Summary
The field of percutaneous valvular interventions is one of the most exciting and rapidly developing within interventional cardiology. Percutaneous pulmonary valve implantation (PPVI) represents the first in human application of these techniques and is a non-surgical option for treating right ventricular outflow tract dysfunction. With the growing numbers of patients with right ventricle to pulmonary artery conduit dysfunction late after repair of congenital heart disease, the importance of a technique with lower morbidity and mortality, good patient acceptance, and efficacy that is comparable with surgery should not be underestimated. Although techniques of percutaneous pulmonary valve implantation were described just a decade ago, thus far two, complementary, stent-mounted devices have been introduced successfully into clincial practice and more than 8000 of these procedures have been performed worldwide. This fact underlines that PPVI has become a feasible, safe, benefitial and cost-effective treatment for both conduit stenosis and regurgitation. Consistently, follow-up data reveal good freedom from both re-operation and re-catheterisation and demonstrate that PPVI can postpone open-heart surgery, thereby potentially reducing the number of operations patients have to undergo within their lifetime. Complications are seen after PPVI but can be avoided in most cases if reasonable precautions are taken into account and correct patient selection criteria are applied. Stent fractures were known to be the most common complication that can require re-intervention in some cases but are increasingly rare when pre-stenting procedures are performed. Moreover, valve competency remains good with significant regurgitation during follow-up only seen in the context of endocarditis. Finally, new devices are under development, which will allow for PPVI in dilated, distensible outflow tracts, to offer this non-surgical treatment option to a larger patient population with congenital heart disease.
Introduction
Since techniques of percutaneous pulmonary valve implantation (PPVI) were first described by Philipp Bonhoeffer and colleagues in 2000[11. Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356(9239):1403-1405. ], over 8000 percutaneous pulmonary valve implants have been performed worldwide[22. Petit CJ. Pediatric transcatheter valve replacement: guests at our own table?. Circulation. 2015;131(22):1943-1945. ]. With 4500 implants in the United States since the original Investigational Device Exemption (IDE) study began in 2007, the procedure has now reached worldwide clinical acceptance and routine interventional procedure status[11. Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356(9239):1403-1405. ]. Several devices have been investigated for purposes of PPVI, but so far only the MELODYTM device (Medtronic, MN, USA) has obtained regulatory approval, making this new percutaneous strategy available to a broader population. The first successful implantation of another device in pulmonary position, the Edwards SAPIEN valve, was reported in 2006 by Garay et al[33. Garay F, Webb J, Hijazi ZM. Percutaneous replacement of pulmonary valve using the Edwards-Cribier percutaneous heart valve: first report in a human patient. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2006;67(5):659-662. ].
PPVI is performed to prolong the lifespan of right ventricle (RV) to pulmonary artery (PA) conduits thereby postponing open-heart surgery in children and adults with congenital heart disease. Over the last 10 years, a marked learning curve in outcome post-PPVI has been demonstrated, with improved safety, efficacy and freedom from redo-surgery or re-intervention for paediatric or adult patients who underwent this procedure [44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. , 55. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960-1970. , 66. Lurz P, Giardini A, Taylor AM, et al. Effect of altering pathologic right ventricular loading conditions by percutaneous pulmonary valve implantation on exercise capacity. The American journal of cardiology. 2010;105(5):721-726. , 77. Lurz P, Bonhoeffer P, Taylor AM. Percutaneous pulmonary valve implantation: an update. Expert review of cardiovascular therapy. 2009;7(7):823-833. , 88. Eicken A, Ewert P, Hager A, et al. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. European heart journal. 2011;32(10):1260-1265. , 99. Boudjemline Y, Brugada G, Van-Aerschot I, et al. Outcomes and safety of transcatheter pulmonary valve replacement in patients with large patched right ventricular outflow tracts. Archives of cardiovascular diseases. 2012;105(8-9):404-413. , 1010. Demkow M, Biernacka EK, Spiewak M, et al. Percutaneous pulmonary valve implantation preceded by routine prestenting with a bare metal stent. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2011;77(3):381-389. , 1111. Gillespie MJ, McElhinney DB. Transcatheter Pulmonary Valve Replacement: A Current Review. Current Pediatrics Reports. 2013;1(2):83-91. , 1212. Butera G, Milanesi O, Spadoni I, et al. Melody transcatheter pulmonary valve implantation. Results from the registry of the Italian Society of Pediatric Cardiology. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2013;81(2):310-316. ].
In the following chapter, the authors review indications, technical aspects and patient selection criteria and discuss early and late clincal results of PPVI using the MelodyTM device (Medtronic, MN, USA) and the SAPIENTM Pulmonic Transcatheter Heart Valve (Edwards Lifesciences LLC, Irvine, CA, USA) as non-surgical treatment options for right ventricular outflow tract (RVOT) dysfunction and finally consider future directions.
The clinical role of PPVI
Over the last 50 years advances in cardiac surgery, intensive care, non-invasive imaging and interventional procedures have led to a substantial increase in life expectancy for many patients with congenital heart disease. Therefore, an ‘interdisciplinary challenging’, heterogeneous population of patients treated by corrective, semi-corrective or palliative surgical procedures, sometimes decades ago, is growing inexorably. Recent studies have shown that the number of adult patients with congenital heart disease is already similar to that of the paediatric population and will continue to grow[1313. Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115(2):163-172. ].
For approximately 20 percent of these patients RVOT dysfunction caused by either significant obstruction, pulmonary regurgitation, or both in combined conditions, becomes clinically evident.
Surgical pulmonary valve replacement using valved conduits (biological valve, xenografts, homografts etc.) has been used to treat RVOT dysfunction and is therefore the most frequent mode of redo-operation in patients with congenital heart disease[1414. Warnes CA. The adult with congenital heart disease: born to be bad?. Journal of the American College of Cardiology. 2005;46(1):1-8. ]. Surgical pulmonary valve replacement is a very safe procedure and can be performed with low morbidity and mortality and acceptable long-term ourcome[1414. Warnes CA. The adult with congenital heart disease: born to be bad?. Journal of the American College of Cardiology. 2005;46(1):1-8. , 1515. O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of Transcatheter and Operative Pulmonary Valve Replacement (from the Pediatric Health Information Systems Database). The American journal of cardiology. 2016;117(1):121-126. ]. However, an important drawback of this treatment is the limited lifespan of such conduits from the RV to the PA. In the literature, this lifespan has been reported to be around 10 years[1616. Tweddell JS, Pelech AN, Frommelt PC, et al. Factors affecting longevity of homograft valves used in right ventricular outflow tract reconstruction for congenital heart disease. Circulation. 2000;102(19 Suppl 3):III130-5. , 1717. Powell AJ, Lock JE, Keane JF, Perry SB. Prolongation of RV-PA conduit life span by percutaneous stent implantation. Intermediate-term results. Circulation. 1995;92(11):3282-3288. , 1818. Oosterhof T, Vliegen HW, Meijboom FJ, Zwinderman AH, Bouma B, Mulder BJ. Long-term effect of pulmonary valve replacement on QRS duration in patients with corrected tetralogy of Fallot. Heart. 2007;93(4):506-509. , 1919. Corno AF. Valved Conduits Right Ventricle to Pulmonary Artery for Complex Congenital Heart Defects. 2012. , 2020. Shinkawa T, Lu CK, Chipman C, Tang X, Gossett JM, Imamura M. The Midterm Outcomes of Bioprosthetic Pulmonary Valve Replacement in Children. Seminars in thoracic and cardiovascular surgery. 2015;27(3):310-318. ]. As a consequence, the majority of patients have to undergo several open-heart procedures during their life. Patient management strategies have been based on delaying surgical intervention for as long as possible, so that the number of open-heart surgeries performed on any individual patient is kept to a minimum. However, this approach bears the risk of delaying surgery beyond a theoretical ‘point of no return’. At this point, RV dysfunction, leading to impaired exercise capacity and an increased risk for sudden death, and which result from chronic adverse RV loading conditions[2121. Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356(9234):975-981. , 2222. Frigiola A, Redington AN, Cullen S, Vogel M. Pulmonary regurgitation is an important determinant of right ventricular contractile dysfunction in patients with surgically repaired tetralogy of Fallot. Circulation. 2004;110(11 Suppl 1):II153-7. , 2323. Carvalho JS, Shinebourne EA, Busst C, Rigby ML, Redington AN. Exercise capacity after complete repair of tetralogy of Fallot: deleterious effects of residual pulmonary regurgitation. British heart journal. 1992;67(6):470-473. , 2424. Meadows J, Powell AJ, Geva T, Dorfman A, Gauvreau K, Rhodes J. Cardiac magnetic resonance imaging correlates of exercise capacity in patients with surgically repaired tetralogy of Fallot. The American journal of cardiology. 2007;100(9):1446-1450. ] might become irreversible. Decision making on the ideal timing of pulmonary valve replacement is still challenging in most cases and represents one of the most controversial issues for cardiologists who take care of children and adults with congenital heart disease[1414. Warnes CA. The adult with congenital heart disease: born to be bad?. Journal of the American College of Cardiology. 2005;46(1):1-8. , 2525. Lurz P, Gaudin R, Taylor AM, Bonhoeffer P. Percutaneous pulmonary valve implantation. Seminars in thoracic and cardiovascular surgery. Pediatric cardiac surgery annual. 2009:112-117. , 2626. Oosterhof T, van Straten A, Vliegen HW, et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116(5):545-551. ].
Attempts have been made to establish RV volume thresholds on magnetic resonance (MR) imaging as predictors for outcome after conduit placement. Cut-off points for RV end-systolic (ESV) and end-diastolic volumes (EDV) in relation to RV ejection fraction have been reported, with some suggesting values for EDV (150-170 mL/m2)[2525. Lurz P, Gaudin R, Taylor AM, Bonhoeffer P. Percutaneous pulmonary valve implantation. Seminars in thoracic and cardiovascular surgery. Pediatric cardiac surgery annual. 2009:112-117. , 2626. Oosterhof T, van Straten A, Vliegen HW, et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116(5):545-551. , 2727. Buechel ER, Dave HH, Kellenberger CJ, et al. Remodelling of the right ventricle after early pulmonary valve replacement in children with repaired tetralogy of Fallot: assessment by cardiovascular magnetic resonance. European heart journal. 2005;26(24):2721-2727. , 2828. Lurz P, Bonhoeffer P, Taylor AM. Percutaneous pulmonary valve implantation: an update. Expert review of cardiovascular therapy. 2009;7(7):823-833. , 2929. Alvarez-Fuente M, Garrido-Lestache E, Fernandez-Pineda L, et al. Timing of Pulmonary Valve Replacement: How Much Can the Right Ventricle Dilate Before it Looses Its Remodeling Potential?. Pediatric cardiology. 2015. ] above which normalisation of RV dimensions is less likely following pulmonary valve replacement. Nevertheless, the impact of the timing of pulmonary valve replacement on RV function, exercise performance and patient long-term survival remains undefined[2828. Lurz P, Bonhoeffer P, Taylor AM. Percutaneous pulmonary valve implantation: an update. Expert review of cardiovascular therapy. 2009;7(7):823-833. ].
To postpone and potentially reduce the number of surgical procedures that patients have to undergo throughout their life, transcatheter stent implantation into stenotic conduits has been carried out[3030. O’Laughlin MP, Slack MC, Grifka RG, Perry SB, Lock JE, Mullins CE. Implantation and intermediate-term follow-up of stents in congenital heart disease. Circulation. 1993;88(2):605-614. , 3131. Aggarwal S, Garekar S, Forbes TJ, Turner DR. Is stent placement effective for palliation of right ventricle to pulmonary artery conduit stenosis?. Journal of the American College of Cardiology. 2007;49(4):480-484. , 3232. Peng LF, McElhinney DB, Nugent AW, et al. Endovascular stenting of obstructed right ventricle-to-pulmonary artery conduits: a 15-year experience. Circulation. 2006;113(22):2598-2605. , 3333. Sugiyama H, Williams W, Benson LN. Implantation of endovascular stents for the obstructive right ventricular outflow tract. Heart. 2005;91(8):1058-1063. ]; though such ‘bare metal stenting’ can potentially convert a pressure overloaded scenario secondary to obstruction into a volume overloaded one, secondary to significant pulmonary incompetence. With the evolution of PPVI, an effective and feasible non-surgical technique has become available that allows treatment of both conduit obstruction and regurgitation. This technique offers a minimally invasive method that has the potential to avoid open-heart surgery for RVOT dysfunction in children and adults by restoring acceptable RV loading conditions.
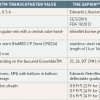
The equipment (Table 1)
MELODY™ TRANSCATHETER PULMONARY VALVE
Device and delivery system
The Edwards SAPIENTM valve (Edwards Lifesciences LLC, Irvine, CA) is radiopaque and consists of three bovine pericardial leaflets hand-sewn into a stainless steel stent (14 or 16mm in length) ( Figure 3.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1947" data-folder="pcr-textbook" data-chapterid="125"> Figure 3). The fabric polyethylene terephtalate sealing cuff covers the lower portion of the stent to facilitate a seal to the implating site to prevent paravalvular leakage. The valve’s tissue is manufacured from three equal sections of bovine pericardium that have been preserved in glutaraldehyde to crosslink the xenograft tissue and preserve its flexibility and strength. The device was initially designed for aortic valve replacement and obtained CE certification for PPVI in 2010. Currently, the successor of the SAPIEN valve, the SAPIEN XT, is commercially available in 23, 26-mm and 29-mm diameter sizes and is crimped onto the designated balloon delivery system (Retroflex) ( Figure 3.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1947" data-folder="pcr-textbook" data-chapterid="125"> Figure 3).
Since 2013, the FDA approved RetroflexTM III represents the latest generation of delivery system prosthesis delivery (Edwards Lifesciences LLC, Irvine, CA.) The system requires a 22-Fr sheath (for the 23-mm valve) or 24-Fr hydrophilic sheath (for the 26-mm and 29-mm valve). According to recent manufacturer’s data, improvements in design (e.g., Edwards eSheath) will offer smaller sheath sizes for the 23-mm valve (18 Fr), the 26-mm valve (19 Fr), and the 29-mm model (16 Fr). The guiding catheter has a control handle on the catheter hub, which can be rotated to deflect the catheter into the tricuspid aperture and through the RVOT. The RetroflexTM III system also contains a retractable nose cone catheter, which facilitates atraumatic delivery. A specialised tool is used to manually crimp the valve over the valvuloplasty balloon[3434. Kenny D, Hijazi ZM, Kar S, et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. Journal of the American College of Cardiology. 2011;58(21):2248-2256. , 3535. Haas NA, Moysich A, Neudorf U, et al. Percutaneous implantation of the Edwards SAPIEN pulmonic valve: initial results in the first 22 patients. Clinical research in cardiology :. official journal of the German Cardiac Society. 2013;102(2):119-128. ].
Technical details regarding the SAPIENTM vale are summarised in Table 1.
SAPIEN™ Pulmonic Transcatheter Heart Valve
Device and delivery system
The Edwards Sapien™ valve (Edwards Lifesciences LLC, Irvine, CA) consists of three bovine pericardial leaflets hand-sewn to a stainless steel stent. There is a fabric sealing cuff covering the lower portion of the stent to facilitate a seal with the calcified conduit and prevent paravalvular leak. Currently, the valve is available in 23 and 26 mm diameter sizes with heights of 14.5 and 16 mm, respectively.
The Retroflex™ II represents the latest generation of catheters for prosthesis delivery (Edwards Lifesciences LLC, Irvine, CA.) The catheter consists of a balloon catheter and a deflectable guiding catheter, and requires either 22 or 24 Fr hydrophilic sheaths for the 23 and 26 mm valves, respectively. The guiding catheter has a control knob on the catheter hub, which can be rotated to deflect the catheter into the tricuspid aperture and through the RV into the RVOT. The Retroflex™ II system also contains a retractable nose cone catheter, which facilitates atraumatic delivery of the system through the ventricle and the calcified/stenotic conduit and or stent.
Patient selection for the MelodyTM and SAPIENTM
CLINICAL CRITERIA
As discussed previously, there are no clear-cut guidelines for when to treat RVOT dysfunction, whether predominantly caused by obstruction or regurgitation. This issue is slightly less complex in the setting of RVOT obstruction, where patients tend to develop symptoms early when significant obstruction is present. According to 2010 European Society of Cardiology (ESC) / Association for European Pediatric Cardiology (AEPC) guidelines for the management of grown-up congenital heart disease patients with RVOT obstruction should be treated if the RV to PA gradient exceeds 60 mmHg or in presence of symtoms due to RVOT obstruction regardless of RVOT gradient[3636. Baumgartner H, Bonhoeffer P, Groot NM de, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). European heart journal. 2010;31(23):2915-2957. ].
The clinical indications for PPVI outlined in the following represent our approach to timing of intervention. This approach is adaptated from indications for RVOT bare metal stenting[3232. Peng LF, McElhinney DB, Nugent AW, et al. Endovascular stenting of obstructed right ventricle-to-pulmonary artery conduits: a 15-year experience. Circulation. 2006;113(22):2598-2605. ].
In cases of RV pressure overload due to RVOT obstruction, patients undergo PPVI if RV pressure exceeds 65 % of systemic pressure in the presence of symptoms. In the absence of symptoms, patients are treated if RV pressure exceeds 75 % of systemic pressure[44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. ].
Indications for RVOT interventions in the context of pulmonary regurgitation are subject to ongoing discussions, especially if patients are asymtomatic. It is common sense to base the indication criteria for transcatheter or surgical treatment on a combined assessment of MRI-derived RV EDV, RV ESV and RV systolic function, cardiopulmonary exercise testing (as an objective measure of patient’s symptoms), and the presence of atrial or ventricular dysrhythmia[2929. Alvarez-Fuente M, Garrido-Lestache E, Fernandez-Pineda L, et al. Timing of Pulmonary Valve Replacement: How Much Can the Right Ventricle Dilate Before it Looses Its Remodeling Potential?. Pediatric cardiology. 2015. ]. According to the 2010 recommendations of the ESC grown up congential heart disease task force, PPVI may be indicated, if patients have severe pulmonary regurgitation (as assessed on echocardiography or MR imaging) and one of the following: (a) severe RV dilatation; (b) severe RV dysfunction; (c) symptoms; (d) impaired exercise capacity[3636. Baumgartner H, Bonhoeffer P, Groot NM de, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). European heart journal. 2010;31(23):2915-2957. ].
In 2011, the American Heart Association (AHA) stated: “It is reasonable to consider the percutaneous pulmonary valve replacement in patient with RV-to-pulmonary artery conduits with moderate to severe pulmonary regurgitation or stenosis provided the patient meets inclusion/exclusion criteria for the available valve”. The AHA writing committee recommended this procedure based on Class IIa evidence (evidence level B)[3737. Feltes TF, Bacha E, Beekman, R. H., 3rd, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011;123(22):2607-2652. ].
There is no absolute lower age limit but an adequate body size (eg, weight > 20 kg) is required to accommodate femoral placement of the introducer[88. Eicken A, Ewert P, Hager A, et al. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. European heart journal. 2011;32(10):1260-1265. ]. As there is no difference in clinical indications Focus Box 1 summarizes clinical indications for MELODYTM and SAPIENTM Transcatheter pulmonary valve implantation.
Clinical and morphological requirements for PPVI using the MelodyTM device
- Clinical indications in the context of RV pressure overload/pulmonary stenosis:
−RV systolic pressure > 65% of systemic pressure in symptomatic patients
−RV systolic pressure > 75% of systemic pressure in asymptomatic patients
- Clinical indications in the context of RV volume overload/pulmonary regurgitation:
−Severe pulmonary regurgiation on echocardiography or MR imaging and
−Severe RV dilatation > 150 ml/m2 or the RV to LV end-diastolic ratio of > 1.7 and/or
−Rapid progressiv RV dilatation and/or
−Severe RV dysfunction and/or
−Symtoms and/or
−Sustained atrial or venticular arrhythmia and/or
−Impaired exercise capacity (<65% compared to norm peak oxygen consumption)
PRE-PROCEDURAL ASSESSMENT PROTOCOL
To establish clinical indication criteria, all patients undergo a standardised assessment protocol. For screening, echocardiography is performed to determine the RVOT gradient and to semi-quantitatively assess the severity of pulmonary regurgitation. Echocardiography is also used to estimate RV pressure (tricuspid valve regurgitant jet) and the RV to systemic pressure ratio (non-invasive blood pressure measurements) but may not being able to equal MRI in the assessment of the RV even when incorporating deformation analysis[3838. Sabate Rotes A, Bonnichsen CR, Reece CL, et al. Long-term follow-up in repaired tetralogy of fallot: can deformation imaging help identify optimal timing of pulmonary valve replacement? Journal of the American Society of Echocardiography :. official publication of the American Society of Echocardiography. 2014;27(12):1305-1310. ]. Therefore, as a crucial part of our assessment, patients undergo cardiac MR imaging unless contra-indicated. We define RV dilatation in the context of pulmonary regurgitation as severe when the indexed RV end-diastolic volume is > 150 ml/m2 or the RV to LV end-diastolic ratio of > 1.5. Indexed RV end-systolic volume and MRI-derived estimation of right ventricular ejection may be included in decission making as published recently by Alvarez-Fuente[2929. Alvarez-Fuente M, Garrido-Lestache E, Fernandez-Pineda L, et al. Timing of Pulmonary Valve Replacement: How Much Can the Right Ventricle Dilate Before it Looses Its Remodeling Potential?. Pediatric cardiology. 2015. ]. It is of note that ventricular volume derived on MR imaging can differ by more than 15 % depending on whether RV trabeculations are included in the volume or whether end-diastolic and end-systolic volumes are defined by the endocardial outline in each of the short-axis cine images, excluding RV trabeculations. In our pratice, RV trabeculations are excluded when volumes are calculated. MR imaging also allows accurate quantification of pulmonary regurgitation using pulmonary artery flow measurements, providing a calculated pulmonary regurgitation fraction. Objective exercise capacity is assessed by cardiopulmonary exercise testing on a bicycle using a ramp protocol. A peak oxygen uptake of less than 65 % of predicted is considered as a significant impairment in exercise capacity. Finally, surface electrocardiograms and Holter ECG monitoring are performed to detect dysrhythmia and define QRS complex duration.
MORPHOLOGICAL CRITERIA
Size and shape of the implantation site (called ‘landing zone’) and its anatomical relation to coronary arteries are decisive morphological criteria which have to be appropriate when considering patients as potential candidates for PPVI.
Size and shape of the ‘landing zone’
The current MELODYTM device is not intended to be dilated to a diameter of more than 22 mm. Patients with (non-dilated) conduits between the RV and pulmonary artery of 22 mm and less offer an ideal environment to perform PPVI. In contrast, native or patched outflow tracts after surgical repair e.g. for Tetralogy of Fallot tend to dilate and to be too large (> 22 mm) and therefore do not provide a secure landing zone for MELODYTM valves. The operative report needs to be studied in detail in order to have a full understanding of the anatomy of the outflow tract. In addition, unless contraindicated, patients undergo MRI prior to the procedure to avoid procedural failure. By the help of MRI cine imaging, maximal and minimal dimensions of the RVOT throughout the cardiac cycle can be determined. Three-dimensional reconstructions of the outflow tract further improve the understanding of the anatomy ( Figure 4.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1948" data-folder="pcr-textbook" data-chapterid="125"> Figure 4). In addition to MR imaging or if results of magnetic resonance imaging are doubtful, balloon sizing of the RVOT is recommended at the time of catheterisation, which will be discussed later on. Apart from a maximum diameter for PPVI, the implantation site should also not be narrowed to ensure optimal opening of the implant at the end of the procedure without unacceptable residual gradients. We recommend to implant valves into conduits or outflow tracts which measure not less than 16 mm in diameter. Rare exceptions to this rule included cases where there was strong evidence from three-dimensional imaging and echo assessment that sufficient space was available to deploy a valve to a reasonable diameter.
Assessment of RVOT shape and dimensions prior to PPVI
- 3-dimensional shape of the RVOT and pulmonary bifurcation
→ MR whole heart imaging and/or MR angiography
- Minimal and maximal diameter diameter of the implantation site
→ Measurements on 2 orthogonal MR cine images through the RVOT
- In case of borderline RVOT dimensions on MR
→ Use of sizing balloons during catheterisation
- Assessment of RVOT distensibility
→ Use of sizing balloons during catheterisation
ILLUSTRATIVE CASE 1
Assessment of coronary artery anatomy
Coronary artery anatomy varies within the broad spectrum of complex congenital heart defects or following surgical re-insertion into the aorta as part of arterial switch operations or similar procedures. In some cases there is relevant proximity of one or more of the relevant coronary artery branches to the main pulmonary artery. This exposes patients considered for PPVI to the risk for coronary artery obstruction due to expansion of the RVOT[3232. Peng LF, McElhinney DB, Nugent AW, et al. Endovascular stenting of obstructed right ventricle-to-pulmonary artery conduits: a 15-year experience. Circulation. 2006;113(22):2598-2605. , 3939. Sridharan S, Coats L, Khambadkone S, Taylor AM, Bonhoeffer P. Images in cardiovascular medicine. Transcatheter right ventricular outflow tract intervention: the risk to the coronary circulation. Circulation. 2006;113(25):e934-5. , 4040. Maheshwari S, Bruckheimer E, Nehgme RA, Fahey JT, Kholwadwala D, Hellenbrand WE. Single coronary artery complicating stent implantation for homograft stenosis in tetralogy of Fallot. Catheterization and cardiovascular diagnosis. 1997;42(4):405-407. ]. It is essential to assess the course of proximal coronary arteries in relation to the RVOT prior to PPVI.
Some centres prefer MRI 3-D whole heart images to judge the anatomical relationship of the coronary arteries and the proposed implantation side ( Figure 5.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1949" data-folder="pcr-textbook" data-chapterid="125"> Figure 5). We recommend to perform selective coronary angiography and particularly aortic root angiography and simultaneous high-pressure balloon inflation within the landing zone (as discussed later) at the time of catheterisation in all patients to rule out the risk of coronary compression ( Figure 6.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1950" data-folder="pcr-textbook" data-chapterid="125"> Figure 6 and Figure 7.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1951" data-folder="pcr-textbook" data-chapterid="125"> Figure 7).
Assessment of risk of coronary compression during and after PPVI
- In all patients
−Coronary artery anatomy and proximity to the RVOT judged on MR whole heart imaging and/or MR angiography
−Aortic root angiography in AP and lateral projection
- In case of any doubts
−Simultaneous high-pressure balloon inflation within the RVOT and selective coronary angiography
ILLUSTRATIVE CASE 2
Patient selection for Sapien™
PATIENT SELECTION
Clinical indications for PPVI using the SAPIENTM valve are in keeping with the ones applied for MelodyTM implants. Indications included right ventricular pressure overload (> 75% of systemic) due to conduit obstruction, significant pulmonary regurgitation, and / or increased right ventricle end diastolic volume (>150 ml/m² as assessed on MR imaging). Applied morphological requirements SAPIENTM valve are: outflow tract of at least 18 mm but not larger than 26 (29) mm with significant discrete narrowing of the conduit[3434. Kenny D, Hijazi ZM, Kar S, et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. Journal of the American College of Cardiology. 2011;58(21):2248-2256. , 4141. Boone RH, Webb JG, Horlick E, et al. Transcatheter pulmonary valve implantation using the Edwards SAPIEN transcatheter heart valve. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2010;75(2):286-294. , 4242. Garay F, Webb J, Hijazi ZM. Percutaneous replacement of pulmonary valve using the Edwards-Cribier percutaneous heart valve: first report in a human patient. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2006;67(5):659-662. ]. In our experience the RVOT shape (after prior pre-stenting) is of much importance when implanting SAPIENTM valves. Due to its ‘engineered’ nature of sutured pericardial tissue, optimal valved stent function in SAPIENTM procedures is guaranteed by a circular RVOT shape. In PPVI procedures with the MELODYTM valve the RVOT shape itself has much less impact on the haemodynamic outcome[4343. Wagner R, Daehnert I, Lurz P. Percutaneous pulmonary and tricuspid valve implantations: An update. World journal of cardiology. 2015;7(4):167-177. ].
The procedure using Melody™
THE CATHETERISATION
Setup and preparations ( Table 2)
PPVI should be performed in a catheterisation laboratory equiped with a mono- or biplane fluoroscopy unit. Although simultaneous surgical backup is not (necessarily) required, PPVI should be performed at institutions with a surgical programme. Ideally, extracorporeal circulation equipment and experience with this technique is available. Autotransfusion kits (such as pleural drainage kits, cellsaver) should be available in cases of acute bleeding. Usually, PPVI is performed using general anaesthesia with endotracheal intubation although the use of conscious sedation is also feasible. Peripheral and central venous and femoral or arterial access for the purposes of continuing haemodynamic monitoring and anaesthetic management are obtained. For PPVI femoral access is preferred, as it allows for an easier working position in the catheterisation laboratory. Preparation of both groins for vascular access allows for quick access change in case of problems. Jugular vein access can also be performed if required[4444. Zampi JD, Berman DP, Bocks ML, et al. Factors associated with the internal jugular venous approach for Melody™ Transcatheter Pulmonary Valve implantation. Cardiology in the young. 2015:1-9. ]. The use of a maximum of 10 Fr sheath for initial venous access is appropriate. A full aseptic technique to surgical standards, initiation of an effective heparinisation at the beginning of the procedure (50 to 100 IU / kg heparin or a standard dose of 5000 IU in adults, repeated as required) and peri-procedural broad-spectrum intravenous antibiotics for endocarditis prophylaxis are recommended. A list of products typically required for Melody PPVI is given in Table 2 (modified to [4545. Association for European Paediatric and Congenital Cardiology. The Melody® TPV Implantation Step-by-Step: A Proctor’s Guide. 2013. ]).
Haemodynamic assessment
Right heart catheterisation is performed according to standard techniques to assess pressures and saturations. Routinely, measurements in the RV, PA and aorta with additional measurements, for example in the branch PAs, made as appropriate. To provide stable position prior to advancing the delivery systems a stiff guidewire (i.e. Amplatz Ultra Stiff guide wire 0.035 in x 260 cm or Lunderquist 0.035 in x 260 cm, Cook Inc., Bloomington, IN) is then positioned into a distal branch PA. Operator concentration is needed to maintain a distal guidewire position with its tip at the level of diaphragm with as few curves as possible and not to interfere with tricuspid valve chordae.
Pulmonary artery (biplane) angiography is performed using appropriate catheters (e.g. balloon tipped, Pigtail or Multi-TrackTM catheter, NuMed Inc., Hopkinton, NY). The catheters tip should be placed just beyond the expected position of the pulmonary valve to allow assessment of the proposed landing zone and estimation of pulmonary regurgitation.
Assessment of morphological suitability
Size of the implantation site
As mentioned before, morphological suitability regarding dimensions and shape of the landing zone is an essential part of pre-procedural planning process. In patients with borderline dimensions regarding the RVOT enlargement derived by MRI imaging, additional invasive assessment during catheterisation might be necessary. Further, distensibiliy of the implantation site can only be assessed at the time of catheterisation.
Distensibility of the site can only be assessed by balloon interrogation and is therefore strongly recommended in cases of expected high distensibility of the RVOT (patch-extended or native outflow tracts) ( Figure 5.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1949" data-folder="pcr-textbook" data-chapterid="125"> Figure 5). We recommend to use soft sizing balloon catheters (e,g PTS® NuMed, Hopkinton, NY). This balloon catheter should be partially inflated when positioned distally to the implantation site and is then slowly retracted across the RVOT back into the RV during simultaneusly use of biplane, orthogonal fluoroscopy. This manoeuvre allows for accurate measures of the landing zone and informs on RVOT distensibilty. The anterior-posterior imaging plane with cranial tilt seems to be ideal to visualise the pulmonary bifurcation and distal end of valved stent. The anterior-posterior cranial +/- left anterior oblique angulation documents the relation to bifurcation. The lateral plane visualises the anterior chest and landing zone for the transcatheter pulmonary valve and seems to be the ideal during stent positioning.
Assessment of coronary artery anatomy
As discussed previously, the risk of coronary compression should be minimised as much as possible before implanting MELODYTM or SAPIENTM valves. To reduce the risk of coronary compression to a minimum, a stepwise approach is recommended. The proximity of the proximal coronary arteries to the RVOT should be assessed on magnetic resonance three-dimensional whole heart images. Furthermore, aortic root angiography should be performed in all patients at the time of catheterisation. On bi-plane projection, the relationship between the coronaries and the pulmonary artery can be assessed. If these two investigations cannot fully rule out the risk for coronary compression, simultaneous high-pressure balloon inflation in the implantation site and selective coronary angiography is performed ( Figure 6.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1950" data-folder="pcr-textbook" data-chapterid="125"> Figure 6) [44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. , 3232. Peng LF, McElhinney DB, Nugent AW, et al. Endovascular stenting of obstructed right ventricle-to-pulmonary artery conduits: a 15-year experience. Circulation. 2006;113(22):2598-2605. , 3939. Sridharan S, Coats L, Khambadkone S, Taylor AM, Bonhoeffer P. Images in cardiovascular medicine. Transcatheter right ventricular outflow tract intervention: the risk to the coronary circulation. Circulation. 2006;113(25):e934-5. , 4040. Maheshwari S, Bruckheimer E, Nehgme RA, Fahey JT, Kholwadwala D, Hellenbrand WE. Single coronary artery complicating stent implantation for homograft stenosis in tetralogy of Fallot. Catheterization and cardiovascular diagnosis. 1997;42(4):405-407. , 4646. Witsenburg M., Gewellig M., Turner M., Daehnert I. The Melody TPV implantation step-by-step: a proctor’s guide. Available at: http://www.aepc.org/@Bin/141218/Melody+Implantation+Step+by+Step_+A+Proctors+Guide_+Rev+1_+May+10_2013. pdf. Accessed January 29, 2016. ] recommend combined aortic root angiogram and balloon dilation to outline coronary anatomy and exclude risk of coronary ostium or coronary compression. In cases of larger distance distance between the landing zone and the coronary arteries low pressure to the balloon is used. If coronary arteries are close, full inflation is indicated to avoid false negative screening to assure that valve insertion is adequately mimicked by the balloon position. It is of importance that an aortic root angiogram may be preferable to selective coronary artery angiogram because intubation of the coronary artery by the catheter may lead to a false negative test. In case of any evidence or doubting regarding the risk of coronary compression the procedure has to be abandoned ( Figure 7.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1951" data-folder="pcr-textbook" data-chapterid="125"> Figure 7) ( ILLUSTRATIVE CASE 2).
Device set-up
Preparations prior to implantation include checking and removing of manufcatures identification labels, hand-flushing, hand-crimping and loading of the valved stent onto the delivery system. Prior to cimping the device should be hand-flushed in at least two bowls filled with sterile, isotonic sodium chloride solution for approximately 30 seconds each to remove residues of the glutaraldehyde fixation and to prove leaflet function. The valved stent should then be hand-crimped over a barrel of a sterile 2ml syringe before being front-loaded onto the delivery system. The blue stitching on the distal portion of the device is matched to the blue portion of the delivery system (“the carrot”) and verified by an independent observer to guarantee correct orientation, as decribed previously. Further hand crimping is needed while advancing the sheath is over the stent device during saline flushing using the side port to exclude air bubbles from the system and facilitating uncovering of the device ( Figure 2).
THE IMPLANT
The AEPC’s MELODYTM proctor guideline[4646. Witsenburg M., Gewellig M., Turner M., Daehnert I. The Melody TPV implantation step-by-step: a proctor’s guide. Available at: http://www.aepc.org/@Bin/141218/Melody+Implantation+Step+by+Step_+A+Proctors+Guide_+Rev+1_+May+10_2013. pdf. Accessed January 29, 2016. ]
After removing of the used aniography catheter (e.g. Multi-Track catheter), the femoral vein is dilated to 22 or 24 French respectively. This will not be necessary if a 18 Fr sheath is used for peri-procedural interventions (as discussed later) as the outer diameter is about 22 Fr. The front-loaded delivery system is introduced into the access site and advanced into the RVOT and landing zone under fluoroscopic guidance. The tip of the guide wire must be seen at all times. If its position has been lost, the advancing of the delivery systems needs to be stopped. Otherwise, the risk of peripheral vascular rupture is high when trying to push the wire back in position whilst carrying the delivery system. The delivery system can be safely removed and repositioned as long as the valve is at least partially covered. The operator has to re-establish stable wire position with the use of catheters before advancing with the delivery system. Coordination between the first and second operator is necessary to safely advance the system into the desired position. Pushing the system at the groin results in moving the system forward or backward (turning is impossible). Driving the wire will determine how the delivery systems end (“the carrot”) moves through its way up to the landing zone. If “the carrot “ make turns well, the wire is kept fixed. If “the carrot” hesitates at entry into the RVOT the operator may push on the delivery system while fixing the wire resulting in backward movement of the tip. Another pushing manoeuvre on the delivery system accompanied by a little pull on the wire may make “the carrot” come loose from the conduits wall and allow advancement.
In rare cases, in which the delivery system cannot be negotiated across the RVOT, cautious partial uncovering of the device can be performed. This manoeuvre provides more flexibility of the very tip of the delivery system and thereby facilitates crossing of very tortuous and stenotic outflow tracts. However, this should only be performed by the experienced operator, since it bares the risk of flaring the distal part of the valved stent with consequent dislodgement of the device. An additional manoeuvre that can be used to advance the delivery system, when it is at the entrance to the conduit, is looping the system within the right atrium. This generates a forward force that often overcomes resistances the system is experiencing and aids passage into the conduit. If the delivery system has been placed too distally, forward pushing of the guidewire rather than backward pulling of the delivery system often helps to withdraw the delivery system slightly.
Once the most distal part of the delivery system has passed the landing zone the wire should be first straightened in the right atrium (the loop keeps tricuspid valve opened) prior proceeding the implantation process. The outer sheath is retracted uncovering the valved stent which sometimes results in forward movement. Contrast media is injected via the side-port to confirm position. It is of importance to note that there are no radiopaque markers on the sheath. This means that complete uncovering of the delivery system can only be confirmed by the marker placed on the proximal portion of the delivery system.
Partial deployment is achieved by hand inflation of the inner balloon. After final confirmation of the position the outer balloon is also hand inflated to complete deployment. The balloons are deflated and the delivery system is then withdrawn. Repeat angiography and pressure measurements are made to confirm a positive outcome ( Figure 8.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1952" data-folder="pcr-textbook" data-chapterid="125"> Figure 8).
ADDITIONAL PERI-PROCEDURAL INTERVENTIONS
Pre-dilatation of the landing zone
The nature and position of RV to PA conduits is heterogenous and cannulation with a large delivery system can be challenging. High pressure balloon systems are used to pre-dilate conduits that are heavily calcified or tortuous in order to facilitate passage of the system (e.g. Mullins high-pressure balloon, NuMed Inc, Hopkinton, NY and or Atlas high-pressure balloon, C.R.Bard Inc.). Further, some operators might argue that pre-dilation of stenotic conduits optimises the haemodynamic result. In our view, the most significant benefit of pre-dilatation is the fact that it provides an ideal assessment of the anatomy. The location of the waist of the balloon, if present, represents the most rigid part of the implantation site and informs on the optimal landing zone for the valved stent. However, above-mentioned advantages of pre-dilatation have to be counterbalanced with the risk of homograft rupture. Notibly, severe bleeding due to homograft rupture is rare with an incidence of ~ 2.5%, partial rupture of the RV to PA conduit during valve implantation is a common finding[4747. Boudjemline Y, Malekzadeh-Milani S, Patel M, et al. Predictors and outcomes of right ventricular outflow tract conduit rupture during percutaneous pulmonary valve implantation: a multicentre study. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. EuroIntervention. 2016;11(9):1053-1062. ]. Whereas this complication has no clinical consequence in the majority of cases due to the covered nature of the stent, this could led to severe bleeding if caused by pre-dilatation. Unfortunately, it is still not understood which patients and RVOTs are at risk for this complication. We believe that the decision for pre-dilatation has to be made individually in each patient, taking the possible advantages and disadvantages into account.
Pre-stenting of the implantation site
Prior to PPVI, pre-stenting with bare metal stents is a feasible option. Observational studies reported that pre-stenting of the conduit before PPVI is associated with a lower risk of development of MelodyTM stent fractures[4848. Nordmeyer J, Lurz P, Khambadkone S, et al. Pre-stenting with a bare metal stent before percutaneous pulmonary valve implantation: acute and 1-year outcomes. Heart. 2011;97(2):118-123. ]. Furthermore, data of a recent meta-analysis including data from 360 patients and 5 studies indicate that pre-stenting of the RVOT prior to PPVI was not only associated with a significant reduction in the overall incidence of stent fractures but also with a lower incidence of stent fractures associated with loss of stent integrity (types II and III stent fractures) and, remarkbly, a significant reduction in the need for re-interventions[4949. Cardoso R, Ansari M, Garcia D, Sandhu S, Brinster D, Piazza N. Prestenting for prevention of melody valve stent fractures: A systematic review and meta-analysis. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2015. ]. It is therefore not surprising that pre-stenting procedures prior to PPVI developed to routine practice and are prefered in the majority of patients since they offer a perfect landmark for correct valve positioning and potentially lead to superior immediate haemodynamic results. Pre-stenting enhances the rigidness of the implantation side and thereby might reduce the risk of stent fractures during follow up. On multivariable retrospective risk factor analysis for the development of stent fractures during follow-up, it appears that a more dynamic RVOT, as seen in a non-calcified RVOT or non-circumferential homografts, is associated with the occurrence of stent fractures[5050. Nordmeyer J, Khambadkone S, Coats L, et al. Risk stratification, systematic classification, and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation. 2007;115(11):1392-1397. ]. This information supports the superior use of pre-stenting to support the implantation site prior to PPVI. An additional bare stent within the RVOT should improve the rigidness of the RVOT and thereby potentially reduce the risk of stent fractures. Randomised trials are needed to confirm the effectiveness of pre-stenting in prevention of stented-valve fractures[4949. Cardoso R, Ansari M, Garcia D, Sandhu S, Brinster D, Piazza N. Prestenting for prevention of melody valve stent fractures: A systematic review and meta-analysis. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2015. ].
For pre-stenting with bare metal stents, we use the balloon-expandable IntraStent® (Max™ LD, EV3, Plymouth, MN). These stents are delivered on BIB® dilatation catheters (NuMED), which are chosen to have smaller nominal balloon diameters than the subsequently used PPVI delivery system and than the original outflow tract size. This approach leaves some degree of residual outflow tract obstruction bearing the advantage of reduced risk for conduit rupture and allows for safer anchoring of the subsequent implanted valved stent. An uncovered stent is also usually used if obstruction of the origin of one of the pulmonary branch arteries (“jailing”) is expected after deployment of the pre-stent. However, as outlined before, any balloon dilatation of stenotic conduits without covered stents bares some risk of significant conduit rupture and bleeding. Especially, in rather small conduits, which are to be dilated up to a diameter bigger than the original size, pre-stenting with covered stents (i.e., CP Stent™, NuMED, Hopkinton, NY) can be performed and should reduce the clinical impact of conduit rupture ( Figure 8.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1952" data-folder="pcr-textbook" data-chapterid="125"> Figure 8). The optimal timing of pre-stenting in relation to definitive PPVI is unknown. Some centres allow stent ingrowth for two to three months, especially if inadequate sealing by covered stents is expected. In our experience, a combined procedure as well as a two-staged procedure are valid options. For the latter, temporal free pulmonary regurgitation after pre-stenting is well tolerated in the vast majority of patients ( Figure 8.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1952" data-folder="pcr-textbook" data-chapterid="125"> Figure 8).
When using the SAPIENTM stented valve for PPVI pre-stenting is also recommended[3535. Haas NA, Moysich A, Neudorf U, et al. Percutaneous implantation of the Edwards SAPIEN pulmonic valve: initial results in the first 22 patients. Clinical research in cardiology :. official journal of the German Cardiac Society. 2013;102(2):119-128. , 5151. Kim DW. Off-Label, On-Target: Transcatheter Pulmonary Valve Implantation With the SAPIEN Valve. JACC. Cardiovascular interventions. 2015;8(14):1828-1830. ]. In these cases pre-stenting with a covered stent also prevent of paravalvular leak due to the relatively short sealing skirt within a geometrically complex and potentially tortuous RV-PA pathway[5151. Kim DW. Off-Label, On-Target: Transcatheter Pulmonary Valve Implantation With the SAPIEN Valve. JACC. Cardiovascular interventions. 2015;8(14):1828-1830. ].
Postdilatation of the valved stent
Post-dilation of the valved stent is indicated if there is a residual gradient of > 20 mm Hg caused by the valve diameter (pre- or post-valve residual stenosis should be ruled out or treated separately when applicable)[4646. Witsenburg M., Gewellig M., Turner M., Daehnert I. The Melody TPV implantation step-by-step: a proctor’s guide. Available at: http://www.aepc.org/@Bin/141218/Melody+Implantation+Step+by+Step_+A+Proctors+Guide_+Rev+1_+May+10_2013. pdf. Accessed January 29, 2016. ]. Cheatham et al. recently underlined that a residual RVOT gradient of > 20 mmHg is related to a significant shorter freedom from re-intervention and re-operation during a 7-year follow up[55. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960-1970. ] which has previously been reported by our group[44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. ]. Therefore, an aggressive approach to a residual RVOT obstruction is required to improve the long-term outcome post-PPVI. Whereas forceful opening of the stenotic implantation site could be achieved by aggressive pre-dilatation or by post-dilatation of the bare metal stent following pre-stenting, we believe that high-pressure post-dilatation to a maximum balloon size of 24 mm of the valved and therefore covered MelodyTM device is the safest option with regard to conduit rupture. It represents our preferred approach. High-pressure balloons such as Mullins-X Ultra High pressure dilation catheter (NuMed, Hopkinton, NY) or Atlas PTA Dilatation catheter (C.R.Bard Inc, Murray Hill, New Jersey) are suitable (see Table 2). Multiple post-dilatations can be considered in the presence of any residual gradient to achieve further expansion of the device and therefore optimise the haemodynamic result. It is of note that we have not seen any damage to the bovine venous valve due to post-dilatation[44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. ].
The procedure using Sapien™
Pre-procedural catheterisation including assessment of the coronary artery anatomy does not differ to what has been described for MelodyTM implants previously. Due to the short length (14 to 16 mm) of the valve as well as the presumed better stability, pre-stenting prior to Edwards SAPIENTM implantation is preferred[3535. Haas NA, Moysich A, Neudorf U, et al. Percutaneous implantation of the Edwards SAPIEN pulmonic valve: initial results in the first 22 patients. Clinical research in cardiology :. official journal of the German Cardiac Society. 2013;102(2):119-128. , 5151. Kim DW. Off-Label, On-Target: Transcatheter Pulmonary Valve Implantation With the SAPIEN Valve. JACC. Cardiovascular interventions. 2015;8(14):1828-1830. , 5252. Wilson WM, Benson LN, Osten MD, Shah A, Horlick EM. Transcatheter Pulmonary Valve Replacement With the Edwards Sapien System: The Toronto Experience. JACC. Cardiovascular interventions. 2015;8(14):1819-1827. ] ( Figure 9.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1953" data-folder="pcr-textbook" data-chapterid="125"> Figure 9).
Acute procedural result
PROCEDURAL COMPLICATIONS
Nature and incidence of complications
Several mono- and multicentre trials consistently reported a low periprocedural complication rate using the MELODY TM pulmonic valve. The London experience reporting of 155 patients [77. Lurz P, Bonhoeffer P, Taylor AM. Percutaneous pulmonary valve implantation: an update. Expert review of cardiovascular therapy. 2009;7(7):823-833. ] and the ‘US MELODY™ Valve Trial’ including 124 patients[3232. Peng LF, McElhinney DB, Nugent AW, et al. Endovascular stenting of obstructed right ventricle-to-pulmonary artery conduits: a 15-year experience. Circulation. 2006;113(22):2598-2605. ] found similar procedural complication rates of six percent. Smaller trials showed an overall early complication rate of up to 11 percent[1212. Butera G, Milanesi O, Spadoni I, et al. Melody transcatheter pulmonary valve implantation. Results from the registry of the Italian Society of Pediatric Cardiology. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2013;81(2):310-316. ]. Data analysis of the ‘MELODY TM Registry’ data distinguishes between major procedural complications in 2.7 percent and 11.9 percent of minor complications in total of 1003 MELODYM procedures[5353. Nordmeyer J, Ewert P, Gewillig M, et al. CURRENT RESULTS OF THE MELODY REGISTRY: AN INTERNATIONAL MULTICENTER REGISTRY OF TRANSCATHETER PULMONARY VALVE IMPLANTATION. Journal of the American College of Cardiology. 2014;63(12_S). ].
Major procedural complications related to MELODY TM PPVI include (sorted by frequency of occurrence) homograft rupture (2.2 percent), perforation of branch pulmonary arteries or guidewire injury to the pulmonary artery (1.7 percent), damage to tricuspid valve (1.6 percent), device dislodgement (0.5 percent), compression of coronary arteries (0.3 percent) or obstruction of pulmonary artery (0.3 percent in London experience).
The ‘Early Phase 1 International Multicenter Clinical Trial’ reporting on SAPIENTM PPVI in 36 patients reported on a successful valve deployment of 97 percent, but seven patients (20.5 percent) experienced adverse events[3434. Kenny D, Hijazi ZM, Kar S, et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. Journal of the American College of Cardiology. 2011;58(21):2248-2256. ]. The major complication was device dislodgement. In none of the patients did homograft rupture occur. All of the SAPIENTM patients received pre-stenting (33.3 percent) or peri-procedural stenting procedures (66,6 percent).
How to avoid and deal with complications
Learning curve
In our experience, we clearly identified a significant learning curve with regards to procedural complications[44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. , 55. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960-1970. ]. Device dislodgement, for example, occurred only in the early experience and can and should be avoided by appropritate assessment of the implantation site as described above. Further, coronary compression should be avoidable in the vast majority of patients, despite the fact that it remains a complex issue.
Conduit rupture
Risk factors for conduit ruptures are still not completely understood, although Boudjemline et al. recently indentified heavy calcifications and the type of conduit (homograft) as independent risk factors[4747. Boudjemline Y, Malekzadeh-Milani S, Patel M, et al. Predictors and outcomes of right ventricular outflow tract conduit rupture during percutaneous pulmonary valve implantation: a multicentre study. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. EuroIntervention. 2016;11(9):1053-1062. ]. It is a complication that is difficult to avoid. No recommendations regarding patient selection and risk reduction can be given except for the avoidance of aggressive dilatation of the RVOT before deployment of a covered stent of the valved stent. Therefore, a structured approach to serious bleeding is paramount to improve patient safety: as a first step, the operator should confirm bleeding. In general, there is bleeding into the pleura rather than into the pericardium, which can be easily recognised by fluoroscopy. If bleeding is confirmed, auto-transfusion should be initiated immediately. Therefore, pleural drainage and auto-transfusion equipment has to be available acutely. In our experience, prompt auto-transfusion allows for re-establishing of a sufficient circulation and planning of any further interventional treatment or watchful observation. A cellsaver could be used during autotransfusion. However, in the very acute setting, the potential benefit of cellsavers has to be counterbalanced with the loss of time and resources in such a dramatic situation. In most cases, after intensive and efficient autotransfusion, bleeding either stops or reduces to an amount, which allows safe transfer to the operating theatre. Acutely, thoracotomy and surgical intervention is not adviced, since decompresion of the chest can exaggregate the bleed and complicates locating the source of bleeding.
Entrapment of the delivery system in the tricupid valve
Significant damage to the tricuspid valve due to entrapment of the delivery system in the tendinous cords of the tricuspid valve can be avoided in most cases. Care should be taken when the tricuspid valve is crossed the first time. Especially for the less experienced operator, the tricuspid valve should be negotiated with a balloon tipped floating catheter to minimize the risk of entrapment of any catheters or wires within the tricuspid subvalvar apparatus.
Injury to branch pulmonary arteries
In order to provide sufficient support for safe advancement of the delivery system, very stiff guidewires have to be used. Manipulation of such wires can lead to branch pulmonary arteries injury or even perforation. To avoid this complication, the operator has to ensure that the guide wire is in a stable position in one of the distal PA branches. Further, if the guidewire happens to move backwards while the delivery system is pushed forward, the guidewire should be repositioned before the delivery system is advanced. The delivery system should only be advanced when the guidewire is placed correctly in one of the distal pulmonary branches. Initial positioning of the guidewire should only be performed via a luminal catheter.
ILLUSTRATIVE CASE 3
Risk of coronary compression
Coronary compression due to RVOT interventions is a well-known and previously described complication in the setting of bare metal stenting of the RVOT[3232. Peng LF, McElhinney DB, Nugent AW, et al. Endovascular stenting of obstructed right ventricle-to-pulmonary artery conduits: a 15-year experience. Circulation. 2006;113(22):2598-2605. ] ( Figure 7.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1951" data-folder="pcr-textbook" data-chapterid="125"> Figure 7) ( ILLUSTRATIVE CASE 2). Approximately five to six percent of all patients that are potential candidates for PPVI will have a coronary artery anatomy which bears the risk for coronary obstruction[1111. Gillespie MJ, McElhinney DB. Transcatheter Pulmonary Valve Replacement: A Current Review. Current Pediatrics Reports. 2013;1(2):83-91. ]. There are several reports of this potential catastrophic complication[5454. Biermann D, Schonebeck J, Rebel M, Weil J, Dodge-Khatami A. Left coronary artery occlusion after percutaneous pulmonary valve implantation. The Annals of thoracic surgery. 2012;94(1):e7-9. , 5555. Mauri L, Frigiola A, Butera G. Emergency surgery for extrinsic coronary compression after percutaneous pulmonary valve implantation. Cardiology in the young. 2013;23(3):463-465. ] which is strongly related to early procedural mortality[88. Eicken A, Ewert P, Hager A, et al. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. European heart journal. 2011;32(10):1260-1265. ]. Although coronary compression should be avoidable in the vast majority of patients, it remains a complex issue. Ruling out the risk for this complication represents one of the most difficult steps in pre-procedural planning for PPVI. As discussed earlier in this chapter, the stepwise approach can minimise the risk of coronary compression. In any case of doubt about the risk of coronary compression, we recommend to abandon the implant in either MELODY TM or SAPIENTM valve procedures. Careful technique and attention to the recommended instructions for use in experienced hands help to avoid these fatal complications.
IMMEDIATE HAEMODYNAMIC OUTCOME ( Table 3 )
The haemodynamic outcome post PPVI within the largest published reports using MELODYTM [44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. , 55. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960-1970. , 88. Eicken A, Ewert P, Hager A, et al. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. European heart journal. 2011;32(10):1260-1265. , 1111. Gillespie MJ, McElhinney DB. Transcatheter Pulmonary Valve Replacement: A Current Review. Current Pediatrics Reports. 2013;1(2):83-91. , 5656. Gillespie MJ, Rome JJ, Levi DS, et al. Melody valve implant within failed bioprosthetic valves in the pulmonary position: a multicenter experience. Circulation. Cardiovascular interventions. 2012;5(6):862-870. , 5757. McElhinney DB, Hellenbrand WE, Zahn EM, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122(5):507-516. ] and using the SAPIENTM device[3434. Kenny D, Hijazi ZM, Kar S, et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. Journal of the American College of Cardiology. 2011;58(21):2248-2256. , 4141. Boone RH, Webb JG, Horlick E, et al. Transcatheter pulmonary valve implantation using the Edwards SAPIEN transcatheter heart valve. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2010;75(2):286-294. , 5252. Wilson WM, Benson LN, Osten MD, Shah A, Horlick EM. Transcatheter Pulmonary Valve Replacement With the Edwards Sapien System: The Toronto Experience. JACC. Cardiovascular interventions. 2015;8(14):1819-1827. ] have already been reviewed[4343. Wagner R, Daehnert I, Lurz P. Percutaneous pulmonary and tricuspid valve implantations: An update. World journal of cardiology. 2015;7(4):167-177. ] and are demonstrated in Table 3. PPVI using the two different devices resulted in a significant reduction in RV pressures, RVOT gradient and RV to systemic pressure ratio. Further, diastolic pulmonary arterial pressures have been shown to rise after deployment, indicating restoration of valvar competence[44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. , 3434. Kenny D, Hijazi ZM, Kar S, et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. Journal of the American College of Cardiology. 2011;58(21):2248-2256. , 5656. Gillespie MJ, Rome JJ, Levi DS, et al. Melody valve implant within failed bioprosthetic valves in the pulmonary position: a multicenter experience. Circulation. Cardiovascular interventions. 2012;5(6):862-870. ]. Angiography prior to and after insertion shows a significant reduction in pulmonary regurgitation in the vast majority of patients. Paravalvar leaks post-procedure are rare[1111. Gillespie MJ, McElhinney DB. Transcatheter Pulmonary Valve Replacement: A Current Review. Current Pediatrics Reports. 2013;1(2):83-91. ] and seen in approximately 1 out of 50 patients. A recent presentation of first data from the multi-centre registry (MELODY TM Registry) involving data of 40 international centres, that have treated thus far more than 1000 patients by PPVI, observed the following: invasively measured right ventricular systolic pressure fell from 62 ± 18 mm Hg to 43 ± 12 mm Hg (p < 0.0001) and there was a significant decrease in the percentage of patients with significant RVOT regurgitation of grade greater than 2 (49 percent to 1 percent, p < 0.0001)[5353. Nordmeyer J, Ewert P, Gewillig M, et al. CURRENT RESULTS OF THE MELODY REGISTRY: AN INTERNATIONAL MULTICENTER REGISTRY OF TRANSCATHETER PULMONARY VALVE IMPLANTATION. Journal of the American College of Cardiology. 2014;63(12_S). ].
Device function during follow-up
RATE OF RE-OPERATION AND TRANSCATHETER REINTERVENTION
Needless to say, the success of PPVI depends on the device function during follow-up. Short- and medium-term results of PPVI with the MELODYTM and SAPIENTM are similar, although more data are available for the former. Long-term outcome data recently became available the MELODY stent-valve[55. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960-1970. ].
Overall mortality of PPVI during follow-up procedures was zero to five percent and was, as far as could be assessed, not related to the device itself.
Failure of the device, either for the MELODYTM or the SAPIENTM , could be related to malfunction of its stent or its sewn valve. While failure of the valve leading to pulmonary regurgitation is seen rarely and only in the context of endocarditis[5858. Buber J, Bergersen L, Lock JE, et al. Bloodstream infections occurring in patients with percutaneously implanted bioprosthetic pulmonary valve: a single-center experience. Circulation. Cardiovascular interventions. 2013;6(3):301-310. , 5959. McElhinney DB, Benson LN, Eicken A, Kreutzer J, Padera RF, Zahn EM. Infective endocarditis after transcatheter pulmonary valve replacement using the Melody valve: combined results of 3 prospective North American and European studies. Circulation. Cardiovascular interventions. 2013;6(3):292-300. , 6060. Cheung G, Vejlstrup N, Ihlemann N, et al. Infective endocarditis following percutaneous pulmonary valve replacement: diagnostic challenges and application of intra-cardiac echocardiography. International journal of cardiology. 2013;169(6):425-429. , 6161. Villafane J, Baker GH, Austin, E. H., 3rd, Miller S, Peng L, Beekman, R., 3rd. Melody pulmonary valve bacterial endocarditis: Experience in four pediatric patients and a review of the literature. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2014. ], the most common reason for re-operation and re-intervention is re-stenosis of the stent portion of the device. Re-stenosis of the stent can be caused by late recoil or lost of radial strength of the device due to stent fractures.
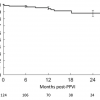
In our assessment of the first 155 PPVI patients with a median follow-up of 28 months, we have demonstrated a freedom from re-operation of 93 (±2), 86 (±3), 84 (±4), and 70 (±13)%, and a freedom from transcatheter re-intervention (second PPVI or balloon dilatation) of 95(±2), 87(±3), 73(±6), and 73(±6)% at 10, 30, 50 and 70 months, respectively[44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. ]. Initially, the most common reason for re-intervention was occurrence of the hammock effect. The ‘hammock’ effect describes a condition in which the venous wall of the valve stent hangs into the lumen causing an effective stenosis[6262. Kenny D, Hijazi ZM. The evolution of transcatheter pulmonary valve replacement. Expert review of cardiovascular therapy. 2013;11(7):795-797. ]. This effect occurred in the first design of devices, as the venous wall was sutured to the stent only at its two extremities. This allowed entrance of blood between the valved stent and the patient’s right ventricular outflow tract. After device modification (with sutures being placed at all struts of the stent) no further cases of the hammock effect have been seen. This condition led to re-intervention in 6 patients out of 21 treated with the early device; three underwent repeated PPVI (new device design) and in 4 the valved-stent was explanted. Overall, stent fractures represent the most common reason for re-intervention after MelodyTM (10 of 155 patients in the London experience and 9 of 124 patients within the US trial). The nature and management of stent fractures are discussed in the following paragraph ( Figure 10.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="1954" data-folder="pcr-textbook" data-chapterid="125"> Figure 10) .
In the cohort of patients, who did not undergo re-operation or transcatheter re-intervention in the London experience, the peak velocity across the device increased only slightly from 1 month to 36 months after the procedure (p=0.07). At 1, 6, 12, and 36 months, peak RVOT velocity was 2.64 ± 0.6 m/s (n=107), 2.7 ± 0.59 m/s (n=86), 2.66 ± 0.5 m/s (n=83), 2.89 ± 0.74 m/s (n=25), respectively. Valvar competence was well maintained during follow-up. On echocardiography, more than mild valvar incompetence was seen only in patients with endocarditis. In total, there were 5 cases of endocarditis in 155 patients with a median follow-up of 28.4 months[6363. Nordmeyer J, Coats L, Lurz P, et al. Percutaneous pulmonary valve-in-valve implantation: a successful treatment concept for early device failure. European heart journal. 2008;29(6):810-815. ]. Out of those 5 patients, 3 were know to had previous episodes of endocarditis.
In the initial US MelodyTM trial data[5757. McElhinney DB, Hellenbrand WE, Zahn EM, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122(5):507-516. ] survival free from RVOT re-intervention was 95.4 ± 2.1% at 1 year and 87.6 ± 4.5% at 2 years. Freedom from a second PPVI was 96.9 ± 2.0% at 1 year and 90.4 ± 4.4% at 2 years. Freedom from PPVI dysfunction was 93.5 ± 2.4% at 1 year and 85.6 ± 4.7% at 2 years. The latest follow-up data from the US Melody trial presented overall freedom from re-intervention after MELODY valve to be 76 ± 4% at 5 years. Freedom from RVOT re-intervention in the cohort of patients who did not develop a type II stent fracture was even higher with 98 ± 1% at 3 years and 91 ± 3% at 5 years. Freedom from device explantation was 92 ± 3% after 60 months post PPVI. By multivariable Cox regression analysis, a higher mean RVOT gradient on discharge echocardiography (HR, 2.6 per 10 mm Hg; 95% CI, 1.1–6.3; P=0.03) and younger age (univariable HR, 0.85; 95% CI, 0.74 to 0.98 per year; P=0.008; multivariable HR, 0.86; 95% CI, 0.73 to 0.99 per year; P=0.01) were associated with shorter freedom from stented valve dysfunction[55. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960-1970. , 5757. McElhinney DB, Hellenbrand WE, Zahn EM, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122(5):507-516. ]. Once again, the latest data from the US Melody trial[55. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960-1970. ] underlined the association between high RVOT gradients on echocardiography post-PPVI and an increased risk for re-intervention which corresponds to our findings that a residual RVOT gradient of > 25 mmHg (invasively measured) results in higher rates of re-intervention during follow-up[44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. ]. Therefore, we believe that rather aggressive post-dilatation should be perfomed especially in very stenotic conduit in order to achieve optimal immediate haemodynamic results and thereby improve long-term outcome in these patients.
Overall, data are available from the major short- and medium-term observational studies with a total of over 450 patients with one- to five-year follow-up[44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. , 88. Eicken A, Ewert P, Hager A, et al. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. European heart journal. 2011;32(10):1260-1265. , 1212. Butera G, Milanesi O, Spadoni I, et al. Melody transcatheter pulmonary valve implantation. Results from the registry of the Italian Society of Pediatric Cardiology. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2013;81(2):310-316. , 5757. McElhinney DB, Hellenbrand WE, Zahn EM, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122(5):507-516. ]: freedom from valve dysfunction or re-intervention was approximately 94 percent at one year follow-up. The patients who did not require re-intervention had consistently mild or less pulmonic valve regurgitation at one-year follow-up. Pulmonary regurgitation decreased from median values of 16 to 27 percent to one to two percent. Median peak velocity over the right ventricular outflow tract was 1.9 to 2.7 m/s at one-year echocardiographic follow-up. Nordmeyer recently reported preliminary but promising MELODYTM Registry data with a one-year freedom for all case events with 92.5 percent and 94.2 percent for PPVI-related events[5353. Nordmeyer J, Ewert P, Gewillig M, et al. CURRENT RESULTS OF THE MELODY REGISTRY: AN INTERNATIONAL MULTICENTER REGISTRY OF TRANSCATHETER PULMONARY VALVE IMPLANTATION. Journal of the American College of Cardiology. 2014;63(12_S). ].
It is worth to underline that Cheatham et al. were also able to present encouraging data with stable mid- and long-term that indicated that the Melody stent-valve remains non-stenotic and non-regurgitant up to 7 years after implantation[55. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960-1970. ]. As for the MELODYTM valve, they are no comparison studies with conventional surgery regarding pulmonary valve replacement by SAPIENTM valves. Data on device function of the SAPIENTM valve are comparatively limited and available from smaller short- and medium-term observational studies with a total of just above 100 patients with right ventricle-to-pulmonary artery conduits[3434. Kenny D, Hijazi ZM, Kar S, et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. Journal of the American College of Cardiology. 2011;58(21):2248-2256. , 4141. Boone RH, Webb JG, Horlick E, et al. Transcatheter pulmonary valve implantation using the Edwards SAPIEN transcatheter heart valve. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2010;75(2):286-294. , 5252. Wilson WM, Benson LN, Osten MD, Shah A, Horlick EM. Transcatheter Pulmonary Valve Replacement With the Edwards Sapien System: The Toronto Experience. JACC. Cardiovascular interventions. 2015;8(14):1819-1827. ] or native RVOT[3535. Haas NA, Moysich A, Neudorf U, et al. Percutaneous implantation of the Edwards SAPIEN pulmonic valve: initial results in the first 22 patients. Clinical research in cardiology :. official journal of the German Cardiac Society. 2013;102(2):119-128. ] or native RVOT interventions only[6464. Demkow M, Ruzyllo W, Biernacka EK, et al. Percutaneous edwards SAPIEN valve implantation for significant pulmonary regurgitation after previous surgical repair with a right ventricular outflow patch. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2014;83(3):474-481. ]. In the largest series of 36 patients – also known as the COMPASSION trial – involving only patients with right ventricle-to-pulmonary artery conduits, successful valve deployment was achieved in 33 of 34 attempts[3434. Kenny D, Hijazi ZM, Kar S, et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. Journal of the American College of Cardiology. 2011;58(21):2248-2256. ]. In cases of native RVOT dysfunction following trans-annular repair of tetralogy of Fallot, a successful valve implantation was achieved in nine of ten eligible patients[6464. Demkow M, Ruzyllo W, Biernacka EK, et al. Percutaneous edwards SAPIEN valve implantation for significant pulmonary regurgitation after previous surgical repair with a right ventricular outflow patch. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2014;83(3):474-481. ]. The COMPASSION trial reports on an early complication rate of 7 / 34 deployed valves primarily due to device migration (three cases, two requiring surgical retrieval)[3434. Kenny D, Hijazi ZM, Kar S, et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. Journal of the American College of Cardiology. 2011;58(21):2248-2256. ]. Other complications included pulmonary hemorrhage (two cases), ventricular fibrillation (one case) and stent migration (one case). In Boone’s report of seven patients with a median follow-up of ten months (range 30 days to 3.5 years), no stent fractures have been described[4141. Boone RH, Webb JG, Horlick E, et al. Transcatheter pulmonary valve implantation using the Edwards SAPIEN transcatheter heart valve. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2010;75(2):286-294. ]. In this series of seven patients, re-catheterisation was necessary in one patient nine months after the initial implant due to re-stenosis of the bare metal stent. Freedom from re-intervention at six month follow-up was 97 percent in the COMPASSION trial while one patient undergoing elective placement of a second valve due to conduit-induced distortion of the initial implant. Haas et al. demonstrated a significant reduction of the RVOT to PA gradient, reduction in right ventricular systolic pressure, increasing of diastolic pulmonary pressure from 6.3 to 14.5 mm Hg as a sign of tremendously decreased pulmonary regurgitation with freedom from re-intervention after six months[3535. Haas NA, Moysich A, Neudorf U, et al. Percutaneous implantation of the Edwards SAPIEN pulmonic valve: initial results in the first 22 patients. Clinical research in cardiology :. official journal of the German Cardiac Society. 2013;102(2):119-128. ]. Wilson recently presented the Canadian expericence in which all patients were pre-stented[5252. Wilson WM, Benson LN, Osten MD, Shah A, Horlick EM. Transcatheter Pulmonary Valve Replacement With the Edwards Sapien System: The Toronto Experience. JACC. Cardiovascular interventions. 2015;8(14):1819-1827. ]. Successful valve deployment was achieved in 24 of 25 patients of whom 2/3 had xenograft and 1/3 homograft implants before. Immediate complication rate was 3 of 25 (26-mm SAPIENTM malposition with relevant paravalvular leakage with the need for re-PPVI with a covered stent and another 26-mm device; loss of wire position and valved stent with the need for surgical explantation; mild hemoptysis without clinical consequences). The immediate haemodynamic outcome is presented in Table 3. After a mean follow-up of 3.5 ± 2.1 years no deaths, stent fractures or cases of endocarditis occurred. Re-intervention rate was low by 1 of the 25 patients in which clinical evident regurgitation of unknown cause occurred at 1 year after implantation of a 26-mm SAPIENTM TPV. The patient was tried to treat by a valve-in-valve procedure with a 22-mm MELODYTM TPV which could not resolve the dysfunction of the RVOT leading ultimately to indication for surgical redo[5252. Wilson WM, Benson LN, Osten MD, Shah A, Horlick EM. Transcatheter Pulmonary Valve Replacement With the Edwards Sapien System: The Toronto Experience. JACC. Cardiovascular interventions. 2015;8(14):1819-1827. ].
However, studies including more patients with longer follow-up are required to inform on follow-up of device performance of SAPIENTM devices in pulmonary position.
THE NATURE AND MANAGEMENT OF STENT FRACTURES
Stent fractures most commonly affect one or more struts and do not impact upon stent integrity (Type I)[5050. Nordmeyer J, Khambadkone S, Coats L, et al. Risk stratification, systematic classification, and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation. 2007;115(11):1392-1397. ]. In this setting, there are no haemodynamic or clinical consequences, but this finding warrants close surveillance. Some patients, however, present with multiple strut fractures and increased RVOT velocity measured by echocardiography. In these patients, the stent fractures have an impact upon the stent integrity and can lead to re-stenosis (Type II), and implantation of a second device within the index device is indicated and, in our experience, can be performed successfully[6363. Nordmeyer J, Coats L, Lurz P, et al. Percutaneous pulmonary valve-in-valve implantation: a successful treatment concept for early device failure. European heart journal. 2008;29(6):810-815. ]. This ‘stent-in-stent’ strategy relieves RVOT obstruction effectively and also, stent integrity can be enhanced, minimising the risk of stent embolisation. From a technical point of view, second PPVI does not differ fundamentally from the initial implant. The first valved stent might even provide a perfect landmark for positioning of the second device, facilitating this procedure.
In only one case did device embolisation into the right pulmonary artery occur. This was due to stent disintegration following stent fractures (Type III)[5050. Nordmeyer J, Khambadkone S, Coats L, et al. Risk stratification, systematic classification, and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation. 2007;115(11):1392-1397. ].
On multivariable risk factor analysis for the development of stent fractures during follow-up, it appears that a more dynamic RVOT, as seen in a non-calcified RVOT or non-circumferential homografts, is associated with the occurrence of stent fractures[5050. Nordmeyer J, Khambadkone S, Coats L, et al. Risk stratification, systematic classification, and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation. 2007;115(11):1392-1397. ]. This information is useful for patient counselling, but also supports our suggestion to use pre-stenting to support the implantation site prior to PPVI[4949. Cardoso R, Ansari M, Garcia D, Sandhu S, Brinster D, Piazza N. Prestenting for prevention of melody valve stent fractures: A systematic review and meta-analysis. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2015. ]. An additional bare stent within the RVOT should improve the rigidness of the RVOT and thereby potentially reduce the risk of stent fractures. As mentioned before, recently we could show that pre-stenting does reduce the risk of stent fractures when corrected for outflow tract type and extent of calcifications[4848. Nordmeyer J, Lurz P, Khambadkone S, et al. Pre-stenting with a bare metal stent before percutaneous pulmonary valve implantation: acute and 1-year outcomes. Heart. 2011;97(2):118-123. ]. In order to detect stent fractures and plan re-interventions, patient should be followed up.
Overall, since (almost) every patient is stented before PPVI, stent fracture is much less seen than 10 years ago and is very rarely of clinical relevance. Nevertheless, patients should be followed up closely with repeat chest radiography and echocardiography to detect stent fractures early and plan re-interventions ( Figure 11.png" data-toggle="modal" data-target="#popup-media" class="media-link" data-media_id="3297" data-folder="pcr-textbook" data-chapterid="125"> Figure 11).
So far, stent fractures for the SAPIENTM stent valve in either aortic or pulmonary position have not been described[5151. Kim DW. Off-Label, On-Target: Transcatheter Pulmonary Valve Implantation With the SAPIEN Valve. JACC. Cardiovascular interventions. 2015;8(14):1828-1830. ].
Valved stent endocarditis
Infective endocarditis (IE) after PPVI has been observed in approximately one to almost five percent of patients during one- to five-year follow-up[5151. Kim DW. Off-Label, On-Target: Transcatheter Pulmonary Valve Implantation With the SAPIEN Valve. JACC. Cardiovascular interventions. 2015;8(14):1828-1830. , 5151. Kim DW. Off-Label, On-Target: Transcatheter Pulmonary Valve Implantation With the SAPIEN Valve. JACC. Cardiovascular interventions. 2015;8(14):1828-1830. , 5151. Kim DW. Off-Label, On-Target: Transcatheter Pulmonary Valve Implantation With the SAPIEN Valve. JACC. Cardiovascular interventions. 2015;8(14):1828-1830. , 5151. Kim DW. Off-Label, On-Target: Transcatheter Pulmonary Valve Implantation With the SAPIEN Valve. JACC. Cardiovascular interventions. 2015;8(14):1828-1830. , 5151. Kim DW. Off-Label, On-Target: Transcatheter Pulmonary Valve Implantation With the SAPIEN Valve. JACC. Cardiovascular interventions. 2015;8(14):1828-1830. , 5151. Kim DW. Off-Label, On-Target: Transcatheter Pulmonary Valve Implantation With the SAPIEN Valve. JACC. Cardiovascular interventions. 2015;8(14):1828-1830. , 5151. Kim DW. Off-Label, On-Target: Transcatheter Pulmonary Valve Implantation With the SAPIEN Valve. JACC. Cardiovascular interventions. 2015;8(14):1828-1830. , 5151. Kim DW. Off-Label, On-Target: Transcatheter Pulmonary Valve Implantation With the SAPIEN Valve. JACC. Cardiovascular interventions. 2015;8(14):1828-1830. , 5151. Kim DW. Off-Label, On-Target: Transcatheter Pulmonary Valve Implantation With the SAPIEN Valve. JACC. Cardiovascular interventions. 2015;8(14):1828-1830. ]. The MELODYTM Registry preliminary data reveal a one-year-post-PPVI rate of 2.7 percent[5353. Nordmeyer J, Ewert P, Gewillig M, et al. CURRENT RESULTS OF THE MELODY REGISTRY: AN INTERNATIONAL MULTICENTER REGISTRY OF TRANSCATHETER PULMONARY VALVE IMPLANTATION. Journal of the American College of Cardiology. 2014;63(12_S). ]. At first glance, the rate of endocarditis after PPVI seems to be similar to the observed rate of endocarditis after surgical right ventricular outflow tract reconstruction, which ranges from approximately zero to five percent of patients during one- to four-year follow-up[6767. van Dijck I, Budts W, Cools B, et al. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart (British Cardiac Society). 2015;101(10):788-793. ]. Uebing et al. recently reviewed available data in a meta-analysis with an overall IE incidence of 4.3 % per patient year after MELODYTM PPVI[6969. Uebing A, Rigby ML. The problem of infective endocarditis after transcatheter pulmonary valve implantation. Heart (British Cardiac Society). 2015;101(10):749-751. ]. However, Villafene stated that a risk comparison between PPVI and surgically implanted bioprosthetic valves is impossible due to fact that infective endocarditis was not being the primary objective in different larger surgical series and thus being underestimated[6161. Villafane J, Baker GH, Austin, E. H., 3rd, Miller S, Peng L, Beekman, R., 3rd. Melody pulmonary valve bacterial endocarditis: Experience in four pediatric patients and a review of the literature. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2014. ]. Trials directly comparing the incidence of endocarditis with the two techniques were lacking until Van Dijck et al. were the first to report on different incidence of IE after PPVI or surgical graft implantation for right ventricular outflow tract dysfunction[6767. van Dijck I, Budts W, Cools B, et al. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart (British Cardiac Society). 2015;101(10):788-793. ]. The group presented data suggesting similar incidence rates of IE following MELODY TM valve and ContegraTM implantation that are significantly higher compared to homograft implantation for RVOT dysfunction. Theoretically, higher flow velocities across the MELODYTM valve or ContegraTM conduit compared to laminar flow patterns in homografts may contribute to different IE rates and may well be as important as differences in valve structure or tissue characteristics[6969. Uebing A, Rigby ML. The problem of infective endocarditis after transcatheter pulmonary valve implantation. Heart (British Cardiac Society). 2015;101(10):749-751. ]. This is attributed to the fact that jet lesions are known to cause endothelial damage that has always been suspected to play a major role in the pathogenesis of IE[6161. Villafane J, Baker GH, Austin, E. H., 3rd, Miller S, Peng L, Beekman, R., 3rd. Melody pulmonary valve bacterial endocarditis: Experience in four pediatric patients and a review of the literature. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2014. ]. Other predisposing factors reported in the literature include prior episodes of endocarditis[44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. , 5858. Buber J, Bergersen L, Lock JE, et al. Bloodstream infections occurring in patients with percutaneously implanted bioprosthetic pulmonary valve: a single-center experience. Circulation. Cardiovascular interventions. 2013;6(3):301-310. , 6565. McElhinney DB, Cheatham JP, Jones TK, et al. Stent fracture, valve dysfunction, and right ventricular outflow tract reintervention after transcatheter pulmonary valve implantation: patient-related and procedural risk factors in the US Melody Valve Trial. Circulation. Cardiovascular interventions. 2011;4(6):602-614. ], male sex[5858. Buber J, Bergersen L, Lock JE, et al. Bloodstream infections occurring in patients with percutaneously implanted bioprosthetic pulmonary valve: a single-center experience. Circulation. Cardiovascular interventions. 2013;6(3):301-310. ], multiple stents[5858. Buber J, Bergersen L, Lock JE, et al. Bloodstream infections occurring in patients with percutaneously implanted bioprosthetic pulmonary valve: a single-center experience. Circulation. Cardiovascular interventions. 2013;6(3):301-310. ], dental treatment[44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. , 5858. Buber J, Bergersen L, Lock JE, et al. Bloodstream infections occurring in patients with percutaneously implanted bioprosthetic pulmonary valve: a single-center experience. Circulation. Cardiovascular interventions. 2013;6(3):301-310. ], previous fungal infection[44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. ], and septic wounds[44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. , 5858. Buber J, Bergersen L, Lock JE, et al. Bloodstream infections occurring in patients with percutaneously implanted bioprosthetic pulmonary valve: a single-center experience. Circulation. Cardiovascular interventions. 2013;6(3):301-310. ]. Relevant residual RV-PA-gradients were found to be related to an increased risk for endocarditis[5959. McElhinney DB, Benson LN, Eicken A, Kreutzer J, Padera RF, Zahn EM. Infective endocarditis after transcatheter pulmonary valve replacement using the Melody valve: combined results of 3 prospective North American and European studies. Circulation. Cardiovascular interventions. 2013;6(3):292-300. , 6969. Uebing A, Rigby ML. The problem of infective endocarditis after transcatheter pulmonary valve implantation. Heart (British Cardiac Society). 2015;101(10):749-751. ]. Villafene recommends early surgical treatment in the presence of relevant restenosis or regurgitation as well as in patients with no clinical improvement under medical treatment[6161. Villafane J, Baker GH, Austin, E. H., 3rd, Miller S, Peng L, Beekman, R., 3rd. Melody pulmonary valve bacterial endocarditis: Experience in four pediatric patients and a review of the literature. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2014. ]. McElhinney et al. suggest that MELODYTM valve replacement is not required during the acute treatment as long as there is no involvement of the valve system[5959. McElhinney DB, Benson LN, Eicken A, Kreutzer J, Padera RF, Zahn EM. Infective endocarditis after transcatheter pulmonary valve replacement using the Melody valve: combined results of 3 prospective North American and European studies. Circulation. Cardiovascular interventions. 2013;6(3):292-300. ]. It is notable that IE was consistently not detected during the peri-procedural period in any of the mentioned studies[6767. van Dijck I, Budts W, Cools B, et al. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart (British Cardiac Society). 2015;101(10):788-793. ]. The earliest case has been reported by our group at 1.9 months post implantion[44. Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972. ]. According to our (limited) clinical experience, stented valve endocarditis could be treated by ESC or AHA-guidelines adopted long-term antibiotic therapy in a reasonable number of cases[55. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960-1970. ]. We recently reported on a case of blood-culture proven Staph. aureus endocarditis after Edwards SAPIEN TM valve implantation[7070. Vollroth M, Daehnert I, Kostelka M, Wagner R. First case of blood-culture proven Staphylococcus aureus endocarditis of a Sapien® XT valve after percutaneous pulmonary valve implantation. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. Cardio-thoracic Surgery. 2015;48(6):e124-5. ], indicating that all patients with prosthetic valves, including those who have undergone either MELODYTM or SAPIENTM PPVI, are considered among those at highest risk for endocarditis and therefore prophylaxis against bacterial endocarditis is strongly recommended for future invasive procedures[6767. van Dijck I, Budts W, Cools B, et al. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart (British Cardiac Society). 2015;101(10):788-793. , 6969. Uebing A, Rigby ML. The problem of infective endocarditis after transcatheter pulmonary valve implantation. Heart (British Cardiac Society). 2015;101(10):749-751. , 7171. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754. ].
At present, within several registry trials in Europe and the US, data on IE after PPVI is collected and analysed, which hopefully will shed more light on the heatedly debated issue of IE following PPVI. In the meantime, we should be careful when treating patients with presumed very high risk of IE and should consent patients accordingly.
Functional outcome
Apart from immediate haemodynamic improvements after PPVI, correction of adverse RV loading conditions resulted in significant improvements in NYHA functional class post-PPVI, irrespective of the treated lesion (predominant stenosis vs. predominant regurgitation)[55. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960-1970. , 66. Lurz P, Giardini A, Taylor AM, et al. Effect of altering pathologic right ventricular loading conditions by percutaneous pulmonary valve implantation on exercise capacity. The American journal of cardiology. 2010;105(5):721-726. , 5656. Gillespie MJ, Rome JJ, Levi DS, et al. Melody valve implant within failed bioprosthetic valves in the pulmonary position: a multicenter experience. Circulation. Cardiovascular interventions. 2012;5(6):862-870. , 5757. McElhinney DB, Hellenbrand WE, Zahn EM, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122(5):507-516. ]. The 7-year-follow-up post Melody trial showed an impovement of NYHA functional class that changed significantly from before implantation to one year (p < 0.001) after. But there was no difference from one year to subsequent follow-up time points up to seven years[55. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960-1970. ].
Functional MR assessment with analysis of right and left ventricular function and calculation of great vessel blood flow performed before and within one month after the PPVI procedure has shown mixed data regarding changes in RV ejection fraction after TPV replacement, with some studies finding no change[88. Eicken A, Ewert P, Hager A, et al. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. European heart journal. 2011;32(10):1260-1265. , 5757. McElhinney DB, Hellenbrand WE, Zahn EM, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122(5):507-516. ] and others reporting on improvements in the acute or short term[1010. Demkow M, Biernacka EK, Spiewak M, et al. Percutaneous pulmonary valve implantation preceded by routine prestenting with a bare metal stent. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2011;77(3):381-389. , 7272. Coats L, Khambadkone S, Derrick G, et al. Physiological and clinical consequences of relief of right ventricular outflow tract obstruction late after repair of congenital heart defects. Circulation. 2006;113(17):2037-2044. , 7373. Coats L, Khambadkone S, Derrick G, et al. Physiological consequences of percutaneous pulmonary valve implantation: the different behaviour of volume- and pressure-overloaded ventricles. European heart journal. 2007;28(15):1886-1893. ]. Our group demonstrated improvements in effective RV stroke volume in both patients with predominant pulmonary stenosis (40.6 ± 11.0 vs. 46.8 ± 8.0 ml/m2; p < 0.001) and with predominant regurgitation (37.1 ± 6.2 vs. 44.7 ± 7.5 ml/m2; p < 0.001)[66. Lurz P, Giardini A, Taylor AM, et al. Effect of altering pathologic right ventricular loading conditions by percutaneous pulmonary valve implantation on exercise capacity. The American journal of cardiology. 2010;105(5):721-726. ]. In the stenotic group, this was due to decreased RV end-systolic volume and improved RV ejection fraction after marked relief of afterload. In contrast, RV ejection fraction remains unchanged in the regurgitant group, with the improvement in RV and LV effective stroke volume due to abolishment of pulmonary regurgitation (reduction in pulmonary regurgitation fraction as assessed by MR imaging: 39.0 ± 8.6 vs. 3.0 ± 4.7 %; p < 0.001). Whereas relief of RV volume overload resulted in a reduction in RV EDV, the total RV stroke volume decreased in these patients with predominant regurgitation. These results are in line with report on surgical pulmonary valve replacement for pulmonary regurgitation[2222. Frigiola A, Redington AN, Cullen S, Vogel M. Pulmonary regurgitation is an important determinant of right ventricular contractile dysfunction in patients with surgically repaired tetralogy of Fallot. Circulation. 2004;110(11 Suppl 1):II153-7. , 2626. Oosterhof T, van Straten A, Vliegen HW, et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116(5):545-551. ]. On cardiopulmonary exercise testing, only patients with a predominant stenotic lesion showed an improvement in peak oxygen uptake. We speculate that significant RVOT obstruction limits the augmentation of cardiac output, which is elicited by exercise, thus reducing exercise capacity. Indeed, we were recently able to demonstrate that reduction in RVOT gradient was the only independent predictor of improved exercise capacity early after PPVI[66. Lurz P, Giardini A, Taylor AM, et al. Effect of altering pathologic right ventricular loading conditions by percutaneous pulmonary valve implantation on exercise capacity. The American journal of cardiology. 2010;105(5):721-726. ]. Further, we postulate that regurgitation is reduced to a minimum (both as percentage and as absolute value) at peak exercise and is not the limiting factor for cardiac output augmentation during exercise. This is likely to be related to shortening of diastole and reduced pulmonary vascular resistance during exercise[66. Lurz P, Giardini A, Taylor AM, et al. Effect of altering pathologic right ventricular loading conditions by percutaneous pulmonary valve implantation on exercise capacity. The American journal of cardiology. 2010;105(5):721-726. ]. Therefore, by purely abolishing regurgitation without improving RV ejection fraction, peak VO2 might not be affected by PPVI acutely in this patient subgroup, explaining the different behavior of the two groups in terms of exercise capacity. Recently, we reported on the ability to recovery from exercise as described by VO² and VCO² decay after maximal exercise. Recovery form exercise after PPVI improves in both groups (predominant stenosis vs predominant regurgitation). These findings could explain the symptomatic improvement observed in patients with predominant regurgitation despite the lack of increased maximal exercise capacity and might have implications for how we judge procedural success[7474. Lurz P, Riede FT, Taylor AM, et al. Impact of Percutaneous Pulmonary Valve Implantation for Right Ventricular Outflow Tract Dysfunction on Exercise Recovery Kinetics. International Journal of Cardiology. 2014. ]. Cheatham et al. found that the maximum workload achieved increased from a median of 105 W (15 – 338 W) before PPVI to 121 W (27 – 271 W) at the one year evaluation (p=0.01). The improvements remained stable at two and three years of follow-up[55. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960-1970. ]. Despite the fact that there were no significant changes in the percent of predicted peak oxygen consumption (percent Vo2max) in the overall population, the ratio of minute ventilation to carbon dioxide production at the anaerobic threshold (Ve/Vco2) improved early after PPVI and remained stable in accordance to the data by Batra[7575. Batra AS, McElhinney DB, Wang W, et al. Cardiopulmonary exercise function among patients undergoing transcatheter pulmonary valve implantation in the US Melody valve investigational trial. American heart journal. 2012;163(2):280-287. ].
Notably, when we looked at RV size and function on MR imaging and exercise capacity one year post PPVI, we found that there was no further change in any parameters from acutely post-PPVI to one year post-PPVI[7676. Lurz P, Nordmeyer J, Giardini A, et al. Early versus late functional outcome after successful percutaneous pulmonary valve implantation: are the acute effects of altered right ventricular loading all we can expect? Journal of the American College of Cardiology. American College of Cardiology. 2011;57(6):724-731. ]. As mentioned above, the ideal timing of any RVOT intervention, whether surgical or percutaneously, remains unknown, since hard and long-term data are still missing. However, based on these results, one might suggest treating patients with pulmonary regurgitation before the onset of RV dysfunction or impaired exercise capacity. Focussing on right to left ventricular interaction, an important physiological consequence of RV enlargement and dysfunction is secondary left ventricular dysfunction in some patients, which might influence exercise capacity. Thus, the impact of PPVI on left ventricular systolic and diastolic function is not a trivial issue. Improved systolic function secondary to PPVI as estimated by ejection fraction on myocardial velocity imaging has been reported in several studies[66. Lurz P, Giardini A, Taylor AM, et al. Effect of altering pathologic right ventricular loading conditions by percutaneous pulmonary valve implantation on exercise capacity. The American journal of cardiology. 2010;105(5):721-726. , 7272. Coats L, Khambadkone S, Derrick G, et al. Physiological and clinical consequences of relief of right ventricular outflow tract obstruction late after repair of congenital heart defects. Circulation. 2006;113(17):2037-2044. ]. Improvement in left ventricular diastolic function has also been reported[2828. Lurz P, Bonhoeffer P, Taylor AM. Percutaneous pulmonary valve implantation: an update. Expert review of cardiovascular therapy. 2009;7(7):823-833. , 7373. Coats L, Khambadkone S, Derrick G, et al. Physiological consequences of percutaneous pulmonary valve implantation: the different behaviour of volume- and pressure-overloaded ventricles. European heart journal. 2007;28(15):1886-1893. , 7777. Lurz P, Nordmeyer J, Coats L, Taylor AM, Bonhoeffer P, Schulze-Neick I. Immediate clinical and haemodynamic benefits of restoration of pulmonary valvar competence in patients with pulmonary hypertension. Heart. 2009;95(8):646-650. ].
Conclusion
The aim of percutaneous pulmonary valve implantation is to prolong the lifespan of conduits, which were surgically placed from the RV to the PA. Currently, intervention-free and explantation-free survival 5 years after percutaneous pulmonary valve implantation is 70 % and > 90 % respectively. This prolonged conduit lifespan, and hence postponed surgery, should reduce the number of multiple open-heart operations over the total lifespan of children and young adults with congenital heart disease and potentially improve life expectancy of these patients. In our opinion, percutaneous insertion of a pulmonary valve represents the non-invasive treatment of choice in patients with dysfunction of their right ventricular outflow tracts, and should be considered superior to insertion of bare metal stents.
Technical limitations still exist related to percutaneous valve implantation especially in patients with dilated native outflow tracts. Although significant improvement has been achieved in early and late outcomes after percutaneous implantation of pulmonary valves, the risk of stent fractures, homograft rupture and stent-valved endocarditis are not yet sufficiently understood. Further investigations are necessary to avoid these complications. Recently presented and very promising mid- and long-term results of PPVI are of importance: One the one hand the data allows clinicians to more confidently counsel paediatric and adult patients and their families when considering PPVI, and on the other give the surgeons good reasons to implant conduits and valve prostheses compatible with future Melody use[22. Petit CJ. Pediatric transcatheter valve replacement: guests at our own table?. Circulation. 2015;131(22):1943-1945. ].
Personal perspective
Many patients are not ideal candidates for PPVI procedures due to their small physical size, limited vascular access or, most important, due to the size and shape of the RVOT[7878. Kreutzer J. Percutaneous pulmonary valve implantation. Revista Argentina de Cardioangiología Intervencionista. 2013;4(2):84-91. , 7979. Ansari MM, Cardoso R, Garcia D, et al. Percutaneous Pulmonary Valve Implantation: Present Status and Evolving Future. Journal of the American College of Cardiology. 2015;66(20):2246-2255. ]. The majority of patients suffering from dysfunction of the RVOT have enlargement of the patch augmented RVOT as part of the initial surgical repair strategy[1111. Gillespie MJ, McElhinney DB. Transcatheter Pulmonary Valve Replacement: A Current Review. Current Pediatrics Reports. 2013;1(2):83-91. ]. This unmet clinical challenge led to the development of novel approaches to treat RVOT dysfunction using existing interventional pulmonic valve technology usually in an off-label use. A small case series reveals an approach to implant or post-dilate the MELODYTM valve using 24 mm balloons. This practice does not compromise function of the engrafted valve and may effectively broaden the pool of eligible patients[1111. Gillespie MJ, McElhinney DB. Transcatheter Pulmonary Valve Replacement: A Current Review. Current Pediatrics Reports. 2013;1(2):83-91. , 8080. Cheatham SL, Holzer RJ, Chisolm JL, Cheatham JP. The Medtronic Melody® transcatheter pulmonary valve implanted at 24-mm diameter--it works. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2013;82(5):816-823. ].
However, RVOT dysfunction with predominant regurgitation and marked dilatation are not be eligible to this approach. Treatment strategies e.g. MELODYTM valve implantation into the branch pulmonary arteries[8181. Robb JD, Harris MA, Minakawa M, et al. Melody valve implantation into the branch pulmonary arteries for treatment of pulmonary insufficiency in an ovine model of right ventricular outflow tract dysfunction following tetralogy of Fallot repair. Circulation. Cardiovascular interventions. 2011;4(1):80-87. , 8282. Gillespie MJ, Dori Y, Harris MA, Sathanandam S, Glatz AC, Rome JJ. Bilateral branch pulmonary artery melody valve implantation for treatment of complex right ventricular outflow tract dysfunction in a high-risk patient. Circulation. Cardiovascular interventions. 2011;4(4):e21-3. ]or a ‘jailing’ procedure of the pulmonary bifurcation by implanting a bare metal stent across the main pulmonary into a pulmary branch have been described as potential options[8383. Boudjemline Y, Legendre A, Ladouceur M, et al. Branch pulmonary artery jailing with a bare metal stent to anchor a transcatheter pulmonary valve in patients with patched large right ventricular outflow tract. Circulation. Cardiovascular interventions. 2012;5(2):e22-5. ].
A hybrid approach combining intra-operative PPVI with simultaneous conduit down-sizing[8484. Bacha EA, Marshall AC, McElhinney DB, del Nido PJ. Expanding the hybrid concept in congenital heart surgery. Seminars in thoracic and cardiovascular surgery. Pediatric cardiac surgery annual. 2007:146-150. ] or direct exposure of the RV or RVOT (e.g. after failed percutaneous attempt, ‘bailout’ procedure) have also proven to be feasible[8585. Cubeddu RJ, Hijazi ZM. Bailout perventricular pulmonary valve implantation following failed percutaneous attempt using the Edwards Sapien transcatheter heart valve. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2011;77(2):276-280. ].
Innovative (experimental) technologies e.g. the self-expanding valves such as Medtronic’s Native Outflow Tract device[8686. Schievano S, Taylor AM, Capelli C, et al. First-in-man implantation of a novel percutaneous valve: a new approach to medical device development. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. EuroIntervention. 2010;5(6):745-750. ] ( Figure 12) or the Venus P Valve (Venus Medtech, Shanghai,China)[8787. Cao Q, Kenny D, Zhou D, et al. Early clinical experience with a novel self-expanding percutaneous stent-valve in the native right ventricular outflow tract. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. Cardiac Angiography & Interventions. 2014;84(7):1131-1137. , 8888. Promphan W, Prachasilchai P, Siripornpitak S, Qureshi SA, Layangool T. Percutaneous pulmonary valve implantation with the Venus P-valve: clinical experience and early results. Cardiology in the young. 2015:1-13. ], infundibular reducer devices[8989. Boudjemline Y, Agnoletti G, Bonnet D, Sidi D, Bonhoeffer P. Percutaneous pulmonary valve replacement in a large right ventricular outflow tract: an experimental study. Journal of the American College of Cardiology. 2004;43(6):1082-1087. ] or newer low-profile pulmonary valves such as the Colibri Heart Valve (Colibri Heart Valve, LLC, CO, USA) indicate future treatment alternatives and hopefully will offer a non-surgical treatment to a much broader patient population[6262. Kenny D, Hijazi ZM. The evolution of transcatheter pulmonary valve replacement. Expert review of cardiovascular therapy. 2013;11(7):795-797. , 7979. Ansari MM, Cardoso R, Garcia D, et al. Percutaneous Pulmonary Valve Implantation: Present Status and Evolving Future. Journal of the American College of Cardiology. 2015;66(20):2246-2255. ].
Online data supplement
Dislodgement of the Melody™ device in a very distensible outflow tract
Compression of the coronary system after PPVI
Perforation of the pulmonary artery during PPVI
Valvular heart disease guidelines 2012
http://www.escardio.org/GUIDELINES-SURVEYS/ESC-GUIDELINES/Pages/valvular-heart-disease.aspx