Summary
Stroke prevention is a major public health priority: the surgical option, carotid endarterectomy (CEA), has been available for more than 50 years and has been the standard treatment for critical carotid stenosis in symptomatic and asymptomatic patients. Carotid artery stenting (CAS) is a new and effective endovascular treatment that avoids the pain and morbidity associated with surgery.
Clinical trials have not yet provided clear evidence of superiority of either treatment for “standard operative risk” patients and have been criticized for their design, patient selection, and very variable physician training and credentialing.
Solid evidence is still needed in many aspects of the management of CAS: we are aware that in the field of this specific interventional therapy, rapid changes in available therapeutic techniques create the situation in which clinical practice tends to follow technical developments without evidence from well-designed randomized trials.
In this gap of clear evidence, carotid artery stenting can be an alternative to surgery only if the peri-procedural complications are equal or less than the ones related to carotid endarterectomy. From this perspective, preprocedural evaluation of CAS technical risks, use of cerebral protection devices, stent selection and proper management of CAS complications are all key-points for managing a safe CAS procedure.
Current indications and contraindications for CAS and clinical data overview
CURRENT INDICATIONS AND CONTRAINDICATIONS
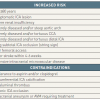
The conditions associated with an increased risk for carotid artery stenting (CAS) are listed in Table 1. As a general rule, the outcomes of CAS are mainly influenced by the anatomy of the patient [11. Roffi M, Mukherjee D, Clair DG. Carotid artery stenting vs. endarterectomy. Eur Heart J. 2009;30:2693-704.
A balanced review and interpretation of existing scientific data on carotid stenting and endarterectomy]. A favourable anatomy at the level of the aortic arch and the supra-aortic vessels makes a successful CAS likely. Conversely, the results of surgery are predominantly influenced by the comorbidities of the patient. The choice between CEA and CAS should therefore be based on local expertise, on the surgical risk of the patient, and on the presence or absence of conditions associated with an increased CAS risk ( Figure 1 ). In order to make an adequate selection of patients for CAS, it is important to know the conditions which increase the risk of the procedure.
CLINICAL DATA OVERVIEW
Randomised clinical trials of CEA vs. CAS
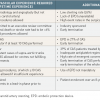
Seven major clinical trials (i.e., comprising over 300 patients) randomising patients for CEA or CAS have been published and/or presented. The SAPPHIRE (Stenting and Angioplasty with Protection in Patients at HIgh Risk of Endarterectomy) trial [22. Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493-501. ] included patients, both symptomatic and asymptomatic, at high risk for surgery. The CAVATAS (CArotid and Vertebral Artery Transluminal Angioplasty Study) [33. CAVATAS investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357: 1729-37. ], SPACE (Stent protected Percutaneous Angioplasty of the Carotid artery versus Endarterectomy) [44. Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, Hennerici M, Jansen O, Klein G, Kunze A, Marx P, Niederkorn K, Schmiedt W, Solymosi L, Stingele R, Zeumer H, Hacke W. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239-47. ], EVA-3S (Endarterectomy Versus Angioplasty in patients with Symptomatic Severe carotid Stenosis) [55. Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, Larrue V, Lievre M, Leys D, Bonneville JF, Watelet J, Pruvo JP, Albucher JF, Viguier A, Piquet P, Garnier P, Viader F, Touze E, Giroud M, Hosseini H, Pillet JC, Favrole P, Neau JP, Ducrocq X. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355: 1660-71. ], and ICSS (International Carotid Stenting Study) enrolled exclusively symptomatic patients [66. Featherstone RL, Brown MM, Coward LJ. International carotid stenting study: protocol for a randomised clinical trial comparing carotid stenting with endarterectomy in symptomatic carotid artery stenosis. Cerebrovasc Dis. 2004;18:69-74. ]. The CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial) included symptomatic and asymptomatic patients at low or intermediate risk for surgery [77. Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11-23. ]. Finally, the results of the ACT I (Asymptomatic Carotid Trial) [88. Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, Wechsler L, Jaff MR, Gray W; ACT I Investigators. Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis. N Engl J Med. 2016 Mar 17;374(11):1011-20. ] have been recently reported and compared CEA and CAS in asymptomatic patients who were not at high risk of surgical complications. Some of the studies were hindered by several limitations, as described in Table 2.
The CAVATAS study, performed in the late nineties and published in 2001, randomised 504 symptomatic patients, at low to moderate risk for surgery, for CEA or carotid angioplasty [33. CAVATAS investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357: 1729-37. ]. The incidence of death or stroke at 30 days was 10.0% in the endovascular group and 9.9% in the surgical group. At 8 years, no difference was observed in ipsilateral stroke or TIA, or any stroke between the two arms [99. Ederle J, Bonati LH, Dobson J, Featherstone RL, Gaines PA, Beard JD, Venables GS, Markus HS, Clifton A, Sandercock P, Brown MM. Endovascular treatment with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid And Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol. 2009;8:898-907. ]. Surgeons criticised the study for the high event rates in the surgical arm if compared to the NASCET and ECST trials. The study was criticised by the interventional community for its low stenting rate (26%) and lack of use of emboli protection devices (EPD). In fact, EPD were unavailable at that time. Stents only became available as of 1994, and their use increased toward the end of the study. A positive aspect of this trial was the independent neurological review that, up to that time, had not been consistently performed in previous studies and registries.
The SAPPHIRE study randomised 334 patients at high surgical risk for CAS with systematic use of EPD or CEA. The trial included asymptomatic (71%) and symptomatic (29%) patients. The primary endpoint – a composite of death, stroke, or myocardial infarction within 30 days after the intervention, or death or ipsilateral stroke between 31 days and 1 year – strongly favoured CAS, occurring in 12.2% of the CAS group and 20.1% of the CEA group (p=0.053). Patients who underwent CAS had significantly fewer myocardial infarctions at 30 days compared with those who had been randomized for CEA (1.9% vs. 6.6%, p=0.04). At 3-year follow-up, CAS and CEA were equally effective in terms of stroke prevention [1010. Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Ansel G, Strickman NE, Wang H, Cohen SA, Massaro JM, Cutlip DE. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-9. ]. The SAPPHIRE trial remains unique in several aspects: the required criteria for endovascular experience to participate in the study (the average experience of interventional physicians with the procedure was 64 cases, the range being from 20 to 700); the prohibition to perform CAS under tutoring conditions; the mandatory use of EPD; and the detection of peri-procedural myocardial infarction by means of systematic cardiac enzyme detection.
The multicentre SPACE study aimed to test the non-inferiority of CAS compared with CEA in symptomatic patients with moderate to severe stenosis. The study terminated after the inclusion of 1,200 patients because of slow enrolment and lack of funding to increase the number of patients to 2,500 to achieve the statistical power for proving non-inferiority of CAS. The study found no difference in the incidence of ipsilateral stroke or death at 30 days between patients allocated to CAS or CEA, with an event rate of 6.8% and 6.3%, respectively. The study failed to prove the non-inferiority of CAS compared with CEA even though the difference between the two treatments was very small (only four events in almost 600 patients per group). Emboli protection devices were used in a minority of CAS patients (27%). At 2 years, the outcomes of the two groups were comparable [1111. Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, Hennerici M, Stingele R, Fiehler J, Zeumer H, Jansen O. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893-902. ].
The French EVA-3S trial aimed to test the non-inferiority of CAS compared with CEA in symptomatic patients with carotid stentosis of 60% or more. The study was stopped prematurely after the inclusion of 527 patients because of a significantly increased event rate among those allocated to endovascular treatment (death or stroke 9.6% in the CAS arm and 3.9% in the CEA arm). At 6 months, the incidence of any stroke or death was 11.7% in the CAS group and 6.1% in the CEA group (p=0.02). The study has been heavily criticised for the minimal requirements in terms of endovascular experience ( Table 2). In the endovascular arm, 39% of patients were treated by a physician in training. At 4-year follow-up, the death or stroke rate still favoured CEA, driven by the 30-day events. However, beyond 30 days, no difference in adverse outcomes between CAS and CEA was observed [1212. Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A, Bossavy JP, Denis B, Piquet P, Garnier P, Viader F, Touzé E, Julia P, Giroud M, Krause D, Hosseini H, Becquemin JP, Hinzelin G, Houdart E, Hénon H, Neau JP, Bracard S, Onnient Y, Padovani R, Chatellier G; EVA-3S Investigators. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7:885-92. ].
The ICSS trial randomised 1,710 symptomatic patients with carotid stenosis of more than 50% in 50 centres in Europe, Australia, New Zealand and Canada between May, 2001, and October, 2008, and was concluded in 2011[66. Featherstone RL, Brown MM, Coward LJ. International carotid stenting study: protocol for a randomised clinical trial comparing carotid stenting with endarterectomy in symptomatic carotid artery stenosis. Cerebrovasc Dis. 2004;18:69-74. ] [1313. Bonati LH, Dobson J, Featherstone RL, Ederle J, van der Worp HB, de Borst GJ, Mali WP, Beard JD, Cleveland T, Engelter ST, Lyrer PA, Ford GA, Dorman PJ, Brown MM; International Carotid Stenting Study investigators. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet. 2015 Feb 7;385(9967):529-38. ]. The pre-specified primary endpoint was the 3-year rate of fatal or disabling stroke in any territory after randomization. Secondary endpoints were any stroke, any stroke or death, any stroke or procedural death, disabling stroke or death, and all-cause death. Patients were followed up for an average of 4.2 years after randomization. The primary endpoint was seen in 52 out of 853 patients in the stenting group (cumulative 5-year risk 6.4%) and in 49 out of 857 patients in the endarterectomy group (cumulative 5-year risk 6.5%), showing that stenting is as effective as endarterectomy in preventing fatal or disabling stroke in patients with symptomatic carotid stenosis. Regarding the secondary endpoints, carotid stenting was associated with a higher procedure-related and long-term risk of any stroke, with a 5-year cumulative risk of 15.2% compared with 9.4% in the endarterectomy group (HR 1.171, 95% CI 1.28-2.30), although functional disability and quality of life did not differ between groups. In the post-procedure period, the main difference between the two groups in terms of risk of stroke was mainly attributable to the occurrence of strokes in the contralateral carotid or vertebrobasilar territory in the stenting group. The authors have no conclusive explanation for this finding. The all-cause mortality did not differ significantly between the groups. The hazard ratio for procedural stroke or procedural death, or ipsilateral stoke during follow-up was lower for patients treated at “larger” centres (enrolling 50 or more patients in the trial, i.e., a minimum average inclusion of only 10 patients per year). Again, some criticisms have been made regarding the level of operator experience required in the ICSS trial. In the study, ‘experienced centres’ were required to have operators who have performed at least 50 stenting procedures, of which only 10 had to be CAS cases. ‘Supervised centres’, where operators did not meet those requirements, could perform tutor-assisted procedures. Additionally, strengthening the criticisms about the low operator experience in this trial, two investigators from separate stenting centres, whose cases were linked to an excess of adverse events (n=11, five with disabling stroke or death), were barred from treating any further patients in the trial and the centres were suspended from further participation, but these patients were included in the trial analyses.
A meta-analysis of the above mentioned trials is described in Figure 2.
Subsequently, the CREST study [77. Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11-23. ], randomising 2,502 patients for CEAor CAS + EPD, was published. The primary endpoint – a composite of stroke, myocardial infarction, or death from any cause during the peri-procedural period or any ipsilateral stroke within 4 years after randomisation – did not differ between the two groups (7.2% in the CAS group and 6.8% in the CEA group). Peri-procedural rates of individual components of the endpoints differed between the stenting group and the endarterectomy group: for death (0.7% vs. 0.3%, p=0.18), for stroke (4.1% vs. 2.3%, p=0.01), and for myocardial infarction (1.1% vs. 2.3%, p=0.03). After this period, the incidences of ipsilateral stroke with stenting and with endarterectomy were similarly low (2.0% and 2.4%, respectively; p=0.85). Although the peri-procedural death or stroke rates were higher with CAS than with CEA, both in symptomatic and in asymptomatic patients, CAS succeeded for the first time within a randomised trial in meeting the recommendation of the American Heart Association of 6.0% for symptomatic patients and 3.0% for asymptomatic patients. Accordingly, the rates observed in the CAS arm of CREST were 6.0% and 2.5%, respectively. The 10-year follow-up results from CREST have recently been published [1414. Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, Moore WS, Hill MD, Mantese VA, Clark WM, Timaran CH, Heck D, Leimgruber PP, Sheffet AJ, Howard VJ, Chaturvedi S, Lal BK, Voeks JH, Hobson RW 2nd; CREST Investigators. Long-Term Results of Stenting versus Endarterectomy for Carotid-Artery Stenosis. N Engl J Med. 2016 Mar 17;374(11):1021-31. ]. The investigators did not find a significant difference between patients who underwent CAS or CEA with respect to the risk of periprocedural stroke, myocardial infarction, or death and subsequent ipsilateral stroke. The rate of postprocedural ipsilateral stroke (primary long-term end point) also did not differ between CAS (6.9%) and CEA (5.6%) (hazard ratio, 0.99; 95% CI, 0.64 to 1.52).
The ACT I trial compared CAS with embolic protection and CEA in asymptomatic patients who were at standard risk for surgical complications. The trial was designed to enroll 1,658 patients but was halted early, after 1,453 patients underwent randomization, because of slow enrollment. The primary composite endpoint of death, stroke, or myocardial infarction within 30 days after the procedure or ipsilateral stroke within 1 year were tested at a non-inferiority margin of 3 percentage points. The results have recently been published [88. Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, Wechsler L, Jaff MR, Gray W; ACT I Investigators. Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis. N Engl J Med. 2016 Mar 17;374(11):1011-20. ], revealing that CAS was non-inferior to CEA with regard to the primary composite endpoint (event rate, 3.8% and 3.4%, respectively; p=0.01 for non-inferiority). The rate of stroke or death within 30 days was less than 3% in both groups (2.9% in the CAS group and 1.7% in the CEA group, p=0.33). The rate of freedom from ipsilateral stroke from 30 days to 5 years was 97.8% in the CAS group and 97.3% in the CEA group (p=0.51). One point to be stressed is the required operator and centre experience in performing both treatments. The study sites and physicians were selected according to a process that included a review of American Board of Medical Specialties certification and training, previous operator experience (25 recent cases of CAS or CEA for each investigator) and recent outcomes. The study also included a lead-in phase in which the operators had to prove proficiency with the study devices in at least two cases. The enrolling sites were also audited for excess adverse events (two sites were temporarily stopped from enrolling patients). One limitation of the study was the reduction of the revised power analysis from 80% (if all 1,658 patients initially planned were recruited) to 75% with the total of 1,453 patients effectively recruited.
The Asymptomatic Carotid Surgery Trial 2 (ACST-2) is a large international randomised trial that compares CEA versus CAS (1:1) in asymptomatic patients with carotid stenosis. ACST-2 is currently recruiting patients from all over the world and intends to recruit at least 5,000. Up to the present, the trial has recruited 2,044 patients, and 3,600 are planned by the end of 2019. Data will be analysed on an intention-to-treat basis; the primary endpoint is peri-procedural risks (MI, stroke and death within the first month) and long-term (up to 5 or more years) prevention of stroke, particularly disabling or fatal stroke in the two treatment groups. The secondary endpoints are economic evaluation of procedural costs, stroke-related healthcare costs and quality of life assessment. The stenting interventionist may be a radiologist, cardiologist, surgeon or physician with specialist training in carotid stenting. Doctors must have performed the particular procedure (CEA or CAS) 25 or more times in order to participate in the trial. In general, physicians should have ≤ 8% stroke and death risk for symptomatic patients and ≤ 4% for asymptomatic patients. The result of this trial will hopefully dispel some doubts about the treatment of asymptomatic patients.
CAS REGISTRIES
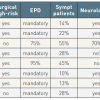
The results of eight CAS registries enrolling over 1,000 patients have been published, for a total of 21,405 patients. All but two were performed in the United States, included patients at high risk for surgery, and the majority of patients included were asymptomatic. The good methodology of the studies is demonstrated by the high proportion of mandatory neurologic assessment pre- and post-procedure and of clinical event committee adjudication of adverse outcomes. The use of EPD was mandatory in five registries and used in the majority of patients in the remaining two. Overall, the studies demonstrate results in the range of current recommendations for CEA even in patients at high risk for surgery, and the results have improved over time ( Table 3 ).
When to intervene in patients with severe carotid disease
- The sole incentive for the treatment of carotid artery stenosis is stroke prevention
- In symptomatic severe carotid disease, updated guidelines provide clear recommendations for individual treatment strategies
- In asymptomatic lesions, the stroke risk associated with any type of prophylactic intervention should not exceed the risk inherent in the natural course of carotid atherosclerosis: 1%-3% per year
- This low risk for clinically asymptomatic carotid disease may become significantly higher in specific subsets (8% to 14% per year)
- For CAS, the experience of hospitals and physicians correlates with patient outcomes
Technical considerations for a safe CAS procedure
The 3% - 6% rule of periprocedural death or stroke rate, for asymptomatic and symptomatic patients, respectively, establishes the upper acceptable complication rate for CAS (CAS) [1515. Roubin GS, Iyer S, Halkin A, Vitek J, Brennan C. Realizing the potential of carotid artery stenting: proposed paradigms for patient selection and procedural technique. Circulation. 2006;113:2021-30. ]. Beyond those boundaries, carotid revascularisation may not be of benefit over medical management. Establishing the level of technical complexity of the procedure by evaluating the clinical presentation of the patient, then anatomical aspects of the vessel, as well as the carotid lesion angiographic and ultrasound characteristics is essential to anticipate the operator skills needed to achieve a safe procedure. Some important concepts related to the safety of the procedure are discussed below.
TAILORED APPROACH CONCEPT
The “tailored approach” to CAS consists of the planning of a specific endovascular strategy for a specific patient. This approach depends on an in-depth knowledge of all characteristics of the endovascular equipment (guiding catheters, guiding sheaths, guidewires, embolic protection devices, balloons and stents) to match precisely the most suitable device to a specific vascular anatomy, carotid lesion, or cerebral circulation pattern as well as to the clinical presentation of the patient [1616. Cremonesi A, Diehm N, Stella A, Gargiulo M, Faggioli G, de Campos Martins EC, Castriota F. Peripheral Arterial Occlusive Disease (chapter 36). ESC Textbook of Cardiovascular Medicine, 2009. Oxford University Press Inc.
, New York]. Our group recently published the results of the “tailored-CASE Registry” which analysed the use of the “tailored approach” in 1,523 procedures [1717. Cremonesi A, Gieowarsingh S, Spagnolo B, Manetti R, Liso A, Furgieri A, Barattoni MC, Ghetti L, Tavazzi L, Castriota F. Safety, efficacy and long-term durability of endovascular therapy for carotid artery disease: the tailored-Carotid Artery Stenting Experience of a single high-volume centre (tailored-CASE Registry). EuroIntervention. 2009;5:589-98. ]. A procedural success rate of 99.6% was achieved. The 30-day all-stroke and death rates were 1.2% (n=14) and 2.7% (n=10) for the asymptomatic and symptomatic patients groups, respectively. The highest-risk population, i.e., symptomatic patients aged more than 80 years, had a 30-day all-stroke and death rate of 4.5%. Based on these findings, the use of the “tailored approach” by experienced operators seems to be a valuable tool to increase the overall safety of CAS.
STENT SCAFFOLDING
Distal embolisation is the major neurological complication not only periprocedurally but also in the postprocedural period up to 30 days [1818. Cremonesi A, Setacci C, Manetti R, de Donato G, Setacci F, Balestra G,Borghesi I, Bianchi P, Castriota F. Carotid angioplasty and stenting: lesion related treatment strategies. EuroIntervention. 2005;1:289-95. ]. Great emphasis is placed on the use of EPD for effective reduction of intraprocedural risk of cerebral embolisation. An important aspect to consider is the fact that the type of stent, in the presence of EPD, may not significantly impact on the risk of intraprocedural complications, but may play a vital role in preventing later events due to plaque prolapse [1919. Müller-Hülsbeck S, Schäfer PJ, Charalambous N, Schaffner SR, Heller M, Jahnke T. Comparison of carotid stents: an in-vitro experiment focusing on stent design. J Endovasc Ther. 2009;16:168-77. ]. Accordingly, whereas during carotid endarterectomy the atheroma and thrombus components are completely excised, during CAS the carotid plaque is compacted to the wall and the plaque material is retained by the scaffolding and wall-coverage properties of the stent. Thus, the risk of plaque prolapse and distal embolisation may be influenced by the stent-cell geometry, not only during the procedure (when EPD are in place), but during the 24-hour postprocedural period and within 30 days post procedure, until re-endothelialisation is completed. This property of the stent is called “intrinsic anti-embolic”.
BRAIN PROTECTION
Carotid artery lesions are sometimes composed of large-burden, friable, thrombotic- containing material and ulcerated plaques which can embolise during endovascular intervention as shown in histopathology analysis [2020. Angelini A, Reimers B, Della Barbera M, Saccà S, Pasquetto G, Cernetti C, Valente M, Pascotto P, Thiene G. Cerebral protection during carotid artery stenting: collection and histopathologic analysis of embolized debris. Stroke. 2002;33:456-61. ] and transcranial Doppler studies [2121. Crawley F, Clifton A, Buckenham T, Loosemore T, Taylor RS, Brown MM. Comparison of hemodynamic cerebral ischemia and microembolic signals detected during carotid endarterectomy and carotid angioplasty. Stroke. 1997;28:2460-4. ]. Macroemboli (defined as emboli >100 μm) are usually associated with clinical events, especially if the size is >200 μm. The effects of microembolisation (defined as emboli <100 μm) are not well known and may include subtle changes in neurocognitive functions. The use of EPD has been associated with a reduction of the embolic load [2222. Al-Mubarak N, Roubin GS, Vitek JJ, Iyer SS, New G, Leon MB. Effect of the distal-balloon protection system on microembolization during carotid stenting. Circulation. 2001;104:1999-2002. ]. Preliminary data indicate that the clinical results of CAS are comparable with the best surgical series, when EPD are routinely applied [2323. Kastrup A, Groschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003;34: 813-9. , 2424. Reimers B, Schlüter M, Castriota F, Tübler T, Corvaja N, Cernetti C, Manetti R, Picciolo A, Liistro F, Di Mario C, Cremonesi A, Schofer J, Colombo A. Routine use of cerebral protection during carotid artery stenting: results of a multicenter registry of 753 patients. Am J Med. 2004;116:217-22. ]. The use of EPD is nowadays considered a standard when pursuing a safe endovascular procedure.
Pre-procedural evaluation of CAS technical risks
A sequential and standardised preprocedural evaluation of the patient’s anatomical and clinical characteristics is essential in identifying the factors that could increase the technical difficulties and risks of CAS to avoid treatment failures or complications. Anticipating adverse scenarios allows the operator to select the most appropriate material and technique, following the concept of the “tailored approach”.
THE “STENTING SEGMENT” CONCEPT
The primary goal of CAS is to prevent a future neurological event. The technical goal of CAS is to cover the entire carotid plaque with the stent, leaving a residual stenosis of less than 30%. Treating carotid lesions by stenting from “normal to normal” arterial segments is a basic concept when performing CAS. A full appreciation of the adverse anatomical scenarios of this “normal to normal” segment is of paramount concern, as this vascular segment will interact with the bare metal/nitinol structure during and after the procedure. “Stenting segment” is defined by the straightened arterial segment that comprises the carotid lesion and the “normal” arterial margins adjacent to the carotid lesion (proximally and distally), which must be covered by stent implantation ( Figure 3 ).
Some basic aspects of the “stenting segment” are shown in Figure 4 . In many cases, a distal curve can be avoided if a very short “normal region” distal to the carotid lesion is considered, so that the stent does not “touch” an accentuated curve ( Figure 4 D-E ). Five millimetres is considered as the shortest length of the “normal” arterial region that should be covered by a well-trained operator. If the distal margin prior to a distal curve is shorter than that, then the curve should be considered part of the “stenting segment” ( Figure 4 G-H ).
As carotid lesions are mainly located at, or very close to, the carotid bifurcation level, the “stenting segment” should always include the carotid bifurcation.
THE “FIVE ARTERIAL ZONES” CONCEPT
Some anatomical classifications, dividing the internal carotid artery (ICA) and its main branches, have been proposed [. , . , . , . , . , . ]. With the introduction of CAS in daily practice, a new anatomical classification is required since some anatomical aspects (such as the common carotid artery [CCA] and aortic arch anatomies) are not considered in those classifications and they have a direct impact on the risks of the CAS procedure. A new nomenclature system is proposed here, based on the delimitation of five arterial zones, each one corresponding to a procedural step of CAS: “The five arterial zones” classification.
After delimiting the “stenting segment”, it is possible to divide the extracranial carotid artery into three main zones: the “proximal vessel” zone (from origin of the left CCA or brachiocephalic trunk [BCFT] to the “stenting segment”); the “stenting segment” zone itself and the “distal vessel” zone (the ICA from the “stenting segment” until the vessel enters the skull). The “five arterial zones” comprise those three zones and the traditional aortic arch and cerebral vessel anatomies (here called “aortic arch” and “cerebral vessel” zones) ( Figure 5 ).
CORRELATING THE “FIVE ARTERIAL ZONES” TO THE “TAILORED CAS” PROCEDURE
Each segment of the “five arterial zones” has a direct correlation to a different step of the CAS procedure. During the analysis of each arterial zone, a basic link must be kept in mind as proposed below:
- The “aortic arch” and “proximal vessel” zones are related to anticipating difficulties during the engagement of the CCA and stabilising the guiding catheter/guiding sheath. The evaluation of this zone should be based on the “aortic arch” zone characteristics:
- Aortic arch classification (Types I, II and III);
- Aortic arch anatomical variations;
- Degree of atherosclerosis/calcification;
- Other conditions such as aneurysms and ulcerations ( Figure 6 ).
- “Proximal vessel” and “distal vessel” zones are related to anticipating difficulties during the proximal or distal EPD placement (respectively) and retrieval. The evaluation of these zones should include:
- Vessel diameter;
- Vessel angulations, tortuosity and kinking;
- Vessel disease (atherosclerosis, stenosis, ulcer, ectasia, aneurysm, or fibro-muscular dysplasia);
- “Guiding catheter landing zone” in the CCA (the arterial segment which is chosen to land the guiding catheter or guiding sheath tip);
- The distal EPD “landing zone” in the ICA.
- “Cerebral vessel” zone is related to anticipating difficulties during the predilatation, stenting and post-dilatation phases. This zone has a direct impact on the choice of the appropriate stent technical features, and influences the choice of the type of cerebral protection device. The evaluation of this zone includes:
- The carotid lesion characteristics:
- Angiographic features;
- Echo Doppler characteristics;
- Patient symptoms: even though not an anatomical or ultrasound aspect, we intentionally organised the patient symptoms in this section because patients with neurological symptoms have carotid lesions with a higher cerebral embolisation potential during CAS procedure;
- Vessel characteristics.
All these aspects are detailed in the Figure 5 .
The “cerebral vessel” zone is linked to verifying the basal cerebral anatomy and collateral flow pattern (integrity of the Circle of Willis) ( Figure 7 ) and the possibility of the use of the proximal EPD. The “cerebral vessel” zone is evaluated according to: (a) vessel diseases (stenosis, aneurysms and arteriovenous malformations) and (b) intracranial vessel anatomy by identifying the basal cerebral arterial anatomy and hemisphere dominance. When an “isolated hemisphere” is suspected, the cerebral collateral flow should be checked by verifying the patency of the anterior and the posterior communicating arteries ( Table 4 ).
Towards a safer carotid stenting
- Recognise in advance all the clinical and anatomical situations which can expose the patient to higher risk for carotid stenting
- The way to access the common carotid artery safely, EPD selection and management, stent selection and implantation are key elements for reducing procedural complications
Cerebral protection devices
CLINICAL RESULTS
The comparison of the efficacy between protected and unprotected CAS by a large randomised trial has never been done and it is difficult to imagine that such a study will ever exist. In this sense, published literature data have given only indirect information about the need for EPD.
EPD capturing visible debris was documented in 60% of CAS procedures by Sprouse et al [3131. Sprouse LR, 2nd, Peeters P, Bosiers M. The capture of visible debris by distal cerebral protection filters during carotid artery stenting: Is it predictable?. J Vasc Surg. 2005;41:950-5. ], and in 66.8% by our group [1818. Cremonesi A, Setacci C, Manetti R, de Donato G, Setacci F, Balestra G,Borghesi I, Bianchi P, Castriota F. Carotid angioplasty and stenting: lesion related treatment strategies. EuroIntervention. 2005;1:289-95. ].
Our group also observed a 79% reduction in the rate of embolic complication with protected CAS [3232. Castriota F, Cremonesi A, Manetti R, Liso A, Oshola K, Ricci E, Balestra G. Impact of cerebral protection devices on early outcome of carotid stenting. J Endovasc Ther. 2002;9:786-92. ].
A review of the global registry in 2003 found a 5.2% and 2.2% rate of stroke and death for unprotected and protected CAS, respectively [3333. Wholey MH, Al-Mubarek N. Updated review of the global carotid artery stent registry. Catheter Cardiovasc Interv. 2003;60:259-66. ].
Unprotected CAS was associated with a 3.9 times higher stroke rate at 30 days as compared to protected CAS in the early phase of the EVA-3S study [3434. Mas JL, Chatellier G, Beyssen B. Carotid angioplasty and stenting with and without cerebral protection: clinical alert from the Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S) trial. Stroke. 2004;35:e18-20. ].
Protected CAS was associated with a significantly lower rate of ipsilateral stroke (1.7% vs. 4.1%, p=0.007) in a German registry [3535. Zahn R, Mark B, Niedermaier N, Zeymer U, Limbourg P, Ischinger T, Haerten K, Hauptmann KE, Leitner ER, Kasper W, Tebbe U, Senges J; Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte (ALKK). Embolic protection devices for carotid artery stenting: better results than stenting without protection?. Eur Heart J. 2004;25:1550-8. ].
Kastrup et al [3636. Kastrup A, Groschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003;34: 813-9. ] reviewed the literature regarding the early outcome of CAS with and without the use of EPD in studies published between 1990 and 2002. The combined 30-day stroke and death rates were 5.5% and 1.8% for unprotected (p=2,537) and protected CAS (p=896) procedures, respectively (p<0.001).
In a recent literature review, Garg et al analysed 134 articles to a total of 12,263 protected and 11,198 unprotected CAS patients [3737. Garg N, Karagiorgos N, Pisimisis GT, Sohal DP, Longo GM, Johanning JM, Lynch TG, Pipinos II. Cerebral protection devices reduce periprocedural strokes during carotid angioplasty and stenting: a systematic review of the current literature. J Endovasc Ther. 2009;16:412-27.
Review]. Using pooled analysis of all 134 reports they found the relative risk for stroke of 0.62 (95% CI: 0.54 to 0.72) in favour of protected CAS. Subgroup analysis revealed a significant benefit for protected CAS in both symptomatic and asymptomatic patients (p<0.05).
Following these data, protected CAS seems to be associated with a reduction of procedural thromboembolic complications.
DISTAL PROTECTION
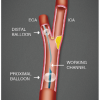
The mechanisms of action of distal EPD are based on the filtering or interruption of the ICA blood flow, by positioning the device distally to the lesion, in a region called the “landing zone” (distal part of the extracranial ICA). The first distal EPD system used consisted of an occlusive balloon that blocked the ICAflow, allowing the treatment of the lesion without cerebral embolisation in the cerebral vessels. This system is rarely used nowadays, widely substituted by filter devices ( Figure 8 ) due to their intuitive use, lesser complexity, efficacy and perhaps because they require less operator experience. Filter devices entrap medium to large-size debris, generally particles which are >100 μm. The filter’s performance can be summarised as crossing profile and capturing capability. The importance of the filter crossing profile is that wire and filter (as a unit or even independent of each other) must cross the lesion area without dislodging friable materials. The filter capture capability is mainly dependent on membrane pore size and proper wall apposition.
Distal EPD, either occlusive balloon or filters, have the limitation of unprotected crossing of the lesion, especially in tight stenoses where the risk of fragment dislodgment and embolisation is increased. Distal occlusion balloons, despite being able to block microemboli and macroemboli by occluding theICA flow, can lead to embolisation via collaterals from the ECA to the middle cerebral artery. In addition, about 5% to 8% of patients develop clamping intolerance due to the interruption of cerebral perfusion. The filter devices fail to trap microemboli, limited mainly by pore size. In tortuous or large distalICA, inadequate wall apposition of the device may allow even macroemboli to bypass the system. The fact that debris may be dislodged (squeezing effect) during the manoeuvres of filter retrieving must be added. Both distal EPD systems may themselves damage the landing zone, becoming a source of embolic materials and other complications such as arterial dissection and spasm.
PROXIMAL PROTECTION
The proximal EPDs work by interrupting or reversing the blood flow in the CCA/ICA. They have the advantages of promoting a protected crossing of the lesion and blocking both macroemboli and microemboli. The fact that there is no manipulation of the device in the distal ICA reduces the risk of arterial spasm, dissection or intimal damage.
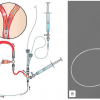
Mo.Ma® (Medtronic, Inc., Minneapolis, MN)
The Mo.Ma system consists of an 8 Fr or 9 Fr sheath with an effective working channel of a 5 Fr and 6 Fr, respectively, with two independently inflatable balloons ( Figure 9 ). The distal balloon is located close to the sheath tips and occludes ECA up to a diameter of 6 mm. The proximal balloon is positioned 7.2 cm proximally to the distal balloon and occludes the CCA up to a diameter of 13 mm. When inflated, both balloons prevent antegrade flow from the CCA and retrograde flow from the ECA. In this situation, the carotid lesion can be crossed and treated under flow blockage, preventing cerebral embolisation. After the lesion treatment, three 20 mm syringes of blood are actively aspirated and checked for debris before deflating the distal and proximal balloons, respectively.
Neuro Protection System™ (NPS; W.L.Gore & Associates, Inc., Flagstaff, AZ, USA)
The Neuro Protection System, derived from the Parodi Anti-Emboli System (PAES; ArteriA Medical Science, San Francisco, CA, USA), promotes passive reverse flow in the ICA. It is composed of two independent systems: the balloon wire and the 11 Fr sheath (9 Fr in the new NPS system), which has an effective working channel of 6 Fr and contains an inflatable balloon at its tip ( Figure 10). The sheath is positioned in the CCA and the balloon wire is inserted through the sheath and positioned at the ECA. When both balloons are inflated, the blood flow through the CCA and ECA is blocked. The proximal part of the sheath is connected to the contralateral femoral vein, allowing flow-reversal of blood from the cerebral circulation (via the Circle of Willis) down the ICA and through the sheath into the venous system. The retrograde blood flow passes through a filter (pore size 180 μm) which collects debris before the blood re-enters the femoral vein. In reverse flow, the carotid lesion can be crossed and treated. At the end of the procedure, 10 ml of blood are actively aspirated and then the balloons are deflated while active suction is applied to retrieve any particles contiguous to the balloon occluder.
There was an announcement on the company site stating this device had not been available for order since 1st October, 2013.
ENROUTE® Transcarotid Neuroprotection System (Silk Road Medical)
The device consists of a novel flow reversal system comprising an arterial sheath, a venous sheath and a flow line (with a flow regulator) ( Figure 15). One dedicated 8F transcervical arterial sheath (with side branch tubing) is designed to be inserted into the ipsilateral common carotid artery via a surgical incision (direct access) above the clavicle (5cm below the carotid bifurcation). The process of inserting the arterial sheath follows the looping of the common carotid artery (proximally to the arterial incision). When tensioned, the loop stops the antegrade flow in the common carotid artery, forcing the blood in the distal common carotid artery, (and consequently from the internal and external carotid arteries) to run retrogradely into the arterial sheath. The venous return sheath is inserted percutaneously into the contralateral femoral vein. The side arm tubing of both sheaths (arterial and venous) is connected to the flow line, creating an arteriovenous shunt. In the flow line, there is an in-line flow controller, which allows the operators to choose the degree of retrograde blood flow (high flow or low flow) through the circuit or even temporary flow blockage. All the phases of lesion manipulation (lesion wiring, pre-dilatation, stenting and post-dilatation) is performed under retrograde flow through the arterial sheath.
Proximal protection during CAS: clinical outcomes
The clinical data on the NPS derived from the two versions of the Parodi system, i.e., the first version PAES and the newest version NPS.
Parodi et al [3838. Parodi JC, Ferreira LM, Lamura R, Sicard G. Results of the first 200 cases of carotid stents using the Arteria device. J Endovasc Ther. 2005;12:1-50. ] 24 reported, in 2005, on the first 200 patients treated under neuroprotection with the PAES system. They achieved a technical success rate of 98.5%, a 30-day stroke and death rate of 1.5% and clamping intolerance of 3%. Some non-randomised studies confirmed the efficacy of the PAES in preventing embolic complications [3939. Adami CA, Scuro A, Spinamano L, Galvagni E, Antoniucci D, Farello GA, Maglione F, Manfrini S, Mangialardi N, Mansueto GC, Mascoli F, Nardelli E, Tealdi D. Use of the Parodi anti-embolism system in carotid stenting: Italian trial results. J Endovasc Ther. 2002;9:147-54. ].
The prospective, multicentre, single-arm EMPiRE Clinical Study investigated the use of the GORE® Flow Reversal System in 245 pivotal high surgical risk patients who underwent CAS. The stroke and death rate was 2.9% and no patient had a major stroke. The death and stroke rates in symptomatic, asymptomatic, and octogenarian subgroups were 2.6%, 3.0% and 2.6%, respectively [4040. Clair DG, Hopkins LN, Mehta M, Kasirajan K, Schermerhorn M, Schönholz C, Kwolek CJ, Eskandari MK, Powell RJ, Ansel GM; for the EMPiRE Clinical Study Investigators. Neuroprotection during carotid artery stenting using the GORE flow reversal system: 30-day outcomes in the EMPiRE Clinical Study. Catheter Cardiovasc Interv. 2010 Sep 17 [Epub ahead of print]. ].
The efficacy of the Mo.Ma device has been tested in some comparative studies and pivotal trials:
- In a comparative study [4141. Schmidt A, Diederich KW, Scheinert S, Bräunlich S, Olenburger T, Biamino G, Schuler G, Scheinert D. Effect of two different neuroprotection systems on microembolization during carotid artery stenting. J Am Coll Cardiol. 2004;44:1966-9. ] between the Mo.Ma system and a distal filter system (EPI FilterWire; Boston Scientific, Natick, MA, USA), by observing microrembolic signals (MES) with transcranial Doppler during CAS procedure, the efficacy of the Mo.Ma device in preventing microembolisation was evaluated according to five procedural steps: 1) positioning the protection system; 2) passage of the stenosis; 3) stent deployment; 4) post-dilatation; 5) retrieval of the protection device. The Mo.Ma system was associated with significantly lower counts during steps 2 to 4 when compared with the filter, whereas no significant differences were observed during the first and last phases.
- The PRIMUS multicentre registry [4242. Coppi G, Moratto R, Silingardi R, Rubino P, Sarropago G, Salemme L, Cremonesi A, Castriota F, Manetti R, Sacca S, Reimers B. PRIAMUS -proximal flow blockage cerebral protectIon during carotid stenting: results from a multicenter Italian registry. J Cardiovasc Surg (Torino). 2005;46:219-27. ] tested the Mo.Ma device in 416 patients who underwent CAS. Technical success was achieved in 99% of cases, mean clamping time was 4.9±1.1 minutes, transient clamping intolerance was observed in 5.8%, and macroscopic debris was retrieved in about 60% of patients. The cumulative incidence of adverse events at 30-day follow-up was 4.6%, with a 0.7% rate of major stroke and death.
- The multicentre ARMOUR trial evaluated the 30-day safety and effectiveness of the Mo.Ma proximal EPD in 262 subjects (37 roll-in and 225 pivotal subjects). Device success was 98.2% and procedural success was 93.2%. The 30-day major cardiac and cerebrovascular events rate was 2.7% with a 30-day major stroke rate of 0.9% [4343. Ansel GM, Hopkins LN, Jaff MR, Rubino P, Bacharach JM, Scheinert D, Myla S, Das T, Cremonesi A; Investigators for the ARMOUR Pivotal Trial. Safety and effectiveness of the INVATEC MO. MA proximal cerebral protection device during carotid artery stenting: results from the ARMOUR pivotal trial. Catheter Cardiovasc Interv. 2010;76:1-8. ].
- Experience of the use of the Mo.Ma device in octogenarians has recently been reported. From 2005 to 2009 a total of 198 octogenarian patients (39.4% were symptomatic) were submitted to CAS. Procedural success was achieved in all cases. The overall 30-day combined stroke/death rate was 2.52% (1.61% incidence in asymptomatic and 3.9% incidence in symptomatic) [4444. Micari A, Stabile E, Cremonesi A, Vadalà G, Castriota F, Pernice V, Sorropago G, Rubino P, Biamino G. Carotid artery stenting in octogenarians using a proximal endovascular occlusion cerebral protection device: a multicenter registry. Catheter Cardiovasc Interv. 2010;76:9-15. ].
The efficacy of the ENROUTE Transcarotid Neuroprotection System has been tested in a multicentre trial:
- The ROADSTER trial [4545. Kwolek CJ, Jaff MR, Leal JI, Hopkins LN, Shah RM, Hanover TM, Macdonald S, Cambria RP. Results of the ROADSTER multicenter trial of transcarotid stenting with dynamic flow reversal. J Vasc Surg. 2015 Nov;62:1227-1234. ] is a prospective, single-arm and multicentre (18 centres) study that tested the safety and efficacy for reverse flow with the Enroute system during carotid stenting in high-risk patients. The trial included symptomatic and asymptomatic patients with stenosis ≥ 50% and 70% respectively. Sixty-seven patients were enrolled in the lead-in phase (up to 5 patients for operator to gain experience) and 141 patients in the pivotal phase (for a total of 208 patients). In the later phase, 26% of patients were asymptomatic and 75% asymptomatic. The choice of carotid stent was left to the description of the operator. The authors reported an acute device and technical success rate of 99%. The all-stroke rate in the pivotal group was 1.4%, stroke and death was 2.8%, and stroke, death and myocardial infarction was 3.5%.
Limitations of proximal devices
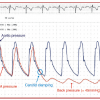
Basically, the drawbacks of proximal EPD compared to distal EPD include: their large size; ischaemic neurological intolerance of patient tolerance is represented by backpressure >30 mmHg (the pressure measured in the system after occlusion of both proximal and distal balloons) ( Figure 11 ). In the presence of clamping intolerance, three strategies can be adopted during the procedure: (1) rushing to check the absence of emboligenic materials to restore cerebral perfusion as soon as possible; (2) performing a step-by-step procedure in which the balloons are inflated, the blood is checked and then the balloon is deflated at each procedural step; and (3) using the “seat-belt and air-bag” technique, i.e., positioning a distal filter under proximal protection and then deflating the proximal EPD balloons.
Increased operator experience is a key factor and directly influences the overall clamping time as seen with the Mo.Ma device (from ten minutes in the study of Diederich et al [4646. Diederich KW, Scheinert D, Schmidt A, Scheinert S, Reimers B, Sievert H, Rabe K, Coppi G, Moratto R, Hoffmann FJ, Schuler GC, Biamino G. First clinical experiences with an endovascular clamping system for neuroprotection during carotid stenting. Eur J Vasc Endovasc Surg. 2004;28:629-33. ] to five minutes in the PRIAMUS registry [4242. Coppi G, Moratto R, Silingardi R, Rubino P, Sarropago G, Salemme L, Cremonesi A, Castriota F, Manetti R, Sacca S, Reimers B. PRIAMUS -proximal flow blockage cerebral protectIon during carotid stenting: results from a multicenter Italian registry. J Cardiovasc Surg (Torino). 2005;46:219-27. ].
ADVANTAGES AND LIMITATIONS OF DIFFERENT PROTECTION SYSTEMS
A direct comparison between proximal and distal EPD in large randomised trials is missing. The choice of the best EPD should be made according to the “tailored approach” concept ( Table 4 ). As a general guideline, proximal EPD should be strongly recommended in patients with angulated or diseased “distal arterial zone” and/or lack of a suitable ICA landing zone. They are also first chosen in angulated “stenting segment” ( as represented in Figure 4G-H ) and/or high embolic-risk or tight lesions. Patient intolerance is the most commonly reported complication related to these devices. In the Mo.Ma registry, a total of 7.1% patients coursed with intolerance. The technique of intermittent balloon deflation was used in 1.9% of patients, and the use of a distal filter was adopted in 0.6% of patients [4747. Reimers B, Sievert H, Schuler GC, Tübler T, Diederich K, Schmidt A, Rubino P, Mudra H, Dudek D, Coppi G, Schofer J, Cremonesi A, Haufe M, Resta M, Klauss V, Benassi A, Di Mario C, Favero L, Scheinert D, Salemme L, Biamino G. Proximal endovascular flow blockage for cerebral protection during carotid artery stenting: results from a prospective multicenter registry. J Endovasc Ther. 2005;12:156-65. ].
Filter devices are the first choice in patients with fibrotic/calcified lesions, contralateral carotid occlusion (not mandatory), ipsilateral isolated hemisphere, occlusion of the external carotid artery and at the beginning of the operator learning curve (due to its intuitive use) ( Figure 12 ). Spasm and slow-flow are the most frequent complications with filter devices, with a reported incidence of up to 3.6% and 7.2%, respectively [4848. Reimers B, Corvaja N, Moshiri S, Saccà S, Albiero R, Di Mario C, Pascotto P, Colombo A. Cerebral protection with filter devices during carotid artery stenting. Circulation. 2001;104:12-5. ]. A manoeuvre that minimises arterial spasm is the use of a gently handled soft-tipped filter wire. Slow-flow occurs when the filter is partially or completely filled by debris. Stop-flow can suggest that a large amount of debris has been captured by the filter, exceeding its capacity to store during the retrieval manoeuvre, incurring the risk of massive embolisation. Aspiration of the blood through dedicated catheters positioned near the filters could be recommended in this situation actively to retrieve the excessive debris volume.
Arterial dissection at the landing zone has been described in 0.5% to 0.9% of cases [4949. Reimers B, Schlüter M, Castriota F, Tübler T, Corvaja N, Cernetti C, Manetti R, Picciolo A, Liistro F, Di Mario C, Cremonesi A, Schofer J, Colombo A. Routine use of cerebral protection during carotid artery stenting: results of a multicenter registry of 753 patients. Am J Med. 2004;116:217-22. , 5050. Cremonesi A, Manetti R, Setacci F, Setacci C, Castriota F. Protected carotid stenting: clinical advantages and complications of embolic protection devices in 442 consecutive patients. Stroke. 2003;34:1936-41. ]. The device retrieval occasionally poses a problem, due to the advancement of the retrieval catheter through tortuous segments or even through the stents. Some useful manoeuvres are moving the patient’s neck (with the goal of changing arterial angulations and resistance points), the use of a buddy-wire or changing the attitude of the system by advancing the guiding catheter close to the stent (this manoeuvre is not possible when using long sheaths).
Cerebral protection during CAS
- Filter protection, advantages: very intuitive device, maintenance of cerebral perfusion
- Filter protection, disadvantages: pass lesion unprotected, need for a suitable landing zone in the ICA, unreliable vessel wall apposition, difficulty of negotiating tortuous anatomies and tight lesions
- Proximal protection, advantages: recommended in challenging anatomies (difficult to access ICAs due to very angulated ICA-CCA take-off, tortuous ICAs, lack of a suitable ICA landing zone)
- Proximal protection, disadvantages: 8-9 Fr introducer sheath compatibility (patient with peripheral diffuse disease), interruption of brain perfusion (intolerance to occlusion takes place in 3-8% of the cases)
- The more risky the plaques and the anatomies you are going to address, the more the use of proximal protection devices is indicated
Self-expanding carotid stents
In this chapter, attention is given to self-expanding carotid stents because the vast majority of carotid lesions are located at the bifurcation level. Balloon-expandable carotid stents were used in the very beginning of CAS mainly to treat complications of carotid angioplasty. Its application in the cervical zone is not justified due to the risk of stent compression by external mechanical forces. The reported compression incidence of the Palmaz stent ranged from 1% to 15% in some series [5151. Wholey MH, Wholey MH, Jarmolowski CR, Eles G, Levy D, Buecthel J. Endovascular stents for carotid artery occlusive disease. J Endovasc Surg. 1997;4:326-38. , 5252. Mathur A, Dorros G, Iyer SS, Vitek JJ, Yadav SS, Roubin GS. Palmaz stent compression in patients following carotid artery stenting. Cathet Cardiovasc Diagn. 1997;41:137-40. , 5353. Johnson SP, Fujitani RM, Leyendecker JR, Joseph FB. Stent deformation and intimal hyperplasia complicating treatment of a post-carotid endarterectomy intimal flap with a Palmaz stent. J Vasc Surg. 1997;25:764-8. ]. The actual indication of balloon-expandable stents is limited to CCA ostial or very proximal (intrathoracic) lesions, because in these regions the risk of external compression is minimised.
FROM FUNCTIONAL CHARACTERISTICS TO STENT SELECTION
From a historical standpoint, the first self-expanding stent dedicated to carotid application was the carotid Wallstent. This braided mesh stent frame is constructed from a single piece of cobalt alloy wire that is woven into a tubular structure. The stent is compressible, allowing it to be constrained within a sheath. A spring-like action allows this structure to expand as the sheath is withdrawn during deployment. Experience with this device has been widely reported, and the braided mesh frame is still today an excellent stent option when performing CAS.
The advantages of this stent include a small and flexible delivery system, a small free cell area with high scaffolding and wall-coverage properties (plaque-covering) and adaptability to the changing diameter across the bifurcation. However, the advantage of its flexibility (when constricted in the delivery system) is offset by the disadvantage of its rigidity after release, which tends to straighten the vessel and, as a consequence, to increase distal tortuosity. In addition, this device also has an unpredictable foreshortening at deployment.
The second important group of self-expanding carotid stents have a nitinol (nickel-titanium alloy) structure. Most stents are made from a nitinol tube that is laser-cut to create a meshed frame comprising sequential aligned annular rings, interconnected in a helical fashion. The thermal expansion properties of nitinol characterise these devices. These properties allow its expansion to the predetermined shape and size when exposed to body temperature. The nitinol group can be divided into four main design categories: open-cell, closed-cell, hybrid design and double-layer stents (detailed below). The nitinol stents are manufactured to assume a cylindrical or tapered shape.
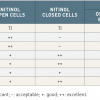
An advantage of the nitinol stents is their minimal foreshortening during deployment, and higher outward radial force when compared with the braided mesh stents. Within the nitinol group, the open-cell stents have a larger free-cell area, higher conformability, flexibility and vessel-wall adaptability when compared to the closed-cell designs. The disadvantages of the open-cell design stents include moderate scaffolding and stent-strut malalignment in complex carotid lesions. The closed-cell nitinol stents have the advantage of higher scaffolding as well as wall-coverage properties and higher outward radial force, when compared to open-cell nitinol stents. The disadvantages of closed-cell stents are device stiffness, both during advancement into the delivery system and after release, and the poor conformability and flexibility. Comparisons of functional characteristics among stent designs are shown in Table 5 .
STENT SELECTION
The choice of stent design in straightforward cases (aortic arch type 1, simple supra-aortic anatomies, straight “stenting segment”, stable fibrous plaques) may not matter. Conversely, challenging anatomical scenarios should lead the operators towards the adoption of a tailored approach ( Table 4 ). Following this concept, the cobalt-alloy braided-mesh and the double-layer nitinol stents are the first choice when reliable plaque coverage and long-acting plaque prolapse prevention (as required by long, soft and not homogeneous lesions) is needed, mainly because of their scaffolding properties. A clear benefit in favour of stent scaffolding and wall coverage for reducing postprocedural neurological complications for symptomatic patients (high risk plaques) was demonstrated by Bosier et al [5454. Bosiers M, de Donato G, Deloose K, Verbist J, Peeters P, Castriota F, Cremonesi A, Setacci C. Does free cell area influence the outcome in carotid artery stenting?. Eur J Vasc Endovasc Surg. 2007;33:135-41; discussion 142-3. ].
Nitinol open-cell stents are the first choice when the carotid bifurcation and plaque complexity (represented by an angled “stenting segment”) require high stent in-vessel flexibility and wall/plaque conformability properties, due to the need for maintenance of the original anatomy of a very tortuous vessel.
When stent outward radial force is required, as in resistant or calcified carotid lesions, nitinol closed-cell stents represent an excellent solution.
INNOVATION IN STENT DESIGN
The Cristallo Ideale™ (Invatec) is a nitinol self-expanding stent that incorporates a hybrid stent architecture solution, characterised by open-cell design in the distal and proximal portions and closed-cell design in the middle segment. With this structure, the stent flexibility and adaptability are warranted by the open cells (in the distal and proximal portions) and the stent high scaffolding and wall-coverage properties are achieved by the closed cells (middle segment). This stent is loaded in a 5 Fr delivery system.
The Cristallo Registry [5555. Cremonesi A, Rubino P, Grattoni C, Scheinert D, Castriota F, Biamino G. Multicenter Experience With a New “Hybrid” Carotid Stent. J Endovasc Ther. 2008;15:186-192. ], an international multicentre prospective registry, has recently evaluated the safety and performance of the hybrid stent in the treatment of ICA stenosis. The primary endpoint was the rate of 30-day major adverse neurological events, defined as major and minor stroke and neurological deaths. Among 124 patients, no major neurological events were found, and two episodes of TIAs (1.6%), both occurring in the post-procedural period, were documented. Secondary endpoints were technical success, which was defined as the ability to deploy the stent at the target lesion with the residual diameter stenosis < 30%, and procedural success, which was defined as technical success free of major procedural neurological events. Technical and procedural success was achieved in all the enrolled patients (100%). Three patients (2.5%) died (non-neurological death) during the study period (two pulmonary-related and one cardiac-related): all these deaths were neither device-related nor procedure-related complications.
The ViVEXX™ (C.R. Bard Inc., Murray Hill, NJ, USA) family of open-cell nitinol design, loaded in a 5 Fr delivery system, offers a broad of range of sizes including a 12 mm diameter option for dealing with very large CCAs.
Another stent that achieved the 5 Fr delivery system (for stents with diameters from 5 to 8 mm) was the “new” Precise stent (Precise® PRO RX®; Cordis Corp., Warren, NJ, USA), an open-cell nitinol stent design which has the smallest free-cell area among the open-cell nitinol stents.
The double-layer nitinol stent category, characterised by double-layer mesh, has been developed recently. One layer is composed of a self-expanding nitinol structure (providing flexible scaffolding), and the second layer, an internal/external mesh, designed to attain high plaque coverage. The mesh layer components vary depending on the two manufacturer: the Roadsaver® Carotid Stent (Terumo) has the micromesh layer in nitinol alloy (both layers in nitinol), and the InspireMD CGuardTM Carotid Embolic Prevention System (InspireMD) has the micromesh composed of Polyethylene Terephthalate. The advantage afforded by the later device is that it acts as a stent and as an embolic preventive system. The CGuard CARENET Trial (Carotid Embolic Protection Using MicroNet) [5656. Schofer J, Musiałek P, Bijuklic K, Kolvenbach R, Trystula M, Siudak Z, Sievert H. A Prospective, Multicenter Study of a Novel Mesh-Covered Carotid Stent: The CGuard CARENET Trial (Carotid Embolic Protection Using MicroNet). JACC Cardiovasc Interv. 2015 Aug 17;8:1229-34. ] included 30 patients in 4 centres in Germany and Poland and evaluated the feasibility of the CGuard system. Protection devices were used in all procedures. Procedural success was 100%, with 0% complications. The 30-day major adverse cerebrovascular or cardiac events rate was 0%. The experience with Roadsaver® Stent is also in its beginning [5757. Hopf-Jensen S, Marques L, Preiß M, Müller-Hülsbeck S. Initial clinical experience with the micromesh roadsaver carotid artery stent for the treatment of patients with symptomatic carotid artery disease. J Endovasc Ther. 2015 Apr;22:220-5. , 5858. Kedev S, Petkoska D, Zafirovska B, Vasilev I, Bertrand OF. Safety of Slender 5Fr Transradial Approach for Carotid Artery Stenting With a Novel Nitinol Double-Layer Micromesh Stent. Am J Cardiol. 2015 Sep 15;116:977-81. ]. It remains to be proven in larger trials whether this attractive new stent category will result in a greater reduction of adverse procedural events than other stent designs.
Stent selection
- Stent scaffolding and conformability play a significant role not only for achieving good procedural results, but also for improving 30-day outcomes
- Stents may exert intrinsic anti-embolic properties
- For open-cell design, the greater the cell size, the higher the potential for plaque penetration and intra-strut prolapse
- The more risky the plaque and the bifurcation anatomy you are going to address, the more the use of high scaffolding and high conformable frames is indicated
Step-by-step technique of CAS
CLINICAL PROTOCOL
Pre-medication
- Dual antiplatelet therapy with aspirin and clopidogrel (or ticlopidine), ideally initiated five days before the procedure and continued for at least 30 days, after which clopidogrel is usually discontinued.
Clinical evaluation
Evaluation of patient atherosclerotic risk factors and comorbidities: age, gender, race, hypertension, dyslipidaemia, diabetes mellitus, current smoking (including previous 5 years), ischaemic heart disease, congestive heart failure, renal insufficiency and severe pulmonary disease.
- Independent neurological evaluation, preferably by a neurologist with expertise in stroke, or by a physician certified in the National Institutes of Health Stroke Scales (NIHSS), is performed to:
- Differentiate symptomatic and asymptomatic patients as those presenting or not, respectively, a history of transient ischaemic attacks (TIA), stroke, or ischaemic ocular symptoms in the last 6 months [5959. Nedeltchev K, Pattynama PM, Biaminoo G, Diehm N, Jaff MR, Hopkins LN, Ramee S, van Sambeek M, Talen A, Vermassen F, Cremonesi A. Standardized definitions and clinical endpoints in carotid artery and supra-aortic trunk revascularization trials. Catheter Cardiovasc Interv. 2010;76:333-44. ].
- Identify urgent clinical situations as “crescendo TIAs” (multiple recurrent TIAs over hours to days) and “stroke in progress”.
- Identify the (1) time from the index event to the treatment, and (2) time from the most recent event to the treatment (if the most recent event is not identical to the index event).
- In patients with a history of stroke, further evaluation should be based on:
- Differentiation in stroke severity as minor (3 or less points on the NIHSS) or major stroke (4 or more points on the NIHSS).
- Evaluation of functional outcomes at 6 months of the stroke as non-disabling, disabling (functional disability of 2 or less points on the modified Rankin scale) or fatal stroke (not the case here).
Pre-procedure image investigation
- Carotid duplex scan.
- Aortic arch + carotid arteries + cerebral arteries angiography/computed tomography angiography (CTA)/magnetic resonance angiography (MRA).
- Brain computed tomography (CT) scan/magnetic resonance imaging (MRI) scan to detect “silent” brain infarctions.
Pre-procedure evaluation of procedural risks
- Evaluation of the “five arterial zones” characteristics to anticipate procedural technical difficulties and risks to plan a tailored approach strategy as commented above.
General procedural measures
- Head restraint and no sedation, with neuro-evaluation during the procedure by simple communication and movement parameters.
- Standard monitoring of vital parameters.
- Heparin IV 70 U/kg (ACT 250-300 seconds).
CAS TECHNIQUE
Vascular access
The femoral artery is the standard access for carotid procedures, but in the presence of extreme vessel tortuosity, arterial disease or occlusion of the femoral/iliac arteries or even of the abdominal aorta, the radial/brachial approach constitutes an alternative access ( Figure 13 ). In this last situation, compatibility of materials should be carefully verified when planning the procedure, and the long sheath technique is highly preferred to working with smaller sheath diameters when compared to the guiding catheter technique. As a general rule, the CCA is approached from the contralateral arm.
Baseline angiographic evaluation
In the absence of a previous aortic arch evaluation (angiography or CTA/MRA scan), aortic arch angiography is performed through a pigtail catheter positioned in the distal part of the ascending aorta, at a 30º to 45º left anterior oblique angulation to visualise best the origin of the supra-aortic vessels. Using the same projection, selective cannulation of the target carotid artery is performed. The right anterior oblique view (≈ 30º) is helpful to visualise the brachiocephalic trunk bifurcation, which facilitates the right CCA catheterisation. The carotid artery (CCA, carotid bifurcation, ECA and ICA) is then evaluated in at least two orthogonal projections to best “open” the ICA and ECA origins and to demonstrate the maximum severity of the lesion. The cerebral vessels angiogram is an essential part of the evaluation as it can reveal unsuspected arteriovenous malformations, cerebral aneurysms and arterial stenosis, and it serves as a comparative parameter with the post intervention cerebral angiogram to identify possible sources of embolisation. The four-vessel cerebral angiography to assess the Circle of Willis patency (through the anterior and posterior communication arteries) is only performed when the use of proximal EPD is planned in patients with contralateral carotid occlusion or when ipsilateral isolated hemisphere is suspected.
Having the “five arterial zones” in mind, a quick “mental” screening of all angiographic aspects and their pitfalls should be carefully analysed to begin the therapeutic procedure securely.
Common carotid engagement
One of the most important technical aspects of the CAS procedure is to obtain a safe and stable engagement of the CCA. Two standard techniques are the guiding catheter and the guiding sheath technique.
Guiding catheter technique: according to the aortic arch configuration, a specific 90-100 cm (8 Fr) guiding catheter is chosen for best access to the supra-aortic vessels.
- A 40º angle soft-tip guiding catheter is used for simple anatomy. The catheter is gently advanced until the mid CCA, over one or multiple soft-angled standard hydrophilic wires positioned just below the carotid bifurcation.
- When dealing with a complex anatomy, such as an aortic arch type 3 or a bovine aortic arch configuration and the proximal left CCA engaged is required, angulated catheter curves such as the hockey-stick guiding catheter should be chosen.
In tortuous vessel anatomies or an adverse aortic arch, a useful technique is the utilisation of multiple 0.035” wires positioned in the target artery to increase the support of the rail along which the catheter is advanced ( Figure 13C ). Another useful manoeuvre is the utilisation of a 0.014” guidewire in the ECA to increase the stability of the entire system during the intervention ( Figure 13E ).
The great advantage of the guiding catheter technique is steerability, not only to engage the CCA, but also to deal with difficulties during the procedure (such as the above-mentioned difficulty in advancing the retrieval catheter through the stent for filter retrieval). Attention should be given to the risk of distal embolisation resulting from catheter manipulation in the aortic arch. This risk can be reduced by meticulous technique, executed with gentle, small, slow movements of the catheter (small amplitude of clock and counter-clockwise catheter rotation), which should always be advanced using the 0.035” wire.
A drawback of this technique is the larger sheaths required (generally two French sizes bigger than the guiding sheath technique) with the attendant risk of local vascular complications.
Guiding sheath placement is a frequently used alternative to guiding catheters.
There are some different techniques to place the guiding sheath securely into the CCA. Described below is the standard technique used by our centre which, in our opinion, has some advantages over other techniques, basically due to the monitored engagement of the ECA with the use of a 5 Fr guiding catheter.
A 100 cm 5 Fr FR4 guiding catheter is advanced over a soft-angled 0.035” standard hydrophilic wire into the CCA. The guiding catheter allows contrast injection even with a 0.035” inside, allowing “check and go”, compared to the “blind technique” when using standard diagnostic catheter and 0.035” wire.
An angiogram is performed to delineate the carotid bifurcation and then the hydrophilic wire is gently advanced into the ECA to be parked finally in a distal branch (avoid the lingual artery due to the risk of life-threatening airway obstruction due to massive lingual haematoma in case of vessel rupture). Maintaining the hydrophilic wire in a fixed position, the guiding catheter is advanced deeply into the ECA until it assumes a stable position. If additional support is needed, a 0.018” wire may also be used to increase the rail support, together with the 0.035” wire. At this point, the standard 0.035” wire is exchanged for a long (260-300 cm) 0.035” support wire.
The guiding catheter is then withdrawn and a long sheath is advanced over the 0.035” support wire into the mid CCA.
In our practice, the insertion of the Mo.Ma device (which incorporates the functional aspect of a proximal EPD into a shuttle sheath) strictly follows all the steps described above, differing only in the advancing of its distal tip into the ECA (through the stiff 0.035” wire), where the distal balloon is checked to prove ECA occlusion.
The advantage of the long sheath technique is the safety when a stable position in the CCA is achieved, and in the smaller required sheath diameter (6 Fr). The disadvantage is its reduced steerability, which can be partially solved with the use of the telescope technique, by using a diagnostic catheter (with the desired curve) through the guiding sheath (over a 0.035” guidewire) first to engage the artery and then, working as a rail, to allow the guiding sheath advancement. Another disadvantage is that, in some tortuous CCA, sheath kinks can occur, and even arterial loops can be accentuated and/or cephalically displaced. In addition, when the ECA is occluded, this technique can become much more risky, requiring an alternative technique which consists of manually creating a “pig tail” format on the soft tip of the 0.035” stiff wire, aiming to leave the “pig tail” tip proximally to the carotid bifurcation and the wire’s stiff part in the mid CCA to provide enough support for sheath advancing.
EPD management
The use of EPD has been discussed in the relevant section. Choosing the appropriate type and correctly handling EPD is one of the key points to successfulCAS.
Predilatation
Predilatation is not an obligatory step when performing CAS, and it is reserved for very tight stenoses, heavily calcified lesions or stenoses with a high tendency to recoil such as fibrotic lesions with high plaque burden ( Figure 14 ). Usually a low profile coronary balloon with a diameter ranging from 2.5 to 3.5 mm and length from 20 to 30 mm is inflated at nominal pressure to minimise the risk of embolisation. Heavily calcified lesions should undergo predilatation with a coronary cutting balloon (with a diameter of 3.5 - 4.0 mm) inflated at moderate pressure (8 atm) ( Figure 12D ). Use of the cutting balloon has been reported during the predilatation phase of the CAS procedure in 178 patients with severe highly calcified de novo lesions with a procedural success rate of 99.4% [6060. Castriota F, de Campos Martins EC, Setacci C, Manetti R, Khamis H, Spagnolo B,Furgieri A, Gieowarsingh S, Parizi ST, Bianchi P, Setacci F, de Donato G,Cremonesi A. Cutting balloon angioplasty in percutaneous carotid interventions. J Endovasc Ther. 2008;15:655-62. ]. The combined all stroke and death rate at 30-day follow-up was 0.6%, with one major stroke.
Pretreatment with 0.5 to 1 mg of intravenous atropine is sometimes required at this stage to prevent activation of vagal efferents and subsequent bradycardia and/or asystole (normally used in the post-dilatation phase). Large doses of atropine are avoided in the elderly, as this can result in confusion and make accurate neurological assessment difficult.
Stent delivery, deployment and post-dilatation
Stent delivery and deployment
When delivering the carotid stent, it is essential to consider the flexibility and trackability of the diverse delivery systems (braided mesh > open-cell > hybrid > closed-cell stent delivery systems). When advancing the stent through the aortic arch, it is advisable to check the behaviour of the guiding catheter/sheath (in a 30º-45º LAO view), which tends to prolapse into the aortic arch with serious consequences.
Normally the self-expanding stent should have an unconstrained diameter 1-2 mm larger than the reference vessel diameter in order to obtain a stable position and reliable wall apposition. An important point to consider is the foreshortening of the stent length, especially when using the braided mesh stents. As stents are delivered from the distal to the proximal part, it is reasonable to release the stent at least 5 mm distally and wait for it to expand and stabilise against the wall before releasing the remainder of the device. In this way, all foreshortening will be concentrated in the proximal portion of the stent, which will assume a higher position when compared to the proximal marker on the delivery system. This means that the operator can control the distal level of the stent implantation and should calculate the level of the stent proximal portion implantation according to (1) stent foreshortening characteristics (braided mesh stent > open-cell stent > hybrid stent > closed-cell stent), and (2) stent and vessel diameter relation (especially with braided mesh stents): as a general rule, the bigger the stent diameter compared to the vessel, the less the foreshortening.
Post-dilatation
The stent post-dilatation is the procedure phase which carries the highest risk of distal embolisation and arterial rupture. It is in this phase that the stent will passively stress (by balloon inflation) the carotid plaque against the arterial wall. To minimise the embolic or arterial rupture risk, the following considerations have been adopted by experienced groups:
- Balloon no larger than 5.5 mm in diameter should be used.
- Inflating the angioplasty balloon to nominal pressure.
- Accepting a residual stenosis <30% (according to the North American Symptomatic Carotid Endarterectomy Trial [NASCET] criteria).
- Persistent contrast via the struts into an ulcer does not require further dilatation as it will spontaneously seal with time.
If the ECA is occluded during the procedure, its recanalisation should be attempted only if the patient becomes symptomatic (jaw or facial pain). In such a case it may be sufficient to restore only TIMI II flow.
Final angiographic evaluation
After the EPD removal, the same baseline angiographic projections are used to acquire the final angiograms. When a distal protection device is used, the landing zone has to be checked carefully to exclude arterial wall damage, spasm or dissection. Ipsilateral intracranial angiography should be routinely performed and compared with the basal angiogram to verify the maintenance of the patency of all vessels. In this way, contrast hold-up in a vessel during prolonged angiographic acquisition is a sign of acute arterial occlusion.
Vascular access management
Standard management of the arterial site should be applied. Early ambulation can be achieved by the use of vascular haemostasis devices after angiographic checking of adequate arterial puncture level (at the common femoral artery) and the absence of diseased arterial segment.
Carotid stenting complications
All major adverse events and vascular complications that occur within 30 days of the procedure are attributed to the CAS procedure. Complications should be divided into intraprocedural complications (occurring after the beginning of the procedure to the point in time when the patient leaves the procedure room) and post-procedural complications, which are split between 24 hours (from the time the patient leaves the procedure room to 24 hours post procedure) and 30 days (from 24 hours to 30 days post procedure) [5959. Nedeltchev K, Pattynama PM, Biaminoo G, Diehm N, Jaff MR, Hopkins LN, Ramee S, van Sambeek M, Talen A, Vermassen F, Cremonesi A. Standardized definitions and clinical endpoints in carotid artery and supra-aortic trunk revascularization trials. Catheter Cardiovasc Interv. 2010;76:333-44. ].
CAROTID SINUS REACTION
Carotid sinus reaction, represented by transient bradycardia or asystole, is a relatively common response during balloon dilatation at the carotid bifurcation (mainly in the postdilatation phase due to the larger balloons used) and pretreatment with atropine is preventative. This response is less commonly observed during the dilatation of restenotic lesions after CEA, where the receptors may have been denervated by surgical dissection. In such cases, atropine is not normally necessary. Hypotension is not uncommon and can be caused by either balloon dilatation and/or the persistent stretching of the self-expanding stent, both acting by stimulating the carotid baroreceptors. Hypotension is managed by adequate intravascular volume expansion.
Treating heavily calcified lesions may course with more pronounced hypotension, which may require small doses of intravenous vasopressors such as metaraminol (0.5 mg). The use of a cutting balloon for pre-treating this lesion subset is associated with less pressure required during the post-dilatation phase, which theoretically generates fewer stimuli to the carotid baroreceptor and consequently lower intensity of hypotensive response [6060. Castriota F, de Campos Martins EC, Setacci C, Manetti R, Khamis H, Spagnolo B,Furgieri A, Gieowarsingh S, Parizi ST, Bianchi P, Setacci F, de Donato G,Cremonesi A. Cutting balloon angioplasty in percutaneous carotid interventions. J Endovasc Ther. 2008;15:655-62. ].
Continued monitoring during the 24-hour post-procedural period is mandatory. Sustained hypotension may require dopamine infusion. It is crucial to look at the differential diagnostic list to exclude, for example, large femoral haematoma, retroperitoneal haemorrhage or myocardial infarction. Some associated conditions such as contralateral carotid disease (severe stenosis or occlusion), intracranial stenosis, vertebrobasilar disease and coronary obstructive disease, do not tolerate hypotension and require a prompt recovery to re-establish a normal haemodynamic status.
ACUTE VESSEL OCCLUSION
A sudden severe spasm, dissection, or thrombus formation in the treated vessel can progress to acute vessel occlusion if not treated, and requires special treatment or it results in stroke.
- Carotid artery spasm at the distal embolic protection device landing zone generally resolves spontaneously within several minutes after device removal. In case of flow-limiting arterial spasm (when the diagnostic of arterial dissection should be excluded), the intra-arterial administration of 100 to 400 μg of nitroglycerine, once blood pressure allows, aids in resolution of the spasm. Low-pressure balloon angioplasty (≤2 atm) is used in cases of persistent arterial spasms.
- Carotid dissection is a rare complication predisposed by many factors, such as severe arterial tortuosity, poor filter position control, use of a distal balloon occlusion device, post-dilatation of distal edge within the ICA, high-pressure balloon inflation, oversized post-dilatation balloon, and aggressive guiding catheter manipulation. It is defined as an intimal disruption of the vessel wall with or without medial or adventitial contrast staining [6161. NASCET Steering Committee. North American symptomatic carotid endarterectomy trial. Methods, patient characteristics, and progress. Stroke. 1991;22:711-20. ]. Additional stent implantation, balloon angioplasty, or a conservative strategy depending on severity and flow, are all management options.
- Acute stent thrombosis: with the adequate use of dual antiplatelet therapy (mentioned earlier), stent thrombosis has become a remarkably rare event. Possible causes are antiplatelet resistance, hypercoagulable state, stent incomplete expansion, unrecognised vessel dissection or severe plaque prolapse. Prompt diagnosis (duplex scan or arteriography) and reperfusion should be instituted to limit the cerebral ischaemic area. Medical (systemic or intra-arterial IIB/IIIA inhibitors or thrombolytic therapy) [6262. Iancu A, Grosz C, Lazar A. Acute carotid stent thrombosis: review of the literature and long-term follow-up. Cardiovasc Revasc Med. 2010;11:110-3. ], endovascular (use of reverse flow system and/or in-stent balloon angioplasty/stenting) [6363. Okazaki T, Satomi J, Satoh K, Hirasawa M, Nagahiro S. Rescue revascularization therapy with a stent-in-stent technique for acute intracranial internal carotid artery occlusion. Neurol Med Chir (Tokyo). 2005;45:253-8. ] and surgical treatment have been reported [6464. Setacci C, de Donato G, Setacci F, Chisci E, Cappelli A, Pieraccini M, Castriota F, Cremonesi A. Surgical management of acute carotid thrombosis after carotid stenting: a report of three cases. A. J Vasc Surg. 2005;42:993-6. ].
DISTAL EMBOLISATION
The routine use of EPDs has reduced distal embolisation to a rare event (0.4 to 1.5%). It is defined as obstruction of the ICA or one of its branches by a foreign substance, plaque, thrombus or air [5959. Nedeltchev K, Pattynama PM, Biaminoo G, Diehm N, Jaff MR, Hopkins LN, Ramee S, van Sambeek M, Talen A, Vermassen F, Cremonesi A. Standardized definitions and clinical endpoints in carotid artery and supra-aortic trunk revascularization trials. Catheter Cardiovasc Interv. 2010;76:333-44. ]. To recognise this complication in good time, the patient’s neurological status should be checked after every step of the procedure. If neurological signs or symptoms appear and persist, the general patient care should be instituted immediately with emphasis on maintaining a viable airway, oxygen administration and recovery of normal blood pressure and intravascular volume status. The assistance of an anaesthesiologist is always required. Whenever possible, the carotid procedure should be quickly concluded and attention directed to the intracranial angiography. The most likely sites of a distal embolus are the distal ICA and the middle cerebral artery, including its branches. Detection of large vessel occlusion is easy, but embolism in the smaller branches may be difficult and will require long acquisition angiograms (to detect any arterial or venous contrast retention) and careful comparison with the basal angiogram, looking for missing vessels. A prompt arterial recanalisation (balloon angioplasty, embolus removal by dedicated retrieval devices, thrombolytic agents or IIb/IIIa inhibitors) should be attempted in larger vessel occlusion, but for an asymptomatic small branch occlusion less invasive interventions are needed, due to the risks of incurring a new cerebral complication (especially with less experienced operators), ensuring only adequate hydration, stable blood pressure and anticoagulation therapy.
INTRACRANIAL HAEMORRHAGE
Cerebral haemorrhage is a rare but life-threatening procedural complication. Severe headache preceding sudden loss of consciousness in the absence of vessel occlusion should alert the operator. A new localised expanding high density area is a sign of cerebral haemorrhage. Once suspected, anticoagulation should be immediately reversed, the neurosurgeon team should be contacted and an emergency CT brain scan performed. Excessive anticoagulation, poorly controlled hypertension, aggressive attempts at intracranial neurovascular rescue, presence of a vulnerable aneurysm or presence of a recent ischaemic stroke (less than 3 weeks) are all conditions associated with cerebral haemorrhage during or after CAS.
HYPERPERFUSION SYNDROME
The hyperperfusion syndrome is a rare complication (below 1%) presented typically with migraine-like headache, nausea, vomiting, confusion, agitation, focal neurological deficits, and epileptic seizures typically occurring in patients with tight carotid stenosis and poor collateral circulation, such as occlusion of the contralateral ICA or an underdeveloped Circle of Willis. Brain imaging may show cerebral oedema or haemorrhage. This complication is related to a long-standing hypoperfusion cerebral status that results in impaired autoregulation of the cerebral microcirculation. Following carotid revascularisation, the increased perfusion pressure overwhelms the ability of the dilated arterioles to constrict. In this sense, bilateral carotid stenting during the same procedure sitting may also contribute, consequently should be discouraged in the general practice.
CAS hyperperfusion syndrome usually develops during the immediate post-procedural period, contrasting with the surgical hyperperfusion syndrome where symptoms develop within a few days. This is probably related to dual antiplatelet agents, heparin administration and the previous use of glycoprotein IIB/IIIA antagonists which are no longer recommended.
CONTRAST-INDUCED ENCEPHALOPATHY
Contrast encephalopathy is a transient neurological syndrome caused by the use of large amounts (≥ 300 ml, strictly dependent on renal function) of the contrast medium, normally linked to a prolonged procedure. The CT scan reveals a contrast enhancement of the basal ganglia and the cortex and no other abnormalities. Because the contrast medium does not cross the blood-brain barrier, this phenomenon may be caused by a fine particulate embolisation or/and excessive local contrast. No abnormalities on intracranial angiography are detected. Symptoms resolve completely within 24 hours in the majority of cases.
CAROTID ARTERY PERFORATION OR RUPTURE
This is an extremely rare event (below 0.3 %) predisposed by aggressive dilatation or oversizing the post-dilatation balloon. It is evidenced by either localised (confined to the tissue immediately surrounding the artery) or non-localised extravasation of the contrast medium. Covered stent or prolonged balloon inflation may be considered in this situation. A useful and often forgotten manoeuvre is the manual compression of the carotid artery at the rupture level, allowing precious time for the operator to act and avoiding local haematoma progression. Maintaining viable airways is crucial, due to the risk of external compression by the haematoma.
IN-STENT RESTENOSIS
Restenosis is one of the possible long-term complications of carotid stenting and is a remarkably rare event (<5%) when compared to coronary artery stenting. This condition is defined as diameter stenosis >50% according to NASCET criteria of the adjacent arterial segments. Mechanisms such as neointimal hyperplasia and stent underexpansion may be involved ( Figure 14 ). In our centre, cutting balloon angioplasty, using a peripheral cutting balloon ranging from 5 mm to 6 mm in diameter inflated at nominal pressure, is the treatment of choice for approaching in-stent restenosis.
Carotid stenting complications
- Carotid stenting is anything but a simple and complication-free procedure
- Interventionalists should be adequately prepared for recognising and managing procedure-related complications
Personal perspective - Alberto Cremonesi
Carotid artery stenting, which has emerged as an alternative therapy to high-risk surgical patients, has become an increasingly important procedure in the optimal management of patients with carotid disease. Highly trained operators are crucial in a field where complication is not well tolerated, and investment in trainee programmes is warranted.
All the experts involved in carotid stenting agree on the importance of “specialty driven curricula” for improving the cognitive background and manual skill of operators directly performing such complex procedures as “primary players”: carotid stenting is anything but a simple procedure.
The role of scientific societies is crucial from this perspective: their official objectives should not only be to standardise and optimise the diagnostic and therapeutic processes, but also to facilitate the management of clinical risk by introducing the “endovascular team concept” (multidisciplinary approach) in order to reduce self-referral and improvisation.
The perception that CAS risks should be anticipated is increasing. The adoption of a systematic pre-procedural evaluation of technical risks by analysing the “five arterial zones” classification serves as an intuitive map to plan a procedure strategy.
The tailored approach was born from operators’ experience and in-depth knowledge of available devices and has been emphasised as the key to achieving a safe practice. Emerging technologies and further innovations pursuing stroke prevention are welcome and should undergo long-term evaluation. All technology improvement, trainee programmes and new efforts should be constantly submitted to critical questioning about whether we are doing our best in terms of patient care.