to-balloon time between 90 and 150 minutes [2121. Nallamothu B, Fox KA, Kennelly BM, Van de Werf F, Gore JM, Steg PG, Granger CB, Dabbous OH, Kline-Rogers E, Eagle KA. Relationship of treatment delays and mortality in patients undergoing fibrinolysis and primary percutaneous coronary intervention. The Global Registry of Acute Coronary Events. Heart. 2007;93:1552-1555. ]. Observational studies correlating treatment delay and mortality after primary PCI should be interpreted with caution. Patients who present early are more likely to be at high risk than late presenters [2222. Aquaro GD, Pingitore A, Strata E, Di Bella G, Palmieri C, Rovai D, Petronio AS, L’Abbate A, Lombardi M. Relation of pain-to-balloon time and myocardial infarct size in patients transferred for primary percutaneous coronary intervention. Am J Cardiol. 2007;100:28-34. ]. Therefore, the apparent similarity of outcome irrespective of time delay may only be the consequence of a worse a priori outcome of high-risk individuals treated early and the better outcome of lower-risk patients treated later. Gersh et al has suggested that, in patients presenting late from symptoms onset, time delay to PCI has a minimal impact on mortality (plateau phase) [2323. Gersh BJ, Stone GW, White HD, Holmes DR, Jr. Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future?. JAMA. 2005;293:979-986. ]. The benefit of time delay reduction may not be similar in all patients but related to risk profile and time of presentation. For ethical reasons, it is impossible to assess properly the role of reducing delay to mechanical reperfusion in a randomised fashion. However, based on current evidence, it is recommended to make as much of an effort as possible to reduce delays to mechanical reperfusion.
PCI-RELATED DELAY
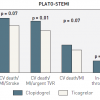
Primary PCI is the preferred form of reperfusion treatment for patients with STEMI [33. Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van ’t Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619. , 44. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa UM, Taggart D, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Kolh P, Alfieri O, Dunning J, Elia S, Kappetein P, Lockowandt U, Sarris G, Vouhe P, Kearney P, von Segesser L, Agewall S, Aladashvili A, Alexopoulos D, Antunes MJ, Atalar E, Brutel de la Riviere A, Doganov A, Eha J, Fajadet J, Ferreira R, Garot J, Halcox J, Hasin Y, Janssens S, Kervinen K, Laufer G, Legrand V, Nashef SA, Neumann FJ, Niemela K, Nihoyannopoulos P, Noc M, Piek JJ, Pirk J, Rozenman Y, Sabate M, Starc R, Thielmann M, Wheatley DJ, Windecker S, Zembala M. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2010;31:2501-2555. , 211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. However, the advantages of an invasive approach over fibrinolytic therapy may be reduced by the additional delay to initiation of mechanical reperfusion due to logistical problems. PCI-related delay is the additional delay necessary to perform primary PCI instead of fibrinolysis administration. This is the theoretical difference between the time of FMC to wire crossing minus the time from FMC to the start of fibrinolytic therapy. The maximum acceptable PCI-related delay, beyond which the benefit of primary PCI is completely counterbalanced by the harmful effect of reperfusion initiation delay, has been analysed in many studies. The majority of them were trial-based but not individual patient-data-based meta-analyses. Kent et al showed that a delay of 50 minutes as threshold yielded equivalent reductions in mortality for both primary PCI and thrombolysis [2424. Kent DM, Lau J, Selker HP. Balancing the benefits of primary angioplasty against the benefits of thrombolytic therapy for acute myocardial infarction: the importance of timing. Eff Clin Pract. 2001;4:214-220. ]. In the analysis of Nallamothu et al [2525. Nallamothu BK, Bates ER. Percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: is timing (almost) everything?. Am J Cardiol. 2003;92:824-826. ], the primary PCI mortality benefit was lost if PCI-related delay exceeded 88 minutes. Pinto et al [2626. Pinto DS, Kirtane AJ, Nallamothu BK, Murphy SA, Cohen DJ, Laham RJ, Cutlip DE, Bates ER, Frederick PD, Miller DP, Carrozza JP, Jr., Antman EM, Cannon CP, Gibson CM. Hospital delays in reperfusion for ST-elevation myocardial infarction: implications when selecting a reperfusion strategy. Circulation. 2006;114:2019-2025. ] showed in a combined analysis of the NRMI-2, 3 and 4 that the maximum acceptable PCI-related delay was much longer, i.e, 114 minutes, and varied considerably depending on various factors like duration of symptoms, age and infarction location. This suggests an individualised rather than a uniform approach for selecting an optimal reperfusion strategy. In the paper by Betriu et al [2727. Betriu A, Masotti M. Comparison of mortality rates in acute myocardial infarction treated by percutaneous coronary intervention versus fibrinolysis. Am J Cardiol. 2005;95:100-101. ], regression analysis showed that the mortality rate for PCI remained the same as that for fibrinolysis when the PCI-related time delay reached 110 minutes. De Luca et al [2828. De Luca G, Cassetti E, Marino P. Percutaneous coronary intervention-related time delay, patient’s risk profile, and survival benefits of primary angioplasty vs lytic therapy in ST-segment elevation myocardial infarction. Am J Emerg Med. 2009;27:712-719. ] found equipoise between primary angioplasty and fibrinolysis at 180 minutes. Only one individual patient data meta-analysis by Boersma et al [2929. Boersma E. Does time matter?. A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J. 2006;27:779-788. ] demonstrated that invasive treatment remains superior over fibrinolysis even for a PCI-related delay of 80-120 minutes (the analysis included only patients with a time delay <120 minutes). In the STREAM Study on STEMI patients presenting early (up to 3 hours from symptoms onset) with an anticipated delay to PCI longer than 60 minutes, despite a relative delay to PCI of 78 minutes early fibrinolysis was not associated with reduction of ischaemic events. Moreover, an increased rate of bleeding was found in patients randomised to fibrinolysis [3030. Armstrong PW, Gershlick AH, Goldstein P, Wilcox R, Danays T, Lambert Y, Sulimov V, Rosell OF, Ostojic M, Welsh RC, Carvalho AC, Nanas J, Arntz HR, Halvorsen S, Huber K, Grajek S, Fresco C, Bluhmki E, Regelin A, Vandenberghe K, Bogaerts K, Van de Werf F. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013;368:1379-1387. ].
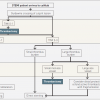
According to available data it is difficult to define the time limit to prefer primary PCI over fibrinolysis precisely. In current ESC STEMI guidelines the absolute time from STEMI diagnosis to PCI-mediated reperfusion rather than a relative PCI-related delay over fibrinolysis has been chosen. It is recommended that an expected maximum delay of 120 min from STEMI diagnosis to primary PCI (wire crossing), should be used in selecting a primary PCI strategy over fibrynolysis. However, as a target for quality assessment, primary PCI should be performed within 90 min after FMC in transferred patients and ≤60 min in patients presenting directly in a PCI-capable hospital For patients presenting in non-PCI centers the delay between arrival to this hospital and discharge (start of transfer with ambulance to the PCI center) should be ≤30 min. Importantly, it is recommended to record and audit delay times and work to achieve and maintain quality targets [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. The organisation of STEMI treatment strategy based on anticipated delay to reperfusion is shown in Figure 3.
TIMING OF ANGIOGRAPHY AND PCI AFTER LYSIS
Routine angiography/PCI
Despite the progress made hitherto, challenges remain. The diffusion of STEMI networks has reduced drastically the number of patients not treatable with primary PCI. The remaining limited patient groups living too far from 24/7 hubs to receive primary PCI in time, however, are disadvantaged by not receiving the most efficacious reperfusion treatment. To this can be added the additional disadvantage that the evidence gathered from the seminal studies of the last decade is not applied routinely or in a timely fashion, and that no new major studies have been conducted or new strategies designed recently. Research into new drugs for this acute indication have also come to a stop in recent years. .
In clinical trials from the 1980s and 1990s, immediate PCI for STEMI following full-dose fibrinolysis (so-called facilitated PCI) was found to be associated with low angiographic efficacy and worse clinical outcome [3131. Topol EJ, Califf RM, George BS, Kereiakes DJ, Abbottsmith CW, Candela RJ, Lee KL, Pitt B, Stack RS, O’Neill WW. A randomized trial of immediate versus delayed elective angioplasty after intravenous tissue plasminogen activator in acute myocardial infarction. N Engl J Med. 1987;317:581-588. , 3232. O’Neill WW, Weintraub R, Grines CL, Meany TB, Brodie BR, Friedman HZ, Ramos RG, Gangadharan V, Levin RN, Choksi N, . A prospective, placebo-controlled, randomized trial of intravenous streptokinase and angioplasty versus lone angioplasty therapy of acute myocardial infarction. Circulation. 1992;86:1710-1717. ]. This might depend on the lack of antiplatelet therapy and the increased prothrombotic state after fibrinolysis [3333. Owen J, Friedman KD, Grossman BA, Wilkins C, Berke AD, Powers ER. Thrombolytic therapy with tissue plasminogen activator or streptokinase induces transient thrombin activity. Blood. 1988;72:616-620. , 3434. Fitzgerald DJ, Catella F, Roy L, FitzGerald GA. Marked platelet activation in vivo after intravenous streptokinase in patients with acute myocardial infarction. Circulation. 1988;77:142-150. ]. In the ASSENT-4 PCI study [3535. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet. 2006;367:569-578. ], a strategy of immediate PCI after fibrinolysis was found to be harmful but many PCI procedures were performed at the time of maximal platelet activation after tenecteplase in patients receiving a suboptimal antiplatelet therapy with only aspirin. Several studies were conducted to assess the role and optimal moment of routine angiography/PCI after fibrinolysis. Some of those trials included an additional transfer to a PCI centre from non-PCI centres after fibrinolysis administration. This strategy represents the so-called pharmaco-invasive approach when fibrinolysis is administered as the initial reperfusion due to the anticipated delay to primary PCI caused by transfer logistics. In the SIAM III trial, a strategy of early angiography and immediate stenting (3.5 ± 2.3 hours after fibrinolysis) was associated with a significant reduction of the combined endpoints of death, re-infarction, target lesion revascularisation (TLR) and other ischaemic events after 30 days and 6 months [3636. Scheller B, Hennen B, Hammer B, Walle J, Hofer C, Hilpert V, Winter H, Nickenig G, Bohm M. Beneficial effects of immediate stenting after thrombolysis in acute myocardial infarction. J Am Coll Cardiol. 2003;42:634-641. ]. Similarly, in the GRACIA-1 study, after the mean time from fibrinolytic agent infusion to coronary angiography of 16.7 ± 5.6 hours, the early angiography/PCI approach was associated with significant risk reduction of the combined endpoint of death, non-fatal re-infarction and revascularisation at 1 year in comparison to the conservative group [3737. Fernandez-Aviles F, Alonso JJ, Castro-Beiras A, Vazquez N, Blanco J, onso-Briales J, Lopez-Mesa J, Fernandez-Vazquez F, Calvo I, Martinez-Elbal L, San Roman JA, Ramos B. Routine invasive strategy within 24 hours of thrombolysis versus ischaemia-guided conservative approach for acute myocardial infarction with ST-segment elevation (GRACIA-1): a randomised controlled trial. Lancet. 2004;364:1045-1053. ]. In the CARESS in AMI trial, transfer of high-risk STEMI patients for early routine PCI soon after the administration of abciximab and half-dose reteplase was shown to reduce the risk of recurrent ischaemia and all ischaemic complications at 30 days in comparison to conservatively treated patients with ischaemia-guided PCI (rescue PCI). The median time from fibrinolysis initiation to angiography in immediate PCI group was 135 minutes [3838. Di Mario C, Dudek D, Piscione F, Mielecki W, Savonitto S, Murena E, Dimopoulos K, Manari A, Gaspardone A, Ochala A, Zmudka K, Bolognese L, Steg PG, Flather M. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet. 2008;371:559-568. ]. Similarly, in the TRANSFER-AMI trial [3939. Cantor WJ, Fitchett D, Borgundvaag B, Ducas J, Heffernan M, Cohen EA, Morrison LJ, Langer A, Dzavik V, Mehta SR, Lazzam C, Schwartz B, Casanova A, Goodman SG. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360:2705-2718. ], enrolling high-risk STEMI patients, the pharmaco-invasive strategy (immediate transfer for PCI within 6 hours of fibrinolysis) was associated with better clinical outcome than standard treatment after fibrinolysis with tenecteplase (rescue PCI for failed reperfusion, with elective PCI encouraged for successfully reperfused patients after 24 hours). The median time from fibrinolysis initiation to angiography was 3 hours. What is important is that all patients received aspirin, heparin, a loading dose of clopidogrel, and, in 73% of patients undergoing immediate PCI, glycoprotein (GP) IIb-IIIa inhibitors [3939. Cantor WJ, Fitchett D, Borgundvaag B, Ducas J, Heffernan M, Cohen EA, Morrison LJ, Langer A, Dzavik V, Mehta SR, Lazzam C, Schwartz B, Casanova A, Goodman SG. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360:2705-2718. ]. In all the above-mentioned studies, no increase in bleeding events was observed. The GRACIA-2 authors report that early routine post-fibrinolysis PCI performed at 3–12 h after initiation of lytic therapy with the mean time to PCI of 6 h (median 4.6 hours) is safe, and results for myocardial perfusion better than primary angioplasty [4040. Fernandez-Aviles F, Alonso JJ, Pena G, Blanco J, onso-Briales J, Lopez-Mesa J, Fernandez-Vazquez F, Moreu J, Hernandez RA, Castro-Beiras A, Gabriel R, Gibson CM, Sanchez PL. Primary angioplasty vs. early routine post-fibrinolysis angioplasty for acute myocardial infarction with ST-segment elevation: the GRACIA-2 non-inferiority, randomized, controlled trial. Eur Heart J. 2007;28:949-960. ]. The safety of PCI early after fibrinolysis was also confirmed in NORDISTEMI, with a high utilisation of a radial approach explaining the very low incidence of access site complications [4141. Bohmer E, Hoffmann P, Abdelnoor M, Arnesen H, Halvorsen S. Efficacy and safety of immediate angioplasty versus ischemia-guided management after thrombolysis in acute myocardial infarction in areas with very long transfer distances results of the NORDISTEMI (NORwegian study on DIstrict treatment of ST-elevation myocardial infarction). J Am Coll Cardiol. 2010;55:102-110. ]. In the meta-analysis of 7 clinical trials [3535. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet. 2006;367:569-578. , 3636. Scheller B, Hennen B, Hammer B, Walle J, Hofer C, Hilpert V, Winter H, Nickenig G, Bohm M. Beneficial effects of immediate stenting after thrombolysis in acute myocardial infarction. J Am Coll Cardiol. 2003;42:634-641. , 3737. Fernandez-Aviles F, Alonso JJ, Castro-Beiras A, Vazquez N, Blanco J, onso-Briales J, Lopez-Mesa J, Fernandez-Vazquez F, Calvo I, Martinez-Elbal L, San Roman JA, Ramos B. Routine invasive strategy within 24 hours of thrombolysis versus ischaemia-guided conservative approach for acute myocardial infarction with ST-segment elevation (GRACIA-1): a randomised controlled trial. Lancet. 2004;364:1045-1053. , 3838. Di Mario C, Dudek D, Piscione F, Mielecki W, Savonitto S, Murena E, Dimopoulos K, Manari A, Gaspardone A, Ochala A, Zmudka K, Bolognese L, Steg PG, Flather M. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet. 2008;371:559-568. , 3939. Cantor WJ, Fitchett D, Borgundvaag B, Ducas J, Heffernan M, Cohen EA, Morrison LJ, Langer A, Dzavik V, Mehta SR, Lazzam C, Schwartz B, Casanova A, Goodman SG. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360:2705-2718. , 4040. Fernandez-Aviles F, Alonso JJ, Pena G, Blanco J, onso-Briales J, Lopez-Mesa J, Fernandez-Vazquez F, Moreu J, Hernandez RA, Castro-Beiras A, Gabriel R, Gibson CM, Sanchez PL. Primary angioplasty vs. early routine post-fibrinolysis angioplasty for acute myocardial infarction with ST-segment elevation: the GRACIA-2 non-inferiority, randomized, controlled trial. Eur Heart J. 2007;28:949-960. , 4242. Armstrong PW. A comparison of pharmacologic therapy with/without timely coronary intervention vs. primary percutaneous intervention early after ST-elevation myocardial infarction: the WEST (Which Early ST-elevation myocardial infarction Therapy) study. Eur Heart J. 2006;27:1530-1538. ] comparing early routine PCI after fibrinolysis vs. standard therapy, lower rates of re-infarction, death and re-infarction at 30 days and 6-12 months and recurrent ischaemia at 30 days were found after early PCI. There was no difference in terms of stroke and major bleedings between the groups [4343. Borgia F, Goodman SG, Halvorsen S, Cantor WJ, Piscione F, Le May MR, Fernandez-Aviles F, Sanchez PL, Dimopoulos K, Scheller B, Armstrong PW, Di Mario C. Early routine percutaneous coronary intervention after fibrinolysis vs. standard therapy in ST-segment elevation myocardial infarction: a meta-analysis. Eur Heart J. 2010;31:2156-2169. ].
After fibrinolysis all patients should be immediately transferred to a PCI-capable center (recommendation Class I; level of evidence A) and routine coronary angiography and, if applicable, PCI are recommended between 2 and 24 h after successful fibrinolysis (recommendation Class I; level of evidence A). [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. It seems to be indisputable that while promptly delivering fibrinolysis the operator should already be planning an immediate transfer to a centre with invasive facilities instead of waiting for fibrinolysis. The absence of negative effects of early angioplasty post-fibrinolysis was confirmed by a sub-analysis of PCI post-fibrinolysis trials [4444. Dimopoulos K, Dudek D, Piscione F, Mielecki W, Savonitto S, Borgia F, Murena E, Manari A, Gaspardone A, Ochala A, Zmudka K, Bolognese L, Steg PG, Flather M, Di Mario C. Timing of events in STEMI patients treated with immediate PCI or standard medical therapy: implications on optimisation of timing of treatment from the CARESS-in-AMI trial. Int J Cardiol. 2012;154:275-281. ]. No studies, however, convincingly demonstrate that a delay between fibrinolysis and PCI after successful reperfusion impairs outcome while the time delay truly affecting prognosis also in these patients remains the time between start of symptoms of chest pain and fibrinolysis.
Rescue angiography/PCI
Rescue PCI for STEMI is defined as intervention performed on an infarct-related coronary artery after unsuccessful fibrinolysis. Accurate identification of patients for whom fibrinolytic therapy has not restored the infarct-related artery patency remains a problem in daily practice. Such assessment is usually done 60-90 minutes after initiation of thrombolysis and is based on clinical symptoms (chest pain relief) and electrocardiographic ST-segment resolution (resolution >50% suggests successful thrombolysis). Rescue PCI strategy has been tested in clinical trials. In the MERLIN trial, 307 patients who failed to achieve 50% electrocardiographic ST-segment resolution at 60 minutes following fibrinolytic therapy were randomised to either rescue PCI or conservative treatment (without repeat administration of fibrinolytic therapy) [4545. Sutton AG, Campbell PG, Graham R, Price DJ, Gray JC, Grech ED, Hall JA, Harcombe AA, Wright RA, Smith RH, Murphy JJ, Shyam-Sundar A, Stewart MJ, Davies A, Linker NJ, de Belder MA. A randomized trial of rescue angioplasty versus a conservative approach for failed fibrinolysis in ST-segment elevation myocardial infarction: the Middlesbrough Early Revascularization to Limit INfarction (MERLIN) trial. J Am Coll Cardiol. 2004;44:287-296. ]. There was no difference in mortality at 30 days, but a lower rate of the composite endpoint (death/re-infarction/stroke/subsequent revascularisation/heart failure) was observed in the rescue PCI arm [4545. Sutton AG, Campbell PG, Graham R, Price DJ, Gray JC, Grech ED, Hall JA, Harcombe AA, Wright RA, Smith RH, Murphy JJ, Shyam-Sundar A, Stewart MJ, Davies A, Linker NJ, de Belder MA. A randomized trial of rescue angioplasty versus a conservative approach for failed fibrinolysis in ST-segment elevation myocardial infarction: the Middlesbrough Early Revascularization to Limit INfarction (MERLIN) trial. J Am Coll Cardiol. 2004;44:287-296. ]. The REACT trial enrolled 427 patients with <50% ST-segment resolution at 90 minutes and showed higher event-free survival at 6 months in patients treated with rescue PCI compared to those randomised to either fibrinolytic re-administration or conservative therapy [4646. Gershlick AH, Stephens-Lloyd A, Hughes S, Abrams KR, Stevens SE, Uren NG, de Belder A, Davis J, Pitt M, Banning A, Baumbach A, Shiu MF, Schofield P, Dawkins KD, Henderson RA, Oldroyd KG, Wilcox R. Rescue angioplasty after failed thrombolytic therapy for acute myocardial infarction. N Engl J Med. 2005;353:2758-2768. ]. In a meta-analysis of 8 trials including 1,177 patients, rescue PCI was associated with no significant reduction in all-cause mortality but showed significant risk reduction in heart failure and re-infarction when compared with conservative treatment [4747. Wijeysundera HC, Vijayaraghavan R, Nallamothu BK, Foody JM, Krumholz HM, Phillips CO, Kashani A, You JJ, Tu JV, Ko DT. Rescue angioplasty or repeat fibrinolysis after failed fibrinolytic therapy for ST-segment myocardial infarction: a meta-analysis of randomized trials. J Am Coll Cardiol. 2007;49:422-430. ]. The effectiveness of rescue angioplasty inspired the design of the STREAM trial, comparing in more than 1,800 patients primary versus an active rescue strategy applied in patients receiving tenecteplase (followed by rapid transfer for rescue PCI in 36.9% of the patients) [3030. Armstrong PW, Gershlick AH, Goldstein P, Wilcox R, Danays T, Lambert Y, Sulimov V, Rosell OF, Ostojic M, Welsh RC, Carvalho AC, Nanas J, Arntz HR, Halvorsen S, Huber K, Grajek S, Fresco C, Bluhmki E, Regelin A, Vandenberghe K, Bogaerts K, Van de Werf F. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013;368:1379-1387. ]. Despite the absence of overall difference in mortality in the two groups, the significant increase in intracranial bleeding in the fibrinolysis group (dose had to be halved in patients over 75 years during the conduct of the trial) and the widespread availability of primary PCI make this alternative unappealing in clinical practice.
According to recent ESC STEMI Guidelines, rescue PCI is indicated immediately when fibrinolysis has failed <50% ST-segment resolution at 60-90 minutes or at any time in the presence of haemodynamic or electrical instability, or worsening ischaemia (recommendation Class I; level of evidence A). Emergency angiography and PCI is indicated in case of recurrent ischaemia or evidence of reocclusion after initial successful fibrinolysis. (recommendation Class I; level of evidence A). [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ].
Angiography and PCI for ST-segment elevation myocardial infarction according to anticipated delay to mechanical reperfusion
- Primary PCI should be performed by an experienced team as soon as possible after STEMI diagnosis
- The preferred time limit from STEMI diagnosis to primary PCI (wire crossing) should be less than 90 minutes. This does not includepatients presenting directly in a PCI-capable hospital where the delay should be ≤60 min.
- Mechanical reperfusion and fibrinolysis are not alternative but complementary treatment modalities. When primary PCI is not available within the recommended time (the best organisational model will fail to achieve this target in rural or mountain areas far from primary PCI centres) fibrinolysis should be administered immediately. Then the patient should immediately be transferred (to avoid further delays in case of persistent chest pain or ECG changes) to a PCI-capable hospital where angiography and PCI should be performed within 24 hours.
Procedure technique
ACCESS SITE SELECTION
The selection of the coronary revascularisation method and the technique of performing primary PCI are not significantly different from those routinely used during elective PCI. Femoral approach is still common but a transradial approach should be preferred to decrease bleeding complications associated with the aggressive antiplatelet and antithrombotic treatment administered in the STEMI setting. The femoral and radial access techniques were described in detail in Chapter 1.04.
Importantly, the transradial approach was shown to be associated not only with a reduction of acute bleeding events, but also with a mortality reduction in STEMI patients. Thus, according to current ESC STEMI Guidelines (I; A) [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ] and ESC/EACTS Myocardial Revascularization Guidelines (IIa; A) [168168. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. ], the use of the transradial access during primary PCI for STEMI should be preferred, if performed by an experienced radial operator. The transradial approach also allows for early ambulation and reduction of total hospitalisation cost. The success rate of primary PCI via the transradial approach is similar to that observed with the femoral approach. Importantly, in experienced centres, the use of radial access in the STEMI setting is not associated with prolongation of door-to-balloon time and procedure time [4848. Pancholy S, Patel T, Sanghvi K, Thomas M, Patel T. Comparison of door-to-balloon times for primary PCI using transradial versus transfemoral approach. Catheter Cardiovasc Interv. 2010;75:991-995. , 4949. Weaver AN, Henderson RA, Gilchrist IC, Ettinger SM. Arterial access and door-to-balloon times for primary percutaneous coronary intervention in patients presenting with acute ST-elevation myocardial infarction. Catheter Cardiovasc Interv. 2010;75:695-699. , 5050. Siudak Z, Zawislak B, Dziewierz A, Rakowski T, Jakala J, Bartus S, Noworolnik B, Zasada W, Dubiel JS, Dudek D. Transradial approach in patients with ST-elevation myocardial infarction treated with abciximab results in fewer bleeding complications: data from EUROTRANSFER registry. Coron Artery Dis. 2010;21:292-297. ]. On the other hand, the use of radial access during more complex procedures is not ideal due to weaker guiding catheter back-up and the limited possibility of larger than 6 Fr guiding catheters being used during the procedure, especially in radial arteries with a small diameter (smaller women, patients with previous transradial procedures) [5151. Bazemore E, Mann JT, III. Problems and complications of the transradial approach for coronary interventions: a review. J Invasive Cardiol. 2005;17:156-159. ]. Cardiogenic shock or haemodynamic compromise should not be considered as absolute contraindications for the transradial approach, provided that the radial artery is palpable, possibly after insertion of an intra-aortic balloon pump (IABP) or left ventricular assist device (LVAD) via the femoral artery.
In a meta-analysis of 12 studies (randomised, case-control, and cohort studies) involving 3,324 patients the transradial approach during primary PCI in comparison to femoral access was associated with a significant reduction of major bleeding, death and the composite endpoint of death, MI, or stroke. However, the fluoroscopic time was longer, and access site crossover was more frequent for the transradial approach [5252. Vorobcsuk A, Konyi A, Aradi D, Horvath IG, Ungi I, Louvard Y, Komocsi A. Transradial versus transfemoral percutaneous coronary intervention in acute myocardial infarction Systematic overview and meta-analysis. Am Heart J. 2009;158:814-821. ].
In the MATRIX study, a total of 8,404 patients with acute coronary syndromes, with or without ST-segment elevation were randomised to radial and femoral access for coronary angiography / PCI [169169. Valgimigli M, Gagnor A, Calabro P, Frigoli E, Leonardi S, Zaro T, Rubartelli P, Briguori C, Ando G, Repetto A, Limbruno U, Cortese B, Sganzerla P, Lupi A, Galli M, Colangelo S, Ierna S, Ausiello A, Presbitero P, Sardella G, Varbella F, Esposito G, Santarelli A, Tresoldi S, Nazzaro M, Zingarelli A, de Cesare N, Rigattieri S, Tosi P, Palmieri C, Brugaletta S, Rao SV, Heg D, Rothenbuhler M, Vranckx P, Juni P. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465-2476. ]. The rates of major adverse cardiovascular events (MACE) as well as net cardiovascular events (combined ischaemic and bleeding events) were lower in the radial access group. The difference was driven by BARC major bleeding unrelated to coronary artery bypass graft surgery (1.6% vs 2.3%, rate ratio (RR) 0.67, 95% confidence interval (CI) 0.49-0.92, p=0.013) and all-cause mortality (1.6% vs 2.2%, RR 0.72, 95% CI 0.53-0.99, p=0.045). Benefits in terms of mortality reduction were confirmed also by an updated meta-analysis of studies comparing radial and femoral approach in acute coronary syndromes [169169. Valgimigli M, Gagnor A, Calabro P, Frigoli E, Leonardi S, Zaro T, Rubartelli P, Briguori C, Ando G, Repetto A, Limbruno U, Cortese B, Sganzerla P, Lupi A, Galli M, Colangelo S, Ierna S, Ausiello A, Presbitero P, Sardella G, Varbella F, Esposito G, Santarelli A, Tresoldi S, Nazzaro M, Zingarelli A, de Cesare N, Rigattieri S, Tosi P, Palmieri C, Brugaletta S, Rao SV, Heg D, Rothenbuhler M, Vranckx P, Juni P. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465-2476. ].
Physicians performing primary PCI should be familiar with both the transradial and femoral approach, as the use of particular approach might be limited due to difficult anatomy or concomitant severe peripheral vascular disease.
CATHETER AND GUIDEWIRE SELECTION
Coronary angiography can be performed with standard diagnostic catheters, although, angiography of the infarct-related artery can also be performed with a guiding catheter in order to save the time needed for catheter exchange. The properties of different guiding catheters and guidewires are described in Chapter 3.03. Standard Judkins and Extra Back-Up guiding catheters are preferred, but other guiding catheters (for example Amplatz), selected based on orifice configuration, anatomy of the artery and location of atherosclerotic lesions, are useful if a more effective guiding catheter back-up is needed. Proper guiding catheter selection increases the success rate and decreases procedure time.
The first attempt of passing through the site of occlusion should be made with soft non-hydrophilic guidewires. Use of soft guidewires limits the risk of distal vessel dissection and facilitates vessel true-lumen finding. If the passage with a soft guidewire is not successful, especially in calcified and tightly narrowed vessels, hydrophilic or polymer-jacketed wires and/or the support of microcatheters should be the second choice.
Aspiration thrombectomy technique
- Use of manual aspiration catheters should be limited to occluded infarct-related arteries or arteries with established distal flow and high thrombus burden after guidewire crossing
- Successful thrombectomy allows better visualisation of the atherosclerotic lesion and distal part of the infarct-related artery
- Aspiration should be started 2 cm before the occlusion or the lesion with thrombus
- Multiple slow-passage technique should be used, with a stop on the thrombus site and continuation of suction distally to the site of occlusion (if possible). Two or three passages are recommended
- The thrombectomy catheter must be removed with aspiration, even into the guiding catheter, and then the blood from the guiding catheter must also be aspirated.
- Suction success should be confirmed by presence of thrombus on the filter
- If greater suction force is needed (bigger arteries, organised thrombus) larger size catheters (7 Fr) or catheters with active thrombus fragmentation can be considered
PRIMARY PCI STRATEGY
Primary PCI should be performed immediately after coronary angiography within the culprit lesion. The optimal technique of primary PCI should be chosen after evaluation of the infarct-related artery flow, thrombus burden and vessel size ( Figure 4 ). Routine use of stents during primary PCI for STEMI is currently recommended. Preferably, the technique of direct stenting (without prior balloon dilatation) should be used if the distal part of the vessel is visible, as it is associated with improvement of reperfusion parameters and a reduced risk of no-reflow phenomenon during primary PCI [5353. Loubeyre C, Morice MC, Lefevre T, Piechaud JF, Louvard Y, Dumas P. A randomized comparison of direct stenting with conventional stent implantation in selected patients with acute myocardial infarction. J Am Coll Cardiol. 2002;39:15-21. , 5454. Napodano M, Ramondo A, Tarantini G, Peluso D, Compagno S, Fraccaro C, Frigo AC, Razzolini R, Iliceto S. Predictors and time-related impact of distal embolization during primary angioplasty. Eur Heart J. 2009;30:305-313. , 5555. Dziewierz A, Siudak Z, Rakowski T, Kleczynski P, Zasada W, Dubiel JS, Dudek D. Impact of direct stenting on outcome of patients with st-elevation myocardial infarction transferred for primary percutaneous coronary intervention (from the EUROTRANSFER registry). Catheter Cardiovasc Interv. 2013. ]. The use of simple manual aspiration catheters as an alternative to standard balloon dilatation may facilitate direct stenting, especially in occluded arteries and vessels with large thrombus burden. However, current ESC STEMI Guidelines recommended manual thrombectomy in STEMI as Class III;A (routine use of thrombus aspiration is not recommended) [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. The strategy of mechanical protection and stent selection in different subsets of patients is described in the following paragraphs.
Only in the setting of cardiogenic shock there is a consensus for attempting multivessel PCI in selected patients with multiple critical lesions [33. Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van ’t Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619. , 44. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa UM, Taggart D, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Kolh P, Alfieri O, Dunning J, Elia S, Kappetein P, Lockowandt U, Sarris G, Vouhe P, Kearney P, von Segesser L, Agewall S, Aladashvili A, Alexopoulos D, Antunes MJ, Atalar E, Brutel de la Riviere A, Doganov A, Eha J, Fajadet J, Ferreira R, Garot J, Halcox J, Hasin Y, Janssens S, Kervinen K, Laufer G, Legrand V, Nashef SA, Neumann FJ, Niemela K, Nihoyannopoulos P, Noc M, Piek JJ, Pirk J, Rozenman Y, Sabate M, Starc R, Thielmann M, Wheatley DJ, Windecker S, Zembala M. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2010;31:2501-2555. , 211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. Consecutive, haemodynamically significant lesions should be treated until haemodynamic stabilisation is achieved. Data concerning safety and efficacy of non-infarct-related artery revascularisation in non-shock patients during primary PCI in the acute setting are still limited. In two small randomised studies there was no excess of in-hospital and one-year mortality and other cardiovascular (CV) events associated with non-infarct-related artery revascularisation during index primary PCI [5656. Di Mario C, Sansa M, Airoldi F, Sheiban I, Manari A, Petronio A, Piccaluga E, De Servi S, Ramondo A, Colusso S, Formosa A, Cernigliaro C, Colombo A, Monzini N, Bonardi MA. Single vs multivessel treatment during primary angioplasty: results of the multicentre randomised HEpacoat for cuLPrit or multivessel stenting for Acute Myocardial Infarction (HELP AMI) Study. Int J Cardiovasc Intervent. 2004;6:128-133. , 5757. Ochala A, Smolka GA, Wojakowski W, Dudek D, Dziewierz A, Krolikowski Z, Gasior Z, Tendera M. The function of the left ventricle after complete multivessel one-stage percutaneous coronary intervention in patients with acute myocardial infarction. J Invasive Cardiol. 2004;16:699-702. ]. However, these studies were underpowered in order to properly assess the potential differences in clinical events. In a more recent study from Politi et al, primary PCI limited to the infarct-related artery was associated with decreased CV event-free survival compared to patients treated with staged or simultaneous revascularisation of non-infarct-related arteries during the index procedure. The PRAMI trial confirmed a mortality benefit after immediate multivessel PCI but the study was very controversial and far from being conclusive because all the patients with culprit lesion only treatment were not triaged for presence and extent of residual ischaemia, a behaviour generally considered as suboptimal care [5858. Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, Berry C, Oldroyd KG. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115-1123. , 170170. Witberg G, Kornowski R. Non-culprit lesion percutaneous coronary intervention during acute myocardial infarction - the road not taken?. Postepy Kardiol Interwencyjnej. 2015;11:71-73. ]. By contrast, data from retrospective registries have shown conflicting results. Some have shown favourable [5959. Qarawani D, Nahir M, Abboud M, Hazanov Y, Hasin Y. Culprit only versus complete coronary revascularization during primary PCI. Int J Cardiol. 2008;123:288-292. ], neutral [6060. Xu F, Chen YG, Li JF, Li GS, Ji QS, Lv RJ, Li RJ, Sun Y, Zhang W, Li L, Zhang Y. Multivessel percutaneous coronary intervention in chinese patients with acute myocardial infarction and simple nonculprit arteries. Am J Med Sci. 2007;333:376-380. , 6161. Khattab AA, bdel-Wahab M, Rother C, Liska B, Toelg R, Kassner G, Geist V, Richardt G. Multi-vessel stenting during primary percutaneous coronary intervention for acute myocardial infarction. A single-center experience. Clin Res Cardiol. 2008;97:32-38. ], and even worse outcomes including increased risk of death [6262. Cavender MA, Milford-Beland S, Roe MT, Peterson ED, Weintraub WS, Rao SV. Prevalence, predictors, and in-hospital outcomes of non-infarct artery intervention during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction (from the National Cardiovascular Data Registry). Am J Cardiol. 2009;104:507-513. , 6363. Corpus RA, House JA, Marso SP, Grantham JA, Huber KC, Jr., Laster SB, Johnson WL, Daniels WC, Barth CW, Giorgi LV, Rutherford BD. Multivessel percutaneous coronary intervention in patients with multivessel disease and acute myocardial infarction. Am Heart J. 2004;148:493-500. , 6464. Roe MT, Cura FA, Joski PS, Garcia E, Guetta V, Kereiakes DJ, Zijlstra F, Brodie BR, Grines CL, Ellis SG. Initial experience with multivessel percutaneous coronary intervention during mechanical reperfusion for acute myocardial infarction. Am J Cardiol. 2001;88:170-3, A6. , 6565. Dziewierz A, Siudak Z, Rakowski T, Zasada W, Dubiel JS, Dudek D. Impact of multivessel coronary artery disease and noninfarct-related artery revascularization on outcome of patients with ST-elevation myocardial infarction transferred for primary percutaneous coronary intervention (from the EUROTRANSFER Registry). Am J Cardiol. 2010;106:342-347. , 6666. Hannan EL, Samadashvili Z, Walford G, Holmes DR, Jr., Jacobs AK, Stamato NJ, Venditti FJ, Sharma S, King SB, III. Culprit vessel percutaneous coronary intervention versus multivessel and staged percutaneous coronary intervention for ST-segment elevation myocardial infarction patients with multivessel disease. JACC Cardiovasc Interv. 2010;3:22-31. ] associated with non-infarct-related artery revascularisation during the index procedure. In the largest of these, which included 3,134 patients treated with non-infarct-related artery PCI during the index procedure (from a total population of 28,936 patients with STEMI), an increased in-hospital mortality was observed after routine multivessel PCI [6262. Cavender MA, Milford-Beland S, Roe MT, Peterson ED, Weintraub WS, Rao SV. Prevalence, predictors, and in-hospital outcomes of non-infarct artery intervention during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction (from the National Cardiovascular Data Registry). Am J Cardiol. 2009;104:507-513. ]. This difference in mortality persisted after adjustment for covariates only in patients with shock, and was no longer significant in patients without shock. Meta-analysis [6767. Bangalore S, Kumar S, Poddar KL, Ramasamy S, Rha JS, Faxon DP. Meta-Analysis of Multivessel Coronary Artery Revascularization Versus Culprit-Only Revascularization in Patients With ST-Segment Elevation Myocardial Infarction and Multivessel Disease. Am J Cardiol. 2011. ] of randomised and non-randomised studies (19 studies, 23 arms) which evaluated 61,764 subjects with STEMI and multivessel disease has shown that simultaneous non-infarct-related artery revascularisation in the acute setting of STEMI is associated with an increase of 30-day mortality, without significant impact on long-term mortality. On the other hand, staged, complete revascularisation during index hospital stay was associated with a reduced risk of death and MACE up to 30 days, as well as at 1 year [6767. Bangalore S, Kumar S, Poddar KL, Ramasamy S, Rha JS, Faxon DP. Meta-Analysis of Multivessel Coronary Artery Revascularization Versus Culprit-Only Revascularization in Patients With ST-Segment Elevation Myocardial Infarction and Multivessel Disease. Am J Cardiol. 2011. ]. These possible benefits of staged, complete revascularisation during index hospital stay were also confirmed by two more recent studies: CvLPRIT [171171. Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, Blackman DJ, Dalby M, Fairbrother KL, Banya W, Wang D, Flather M, Hetherington SL, Kelion AD, Talwar S, Gunning M, Hall R, Swanton H, McCann GP. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963-972. ] and PRIMULTI [172172. Engstrom T, Kelbaek H, Helqvist S, Hofsten DE, Klovgaard L, Holmvang L, Jorgensen E, Pedersen F, Saunamaki K, Clemmensen P, De Backer O, Ravkilde J, Tilsted HH, Villadsen AB, Aaroe J, Jensen SE, Raungaard B, Kober L. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665-671. ]. The first one was not able to show reductions in “hard” endpoints, and the benefit in the composite endpoint was solely driven by repeat revascularisations [171171. Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, Blackman DJ, Dalby M, Fairbrother KL, Banya W, Wang D, Flather M, Hetherington SL, Kelion AD, Talwar S, Gunning M, Hall R, Swanton H, McCann GP. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963-972. ]. On the other hand, in the cardiac magnetic resonance (CMR) substudy, multivessel PCI in the setting of STEMI led to a small increase in CMR-detected non-infarct-related artery MI, but total infarct size was not different from an infarct-related artery-only revascularisation strategy [173173. McCann GP, Khan JN, Greenwood JP, Nazir S, Dalby M, Curzen N, Hetherington S, Kelly DJ, Blackman DJ, Ring A, Peebles C, Wong J, Sasikaran T, Flather M, Swanton H, Gershlick AH. Complete Versus Lesion-Only Primary PCI: The Randomized Cardiovascular MR CvLPRIT Substudy. J Am Coll Cardiol. 2015;66:2713-2724. ]. In the second study, 627 patients with STEMI and multivessel PCI after successful primary PCI of the infarct-related artery were randomized either to no further invasive treatment or complete fractional-flow reserve (FFR)-guided revascularisation before discharge [172172. Engstrom T, Kelbaek H, Helqvist S, Hofsten DE, Klovgaard L, Holmvang L, Jorgensen E, Pedersen F, Saunamaki K, Clemmensen P, De Backer O, Ravkilde J, Tilsted HH, Villadsen AB, Aaroe J, Jensen SE, Raungaard B, Kober L. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665-671. ]. Complete FFR-guided revascularisation significantly reduced the risk of future events as compared with no further invasive intervention after primary PCI. This effect was driven by significantly fewer repeat revascularisations, because all-cause mortality and non-fatal re-infarction did not differ between groups. Recently published results of CULPRIT-SHOCK study provide new insights on the management of patients with multivessel disease and acute myocardial infarction complicated by cardiogenic shock. In this study, 706 patients with multivessel disease were randomized to one of two initial revascularization strategies: either PCI of the culprit lesion only, with the option of staged revascularization of nonculprit lesions, or immediate multivessel PCI. At 30 days, the risk of death or renal-replacement therapy (the composite primary end point) was higher in the multivessel PCI group as compared to the culprit-lesion-only PCI group (55.4% vs. 45.9%; P=0.01). Similarly, the risk of death was lower in patients with PCI limited only to the culprit-lesion during the index procedure [212212. Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, Fach A, Lapp H, Piek JJ, Noc M, Goslar T, Felix SB, Maier LS, Stepinska J, Oldroyd K, Serpytis P, Montalescot G, Barthelemy O, Huber K, Windecker S, Savonitto S, Torremante P, Vrints C, Schneider S, Desch S, Zeymer U; CULPRIT-SHOCK Investigators. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N Engl J Med. 2017;377:2419-2432. ].
In summary, based on the ESC STEMI Guidelines [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ] non-infarct-related artery PCI during the index procedure should be considered in patients with cardiogenic shock (IIa; C). Routine revascularization of non-culprit lesions should be considered in STEMI patients with multivessel disease before hospital discharge (IIa; A). We might expect that those recommendations, especially for cardiogenic shock will be revaluated based on the results of the CULPRIT-SHOCK study.
THROMBECTOMY, PROXIMAL AND DISTAL PROTECTION
Suboptimal myocardial reperfusion may occur in a relatively large proportion of patients undergoing primary PCI for STEMI despite optimal restoration of epicardial flow, with subsequent unfavourable short and long-term outcome [6868. De Luca G, van ’t Hof AW, Ottervanger JP, Hoorntje JC, Gosselink AT, Dambrink JH, Zijlstra F, de Boer MJ, Suryapranata H. Unsuccessful reperfusion in patients with ST-segment elevation myocardial infarction treated by primary angioplasty. Am Heart J. 2005;150:557-562. ]. Among the factors accounting for poor myocardial reperfusion after primary angioplasty, in recent years mounting interest has emerged regarding the role of distal embolisation [6969. De Luca G, Suryapranata H, Chiariello M. Prevention of distal embolization in patients undergoing mechanical revascularization for acute myocardial infarction. A review of current status. Thromb Haemost. 2006;96:700-710. , 7070. De LG, Gibson CM, Bellandi F, Noc M, Maioli M, Zorman S, Zeymer U, Gabriel HM, Emre A, Cutlip D, Arntz HR, Dudek D, Rakowski T, Gyongyosi M, Huber K, van’t Hof AW. Impact of distal embolization on myocardial perfusion and survival among patients undergoing primary angioplasty with glycoprotein IIb-IIIa inhibitors: insights from the EGYPT cooperation. J Thromb Thrombolysis. 2010;30:23-28. ] and therefore in mechanical devices to prevent such a complication ( Table 2 ).
Distal protection devices
Several distal protection devices ( Table 2 , Figure 5 ) have proved their beneficial effects in PCI of saphenous venous bypass grafts. Several randomised trials have been conducted in primary PCI.
A) Distal occlusive devices
The promising results with the PercuSurge (Medtronic, Inc, Minneapolis, MN, USA), observed in initial studies on PCI of saphenous venous bypass grafts, have not been confirmed in STEMI by the large randomised EMERALD trial [7171. Stone GW, Webb J, Cox DA, Brodie BR, Qureshi M, Kalynych A, Turco M, Schultheiss HP, Dulas D, Rutherford BD, Antoniucci D, Krucoff MW, Gibbons RJ, Jones D, Lansky AJ, Mehran R. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA. 2005;293:1063-1072. ], where a total of 501 patients were randomised to PercuSurge GuardWire (Medtronic) (n=252) or conventional angioplasty (n=249). Despite atherothrombotic debris being found in 78% of patients, no benefits were observed in terms of myocardial perfusion, whereas infarct size was paradoxically increased with the device. Similar findings were observed in the ASPARAGUS [7272. Fujita N, Suwa S, Koyama S, Saito M, Muramatsu T. The efficacy of distal embolic protection device during an acute myocardial infarction: Early and long-term results. Am J Cardiol. 2004;94 (suppl 6A):34E. ] trial, where 341 patients were randomised to PercuSurge GuardWire (Medtronic) (n=173) or conventional primary angioplasty (n=168). However, in both trials this device did not increase the risk of coronary perforation or other mechanical complications. Due to the absence of benefits, these devices are not recommended in the setting of STEMI.
B) Filters
The use of intracoronary filters ( Figure 5 ) has been shown to improve outcome in elective patients undergoing elective PCI of a saphenous venous bypass graft. Also in this case, the promising results observed with initial non-randomised trials have not been confirmed by randomised trials. In the PROMISE trial [7373. Gick M, Jander N, Bestehorn HP, Kienzle RP, Ferenc M, Werner K, Comberg T, Peitz K, Zohlnhofer D, Bassignana V, Buettner HJ, Neumann FJ. Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation. 2005;112:1462-1469. ], 200 patients were randomised to FilterWire EZ™ (Boston Scientific, Natick, MA, USA) or conventional angioplasty. The use of filters did not lead to improvements in terms of myocardial perfusion (as evaluated by Doppler flow wire) and infarct size (as evaluated by cardiac magnetic resonance).
A pooled analysis of all trials on distal protection devices (7 trials, with a total of 1,353 patients)[7474. De Luca G, Suryapranata H, Stone GW, Antoniucci D, Neumann FJ, Chiariello M. Adjunctive mechanical devices to prevent distal embolization in patients undergoing mechanical revascularization for acute myocardial infarction: a meta-analysis of randomized trials. Am Heart J. 2007;153:343-353. ]showed that despite benefits in terms of myocardial perfusion (myocardial blush grade (MBG) 3: 50.2% vs. 39%, odds ratio (OR) = [95% CI] = 1.96 [1.18-3.26], p=0.009 (random effect model), phet = 0.02), no advantages were observed in terms of 30-day mortality (2.0% vs. 3.4%, OR [95% CI] = 0.61 [0.3-1.25], p=0.18 (random effect model), phet = 0.92) ( Figure 6 ). Due to the absence of benefits, these devices are not recommended in the setting of STEMI.
Proximal protection devices
The Proxis Embolic Protection System (Velocimed, Maple Grove, MN, USA), has recently been introduced to obtain complete protection from distal embolisation during PCI. In fact, this system may overcome some of the limitations of distal protection devices, such as the need of a distal “landing zone” of adequate calibre, incomplete protection in the case of large branches proximal to the distal protection device, and difficulties due to a complex anatomy such as vessel tortuosity or calcifications. This catheter, in fact, is deployed proximally to the target lesion, with complete interruption of antegrade blood flow before crossing the lesion. Unlike distal protection devices, this system is able to retrieve embolic materials of any size and composition.
In the PREPARE trial [7575. Haeck JD, Koch KT, Bilodeau L, van der Schaaf RJ, Henriques JP, Vis MM, Baan J, Jr., Van der Wal AC, Piek JJ, Tijssen JG, Krucoff MW, de Winter RJ. Randomized comparison of primary percutaneous coronary intervention with combined proximal embolic protection and thrombus aspiration versus primary percutaneous coronary intervention alone in ST-segment elevation myocardial infarction: the PREPARE (PRoximal Embolic Protection in Acute myocardial infarction and Resolution of ST-Elevation) study. JACC Cardiovasc Interv. 2009;2:934-943. ], Haeck et al have randomised 141 STEMI patients to PROXIS and 143 to conventional primary PCI. Despite significant advantages in terms of immediate ST-resolution (66% vs. 50%, p=0.009), no difference was observed in terms of ST-resolution at 90 minutes (81% vs. 74%, p=0.23), MBG 3 (81% vs. 83%, p=0.93), distal embolisation (10% vs. 14%, p=0.36) and clinical outcome. Future larger trials are certainly needed to evaluate the benefits in terms of myocardial perfusion and clinical outcome with this device. Due to the paucity of data, no recommendation is provided on this device in current guidelines.
Thrombectomy devices
The use of thrombectomy devices ( Figure 7) seems attractive to overcome some of the limitations of distal protection devices, such as the need of a “landing zone” and to cross the lesion which may cause distal embolisation. Several thrombectomy devices have been proposed to prevent distal embolisation, such as AngioJet® (Medrad Inc, Minneapolis, MN, USA), X-SIZER® (eV3, Inc, Plymouth, MN, USA), Rescue (Boston Scientific-Scimed, Inc, Maple Grove, MN, USA – not in use anymore), Export® Catheter (Medtronic Inc, Santa Rosa, CA, USA), Diver C.E.® (Invatec S.p.a, Roncadelle, Italy), Pronto® Catheter (Vascular Solutions, Inc, Minneapolis, MN, USA), Rinspiration® System (Kerberos Proximal Solutions, Cupertino, CA, USA), TVAC (Thrombus vacuum aspiration catheter; Nipro, Osaka, Japan), Eliminate (Terumo, Tokyo, Japan), Fetch-2® (Medrad Inc., Minneapolis, MN, USA), XpressWay ® (Kaneka, Osaka, Japan), QuickCat® extraction catheter (Spectranetics, Colorado Springs, CO, USA) ( Figure 7 ).
Several trials have been conducted with different devices leading to conflicting results. Mostly negative results have been observed in two large trials with mechanical thrombectomy [7676. Ali A, Cox D, Dib N, Brodie B, Berman D, Gupta N, Browne K, Iwaoka R, Azrin M, Stapleton D, Setum C, Popma J. Rheolytic thrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarction: 30-day results from a multicenter randomized study. J Am Coll Cardiol. 2006;48:244-252. , 7777. Kaltoft A, Bottcher M, Nielsen SS, Hansen HH, Terkelsen C, Maeng M, Kristensen J, Thuesen L, Krusell LR, Kristensen SD, Andersen HR, Lassen JF, Rasmussen K, Rehling M, Nielsen TT, Botker HE. Routine thrombectomy in percutaneous coronary intervention for acute ST-segment-elevation myocardial infarction: a randomized, controlled trial. Circulation. 2006;114:40-47. ].
In the AIMI multicentre trial [7676. Ali A, Cox D, Dib N, Brodie B, Berman D, Gupta N, Browne K, Iwaoka R, Azrin M, Stapleton D, Setum C, Popma J. Rheolytic thrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarction: 30-day results from a multicenter randomized study. J Am Coll Cardiol. 2006;48:244-252. ], a total of 480 patients were randomised to rheolytic thrombectomy with AngioJet® (Medrad Inc.) vs. conventional primary PCI. The primary endpoint was infarct size estimated by technetium-99m sestamibi. Paradoxically this trial showed a larger infarct size and higher mortality in patients treated with thrombectomy in comparison with conventional primary PCI. However, several factors may explain the negative results of this trial, including the low rate of anterior infarction (around 35%), a larger unjustified use of a temporary pacemaker in patients randomised to thrombectomy (58% vs. 19%), the large prevalence of preprocedural recanalisation (preprocedural thrombolysis in myocardial infarction (TIMI) 3 flow was more frequently observed in the control group [2727. Betriu A, Masotti M. Comparison of mortality rates in acute myocardial infarction treated by percutaneous coronary intervention versus fibrinolysis. Am J Cardiol. 2005;95:100-101. ] than in patients randomised to thrombectomy [1919. Brodie BR, Stuckey TD, Muncy DB, Hansen CJ, Wall TC, Pulsipher M, Gupta N. Importance of time-to-reperfusion in patients with acute myocardial infarction with and without cardiogenic shock treated with primary percutaneous coronary intervention. Am Heart J. 2003;145:708-715. ]), and the very low rate of patients with evidence of thrombus.
In a Danish single-centre trial [7777. Kaltoft A, Bottcher M, Nielsen SS, Hansen HH, Terkelsen C, Maeng M, Kristensen J, Thuesen L, Krusell LR, Kristensen SD, Andersen HR, Lassen JF, Rasmussen K, Rehling M, Nielsen TT, Botker HE. Routine thrombectomy in percutaneous coronary intervention for acute ST-segment-elevation myocardial infarction: a randomized, controlled trial. Circulation. 2006;114:40-47. ], a total of 215 STEMI patients were randomised to mechanical thrombectomy by the Rescue catheter (Boston Scientific-Scimed) or conventional primary angioplasty. Also in this study, patients were not selected on the basis of angiographic evidence of thrombus. Enzymatic infarct size, the primary study endpoint, was, in accordance with the AIMI trial, paradoxically larger in patients randomised to thrombectomy. No benefits were observed in terms of ST-segment resolution.
Different findings have been observed in the recently conducted JETSTENT trial [7878. Migliorini A, Stabile A, Rodriguez AE, Gandolfo C, Rodriguez Granillo AM, Valenti R, Parodi G, Neumann FJ, Colombo A, Antoniucci D. Comparison of AngioJet rheolytic thrombectomy before direct infarct artery stenting with direct stenting alone in patients with acute myocardial infarction. The JETSTENT trial. J Am Coll Cardiol. 2010;56:1298-1306. ], where the AngioJet® was tested in patients with large thrombotic burden. The device was associated with a significant improvement in ST-segment resolution (primary endpoint) and MACE at 6 months, even though no difference was observed in scintigraphic infarct size (primary endpoint). A pooled analysis of RCTs on mechanical devices did not show better quality reperfusion, and despite improvement in ST-segment resolution, no difference was observed in mortality ( Figure 6 ) [7979. De Luca G, Navarese EP, Suryapranata H. A meta-analytic overview of thrombectomy during primary angioplasty. Int J Cardiol. 2013;166:606-612. ].
Several randomised studies have shown that manual thrombectomy devices significantly improve myocardial perfusion (evaluated by MBG and ST-segment resolution) and reduce distal embolisation.
In the large TAPAS trial [8080. Svilaas T, Vlaar PJ, van dH, I, Diercks GF, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557-567. ], more than 1,000 STEMI patients were randomised before angiography to routine manual thrombectomy (Export Catheter; Medtronic) or conventional primary PCI. The vast majority of patients received GP IIb-IIIa inhibitors. This study showed significant benefits in myocardial perfusion (evaluated by MBG and ST-segment resolution) and significant benefits in one-year survival with manual thrombectomy.
In a first meta-analysis of 9 randomised trials on manual thrombectomy devices including 2,401 patients [8181. De Luca G, Dudek D, Sardella G, Marino P, Chevalier B, Zijlstra F. Adjunctive manual thrombectomy improves myocardial perfusion and mortality in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction: a meta-analysis of randomized trials. Eur Heart J. 2008;29:3002-3010. ], manual thrombectomy devices were associated with significant benefits in 30-day survival, explained by the improvement of epicardial and myocardial perfusion and reduction in distal embolisation However, the benefits seemed to be less pronounced in a more updated meta-analysis ( Figure 6) [7979. De Luca G, Navarese EP, Suryapranata H. A meta-analytic overview of thrombectomy during primary angioplasty. Int J Cardiol. 2013;166:606-612. ]
The beneficial effects from manual thrombectomy have not been confirmed by the recently published large TASTE randomized trial [8282. Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angeras O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Karegren A, Nilsson J, Robertson L, Sandhall L, Sjogren I, Ostlund O, Harnek J, James SK. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587-1597. ]. In fact at 30-day follow-up death from any cause occurred in 2.8% of the patients in the thrombus-aspiration group (103 of 3,621), as compared with 3.0% in the PCI-only group (110 of 3,623) (hazard ratio (HR), 0.94; 95% CI, 0.72-1.22, p=0.63). No significant differences were observed between the groups with respect to the rate of stroke or neurologic complications at the time of discharge (p=0.87). However, the study nested in a registry approach of this Swedish group did not prevent the exclusion of about 40% of the initial screened population, contributing to a large selection bias. In fact, with a mortality rate as low as 3%, we could have not expected so much absolute beneficial effects. Finally, the study was conducted as a registry, without any event verification, especially for any secondary endpoint.
No beneficial effects from routine thrombectomy have been confirmed in the last large TOTAL trial [174174. Jolly SS, Cairns JA, Yusuf S, Rokoss MJ, Gao P, Meeks B, Kedev S, Stankovic G, Moreno R, Gershlick A, Chowdhary S, Lavi S, Niemela K, Bernat I, Cantor WJ, Cheema AN, Steg PG, Welsh RC, Sheth T, Bertrand OF, Avezum A, Bhindi R, Natarajan MK, Horak D, Leung RC, Kassam S, Rao SV, El-Omar M, Mehta SR, Velianou JL, Pancholy S, Dzavik V. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet. 2016;387:127-135. ]. In this prospective randomised trial 10 732 patients with STEMI were randomly assigned to thrombectomy followed by PCI (n=5372) or to PCI alone (n=5360). This trial did not show a difference at 1 year in the primary outcome of cardiovascular death, MI, cardiogenic shock, or heart failure (HR 1.00 [95% CI 0.87-1.15], p=0.99), and cardiovascular death (HR 0.93 [95% CI 0.76-1.14], p=0.48), despite the improvements in the surrogate outcomes of ST segment resolution and distal embolisation. The key safety outcome, stroke within 1 year, occurred in 60 patients (1.2%) in the thrombectomy group compared with 36 (0.7%) in the PCI alone group (HR 1.66 [95% CI 1.10-2.51], p=0.015).
In conclusion, randomised trials conducted so far on mechanical thrombectomy devices have failed to show benefits in terms of infarct size and myocardial perfusion. The TASTE and TOTAL trials, the largest trials so far conducted, have recently questioned the beneficial effects from routine manual thrombectomy devices.
A recent meta-analysis [213213. Jolly SS, James S, Džavík V, Cairns JA, Mahmoud KD, Zijlstra F, Yusuf S, Olivecrona GK, Renlund H, Gao P, Lagerqvist B, Alazzoni A, Kedev S, Stankovic G, Meeks B, Frøbert O. Thrombus Aspiration in ST-Segment-Elevation Myocardial Infarction: An Individual Patient Meta-Analysis: Thrombectomy Trialists Collaboration. Circulation 2017;135:143-152. ] based on individual patients’ data of three large randomized trials (TAPAS, TASTE, and TOTAL) included 19 047 patients, of whom 18 306 underwent PCI and were included in the primary analysis. Cardiovascular death at 30 days occurred in 221 of 9155 patients (2.4%) randomized to thrombus aspiration and 262 of 9151 (2.9%) randomized to PCI alone (HR 0.84 [95% CI, 0.70-1.01; P=0.06). Stroke or transient ischemic attack occurred in 66 (0.8%) randomized to thrombus aspiration and 46 (0.5%) randomized to PCI alone (P=0.06). There were no significant differences in reMI, stent thrombosis, heart failure, or target vessel revascularization (TVR). In the subgroup with high thrombus burden (TIMI thrombus grade ≥3), thrombus aspiration was associated with fewer cardiovascular deaths (170 [2.5%] vs. 205 [3.1%]; P=0.03) and with more strokes or transient ischemic attacks (55 [0.9%] vs. 34 [0.5%]; P=0.04). However, the interaction P values were 0.32 and 0.34, respectively.
However, independently from the conclusions of these two large trials, a thrombus-based strategy may still be suggested and considered in case of evident thrombus. In fact, thrombus removal is a key element in optimal stent selection and implantation, by minimizing the occurrence of coronary vasospasm (that may reduce the risk of stent undersizing and stent malapposition), and reducing the risk of thrombus entrapment between the struts and the wall that contribute to late stent malapposition. The use of mechanical devices may certainly be considered in case of large, extensive thrombus burden.
According to Current ESC guidelines recommendation [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ] routine thrombus aspiration is not recommended (Class III; level of evidence A).
STENT SELECTION (BARE METAL, DRUG-ELUTING, MESH-COVERED STENTS)
A previous meta-analysis of patients undergoing primary PCI has shown the benefits of stenting compared to balloon angioplasty alone in terms of reducing TVR, though no definite impact on death and re-infarction was present [8383. De Luca G, Suryapranata H, Stone GW, Antoniucci D, Biondi-Zoccai G, Kastrati A, Chiariello M, Marino P. Coronary stenting versus balloon angioplasty for acute myocardial infarction: a meta-regression analysis of randomized trials. Int J Cardiol. 2008;126:37-44. ].
Several randomised trials have been conducted on drug-eluting stents (DES) in STEMI, and long-term follow-up data have recently been published. Tebaldi et al [8484. Tebaldi M, Arcozzi C, Campo G, Percoco G, Ferrari R, Valgimigli M. The 5-year clinical outcomes after a randomized comparison of sirolimus-eluting versus bare-metal stent implantation in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2009;54:1900-1901. ], recently reported 5-year follow-up from the STRATEGY trial, where sirolimus-eluting stent (SES) plus tirofiban was compared to bare metal stent (BMS) BxVelocity® (Cordis, Johnson & Johnson, Warren, NJ, USA) plus abciximab in 175 STEMI patients. At 5 years, the cumulative incidence of MACE (death, MI, or TVR) showed a lower trend in the tirofiban-SES group (29.9% vs. 43.2%, p=0.067). All-cause mortality and the composite of death or MI were similar in the tirofiban-SES versus the abciximab-BMS group, whereas the need for TVR remained markedly reduced (10.3% vs. 26.1%, p=0.007) in the tirofiban-SES group. The benefits from SES in terms of reducing clinical and angiographic restenosis without an increase in death or MI have been confirmed at long-term follow-up (4 and 3 years) in subsequent moderate-sized randomised trials such as the TYPHOON [8585. Spaulding C, Teiger E, Commeau P, Varenne O, Bramucci E, Slama M, Beatt K, Tirouvanziam A, Polonski L, Stella PR, Clugston R, Fajadet J, de B, X, Bode C, Carrie D, Erglis A, Merkely B, Hosten S, Cebrian A, Wang P, Stoll HP, Henry P. Four-year follow-up of TYPHOON (trial to assess the use of the CYPHer sirolimus-eluting coronary stent in acute myocardial infarction treated with BallOON angioplasty). JACC Cardiovasc Interv. 2011;4:14-23. ] and SESAMI [8686. Violini R, Musto C, De Felice F, Nazzaro MS, Cifarelli A, Petitti T, Fiorilli R. Maintenance of long-term clinical benefit with sirolimus-eluting stents in patients with ST-segment elevation myocardial infarction 3-year results of the SESAMI (sirolimus-eluting stent versus bare-metal stent in acute myocardial infarction) trial. J Am Coll Cardiol. 2010;55:810-814. ] trials, respectively.
In the PASSION trial [8787. Vink MA, Dirksen MT, Suttorp MJ, Tijssen JG, van Etten J, Patterson MS, Slagboom T, Kiemeneij F, Laarman GJ. 5-year follow-up after primary percutaneous coronary intervention with a paclitaxel-eluting stent versus a bare-metal stent in acute ST-segment elevation myocardial infarction: a follow-up study of the PASSION (Paclitaxel-Eluting Versus Conventional Stent in Myocardial Infarction with ST-Segment Elevation) trial. JACC Cardiovasc Interv. 2011;4:24-29. ], a paclitaxel-eluting stent (PES) was compared to the EXPRESS™ stent (Boston Scientific, Maple Grove, MN, USA) in 619 STEMI patients. Despite the safety of PES in terms of death and stent thrombosis as compared to BMS, at 5-year follow-up only a weak trend was present towards a reduction in TVR (7.3% vs. 10.5%, p=0.16). The relatively less favourable outcomes of PES in this trial compared to SES in the previous trials may relate to either the less marked reduction of neointimal hyperplasia with paclitaxel compared to sirolimus or a better outcome with the control stent in the PASSION trial compared to the Bx Velocity® (Cordis) in the SES trials. Also of note, routine angiographic follow-up was not performed in the PASSION trial.
Data from the PASEO trial [8888. Di Lorenzo E, Sauro R, Varricchio A, Carbone G, Cortese G, Capasso M, Lanzillo T, Manganelli F, Mariello C, Siano F, Pagliuca MR, Stanco G, Rosato G, De Luca G. Long-Term outcome of drug-eluting stents compared with bare metal stents in ST-segment elevation myocardial infarction: results of the paclitaxel- or sirolimus-eluting stent versus bare metal stent in Primary Angioplasty (PASEO) Randomized Trial. Circulation. 2009;120:964-972. ] showed that at 5-year follow-up both SES and PES were equally associated with a significant reduction in TVR and MACE, without any concern in terms of death and in-stent thrombosis.
Some words of caution recently came from the DEDICATION trial [8989. Kaltoft A, Kelbaek H, Thuesen L, Lassen JF, Clemmensen P, Klovgaard L, Engstrom T, Botker HE, Saunamaki K, Krusell LR, Jorgensen E, Tilsted HH, Christiansen EH, Ravkilde J, Kober L, Kofoed KF, Terkelsen CJ, Helqvist S. Long-term outcome after drug-eluting versus bare-metal stent implantation in patients with ST-segment elevation myocardial infarction: 3-year follow-up of the randomized DEDICATION (Drug Elution and Distal Protection in Acute Myocardial Infarction) Trial. J Am Coll Cardiol. 2010;56:641-645. ], where a significant reduction in TVR (8.9% vs. 19.8%), but a significantly higher risk of cardiac death with DES as compared to BMS (6.1% vs. 1.9%, p=0.013), was observed at 3-year follow-up.
Two-year follow-up data of the HORIZONS-AMI trial [9090. Stone GW, Parise H, Witzenbichler B, Kirtane A, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Mockel M, Lansky AJ, Mehran R. Selection criteria for drug-eluting versus bare-metal stents and the impact of routine angiographic follow-up: 2-year insights from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. J Am Coll Cardiol. 2010;56:1597-1604. ] have recently been published. Compared with BMS, PES showed similar outcome in terms of death, re-infarction and in-stent thrombosis, while the 24-month TLR rate was significantly reduced from 14.2% to 8.3% (p<0.0001). The difference was more pronounced in patients undergoing routine angiographic follow-up [9090. Stone GW, Parise H, Witzenbichler B, Kirtane A, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Mockel M, Lansky AJ, Mehran R. Selection criteria for drug-eluting versus bare-metal stents and the impact of routine angiographic follow-up: 2-year insights from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. J Am Coll Cardiol. 2010;56:1597-1604. ] and in patients with identified high-risk features for TLR, such as insulin-treated diabetes mellitus, reference vessel diameter ≤3.0 mm, and lesion length ≥30 mm. In patients with 2 or 3 of these baseline risk factors, PES compared with BMS markedly reduced 12-month TLR (19.8% vs. 8.1%, p=0.003), with only modest benefits in patients with 1 of these risk factors (7.3% vs. 4.3%, p=0.02) and no benefits in patients with no risk factor (3.3% vs. 3.2%, p=0.93).
A recent meta-analysis (DESERT) based on individual data with long-term follow-up from 6,298 patients [9191. De Luca G, Dirksen MT, Spaulding C, Kelbaek H, Schalij M, Thuesen L, van der Hoeven B, Vink MA, Kaiser C, Musto C, Chechi T, Spaziani G, Diaz de la Llera L, Pasceri V, Di Lorenzo E, Violini R, Cortese G, Suryapranata H, Stone GW. Drug-eluting vs bare-metal stents in primary angioplasty: a pooled patient-level meta-analysis of randomized trials. Arch Intern Med. 2012;172:611-621. ] showed, at a mean follow-up of 3 years, that 1st generation DES significantly reduced TVR compared with BMS, without increasing mortality, re-infarction and overall stent thrombosis. However, very late re-infarction and stent thrombosis, occurring 1 year after revascularisation, were significantly increased with DES.[9292. Piccolo R, Cassese S, Galasso G, De RR, D’Anna C, Piscione F. Long-term safety and efficacy of drug-eluting stents in patients with acute myocardial infarction: a meta-analysis of randomized trials. Atherosclerosis. 2011;217:149-157. ]Large interests have been recently focused on new generation DES, with more biocompatible polymer, bioabsorbable polymers, or even polymer-free. In fact, it was well known that the polymer was, due to persistent inflammation, one of the determinants of late stent thrombosis. Several trials have been conducted in the setting of STEMI.
The COMFORTABLE-AMI [9393. Raber L, Kelbaek H, Ostojic M, Baumbach A, Heg D, Tuller D, von Birgelen C, Roffi M, Moschovitis A, Khattab AA, Wenaweser P, Bonvini R, Pedrazzini G, Kornowski R, Weber K, Trelle S, Luscher TF, Taniwaki M, Matter CM, Meier B, Juni P, Windecker S. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308:777-787. ] compared the biolimus-eluting stent with biodegradable polymer (BES) vs BMS in 1,500 patents. At 1-year follow-up major adverse cardiac events occurred in 24 patients (4.3%) receiving BES and 49 patients (8.7%) receiving BMS (p=0.004). The difference was driven by a lower risk of target vessel-related re-infarction (0.5% vs. 2.7%, p=0.01) and ischaemia-driven TLR (1.6% vs. 5.7%, p<0.001) in patients receiving BES compared with those receiving BMS.
At 2 years [175175. Raber L, Kelbaek H, Taniwaki M, Ostojic M, Heg D, Baumbach A, von Birgelen C, Roffi M, Tuller D, Engstrom T, Moschovitis A, Pedrazzini G, Wenaweser P, Kornowski R, Weber K, Luscher TF, Matter CM, Meier B, Juni P, Windecker S. Biolimus-eluting stents with biodegradable polymer versus bare-metal stents in acute myocardial infarction: two-year clinical results of the COMFORTABLE AMI trial. Circ Cardiovasc Interv. 2014;7:355-364. ], differences in the primary end point of cardiac death, target-vessel MI, and TLR continued to diverge in favour of BES-treated patients (5.8%) compared with BMS-treated patients (11.9%) (p<0.001) with a significant risk reduction during the second year of follow-up (p=0.049). Differences in the primary end point were driven by a reduction inTLR (3.1% vs. 8.2%, p<0.001) and target-vessel re-infarction (1.3% vs. 3.4%, p=0.023). The composite of death, any re-infarction and revascularisation (14.5% vs. 19.3%. p=0.03), and cardiac death or target-vessel MI (4.2% versus 7.2%, p=0.036) were less frequent among BES-treated patients compared with BMS-treated patients.
In the EXAMINATION trial [9494. Sabate M, Cequier A, Iniguez A, Serra A, Hernandez-Antolin R, Mainar V, Valgimigli M, Tespili M, den Heijer P, Bethencourt A, Vazquez N, Gomez-Hospital JA, Baz JA, Martin-Yuste V, van Geuns RJ, Alfonso F, Bordes P, Tebaldi M, Masotti M, Silvestro A, Backx B, Brugaletta S, van Es GA, Serruys PW. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380:1482-1490. ] 1,498 patients with STEMI were randomly assigned to receive EES or BMS. At 1-year follow-up the primary endpoint (patient-oriented combined endpoint of all-cause death, any recurrent MI, and any coronary revascularisation) was similar in both groups (11.9% in the EES group vs. 14.2% in the BMS group, p=0.19. However,EES was associated with significantly lower rates of TLR and TVR (2.1% vs. 5.0%, p=0.003, and 3.7% vs. 6.8%, p=0.0077) and stent thrombosis (0.5% vs. 1.9% for definite and 0.9% vs. 2.5% for combined definite or probable stent thrombosis, both p=0.019).
At 5 years, complete clinical follow-up data were obtained for 731 patients treated with EES and 727 treated with BMS (97% of both groups) [176176. Sabate M, Brugaletta S, Cequier A, Iniguez A, Serra A, Jimenez-Quevedo P, Mainar V, Campo G, Tespili M, den Heijer P, Bethencourt A, Vazquez N, van Es GA, Backx B, Valgimigli M, Serruys PW. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet. 2016;387:357-366. ]. The patient-oriented endpoint occurred in 159 (21%) patients in the EES group versus 192 (26%) in the BMS group (p=0.033). This difference was mainly driven by a reduced rate of all-cause mortality (9% vs. 12%, p=0.047).
In the XAMI (XienceV Stent vs Cypher Stent in Primary PCI for Acute Myocardial Infarction) [9595. Hofma SH, Brouwer J, Velders MA, van’t Hof AW, Smits PC, Quere M, de Vries CJ, van Boven AJ. Second-generation everolimus-eluting stents versus first-generation sirolimus-eluting stents in acute myocardial infarction. 1-year results of the randomized XAMI (XienceV Stent vs. Cypher Stent in Primary PCI for Acute Myocardial Infarction) trial. J Am Coll Cardiol. 2012;60:381-387. ] trial a total of 625 patients with AMI were randomised (2:1) to receive EES or SES. Primary endpoint was major adverse cardiac events (MACE) at 1 year consisting of cardiac death, nonfatal MI, or any TVR. EES was associated with a significant reduction in the primary endpoint (4% vs. 7.7%, p=0.048), whereas no difference was observed in terms of cardiac mortality (1.5% vs. 2.7%, p = 0.36), and definite and/or probable stent thrombosis (1.2% vs. 2.7%, p=0.21). At three-year follow-up [177177. Hofma SH, Smits PC, Brouwer J, Velders MA, van ’t Hof AW, Quere M, de Vries CJ, van Boven AJ. Long-term follow-up of second-generation everolimus-eluting stents versus first-generation sirolimus-eluting stents in acute myocardial infarction: three-year results of the XAMI trial. EuroIntervention. 2015;10:1280-1283. ], the primary endpoint was 8.0% for EES and 10.5% for SES (p=0.30). Cardiac death was low and comparable in both groups (2.5% vs. 2.7%; p=0.86), as was definite/probable stent thrombosis (2.3% vs. 3.2%, p=0.60).In the RACES MI 500 patients with STEMI were randomized to EES (n = 250) or SES (n = 250). At 2-year follow-up, no significant difference was observed between EES and SES in major adverse cardiac events (16% vs. 20.8%, p=0.17), cardiac death (4.4% vs. 5.6%, p=0.53), recurrent MI (6.4% vs. 10%, p=0.13), and TVR (4.8% vs. 4.8%, p=0.99) [178178. Di Lorenzo E, Sauro R, Varricchio A, Capasso M, Lanzillo T, Manganelli F, Carbone G, Lanni F, Pagliuca MR, Stanco G, Rosato G, Suryapranata H, De Luca G. Randomized comparison of everolimus-eluting stents and sirolimus-eluting stents in patients with ST elevation myocardial infarction: RACES-MI trial. JACC Cardiovasc Interv. 2014;7:849-856. ]. However, EES was associated with a significant reduction in stent thrombosis (1.6% vs. 5.2%, p=0.035). At 5-year follow-up, EES was associated with a significant reduction in MACE (23.8 vs. 34.1%, p=0.028), Stent thrombosis (2.5% vs. 7.7%, p=0.009), without any difference in death (8.7% vs. 11.4%, adjusted p=0.47), re-infarction (9.3% vs 13.1%; adjusted p=0.18) or TVR (8.6% vs 12.3%, adjusted p=0.31) [179179. Di Lorenzo E, Sauro R, Capasso M, Lanni F, Lanzillo T, Carbone G, Manganelli F, Palmieri V, Serino V, Pagliuca MR, Rosato G, Suryapranata H, De Luca G. Long-term results of the randomized comparison of everolimus-eluting stents and sirolimus-eluting stent in patients with ST elevation myocardial infarction (RACES-MI trial). Int J Cardiol. 2016;202:177-182. ].
The beneficial effects and a significant reduction in stent thrombosis with new generation DES have been confirmed in a recent network meta-analysis [9696. Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Sabate M, Valgimigli M, Frati G, Kedhi E, Smits PC, Kaiser C, Genereux P, Galatius S, Kirtane AJ, Stone GW. Clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2013;62:496-504. ].
Due to the available data, current guidelines strongly recommend the use of new generation DES over BMS in the setting of STEMI (Class I; level of evidence A) [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. However, it should be kept in mind the avoidance of stent implantation in the presence of residual thrombus. Thrombectomy is strongly encouraged in order to avoid, after thrombus dissolution, late stent malapposition. Furthermore, thrombus removal may reduce vasospasm associated with platelet humoral factors and therefore help to evaluate the right vessel size. Nitrate administration is strongly encouraged to reduce vasospasm further.
Large attention has also been paid to the use of BVS in the setting of STEMI and several studies have recently been published.
The BVS-EXAMINATION study [180180. Brugaletta S, Gori T, Low AF, Tousek P, Pinar E, Gomez-Lara J, Scalone G, Schulz E, Chan MY, Kocka V, Hurtado J, Gomez-Hospital JA, Munzel T, Lee CH, Cequier A, Valdes M, Widimsky P, Serruys PW, Sabate M. Absorb bioresorbable vascular scaffold versus everolimus-eluting metallic stent in ST-segment elevation myocardial infarction: 1-year results of a propensity score matching comparison: the BVS-EXAMINATION Study (bioresorbable vascular scaffold-a clinical evaluation of everolimus eluting coronary stents in the treatment of patients with ST-segment elevation myocardial infarction). JACC Cardiovasc Interv. 2015;8:189-197. ] included 290 consecutive STEMI patients treated by BVS, compared with either 290 STEMI patients treated with EES or 290 STEMI patients treated with bare-metal stents (BMS) from the EXAMINATION trial, by applying propensity score matching. The primary endpoint was a device-oriented endpoint (DOCE), including cardiac death, target-vessel MI, and TLR, at 1-year follow-up. Device thrombosis, according to the Academic Research Consortium criteria, was also evaluated. The cumulative incidence of DOCE did not differ between the BVS and EES or BMS groups either at 30 days (3.1% vs. 2.4%, p=0.593; vs. 2.8%, p=0.776, respectively) or at 1 year (4.1% vs. 4.1%, p=0.994; vs. 5.9%, p=0.306, respectively). Definite/probable BVS thrombosis rate was numerically higher either at 30 days (2.1% vs. 0.3%, p=0.059; vs. 1.0%, p=0.324, respectively) or at 1 year (2.4% vs. 1.4%, p=0.948; vs. 1.7%, p=0.825, respectively), as compared with EES or BMS. This study showed that at 1-year follow-up, STEMI patients treated with BVS showed similar rates of DOCE compared with STEMI patients treated with EES or BMS, although the rate of scaffold thrombosis, mostly clustered in the early phase, was not negligible. Larger studies with longer follow-up are needed to confirm this findings.
The PRAGUE-19 [181181. Widimsky P, Petr R, Tousek P, Maly M, Linkova H, Vrana J, Hajsl M, Budesinsky T, Lisa L, Kocka V. One-Year Clinical and Computed Tomography Angiographic Outcomes After Bioresorbable Vascular Scaffold Implantation During Primary Percutaneous Coronary Intervention for ST-Segment-Elevation Myocardial Infarction: The PRAGUE-19 Study. Circ Cardiovasc Interv. 2015;8. , 182182. Tousek P, Kocka V, Maly M, Kozel M, Robert P, Hajsl M, Jarkovsky J, Widimsky P. Two-year follow-up after bioresorbable vascular scaffold implantation in STEMI patients - Results from PRAGUE-19 study. Int J Cardiol. 2016;209:20-21. ] is a prospective multicentre single-arm study enrolling consecutive STEMI patients undergoing primary PCI with intention-to-implant BVS. A total of 343STEMI patients were screened during 15 months enrolment period, and 70 patients fulfilled entry criteria and BVS was successfully implanted in 96% of them. Patients underwent computed tomographic angiographic control 1 year after BVS implantation. Restenosis was defined as ≥75% area stenosis within the scaffolded segment. Three events were potentially related to BVS: 1 in-stent restenosis (treated 7 months after primary PCI with drug-eluting balloon), 1 stent thrombosis (treated 2 weeks after primary PCI by balloon dilatation-this patient stopped all medications after primary PCI), and 1 sudden death at home 9 months after primary PCI. Overall, 1-year mortality was 2.9%. Computed tomographic angiography performed in 59 patients showed a binary restenosis rate of 2%. No differences in primary composite endpoint during the 2 year follow-up have been found between theBVS and control group (7.5% vs. 18.9%, p=0.141) and no additional definitive/probable stent thrombosis occurred in both groups [182182. Tousek P, Kocka V, Maly M, Kozel M, Robert P, Hajsl M, Jarkovsky J, Widimsky P. Two-year follow-up after bioresorbable vascular scaffold implantation in STEMI patients - Results from PRAGUE-19 study. Int J Cardiol. 2016;209:20-21. ].
The ABSORB-STEMI TROFI II [183183. Sabate M, Windecker S, Iniguez A, Okkels-Jensen L, Cequier A, Brugaletta S, Hofma SH, Raber L, Christiansen EH, Suttorp M, Pilgrim T, van Es GA, Sotomi Y, Garcia-Garcia HM, Onuma Y, Serruys PW. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J. 2016;37:229-240. ] was a multicentre non-inferiority randomized trial. including 191 STEMI patients undergoing primary PCI who were randomly assigned 1:1 to Absorb (n = 95) or EES (n = 96). The primary endpoint was the 6-month optical frequency domain imaging healing score (HS) based on the presence of uncovered and/or malapposed stent struts and intraluminal filling defects. Main secondary endpoint included DOCE according to the Academic Research Consortium At 6 months, HS was lower in the Absorb arm when compared with EES arm [1.74 (2.39) vs. 2.80 (4.44); difference (90% CI) -1.06 (-1.96, -0.16); p for non-inferiority <0.001]. Device-oriented composite endpoint was also comparably low between groups (1.1% Absorb vs. 0% EES). One case of definite subacute stent thrombosis occurred in the Absorb arm (1.1% vs. 0% EES, p=ns). Therefore, this study concluded that Absorb implantation in the setting of STEMI resulted in a nearly complete arterial healing which was comparable with that of metallic EES at 6 months.
However, due to the increased risk of target lesion failure and device thrombosis observed in several randomized trials [214214. Kereiakes DJ, Ellis SG, Metzger C, Caputo RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO, Popma JJ, McGreevy R, Zhang Z, Simonton C, Stone GW; ABSORB III Investigators. 3-Year Clinical Outcomes With Everolimus-Eluting Bioresorbable Coronary Scaffolds: The ABSORBIII Trial. J Am Coll Cardiol. 2017;70:2852-2862. , 215215. Ali ZA, Gao RF, Kimura T, Onuma Y, Kereiakes DJ, Ellis SG, Chevalier B, Vu MT, Zhang Z, Simonton CA, Serruys PW, Stone GW. Three-Year Outcomes With the Absorb Bioresorbable Scaffold: Individual-Patient-Data Meta-Analysis From the ABSORB Randomized Trials. Circulation. 2018;137:464-479. ], the Absorb stent has been removed from the market and Abbott has stopped its production [216216. https://www.tctmd.com/news/no-more-absorb-bvs-abbott-puts-stop-sales ].
In case of large thrombus burden, the use of a mesh-covered stent may be considered to trap the thrombus and reduce distal embolisation. Recent experiences [9797. Dudek D, Dziewierz A, Rzeszutko L, Legutko J, Dobrowolski W, Rakowski T, Bartus S, Dragan J, Klecha A, Lansky AJ, Siudak Z, Zmudka K. Mesh covered stent in ST-segment elevation myocardial infarction. EuroIntervention. 2010;6:582-589. , 9898. Piscione F, Danzi GB, Cassese S, Esposito G, Cirillo P, Galasso G, Rapacciuolo A, Leosco D, Briguori C, Varbella F, Tuccillo B, Chiariello M. Multicentre experience with MGuard net protective stent in ST-elevation myocardial infarction: safety, feasibility, and impact on myocardial reperfusion. Catheter Cardiovasc Interv. 2010;75:715-721. , 9999. Dudek D, Dziewierz A, Kleczynski P, Giszterowicz D, Rakowski T, Sorysz D, Rzeszutko L, Legutko J, Bartus S, Dragan J, Klecha A, Siudak Z, Zmudka K. Long-term follow-up of mesh-covered stent implantation in patients with ST-segment elevation myocardial infarction. Kardiol Pol. 2014. ] showed the safety of MGuard™ (MGS, Inspire-MD, Tel Aviv, Israel) implantation in the setting of primary PCI. In a recent randomised trial [100100. Stone GW, Abizaid A, Silber S, Dizon JM, Merkely B, Costa RA, Kornowski R, Abizaid A, Wojdyla R, Maehara A, Dressler O, Brener SJ, Bar E, Dudek D. Prospective, Randomized, Multicenter Evaluation of a Polyethylene Terephthalate Micronet Mesh-Covered Stent (MGuard) in ST-Segment Elevation Myocardial Infarction: The MASTER Trial. J Am Coll Cardiol. 2012;60:1975-1984. , 184184. Dudek D, Dziewierz A, Brener SJ, Abizaid A, Merkely B, Costa RA, Bar E, Rakowski T, Kornowski R, Dressler O, Abizaid A, Silber S, Stone GW. Mesh-covered embolic protection stent implantation in ST-segment-elevation myocardial infarction: final 1-year clinical and angiographic results from the MGUARD for acute ST elevation reperfusion trial. Circ Cardiovasc Interv. 2015;8:e001484. ] a total of 433 patients with STEMIpresenting within 12 hours of symptom onset undergoing PCI were randomized to the MGuard (n=217) or commercially available BMS or DES (n=216). The primary endpoint was the rate of complete (≥70%) ST-segment resolution measured 60 to 90 min post-procedure, which was significantly improved in the patients randomised to the MGuard stent compared with control patients (57.8% vs. 44.7%, p=0.008). MGuard stent resulted in superior rates of Thrombolysis In Myocardial Infarction 3 flow (91.7% vs. 82.9%, p=0.006), with a trend towards reduction in mortality (0% vs. 1.9%, p=0.06). Major adverse cardiac events at 1 year were higher with the MGuard, driven by greater ischaemia-driven TLR (8.6% vs. 0.9%, p=0.0003) [184184. Dudek D, Dziewierz A, Brener SJ, Abizaid A, Merkely B, Costa RA, Bar E, Rakowski T, Kornowski R, Dressler O, Abizaid A, Silber S, Stone GW. Mesh-covered embolic protection stent implantation in ST-segment-elevation myocardial infarction: final 1-year clinical and angiographic results from the MGUARD for acute ST elevation reperfusion trial. Circ Cardiovasc Interv. 2015;8:e001484. ]. Conversely, mortality tended to be lower with the MGuard at 30 days (0% vs. 1.9%, p=0.04) and at 1 year (1.0% vs. 3.3%, p=0.09). Late lumen loss at 13 months in the MGuard was 0.99±0.80 mm, and binary restenosis was 31.6%.The MASTER II study was aimed to confirm non-inferiority of the MGuard Prime (cobalt-chromium version) compared to commercially available BMS/DES in 1,114 patients undergoing primary PCI for STEMI. However, enrolment was voluntarily suspended after 310 patients had been randomized because of a higher than expected rate of stent dislodgement with the MGuard Prime (3.2% of dislodgment rate). No endpoint events due to a stent dislodgement were observed. The issue was addressed with a manufacturing change, but sponsor finally elected to terminate enrolment because of slow recruitment. Additional randomized clinical trials powered for clinical endpoints are needed to weigh the competing benefits (potentially improved myocardial reperfusion, reduced infarct size and greater survival) and risks (potentially greater restenosis) of the MGuard as an alternative to metallic stents in patients with STEMI [217217. Dziewierz A, Dudek D. Choosing the right stent for patients with ST-segment elevation myocardial infarction: the evidence-based approach. Minerva Cardioangiol. 2016;64:265-83. ].
In addition, great interest has been focused in recent years on self-expanding nitinol stents, such as Stentys (Stentys S.A, Paris, France). In fact, this may potentially overcome the risk of late stent malapposition and reduce the risk of distal embolisation. A study including 25 STEMI patients treated with Stentys [101101. Amoroso G, van Geuns RJ, Spaulding C, Manzo-Silberman S, Hauptmann KE, Spaargaren R, Garcia-Garcia HM, Serruys PW, Verheye S. Assessment of the safety and performance of the STENTYS self-expanding coronary stent in acute myocardial infarction: results from the APPOSITION I study. EuroIntervention. 2011;7:428-436. ] showed by intravascular ultrasound a significant vasodilatation distal to the culprit lesion at three-day follow-up (+19%), with a similar expansion of the implanted stent (+18%), without any cases of late stent malapposition at six months.
In the APPOSITION II trial [185185. van Geuns RJ, Tamburino C, Fajadet J, Vrolix M, Witzenbichler B, Eeckhout E, Spaulding C, Reczuch K, La Manna A, Spaargaren R, Garcia-Garcia HM, Regar E, Capodanno D, Van Langenhove G, Verheye S. Self-expanding versus balloon-expandable stents in acute myocardial infarction: results from the APPOSITION II study: self-expanding stents in ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2012;5:1209-1219. ] eighty STEMI patients were randomized to receive a self-expanding stent (STENTYS) (n = 43) or a balloon-expandable stent (VISION, Abbott Vascular, Santa Clara, California; or Driver, Medtronic, Minneapolis, Minnesota) (n = 37). The primary endpoint was the proportion of stent strut malapposition at 3 days after implantation measured by optical coherence tomography. Secondary endpoints included major adverse cardiac events (cardiac death, recurrent MI, emergent bypass surgery, or clinically drivenTLR). At 3 days after implantation, on a per-strut basis, a lower rate of malapposed stent struts was observed by optical coherence tomography in the self-expanding stent group than in the balloon-expandable group (0.58% vs. 5.46%, p <0.001). On a per-patient basis, none of the patients in the self-expanding stent group versus 28% in the balloon-expandable group presented ≥5% malapposed struts (p<0.001). At 6 months, major adverse cardiac events were 2.3% versus 0% in the self-expanding and balloon-expandable groups, respectively (p=NS).
The clinical significance of improved early stent apposition has been assessed in the APPOSITION III study [186186. Koch KT, Grundeken MJ, Vos NS, IJsselmuiden AJ, van Geuns RJ, Wessely R, Dengler T, La Manna A, Silvain J, Montalescot G, Spaargaren R, Tijssen JG, Amoroso G. One-year clinical outcomes of the STENTYS Self-Apposing coronary stent in patients presenting with ST-segment elevation myocardial infarction: results from the APPOSITION III registry. EuroIntervention. 2015;11:264-271. ]. In this prospective registry 965 patients with STEMI were treated either with paclitaxel-eluting STENTYS or bare-metal STENTYS. At one year, cardiac death rate was 2.0% with target-vessel MI rate of 1.3%. The rates of ischaemia-driven TLR and definite/probable stent thrombosis were 7.4% and 3.5%, respectively. An interim safety analysis of in-hospital outcomes in the first 400 patients showed higher event rates if post-dilation was not performed, and post-dilations became highly recommended in the remaining cohort. One-year target-vessel MI rate (0.8% vs. 2.5%, p=0.027) and definite stent thrombosis (1.9% vs. 5.0%; p=0.010) rates were significantly lower if post-dilation was performed, with the divergence occurring at <30 days. The first randomized trial with STENTYS inSTEMI powered for the assessment of clinical endpoints was APPOSITION V [187187. Grundeken MJ, Lu H, Mehran R, Cutlip DE, Leon MB, Yeung A, Koch KT, Montalescot G, van Geuns RJ, Spaargaren R, Buchbinder M. APPOSITION V: STENTYS coronary stent system clinical trial in subjects with ST-segment elevation myocardial infarction--rationale and design. Am Heart J. 2014;168:652-660. ]. In this study patients were randomised (2:1) to the bare-metal STENTYS stent and the balloon-expandable bare-metal MULTI-LINK VISION stent. Unfortunately, the target population of 318 patients was not reached as the study was prematurely stopped due to slow enrolment. However, despite some possible advantages of self-expanding stents in the treatment of STEMI, their clinical effectiveness in comparison to other stent designs should be confirmed in large scale, randomised clinical trial.
IABP & LEFT VENTRICULAR ASSIST DEVICES
A meta-analysis of 7 randomised studies on STEMI patients, confirmed that the routine use of IABP in non-shock STEMI patients showed neither a 30-day survival benefit nor improved left ventricular ejection fraction, while being associated with significantly higher stroke and bleeding rates [102102. Sjauw KD, Engstrom AE, Vis MM, van der Schaaf RJ, Baan J, Jr., Koch KT, de Winter RJ, Piek JJ, Tijssen JG, Henriques JP. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines?. Eur Heart J. 2009;30:459-468. ]. Similarly, in the CRISP AMI study, the routine use of IABP during primary PCI among patients with acute anterior STEMI without shock was not associated with reduction of infarct size assessed by cardiac magnetic resonance [103103. Patel MR, Smalling RW, Thiele H, Barnhart HX, Zhou Y, Chandra P, Chew D, Cohen M, French J, Perera D, Ohman EM. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA. 2011;306:1329-1337. ]. It should be stressed that IABP is contraindicated in patients with severe aortic insufficiency or aortic dissection. Importantly, based on the results of IABP-SHOCK II study the routine use of IABP even in patients with cardiogenic shock is questioned. In this study, no difference in 30-day as well as 12-month mortality was observed between patients with cardiogenic shock treated with and without IABP [104104. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Bohm M, Ebelt H, Schneider S, Schuler G, Werdan K. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287-1296. , 105105. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, de Waha A, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Lauer B, Bohm M, Ebelt H, Schneider S, Werdan K, Schuler G. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382:1638-1645I. ]. Based on the IABP-SHOCK II study results, the recommendation for the routine use of IABP in cardiogenic shock was downgraded to III B recommendation (not recommended) by ESC STEMI Guidelines [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. On the other hand, IABP insertion should be considered in patients with haemodynamic instability / cardiogenic shock due to mechanical complications of MI [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ].
Several other LVADs which can be inserted percutaneously have been tested in the STEMI setting. However, the use of percutaneous centrifugal pumps [106106. Thiele H, Sick P, Boudriot E, Diederich KW, Hambrecht R, Niebauer J, Schuler G. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26:1276-1283. ] (Tandem Heart; Cardiac Assist, Inc, Pittsburgh, PA, USA) and microaxial propeller pumps (Impella; Abiomed, Inc, Danvers, MA, USA) [107107. Seyfarth M, Sibbing D, Bauer I, Frohlich G, Bott-Flugel L, Byrne R, Dirschinger J, Kastrati A, Schomig A. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52:1584-1588. ] was not associated with significant 30-day mortality reduction, despite initial early haemodynamic recovery in shock patients. A meta-analysis summarising the data of 100 patients from three randomised clinical trials showed no difference in 30-day mortality and a trend towards more adverse events, such as bleeding and vascular complications in the group receiving percutaneous assist devices [108108. Cheng JM, den Uil CA, Hoeks SE, van der Ent M, Jewbali LS, Van Domburg RT, Serruys PW. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009;30:2102-2108. ]. In patients with STEMI and severe or profound cardiogenic shock Engström at al. [188188. Engstrom AE, Cocchieri R, Driessen AH, Sjauw KD, Vis MM, Baan J, de Jong M, Lagrand WK, van der Sloot JA, Tijssen JG, de Winter RJ, de Mol BA, Piek JJ, Henriques JP. The Impella 2.5 and 5.0 devices for ST-elevation myocardial infarction patients presenting with severe and profound cardiogenic shock: the Academic Medical Center intensive care unit experience. Crit Care Med. 2011;39:2072-2079. ]have suggested improved survival in patients who received immediate Impella 5.0 treatment, as well as in patients who were upgraded from 2.5 to 5.0 support, when compared to patients who received only Impella 2.5 support. More recent percutaneous, catheter-based Impella device, so called Impella CP may provide a maximum blood flow of 4 L/min. It can be implanted via a 9 French catheter into the left ventricle. Catheter-based technology and reduced catheter size may facilitate device implantation and reduce the risk of vascular complications. So far, small randomized IMPRESS in Severe Shock study, did not confirm a mortality benefit associated with the use of Impella CP (vs. IABP) in acute myocardial infarction complicated by cardiogenic shock [218218. Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, de Winter RJ, Piek JJ, Lagrand WK, de Mol BA, Tijssen JG, Henriques JP. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2017;69:278-287. ]. Another larger study (the DanShock trial) on this issue is ongoing. In patients who develop acute right heart failure or decompensation following LVAD implantation, MI, or heart transplant Impella RP dedicated for right-sided percutaneous support can be used. In addition, the SHIELD I study have confirmed the safety and efficacy of a novel percutaneous LVAD - HeartMate PHP™ (Percutaneous Heart Pump) from Thoratec Corporation / St. Jude Medical, Inc (Pleasanton, CA, USA) during high-risk PCI. On the other hand, the device has not been tested yet in patients with cardiogenic shock. Some promising results were reported for patients in cardiogenic shock or after out-of-hospital cardiac arrest treated with veno-arterial extracorporeal membrane oxygenation (VA ECMO) [189189. Chung SY, Sheu JJ, Lin YJ, Sun CK, Chang LT, Chen YL, Tsai TH, Chen CJ, Yang CH, Hang CL, Leu S, Wu CJ, Lee FY, Yip HK. Outcome of patients with profound cardiogenic shock after cardiopulmonary resuscitation and prompt extracorporeal membrane oxygenation support. A single-center observational study. Circ J. 2012;76:1385-1392. , 190190. Esper SA, Bermudez C, Dueweke EJ, Kormos R, Subramaniam K, Mulukutla S, Sappington P, Waters J, Khandhar SJ. Extracorporeal membrane oxygenation support in acute coronary syndromes complicated by cardiogenic shock. Catheter Cardiovasc Interv. 2015;86 Suppl 1:S45-S50. , 191191. Sheu JJ, Tsai TH, Lee FY, Fang HY, Sun CK, Leu S, Yang CH, Chen SM, Hang CL, Hsieh YK, Chen CJ, Wu CJ, Yip HK. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med. 2010;38:1810-1817. ]. VA ECMO may provide immediate and adequate systemic circulation and oxygenation. From a practical point of view, VA ECMO procedure is also much more simple and requires a shorter time to complete as compared with LVAD [192192. Noc M. Prime time for veno-arterial extracorporeal membrane oxygenation in 24-7 interventional cardiology center?. Postepy Kardiol Interwencyjnej. 2015;11:285-287. ]. Sheu et al. [191191. Sheu JJ, Tsai TH, Lee FY, Fang HY, Sun CK, Leu S, Yang CH, Chen SM, Hang CL, Hsieh YK, Chen CJ, Wu CJ, Yip HK. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med. 2010;38:1810-1817. ] have confirmed improved clinical outcomes, including mortality reduction for patients with STEMI complicated by profound cardiogenic shock who undergone primary PCI supported with VA ECMO. More importantly, treatment with VA ECMO can be initiated in primary care hospitals or even in pre-hospital setting and continued in reference centres [193193. Beurtheret S, Mordant P, Paoletti X, Marijon E, Celermajer DS, Leger P, Pavie A, Combes A, Leprince P. Emergency circulatory support in refractory cardiogenic shock patients in remote institutions: a pilot study (the cardiac-RESCUE program). Eur Heart J. 2013;34:112-120. ].
According to ESC STEMI Guidelines, short-term mechanical circulatory support may be considered in patients in refractory shock (IIb; C) [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. Different devices such as Impella, Tandem Heart, as well as VA ECMO would promptly stabilise a patient, allow subsequent PCI in a more stable condition and “buy time” for myocardial recovery or serve as a bridge to a long-term assist device or heart transplantation [192192. Noc M. Prime time for veno-arterial extracorporeal membrane oxygenation in 24-7 interventional cardiology center?. Postepy Kardiol Interwencyjnej. 2015;11:285-287. ]. Interestingly, in the AMICS registry survival benefit was higher in patients in whom mechanical circulatory support was initiated early [219219. Basir MB, Schreiber TL, Grines CL, Dixon SR, Moses JW, Maini BS, Khandelwal AK, Ohman EM, O’Neill WW. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am J Cardiol. 2017;119:845-851. ]. Thus, suggesting the importance of shortened onset of “shock to support” times in a similar fashion to “door-to-balloon” times in STEMI.
In patients with no contraindication for cardiac transplantation, especially younger patients, LVAD therapy can be implemented as a bridge to transplantation. In patients not eligible for transplant, LVADs may be inserted as a bridge to recovery or with the goal of destination therapy.
Adjunctive pharmacotherapy
ANTIPLATELET THERAPY BEFORE, DURING AND AFTER ANGIOPLASTY
Optimal inhibition of platelet aggregation is essential in STEMI patients undergoing mechanical reperfusion. In addition to acetylsalicylic acid, several therapies have been proposed in the acute and chronic phase.
P2Y12 inhibitors
Clopidogrel
Clopidogrel, a second-generation thienopyridine which irreversibly binds to an adenosine diphosphate P2Y12 receptor, has largely replaced the first-generation thienopyridine (ticlopidine) due to its similar efficacy but better tolerability profiles. Despite the absence of clinical trials specific to the STEMI population, combination therapy including aspirin and clopidogrel is considered the standard of care for patients receiving primary PCI. Several strategies have been evaluated in patients undergoing PCI to overcome some limitations of clopidogrel, especially in the settings of primary PCI, including slow onset of action and large inter-patient variability. Enhancing the loading dose of clopidogrel (600-900 mg) shortens the delayed onset of platelet inhibition compared to a 300 mg loading dose of clopidogrel and reduces the rate of suboptimal responders and further decreases the release of troponin prior to PCI [109109. Montalescot G, Sideris G, Meuleman C, Bal-Dit-Sollier C, Lellouche N, Steg PG, Slama M, Milleron O, Collet JP, Henry P, Beygui F, Drouet L. A randomized comparison of high clopidogrel loading doses in patients with non-ST-segment elevation acute coronary syndromes: the ALBION (Assessment of the Best Loading Dose of Clopidogrel to Blunt Platelet Activation, Inflammation and Ongoing Necrosis) trial. J Am Coll Cardiol. 2006;48:931-938. ]. The same results were found in patients undergoing PCI and treated chronically with clopidogrel [110110. Collet JP, Silvain J, Landivier A, Tanguy ML, Cayla G, Bellemain A, Vignolles N, Gallier S, Beygui F, Pena A, Montalescot G. Dose effect of clopidogrel reloading in patients already on 75-mg maintenance dose: the Reload with Clopidogrel Before Coronary Angioplasty in Subjects Treated Long Term with Dual Antiplatelet Therapy (RELOAD) study. Circulation. 2008;118:1225-1233. ]. More details concerning the phenomenon of non-response to clopidogrel and its clinical relevance are provided in Chapter 3.25. The effects of a high loading dose (600 mg vs. 300 mg) and a maintenance dose (150 mg vs. 75 mg) have recently been shown to provide benefits in terms of thrombotic complications. More recent ESC guidelines on PCI [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ], recommend (recommendation Class I; level of evidence B) the administration of a 600 mg loading dose (with a daily dose of 75 mg) of clopidogrel at the FMC in STEMI patients who are clinically eligible for primary PCI only when prasugrel or ticagrelor are not available or are contraindicated. If indicated, after primary PCI, 75 mg of clopidogrel is recommended for up to 12 months in association with aspirin, unless there are contraindications such as excessive risk of bleeding (recommendation Class I; level of evidence A).
Third-generation thienopyridine: Prasugrel
In order to overcome the evident limitations of clopidogrel, new oral adenosine diphosphate antagonists have recently been developed. Prasugrel is still a prodrug, as much as clopidogrel. However, its shorter metabolic pathway increases the efficacy of the drug, which is able to obtain a faster and more complete inhibition of platelet aggregation as compared to clopidogrel. The randomised, double-blind TRITON-TIMI 38 trial included 13,608 patients with acute coronary syndrome eligible for PCI and patients were randomised to receive either prasugrel (60 mg loading dose plus maintenance at 10 mg/day) or clopidogrel (300 mg loading dose plus maintenance at 75 mg/day) on top of aspirin. In the STEMI cohort [111111. Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, Antman EM. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373:723-731. ] (3,534 patients undergoing primary PCI [within 12 h of onset of symptoms] or secondary PCI [12 h to 14 days after onset of symptoms]), prasugrel was superior to clopidogrel in the primary endpoint (a composite of CV death, non-fatal MI, or non-fatal stroke) at both 30 days (hazard ratio [HR] 0.68, 95% CI: 0.54–0.87, p=0.002) and 15 months (HR 0.79, 95% CI: 0.65–0.97, p=0.019) ( Figure 8 ).
The incidence of definite or probable stent thrombosis, as defined by Academic Research Consortium criteria, was also significantly lower with prasugrel at both 30 days and 15 months. In contrast to the overall study population, the incidence of TIMI major bleeding unrelated to coronary artery bypass grafting (CABG) in the STEMI cohort did not differ significantly between prasugrel and clopidogrel at 30 days (HR 0.74, 95% CI: 0.39–1.38, p=0.34) or 15 months (HR 1.11, 95% CI: 0.70–1.77, p=0.65).
Several non randomised experiences have shown the safety and feasibility of switching from clopidogrel to prasugrel [194194. Verdoia M, Barbieri L, Suryapranata H, De Luca G. Switching from Clopidogrel to Prasugrel in patients undergoing PCI: A meta-analytic overview. Platelets. 2016;27:93-104. ], whereas the clinical benefits of 5 mg prasugrel in elderly patients with ACS will be definitively clarified by the ongoing Elderly-2 trial.
If no contraindication does exist, current guidelines [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ] recommend administration of Prasugrel (60 mg loading dose, 10 mg daily dose) at FMC (Class I; level of evidence B), and its continuation for up to 12 months in association with aspirin, unless there are contraindications such as excessive risk of bleeding (recommendation Class I; level of evidence A).
Ticagrelor
The oral, reversible P2Y12 receptor antagonist ticagrelor is the first of a new chemical class of antiplatelet agents, the cyclopentyl-triazolo-pyrimidines. Unlike the thienopyridines, ticagrelor reversibly blocks the platelet P2Y12 receptor. This agent allows for a nearly complete inhibition of adenosine diphosphate-induced platelet aggregation ex vivo and as a direct-acting compound does not require any metabolic activation.
In the PLATO randomised trial [112112. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057. ], including about 18,000 moderate to high-risk acute coronary syndrome patients, including STEMI scheduled for primary PCI, ticagrelor at a dose regimen of 180 mg followed by 90 mg BID dose has been shown to provide, as compared to clopidogrel (300/600 mg loading dose / 75 mg maintenance dose), significant benefits in terms of mortality and in-stent thrombosis, without increasing the risk of major bleeding complications. The results have been confirmed in the STEMI cohort of patients [113113. Steg PG, James S, Harrington RA, Ardissino D, Becker RC, Cannon CP, Emanuelsson H, Finkelstein A, Husted S, Katus H, Kilhamn J, Olofsson S, Storey RF, Weaver WD, Wallentin L. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: A Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122:2131-2141. ] ( Figure 9 ).
Recently several pharmacokinetic and pharmacodymamic studies have conducted on ticagrelor. Increased dosage of ticagrelor may fasten the onset and the optimal inhibition of platelet aggregation as much as administration of crushed tablets,that may improve intestinal absorption [195195. Franchi F, Rollini F, Cho JR, Bhatti M, DeGroat C, Ferrante E, Dunn EC, Nanavati A, Carraway E, Suryadevara S, Zenni MM, Guzman LA, Bass TA, Angiolillo DJ. Impact of Escalating Loading Dose Regimens of Ticagrelor in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: Results of a Prospective Randomized Pharmacokinetic and Pharmacodynamic Investigation. JACC Cardiovasc Interv. 2015;8:1457-1467. , 196196. Alexopoulos D, Barampoutis N, Gkizas V, Vogiatzi C, Tsigkas G, Koutsogiannis N, Davlouros P, Hahalis G, Nylander S, Parodi G, Xanthopoulou I. Crushed Versus Integral Tablets of Ticagrelor in ST-Segment Elevation Myocardial Infarction Patients: A Randomized Pharmacokinetic/Pharmacodynamic Study. Clin Pharmacokinet. 2016;55:359-367. , 197197. Parodi G, Xanthopoulou I, Bellandi B, Gkizas V, Valenti R, Karanikas S, Migliorini A, Angelidis C, Abbate R, Patsilinakos S, Baldereschi GJ, Marcucci R, Gensini GF, Antoniucci D, Alexopoulos D. Ticagrelor crushed tablets administration in STEMI patients: the MOJITO study. J Am Coll Cardiol. 2015;65:511-512. ]. However, large randomized trials are certainly needed to demonstrate additional benefits in outcome.
If no contraindication does exist, current guidelines [168168. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. ] recommend administration of Ticagrelor (180 mg loading dose, 90 mg twice daily) at FMC (Class I; level of evidence B), and its continuation for up to 12 months in association with aspirin, unless there are contraindications such as excessive risk of bleeding (recommendation Class I; level of evidence A).
Prasugrel vs. ticagrelor
Based on TRITON-TIMI 38 STEMI and PLATO-STEMI, prasugrel appears more protective against stent thrombosis ( Figure 10), especially in the early phase. Small randomised trials have compared the pharmacodynamic effects of ticagrelor vs prasugrel in STEMI patients undergoing primary angioplasty, showing no significant difference between the two groups [114114. Parodi G, Valenti R, Bellandi B, Migliorini A, Marcucci R, Comito V, Carrabba N, Santini A, Gensini GF, Abbate R, Antoniucci D. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J Am Coll Cardiol. 2013;61:1601-1606. , 115115. Alexopoulos D, Xanthopoulou I, Gkizas V, Kassimis G, Theodoropoulos KC, Makris G, Koutsogiannis N, Damelou A, Tsigkas G, Davlouros P, Hahalis G. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2012;5:797-804. , 116116. Deharo P, Bassez C, Bonnet G, Pankert M, Quilici J, Lambert M, Verdier V, Morange P, Alessi MC, Bonnet JL, Cuisset T. Prasugrel versus ticagrelor in acute coronary syndrome: A randomized comparison. Int J Cardiol. 2013;170:e21-e22. ] [198198. Parodi G, Bellandi B, Valenti R, Migliorini A, Marcucci R, Carrabba N, Giurlani L, Gensini GF, Abbate R, Antoniucci D. Comparison of double (360 mg) ticagrelor loading dose with standard (60 mg) prasugrel loading dose in ST-elevation myocardial infarction patients: the Rapid Activity of Platelet Inhibitor Drugs (RAPID) primary PCI 2 study. Am Heart J. 2014;167:909-914. , 199199. Lee YS, Jin CD, Kim MH, Guo LZ, Cho YR, Park K, Park JS, Park TH, Kim YD. Comparison of Prasugrel and Ticagrelor Antiplatelet Effects in Korean Patients Presenting With ST-Segment Elevation Myocardial Infarction. Circ J. 2015;79:1248-1254. , 200200. Laine M, Gaubert M, Frere C, Peyrol M, Thuny F, Yvorra S, Chelini V, Bultez B, Luigi S, Mokrani Z, Bessereau J, Toesca R, Champenois A, Dignat-George F, Paganelli F, Bonello L. COMparison of Platelet reactivity following prAsugrel and ticagrelor loading dose in ST-Segment elevation myocardial infarctION patients: The COMPASSION study. Platelets. 2015;26:570-572. ]. There are no studies directly testing the impact of ticagrelor vs. prasugrel on outcome in patients with STEMI. An adjusted indirect comparison meta-analysis [117117. Biondi-Zoccai G, Lotrionte M, Agostoni P, Abbate A, Romagnoli E, Sangiorgi G, Angiolillo DJ, Valgimigli M, Testa L, Gaita F, Sheiban I. Adjusted indirect comparison meta-analysis of prasugrel versus ticagrelor for patients with acute coronary syndromes. Int J Cardiol. 2011;150:325-331. ] of prasugrel versus ticagrelor for patients with acute coronary syndromes (three trials with 32,893 patients included) have shown that both drugs are superior to clopidogrel for acute coronary syndrome.
The Prasugrel Versus Ticagrelor in Patients With Acute Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention (PRAGUE-18) trial (New Reference 5)planned to randomize 2500 across 14 sites to either prasugrel or ticagrelor, initiated before percutaneous coronary intervention. The primary end point was defined as death, reMI, urgent TVR, stroke, or serious bleeding requiring transfusion or prolonging hospitalization at 7 days (to reflect primarily the in-hospital phase). The study was prematurely terminated for futility. The occurrence of the primary end point did not differ between groups receiving prasugrel and ticagrelor (4.0% and 4.1%, respectively; OR 0.98; 95%CI, 0.55–1.73; P=0.939). No significant difference was found in any of the components of the primary end point. The occurrence of key secondary end point within 30 days, composed of cardiovascular death, nonfatal myocardial infarction, or stroke, did not show any significant difference between prasugrel and ticagrelor (2.7% and 2.5%, respectively; OR 1.06; 95%CI 0.53–2.15; P=0.864). The results were confirmed at 1 year follow-up, event though a large proportion of patients had to shift to clopidogrel, since the study drug was provided only to up 7 days [220220. Motovska Z, Hlinomaz O, Miklik R, Hromadka M, Varvarovsky I, Dusek J, Knot J, Jarkovsky J, Kala P, Rokyta R, Tousek F, Kramarikova P, Majtan B, Simek S, Branny M, Mrozek J, Cervinka P, Ostransky J, Widimsky P; PRAGUE-18 Study Group. Prasugrel Versus Ticagrelor in Patients With Acute Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention: Multicenter Randomized PRAGUE-18 Study. Circulation. 2016;134:1603-1612. ].
A recent network meta-analysis of 37 studies, showed superiority of prasugrel to clopidogrel and ticagrelor at short-term and 1-year follow-up [221221. Rafique AM, Nayyar P, Wang TY, Mehran R, Baber U, Berger PB, Tobis J, Currier J, Dave RH, Henry TD. Optimal P2Y12 Inhibitor in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: A Network Meta-Analysis. JACC Cardiovasc Interv. 2016;9:1036-46. ]. Outcomes at 1 month (22 studies and 60,783 patients) suggest that prasugrel was associated with: lower MACE than clopidogrel (standard dose OR 0.59, 95%CI: 0.50 to 0.69; high-dose OR: 0.60, 95% CI: 0.51 to 0.71; upstream OR: 0.79, 95% CI: 0.66 to 0.94), and ticagrelor (standard dose OR: 0.69, 95% CI: 0.56 to 0.84; upstream OR: 0.72, 95% CI: 0.50 to 1.05); lower mortality and MI than clopidogrel and standard ticagrelor; lower stroke risk than standard clopidogrel and standard or upstream ticagrelor; and lower stent thrombosis than standard or upstream clopidogrel. At 1-year (10 studies, n = 40,333) prasugrel was associated with lower mortality and MACE than other P2Y12 inhibitors. MACE was particularly lower with prasugrel in studies where patients received bivalirudin, DES, and but not GP IIb-IIIa inhibitor. Based on these data, some more consideration should certainly be given to prasugrel as compared to Ticagrelor in the setting of primary angioplasty. An ongoing large randomised trial (ISAR-REACT 5) will hopefully provide some additional data on this issue [118118. Schulz S, Angiolillo DJ, Antoniucci D, Bernlochner I, Hamm C, Jaitner J, Laugwitz KL, Mayer K, von Merzljak B, Morath T, Neumann FJ, Richardt G, Ruf J, Schomig G, Schuhlen H, Schunkert H, Kastrati A. Randomized Comparison of Ticagrelor versus Prasugrel in Patients with Acute Coronary Syndrome and Planned Invasive Strategy-Design and Rationale of the Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 Trial. J Cardiovasc Transl Res. 2013. ].
Optimal timing and duration of administration
Early upstream administration of oral ADP antagonists have always been highly recommended in all previous STEMI guidelines, despite the absence of dedicated randomized trials. The ATLANTIC trial [201201. Montalescot G, van ’t Hof AW, Lapostolle F, Silvain J, Lassen JF, Bolognese L, Cantor WJ, Cequier A, Chettibi M, Goodman SG, Hammett CJ, Huber K, Janzon M, Merkely B, Storey RF, Zeymer U, Stibbe O, Ecollan P, Heutz WM, Swahn E, Collet JP, Willems FF, Baradat C, Licour M, Tsatsaris A, Vicaut E, Hamm CW. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. 2014;371:1016-1027. ] was the first randomized trial evaluating the benefits in terms of reperfusion from upstream vs downstream administration of ticagrelor. A total of 1862 STEMI patients were included and randomly assigned 1:1 to upstream or periprocedural administration of ticagrelor. The median time from randomization to angiography was 48 minutes, and the median time difference between the two treatment strategies was 31 minutes. The two co-primary end points (the proportion of patients with less than 70% ST-segment resolution before percutaneous coronary intervention (PCI) and the proportion of patients with TIMI flow < 3 in the infarct-related artery at initial angiography) did not differ significantly between the prehospital and in-hospital groups. The absence of ST-segment elevation resolution of 70% or greater after PCI (a secondary end point) was reported for 42.5% and 47.5% of the patients, respectively. The rates of major adverse cardiovascular events did not differ significantly between the two study groups. Rates of major bleeding events were low and virtually identical in the two groups, regardless of the bleeding definition used. The negative outcome of the ATLANTIC trial does not support routine upstream administration of Ticagrelor in the setting of STEMI.
Still debated is the optimal duration of dual antiplatelet therapy after ACS, including STEMI patients. In fact, the recommendation of 12 months was mainly based on the old PCI CURE trial [202202. Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, Fox KA. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527-533. ], where a relevant proportion of the benefits were observed before angiography. In fact, patients were included in the trial and randomized to placebo (with subsequently postprocedural clopidogrel up to 30 days) vs clopidogrel (up to 1 year) much before angiography. In addition, improvement in DES technology has allowed a shorter duration of dual antiplatelet therapy, with even 1 month in case of polymer-free DES.
Several meta-analyses have been performed [203203. Verdoia M, Schaffer A, Barbieri L, Montalescot G, Collet JP, Colombo A, Suryapranata H, De Luca G. Optimal Duration of Dual Antiplatelet Therapy After DES Implantation: A Meta-Analysis of 11 Randomized Trials. Angiology. 2016;67:224-238. , 204204. Spencer FA, Prasad M, Vandvik PO, Chetan D, Zhou Q, Guyatt G. Longer- Versus Shorter-Duration Dual-Antiplatelet Therapy After Drug-Eluting Stent Placement: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163:118-126. , 205205. Giustino G, Baber U, Sartori S, Mehran R, Mastoris I, Kini AS, Sharma SK, Pocock SJ, Dangas GD. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2015;65:1298-1310. ] demonstrating increased rates of all-cause mortality with prolonged dual antiplatelet therapy compared with shorter dual antiplatelet therapy after DES, due to greater non-cardiovascular mortality with prolonged dual antiplatelet therapy not offset by a concomitant reduction in cardiac mortality. It must be recognized that no trial was strictly focused on ACS patients, who were excluded from many trials.
The recent DAPT STEMI trial (Late braking trial TCT 2017), including more than 800 STEMI patients free of events at 6 months follow-up, showed that 6 months DAPT was not inferior to 12 months DAPT up to 2 year follow-up.
The REDUCE trial (Late braking trial TCT 2017) [206206. Suryapranata H, De Luca G. Short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with the COMBO dual-therapy stent: what we plan to learn from REDUCE. Future Cardiol. 2015;11:17-20. ] included 1500 randomly assigned ACS patients (about 50% STEMI) and showed non inferiority of 3 vs 12 months DAPT in the primary endpoint (composite of all cause death, MI, ST, stroke, TVR or bleeding (BARC II, III, V): 8.2% vs 8.4%, OR [95% CI] = 0.97 [0.67-1.41], p <0.001). A shorter 3 months DAPT treatment, therefore, may be potentially considered.
Until the results of larger randomized trial will become available, 1-year DAPT is still recommended. However, with new generation DES, based on recent randomized trials, a shorter duration of DAPT may be considered, especially in patients at high-risk of major bleeding complications.
Cangrelor
Cangrelor is an intravenous ADP antagonist with quick fast-on and fast-off effects (due to rapid reversibility). This drug is of potential interest for STEMI. In fact, several recent studies have shown that even new oral ADP antagonists have a delay in onset of action, with less than 50% of patients with optimal inhibition at 2 hours and peak effect that may be observed later than 4 hours after administration. No randomised trials have strictly been conducted in the setting of STEMI. However, the large CHAMPION series, including also STEMI patients in CHAMPION PCI and CHAMPION Phoenix, showed by pooled analysis a significant reduction in stent thrombosis at 30 days (0.9% vs 1.3%, p=0.0027) [119119. Steg PG, Bhatt DL, Hamm CW, Stone GW, Gibson CM, Mahaffey KW, Leonardi S, Liu T, Skerjanec S, Day JR, Iwaoka RS, Stuckey TD, Gogia HS, Gruberg L, French WJ, White HD, Harrington RA. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet. 2013;382:1981-1992. ]. Future large trials are certainly needed to evaluate the safety and benefits of cangrelor in primary PCI, especially as upstream therapy with the aim of early recanalization, in the era of new oral ADP antagonists.
Glycoprotein IIb-IIIa inhibitors
Among the three molecules ( Table 3 ), most of the trials have been focused on abciximab, showing that its adjunctive administration may reduce the risk of mortality and re-infarction [120120. De Luca G, Suryapranata H, Stone GW, Antoniucci D, Tcheng JE, Neumann FJ, Van de Werf F, Antman EM, Topol EJ. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. JAMA. 2005;293:1759-1765. ]. The use of this therapy has waned by the introduction of new ADP-antagonists [120120. De Luca G, Suryapranata H, Stone GW, Antoniucci D, Tcheng JE, Neumann FJ, Van de Werf F, Antman EM, Topol EJ. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. JAMA. 2005;293:1759-1765. ]. However, recent data have clearly shown a delayed onset of action of both prasugrel and ticagrelor [121121. Valgimigli M, Tebaldi M, Campo G, Gambetti S, Bristot L, Monti M, Parrinello G, Ferrari R. Prasugrel versus tirofiban bolus with or without short post-bolus infusion with or without concomitant prasugrel administration in patients with myocardial infarction undergoing coronary stenting: the FABOLUS PRO (Facilitation through Aggrastat By drOpping or shortening Infusion Line in patients with ST-segment elevation myocardial infarction compared to or on top of PRasugrel given at loading dOse) trial. JACC Cardiovasc Interv. 2012;5:268-277. ]. In addition, the negative results of the ATLANTIC trial [201201. Montalescot G, van ’t Hof AW, Lapostolle F, Silvain J, Lassen JF, Bolognese L, Cantor WJ, Cequier A, Chettibi M, Goodman SG, Hammett CJ, Huber K, Janzon M, Merkely B, Storey RF, Zeymer U, Stibbe O, Ecollan P, Heutz WM, Swahn E, Collet JP, Willems FF, Baradat C, Licour M, Tsatsaris A, Vicaut E, Hamm CW. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. 2014;371:1016-1027. ] have renewed the interest for GP IIb-IIIa inhibitors. Time-to-treatment has been shown to have a negative impact on survival even with mechanical reperfusion [2020. De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223-1225. ], and therefore further benefits may be expected from early pharmacological reperfusion. Despite the negative results of the largest trial so far conducted, the FINESSE trial [122122. Ellis SG, Tendera M, de Belder MA, van Boven AJ, Widimsky P, Janssens L, Andersen HR, Betriu A, Savonitto S, Adamus J, Peruga JZ, Kosmider M, Katz O, Neunteufl T, Jorgova J, Dorobantu M, Grinfeld L, Armstrong P, Brodie BR, Herrmann HC, Montalescot G, Neumann FJ, Effron MB, Barnathan ES, Topol EJ. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med. 2008;358:2205-2217. ], an individual patient’s data meta-analysis of several randomised trials [123123. De Luca G, Gibson CM, Bellandi F, Murphy S, Maioli M, Noc M, Zeymer U, Dudek D, Arntz HR, Zorman S, Gabriel HM, Emre A, Cutlip D, Biondi-Zoccai G, Rakowski T, Gyongyosi M, Marino P, Huber K, van’t Hof AW. Early glycoprotein IIb-IIIa inhibitors in primary angioplasty (EGYPT) cooperation: an individual patient data meta-analysis. Heart. 2008;94:1548-1558. ], and several registries [124124. Dudek D, Siudak Z, Janzon M, Birkemeyer R, ma-Lopez G, Lettieri C, Janus B, Wisniewski A, Berti S, Olivari Z, Rakowski T, Partyka L, Goedicke J, Zmudka K. European registry on patients with ST-elevation myocardial infarction transferred for mechanical reperfusion with a special focus on early administration of abciximab -- EUROTRANSFER Registry. Am Heart J. 2008;156:1147-1154. , 125125. Ortolani P, Marzocchi A, Marrozzini C, Palmerini T, Saia F, Taglieri N, Baldazzi F, Dall’Ara G, Nardini P, Gianstefani S, Guastaroba P, Grilli R, Branzi A. Long-term effectiveness of early administration of glycoprotein IIb/IIIa agents to real-world patients undergoing primary percutaneous interventions: results of a registry study in an ST-elevation myocardial infarction network. Eur Heart J. 2009;30:33-43. ] data have shown clear benefits in terms of myocardial perfusion and survival from early abciximab administration. Recently, the ON-TIME II trial [126126. van’t Hof AW, Ten Berg J, Heestermans T, Dill T, Funck RC, van Werkum W, Dambrink JH, Suryapranata H, van Houwelingen G, Ottervanger JP, Stella P, Giannitsis E, Hamm C. Prehospital initiation of tirofiban in patients with ST-elevation myocardial infarction undergoing primary angioplasty (On-TIME 2): a multicentre, double-blind, randomised controlled trial. Lancet. 2008;372:537-546. ] showed significant benefits from early high-dose tirofiban administration before transfer for primary angioplasty as compared to placebo.
The use of GP IIb-IIIa inhibitors in the last years has also been waned by initial trials on Bivalirudin. However, it is known that thrombus composition may change in the hours after the beginning of the occlusion, with more platelets in the early phase (< 3 hours) and more fibrin in the late phase (> 6 hours).
A recent analysis from the HORIZONS trial [222222. Schoos MM, De Luca G, Dangas GD, Clemmensen P, Ayele GM, Mehran R, Stone GW.Impact of time to treatment on the effects of bivalirudin vs. glycoprotein IIb/IIIa inhibitors and heparin in patients undergoing primary percutaneous coronary intervention: insights from the HORIZONS-AMI trial. EuroIntervention. 2016;12:1144-1153. ], sought to examine whether the beneficial effects of bivalirudin, as compared to Gp IIb-IIIa inhbitors are dependent on time to treatment. Among patients with an SBT ≤3 hours, bivalirudin resulted in higher 30-day rates of MACE compared to UFH plus a GPI. Non-significant differences were observed in patients with an SBT >3 hours. Similar results were found for MACE at three years and stent thrombosis and reinfarction at 30 days and three years. By multivariable analysis, bivalirudin was an independent predictor of MACE at 30 days and three years in patients with an SBT ≤3 hours, but not in patients with SBT >3 hours. Therefore, the authors conlcuded that Bivalirudin compared to UFH plus a GPI is associated with an increased rate of stent thrombosis and MACE in patients with short SBTs, but not in those with longer SBTs. These data further support the use of Gp IIb-IIIa inhbitors, especially in the early phase of STEMI.
Initial non-randomised studies have suggested greater benefits from selective intracoronary administration. The recently published CICERO trial [127127. Gu YL, Kampinga MA, Wieringa WG, Fokkema ML, Nijsten MW, Hillege HL, van den Heuvel AF, Tan ES, Pundziute G, van der Werf R, Hoseyni GS, van der Horst I, Zijlstra F, de Smet BJ. Intracoronary versus intravenous administration of abciximab in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention with thrombus aspiration: the comparison of intracoronary versus intravenous abciximab administration during emergency reperfusion of ST-segment elevation myocardial infarction (CICERO) trial. Circulation. 2010;122:2709-2717. ] has shown no benefits in infarct size and clinical outcome. Similarly, the AIDA STEMI trial, including more than 2,000 patients, did not prove any benefit in reduction of clinical endpoints relating to intracoronary over intravenous administration of abciximab. In the recently reported INFUSE AMI trial intralesional administration of abciximab using the ClearWay™ RX infusion catheter (Atrium Medical, Hudson, NH, USA) was associated with significant, however, modest reduction of the infarct size assessed by cardiac magnetic resonance at 30 days after first, anterior wall STEMI in comparison to no abciximab administration. The highest benefit was observed when local infusion of abciximab was combined with prior thrombus aspiration [128128. Stone GW, Maehara A, Witzenbichler B, Godlewski J, Parise H, Dambrink JH, Ochala A, Carlton TW, Cristea E, Wolff SD, Brener SJ, Chowdhary S, El-Omar M, Neunteufl T, Metzger DC, Karwoski T, Dizon JM, Mehran R, Gibson CM. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA. 2012;307:1817-1826. ].
Several meta-analyses [129129. De Luca G, Ucci G, Cassetti E, Marino P. Benefits from small molecule administration as compared with abciximab among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: a meta-analysis. J Am Coll Cardiol. 2009;53:1668-1673. , 130130. Gurm HS, Tamhane U, Meier P, Grossman PM, Chetcuti S, Bates ER. A comparison of abciximab and small-molecule glycoprotein IIb/IIIa inhibitors in patients undergoing primary percutaneous coronary intervention: a meta-analysis of contemporary randomized controlled trials. Circ Cardiovasc Interv. 2009;2:230-236. ] have shown similar results between high-dose tirofiban (25 ug/kg) (5 trials), eptifibatide (double-bolus) (1 trial) as compared to abciximab. However, no trial was adequately powered to evaluate any difference in hard endpoints such as mortality. The need for a second bolus (10 minutes after the first one) of eptifibatide may be less user-friendly in the setting of primary PCI.
Current recommendations for antiplatelet drugs in patients with STEMI are shown in Table 3.
ANTITHROMBOTIC THERAPY BEFORE, DURING AND AFTER ANGIOPLASTY
Unfractionated heparin & bivalirudin
Based on current recommendations[168168. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. ], anticoagulation in patients with STEMI includes unfractionated heparin (UFH) (50-60 IU/kg i.v. bolus if the usage of GP IIb–IIIa inhibitor is planned or 70-100 IU/kg i.v. bolus without GP IIb–IIIa inhibitor), bivalirudin (0.75 mg/kg i.v. bolus followed by 1.75mg/kg/h i.v. infusion), or enoxaparin (0.5 mg/kg i.v. followed by s.c. treatment). The anticoagulation should be selected according to both ischaemic and bleeding risks, and according to the efficacy-safety profile of the chosen agent (I;C) [168168. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. ].
UFH can be administered before cath lab admission; however, dose of i.v. bolus dose should not exceed 5,000 IU. Before primary PCI, activated clotting time (ACT) should be checked and additional boluses of UFH, if required, should be given to achieve an ACT target range between 200 and 250 seconds if GP IIb-IIIa inhibitors are used, and ACT between 250 and 300 seconds in patients without GP IIb-IIIa inhibitors. Monitoring of ACT and dose adjustment is not required in patients treated with bivalirudin, except patients with severe renal dysfunction in whom reduction of i.v. infusion dose to 1.0 mg/kg/h should be considered [44. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa UM, Taggart D, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Kolh P, Alfieri O, Dunning J, Elia S, Kappetein P, Lockowandt U, Sarris G, Vouhe P, Kearney P, von Segesser L, Agewall S, Aladashvili A, Alexopoulos D, Antunes MJ, Atalar E, Brutel de la Riviere A, Doganov A, Eha J, Fajadet J, Ferreira R, Garot J, Halcox J, Hasin Y, Janssens S, Kervinen K, Laufer G, Legrand V, Nashef SA, Neumann FJ, Niemela K, Nihoyannopoulos P, Noc M, Piek JJ, Pirk J, Rozenman Y, Sabate M, Starc R, Thielmann M, Wheatley DJ, Windecker S, Zembala M. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2010;31:2501-2555. ]. Bivalirudin can also be used in patients initially treated with UFH; however, bivalirudin should be administered >30 minutes from the last UFH dose. There is no need for prolonged use of antithrombotic agents after successful primary PCI for STEMI, except for a few clinical indications (left ventricle aneurysm and/or thrombus, atrial fibrillation, prolonged bed rest, deferred sheath removal, use of IABP).
No specific randomised clinical trial has been conducted to address the impact of UFH on the safety and efficacy of primary PCI. UFH is currently recommended based on the opinion of experts (I C, Table 3 ) [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. Recommendation for the use of bivalirudin is based on the results of the several randomised studies. In the HORIZONS-AMI trial [132132. Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218-2230. , 133133. Mehran R, Lansky AJ, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Wong SC, Nikolsky E, Gambone L, Vandertie L, Parise H, Dangas GD, Stone GW. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet. 2009;374:1149-1159. ], among patients undergoing primary PCI for STEMI, bivalirudin monotherapy (n=1,800) was associated with a significantly lower 30-day rate of net adverse clinical events in comparison to patients treated with a combination of UFH and GP IIb-IIIa inhibitors (n=1,802). The observed benefit was primarily driven by a lower risk of non-CABG major bleeding. There was no difference in 30-day major adverse CV event rates between groups. These 30-day clinical outcomes were maintained at 3-year follow-up. Importantly, bivalirudin monotherapy compared to a combination of UFH and GP IIb-IIIa inhibitors was associated with significant cardiac and all-cause mortality reduction during short-term (30-day), as well as long-term (up to 3 years) follow-up. Although there was an increased risk of acute stent thrombosis (<24 hours) in the bivalirudin group, no significant increase was present by 30 days and 3 years.
Results of the EUROMAX study have confirmed the safety and efficacy of bivalirudin administration during transfer for primary PCI. In this study, 2,218 patients with STEMI were randomized to receive either bivalirudin or UFH/enoxaparin with optional GP IIb-IIIa inhibitors (control group). Initiation of bivalirudin before transfer for primary PCI was associated with reduction of non-CABG-related major bleeding as well as a composite endpoint of death and non-CABG-related major bleeding at 30 days as compared to control group. However, the higher risk of stent thrombosis was observed in patients treated with bivalirudin[134134. Steg PG, van ’t HA, Hamm CW, Clemmensen P, Lapostolle F, Coste P, Ten Berg J, Van Grunsven P, Eggink GJ, Nibbe L, Zeymer U, Campo dell’Orto M, Nef H, Steinmetz J, Soulat L, Huber K, Deliargyris EN, Bernstein D, Schuette D, Prats J, Clayton T, Pocock S, Hamon M, Goldstein P. Bivalirudin started during emergency transport for primary PCI. N Engl J Med. 2013;369:2207-2217. ].
In a more recent, single-centre, randomised HEAT-PPCI study a total of 1,829 patients with STEMI were randomised to receive bivalirudin or UFH [209209. Shahzad A, Kemp I, Mars C, Wilson K, Roome C, Cooper R, Andron M, Appleby C, Fisher M, Khand A, Kunadian B, Mills JD, Morris JL, Morrison WL, Munir S, Palmer ND, Perry RA, Ramsdale DR, Velavan P, Stables RH. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet. 2014;384:1849-1858. ]. The study represents contemporary practice with restriction of GPIIb/IIIa inhibitors to bail-out situations (in 15% of the randomised patients population), the frequent use of novel P2Y12 inhibitors (89% of the patients), radial approach and predominant DES implantation. A composite endpoint of all-cause mortality, stroke, recurrent MI and unplanned TLR at 28 days was higher for the bivalirudin than for the UFH group (8.7% vs. 5.7%, p=0.01) including an increase in stent thrombosis (3.4% vs. 0.9%, p=0.001). More importantly no difference in mortality and major bleeding was confirmed [209209. Shahzad A, Kemp I, Mars C, Wilson K, Roome C, Cooper R, Andron M, Appleby C, Fisher M, Khand A, Kunadian B, Mills JD, Morris JL, Morrison WL, Munir S, Palmer ND, Perry RA, Ramsdale DR, Velavan P, Stables RH. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet. 2014;384:1849-1858. ].
Another study, the BRAVE 4 study [210210. Schulz S, Richardt G, Laugwitz KL, Morath T, Neudecker J, Hoppmann P, Mehran R, Gershlick AH, Tolg R, Anette FK, Abdel-Wahab M, Kufner S, Schneider S, Schunkert H, Ibrahim T, Mehilli J, Kastrati A. Prasugrel plus bivalirudin vs. clopidogrel plus heparin in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2014;35:2285-2294. ], tested whether a strategy of prasugrel plus bivalirudin (n=269) was superior to a strategy with clopidogrel plus UFH (n=275) during primary PCI for STEMI. The study was interrupted due slow recruitment. No difference in the primary endpoint of a composite of death, MI, unplanned TVR, stent thrombosis, stroke or major bleeding at 30 days. Also, other secondary ischaemic and bleeding endpoints did not differ between groups.
The Matrix study, randomly assigned 7213 patients with an acute coronary syndrome undergoing PCI to receive either bivalirudin or unfractionated heparin [223223. Valgimigli M, Frigoli E, Leonardi S, Rothenbühler M, Gagnor A, Calabrò P, Garducci S, Rubartelli P, Briguori C, Andò G, Repetto A, Limbruno U, Garbo R, Sganzerla P, Russo F, Lupi A, Cortese B, Ausiello A, Ierna S, Esposito G, Presbitero P, Santarelli A, Sardella G, Varbella F, Tresoldi S, de Cesare N, Rigattieri S, Zingarelli A, Tosi P, van ’t Hof A, Boccuzzi G, Omerovic E, Sabaté M, Heg D, Jüni P, Vranckx P; MATRIX Investigators. Bivalirudin or Unfractionated Heparin in Acute Coronary Syndromes. N Engl J Med. 2015;373:997-1009. ]. Patients in the bivalirudin group were subsequently randomized to receive or not to receive a post-PCI bivalirudin infusion. The rate of a composite of death, myocardial infarction, or stroke was comparable between groups (bivalirudin vs. UFH: 10.3% vs. 10.9%; P = 0.44). Similarly, no difference in the rate of net adverse clinical events (a composite of major bleeding or a major adverse cardiovascular event) was observed. In addition, the rate of the composite of urgent TVR, definite stent thrombosis, or net adverse clinical events was not significantly lower with a post-PCI bivalirudin infusion than with no post-PCI infusion.
The most recent large randomised study on the impact of bivalirudin during primary PCI for STEMI was VALIDATE-SWEDEHEART study, In this study, a total of 6006 patients (3005 with STEMI and 3001 with NSTEMI) were enrolled [224224. Erlinge D, Omerovic E, Fröbert O, Linder R, Danielewicz M, Hamid M, Swahn E, Henareh L, Wagner H, Hårdhammar P, Sjögren I, Stewart J, Grimfjärd P, Jensen J, Aasa M, Robertsson L, Lindroos P, Haupt J, Wikström H, Ulvenstam A, Bhiladvala P, Lindvall B, Lundin A, Tödt T, Ioanes D, Råmunddal T, Kellerth T, Zagozdzon L, Götberg M, Andersson J, Angerås O, Östlund O, Lagerqvist B, Held C, Wallentin L, Scherstén F, Eriksson P, Koul S, James S. Bivalirudin versus Heparin Monotherapy in Myocardial Infarction. N Engl J Med. 2017;377:1132-1142. ]. At 180 days, no differences in the rates of myocardial infarction, major bleeding and death were obserwed.
As the recent trials comparing bivalirudin with UFH without systematic use of GPIIb/IIIa antagonists uphold concerns over an excess risk for acute stent thrombosis with bivalirudin the recommendation for bivalirudin during primary PCI for STEMI were downgraded from I;B (preferred) [33. Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van ’t Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619. ] to IIa;A [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ] recommendation. No additional protective efect of prolonged bivalirudin infusion was confirmed. On the other hand, bivalirudin is unanimously recommended as a replacement for heparin in the case of heparin-induced thrombocytopaenia.
Fondaparinux
The use of fondaparinux in the setting of primary PCI in patients with STEMI is not currently recommended (III B) [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]. The clinical safety and efficacy of fondaparinux has been tested in 12,092 STEMI patients enrolled in the randomised, double-blind, multicentre OASIS-6 trial [135135. van Rees V, Peters RJ, Yusuf S, Afzal R, Chrolavicius S, O’Donnell M, Mehta SR, Pluta W, Sacha J, Eikelboom JW. Efficacy and safety of fondaparinux in patients with ST-segment elevation myocardial infarction across the age spectrum. Results from the Organization for the Assessment of Strategies for Ischemic Syndromes 6 (OASIS-6) trial. Am Heart J. 2010;160:1049-1055. ]. Significant benefits of fondaparinux were observed in patients receiving thrombolytic therapy and those not receiving any reperfusion therapy. On the other hand, there was a trend to harm among 3,768 patients undergoing primary PCI in terms of death and re-infarction at 30 days, with a higher rate of guiding catheter thrombosis and more coronary complications (abrupt coronary artery closure, new angiographic thrombus, catheter thrombus, no-reflow, dissection, or perforation) with fondaparinux [135135. van Rees V, Peters RJ, Yusuf S, Afzal R, Chrolavicius S, O’Donnell M, Mehta SR, Pluta W, Sacha J, Eikelboom JW. Efficacy and safety of fondaparinux in patients with ST-segment elevation myocardial infarction across the age spectrum. Results from the Organization for the Assessment of Strategies for Ischemic Syndromes 6 (OASIS-6) trial. Am Heart J. 2010;160:1049-1055. ]. Fondaparinux use was associated with lower rates of major bleeding events in all patient groups except primary PCI patients. The observed higher intracatheter thrombosis rate is explained by the fact that fondaparinux and other pentasaccharides, in contrast to UFH, are ineffective in blocking the contact activation of the coagulation pathway. The existence of such pathogenetic mechanisms, as well as data from OASIS-6 and the more recent FUTURA-OASIS-8 study [136136. Steg PG, Jolly SS, Mehta SR, Afzal R, Xavier D, Rupprecht HJ, Lopez-Sendon JL, Budaj A, Diaz R, Avezum A, Widimsky P, Rao SV, Chrolavicius S, Meeks B, Joyner C, Pogue J, Yusuf S. Low-dose vs standard-dose unfractionated heparin for percutaneous coronary intervention in acute coronary syndromes treated with fondaparinux: the FUTURA/OASIS-8 randomized trial. JAMA. 2010;304:1339-1349. ], support the need for full-dose UFH administration during primary PCI for STEMI in patients pre-treated with fondaparinux.
Enoxaparin
There is a growing amount of data concerning the safety and feasibility of i.v. administration of enoxaparin during primary PCI for STEMI. In the STEEPLE study [137137. Montalescot G, White HD, Gallo R, Cohen M, Steg PG, Aylward PE, Bode C, Chiariello M, King SB, III, Harrington RA, Desmet WJ, Macaya C, Steinhubl SR. Enoxaparin versus unfractionated heparin in elective percutaneous coronary intervention. N Engl J Med. 2006;355:1006-1017. ], enoxaparin administration at the dose of 0.5 mg/kg i.v. bolus in elective patients was associated with significant reduction of the risk of major bleeding in comparison to UFH. Such clinical benefit was not confirmed for higher dose (0.75 mg/kg) of enoxaparin. In the randomised, open-label, multicentre ATOLL trial, the use of 0.5 mg/kg i.v. bolus of enoxaparin during primary PCI for STEMI was associated with significant reduction of the risk of composite ischaemic endpoint of death, recurrent MI and urgent revascularisation during 30-day follow-up in comparison to UFH. There was no difference in the non-CABG major bleeding rate between groups. Based on results of the ATOLL study and meta-analysis of randomized studies [BMJ 2012;344:e553], intravenous enoxaparin (with or without routine GP IIb-IIIa inhibitor) should be considered as an alternative to UFH as anticoagulant to primary PCI according to ESC STEMI guidelines (IIa; A) [211211. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. ]
Current recommendations for antithrombotic drugs in patients with STEMI are shown in Table 3 .
ROLE OF LYTICS IN STEMI TREATMENT
Introduction and definitions: complementarity of the two treatment modalities
Fibrinolysis offers an opportunity for early restoration of flow in patients too far from a primary PCI centre to reach it within 90-120 minutes. Fibrinolysis is not a stand-alone treatment because a mechanical intervention is needed to obtain coronary reperfusion in patients with failed thrombolysis (rescue PCI) and to decrease the risk of further ischaemic events in patients with successful thrombolysis (urgent early PCI after fibrinolysis). In the attempt to minimise the time delay to reperfusion, the administration of fibrinolysis has been advocated in all patients undergoing PCI (facilitated PCI). The definition was stretched to indicate whatever concomitant, more powerful antiplatelet regimen was added to the conventional aspirin and clopidogrel. Especially after the introduction of prasugrel and ticagrelor, preferred in STEMI because of their rapid action, and the reduced use of GP IIb-IIIa inhibitors produced by the negative results of HORIZONS-AMI, BRAVE 3 and other trials, the approach we recommend is to stick to the more logical definition of facilitated PCI, limited to fibrinolytics. Since an excess bleeding risk was consistently induced by fibrinolysis before PCI, with no improvement in other adverse events, for patients who can receive primary PCI promptly, fibrinolysis plays no role in optimal PCI candidates able to receive this treatment within 90-120 minutes.
Facilitated angioplasty must be distinguished by the use of fibrinolysis for those patients for whom primary PCI is not available within 90-120 minutes. In the past, those patients were considered as a medically treated cohort and the indications to PCI were established based on clinical criteria and mainly limited to patients with evidence of residual ischaemia. This conventional conservative approach is challenged by the fact that more than 1/3 of the patients with STEMI undergoing fibrinolysis do not show signs of reperfusion, and that, even when reperfusion is achieved, they have a considerable risk of recurrent MI and ischaemia because of late occlusion of the severe unstable residual stenosis which caused the initial episode. Since fibrinolysis is not an alternative to transfer for PCI, the organisational model of a territorial network for the treatment of STEMI patients should not be limited to the areas around primary PCI hospitals but should be expanded to provide a timely access to hospitals with 24/7 interventional facilities also to patients coming from areas too far away for a timely primary PCI treatment and who should be treated with fibrinolysis locally and then transferred for rescue PCI or for an urgent early invasive management.
Which fibrinolytic agent should we use?
Interventional cardiologists should be well aware of the benefit of fibrinolytic therapy because, as for multivessel or left main PCI which indirectly established their prognostic benefit versus medical treatment by being compared to bypass surgery, the evidence of superiority of primary PCI has been compared to the previous gold standard, i.e, fibrinolysis. Fibrinolysis prevents approximately 30 early deaths per 1,000 patients treated within 6 h after symptom onset [138138. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet. 1994;343:311-322. ]. Overall, the largest absolute benefit is seen among patients with the highest risk, but the proportional benefit is similar. The benefit is also seen in the elderly: in a subgroup of 3,300 patients over the age of 75 presenting within 12 h of symptom onset and with either ST-segment elevation or bundle-branch block, mortality rates were significantly reduced by fibrinolytic therapy.
Unfortunately, contraindications and especially the risk of bleeding complications are so high after 75 years that few of these patients can benefit from the potential advantage of fibrinolytic therapy. As with and more so than for primary PCI, time is the most crucial factor for the success of fibrinolysis. While for primary PCI a time delay only limits the ability to recover myocardial function, the organisation of the occlusive coronary thrombus reduces the penetration in the thrombus and the ability to break the links of the fibrin strands. These two reasons explain why guidelines tend to have different time indications for fibrinolytics and primary PCI, with the first recommended almost exclusively in the first 6 hours after symptom onset because beyond that time and certainly beyond 12 hours the limited success rate does not justify the preliminary use of fibrinolysis which will improve only marginally the relative delay of primary PCI. Conversely, before 3 and especially before 1 or 2 hours from symptom onset, the greater success rate of fibrinolysis and the greater clinical relevance of even small time delays to reperfusion in the golden hours when every minute counts for myocardial recovery suggest a liberal use of fibrinolytics, especially in larger anterior MI and in groups at low risk. Unlike primary PCI, fibrinolysis can be administered before hospital admission, provided the diagnosis is firmly established and the medical/paramedical crew is certified and trained for use. A meta-analysis of studies in which >6,000 patients were randomised to pre-hospital or in-hospital thrombolysis has shown significant reduction (17%) in early mortality with pre-hospital treatment [139139. Morrison LJ, Verbeek PR, McDonald AC, Sawadsky BV, Cook DJ. Mortality and prehospital thrombolysis for acute myocardial infarction: A meta-analysis. JAMA. 2000;283:2686-2692. ]. In a meta-analysis of 22 trials, a much larger mortality reduction was found in patients treated within the first 2 h than in those treated later [140140. Boersma E, Maas AC, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet. 1996;348:771-775. ]. More recent post hoc analyses of several randomised trials and data from registries have confirmed the clinical usefulness of pre-hospital fibrinolysis [3535. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet. 2006;367:569-578. , 141141. Steg PG, Bonnefoy E, Chabaud S, Lapostolle F, Dubien PY, Cristofini P, Leizorovicz A, Touboul P. Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: data from the CAPTIM randomized clinical trial. Circulation. 2003;108:2851-2856. , 142142. Danchin N, Coste P, Ferrieres J, Steg PG, Cottin Y, Blanchard D, Belle L, Ritz B, Kirkorian G, Angioi M, Sans P, Charbonnier B, Eltchaninoff H, Gueret P, Khalife K, Asseman P, Puel J, Goldstein P, Cambou JP, Simon T. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the french registry on acute ST-elevation myocardial infarction (FAST-MI). Circulation. 2008;118:268-276. , 143143. Kalla K, Christ G, Karnik R, Malzer R, Norman G, Prachar H, Schreiber W, Unger G, Glogar HD, Kaff A, Laggner AN, Maurer G, Mlczoch J, Slany J, Weber HS, Huber K. Implementation of guidelines improves the standard of care: the Viennese registry on reperfusion strategies in ST-elevation myocardial infarction (Vienna STEMI registry). Circulation. 2006;113:2398-2405. ]. Most of these studies reported outcome data similar to those of primary PCI, provided early angiography and PCI were performed in those who needed intervention.
Fibrinolytic therapy is associated with a small but significant increase in strokes, mainly occurring on the first day after treatment [138138. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet. 1994;343:311-322. ]. Early strokes are almost exclusively caused by cerebral haemorrhages, while later strokes are more frequently thrombotic or embolic and can also develop after primary PCI. Old age, low weight, female gender, prior cerebrovascular disease, and systolic and diastolic hypertension on admission predict the development of intracranial haemorrhages [144144. Gore JM, Granger CB, Simoons ML, Sloan MA, Weaver WD, White HD, Barbash GI, Van de WF, Aylward PE, Topol EJ, . Stroke after thrombolysis. Mortality and functional outcomes in the GUSTO-I trial. Global Use of Strategies to Open Occluded Coronary Arteries. Circulation. 1995;92:2811-2818. ]. In the latest trials, intracranial bleeding occurred in 0.9%-1.0% of the total population studied [145145. Van de Werf F, Adgey J, Ardissino D, Armstrong PW, Aylward P, Barbash G, Betriu A, Binbrek AS, Califf R, Diaz R, Fanebust R, Fox K, Granger C, Heikkila J, Husted S, Jansky P, Langer A, Lupi E, Maseri A, Meyer J, Mlczoch J, Mocceti D, Myburgh D, Oto A, Paolasso E, Pehrsson K, Seabra-Gomes R, Soares-Piegas L, Sugrue D, Tendera M, Topol E, Toutouzas P, Vahanian A, Verheugt F, Wallentin L, White H. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354:716-722. , 146146. A comparison of reteplase with alteplase for acute myocardial infarction. The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO III) Investigators. N Engl J Med. 1997;337:1118-1123. ]. Major non-cerebral bleeds (bleeding complications requiring blood transfusion or those which are life-threatening) can occur in 4%-13% of the patients treated [145145. Van de Werf F, Adgey J, Ardissino D, Armstrong PW, Aylward P, Barbash G, Betriu A, Binbrek AS, Califf R, Diaz R, Fanebust R, Fox K, Granger C, Heikkila J, Husted S, Jansky P, Langer A, Lupi E, Maseri A, Meyer J, Mlczoch J, Mocceti D, Myburgh D, Oto A, Paolasso E, Pehrsson K, Seabra-Gomes R, Soares-Piegas L, Sugrue D, Tendera M, Topol E, Toutouzas P, Vahanian A, Verheugt F, Wallentin L, White H. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354:716-722. , 147147. Berkowitz SD, Granger CB, Pieper KS, Lee KL, Gore JM, Simoons M, Armstrong PW, Topol EJ, Califf RM. Incidence and predictors of bleeding after contemporary thrombolytic therapy for myocardial infarction. The Global Utilization of Streptokinase and Tissue Plasminogen activator for Occluded coronary arteries (GUSTO) I Investigators. Circulation. 1997;95:2508-2516. ]. In patients undergoing subsequent PCI, the most common sources of bleeding are procedure-related.
Administration of streptokinase may be associated with hypotension, but severe allergic reactions are rare. Streptokinase should not be re-administered because of antibodies which can impair its activity and because of the risk of allergic reactions. The advantages provided by new fibrin-specific lytics are small but well demonstrated. In the GUSTO trial, which included >30,000 patients, an accelerated infusion of the fibrin-specific agent t-PA (tissue plasminogen activator; alteplase) resulted in 10 fewer deaths per 1,000 patients [148148. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med. 1993;329:673-682. ]. Double-bolus r-PA (reteplase) does not offer any further mortality reduction over accelerated t-PA except for its ease of administration [146146. A comparison of reteplase with alteplase for acute myocardial infarction. The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO III) Investigators. N Engl J Med. 1997;337:1118-1123. ]. Single-bolus weight-adjusted tenecteplase is equivalent to accelerated t-PA for 30-day mortality and is associated with a significantly lower rate of non-cerebral bleedings and less need for blood transfusion [145145. Van de Werf F, Adgey J, Ardissino D, Armstrong PW, Aylward P, Barbash G, Betriu A, Binbrek AS, Califf R, Diaz R, Fanebust R, Fox K, Granger C, Heikkila J, Husted S, Jansky P, Langer A, Lupi E, Maseri A, Meyer J, Mlczoch J, Mocceti D, Myburgh D, Oto A, Paolasso E, Pehrsson K, Seabra-Gomes R, Soares-Piegas L, Sugrue D, Tendera M, Topol E, Toutouzas P, Vahanian A, Verheugt F, Wallentin L, White H. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354:716-722. ]. This single-bolus weight-adjusted drug is particularly advantageous in the pre-hospital setting and has gained popularity in most European countries.
For patients screened in the dedicated mobile units where pre-hospital diagnosis can be established, and for patients arriving directly to the hospital, a realistic aim is to initiate fibrinolysis within 30 minutes (door-to-needle time) with a maximum tolerated delay of 60 minutes.
Pre-hospital fibrinolysis
It is reasonable to initiate fibrinolytic therapy as fast as possible after the confirmation of STEMI diagnosis. In many networks, administration of fibrinolytic therapy in a pre-hospital setting was introduced to decrease time from symptom onset to treatment. This strategy is especially feasible in locations where fibrinolytic therapy is administered by paramedics [149149. Welsh RC, Travers A, Senaratne M, Williams R, Armstrong PW. Feasibility and applicability of paramedic-based prehospital fibrinolysis in a large North American center. Am Heart J. 2006;152:1007-1014. ] or general practitioners. In addition, it may be a valuable option in rural or congested urban areas where transportation times are very long, as well as in areas in which primary PCI facilities are not immediately available. Fibrinolytics used as i.v. bolus should be preferred over i.v. infusion to facilitate pre-hospital management. Importantly, following pre-hospital fibrinolysis, the ambulance should transport the patient to a 24 h a day/7 days a week primary PCI facility.
A number of studies have demonstrated that pre-hospital fibrinolysis may allow a decrease in both time from symptom onset to treatment [139139. Morrison LJ, Verbeek PR, McDonald AC, Sawadsky BV, Cook DJ. Mortality and prehospital thrombolysis for acute myocardial infarction: A meta-analysis. JAMA. 2000;283:2686-2692. , 149149. Welsh RC, Travers A, Senaratne M, Williams R, Armstrong PW. Feasibility and applicability of paramedic-based prehospital fibrinolysis in a large North American center. Am Heart J. 2006;152:1007-1014. , 150150. Morrow DA, Antman EM, Sayah A, Schuhwerk KC, Giugliano RP, deLemos JA, Waller M, Cohen SA, Rosenberg DG, Cutler SS, McCabe CH, Walls RM, Braunwald E. Evaluation of the time saved by prehospital initiation of reteplase for ST-elevation myocardial infarction: results of The Early Retavase-Thrombolysis in Myocardial Infarction (ER-TIMI) 19 trial. J Am Coll Cardiol. 2002;40:71-77. ] and from symptom onset to successful reperfusion [150150. Morrow DA, Antman EM, Sayah A, Schuhwerk KC, Giugliano RP, deLemos JA, Waller M, Cohen SA, Rosenberg DG, Cutler SS, McCabe CH, Walls RM, Braunwald E. Evaluation of the time saved by prehospital initiation of reteplase for ST-elevation myocardial infarction: results of The Early Retavase-Thrombolysis in Myocardial Infarction (ER-TIMI) 19 trial. J Am Coll Cardiol. 2002;40:71-77. ]. Moreover, several studies have shown improved outcomes, including mortality reduction associated with pre-hospital-initiated fibrinolysis in comparison to in-hospital fibrinolysis [139139. Morrison LJ, Verbeek PR, McDonald AC, Sawadsky BV, Cook DJ. Mortality and prehospital thrombolysis for acute myocardial infarction: A meta-analysis. JAMA. 2000;283:2686-2692. , 151151. Danchin N, Blanchard D, Steg PG, Sauval P, Hanania G, Goldstein P, Cambou JP, Gueret P, Vaur L, Boutalbi Y, Genes N, Lablanche JM. Impact of prehospital thrombolysis for acute myocardial infarction on 1-year outcome: results from the French Nationwide USIC 2000 Registry. Circulation. 2004;110:1909-1915. ], as well as to primary PCI [151151. Danchin N, Blanchard D, Steg PG, Sauval P, Hanania G, Goldstein P, Cambou JP, Gueret P, Vaur L, Boutalbi Y, Genes N, Lablanche JM. Impact of prehospital thrombolysis for acute myocardial infarction on 1-year outcome: results from the French Nationwide USIC 2000 Registry. Circulation. 2004;110:1909-1915. ]. In the CAPTIM study, pre-hospital fibrinolysis (with transfer to an interventional facility for possible rescue PCI) was associated with similar rates of the composite primary endpoint (death, non-fatal re-infarction, and non-fatal disabling stroke within 30 days) or mortality in comparison to primary PCI [152152. Bonnefoy E, Lapostolle F, Leizorovicz A, Steg G, McFadden EP, Dubien PY, Cattan S, Boullenger E, Machecourt J, Lacroute JM, Cassagnes J, Dissait F, Touboul P. Primary angioplasty versus prehospital fibrinolysis in acute myocardial infarction: a randomised study. Lancet. 2002;360:825-829. ]. However, post hoc analysis of data from the CAPTIM study revealed that patients randomised <2 hours after symptom onset had a strong trend towards lower 30-day mortality with pre-hospital thrombolysis compared with those randomised to primary PCI (2.2% vs. 5.7%, p=0.058), whereas mortality was similar in patients randomised ≥2 hours [141141. Steg PG, Bonnefoy E, Chabaud S, Lapostolle F, Dubien PY, Cristofini P, Leizorovicz A, Touboul P. Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: data from the CAPTIM randomized clinical trial. Circulation. 2003;108:2851-2856. ]. After 5 years of follow-up, the difference in mortality in patients randomised within 2 hours reached statistical significance (5.8% vs. 11.1%, respectively, p=0.04) [153153. Bonnefoy E, Steg PG, Boutitie F, Dubien PY, Lapostolle F, Roncalli J, Dissait F, Vanzetto G, Leizorowicz A, Kirkorian G, Mercier C, McFadden EP, Touboul P. Comparison of primary angioplasty and pre-hospital fibrinolysis in acute myocardial infarction (CAPTIM) trial: a 5-year follow-up. Eur Heart J. 2009;30:1598-1606. ]. The advantage of pre-hospital fibrinolysis over primary PCI in terms of reduction of 1-year mortality in early presenters (patients randomised within 2 hours from symptom onset) was also confirmed by combined analysis of data from CAPTIM and WEST studies (2.8% vs. 6.9%, respectively, p=0.021) [154154. Westerhout CM, Bonnefoy E, Welsh RC, Steg PG, Boutitie F, Armstrong PW. The influence of time from symptom onset and reperfusion strategy on 1-year survival in ST-elevation myocardial infarction: a pooled analysis of an early fibrinolytic strategy versus primary percutaneous coronary intervention from CAPTIM and WEST. Am Heart J. 2011;161:283-290. ]. However, whether pre-hospital fibrinolysis is associated with a similar or better clinical outcome than primary PCI in patients presenting early has not been studied prospectively in an adequately sized randomised fashion. The STREAM study comparing primary PCI against an active rescue strategy did not show a difference in mortality between both strategies [3030. Armstrong PW, Gershlick AH, Goldstein P, Wilcox R, Danays T, Lambert Y, Sulimov V, Rosell OF, Ostojic M, Welsh RC, Carvalho AC, Nanas J, Arntz HR, Halvorsen S, Huber K, Grajek S, Fresco C, Bluhmki E, Regelin A, Vandenberghe K, Bogaerts K, Van de Werf F. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013;368:1379-1387. , 155155. Armstrong PW, Gershlick A, Goldstein P, Wilcox R, Danays T, Bluhmki E, Van de Werf F. The Strategic Reperfusion Early After Myocardial Infarction (STREAM) study. Am Heart J. 2010;160:30-35. ].
Treatment after fibrinolysis
In cases of failure of a fibrinolytic treatment re-administration of a second dose of fibrinolysis was not shown to be beneficial [4646. Gershlick AH, Stephens-Lloyd A, Hughes S, Abrams KR, Stevens SE, Uren NG, de Belder A, Davis J, Pitt M, Banning A, Baumbach A, Shiu MF, Schofield P, Dawkins KD, Henderson RA, Oldroyd KG, Wilcox R. Rescue angioplasty after failed thrombolytic therapy for acute myocardial infarction. N Engl J Med. 2005;353:2758-2768. ] and patients should be immediately transferred for rescue angioplasty based on data presented above. .The approach for patients in whom it is likely that fibrinolysis was successful (>50% ST-segment resolution at 60-90 minutes, typical reperfusion arrhythmia, disappearance of chest pain) is more controversial. A strategy of routine early angiography is recommended if there are no contraindications [33. Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van ’t Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619. , 44. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa UM, Taggart D, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Kolh P, Alfieri O, Dunning J, Elia S, Kappetein P, Lockowandt U, Sarris G, Vouhe P, Kearney P, von Segesser L, Agewall S, Aladashvili A, Alexopoulos D, Antunes MJ, Atalar E, Brutel de la Riviere A, Doganov A, Eha J, Fajadet J, Ferreira R, Garot J, Halcox J, Hasin Y, Janssens S, Kervinen K, Laufer G, Legrand V, Nashef SA, Neumann FJ, Niemela K, Nihoyannopoulos P, Noc M, Piek JJ, Pirk J, Rozenman Y, Sabate M, Starc R, Thielmann M, Wheatley DJ, Windecker S, Zembala M. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2010;31:2501-2555. ]. Various randomised trials [3838. Di Mario C, Dudek D, Piscione F, Mielecki W, Savonitto S, Murena E, Dimopoulos K, Manari A, Gaspardone A, Ochala A, Zmudka K, Bolognese L, Steg PG, Flather M. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet. 2008;371:559-568. , 3939. Cantor WJ, Fitchett D, Borgundvaag B, Ducas J, Heffernan M, Cohen EA, Morrison LJ, Langer A, Dzavik V, Mehta SR, Lazzam C, Schwartz B, Casanova A, Goodman SG. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360:2705-2718. , 4141. Bohmer E, Hoffmann P, Abdelnoor M, Arnesen H, Halvorsen S. Efficacy and safety of immediate angioplasty versus ischemia-guided management after thrombolysis in acute myocardial infarction in areas with very long transfer distances results of the NORDISTEMI (NORwegian study on DIstrict treatment of ST-elevation myocardial infarction). J Am Coll Cardiol. 2010;55:102-110. ] and two contemporary meta-analyses [4343. Borgia F, Goodman SG, Halvorsen S, Cantor WJ, Piscione F, Le May MR, Fernandez-Aviles F, Sanchez PL, Dimopoulos K, Scheller B, Armstrong PW, Di Mario C. Early routine percutaneous coronary intervention after fibrinolysis vs. standard therapy in ST-segment elevation myocardial infarction: a meta-analysis. Eur Heart J. 2010;31:2156-2169. , 156156. Collet JP, Montalescot G, Le May M, Borentain M, Gershlick A. Percutaneous coronary intervention after fibrinolysis: a multiple meta-analyses approach according to the type of strategy. J Am Coll Cardiol. 2006;48:1326-1335. ] have shown that early routine post-thrombolysis angiography with subsequent PCI if required (“drip & ship”) reduces the rates of re-infarction and recurrent ischaemia both at 30 days and 1 year compared with a conventional “watch & wait” strategy with angiography and revascularisation only in patients with spontaneous or induced severe ischaemia or left ventricular dysfunction.
NO-REFLOW TREATMENT IN THE STEMI SETTING
Principles of no-reflow prevention and treatment are described in Chapter 3.24. In the case of no-reflow phenomenon occurrence during primary PCI, intracoronary administration of adenosine and verapamil could be useful. The direct nitric oxide donor sodium nitroprusside (used as repeated intracoronary boluses of 50-100 μg, up to 1,000 μg under blood pressure control) seems to be the most effective in improvement of microcirculatory flow. In some cases, 2 or 3 of these drugs may be combined. In cases of pharmacotherapy resistant no-reflow phenomenon, the use of IABP may be justified.
Angiographic methods of reperfusion assessment
Immediate results of reperfusion with primary PCI may be analysed at epicardial and myocardial level. In daily clinical practice, epicardial flow is assessed according to 4 grades - qualitative TIMI flow grade scale (Chapter 2.06). The scale describes the presence and speed of epicardial vessel filling with contrast [157157. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med. 1985;312:932-936. ]. Another tool, used more for research, is the TIMI frame count (TFC), which is quantitative flow assessment, so it is less subjective than the TIMI flow grade [158158. Gibson CM, Cannon CP, Daley WL, Dodge JT, Jr., Alexander B, Jr., Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, Braunwald E. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879-888. ]. TFC is based on the calculation of the number of cine frames required for contrast first to reach standardised distal coronary landmarks in the infarct-related artery (Chapter 2.06). Another two scales were proposed for assessment of reperfusion based on myocardial level. The TIMI myocardial perfusion grade scale (TMPG) was described by the TIMI group based on studies of fibrinolysis treatment [159159. Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, McCabe CH, Van de WF, Braunwald E. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000;101:125-130. ]. In the TMPG scale, the filling and clearance of contrast in the myocardium is analysed distal to the culprit lesion. The myocardial blush grade (MBG) scale was proposed by the Zwolle group based on primary PCI studies [160160. van ’t Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97:2302-2306. ]. MBG assessment is based on analysis of contrast density in the microcirculation (Chapter 2.06). All of the above-mentioned angiographic scales correlate well with patient clinical outcome, including mortality.
Electrocardiographic methods of reperfusion assessment - ST-segment resolution
ST-segment assessment after reperfusion therapy is a commonly used parameter which correlates well with clinical outcome including long-term mortality. Electrocardiographic (ECG) ST-segment changes reflect myocardial rather than epicardial flow. This provides complementary information beyond the angiographic parameters. ECG ST-segment resolution (STR) analysis is one of the most popular methods of reperfusion assessment and it is widely used in clinical practice [161161. Schroder R. Prognostic impact of early ST-segment resolution in acute ST-elevation myocardial infarction. Circulation. 2004;110:e506-e510. ]. There are at least three methods of ST-segment analysis. The most popular is the resolution of the sum of ST-segment elevation in ECG at a given time point after primary PCI compared to baseline ECG [162162. Schroder R, Wegscheider K, Schroder K, Dissmann R, Meyer-Sabellek W. Extent of early ST segment elevation resolution: a strong predictor of outcome in patients with acute myocardial infarction and a sensitive measure to compare thrombolytic regimens. A substudy of the International Joint Efficacy Comparison of Thrombolytics (INJECT) trial. J Am Coll Cardiol. 1995;26:1657-1664. ]. Two others are single-lead STR and maximum ST-segment elevation after primary PCI [163163. Schroder K, Wegscheider K, Zeymer U, Tebbe U, Schroder R. Extent of ST-segment deviation in a single electrocardiogram lead 90 min after thrombolysis as a predictor of medium-term mortality in acute myocardial infarction. Lancet. 2001;358:1479-1486. , 164164. Schroder K, Wegscheider K, Zeymer U, Neuhaus KL, Schroder R. Extent of ST-segment deviation in the single ECG lead of maximum deviation present 90 or 180 minutes after start of thrombolytic therapy best predicts outcome in acute myocardial infarction. Z Kardiol. 2001;90:557-567. ]. These last two methods are simpler but both have good predictive accuracy, even better than the sum STR model. In the study of Brodie et al, STR and maximum ST-segment elevation after PCI were analysed in more than 1,000 patients showing good correlation with clinical outcome [165165. Brodie BR, Stuckey TD, Hansen C, Versteeg DS, Muncy DB, Moore S, Gupta N, Downey WE. Relation between electrocardiographic ST-segment resolution and early and late outcomes after primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. 2005;95:343-348. ]. Similarly, analysis of the CADILLAC population confirmed the value of these parameters as predictors of outcome [166166. McLaughlin MG, Stone GW, Aymong E, Gardner G, Mehran R, Lansky AJ, Grines CL, Tcheng JE, Cox DA, Stuckey T, Garcia E, Guagliumi G, Turco M, Josephson ME, Zimetbaum P. Prognostic utility of comparative methods for assessment of ST-segment resolution after primary angioplasty for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J Am Coll Cardiol. 2004;44:1215-1223. ]. In both studies, the time window for ECG after PCI was liberal and defined as less than 4 hours. In the APEX-AMI ECG analysis, ST-segment parameters were assessed based on ECG performed early (30 minutes) after PCI. All correlated well with 90-day clinical events (death, shock, heart failure) [167167. Buller CE, Fu Y, Mahaffey KW, Todaro TG, Adams P, Westerhout CM, White HD, van ’t Hof AW, Van de Werf FJ, Wagner GS, Granger CB, Armstrong PW. ST-segment recovery and outcome after primary percutaneous coronary intervention for ST-elevation myocardial infarction: insights from the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial. Circulation. 2008;118:1335-1346. ]. In studies of thrombolytic therapy, ECG parameters were analysed in a predefined time window which was driven by the pharmacokinetics of a certain agent. However, in clinical practice early reperfusion assessment after thrombolysis is very important because it facilitates decision-making about the potential use of rescue PCI treatment (see Rescue PCI).
Conclusion
Primary PCI is the preferred method of reperfusion in patients with STEMI. To overcome reperfusion delay caused by logistical problems, regional networks should be implemented based on cooperation of primary PCI centres with ambulances and non-PCI-capable hospitals. With appropriate planning, only in a small minority of patients the anticipated delay to primay PCI will be so long to require pre- or in-hospital fibrinolytic therapy as a bridge to invasive therapy. PCI for STEMI carries a higher risk of complications including distal embolisation or no-reflow which influence clinical outcome. Modern pharmacotherapy and new stent designs allow a reduction in the risk of such complications and an improvement in immediate and long-term results of primary PCI.
Personal perspective – Dariusz Dudek
Due to rapid evolution in the invasive treatment of acute MI, such an approach became a separate part of interventional cardiology. Currently, management of patients with STEMI is not only a mechanical restoration of the flow within the infarct-related artery, but it also includes a system of early diagnosis and rapid transfer to PCI-capable centres, selection of optimal adjunctive pharmacotherapy and finally PCI procedure with dedicated devices. All these components are necessary for the eventual success of the treatment. The networking concept which was introduced to overcome the time delay from FMC to the primary PCI centre is currently accepted as an optimal model of STEMI treatment organisation. Well established cooperation of a primary PCI centre, non-PCI hospitals and emergency medical systems is crucial for rapid diagnosis and transfer. The second key component of optimal treatment of STEMI is selection of adjunctive pharmacotherapy, especially antiplatelet and antithrombotic agents. New drugs allow a reduction in rates of clinical events including mortality, but this benefit may be reduced by the higher risk of bleedings in some patients. That is why adjunctive pharmacotherapy should be tailored to the individual patient, based on the assessment of ischaemic and bleeding risk. Such an approach consists not only of deciding on optimal agent choice, but also on the moment (before transfer, in the cath lab before angiography, or in the cath lab after angiography/PCI) and the means of administration (intravenous, intracoronary or intralesion administration), and its application is only possible when performed by experienced medical staff. Special technologies such as aspiration thrombectomy, new stent designs (mesh-covered stents, self-expandable stents, bioabsorbable vascular scaffolds) and local drug delivery systems may have been developed to improve the results of primary PCI but for many of them results of trials have been inconclusive or negative so that their use should be carefully limited to specific patient characteristics and lesion morphology. Also, the broader application of intravascular imaging, especially optical coherence tomography may allow for better procedure planning and understanding the pathophysiology of STEMI. Therefore, operator experience is fundamental for procedural success. In patients with multivessel disease presenting with STEMI, the index procedure should be limited to PCI within the infarct-related artery. However, complete revascularization during index hospital stay should be strongly encouraged. FFR/iFR-guided revascularization seems to be particularly useful in this setting. Further studies targeted on the highest risk patients, including patients with cardiogenic shock and/or after cardiac arrest are required. These should focus on the assessment of the value of LVAD and ECMO in those patients. Apart from achieving the successful reperfusion on the epicardial level, the primary target nowadays seems to be the prevention of reperfusion injury. It might be achieved partly with several pharmacological strategies and ischemic pre- and postconditioning. In addition, new mechanical solutions targeting on the improvement of reperfusion and reduction of infarct size, for instance, pressure-controlled intermittent coronary sinus occlusion are being introduced now. All these factors make the primary PCI approach different from a planned intervention. That is why interventional STEMI treatment should be performed in experienced, well equipped centres working in a network system and providing 24/7 primary PCI programmes.