Treatment and Prevention
MEDICAL THERAPIES
Treatment strategies for atrial fibrillation center around two concepts: management of symptoms and prevention of complications. In terms of symptoms, management can either focus on rate or rhythm control. Rate control strategies include beta-blockers, calcium channel blockers, digoxin, and in refractory cases, atrioventricular node ablation with pacemaker implantation. Rhythm control strategies include medical therapies such as sotolol, dofetilide, amiodarone, dronedarone, electrical cardioversion or pulmonary vein isolation.
Regardless of the treatment strategy for rate or rhythm control, treatment efforts must also focus on prevention of thromboembolic events. Atrial fibrillation increases the risk of cerebral ischemic events 5-fold across the entire spectrum of age, and it accounts for approximately 1.5% of events in patients under age 50 to greater than 20% of events in patients greater than age 80.[44. Lloyd-Jones D, Adams RJ, Brown TM et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010;121:e46-e215. ] Regardless of whether it is paroxysmal or permanent, the annual rate of cerebral ischemic events in patients with atrial fibrillation approaches 4.5% per year.[55. Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol 2000;35:183-7. ] In fact, even subclinical atrial fibrillation lasting 6 minutes or longer is associated with an increased risk of ischemic events.[66. Healey JS, Connolly SJ, Gold MR et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120-9. ]
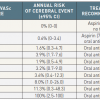
Although the overall risk of cerebral ischemic events in patients with atrial fibrillation is understood on the population level, it is both practical and important to make attempts at individualizing the risk for a particular patient. A number of risk models have been proposed. The first is the CHADS2 score.[77. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864-70. ] In this model, a patient is assessed according to 5 established risk factors ( Table 1) including age >75, history of hypertension, history of congestive heart failure, diabetes, and prior embolic event. Each risk factor is worth one point with the exception of a prior embolic event, which is worth two points. The resultant score ranges from 0-6 and corresponds to a significant increase in risk for each incremental score. The absolute risk varies by series. In one study of 1733 patients with atrial fibrillation not on warfarin, the annualized risk of cerebral ischemic events ranged from 1.9% for a CHADS2 score of 0 to 18.2% for a CHADS2 score of 6.[77. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864-70. ] In another, larger series of 11,526 patients with atrial fibrillation not on warfarin, the annualized risk of events ranged from 0.49% for a CHADS2 score of 0 to 6.9% for a CHADS2 score of 6.[88. . Go AS, Hylek EM, Chang Y et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 2003;290:2685-92. ] Although these two studies highlight variability in cerebral ischemic event risk across different populations, the unifying concept that higher CHADS2 scores portend higher event risk remains true.
Although the CHADS2 score is well studied and commonly applied, it is uncertain whether this score adequately stratifies low risk patients. This point is highlighted by the fact that even low risk patients (those with CHADS2 scores of 1) can benefit from oral anticoagulation with warfarin or the non-vitamin K oral anticoagulant medications discussed below. Furthermore, greater emphasis on certain risk factors and the addition of other known risk factors for cerebral ischemic events may further refine risk stratification. The CHA2DS2VASc score ( Table 2) is a 9-point scale incorporating the factors in the CHADS2 system (congestive heart failure, hypertension, age >75, diabetes and prior stroke x2) placing increased emphasis on age (65-74 merits 1 point while ³75 merits 2 points). Additionally, it adds as risk factors vascular disease and female gender. Based on an analysis of 1084 patients Lip et al. validated this risk model and demonstrated incremental risk of embolic events with a rising score ( Table 2).[99. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2009;137:263-72. ] This score has been further validated in a trial of over 7000 patients.[22. Camm AJ, Kirchhof P, Lip GY et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429. ] While the CHA2DS2VASc score is a more comprehensive system, and it has become the standard in assessing thromboembolism risk, but it is worth recognizing that small numbers at either end of the risk spectrum may under or overestimate absolute risk.
It is also important to balance the risk of cerebral ischemic events against the risk of bleeding. Numerous algorithms have been proposed, but the most commonly utilized is the HAS-BLED score ( Table 3), incorporating hypertension, abnormal liver/kidney function, prior stroke, prior bleeding, labile INRs, elderly and drug/alcohol abuse on a 9 point scale.[1010. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093-100. ] Scores of ≥ 3 are associated with increased bleeding risk, but it is important to note that often, similar factors (such as age and hypertension) increase both the bleeding risk and the risk of cerebral ischemic events, and often, the risk related to bleeding is outweighed by the risk of stroke.[1111. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation 2012;125:2298-307. ] Decision algorithms designed to balance these risks have been proposed[1212. Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation 2012;126:860-5. ], and both the 2012 updated European guidelines and the 2014 ACC/AHA/HRS guidelines recommend thoughtful anticoagulation with close monitoring in patients at elevated risk for bleeding.[33. January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1-76. , 1313. Camm AJ, Lip GY, De Caterina R et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719-47. ]
Multiple anti-coagulation strategies to prevent cerebral ischemic events have been studied ( Table 4), including aspirin, clopidogrel, warfarin and the target specific anticoagulant medications: dabigatran, rivaroxaban, apixaban and edoxaban. Both aspirin and warfarin have been compared to placebo. In 1999, Hart et al. published a meta-analysis of studies comparing aspirin to placebo, warfarin to placebo and aspirin to warfarin in patients with atrial fibrillation.[1414. Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999;131:492-501. ] Across the studies comparing aspirin to placebo with the primary endpoint of cerebrovascular accident, therapy with aspirin resulted in a relative risk reduction of 22% and an absolute risk reduction of 1.5% per year respectively for primary prevention and 2.5% per year for secondary prevention. In the studies comparing warfarin to placebo, warfarin resulted in a relative risk reduction of 62% with absolute reductions of 2.7% per year for primary prevention and 8.4% per year for secondary prevention. In 5 studies comparing aspirin to warfarin, warfarin resulted in a 34% relative risk reduction, proving superior to aspirin in the reduction of cerebral events; however, it should be noted that the risk of both intracranial and extracranial hemorrhage was higher with warfarin compared to aspirin.[1414. Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999;131:492-501. ]
Clopidogrel has also been studied in patients with atrial fibrillation. The Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE) trials evaluated the efficacy and safety of dual antiplatelet therapy in patients with atrial fibrillation. The ACTIVE W trial evaluated dual antiplatelet therapy versus warfarin in 6726 patients with atrial fibrillation and at least one other risk factor for thromboembolic events with a primary endpoint of cerebral events, non-cerebral embolic events, myocardial infarction or vascular death.[1515. Connolly S, Pogue J, Hart R et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006;367:1903-12. ] This study was stopped early, as therapy with dual antiplatelet therapy was associated with a significantly higher event rate (relative risk 1.44, 95% CI 1.18-1.76, p=0.003) compared to warfarin alone.[1515. Connolly S, Pogue J, Hart R et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006;367:1903-12. ]
In the ACTIVE A trial, 7554 patients with atrial fibrillation at increased risk of stroke who were considered unsuitable candidates for warfarin therapy were randomized to aspirin plus clopidogrel versus aspirin alone. Over a mean of 3.6 years of follow-up, patients treated with dual antiplatelet therapy had a significantly lower risk of the composite primary endpoint consisting of cerebral event, non-cerebral embolic event, myocardial infarction or vascular death (relative risk 0.89, 95% CI 0.81-0.98, p=0.01) at the cost of an increased risk of bleeding (relative risk 1.57, 95% CI 1.29-1.92, p<0.01).[1616. Connolly SJ, Pogue J, Hart RG et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009;360:2066-78. ] As a result of these and other studies, dual antiplatelet therapy has not gained widespread acceptance for thromboembolic prevention in patients with atrial fibrillation except for patients in whom warfarin is contraindicated.
Although long-term anti-coagulation with warfarin is efficacious, numerous drawbacks exist. The narrow therapeutic window of warfarin forces a delicate balance between lack of efficacy and a significantly elevated risk of bleeding, therefore requiring frequent blood tests. Additionally, numerous food and drug interactions exist, thus making chronic management of multisystem disease difficult. Finally, in patients at risk for falling, anti-coagulation itself may incur more risk than the thromboembolic event it is prescribed to prevent.
Highlighting these issues, warfarin therapy has been associated with bleeding rates upwards of 10% per year, leading to a regulatory “black box warning”.[1717. Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med 2007;167:1414-9. ] Additionally, up to 40% of patients with atrial fibrillation have contraindications to anticoagulation therapy.[1818. Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin?. Arch Intern Med 2000;160:41-6. ] Warfarin is often underutilized or associated with difficulties finding appropriate dosage. Among patients who are at moderate or high risk for ischemic stroke and considered good candidates for warfarin therapy, it is prescribed in only 40% of patients[1919. Brass LM, Krumholz HM, Scinto JM, Radford M. Warfarin use among patients with atrial fibrillation. Stroke 1997;28:2382-9. ], and even in the controlled Stroke Prevention Using and Oral Thrombin Inhibitor in Atrial Fibrillation (SPORTIF III and V) trial settings, up to 30% of patients where subtherapeutic and 15% supratherapeutic on warfarin.[2020. White HD, Gruber M, Feyzi J et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med 2007;167:239-45. ] Finally, in a study of 41900 patients with chronic atrial fibrillation, only 50% of patients treated with aspirin and 70% of patients treated with warfarin remained on this therapy at one year,[2121. Gallagher AM, Rietbrock S, Plumb J, van Staa TP. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis?. J Thromb Haemost 2008;6:1500-6. ] further highlighting difficulties with anti-coagulation.
Despite these issues, vitamin K antagonists remain the most commonly employed medical thromboembolic prevention in atrial fibrillation, particularly in patients at elevated risk of thromboembolism. According to the ESC guidelines, assessing the individualized risk of cerebral events in patients with atrial fibrillation and the risks of chronic therapy with warfarin is paramount.[22. Camm AJ, Kirchhof P, Lip GY et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429. , 1313. Camm AJ, Lip GY, De Caterina R et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719-47. ] As outlined in Table 2, patients at low risk for cerebral events (CHA2DS2VASc score of 0) can be treated with aspirin alone or no therapy at all. Those at higher risk should be treated with oral anticoagulation unless otherwise contraindicated due to excessive bleeding risk.[22. Camm AJ, Kirchhof P, Lip GY et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429. , 1313. Camm AJ, Lip GY, De Caterina R et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719-47. , 2222. Gage BF, van Walraven C, Pearce L et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation 2004;110:2287-92. ]
The non-vitamin K oral anticoagulants, dabigatran, rivaroxaban and apixaban have gained considerable interest for thromboembolic event prevention. The first such agent was dabigatran, studied in the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) Trial evaluating 18,113 patients with atrial fibrillation and an increased risk of stroke (mean CHADS2 score of 2). Patients were treated with warfarin or dabigatran (110 or 150mg twice daily) with a primary endpoint of cerebral or systemic embolic events in a non-inferiority design.[2323. Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. ] Both doses of dabigatran proved non-inferior to warfarin; however, dabigatran at 150 mg twice daily proved superior to warfarin with regard to both the primary endpoint (relative risk 0.66, 95% CI 0.53-0.82, p<0.001) as well net clinical benefit, including the primary endpoint, bleeding and death (relative risk 0.91, 95% CI 0.82-1.00, p=0.04).[2323. Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. ] This benefit appears to be even greater in patients with poor anticoagulation control with warfarin, but it should be noted that medication discontinuation rates were significantly higher with either dose of dabigatran than with warfarin at both one year (14.5% dabigatran 110mg, 15.5%, dabigatran 150mg, 10.2% warfarin, p<0.01) and two-years (20.7% dabigatran 110mg, 21.2%, dabigatran 150mg, 16.6% warfarin, 0<0.01).[2424. Wallentin L, Yusuf S, Ezekowitz MD et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010. ]
Rivaroxaban was studied in the 14,226 patient Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for the Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Patients were randomized to either warfarin targeted to an INR of 2.0-3.0 or rivaroxaban 20 mg daily (15 mg daily for creatinine clearance 30-49 ml/min) with a primary composite endpoint of stroke and systemic embolism.[2525. Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus warfarin in atrial fibrillation. N Engl J Med 2011;365:883-91. ] In the primary, as treated analysis, there were similar rates of stroke and systemic embolism (1.7%/year rivaroxaban vs. 2.2%/year warfarin, HR 0.79, 95% CI 0.66-0.96, p<0.001 for non-inferiority). There were similar rates of bleeding between the two groups, but intracranial (0.5% vs. 0.7%, p=0.02) and fatal bleeding (0.2% vs. 0.5%, p=0.0.03) were both significantly lower in the rivaroxaban treated patients.[2525. Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus warfarin in atrial fibrillation. N Engl J Med 2011;365:883-91. ]
The Apixaban for Reduction of Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial evaluated 18,201 patients with atrial fibrillation and at least one risk factor for cerebral embolic events to warfarin targeted to INR 2.0-3.0 or apixaban 5 mg twice daily with a primary endpoint of stroke or ischemic embolism over a median 1.8 year follow-up. In this study, the primary endpoint occurred in 1.27%/yr. in the apixaban group compared to 1.6%/year in the warfarin group (HR 0.79, 95% CI 0.66-0.95, p<0.001 for non-inferiority, p=0.01 for superiority).[2626. Granger CB, Alexander JH, McMurray JJ et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. ] Apixaban was also associated with reductions in major bleeding (2.1%/year apixaban vs. 3.1 %/year warfarin, p<0.001) and all-cause mortality (3.5% apixaban vs. 3.9% warfarin, p=0.047).[2626. Granger CB, Alexander JH, McMurray JJ et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. ]
The Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) randomized 21,105 patients with nonvalvular atrial fibrillation to warfarin (n=7036) or edoxaban at either 60 mg (n=7035) or 30 mg (n=7034) with a primary efficacy endpoint of stroke or systemic embolic event and a primary safety endpoint of major bleeding.[2727. Giugliano RP, Ruff CT, Braunwald E et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104. ] In this noninferiority designed trial, stroke or systemic embolism occurred in 1.50% patients/year with warfarin versus 1.18% patients/year with edoxaban 60 mg (HR 0.79; 97.5% CI 0.63-0.99; p<0.001 for noninferiority) and 1.61% patients/year with edoxaban 30 mg (HR1.07; 97.5% CI, 0.87-1.31; p=0.005 for noninferiority). Bleeding events occurred at a rate of 3.43% patients/year with warfarin versus 2.75% patients/year with edoxaban 60 mg (HR 0.80; 95% CI, 0.71 to 0.91; p<0.001) and 1.61% patients/year with
Edoxaban 30 mg (HR 0.47; 95% CI, 0.41 to 0.55; P<0.0p1). Additionally, non-cardiovascular death, a prespecified secondary endpoint, occurred in 3.17% patients/year with warfarin versus 2.74% with edoxaban 60 mg (HR 0.86; 95% CI, 0.77 to 0.97; p=0.01), and 2.71% with edoxaban 30 mg (HR 0.85; 95% CI, 0.76 to 0.96; p=0.008).[2727. Giugliano RP, Ruff CT, Braunwald E et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104. ]
While the target specific oral anticoagulants appear to be both safe and efficacious for patients with non-valvular atrial fibrillation, it is important to note that the clinical trials involve carefully selected patients, and these agents will require post-market analysis to establish their relative utility in more general populations. Additionally, these agents were studied against warfarin, so head-to-head comparisons are lacking. Nevertheless, the preponderance of evidence suggests that oral anticoagulation is superior to antiplatelet therapy alone, and these newer agents may have advantages over warfarin in appropriately selected patients.
Medical therapy
- While symptomatic management focuses on rate and/or rhythm control, prevention of complications focuses on stroke prevention
- Cerebrovascular thromboembolic events are the most feared complication of atrial fibrillation, and the risk of stroke can be individualized
- The CHADS2 and CHA2DS2VASc are the two most commonly used risk models
- Coumadin is the cornerstone of thromboembolic event prevention in atrial fibrillation, although non-vitamin K oral anticoagulant medications, such as dabigatran, rivaroxaban, apixaban and edoxaban may have similar efficacy and improved safety profiles
- For patients with CHA2DS2VASc scores of 0, either aspirin or no therapy is indicated. Scores of 1 or greater warrant oral anticoagulation.
MECHANICAL THERAPIES
When thromboembolic events occur in patients with atrial fibrillation, the majority of thrombi originate in the left atrial appendage (LAA). More than 90% of all thrombi in patients with non-rheumatic atrial fibrillation originate in the LAA.[2828. Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755-9. ] In view of the numerous issues with anti-coagulation therapy and the relative simplicity of this anatomical target, exclusion of the LAA, either by surgical or percutaneous means, has garnered interest in recent years.
Mechanical therapy
- The great majority of thromboembolic events in patients with atrial fibrillation arise from thrombus in the left atrial appendage
- Occlusion of the left atrial appendage may significantly reduce the risk of events, particularly in patients in whom anti-coagulation is contraindicated
- Surgical means of left atrial appendage exclusion have been evaluated with mixed results, particularly owing to incomplete closure and residual flow into and out of the appendage
- Percutaneous techniques are emerging as an effective means of LAA occlusion.
- Currently approved devices for LAA occlusion include Watchman, Amplatzer Amulet, Wavecrest, Cardia and L’Ambre.
LEFT ATRIAL APPENDAGE ANATOMY
The LAA is an embryonic remnant of the left atrium. This structure is located anterolaterally in the atrioventricular groove and is lined with trabeculated pectinate muscles. There are wide variations in its shape, number of lobes, length, volume and orifice diameter.[2929. Hara H, Virmani R, Holmes DR, Jr. et al. Is the left atrial appendage more than a simple appendage?. Catheter Cardiovasc Interv 2009;74:234-42. ] In an autopsy study of 500 normal hearts, Veinot et al. found that the LAA was commonly bent or spiral in shape, and 54% of LAA had two lobes, whereas 23% had 3 lobes and another 20% had only one lobe.[3030. Veinot JP, Harrity PJ, Gentile F et al. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation 1997;96:3112-5. ] Volumes ranged from 0.7-19.2 cc, length ranged from 16-51 mm and orifice diameter ranged from 10-40 mm.[3030. Veinot JP, Harrity PJ, Gentile F et al. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation 1997;96:3112-5. ]
Given the wide variation in LAA anatomy, it stands to reason that imaging techniques must adequately visualize the LAA in order to successfully occlude it. Direct surgical visualization is certainly possible, as is non-invasive visualization with transesophageal or intracardiac echocardiography, computed tomography and magnetic resonance imaging. With regard to procedural guidance, transesophageal echocardiography (TEE) and fluoroscopy remain the primary modalities for assessment of anatomic variation, device positioning, assessment of successful deployment and monitoring of complications.
SURGICAL LAA EXCLUSION
As an operative procedure, surgical exclusion of the LAA dates back to the 1940’s. In recent years, the procedure has become increasingly common when patients with atrial fibrillation undergo otherwise indicated cardiac surgery. Despite common practice, there exist little published data to support this practice. The only randomized trial to date is the Left Atrial Appendage Occlusion Study (LAAOS) published in 2005.[3131. Healey JS, Crystal E, Lamy A et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J 2005;150:288-93. ] In this trial, 77 patients with atrial fibrillation and risk factors for cerebral events undergoing coronary artery bypass surgery were randomized 2:1 to LAA exclusion or no treatment. Of the 52 patients randomized to exclusion, 44 underwent repeat TEE. Only 5/11 (45%) of patients who had the LAA closed with sutures had successful closure on follow-up TEE, while 24/33 (72%) of patients who underwent combined suture and staple closure had complete closure.[3131. Healey JS, Crystal E, Lamy A et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J 2005;150:288-93. ] The study was not powered to assess events, but the results suggest suboptimal closure rates despite the employment of multiple techniques.
A more recently published meta-analysis evaluated the literature on surgical LAA closure.[3232. Dawson AG, Asopa S, Dunning J. Should patients undergoing cardiac surgery with atrial fibrillation have left atrial appendage exclusion?. Interact Cardiovasc Thorac Surg 2010;10:306-11. ] Specifically examining five clinical trials, including the LAAOS trial, the authors concluded that insufficient evidence exists to support routine closure of the LAA. In fact, of these five trials, only one showed benefit, while three were neutral and one showed harm. The authors suggest that incomplete closure (average 55-65%) may account for the discouraging results.[3232. Dawson AG, Asopa S, Dunning J. Should patients undergoing cardiac surgery with atrial fibrillation have left atrial appendage exclusion?. Interact Cardiovasc Thorac Surg 2010;10:306-11. ] This point is especially relevant as surgical LAA occlusion renders the patient at risk due to increased procedural time and incomplete closure does not eliminate the potential for thromboembolic events
PERCUTANEOUS LAA OCCLUSION
The primary downside to surgical LAA occlusion is that it holds little attraction as a stand-alone procedure. The trials investigating its utility included only patients undergoing cardiac surgery for another indication. Percutaneous means to occlude the LAA are intuitively attractive as they may be equally (or more) effective with substantially less physiologic insult to the patient and an overall improved safety profile compared to surgical closure or even medical therapy. A number of devices have thus far been developed for occlusion of the left atrial appendage. Initial devices included the Percutaneous Left Atrial Appendage Occluder (PLAATO, eV3, Inc., Plymouth, MA, USA) and the Watchman Left Atrial Appendage System (Boston Scientific Corporation, Marlborough MA, USA). Additionally, Amplatzer ASD and VSD devices (St. Jude Medical, St. Paul, MN, USA), although originally not intended to occlude the left atrial appendage, have been utilized to occlude the LAA. The Amplatzer Amulet left atrial appendage occluder is an iterative design of the ACP, specifically developed for atrial appendage occlusion, and it has recently become available in a number of countries. Additionally, the WaveCrest (Coherex Medical, Salt Lake City, UT, USA), UltraSept (Cardia Inc, Eagan, MN, USA) and L’Ambre (LifeTech Shenzen, China) devices are three newer devices with CE mark approval. Finally, the Lariat device (SentreHEART, Redwood City, CA) provides an extracardiac means to ligate/occlude the left atrial appendage.
TECHNICAL CONSIDERATIONS
Regardless of the particular device deployed, procedural aspects related to percutaneous LAA occlusion are similar. First, the LAA should be evaluated by TEE for existing thrombus ( Figure 2). If thrombus does exist, the procedure should be postponed, and oral anticoagulation should continue until the thrombus resolves. Once the appendage is found to be free of thrombus, transseptal puncture techniques are used to gain access to the left atrium. The location of the transseptal puncture is important and somewhat dependent on the device to be implanted. Once transeptal access is gained, a sheath is placed across the septum and a pigtail catheter is inserted into the LAA. This technique minimizes trauma to the LAA on engagement. Multiple fluoroscopic and echocardiographic measurements are made to accurately define the diameter, angle and depth and specific anatomy of the atrial appendage (Figures 3-5). After the appropriate size is determined, the device is positioned in the LAA, and the delivery sheath is retracted, thus deploying the device. Upon determination of successful deployment, the delivery sheath is detached from the device. At this point, the sheath is withdrawn into the right atrium.
General technical considerations
- Fluoroscopic and TEE guidance are imperative for successful procedure
- Location of transeptal puncture may vary by device shape and ensures coaxial alignment, potentially maximizing efficacy and minimizing complications
- LAA measurements taken by TEE and fluoroscopy with RAO caudal often providing the largest LAA dimensions
- A pigtail catheter with a soft wire is advanced past the transeptal delivery sheath to facilitate safe LAA engagement.
PLAATO
The PLAATO ( Figure 6) device, although no longer in production, was the first successfully implanted LAA occlusion device in humans.[3333. Sievert H, Lesh MD, Trepels T et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation 2002;105:1887-9. ] The device consisted of a self-expandable nitinol cage 18 to 32 mm in diameter and was coated with expanded polytetrafluoroethylene. In 2005, Ostermayer et al. reported the results of a nonrandomized multicentre trial in 111 patients with non-rheumatic atrial fibrillation of at least 3 months duration who had contraindications to therapy with warfarin.[3434. Ostermayer SH, Reisman M, Kramer PH et al. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J Am Coll Cardiol 2005;46:9-14. ] The PLAATO system was successfully implanted in 97.3% of patients (108/111). Four patients developed pericardial effusions or cardiac tamponade, and pericardiocentesis was necessary in three of them. One patient experienced a hemothorax and another a pleural effusion. At 6 months follow-up, successful LAA occlusion was demonstrated in 98% of the patients with a transesophageal echocardiogram. One patient developed a laminar thrombus on the device, detected at routine 6-month follow-up. During a follow-up period of 91 patient-years, two patients had a stroke, leading to an annual stroke rate of 2.2% after successful left atrial appendage occlusion. This represents a 65% relative risk reduction compared to a CHADS2 score predicted stroke rate of 6.3% in this patient cohort.[3434. Ostermayer SH, Reisman M, Kramer PH et al. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J Am Coll Cardiol 2005;46:9-14. ] Extending results out to 5-years, Block et al. reported on 64 patients entered into the North American cohort of this study.[3535. Block PC, Burstein S, Casale PN et al. Percutaneous left atrial appendage occlusion for patients in atrial fibrillation suboptimal for warfarin therapy: 5-year results of the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) Study. JACC Cardiovasc Interv 2009;2:594-600. ] Over 239 patient years of follow-up, the annualized cerebral events rate in this population (mean CHADS2 score of 2.6) was 3.8% while the expected rate was 6.6%.[3535. Block PC, Burstein S, Casale PN et al. Percutaneous left atrial appendage occlusion for patients in atrial fibrillation suboptimal for warfarin therapy: 5-year results of the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) Study. JACC Cardiovasc Interv 2009;2:594-600. ]
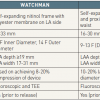
Watchman
The Watchman ( Figure 7) implant consists of a self-expanding Nitinol frame covered by a 160 µm polyester membrane on its left atrial side. Fixation barbs around the mid-perimeter secure the occluder to the wall of the left atrial appendage. The device is available in diameters from 21–33 mm.
From a technical standpoint, a few specific steps must be taken to ensure safe and effective implantation ( Figure 8). First, measurement of the LAA length (taken from the inferior LAA ridge to anterior upper lobe) is paramount for inclusion/exclusion purposes. In this case the LAA must be at least 19 mm in length. Next, measurement of the LAA width enables accurate device sizing, where the goal is to achieve device compression of 8-20% at the inferior ridge. As a result, LAA width can range from 17 mm (20% compression of a 21 mm device, to 31 mm (8% compression of a 33 mm device).
In 2007, Sick et al reported the initial experience of 75 patients.[3636. Sick PB, Schuler G, Hauptmann KE et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2007;49:1490-5. ] It was successfully implanted in 66/75 (88%) of patients with 93% complete sealing of the LAA at 45 days. Device embolization occurred in two of the initial patients. This led to redesign of the fixation barbs with no further instances with suggestion of improvement in embolization risk. Additional adverse events included cardiac tamponade in two patients, air embolism, in one patient, and delivery wire fracture requiring surgery in one patient. Four patients developed a flat layer of thrombus on the device at 6 months, and these resolved with additional anticoagulation. Two patients experienced a transient ischemic attack and there were no cerebral events during follow-up.[3636. Sick PB, Schuler G, Hauptmann KE et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2007;49:1490-5. ]
In the PROTECT AF (Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority) trial, 707 patients with atrial fibrillation and a CHADS2 score of ³ 1 were randomized to LAA occlusion with the Watchman device and planned discontinuation of warfarin after 45 days versus continued therapy with warfarin.[3737. Holmes DR, Reddy VY, Turi ZG et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534-42. ] Randomization was 2:1 favouring LAA occlusion. The study had a non-inferiority design with a composite primary efficacy endpoint of cerebral events, cardiovascular death and embolic event. The primary safety endpoint was a composite of haemorrhagic, pericardial effusion and device embolization. The device was successfully implanted in 88% (408/463) of patients, and at 45 days 86% of these patients were able to discontinue warfarin.
The results of this trial have since been extended out to 2.3 years[3838. Reddy VY, Doshi SK, Sievert H et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2. 3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation 2013;127:720-9. ] with a favourable impact on quality of life.[3939. Alli O, Doshi S, Kar S et al. Quality of life assessment in the randomized PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) trial of patients at risk for stroke with nonvalvular atrial fibrillation. J Am Coll Cardiol 2013;61:1790-8. ] Continued efficacy of the Watchman device was demonstrated at 4-year follow-up. In the 463 Watchman treated patients compared to the 244 medically treated patients the primary endpoint was noninferior (2.3% vs. 3.8%, relative risk 0.60, 95% CI 0.41-1.05). Interestingly, all-cause mortality (3.2% vs. 4.8%, relative risk 0.66, 95% CI 0.45-0.98, p = 0.0379) and cardiovascular mortality (1.0% vs. 2.4%; relative risk 0.40, 95% CI 0.23-0.82, p= 0.0045) were lower in Watchman treated patients.[4040. Reddy VY, Sievert H, Halperin J et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014;312:1988-98. ]
In view of lingering safety concerns from the PROTECT AF trial, the Prospective Randomized Evaluation of the Watchman LAA Closure Device In Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy (PREVAIL) trial randomized 407 patients with nonvalvular atrial fibrillation and a mean CHADS2 score of 2.6±1.0 to either LAA occlusion with the Watchman device (n=269) or medical therapy (n=138) with a primary combined endpoint of stroke, systemic embolism and cardiac/unexplained death.[4141. Holmes DR, Jr., Kar S, Price MJ et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1-12. ] At 18-months, the primary endpoint rate was 0.064 in the device group versus 0.063 in the control group (RR 1.07, 95% CI 0.57 to 1.89), and this did not meet the prespecified margin to satisfy noninferiority. The second noninferiority endpoint of stroke and system embolism >7 days post randomization was satisfied. On balance, this trial was limited by extremely low event rates in both groups, and the rates of pericardial effusion requiring surgical repair decreased in this trial to 0.4% (compared to 1.6% in PROTECT AF). These findings led to the general conclusion that LAA occlusion is both safe and effective.[4141. Holmes DR, Jr., Kar S, Price MJ et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1-12. ]
A patient level meta-analysis evaluating bleeding outcomes for the 1,114 patients enrolled in PROTECT AF and PREVAIL over a median 3.1 years of follow-up was recently published by Price and colleagues.[4242. Price MJ, Reddy VY, Valderrabano M et al. Bleeding Outcomes After Left Atrial Appendage Closure Compared With Long-Term Warfarin: A Pooled, Patient-Level Analysis of the WATCHMAN Randomized Trial Experience. JACC Cardiovasc Interv 2015;8:1925-32. ] In this analysis, there were similar overall bleeding rates between patients treated with the Watchman device compared to those treated with medical therapy (3.5 vs. 3.6 events per 100 patient years, RR 0.95, 95% CI 0.66-1.40, p=0.84). However, when considering events occurring > 7-days post randomization, there were significantly less events in the Watchman patients (1.8 vs. 3.6 events per 100 patient years, RR 0.49, 95% CI 0.32 – 0.75, p=0.001) with an even more significant difference noted after 6 months.[4242. Price MJ, Reddy VY, Valderrabano M et al. Bleeding Outcomes After Left Atrial Appendage Closure Compared With Long-Term Warfarin: A Pooled, Patient-Level Analysis of the WATCHMAN Randomized Trial Experience. JACC Cardiovasc Interv 2015;8:1925-32. ] This study demonstrates that the initial procedural risk is more than balanced by long-term benefit with regard to bleeding.
The overall results of PROTECT AF and PREVAIL studies suggest that LAA occlusion with the Watchman device is a reasonable alternative to warfarin therapy in patients with atrial fibrillation and elevated risk of cerebral events. Patients with a contraindication to warfarin were evaluated in the ASA Plavix Feasibility with Watchman Left Atrial Appendage Closure Technology trial. In this study, 150 patients with nonvalvular atrial fibrillation and a CHADS2 score of 2.8 ± 1.2 and a contraindication to oral anti-coagulation (93% with history of hemorrhage or other bleeding tendency) were enrolled and followed for a mean 14.4 months post procedure. Compared to an expected rate for ischemic stroke of 7.3%/year, patients undergoing LAA Occlusion with the Watchman device had ischemic stroke rates of 1.7%/year.[4343. Reddy VY, Mobius-Winkler S, Miller MA et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol 2013;61:2551-6. ] One additional patient suffered a hemorrhagic stroke. Adverse events occurred in 8.7% of patients and included bleeding, pericardial effusion and device thrombus/embolization.[4343. Reddy VY, Mobius-Winkler S, Miller MA et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol 2013;61:2551-6. ] These results suggest that the Watchman device is safe and effective for patients with contraindications to warfarin therapy.
Watchman
- Available sizes: 21-33 mm in 3 mm increments with sizing aimed to achieve 8-20% compression of the device at the inferior ridge of the LAA.
- Minimum LAA length is 19 mm as each device is of similar length
- Treatable LAA width 17-31 mm.
Amplatzer devices
LAA occlusion has also been performed using the Amplatzer Septal Occluder. Meier at al. reported on 16 patients who underwent LAA occlusion with this device.[4444. Meier B, Palacios I, Windecker S et al. Transcatheter left atrial appendage occlusion with Amplatzer devices to obviate anticoagulation in patients with atrial fibrillation. Catheter Cardiovasc Interv 2003;60:417-22. ] Implantation was successful in 15 patients with one patient experiencing device embolization. During follow-up, complete occlusion was noted in all 15 patients with no further complicating events.[4444. Meier B, Palacios I, Windecker S et al. Transcatheter left atrial appendage occlusion with Amplatzer devices to obviate anticoagulation in patients with atrial fibrillation. Catheter Cardiovasc Interv 2003;60:417-22. ]
The Amplatzer Cardiac Plug ( Figure 9a) is a device developed specifically for atrial appendage occlusion. It consists of a distal lobe with a proximal disk connected by a flexible central waist. Procedurally, the distal lobe is positioned within the left atrial appendage and the proximal disc is angled to fully cover the orifice of the left atrial appendage. Six pairs of hooks are attached to the distal body enabling the occluder to engage the wall of the left atrial appendage. The device is fully repositionable and recapturable. Device sizing is with reference to the distal lobe and ranges from 16-30 mm in 2 mm increments. The proximal disks are 4 mm larger for lobe sizes 16-22 mm and 6 mm larger for lobe sizes 24-30 mm. Appropriate sizing is 10-20% larger than the minimum appendage orifice measurement by TEE.
The initial European experience with ACP was reported for 143 European patients with atrial fibrillation. Device implantation was successful in 132/137 (96%) attempted implants. Serious adverse events within 24 hours occurred in 10 (7%) of patients. These events included 3 cerebral events, 2 device embolizations and 5 pericardial effusions requiring drainage.[4545. Park JW, Bethencourt A, Sievert H et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv 2011;77:700-6. ] More recently, Kefer et al. reported their experience of 60 patients with atrial fibrillation (CHADS2VASc 4.4±1.8, HAS-BLED 3.3±1.3) undergoing LAA appendage occlusion with the ACP device. The device was successfully implanted in 59 patients with 3 cases of tamponade, 3 smaller effusions, 2 cases of air embolism and 1 pseudoaneurysm. Over 1 year follow-up, the rate of stroke was 2.14%/yr.[4646. Kefer J, Vermeersch P, Budts W et al. Transcatheter left atrial appendage closure for stroke prevention in atrial fibrillation with Amplatzer cardiac plug: the Belgian Registry. Acta cardiologica 2013;68:551-8. ] For patients with contraindications to anticoagulation, Wiebe et al. reported their experience with 60 patients (CHADS2VASc 4.3±1.7, HAS-BLED 3.3±1.0) undergoing LAA occlusion with the ACP device. The procedure was successful in all but 3 patients. Over a mean of 1.8 years follow-up (1.0-2.8), no patients experienced stroke.[4747. Wiebe J, Bertog S, Franke J et al. Safety of percutaneous left atrial appendage closure with the amplatzer cardiac plug in patients with atrial fibrillation and contraindications to anticoagulation. Catheter Cardiovasc Interv 2014;83:796-802. ] Most recently, Tzikas et al reported experience with the first generation Amplatzer cardiac plug in 22 centers involving 1,047 patients. Procedure success rate was high at 97%, and overall periprocedural complications were low at 4.3% (death, tamponade, stroke, bleeding, myocardial infarction, and device failure). On follow up, the observed stroke rate was 2.3% versus an expected 5.62% based on CHADS2VASc score risk stratification with an overall reduction of 59%.[4848. Tzikas A, Shakir S, Gafoor S et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention 2016;11:1170-9. ]
Amulet:
The Amulet ( Figure 9b) is an iterative design advance on the original ACP device (which received CE approval in 2008). Advantages of the Amulet include preloading for enhanced ease of use, availability 31 and 34 mm sizes that allows for closure of a wider range of LAA diameters (11- 31 mm), a longer waist and greater number of fixation wires to enhance stability and a recessed to pin to minimize thrombus formation. The first-in-man evaluation included 25 patients with atrial fibrillation and a mean CHA2DS2VASc score of 4.3 +/- 1.7 undergoing LAA occlusion with the Amulet device. Procedural success was 96% with no procedural complications and complete closure in all patients. One patient experienced device related thrombus.[4949. Freixa X, Abualsaud A, Chan J et al. Left atrial appendage occlusion: initial experience with the Amplatzer Amulet. Int J Cardiol 2014;174:492-6. ] These findings led to CE approval in early 2013. More recently, a head-to-head comparison of the Amulet and ACP devices was performed in 59 patients (31 ACP, 28 Amulet). There were no differences in procedural success or device related complications, but less patients in the Amulet group had any form of leak on follow-up TEE (8% Amulet vs. 48% ACP, p=0.01).[5050. Abualsaud A, Freixa X, Tzikas A et al. Side-by-Side Comparison of LAA Occlusion Performance With the Amplatzer Cardiac Plug and Amplatzer Amulet. J Invasive Cardiol 2016;28:34-8. ] Currently, enrollment is ongoing for a randomized trial comparing Amulet vs. Watchman devices in 1600 patients in 150 centers globally with atrial fibrillation and a compelling reason for avoiding OACs (www.clinicaltrials.gov: NCT02879448). Primary end point will include death, bleeding, and periprocedural complications with a composite safety endpoint of complications, death, and bleeding at 12 months. Another co primary end point will be stroke and embolism. The study is planned to conclude in 2020 with a minimum of five year follow up for implanted patients.
WaveCrest:
The WaveCrest (Coherex Medical, Salt Lake City, UT, USA) is a next generation occlusion device ( Figure 10a) that recently received a CE mark in Europe. The device is designed to allow occlusion at the ostium with little device protrusion into the appendage (the device is short, therefore, appendage depth is rarely a limiting factor). It has retractable distal anchors distributed around the perimeter of the occluded designed to conform to irregular anatomy and maximize stability. The PTFE outer coating is both occlusive and non-thrombogenic. Additionally, the ability to inject distally allows for assessment of occlusion and stability by angiography. The device is available in 3 sizes: 22, 27 and 32 mm. In the initial trial of 73 patients, 68 (93%) had acute procedural success with 2 cases of pericardial effusion requiring drainage. At 45-day follow-up, successful closure (defined as less than a 3 mm residual leak) was present in 67 (92%) of patients.[5151. Whisenant B. WaveCrest. Transcatheter Cardiovascular Therapeutics. Washington, DC, 2014. ]
UltraSept:
The UltraSept device (Cardia Inc, Eagan, MN, USA) is another next generation device ( Figure 10b) recently receiving a CE mark in Europe. The device is available in sizes ranging from 16-32 mm (the outer occluding “sail” is 6 mm larger than the bulb) and is delivered through either a 10 or 12 French sheath. It is articulated with titanium centerposts and a nitinol bulb with the design intended to conform to various LAA shapes with minimal tension on the system. The device is completely repositionable and retrievable.[5252. Ibrahim R. ULTRASEPT LAA Closure Device (Cardia). Transcatheter Cardiovascular Therapeutics. San Francisco, CA, 2015. ]
The L’Ambre device (LifeTech, Shenzen, China) is the third novel device ( Figure 10cc) with recent CE approval. This device is a dual membrane design (umbrella and cover) available in two configurations depending on the size and lobality of the LAA to be occluded. Seventeen different combinations are available with sizes ranging from 16-36 mm for the umbrella and 22-40 mm for the cover. The occluder is delivered through an 8-10 French sheath depending on the particular size chosen. The CE mark study was based on a 60 patient experience demonstrating 100% success with complete closure in all cases and a 3.3% adverse event rate (1 patient died secondary to LAA wire perforation and another developed a pericardial effusion).[5353. Sievert H. Clinical experience with the L’Ambre left atrial appendage occlusion system. Joint Interventional Meeting. Milan, Italy, 2017. ]
Lariat Device:
While the above devices occlude the left atrial appendage via intracardiac device implantation, the Lariat device (SentreHEART, Redwood City, CA, Figures 11 and 12) occludes the LAA via an epicardial suture. In this procedure, epicardial access is obtained via a subxyphoid approach and a 14F epicardial guide is inserted. Transeptal access is gained via standard techniques and the left atrium is visualized under cineangiography. A 20 mm balloon tipped catheter is advanced over a 0.025” magnet tipped guide wire to the left atrial appendage. Next, a 0.035” magnet tipped epicardial guide wire is advanced through the epicardial sheath to the appendage so that the two magnets connect. Once this is accomplished, the Lariat snare is advanced over the 0.35” epicardial guide wire into position around the LAA. A balloon tipped catheter may be advanced to assist in positioning over the ostium of the LAA. As the snare is closed, under TEE and fluoroscopic imaging, flow in the LAA is assessed. When fully occluded, the suture is closed, the snare is removed and a suture cutter is advanced. A pericardial drain is exchanged over the 0.035” guide wire and the remaining equipment is removed. The drain is removed as appropriate.
The Lariat device was evaluated in 89 patients with nonvalvular atrial fibrillation, a CHADS2 score ≥ 1 and contraindications to oral anticoagulation.[5454. Bartus K, Han FT, Bednarek J et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013;62:108-18. ] Important exclusions included both clinical and anatomic features as demonstrated by pre-procedural CT scan. Clinical exclusions consisted of history of pericarditis or cardiac surgery, pectus excavatum, prior thoracic radiation, ejection fraction less than 30%, myocardial infarction within 3 months, NYHA Class IV heart failure or thromboembolic event within 30 days. Anatomic exclusions included a LAA diameter > 40 mm, multilobed LAA in different planes exceeding 40 mm, a posteriorly rotated heart or a superiorly oriented LAA such that the apex extends behind the pulmonary trunk. Of the 89 patients treated, 85 were successfully closed with immediate complete occlusion in 81 with no evidence of flow by TEE. Adverse events included late pericardial effusion (n=1), pericarditis (n=2), sudden death (n=2) and non-embolic stroke (n=2). At one-month follow-up, complete closure rates were 91%.[5454. Bartus K, Han FT, Bednarek J et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013;62:108-18. ] Longer-term follow up and randomized, controlled trials are necessary in order to establish the ultimate role of this device.
Subsequently, results from the U.S. transcatheter LAA ligation consortium were published (154 patients) that showed a major complication rate of 9.7% (primarily related to pericardial bleeding) and, in 2015, the FDA released a serious adverse effects warning for the Lariat device.[5555. Price MJ, Gibson DN, Yakubov SJ et al. Early safety and efficacy of percutaneous left atrial appendage suture ligation: results from the U.S. transcatheter LAA ligation consortium. J Am Coll Cardiol 2014;64:565-72. ] However, more recent data demonstrated a better safety profile than that reported in the aforementioned study (58 patients, no peri-procedural complications with the exception of one late pericardial effusion, complete closure rate of 92%, all leaks were <3 mm).[5656. Bartus K, Gafoor S, Tschopp D et al. Left atrial appendage ligation with the next generation LARIAT(+) suture delivery device: Early clinical experience. Int J Cardiol 2016;215:244-7. ] The better safety profile is likely related to increasing experience with the system as well as with changes in pericardial access technique including the routine use of a micro-puncture system. Currently the randomized controlled aMAZE (LAA ligation Adjunctive to PVI for Persistent or Longstanding Persistent Atrial Fibrillation) trial is ongoing (www.clinicaltrials.gov:NCT02513797). In this trial, patients with atrial fibrillation are randomized to pulmonary vein isolation alone or pulmonary vein isolation in combination with LARIAT LAA suture closure. The endpoint is the incidence of recurrent atrial fibrillation and safety. The plan is to enroll 600 patients.
Amplatzer® Amulet
- Transeptal puncture ideally anterior and inferior within fossa to ensure coaxial device alignment.
- Device sizes available are 16-34 mm in 2 mm increments with sizing 10-20% larger than landing zone by TEE.
- Minimum LAA length is 10 mm.
- Treatable LAA width 11-31 mm.
Lariat
- Suture mediated via epicardial access.
- Maximum LAA diameter is 40 mm.
- Maximum LAA height is 20 mm.
- Maximum LAA length is 70 mm.
- Anatomic exclusions include posterior or superior LAA rotation behind pulmonary trunk.
- Prior cardiac surgery is clinical exclusion.