Basics
THE PRINCIPLE OF LASER ANGIOPLASTY
LASER stands for light amplification by stimulated emission of radiation. Laser energy is produced when an active medium (e.g., XeCl) is excited by electrical energy and emits monochromatic, coherent light. Laser energy can be emitted as a continuous or pulsed wave [66. Deckelbaum LI, Isner JM, Donaldson RF, Laliberte SM, Clarke RH, Salem DN. Use of pulsed energy delivery to minimize tissue injury resulting from carbon dioxide laser irradiation of cardiovascular tissues. J Am Coll Cardiol. 1986;7:898-908. ]. The only approved laser system for coronary application is a pulsed excimer (excited dimer) laser system utilising XeCl as the active medium with a wavelength of 308 nm. Excimer laser energy has been shown to ablate inorganic material by photochemical mechanisms that break molecular bonds without generation of excessive heat [77. Grundgest WS, Litvack F, Forrester JS, Goldenberg T, Swan HJ, Morgenstern L, Fishbein M, McDermid IS, Rider DM, Pacala TJ, et al. Laser ablation of human atherosclerotic plaque without adjacent tissue injury. J Am Coll Cardiol. 1985;5:929-33.
An essential resource to understand LASER therapy in atherosclerotic plaques., 88. Isner JM, Donaldson RF, Deckelbaum LI, Clarke RH, Laliberte SM, Ucci AA, Salem DN, Konstam MA. The excimer laser: gross, light microscopic and ultrastructural analysis of potential advantages for use in laser therapy of cardiovascular disease. J Am Coll Cardiol. 1985;6:1102-1109. ]. In vivo, these photochemical mechanisms, together with localised thermal and mechanical effects, result in an atheroablation which can be documented by intravascular ultrasound studies in humans [99. Honye J, Mahon DJ, Nakamura S, Wallis J, al-Zarka A, Saito S, Berns M, Tobis JM. Intravascular ultrasound imaging after excimer laser angioplasty. Cathet Cardiovasc Diagn. 1994;32:213-222. , 1010. Mintz GS, Kovach JA, Javier SP, Pichard AD, Kent KM, Poma JJ, Salter LF, Leon MB. Mechanisms of lumen enlargement after excimer laser coronary angioplasty: An intravascular ultrasound study. Circulation. 1995; 3408-3414. ]. ELCA has been shown to have no influence on immediate elastic recoil compared to PTCA [1111. Strikwerda S, van Swijndregt EM, Melkert R, Serruys PW. Quantitative angiographic comparison of elastic recoil after coronary excimer laser-assisted balloon angioplasty and balloon angioplasty alone. J Am Coll Cardiol. 1995;25:378-386. ].
During its technical evolution it was realised that ultraviolet laser energy is avidly absorbed in blood or contrast medium, inducing significant acoustic effects generating fast-expanding and imploding vapour bubbles which produce rapid microsecond dilatation followed by invagination of the adjacent arterial segment.
This can induce tissue disruption with extensive medial necrosis, intramural haemorrhage and subluminal infiltration of leucocytes ( Figure 1), resulting in dissections or even vessel perforations [1212. van Leeuwen TG, Meertens JH, Velema E, Post MJ, Borst C. Intraluminal vapor bubble induced by excimer laser pulse causes microsecond arterial dilation and invagination leading to extensive wall damage in the rabbit. Circulation. 1993;87:1258-63.
Animal work that explains the potential for dissections and perforations to occur as major complications during ELCA.]. Therefore, excimer laser coronary angioplasty (ELCA) should only be performed after displacement of blood or contrast medium from the coronary artery by a continuous saline flush during laser activation [1313. Deckelbaum LI, Natarajan MK, Bittl JA, Rohlfs K, Scott J, Chisholm R, Bowman KA, Strauss BH. Effect of intracoronary saline infusion on dissection during excimer laser coronary angioplasty: a randomized trial. The Percutaneous Excimer Laser Angioplasty (PELCA) Investigators. J Am Coll Cardiol. 1995; 26: 1264-9.
Study illustrating the importance of saline infusion during ELCA., 1414. Tcheng JE. Saline infusion in excimer laser coronary angioplasty. Semin Interv Cardiol. 1996;1:135-141. ]. Furthermore, pulsed energy delivery is used reduce laser-induced pathological tissue injury [1515. Deckelbaum LI, Isner JM, Donaldson RF, Clarke RH, Laliberte S, Aharon AS, Bernstein JS. Reduction of laser-induced pathologic tissue injury using pulsed energy delivery. Am J Cardiol. 1985;56:662-667. ].
Mechanisms of ELCA
- Photochemical mechanisms that ablate inorganic material
- Breaking of molecular bonds
- Generation of fast-expanding and imploding vapour bubbles creating dissections or even perforation especially when ELCA is activated in blood or contrast medium
- The Dotter effect
THE EXCIMER LASER SYSTEM AND CATHETERS
The CVX-300® ELCA system (Spectranetics Inc., Colorado Springs, CO, USA) utilises a XeCl LASER unit and laser catheters for coronary or peripheral application ( Figure 2).
The only currently available ELCA system for coronary application is supplied by the Spectranetics Corporation. Its laser unit uses XeCl as the active medium at a wavelength of 308 nm and it generates a catheter energy output up to 80 mJ/mm2 with a maximum repetition rate of 40 Hz, and a pulse width of 125 - 200 ns. This energy output is described by the term fluence. The system has a warm-up time of 5 minutes and requires calibration.
Laser catheters are available as over-the-wire or rapid exchange devices and consist of different amounts of 50 μm fibres, which are either concentrically or eccentrically ( Figure 3) arranged around a 0.014 inch guidewire (0.018 inch for the 2.0 mm eccentric laser catheter version).
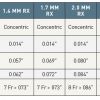
The 0.9 mm catheter has a maximum tip outer diameter of 0.038 inch and is compatible with 5 French (Fr) guide catheters, while the 1.4 mm catheter has a maximum tip outer diameter of 0.057 inch and requires a 7 Fr guide catheter. The 1.7 mm catheter has a maximum tip outer diameter of 0.069 inch and is still 7 Fr compatible whilst the 2.0 mm catheter with a maximum tip outer diameter of 0.079 to 0.080 requires an 8 Fr guide catheter. The recommended laser catheter size should not exceed 0.5 to 0.6 times the reference vessel diameter. Since ELCA catheters are contact lasers, the maximum tip outer diameter defines the maximum achievable lumen with a single passage. The eccentric versions of the ELCA catheters were developed to increase the size of ablation and to address eccentric coronary lesions. By fluoroscopically guided rotation of this eccentric version the operator can target the lasing part of the catheter tip towards the target plaque burden ( Figure 4). Despite being bulky in comparison to other coronary catheters the versions of ELCA catheter which are available provide a contemporaneous pushability and trackability. Table 1 summarises the data relating to ELCA catheters which are available for coronary application.
Further developments have included improvements in laser catheter design allowing a more homogenous distribution of light to reduce vessel wall trauma during ELCA [1616. Gijsbers GH, Hamburger JN, Serruys PW. Homogeneous light distribution to reduce vessel trauma during excimer laser angioplasty. Semin Interv Cardiol. 1996;1:143-148. ]. This achievement has been made possible by thicker laser fibres, as well as the exclusion of dead space around multiple fibres in the ELCA catheter. As a result, energy density can be reduced by 70% without losing efficacy in tissue crossing or penetration.
EXCIMER LASER ANGIOPLASTY PROTOCOL
Once there is an indication to use ELCA, the laser unit should be set up and the warm-up time started. Following the initial set-up,, the laser catheter with the intended size needs to be calibrated. During this calibration process laser eye protection glasses must be worn by the patient and all personnel in the catheter laboratory. This is not necessary during laser activation within the patient´s body.
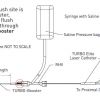
After a guide catheter with appropriate dimensions to accommodate the laser catheter size is positioned in the ostium of the target coronary artery, a 0.014 inch (or smaller) guidewire should be passed across the target lesion under fluoroscopic control in the conventional way. The laser catheter is then advanced over the wire to come into contact with the target lesion. Before laser activation, the guide catheter is flushed with at least 10 to 20 mls of saline to wash-out blood or contrast medium. The first passes should be performed with a fluence of 45 mJ/mm2 and a repetition rate of 25 Hz. If resistance occurs, fluence and repetition rates can be increased to a maximum fluence of 80 mJ/mm2 and a repetition rate of 80 Hz depending on the laser catheter used. The system automatically defines the maximum lasing period of 5 or 10 seconds followed by a 5 or 10 second regeneration period during which the system cannot be activated. During each lasing pass continuous saline infusion via the guide catheter at 1 to 2 ml per second is mandatory. When over-the-wire laser catheters are used, saline injection can be performed manually, or via an indeflator through the wire lumen if the guidewire size does not exceed 0.014 inch ( Figure 5). During laser activation the catheter is advanced gently, and slowly, at less than 0.5 to 1 mm per second, through the target lesion under fluoroscopic guidance. This slow advancement is necessary to allow for optimised tissue ablation, whilst if the catheter is advanced too fast there is almost a pure ‘’Dotter effect’’ (mechanical displacement of tissue). The operator should start and prematurely terminate the laser pass if necessary while allowing another person to activate fluoroscopy. Larger laser catheters, including the eccentric versions, can be used to increase the post-ablation lumen, while some push or pull on the guidewire with subsequent passes of the same catheter size can also result in a modest increase in lumen.
When ELCA is finished the intervention can be completed, typically by additional balloon angioplasties or stent implantation if indicated.
Essentials of the ELCA procedure
- Obtain good guide catheter support
- Choose guide catheter sizes according to the planned ELCA catheter size
- Secure correct guidewire positioning in the vessel lumen
- Allow a laser unit warm-up time of five minutes
- Choose ELCA catheter diameter to vessel diameter ratio of 0.5 to 0.6
- Flush guide catheter with at least 10 to 20 mls saline before laser activation
- Start with low fluence of 45 mJ/mm2 at a repetition rate of 25 Hz and increase if necessary
- During laser activation flush guide catheter with 1 to 2 ml per second
- During laser activation advance ELCA catheter slowly (0.5 to 1 mm per second)
ADJUNCTIVE PHARMACOTHERAPY
Patients undergoing ELCA should be pre-treated with dual antiplatelet therapy as per ESC guidance, including aspirin (100 to 500 mg) and clopidogrel (300 or 600 mg loading dose, 75 mg maintenance dose), at least 6 hours (in elective patients) before the procedure. In primary PCI, including ELCA for the treatment of ST elevation or non-ST elevation myocardial infarction, ESC guideline recommended dual antiplatelet loading applies. Newer and more potent antiplatelet therapies, including glycoprotein IIb/IIIa inhibitors and prasugrel or ticagrelor, can be administered as indicated although no particular data are available for these drugs in the context of ELCA. At the time of the procedure, unfractionated heparin is administered to achieve a target activated clotting time (ACT) of 300 sec. A liberal use of intracoronary nitrate is recommended to avoid periprocedural coronary vasospasm. In general, there is no specific adjunctive pharmacotherapy required when ELCA is attempted. The essential role of a continuous saline flush during laser application has been emphasised before.
Clinical studies with ELCA
After several small observational studies documented the feasibility of ELCA in the treatment of stable coronary lesions with procedural success rates of about 90% [1717. Strikwerda S, Montauban van Swijndregt E, Foley DP, Boersma E, Umans VA, Melkert R, Serruys PW. Immediate and late outcome of excimer laser and balloon coronary angioplasty: a quantitative angiographic comparison based on matched lesions. J Am Coll Cardiol. 1995;26:939-946. ], two randomised trials were conducted to compare ELCA with PTCA. In the multicentre AMRO study [1818. Appelman YE, Piek JJ, Strikwerda S, Tijssen JG, de Feyter PJ, David GK, Serruys PW, Margolis JR, Koelemay MJ, Montauban van Swijndrecht EW, Koolen JJ. Randomised trial of excimer laser angioplasty versus balloon angioplasty for treatment of obstructive coronary artery disease. Lancet. 1996;347:79-84.
Seminal randomised study comparison of ELCA versus PTCA.], 308 patients with stable angina were found to have no significant differences in angiographic success (79% versus 80%), however there was a 10-fold higher transient occlusion rate in the ELCA group (0.7% versus 7%) in periprocedural complications, and a trend towards higher restenosis rate in the ELCA group (41.3% versus 51.6%) at 6 months, but no difference in angiographic net lumen gain (0.40 mm versus 0.48 mm). Patients with tandem lesions, lesions in vessels < 2.5 mm, or lesion location in the left circumflex coronary artery appear to have significantly poorer outcomes with ELCA compared to PTCA [1919. Appelman YE, Piek JJ, Redekop WK, de Feyter PJ, Koolen JJ, David GK, Strikwerda S, Tijssen JG, Serruys PW, van Swijndregt E, van Gemert MJ, Lie KI. Clinical events following excimer laser angioplasty or balloon angioplasty for complex coronary lesions: subanalysis of a randomised trial. Heart. 1998;79:34-38. , 2020. Appelman YE, Piek JJ, van der Wall EE, Redekop WK, van Royen EA, Fioretti PM, de Feyter PJ, Koolen JJ, Strikwerda S, Serruys PW, David GK, Tijssen JG, Lie KI. Evaluation of the long-term functional outcome assessed by myocardial perfusion scintigraphy following excimer laser angioplasty compared to balloon angioplasty in longer coronary lesions. Int J Card Imaging. 2000;16:267-277. ]. The ERBAC study [2121. Reifart N, Vandormael M, Krajcar M, Göhring S, Preusler W, Schwarz F, Störger H, Hofmann M, Klöpper J, Müller S, Haase J. Randomized comparison of angioplasty of complex coronary lesions at a single center, Excimer Laser, Rotational Atherectomy and Balloon Angioplasty Comparison (ERBAC) Study. Circulation. 1997;96:91-98.
A comparison of ELCA, PTCA and rotational atherectomy in complex coronary lesions.] was originally a three arm trial comparing PTCA with ELCA and rotational atherectomy in 685 patients with stable angina. Procedural success rates were comparable, with 77% and 80% between the ELCA and PTCA groups as well as in-hospital complication rates of 4.3% and 3.1%. The periprocedural cross-over rate was almost three times higher with ELCA (5.0% versus 15.1%). At 6-month follow-up the target vessel revascularisation rate was significantly higher in the ELCA group compared to the PTCA group (46.0% versus 31.9%). The results of both studies must be interpreted bearing in mind that saline flushing at that time was not part of the lasing protocol.
In a large registry of 9,222 patients with different percutaneous coronary interventional techniques, including 500 patients with laser angioplasty and 4,104 patients with PTCA, a significantly higher likelihood of developing restenosis with an odds ratio of 1.55 was documented [2222. Bittl JA, Chew DP, Topol EJ, Kong DF, Califf RM. Meta-analysis of randomized trials of percutaneous transluminal coronary angioplasty versus atherectomy, cutting balloon atherectomy, or laser angioplasty. J Am Coll Cardiol. 2004;43:936-42.
A meta-analysis comparing safety and outcome of ELCA versus PTCA, atherectomy, or cutting balloon angioplasty.].
Since these results did not support the broad use of ELCA and in parallel, coronary stents showed better angiographic, acute, and long-term clinical results compared to PTCA, the application of ELCA was limited to certain subgroups of lesions despite technological and procedural improvement of this technology.
Lesion subsets
THE UNDILATABLE LESION
In some lesions resistance is so high that an adequately sized balloon is not able to expand completely despite high pressures of up to 20 atm or more ( Figure 6). These so-called undilatable lesions are a target for ELCA [2323. Amed WG, Al-Anazi MM, Bittl JA. Excimer laser-facilitated angioplasty for undilatable coronary narrowings. Am J Cardiol. 1996;78:1045-1047. , 2424. Bilodeau L, Fretz EB, Taeymans Y, Koolen J, Taylor K, Hilton DJ. Novel use of a high-energy excimer laser catheter for calcified and complex coronary artery lesions. Catheter Cardiovasc Interv. 2004;62:155-61. ]. It is not only the ablative aspect of ELCA but also the creation of fast-expanding and imploding vapour bubbles, despite saline flush, that create microfissures to the obstructive plaque which allow complete balloon inflation during post-dilatation. Without such forms of technology, PCI of undilatable lesions would not be possible. Thus ELCA competes for this indication with rotational atherectomy or cutting-balloon catheters.
CHRONIC TOTAL CORONARY OCCLUSIONS
Even after successful wire passage across a chronic total coronary occlusion (CTO), PCI is sometimes limited similarly by the inability to pass with a balloon catheter . In this particular setting ELCA is a valuable option since, in contrast to rotational atherectomy, it can be advanced along standard guidewires ( Figure 7 parts 1 and 2). The laser can create a channel through the CTO which allows a balloon catheter to pass afterwards in order to facilitate final vessel reconstruction by stent implantation [2424. Bilodeau L, Fretz EB, Taeymans Y, Koolen J, Taylor K, Hilton DJ. Novel use of a high-energy excimer laser catheter for calcified and complex coronary artery lesions. Catheter Cardiovasc Interv. 2004;62:155-61. , 2525. Gruberg L, Mehran R, Dangas G, Hong MK, Mintz GS, Kornowski R, Lansky AJ, Kent KM, Pichard AD, Satler LF, Stone GW, Leon MB.. Effects of plaque debulking and stenting on short term and long-term outcomes after revascularization of chronic total occlusions: comparison of debulking (laser or RCA) plus stenting alone in the treatment of chronic total occlusions. J Am Coll Cardiol. 2000;35:151-156. ]. Meticulous attention should be paid to a correct distal intraluminal wire position before ELCA is performed in order to avoid perforations.
A subgroup analysis of the randomised AMRO trial in patients with functional or total coronary occlusions >10 mm evaluated the safety and efficacy of ELCA plus PTCA versus PTCA alone [2626. Appelman YE, Koolen JJ, Piek JJ, Redekop WK, de Feyter PJ, Strikwerda S, David GK, Serruys PW, Tijssen JG, van Swijnregt E, Lie KI. Excimer laser angioplasty versus balloon angioplasty in functional and total coronary occlusions. Am J Cardiol. 1996;78:757-762. ]. Angiographic success rates were similar in both groups at 65% and 61%. Net lumen gain at six months was also not significantly different (0.81±0.74 mm versus 1.04±0.68 mm), whilst there was at least a trend towards a lower restenosis rate in the PTCA group alone (48.5% versus 66.7%; p=0.15).
A special 0.018 inch laser wire was introduced to address those CTOs which cannot be passed with standard or dedicated crossing wires [2727. Hamburger JN, Gijsbers GH, Ozaki Y, Ruygrok PN, de Feyter PJ, Serruys PW. Recanalization of chronic total coronary occlusions using a laser guide wire: a pilot study. J Am Coll Cardiol. 1997;30:649-656. , 2828. Hamburger JN, Serruys PW, Scabra-Gomes R, Simon R, Koolen JJ, Fleck E, Mathey D, Sievert H, Rutsch W, Buchwald A, Marco J, Al-Kasab SM, Pizulli L, Hamm C, Corcos T, Reifart N, Hanrath P, Taeymans Y. Recanalization of total coronary occlusions using a laser guidewire (the European TOTAL Surveillance Study). Am J Cardiol. 1997;80:1419-1423. ]. This wire was quite rigid and wire exits during the crossing attempt were not infrequent.
In the Total Occlusion Trial with Angioplasty by using Laser guidewire (TOTAL), 303 patients with CTOs were randomly assigned to the laser guidewire or conventional guidewires [2929. Serruys PW, Hamburger JN, Koolen JJ, Fajadet J, Haude M, Klues H, Seabra-Gomes R, Corcos T, Hamm C, Pizulli L, Meier B, Mathey D, Fleck E, Taeymans Y, Melkert R, Teunissen PW, Simon R. Total occlusion trial with angioplasty by using laser guidewire. The TOTAL trial. Eur Heart J. 2000;21:1797-805.
A multicentre trial on the use of a LASER wire for the recanalisation of chronic total coronary occlusions.]. Treatment success as the primary endpoint, defined as successful crossing within 30 minutes of fluoroscopic time, was not different between both arms (52.8% in the laser wire group versus 47.2% with conventional wires). Angiographic and clinical results up to 12 months were not different with 79% of patients receiving a stent. This device is no longer available and has been replaced by dedicated mechanical crossing wires and techniques [3030. García-García HM, Kukreja N, Daemen J, Tanimoto S, van Mieghem C, Gonzalo N, van Weenen S, van der Ent M, Sianos G, de Feyter P, Serruys PW. Contemporary treatment of patients with chronic total occlusion: critical appraisal of different state-of-the-art techniques and devices. EuroIntervention. 2007;3:188-196. ].
Despite a lack of data supporting the broad use of ELCA for this indication, it can still be a valuable option for patients in whom conventional techniques failed to recanalise a CTO [3131. Shen ZJ, García-García HM, Schultz C, van der Ent M, Serruys PW. Crossing of a calcified “balloon uncrossable” coronary chronic total occlusion facilitated by a laser catheter: a case report and review recent four years’ experience at the Thoraxcenter. Int J Cardiol. 2010;145:251-254. ].
IN-STENT RESTENOSIS
The development of in-stent restenosis after the implantation of bare metal stents occurs in 10% to 20% of treated lesions. Long stented segments especially have a higher likelihood of developing in-stent restenosis. ELCA has been tested in this setting as a treatment option to debulk first before adjunctive PTCA in contrast to PTCA alone [3232. Mehran R, Mintz GS, Satler LF, Pichard AD, Kent KM, Bucher TA, Popma JJ, Leon MB. Treatment of in-stent restenosis with excimer laser coronary angioplasty: mechanisms and results compared with PTCA alone. Circulation. 1997; 96: 2183-2189. , 3333. Köster R, Hamm CW, Seabra-Gomes R, Hermann G, Sievert H, Macaya C, Fleck E, Fischer K, Bonier JJ, Fajadet J, Waigand J, Kuck KH, Henry M, Morice MC, Pizulli L, Webb-Peploe MM, Buchwald AB, Ekström L, Grube E, Al Kasab S, Colombo A, Sanati A, Ernst SM, Serruys PW. Laser angioplasty of restenosed coronary stents: results of a multicenter surveillance trial. The Laser Angioplasty of Restenosed Stents (LARS) Investigators. J Am Coll Cardiol. 1999; 34: 25-32. , 3434. Hamburger JN, Foley DP, de Feyter PJ, Wardeh AJ, Serruys PW Six-month outcome after excimer laser coronary angioplasty for diffuse in-stent restenosis in native coronary arteries. Am J Cardiol. 2000; 86: 390-394. , 3535. Köster R, Kähler J, Terres W, Reimers J, Baldus S, Hartig D, Berger J, Meinertz T, Hamm CW. Six-month clinical an angiographic outcome after successful excimer laster angioplasty for in-stent restenosis. Am J Coll Cardiol. 2000; 36: 69-74. , 3636. Dahm JB, Kuon E, Vogelgesang D, Hummel A, Möx B, Staudt A, Felix SB. Relation of degree of laser debulking of in-stent restenosis as a predictor of restenosis rate. Am J Cardiol. 2002; 90: 68-70. , 3737. Dahm JB, Kuon E. High-energy eccentric excimer laser angioplasty for debulking diffuse in-stent restenosis leads to better acute and 6-month follow-up results. J Invasive Cardiol. 2000; 12: 335-342. ]. Figure 8 illustrates an example. Intravascular ultrasound-based data documented effective ablation of neointimal tissue [3232. Mehran R, Mintz GS, Satler LF, Pichard AD, Kent KM, Bucher TA, Popma JJ, Leon MB. Treatment of in-stent restenosis with excimer laser coronary angioplasty: mechanisms and results compared with PTCA alone. Circulation. 1997; 96: 2183-2189. ]. Nevertheless, acute procedural as well as long-term angiographic and clinical results for ELCA plus PTCA were not superior to the PTCA alone strategy.
In a meta-analysis of 3,012 patients with in-stent restenosis, 474 patients receiving ELCA as the treatment presented the highest probability, 34.8%, to develop major cardiac events (death, myocardial infarction or revascularisation) at follow-up [3838. Radke PW, Kaiser A, Frost C, Sigwart U. Outcome after treatment of coronary in-stent restenosis: results from a systematic review using meta-analysis techniques. Eur Heart J. 2003;24:266-73.
A meta-analysis comparing different interventional techniques(including ELCA) for the treatment of in-stent restenosis.].
Today, ELCA is rarely used even in diffuse in-stent restenosis with a large neointimal burden. Drug-eluting stents, or more recently, drug-eluting balloons have shown superior angiographic and clinical results for the treatment of this condition compared to PTCA alone (  View chapter and
View chapter and  View chapter ).
View chapter ).
This is underlined in the most recent guidelines on myocardial revascularisation of the European Society of Cardiology, where ELCA is cited explicitly as not being useful for the treatment of in-stent restenosis [3939. Wijns W, Kohl P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D; ESC Committee for Practice Guidelines, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P; EACTS Clinical Guidelines Committee, Kolh P, Alfieri O, Dunning J, Elia S, Kappetein P, Lockowandt U, Sarris G, Vouhe P, Kearney P, von Segesser L, Agewall S, Aladashvili A, Alexopoulos D, Antunes MJ, Atalar E, Brutel de la Riviere A, Doganov A, Eha J, Fajadet J, Ferreira R, Garot J, Halcox J, Hasin Y, Janssens S, Kervinen K, Laufer G, Legrand V, Nashef SA, Neumann FJ, Niemela K, Nihoyannopoulos P, Noc M, Piek JJ, Pirk J, Rozenman Y, Sabate M, Starc R, Thielmann M, Wheatley DJ, Windecker S, Zembala M. Guidelines on myocardial revascularization. The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2010; 31: 2501–2555. ].
THROMBUS-CONTAINING LESION
Experimental work has shown that ultraviolet excimer laser fluence at a 308 nm wavelength decreases platelet aggregation and reduces platelet force development capability [4040. Topaz O, Minisi A J, Morris C, Mohanty PK and Carr ME Jr. Photoacoustic fibrinolysis: pulsed-wave, mid-infrared laser-clot interaction. J. Thrombosis Thrombolysis. 1996;3:209-214. , 4141. Topaz O, Morris C, Minisi AJ, Mohanty PK and Carr ME Jr. Enhancement of t-PA induced fibrinolysis with laser energy: in-vitro observations. Lasers Med. Sci. 1999;14:123-128. ]. In addition to its ablative properties, ELCA has been used to address thrombus-containing lesions primarily in patients with acute coronary syndromes [4242. Topaz O, Bernardo N L, Shah R, McQueen R, Desai P, Janin Y, Lansky AJ and Carr ME Jr. Effectiveness of excimer laser coronary angioplasty in acute myocardial infarction or in unstable angina pectoris. Am J Cardiol. 2001;87:849-855. , 4343. Topaz O, Ebersole D, Das T, Alderman EL, Madyoon H, Vora K, Baker JD, Hilton D, Dahm JB. Excimer laser angioplasty in acute myocardial infarction (the CARMEL multicenter trial). Am J Cardiol. 2004;93:694-701.
Clinical evaluation of ELCA for the treatment of acute myocardial infarction., 4444. Dahm JB, Ebersole D, Das T, Madyoon H, Vora K, Baker J, Hilton D, Topaz O. Prevention of distal embolization and no-reflow in patients with acute myocardial infarction and total occlusion in the infarct-related vessel: a subgroup analysis of the cohort of acute revascularization in myocardial infarction with excimer laser-CARMEL multicenter study. Catheter Cardiovasc Interv. 2005; 64:67-74. , 4545. Dorr M, Vogelgesang D, Hummel A, Staudt A, Robinson DM, Felix SB, Dahm JB. Excimer laser thrombus elimination for prevention of distal embolization and no-reflow in patients with acute ST elevation myocardial infarction Results from the randomized Laser AMI study. Int J Cardiol. 2007; 116: 20-26. , 4646. LarosaC, Ricco A, Cosentino N, Marino M, Mongiardo R, Niccoli G. A case of very late thrombosis of bare metal stent successfully treated with Excimer Laser Coronary Angioplasty. Int J Cardiol. 2010.145:e60-63. , 4747. Ambrosini V, Cioppa A, Salemme L, Tessorino T, Sorropago G, Popusoi G, Stabile E, Medolla A, Cangella F, Agrusta M, Picano E, Rubino P. Excimer laser in acute myocardial infarction: single centre experience on 66 patients. Int J Cardiol. 2008;127:98-102. ]. In the CARMEL trial, ELCA showed a significant mean increase in TIMI flow from 1.2 at baseline to 2.8. The same finding was observed in the subgroup of patients with very large thrombus burden [4444. Dahm JB, Ebersole D, Das T, Madyoon H, Vora K, Baker J, Hilton D, Topaz O. Prevention of distal embolization and no-reflow in patients with acute myocardial infarction and total occlusion in the infarct-related vessel: a subgroup analysis of the cohort of acute revascularization in myocardial infarction with excimer laser-CARMEL multicenter study. Catheter Cardiovasc Interv. 2005; 64:67-74. ]. Nevertheless, laser-induced major dissections occurred in 3% despite saline flushing, whilst laser-induced distal embolisation or no reflow were rare at 0.6% and 0.6% respectively. In a small randomised trial of 27 patients with ST segment elevation, myocardial infarction ELCA as an adjunct to PTCA plus stenting did not impact post-procedural TIMI flow or surrogate parameters of microvascular perfusion such as myocardial blush score or final corrected TIMI frame count [4545. Dorr M, Vogelgesang D, Hummel A, Staudt A, Robinson DM, Felix SB, Dahm JB. Excimer laser thrombus elimination for prevention of distal embolization and no-reflow in patients with acute ST elevation myocardial infarction Results from the randomized Laser AMI study. Int J Cardiol. 2007; 116: 20-26. ].
SAPHENOUS VEIN GRAFT LESIONS
In an observational study ELCA alone (17%) or as an adjunct to PTCA (83%) was tested in the treatment of 106 patients with 125 saphenous vein graft bypass (SVG) lesions [4848. Strauss BH, Natarajan MK, Batchelor WB, Yardley DE, Bittl JA, Sanborn TA, PowerJA, Watson LE, Moothart R, Tcheng JE. Early and late quantitative angiographic results of vein graft lesions treated by excimer laser with adjunctive balloon angioplasty Circulation. 1995;92:348-356. ]. Despite angiographic improvement in lesion dimensions, periprocedural dissections occurred in 45% of lesions after ELCA treatment, including graft occlusion in 7%. The angiographic success rate was 54% after ELCA which increased to 91% after adjunct PTCA. In-hospital rates of death, myocardial infarction or bypass surgery were 0.9%, 4.5% and 0.9% respectively. At follow-up, the restenosis rate was 52%, including graft occlusions in 24%. Event-free survival was 48.2% at 1 year with a mortality rate of 8.6%.
Similar angiographic results were documented in a matched control study of ELCA plus PTCA versus PTCA alone in SVG lesions with no difference in acute recoil or acute lumen gain [4949. Natarajan MK, Bowman KA, Chisholm RJ, Adelman AG, Isner JM, Chokshi SK, Strauss BH. Excimer laser angioplasty vs. balloon angioplasty in saphenous vein bypass grafts: quantitative angiographic comparison of matched lesions. Cathet Cardiovasc Diagn. 1996;38:153-158. ]. Even in the subgroup of patients with aorto-ostial SVG lesions, ELCA plus PTCA did not show superior angiographic or clinical results over PTCA alone [5050. Ahmed JM, Hing MK, Mehran R, Mintz GS, Lansky AJ, Pichard AD, Satler LF, Kent KM, Wu H, Stone GW, Leon MB. Comparison of debulking followed by stenting versus stenting alone for saphenous vein graft aortoostial lesions: immediate and one-year clinical outcomes. J Am Coll Cardiol. 2000;35:1560-1568. ].
Consequently, it may be argued that supportive angiographic or clinical data is lacking for a broad use of ELCA in the setting of SVG lesions.
Currently, distal or proximal protection devices have gained broad acceptance as a means of avoiding embolisation in this lesion subset. The potential of ELCA in this context has never been tested against these protection devices.
Potential indications for ELCA
- The undilatable lesion
- CTO with successful wire crossing
- (diffuse) in-stent stenosis
- Thrombus-containing lesions
- Saphenous vein graft lesions