Atrial septal defect
CLINICAL PRESENTATION AND DIAGNOSIS
About 10% of all patients with congenital heart disease have an atrial septal defect (ASD). Patients with ASD are more often female than male. The left-to-right shunt (LR shunt) results in a volume overload of the right ventricle (RV) and the pulmonary circulation. The shunt volume depends on the defect size, and the compliance and pressure of RV and left ventricle (LV). A reduction in LV compliance (e.g., due to coronary artery disease, arterial hypertension, cardiomyopathy, aortic valve disease, mitral valve disease) can raise the left atrial (LA) pressure, which may result in a higher shunt volume. After childhood as patients age, an increase in RV compliance results in symptoms such as dyspnoea, pulmonary infections, and atrial arrhythmias such as atrial fibrillation or atrial flutter [11. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke-Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas P, Widimsky P, McDonagh T, Swan L, Andreotti F, Beghetti M, Borggrefe M, Bozio A, Brecker S, Budts W, Hess J, Hirsch R, Jondeau G, Kokkonen J, Kozelj M, Kucukoglu S, Laan M, Lionis C, Metreveli I, Moons P, Pieper PG, Pilossoff V, Popelova J, Price S, Roos-Hesselink J, Uva MS, Tornos P, Trindade PT, Ukkonen H, Walker H, Webb GD, Westby J; Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. 2010;31:2915-57.
This is an excellent guideline regarding the management of grown-up congenital heart disease, 22. Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation. 2006;114:1645-53.
Great paper dealing with the classification, pathophysiology and treatment of atrial septal defects in adult patients]. By contrast, a decrease in RV compliance results in a decrease in LR shunting or even in right-to-left shunts (RL shunt). This situation may occur due to pulmonary stenosis, pulmonary hypertension or tricuspid valve disease. Systemic embolism may occur due to atrial fibrillation or paradoxical embolism.
Echocardiography allows the diagnosis of an ASD and localisation of the defect. Signs of RV volume overload on trans-thoracic echocardiography (TTE) is very suspicious for the presence of an ASD and necessitates a detailed diagnostic work-up. Transoesophageal echocardiography (TOE) is mandatory under such circumstances since TTE does not localise all defect types (e.g., sinus venosus defect, Figure 1). TOE, however, does enable localisation as well as the evaluation of defect size, enlargement of heart chambers and evidence of sufficient rims for percutaneous closure ( MOVING IMAGE 1-1 MOVING IMAGE 1-2).
Atrial septal defect
- 10% of patients with congenital heart disease have an atrial septal defect (ASD)
- Different types of ASD can be differentiated by TEE:
- Secundum ASD: 80% of ASDs, located in the region of the fossa ovalis and its surroundings
- Primum ASD: 15%
- Diagnostic cardiac catheterisation is required for percutaneous closure of the ASD and for clarification of non-conclusive non-invasive results
- The success rate for percutaneous closure of secundum ASDs is very high with a complete closure rate of more than 95%
- The risk of complications with device closure of secundum ASDs is low with a frequency of less than 0.5%
- Antiplatelet therapy is usually given for 3-6 months with a minimum of 100 mg aspirin daily
Three-dimensional (3D) TOE enables exact visualisation of the ASD morphology ( MOVING IMAGE 2) and is especially useful in complex cases [33. Johri AM, Witzke C, Solis J, Palacios IF, Inglessis I, Picard MH, Passeri JJ. Real-time three-dimensional transesophageal echocardiography in patients with secundum atrial septal defects: outcomes following transcatheter closure. J Am Soc Echocardiogr. 2011;24:431-7. ].
Types of ASD
Several types of ASD can be differentiated by transoesophageal echocardiography ( Figure 1).
- Secundum ASD: 80% of ASDs, located in the region of the fossa ovalis and its surroundings.
- Primum ASD: 15%, located near the crux, the atrioventricular (AV) valves are typically malformed resulting in various degrees of regurgitation.
- Superior sinus venosus defect: 5%, located near the superior vena cava entry, associated with partial or complete connection of right pulmonary veins to right atrium or superior vena cava (SVC).
- Inferior sinus venosus defect: <1%, located near the inferior vena cava entry.
- Unroofed coronary sinus: <1%, separation from the left atrium can be partially or completely missing.
ASDs can be associated with other defects, such as anomalous pulmonary venous connection, persistent left superior vena cava, pulmonary valve stenosis, and mitral valve prolapse. These associated diseases have to be excluded prior to closure of an ASD. To exclude a pulmonary venous anomaly, cardiac magnetic resonance (CMR) imaging or computed tomography (CT) is a helpful tool [44. Festa P, Ait-Ali L, Cerillo AG, De Marchi D, Murzi B. Magnetic resonance imaging is the diagnostic tool of choice in the preoperative evaluation of patients with partial anomalous pulmonary venous return. Int J Cardiovasc Imaging. 2006;22:685-93. , 115115. Han F, Kiparizoska S, Campbell W, Richards C, Kogon B, Holloway M, Watson C, Kerut EK, McMullan M. The case of the missing pulmonary vein: A focused update on anomalous pulmonary venous connection in congenital cardiovascular disease. Echocardiography. 2019 Oct 1. doi: 10. 1111/echo. 14490. ]. Furthermore, CMR allows for the evaluation of shunts, RV, and RA volumes [55. Burgstahler C, Wöhrle J, Kochs M, Nusser T, Löffler C, Kunze M, Höher M, Gawaz MP, Hombach V, Merkle N. Magnetic resonance imaging to assess acute changes in atrial and ventricular parameters after transcatheter closure of atrial septal defects. J Magn Reson Imaging. 2007;25:1136-40. ].
INDICATION FOR ASD CLOSURE
The status of the RV reflects the haemodynamic burden and defines whether an ASD is significant. Diagnostic cardiac catheterisation is required for percutaneous closure of the ASD and for clarification of inconclusive non-invasive results. An elevated pulmonary artery (PA) pressure alone does not preclude closure. Pulmonary vascular resistance should be calculated in patients with elevated PA pressure [66. Steele PM, Fuster V, Cohen M, Ritter DG, McGoon DC. Isolated atrial septal defect with pulmonary vascular obstructive disease--long-term follow-up and prediction of outcome after surgical correction. Circulation. 1987;76:1037-42. ]. In selected patients haemodynamic evaluation with test balloon occlusion of the ASD (e.g., a patient with RV dysfunction with some RL shunt or an older patient with LV dysfunction with CHF symptoms) [77. Sánchez-Recalde A, Oliver JM, Galeote G, González A, Calvo L, Jiménez-Valero S, Moreno R, López-Sendón JL. Atrial septal defect with severe pulmonary hypertension in elderly patients: usefulness of transient balloon occlusion. Rev Esp Cardiol. 2010;63:860-4. ], or vasoreactivity of the pulmonary circulation is necessary in order to allow a proper decision as to whether the patient would benefit from ASD closure.
ASD closure leads to a symptomatic improvement (right heart failure, exercise capacity, dyspnoea) and regression of RV size and pulmonary hypertension, even in older patients [116116. Wang S, Pan J, Xiao B, Tang Y, Lan J, Zheng X, Yang C, Xu D, Zhang J. Immediate and short-term effects of transcatheter device closure of large atrial septal defect in senior people. Congenit Heart Dis. 2019 Sep 12. doi: 10. 1111/chd. 12844. ]. Regression of pulmonary hypertension within 3 months after ASD closure was associated with a similar survival to those patients without pulmonary hypertension at time of closure [112112. Ranard LS, Mallah WE, Awerbach JD, Abernethy A, Halane M, Qureshi AM, Krasuski RA. Impact of Pulmonary Hypertension on Survival Following Device Closure of Atrial Septal Defects. Am J Cardiol. 2019;124:1460-1464. ], whereas remaining increased pulmonary pressure was associated with a higher event rate.
A patient’s benefit from closure is not dependent on age [88. Humenberger M, Rosenhek R, Gabriel H, Rader F, Heger M, Klaar U, Binder T, Probst P, Heinze G, Maurer G, Baumgartner H. Benefit of atrial septal defect closure in adults: impact of age. Eur Heart J. 2011;32:553-60.
This papers links the effect of age on the clinical benefit of atrial septal defect closure in adults]. However, the best outcome is achieved in young patients with less functional impairment [99. Murphy JG, Gersh BJ, McGoon MD, Mair DD, Porter CJ, Ilstrup DM, McGoon DC, Puga FJ, Kirklin JW, Danielson GK. Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years. N Engl J Med. 1990;323:1645-50. , 1010. Roos-Hesselink JW, Meijboom FJ, Spitaels SE, van DR, van Rijen EH, Utens EM, Bogers AJ, Simoons ML. Excellent survival and low incidence of arrhythmias, stroke and heart failure long-term after surgical ASD closure at young age. A prospective follow-up study of 21–33 years. Eur Heart J. 2003;24:190-7. ]. Indications for ASD closure according to the ESC guidelines are listed in Table 1. If technically feasible, secundum ASD should be closed by implantation of an occluder.
Surgical repair should be performed in cases not eligible for closure by occluder implantation. The risk of procedural complications is low and life expectancy returns to normal if closure is performed in adolescence or childhood [99. Murphy JG, Gersh BJ, McGoon MD, Mair DD, Porter CJ, Ilstrup DM, McGoon DC, Puga FJ, Kirklin JW, Danielson GK. Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years. N Engl J Med. 1990;323:1645-50. , 1010. Roos-Hesselink JW, Meijboom FJ, Spitaels SE, van DR, van Rijen EH, Utens EM, Bogers AJ, Simoons ML. Excellent survival and low incidence of arrhythmias, stroke and heart failure long-term after surgical ASD closure at young age. A prospective follow-up study of 21–33 years. Eur Heart J. 2003;24:190-7. ].
TECHNIQUE OF PERCUTANEOUS ASD CLOSURE
The success rate for percutaneous closure of secundum ASDs is very high with a complete closure rate of more than 95% [1111. Majunke N, Bialkowski J, Wilson N, Szkutnik M, Kusa J, Baranowski A, Heinisch C, Ostermayer S, Wunderlich N, Sievert H. Closure of atrial septal defect with the Amplatzer septal occluder in adults. Am J Cardiol. 2009;103:550-4. ]. The most important key to success is to perform a pre-interventional careful check to confirm whether the ASD is suitable for percutaneous closure via device implantation. With TTE and TOE multiple points should be evaluated: defect morphology and position, fenestrations, multiple defects, RV size and function, tricuspid valve function, maximal diameter of ASD, exclusion of primum or sinus venosus defect, ideal rims of about 5 mm (with the exception of the aortic rim), and a total septal length not exceeding the diameter of the LA disc chosen. There are challenging cases such as ASD with deficient superior anterior rim, with an aneurysm, with multiple holes, with deficient inferior anterior rim, all of which if present should be treated by a team with sufficient experience.
The procedure for percutaneous ASD closure will be described for the Amplatzer® Septal Occluder (St. Jude Medical Inc., St. Paul, MN, USA), which is the most implanted device type for ASD closure ( MOVING IMAGE 3-1 MOVING IMAGE 3-2). Other device types have also been used for selected patients with proper ASD morphology such as Figulla ASD Occluder (Occlutech AB, Helsingboarg, Sweden) [1313. Halabi A, Hijazi ZM. A new device to close secundum atrial septal defects: first clinical use to close multiple defects in a child. Catheter Cardiovasc Interv. 2008;71:853-6. , 1414. Cansel M, Pekdemir H, Yagmur J, Tasolar H, Ermis N, Kurtoglu E, Acıkgoz N, Atas H, Ozdemir R. Early single clinical experience with the new Figulla ASD Occluder for transcatheter closure of atrial septal defect in adults. Arch Cardiovasc Dis. 2011;104:155-60. ], Cardia ASD device (Cardia Inc., Eagan, MN, USA) [1515. Goy JJ, Stauffer JC, Yusoff Z, Wong AR, Owlya R, Perret F, Siegenthaler M, Savcic M, Ménétrey R, Seydoux C. Percutaneous closure of atrial septal defect type ostium secundum using the new Intrasept occluder: initial experience. Catheter Cardiovasc Interv. 2006;67:265-7. , 1616. Stolt VS, Chessa M, Aubry P, Juliard JM, Schraeder R, Berger A, Goy JJ. Closure of ostium secundum atrial septum defect with the Atriasept occluder: early European experience. Catheter Cardiovasc Interv. 2010;75:1091-5. ], Helex® Septal Occluder (Gore Medical, Flagstaff, AZ, USA) [1717. Latson LA, Jones TK, Jacobson J, Zahn E, Rhodes JF. Analysis of factors related to successful transcatheter closure of secundum atrial septal defects using the HELEX septal occluder. Am Heart J. 2006;151:1129.
e7-11, 1818. Jones TK, Latson LA, Zahn E, Fleishman CE, Jacobson J, Vincent R, Kanter K. Multicenter Pivotal Study of the HELEX Septal Occluder Investigators. Results of the U.S. multicenter pivotal study of the HELEX septal occluder for percutaneous closure of secundum atrial septal defects. J Am Coll Cardiol. 2007;49:2215-21. ], or in previous years STARFlex device (NMT Medical Inc., Boston, MA, USA; not available any more and thus of historical interest only) [1919. Ruiz CE, Cohen HA, Nugent AW, Kramer P. Long-term follow-up of the STARFlex device for closure of secundum atrial septal defect. Catheter Cardiovasc Interv. 2009;74:525-6. ].
Recent prospective comparison of 3 different devices for ASD closure (Amplatzer, Cera (Lifetech Scientific, Shenzhen, China), Figulla) showed similar clinical results within mid-term follow-up ranging from 12-47 months [119119. Bhattacharjya S, Pillai LS, Doraiswamy V, Satyanarayana RM, Chandrasekaran R, Pavithran S, Sivakumar K. Prospective concurrent head-to head comparison of three different types of nitinol occluder device for transcatheter closure of secundum atrial septal defects. EuroIntervention. 2019 Jul 20;15(4):e321-e328. ], whereas another study including 158 patients showed a higher rate of successful device placement with the first attempt with the Figulla compared to the Amplatzer ASD device [124124. Kenny D, Eicken A, Dähnert I, Boudjemline Y, Sievert H, Schneider MB, Gori T, Hijazi ZM. A randomized, controlled, multi-center trial of the efficacy and safety of the Occlutech Figulla Flex-II Occluder compared to the Amplatzer Septal Occluder for transcatheter closure of secundum atrial septal defects. Catheter Cardiovasc Interv. 2019 Feb 1;93(2):316-321. ].
TOE or intracardiac echocardiography (ICE) usually guides the implantation procedure and allows for the assessment of the precision and stability of the device position. Venous access is usually obtained from the right femoral vein. The ASD is passed by e.g. a multipurpose catheter and placed in the left upper pulmonary vein (LUPV). In case of an anomalous venous return of one of the pulmonary veins surgical treatment has to be favoured. Through the multipurpose catheter an extra-stiff or super-stiff wire is placed in the LUPV. The catheter is removed and then a sizing balloon can be placed across the defect in order to size the ASD with the stop flow technique [1212. Amin Z, Hijazi ZM, Bass JL, Cheatham JP, Hellenbrand WE, Kleinman CS. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv. 2004;63:496-502. ]. The balloon is inflated until the shunt seen on Colour Doppler disappears. The balloon is deflated until the shunting re-appears and then re-inflated until the shunt is eliminated. Measurement of balloon size by ultrasound or X-ray determines the stop-flow diameter of the ASD. The difference between both values should not exceed 5%, otherwise the measurements should be repeated to allow proper device selection. Experienced centres do not always use sizing balloons for device selection. The balloon-sized diameter of the ASD can deviate substantially from prior echocardiographic measurements, since most of the defects are oval and a flimsy septum is pushed away. During balloon inflation the atrial septum should be carefully checked for evaluation of additional defects. An oversizing of the defect by inflation of the balloon until appearance of a waist will subsequently overstretch the ASD and may result in septal rupture or selection of larger devices with a potential higher risk of complications. Some experienced centers use ICE to eliminate the need for balloon sizing [118118. Rigatelli G, Nghia NT, Zuin M, Conte L, D'Elia K, Nanjundapa A. Very long-term outcomes of transcatheter secundum atrial septal defect closure
using intracardiac echocardiography without balloon sizing. Clin Radiol. 2019;74:732.e17-732.e22 ].
Selection of device size depends on multiple factors. Usually the waist of the device is selected 1-2 mm larger than the diameter of the sizing balloon obtained by stop-flow technique. When the aortic rim is small or absent, or the atrial septum around the secundum ASD is very floppy, a device 2-4 mm larger should be selected in order to achieve a stable position of the occluder. An important issue (especially in children) is to measure the total length of the atrial septum. The LA must be able to accommodate the device. Device selection as well as implantation has to be performed carefully, especially in challenging ASDs such as those with deficient aortic and/or posterior rims, floppy rims, multiple ASDs or large ASDs. In these scenarios the standard deployment procedure may not be adequate.
While the guidewire is left in place, the sizing balloon is retracted for delivery sheath placement in the LUPV. Careful back bleeding through the delivery sheath minimises the risk of air embolism, and so the sheath has to be located below the level of the heart. Then the occluder is advanced to the tip of the delivery sheath. After or during withdrawal of the delivery sheath from the LUPV in the mid LA the left atrial disc is opened. The system is pinched and pulled in order to attach the LA disc at the atrial septum. TOE or ICE will nicely show the atrial septum and the position of the LA disc. The middle part of the occluder is opened and then the right disc is released and attached to the atrial septum. A gentle push and pull of the device (‘Minnesota Wiggle’) will enable one to check for device stability. After the device is deployed there should be a careful evaluation by TOE or ICE for interrogation of rims, function of AV valves and potential contact of the device with the atrial roof in order to evaluate the risk of long-term complications, such as dysfunction of valves or erosion of the atrial roof. Furthermore, residual shunting as well as screening for additional defects should be done with colour Doppler. If the device fits nicely in the ASD and noticeable problems have been excluded, the device can be released. Since the tension on the delivery cable often modifies the orientation of the atrial septum with the Amplazter device, the occluder will shift into the final position after device release (other devices using a forceps for the connection are associated with less tension on the delivery cable).
In large defects, the RA disc should be opened several mm from the septum. Furthermore, the tension on the delivery cable should not be too strong, otherwise a prolapse of the LA disc in the RA may occur. For difficult situations clockwise rotation on the delivery sheath will change the position of the device. An alternative is the use of sheaths with another tip configuration e.g. the Hausdorff sheath. Deployment of the LA disc in LUPV has been described for large defects [2020. Fu YC, Cao QL, Hijazi ZM. Device closure of large atrial septal defects: technical considerations. J Cardiovasc Med (Hagerstown). 2007;8:30-3. , 2121. Amin Z. Transcatheter closure of secundum atrial septal defects. Catheter Cardiovasc Interv. 2006;68:778-87.
Very good paper regarding closure of atrial septal defects with many tips and tricks in challenging cases]. For deficient posterior rim, a deployment of the LA disc in the RUPV or the left atrial roof technique is helpful [2121. Amin Z. Transcatheter closure of secundum atrial septal defects. Catheter Cardiovasc Interv. 2006;68:778-87.
Very good paper regarding closure of atrial septal defects with many tips and tricks in challenging cases]. For closure of large ASDs a balloon-assisted technique can be used. The inflated balloon in the ASD prevents the LA disc to prolapse into the RA. The LA disc and sheath are pulled towards the atrial septum, and the waist and RA disc released. Then the balloon is gradually deflated. During this deflation the RA disc is moved towards the LA disc by pushing the delivery cable. Balloon and guidewire are subsequently removed. This technique requires an additional venous access [2222. Dalvi BV, Pinto RJ, Gupta A. New technique for device closure of large atrial septal defects. Catheter Cardiovasc Interv. 2005;64:102-7. ] but is associated with a high 87% success rate in patients with ASD defects larger than 30mm [125125. Ananthakrishna Pillai A, Upadhyay A, Gousy S, Handa A. Impact of modified techniques of transcatheter closure in large atrial septal
defects (⩾30 mm) with anatomic complexities. Cardiol Young. 2018 Oct;28(10):1122-1133
Impact of modified techniques of transcatheter closure in large atrial septal]. In the case of insufficient superior rims an additional guidewire placed in the LUPV helps to maintain the LA disc in the left atrium [100100. Kanazawa H, Kawamura A, Kimura M, Akita K, Yashima F, Arai T, Kawakami T, Hayashida K, Tsuruta H, Yuasa S, Itabashi Y, Murata M, Maekawa Y, Fukuda K. A Novel Wire-Assisted Technique for Closing Large Atrial Septal Defects: New Concepts of Closure Mechanism. JACC Cardiovasc Interv. 2016, doi: 10. 1016/j. jcin. 2015. 12. ].
Important variations
Careful evaluation of the atrial septum by TOE or ICE will allow the detection of multiple defects. The implantation of 2 or even 3 devices will allow closure of these complex ASDs. In order to test whether a single device strategy will be sufficient, a sizing balloon can be inflated in the defect. If both ASDs are very close, a single ASD device can be used. If the distance is larger than 5 mm, the defects are usually handled as separate holes and need one device per defect. If the distance between the defects is less than 5 mm, the larger one can be closed by an ASD device followed by a non-self-centering device (e.g., Cribriform Occluder) as a second device. For a multiple perforated atrial septum a Cribriform Occluder can be used. ICE or TOE with 3D imaging will help to place the guiding catheter and delivery sheath through the central defect. For a strategy with one occluder, the size of device should be double the distance from the central defect to the edge of the most remote defect.
In general, the following ASD morphologies are not suitable for device implantation: large ASDs with a diameter equal to or greater than 38 mm, those with absent inferior rims, those with complete absent rims in more than 2 areas, those in which the device is too large to fit in the atria, sinus venosus defect, coronary sinus defects, very short AV valve rim, and ASDs with associated partial anomalous pulmonary venous drainage. In these scenarios surgery is the best option.
Eisenmenger’s syndrome
The general risk of Eisenmenger in ASD patients is about 10%. In these patients the primary goal is to improve quality of life. Management of these patients is complex. There is no optimal standardised approach. Treatment with the endothelin receptor antagonist (ERA) bosentan or phosphodiesterase type-5 (PDE-5) inhibitors may improve patients’ exercise capacity and reduce RL shunt. Careful haemodynamic evaluation may allow partial closure of the ASD with subsequent re-evaluation and further treatment with ERA and PDE-5 inhibitors. The ESC Guidelines for the diagnosis and treatment of pulmonary hypertension [2323. Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30:2493-537. , 101101. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barberà J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol Ç, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Völler H, Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67-119. ] give more detailed information.
COMPLICATIONS
The risk of complications with device closure of secundum ASDs is low with a frequency of less than 0.5% in large series [2424. Butera G, Carminati M, Chessa M, Youssef R, Drago M, Giamberti A, Pome` G, Bossone E, Frigiola A. Percutaneous versus surgical closure of secundum atrialseptal defect: comparison of early results and complications. Am Heart J. 2006;151:228-34. , 2525. Fischer G, Stieh J, Uebing A, Hoffmann U, Morf G, Kramer HH. Experience with transcatheter closure of secundum atrial septal defects using the Amplatzerseptal occluder: a single centre study in 236 consecutive patients. Heart. 2003;89:199-204. ]. Careful preparation and handling of sheath and flushing has to be performed to avoid air or clot embolisation or cardiac perforation by the guidewire or delivery sheath. A proper measurement with the stop-flow technique avoids an undersizing of the device with the risk of subsequent embolisation. Device embolisation has been described early [2626. Piatkowski R, Kochanowski J, Scislo P, Kochman J, Opolski G. Dislocation of Amplatzer septal occluder device after closure of secundum atrial septal defect. J Am Soc Echocardiogr. 2010;23:1007.
e1-2] and late [2727. Zorger N, Steinbauer M, Luchner A. Percutaneous removal of embolized Amplatzer occluder from the abdominal aorta: a different type of belly-button. Eur Heart J. 2008;29:1791. ] to the right [2626. Piatkowski R, Kochanowski J, Scislo P, Kochman J, Opolski G. Dislocation of Amplatzer septal occluder device after closure of secundum atrial septal defect. J Am Soc Echocardiogr. 2010;23:1007.
e1-2] as well as to the left side of the heart ( MOVING IMAGE 4-1 MOVING IMAGE 4-2) [2727. Zorger N, Steinbauer M, Luchner A. Percutaneous removal of embolized Amplatzer occluder from the abdominal aorta: a different type of belly-button. Eur Heart J. 2008;29:1791. ]. In the event of device embolisation, the device should be captured by snares via an additional venous or arterial access and percutaneously removed.
Excessive coughing or jumping soon after device implantation should be avoided [2828. Schoof S, Norozi K, Breymann T, Wessel A, Bertram H. Cough. A potentially life-threatening condition after interventional closure of atrial septal defect. Circ Cardiovasc Imaging. 2009;2:e30-1. ]. Device embolisation or dislocation ( MOVING IMAGE 5) may be due to unrecognised deficient rims around the ASD. If the device is rotated during advancement within the delivery sheath (or the device is too small compared with the size of the delivery sheath) the left disc may twist and form a Cobra head after deployment [2929. Cooke JC, Gelman JS, Harper RW. Cobra head malformation of the Amplatzer septal occluder device: An avoidable complication of percutaneous ASD closure. Catheter Cardiovasc Interv. 2001;52:83-85. , 3030. Waight DJ, Hijazi ZM. Amplatzer devices: Benign cobra head malformation. Catheter Cardiovasc Interv. 2001;52:86-7. , 3131. Aaron S, Mainzer G, Lorber A. Taming the “Cobra”: An approach to “cobra” like formation seen in the Occlutech atrial septal defect and patent foramen ovale occluders. Catheter Cardiovasc Interv. 2011, doi:10. 1002/ccd. 23303. ]. After recapturing, the device usually has to be replaced.
A gentle inflation of the sizing balloon avoids dilation and rupture of the atrial septum, which results in a larger defect. A careful step-by-step implantation procedure avoids entrapment of RA structures. Sufficient anticoagulation (ACT >250 sec) during the procedure and starting antiplatelet therapy the day before implantation with a loading dose will minimise the risk of development of thrombi on the device or guidewire and thus the potential risk of stroke. Careful venous puncture and anticoagulation as well as compression after the procedure will avoid bleeding complications at the puncture site. In some centres pre-closure of the venous access site is performed [3232. Mahadevan VS, Jimeno S, Benson LN, McLaughlin PR, Horlick EM. Pre-closure of femoral venous access sites used for large-sized sheath insertion with the Perclose device in adults undergoing cardiac intervention. Heart. 2008;94:571-2. ]. Periprocedural administration of antibiotics reduces the risk of endocarditis, which is a very rare complication. Arrhythmias, such as supraventricular extrasystoles or atrial fibrillation occurring early after intervention, are mostly transient. In ASD patients with LV dysfunction the decrease in LR shunt acutely increases LV preload and increases cardiac output at the cost of LA hypertension with the risk of developing acute pulmonary oedema. The occurrence of an AV block in large devices has been described [3333. Al-Anani SJ, Weber H, Hijazi ZM. Atrioventricular block after transcatheter ASD closure using the Amplatzer septal occluder: risk factors and recommendations. Catheter Cardiovasc Interv. 2010;75:767-72. ], as well as resolution of AV block after device explantation [3434. Clark JB, Chowdhury D, Pauliks LB, Weber HS. Resolution of heart block after surgical removal of an amplatzer device. Ann Thorac Surg. 2010;89:1631-3. ].
There is a minimal risk (0.07%) of late perforation due to erosion of the atrial wall or aorta, which requires surgical repair [1212. Amin Z, Hijazi ZM, Bass JL, Cheatham JP, Hellenbrand WE, Kleinman CS. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv. 2004;63:496-502. , 3535. Divekar A, Gaamangwe T, Shaikh N, Raabe M, Ducas J. Cardiac perforation after device closure of atrial septal defects with the Amplatzer septal occluder. J Am Coll Cardiol. 2005;45:1213-8. , 3636. Sarris GE, Kirvassilis G, Zavaropoulos P, Belli E, Berggren H, Carrel T, Comas JV, Corno AF, Daenen W, Di Carlo D, Ebels T, Fragata J, Hamilton L, Hraska V, Jacobs J, Lazarov S, Mavroudis C, Metras D, Rubay J, Schreiber C, Stellin G. Surgery for complications of trans-catheter closure of atrial septal defects: a multi-institutional study from the European Congenital Heart Surgeons Association. Eur J Cardiothorac Surg. 2010;37:1285-90.
Multicenter record of patients with complications after percutaneous ASD closure, 121121. Abe T, Tsukano S, Tosaka Y. Pericardial tamponade due to erosion of a Figulla Flex II device after closure of
an atrial septal defect. Catheter Cardiovasc Interv. 2019 Jun 13. doi: 10.1002/ccd.28367.
Pericardial tamponade due to erosion of a Figulla Flex II device after closure of]. This risk is increased if the device is oversized in relation to the size of the ASD. Deficient rims and rotation of device post-implantation have also been associated with late perforation. Cardiac perforation has been reported early after device implantation [3737. Maimon MS, Ratnapalan S, Do A, Kirsh JA, Wilson GJ, Benson LN. Cardiac perforation 6 weeks after percutaneous atrial septal defect repair using an Amplatzer septal occluder. Pediatrics. 2006;118:e1572-5. ] and up to 4 years [3838. Cazzaniga M, Bermudez-Canete R, Sanchez I, Gomez R, Maître MJ. Late cardiac perforation after transcatheter closure of an atrial septal defect using the Amplatzer septal occluder. Rev Fed Arg Cardiol. 2010;39:311-13. , 3939. Murphy JC, Walsh SJ, Spence MS. Late aortic perforation with an Atriasept device resulting in life-threatening tamponade. Catheter Cardiovasc Interv. 2010;76:132-4. ]. Potential complications such as allergic reactions [4040. Singh HR, Turner DR, Forbes TJ. Nickel allergy and the amplatzer septal occluder. J Invasive Cardiol. 2004;16:681-2. ], including severe bronchospasms [4141. Khodaverdian RA, Jones KE. Metal Allergy to Amplatzer occluder device presented as severe bronchospasm. Ann Thorac Surg. 2009;88:2021-22. ] or aortic insufficiencies, have been described [4242. Schoen SP, Boscheri A, Lange SA, Braun MU, Fuhrmann J, Kappert U, Strasser RH. Incidence of aortic valve regurgitation and outcome after percutaneous closure of atrial septal defects and patent foramen ovale. Heart. 2008;94:844-7. ]. Detailed CMR analysis after occluder implantation demonstrated no risk of aortic insufficiency [4343. Wöhrle J, Kochs M, Spiess J, Nusser T, Hombach V, Merkle N. Impact of percutaneous device implantation for closure of patent foramen ovale on valve insufficiencies. Circulation. 2009;119:3002-8.
Detailed analysis with seriel cardiac magnetic resonance imaging ruling out an impact of percutaneous device implantation for patent foramen ovale on aortic valve insufficieny]. With TOE, thrombi have been detected during follow-up, which can usually be resolved by anticoagulation with warfarin [4444. Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, Horvath K, Sievert H. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol. 2004;43:302-9.
This paper links the occurrence of thrombus formation to the type of occluder and details the subsequent therapy]. In rare cases, patients with thrombi were treated by surgery, especially for mobile thrombi located on the LA disc [4444. Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, Horvath K, Sievert H. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol. 2004;43:302-9.
This paper links the occurrence of thrombus formation to the type of occluder and details the subsequent therapy]. During long-term follow-up wire fracture in some devices [4545. Qureshi AM, Mumtaz MA, Latson LA. Partial prolapse of a HELEX device associated with early frame fracture and mitral valve perforation. Catheter Cardiovasc Interv. 2009;74:777-82. , 4646. Fagan T, Dreher D, Cutright W, Jacobson J, Latson L; GORE HELEX Septal Occluder Working Group. Fracture of the GORE HELEX septal occluder: associated factors and clinical outcomes. Catheter Cardiovasc Interv. 2009;73:941-8. ] and persistent residual shunting have been described without haemodynamic relevance. Other rare complications are development of mitral regurgitation due to oversized mismatched device implantation [4747. Li W, Han W, Yu C, Zhang C, Tu Z, Wu S, Huber CH, Ma L. Severe mitral valve insufficiency after transcatheter atrial septal defect closure with the amplatzer septal occluder: a device-related complication. J Card Surg. 2009;24:672-4. ] and development of pulmonary vein stenosis [4848. Moiduddin N, Cheatham JP, Hoffman TM, Phillips AB, Kovalchin JP. Amplatzer septal occluder associated with late pulmonary venous obstruction requiring surgical removal with acquired aorta to left atrial fistula. Am J Cardiol. 2009;103:1039-40. ] or device explantation due to severe migraine associated with nickel allergy [117117. Fernandes P, Sharma SR, Magee A, Michielon G, Fraisse A. Severe Migraine Associated With Nickel Allergy Requiring Surgical Removal of
Atrial Septal Device. Ann Thorac Surg. 2019 Sep;108(3):e183-e184. ]. Another rare complication is a fracture of the ASD device associated with mitral valve perforation with subsequent need of surgical removal [120120. Werner RS, Prêtre R, Maisano F, Wilhelm MJ. Fracture of a Transcatheter Atrial Septal Defect Occluder Device Causing Mitral
Valve Perforation. Ann Thorac Surg. 2019 Jul;108(1):e29-e30.
Fracture of a Transcatheter Atrial Septal Defect Occluder Device Causing Mitral].
FOLLOW-UP THERAPY
Antiplatelet therapy is usually recommended for 3-6 months with a minimum of 100 mg aspirin daily [142142. Diener HC; die Deutsche Gesellschaft für Neurologie (DGN), Grau AJ; die Deutsche Schlaganfall-Gesellschaft (DSG), Baldus S; die Deutsche Gesellschaft für Kardiologie‑, Herz- und Kreislaufforschung (DGK), Ghanem A, Gröschel K, Liebetrau C, Massberg S, Möllmann H, Nef H, Sander D, Weimar C, Wöhrle J, Mattle H; die Schweizerische Neurologische Gesellschaft. Cryptogenic stroke and patent foramen ovale : S2e guidelines. Nervenarzt. 2018;89:1143-1153. ]. In many centres dual antiplatelet therapy with aspirin and clopidogrel (75 mg per day) is prescribed for 3 months. There is no need for long-term antiplatelet therapy. TTE or TOE during follow-up will allow evaluation of the proper position of the device, residual shunting, RV size and function, pericardial effusion, thrombotic complications as well as proper function of mitral, tricuspid and aortic valves without obstruction of pulmonary veins or venae cavae. The occlusion rate will increase during the following years up to almost 100% [4949. Bialkowski J, Kusa J, Szkutnik M, Kalarus Z, Banaszak P, Bermúdez-Cañete R, Fernández Pineda L, Zembala M. Percutaneous catheter closure of atrial septal defect. Short-term and mid-term results. Rev Esp Cardiol. 2003;56:383-8. ].
During long-term follow-up patients may need additional left heart structural interventions e.g. MitraClip, closure of left atrial appendage or pulmonary vein isolation. With multimodality imaging and detail anatomical considerations transseptal puncture through the native septum or through the ASD device using balloon dilatation is feasible and safe [113113. Yap J, Chen S, Stripe BR, Smith TWR, Rogers JH, Singh GD. Transseptal access for left heart structural interventions in the setting of
prior atrial septal defect closure. Catheter Cardiovasc Interv. 2019 Oct 22. doi: 10.1002/ccd.28548.
Transseptal access for left heart structural interventions in the setting of, 126126. Sang CH, Dong JZ, Long DY, Yu RH, Bai R, Salim M, Tang RB, Ning M, Jiang CX, Liu N, Li SN, Wen SN, Wu JH, Chen K, Chen
YW, Ma CS. Transseptal puncture and catheter ablation of atrial fibrillation in patients with atrial septal occluder: initial experience of a single centre. Europace. 2018 Sep 1;20(9):1468-1474. ].
Patent foramen ovale
Patent foramen ovale (PFO) has been associated with "cryptogenic" ischaemic events such as stroke, transient ischaemic attack, peripheral embolism, and also with migraine and decompression illness. Detection of PFO is usually performed by TOE with a bubble test after a sustained Valsalva manoeuvre or other maneuovers (cough, sniff, etc.), which is superior compared to TTE [5050. Maffè S, Dellavesa P, Zenone F, Paino AM, Paffoni P, Perucca A, Kozel D, Signorotti F, Bielli M, Parravicini U, Pardo NF, Cucchi L, Aymele AG, Zanetta M. Transthoracic second harmonic two- and three-dimensional echocardiography for detection of patent foramen ovale. Eur J Echocardiogr. 2010;11:57-63. ] and CMR [5151. Nusser T, Höher M, Merkle N, Grebe OC, Spiess J, Kestler HA, Rasche V, Kochs M, Hombach V, Wöhrle J. Cardiac magnetic resonance imaging and transesophageal echocardiography in patients with transcatheter closure of patent foramen ovale. J Am Coll Cardiol. 2006;48:322-9. ] if the patient is not heavily sedated. With either technique, the performance of a proper Valsalva manoeuvre is crucial in order to increase RA over LA pressure and to open the PFO [5252. Cheng TO. The proper conduct of Valsalva maneuver in the detection of patent foramen ovale. J Am Coll Cardiol. 2005;45:1145-6. ].
The present European position paper recommends the event to be classified as PFO-related instead of “cryptogenic”, which should be used in patients with cryptogenic ischemic left circulation embolism without the evidence of a PFO [127127. Pristipino C, Sievert H, D’Ascenzo F, Mas JL, Meier B, Scacciatella P, Hildick-Smith D, Gaita F, Toni D, Kyrle P, Thomson J, Derumeaux G, Onorato E, Sibbing D, Germonpré P, Berti S, Chessa M, Bedogni F, Dudek D, Hornung M, Zamorano J; European Association of Percutaneous Cardiovascular Interventions (EAPCI); European Stroke Organisation (ESO); European Heart Rhythm Association (EHRA); European Association for Cardiovascular Imaging (EACVI); Association for European Paediatric and Congenital Cardiology (AEPC); ESC Working group on GUCH; ESC Working group on Thrombosis; European Haematological Society (EHA). European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. EuroIntervention. 2019 Jan 20;14(13):1389-1402. ].
The fossa ovalis is the remnant of the PFO after anatomical closure, which usually occurs within the first two years of life [5353. Anderson RH, Brown NA, Webb S. Development and structure of the atrial septum. Heart. 2002;88:104-110. ]. The lack of closure results in a PFO with a significant variance in terms of size and anatomy. The prevalence of PFO decreases with age, from 34% at the age of 30 to 20% at the age of 90 [5454. Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17-20. ]. The evidence of a persistent PFO is not in itself a pathological finding. The PFO plays a pathophysiological role by facilitating the transport of corpuscular components or vasoactive substances in the blood from RA to LA. In general, the mean pressure in LA exceeds the mean pressure in RA. A transient, physiological reversal of the pressure difference between the two atrial chambers is present during each heartbeat in early diastole and during the isovolumetric contraction of the RV. The RA pressure may physiologically exceed the LA pressure during inspiration, coughing, sneezing, pressing or Valsalva manoeuvre, with a consequent increase of shunt flow from RA to LA, which is haemodynamically irrelevant. This RL shunt becomes clinically relevant due to transportation of particles from RA in the systemic circulation. Symptoms may vary depending on the affected vessel territory, leading to stroke in cerebral arteries or to myocardial infarction in coronary arteries, mesenterical infarction and other disorders.
In patients with pulmonary hypertension the shunt volume from RA to LA can be significantly increased. In this scenario, the shunt per se may become clinically relevant. A continuous shunt, in turn, increases the risk of the passage of small particles.
Patent foramen ovale
- Patent foramen ovale (PFO) is associated with cryptogenic ischaemic events
- Detection of PFO is usually performed by TEE or TTE
- Stroke is a leading cause of death and the leading cause of long-term disability
- The presence of an atrial septal aneurysm (ASA) is more frequent in patients with cryptogenic stroke compared to the general population
- Patients with platypnoe orthodeoxia are also linked to PFO
- The presence of a PFO increases the probability of decompression illness
- Several devices are approved for PFO closure in Europe
- Most of the devices have a double umbrella design
“PARADOXICAL” EMBOLISM
Stroke is a leading cause of death and the leading cause of long-term disability. In 30% to 40% of stroke patients no cause (e.g., atrial fibrillation, stenosis of the carotid arteries, aortic plaques) is detected at the time of manifestation of stroke. In some patients atrial fibrillation may be detected within the next weeks or months by use of several Holter ECGs or event recorder, reducing the number of patients with true cryptogenic events. In patients with cryptogenic stroke, the presence of a PFO is more frequent compared with a healthy population or patients with non-cryptogenic stroke [5555. Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357:2262-8. ]. The presence of an atrial septal aneurysm (ASA) is also more frequent in patients with cryptogenic stroke compared to the general population. The reason for the cerebral ischaemia in cryptogenic stroke patients includes the passage of small thrombi or thrombotic particles through the PFO from RA to LA. The term cryptogenic for paradoxical embolism via PFO in stroke patients has become a discussion point [127127. Pristipino C, Sievert H, D’Ascenzo F, Mas JL, Meier B, Scacciatella P, Hildick-Smith D, Gaita F, Toni D, Kyrle P, Thomson J, Derumeaux G, Onorato E, Sibbing D, Germonpré P, Berti S, Chessa M, Bedogni F, Dudek D, Hornung M, Zamorano J; European Association of Percutaneous Cardiovascular Interventions (EAPCI); European Stroke Organisation (ESO); European Heart Rhythm Association (EHRA); European Association for Cardiovascular Imaging (EACVI); Association for European Paediatric and Congenital Cardiology (AEPC); ESC Working group on GUCH; ESC Working group on Thrombosis; European Haematological Society (EHA). European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. EuroIntervention. 2019 Jan 20;14(13):1389-1402. ]. The event should be classified as PFO-related instead of “cryptogenic”.
In patients with atrial fibrillation the evidence of a thrombotic mass is not necessary to prove that the rhythm disorder is responsible for the stroke. The same is true for a patients with stroke and PFO without other reasons for the event. Other potential sources of embolism in cryptogenic stroke include the mitral and aortic valves, the left atrium and ventricle (thrombotic mass, calcification, mitral annulus calcification, endocarditis, etc.). A new clinical construct termed embolic stroke of undetermined source (ESUS) was recently introduced by the Cryptogenic Stroke/ESUS International Working Group as a potential therapeutic relevant entity with a possible indication for anticoagulation [102102. Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, Sacco RL, Connolly SJ; Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429-38. ]. Recent data support the use of anticoagulation in those patients compared to medical therapy. In a meta-analysis combining the data from NAVIGATE-ESUS, PICSS and CLOSE the risk of ischemic stroke with significantly reduced with anticoagulation compared with antiplatelet therapy [131131. Kasner SE, Swaminathan B, Lavados P, Sharma M, Muir K, Veltkamp R, Ameriso SF, Endres M, Lutsep H, Messé SR, Spence JD, Nedeltechev K, Perera K, Santo G, Olavarria V, Lindgren A, Bangdiwala S, Shoamanesh A, Berkowitz SD, Mundl H, Connolly SJ, Hart RG; NAVIGATE ESUS Investigators. Rivaroxaban or aspirin for patent foramen ovale and embolic stroke of undetermined source: a prespecified subgroup analysis from the NAVIGATE ESUS trial. Lancet Neurol. 2018 Dec;17(12):1053-1060. ].
There are numerous case reports in which large thrombi were detected by echocardiography in the PFO [5656. Kearney LG, Srivastava PM. Thrombus entrapped in a patent foramen ovale: a potential source of pulmonary and systemic embolism. Heart Lung. Circ 2010;19:58-60. , 5757. Mascarenhas V, Kalyanasundaram A, Nassef LA, Lico S, Qureshi A. Simultaneous massive pulmonary embolism and impending paradoxical embolism through a patent foramen ovale. J Am Coll Cardiol. 2009;53:1338. ]. Nevertheless, in patients with cryptogenic stroke/ESUS and PFO, no thrombus can usually be detected in the PFO. With careful clinical and sonographic evaluation, deep vein thrombosis can be verified in about 10% [5858. Lethen H, Flachskampf FA, Schneider R, Sliwka U, Köhn G, Noth J, Hanrath P. Frequency of deep vein thrombosis in patients with patent foramen ovale and ischemic stroke or transient ischemic attack. Am J Cardiol. 1997;80:1066-9. ]. Using magnetic resonance angiography the prevalence of pelvic vein thrombosis was 20% in cryptogenic stroke patients compared to 4% in patients with non-cryptogenic stroke (p=0.025) [5959. Cramer SC, Rordorf G, Maki JH, Kramer LA, Grotta JC, Burgin WS, Hinchey JA, Benesch C, Furie KL, Lutsep HL, Kelly E, Longstreth WT Jr. Increased pelvic vein thrombi in cryptogenic stroke: results of the Paradoxical Emboli From Large Veins in Ischemic Stroke (PELVIS) Study. Stroke. 2004;35:46-50. ]. A small thrombus with a diameter of about 3 mm can occlude the middle cerebral artery. Similarly a very small thrombus of 1 mm in diameter may occlude the cortical branches. At autopsy, mean PFO diameter was measured at 4.9 mm [5454. Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17-20. ], large enough to allow the passage of such small particles. Such small thrombotic particles do not have to originate from deep vein thrombosis, but may also occur spontaneously in superficial veins during immobility (e.g., flight, car ride) or minor trauma. Stroke patients with a history of immobility, defined as a trip over 4 hours in a sitting position, had a PFO significantly more often than stroke patients without a history of travel (45% versus 11%) [6060. Heckmann JG, Stadter M, Reulbach U, Duetsch M, Nixdorff U, Ringwald J. Increased frequency of cardioembolism and patent foramen ovale in patients with stroke and a positive travel history suggesting economy class stroke syndrome. Heart. 2006;92:1265-8. ].
The presence of an atrial septal aneurysm (ASA) facilitates the passage of thrombotic material from RA to LA. During a Valsalva manoeuvre with RA pressure exceeding the LA pressure, the ASA shifts to the LA increasing the size of the PFO. Furthermore, the ASA and PFO form a funnel, which enables thrombotic particles to enter the systemic circulation ( MOVING IMAGE 6-1 MOVING IMAGE 6-2). There is no standard definition for the presence of an ASA. With different types of measurement (M-mode, 2D echocardiography summarising the shift of the atrial septum in RA and LA [6161. Mügge A, Daniel WG, Angermann C, et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91:2785–92. ]), a mobility of more than 10 mm of the atrial septum is accepted in most institutions.
After passage of the PFO the thrombotic material show most often cerebral symptoms. On the one hand, the anatomy of the brachiocephalic trunk and left carotid artery at the outer curvature of the aortic arch facilitates thrombi to enter the cerebral circulation. On the other hand, ischaemia of a cerebral artery is much more clinically relevant compared with an often asymptomatic ischaemia in the kidney or liver. Asymptomatic myocardial infarctions were detected in about 10% of patients by delayed enhancement in CMR imaging in patients with cryptogenic stroke [6262. Wöhrle J, Kochs M, Hombach V, Merkle N. Prevalence of myocardial scar in patients with cryptogenic cerebral ischemic events and patent foramen ovale. JACC Cardiovasc Imaging. 2010;3:833-9. ]. The presence of prothrombotic coagulation - primarily Factor V Leiden mutation or prothrombin mutation - is an independent risk factor for cryptogenic stroke [6363. Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation. 2005;112:1063-72. ]. The thrombophilic diathesis increases the probability of the formation of thrombotic particles and deep vein thrombosis.
Meta-analysis of non-randomised trials has demonstrated that device implantation for PFO closure is superior to medical treatment [6464. Wöhrle J. Closure of patent foramen ovale after cryptogenic stroke. Lancet. 2006;368:350-2.
Nice meta-analysis regarding the impact of percutaneous closure of patent foramen ovale compared with medical therapy]. The optimal medical treatment strategy in patients with PFO after cryptogenic ischaemic event has not been defined, but recent data point out that anticoagulation may be superior to antiplatelet therapy. Published trials with anticoagulation or aspirin treatment have always demonstrated a lower event rate with anticoagulation [6565. Cujec B, Mainra R, Johnson DH. Prevention of recurrent cerebral ischemic events in patients with patent foramen ovale and cryptogenic strokes or transient ischemic attacks. Can J Cardiol. 1999;15:57-64. , 6666. Windecker S, Wahl A, Nedeltchev K, et al. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J Am Coll Cardiol. 2004;44:750-8. , 6767. Schluchlenz HW, Weihs W, Berghold A, Lechner A, Schmidt R. Secondary prevention after cryptogenic cerebrovascular events in patients with patent foramen ovale. Int J Cardiol. 2005;101:77-82. , 6868. Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Eff ect of medical treatment in stroke patients with patent foramen ovale. Circulation. 2002;105:2625-31. ]. With antiplatelet therapy patients with PFO and ASA were at increased risk of recurrent events [6969. Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste; Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740-6.
One of the most cited papers regarding treatment of patent foramen ovale, stressing the importance of an atrial septal aneurysm], whereas after device implantation the initial presence of an ASA had no more impact on long-term follow-up [7070. Wahl A, Krumsdorf U, Meier B, Sievert H, Ostermayer S, Billinger K, Schwerzmann M, Becker U, Seiler C, Arnold M, Mattle HP, Windecker S. Transcatheter treatment of atrial septal aneurysm associated with patent foramen ovale for prevention of recurrent paradoxical embolism in high-risk patients. J Am Coll Cardiol. 2005;45:377-80. This paper demonstrates that the initial presence of an atrial septal aneurysm in combination with a PFO is no more a risk factor after device implantation.
A residual right-to-left shunt was a predictor for recurrence].Several randomised trials compared device implantation with best medical therapy in patients with cryptogenic ischaemic events [7171. Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L; CLOSURE I Investigators. http://www.ncbi.nlm.nih.gov/pubmed/22417252 Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-9. , 9393. Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-100. , 9494. Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, Wahl A, Windecker S, Jüni P; PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-91. , 134134. Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim DH, Song JM, Kang DH, Kwon SU, Kang DW, Lee D, Kwon HS, Yun SC, Sun BJ, Park JH, Lee JH, Jeong HS, Song HJ, Kim J, Park SJ. Cryptogenic Stroke and High-Risk Patent Foramen Ovale: The DEFENSE-PFO Trial. J Am Coll Cardiol. 2018 May 22;71(20):2335-2342. , 136136. Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, Arquizan C, Béjot Y, Vuillier F, Detante O, Guidoux C, Canaple S, Vaduva C, Dequatre-Ponchelle N, Sibon I, Garnier P, Ferrier A, Timsit S, Robinet-Borgomano E, Sablot D, Lacour JC, Zuber M, Favrole P, Pinel JF, Apoil M, Reiner P, Lefebvre C, Guérin P, Piot C, Rossi R, Dubois-Randé JL, Eicher JC, Meneveau N, Lusson JR, Bertrand B, Schleich JM, Godart F, Thambo JB, Leborgne L, Michel P, Pierard L, Turc G, Barthelet M, Charles-Nelson A, Weimar C, Moulin T, Juliard JM, Chatellier G; CLOSE Investigators. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N Engl J Med. 2017;377:1011-1021. , 137137. Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med. 2017;377:1022-1032. , 138138. Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick-Smith D, Spence JD, Thomassen L; Gore REDUCE Clinical Study Investigators. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med. 2017;377:1033-1042. ]. In the first published CLOSURE-I trial 851 PFO patients were included comparing best medical treatment with device implantation for PFO closure. This study was designed as a superiority trial [7171. Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L; CLOSURE I Investigators. http://www.ncbi.nlm.nih.gov/pubmed/22417252 Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-9. ] and did not meet the primary endpoint. However, in every subgroup, recurrence rate was lower in patients with PFO device implantation compared with medical treatment although the follow-up was limited to 2 years and the sample size was adjusted several times due to difficulties in patient recruitment. In the PC-trial 314 patients were randomised to PFO closure with the Amplatzer PFO device compared with medical therapy. After a mean follow-up period of 4 years the composite primary endpoint (death, non-fatal stroke, TIA, peripheral embolism) occurred in 3.4% in the device group and in 5.2% in the medical treated population (p=0.34). Both stroke and TIA rates were numerically lower in the device group versus the medical treated population (0.5% versus 2.4%, p=0.14 ; 2.5% versus 3.3%, p=0.56) [9494. Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, Wahl A, Windecker S, Jüni P; PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-91. ]. The RESPECT study included 980 patients. Patients randomised to device closure were treated with the Amplatzer PFO device. Best medical treatment included antiplatelet medication in 75% and warfarin in 25% of patients. In the intention-to-treat analysis there was a trend towards a lower event rate with device implantation compared to medical treatment (hazard ratio with closure 0.49, 95% confidence interval (CI) 0.22-1.11; p=0.08). In the per-protocol and as treated cohort the event rate was significantly lower with Amplatzer PFO device compared with best medical treatment (per protocol hazard ratio 0.37 ; 95% CI 0.14-0.96, p=0.03 ; as treated hazard ratio 0.27 ; 95% CI 0.10-0.75, p=0.007). There were three subgroups with a significant reduction of the primary endpoint according to the intention-to-treat analysis. Patients with a substantial shunt at baseline, patients with atrial septal aneurysm at baseline and patients with antiplatelet therapy as planned medical regimen showed significantly lower event rates with Amplatzer PFO device implantation compared with medical treatment. Further randomized Trials with long-term follow-up reported consistently a significant reduction of recurrent stroke patients undergoing PFO closure compared to medical therapy [134134. Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim DH, Song JM, Kang DH, Kwon SU, Kang DW, Lee D, Kwon HS, Yun SC, Sun BJ, Park JH, Lee JH, Jeong HS, Song HJ, Kim J, Park SJ. Cryptogenic Stroke and High-Risk Patent Foramen Ovale: The DEFENSE-PFO Trial. J Am Coll Cardiol. 2018 May 22;71(20):2335-2342. , 136136. Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, Arquizan C, Béjot Y, Vuillier F, Detante O, Guidoux C, Canaple S, Vaduva C, Dequatre-Ponchelle N, Sibon I, Garnier P, Ferrier A, Timsit S, Robinet-Borgomano E, Sablot D, Lacour JC, Zuber M, Favrole P, Pinel JF, Apoil M, Reiner P, Lefebvre C, Guérin P, Piot C, Rossi R, Dubois-Randé JL, Eicher JC, Meneveau N, Lusson JR, Bertrand B, Schleich JM, Godart F, Thambo JB, Leborgne L, Michel P, Pierard L, Turc G, Barthelet M, Charles-Nelson A, Weimar C, Moulin T, Juliard JM, Chatellier G; CLOSE Investigators. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N Engl J Med. 2017;377:1011-1021. , 137137. Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med. 2017;377:1022-1032. , 138138. Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick-Smith D, Spence JD, Thomassen L; Gore REDUCE Clinical Study Investigators. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med. 2017;377:1033-1042. ]. Recent meta-analyses based on randomized trials demonstrated a substantial benefit for patients undergoing PFO closure compared to medical therapy with a significant reduced risk for recurrent stroke [129129. Nasir UB, Qureshi WT, Jogu H, Wolfe E, Dutta A, Majeed CN, Tan WA. Updated meta-analysis of closure of patent foramen ovale versus medical therapy after cryptogenic stroke. Cardiovasc Revasc Med. 2019 Mar;20(3):187-193. , 130130. Qiu B, Cai Y, Wang D, Lin J, Fan Y. Closure versus Medical Therapy for Patent Foramen Ovale in Patients with Cryptogenic Stroke: An Updated Meta-Analysis of Randomized Controlled Trials. J Stroke Cerebrovasc Dis. 2018;27:3463-3472. , 132132. Giacoppo D, Caronna N, Frangieh AH, Michel J, Andò G, Tarantini G, Kasel AM, Capodanno D, Byrne RA. Long-term effectiveness and safety of transcatheter closure of patent foramen ovale compared with antithrombotic therapy alone: a meta-analysis of six randomised clinical trials and 3,560 patients with reconstructed time-to-event data. EuroIntervention. 2018 Oct 20;14(8):857-867. doi: 10. 4244/EIJ-D-18-00341. , 133133. Reinthaler M, Ozga AK, Sinning D, Curio J, Al-Hindwan HS, Bäckemo Johansson J, Jung F, Lendlein A, Rauch G, Landmesser U. Revival of transcatheter PFO closure: A meta-analysis of randomized controlled trials - impact of shunt size and age. Am Heart J. 2018 Jul;201:95-102. ] but an increased risk of new onset atrial fibrillation [129129. Nasir UB, Qureshi WT, Jogu H, Wolfe E, Dutta A, Majeed CN, Tan WA. Updated meta-analysis of closure of patent foramen ovale versus medical therapy after cryptogenic stroke. Cardiovasc Revasc Med. 2019 Mar;20(3):187-193. , 132132. Giacoppo D, Caronna N, Frangieh AH, Michel J, Andò G, Tarantini G, Kasel AM, Capodanno D, Byrne RA. Long-term effectiveness and safety of transcatheter closure of patent foramen ovale compared with antithrombotic therapy alone: a meta-analysis of six randomised clinical trials and 3,560 patients with reconstructed time-to-event data. EuroIntervention. 2018 Oct 20;14(8):857-867. doi: 10. 4244/EIJ-D-18-00341. ]. A substantial shunt at baseline as well as the presence of an atrial septal aneruysm were well known risk factors for recurrent events in patients with cryptogenic stroke.
Long-term follow-up after device implantation for PFO closure to prevent a second event is essential to demonstrate non-inferiority compared with life-long medical treatment in PFO patients with cryptogenic ischaemic events or to demonstrate differences between various types of occluders. Several studies with long-term follow-up demonstrated that the risk of recurrent events after device implantation is low [9999. Eeckhout E, Martin S, Delabays A, Michel P, Girod G. Very long-term follow-up after percutaneous closure of patent foramen ovale. EuroIntervention. 2015;10:1474-9. , 110110. Mirzaali M, Dooley M, Wynne D, Cooter N, Lee L, Haworth P, Saha R, Gainsborough N, Hildick-Smith D. Patent foramen ovale closure following cryptogenic stroke or transient ischaemic attack: Long-term follow-up of 301 cases. Catheter Cardiovasc Interv. 2015;86:1078-84. , 111111. Luani B, Markovic S, Krumsdorf U, Rottbauer W, Wöhrle J. Efficacy of different devices for transcatheter closure of patent foramen ovale assessed by serial transoesophageal echocardiography and rates of recurrent cerebrovascular events in a long-term follow-up. EuroIntervention. 2015;11:85-91. , 128128. Seeger J, Uber A, Wöhrle J. Long-Term Outcome After Percutaneous Closure of Patent Foramen Ovale for
Cryptogenic Ischemic Events. J Invasive Cardiol. 2019;31:E242-E248. , 141141. Wintzer-Wehekind J, Alperi A, Houde C, Côté JM, Asmarats L, Côté M, Rodés-Cabau J. Long-Term Follow-Up After Closure of Patent Foramen Ovale in Patients With Cryptogenic Embolism. J Am Coll Cardiol. 2019;73:278-287. ]. After device implantation the risk of an embolic event was low with 0.28% annual/patient risk demonstrated in 232 consecutive patients followed for 7.6±2.4 years [9999. Eeckhout E, Martin S, Delabays A, Michel P, Girod G. Very long-term follow-up after percutaneous closure of patent foramen ovale. EuroIntervention. 2015;10:1474-9. ]. With 660 patients randomised to three different occluders there were significant differences with respect to complete closure after single device implantation and with respect to the primary endpoint (stroke, TIA, or amaurosis fugax) after 5 years of follow-up [9898. Hornung M, Bertog SC, Franke J, Taaffe M, Wunderlich N, Vaskelyte L, Hofmann I, Sievert H. Long-term results of a randomized trial comparing three different devices for percutaneous closure of a patent foramen ovale. Eur Heart J 2013. 34:3362-9. ].
OTHER DISORDERS LINKED TO PFO
Patients with platypnoea-orthodeoxia experience dyspnoea in an upright position while symptoms improve in the supine position (platypnoea). When standing, oxygen saturation is low (orthodeoxia) and it increases in recumbency. Platypnoea-orthodeoxia is caused by a position-dependent RL shunt even with normal pulmonary arterial pressure. In the upright position, there is a shift of the atrial septum facilitating a RL shunt through the PFO. A Eustachian valve may direct the blood from the inferior vena cava to the PFO. The shift of the atrial septum may be based on a dilation of the aortic root, elongation of the aorta kyphoscoliosis, or compression fractures of the vertebrae, but a pericardial effusion, a right-sided pneumonectomy, constrictive pericarditis, or paralysis of the diaphragm have also been linked to these symptoms [7373. Hara H, Virmani R, Ladich E, Mackey-Bojack S, Titus J, Reisman M, Gray W, Nakamura M, Mooney M, Poulose A, Schwartz RS. Patent foramen ovale: current pathology, pathophysiology, and clinical status. J Am Coll Cardiol. 2005;46:1768-76. , 7474. Johansson MC, Eriksson P, Dellborg M. The significance of patent foramen ovale. A current review of associated conditions and treatment. Int J Cardiol. 2009;13:17-24. ]. Patients with platypnoea-orthodeoxia can be cured by PFO occluder implantation and elimination of RL shunting.
The presence of a PFO increases the probability of decompression illness. The hydrostatic water pressure leads to a redistribution of more than 500 ml of blood in the thoracic vessels [7575. Muth CM, Tetzlaff K. Tauchen und Herz. Herz. 2004;29:406-13. ]. The volume increase in the right heart leads to an increase in right atrial pressure with an easier passage of blood from RA to LA including nitrogen bubbles possibly resulting in decompression illness. Even in asymptomatic sport divers multiple cerebral ischaemic lesions were documented by magnetic resonance imaging significantly more often in divers with PFO than in divers without PFO [7676. Schwerzmann M, Seiler C, Lipp E, Guzman R, Lövblad KO, Kraus M, Kucher N. Relation between directly detected patent forame ovale and ischemic brain lesions in sport divers. Ann Intern Med. 2001;134:21-4. ]. The risk of decompression illness is 5 times higher in divers with PFO compared with divers without PFO (5 versus 1 event per 10,000 dives) [7777. Torti SR, Billinger M, Schwerzmann M, Vogel R, Zbinden R,Windekcer S, Seiler C. Risk of decompression illness among 230 divers in relation to the presence and size of patent foramen ovale. Eur Heart J. 2004;25:1014-20. ]. Professional divers with PFO should undergo PFO closure as well as any divers after a decompression illness who intend to continue diving.
OTHER DISORDERS WITH A POSSIBLE LINK TO PFO
Other disorders such as migraine or high-altitude pulmonary oedema (HAPE) have been described in the context of a PFO. A PFO in patients with HAPE is 4 to 5 times more frequent than in patients without HAPE [7878. Allemann Y, Hutter D, Lipp E, Sartori C, Duplain H, Egli M, Cook S, Scherrer U, Seiler C. Patent foramen ovale and high-altitude pulmonary edema. JAMA. 2006;296:2954-8. ]. At high altitudes the detection of a PFO is associated with lower arterial oxygen saturation and a higher systolic pulmonary artery pressure. The size of the PFO is directly related to the amount of arterial hypoxia. The indication for closure of PFO here is analogous to the indication for closure in divers. The risk of acute mountain sickness was significantly higher in patients with compared to patients without PFO (63% vs 39%, p=0.034) [140140. West BH, Fleming RG, Al Hemyari B, Banankhah P, Meyer K, Rozier LH, Murphy LS, Coluzzi AC, Rusheen JL, Kumar P, Elashoff D, Tobis JM. Relation of Patent Foramen Ovale to Acute Mountain Sickness. Am J Cardiol. 2019;123:2022-2025. ].
The prevalence of PFO in patients with migraine is 2.5 times higher than in patients without migraine [7979. Schwedt TJ. The migraine association with cardiac anomalies, cardiovascular disease, and stroke. Neurol Clin. 2009;27:513-23. ]. The incidence of PFO in combination with migraine was reported in 40% to 70% compared to 25% in the general population. Conversely, people with PFO are 5 times more likely to have migraine than people without PFO. The occurrence of migraine was described in up to 65% of people with PFO versus 13% in the general population. In particular, migraine with aura is present in 13% to 50% of patients with PFO compared with 4% in the general population. Migraine is a complex disorder with multiple participating factors and cannot be reduced to one single cause. For patients with PFO and RL shunt, the passage of small thrombotic particles with focal cerebral ischaemia or the passage of vasoactive substances via the PFO has been linked to the clinical presentation of migraine. In patients with migraine and aura, a higher frequency of RL shunt was demonstrated by transcranial Doppler sonography compared to migraine patients without aura or healthy subjects [8080. Anzola GP, Magoni M, Guindani M, Rozzini L, Dalla Volta G. Potential source of cerebral embolism in migraine with aura: a transcranial Doppler study. Neurology. 1999;52:1622-5. ]. Non-randomised trials showed an improvement in symptoms after PFO closure in migraine patients [8282. Trabattoni D, Fabbiocchi F, Montorsi P, Galli S, Teruzzi G, Grancini L, Gatto P, Bartorelli AL. Sustained long-term benefit of patent foramen ovale closure on migraine. Catheter Cardiovasc Interv. 2011;77:570-4. , 8383. Wahl A, Praz F, Tai T, Findling O, Walpoth N, Nedeltchev K, Schwerzmann M, Windecker S, Mattle HP, Meier B. Improvement of migraine headaches after percutaneous closure of patent foramen ovale for secondary prevention of paradoxical embolism. Heart. 2010;96:967-73. ]. However, in two randomised studies (147 patients and 107 patients) comparing medical therapy with occluder implantation in order to close the PFO in migraine patients, no reduction of symptoms was observed [8181. Dowson A, Mullen MJ, Peatfield R, Muir K, Khan AA, Wells C, Lipscombe SL, Rees T, De Giovanni JV, Morrison WL, Hildick-Smith D, Elrington G, Hillis WS, Malik IS, Rickards A. Migraine Intervention with STARFlex Technology (MIST) trial: a prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation. 2008;117:1397-404. , 139139. Mattle HP, Evers S, Hildick-Smith D, Becker WJ, Baumgartner H, Chataway J, Gawel M, Göbel H, Heinze A, Horlick E, Malik I, Ray S, Zermansky A, Findling O, Windecker S, Meier B. Percutaneous closure of patent foramen ovale in migraine with aura, a randomized
controlled trial. Eur Heart J. 2016;37:2029-36 ]. In another randomized trial including 230 patients PFO closure did also not demonstrate a reduction in responder rate in patients with frequent migraine (PREMIUM study) compared to medical therapy [135135. Tobis JM, Charles A, Silberstein SD, Sorensen S, Maini B, Horwitz PA, Gurley JC. Percutaneous Closure of Patent Foramen Ovale in Patients With Migraine: The PREMIUM Trial. J Am Coll Cardiol. 2017;70:2766-2774. ].
DEVICES FOR PFO CLOSURE
Several devices are approved for PFO closure in Europe. Most of the devices have a double umbrella design with deployment of the LA disc, alignment of the LA disc at the left side of the atrial septum and then deployment of the RA disc ( MOVING IMAGE 7-1, MOVING IMAGE 7-2, MOVING IMAGE 7-3, MOVING IMAGE 7-4, MOVING IMAGE 7-5, MOVING IMAGE 7-6, MOVING IMAGE 7-7-1, MOVING IMAGE 7-7-2, MOVING IMAGE 7-8-1, MOVING IMAGE 7-8-2, MOVING IMAGE 7-9, MOVING IMAGE 7-10, MOVING IMAGE 7-11). Such double umbrella devices are e.g. the Amplatzer® PFO Occluder (AGA Medical Corporation, Plymouth, MN, USA), Helex® Septal Occluder (W.L. Gore & Associates, Inc., Flagstaff, AZ, USA), Solysafe® Septal Occluder (Carag AG, Baar, Switzerland), Occlutech device (Occlutech AB, Helsingborg, Sweden) and Cardia devices (Cardia, Eagan, MN, USA). This list may not be complete. For PFOs with long tunnels, specially designed devices had been developed (but are no longer available or did not get approval by the FDA) such as the Premere occluder (St. Jude Medical, Inc., St. Paul, MN, USA, ( MOVING IMAGE 8-1, MOVING IMAGE 8-2, MOVING IMAGE 8-3, MOVING IMAGE 8-4, MOVING IMAGE 8-5, MOVING IMAGE 8-6, MOVING IMAGE 8-7), the PFO SeptRx® device (SeptRx, Inc., Fremont, CA, USA) or the FlatStent™ PFO closure system (Coherex Medical, Salt Lake City, UT, USA, ( MOVING IMAGE 9-1, MOVING IMAGE 9-2, MOVING IMAGE 9-3, MOVING IMAGE 9-4). Possible advantages of intra-tunnel devices are minimal material in the LA and no, or minimal, distortion of the atrial septum with a subsequent lower risk of atrial arrhythmias or thrombus development on the device. However no clinical trial has demonstrated the superiority of in-tunnel devices compared with double umbrella occluders.
Especially for the double umbrella devices the implantation procedure is very similar to the implantation of an ASD device, whereas for other occluders the implantation technique can substantially differ ( MOVING IMAGE 7-1, MOVING IMAGE 7-2, MOVING IMAGE 7-3, MOVING IMAGE 7-4, MOVING IMAGE 7-5, MOVING IMAGE 7-6, MOVING IMAGE 7-7-1, MOVING IMAGE 7-7-2, MOVING IMAGE 7-8-1, MOVING IMAGE 7-8-2, MOVING IMAGE 7-9, MOVING IMAGE 7-10, MOVING IMAGE 7-11, MOVING IMAGE 8-1, MOVING IMAGE 8-2, MOVING IMAGE 8-3, MOVING IMAGE 8-4, MOVING IMAGE 8-5, MOVING IMAGE 8-6, MOVING IMAGE 8-7, MOVING IMAGE 9-1, MOVING IMAGE 9-2, MOVING IMAGE 9-3, MOVING IMAGE 9-4). If the PFO cannot be easily passed by the multipurpose catheter, a guidewire (e.g., large ASA) can be used to enter the LA ( MOVING IMAGE 7-1, MOVING IMAGE 7-2, MOVING IMAGE 7-3, MOVING IMAGE 7-4, MOVING IMAGE 7-5, MOVING IMAGE 7-6, MOVING IMAGE 7-7-1, MOVING IMAGE 7-7-2, MOVING IMAGE 7-8-1, MOVING IMAGE 7-8-2, MOVING IMAGE 7-9, MOVING IMAGE 7-10, MOVING IMAGE 7-11). For routine cases no echocardiographic imaging is required to implant the double-umbrella device [8585. Meier B. Closure of patent foramen ovale: technique, pitfalls, complications, and follow up. Heart. 2005;91:444-8. ]. Echocardiographic guidance is recommended in complex cases (large atrial setpal aneurysm, multiple perforations) in order to visualise the PFO while implanting the device.
In PFOs with a long stiff tunnel an incomplete opening of the right-sided disc can occur. In such anatomic situations, classical double umbrella devices can be used after ‘de-tunnelisation’ technique [8686. Spence MS, Khan AA, Mullen MJ. Balloon assessment of patent foramen ovale morphology and the modification of tunnels using a balloon detunnelisation technique. Catheter Cardiovasc Interv. 2008;71:222-8. ] by balloon dilation of the PFO, resulting in a shorter tunnel length but larger PFO diameter. For these PFOs transseptal puncture technique with device implantation has been described [8787. McMahon CJ, El Said HG, Mullins CE. Use of the transseptal puncture in transcatheter closure of long tunnel-type patent foramen ovale. Heart. 2002;88:E3. ], but the closure rate with this technique was limited [8888. Tande AJ, Knickelbine T, Chavez I, Mooney MR, Poulose A, Harris KM. Transseptal technique of percutaneous PFO closure results in persistent interatrial shunting. Catheter Cardiovasc Interv. 2005;65:295-300. ].
COMPLICATIONS AND RESIDUAL SHUNTING
For experienced operators the occurrence of adverse events or complications with device implantation for PFO closure is minimal. In principle, types of complication do not differ from those described for device implantation for ASD closure ( MOVING IMAGE 10-1, MOVING IMAGE 10-2, MOVING IMAGE 10-3, MOVING IMAGE 10-4, MOVING IMAGE 10-5, MOVING IMAGE 10-6). Post- procedural annual follow-up should include assessment of residual shunting. In a study including more than 500 patients, the risk of recurrent TIA, ischaemic stroke or peripheral embolism was 3.4 times higher in patients with residual shunting as compared to patients without residual shunting [8989. Wahl A, Kunz M, Moschovitis A, Nageh T, Schwerzmann M, Seiler C, Mattle HP, Windecker S, Meier B. Long-term results after fluoroscopy-guided closure of patent foramen ovale for secondary prevention of paradoxical embolism. Heart. 2008;94:336-41. ]. Optimal treatment for these patients has not been defined. Another procedure with a second device implantation is possible [9090. Diaz T, Cubeddu RJ, Rengifo-Moreno PA, Cruz-Gonzalez I, Solis-Martin J, Buonanno FS, Inglessis I, Palacios IF. Management of residual shunts after initial percutaneous patent foramen ovale closure: a single center experience with immediate and long-term follow-up. Catheter Cardiovasc Interv. 2010;76:145-50. , 9191. Majunke N, Wallenborn J, Baranowski A, Wunderlich N, Sievert H. Device closure of residual shunt after percutaneous closure of patent foramen ovale. EuroIntervention. 2010;5:833-7. , 9292. Rajani R, Lee L, Sohal M, Khawaja MZ, Hildick-Smith D. Redo patent foramen ovale closure for persistent residual right-to-left shunting. EuroIntervention. 2011;6:735-9. ] but also associated with additional risk of complications [122122. Sigler M, Horke A, Paul T, Uhlemann F. Solysafe Device Pushed Away by Amplatzer Septal Occluder After Closure of a
Residual Atrial Septal Defect. JACC Cardiovasc Interv. 2019 Jun 10;12(11):e95-e96
Solysafe Device Pushed Away by Amplatzer Septal Occluder After Closure of a]. However, a second or third device implantation for closure of a residual shunt via the PFO should not be considered in patients with a small RL bubble passage, since within the first 2 years after device implantation there is a continuous increase in closure rates.



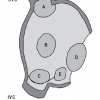

The online data supplement do not correspond to the ASD closure cour but to the alcoolisation of the hypertrophic cardiomyopathy...
Thank you for your message. The modification has been made. Best regards.
thanks