Summary
Stem cell-based cardiovascular regenerative medicine offers a disruptive therapy to achieve structural and functional repair of the myocardium. Initial clinical experience indicates that this therapy is feasible and safe, yet structural and functional benefit still remains rather modest. A common underlying pitfall appears to be variable biodistribution and low rates of cell retention by the recipient myocardial tissue. In addition, clinical studies indicate a progressive decrease in myocardial signals after delivery of labelled stem cells, a finding consistent with rapid cell death or washout within hours of administration. There is ongoing discussion on the most appropriate technique or device for stem cell delivery. The choice depends on the assessment of the underlying pathology, the acuteness of myocardial injury or associated interventional procedure as well as the actual cell type to be delivered. Whilst in the setting of STEMI, intracoronary cell transfer is preferred, direct endomyocardial delivery is the method of choice as a stand-alone procedure in ischaemic heart failure. This chapter describes in detail the respective strategies and devices used for cell delivery as well as novel approaches to improve cell retention.
Introduction
Stem cell-based cardiac regeneration is an emerging therapy which may halt or even reverse the events responsible for the progression of heart failure (details see chapter Mozid et al, Cell based therapy). Initial clinical experience with cell therapy demonstrates that the approach is feasible and safe [11. Abdel-Lattif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989-97. , 22. Lipinski MJ, Biondi-Zoccia GG, Abatte A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761-7.
Both references provide the meta-analyses of early clinical cell therapy trials. These analyses underline the safety of the cell therapy and point at the efficacy on the surrogate end-points after cell therapy.]. However, structural and functional benefit (albeit statistically significant across studies and there being sound evidence of the concept supporting cell therapy) remains rather modest in magnitude [11. Abdel-Lattif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989-97. , 22. Lipinski MJ, Biondi-Zoccia GG, Abatte A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761-7.
Both references provide the meta-analyses of early clinical cell therapy trials. These analyses underline the safety of the cell therapy and point at the efficacy on the surrogate end-points after cell therapy.]. Clinical trial experience has identified several factors which may influence the outcome of therapy [33. Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208-16.
A review highlighting the concept of cell therapy in patients with recent myocardial infarction. The review describes in detail the biology, and purported mechanisms of cell therapy., 44. Bartunek J, Vanderheyden M, Hill J, Terzic A. Cells as biologics for cardiac repair in ischaemic heart failure. Heart. 2010;96:792-800.
A review article analysing the current status of the clinical adoption of cardiac regenerative strategies. It also summarises hurdles that cardiac regeneration must overcome and potential solutions to facilitate its further clinical translation.]. These include patient-specific variables such as functionality of autologous cells, choice of cell type or patient-independent parameters, for example stem cell processing [33. Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208-16.
A review highlighting the concept of cell therapy in patients with recent myocardial infarction. The review describes in detail the biology, and purported mechanisms of cell therapy., 44. Bartunek J, Vanderheyden M, Hill J, Terzic A. Cells as biologics for cardiac repair in ischaemic heart failure. Heart. 2010;96:792-800.
A review article analysing the current status of the clinical adoption of cardiac regenerative strategies. It also summarises hurdles that cardiac regeneration must overcome and potential solutions to facilitate its further clinical translation.]. These findings will guide the design of future hypothesis- driven, as well as larger outcome-driven, clinical trials [44. Bartunek J, Vanderheyden M, Hill J, Terzic A. Cells as biologics for cardiac repair in ischaemic heart failure. Heart. 2010;96:792-800.
A review article analysing the current status of the clinical adoption of cardiac regenerative strategies. It also summarises hurdles that cardiac regeneration must overcome and potential solutions to facilitate its further clinical translation.]. Notwithstanding the aforementioned, a common feature has been the apparently low cell retention rates and discussions surrounding the optimal method of cell delivery [55. Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheter-based delivery of cells to the heart. Nat Clin Prac Cardiovasc Med. 2006;3:Suppl 1:S57-64. , 66. Bartunek J, Sherman W, Vanderheyden M, Fernandez-Aviles F, Wijns W, Terzic A. Delivery of biologics in cardiovascular regenerative medicine. Clin Pharmacol Ther. 2009;85:548-52.
A review article detailing issues with cell delivery in the clinical setting and a framework of future translational research aimed at improved cell retention and survival., 77. Dib N, Khawaja H, Varner S, McCarthy M, Cambell. Cell Therapy for cardiovascular disease: A comparison of methods of delivery. J of Cardiovasc Trans Res. 2011; 4:177-81. ].
Using current techniques, delivery of stem cell-based biologics demonstrates variable retention rates, typically not exceeding 5% to 10% of the injected dose, regardless of the method of administration [88. Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198-202. , 99. Blocklett D, Toungouz M, Berkenboom G, Lambermont M, Unger P, Preumont N, Stoupel E, Egrise D, Degaute JP, Goldman M, Goldman S. Myocardial homing of nonmobilized peripheral-blood CD34+ cells after intracoronary injection. Stem Cells. 2006;24:333-6. , 1010. Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med. 2006;47:1295-301. , 1111. Schots R, De Keulenaer G, Schoors D, Caveliers V, Dujardin M, Verheye S, Van Camp G, Franken PR, Roland J, Van Riet I, Everaert H. Evidence that intracoronary- injected CD133+ peripheral blood progenitor cells home to the myhocardium in chronic postinfarction heart failure. Exp Hematol. 2007;35:184-9. , 1212. Penicka M, Lang O, Widimsky P, Kobylka P, Kozak T, Vanek T, Dvorak J, Tintera J, Bartunek J. One-day kinetics of myocardial engraftment after intracoronary injection of bone marrow mononuclear cells in patients with acute and chronic myocardial infarction. Heart. 2007;93:837-41. , 1313. Schächinger V, Aicher A Döbert N, Röver R, Diener J, Fichtischerer S, Assmus B, Seeger FH, Menzel C, Brenner W, Dimmeler S, Zeiher AM. pilot trial on determinants of progenitor cell recruitment to the infarcted myocardium. Circulation. 2008;118:1425-32.
This study is the most comprehensive study undertaken to date describing acute cell retention and kinetics after coronary cell delivery.] ( Table 1 ). Biodistribution is also variable, depending in part on the cell type [88. Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198-202. ], with potentially large numbers of cells finding their way to remote organs such as the lungs, liver or spleen. The consequences of extracardiac seeding of stem cells are not entirely clear and bio-vigilance monitoring has been incorporated in the development of stem-cell based interventions as was done earlier in traditional small molecule drug development. In addition, studies indicate a progressive decrease in myocardial signals after delivery of labelled stem cells [1010. Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med. 2006;47:1295-301. , 1212. Penicka M, Lang O, Widimsky P, Kobylka P, Kozak T, Vanek T, Dvorak J, Tintera J, Bartunek J. One-day kinetics of myocardial engraftment after intracoronary injection of bone marrow mononuclear cells in patients with acute and chronic myocardial infarction. Heart. 2007;93:837-41. , 1313. Schächinger V, Aicher A Döbert N, Röver R, Diener J, Fichtischerer S, Assmus B, Seeger FH, Menzel C, Brenner W, Dimmeler S, Zeiher AM. pilot trial on determinants of progenitor cell recruitment to the infarcted myocardium. Circulation. 2008;118:1425-32.
This study is the most comprehensive study undertaken to date describing acute cell retention and kinetics after coronary cell delivery.], a finding consistent with rapid cell death or washout within hours of administration. This limitation does not necessarily invalidate the efficacy of stem cells. It rather suggests that reparative mechanisms involve additional paracrine or immunomodulatory processes that may not require long term preservation of intact cells. The present chapter will review current techniques of cell delivery and outline the path for further development to improve cell retention and cell survival upon delivery. In Table 2 there is a summary of the current techniques for cell delivery. Cell therapy can be delivered indirectly by peripheral injection, or by mobilisation using cytokine therapy. Delivery into the heart is also possible, either via the coronary arteries, coronary sinus or direct intramyocardial injection. Here, we focus on the invasive percutaneous interventional techniques of coronary and direct myocardial cell delivery.
Cardiac stem cell therapy
- Cell therapy is an emerging therapy used as an adjunctive treatment after STEMI, chronic myocardial ischaemia and in patients with ischaemic or non-ischaemic congestive heart failure
- Factors affecting the final biological and functional therapeutic outcome are multiple and relate to patient-specific variables, such as functionality of autologous cells, choice of cell type or patient-independent parameters, such as stem cell processing
- Low rates of cell retention and variable biodistribution are common features of current clinical experience which will require attention in the future clinical adoption of cellular strategies
Percutaneous coronary cell delivery
A coronary therapeutic approach is aimed at augmenting endogenous myocardial salvage by facilitating the transendothelial passage of stem cells and their homing to the site of the injured myocardium. It is based on a combination of factors which increase myocardial cell retention after myocardial injury. They include cell adhesion onto the endothelial layer, transendothelial(vascular) passage and migration, and the presence of chemoattractive and adhesive forces in the myocardium, leading ultimately to cell retention in the perivascular region and within the myocardium itself. This technique has been the method of choice for nearly all studies in patients with STEMI. Similarly it has also been applied in a few studies in chronic myocardial ischaemia or ischaemic LV dysfunction. The success of coronary transfer requires that target tissue be subtended by an angiographically patent coronary artery or identifiable collateral vessel. The potential advantage of such coronary cell transfer is the homogenous distribution of the cells in the subtended target territory. The optimal time window for the coronary cells transfer depends on the balance between multiple putative and detrimental factors governing the infarction healing process. The balance appears to be most favourable between day 3 and 7 post-infarction [1414. Bartunek J, Wijns W, Heyndrickx G, Vanderheyden M. Timing of intracoronary bone-marrow-derived stem cell transplantation after ST-elevation myocardial infarction. Nat Clin Prac Cardiovasc Med. 2006;Suppl 1:S52-56. ]. However, the kinetics and timing of coronary arterial cell delivery have received little attention in preclinical studies and the transition from isolated small animal studies to humans has been rapid. Consequently, much of what is known regarding cell retention after intracoronary administration has been learned from clinical studies. These studies were associated with a positive functional outcome if cell injection was performed beyond day three [11. Abdel-Lattif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989-97. , 22. Lipinski MJ, Biondi-Zoccia GG, Abatte A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761-7.
Both references provide the meta-analyses of early clinical cell therapy trials. These analyses underline the safety of the cell therapy and point at the efficacy on the surrogate end-points after cell therapy.]. On the other hand, efficacy of coronary cell transfer in the later stages or in a chronic setting is still the matter of ongoing research and requires a better understanding of humorally mediated homing. Coronary cell transfer may be associated with a risk of microemboli in the case of large cells or highly viscous suspensions.
Guide catheters (6 Fr or 5 Fr) are preferred for selective cell infusion, given the calibre of their internal diameters (ID). The technique typically utilises off-the-shelf devices such as over-the-wire (OTW) angioplasty balloon catheters. The basic set-up is similar to balloon angioplasty with full anticoagulation as well as haemodynamic and ECG monitoring. The most commonly applied technique of coronary cell transfer is anterograde delivery using selective coronary injections.
Cells are injected through the central lumen of the delivery catheter whilst either maintaining coronary flow (non-occlusive) or interrupting it with balloon occlusion (“stop-flow” method). In both cases, care is taken so that the delivery catheter is flushed with saline prior to its use in order to prevent intraluminal thrombus formation. In the case of the non-occlusive method, an angioplasty balloon catheter (or a specialty catheter) is used for subselective injection into the coronary vessel. The position of the catheter is determined by the extent of the target territory. The internal lumen is connected at its proximal end to the syringe containing the cell product. The cell product is then introduced either as a continuous infusion or by repeated slow hand injections. In the case of hand injection, the cell product is loaded securely via a Luer lock using a small volume syringe, which thus allows for smooth and controlled injections.
Some more recent animal work suggests that continuous infusion may be more effective [1515. Doyle B, Kemp BJ, Chareonthaitawee P, Reed C, Schmeckpeper J, Sorajja P, Russell S, Araoz P, Riederer SJ, Caplice NM. Dynamic tracking during intracoronary injection of 18F-FDG-labeled progenitor cell therapy for acute myocardial infarction. J Nucl Med. 2007;48:1708-1714. ]. However, “stop-flow” is the predominant method in clinical trials. This method is preferred especially after myocardial infarction ( Figure 1 ). A balloon angioplasty OTW catheter is placed at the site of the stented segment at the location of the culprit lesion of the infarct-related coronary artery. Intracoronary nitrates should be administered prior to wire and balloon placement. Care should be taken to avoid an additional injury of the stented segment or surrounding vessel wall. Accordingly, balloon length should be smaller than the nominal length of the stent. The size should be comparable or it may be at most 0.5 mm larger than the nominal size of the implanted stent. Likewise, low pressure balloon inflations are applied that typically do not exceed the nominal inflation pressure. Before cell injection, test inflation followed by test contrast can be performed to document the coronary artery occlusion at the desired segment. Cell product can be loaded via 3-way stop-cock connected to both to a small volume Luer lock syringe (3 ml) and a larger Luer lock syringe (10 or 20 ml) The system is then attached to the proximal internal lumen of the OTW balloon catheter. After balloon inflation cells are injected typically in boluses of ~ 3 ml over ~ 1 minute period with a total occlusive time of 3 minutes. This timing is empirically chosen and is presumed to prolong the contact between infused cells and subtended endothelial surface of the coronary macro and microcirculation. Following a 3 minute period, reperfusion is allowed for 2 to 3 minutes. The “stop-flow” cycle is repeated until all the desired cell product is transferred. During balloon occlusion, the patient is monitored for signs of ischaemia, arrhythmias or haemodynamic instability. If severe arrhythmias, severe symptomatic ischaemia or major haemodynamic instability are observed during balloon inflation, the procedure should be interrupted and injections modified according to patient tolerance. At the end of the procedure, vessel patency and flow in the coronary artery are checked angiographically.
Addressing the challenge of low retention rates, a new coronary cell delivery method using the periadventitial space as target area was developed using a dedicated micro-infusion catheter (Cricket™; Mercator Medsystems Inc, San Leando, CA, USA). This is task specific catheter, tipped with a balloon-sheathed microneedle, and is guided and inflated in a manner similar to an angioplasty catheter, but with far lower expansion pressures. The closed balloon provides a protective covering for a tiny injection needle as it is guided safely through the vasculature. When the desired injection site is reached, the balloon is inflated with saline, securing the system for injection and sliding the microneedle through the vessel wall. This catheter has been used in at least one US-based clinical study on the injection of allogeneic biologics in patients with recent myocardial infarction. The potential advantage of the catheter is a high retention rate after delivering the biologics to the periadventitial space, which serves as a reservoir for the target area. A further advantage is that by avoiding coronary transfer it may be suitable for delivering biologics comprised of large cells. There is a concern regarding potential vessel damage in the presence of atherosclerotic lesions, nevertheless, initial experience so far has not revealed major clinical safety issues in the clinical setting (personal communication, Athersys).
In addition to antegrade coronary cell transfer, retrograde infusion of stem cells through the coronary sinus and coronary venous system has been achieved in experimental models [1616. Suzuki K, Murtuza B, Fukushima S, Smolenski RT, Varela-Carver A, Coppen SR, Yacoub MH. Targeted cell delivery into infarcted rat hearts by retrograde intracoronary infusion: distribution, dynamics, and influence on cardiac function. Circulation. 2004;110:II225-II230. ]. Theoretically this approach should also be safer than intracoronary or intramyocardial injection. There have been sporadic case reports demonstrating the safety of this technique [1717. Murad-Netto S, Moura R, Romeo LJ, Manoel NA, Duarte N, Barreto F, Jensen A, Vina RF, Vraslovik F, Oberdan A, Benetti F, Saslavsky J, Vina MF, Amino JG. Stem cell therapy with retrograde coronary perfusion in acute myocardial infarction. A new technique. Arq Bras Cardiol. 2004;83:352-4. ] but so far there have not been any published clinical trials. In this procedure, the coronary sinus is cannulated using a guidewire and an angioplasty balloon is advanced to the selected cardiac vein related to the coronary artery territory to be treated. Venous blood flow is then stopped, by prolonged balloon inflation for approximately 15 minutes, while the stem cells are infused under pressure. There is, as yet, no clinical efficacy data regarding this technique and the main limitations to this approach are the technical difficulties related to the more variable coronary venous anatomy (as compared to the coronary arterial tree) which can at times be difficult to negotiate with guidewires and balloons. This technique may not be possible in patients who already have a coronary sinus device in situ, such as a LV lead for resynchronisation therapy or a coronary sinus annuloplasty device, although cells could be administered as an adjunct to the implantation of such devices.
Coronary cell delivery
- Coronary cell transfer using the “stop-flow” method is the preferred method of delivery early after STEMI
- The optimal time window for coronary delivery depends on the balance between multiple putative and detrimental factors governing the infarction healing which appears to be most favourable between day 3 and 7 after the infarction
- Over-the-wire balloon catheter are the most widely used device for cell transfer
- New devices such as an infusion catheter allowing the periadventitial cell delivery may offer higher retention rates. Early experience suggest a favourable safety profile of the device
- The clinical efficacy of retrograde coronary venous infusion remains undetermined
Percutaneous intramyocardial cell delivery
Direct myocardial (intramyocardial) injection is the preferred choice of cell delivery in the setting of ischaemic heart failure or chronic myocardial ischaemia. It can be performed transepicardially, typically as adjunct to coronary bypass surgery, or percutaneously with endoventricular injection as a standalone procedure. The percutaneous approach can be performed using trans-coronary-venous intramyocardial (TVIM) injection or using the endoventricular approach. Trans-coronary-venous intramyocardial (TVIM) injection can be performed using the TransAccess™ catheter system (Transvascular Inc., Menlo Park, CA, USA) which is placed percutaneously into the coronary sinus and target vein. Then, using intravascular ultrasound (IVUS) guidance, a 24-gauge nitinol needle is extended to perform transvenous myocardial puncture. A 27-guage microinfusion catheter (IntraLume™; Medtronic Vascular, Inc., Santa Rosa, CA, USA) is then advanced though the needle to the targeted areas for cell delivery. Initial studies have confirmed the feasibility and safety of this approach in porcine models [1818. Thompson CA, Nasseri BA, Makower J, Houser S, McGarry M, Lamson T, Pomerantseva I, Chang JY, Gold HK, Vacanti JP, Oesterle SN. Percutaneous transvenous cellular cardiomyoplasty. A novel nonsurgical approach for myocardial cell transplantation. J Am Coll Cardiol. 2003;41:1964-71. ]. A Phase 1 clinical trial has confirmed the safety and efficacy of this delivery method of skeletal myoblasts for the treatment of chronic ischaemic LV systolic dysfunction [1919. Siminiak T, Fiszer D, Jerzykowska O, Grygielska B, Rozwadowska N, Kalmucki P, Kurpisz M. Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: the POZNAN trial. Eur Heart J. 2005;26:1188-95. ]. Furthermore, an animal study has demonstrated that the TVIM injection of BM derived mononuclear cells may lead to a greater retention of the cellular product at the target area as compared to intracoronary infusion [2020. George JC, Goldberg J, Joseph M, Abdulhameed N, Crist J, Das H, Pompili VJ. Transvenous intramyocardial cellular delivery increases retention in comparison to intracoronary delivery in a porcine model of acute myocardial infarction. J Interv Cardiol. 2008;21:424-31. ]. A theoretical advantage over the transendocardial approach, in which cells are injected perpendicularly into the left ventricular wall, is that the TVIM approach allows parallel cell injection which may result in greater cell retention [2121. Perin EC, Lopez J. Methods of stem cell delivery in cardiac diseases. Nat Clin Pract Cardiovasc Med. 2006;3:S110-S113. ]. The main limitations to this approach are related due to variable coronary venous anatomy which can at times be challenging to access with guidewires, thus making TVIM one of the more technically demanding procedures in this field.
Percutaneous 8Fr femoral arterial access with retrograde left ventricular approach (with the catheter passing the aortic valve into the left ventricle) is currently the preferred choice of direct myocardial delivery. This approach can also be applied safely to high risk patients without the need for general anaesthesia or sternotomy by targeting the injections into the desired myocardial areas and, in principle, may also allow a repeat injection as part of a serial treatment strategy. However, the technique requires training. There is a learning curve which in early cases may result in an uneven distribution of the cell injections and is associated with, an albeit minimal, risk of perforation or tissue injury. In addition, the percutaneous myocardial delivery requires either fluoroscopic or dedicated three-dimensional imaging guidance.
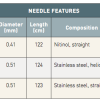
Three injection catheters have been used so far in clinical trials of cell delivery ( Table 3 ): the Helix™ infusion catheter (BioCardia Inc., San Francisco, CA, USA), MyoCath™ (Bioheart Inc., Sunrise, FL, USA) and MyoStar™ (Biologics Delivery Systems, Diamond Bar, CA, USA). BioCardia’s Helix bio-therapeutic delivery catheter system ( Figure 2 ) consists of a helical 25 gauge needle, expandable up to 4 mm. Under fluoroscopic guidance, the system is advanced to the left ventricle over the guidewire with the Morph™ guide catheter (BioCardia Inc.). The deflectable system allows three degrees of freedom to facilitate operator control. The helical, corkscrew needle is rotated into the heart tissue to provide active fixation during therapeutic delivery, similar to the active fixation electrodes used in cardiac pacing. The position and fixation of the needle is checked by injecting small bolus of a contrast through the second barrel contrast port located at the base of the helical needle.
The MyoCath™ is an injection catheter with a deflectable tip, a single port and a 25 gauge needle, which is expandable up to 6 mm. The catheter is advanced with the needle in the retracted position and the tip deflected. Both positioning and injections are performed under fluoroscopic guidance. As an alternative to fluoroscopic guidance, image fusion-based guidance or 3D echocardiography is currently being tested and may allow for real-time navigation.
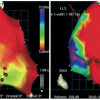
The MyoStar™ catheter is integrated with the NOGA® XP navigational system of electromechanical guidance (Cordis, Johnson & Johnson, Warren, NJ, USA). Cell delivery is performed after obtaining a diagnostic endocardial map of the left ventricle. The NOGA® XP System produces a 3D electromechanical map by analysing parameters generated from intracardiac electrograms acquired at multiple endocardial locations which characterise mechanical, dynamic and electrical functions of the mapped cardiac chamber ( Figure 3 and Figure 4 ). Both diagnostic (NOGASTAR®; Cordis, Johnson & Johnson) and injection catheters can be manipulated with six degrees of freedom (x, y, z, roll, pitch and yaw) within the left ventricle. In the first step, data are gathered from the location sensor and the electrical signal from the tip electrode of the diagnostic catheter. The system then measures the position and distance of each point in relation to the other points, as well as local electrical activity and mechanical movement associated with each point. The derived data generated can determine the end-diastolic and end-systolic volumes and ejection fraction. In real-time, each acquired point provides data on the local activation time, unipolar and bipolar voltage amplitudes. Mechanical information is computed from the average change in distance between the index catheter point and points from end-diastole to end-systole, and presented as a linear local shortening (LLS). The map which is then constructed provides views from multiple positions, including a bull’s-eye segmental view used to review the even distribution of regional points. Volumetric and electromechanical data correlate with other imaging modalities such as perfusion or MRI studies. After a diagnostic map has been constructed, the target area is delineated based on the combined information from unipolar voltage and LLS data. Normal myocardium exhibits unipolar voltages greater than 10 to 12 mV and LLS above 9% to 10%. Akinetic regions with LLS < 2% to 3% and unipolar voltage lower than 4 mV are considered to represent the infarct scar. Generally, areas of reduced LLS with unipolar voltage in the range of 6 to 9 mV represent viable myocardium being the target of therapeutic intervention. Endoventricular injections are then performed with the MyoStar™ catheter, which retains the identical diagnostic properties but it is also equipped with a 27 gauge adjustable needle. Injections must be performed by a dedicated team, preferably with 2 operators and a technician behind the NOGA workstation. The needle length is adjusted on a proximal handle consisting of an adjustable injector thumb knob, a hub for connection with the injection syringe. During injections the quality criteria are set to ensure the optimal biologics delivery and retention. They include the catheter stability, confirmation of the unipolar voltage in the desired range. Each injection site is marked on the controlling map to ensure the even spread of the injections to the target area.
Though the navigation is designed to be non-fluoroscopic, x-ray guidance typically facilitates the inter-region manipulation of the catheter. To ensure accuracy, it is critical to avoid any positional shift of the heart during the procedure. Accordingly, deep sedation or even general anaesthesia is required. Navigation and accuracy can also be compromised by catheter-induced bundle branch block or variable cycle length. The diagnostic performance as well as the interventional guidance to establish electrical and mechanical thresholds can be difficult in large ventricles with severely depressed global function. In such cases, unipolar voltage thresholds to delineate viable areas may need to be reduced, often to 4 to 5 mV. Other limitations are the time needed to construct the diagnostic map prior to the injection and the use of 2 catheters for completing the procedure, driving up the cost of the cell therapy.
Endomyocardial delivery is being utilised in the growing number of cell therapy trials which will ultimately establish the procedural performance of the individual catheters [55. Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheter-based delivery of cells to the heart. Nat Clin Prac Cardiovasc Med. 2006;3:Suppl 1:S57-64. , 66. Bartunek J, Sherman W, Vanderheyden M, Fernandez-Aviles F, Wijns W, Terzic A. Delivery of biologics in cardiovascular regenerative medicine. Clin Pharmacol Ther. 2009;85:548-52.
A review article detailing issues with cell delivery in the clinical setting and a framework of future translational research aimed at improved cell retention and survival., 77. Dib N, Khawaja H, Varner S, McCarthy M, Cambell. Cell Therapy for cardiovascular disease: A comparison of methods of delivery. J of Cardiovasc Trans Res. 2011; 4:177-81. , 2222. Psaltis PJ, Zannettino AC, Gronthos S, Worthley SG. Intramyocardial navigation and mapping for stem cell delivery. J Cardiovasc Transl Res. 2010;3:135-46.
A detailed and educative description of endoventricular myocardial cell delivery using electromechanical guidance.]. In all catheters, the injection strategy and target areas should be well planned in advance using the available clinical information. The selection of the individual catheter and the size should be carefully chosen and periprocedural safety monitoring is mandatory. The injection technique is similar for catheters with manually retractable needles such as MyoCath™ or MyoStar™. The needle extension is performed swiftly after having made stable and perpendicular contact with the vessel wall as documented under fluoroscopic or electromechanical guidance. The injection of the biologics should be performed slowly to allow for the fluid absorption by the myocardium. The volume varies between 0.1 mL and 0.5 mL per injection with maximum of biologics up to 10 mL in some studies. After delivery of the designated volume per injection, the needle should remain settled for few seconds and retracted with some delay to prevent backflow and expulsion of the biologics due to myocardial squeezing. In all cases, post-procedural monitoring is comparable to other interventional procedures, including a recommended monitoring of cardiac enzymes.
Direct intramyocardial cell delivery
- Percutaneous endoventricular delivery, as a standalone procedure, can be used in ischaemic heart failure under fluoroscopic or electromechanical guidance
- Three injection catheters have been used so far in clinical trials of cell delivery: the Helix™ infusion catheter (BioCardia Inc., San Francisco, CA, USA) with a helical needle and MyoCath™ (Bioheart Inc.) and MyoStar™ (Biologics Delivery Systems) with a straight needle
- The injection strategy and target areas should be well planned in advance using the available clinical information
- Injections should be performed by a dedicated team after prior training. The needle extension must be performed swiftly after having established stable and perpendicular contact with the vessel wall as documented under fluoroscopic or electromechanical guidance. The injection of the biologics should be performed slowly to allow for the fluid absorption by the myocardium
Delivery of biologics and hurdles in clinical translation
Suboptimal engraftment and retention represents the Achilles’ heel in the clinical translation of the cell therapy. The avenues for the future development include the biology of the myocardial niche-cell and device-niche interaction [55. Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheter-based delivery of cells to the heart. Nat Clin Prac Cardiovasc Med. 2006;3:Suppl 1:S57-64. , 66. Bartunek J, Sherman W, Vanderheyden M, Fernandez-Aviles F, Wijns W, Terzic A. Delivery of biologics in cardiovascular regenerative medicine. Clin Pharmacol Ther. 2009;85:548-52.
A review article detailing issues with cell delivery in the clinical setting and a framework of future translational research aimed at improved cell retention and survival.] ( Figure 5 ). With regard to niche-cell interaction, chemoattractive forces are generally favourable for cells to adhere to vascular or structural myocardial compartments early after acute ischaemic injury. Yet, it is controversial as to whether these signals are present in the setting of remodelled and dysfunctional myocardium. A better understanding of the signalling profiles of diseased myocardium relative to the extent of remodelling and dysfunction [1313. Schächinger V, Aicher A Döbert N, Röver R, Diener J, Fichtischerer S, Assmus B, Seeger FH, Menzel C, Brenner W, Dimmeler S, Zeiher AM. pilot trial on determinants of progenitor cell recruitment to the infarcted myocardium. Circulation. 2008;118:1425-32.
This study is the most comprehensive study undertaken to date describing acute cell retention and kinetics after coronary cell delivery.] is important in predicting the amount of cell adhesion and migration likely to occur after stem cell administration. Armed with this information, efforts aimed at priming the tissue for more efficacious retention can be established, such as application of low energy shockwaves. Alternative strategies to augment cell retention such as by the pharmacological priming of the cells using genetic modifications or the use of resorbable scaffolds are being investigated. Besides enhancing the immediate engraftment, these strategies are also relevant with regard to improved cell survival, a major prerequisite for any sustained therapeutic benefit. Likewise, when considering the extent of the negative remodelling, including wall thinning, excessive fibrosis replacement integration of “non-cellular” components with biologics is likely to be critical to balance off a number of factors which adversely affect cell survival and retention. Cell scaffolding alone or in combination with various self-assembling matrices used as a delivery platform may add not only to the mechanistic support of cells or thinned tissue, but also could be helpful to enhance cell maturation by applying a multidimensional spatial relationship or by serving as a carrier of nutrients.
In the setting of chronically ischaemic myocardium, tissue heterogeneity due to fibrosis and non-viable myocardium profoundly affects cell-tissue-device interaction. The interplay between these components is variable and dependent on their unique properties and it is for the delivery system to assure effective administration of biologics with minimal trauma to cells or tissue. In this regard, mathematical modelling incorporating key mechanical facets of cell-tissue-device interaction may help to predict the spatio-temporal dynamics following myocardial cell delivery ( Figure 6 ) and define the optimal needle design. This strategy has recently been deployed (personal communication; Jean-Pierre Latere, Cardio3 BioSciences, Mont-Saint-Guibert, Belgium) and led to a novel needle design currently undergoing preclinical testing. Consistent with the mathematical modelling, the needle design promotes cells dispersion while limiting immediate washout. Likewise, in the context of cell-tissue-device interaction, better understanding is needed to define the optimal relationship between the dose, concentration and volume of biologics and maximal retention and therapeutic effect. It remains unclear what is the optimal cell dose/volume for individual and total number of injections and what number of injections may lead to better functional benefit without increasing the procedural risk. This may be critical for a proper definition of the dose-dependent response and answer the question of whether repeat injections to deliver the target dose of the biologics may be appropriate rather than a one-procedure treatment with injection of high dose/high volume biologic. In this regard, an experimental study has indicated that multisite injection with smaller volumes (‘microdepots’) resulted in superior efficacy as compared to delivery of the same dose but given in the smaller number of injections with large volume [2323. Ott HC, Kroes R, Bonaros B, Marksteiner R, Margreiter E, Schachner T, Laufer G, Hering S. Intramyocardial microdepot injection increases the efficacy of skeletal myoblast transplantation. Eur J Cardiothorac Surg. 2005;27:1017-21.
This experimental study demonstrates that multisite injection with smaller volumes of the biologic per individual injection is superior to injections of larger volumes despite an identical total dose.].
Moreover, the choice of appropriate imaging and guidance to the desired target myocardial areas remains to be further explored. Real time electromechanical guidance is currently the benchmark technology. However, MRI-guided approach has been successfully tested in animal models [2424. Dick AJ, Guttman MA, Raman VK, Peters DC, Pessanha BS, Hill JM, Smith S, Scott G, McVeigh ER, Lederman RJ. Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in swine. Circulation. 2003;108:2899-904. , 2525. Tomkowiak MT, Klein AJ, Vigen KK, Hacker TA, Speidel MA, Van Lysel MS, Raval AN. Targeted Transendocardial Therapeutic Delivery Guided by MRI-X-ray Image Fusion. Catheter Cardiovasc Interv. 2011;78:468-78. ] and has the capacity to enable disease-specific targeting. Advances in imaging technology such as image fusion, intracardiac ultrasound or other imaging modalities allowing 3D orientation, such as CT, are likely to be progressively explored to ensure precision in targeting in the designated myocardial areas.
Future development of cell delivery techniques
- Strategies to improve cell delivery and retention include better understanding and exploration of the myocardial niche-cell and device-niche interaction
- Tissue priming, or cell priming including the use of cell scaffolds, are under testing to improve cell retention and survival
- Mathematical modelling, hand in hand with experimental in vitro and in vivo testing, is a sound platform to validate various needle designs to improve cell retention
Conclusions
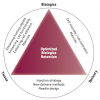
In summary, in an attempt to optimise the effects of stem cells, novel biologic products are being developed to increase the responsiveness of cells to tissue signalling and to minimise their susceptibility to attrition. Advances in stem cell delivery systems must keep pace if progress in cardiovascular regenerative medicine is to be assured. Development strategies should address the main aspects of delivery in a concerted manner, ensuring that integration of the three components of biologics-based tissue repair (underlying disease, cell preparation and delivery method) is preserved ( Figure 5 ) [55. Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheter-based delivery of cells to the heart. Nat Clin Prac Cardiovasc Med. 2006;3:Suppl 1:S57-64. , 66. Bartunek J, Sherman W, Vanderheyden M, Fernandez-Aviles F, Wijns W, Terzic A. Delivery of biologics in cardiovascular regenerative medicine. Clin Pharmacol Ther. 2009;85:548-52.
A review article detailing issues with cell delivery in the clinical setting and a framework of future translational research aimed at improved cell retention and survival., 77. Dib N, Khawaja H, Varner S, McCarthy M, Cambell. Cell Therapy for cardiovascular disease: A comparison of methods of delivery. J of Cardiovasc Trans Res. 2011; 4:177-81. ]. The interplay between these components is variable and dependent on their unique properties and it is for the delivery system to assure effective biologics administration, with minimal trauma to cells or tissue. Importantly, preclinical and clinical development will also depend on the interventional skills of the operators. Intramyocardial injection catheters function within a different framework than vascular devices and, while a fair body of experience exists, most have seen limited use in clinical trials to date. Even though none are overly demanding in concept or mechanics, learning curves are still being charted for all of them, especially for those in which advanced myocardial disease is being targeted [2626. Dib N, Menasche P, Bartunek JJ, Zeiher AM, Terzic A, Chronos NA, Henry TD, Peters NS, Fernández-Avilés F, Yacoub M, Sanborn TA, Demaria A, Schatz RA, Taylor DA, Fuchs S, Itescu S, Miller LW, Dinsmore JH, Dangas GD, Popma JJ, Hall JL, Holmes DR Jr; International Society for Cardiovascular Translational Research. Recommendations for successful training on methods of delivery of biologics for cardiac regeneration: a report of the International Society for Cardiovascular Translational Research. JACC Cardiovasc Interv. 2010;3:265-75.
This recommendation describes teaching objectives and requirements for training in cell delivery techniques.].
Personal perspective - Jozef Bartunek
Cardiac regeneration therapy is an emerging intervention that is based on sound biological concepts and experimental evidence and holds the promise to be a new generation-disruptive therapy for ischaemic heart failure. It is reassuring that early randomised controlled clinical trials demonstrate a favourable safety profile allowing further clinical development. The largest clinical experience has been gathered from cell delivery as an adjunct therapy early after reperfused STEMI and accumulating data in this setting does suggest a therapeutic benefit. Clinical experience in ischaemic heart failure is still limited to small studies. The importance of these early trials is in identification of the various factors that determine the therapeutic response and should be considered in the design of future larger trials. The cell delivery appears an “Achilles’ heel” in regenerative efforts. While advances in cell biology or non-cellular scaffolds are likely to propel further the regenerative strategies, cell delivery will need to follow. Current devices, though associated with a detectable therapeutic benefit, are also associated with low myocardial cell retention. Though this does not contradict the paracrine paradigm as the main mechanism underlying the therapeutic benefit, the efficacy of cell delivery needs to be improved to maximise the potential of various biologics. Though it should also be acknowledged that myocardial delivery will inevitably suffer from pitfalls related to unintentional injection into the myocardial vasculature associated with rapid washout, further development should focus on device improvement targets to increase the immediate retention without additional myocardial damage. The targeting and guidance of cell delivery is also suboptimal. Currently, electromechanical mapping offers the most comprehensive guidance of cell delivery including the identification of the designated areas, guidance and catheter steering as well as assurance of effective myocardial delivery. New imaging modalities such as MRI, CT or 3D echocardiography and their combination are being tested as alternative strategies. We are also falling short in understanding the optimal relationship between the dose, concentration and volume of biologics needed for the maximal therapeutic effect. It remains unclear what is the optimal cell dose/volume for individual or total number of injections, what number of injections may lead to better functional benefit without increasing the procedural risk. This is critical for the design of future clinical trials for the proper definition of the dose-dependent response as well as the question as to whether repeat injections to deliver the target dose of the biologics may be suitable rather than one-procedure treatment with injection of high dose/high volume biologic.
Online data supplement
MOVING IMAGE 1
NOGA guided delivery.
MOVING IMAGE 2
Reinjection part 1 , Reinjection part 2 and Reinjection part 3.