Clinically tested BRS
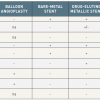
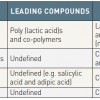
A number of manufacturing companies have developed scaffolds that after favorable outcomes in preclinical testing have transitioned to clinical studies ( Table 4). The front-runner in this process is the Absorb scaffold, which is presently the only BRS with randomized data for clinical outcome versus a new generation DES and has an estimated number of devices implanted in the order of 200,000[6868. Byrne RA, Stefanini GG, Capodanno D, Onuma Y, Baumbach A, Escaned J, Haude M, James S, Joner M, Juni P, Kastrati A, Oktay S, Wijns W, Serruys PW and Windecker S. Report of an ESC-EAPCI Task Force on the evaluation and use of bioresorbable scaffolds for percutaneous coronary intervention: executive summary. EuroIntervention. 2018;13:1574-1586. ]. However, following the negative results of the clinical trials (discussed later), the manufacturer decided on a worldwide halt to sales of the Absorb scaffold as of September 14, 2017.
IGAKI-TAMAI STENT
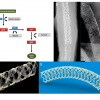
The Igaki-Tamai PLLA coronary stent was the first fully bioresorbable stent to be implanted in humans with complete degradation taking 18-24 months. The stent had a helical zigzag design, which differed from previous knitted patterns ( Figure 4). This resulted in less vessel wall injury during implantation and therefore less initial thrombus formation and reduced intimal hyperplasia.[6969. Tamai H, Igaki K, Kyo E, Kosuga K, Kawashima A, Matsui S, Komori H, Tsuji T, Motohara S and Uehata H. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation. 2000;102:399-404. ] The stent was mounted on a standard angioplasty balloon and was both thermal self-expanding and balloon expandable. Self-expansion occurred in response to heating the PLLA, which was achieved by using heated contrast (up to 70 °C) to inflate the delivery balloon. Stent expansion was further optimised by inflating the delivery balloon to 6-14 atm for 30 seconds, and the stent’s nominal size was ultimately achieved by continued self-expansion at 37°C in the 20-30 minutes after stent deployment. The stent had a standard length of 12 mm, and was available in diameters of 3 mm, 3.5 mm and 4 mm; the stent strut thickness was 0.17 mm. An 8F guiding catheter was required because the stent was initially constrained by a sheath that was removed once it was across the lesion. At either end of the stent to aid visualisation were two radio-opaque cylindrical gold markers (0.6 mm high by 0.18 mm in diameter) ( Figure 4). The first in man (FIM) study of the Igaki-Tamai stent (15 patients, 19 lesions, 25 stents), demonstrated no major adverse cardiovascular events (MACE) or ST within 30 days, and one repeat PCI at 6 months follow-up. Encouragingly, the loss index (late loss/acute gain) was 0.48mm, which was comparable to BMS, and demonstrated for the first time that BRS did not induce an excess of intimal hyperplasia. Furthermore, intravascular ultrasound (IVUS) imaging demonstrated no significant stent recoil at day one, and continued stent expansion was observed in the first three months of follow-up. The mean stent cross sectional area increased from 7.42±1.51 mm2 at baseline to 8.18±2.42 mm2 (P=0.086) at 3 months, and 8.13±2.52 mm2 at 6 months. [6969. Tamai H, Igaki K, Kyo E, Kosuga K, Kawashima A, Matsui S, Komori H, Tsuji T, Motohara S and Uehata H. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation. 2000;102:399-404. ] A second larger study of 50 elective patients (63 lesions, 84 stents) also showed promising results. IVUS performed at 3 years follow-up demonstrated the complete absence of stent struts, whilst angiographic analysis demonstrated a mean diameter stenosis of 25%, compared to 38%, 29% and 26% at 6, 12, and 24 months respectively. Clinical outcomes at 4-year follow-up showed rates of overall and MACE-free survival rates of 97.7% and 82.0%, respectively. [7070. Tsuji T, Tamai H, Igaki K, Hsu Y-S, Kosuga K, Hata T, OKada M, Nakamura T and Fujita S. Four-year Follow-up of the Biodegradable Stent (IGAKI-TAMAI Stent). Circ J. 2004;68:135. ]
At 10-year clinical follow-up, freedom from cardiac death, non-cardiac death and MACE were 98%, 87%, and 48% respectively.[7171. Nishio S. Long-term (>10 years) clinical outcomes of first-in-man biodegradable poly-l-lactic acid coronary stents. Paper presented at: EuroPCR; 2010; Paris. ] In the limited cases with serial angiographic follow-up, the MLD was stable: the mean MLD was 2.01 mm at 1 year and 2.06 mm at 10 years. There were two ST events: 1 subacute event occurring at day 5 possibly due to inadequate heparinisation at the time of PCI, and one very late ST event occurring in the sirolimus-eluting metallic stent which was later implanted proximal to the previously placed Igaki-Tamai stent. Angiographic and OCT images of the stent at 10-year follow-up in one anecdotal case are shown in Figure 4.
Despite these impressive results, the failure of the stent to progress was primarily related to the use of heat to induce self-expansion. There were concerns that this could cause necrosis of the arterial wall leading to excessive intimal hyperplasia or increased platelet adhesion leading to ST. [7272. Post MJ, de Graaf-Bos AN, van Zanten HG, de Groot PG, Sixma JJ and Borst C. Thrombogenicity of the human arterial wall after interventional thermal injury. J Vasc Res. 1996;33:156-63. ] None of these concerns were substantiated in the initial studies, however only low-risk patients were enrolled. After completion of the PERSEUS study,[7373. Biamino G, Schmidt A and Scheinert D. Treatment of SFA lesions with PLLA biodegradable stents: results of the PERSEUS study. J Endovasc Ther. 2005;12:5. ] the stent became available in Europe for peripheral use, however there are plans to review its use in coronary arteries. At present the stent has no drug elution, although preclinical studies of the polymeric stent eluting the tyrosine kinase antagonist ST 638 showed promising results.[5353. Yamawaki T, Shimokawa H, Kozai T, Miyata K, Higo T, Tanaka E, Egashira K, Shiraishi T, Tamai H, Igaki K and Takeshita A. Intramural delivery of a specific tyrosine kinase inhibitor with biodegradable stent suppresses the restenotic changes of the coronary artery in pigs in vivo. J Am Coll Cardiol. 1998;32:780-6. ]
MAGNESIUM ALLOY
Magnesium (Mg) is the fourth commonest cation within the human body; the total body content is ~20g, with 350mg required daily. It is essential for the synthesis of over 300 enzymes and is a co-factor for ATPase. A high dose infusion of Mg can cause vasodilatation; the promotion and recruitment of collaterals during ischaemia; and can function as a direct inhibitor of stent thrombosis.
The absorbable metallic stent (AMS-1) (Biotronik, Berlin, Germany) is the first metallic biodegradable stent, composed of 93% magnesium (approximate weight of 3.0x10 mm is 3 mg) and 7% rare earth metals ( Figure 5A-B). The first generation AMS-1 stent, which is balloon-expandable, is available in diameters of 3.0-3.5 and lengths of 10-15 mm. It has a high mechanical strength; and has comparable properties to stainless steel stents in view of its low elastic recoil (<8%), high collapse pressure (0.8 bar) and minimal shortening after inflation (<5%).[7474. Erbel R, Di Mario C, Bartunek J, Bonnier J, de Bruyne B, Eberli FR, Erne P, Haude M, Heublein B, Horrigan M, Ilsley C, Bose D, Koolen J, Luscher TF, Weissman N and Waksman R. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007;369:1869-75. ] The degradation of Mg produces an electronegative charge that results in the stent being hypo-thrombogenic.[7575. Heublein B, Rohde R, Kaese V, Niemeyer M, Hartung W and Haverich A. Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology?. Heart. 2003;89:651-6. ] In the porcine model, the AMS-1 has been shown to be rapidly endothelialised, and within 60 days is largely degraded into inorganic salts, with little associated inflammatory response.[7676. Waksman R, Pakala R, Kuchulakanti PK, Baffour R, Hellinga D, Seabron R, Tio FO, Wittchow E, Hartwig S, Harder C, Rohde R, Heublein B, Andreae A, Waldmann KH and Haverich A. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries. Catheter Cardiovasc Interv. 2006;68:607-17; discussion 618-9. discussion 618-9. ] After promising initial preclinical trials, and successful deployment in 20 patients with critical limb ischaemia,[7777. Peeters P, Bosiers M, Verbist J, Deloose K and Heublein B. Preliminary results after application of absorbable metal stents in patients with critical limb ischemia. J Endovasc Ther. 2005;12:1-5. ] the PROGRESS AMS trial was performed. This was a multicenter, non-randomized, prospective study, which assessed the efficacy and safety of the stent in 63 patients (71 stents) with single de novo lesions.
The study reached the primary endpoint, a composite of cardiac death, non-fatal MI, and clinically driven TLR (=MACE) at 4 months, by achieving a rate of MACE of 23.8%; the rate of MACE at 12 months was 26.7%. The study demonstrated the safety of the AMS-1 with no reported death, MI or ST during 12 months follow-up; in addition, there was a return of vessel vaso-reactivity. Unfortunately, the rate of any TLR was disappointing: 23.8% at 4 months, and 45% at 12 months. [7474. Erbel R, Di Mario C, Bartunek J, Bonnier J, de Bruyne B, Eberli FR, Erne P, Haude M, Heublein B, Horrigan M, Ilsley C, Bose D, Koolen J, Luscher TF, Weissman N and Waksman R. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007;369:1869-75. ] QCA and IVUS have both provided important information regarding the mechanism of this restenosis, all of which have had important implications on future stent designs. QCA showed an acute gain post stenting of 1.41 mm (SD 0.46 mm), and a reduction in the mean diameter stenosis from 61.5% pre-stenting (SD 13.1%), to 12.7% (SD 5.6%) post-stenting. At 4 months follow-up the mean diameter stenosis was 48.4% (SD 17.0%) and the in-stent late loss was 1.08 mm (SD 0.49 mm). Immediately after post-stenting balloon dilatation, which was required in 42 patients, IVUS demonstrated that the mean stent cross sectional area (6.2±1.5 mm2), and mean stent volume (116.5±40.2 mm3) were both lower than that seen with standard metallic stents deployed in similar sized vessels. These results were consistent with QCA results, and were probably related to both the lower radial force of the Mg alloy compared to stainless steel, and by immediate vessel recoil after stent implantation. At four months follow-up IVUS demonstrated that only small remnants of the original struts were visible, which were all well embedded into the intima. IVUS also showed a 42% reduction in the area delineated by the external elastic membrane, suggesting that early vessel recoil was indeed the primary cause of the high late loss, and restenosis. This vessel recoil was attributable to the loss of radial force from the early, rapid AMS-1 stent degradation, such that no stent support was available to oppose constrictive remodeling; a natural response of the vessel to injury. Exacerbating the problem was evidence to suggest that stent degradation was possibly faster than previously anticipated. A repeat IVUS in one patient only three weeks post AMS-1 implantation showed 50% of struts were already degraded.[7878. Waksman R, Erbel R, Di Mario C, Bartunek J, de Bruyne B, Eberli FR, Erne P, Haude M, Horrigan M, Ilsley C, Bose D, Bonnier H, Koolen J, Luscher TF and Weissman NJ. Early- and long-term intravascular ultrasound and angiographic findings after bioabsorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc Interv. 2009;2:312-20. ] Other factors besides constrictive recoil contributing to the luminal loss seen at follow-up were intra-stent tissue growth (intra-stent neointima) (41%) and tissue growth behind the stent struts (extra-stent neointima) (13.5%) ( Figure 5E). Reassuringly long-term data from angiographic and IVUS performed in the eight patients who did not experience an event at 4 months have shown that there was no evidence of either later recoil or late development of neointima. In fact, in some patients evidence was seen of neointimal regression, and/or an increase in vessel and lumen volume.[7878. Waksman R, Erbel R, Di Mario C, Bartunek J, de Bruyne B, Eberli FR, Erne P, Haude M, Horrigan M, Ilsley C, Bose D, Bonnier H, Koolen J, Luscher TF and Weissman NJ. Early- and long-term intravascular ultrasound and angiographic findings after bioabsorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc Interv. 2009;2:312-20. ] Of note, the AMS stent is proven to be MRI compatible[7979. Eggebrecht H, Rodermann J, Hunold P, Schmermund A, Bose D, Haude M and Erbel R. Images in cardiovascular medicine. Novel magnetic resonance-compatible coronary stent: the absorbable magnesium-alloy stent. Circulation. 2005;112:e303-4. ] and furthermore, the vasodilator function after nitroglycerin injection was restored in the treated segment at follow-up.[8080. Ghimire G, Spiro J, Kharbanda R, Roughton M, Barlis P, Mason M, Ilsley C, Di Mario C, Erbel R, Waksman R and Dalby M. Initial evidence for the return of coronary vasoreactivity following the absorption of bioabsorbable magnesium alloy coronary stents. EuroIntervention. 2009;4:481-4. ]
The results from the patients enrolled in studies of the AMS-1 stent demonstrated it was safe for use in both coronary and peripheral vessels.[7474. Erbel R, Di Mario C, Bartunek J, Bonnier J, de Bruyne B, Eberli FR, Erne P, Haude M, Heublein B, Horrigan M, Ilsley C, Bose D, Koolen J, Luscher TF, Weissman N and Waksman R. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007;369:1869-75. , 7777. Peeters P, Bosiers M, Verbist J, Deloose K and Heublein B. Preliminary results after application of absorbable metal stents in patients with critical limb ischemia. J Endovasc Ther. 2005;12:1-5. , 8181. Bosiers M, Peeters P, D’Archambeau O, Hendriks J, Pilger E, Duber C, Zeller T, Gussmann A, Lohle PN, Minar E, Scheinert D, Hausegger K, Schulte KL, Verbist J, Deloose K and Lammer J. AMS INSIGHT--absorbable metal stent implantation for treatment of below-the-knee critical limb ischemia: 6-month analysis. Cardiovasc Intervent Radiol. 2009;32:424-35. ] The stent was resorbed as intended, with no undue safety concerns. The disappointingly high TLR rate compared to standard BMS and DES have lead to modifications in the future stent’s design. These aim to prolong degradation and enable drug elution, and thereby reduce restenosis that was partly due to negative remodeling, and partly due to an excessive healing response. Two new stents have been developed: AMS- 2 and AMS-3 ( Figure 5C-D). The AMS-2 stent is designed to overcome some of the problems of excessive vessel recoil seen with AMS-1. It provides prolonged mechanical stability, which has been achieved by using a different magnesium alloy, which has not only a higher collapse pressure of 1.5bar, compared to 0.8bar with AMS-1, but also a slower degradation time. In addition, the stent surface has been modified; the stent strut thickness has been reduced from 165μm to 125 μm and the shape of the strut in crosssection has been altered from rectangular to square (improving radial strength). These changes have in animal models prolonged scaffolding and stent integrity, improved radial strength and reduced neointima proliferation.
The AMS-3 stent (DREAMS 1), used a refined, WE43 alloy (93% magnesium and 7% rare earth elements) with slower bioresorption time (9-12 months), higher radial strength, 125 microns struts with rectangular shape and paclitaxel elution for 3 months. It was tested in BIOSOLVE I study, which has shown 100% procedural success with device. The device has shown good safety profile with no death or stent thrombosis at 12 months and only one MI [8282. Haude M, Erbel R, Erne P, Verheye S, Degen H, Bose D, Vermeersch P, Wijnbergen I, Weissman N, Prati F, Waksman R and Koolen J. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet. 2013. ]. Regarding efficacy end points, the new design has also shown considerable improvements[8282. Haude M, Erbel R, Erne P, Verheye S, Degen H, Bose D, Vermeersch P, Wijnbergen I, Weissman N, Prati F, Waksman R and Koolen J. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet. 2013. ] with angiographic in-stent LLL of 0.52±0.49mm at 1-year [8383. Haude M, Erbel R, Erne P, Verheye S, Degen H, Bose D, Vermeersch P, Wijnbergen I, Weissman N, Prati F, Waksman R and Koolen J. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet (London, England). 2013;381:836-44. ], while at 3 years TLR rates reached 6.6%, with no cases of CD, TV-MI or ScT[8383. Haude M, Erbel R, Erne P, Verheye S, Degen H, Bose D, Vermeersch P, Wijnbergen I, Weissman N, Prati F, Waksman R and Koolen J. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet (London, England). 2013;381:836-44. , 8484. Haude M. Two-year clinical data and multi-modality imaging results up to 1-year follow-up of the BIOSOLVE-I study with the paclitaxel-eluting bioabsorbable magnesium scaffold (DREAMS). Paper presented at: EuroPCR; 2013; Paris, France. ].
A newer iteration (2nd generation DREAMS, marketed as Magmaris) made of the same alloy and design but with a strut thickness of 150 µm has been recently developed. This device elutes sirolimus instead of paclitaxel, has tantalum radiopaque markers at both ends and a modified, electropolished strut cross-sectional profile. These modifications result not only in slower dismantling and resorption rate but also improved visibility, higher bending flexibility, increased deployment diameter and higher acute radial force and fracture resistance compared to the previous generations. Magmaris was evaluated in the international multicenter FIM BIOSOLVE II trial (n=123). In-scaffold LLL was 0.27±0.37mm at 6 months (primary end-point) and 0.39±0.27 mm at 12-months follow-up. TLF -a composite of CD, TV-MI, clinically indicated TLR (CI-TLR) and CABG- occurred in 4 patients (3%), consisting of 1 CD, 1 TV-MI, and 2 CI-TLR[8585. Haude M, Ince H, Abizaid A, Toelg R, Lemos PA, von Birgelen C, Christiansen EH, Wijns W, Neumann FJ, Kaiser C, Eeckhout E, Lim ST, Escaned J, Garcia-Garcia HM and Waksman R. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet. 2016;387:31-9. ]. Long-term results of the Magmaris BRS have been recently published in 184 patients, including pooled follow-up data at 24 months from BIOSOLVE II and at 6 months from the pilot BIOSOLVE III trial (n=61), demonstrating TLF rates of 5.6% at 2 years[8686. Haude M, Ince H, Kische S, Abizaid A, Tolg R, Alves Lemos P, Van Mieghem NM, Verheye S, von Birgelen C, Christiansen EH, Wijns W, Garcia-Garcia HM and Waksman R. Sustained safety and clinical performance of a drug-eluting absorbable metal scaffold up to 24 months: pooled outcomes of BIOSOLVE-II and BIOSOLVE-III. EuroIntervention. 2017;13:432-439. ]. Most importantly, up to now no cases of definite or probable ScT have been reported in patients treated with any of the 3 iterations of this magnesium-based BRS.
TYROSINE POLYCARBONATE: THE REVA STENT
The Reva Medical (San Diego, CA) BRS is a tyrosine poly (desaminotyrosyltyrosine ethyl ester) carbonate stent, which degrades, as summarized in Figure 6A, into water, carbon dioxide, and ethanol; in addition, tyrosine is metabo- lized by the Krebs cycle. The first two iterations of the first-generation of the Reva Medical BRS ( Figure 6B, C), showed high TLR and low technical success rates in the RESORB FIM trial (bare form)[8787. Abizaid A. The REVA Tyrosine-Derived Polycarbonate Bioabsorbable Stent: Lessons Learned and Future Directions. Presented at TCT 2009, San Francisco, USA. ] and the RESTORE I trial (sirolimus-eluting version, ReZolve BRS) respectively[8787. Abizaid A. The REVA Tyrosine-Derived Polycarbonate Bioabsorbable Stent: Lessons Learned and Future Directions. Presented at TCT 2009, San Francisco, USA. ]. A third iteration, ReZolve BRS 2 ( Figure 6D), is being tested in the RESTORE II trial. Preliminary results in 67 patients at 6 months showed 3 MACE cases and the trial is on-going[8888. Muller D. RESTORE II trial: clinical results from the ReZolve2 sirolimus-eluting bioresorbable scaffold. Presented at EuroPCR 2014, Paris, France. ]. However, the problematic deliverability of the device pushed the company to feature a conventional balloon-expandable system for its next and current iteration of the scaffold, named Fantom ( Figure 6E). The most characteristic feature of the Fantom BRS is that it contains iodine covalently bound to its desamino-tyrosine polycarbonate backbone, which makes it intrinsically radio-opaque and may decrease the need for intravascular imaging during follow-up. Furthermore, it has a wide expandability range while the time required for full reabsorption is significantly longer compared to PLLA based scaffolds (3 years), but more than 80% molecular weight loss takes place within the first year. Clinically, the Fantom BRS was initially tested in a small FIM trial[8989. Ribamar JC Jr. Initial results of the FANTOM I trial: a first-in-man evaluation of a novel, radiopaque sirolimus-eluting BRS. Presented at EuroPCR 2016, Paris, France. ] and subsequently in the FANTOM II trial which included 240 patients split in two cohorts. Acute technical and procedural success was observed in 95.8% and 99.1% respectively of the cohort A patients. The 6 months angiographic and clinical primary endpoints for cohort A have been formally published [9090. Abizaid A, Carrie D, Frey N, Lutz M, Weber-Albers J, Dudek D, Chevalier B, Weng SC, Costa RA, Anderson J and Stone GW. 6-Month Clinical and Angiographic Outcomes of a Novel Radiopaque Sirolimus-Eluting Bioresorbable Vascular Scaffold: The FANTOM II Study. JACC Cardiovasc Interv. 2017;10:1832-1838. ]. Recently, 12 months results for the entire cohort showing a MACE rate of 4.2% with a single ScT (subacute)[9191. Abizaid A. The FANTOM II Study: First Report for the 12-month clinical outcomes of the Fantom sirolimus-eluting bioresorbable scaffold. Presented at EuroPCR 2017, Paris, France. ] and 24 months results for 125 patients showing a MACE rate of 5.6% and a single very late ScT were reported[9292. Costa RA. FANTOM II Trial: Safety & Performance Study of the Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – First Report on Initial 24 Month Outcomes. Presented at TCT 2017, Denver, USA. ]. In addition to the latest clinical follow up, a 25-patients’ subset in the trial underwent angiographic imaging, showing a final in-scaffold late loss of 0.25 mm, which is in the desired range of 0.2 mm to 0.4 mm. CE Mark approval for the Fantom scaffold was received in April 2017, while cohort C of the FANTOM II trial is currently enrolling patients with more complex lesions. Reva has also announced a new iteration, named Fantom Encore, with a strut thickness of 95 microns in 2.5 mm diameter; its performance results are awaited.
POLY (ANHYDRIDE ESTER) SALICYLIC ACID: THE IDEAL™ STENT
The IDEALTM biodegradable stent (Bioabsorbable Therapeutics, Inc. Menlo Park, CA, USA) consists of a backbone of poly-anhydride ester based on salicylic acid and adipic acid anhydride, and an 8.3μg/mm coating of sirolimus ( Figure 7), potentially giving the stent both anti-inflammatory and anti-proliferative properties. The vascular compatibility and efficacy of this biodegradable salicylate-based polymer has previously been demonstrated in the porcine model. Most notably the polymer was associated with reduced inflammation when compared to a standard BMS and Cypher stent. [9393. Jabara R, Chronos N and Robinson K. Novel bioabsorbable salicylate-based polymer as a drug-eluting stent coating. Catheter Cardiovasc Interv. 2008;72:186-94. ] This was very likely to be due to the anti-inflammatory properties of salicylic acid following absorption by the vessel wall after its release. Drug elution was found to be complete after 30 days, whilst complete stent degradation occurred over a 9-12 month period. The 8F compatible, balloon expandable stent is radio-opaque, and does not require any special storage. Its radial strength is greater than a BMS, but considerably less than the Cypher stent. Histological analyses from pre-clinical studies of the IDEAL stent in pigs have confirmed the absence of excessive thrombosis, or inflammatory reaction, and satisfactory healing of the vessel. Furthermore, the promising mechanical properties of the stent were confirmed with well apposed stent struts observed at follow-up. [9494. Jabara R, Pendyala L, Geva S, Chen J, Chronos N and Robinson K. Novel fully bioabsorbable salicylate-based sirolimus-eluting stent. EuroIntervention. 2009;5:F58-F64. ] In July 2009, the 11 patients enrolled in the multi-centre FIM Whisper study completed their 12 month follow-up. Primary results have shown stent safety, and confirmed structural integrity of the stent with no evidence of acute or chronic recoil. Unfortunately, insufficient neo-intimal suppression has been demonstrated.[9494. Jabara R, Pendyala L, Geva S, Chen J, Chronos N and Robinson K. Novel fully bioabsorbable salicylate-based sirolimus-eluting stent. EuroIntervention. 2009;5:F58-F64. ] This is likely to be the consequence of inadequate drug dosing, particularly when considering that the surface area dose of sirolimus is only a quarter of that found on the Cypher stent. The rapid elution of sirolimus may also be a contributing factor.
A second-generation stent has been developed, with a higher dose of sirolimus, and a slower drug release pattern. Furthermore, the stent design has been optimized; which has resulted in a reduced crossing profile (6F compatible), and thinner struts (175μm). Although the program was on hold in early 2009, the program was resumed and the new device is currently undergoing preclinical evaluation [9595. Jabara R. Bio-mechanical properties and ABC of bioresorption of adipic acid. Paper presented at: PCR focus group on bioresorbable vascular scaffolds; 2012; Rotterdam, The Netherlands. ].
Novolimus-eluting PLLA scaffold: The DESolve scaffold
The DESolve family of PLLA-based BRS (Elixir Medical, Sunnyvale, CA, US) includes the 1st generation device with 150 μm strut thickness and the 2nd generation device which has a strut thickness of 120 μm and elutes novolimus, an active metabolite of sirolimus. In both iterations, the scaffold is coated with a matrix of polylactide-based biodegradable polymer and antiproliferative drug, which is applied using a proprietary technique[9696. Sotomi Y, Onuma Y, Collet C, Tenekecioglu E, Virmani R, Kleiman NS and Serruys PW. Bioresorbable Scaffold: The Emerging Reality and Future Directions. Circulation research. 2017;120:1341-1352. ]. The important features of the DESolve BRS are intrinsic self-correcting deployment properties that become operative in the event of minor strut malapposition and ability to over-expand across a wide range of diameters without risk of strut fracture[9797. Ormiston JA, Webber B, Ubod B, Darremont O and Webster MW. An independent bench comparison of two bioresorbable drug-eluting coronary scaffolds (Absorb and DESolve) with a durable metallic drug-eluting stent (ML8/Xpedition). EuroIntervention. 2015;11:60-7. ]. The first iteration was initially tested as a myolimus eluting device, in the small (n=16), FIM DESolve I trial, which showed encouraging imaging and clinical results at 6 and 12 months respectively[9898. Verheye S, Ormiston JA, Stewart J, Webster M, Sanidas E, Costa R, Costa JR, Jr., Chamie D, Abizaid AS, Pinto I, Morrison L, Toyloy S, Bhat V, Yan J and Abizaid A. A next-generation bioresorbable coronary scaffold system: from bench to first clinical evaluation: 6- and 12-month clinical and multimodality imaging results. JACC Cardiovasc Interv. 2014;7:89-99. ]. Subsequently, the novolimus eluting version was tested in the larger (n=126), single arm DESolve NX trial, in which the primary end point of in-stent LLL at 6 months was 0.21±0.32 mm (113 patients with paired analysis) with 98.7% struts coverage at the same time point. At 5 years, the rates of MACE, TV-MI, CD and clinically indicated TLR were 9%, 1.6%, 3.3% and 4.1%, respectively. No cases of definite ScT were observed [9999. Verheye S. Prospective, Multi-Center Evaluation of the DESolve Novolimus-Eluting Bioresorbable Coronary Scaffold: Imaging Outcomes and 5-Year Clinical and Imaging Results. Presented at TCT 2017, Denver, USA. ]. A single, retrospective, comparative analysis between this device and the Absorb BRS using propensity-score matching is also available, showing similar outcomes between the two devices with regards to 1-year rates of TLF, TLR, CD and definite ScT[100100. Wiebe J, Dorr O, Ilstad H, Husser O, Liebetrau C, Boeder N, Bauer T, Mollmann H, Kastrati A, Hamm CW and Nef HM. Everolimus- Versus Novolimus-Eluting Bioresorbable Scaffolds for the Treatment of Coronary Artery Disease: A Matched Comparison. JACC Cardiovasc Interv. 2017;10:477-485. ]. The second iteration was tested in the relatively small (n=50), single arm DESolveCx trial, showing in-scaffold LLL of 0.19±0.25 mm at 6 months and no MACE at 12 months’ follow-up[101101. Verheye S. Desolve Nx, Cx, and Amity: A Family of Progressively Thinner-Strut PLLA-Based BRS With Novel Properties. Presented at TCT 2017, Denver, USA. ]. Both iterations have CE mark while the company is currently developing a 3rd generation device (Desolve NXT plus) with 120 strut thickness and a contoured strut design for enhanced acute performance.
Arterial remodelling technologies (ART) PLLA stent
The ART stent (Noisy le Roi, France) is a poly-lactic acid (PLA) BRS which has a unique combination of L and D isomers resulting in a highly biocompatible and haemocompatible stent. It is produced using molecular weight preserving technology, which preserves its mechanical properties and avoids premature degradation. The balloon expandable 6F compatible ART stent provides suitable mechanical strength for 5- 7 months to resist vessel recoil, and complete monomer resorption occurs within 18 months. In preclinical studies positive remodelling (vessel enlargement) has been demonstrated to occur between 3 and 6 months. The device has no antiproliferative drug and the developers believe that it is not necessary as the positive remodelling will accommodate intervention-induced intimal hyperplasia and in addition intimal hyperplasia reaches a peak at about 6 months then regresses [102102. Virmani R, Kolodgie F, Farb A and Lafont A. Drug eluting stents: are human and animal studies comparable?. Heart. 2003;89:133-138. ]. Absence of antiproliferative coating may also permit more rapid return of normal endothelial coverage, function and maintain an efficient endothelial barrier limiting neoatherosclerosis [103103. Nakazawa G, Finn A, Vorpahl M, Ladich E, Kolodgie F and Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. Journal of the American College of Cardiology 2011;57:390-398. , 104104. Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani S, Ladich E, Kolodgie F, Finn A and Virmani R. The Pathology of Neoatherosclerosis in Human Coronary Implants. Bare-Metal and Drug-Eluting Stents. Journal of the American College of Cardiology. 2011;57:1314-1322. ]. The first-in-man trial, ART-DIVA is undergoing to evaluate the safety of the ART scaffold for the treatment of patients with single de novo lesion of a native coronary artery with mandatory balloon predilatation. The scaffold received CE Mark in May 2015, but was never marketed in Europe and in February 2017 Terumo announced the termination of the product’s co-development with ART.
First-in-man trials
- Since 1999, Igaki-Tamai stent (PLLA), absorbable metallic stent (Magnesium), REVAstent (tyrosine polycarbonate) and IDEAL stent (adipic acid and salicylate) have been tested in first-in-man trials
- While these first-in-man trials consistently showed the safety of these devices, high revascularisation rates remained a clinical issue. The second-generation BRSs with drug elution (Magnesium, REVA and IDEAL) have been developed and are being/will be tested in clinical trials
EVEROLIMUS-ELUTING PLLA scaffold: the ABSORB BVS scaffold
The BVS stent design is characterized by a crossing profile of 1.4 mm with circumferential hoops of PLLA. The struts are 150 microns thick and are either directly joined or linked by straight bridges ( Figure 8). Both ends of the stent have two adjacent radio-opaque platinum markers. The radial strength, measured in a water bath at 37°C using IVUS and by flat plate compression of 10, 15 and 25 % is 0.048±0.007 N/mm2, 0.070±0.008 N/mm2 and 0.106±0.009 N/mm2, while comparative values for a contemporary bare-metal stent (Vision coronary stent, Abbott Vascular, Santa Clara, CA, USA) is 0.073±0.011 N/mm2, 0.114±0.012 N/mm2 and 0.155±0.012 N/mm2, respectively.[105105. Ormiston JA, Serruys PW, Regar E, Dudek D, Thuesen L, Webster MW, Onuma Y, Garcia-Garcia HM, McGreevy R and Veldhof S. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899-907. ]
The backbone of BVS device is made of semicrystalline polymer called Poly-L-lactic acid.[105105. Ormiston JA, Serruys PW, Regar E, Dudek D, Thuesen L, Webster MW, Onuma Y, Garcia-Garcia HM, McGreevy R and Veldhof S. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899-907. ] The coating consists of poly D,L-lactide, which is a random copolymer of D- and L-lactic acid with lower crystallinity than the BVS backbone. The coating contains and controls the release of the anti-proliferative drug, everolimus. Both PLLA and PDLLA are fully bioresorbable. During bioresorption, the long chains of PLLA and PDLLA are progressively shortened as ester bonds between lactide repeat units are hydrolyzed, and small particles less than 2 µm in diameter are phagocytosed by macrophages. Ultimately, PLLA and PDLLA degrade to lactic acid, which is metabolized via the Krebs cycle. In a porcine coronary artery model, mass decreased with time; 30% at 12 months increasing to 60% at 18 months and to 100% at 24 months post implantation.
ABSORB COHORT A AND B TRIALs
The ABSORB BRS has a PLLA-based backbone with a PDLLA coating. The later contains everolimus with a coating to drug ratio of 1:1 and controls its release in a purely diffusion-related fashion[9696. Sotomi Y, Onuma Y, Collet C, Tenekecioglu E, Virmani R, Kleiman NS and Serruys PW. Bioresorbable Scaffold: The Emerging Reality and Future Directions. Circulation research. 2017;120:1341-1352. ]. There have been two iterations of this device. The first-generation, 1.0, was tested in the FIM ABSORB A trial (n = 30), demonstrating very late lumen enlargement (from 6 months to 2 years), restoration of vasomotion and endothelial function at 2 years[4040. Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miquel-Hebert K, Veldhof S, Webster M, Thuesen L and Dudek D. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897-910. , 106106. Garcia-Garcia HM, Gonzalo N, Pawar R, Kukreja N, Dudek D, Thuesen L, Ormiston JA, Regar E and Serruys PW. Assessment of the absorption process following bioabsorbable everolimus-eluting stent implantation: temporal changes in strain values and tissue composition using intravascular ultrasound radiofrequency data analysis. A substudy of the ABSORB clinical trial. EuroIntervention. 2009;4:443-8. ], with a MACE rate of 3.4% and no ScT at 5 years[107107. Onuma Y, Dudek D, Thuesen L, Webster M, Nieman K, Garcia-Garcia HM, Ormiston JA and Serruys PW. Five-year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB cohort A trial. JACC Cardiovasc Interv. 2013;6:999-1009. ]. At 6-month follow-up, the angiographic in-stent late lumen loss (LLL) was 0.44 mm, attributed mainly to a mild reduction of the stent area (-11.8%) as measured by IVUS (chronic recoil). The neointimal area was small (0.30 mm2), with a minimal area obstruction of 5.5%, demonstrating effective suppression of restenosis by everolimus[105105. Ormiston JA, Serruys PW, Regar E, Dudek D, Thuesen L, Webster MW, Onuma Y, Garcia-Garcia HM, McGreevy R and Veldhof S. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899-907. ]. In contrast to the radio-opaque metallic stents that hinder in-stent luminal assessment with MSCT because of blooming artefacts, the polymeric BVS stent is radiolucent except for two metallic markers located at both extremities of the stent that facilitates the luminal and length assessment of the scaffold with MSCT ( Figure 8). Due to the non-invasive nature of MSCT, 25 patients underwent MSCT imaging 18 months after the index procedure, which represents a larger number of patients as compared to the patients who underwent conventional coronary angiography at 24 months (n=19). Out of the 25 patients who underwent MSCT, quantitative analysis was feasible in 24. According to MSCT measurements, the mean luminal area was 5.2 ± 1.3mm2, the minimal lumen area was 3.6 ± 0.9 mm2 and the mean area stenosis was 34 ±15%. The calculated mean diameter stenosis was 19 ± 9 % and was in fact not too much at variance with the results of invasive quantitative coronary angiography (% diameter stenosis, 27±11%).
At 5 years, 18 patients underwent MSCT angiography. All scaffolds were patent with an median minimal lumen area of 3.25 mm2 (interquartile range [IQR] 2.20, 4.33). Non-invasive FFR analysis was feasible in 13 of the 18 scans, which yielded a median distal FFR of 0.83 [IQR: 0.81, 0.94] ( Figure 9). [107107. Onuma Y, Dudek D, Thuesen L, Webster M, Nieman K, Garcia-Garcia HM, Ormiston JA and Serruys PW. Five-year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB cohort A trial. JACC Cardiovasc Interv. 2013;6:999-1009. ] The feasibility and accuracy of the use of MSCT in analyzing radiolucent biodegradable stents with a possibility of functional assessment may therefore usher in a new era of non-invasive evaluation of patients treated with radiolucent stents.[4040. Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miquel-Hebert K, Veldhof S, Webster M, Thuesen L and Dudek D. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897-910. , 108108. Bruining N, Tanimoto S, Otsuka M, Weustink A, Ligthart J, de Winter S, van Mieghem C, Nieman K, de Feyter PJ, van Domburg RT and Serruys PW. Quantitative multi-modality imaging analysis of a bioabsorbable poly-L-lactic acid stent design in the acute phase: a comparison between 2- and 3D-QCA, QCU and QMSCT-CA. EuroIntervention. 2008;4:285-91. ]
The underlying principle of palpography is that softer tissue is more readily deformed than harder or scaffolded tissue when pulsatile arterial pressure is applied.[109109. Serruys PW, Garcia-Garcia HM, Buszman P, Erne P, Verheye S, Aschermann M, Duckers H, Bleie O, Dudek D, Botker HE, von Birgelen C, D’Amico D, Hutchinson T, Zambanini A, Mastik F, van Es GA, van der Steen AF, Vince DG, Ganz P, Hamm CW, Wijns W and Zalewski A. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118:1172-82. , 110110. Schaar JA, De Korte CL, Mastik F, Strijder C, Pasterkamp G, Boersma E, Serruys PW and Van Der Steen AF. Characterizing vulnerable plaque features with intravascular elastography. Circulation. 2003;108:2636-41. , 111111. Schaar JA, Regar E, Mastik F, McFadden EP, Saia F, Disco C, de Korte CL, de Feyter PJ, van der Steen AF and Serruys PW. Incidence of high-strain patterns in human coronary arteries: assessment with three-dimensional intravascular palpography and correlation with clinical presentation. Circulation. 2004;109:2716-9. ] The rationale of this analysis in the present study was to detect some subtle changes in strain resulting from scaffolding and late bioresorption of the stent. The deformability of a vessel wall is quantified using back-scattering radiofrequency analysis of signals at different diastolic pressure levels. This allows for the reconstruction of a color-coded ‘strain’ image in which harder (low strain in blue color) and softer (high strain in yellow color) regions of the coronary arteries can be identified, with radial strain values ranging between 0% and 2%.
At 2-year follow-up, the cumulative strain values showed no significant interval changes between 6 months and 2 years follow-up (0.28± 0.12 vs. 0.31± 0.17, p=0.80). These findings suggest that even 2 years after implantation of BVS, plaque deformability remains diminished and less prone to rupture. ( Figure 10)
In the ABSORB trial, multiple imaging modalities/analyses were used in an attempt to evaluate the bioabsorption of the polymeric struts [4040. Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miquel-Hebert K, Veldhof S, Webster M, Thuesen L and Dudek D. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897-910. , 105105. Ormiston JA, Serruys PW, Regar E, Dudek D, Thuesen L, Webster MW, Onuma Y, Garcia-Garcia HM, McGreevy R and Veldhof S. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899-907. , 112112. Bruining N, Verheye S, Knaapen M, Somers P, Roelandt JR, Regar E, Heller I, de Winter S, Ligthart J, Van Langenhove G, de Feijter PJ, Serruys PW and Hamers R. Three-dimensional and quantitative analysis of atherosclerotic plaque composition by automated differential echogenicity. Catheter Cardiovasc Interv. 2007;70:968-78. ]. From pre- to post-stenting, there was an increase in the mean “DC” (9.8% vs. 25.4%, p<0.001) and “necrotic core (NC)” (15.5% vs. 30.5%, p=0.001) (n=13).[113113. García-García HM, Gonzalo N, Pawar R, Kukreja N, Dudek D, Thuesen L, Ormiston JA, Regar E and Serruys PW. Assessment of the absorption process following bioabsorbable everolimus-eluting stent implantation: temporal changes in strain values and tissue composition using intravascular ultrasound radiofrequency data analysis. A substudy of the ABSORB clinical trial. EuroInterv. 2008;4:443-448. ] However, at 6-month follow-up, VH showed a relative 30% decrease in “DC” (29.7% vs. 21.2%, p<0.001) and a nearly 20% decrease in “NC” (26.9% vs. 21.9%, p=0.005) (n=27). Between 6 months and 2-year follow-up, IVUS-VH assessments demonstrated no significant differences in percentage of each plaque component ( Figure 10).[4040. Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miquel-Hebert K, Veldhof S, Webster M, Thuesen L and Dudek D. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897-910. ] Serial OCT data obtained immediately after stent implantation, at 6 months and 2-year follow-up were available in 7 patients from the ITT population[4040. Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miquel-Hebert K, Veldhof S, Webster M, Thuesen L and Dudek D. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897-910. ]. One of the main findings was a reduction in the number of apparent struts over time. The total number of apparent struts decreased from 403 at baseline to 368 at 6 months follow-up and to 264 at 2-year follow-up (35% reduction over two years). Strut appearance at 2 years is shown in Figure 11. The main observation provided by Grey-scale IVUS was the significant increase in minimal luminal area and average luminal area/volume together with a significant decrease in plaque area/volume between 6 months and 2-year follow-up. With the exception of the minimal luminal area (decreasing from 5.09 to 4.35 mm2, p=0.034), there were no apparent differences in the vessel area, average luminal area, plaque area as well as lumen area stenosis between the immediate post-procedure and 6-month follow-up measurements. Of note, the vessel area/volumes remained constant during follow-up suggesting the absence of significant remodeling; late enlargement of the lumen was however observed in OCT analysis (n=7).
To study vasomotion, either the endothelium independent vasoconstrictor methylergonovine maleate (methergin), or the endothelium dependent vasoactive agent, acetylcholine were administered at the time of 2-year angiographic follow-up. Mean lumen diameters were measured by QCA after baseline saline infusion and after administration of methergine/acetylcholine. Both tests were terminated by intracoronary administration of 200 micrograms of nitroglycerin. In the methergin group (n=7), there was significant vasoconstriction in proximal (pre 2.70±0.43mm vs. post Met 2.49±0.46mm, p=0.02) and scaffolded segments (pre 2.64±0.22mm vs. post Met 2.44±0.33mm, p=0.03). In the acetylcholine group (n=9), 5 patients exhibited vasodilation in the scaffolded segment ( Figure 12). These results suggested that there was restoration of vasomotor function in the scaffolded segment, an observation that has obviously never been made after metallic stent implantation.
At 5 years, clinical follow-up was obtained from 29 out of 30 enrolled patients.[6767. Onuma Y, Serruys PW, Ormiston JA, Regar E, Webster M, Thuesen L, Dudek D, Veldhof S and Rapoza R. Three-year results of clinical follow-up after a bioresorbable everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB trial. EuroIntervention. 2010;6:447-53. ], [114114. Dudek D, Onuma Y, Ormiston JA, Thuesen L, Miquel-Hebert K and Serruys PW. Four-year clinical follow-up of the ABSORB everolimus-eluting bioresorbable vascular scaffold in patients with de novo coronary artery disease: the ABSORB trial. EuroIntervention. 2012;7:1060-1. , 115115. Onuma Y, Nieman K, Webster M, Thuesen L, Dudek D, Ormiston JA and Serruys PW. Five-year clinical outcomes and non-invasive angiographic imaging results with functional assessment after bioresorbable everolimus-eluting scaffold implantation in patients with de novo coronary artery disease. Paper presented at: Transcatheter Cardiovascular Therapeutics; 2012; Miami Beach, FL. , 116116. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW and Serruys PW. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-51. ] There was only one non-Q wave myocardial infarction (peak troponin 2.21ng/ml) related to the treatment of a non-flow-limiting stenosis (QCA DS 42%) in a patient implanted with the BVS 46 days earlier. Furthermore, this patient experienced a single episode of angina at rest without any electrocardiographic evidence of ischemia.
Otherwise, there were no new MACE events between 6 months and 5 years, and no instances of stent thrombosis as defined by the protocol or ARC definitions. In total, the MACE rate at 5 years was 3.4%.[116116. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW and Serruys PW. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-51. ]
During the ABSORB cohort A trial, the mechanical properties of the polymeric stent were assessed. The acute recoil was evaluated in angiography as the difference between the mean luminal diameter of the scaffolded vessel and the diameter at maximal balloon inflation. The mean percent acute recoil was 6.85±6.96% for BVS in the Absorb cohort A trial, while it was 4.27 ±7.08% for Xience V stent (Abbott Vascular, Santa Clara, USA) in the SPIRIT I trial. [117117. Tanimoto S, Serruys PW, Thuesen L, Dudek D, de Bruyne B, Chevalier B and Ormiston JA. Comparison of in vivo acute stent recoil between the bioabsorbable everolimus-eluting coronary stent and the everolimus-eluting cobalt chromium coronary stent: insights from the ABSORB and SPIRIT trials. Catheter Cardiovasc Interv. 2007;70:515-23. ] This suggests that the scaffolding properties of the BVS are slightly weaker than in the Xience V stent.
The late recoil assessed by means of IVUS was defined as a reduction of the stent area from post procedure to 6-month follow-up.[118118. Tanimoto S, Bruining N, van Domburg RT, Rotger D, Radeva P, Ligthart JM and Serruys PW. Late stent recoil of the bioabsorbable everolimus-eluting coronary stent and its relationship with plaque morphology. J Am Coll Cardiol. 2008;52:1616-20. ] At 6 months, the lumen area was reduced by 16.6%, while the late recoil was 11.7%. This suggested that approximately two-thirds of the luminal area reduction was caused by late recoil. ( Figure 13) To enhance the mechanical strength of the struts and to reduce acute and late recoil[7878. Waksman R, Erbel R, Di Mario C, Bartunek J, de Bruyne B, Eberli FR, Erne P, Haude M, Horrigan M, Ilsley C, Bose D, Bonnier H, Koolen J, Luscher TF and Weissman NJ. Early- and long-term intravascular ultrasound and angiographic findings after bioabsorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc Interv. 2009;2:312-20. ], the strut design and the manufacturing process of the polymer were modified in the revised version: BVS 1.1. Firstly, the new design has in-phase zigzag hoops linked by bridges that allow a more uniform strut distribution, reduce maximum circular unsupported surface area (MCUSA) and provide more uniform vessel wall support and drug transfer ( Figure 8).[119119. Okamura T, Garg S, Gutiérrez-Chico J, Shin E, Onuma Y, García-García H, Rapoza R, Sudhir K, Regar E and Serruys P. In vivo evaluation of stent strut distribution patterns in the bioabsorbable everolimus-eluting device: an OCT ad hoc analysis of the revision 1.0 and revision 1.1 stent design in the ABSORB clinical trial. EuroIntervention. 2010:932-938. ] Secondly, a modified manufacturing process has resulted in a slower hydrolysis (in vivo degradation) rate of the polymer, thus preserving its mechanical integrity for a longer period of time [120120. Serruys PW, Onuma Y, Ormiston JA, Bruyne Bd, Regar E, Dudek D, Thuesen L, Smits PC, Chevalier B, McClean D, Koolen J, Windecker S, Whitbourn R, Meredith I, Dorange C, Veldhof S, Hebert KM, Rapoza R and Garcia-Garcia HM. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: 6-month clinical and imaging outcomes Circulation. 2010:Submitted. ].
The BVS revision 1.1 was tested in 101 patients of the ABSORB Cohort B study. This cohort was subdivided in two subgroups of patients: the first group (B1) had to undergo invasive imaging with QCA, IVUS, IVUS-VH and OCT at 6 and 24 months whereas the second group (B2) underwent invasive imaging at 12 and will repeat at 36 months.[107107. Onuma Y, Dudek D, Thuesen L, Webster M, Nieman K, Garcia-Garcia HM, Ormiston JA and Serruys PW. Five-year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB cohort A trial. JACC Cardiovasc Interv. 2013;6:999-1009. ], [121121. Ormiston JA, Serruys PW, Onuma Y, van Geuns RJ, de Bruyne B, Dudek D, Thuesen L, Smits PC, Chevalier B, McClean D, Koolen J, Windecker S, Whitbourn R, Meredith I, Dorange C, Veldhof S, Hebert KM, Rapoza R and Garcia-Garcia HM. First serial assessment at 6 months and 2 years of the second generation of absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study. Circ Cardiovasc Interv. 2012;5:620-32. , 122122. Serruys PW, Onuma Y, Dudek D, Smits PC, Koolen J, Chevalier B, de Bruyne B, Thuesen L, McClean D, van Geuns RJ, Windecker S, Whitbourn R, Meredith I, Dorange C, Veldhof S, Hebert KM, Sudhir K, Garcia-Garcia HM and Ormiston JA. Evaluation of the second generation of a bioresorbable everolimus-eluting vascular scaffold for the treatment of de novo coronary artery stenosis: 12-month clinical and imaging outcomes. J Am Coll Cardiol. 2011;58:1578-88. , 123123. Serruys PW, Onuma Y, Ormiston JA, de Bruyne B, Regar E, Dudek D, Thuesen L, Smits PC, Chevalier B, McClean D, Koolen J, Windecker S, Whitbourn R, Meredith I, Dorange C, Veldhof S, Miquel-Hebert K, Rapoza R and Garcia-Garcia HM. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: six-month clinical and imaging outcomes. Circulation. 2010;122:2301-12. ] Between one and three years, late luminal loss remained unchanged (6 months: 0.19 mm, 1 year: 0.27 mm, 2 years: 0.27 mm, 3 years: 0.29 mm) and the in-segment angiographic restenosis rate for the entire cohort B (n=101) at three years was 6%. On IVUS, mean lumen, scaffold, plaque and vessel area showed enlargement up to two years. Mean lumen and scaffold area remained stable between two and three years whereas significant reduction in plaque behind the struts occurred with a trend toward adaptive restrictive remodelling of EEM ( Figure 14). Hyperechogenicity of the vessel wall, a surrogate of the bioresorption process, decreased from 23.1% to 10.4% with a reduction of radiofrequency backscattering for dense calcium and necrotic core (p<0.001). At three years, the count of strut cores detected on OCT increased significantly, probably reflecting the dismantling of the scaffold; 98% of struts were covered. In the entire cohort B (n=101), the three-year major adverse cardiac event rate was 10.0% without any scaffold thrombosis. [120120. Serruys PW, Onuma Y, Ormiston JA, Bruyne Bd, Regar E, Dudek D, Thuesen L, Smits PC, Chevalier B, McClean D, Koolen J, Windecker S, Whitbourn R, Meredith I, Dorange C, Veldhof S, Hebert KM, Rapoza R and Garcia-Garcia HM. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: 6-month clinical and imaging outcomes Circulation. 2010:Submitted. ] Between 6 months/1 year and 5 years angiographic luminal late loss (LLL) remained unchanged; B1 0.14±19mm vs 0.13±0.33mm, p=0.7953, B2 0.23±0.28mm vs 0.18±0.32mm, p=0.5685. When patients with a TLR were included LLL was 0.15±0.20 mm vs 0.15±0.24 mm, p=0.8275 for B1 and 0.30±0.37 mm vs 0.32±0.48 mm, p=0.8204 for B2. At 5 years, in-scaffold and in-segment binary restenosis was 7.8% (5/64) and 12.5% (8/64).
On IVUS the minimum lumen area of B1 decreased from 5.23±0.97 mm2 at 6 months to 4.89±1.81mm2 at 5 years, p=0.04 but remained unchanged in B2 (4.95±0.91mm2 at 1 year to 4.84±1.28 mm2 at 5 years, p=0.5). At 5 years the struts were no longer discernable by OCT and IVUS. On OCT the minimum lumen area (MLA) in B1 decreased from 4.51±1.28mm2 at 6 months to 3.65±1.39mm2 at 5years, p=0.01, but remained unchanged in B2, 4.35±1.09mm2 at 1 year and 4.12±1.38mm2 at 5 years, p=0.24. Overall, the 5-year Major Adverse Cardiac Event (MACE) rate was 11.0% without any scaffold thrombosis [124124. Serruys PW, Ormiston J, van Geuns RJ, de Bruyne B, Dudek D, Christiansen E, Chevalier B, Smits P, McClean D, Koolen J, Windecker S, Whitbourn R, Meredith I, Wasungu L, Ediebah D, Veldhof S and Onuma Y. A Polylactide Bioresorbable Scaffold Eluting Everolimus for Treatment of Coronary Stenosis: 5-Year Follow-Up. J Am Coll Cardiol. 2016;67:766-76. ].
Randomized trials and registries
The promising results of this second-generation bioresorbable drug-eluting scaffold (BVS 1.1) constitute the proof of concept that this device can adequately revascularize coronary vessels and prevent restenosis. After the encouraging results of ABSORB B, a number of large registries were initiated [125125. Abizaid A, Ribamar Costa J, Jr., Bartorelli AL, Whitbourn R, van Geuns RJ, Chevalier B, Patel T, Seth A, Stuteville M, Dorange C, Cheong WF, Sudhir K, Serruys PW and investigators AE. The ABSORB EXTEND study: preliminary report of the twelve-month clinical outcomes in the first 512 patients enrolled. EuroIntervention. 2015;10:1396-401. , 126126. Capodanno D, Gori T, Nef H, Latib A, Mehilli J, Lesiak M, Caramanno G, Naber C, Di Mario C, Colombo A, Capranzano P, Wiebe J, Araszkiewicz A, Geraci S, Pyxaras S, Mattesini A, Naganuma T, Munzel T and Tamburino C. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention. 2015;10:1144-53. , 127127. Puricel S, Cuculi F, Weissner M, Schmermund A, Jamshidi P, Nyffenegger T, Binder H, Eggebrecht H, Munzel T, Cook S and Gori T. Bioresorbable Coronary Scaffold Thrombosis: Multicenter Comprehensive Analysis of Clinical Presentation, Mechanisms, and Predictors. J Am Coll Cardiol. 2016;67:921-31. ] to evaluate results in real world patients, but most importantly, a series of randomized controlled trials (RCTs) comparing the Absorb BRS vs a CoCr-based, everolimus eluting stent (Xience, Abbot Vascular) were conducted, with on-going follow-up. Currently, 2-3 years’ interim analysis results are available for all of these trials and for most even longer follow-up results have been presented, although some of them have not been formally published in peer reviewed journals. The planned follow-up extends to 5 years for all ABSORB RCTs, except for ABSORB Japan (4 years) and TROFI II (3 years). In general, these trials included relatively simple lesions –i.e. excluded moderately or heavily calcified or thrombotic lesions and excessive vessel tortuosity- while intravascular imaging guidance was used only in 23.9% of the BRS-treated patients[128128. Ali ZA, Serruys PW, Kimura T, Gao R, Ellis SG, Kereiakes DJ, Onuma Y, Simonton C, Zhang Z and Stone GW. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease: a systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet. 2017;390:760-772. ] with no collection of the manner in which it was applied to guide device implantation[129129. Tanaka A, Jabbour RJ, Latib A and Colombo A. Bioresorbable vascular scaffolds: From patient selection to optimal scaffold implantation; tips and tricks to minimize device failure. Catheter Cardiovasc Interv. 2016;88:10-20. ]. The latest available results of these trials are presented in Table 5. In ABSORB China, enrolling 480 patients (1:1) with a primary end-point of angiographic in-segment LLL at 12 months, Absorb BRS was non-inferior to Xience with regards to the primary end-point, while at 3 years there were no statistically significant differences between the two devices with respect to all clinical end-points[130130. Xu B, Yang Y, Han Y, Huo Y, Wang L, Qi X, Li J, Chen Y, Kuo HC, Ying SW, Cheong WF, Zhang Y, Su X, Popma JJ, Gao R and Stone G. Comparison of everolimus-eluting bioresorbable vascular scaffolds and metallic stents: three-year clinical outcomes from the ABSORB China randomised trial. EuroIntervention. 2017 [Epub ahead of print]. ]. In ABSORB Japan, enrolling 400 patients (2:1) with a primary end-point of target lesion failure (TLF) at 12 months [a composite of CD, target-vessel MI (TV-MI), and ID-TLR], Absorb BRS was demonstrated to be non-inferior to Xience [131131. Kimura T, Kozuma K, Tanabe K, Nakamura S, Yamane M, Muramatsu T, Saito S, Yajima J, Hagiwara N, Mitsudo K, Popma JJ, Serruys PW, Onuma Y, Ying S, Cao S, Staehr P, Cheong WF, Kusano H, Stone GW and Investigators AJ. A randomized trial evaluating everolimus-eluting Absorb bioresorbable scaffolds vs. everolimus-eluting metallic stents in patients with coronary artery disease: ABSORB Japan. Eur Heart J. 2015;36:3332-42. ], while TLF rates were not significantly different between the two devices at both 2 and 3 years of follow-up [132132. Onuma Y, Sotomi Y, Shiomi H, Ozaki Y, Namiki A, Yasuda S, Ueno T, Ando K, Furuya J, Igarashi K, Kozuma K, Tanabe K, Kusano H, Rapoza R, Popma JJ, Stone GW, Simonton C, Serruys PW and Kimura T. Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent: insights from the randomised ABSORB Japan trial. EuroIntervention. 2016;12:1090-1101. , 133133. Kozuma K. ABSORB Japan Results: 3-year Clinical and Angiographic Results. Presented at EuroPCR 2017, Paris, France. ]. ABSORB II, enrolled 501 patients in a 2:1 fashion and the primary endpoint was superiority of the Absorb BRS versus the Xience DES in angiographic vasomotor reactivity at 3 years with a co-primary endpoint being the non-inferiority of angiographic late luminal loss. The trial, which is the longest randomized comparison to date, did not meet its co-primary endpoints. More troubling, however, was the fact that although the study was not powered for clinical endpoints, the Absorb BRS demonstrated significantly higher rates of the secondary TLF endpoint at 3 years (10% vs 5%, p=0.04), driven by increased rates of TV-MI (6% vs 1%; p=0.01)[134134. Serruys PW, Chevalier B, Sotomi Y, Cequier A, Carrie D, Piek JJ, Van Boven AJ, Dominici M, Dudek D, McClean D, Helqvist S, Haude M, Reith S, de Sousa Almeida M, Campo G, Iniguez A, Sabate M, Windecker S and Onuma Y. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. 2016;388:2479-2491. ], despite similar rates of this end-point at shorter follow-ups: 1[135135. Serruys PW, Chevalier B, Dudek D, Cequier A, Carrie D, Iniguez A, Dominici M, van der Schaaf RJ, Haude M, Wasungu L, Veldhof S, Peng L, Staehr P, Grundeken MJ, Ishibashi Y, Garcia-Garcia HM and Onuma Y. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2015;385:43-54. ] and 2[136136. Chevalier B, Onuma Y, van Boven AJ, Piek JJ, Sabate M, Helqvist S, Baumbach A, Smits PC, Kumar R, Wasungu L and Serruys PW. Randomised comparison of a bioresorbable everolimus-eluting scaffold with a metallic everolimus-eluting stent for ischaemic heart disease caused by de novo native coronary artery lesions: the 2-year clinical outcomes of the ABSORB II trial. EuroIntervention. 2016;12:1102-1107. ] years. Patient-oriented composite end-point and definite ScT rates were not statistically different between the two devices, but rates of definite or probable ScT were higher for Absorb (3% vs 0%, p=0.03)[134134. Serruys PW, Chevalier B, Sotomi Y, Cequier A, Carrie D, Piek JJ, Van Boven AJ, Dominici M, Dudek D, McClean D, Helqvist S, Haude M, Reith S, de Sousa Almeida M, Campo G, Iniguez A, Sabate M, Windecker S and Onuma Y. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. 2016;388:2479-2491. ]. Interestingly, the recently published 4 years’ data in 428 patients, documented non significantly different rates of TLF (11.1% vs. 6.0%, p=0.05), while no new events of ScT were observed in the landmark analysis from 3 to 4-years[137137. Chevalier B, Cequier A, Dudek D, Haude M, Carrie D, Sabate M, Windecker S, Reith S, de Sousa Almeida M, Campo G, Iniguez A, Onuma Y and Serruys PW. Four-year follow-up of the randomised comparison between an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II trial). EuroIntervention. 2017. ]. ABSORB III is the largest RCT reported today and the pivotal trial of US premarket approval of the Absorb BRS. It enrolled 2008 patients randomized in a 2:1 fashion and its primary end-point was TLF (CD, TV-MI or ID-TLR) at 1 year. Although Absorb was non-inferior to Xience in both intention-to-treat and as treated analyses with regards to the primary end-point[138138. Ellis SG, Kereiakes DJ, Metzger DC, Caputo RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO, Popma JJ, McGreevy R, Zhang Z, Simonton C, Stone GW and Investigators AI. Everolimus-Eluting Bioresorbable Scaffolds for Coronary Artery Disease. N Engl J Med. 2015;373:1905-15. ], at 2 years, TLF rates were significantly higher for Absorb BRS (11.0% vs. 7.9%, p=0.03) and this result was driven by the higher rates of TV-MI (7.3% vs. 4.9%, p=0.04)[139139. Ellis S KD, Stone G. Everolimus-eluting Bioresorbable Vascular Scaffolds in Patients with Coronary Artery Disease: ABSORB III Trial 2-Year Results Presented at the ACC Annual Scientific Session, Washington, DC, March 18, 2017. ]. At 3 years, TV-MI rates remained significantly higher for Absorb BRS and additionally, the numeric higher tendency in device thrombosis against Absorb observed at 2 years (1.9% vs. 0.8%) became a statistically significant difference (2.3% vs. 0.7%; p = 0.01). However, TLF rates were non significantly higher both through 3 years (13.4% vs 10.4%, p = 0.06) and between 1 and 3 years (7.0% vs 6.0%, p = 0.39)[140140. Kereiakes DJ, Ellis SG, Metzger C, Caputo RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO, Popma JJ, McGreevy R, Zhang Z, Simonton C and Stone GW. 3-Year Clinical Outcomes With Everolimus-Eluting Bioresorbable Coronary Scaffolds: The ABSORB III Trial. J Am Coll Cardiol. 2017;70:2852-2862. ]. Patients with thrombotic events in both treatment periods were taking dual antiplatelet therapy (DAPT) at the time of the events, the majority of which occurred in appropriately sized vessels. However, in BRS-assigned patients, treatment of vessels with diameter <2.25 mm was an independent predictor of 3-year TLF and ScT, driven by the relationship at 1 year. The AIDA investigator-initiated trial enrolled 1845 patients in a 1:1 fashion and was designed to evaluate the non-inferiority of Absorb vs. Xience at 2 years with regards to TLF (a composite of CD, TV-MI or TLR). The data and safety monitoring board of the trial recommended early reporting of the study results, due to safety concerns, after a median follow-up of 707 days. By then, no significant differences in the rates of the primary end point were observed (11.7% vs. 10.7%, p=0.43) and this was also the case for CD and TLR. However, a higher incidence of TV-MI (5.5% vs. 3.2%, p = 0.04) was observed, driven by increased rates of definite or probable ScT for the Absorb BRS (3.5% vs. 0.9%, p<0.001). Furthermore, no major predictors of ScT were found[141141. Wykrzykowska JJ, Kraak RP, Hofma SH, van der Schaaf RJ, Arkenbout EK, AJ IJ, Elias J, van Dongen IM, Tijssen RYG, Koch KT, Baan J, Jr., Vis MM, de Winter RJ, Piek JJ, Tijssen JGP and Henriques JPS. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N Engl J Med. 2017;376:2319-2328. ]. EVERBIO II had the most liberal inclusion criteria in the ABSORB RCT family. In this single-center study, a total of 240 patients were randomized in a 1:1:1 fashion to Absorb BRS, everolimus-eluting persistent polymer DES (EES) and biolimus-eluting bioabsorbable polymer DES (BES) with the primary endpoint being angiographic in-device late loss at 9 months. The rates of the primary endpoint were similar for BVS vs. EES/BES, while a post-hoc non-inferiority analysis showed non-inferiority (p<0.001) of the BVS for the same end point. Overall, event rates were very low and the study was underpowered regarding clinical end-points. However, in the comparison between BRS and BES, device related adverse events at 2 years were significantly higher for Absorb[142142. Arroyo D, Gendre G, Schukraft S, Kallinikou Z, Muller O, Baeriswyl G, Stauffer JC, Goy JJ, Togni M, Cook S and Puricel S. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds: Two-year clinical outcomes of the EVERBIO II trial. Int J Cardiol. 2017;243:121-125. ]. TROFI II was another relatively small RCT (n=191), whose importance lies on the fact that it included only patients with STEMI undergoing primary percutaneous coronary revascularization. The primary endpoint of the 6-month optical frequency domain imaging healing score (HS) was based on the presence of uncovered and/or malapposed stent struts and intraluminal filling defects and was lower in the Absorb arm (Pnon-inferiority < 0.001)[143143. Sabate M, Windecker S, Iniguez A, Okkels-Jensen L, Cequier A, Brugaletta S, Hofma SH, Raber L, Christiansen EH, Suttorp M, Pilgrim T, Anne van Es G, Sotomi Y, Garcia-Garcia HM, Onuma Y and Serruys PW. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J. 2016;37:229-40. ]. At 2 years, the rates of all clinical secondary end-points were similar between the two devices[144144. Windecker S. Comparison of the ABSORB Everolimus Eluting Bioresorbable Vascular Scaffold System with a Drug Eluting Metal Stent (XienceTM) in acute ST-elevation Myocardial Infarction: 2-years results of TROFI II. Presented at TCT 2016, Washington, USA. ]. Again, event rates were too low to allow any meaningful statistical comparisons or clinical correlations.
Amaranth
The Amaranth scaffold family (Amaranth Medical, CA) includes BRS with unique polymer production features that result in enhanced radial force and over-expansion capabilities with increased fracture resistance. The first generation device in this group is the Fortitude scaffold (150 microns), which was initially tested as a non-drug eluting version in the MEND I (n=13) trial and subsequently as a sirolimus-eluting version with encouraging results in the RENASCENT-I, MEND II[145145. Esposito G. From Fortitude 150 to Aptitude 115: Clinical update. Presented at EuroPCR 2017, Paris, France. ] and FORTITUDE (n=63) trials[146146. Colombo A. FORTITUDE: Nine-Month Clinical, Angiographic, and OCT Results With an Amorphous PLLA-Based Sirolimus-Eluting Bioresorbable Vascular Scaffold in Patients With Coronary Artery Disease. Presented at TCT 2016, Washington, USA. , 147147. Juan FG. Fortitude, Aptitude, and Magnitude: Progressively Thin-Strut BRS Based on Ultra-High MW Amorphous PLLA. Presented at TCT 2017, Denver, USA. ], with the latter showing 5.3% TLF and 1.8% ScT rates at 2 years. It was then further miniaturized, leading to two newer iterations, the Aptitude and Magnitude scaffolds, with strut thickness of 115 and 98 microns respectively. The single arm RENASCENT-II trial enrolled 60 patients treated with the Aptitude scaffold and has reported on its primary endpoints of safety and efficacy at 9 months[148148. Antonio Colombo. 9-Month Clinical and Imaging Outcomes of a Thin Wall (115 Microns) Novel Ultra-High Molecular Weight Poly-L-Lactide BRS. A Prospective Multicenter International Investigation: The RENASCENT II Study. Presented at EuroPCR 2017, Paris, France. ]. In-scaffold LLL was 0.34±0.36 mm, TLF rates were 3.4% driven by 2 cases of TV-MI, while no ScT were observed. Clinical success was 98.3%, while OCT demonstrated 97% strut coverage and low rate of malapposition. CE mark approval has been submitted. The on-going FIM RENASCENT-III trial with complete enrolment of 70 patients will address the outcomes of the Magnitude scaffold, the world’s first clinically tested sub-100-micron BRS, and the results regarding its 9-month primary end-point are expected in May 2018. Preliminary results at 30 days for the entire cohort have shown 3 TVF cases and no ScT[147147. Juan FG. Fortitude, Aptitude, and Magnitude: Progressively Thin-Strut BRS Based on Ultra-High MW Amorphous PLLA. Presented at TCT 2017, Denver, USA. ]
MeRes 100
MeRes BRS (Meril Life Sciences, Vapi, Gujarat, India) is a PLLA-based, sirolimus-eluting BRS with hybrid geometry structure that provides high radial strength, thin struts and tri-axial radiopaque markers. MeRes Ι FIM study (n=108) was performed in 16 medical centres of India [5858. Cocca M, Lorenzo MLD, Malinconico M and Frezza V. Influence of crystal polymorphism on mechanical and barrier properties of poly(l-lactic acid). European Polymer Journal. 2011;47:1073-1080. ] and showed in-scaffold LLL of 0.15±0.23 mm with “virtually complete" strut coverage (99.3%) at 6 months and very low MACE rates at 1 year (0.93%, 1 ischemia-driven TLR with no ST). Based on the impressive results of the FIM trial, the company has initiated an ambitious programme of further trials being performed in non-US centres, but with worldwide participation, including MeRes-I extend and MeRes-100 China, a pivotal RCT of MeRes100 vs Xience (1:1) with a goal of enrolling 470 patients.
Mirage
Mirage (Manli Cardiology Singapore) is a PLLA-based sirolimus-eluting scaffold, that incorporates a unique helix coil design for high flexibility, has relatively short bioresorption time, high scaffold dislodging force and high radial strength, while it can be stored at room temperature (as long as it is below 25oC) and has been shown to be “MR conditional”[149149. Shellock FG and Giangarra CJ. In vitro assessment of 3-T MRI issues for a bioabsorbable, coronary artery scaffold with metallic markers. Magnetic resonance imaging. 2014;32:163-7. ]. Clinically, it was evaluated in a single blinded, randomized clinical trial of 60 patients who were randomized to either the Mirage (n=31) or the Absorb (n=29) BRS. The primary end-point of in-scaffold LLL at 12 months (0.48±0.49 mm for Mirage), as well as the rates of all clinical end-points were similar between the two study groups, despite the fact that diameter stenoses 1 year after implantation on angiography and OCT were significantly higher with Mirage[150150. Tenekecioglu E, Serruys PW, Onuma Y, Costa R, Chamie D, Sotomi Y, Yu TB, Abizaid A, Liew HB and Santoso T. Randomized Comparison of Absorb Bioresorbable Vascular Scaffold and Mirage Microfiber Sirolimus-Eluting Scaffold Using Multimodality Imaging. JACC Cardiovasc Interv. 2017;10:1115-1130. ]. Clinical follow-up of this trial is scheduled yearly for 5 years, but the company will begin a new study, incorporating small changes to further enhance the performance of this device.
Firesorb
Firesorb (Shanghai MicroPort Medical) is a sirolimus-eluting BRS, with similar structure with the Absorb BRS but significantly lower strut thickness and antiproliferative drug dose (60%). It has been tested in the FIM FUTURE I trial, a single-center Chinese study in 45 patients randomly assigned to 2 cohorts (2:1), undergoing multimodality intravascular imaging at 6 months and 1 year respectively. TLF and ScT rates were 0% both at 30 days (primary end-point) and at 1 year, with only 1 patient undergoing revascularization for a non-target MI the day after the index procedure. At 6 months, in scaffold LLL was 0.15±0.11 mm and struts coverage 98.4%. At 12 months, the latter was significantly increased to 99.0%, while there were no other significant differences between the two cohorts in terms of imaging findings[151151. Xu B. FIRESORB PLLA-Based Sirolimus-Eluting Scaffold: 6-Month FUTURE-I Results. Presented at TCT 2016, Washington, USA. ]. In September 2017, the manufacturing company announced the enrolment of the first patient in the FUTURE II RCT which is designed to enrol 610 patients and test the Firesorb BRS against the Xience stent (NCT02890160).
Xinsorb
The Xinsorb BRS (Shandong Huaan Biotechnology Co., Ltd., Hangzhou, Zhejiang, People’s Republic of China) is a sirolimus-eluting BRS, with a backbone composed of a mixture of PLA, PCL and PGA and a coating of PDLLA mixed with PLLA. The device is stored at 4°C [152152. Chen JH, Wu YZ, Shen L, Zhang F, Yao ZF, Yin JS, Ji M, Wang QB, Ge L, Qian JY, Hu X, Xie J and Ge JB. First-in-man Implantation of the XINSORB Bioresorbable Sirolimus-eluting Scaffold in China. Chinese medical journal. 2015;128:1275-6. ]. The manufacturing company has completed a small (n=30) FIM trial in China, in which imaging findings at 6 months were favourable[153153. Wu Y, Shen L, Ge L, Wang Q, Qian J, Zhang F, Yao K, Huang D, Chen Y and Ge J. Six-month outcomes of the XINSORB bioresorbable sirolimus-eluting scaffold in treating single de novo lesions in human coronary artery. Catheter Cardiovasc Interv. 2016;87 Suppl 1:630-7. ] and no MACE were observed, but extended clinical follow-up to 30 months demonstrated a MACE rate of 3.3%, with one confirmed case of ScT and 2 cases of ID-TLR[154154. Ge L. XINSORB Scaffold: Current Status and Updates. Presented at TCT 2016, Washington, USA. ]. Based on the encouraging initial results of the FIM trial, a RCT versus a biodegradable-polymer DES of 400 patients and a single-arm registry of 800 patients were subsequently initiated. Both of these trials have completed enrolment in June 2016 and are currently in the data collection phase.
NeoVas
This sirolimus-eluting, PLLA-based BRS with relatively thick struts (NeoVas, Lepu Medical, Beijing, China) was initially tested in an on-going follow-up FIM study of 31 patients that at 6 months reported TLF rates of 3.2%, no ScT and angiographic in-scaffold LLL of 0.26±0.32 mm with 95.7% strut coverage[155155. Zhang YJ, Wang XZ, Fu G, Jing QM, Wang G, Jin CY, Xie LH, Cai JZ, Xu B and Han YL. Clinical and multimodality imaging results at 6 months of a bioresorbable sirolimus-eluting scaffold for patients with single de novo coronary artery lesions: the NeoVas first-in-man trial. EuroIntervention. 2016;12:1279-1287. ]. Most importantly, the NeoVas BRS was subsequently tested in a 1:1 design RCT of 560 patients versus the Xience stent, that has completed one-year clinical and angiographic follow-up in 99.8% and 87.9% of the patients respectively[156156. Han YL, Xu B, Fu GS, Wang XZ, Xu K, Guan CD and Stone GW. A randomized trial comparing a novel sirolimus-eluting bioresorbable scaffold with everolimus-eluting metallic stents in patients with coronary artery disease. European Heart Journal. 2017;38. ] and upon announcement of the results will become the first BRS to present controlled data after ABSORB.
Iron based alloy
Iron was the first metal to be considered for use in a BRS. In 2001 Peuster et al. developed a corrodible iron stent (NOR-1) containing 41mg of pure iron, which represents a month’s normal oral iron intake in a common human diet[157157. Peuster M, Wohlsein P, Brugmann M, Ehlerding M, Seidler K, Fink C, Brauer H, Fischer A and Hausdorf G. A novel approach to temporary stenting: degradable cardiovascular stents produced from corrodible metal-results 6-18 months after implantation into New Zealand white rabbits. Heart. 2001;86:563-9. ]. The stents which have a degradation period of >1 year were implanted into 16 rabbits, all of which experienced no adverse events or thromboembolic complications during 6-18 months follow-up. Importantly there was no evidence of any toxic effects of iron or iron related organ damage. Furthermore, no significant neointimal proliferation, or inflammatory response was seen on histological examination.[157157. Peuster M, Wohlsein P, Brugmann M, Ehlerding M, Seidler K, Fink C, Brauer H, Fischer A and Hausdorf G. A novel approach to temporary stenting: degradable cardiovascular stents produced from corrodible metal-results 6-18 months after implantation into New Zealand white rabbits. Heart. 2001;86:563-9. ] A further study in 29 mini pigs compared the iron alloy stent with a BMS, and demonstrated comparable neointimal proliferation at 12 months with no evidence of toxicity.[158158. Peuster M, Hesse C, Schloo T, Fink C, Beerbaum P and von Schnakenburg C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials. 2006;27:4955-62. ] This device was tested in 2009 in the FIM WHISPER study (n=11). Although the trial has not been fully reported, IVUS and OCT showed higher-than-expected intimal hyperplasia that was attributed to the too rapid elution and the too low surface area dose of the antiproliferative drug[159159. Jabara R, Pendyala L, Geva S, Chen J, Chronos N and Robinson K. Novel fully bioabsorbable salicylate-based sirolimus-eluting stent. EuroIntervention. 2009;5 Suppl F:F58-64. ]. A new iteration has been developed with higher dose and slower release kinetics of the drug and also thinner struts. It is currently undergoing pre-clinical evaluation.
BRS technologies in preclinical phase
ArterioSorb
The ArterioSorb™ scaffold (Arterius Ltd, Leeds, United Kingdom) is manufactured using a patented die-drawing processing technique that results in improved radial strength and a thin strut thickness (95µm). The poly-L-lactic acid (PLLA) that is processed further to manufacture ArterioSorb™ using a novel technology enables the cost-effective production with high strength and stiffness and greater flexibility. The test device ArterioSorbÔ (Arterius Ltd., Leeds, UK) is composed of an 8-cell open-cell design with smaller cells at the centre to improve radial strength and cell connectors distributed in a spiral design. The scaffold is coated with a bioabsorbable (PDLA) polymer containing the active drug sirolimus. Bench testing has shown good radial strength of ArterioSorbÔ following IRIS testing of the radial force of the expanded ArterioSorb™.
HARTSORB™ stent
HARTLON (Wilmington, Delaware, USA) has developed a laboratory-scale process for manufacturing a bioresorbable scaffold that is different than previous bioresorbable scaffolds because the HARTSORB stent has struts that are comprised of a novel microporous, fibrillated polymeric material instead of a solid polymeric material. The microporous, fibrillated struts offer superior resistance against vessel recoil and strut fracture. The stronger fibrillated material offers the potential to reduce the strut thickness thereby maximizing arterial lumen capacity. The struts include very small fibrils of poly-L-lactic acid (PLLA) oriented in multiple axes between nodes of PLLA that increase the radial strength of the scaffold. The void space between the fibrils increases the toughness of the scaffold thereby reducing the potential of strut fracture during deployment or scaffold overlapping. However, as there is a limitation that fibrillated PLLA loses orientation when forming network during heating, the company has shifted to the development of ultra-high molecular weight PLLA polymer to form a scaffold with high tensile strength and ductility.
FROM INITIAL TRIALS AND PROMISES TO CURRENT STATUS AND FUTURE OUTLOOK
Seventeen years after the first implantation of a BRS in human, there are still few data to support the theoretical advantages of these devices. Although improvements in vasomotion within the treated coronary segment were documented using physiological testing in observational studies[8383. Haude M, Erbel R, Erne P, Verheye S, Degen H, Bose D, Vermeersch P, Wijnbergen I, Weissman N, Prati F, Waksman R and Koolen J. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet (London, England). 2013;381:836-44. , 124124. Serruys PW, Ormiston J, van Geuns RJ, de Bruyne B, Dudek D, Christiansen E, Chevalier B, Smits P, McClean D, Koolen J, Windecker S, Whitbourn R, Meredith I, Wasungu L, Ediebah D, Veldhof S and Onuma Y. A Polylactide Bioresorbable Scaffold Eluting Everolimus for Treatment of Coronary Stenosis: 5-Year Follow-Up. J Am Coll Cardiol. 2016;67:766-76. ], in ABSORB B at 5 years the improvement in vasomotion did not reach the functionality of the native vessels[160160. Dudek D, Rzeszutko L, Onuma Y, Sotomi Y, Depukat R, Veldhof S, Ediebah D, Staehr P, Zasada W, Malinowski KP, Kaluza GL and Serruys PW. Vasomotor Response to Nitroglycerine Over 5 Years Follow-Up After Everolimus-Eluting Bioresorbable Scaffold Implantation. JACC Cardiovasc Interv. 2017;10:786-795. ] and the randomized controlled ABSORB II trial failed to show this improvement at 3 years for the Absorb BRS against the Xience stent[134134. Serruys PW, Chevalier B, Sotomi Y, Cequier A, Carrie D, Piek JJ, Van Boven AJ, Dominici M, Dudek D, McClean D, Helqvist S, Haude M, Reith S, de Sousa Almeida M, Campo G, Iniguez A, Sabate M, Windecker S and Onuma Y. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. 2016;388:2479-2491. ]. The latter trial on the other hand demonstrated that expansive vessel wall remodelling was more frequent and intense with Absorb at 3 years[161161. Serruys PW, Katagiri Y, Sotomi Y, Zeng Y, Chevalier B, van der Schaaf RJ, Baumbach A, Smits P, van Mieghem NM, Bartorelli A, Barragan P, Gershlick A, Kornowski R, Macaya C, Ormiston J, Hill J, Lang IM, Egred M, Fajadet J, Lesiak M, Windecker S, Byrne RA, Raber L, van Geuns RJ, Mintz GS and Onuma Y. Arterial Remodeling After Bioresorbable Scaffolds and Metallic Stents. J Am Coll Cardiol. 2017;70:60-74. ], supporting: (i) previous, non-randomized observations that showed that at 6 to 12 months after BRS implantation coronary geometry tends to revert to its pre-implant level [162162. Gomez-Lara J, Brugaletta S, Farooq V, van Geuns RJ, De Bruyne B, Windecker S, McClean D, Thuesen L, Dudek D, Koolen J, Whitbourn R, Smits PC, Chevalier B, Morel MA, Dorange C, Veldhof S, Rapoza R, Garcia-Garcia HM, Ormiston JA and Serruys PW. Angiographic geometric changes of the lumen arterial wall after bioresorbable vascular scaffolds and metallic platform stents at 1-year follow-up. JACC Cardiovasc Interv. 2011;4:789-99. ], and (ii) recent observations showing persistence of in-scaffold late lumen enlargement with normalization of the FFRCT up to 6 years[163163. Onuma Y, Collet C, van Geuns RJ, de Bruyne B, Christiansen E, Koolen J, Smits P, Chevalier B, McClean D, Dudek D, Windecker S, Meredith I, Nieman K, Veldhof S, Ormiston J, Serruys PW and Investigators A. Long-term serial non-invasive multislice computed tomography angiography with functional evaluation after coronary implantation of a bioresorbable everolimus-eluting scaffold: the ABSORB cohort B MSCT substudy. European heart journal cardiovascular Imaging. 2017;18:870-879. ]. It should be noted however, that LLL was generally larger in the Absorb compared to the Xience group. Although the altered vessel geometry and biomechanics can result in chronic irritation and flow disturbances that may contribute to neointimal proliferation and adverse events[164164. Gyongyosi M, Yang P, Khorsand A and Glogar D. Longitudinal straightening effect of stents is an additional predictor for major adverse cardiac events. Austrian Wiktor Stent Study Group and European Paragon Stent Investigators. J Am Coll Cardiol. 2000;35:1580-9. , 165165. Wentzel JJ, Whelan DM, van der Giessen WJ, van Beusekom HM, Andhyiswara I, Serruys PW, Slager CJ and Krams R. Coronary stent implantation changes 3-D vessel geometry and 3-D shear stress distribution. Journal of biomechanics. 2000;33:1287-95. ], the clinical impact of these observations remains uncertain. This also is the case for the scarce data showing late increases in scaffold-free ostial area in jailed side branches[166166. Onuma Y, Grundeken MJ, Nakatani S, Asano T, Sotomi Y, Foin N, Ng J, Okamura T, Wykrzykowska JJ, de Winter RJ, van Geuns RJ, Koolen J, Christiansen E, Whitbourn R, McClean D, Smits P, Windecker S, Ormiston JA and Serruys PW. Serial 5-Year Evaluation of Side Branches Jailed by Bioresorbable Vascular Scaffolds Using 3-Dimensional Optical Coherence Tomography: Insights From the ABSORB Cohort B Trial (A Clinical Evaluation of the Bioabsorbable Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions). Circ Cardiovasc Interv. 2017;10. ] -despite initial higher post-procedural incidence of side-branch occlusion compared to DES[167167. Muramatsu T, Onuma Y, Garcia-Garcia HM, Farooq V, Bourantas CV, Morel MA, Li X, Veldhof S, Bartorelli A, Whitbourn R, Abizaid A and Serruys PW. Incidence and short-term clinical outcomes of small side branch occlusion after implantation of an everolimus-eluting bioresorbable vascular scaffold: an interim report of 435 patients in the ABSORB-EXTEND single-arm trial in comparison with an everolimus-eluting metallic stent in the SPIRIT first and II trials. JACC Cardiovasc Interv. 2013;6:247-57. ]- and facilitation of non-invasive surveillance imaging with CT[168168. Tanabe K, Popma JJ, Kozuma K, Saito S, Muramatsu T, Nakamura S, Namiki A, Morino Y, Hagiwara N, Uematsu M, Kawasaki T, Fujii K, Serruys PW, Onuma Y, Ying S, Kusano H, Stone GW and Kimura T. Multi-slice Computed Tomography Assessment of Everolimus-Eluting Absorb Bioresorbable Scaffold in Comparison with Metallic Drug-Eluting Stents from the ABSORB Japan randomized Trial. EuroIntervention. 2017. ]. On the contrary, sufficient mid-term clinical data are now available to support the fact that Absorb BRS is inferior to new generation DES[134134. Serruys PW, Chevalier B, Sotomi Y, Cequier A, Carrie D, Piek JJ, Van Boven AJ, Dominici M, Dudek D, McClean D, Helqvist S, Haude M, Reith S, de Sousa Almeida M, Campo G, Iniguez A, Sabate M, Windecker S and Onuma Y. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. 2016;388:2479-2491. , 139139. Ellis S KD, Stone G. Everolimus-eluting Bioresorbable Vascular Scaffolds in Patients with Coronary Artery Disease: ABSORB III Trial 2-Year Results Presented at the ACC Annual Scientific Session, Washington, DC, March 18, 2017. , 141141. Wykrzykowska JJ, Kraak RP, Hofma SH, van der Schaaf RJ, Arkenbout EK, AJ IJ, Elias J, van Dongen IM, Tijssen RYG, Koch KT, Baan J, Jr., Vis MM, de Winter RJ, Piek JJ, Tijssen JGP and Henriques JPS. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N Engl J Med. 2017;376:2319-2328. ] and both FDA[169169. FDA. Update on increased rate of major adverse cardiac events observed in patients receiving Abbott Vascular’s Absorb GT1 bioresorbable vascular scaffold: letter to healthcare providers. https://wwwfdagov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm547256htm 2017;Published and accessed on: October 31, 2017. ]and ESC-EAPCI[6868. Byrne RA, Stefanini GG, Capodanno D, Onuma Y, Baumbach A, Escaned J, Haude M, James S, Joner M, Juni P, Kastrati A, Oktay S, Wijns W, Serruys PW and Windecker S. Report of an ESC-EAPCI Task Force on the evaluation and use of bioresorbable scaffolds for percutaneous coronary intervention: executive summary. EuroIntervention. 2018;13:1574-1586. ] have issued documents reflecting on this position. Paradoxically, the greatest promise turned out as the Achilles foot of this emblematic first generation BRS. In all ABSORB RCTs, published rates of device thrombosis were numerically higher for Absorb, regardless of the statistical significance of this difference, while a meta-analysis of the first six trials reported a two-fold increase in the risk of device thrombosis with Absorb at just 1 year of follow-up, despite similar rates of all other clinical end-points[170170. Cassese S, Byrne RA, Ndrepepa G, Kufner S, Wiebe J, Repp J, Schunkert H, Fusaro M, Kimura T and Kastrati A. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. Lancet. 2016;387:537-44. ]. Moreover, several recent meta-analyses summarizing mid-term outcomes at 2 years[128128. Ali ZA, Serruys PW, Kimura T, Gao R, Ellis SG, Kereiakes DJ, Onuma Y, Simonton C, Zhang Z and Stone GW. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease: a systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet. 2017;390:760-772. ], [171171. Collet C, Asano T, Miyazaki Y, Tenekecioglu E, Katagiri Y, Sotomi Y, Cavalcante R, de Winter RJ, Kimura T, Gao R, Puricel S, Cook S, Capodanno D, Onuma Y and Serruys PW. Late thrombotic events after bioresorbable scaffold implantation: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2017;38:2559-2566. , 172172. Ha FJ, Nerlekar N, Cameron JD, Bennett MR, Meredith IT, West NE and Brown AJ. Midterm Safety and Efficacy of ABSORB Bioresorbable Vascular Scaffold Versus Everolimus-Eluting Metallic Stent: An Updated Meta-Analysis. JACC Cardiovasc Interv. 2017;10:308-310. , 173173. Mahmoud AN, Barakat AF, Elgendy AY, Schneibel E, Mentias A, Abuzaid A and Elgendy IY. Long-Term Efficacy and Safety of Everolimus-Eluting Bioresorbable Vascular Scaffolds Versus Everolimus-Eluting Metallic Stents: A Meta-Analysis of Randomized Trials. Circ Cardiovasc Interv. 2017;10. , 174174. Sorrentino S, Giustino G, Mehran R, Kini AS, Sharma SK, Faggioni M, Farhan S, Vogel B, Indolfi C and Dangas GD. Everolimus-Eluting Bioresorbable Scaffolds Versus Everolimus-Eluting Metallic Stents. J Am Coll Cardiol. 2017;69:3055-3066. , 175175. Montone RA, Niccoli G, De Marco F, Minelli S, D’Ascenzo F, Testa L, Bedogni F and Crea F. Temporal Trends in Adverse Events After Everolimus-Eluting Bioresorbable Vascular Scaffold Versus Everolimus-Eluting Metallic Stent Implantation: A Meta-Analysis of Randomized Controlled Trials. Circulation. 2017;135:2145-2154. , 176176. Zhang XL, Zhu QQ, Kang LN, Li XL and Xu B. Mid- and Long-Term Outcome Comparisons of Everolimus-Eluting Bioresorbable Scaffolds Versus Everolimus-Eluting Metallic Stents: A Systematic Review and Meta-analysis. Annals of internal medicine. 2017;167:642-654. ] ( Figure 15) and one also in 3 years[177177. Ali ZA, Gao RF, Kimura T, Onuma Y, Kereiakes DJ, Ellis SG, Chevalier B, Vu MT, Zhang Z, Simonton CA, Serruys PW and Stone GW. Three-Year Outcomes With the Absorb Bioresorbable Scaffold: Individual-Patient-Data Meta-Analysis From the ABSORB Randomized Trials. Circulation. 2017. ] ( Figure 16) reported concordant findings, with a higher risk of both thrombosis and TLF for the Absorb BRS, the latter driven mainly by increased rates of TV-MI and ID-TLR. Current evidence also suggests no late advantage in terms of clinical efficacy, including relief of angina pectoris.
It should be emphasized that the advantages of BRS are not fully demonstrated until bioresorption is complete and long-term results, i.e. > 3 years, are still needed to complete the picture. In this regard, the 4 years results of ABSORB II although they don’t represent the beginning of a clinical benefit, may be interpreted at least as the end of the increased mid-term thrombotic risk. The ultimate judge of the Absorb BRS will probably be the on-going ABSORB IV trial, that has just recently reported 30 days’ results in 2604 patients[178178. Stone GW. Outcomes of Absorb bioresorbable scaffolds with improved technique in an expanded patient population: the ABSORB IV randomized trial. Presented at TCT 2017, Denver, USA. ]. Since ABSORB III and IV will be pooled together for a landmark clinical endpoint analysis between 3 and >5 (7-10) years, we still need to wait many years before understanding if a long-term clinical benefit can come from BRS. However, even if such a final benefit can be reached for Absorb, it will have to pass through the “Symplegades” of increased mid-term thrombotic risk. This risk was concordant across the early (<30 days), late (30 days to 1 year), and very late (>1 year) periods[171171. Collet C, Asano T, Miyazaki Y, Tenekecioglu E, Katagiri Y, Sotomi Y, Cavalcante R, de Winter RJ, Kimura T, Gao R, Puricel S, Cook S, Capodanno D, Onuma Y and Serruys PW. Late thrombotic events after bioresorbable scaffold implantation: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2017;38:2559-2566. ], [173173. Mahmoud AN, Barakat AF, Elgendy AY, Schneibel E, Mentias A, Abuzaid A and Elgendy IY. Long-Term Efficacy and Safety of Everolimus-Eluting Bioresorbable Vascular Scaffolds Versus Everolimus-Eluting Metallic Stents: A Meta-Analysis of Randomized Trials. Circ Cardiovasc Interv. 2017;10. , 174174. Sorrentino S, Giustino G, Mehran R, Kini AS, Sharma SK, Faggioni M, Farhan S, Vogel B, Indolfi C and Dangas GD. Everolimus-Eluting Bioresorbable Scaffolds Versus Everolimus-Eluting Metallic Stents. J Am Coll Cardiol. 2017;69:3055-3066. , 175175. Montone RA, Niccoli G, De Marco F, Minelli S, D’Ascenzo F, Testa L, Bedogni F and Crea F. Temporal Trends in Adverse Events After Everolimus-Eluting Bioresorbable Vascular Scaffold Versus Everolimus-Eluting Metallic Stent Implantation: A Meta-Analysis of Randomized Controlled Trials. Circulation. 2017;135:2145-2154. ], an observation in favour of an inherent thrombogenicity of the scaffold. The latter is partially supported by the results of the AIDA trial and seems to originate from the rather thick struts of this first generation device, although issues inherent to the mechanical disintegration of the scaffold during the resorption process may also contribute[171171. Collet C, Asano T, Miyazaki Y, Tenekecioglu E, Katagiri Y, Sotomi Y, Cavalcante R, de Winter RJ, Kimura T, Gao R, Puricel S, Cook S, Capodanno D, Onuma Y and Serruys PW. Late thrombotic events after bioresorbable scaffold implantation: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2017;38:2559-2566. , 173173. Mahmoud AN, Barakat AF, Elgendy AY, Schneibel E, Mentias A, Abuzaid A and Elgendy IY. Long-Term Efficacy and Safety of Everolimus-Eluting Bioresorbable Vascular Scaffolds Versus Everolimus-Eluting Metallic Stents: A Meta-Analysis of Randomized Trials. Circ Cardiovasc Interv. 2017;10. , 179179. Chan CY, Wu EB and Yan BP. Very Late Bioresorbable Scaffold Thrombosis Caused by Intraluminal Scaffold Dismantling. JACC Cardiovasc Interv. 2016;9:1844-7. ]. Whether the risk of ScT can be mitigated by a specific implantation technique, by avoiding very small vessels, or by prolonging dual antiplatelet therapy (DAPT) is subject to debate. Extension of DAPT duration until BRS resorption is completed is widely recommended, however this is based more on expert’s consensus[180180. Mukherjee D. Device Thrombosis with Bioresorbable Scaffolds. N Engl J Med. 2017;376:2388-2389. ] rather than on solid evidence [181181. Felix CM, Vlachojannis GJ, AJJ IJ, Fam JM, Smits PC, Lansink WJ, Diletti R, Zijlstra F, Regar ES, Boersma E, Onuma Y and van Geuns RJM. Potentially increased incidence of scaffold thrombosis in patients treated with Absorb BVS who terminated DAPT before 18 months. EuroIntervention. 2017;13:e177-e184. ] and is subject to a potential offset of its beneficial effect by the accompanying increased bleeding risk. Regarding the effects of meticulous adherence to good implantation techniques, briefly summarized in the acronym PSP (pre-dilate, sizing, post-dilate)[129129. Tanaka A, Jabbour RJ, Latib A and Colombo A. Bioresorbable vascular scaffolds: From patient selection to optimal scaffold implantation; tips and tricks to minimize device failure. Catheter Cardiovasc Interv. 2016;88:10-20. ], there are indeed data suggesting improved outcomes[127127. Puricel S, Cuculi F, Weissner M, Schmermund A, Jamshidi P, Nyffenegger T, Binder H, Eggebrecht H, Munzel T, Cook S and Gori T. Bioresorbable Coronary Scaffold Thrombosis: Multicenter Comprehensive Analysis of Clinical Presentation, Mechanisms, and Predictors. J Am Coll Cardiol. 2016;67:921-31. , 182182. Colombo A and Azzalini L. Optimal implantation is the way to prevent scaffold thrombosis: a hypothesis to be tested. EuroIntervention. 2017;13:e142-e144. , 183183. Stone GW, Abizaid A, Onuma Y, Seth A, Gao R, Ormiston J, Kimura T, Chevalier B, Ben-Yehuda O, Dressler O, McAndrew T, Ellis SG, Kereiakes DJ and Serruys PW. Effect of Technique on Outcomes Following Bioresorbable Vascular Scaffold Implantation: Analysis From the ABSORB Trials. J Am Coll Cardiol. 2017;70:2863-2874. ]. However, these data were derived from post-hoc analyses and have not been prospectively validated. In ABSORB-IV, where pre and post-dilatation were performed in 99.8% and 82.6% of the lesions respectively, overall rates of device thrombosis at 30 days were markedly lower compared to ABSORB III at the same time point (0.6% vs 1.1% for Absorb BRS) and Absorb was non-inferior to Xience with regards to the primary end-point of TLF, but again with numerically higher rates of ScT. Furthermore, it has been estimated that only half of patients with late or very late ScT might benefit from meticulous implantation techniques[184184. Yamaji K, Raber L and Windecker S. What determines long-term outcomes using fully bioresorbable scaffolds - the device, the operator or the lesion?. EuroIntervention. 2017;12:1684-1687. ], which in addition result in a significant lengthening of the procedure[185185. Colombo A and Azzalini L. Bioresorbable scaffolds: reflections after a setback - losing a battle does not mean losing the war! EuroIntervention. 2017;13:785-786. ]. Finally, small vessel diameter, which was an exclusion criterion in the ABSORB RCTs series, has consistently shown to be a predictor of adverse events[128128. Ali ZA, Serruys PW, Kimura T, Gao R, Ellis SG, Kereiakes DJ, Onuma Y, Simonton C, Zhang Z and Stone GW. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease: a systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet. 2017;390:760-772. , 183183. Stone GW, Abizaid A, Onuma Y, Seth A, Gao R, Ormiston J, Kimura T, Chevalier B, Ben-Yehuda O, Dressler O, McAndrew T, Ellis SG, Kereiakes DJ and Serruys PW. Effect of Technique on Outcomes Following Bioresorbable Vascular Scaffold Implantation: Analysis From the ABSORB Trials. J Am Coll Cardiol. 2017;70:2863-2874. ], but the effect of this observation in the results of these trials can only be speculated. It is conceivable that even if the aforementioned solutions become accepted as a necessary short-term price to pay in order to achieve the potential long-term benefits of BRS, it will be a Pyrrhic victory for Absorb in an era where DES are both “operator-friendly” in terms of implantation and related with very short DAPT durations. Reflecting on this, Abbott has discontinued the sales of Absorb in both USA and Europe and restricted its use in Europe in registries, maintaining a rigorous follow-up of the on-going RCTs in the hope of more favorable long-term results[186186. King SB, 3rd and Gogas BD. Can the Vanishing Stent Reappear?.: Fix the Technique, or Fix the Device?. J Am Coll Cardiol. 2017. ]. For the BRS technology overall however, at present hope seems to lie more on the development of second generation devices, and even Abbott is developing a new generation, sub-100 μm scaffold named Falcon[186186. King SB, 3rd and Gogas BD. Can the Vanishing Stent Reappear?.: Fix the Technique, or Fix the Device?. J Am Coll Cardiol. 2017. ]. Currently, the distinction between first and second-generation devices is based predominately on strut thickness, although a number of other characteristics still need improvement to achieve an ideal BRS platform, including over-expansion capability, visibility, deliverability and storage conditions. A number of second generation devices have shown encouraging results in clinical trials[187187. Onuma Y and Serruys PW. Rather Thick, Yet Antithrombogenic: Is the Magmaris Scaffold a New Hope for Bioresorbable Coronary Scaffold?. Circ Cardiovasc Interv. 2017;10. ] and coupled with clear differences in material technology from the archetypical Absorb BRS challenge the class effect of its so far unfavourable results. The evaluation of these devices will not be possible until randomized data become available and many experts doubt if an appropriate challenger is currently available to go against a mature technology with solid results, like DES[185185. Colombo A and Azzalini L. Bioresorbable scaffolds: reflections after a setback - losing a battle does not mean losing the war! EuroIntervention. 2017;13:785-786. ]. In spite of this, a number of companies worldwide have initiated ambitious RCTs for their products, that will determine if BRS evolutional history will follow the pattern of DES, in terms of success through further development, or another scenario will prevail.
Fully bioresorbable, everolimus-eluting scaffold
- Fully bioresorbable, everolimus-eluting scaffold (ABSORB 1.0) has been tested in the ABSORB cohort A study since 2006
- The first-in-man ABSORB trial, with multi-modality imaging showed:
1. favourable clinical outcomes (MACE rate 3.4% at 4 years)
2. occurrence of bioresorption (IVUS-VH, echogenicity and OCT),
3. late lumen enlargement (IVUS and OCT)
4. restoration of vasomotion in the scaffolded segment
- At that time, elimination of scaffold shrinkage at 6 months remained a challenge



 (1)
(1) (3)
(3)