Summary
Balloons, assisted by stents, are the established tools to widen coronary stenoses. This applies to chronic coronary disease as well as to acute coronary syndromes. The goals of good balloon performance are effective dilatation (in terms of a sustained result), patient safety, promptness of action and efficiency. The earlier experience and research of pioneers is now standard knowledge imparted in educational programmes. It should be kept in mind, however, that a balloon is always part of a catheter system, namely a foreign body interacting with tissues and blood fluid. The materials, polymers and metals, also interact with each other, sometimes in an unpredictable manner. The use of balloon catheters therefore requires clear knowledge of the disease being treated and also of the technical and biological interactions with intracoronary equipment.
Introduction
Balloon catheters are the basis for everyday catheter laboratory work. The construction of standard products is well established and the principal function of the balloon has not changed much in the last 15 years. The materials, however, are better now, and stents and drugs have been added which aim to stabilise the dilatatory effect of the balloon, such that nearly every PCI is now finished with a stent. With the treatment of ever increasingly complicated atherosclerotic disease, predilatation prior to stent deployment and to some degree post-dilatation are now the main indications.
The standard construction principle for intracoronary catheters is the Monorail system, which is best understood from its function. Since balloon dilatation and the Monorail principle are often the basis of a multitude of intracoronary techniques, we will refer to relevant chapters of this book where necessary.
The focus of this chapter’s contribution is to understand the general procedures and risks associated with the use of balloons. First and foremost, however, it must be remembered that it is the responsibility of the operator to ensure, before starting any PCI, that the diagnosis, indication, and informed consent, as well as all other prerequisites, are satisfactory.
Balloon catheter design
THE NON-COMPLIANT BALLOON CONCEPT (GRÜNTZIG)
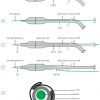
The evolution of balloon catheter systems was dominated by a consecutive series of essential developments: non-compliant balloons, miniaturisation, steerable catheters, catheter exchange and the combination of these developments in one catheter ( Figure 1 ).
Grüntzig’s solution to treat stenoses in atherosclerotic arteries was a non-compliant “sausage-like” balloon where pressure is transformed into radial force during inflation, evenly distributed over the balloon and directed toward the circumference. The balloon membrane is strained according to Laplace’s law and thus requires strong polymers. As long as the balloon is not too large, it delivers high radial pressures to the stenotic area until the tissue is sufficiently stretched or “cracked”. Other techniques, such as debulking or ablation, with a few exceptions, have not proved to be equivalent or superior to PCI (  View chapter ,
View chapter ,  View chapter and
View chapter and  View chapter ).
View chapter ).
BALLOON CATHETER SYSTEMS
Early evolution
The balloon is part of a catheter system, consisting of the balloon itself, a stiff yet flexible catheter shaft and a guidewire for delivery [11. Gruentzig AR, Transluminal dilatation of coronary artery stenosis. Lancet. 1978;1:263. , 22. Bertrand ME, Kaltenbach M. The steerable catheters, in Bertrand ME, ed. The Evolution of Cardiac Catheterization and Interventional Cardiology. ESC/Iatric Press; 2006:91-96.
Description of the development of the first steerable coronary catheter systems]. The shaft contains a lumen for balloon inflation and deflation (and originally contained a second lumen for distal pressure measurement). With the pioneering Grüntzig catheters, a great idea was realised but they were technically challenging to use. A major problem was to reach and to pass the stenosis because catheters themselves offered minimal torque control and were not steerable ( Figure 2 [A] ).
A great number of subsequent balloon designs have been in use over various time periods, such as step-up balloons or tapered balloons and the coronary perfusion catheter “CPC Mainz”, which was the first design to maintain a continuous blood flow during balloon inflation [33. Erbel R, Clas W, Busch U, von Seelen W, Brennecke R, Blömer H, Meyer J. New balloon catheter for prolonged percutaneous transluminal coronary angioplasty and bypass flow in occluded vessels. Cathet Cardiovasc Diagn. 1986;12;116-23. ] and thus reduce ischaemia. In cases of occluding coronary dissections the patient could be stabilised and transferred to the operating room for emergency bypass surgery.
Miniaturisation
The first guide catheters had outer diameters of 9 Fr (3 mm) and thickly constructed walls, the balloon catheter shafts had diameters of 4 or 4.3 Fr, the balloon polymers were stiff and the deflated profiles high [44. Vandormael M, Ischinger T, Roth R. Angioplasty equipment and supplies: Technical considerations. In: T. Ischinger, Practice of coronary angioplasty, Springer. 1986;93-127. ]. The contrast flow through the guide catheter with a thick balloon catheter inside was low and stenosis visualisation inadequate. Effective steps towards smaller catheters were achieved in 1982 with the semi-steerable “Hartzler catheters” [55. Vandormel M, Ischinger T, Roth R. Angioplasty equipment and supplies: Technical considerations. In: T. Ischinger, Practice of coronary angioplasty, Springer. 1986;104. ], later with the “balloon on the wire” [66. King SB. Balloon-on-wire probe system for coronary angioplasty. In: Vogel JHK and King SB. Interventional Cardiology: future directions. Mosby. 1989:300-6. ], and in 1985 with steerable Monorail catheters with shaft diameters under 3 Fr. The later low-profile designs were achieved by dispensing with the facility for distal pressure measurement and with improved construction materials ( Figure 2 [B] ).
Guidewires: steerability and exchange
The first technical advance to help a catheter navigate the vascular tree was made by Simpson who introduced a steerable guidewire, contained within the pressure lumen of the balloon catheter itself [77. Simpson J, Baim D, Robert E, Harrison D A new catheter system for coronary angioplasty. Am J Cardiol. 1982;49:1216-22. ]. This guidewire was longer than the catheter, had a flexible tip protruding from the distal pressure lumen and could be rotated by hand from the proximal end. Kaltenbach modified this approach and improved the exchange of balloons with the help of the “long wire technique” extending standard-length guidewires to more than 3 m [88. Kaltenbach M The long wire technique – a new technique for steerable balloon catheter dilatation of coronary artery stenoses. Eur Heart J. 1984;5:1004-09. ]. This was an important step forward but this “over the wire” (OTW) procedure is time-consuming, insecure and normally requires two operators. Nevertheless, its main advantages are good pushability, trackability and the ability to exchange guidewires as well as inject fluids distally (drugs, cell suspensions and contrast medium). This is especially helpful with chronic total occlusion recanalisations. A further, less frequently used, OTW application is transseptal myocardial ablation whereby a septal perforator artery is selected and alcohol injected while simultaneously preventing reflux into the main parent vessel. Some other devices, such as rotablators, also have to use OTW technology ( Figure 2 [C] ).
Monorail system (Rx or SOE)
In 1985, the advent of the Monorail catheter effectively simplified PCI and contributed to the overall propagation of PCI as a technique [99. Bonzel T, Wollschlaeger H, Just H. A new catheter system for coronary angioplasty (PTCA) with exchangeable intracoronary catheters, high flow contrast agent and improved steerability. Biomed Tech. 1986;31:195-200 (in German). , 1010. Bonzel T, Wollschlaeger H, Kasper W, Meinertz T, Just H. The sliding rail system (monorail): description of a new technique for intravascular instrumentation and its application to coronary angioplasty. Z Kardiol. 1987;76:119-22. ]. The essential feature is that a standard-length guidewire runs only in the distal balloon part of the catheter within a guidewire lumen, and exits the shaft a little proximal to the balloon itself to run outside the balloon catheter for most of the remaining wire length. A vast number of balloon devices have used the Monorail technology [1111. Bonzel T. The monorail technique, in Bertrand ME, ed. The Evolution of Cardiac Catheterization and Interventional Cardiology. ESC/Iatric Press; 2006: 97-104.
Description of the monorail technique for coronary dilatation and for a number of further applications]. This includes the first purely diagnostic intracoronary catheter, which was specifically designed for a study on flow-dependent coronary vasodilatation [1212. Drexler H, Zeiher AM, Wollschläger H, Meinertz T, Just H, Bonzel T. Flow-dependent coronary artery dilatation in humans. Circulation. 1989;80:466-74. A study of the flow-dependent coronary dilatation in humans, mediated through the endothelium after a maximal pharmacologically-induced flow increase.
For this study, modified diagnostic monorail catheters were used]. The Monorail principle is now used for numerous other techniques such as angioscopy, intravascular ultrasound and also the measurement of fractional flow reserve (FFR).
The Monorail construction significantly changes balloon catheter system handling compared to the earlier OTW designs. To operate it, a fully steerable guidewire is first inserted across the stenosis, and then the balloon is loaded onto the wire. The proximal end of the guidewire (rail) can be fixed with one hand while the balloon catheter is advanced or may be removed and replaced with the other hand, e.g., for a stented balloon. The exchange can be performed safely within seconds. Hence Monorail catheters are also called “single operator exchange” (SOE) or “rapid exchange” (Rx) catheters ( Figure 2 [D] ).
Balloon-expandable stents
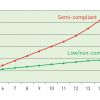
Most stent systems rely on balloon catheters to deploy stents against the vessel wall. Stents are embedded or crimped onto the folded balloons by the manufacturer. The pressure required to deploy a stent should be at least the nominal pressure of the balloon ( Figure 3 ). Stent systems typically have only one or two platform sizes across their displayed size range and the balloon size determines the initial deployment diameter. Balloon polymers are semi-compliant, and frequently increased pressures are needed to overcome hard stenoses and stent recoil. The balloon compliance is shown on a manufacturer’s pressure chart, which comes with each catheter (  View chapter ) ( Figure 4 [A] and Figure 4 [B] ).
View chapter ) ( Figure 4 [A] and Figure 4 [B] ).
Drug-coated balloons (DCB) and Drug-eluting stents (DES)
One of the major drawbacks of pure balloon angioplasty, but also of bare metal stents, is restenosis. Local application of antiproliferative drugs is an effective means to prevent this vascular response to injury induced by PCI [1313. Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnàr F, Falotico R, for the RAVEL Study Group. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773-80. ]. These drugs can be carried into the stenosis using polymeric or metal drug-eluting stents (DES), on balloon surfaces (drug-coated balloon, DCB) or within the balloon fluid itself, from where they can be released through micropores in the balloon membrane. The mechanism of delivery onto the diseased vessel wall involves both close contact of drug and vessel wall and the pressure generated by the fluid and the expanded balloon (  View chapter and
View chapter and  View chapter).
View chapter).
Balloon catheter systems
- Monorail balloon catheters (SOE, Rx)
- Over-the-wire balloon catheters
- High-pressure balloon catheters
- Low-profile balloon catheters
- Balloon-expandable stent catheters
- Drug-coated balloon catheters (DCB)
- Porous balloon catheters
- Bifurcation balloon catheters
- Focused force (scoring) balloon catheters
Technical information
BALLOON CATHETERS
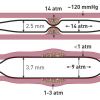
Single Monorail balloon catheters with shaft diameters between 2 and 2.5 Fr fit easily through 5 Fr (1.7 mm) guide catheters and two balloons fit through 6 Fr (1.98 mm) guide catheters for kissing balloon techniques. The effective or usable length of standard balloon catheters is usually 140 cm (for bypasses 150 cm). Guidewire length is normally 175 cm with the diameter standardised at 0.014 of an inch. Modern standard or “workhorse” balloons are semi-compliant with a balloon length of 1-3 cm and diameters between 2 and 4 mm at the nominal pressure (NP). Stents are minimally shorter than their corresponding delivery balloon and are deployed at the nominal or higher pressures. Below the rated burst pressure (RBP) the diameter can be moderately increased by about 0.05 mm per atm obviating the need for multiple upsizing and balloon exchanges ( Figure 5 ).
The balloon polymer has been changed several times since the original design and is typically now a nylon derivate. Different balloon materials, however, can be used (depending on the primary function of the device) for pressure dilatation, for stent delivery or for drug delivery. In order to reduce friction all parts have lubricious surfaces, which can also on occasion be a disadvantage as this feature can make a balloon more likely to slip in smooth lesions and induce a “geographic miss” (typically seen during angioplasty of in-stent restenosis).
Balloons with diameters between 2.5 and 3.5 mm are the usual working range sufficient for the majority of procedures.
Small balloons (diameters of 1-2 mm) with small tip shapes and smooth transition zones (between tip and balloon) can pass nearly all forms of stenoses, tortuous vessels and stents. They are used in large vessels for predilation before stent insertion, in tight stenoses or total occlusions and in distal vessels and side branches for pure balloon angioplasty. One exception is the hard, calcified or stent-strut-induced stenosis, which is better suited for rotational atherectomy (“rotablation’’). One important feature during the positioning of small balloons is that the dual radiopaque markers (seen at either end of a typical balloon) are replaced by a single centrally placed marker.
Larger balloons (diameters of 4 mm or greater) are mostly used as stent delivery balloons for direct stenting. The very largest diameters are typically reserved for atherosclerotically dilated (ectatic) segments and venous bypass grafts, the latter often in combination with embolic protection devices.
As a consequence of non-compliance, vessels of a certain diameter also require balloons of a predefined diameter. This necessitates the storage of a sufficient number of balloons and stents of various lengths and diameters.
European/US regulations have to be followed for the production, testing, certification (CE/FDA) and device specifications, which are supplied together with catheters [1414. . . International Standard ISO 10555-1 1995-06-15 (Part 4 balloon dilatation catheters). , 1515. . . International Standard ISO 25539-2 2008-09-01 (Part 2 vascular stents). ]. They are sometimes complemented by national regulations. The information is usually contained on the label and in the user information card which is included in the catheter packing. This relates to general topics such as lot number and sterilisation dates and to parameters for the procedural application as well as a pressure dimension chart. In small balloons (without stents) the tip profile is often stated instead of the usual crossing profile.
Information supplied with balloon catheters in Europe
- Pressure-diameter curves
- Balloon diameter
- Shaft diameter
- Effective catheter length (without hub)
- Expiry date or use by date
- Indication of sterility
- Lot designation
- Instructions for use and warnings
- SI units
- CE certification
The treatment of calcified lesions and intrastent restenosis remains a challenge for the interventionalist, and thereby still generates research for new balloon angioplasty technologies.
When conventional non-compliant balloons fail to achieve an adequate post-dilatation luminal gain after stent implantation, super high pressure balloons can be an effective alternative. The OPN NC® (SIS Medical AG, Winterhur, Switzerland) is a rapid-exchange catheter that incorporates a twin-layer balloon construction, which allows very high pressure inflations and ensures uniform expansion over a wide range of pressures. The balloon is therefore highly non-compliant with a RBP of 35 atm, and it has been used in several indications including the pre-dilatation of heavily resistant lesions, optimization of stent deployment and treatment of intrastent restenosis.
Another approach to prevent restenosis but also to open up calcified lesions is the concept of “focused force”. In an atherosclerotic lesion sharp blades of a Cutting balloon® (Boston Scientific, Natick, MA, USA) cut longitudinally into the plaque and thus create a controlled trauma instead of a large tear. Cutting balloon angioplasty was developed in the early 1990s and has been approved as a treatment modality for “high-pressure balloon resistant lesions” including de novo lesions and in-stent restenoses. The Cutting balloon features three or four atherotomes (microsurgical blades), which are 3-5 times sharper than conventional surgical blades. There is a unique design which protects the vessel and the balloon from the edges of the atherotomes when it is deflated. However, for prevention of restenosis in de novo lesions, Cutting balloon angioplasty in limited studies did not show itself to be superior to balloon angioplasty, but it may be favoured in in-stent restenosis [1616. Lee MS, Singh V, Nero TJ, Wilentz JR. Cutting balloon angioplasty. J Invasive Cardiol. 2002;14:552-6. ].
Instead of microblades, the latest balloon device designs include the Scoreflex™, AngioSculpt® and Grip™ balloons. The former consists of two opposite interdigitating wires on the balloon surface. A mobile guidewire and a second fixed wire are used to focus the force applied during expansion and thus break up hard and calcified lesions with acceptable pressures (Scoreflex™, OrbusNeich, Hong Kong, China). This design resembles a more common approach used by operators, namely to position, during inflation of a normal or high-pressure balloon, an additional guidewire (Acrostak, Zurich, Switzerland) next to the balloon within a resistant stenosis. The AngioSculpt® (Biotronik, Bülach, Switzerland) features a nitinol scoring element made of three blades wrapping around the balloon and focusing dilation forces. The Grip™ (Acrostak, Zurich, Switzerland) consists of a balloon with four lines of knobs which help anchor the balloon to the lesion during inflation.
Novel devices were introduced to decrease vessel trauma related to balloon angioplasty, which can be associated with acute complications following inflation and deflation of standard balloons. The Chocolate® (Medtronic, Fridley, MN, USA) is an over-the-wire balloon catheter characterized by a mounted nitinol-constraining structure over the balloon allowing uniform inflation and rapid deflation. Moreover, the nitinol structure creates small balloon segments (”pillows”) during balloon inflation, intended to reduce radial stress on arterial walls and minimize vessel trauma. The Chocolate® PTA Balloon Catheter demonstrated to be safe and effective in treating symptomatic peripheral arterial disease.
A new approach of vessel preparation in calcified lesions, lithoplasty, was recently developed. The Lithoplasty System® (Shockwave Medical, Fremont, CA, USA) uses unfocused acoustic pulse waves to disrupt both superficial and deep calcium within the target lesion. Built on an angioplasty balloon platform, the lithoplasty catheter incorporates multiples emitters that deliver circumferential pressure pulses through a low pressure inflated balloon (4 to 6 atm). The efficacy and safety of lithoplasty was evaluated in two studies. DISRUPT-PAD (Safety and Performance Study of the Shockwave Lithoplasty System) and DISRUPT-CAD (Shockwave Coronary Rx Lithoplasty Study) both demonstrated a low rate of major cardiovascular events within a short-term follow-up period and minimal adverse events following treatment of peripheral and coronary artery disease.
Balloon catheter parameters (typical measurements indicated, but these may vary with manufacturers)
- Balloon diameter (2-4 mm or more; low profile 0.85-1.5 mm)
- Balloon lengths (between 10 mm and 30 mm or more)
- Effective or usable catheter (length 140 cm)
- Proximal shaft diameter (2 French Monorail)
- Proximal shaft diameter (3 French OTW)
- Distal shaft diameter (2.5 French Monorail)
- Crossing profile
- Tip or catheter entry profile (0.017 inches)
- Nominal pressure (5-7 atm)
- Rated burst pressure (12-14 atm, high-pressure balloon 18 atm or more)
- Compliance (semi-compliant, high-pressure non-compliant)
Specific information and parameters concerning the dimension and function of balloon catheters are:
- Nominal pressure (NP): the pressure at which the balloon reaches its nominal diameter in a water bath during bench testing. The nominal pressure is usually the lowest pressure applied.
- Rated burst pressure (RBP): the maximum pressure at which 99.9% of the balloons will not burst. This statement is correct with a 95% confidence. The RBP is determined by performing a standardised test according to an FDA protocol.
- Compliance: a description of the incremental increase in balloon diameter corresponding to an incremental increase in inflation pressure.
- High-pressure balloon: indicative of a balloon with a high rated burst pressure (usually above 18 or 20 atm).
- Tip profile (or lesion entry profile): the diameter of the most distal part of the balloon catheter especially in small balloons designed for crossing very narrow stenoses.
- Crossing profile: the diameter of the folded balloon without or with stent as it comes packaged or before the balloon has been inflated.
- Secondary crossing profile: the (recross) diameter of a deflated balloon having been previously inflated, which is usually bigger than the “crossing profile”. The term “rewrap” also refers to the ability of the balloon to deflate to a small diameter for a small secondary crossing profile.
- Deflation time: the time needed to evacuate contrast and saline from an inflated balloon. Standard deflation times are in the range of a few seconds, but optimal deflation and infolding can take more than a minute of negative pressure, depending upon the size and length of a balloon.
The performance characteristics of balloon catheters are (with or without a stent) not specifically standardised but can be described thus:
- Trackability is the ability to negotiate vessel tortuosities and angulations. It depends on the friction (coating) of all catheter parts, on the shape, profile, and flexibility of the catheter tip, the crossing profile and flexibility of the balloon and stent, and on the guidewire and guide catheter friction and support.
- Pushability is the ability of a balloon catheter to transmit the proximally applied force to its distal tip. Catheters can lose pushability in tortuous anatomy by increased contact, friction and buckling. The pushability also depends on the stiffness and friction of the shaft, guidewire and supporting guide catheter.
- Crossability describes the ease with which a balloon can traverse a lesion, especially if the lesion is very narrow, calcified or situated in an unfavourable, e.g., angled coronary segment. The crossability depends on the crossing profile and flexibility of the balloon, the construction and surface friction of the tip and the pushability.
- The ease required to pull back a used (inflated and then deflated) balloon catheter into the guiding catheter depends on the secondary crossing profile and flexibility of the balloon, and largely on properties favourable for trackability and pushability.
Clinical studies regarding the clinical effectiveness of catheter devices are not yet generally required. The majority of catheters in the catheter laboratory are Monorail catheters with balloons carrying BMS or DES in multiple configurations.
PRESSURE DEVICES (INDEFLATORS)
Balloon pressures of up to and greater than 20 atm can be generated by handheld devices: these consist of a hand grip, syringe, pressure lock and pressure scale. The syringe is filled with a mixture of saline and contrast medium in equal proportion (1:1 mixture). Higher concentrations will increase the viscosity and the balloon deflation time. Lower concentrations decrease deflation time but also the visibility of the inflated balloon. These devices can be used to inflate or deflate the balloon and are thus frequently referred to as “indeflators”.
Units (Data may vary depending on manufacturers or product lines)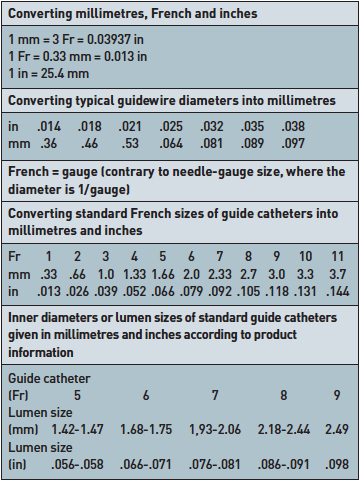
How to use balloon catheter systems
INTRODUCTORY REMARKS
Modern equipment allows operators to perform simple procedures correctly in a few minutes. This furthers the ability to “tackle” ever more complicated lesions, where unlimited combinations of guiding catheters, balloon catheters with or without stents, guidewires and other devices can be used. The use of balloons with and without stents is similar for approaches from the femoral or radial arteries and for native or bypass vessels (where they are typically combined with distal protection devices) (  View chapter ).
View chapter ).
To help the operator with PCI indications and the use of balloons and stents, numerous evidence-based guidelines have been compiled in European or North American interventional societies [1717. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, Morrison DA, O’Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; ACC/AHA Task Force on Practice Guidelines; ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2006;47:e1-121. Combined guideline updates for percutaneous coronary intervention of the American College of Cardiology (ACC), the American Heart Association (AHA) and the Society for Cardiac Angiography and Interventions (SCAI) for 2005 (121 pages). , 1818. King SB 3rd, Aversano T, Ballard WL, Beekman RH 3rd, Cowley MJ, Ellis SG, Faxon DP, Hannan EL, Hirshfeld JW Jr, Jacobs AK, Kellett MA Jr, Kimmel SE, Landzberg JS, McKeever LS, Moscucci M, Pomerantz RM, Smith KM, Vetrovec GW, Creager MA, Hirshfeld JW Jr, Holmes DR Jr, Newby LK, Weitz HH, Merli G, Piña I, Rodgers GP, Tracy CM; American College of Cardiology Foundation; American Heart Association; American College of Physicians Task Force on Clinical Competence and Training (writing Committee to Update the 1998 Clinical Competence Statement on Recommendations for the Assessment and Maintenance of Proficiency in Coronary Interventional Procedures). ACCF/AHA/SCAI 2007 update of the clinical competence statement on cardiac interventional procedures: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training (writing Committee to Update the 1998 Clinical Competence Statement on Recommendations for the Assessment and Maintenance of Proficiency in Coronary Interventional Procedures). J Am Coll Cardiol. 2007;50:82-108. Combined update of the clinical competence statement on cardiac interventional procedures from the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA) and the Society for Cardiac Angiography and Interventions (SCAI) from 2007 (26 pages). , 1919. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Flavio L, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization. Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2010;31:2501-55. Combined guidelines on myocardial revascularisation from the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) with the special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI) from 2010 (54 pages). ]. However, for most technical approaches randomised studies are not available or do not often lend themselves to solving the problems encountered by the operator. Thus, a combination of knowledge, education, institutional and course-related experience and consensus decisions play an important role in guiding contemporary good medical practice in PCI [1919. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Flavio L, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization. Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2010;31:2501-55. Combined guidelines on myocardial revascularisation from the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) with the special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI) from 2010 (54 pages). ].
GUIDE CATHETERS AND GUIDEWIRES  View chapter
View chapter
Before balloon catheter insertion, detailed coronary anatomy and stenosis characteristics should be studied and the strategy for PCI in relation to material selection and procedural sequence should be planned. In standard technical situations Monorail catheters are the systems of choice. For balloon and stent dilatation of simple lesions a 6 Fr guide catheter is favoured for contemporary practice; however, a 5 Fr catheter can be used as an alternative. After its placement, the selected guidewire (which can include a pressure wire if an FFR study is being conducted), is carefully advanced under fluoroscopy through the coronary artery and steered across the stenosis. Particularly in complicated high-grade stenoses, controlled wiring under contrast image guidance should be preferred over a “trial and error” crossing to avoid plaque disruption and the risk of acute closure. A variety of guidewires and supporting methods can be applied. After crossing the stenosis, the wire should be advanced to the distal segment of the main vessel. Vessel perforation is a potential hazard of hydrophilic wires and their use should be limited to specific situations.
For complex techniques (such as kissing balloons) and lesions, 6 Fr guide catheters (or larger) are mandatory. If used routinely from the beginning of the procedure, these catheters offer a wider range of therapeutic options without the need for the operator to exchange guide catheters over a long wire.
BALLOON CATHETERS (MONORAIL)
Balloon preparation
The purpose of balloon preparation is the evacuation of air from within the balloon chamber to obtain a small deflated profile, good visibility of the inflated balloon, and as a precaution against balloon rupture. If a balloon is prepared for the passage across a very tight stenosis or for a high-pressure dilatation in the range of the RBP or more, the greatest possible evacuation should be attempted. The smallest crossing profile is obtained when air is completely evacuated by suction from the folded balloon as it is supplied by the manufacturer. Any inflation of the balloon creates a larger second deflated (or crossing) profile. The balloon is best evacuated with an empty large (10-20 ml) plastic syringe, with which maximal negative suction can be applied. The balloon can then be advanced on negative pressure across the stenosis. Only for actual dilatation itself is the balloon filled with a saline and contrast mixture. The inflation pressure must be observed carefully on the scale and must usually be kept between NP and RBP. Air trapped in the balloon may create a major complication when a balloon ruptures, for a significant volume of air can be delivered from an indeflator into the coronary system.
Predilatation
The method of dilatation, whether direct stenting or predilatation, can be planned in advance but can also be modified by information derived from wiring. If predilatation is intended, normally a balloon longer than the stenosis is selected, mounted on the guidewire and advanced across the stenosis. This is commonly a balloon 0.5 mm smaller than the reference vessel diameter, inflated in the stenosis only for a few seconds at high pressure. This aims to create a sufficient lumen for subsequent stent passage. The lumen created by a lower profile balloon (<2.0 mm diameter) frequently has to be dilated further for a stent passage. In a vessel of 3 mm or larger, a smaller balloon helps to avoid dissection and will not traumatise the pre- and post-stenotic segments. However, in calcified lesions predilatation with a larger balloon or a non-compliant balloon is mandatory to guarantee a complete opening of the stenosis before a stent is deployed. This can also be done with a cutting or focused force balloon. The length of the predilating balloon has to be adjusted carefully to the length of the intended stent.
Balloon deflation
The deflation of the balloon, which normally takes a few seconds, is much longer when a second deflated profile is obtained or when the balloon is large and/or long. Allowing for full deflation is especially necessary when the balloon has to be retracted through stent struts or heavily calcified stenoses and tortuous vessels or when the balloon is trapped within an insufficiently expanded stent. In the latter case, a repeat and extended inflation with higher pressure may solve the problem. Sometimes it is helpful to push the deflated balloon distally before retracting. If excessive force is applied, either deep engagement of the guide catheter can result in causing inadvertent dissection or, in extreme cases, balloon fragments may shear off the catheter shaft and have to be recovered surgically.
Balloon exchange
When the guidewire is in a stable position with its tip distal to the stenosis and its proximal end is secured, the deflated balloon is gently retracted. Fluoroscopy is often applied to observe the balloon fold in and leave the stenosis but it is not needed during balloon passage of the guide catheter. Advancing a balloon is performed in a similar way. However, large deflated balloons, when pushed forward, can suck air into the guide catheter, mainly if they are in 5 Fr catheters. To avoid air embolus, back bleeding out of the open valve connected with the proximal guide catheter entry should occur before any flushing or contrast injection is started.
Post-dilatation
After stent implantation, particularly in calcified lesions, post-dilatation should be performed to ensure an optimal stent expansion and prevent stent malapposition. Non-compliant high-pressure balloons are the preferred option with a 1:1 ratio according to the coronary artery diameter. In case of a fibrocalcific lesion with significant residual waist, high-pressure NC balloons (up to 35 atm) can be utilised, e.g., OPN NC® (SIS Medical, Winterthur, Switzerland). Post-dilatation balloons are usually selected shorter than the stent intended to be optimised to prevent edge dissections.
Plain balloon angioplasty
Plain balloons are predominantly used for pre- and post-dilatation of normally sized native vessels and of stent stenoses and for final dilatation of small side branches in bifurcation lesions and in distal vessels. Other situations for plain balloons, usually with the OTW technique, are recanalisations of total occlusions, tortuous passages and septal ablation.
Kissing balloon inflation
Final kissing balloon technique (FKB) is strongly recommended when treating bifurcation lesions, particularly with a two-stent technique. Two balloons are inflated simultaneously to achieve an adequate stent apposition, particularly at the ostium of the side branch and the opposite wall of the main branch. FKB should be carried out with balloons selected according to the diameters of the side branch and the distal main branch. These balloons should be short enough to remain within stents and avoid proximal or distal dissections. Non-compliant balloons may allow better in-stent deployment and limit the increase in the diameter of the balloon inflated at high pressure in the SB. Non-compliant balloons can be inflated alternately at high pressure then simultaneously at nominal pressure. This technique seems associated in vitro with homogeneous and optimal stent expansion.
OVER-THE-WIRE BALLOON CATHETERS (OTW)
Nowadays, the use of OTW balloons is restricted to special situations or instruments (see earlier text). Often the combined wire and catheter are advanced, while in some instances the wire is advanced first as described with the Monorail technique. The wire can be a long wire or a short wire prolonged with a docking extension wire. When the catheter tip is situated in the coronary vessel, the wire can be temporarily removed for the injection of dye or drugs through the guidewire lumen or for a wire exchange. During exchange procedures of the catheter, fluoroscopy is required to help safeguard that the wire tip remains distal to the stenosis. Delicate handling is paramount especially in stressful clinical situations. OTW catheters may provide an advantage in the recanalisation of thrombotic or chronic occlusions (  View chapter ).
View chapter ).
With OTW systems the guidewire can be lengthened with an extension wire connected firmly to the proximal end of the normal guidewire (known as a docking extension). For the exchange, an assistant has to hold this 3 m wire straight, while the main operator carefully retracts the catheter. The position of the wire tip distal to the stenosis has to be controlled with the aid of fluoroscopy. If this procedure is performed frequently, all operators are well trained; if not, typical, potentially hazardous, risks are disconnecting the extension wire and inadvertently losing wire position and retracting the wire tip proximal to the stenosis ( Figure 6 ). Instead of using a docking extension many operators use an indeflator and “saline flush method” to “blow off” the OTW balloon system (  View chapter ). To do this the balloon is withdrawn until its proximal hub is at the limit of the guidewire. The O-ring of the Tuohy connector is opened. An indeflator is then attached and inflated to 20 atm. This action propels the balloon system, which must be safely within the guide catheter, backwards and out of the O-ring without disturbing the position of the intra-coronary guidewire.
View chapter ). To do this the balloon is withdrawn until its proximal hub is at the limit of the guidewire. The O-ring of the Tuohy connector is opened. An indeflator is then attached and inflated to 20 atm. This action propels the balloon system, which must be safely within the guide catheter, backwards and out of the O-ring without disturbing the position of the intra-coronary guidewire.
Another option for OTW balloon exchange is the trapping balloon technique. The OTW balloon is pulled back in the guiding catheter within the ascending aorta. A standard rapid exchange balloon is then advanced beside the OTW one, and placed more distally but remaining within the guiding catheter. This balloon is then inflated at nominal pressure to trap the guidewire against the inner wall of the guiding catheter while safely withdrawing the OTW balloon. The trapping balloon technique is conceivable if the GC is large enough to accommodate simultaneously an Rx and an OTW balloon.
Advantageous applications of OTW technology
- Total occlusion or tortuosity passage
- Distal wire exchange
- Distal drug or contrast medium injection
- Rotablation
- Septal ablation
Obtaining a good result
EFFECTS OF DILATATION
The radial pressure-driven expansion of the balloon breaks up and reshapes plaques and injures the intima and media. The effected tears prevent some of the elastic recoil of the first minutes. By dilation, the outer circumference of the vessel is enlarged, but the adventitia is normally not injured. Parts of the altered vessel wall may “glue” together in a new arrangement and the stenosis remains dilated. The usually small residual stenosis is haemodynamically insignificant; however, it is unstable and can provoke acute vessel closure up to 48 hours, rarely later, as well as restenosis over the long term. The balloon can be selected slightly larger than the vessel, but should not be oversized (>1.1:1 balloon:artery ratio) to avoid significant mismatch with the attendant risk of extensive coronary dissection or vessel rupture. At the shoulders of the balloon, predominantly in atheromatous vessels, a snow-plough effect may result in plaque shift creating a new collateral stenosis or side branch occlusion. Stents stabilise the disrupted plaque or vessel wall, prevent elastic recoil and thus both improve the immediate dilation result and reduce the restenosis rate (  View chapter ).
View chapter ).
ANGIOGRAPHIC EVALUATION
With modern catheter and x-ray equipment, PCI results are normally evaluated visually or by digital analysis (i.e., without additional catheter-based tools). A first result may be observed by contrast injections with the deflated balloon within the deployed stent. For a final assessment, the balloon can be moved into the proximal part of the dilated vessel or into the straight part of the guide catheter shaft, or can even be removed completely. However, upon contrast injection, the balloon should never remain in the distal guide catheter opening where the resultant jet may cause plaque dissection. The vessel lumen has to be filled completely to outline clearly the contour of the lumen. For a good angiographic result the vessel wall and stent show a smooth transition without a step up or step down contour. Contour mismatch may result frequently from increased coronary vascular tone or spasm, especially adjacent to the stent, and is usually resolved after intracoronary nitroglycerine injections. Ultrasound imaging of vessel wall and plaque can be used to judge the final result and may be useful in particular situations (  View chapter and
View chapter and  View chapter ). Dissection flaps, in particular upstream flaps, plaque shift and residual stenoses in neighbouring segments may also lead to additional stenting but should not automatically generate an unnecessary and potentially deleterious extension of the procedure. At least two projections are required for a final judgement. In complex or unclear situations it is strongly recommended to seek the opinion of a second experienced operator.
View chapter ). Dissection flaps, in particular upstream flaps, plaque shift and residual stenoses in neighbouring segments may also lead to additional stenting but should not automatically generate an unnecessary and potentially deleterious extension of the procedure. At least two projections are required for a final judgement. In complex or unclear situations it is strongly recommended to seek the opinion of a second experienced operator.
DEFINING SUCCESS
Angiographic success
With plain balloon angioplasty, angiographic success depended largely on a non-complex stenosis anatomy. Today, with the advent of stents, only “high-risk” type C lesions are important determinants for obtaining a satisfying short and long-term result [1717. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, Morrison DA, O’Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; ACC/AHA Task Force on Practice Guidelines; ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2006;47:e1-121. Combined guideline updates for percutaneous coronary intervention of the American College of Cardiology (ACC), the American Heart Association (AHA) and the Society for Cardiac Angiography and Interventions (SCAI) for 2005 (121 pages). ]. With plain balloon dilatation, “angiographic success” was originally defined as a diameter reduction of at least 20%, with a final stenosis diameter of less than 50% of the vessel diameter; but nowadays, with stents, a final diameter of 20% or less has been the benchmark of an optimal result (in some studies 30% has been used). Success also includes a normal distal opacification of TIMI grade 3. Rates of angiographic success in stenoses have been 72%-74% with balloon only angioplasty. They are now 82%-98% with stents, and are comparable in stable CAD and in ACS [1919. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Flavio L, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization. Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2010;31:2501-55. Combined guidelines on myocardial revascularisation from the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) with the special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI) from 2010 (54 pages). ] ( Figure 7 ).
Procedural success
This is defined as angiographic success achieved without major complications or adverse cardiac and cerebrovascular events (MACCE) during hospitalisation [2020. Williams DO, Holubkov R, Yeh W, Bouhassa M, Al-Bassam M, Block P, Coady P, Cohen H, Cowley M, Dorros G, Faxon D, Holmes D, Jacobs A, Kelsey S, King S, Myler R, Slater J, Stanek V, Vlachos H, Detre K. Percutaneous coronary intervention in the current era compared with 1985-1986: the National Heart, Lung, and Blood Institute Registries. Circulation. 2000;102:2945-51. ]. For details of in-hospital complications,  View chapter .
View chapter .
Clinical success
This definition varies depending on the specified time period observed after the procedure. A 30-day observation period is reasonable for the description of a “short-term clinical success” which includes the angiographic and procedural success and the relief of symptoms in the absence of adverse effects. For a long-term clinical success the additional parameter of freedom from clinical restenosis (defined as persistent absence of symptoms) is the minimum requirement. In other studies additional non-invasive or invasive parameters have been used [1717. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, Morrison DA, O’Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; ACC/AHA Task Force on Practice Guidelines; ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2006;47:e1-121. Combined guideline updates for percutaneous coronary intervention of the American College of Cardiology (ACC), the American Heart Association (AHA) and the Society for Cardiac Angiography and Interventions (SCAI) for 2005 (121 pages). ].
Restenosis
Restenosis rates are lower when stents are applied as compared to balloons alone, however there is no proof of a reduced mortality. Restenosis rates are also lower with DES as compared to BMS (  View chapter ).
View chapter ).
For a general orientation restenosis rates after six months are for pure balloons between 20-50% (in complicated stenoses), for BMS 15-30% and for DES 5-20%. These numbers are derived from multiple studies in a wide spectrum of clinical and angiographic situations (  View chapter and
View chapter and  View chapter ) [1717. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, Morrison DA, O’Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; ACC/AHA Task Force on Practice Guidelines; ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2006;47:e1-121. Combined guideline updates for percutaneous coronary intervention of the American College of Cardiology (ACC), the American Heart Association (AHA) and the Society for Cardiac Angiography and Interventions (SCAI) for 2005 (121 pages). , 1919. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Flavio L, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization. Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2010;31:2501-55. Combined guidelines on myocardial revascularisation from the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) with the special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI) from 2010 (54 pages). , 2121. Brophy JM, Belisle P, Joseph L. Evidence for use of coronary stents. A hierarchical bayesian meta-analysis. Ann Intern Med. 2003;138:777-86. , 2222. Al Suwaidi J, Holmes DRJr, Salam AM, Lennon R, Berger PB. Impact of coronary artery stents on mortality and nonfatal myocardial infarction: meta-analysis of randomized trials comparing a strategy of routine stenting with that of balloon angioplasty. Am Heart J. 2004;147:815-22. ]. The results from BMS have also improved with new materials and ultra-low strut profiles.
View chapter ) [1717. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, Morrison DA, O’Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; ACC/AHA Task Force on Practice Guidelines; ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2006;47:e1-121. Combined guideline updates for percutaneous coronary intervention of the American College of Cardiology (ACC), the American Heart Association (AHA) and the Society for Cardiac Angiography and Interventions (SCAI) for 2005 (121 pages). , 1919. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Flavio L, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization. Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2010;31:2501-55. Combined guidelines on myocardial revascularisation from the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) with the special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI) from 2010 (54 pages). , 2121. Brophy JM, Belisle P, Joseph L. Evidence for use of coronary stents. A hierarchical bayesian meta-analysis. Ann Intern Med. 2003;138:777-86. , 2222. Al Suwaidi J, Holmes DRJr, Salam AM, Lennon R, Berger PB. Impact of coronary artery stents on mortality and nonfatal myocardial infarction: meta-analysis of randomized trials comparing a strategy of routine stenting with that of balloon angioplasty. Am Heart J. 2004;147:815-22. ]. The results from BMS have also improved with new materials and ultra-low strut profiles.
Adverse effects of balloon catheter systems
This section deals with technical adverse effects and complications connected with balloons and their application. For more detailed information, rare complications and their therapy,  View chapter and
View chapter and  View chapter. It should also be kept in mind that complications may be related to problems in indication, informed consent and in the underuse or overuse of the available equipment.
View chapter. It should also be kept in mind that complications may be related to problems in indication, informed consent and in the underuse or overuse of the available equipment.
BALLOON RUPTURE
Balloon rupture is the most typical balloon-related adverse event. Complications may derive from vessel perforation, air embolus or trapped balloon flaps. Rupture may occur with normal pressures, when calcium spicules are cutting into the balloon membrane, with high pressure in the range of or beyond the RBP, and rarely in defective balloons. Since fluid is not compressible, balloon rupture is accompanied by an immediate pressure drop; however, compressed residual air within the balloon will expand producing a high energy jet. Rupture at the proximal and distal balloon bonding sites generates a longitudinally propagated force which will rarely cause damage. More often, the balloon membrane ruptures at its largest circumference, resulting in a radial jet, which can severely injure the vessel wall, leading to cardiac tamponade, characterised by rapidly progressive hypotension and shock. A rupture can first be recognised from the pressure drop indicated on the indeflator scale or from irregular contrast flow coming from the balloon and visible on fluoroscopy. Immediate pericardiocentesis is the treatment of choice for tamponade. In some instances, small micro-holes in the membrane allow for a discrete jet to perforate the wall. This may initially remain clinically unrecognised, with a delayed haemopericardium presenting after several hours. Similar to guidewire perforations, this risk is increased in the presence of intensified antithrombotic therapy. Late pressure loss therefore should prompt the exclusion of a pericardial effusion by echocardiography. In patients with previous heart surgery, pericardial adhesion can also prevent major blood exit into a haemopericardium and thus modify the clinical presentation.
Air embolus is usually mild when only one or two millilitres are released from within the balloon chamber but sometimes it is not recognised on the spot when major amounts of air from within the indeflator are injected. Retrieving ruptured balloons may be challenging ( Figure 8 ).
BALLOON TRAPPING
Balloon trapping may occur in calcified lesions and stent struts and can result in membrane fragments shearing off or in a break of the complete balloon part of the catheter. One possible reason is incomplete deflation or folding of the balloon with a large “second” deflated profile. Complete deflation and optimal visualisation are the first measures to take, followed by various gentle manipulations. In some instances surgical removal may be required.
VESSEL OCCLUSION
Vessel occlusion during the attempted first passage of the balloon, more often of the guidewire, can trigger vessel closure with the attendant risks of myocardial infarction, ventricular arrhythmia and cardiac death. This is one of the few serious complications in coronary angioplasty that cannot be avoided entirely. Precautions include careful handling of all equipment, an adequate balloon choice, the use of small well-prepared balloons (see above), sufficient guide catheter support and adequate antithrombotic therapy.
CORONARY DISSECTION AND PERFORATION
Balloon-induced dissection can occur during dilatation of a plaque, during balloon passage within the proximal vessel segment, and can be induced by deep guide catheter engagement into a coronary ostium when the balloon is retracted. Rarer causes of dissection include the rapid retraction of an insufficiently deflated balloon with sharp polymer edges, or a high-pressure contrast jet exiting a ruptured balloon as generated by an indeflator. This may also happen upon manual injection, when a deflated balloon is partly occluding the guide catheter ostium creating a more focused jet. The last form of dissection can initially remain unrecognised, particularly in the left main stem, owing to the dissection flap being splinted by the guide catheter with serious consequences upon disengagement.
Longitudinal intimal dissection can be induced after dilatation and even after stent deployment, particularly in the proximal right coronary artery. If any such situation is suspected, it is essential to keep the guidewire in place for a while and repeat angiography, e.g., after 10 min.
Intimal dissection and perforation are also encountered in CTO recanalisation procedures. Precautions here involve taking special care that a balloon is not inflated in the false lumen. The incidence of perforation in this subset of balloon angioplasty has been reported variably to vary between 0.10% and 1.14% [1717. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, Morrison DA, O’Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; ACC/AHA Task Force on Practice Guidelines; ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2006;47:e1-121. Combined guideline updates for percutaneous coronary intervention of the American College of Cardiology (ACC), the American Heart Association (AHA) and the Society for Cardiac Angiography and Interventions (SCAI) for 2005 (121 pages). , 2323. Ellis SG, Ajluni S, Arnold AZ, Popma JJ, Bittl JA, Eigler NL, Cowley MJ, Raymond RE, Safian RD, Whitlow PL. Increased coronary perforation in the new device era: incidence, classification, management, and outcome. Circulation. 1994;90:2725-30. ]. For further details,  View chapter .
View chapter .
EMBOLISATION
Distal coronary embolisations of large thrombi can occur with balloons and stents, especially in degenerated vein grafts. Shifting of large thrombi with the balloon into large side branches can be immediately life-threatening, especially when LAD and LCx are occluded. The use of balloons and stents in combination with distal protection devices in venous bypass grafts follows specific rules (  View chapter). Microembolisation in native vessels goes mostly unrecognised but is a major topic of research.
View chapter). Microembolisation in native vessels goes mostly unrecognised but is a major topic of research.
Another threat is systemic embolisation of material extracted from coronary arteries, especially when they are filled with loose arterosclerotic or thrombotic material (or out of the aorta)
(  View chapter ).
View chapter ).
Possible balloon-related complications
- Plaque rupture/dissection
- Stent loss
- Vessel occlusion/thrombus
- Balloon rupture
- Balloon trapping
- Balloon shearing off
- Coronary embolilsation
- Systemic embolisation
Conclusions
Balloon angioplasty is the established basis for intracoronary therapy. Plaque ablation or debulking techniques have not been found to be superior or even equivalent to balloon dilatation in the majority of lesion and patient subsets. Instead, new solutions have been developed for the acute and long-term stabilisation of the mechanical balloon effects, namely stents with or without antiproliferative drugs. The large variety of possible approaches to complicated stenoses has been significantly facilitated by the Monorail catheter technique. For the procedural steps, extensive practical experience has been accumulated which can best be utilised through continuous learning from live courses, scientific meetings and multimedia resources.
Personal perspective – Tassilo Bonzel
Ever since the beginning of coronary interventions, it is impressive to see that balloon angioplasty, established for more than 30 years now, remains as yet unchallenged. It is also impressive that the technique required to use balloon catheters similarly remains unchanged. However, the number and variety of different procedural options can sometimes be overlooked and thus more difficult to apply critically to an individual clinical problem. Evidence-based medicine is, and will always be, the unassailable foundation for therapeutic guidelines. For individual recommendations and practical approaches, however, as recommended in recent guidelines [1919. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Flavio L, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization. Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2010;31:2501-55. Combined guidelines on myocardial revascularisation from the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) with the special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI) from 2010 (54 pages). , 2323. Ellis SG, Ajluni S, Arnold AZ, Popma JJ, Bittl JA, Eigler NL, Cowley MJ, Raymond RE, Safian RD, Whitlow PL. Increased coronary perforation in the new device era: incidence, classification, management, and outcome. Circulation. 1994;90:2725-30. ], consensus decisions via a multidisciplinary heart team, and not singular opinions, have to gain wider acceptance.