Summary
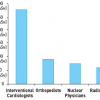
Fluoroscopically-guided diagnosis and intervention amongst adult cardiology patients account for 12% of all radiological examinations performed, and 48% of their total collective dose. On average, a diagnostic invasive coronary angiogram corresponds to a radiation exposure for the patient of about 350 chest x-rays (range 100-800), while coronary intervention and cardiac radiofrequency ablation correspond to 750 chest x-rays (range 350-2,650). Interventional cardiologists have an exposure per annum two to three times higher than that of radiologists. This equates to an annual exposure of 250 chest x-rays per practitioner each year. A reduction in occupational doses by a factor of ten can be achieved simply by an intensive training programme. European law mandates that it is the responsibility of all physicians to minimise the hazard of radiation injury to their patients, to other staff and to themselves. This is further reinforced by professional guidelines. A well-trained interventional cardiologist should not be afraid of radiation, but must fear, instead, radiation “unawareness”.
Radiation in context
Radiation is an essential part of daily life. From birth, we are exposed to radiation from cosmic rays in our surroundings as well as from food and drink that contain traces of radioactivity. In fact, even the human body contains small amounts of radioactivity (in the form of radioisotopes of potassium, caesium and radium). Ionising radiation used in medical practice in the form of x-rays is similar in nature to the radiation (gamma radiation) to which we are exposed to in daily life.
Radiation in context
- X-rays are a proven carcinogen
- Effective dose from medical radiation is 150 chest x-rays per head per year
- In cardiology patients, about 50% of the cumulative dose is from invasive cardiology
- Interventional cardiologists receive annual exposure 3-times higher than radiologists
- Unnecessary medical radiation exposure is an avoidable health burden
SOURCES OF RADIATION EXPOSURE
The use of ionising radiation in diagnostic examinations and interventional procedures is the largest man-made source of radiation exposure. The radiation dose received by the whole body is expressed in millisieverts (mSv) as a unit of dose quantity that is called the “effective dose”. Roughly 1 mSv of effective dose is equivalent to an exposure to 50 PA chest x-rays (although the precise figure varies with the technology employed and the technique used). Another way to understand the concept of radiation quantity is to compare it with the radiation that we receive from natural sources such as cosmic radiation, radiation within the earth, building materials, and food.
Global estimates of the average annual dose humans receive from natural sources is 2.4 mSv (with variation dependent on geographical location) ( Figure 1 ) [11. UNSCEAR 2008 Report: Sources and effects of ionizing radiation. Volume I. Annex A http://www.unscear.org/docs/reports/2008/09-86753_Report_2008_Annex_A.pdf and Annex B http://www.unscear.org/docs/reports/2008/09-86753_Report_2008_Annex_B.pdf. ]. At some sites, the average annual dose is in the range of 3 to 15 mSv, whereas in other locations it can be as low as 1 mSv. For the United States in 1987, the radiation dose received by the population from medical sources of radiation was about one fifth of that from natural radiation (3.0 mSv), whereas, by 2006, it was almost about equal to natural background radiation. Currently, it is seen to be rising at a rate of 10% per year [22. Picano E. Sustainability of medical imaging. BMJ. 2004;328:578-80.
Overview of medical imaging and radiation protection awareness. Medical radiation from x-rays and nuclear medicine is the largest man-made source of radiation exposure, yet observational data suggest doctors and patients are often unaware of long-term risks of this exposure.]. According to an UNSCEAR report, CT constitutes 5% of the frequency of radiological examinations and contributes 35% to the collective dose, whereas angiography and interventional procedures constitute around 1% in frequency, but contribute 10% to this collective dose [11. UNSCEAR 2008 Report: Sources and effects of ionizing radiation. Volume I. Annex A http://www.unscear.org/docs/reports/2008/09-86753_Report_2008_Annex_A.pdf and Annex B http://www.unscear.org/docs/reports/2008/09-86753_Report_2008_Annex_B.pdf. ]. For the USA population, in absolute terms, the contribution per caput dose per year from radiological procedures (cardiology and radiology) alone was 0.43 mSv (22 chest x-rays) in 2006 [33. Mettler FA Jr, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, Lipoti JA, Mahesh M, McCrohan JL, Stabin MG, Thomadsen BR, Yoshizumi TT. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources--1950-2007. Radiology. 2009;253:520-31. ].
Radiation is a proven carcinogen [44. BEIR VII Health Risks from Exposure to Low levels of Ionizing Radiation, Phase 2. http://dels-old.nas.edu/dels/rpt_briefs/beir_vii_final.pdf.
This landmark document of the National Research Council describes the best available estimates of risk associated with radiation exposure. It updates the previous 1990 document and increases estimates of cancer risk for any given dose.], and medical exposure to ionising radiation may elevate a person’s lifetime risk of developing cancer [55. White Paper: Initiative to Reduce Unnecessary Radiation Exposure from Medical Imaging. Food and Drug Administration. Feb. 9, 2010.
Consensus paper highlighting that every patient should receive an appropriate imaging exam, at an appropriate time, with the lowest appropriate radiation dose.]. The attributable risk of developing cancer following medical radiation exposure is estimated to be around 5% [66. Berrington de González A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 2004;363:345-51. , 77. Picano E. Risk of cancer from diagnostic X-rays. Lancet. 2004;363:1909-10. ], with an estimated 29,000 future cancers (2% of all cancers) in the USA related to CT scans alone [88. Berrington de González A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, Land C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-7. ]. A balanced public health approach seeks to support the benefits of these often life-saving medical imaging procedures while simultaneously minimising the risks [99. Food and Drug Administration. Feb. 9, 20 ].
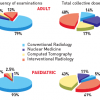
THE CONTRIBUTION OF INTERVENTIONAL CARDIOLOGY TO GLOBAL RADIOLOGICAL EXPOSURE
In the USA, cardiac catheterisation procedures increased from 2.45 million in 1993 to 3.41 million in 1997 and to 4.6 million in 2006. These now account for about 28% of the fluoroscopic procedures and 53% of the collective effective dose from all fluoroscopic-guided interventional procedures in healthcare. Interventional cardiology thus accounted for 0.23 mSv (12 chest x-rays) per head for the average citizen in the USA [33. Mettler FA Jr, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, Lipoti JA, Mahesh M, McCrohan JL, Stabin MG, Thomadsen BR, Yoshizumi TT. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources--1950-2007. Radiology. 2009;253:520-31. ]. In 2006 in Europe, the estimated number of coronary angiographic examinations per million of the population was just under 5,000, with over 2,300 PCI procedures per million and 918 pacemaker insertions per million [1010. 10. Faulkner K, Werduch A. An estimate of the collective dose to the European population from cardiac X-ray procedures. Br J Radiol. 2008;81:955-62. ]. In adult cardiology patients, interventional cardiology procedures accounted for 12% of the examinations, and 48% of their total collective dose [1111. Bedetti G, Botto N, Andreassi MG, Traino C, Vano E, Picano E. Cumulative patient effective dose in cardiology. Br J Radiol. 2008;81:699-705. ] ( Figure 2 ). For patients with acute myocardial infarctions undergoing diagnostic cardiac catheterisation and percutaneous coronary interventions, these procedures accounted for 67% of their average cumulative radiation exposure [1212. Kaul P, Medvedev S, Hohmann SF, Douglas P, Peterson ED, Patel MR Ionizing radiation exposure to patients admitted with acute myocardial infarction in the United States. Circulation. 2010;122:2160-69. ].
In children with congenital heart disease, invasive catheterisation (diagnostic and interventional) accounted for 6% of all radiological examinations and 84% of their collective dose in a study carried out in an Italian paediatric hospital [1313. Ait-Ali L, Andreassi MG, Foffa I, Spadoni I, Vano E, Picano E. Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease. Heart. 2010;96:269-74. ].
At the same time, the number of professional staff members exposed in the catheterisation laboratory as a part of their work continues to rise. According to 2008 UNSCEAR estimates, the number of occupationally exposed workers totals 22.8 million (excluding military personnel), and 7.35 million of these are medical workers [11. UNSCEAR 2008 Report: Sources and effects of ionizing radiation. Volume I. Annex A http://www.unscear.org/docs/reports/2008/09-86753_Report_2008_Annex_A.pdf and Annex B http://www.unscear.org/docs/reports/2008/09-86753_Report_2008_Annex_B.pdf. ]. Amongst the most affected are the cardiac catheterisation laboratory staff (physicians, nurses and technicians). For example, data from the Italian Tuscany region (n=5164) demonstrate that 10% of this monitored healthcare workforce (including all medical and surgical specialties) have high dosimetric values of exposure. Catheter laboratory personnel represented 31% having an exposure >1 mSv , and 67% of those with a yearly exposure > 6 mSv [1414. Venneri L, Rossi F, Botto N, Andreassi MG, Salcone N, Emad A, Lazzeri M, Gori C, Vano E, Picano E. Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: insights from the National Research Council’s Biological Effects of Ionizing Radiation VII Report. Am Heart J. 2009;157:118-24.
Occupational data describing that on the basis of contemporary dosimetry data, a high volume interventional cardiologist’s projected additional lifetime attributable risk for cancer is in the order of 1 in 100 at retirement.].
RECOMMENDATIONS OF REGULATORY BODIES AND SCIENTIFIC SOCIETIES
Advances in imaging technology have led to an explosive growth of cardiovascular imaging and interventional fluoroscopy. As pointed out by the European Commission in their referral guidelines for imaging (released in 2001 and updated in 2008), this poses questions of overuse or inappropriate use of new technologies, with the potential for harm, unnecessary waiting lists for other patients, and an increase in cost for the individual and society as well as the possiblity of poorer quality of care [1515. European Commission on Radiation Protection 118: Referral guidelines for imaging.
(last accessed 27 November 2010).]. In 2005, the ACC/AHA/ACP Task Force clearly specified that “responsibility is on all physicians to minimise the radiation injury hazard to their patient, to their professional staff, and to themselves” [1616. Hirshfeld, JW Jr, Balter S, Brinker JA, Kern MJ, Klein LW, Lindsay BD, Tommaso CL, Tracy CM, Wagner LK, Creager MA, Elnicki M, Lorell BH, Rodgers GP, Weitz HH. ACCF/AHA/HRS/SCAI clinical competence statement on physician knowledge to optimize patient safety and image quality in fluoroscopically guided invasive cardiovascular procedures: A Report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. Circulation. 2005;111:511-32.
Guidelines outlining the responsibility of all physicians to minimise the radiation injury hazard to their patients, to their professional staff and to themselves.]. This advice was repeated in April 2010 by the American President’s Cancer Panel, thus all possible actions should be taken by health care providers to minimise radiation exposure from medical sources, as radiation is recognised as one of the six major causes of environmental cancer. Radiation protection principles should be applied universally in clinical practice, especially where the dose and the risk are highest – such as in interventional cardiology. Practitioners have a considerable role to play in reducing the concentration of ionising radiation used in the catheterisation laboratory and “needlessly increase health care costs, cripple our Nation’s productivity and devastate our lives” [1717. President’s Cancer Panel: Environmentally caused cancers are “grossly underestimated” and “needlessly devastate American lives”.
Paper outlining how medical sources of radiation are amongst the top 6 major sources of environmental contaminants, and that actions must be taken to minimise radiation exposure.].
Physical and biological basis of radiation damage
PHYSICAL BASIS
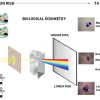
Medical examinations and procedures use x-rays or radioisotopes that mostly emit gamma rays. Radiation is said to be ionising when it strikes another object and displaces (“knocks off”) an electron from its orbital shell ( >link ). If radiation does not have enough energy to displace an electron, it is non-ionising. Ionising radiations (x-rays and gamma rays) are only one part of the electromagnetic spectrum ( Figure 3 ). X-rays being a form of electromagnetic radiation, have many characteristics similar to visible light, as well as some important differences attributable to their greater energy. Physically, all electromagnetic radiation such as x-rays or gamma rays consist of photons that have no charge and no mass, but are simply carriers of energy in the form of discrete packets described as quanta. An x-ray photon in the diagnostic range contains 5,000 to 75,000 times as much energy as a visible light photon [1717. President’s Cancer Panel: Environmentally caused cancers are “grossly underestimated” and “needlessly devastate American lives”.
Paper outlining how medical sources of radiation are amongst the top 6 major sources of environmental contaminants, and that actions must be taken to minimise radiation exposure.]. A stronger x-ray source produces more photons per unit time than does a weaker source. Radiation levels and doses can be characterised by using a number of different parameters.
Besides x-rays and gamma rays, there are a number of other forms of radiation in the electromagnetic spectrum (e.g., visible light, infrared waves, radio waves). These do not have the ability to ionise atoms. According to the general equation E = h∙υ, radiation energy (E) is directly proportional to the frequency (υ). It is only high-energy radiation that can cause ionisation of the cellular DNA and result in the production of free radicals. The use of high-energy radiation offers several diagnostic advantages, including the ability to penetrate body tissues and produce high-resolution images. Other technologies employed in cardiac imaging include magnetic resonance, which uses (low frequency) radio waves of the electromagnetic spectrum combined with the magnetic field, and ultrasound, which employs acoustic (mechanical) waves. Neither of these forms of physical energy is capable of producing ionisation.
BIOLOGICAL BASIS
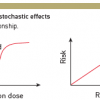
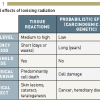
There are two main biological effects of radiation: tissue reactions (deterministic effects), which cause a very predictable change to tissue, and stochastic effects, which relate to the potential for future harm to the tissue and the body ( >link ).
Tissue reactions happen when the dose exceeds a specific threshold. Cataract formation is an example of such a tissue reaction. The severity of tissue reactions, rather than their probability of occurrence, is proportional to the dose imparted to the tissue ( Table 1 ).
Stochastic effects refer to the potential for cancer occurrence and owe their name to the random (stochastic) nature of radiation’s interaction with matter. Stochastic effects are thought to have no dose threshold for occurrence (the “linear, non-threshold theory”). Theoretically, a single mutation of the DNA may cause a carcinogenic effect. However, it is important to understand that many cells may undergo mutation and yet no cancer result due to cellular repair mechanisms. The probability of stochastic effects is considered to be proportional to the imparted dose, no matter how low that dose might be. This probability of occurrence is additive and proportional to the dose, whereas the severity of the ensuing cancer does not depend on the amount of imparted dose. Ionising radiation also has the potential to cause another type of stochastic effect called “hereditary anomalies”. Such effects have until now, however, not been observed to occur in humans, although they have been documented in non-human species ( >link ).
Tissue reactions such as skin erythema or cataract have a threshold dose below which the effect is not observed ( Figure 4 ). Some interventional procedures with long screening times and multiple image acquisition (e.g., percutaneous coronary intervention, radio-frequency ablation, etc.) may give rise to tissue reactions primarily in patients, but also in staff. A stochastic effect is a probabilistic event and there is no known threshold dose. Stochastic effects occur when the cell is modified by damage to its DNA but remains viable, the harm eventually being expressed through cell proliferation ( Figure 5 ). Carcinogenic effect, after a latency period of many years (2 to 10 years for leukaemia, 10 to 40 years for solid tumours), is the main stochastic effect that concerns us. The reduction of the risk of cancer is therefore at the core of the radiation protection system for staff and patients. At low levels of radiation exposure it is possible to predict the proportion of a given population of exposed persons who may be affected, but impossible to predict which particular individual will fall ill. The likelihood of inducing the effect, but not the severity, increases in relation to dose and may differ among individuals [1818. Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290-305. ].
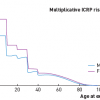
The current risk estimates are based on several authoritative reports: ICRP-103 [1919. The 2007 Recommendations of the International Commission on Radiological Protection. Publication 103. Ann ICRP. 2007;37:1-332. ], UNSCEAR 2008 [11. UNSCEAR 2008 Report: Sources and effects of ionizing radiation. Volume I. Annex A http://www.unscear.org/docs/reports/2008/09-86753_Report_2008_Annex_A.pdf and Annex B http://www.unscear.org/docs/reports/2008/09-86753_Report_2008_Annex_B.pdf. ] and the Seventh Report of the Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation (BEIR VII Report [Biological Effects of Ionising Radiation]) [44. BEIR VII Health Risks from Exposure to Low levels of Ionizing Radiation, Phase 2. http://dels-old.nas.edu/dels/rpt_briefs/beir_vii_final.pdf.
This landmark document of the National Research Council describes the best available estimates of risk associated with radiation exposure. It updates the previous 1990 document and increases estimates of cancer risk for any given dose.]. These are estimates based on a large number of studies, including 87,000 Hiroshima and Nagasaki atomic bomb survivors and 407,000 nuclear power plant workers. The epidemiological evidence linking increased cancer risk to radiation exposure is now considered conclusive for a dose level above 100 mSv of effective dose (which is sometimes reached by a patient in multiple examinations and procedures). ICRP and BEIR VII endorsed the linear no-threshold model, accepting that there is no safe dose and that the greater the dose, the higher the risk. For any given radiation exposure, the cancer risk is higher in females (by about 38%) than males at all ages, three to four-fold higher in children than adults, and about 50% less in an 80-year-old when compared to a 50-year-old person ( Figure 6 ) [2020. Picano E. Informed consent and communication of risk from radiological and nuclear medicine examinations: how to escape from a communication inferno. BMJ. 2004;329:849-51.
Overview of informed consent issues relating to interventional and diagnostic imaging procedures and their projected risks.]. Any subsequent radiation-induced cancer is clinically indistinguishable from a spontaneously occurring cancer.
Although the risk from medical radiological procedures is low, and certainly outweighed by the benefits if the procedure is appropriate, it is not negligible. If 100 subjects are exposed to 100 mSv (corresponding to 5,000 chest x-rays), 42 of these subjects may have a spontaneous cancer (independent of radiation exposure) while one may have a radiation-induced cancer. Of note these attributable risk estimates have a considerable two to three-fold margin of uncertainty, with a generally accepted range (95% confidence interval) between 1 in 30 to 1 in 300 ( Figure 7 ).
Several genetic, environmental and dietary variables can affect the variability of damage observed at any given level of radiation, and research is now shifting from epidemiological estimates to personalised measurements of DNA and chromosomal damage [2121. Adams FH, Norman A, Bass D, Oku G. Chromosome damage in infants and children after cardiac catheterization and angiocardiography. Pediatrics. 1978;2:312–316. , 2222. Andreassi MG, Ait-Ali L, Botto N, Manfredi S, Mottola G, Picano E. Cardiac catheterization and long-term chromosomal damage in children with congenital heart disease. Eur Heart J. 2006;27:2703-8. ]. Similarly there is a focus on identifying inter-individual differences that could modulate radiation risks in order to obtain better estimates of the extent of the differences [2323. Beels L, Bacher K, De Wolf D, Werbrouck J, Thierens H. gamma-H2AX foci as a biomarker for patient X-ray exposure in pediatric cardiac catheterization: are we underestimating radiation risks? Circulation. 2009;120:1903-9. , 2424. Andreassi MG, Cioppa A, Botto N, Joksic G, Manfredi S, Federici C, Ostojic M, Rubino P, Picano E. Somatic DNA damage in interventional cardiologists: a case-control study. FASEB J. 2005;19:998-9. ]. Radiation-associated chromosomal damage in interventional cardiologists is amplified by smoking and by genetic polymorphisms of genes involved in DNA repair [2525. Andreassi MG. Radiation risk from pediatric cardiac catheterization: friendly fire on children with congenital heart disease. Circulation. 2009;120:1847-9. ]. If the risk is personalised it will be possible to implement specifically tailored preventive and chemo-preventive strategies ( Figure 8 ).
Radiation doses and risks to patients
Average dose comparisons for common examinations (by equivalent chest x-rays)
- 250: diagnostic coronary angiography
- 500: abdominal arteriography
- 500-2500: coronary stenting or cardiac radiofrequency ablation
- >2500: dilation of chronic total occlusion of coronary artery
Exposure doses for high volume interventional cardiologist
- Average: 250 chest x-rays per operator per year (below apron)
- Left side > right side
- Reduced by 90% with proper protection policy
DOSES OF COMMON PROCEDURE
The effective dose for a radiological investigation provides a rough estimate of exposure that is related to the total radiation risk. This is the case no matter how the radiation dose is distributed around the body, and provided that the representative patients or patient populations from which the effective doses are derived are similar with regard to age and sex. Typical effective doses for common invasive cardiovascular procedures are reported in ( Table 2 ) [1717. President’s Cancer Panel: Environmentally caused cancers are “grossly underestimated” and “needlessly devastate American lives”.
Paper outlining how medical sources of radiation are amongst the top 6 major sources of environmental contaminants, and that actions must be taken to minimise radiation exposure., 2626. Gerber TC, Carr JJ, Arai AE, Dixon RL, Ferrari VA, Gomes AS, Heller GV, McCollough CH, McNitt-Gray MF, Mettler FA, Mieres JH, Morin RL, Yester MV. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009;119:1056-65.
A useful resource describing contemporary reference doses for common cardiology examinations., 2727. Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008; 248:254-63. , 2828. Picano E, Vañó E, Rehani MM, Cuocolo A, Mont L, Bodi V, Bar O, Maccia C, Pierard L, Sicari R, Plein S, Mahrholdt H, Lancellotti P, Knuuti J, Heidbuchel H, Di Mario C, Badano LP. The appropriate and justified use of medical radiation in cardiovascular imaging: a position document of the ESC Associations of Cardiovascular Imaging, Percutaneous Cardiovascular Interventions and Electrophysiology. Eur Heart J 2014; 35; 665–72. , 2929. Heidbuchel H, Wittkampf F, Vano E, Ernst S, Schilling R, Picano E, Mont L. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace. 2014, 16: 946-64. , 3030. Hirshfeld JW Jr, Ferrari VA, Bengel FM, Bergersen L, Chambers CE, Einstein AJ, Eisenberg MJ, Fogel MA, Gerber TC, Haines DE, Laskey WK, Limacher MC, Nichols KJ, Pryma DA, Raff GL, Rubin GD, Smith D, Stillman AE, Thomas SA, Tsai TT, Wagner LK, Wann LS. 2018 ACC/HRS/NASCI/SCAI/SCCT Expert Consensus Document on Optimal Use of Ionizing Radiation in Cardiovascular Imaging: Best Practices for Safety and Effectiveness: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018 Jun 19;71(24):e283-e351. , 3131. Bacher K, Bogaert E, Lapere R, De Wolf D, Thierens H. Patient-specific dose and radiation risk estimation in pediatric cardiac catheterization. Circulation. 2005;111:83-9. ].
The age and gender distribution of both catheter laboratory workers and that of the general population can be quite different from that of the population undergoing medical procedures using ionising radiation. Differences will also occur from one type of medical procedure to another, depending on the prevalence of those individuals with the medical condition being evaluated. Thus, risk assessment for medical use of radiation is best evaluated using appropriate risk factors for the individual tissues at risk, as well as for the age and sex distribution of the individuals undergoing the medical procedures [3232. Radiological Protection in Medicine. Publication 105. Ann ICRP. 2008;37:1-63. ].
Effective doses for some common interventional procedures and diagnostic examinations vary widely, on occasions by as much as a factor of 1,000. Some low dose examinations are equivalent to between 1 and 2 days of natural background radiation (e.g., 0.02 mSv for a chest radiography) whereas others are equivalent to several years of exposure (e.g., for abdominal CT). Even within the same procedural category, for instance, percutaneous coronary intervention, the dose varies by a factor of 10 [2828. Picano E, Vañó E, Rehani MM, Cuocolo A, Mont L, Bodi V, Bar O, Maccia C, Pierard L, Sicari R, Plein S, Mahrholdt H, Lancellotti P, Knuuti J, Heidbuchel H, Di Mario C, Badano LP. The appropriate and justified use of medical radiation in cardiovascular imaging: a position document of the ESC Associations of Cardiovascular Imaging, Percutaneous Cardiovascular Interventions and Electrophysiology. Eur Heart J 2014; 35; 665–72. ]. Therefore, the reporting of ranges for effective doses, rather than single values with decimal precision, better reflects the reality that definite quantitative doses for any examination are not really accurate. In interventional cardiology, radiation dose is estimated by two quantities, the dose area product (DAP), whose current name is kerma area product (KAP). This value is the absorbed dose to air (air kerma) multiplied by the x-ray beam cross-sectional area at the point of measurement, and is expressed in Gy cm2 [1616. Hirshfeld, JW Jr, Balter S, Brinker JA, Kern MJ, Klein LW, Lindsay BD, Tommaso CL, Tracy CM, Wagner LK, Creager MA, Elnicki M, Lorell BH, Rodgers GP, Weitz HH. ACCF/AHA/HRS/SCAI clinical competence statement on physician knowledge to optimize patient safety and image quality in fluoroscopically guided invasive cardiovascular procedures: A Report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. Circulation. 2005;111:511-32.
Guidelines outlining the responsibility of all physicians to minimise the radiation injury hazard to their patients, to their professional staff and to themselves.]. According to a clinical competence statement of the 2005 ACC/AHA, the “DAP delivery to a patient during a procedure is both a measure of stochastic risk and a potential quality indicator [1616. Hirshfeld, JW Jr, Balter S, Brinker JA, Kern MJ, Klein LW, Lindsay BD, Tommaso CL, Tracy CM, Wagner LK, Creager MA, Elnicki M, Lorell BH, Rodgers GP, Weitz HH. ACCF/AHA/HRS/SCAI clinical competence statement on physician knowledge to optimize patient safety and image quality in fluoroscopically guided invasive cardiovascular procedures: A Report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. Circulation. 2005;111:511-32.
Guidelines outlining the responsibility of all physicians to minimise the radiation injury hazard to their patients, to their professional staff and to themselves.]. Physicians should be made aware of the exposures they deliver to their patients and how they compare to established norms” [1717. President’s Cancer Panel: Environmentally caused cancers are “grossly underestimated” and “needlessly devastate American lives”.
Paper outlining how medical sources of radiation are amongst the top 6 major sources of environmental contaminants, and that actions must be taken to minimise radiation exposure.]. From DAP (a measure of the amount of radiation), the effective dose in mSv (used as a rough estimate for the risk) can be derived with a conversion factor (for adults): mSv = DAP x 0.183 [3232. Radiological Protection in Medicine. Publication 105. Ann ICRP. 2008;37:1-63. ]. In paediatric catheterisation, the conversion factor is higher and very dependent on the patient’s age: mSv = DAP x 3.7 for newborns; 1.9 for 1 year; 1.0 for 5 years; 0.6 for 10 years; 0.4 for 15 years [3333. Karambatsakidou A, Sahlgren B, Hansson B, Lidegran M, Fransson A. Effective dose conversion factors in paediatric interventional cardiology. Br J Radiol. 2009;82:748-55. ]. DAP readings may easily be included in patient records, could be correlated to staff doses, and may be useful for comparison with pre-established procedure specific reference values [3434. Bashore TM, Bates ER, Berger PB, Clark DA, Cusma JT, Dehmer GJ, Kern MJ, Laskey WK, O’Laughlin MP, Oesterle S, Popma JJ, O’Rourke RA, Abrams J, Bates ER, Brodie BR, Douglas PS, Gregoratos G, Hlatky MA, Hochman JS, Kaul S, Tracy CM, Waters DD, Winters WL Jr; American College of Cardiology. Task Force on Clinical Expert Consensus Documents. American College of Cardiology/Society for Cardiac Angiography and Interventions Clinical Expert Consensus Document on cardiac catheterization laboratory standards. A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:2170-214. ], introduced by ICRP as “Diagnostic Reference Levels” or reference levels [1919. The 2007 Recommendations of the International Commission on Radiological Protection. Publication 103. Ann ICRP. 2007;37:1-332. , 3131. Bacher K, Bogaert E, Lapere R, De Wolf D, Thierens H. Patient-specific dose and radiation risk estimation in pediatric cardiac catheterization. Circulation. 2005;111:83-9. ].
HOW TO MINIMISE PATIENT RISK IN THE CATHETERISATION LAB: THE OPTIMISATION PROCESS
Decreasing patient dose will result in a proportional decrease in scatter dose to the operator. Therefore, techniques that reduce patient dose will generally also reduce occupational dose. This is a “win-win” situation: operator and patient both benefit ( Table 3 ). Preventive action should start with awareness on the part of the director of the catheterisation laboratory, who should oversee the use of proper, well-maintained equipment, provide guidelines for the staff, monitor performance, give feedback, review problem cases and interact with radiation physicists [3535. Miller DL, Vañó E, Bartal G, Balter S, Dixon R, Padovani R, Schueler B, Cardella JF, de Baère T; Cardiovascular and Interventional Radiology Society of Europe; Society of Interventional Radiology. Occupational radiation protection in interventional radiology: a joint guideline of the Cardiovascular and Interventional Radiology Society of Europe and the Society of Interventional Radiology. Cardiovasc Intervent Radiol. 2010;33:230-9. ].
At a more technical level, it is important to minimise fluoroscopy time, and this can be accomplished efficiently by reviewing the last-image-hold for study or the archived fluoroscopy runs. Even more important is to minimise the number of cineangiographic images acquired, since radiation exposure rate with cine is 10 to 30 times higher than with fluoroscopy [3636. Vano E, Ubeda C, Fernandez JM, Sanchez RM, Prieto C. Dose assessment during the commissioning of flat detector imaging systems for cardiology. Radiat Prot Dosimetry. 2009;136:30-7. ]. For documentation, use stored last-image-hold images instead of acquiring additional images. It is also important to use good imaging-chain geometry, so that the patient is positioned as far as possible from the x-ray tube, and the image receptor is as close as possible to the patient. Incorrect positioning of the patient may contribute to a radiation injury. For example, inadvertent positioning of the arm over the x-ray source can lead to severe skin injury and muscle necrosis. The restriction of radiation beam size can be achieved by adjusting collimator blades tightly to the area of interest, reducing patient dose and improving image quality by reducing scatter. A useful purpose is served by the proper use of wedge filters, which cuts down radiation over a selected part in the field. One should use the smallest amount of magnification by choosing a larger field of view. It is also advisable to use available dose reduction technologies, including a low fluoroscopy dose rate option, low frame rate pulsed fluoroscopy, and removal of the anti-scatter grid for small patients in paediatrics. The logic behind low frame rate acquisition is simple. If only 15 instead of 30 x-ray pulses are produced per second, then the dose to the patient can effectively be halved. However, if only 15 images of the dynamics of motion are available per second, then the dynamics of motion are less well-defined and the image may appear to be more “sticky” or “jerky” due to loss of temporal resolution. However, 15 frames per second are adequate for many, if not most, parts of the procedure. High radiation dose procedures should be performed with fluoroscopic systems that incorporate recommended dose-reduction technology and comply with the most current International Electrotechnical Commission Standards. Institutions should be encouraged to purchase equipment that meets international or national standards for interventional laboratories.
In primary PCI it is commonly repeated that “time is muscle” – a timely intervention saves muscle. In the same way, for catheterisation laboratory workers, there must be a consideration that “radiation can cause cancer”. By so doing, both technology and culture can work together to minimise the exposure that is compatible with diagnostic levels of imaging.
From a strictly medical viewpoint, left ventriculography should be spared whenever possible so as to avoid the extra risk due to nephrotoxic contrast and radiation (2 to 3 mSv) [3232. Radiological Protection in Medicine. Publication 105. Ann ICRP. 2008;37:1-63. ]. The transfemoral approach is about 20% to 30% less radiation-expensive than the radial approach [3737. Brueck M, Bandorski D, Kramer W, Wieczorek M, Höltgen R, Tillmanns H. A randomized comparison of transradial versus transfemoral approach for coronary angiography and angioplasty. JACC Cardiovasc Interv. 2009; 2:1047-54. ]. Children are a special case as they are relatively more radiosensitive than adults and have a longer mean life expectancy. In addition, doses for some paediatric procedures per se are high. Patients with repaired congenital heart disease are a large and growing population, estimated to be 1 million in the USA by 2020. The long-term outcome of the underlying cardiac disease has been dramatically improved by interventions in the last decade, and now their excellent survival is the rule rather than the exception. Therefore, today when managing serious conditions such as a complex congenital heart disease, patients must be protected from risks that may manifest clinically years and even decades later. The x-ray equipment should be set up with specific programmes for paediatric cases. For small children, the grid should be removed and an air gap used to minimise image degradation due to scatter. The occupational doses to staff in general tend to be high compared with other radiological procedures. Although it is difficult to use satisfactory mobile screens when the patient is small, as they tend to obstruct access to the patient, every attempt should be made to heighten radiation awareness and training for all personnel, including anaesthetists, who may not be familiar with a radiation environment [3535. Miller DL, Vañó E, Bartal G, Balter S, Dixon R, Padovani R, Schueler B, Cardella JF, de Baère T; Cardiovascular and Interventional Radiology Society of Europe; Society of Interventional Radiology. Occupational radiation protection in interventional radiology: a joint guideline of the Cardiovascular and Interventional Radiology Society of Europe and the Society of Interventional Radiology. Cardiovasc Intervent Radiol. 2010;33:230-9. ].
BENEFITS OF USING DIAGNOSTIC REFERENCE LEVELS
The European Medical Exposures Directive considers interventional radiology and cardiology to be special practices that involve high doses to patients and require strict monitoring to ensure the best quality assurance programmes as well as quality control measures are followed. The Directive also states that member states of the European Union should promote the establishment and use of diagnostic reference levels for patient doses.
The DRLs have been shown to be a good tool to achieve optimisation. Although DRLs were initially defined for diagnostic examinations, there has been extension of their use to interventional procedures (IAEA Safety Report Series No. 59). ICRP has recently recommended the use of reference levels in interventional procedures. In Publication 105 [3131. Bacher K, Bogaert E, Lapere R, De Wolf D, Thierens H. Patient-specific dose and radiation risk estimation in pediatric cardiac catheterization. Circulation. 2005;111:83-9. ] it is stated: “For fluoroscopically guided interventional procedures, diagnostic reference levels, in principle, could be used to promote the management of patient doses with regard to avoiding unnecessary stochastic radiation risks. However, the observed distribution of patient doses is very wide, even for a specified protocol, because the duration and complexity of the fluoroscopic exposure for each performance of a procedure is strongly dependent on the individual clinical circumstances. A potential approach is to take into consideration not only the usual clinical and technical factors, but also the relative complexity of the procedure”.
The first step in optimising patient doses is to know what procedural doses are using audit. The second step is to know what dose constitutes good or optimised practice (by obtaining feedback from dose reference values specific for that examination). The next step is to investigate the procedures where there are ‘‘outliers’’. This whole process can only improve the quality of care delivered, and the safety of the cardiac catheterisation lab, and is in line with European legislation [3838. Vano E, Sanchez R, Fernandez JM, Gallego JJ, Verdu JF, de Garay MG, Azpiazu A, Segarra A, Hernandez MT, Canis M, Diaz F, Moreno F, Palmero J. Patient dose reference levels for interventional radiology: a national approach. Cardiovasc Intervent Radiol. 2009;32:19-24. ]. Finally optimisation at the individual patient level using well-known principles and approaches for radiation protection results in a radiation dose and exposure as low as reasonably achievable.
As an example, ( Figure 9 ) shows dose distribution for a lower limb arteriography. Reference values correspond to the third quartile. As suggested by the IAEA, patient doses reaching twice the reference levels should be considered the trigger value for any individual analysis as well as follow-up for potential skin injuries in therapeutic procedures [3838. Vano E, Sanchez R, Fernandez JM, Gallego JJ, Verdu JF, de Garay MG, Azpiazu A, Segarra A, Hernandez MT, Canis M, Diaz F, Moreno F, Palmero J. Patient dose reference levels for interventional radiology: a national approach. Cardiovasc Intervent Radiol. 2009;32:19-24. ].
TISSUE REACTIONS (DETERMINISTIC EFFECTS): SKIN INJURIES
Tissue reactions (deterministic effects) in cardiology may be observed during intensive fluoroscopic procedures. X-ray radiation generated during fluoroscopy is attenuated rapidly in tissue, so the maximum dose is delivered to the skin at the point the x-ray beam enters the patient. Only about 1% of the x-rays that enter a ‘‘23-cm-thick patient’’ actually emerge on the other side and produce the image at the detector.
Skin injury is deterministic in nature, as there is a threshold below which the effect is no longer observed. As the dose increases above the threshold, the severity of the effect increases rapidly. Guidance regarding threshold doses for tissue reactions on skin is presented in ( Table 4 ) along with the corresponding single radiation dose exposure [3939. Balter S, Hopewell JW, Miller DL, Wagner LK, Zelefsky MJ. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-41. ]. Patient skin injuries may occur when fluoroscopic procedures exceed 20 minutes using high-contrast fluoroscopy mode, or 60 minutes in low level fluoroscopy. This may happen typically in complex percutaneous coronary interventions and radiofrequency ablation (especially atrial fibrillation ablation and chronic total occlusion PCI). Tissue injury following fluoroscopic-guided procedures ( Figure 10 ) is often unrecognised as it occurs weeks after the procedure (see below).
In its Publication 85, the International Commission on Radiological Protection (ICRP) recommends that if there is any significant risk of radiation-induced injury, the patient should be warned and the patient’s personal physician (general practitioner) informed [3131. Bacher K, Bogaert E, Lapere R, De Wolf D, Thierens H. Patient-specific dose and radiation risk estimation in pediatric cardiac catheterization. Circulation. 2005;111:83-9. , 3636. Vano E, Ubeda C, Fernandez JM, Sanchez RM, Prieto C. Dose assessment during the commissioning of flat detector imaging systems for cardiology. Radiat Prot Dosimetry. 2009;136:30-7. ]. Patient doses therefore must be measured, recorded and acted upon if a high dose exposure has occurred.
Significantly, injuries are not limited solely to the use of older equipment, but can occur when poor technique is employed with newer and digital equipment capable of delivering higher doses. Furthermore, in contrast to the familiar thermal burn, which is associated with a recognisable source of heat and instantaneous pain, radiation burns remain asymptomatic and often go unrecognised [4040. Vlietstra RE, Wagner LK. X-ray burns--painful, protracted, and preventable. Clin Cardiol. 2008;31:145-7. ]. They usually occur on the patient’s back (where the x-rays are delivered) and since they develop several weeks after the procedure their association with cardiac interventions may not be considered, and many severe cases come to light only through litigation. A case is filed in the US courts every 4 to 5 weeks by patients who have suffered such injuries [4141. Rehani MM Srimahachota S. Skin injuries in interventional procedures. Radiation Protection Dosimetry 2011, pp. 1–5. ]. It is a legal requirement in almost every country around the world to report significant radiological incidents and accidents that occur during or as a direct result of using ionising radiation for a medical procedure. But in practice, however, such reporting hardly ever happens. Very few countries have a functioning reporting system because no one wants to be blamed for patients’ radiation related burns, hair loss or skin injury. However, this information is essential in order that lessons may be learned. The IAEA has set up its own international reporting system called SAFRAD (SAFety in RADiological procedures). Because the SAFRAD system is anonymous and the IAEA will not supply identifiable data to governmental authorities or other third parties, thus protecting practitioners from any legal repercussions or liability [4242. SAFRAD - SAFety in RADiological procedures. (http://rpop.iaea.org/safrad). ]. STOCHASTIC RISK: CANCER According to BEIR VII estimates the estimated attributable lifetime risk of cancer for exposed adult patients is 1 in 750 following a typical cardiac radiofrequency ablation or percutaneous coronary intervention procedure [44. BEIR VII Health Risks from Exposure to Low levels of Ionizing Radiation, Phase 2. http://dels-old.nas.edu/dels/rpt_briefs/beir_vii_final.pdf.
This landmark document of the National Research Council describes the best available estimates of risk associated with radiation exposure. It updates the previous 1990 document and increases estimates of cancer risk for any given dose.]. The range of estimated effective doses (7 to 51 mSv) translates into a range of risks (1/1,500 to 1/300, shown in ( Figure 11 )) due to the linear correlation between doses and risks.
The risks of radiation
- Deterministic (skin effects, from epilation in doctors to dermal necrosis in patients)
- Stochastic (cancer in doctors and patients)
- Stochastic, but traditionally considered deterministic (eye cataract in 50% of doctors)
Radiation doses and risks to healthcare workers
ANNUAL LIMITS AND ABSORBED DOSES: FROM DOSIMETRY TO mSv
The setting of occupational dose limits requires that national authorities address the question of what level of risk is deemed acceptable. Thus, the acceptability of any radiation risk is clearly also a political and social question. Setting dose limits too high could expose professionals to an unnecessary level of personal health risk, conversely setting dose limits too low may result in the misallocation of valuable resources for radiation protection purposes.
The current occupational effective dose limit, as recommended by the ICRP in their publication 103 (2007), is 20 mSv/year averaged over a 5 year period [1919. The 2007 Recommendations of the International Commission on Radiological Protection. Publication 103. Ann ICRP. 2007;37:1-332. ]. It is noteworthy that the recommended limits on annual occupational exposure have shown a trend for a continual decrease from the beginning of the century to the present day. The dose limit was 500 mSv in 1931, 50 mSv in 1958 and is now 100 mSv over any 5 year period, with a maximum of 50 mSv in any one year [1919. The 2007 Recommendations of the International Commission on Radiological Protection. Publication 103. Ann ICRP. 2007;37:1-332. ]. Interventional cardiologists according to current observations have an exposure per-operator per-year two to three times higher than that of radiologists ( Figure 12 ) [4343. Vañó E, González L, Guibelalde E, Fernández JM, Ten JI. Radiation exposure to medical staff in interventional and cardiac radiology. Br J Radiol. 1998;71:954-60. ]. The head and neck, the hands and the legs are the most exposed segments ( Figure 13 ) and this has a bearing on the exact estimation of dose using dosimetry. There are three basic approaches. A single dosimeter worn under the lead apron will yield a reasonable estimate of effective dose in most instances ( Figure 14 ). Wearing an additional dosimeter at the neck level, external to lead protection (as recommended by ICRP in its publication 85), provides an indication of head (eye) dose [3939. Balter S, Hopewell JW, Miller DL, Wagner LK, Zelefsky MJ. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-41. , 4444. NCRP report 122. Use of personal monitors to estimate effective dose equivalent and effective dose to workers for external exposure to low-LET radiation. Bethesda (MD): NRCP; 1995. ]. It is also possible to combine the two readings to provide an improved estimate of effective dose. It is recommended that interventional departments develop a two-dosimeter policy. Department doses should be analysed and high doses and outliers investigated. Some interventional cardiologists may be disinclined to wear their dosimeters often, or use one rather than two dosimeters. Reasons for non-compliance include, the avoidance of recalls from the health physics departments (or Regulatory Authority), or the threat of a limitation in their catheter laboratory practice, or simply because they are not convinced of its value [4545. Padovani R, Le Heron J, Cruz-Suarez R, Duran A, Lefaure C, Miller DL, Sim HK, Vano E, Rehani M, Czarwinski R. International project on individual monitoring and radiation exposure levels in interventional cardiology. Radiat Prot Dosimetry. 2011;144:437-41. ]. This behaviour can have consequences. In the case of radiation related effects there can be substantial injury, primarily cancer and cataract, and physical dosimetry is essential to establish a legally critical probabilistic link between exposure and health damage. If the dosimeter results are not available, legal and financial compensation claims from the healthcare worker may be denied.
Practitioners need to know their occupational dose in order to ensure that they are working safely. The dose data will not be accurate unless dosimeters are always worn in the correct configuration. Additional restrictions that apply to the occupational exposure of pregnant women must also be adhered to (see below).
DETERMINISTIC RISKS: CATARACTS
The radiation protection standards formulated by the United States National Council on Radiation Protection and Measurements (NCRP) and the International Commission on Radiological Protection (ICRP) are all based on the belief that lens opacities (cataracts) are deterministic radiation-induced effects and appear only if a dose threshold is exceeded. Cataract, or opacification of the lens, is frequently associated with visual impairment and are classified according to their anatomical location into three main categories: nuclear, cortical, and posterior subcapsular [4545. Padovani R, Le Heron J, Cruz-Suarez R, Duran A, Lefaure C, Miller DL, Sim HK, Vano E, Rehani M, Czarwinski R. International project on individual monitoring and radiation exposure levels in interventional cardiology. Radiat Prot Dosimetry. 2011;144:437-41. ]. Amongst age-related cataracts, posterior subcapsular is the least common, however it is the one most frequently associated with ionising radiation exposure. The mechanism of radiation-induced cataract formation remains incompletely unserstood. There is a transparent layer of cells covering the interior frontal side of the capsule that covers the lens. This layer maintains the function of the lens by slowly growing toward the centre, achieved through cell division at the periphery. Because radiation is especially harmful to dividing cells, exposed cells at the equator are most prone to damage. For unknown reasons, damaged cells move toward the rear of the lens before converging on the centre. Such cells prevent light from travelling along the central axis of the lens resulting in visual impairment. Because of their location, relatively minor posterior subcapsular cataracts can have great impact on vision. The estimated eye dose is around 0.5 mGy/procedure in cardiac catheterisation laboratories when no eye protection is used. Until recently, the dose threshold for radiation-induced lens opacities was considered 2 Gy for a single dose exposure or 5 Gy for a fractionated dose [1919. The 2007 Recommendations of the International Commission on Radiological Protection. Publication 103. Ann ICRP. 2007;37:1-332. ]. However, several epidemiological studies among radiation workers at Chernobyl, atomic bomb survivors, astronauts, residents of contaminated buildings and surveys of staff in interventional operating rooms indicate that there is an increased incidence of lens opacities at single doses below 0.5 Gy, and even suggests a stochastic hypothesis (non-threshold effect) [4646. Rehani MM, Vano E, Ciraj-Bjelac O, Kleiman NJ. Radiation and cataract. Radiat Prot Dosimetry. 2011 Jul 14. , 4747. Vano E, Kleiman NJ, Duran A, Rehani MM, Echeverri D, Cabrera M. Radiation cataract risk in interventional cardiology personnel. Radiat Res. 2010;174:490–495. , 4848. Ciraj-Bjelac O, Rehani MM, Sim KH, Liew HB, Vano E, Kleiman NJ. Risk for radiationinduced cataract for staff in interventional cardiology: is there reason for concern? Catheter Cardiovasc Interv. 2010;76:826–834. ].
Whether deterministic or stochastic in nature, lens opacities have been documented in up to 50% of interventional cardiologists [4646. Rehani MM, Vano E, Ciraj-Bjelac O, Kleiman NJ. Radiation and cataract. Radiat Prot Dosimetry. 2011 Jul 14. ]. The reasons for this high prevalence are three-fold: firstly the operator’s eyes are exposed to scattered x-rays, secondly the frequent failure of cardiologists to use protective leaded eyewear [4646. Rehani MM, Vano E, Ciraj-Bjelac O, Kleiman NJ. Radiation and cataract. Radiat Prot Dosimetry. 2011 Jul 14. ]; and thirdly, the legal occupational dose limits were set too high to have created concern. On April 21, 2011, the ICRP dramatically reduced the earlier dose limit of 150 mSv a year for the lens of the eye to the present 20 mSv a year, averaged over a defined period of five years, with no single year exceeding 50 mSv [4949. ICRP Statement on Tissue Reactions. Approved by the Commission on April 21, 2011. ].
STOCHASTIC RISK: CANCER
The current risk estimates of radiobiological risk by the International Commission of Radiological Protection includes only oncogenic risk and the much lower risk of heritable disease (estimated by the ICRP to be not greater than 10% of the radiation-induced carcinogenic risk) [1919. The 2007 Recommendations of the International Commission on Radiological Protection. Publication 103. Ann ICRP. 2007;37:1-332. ]. For the most experienced (and most exposed) staff working in the cardiac catheterisation laboratory, with a range of exposures between 2 and 5 mSv per year [4343. Vañó E, González L, Guibelalde E, Fernández JM, Ten JI. Radiation exposure to medical staff in interventional and cardiac radiology. Br J Radiol. 1998;71:954-60. ], a typical cumulative 30-year radiological exposure around the equivalent of 100 mSv is associated with a non-negligible lifetime attributable risk in the order of magnitude of 1 cancer (fatal and non-fatal) in 100 exposed subjects and 1 fatal cancer in 200 exposed subjects [1414. Venneri L, Rossi F, Botto N, Andreassi MG, Salcone N, Emad A, Lazzeri M, Gori C, Vano E, Picano E. Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: insights from the National Research Council’s Biological Effects of Ionizing Radiation VII Report. Am Heart J. 2009;157:118-24.
Occupational data describing that on the basis of contemporary dosimetry data, a high volume interventional cardiologist’s projected additional lifetime attributable risk for cancer is in the order of 1 in 100 at retirement.]. In general, an occupation with <1 fatality per 10,000 workers per year is considered a safe occupation by the National Council of Radiological Protection. This safety limit is largely exceeded if an active interventional cardiologist does not strictly follow protection rules.
The cancer risk from medical radiation is not only based on extrapolations and estimates but also from direct epidemiological evidences in populations exposed to medical radiation. In a study on 24, 833 adults with congenital heart disease, the risk of cancer increased by more than 250% if the patient had received ≥ 6 procedures with ionizing tests [5050. Cohen S, Liu A, Gurvitz M, Guo L, Therrien J, Laprise C, Kaufman JS, Abrahamowicz M, Marelli AJ. Exposure to Low-Dose Ionizing Radiation From Cardiac Procedures and Malignancy Risk in Adults With Congenital Heart Disease. Circulation. 2018 Mar 27;137:1334-1345. ]. In adults with ischemic heart disease, the number of coronary angioplasties per patient is associated with a progressively increased risk of cancer [5151. Lawler PR, Afilalo J, Eisenberg MJ, Pilote L. Comparison of cancer risk associated with low-dose ionizing radiation from cardiac imaging and therapeutic procedures after acute myocardial infarction in women versus men. Am J Cardiol. 2013 Nov 15;112(10):1545-50. ]. The pattern of cancer localization, both in children and adults, is consistent with the radiobiological concept that red bone marrow (leukemias), lungs, stomach, esophagus and female breast are highly radio-sensitive organs and receive the highest organ dose from invasive cardiology testing, which are the largest sources of exposure in cardiac patients, especially those with ischemic heart disease [1111. Bedetti G, Botto N, Andreassi MG, Traino C, Vano E, Picano E. Cumulative patient effective dose in cardiology. Br J Radiol. 2008;81:699-705. ].
Radiation-induced cancer remains the most alarming professional hazard from fluoroscopic-guided interventions, especially left-sided brain tumors in interventional cardiologists, who stand with their left side adjacent to the radiation source [5252. Roguin A, Goldstein J, Bar O, Goldstein JA. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol. 2013;111:1368–1372. ]. The brain has long been considered a radio-resistant organ, but it is not, and brain cancer due to radiation can be considered a professional disease.
In recent years, mortality and clinical data derived from studies of the Japanese atomic bomb survivors has also suggested associations between radiation dose and mortality from non-cancerous diseases, specifically heart disease and stroke, as well as for diseases of the respiratory system. These increased non-cancer disease risks have been seen at high and medium doses, but remain uncertain for doses lower than about 500 mSv (50 rems) [1313. Ait-Ali L, Andreassi MG, Foffa I, Spadoni I, Vano E, Picano E. Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease. Heart. 2010;96:269-74. , 4141. Rehani MM Srimahachota S. Skin injuries in interventional procedures. Radiation Protection Dosimetry 2011, pp. 1–5. ].
HEALTH EFFECTS OF PRENATAL RADIATION EXPOSURE AND DOSE LIMITS FOR THE PREGNANT WORKER AND PATIENT
Radiation exposure during childbearing age is listed as a reason for altering a career plan in cardiology to a minimally exposed field in 25% of women [5353. Best PJ, Skelding KA, Mehran R, Chieffo A, Kunadian V, Madan M, Mikhail GW, Mauri F, Takahashi S, Honye J, Hernández-Antolín R, Weiner BH; Society for Cardiovascular Angiography & Interventions’ Women in Innovations (WIN) Group. SCAI consensus document on occupational radiation exposure to the pregnant cardiologist and technical personnel. EuroIntervention. 2011;6:866-74. ].
Pregnant cardiologists and pregnant patients
- In utero exposure to ionising radiation can be teratogenic and carcinogenic
- Teratogenic effects are deterministic and require a threshold >50 mSv
- The stochastic risk of cancer (mainly leukaemia) is increased with increasing doses, but no safe limit exists
- Occupational dose limit is <5 mSv for the entire pregnancy for NCRP (in USA)
- A more restrictive limit of <1 mSv for the entire pregnancy is set by ICRP and IAEA (in Europe)
- A 5 mSv dose to the foetus increases the lifetime cancer risk of 1 in 500 (above the normal lifetime cancer risk of 40% to 50%)
- More data are needed to assess (cancer and non-cancer, acute and long term) effects of radiation exposure of mothers (and fathers!) on offspring
- The best protection and surveillance for pregnant operator is achieved with double thickness of protection garment; specific maternity lead apron; and monthly monitoring of the radiation exposure under the lead at waist level
Intra-uterine exposure to ionising radiation can be both teratogenic and carcinogenic [5454. Shaw P, Duncan A, Vouyouka A, Ozsvath K. Radiation exposure and pregnancy. J Vasc Surg. 2011;53:28S-34S. ]. Teratogenic effects are deterministic in nature, and consist of intra-uterine growth retardation, microcephaly, reduced intelligence quotient and congenital malformations. Their occurrence requires that a threshold of 50 mSv is exceeded [5555. National Council on Radiation Protection and Measurements. Limitation and exposure to ionizing radiation. NCRP opinion no. 299. Obstet Gynecol. 2004;104:647-651. ]. In practice this should not breached provided that good radiation safety practices are used and dose limits are respected. The main stochastic effects from radiation exposure to the embryo consist of the risk of cancer. Exposure to diagnostic radiography in utero has been associated with an increased risk of childhood cancer, particularly leukaemia, and especially acute myeloid leukaemia [5656. Stewart AWJ, Giles D, Hewitt D. Malignant disease in childhood and diagnostic irradiation in utero. Lancet. 1956;2:447. , 5757. Rajaraman P, Simpson J, Neta G, Berrington de Gonzalez A, Ansell P, Linet MS, Ron E, Roman E. Early life exposure to diagnostic radiation and ultrasound scans and risk of childhood cancer: case-control study. BMJ. 2011;342:d472. ], also for an estimated dose range below 5 mSv [5858. Sarkozy A, De Potter T, Heidbuchel H, Ernst S, Kosiuk J, Vano E, Picano E, Arbelo E, Tedrow U; ESC Scientific Document Group. Occupational radiation exposure in the electrophysiology laboratory with a focus on personnel with reproductive potential and during pregnancy: A European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS). Europace. 2017 19:1909-1922 ]. Therefore, additional dose restrictions apply to the pregnant worker, although with substantial variability in different countries due to the delicate balance between the legal rights of the pregnant healthcare worker and of the foetus as a member of the public [5353. Best PJ, Skelding KA, Mehran R, Chieffo A, Kunadian V, Madan M, Mikhail GW, Mauri F, Takahashi S, Honye J, Hernández-Antolín R, Weiner BH; Society for Cardiovascular Angiography & Interventions’ Women in Innovations (WIN) Group. SCAI consensus document on occupational radiation exposure to the pregnant cardiologist and technical personnel. EuroIntervention. 2011;6:866-74. ].
In the USA, the NCRP recommends “pregnant workers can continue to work in the catheterisation laboratory if they so choose. Foetal exposure, as measured by a waist dosimeter, should be < 0.5 rem (5 mSv) for the entire pregnancy” [3434. Bashore TM, Bates ER, Berger PB, Clark DA, Cusma JT, Dehmer GJ, Kern MJ, Laskey WK, O’Laughlin MP, Oesterle S, Popma JJ, O’Rourke RA, Abrams J, Bates ER, Brodie BR, Douglas PS, Gregoratos G, Hlatky MA, Hochman JS, Kaul S, Tracy CM, Waters DD, Winters WL Jr; American College of Cardiology. Task Force on Clinical Expert Consensus Documents. American College of Cardiology/Society for Cardiac Angiography and Interventions Clinical Expert Consensus Document on cardiac catheterization laboratory standards. A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:2170-214. ]. In other countries, tighter restrictions apply. After a worker has declared her pregnancy, the ICRP recommends that the additional dose to the embryo/foetus does not exceed about mSv during the remainder of the pregnancy [1919. The 2007 Recommendations of the International Commission on Radiological Protection. Publication 103. Ann ICRP. 2007;37:1-332. ]. The IAEA Basic Safety standards present similar recommendations. On the basis of these recommendations, radiation safety limits for pregnant workers are <1 mSv in UK, Spain and most European countries. In Italy the national law (DLgs 26/03/2001, number 151) requires a woman working with radiation to communicate her pregnancy to the hospital director or to the chief of the practice after which the worker is absolutely forbidden to enter the exposed zone throughout the pregnancy [5353. Best PJ, Skelding KA, Mehran R, Chieffo A, Kunadian V, Madan M, Mikhail GW, Mauri F, Takahashi S, Honye J, Hernández-Antolín R, Weiner BH; Society for Cardiovascular Angiography & Interventions’ Women in Innovations (WIN) Group. SCAI consensus document on occupational radiation exposure to the pregnant cardiologist and technical personnel. EuroIntervention. 2011;6:866-74. ]. For pregnant operators, it is important to wear a double thickness of protective garments and to strictly comply with accepted strategies to reduce radiation exposure [5353. Best PJ, Skelding KA, Mehran R, Chieffo A, Kunadian V, Madan M, Mikhail GW, Mauri F, Takahashi S, Honye J, Hernández-Antolín R, Weiner BH; Society for Cardiovascular Angiography & Interventions’ Women in Innovations (WIN) Group. SCAI consensus document on occupational radiation exposure to the pregnant cardiologist and technical personnel. EuroIntervention. 2011;6:866-74. , 5454. Shaw P, Duncan A, Vouyouka A, Ozsvath K. Radiation exposure and pregnancy. J Vasc Surg. 2011;53:28S-34S. ].
For the pregnant patient, if possible, radiation-guided procedures and examinations should be delayed until at least the completion of the period of major organogenesis (>12 weeks gestation). Most medical procedures do not expose the foetus to doses >50 mSv, but any small increase in the risk of childhood cancer should be avoided. With a maternal exposure of 15 mSv for a PCI or radiofrequency catheter ablation, the corresponding foetal exposure is 3 mSv. With a maternal exposure of 7 mSv for a coronary angiography or a chest CT, the corresponding foetal exposure is 1.5 mSv and 0.3 mSv respectively [5959. Endorsed by the European Society of Gynecology (ESG), the Association for European Paediatric Cardiology (AEPC), and the German Society for Gender Medicine (DGesGM); Authors/Task Force Members, Regitz-Zagrosek V, Lundqvist CB, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L; ESC Committee for Practice Guidelines (CPG), Bax J, Auricchio A, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Knuuti J, Kolh P, McDonagh T, Moulin C, Poldermans D, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Torbicki A, Vahanian A, Windecker S; Document Reviewers, Baumgartner H, Deaton C, Aguiar C, Al-Attar N, Garcia AA, Antoniou A, Coman I, Elkayam U, Gomez-Sanchez MA, Gotcheva N, Hilfiker-Kleiner D, Kiss RG, Kitsiou A, Konings KT, Lip GY, Manolis A, Mebaaza A, Mintale I, Morice MC, Mulder BJ, Pasquet A, Price S, Priori SG, Salvador MJ, Shotan A, Silversides CK, Skouby SO, Stein JI, Tornos P, Vejlstrup N, Walker F, Warnes C. ESC Guidelines on the management of cardiovascular diseases during pregnancy: The Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:3147-97. ]. The 2017 consensus document on occupational exposure of the European Heart Rhythm Association recommends that "pregnant medical workers may continue to work in a radiation environment if they wish to provided that the foetal exposure dose can be kept below 1 mSv in the EU and 5 mSv in the USA during the course of pregnancy and requiring the employer to review the exposure conditions of pregnant women carefully" [5858. Sarkozy A, De Potter T, Heidbuchel H, Ernst S, Kosiuk J, Vano E, Picano E, Arbelo E, Tedrow U; ESC Scientific Document Group. Occupational radiation exposure in the electrophysiology laboratory with a focus on personnel with reproductive potential and during pregnancy: A European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS). Europace. 2017 19:1909-1922 ].
HOW TO MAXIMISE PROTECTION FOR DOCTORS: TIME; DISTANCE; SHIELDING
IAEA has recently prepared an extremely useful poster with the so-called 10 “pearls of wisdom” of radiation protection for staff engaged in fluoroscopically-guided procedures ( >link ). In summary, the three key methods for reducing radiation exposure are based on time, distance and shielding [6060. Valentin J. Avoidance of radiation injuries from medical interventional procedures. Ann ICRP. 2000;30:7-67. , 6161. Vano E. Radiation exposure to cardiologists: how it could be reduced. Heart. 2003; 89:1123-4. ] ( Figure 15 ).
Time: Every effort should be made by the primary operator in the catheterisation laboratory to minimise fluoroscopy and cine-acquisition screening time. Radiation in the catheterisation laboratory is generated using two different modes: fluoroscopy or cine angiography (cine). Fluoroscopy is used for catheter placement and represents on average 95% of the total x-ray operation time, but only causes 40% of the total radiation exposure to staff and patients. Cine is used to acquire diagnostic images and to generate a permanent record of the procedure. Although it represents only 5% of the total x-ray tube operation time, it accounts for 60% of the total radiation exposure to staff and patients. This is primarily due to the use of relatively high-dose rapid sequence screening required to record on film. Only essential staff should be in the catheterisation laboratory during radiation exposure. All persons not required in the room should leave the room during serial radiographic exposure [6060. Valentin J. Avoidance of radiation injuries from medical interventional procedures. Ann ICRP. 2000;30:7-67. ].
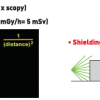
Distance: Increasing the distance from the radiation beam decreases the risk of exposure. It is essential to use the inverse square law to advantage and whenever possible move away from the x-ray source as far as safety allows. At a 1 step distance from the beam, the relative exposure rate is 100; at 2 steps it falls to 25; at 3 steps to 11; at 4 steps to 6. Doubling the distance between the primary beam and the operator reduces exposure by a factor of four. Oblique views (left and right anterior oblique) and steep angulations increase radiation
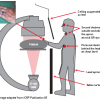
exposure but are often employed to improve visualisation. Sixty degree angulations give up to three times the dose than do thirty degree angulations. The second operator or assistant is generally less exposed to radiation when compared to the first operator, but they are certainly more at risk than other staff in the room. Heads should be kept outside the primary beam unless totally unavoidable. Hands inside the central area of the primary beam will increase exposure factors (kV, mA) and doses to patients and staff. It is also important to consider that only 1% to 5% of radiation falling on the patient’s body exits the other side. The operator should therefore stand on the side of transmitted beam (detector side), which contains only 1% to 5% of the incident radiation and scatter. The x-rays tube should be kept under the patient table and not over it. “Under-couch” systems provide better protection from scattered dose ( Figure 16 ).
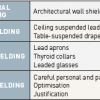
Shielding. Protective shielding includes structural shielding, mobile shielding and personal shielding ( Table 5). Lead shields and shielding will significantly reduce the risk of exposure, but only if appropriately used and in proper working order. Protective equipment includes lead aprons, thyroid collars and leaded glasses for eye protection. The use of ceiling suspended screens, lateral shields and table curtains provides more than 90% protection from scattered radiation in fluoroscopy. Ceiling mounted lead screens, in particular, reduce radiation exposure to the brain. The staff wear a protective apron of at least 0.25 mm lead equivalent, but with overlap on front to make it 0.5 mm on the front and 0.25 mm on the back (provides >90% protection). All such protective clothing should bear an identifying mark and should be examined at yearly intervals. Defective items should be withdrawn from use. The most effective ‘‘shielding’’, however, is the operator’s knowledge of radiation risk [6161. Vano E. Radiation exposure to cardiologists: how it could be reduced. Heart. 2003; 89:1123-4. ].
How to improve the radiological climate in the catheter laboratory
The International Atomic Energy Agency recently launched a campaign to increase the justification of radiological examination through the “3A’s” strategy: Awareness, Appropriateness and Audit [6262. Malone J, Craven C, Guliera R, Horton P, Järvinen H, Mayo J, O’Reilly G, Picano E, Remedios D. Justification of diagnostic medical exposures, some practical issues. Report of an International Atomic Energy Agency (IAEA) Consultation. Br J Radiol. 2011 Feb 22. [Epub ahead of print]. ]. This strategy deployed in the catheterisation laboratory has the potential to yield surprising safety dividends. In the USA, the issue of radiological responsibility was addressed with the Image Gently, Step Lightly Campaign, especially focused on the risks of unnecessary and excessive medical radiation from interventional radiology administered to paediatric patients [6363. Sidhu M, Goske MJ, Connolly B, Racadio J, Yoshizumi TT, Strauss KJ, Coley BD, Utley T. Image Gently, Step Lightly: promoting radiation safety in pediatric interventional radiology. AJR Am J Roentgenol. 2010;195:W299-301. ].
The stakeholders and key players are interventional cardiologists and radiologists (responsible for Awareness; Audit; and Appropriateness); industry (necessary to steer technological upgrades towards better safety) and the scientific societies (responsible for training and certification).
How to maximise protection- Time (cine mode gives 10-times more radiation exposure than fluoroscopy)
- Distance (inverse square law)
- Shielding (structural, mobile, personal and cultural)
INCREASED RADIATION AWARENESS OF STAFF AND PATIENTS
Extensive recent data show a substantial lack of awareness of radiological doses and risks, not only of patients but of prescribing and practicing doctors as well. Results of recent surveys show that most doctors grossly underestimate the radiation doses (usually by up to 500 times) and corresponding cancer risks for most commonly requested tests [6464. Lee CI, Haims AH, Monico EP, Brink JA, Forman HP: Diagnostic CT scans: assessment of patient, physician, and radiologist awareness of radiation dose and possible risks. Radiology. 2004;231:393-398. , 6565. Correia MJ, Hellies A, Andreassi MG, Ghelarducci B, Picano E. Lack of radiological awareness among physicians working in a tertiary-care cardiological centre. Int J Cardiol. 2005;103:307. ]. Paradoxically, about 80% of interventional cardiology fellows did not know their own radiation exposure [6666. Kim C, Vasaiwala S, Haque F, Pratap K, Vidovich MI. Radiation safety among cardiology fellows. Am J Cardiol. 2010;106:125-8. ]. Yet, knowledge is the best “protection” against radiation exposure. A reduction of occupational doses by a factor of 10 can be achieved simply by an intensive training programme in radiation protection [6767. Vaño E, Gonzalez L, Fernandez JM, Alfonso F, Macaya C. Occupational radiation doses in interventional cardiology: a 15-year follow-up. Br J Radiol. 2006;79:383-8.
Occupational data demonstrating interventional cardiologists are 3 times more exposed than diagnostic radiologists. Radiation protection knowledge is the best shield against radiation damage, and can reduce by 90% operator exposure.] and by a factor of 100% by implementing radiation protective devices, capable of reducing operator exposure to 0.8% of typical levels in advanced catheterisation laboratories [6868. Kuon E. Radiation exposure in invasive cardiology. Heart. 2008;94:667-74. ]. Some of this potential damage in professionally exposed staff is obviously unavoidable, due to the high radiation burden of the procedures, with the operator close to the radiation source, and not infrequently with the patient in clinically critical condition. However, another part of the exposure is avoidable, and is due to exposure deriving from inappropriate instructions and lack of implementation of safety principles in the catheter laboratory.
AUDIT OF DOSES (OF LABORATORY AND OPERATORS)
Because DAP is determined both by operator behaviour and by variables outside the operator’s control, “it is impossible to identify a value for DAP that denotes a boundary between appropriate and inappropriate practices” [1616. Hirshfeld, JW Jr, Balter S, Brinker JA, Kern MJ, Klein LW, Lindsay BD, Tommaso CL, Tracy CM, Wagner LK, Creager MA, Elnicki M, Lorell BH, Rodgers GP, Weitz HH. ACCF/AHA/HRS/SCAI clinical competence statement on physician knowledge to optimize patient safety and image quality in fluoroscopically guided invasive cardiovascular procedures: A Report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. Circulation. 2005;111:511-32.
Guidelines outlining the responsibility of all physicians to minimise the radiation injury hazard to their patients, to their professional staff and to themselves.]. Few data are available which permit benchmarking for these parameters. However, we know that for the same procedure, in the same laboratory, dose variations can occur that are up to 10 times the median value. Coronary intervention can be associated with a dose of 1.000 chest x-rays, ranging from 300 to 7.000 chest x-rays in a series of 50 consecutive procedures. This means that the use of a “reference value” for doses is not appropriate for individual patients or procedures (as stated by ICRP) [1919. The 2007 Recommendations of the International Commission on Radiological Protection. Publication 103. Ann ICRP. 2007;37:1-332. ]. Each laboratory and each operator should very carefully assess his/her standing compared to benchmark values. The Step Lightly checklist for paediatric interventional radiology can also be useful in adult procedures, always keeping in mind reducing radiation doses along with effective completion of the procedure. Not all the steps may be possible in each case, depending on patient size, technical challenge, and the critical nature of the procedure [6969. Sidhu M, Coley BD, Goske MJ, Connolly B, Racadio J, Yoshizumi TT, Utley T, Strauss KJ. Image Gently, Step Lightly: increasing radiation dose awareness in pediatric interventional radiology. Pediatr Radiol. 2009;39:1135-8. ].
APPROPRIATENESS OF PROCEDURE: THE JUSTIFICATION PRINCIPLE
Various professional organisations, including the American College of Cardiology and the European Society of Cardiology have developed and are working on the dissemination of imaging referral criteria called “appropriateness criteria” [7070. Brindis R, Douglas PS. President’s page: The ACC encourages multi-pronged approach to radiation safety. J Am Coll Cardiol. 2010;56:522-4. ]. Existing criteria for appropriate ordering of medical imaging exams have not yet been broadly adopted by the practicing medical community, and for instance in Germany (which has the highest per capita rate of invasive cardiological procedures in Europe) 23% of diagnostic angiography and coronary interventions were rated as “equivocal” or “inappropriate” [7171. Brause M, Grande G, Mannebach M, Badura B.The impact of social and institutional characteristics of the appropriateness of invasive cardiological procedures. Med Klin. 2006;101:226-34. ].
The acute and long-term risks of an interventional procedure are the same, whether it is appropriately or inappropriately performed. In 2010 the FDA recommended supporting various professional organisations, including the ACR and ACC, in their effort to develop and adopt appropriate use criteria for interventional fluoroscopy. They suggested that an electronic decision support tool for ordering imaging procedures could incorporate these criteria to improve quality and consistency in clinical decision making. This crucial aspect remains an important field for a proactive role in scientific societies. At least in Europe, this striving for appropriateness is also propelled by the law which clearly states that “if an exposure cannot be justified, it should be prohibited” (article 3 of Euratom 97/43 directive), and that “both the prescriber and the practitioner are responsible for the justification of the test exposing the patient to ionising radiation” (article 5 of Euratom 97/43 directive). Any responsible prescription of invasive cardiology tests should strictly adhere to the principle of justification.
ROLE OF INDUSTRY
Recently, the American College of Radiology’s Blue Ribbon Panel on Radiation Dose in Medicine released a White Paper with a proposed plan of action aimed at preventing the inappropriate use of radiation imaging as well as optimising studies that are performed to obtain the best image quality with the lowest radiation dose. Vendors are an essential part of this team effort. In fact, professional societies should work with the International Electrotechnical Commission and the National Electrical Manufacturers Association (NEMA) to encourage vendors to ensure that their application specialists are familiar with these imaging protocols, establishing the standard as low as reasonably achievable for their new equipment. Professional societies should continue working with manufacturers’ associations to encourage vendors to standardise digital equipment using ionising radiation so that it automatically captures complete dose information for each examination as recommended by the ICRP [7272. Managing patient dose in digital radiology. ICRP Publication 93. Ann ICRP. 2004;34:1-73. ]. For example, in the fluoroscopy mode of an interventional unit, voltage, tube current, pulse width, pulse rate, filter material and thickness, focal spot size, exposure level at the image receptors, field of view, and at least a half dozen image-processing parameters can affect not only image quality but also radiation dose. Too often, practicing cardiologists unrealistically expect vendors’ application specialists to have “all the answers”. Vendors need to continually train and update their application specialists to make sure that improved and validated parameter choices are introduced to the entire customer base [7373. Vano E, Ubeda C, Leyton F, Miranda P, Gonzalez L. Staff radiation doses in interventional cardiology: correlation with patient exposure. Pediatr Cardiol. 2009;30:409-13. ].
Imaging equipment needs to display and record more information about patient dose. Although the US Food and Drug Administration (FDA) mandated the display of radiation air Kerma and cumulative air Kerma on new fluoroscopic equipment, a single, standardised exposure indicator has not been adopted universally by vendors. This confuses imaging staff members, prevents comparison of exposure indices among rooms and institutions and impedes image quality analysis. The displayed radiation dose information should automatically be recorded without operator intervention so as to eliminate errors and incomplete records that may result from recording this information manually [7373. Vano E, Ubeda C, Leyton F, Miranda P, Gonzalez L. Staff radiation doses in interventional cardiology: correlation with patient exposure. Pediatr Cardiol. 2009;30:409-13. ].
RADIATION PROTECTION TRAINING AND CERTIFICATION
In theory, training programmes and the qualifying examination require an interventional cardiologist to be knowledgeable about specialised equipment, techniques, and devices used to perform PCI competently, including the theoretical and practical aspects of x-ray imaging, radiation physics and safety as well as concerning other equipment to generate digital images; quality control of images; image archiving; consequences of exposure of patients and personnel to ionising radiation and methods of reducing patient and staff radiation exposure.
In practice, few training programmes or hospitals have well-developed policies regarding radiation risk and exposure.
The ACC conducted a survey that found that only 19% of the respondents referred to hospital or training policies and 29% had never obtained such information [7474. Limacher MC, Douglas PS, Germano G, Laskey WK, Lindsay BD, McKetty MH, Moore ME, Park JK, Prigent FM, Walsh MN. ACC expert consensus document. Radiation safety in the practice of cardiology. American College of Cardiology. J Am Coll Cardiol. 1998;31:892-913. ]. Interventional cardiologists are often inadequately trained in radiation safety and radiobiology. In Europe, the Ionising Radiation (Medical Exposure) Regulations require a stricter approach to controlling exposure to prevent unnecessary radiation detriment. The requirement is for interventionists to be experts in the use of radiation, with mandatory practical and theoretical training. The latter should be specific and additional to that undertaken for diagnostic radiology [7373. Vano E, Ubeda C, Leyton F, Miranda P, Gonzalez L. Staff radiation doses in interventional cardiology: correlation with patient exposure. Pediatr Cardiol. 2009;30:409-13. ].
Outcome audits should be undertaken periodically with the aim of modifying the selection of patients, choice of technique,
and operational procedures to improve clinical outcomes and reduce complication rates, including any related to ionising radiation. DAP (or KAP) and sometimes the corresponding rough estimation of mSv value should be displayed during the examination, present in the written report, and understood. The dose delivered should be part of the case record of each individual operator, and an aspect – not necessarily minor – of the laboratory performance. Radiation protection should probably be taught and certified separately within the interventional cardiology curriculum so that one can temporarily lose their radiological certification for simply failing to meet minimum radiation protection standards (such as single high exposures or high rates of radiation-induced complications.) Re-accreditation should be allowed after a period of re-training [7575. Guidelines on education and training in radiation protection for medical exposures. Radiation Protection 116, European Commission. Directorate General Environment, Nuclear Safety and Civil Protection. Luxembourg, 2000. ].
Radiation protection in the cardiac catheter laboratory:the 3 A’s strategy- Awareness of risks
- Appropriateness of examinations
- Audit of dose to patient and operators
Conclusion
The perception of radiation in the cardiac catheterisation laboratory is rapidly changing. In the words of an eminent interventional cardiologist, Rita Watson, “increasingly, we have become casual regarding our own exposure in the cathlab. We forget to wear the dosimeters. We pay little heed to monthly or cumulative reports of radiation exposure. While some institutions require a course in radiation safety prior to issuing the radiation dosimetry badges to new fellows, they represent the exception. Not infrequently, there is a macho disregard for radiation protection” [7676. Watson RM. Radiation exposure: clueless in the cath lab, or sayonara ALARA. Cathet Cardiovasc Diagn. 1997;42:126-7. ]. This cultural approach has led to serious, avoidable ill-health for invasive cardiologists, staff and patients. Now something has changed in the mainstream cardiology culture. The ESC 2014 position paper on medical radiation states that "the priority given to radioprotection in every cardiology department is an effective strategy for primary prevention of cancer, a strong indicator of the quality of the cardiology division and the most effective shielding to enhance the safety of operator, patient and staff"[2828. Picano E, Vañó E, Rehani MM, Cuocolo A, Mont L, Bodi V, Bar O, Maccia C, Pierard L, Sicari R, Plein S, Mahrholdt H, Lancellotti P, Knuuti J, Heidbuchel H, Di Mario C, Badano LP. The appropriate and justified use of medical radiation in cardiovascular imaging: a position document of the ESC Associations of Cardiovascular Imaging, Percutaneous Cardiovascular Interventions and Electrophysiology. Eur Heart J 2014; 35; 665–72. ]. The American College of Cardiology 2018 radiation safety position paper explicitly states that "good cardiology training should create a culture of respect for radiation hazard and a commitment to minimize exposure and maximize protection"[3030. Hirshfeld JW Jr, Ferrari VA, Bengel FM, Bergersen L, Chambers CE, Einstein AJ, Eisenberg MJ, Fogel MA, Gerber TC, Haines DE, Laskey WK, Limacher MC, Nichols KJ, Pryma DA, Raff GL, Rubin GD, Smith D, Stillman AE, Thomas SA, Tsai TT, Wagner LK, Wann LS. 2018 ACC/HRS/NASCI/SCAI/SCCT Expert Consensus Document on Optimal Use of Ionizing Radiation in Cardiovascular Imaging: Best Practices for Safety and Effectiveness: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018 Jun 19;71(24):e283-e351. ]. It is becoming increasingly clear that long-term radiation-induced cancer risk should be included on the risk side of the risk-benefit balance of medical decision making, and therefore a clear perception of the radiation issue is vital for both patients and doctors. Effective radiation exposure per episode of care represents a potential safety metric for patients with common clinical conditions and the cumulative occupational radiation exposure is linearly correlated to increased genomic instability and reduced antioxidant capacity [7777. Siama Z, Zosangzuali M, Vanlalruati A, Jagetia GC, Pau KS, Kumar NS. Chronic low dose exposure of hospital workers to ionizing radiation leads to increased micronuclei frequency and reduced antioxidants in peripheral blood lymphocytes. Int J Radiat Biol. 2019 Jan 22:1-37. ]. As an example, recent recognition of the dramatic increase in cataracts in exposed workers has led to a lowering of the allowed dose limits [4949. ICRP Statement on Tissue Reactions. Approved by the Commission on April 21, 2011. ]. As recently stated by the 2009 Joint Inter-Society Task Force on Occupational Hazards, in the interventional laboratory “if the same level of ingenuity and commitment that produced the incredible innovations that have transformed the practice of interventional medicine were applied to enhancing workplace safety, the career of an interventionist would undoubtedly be more comfortable, healthier and longer” [7878. Klein LW, Miller DL, Balter S, Laskey W, Haines D, Norbash A, Mauro MA, Goldstein JA; Joint Inter-Society Task Force on Occupational Hazards in the Interventional Laboratory. Occupational health hazards in the interventional laboratory: time for a safer environment. J Vasc Interv Radiol. 2009; 20:S278-83.
Consensus document describing the occupational risks of interventional practice and suggested innovations to enhance workplace safety.].
The unprecedented radiation exposure of the interventional cardiologists and staff in the cardiac catheterisation laboratory represents a challenge and an opportunity for the interventional community. The present situation must be seen as an opportunity, since a great improvement in practice quality can be achieved with a very limited and targeted training effort in radiation protection. The blueprints of certification and recertification for interventional cardiologists should specify radiation safety subject matter. Spectacular improvements in image engineering took place in the last decade and allow to have better images with substantially lower doses. Obsolete imaging equipment should be renovated or replaced to optimize the dose that must be included, in Europe, by law (Euratom directive) starting February 7th 2018, in all reports from the cardiac catheterisation laboratory [7979. Tsapaki V, Balter S, Cousins C, Holmberg O, Miller DL, Miranda P, Rehani M, Vano E. The International Atomic Energy Agency action plan on radiation protection of patients and staff in interventional procedures: Achieving change in practice. Phys Med. 2018 Aug;52:56-64. ]. Manufacturers play an important role in enhancing patient safety, since low dose technologies are still too expensive and automated patient dose reporting and real-time skin dose maps still under development [2828. Picano E, Vañó E, Rehani MM, Cuocolo A, Mont L, Bodi V, Bar O, Maccia C, Pierard L, Sicari R, Plein S, Mahrholdt H, Lancellotti P, Knuuti J, Heidbuchel H, Di Mario C, Badano LP. The appropriate and justified use of medical radiation in cardiovascular imaging: a position document of the ESC Associations of Cardiovascular Imaging, Percutaneous Cardiovascular Interventions and Electrophysiology. Eur Heart J 2014; 35; 665–72. , 3030. Hirshfeld JW Jr, Ferrari VA, Bengel FM, Bergersen L, Chambers CE, Einstein AJ, Eisenberg MJ, Fogel MA, Gerber TC, Haines DE, Laskey WK, Limacher MC, Nichols KJ, Pryma DA, Raff GL, Rubin GD, Smith D, Stillman AE, Thomas SA, Tsai TT, Wagner LK, Wann LS. 2018 ACC/HRS/NASCI/SCAI/SCCT Expert Consensus Document on Optimal Use of Ionizing Radiation in Cardiovascular Imaging: Best Practices for Safety and Effectiveness: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018 Jun 19;71(24):e283-e351. ]. Technology now exists allowing radiation dose tracking for the patient and the professionally exposed worker. The radiation dose tracking would be important to estimate the individual risk for cancer and also to better understand the dose-response curve for cancer and non-cancer effects of radiation [3030. Hirshfeld JW Jr, Ferrari VA, Bengel FM, Bergersen L, Chambers CE, Einstein AJ, Eisenberg MJ, Fogel MA, Gerber TC, Haines DE, Laskey WK, Limacher MC, Nichols KJ, Pryma DA, Raff GL, Rubin GD, Smith D, Stillman AE, Thomas SA, Tsai TT, Wagner LK, Wann LS. 2018 ACC/HRS/NASCI/SCAI/SCCT Expert Consensus Document on Optimal Use of Ionizing Radiation in Cardiovascular Imaging: Best Practices for Safety and Effectiveness: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018 Jun 19;71(24):e283-e351. ]. The facility director is accountable of the overall radiological performance, which can be monitored by tabulating patient doses per procedure and personnel doses to ascertain that these levels are within reference limits, with corrective action in presence of outliers [7979. Tsapaki V, Balter S, Cousins C, Holmberg O, Miller DL, Miranda P, Rehani M, Vano E. The International Atomic Energy Agency action plan on radiation protection of patients and staff in interventional procedures: Achieving change in practice. Phys Med. 2018 Aug;52:56-64. ]. Furthermore, close monitoring of relatively highly exposed interventional cardiologists may provide important insights into the still elusive mechanisms of biological adaptation to chronic exposure to low dose radiation. It is becoming increasingly clear that a level of radiation exposure which is considered “safe” by regulating standards can induce a profound biochemical and cellular adaptation, of as yet uncertain clinical meaning, such as increased oxidant stress and increased cellular signs of apoptosis [8080. Russo GL, Tedesco I, Russo M, Cioppa A, Andreassi MG, Picano E. Cellular adaptive response to chronic radiation exposure to interventional cardiologists. Eur Heart J. 2012;33:408-14. ]. In high risk population, a combined conventional epidemiology and molecular epidemiology approach may allow to detect early signs of cancer and non cancer disease [8181. Andreassi MG, Piccaluga E, Gargani L, Sabatino L, Borghini A, Faita F, Bruno RM, Padovani R, Guagliumi G, Picano E. Subclinical carotid atherosclerosis and early vascular aging from long-term low-dose ionizing radiation exposure: a genetic, telomere, and vascular ultrasound study in cardiac catheterization laboratory staff. JACC Cardiovasc Interv. 2015; 20;:616-27. , 8282. Borghini A, Vecoli C, Mercuri A, Piccaluga E, Guagliumi G, Picano E, Andreassi MG. Low-dose exposure to ionizing radiation deregulates the brain-specific miR-134 in interventional cardiologists (Research letter). Circulation. 2017; 136:2516–2518. ].
How to maximise protection
- Ask the patient or family about previous radiation (link to imaging record). Answer questions about radiation safety (link to patient brochure)
- Use ultrasound when possible
- Position hanging table shields and overhead lead shields prior to procedure with reminders during the case as needed
- Operators and personnel wear well fitted lead aprons, thyroid shield and leaded eye wear
- Use pulse rather than continuous fluoroscopy when possible, and with as low a pulse as possible
- Position and collimate with fluoroscopy off, tapping on the pedal to check position
- Collimate tightly. Exclude eyes, thyroid, breast, gonads when possible
- Operator and personnel hands out of the beam
- Step lightly: tap on pedal and review anatomy on last image hold rather than with live fluoroscopy when possible; minimise live fluoroscopy time
- Minimise use of electronic magnification; use digital zoom whenever possible
- Acknowledge fluoroscopy timing alerts during procedure
- Use last image hold whenever possible instead of exposures
- Adjust acquisition parameters to achieve lowest dose necessary to accomplish procedure: use lowest dose protocol possible for patient size, lower frame rate, minimise magnification, reduce length of run
- Plan and communicate number and timing of acquisitions, contrast parameters, patient positioning and suspension of respiration with radiology and sedation team in advance to minimise improper or unneeded runs
- Move table away from x-ray tube in both planes. Move patients as close to detector in both planes
- Use power injector or extension tubing if hand injecting
- Move personnel away from table or behind protective shields during acquisitions
- Minimise overlap of fields on subsequent acquisitions
- After procedure: record and review dose
Personal perspective – Eugenio Picano
One of the most important ethical concerns when practicing medicine is primum non nocere. So, it is incumbent upon us to strive for better ways to minimise radiation and its consequent risks. A good cardiologist – and even more so, a good interventional cardiologist – cannot be afraid of radiation but must be very afraid of radiation “unawareness”.